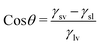Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biological design and inspiration of bactericidal hierarchical interfaces
Mahreen
Muneer
ab,
Hariprasad Parayil
Kalappurackal
 bc,
Akshay
Balachandran
bc,
Akshay
Balachandran
 ab and
Saifullah
Lone
ab and
Saifullah
Lone
 *ab
*ab
aDepartment of Chemistry, National Institute of Technology (NIT), J&K, Srinagar, 190006, India. E-mail: saifullah.lone@nitsri.ac.in
biDREAM (Interdisciplinary Division for Renewable Energy & Advanced Materials), Laboratory for Bioinspired Research on Advanced Interface and Nanomaterials (BRAINS), NIT, J&K, Srinagar, 190006, India
cDepartment of Physics, NIT, J&K, Srinagar, 190006, India
First published on 22nd May 2024
Abstract
Surfaces act as reservoirs for the proliferation of microorganisms, including bacteria and viruses, that can be transmitted to individuals who come into contact with them. The phenomenon is known as “fomite transmission”, where pathogens can survive on surfaces for varying periods, depending on the material and environmental conditions. Fomite transmission plays a significant role in spreading infectious diseases. This transmission route is particularly relevant in high-traffic environments like healthcare facilities, public transportation, schools, etc. Developing surfaces with bactericidal or antiviral properties and designing spaces to minimize surface contact are strategies to reduce the risk of fomite transmission. This is where the concept of nature-inspired bactericidal surfaces becomes valuable. Nature offers sustainable surface design for preventing bacterial colonization and growth. Crafting nature-inspired bactericidal surfaces can lead to the development of materials that can help prevent the spread of infections, reduce the need for frequent cleaning, and potentially contribute to healthcare and hygiene applications. To minimize human health and environmental issues, instead of using harmful disinfectants regularly in public places, nanoengineered surfaces with antipathogen features could alternatively halt microbial growth to prevent the risk of establishing a surface-contamination network. In infectious disease control, this work aims to provide a detailed overview and perspective on the importance of developing nature-inspired bactericidal surfaces to combat surface contamination issues. This approach holds the potential to offer more sustainable and practical solutions compared to traditional methods of using disinfectants and harsh chemicals.
1. Introduction
The harrowing memories of contagious COVID-19 serve as a startling reminder of the healthcare vulnerabilities of the developed and emerging world.1 This viral outbreak will not be the last pandemic that could test our preparations for preventing/or curing widespread potential viral/or bacterial outbreaks.2 And, what some countries underwent during the COVID-19 outbreak was nothing short of an apocalypse. Annually, bacterial infections cause seventeen million mortalities in the world, making infection the second leading cause of death in humans. The central process of infection is bacterial colonization of surfaces. One of the most concerned fields is the medical industry, where occupational infections occur during healthcare delivery for other diseases, even after discharging the patients.3,4 Particularly, seven out of every hundred hospitalized patients in developed nations and ten out of every hundred in economically challenging countries catch a healthcare-associated infection.5 And, they only gain attention when reaching up to pandemic proportions. Therefore, a broader understanding of future pandemics, in-depth study, and identification of the physical parameters/or environments that influence (i.e., factors aiding/restricting) the infection surge could reduce the loss of precious lives.Surface contamination is well-documented as one of the critical factors in many outbreak reports.6 People always physically interact with surfaces/or items around them. In a contaminated environment, a proportion of people touch the contaminated surface, and each person touches other surfaces in different environments and locations; eventually, a surface-touch network is established. The network allows the pathogen to spread across and create a surface-contamination network.7 In other words, a surface-touch network gives rise to an unending surface-contamination network. Depending upon the cleaning process and the surface being cleaned, both cleaning agents (i.e., soap/or detergent) and disinfectants lower the contamination or bacteria on the surfaces.8 Adapting to effective hand hygiene, the World Health Organization (WHO) recommends using two alcohol-based formulae to reduce pathogen spread and infectivity. Ethanol, isopropanol, and various forms of hydrogen peroxide make up the majority of alcohol-based hand sanitizers. Nonetheless, these chemicals are toxic to human health when released through evaporation, and accidental ingestion of these chemicals could cause portal vein thrombosis, severe respiratory/or central nervous system disorders, arrhythmia, ketoacidosis, hyperglycemia, and possibly cardiac arrest.9 To minimize human health and environmental issues, instead of using harmful disinfectants regularly in public places, nanoengineered surfaces with antipathogen features could alternatively halt microbial growth to prevent the risk of establishing a surface-contamination network.
Being cosmopolitan in distribution, bacteria dwell, adapt, and thrive under adverse environmental conditions. Bacteria around us have existed for over 3.5 billion years, and it only takes about thirty minutes to multiply due to random natural mutations. Bacteria are well-suited to fast evolution because their populations are so large, and they reproduce so quickly that the probability of one cell bearing a resistant mutation is significantly high. Yet, the chances of each cell being resistant are extremely low.10,11 Antibiotic resistance is an example of evolution in action. Therefore, only a small percentage of bacteria in a population could develop antibiotic resistance. There is minimal mutation, resulting in highly minor variations in each bacterium's DNA. One or a few of them, by chance, have a mutation that allows them to survive antibiotics. These bacteria multiply quickly and seize control of the entire population.12 Thus, given the high antibiotic tolerance and proliferation of multidrug-resistant bacterial infections facilitated by the resistance genes, treating biomaterial-associated infections and eradicating biofilms in clinics pose serious challenges.13
Furthermore, other viral infections like COVID-19 cause patients to develop various bacterial infections, some of which exhibit antimicrobial resistance and contribute to significantly worse outcomes. People have died as a result of antibiotic-resistant illnesses. If current trends continue, by 2050, ten million people will die annually as a result of these resistant diseases, equating to one person dying every second.14 This is a typical biological “arms race”, with bacteria rapidly creating antibiotic resistance mechanisms and humans developing new antibacterial weapons.15 Thus, the new scenario will have to win the “arms race” by developing new modifications against the diseases resistant to existing antibiotics. Otherwise, we end up being susceptible to bacterial infection. According to the National Institutes of Health (NIH), biofilm formation is linked to 65% and 80% of all microbial and chronic illnesses, respectively.16–18
Innovative technologies have been employed to develop antifouling, bactericidal, or antibiofilm biomaterials and micro/or nanoscale surfaces to confront this unresolved bacterial menace. Antibiofouling textures can be antiadhesive or bactericidal, which resist initial bacterial attachment by obstructing the cell-on-contact or result in cell death. The mechano-bactericidal nanosurfaces resist bacteria's cellular attachment due to the unfavorable hierarchical surface topography.19–21 In this connection, owing to their protracted evolution and adaptability, some intriguing surfaces in plant and animal kingdoms have undergone significant topographical alterations at the micro-and nanoscale with antipathogen properties to survive in hostile environments. Inspired by nature, green fabrication techniques for micro/nanoscale (i.e., hierarchical) surfaces have been established. Notably, surface roughness, wettability, surface energy, and adhesion are the critical features under examination while designing superior antipathogen surfaces. Mimicking nature by producing repeating hierarchical units without being dependent on harmful chemicals is advantageous. And, given the edge over other tedious, expensive, and environmentally degrading techniques, scientists are always tempted to emulate the antibacterial behavior of naturally existing surfaces.22
Depending upon the modes of operation, bactericidal surfaces are of three types (Fig. 1): (a) anti-adhesive – minimizes the adhesion of bacteria with a solid surface to prevent contamination; (b) contact active – reduces the bacterial contamination by attaching an antibacterial agent to the surface based on a biocide attach/release mechanism – coordinates the contact-release of poisonous chemicals to the surface linked bacteria (e.g.: toad skin), and (c) topographically hierarchical surfaces – produce unfavorable micro/nanoscale repeating units of structures to repel/or kill bacterial growth. The discovery of antibacterial capabilities caused by the micro- and nanoscale hierarchical textures on the surface of several species in nature is unarguably one of the promising fields of surface biomimetics to advance the non-toxic antimicrobial surfaces. This review will highlight micro or nano-topographical surface patterns with antibacterial properties (Fig. 1). We will discuss these surfaces under two categories: plant-based and animal-based. Moreover, we will discuss the operational principle of bactericidal action on hierarchical surfaces. Finally, we will present an outlook and future perspective on bioinspired bactericidal surfaces.
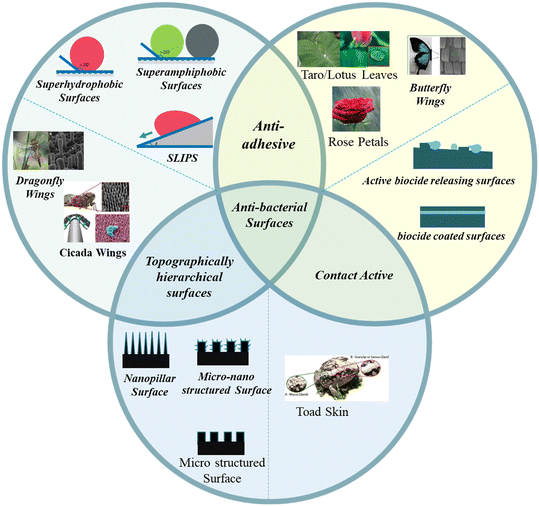 | ||
| Fig. 1 Schematic representation of natural and artificial antibacterial surfaces. Images of topographically hierarchical surfaces are given in the left circle (schematic representation in the bottom circle).25–27,31,32 Copyright 2018, American Chemical Society; copyright 2017, American Chemical Society; copyright 2020, Elsevier; copyright 2017, Royal Society of Chemistry; copyright 2021, Elsevier. Images of natural anti-adhesive surfaces are given in the right circle (schematic representation in the left circle).32,33,40,50,55 Copyright 2017, Elsevier; copyright 2014, Royal Society of Chemistry; copyright 2020, Wiley; copyright 2008, American Chemical Society; copyright 2019, Springer. Images of natural contact active surfaces are given in the bottom circle.87 Copyright 2017, Springer. | ||
2. Examples from the insect and animal world
2.1. The nanopatterned bactericidal surface of the cicada wing
The unique class of superhydrophobic bactericidal surfaces is ubiquitous; the cicada insect offers one such prominent model (Fig. 2, a). The inset shows large-sized wings necessary for flight and antireflection purposes. However, the insect has shorter extremities to clean the wings that remain exposed to various contaminants (for instance, soil, industrial dust particles, pollens, and pathogens). Thus, self-cleaning of contamination from wings becomes important for cicadas. More importantly, the insect bears an idiosyncratic nanopattern on its wing surface, which kills pathogens simply by physical contact without discharging any chemicals (Fig. 2a ii). The intriguing nanopattern is a prototype for crafting sustainable, functional surfaces with improved resistance to contamination/or infection. Due to these distinctive antibacterial traits, the cicada wings have gained considerable scientific scrutiny. The wings, predominantly composed of chitin, protein, and wax with fine nanostructures on top, empower them to adapt to various surroundings. The cicada wing's nanopatterned bactericidal surface combats bacterial development through a multifaceted mechanism. The surface is covered in a multitude of nanopillars that, when in contact with bacterial cell membranes, physically puncture them, causing damage and lysis. Furthermore, the nanopatterned surface provides a large surface area, minimizing bacterial adherence and growth. Together, these attributes form a highly effective defense strategy against microbial infections, inhibiting proliferation of bacteria and enhancing cicada survival in a multitude of conditions.23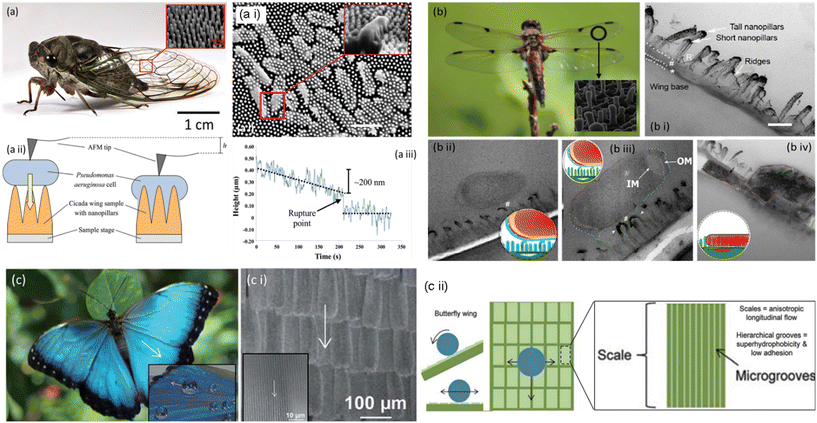 | ||
| Fig. 2 Pictorial representation of a cicada and the SEM image of nanopillar structures on its wings. (a i) A cicada wing possesses Pseudomonas aeruginosa cells. The nanopillar structures on the wing's surface directly penetrate cells (inset), scale bar = 1 μm. (a ii) An AFM tip was placed on top of a Pseudomonas aeruginosa cell, in contact with the wing surface, to determine its downward movement. (a iii) The tip was lowered by 200 nm over 220 s, resulting in a sudden short decrease indicating cell rupture.25–27 Copyright 2020, Elsevier; copyright 2017, Royal Society of Chemistry; copyright 2012 Wiley. b) Pictorial and SEM representation of a dragonfly and nanopillars on its wings, (b i) the TEM image depicts a cross-sectional view of the wing and its nanotopography. Scale bar = 200 nm. (b ii) TEM micrographs showing bacteria–nanopillar interaction at the interface. A longitudinal cross section of the E. coli bacterium reveals the separation of the inner membrane (IM) and outer membrane (OM) at its polar ends. (b iii) A longitudinal cross section of a bacterium on a dragonfly wing. The bacterium's polar end exhibits increased membrane separation. (b iv) The bacterium has lost its spherical shape and volume, resulting in nanopillar topography.30,31 Copyright 2018, American Chemical Society; copyright 2017, American Chemical Society. (c) Butterfly wing effect; hierarchical structures composed of coordinated shingle-like scales that provide anisotropic flow. (c i) SEM images of butterfly wings; the arrows represent the direction of anisotropic fluid flow. (c ii) Water flow control model of butterfly wings. Arrows depict fluid flow patterns in both transverse and longitudinal directions.36 Copyright 2013, Royal Society of Chemistry. | ||
Ivanova et al.22 reported the ability of cicada wing surfaces to slay P. aeruginosa cells within three minutes of physical contact. Such profound bactericidal properties encouraged researchers to concentrate on replicating it on diverse substrates. Pogodin et al. provided a biophysical model of the interaction between bacterial cells and a nano-pillared surface on a cicada. According to the paradigm, bacterial resistance to the bactericidal characteristics of the wing surface is determined mainly by mechanical features, particularly cell stiffness. In this way, contrary to Gram-negative cells, cicada wing surfaces had less of a bactericidal impact on Gram-positive bacteria despite their greater cell stiffness.24–28
2.2. The nanopatterned bactericidal surface of the dragonfly wing
The Anisoptera class of dragonfly species is distinguished by their long bodies and two narrow pairs of elaborately veined, membranous wings that, although primarily transparent, may have colored patterning. The aerodynamic prowess of dragonflies is well- known, and their wings have received substantial macro-scale research. The dragonfly's ability to maintain its aerodynamic performance is crucial to its survival. Thus, a clean (i.e., contamination-free) and lightweight wing becomes essential. Besides that, dragonflies rest with their wings splayed horizontally instead of vertically (except for one tiny family, Epiophlebiidae). It is attainable by readily self-cleaning the dust particles from the wing surface and preventing bacterial growth under wet conditions.22The wing surface achieves self-cleaning and antibacterial properties through a perfect arrangement of micro/nanoscale structures to repel contamination in dry/wet states. The dragonfly wing is a typical example of a superhydrophobic surface with a contact angle (CA) of 153°. Scientists discovered a curious phenomenon when exploring the intricate surface structure of dragonfly wings. The fine nano-textured surface of the wings, which resembles a bed of spikes and physically punctures the bacterial cell wall to kill it as in the case of cicada wings, has been widely assumed to be the reason that the wings were able to destroy the bacteria (Fig. 2b ii and iii). On close investigation, it was apparent that these spikes had varied heights rather than the expected uniform length. We uncovered another prominent finding by studying how the bacteria interacted with the surface. The bacterial cell wall never makes direct physical contact with the surface. Instead, the bacterial release's structural elements serve as an adhesive to bind the microorganisms to the wing. The varied heights of the spikes on the nano-textured surface serve as the key to the problem. The bacteria don't instantly disintegrate when they touch the wings; if they stayed still, they might persist. As soon as they begin to move, the spines cling firmly to the bacterial “glue”, and the bacteria are fatally torn apart by the shearing forces, expelling their cellular contents (Fig. 2b iv).28,29
The minuscule spike-like structures, which are inaccessible to human eyes, damage various bacteria, including Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. These pathogenic microorganisms are frequently associated with different tertiary care infections.29–31
2.3. The nanopatterned bactericidal surface of the butterfly wing
Butterfly wings achieve a sustainable anti-biofouling surface by integrating the anisotropic flow effects from shark skin with the superhydrophobic attributes of lotus and taro leaves.31 The butterfly wing comprises slender layers of chitin protein, with tiny scales on top exhibiting multiple functions depending on the butterfly species. These scales produce dazzling hues and effectively protect and insulate the insects, facilitating airflow over their wings during flight. Additionally, the butterfly's scales may contribute to heat absorption, assisting cold-blooded butterflies in raising their core temperature for optimal bodily functions.Since any moisture or dirt with microorganisms that adhere to a butterfly wing during a rainstorm could impact its weight and make it harder for it to fly, hydrophobicity is crucial for butterfly wings, enabling them to withstand the weather. The nano- and microstructures of butterfly wings give it a lotus-like hydrophobic and self-cleaning efficiency. The butterfly scale ridges trap a pocket of air beneath the water droplets that settle on them. These arrays of scales are covered with hierarchal micro-grooves that generate a high contact angle (148°), allowing water droplets to roll down the wing's surface axially, facilitating self-cleaning (Fig. 2c; i–iv). Since the interactions between water molecules are more potent than those between water and air, water droplets cannot enter the hydrophobic pockets. Moreover, internal pressure within the air pockets prevents water from penetrating.32
Aligned shingle-like scales on the wing, ranging from 30–50 m in width and 58–146 m in length, spur on this anisotropic behavior. Anisotropic flow fosters low drag and water repellence and, when associated with superhydrophobic features, produces a surface with low drag, anti-biofouling, and low bacterial adhesion features. The investigated wing isolates have mild to very high antibacterial activities, according to antibacterial experiments using the entomopathogenic bacterial species Pseudomonas fluorescens, Bacillus subtilis, and Bacillus amyloliquefaciens.33–35
2.4. The nanopatterned bactericidal surface of shark skin
Sharks have undergone extensive evolution to become enviable predators. Shark skin features self-cleaning, anti-biofouling, hydrophobic, drag-reducing, and aerodynamic properties on its surface. Scientists have attempted to emulate the texture of shark skin, which helps propel water past the shark with the least drag, for applications ranging from boats and cars to swimwear. The ability of shark skin to resist biofouling and self-clean is due to the micro-structured riblets that line its dermal denticles (Fig. 3a and a i). The minuscule scales that makeup shark skin are triangular, typically 200–500 m long, with fine, regularly spaced ridges (30–100 μm) aligned along the body axis. Prior research has shown that the scales can modify the water flow near the skin and possibly alleviate drag on the body. The same technique could assist in the prevention of biofouling since hastily moving water close to the skin's surface would shorten the time microorganisms have to colonize the surface while also assisting in the washing away of organisms. The mechanism behind the nanopatterned bactericidal surface of shark skin involves the unique microscopic features found on its surface, such as ridges and scales. These traits result in a texture that is uneven at the nanoscale. When bacteria come into interface with the shark skin's surface, they encounter minute ridges and scales. The skewed and abrasive structure of shark skin impedes the bacterial cell membrane. As bacteria attempt to cling to the surface, their cell membranes become entangled and stretched over the uneven surface features. This physical interaction creates mechanical stress on the bacterial cell membrane, resulting in distortion and eventual rupture.35,36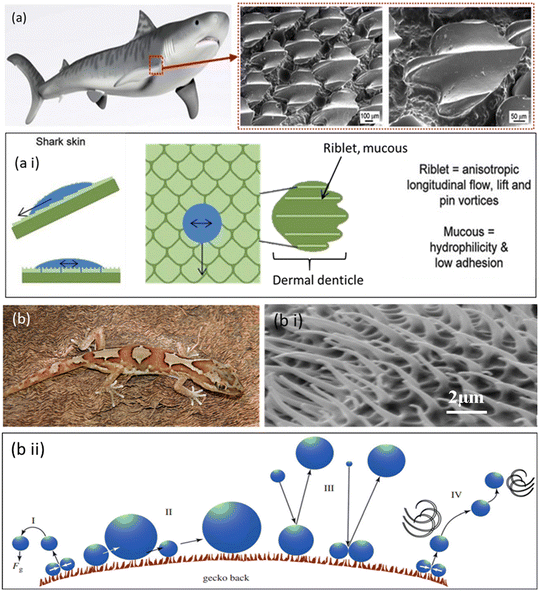 | ||
| Fig. 3 (a) Pictorial and SEM images of the shark skin surfaces. (a i) Water flow control model of shark skin. Arrows depict fluid flow patterns in both transverse and longitudinal directions.35,40 Copyright 2020, Springer Science; copyright 2013, Royal Society of Chemistry. (b) Pictorial representation of a gecko. (b i) SEM image representing micro/nanostructures on the dorsal surface of a gecko, consisting of spinules and a base layer patterning. (b ii) Systematic representation of anti-adhesive and antibacterial properties of gecko skin. (I) Self-propelled mechanism of droplets from the skin surface, (II) coalescence of droplets propelled along the surface, (III) altering droplets from fog or other falling droplets enhancing self-propulsion and (IV) wind-assisted evacuation.44 Copyright 2015, Royal Society. | ||
Another theory asserts that the surface roughness and minuscule contours of shark scales prevent the settling of microbes. Marine fouling species, soft (such as anemones and algae) and hard (like barnacles and mussels), can undermine ecological integrity in the marine ecosystem. In the water, sharks face difficulty in adhering zoospores and bacteria to surfaces smaller than their size, resulting in a significant decrease in bacterial adherence and demonstrating a potent antibacterial effect. To replicate the antibacterial features of shark skin, research should optimize shark skin replicas and straightforward models, highlighting that microbes prefer to colonize specific groove widths and depths.37–40
2.5. The nanopatterned bactericidal surface of geckos
Geckos are reptiles that dwell in temperate areas all over the world. Notably, geckos' feet (in particular, their extraordinary adhesion properties) have received much attention. Strong adhesive qualities allow gecko feet to adhere to many surfaces preferentially. Setae, a periodic array of hierarchically arranged keratinous hairs, are the reason for this phenomenon. The hairs have a diameter of 5000 μm, a length of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 130
000 to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm, and are divided into hundreds of 200 to 500 nm wide nanoscale spatula. Small van der Waals forces induced by each spatula add up to substantial adhesion and anti-wetting capabilities (Fig. 3b; i and ii).39,40 Geckos are not known for grooming their feet, but they keep them sticky between moults for months. Due to the inherent self-cleaning ability of their setal nanostructures, geckos can keep their feet clean even though they have sticky toes. In this approach, geckos with dirty feet could reclaim their ability to adhere to vertical surfaces after just a few steps. Self-cleaning transpired in setae arrays which was distinct from that of the gecko. According to contact mechanical models, self-cleaning occurs due to an energy disequilibrium between the adhesive forces that drive dirt to a surface and those that attract the same dirt to one or more spatulae. Since the feet of some gecko species have become the focus of extensive study, the remaining areas of the lizard body have received minimal attention regarding microstructure, particularly studies divulging skin functions. This is somewhat surprising considering that the gecko has an intriguing microstructure on the dorsal and ventral regions, consisting of tiny hairs (commonly referred to as spines, spinules, or microspinules) spaced 0.2–0.7 μm apart and up to several microns in height. The gecko spines are water-repellent, and it has been claimed that they also function as a self-cleaning surface, where rain may wash away particles and so use the lotus effect to eliminate dirt and contaminants. The gecko spines exhibit water-resistant features owing to the presence of a distinctive arrangement of surface hydrophobic nanostructures. These water-repellent qualities prevent water and other liquids from clinging to the spine surface, allowing liquid pollutants, such as water droplets harboring bacteria or other germs, to simply roll off. So, the synergy of water-repellent qualities and nanostructured surface of the gecko spines allows them to operate as a self-cleaning surface, minimizing the growth of pollutants and germs on the gecko's skin and optimizing the gecko's ability to survive microbial conditions.41
000 μm, and are divided into hundreds of 200 to 500 nm wide nanoscale spatula. Small van der Waals forces induced by each spatula add up to substantial adhesion and anti-wetting capabilities (Fig. 3b; i and ii).39,40 Geckos are not known for grooming their feet, but they keep them sticky between moults for months. Due to the inherent self-cleaning ability of their setal nanostructures, geckos can keep their feet clean even though they have sticky toes. In this approach, geckos with dirty feet could reclaim their ability to adhere to vertical surfaces after just a few steps. Self-cleaning transpired in setae arrays which was distinct from that of the gecko. According to contact mechanical models, self-cleaning occurs due to an energy disequilibrium between the adhesive forces that drive dirt to a surface and those that attract the same dirt to one or more spatulae. Since the feet of some gecko species have become the focus of extensive study, the remaining areas of the lizard body have received minimal attention regarding microstructure, particularly studies divulging skin functions. This is somewhat surprising considering that the gecko has an intriguing microstructure on the dorsal and ventral regions, consisting of tiny hairs (commonly referred to as spines, spinules, or microspinules) spaced 0.2–0.7 μm apart and up to several microns in height. The gecko spines are water-repellent, and it has been claimed that they also function as a self-cleaning surface, where rain may wash away particles and so use the lotus effect to eliminate dirt and contaminants. The gecko spines exhibit water-resistant features owing to the presence of a distinctive arrangement of surface hydrophobic nanostructures. These water-repellent qualities prevent water and other liquids from clinging to the spine surface, allowing liquid pollutants, such as water droplets harboring bacteria or other germs, to simply roll off. So, the synergy of water-repellent qualities and nanostructured surface of the gecko spines allows them to operate as a self-cleaning surface, minimizing the growth of pollutants and germs on the gecko's skin and optimizing the gecko's ability to survive microbial conditions.41
The hairy structures that establish a 150° contact angle have bactericidal effects on some Gram-positive and Gram-negative bacteria. The micro-/nanostructure of the skin displayed very little adherence to contaminants. Besides, the topography produces a superhydrophobic, anti-wetting barrier that can be self-cleaned by the collision or rolling of low-velocity droplets with a range of sizes from microns to several millimeters. Tiny water droplets (10–100 m) can self-propel off the surface, increasing their portability and cleaning efficacy. Also, they can easily access troughs between the scales for effective self-cleaning.42–44
3. Examples from the plant world
3.1. Taro leaves
Due to their hierarchical micro- and nanopatterned surface, taro leaves (Colocasia esculenta) have anti-biofouling, hydrophobic, and self-cleaning properties (Fig. 4, a i). The foremost surface structure of taro leaves consists of hierarchical, waxy nanoscale epicuticular crystals encapsulating microscale ellipsoidal bumps (10–30 μm in diameter). These bumps make the surface more slippery by increasing the contact angle (90–150°) with the surface. As a result, water droplets on the surface attract dirt particles and bacteria more strongly than the surface on its own. Then, the leaf is concurrently cleaned as the water droplet rolls off the leaf with the dirt and contaminants.47 A study conducted by Ma et al. revealed the antibacterial effect of the taro leaf surface with a high density of nanostructures.45 Even under different water conditions, air must permanently be entrapped between the nanostructures for this process to function. Both surface roughness and wettability have an impact on this characteristic. Compared to low-density patterns, nanostructures with highly dense patterns reduce the underwater bacterial and particle adhesion rate (Fig. 4, a ii and a iii).46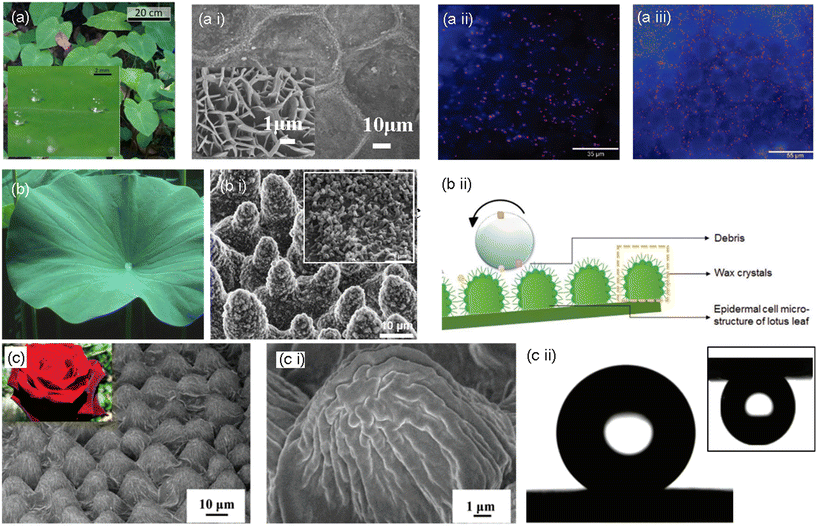 | ||
| Fig. 4 (a) Pictorial representation of taro leaves and their superhydrophobic property (inset), a i) SEM images of the taro leaf surface, magnified image shown in the inset.46 Copyright 2020, Nature. Pseudomonas aeruginosa adhesion on a taro leaf under a ii) non-wet and a iii) wet conditions.45 Copyright 2011 American Chemical Society. (b) Pictorial representation of a lotus leaf, (b i) SEM images of the surface topography of the lotus leaf with a magnified section showing wax crystals, and (b ii) schematic representation of the self-cleaning effect of the lotus leaf.49,50 Copyright 2020, Wiley; copyright 2008, American Chemical Society. (c) Optical image of rose and SEM image of rose petals. (c i) Magnified surface microstructure of a rose petal; (c ii) water droplet on the rose petal surface showing superhydrophobic property and pinning effect in the upside down position (inset).50 Copyright 2008, American Chemical Society. | ||
3.2. Lotus leaves
One of the most well-known superhydrophobic surfaces, the lotus leaf surface, exhibits outstanding anti-biofouling characteristics. This is primarily due to the persistent airframe trapped at liquid/solid interfaces, significantly minimizing the interface area and preventing biofouling. Natural lotus leaves exhibit innate bactericidal activity against adherent bacteria and have a high degree of microbial repellency. As per the tested hypothesis, the bactericidal activity of this superhydrophobic surface may be the effect of a mechanical killing mechanism since the lotus leaf structures comprise micro-sized papillae and nano-sized outermost wax tubes with a similar aspect ratio to the bactericidal nanopillars (Fig. 4, b ii). Scientists define this property of the lotus leaf as the “lotus effect”.47As the upper surface of the lotus leaf is continually in touch with the surrounding water in the habitat, it is vital to avoid micro attachment. The adhesion of tiny droplets can also induce biofouling and surface degradation. The super-repellency of the lotus leaf (the non-fouling feature) towards the bacterial medium and its mechanical bactericidal action against the adhered bacteria are divulged to have synergistic antibacterial effects. It inspired the development of a hierarchically structured superhydrophobic surface with packed nanoneedles and regularly spaced micro-pillar arrays, which showed remarkable antibacterial activities against Escherichia coli.48,49
3.3. Rose petals
The “petal effect” is another well-known super hydrophobicity phenomenon. The “petal effect” surfaces exhibit a superhydrophobic state with a static water contact angle larger than 150°, while they also exhibit considerable adhesion to water. The “petal effect” structure has a very large contact angle hysteresis. The microstructure of rose petals reveals hierarchical micro-bumps with many nano-folds observed on top of a single micro-bump. Water is within the interface with this complex hierarchical micro-/nanostructure at an angle of 152°. Although inverted, the water droplet still adheres to the surface without moving, exemplifying a significant adhesion force.50–52The micro-bumps and nano-folds on rose petals have a higher pitch value (center–center distance between micro-bumps) and steeper micro-valleys than those on a lotus leaf (Fig. 4, c–c ii). With these specifications, Wenzel-state superhydrophobicity is developed, wherein water may easily pass through the bottom of a pillar structure. The surface of the rose petals exhibits an enhanced adherence to the water droplet since the water droplet makes a complete interface with the microstructure, and the structures hold the left part without penetration. On the other hand, more air pockets develop between microstructures with higher pillars and lower pitch values. This arrangement eventually produces a superhydrophobic surface with a lower contact angle hysteresis and a lower adhesion by preventing liquid penetration and reducing the contact area between water and the surface. The superhydrophobicity and superb-self-cleaning surface phenomena are rationalized by the Cassie–Baxter state.53–55
4. Bacterial repellent mechanisms
Antibacterial surfaces can be divided into surfaces that resist bacteria and inhibit bacteria on contact based on the underlying mechanisms (Fig. 5). Bacteria can discern mechanical cues from surfaces, such as surface texture. In general, bacteria are affected by hydrodynamics at the micro level, whereas nanoscale characteristics are affected by physicochemical forces and cell membrane deformation at the nanoscale.56,57 The micro/nano-topography of the substrate, or its roughness, has been identified as the most paramount surface characteristic for regulating microbial adhesion and the early stages of biofilm formation. To optimize antifouling behavior, the surface should have adequately spaced-apart features to prevent bacteria from penetrating between them and be sufficiently large to minimize the number of potential attachment points.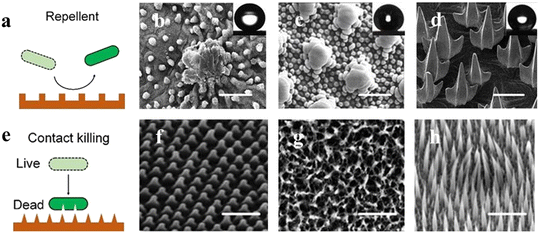 | ||
| Fig. 5 Nature bacteria-repellent surface and contact-killing surfaces. (a) Graphical representation of the nature-repellent surface, (b–d) SEM images representing the microstructures on the lotus leaf (micropapilla) scalebar; 50 μm, springtail skin (doubly-reentrant) scalebar; 2 μm, and sharkskin (microdenticle) scalebar; 100 μm. (e) Graphical representation of the nature-contact killing surface, (f–h) SEM images representing the nanostructures on the cicada wing (nanocone) scalebar; 2 μm, dragonfly wing (nanopillar) scalebar; 200 nm, lizard skin (microspine) scalebar; 2.5 μm.57 Copyright 2022, Multidisciplinary Digital Publishing Institute. | ||
4.1. Bacterial repellent mechanism of superhydrophobic surfaces
The surface is crucial in incentivizing or inhibiting bacterial adherence because it is the point of contact between bacteria and the bulk of a material. Superhydrophobic or “self-cleaning” surfaces, frequently found on plant leaves, insect cuticles, fish skin, etc., allow these species to limit biofouling passively. For instance, superhydrophobicity and bacterial repellence were initially inferred in lotus leaves.58 The underpinning mechanism was the combination of low surface energy and multiscale texture of surface lipid hierarchies, which enabled the surface to exhibit a greater water contact angle (>150°) and a low sliding angle (<10°) and trapped significant amounts of air cushion. Such surfaces would preclude bacteria from developing biofilms by removing bacteria that were colonizing them.59The contact angle is the concept used to represent the state of interaction between liquids and surfaces. The contact angle measurement indicates the wettability features of the surface of a material with a more than 150° contact angle being superhydrophobic, a contact angle between 90° and 150° being hydrophobic, between 10° and 90° being hydrophilic, and <10° being superhydrophilic. The contact angle is measured between the tangential line on the liquid surface near the solid–gas–liquid three-phase contact line and the horizontal direction.60–63 This angle is formed because of the surface tension of the interface between the solid, the liquid, and the gas. Young's equation that gives the relation between the surface tension of the three phases and the contact angle is:
Low surface energy materials can thus make up hydrophobic surfaces. Another factor contributing to hydrophobicity is the surface nano/microscale roughness. Microscale interface protrusions in the surface have superhydrophobic and low-adhesion characteristics that make it permissible for water droplets to slide off with the surface debris. The “lotus effect” refers to the process by which water droplets coalesce when they roll off the water-repellent surfaces of lotus leaves (Nelumbo nucifera), accumulating dirt and debris in them. Thus, due to this “constant cleaning” effect of these surfaces, any contaminations that aid in forming bacterial biofilm are eliminated. Although water has a high interfacial tension, bacteria could pass through the air–liquid contact. The wide, stable air–liquid interface on the surface topography makes it crucial for bacteria to track down navigable sites for cell anchoring.65
The prominent surface attributes that equate with one another are surface wettability and surface roughness/topography. The presence of tiny air pockets on the surface that lead to incomplete wetting often get lodged in the pores and grooves of a hydrophobic surface when the roughness of the surface increases. Liquids on this junction are easily removed and cannot access the surface grooves. By trapping an air layer (bubbles) between the surface morphology, micro-features minimize the surface area exposed for bacterial adhesion, resulting in an anti-biofouling effect. The Cassie–Baxter state, which is a result of this phenomenon, is just what causes superhydrophobic surfaces to be propelled by a combination of surface roughness and wettability. The CA of a droplet resting on top of a microstructure is depicted in this model, generating an air layer surrounding the microstructure and beneath the droplet. In other words, the Cassie–Baxter can describe a (super)hydrophobic surface; usually, a small amount of air is on the surface underneath the drop. The equation explaining the Cassie–Baxter model is
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θw = fsl θw = fsl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ1 + fla θ1 + fla![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 θ0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θw = r
θw = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θy, where θW is the apparent contact angle applied for rough surfaces, θy is the Young's contact angle, and r is the roughness ratio).66–71
θy, where θW is the apparent contact angle applied for rough surfaces, θy is the Young's contact angle, and r is the roughness ratio).66–71
Due to the retention of an air barrier that minimizes the surface area available for bacterial adherence, superhydrophobic materials can impede bacterial adsorption and growth on implantable components, such as catheters and pipes, during the pivotal post-operative period. Superhydrophobic surfaces exhibit excellent antithrombotic properties. These antithrombotic surfaces can prevent the deposition of unwanted substances in the blood, such as platelets. This adhesion of substances in implants will cause the formation of thrombi, which has serious consequences. Sun et al.72 tested these antithrombic properties by placing platelets on a superhydrophobic surface and a smooth film surface. Platelets formed a spherical shape on a superhydrophobic surface without getting adhered to it. Meanwhile, a smooth surface favored the adhesion of the platelets with the formation of pseudopods. This proved the capability of superhydrophobic implant surfaces to prevent infections and thrombosis without any drugs.
A superhydrophobic surface cannot prevent all kinds of infectious agents. Therefore, combining superhydrophobic properties with antimicrobial materials can increase the effectiveness of such surfaces against infections. For example, superhydrophobicity induced on materials like copper/stainless-steel surfaces exhibits better anti-biofouling properties.69–72
4.2. Bactericidal mechanism of nanotextured surfaces
To evaluate the possible antibacterial behavior of superhydrophobic surfaces (contact angle > 150°), which have been found to hinder or inhibit adherence of bacterial strains like S. aureus and P. aeruginosa, surface wettability studies are a significant metric. Numerous research teams have created antimicrobial surfaces based on the cellular repulsion phenomena that taro and lotus leaves exhibit. However, most Gram-negative microorganisms have exhibited a super-repulsive nature. In contrast, Gram-positive microorganisms tend to attach to these surfaces (Fig. 6), convoluting the microbial repulsion mechanism on superhydrophobic surfaces. Recent research has demonstrated a paradigm shift toward nano-textured surfaces, where microbial membrane disruption by cellular adherence is the root of cell death.73,74 However, a recent discovery implies that topography-induced antibacterial mechanisms are not limited to self-cleaning in nature. As demonstrated by a recent biomimetic discovery, Psaltoda claripennis, a cicada species, has enormously bactericidal wings. Ivanova et al. hypothesized that this bactericidal characteristic, which differs from its microbial-repelling nature owing to self-cleaning, is mediated by the physical interaction of highly organized arrays of wing nanopillars with bacteria (Fig. 5).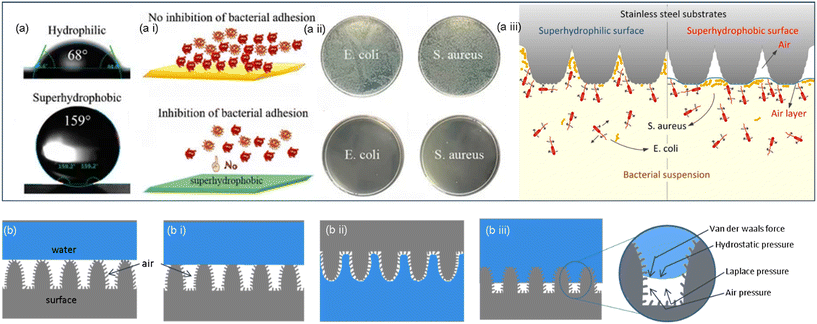 | ||
| Fig. 6 (a) Schematic illustration of the water contact angle in hydrophilic and superhydrophobic surfaces. (a i) Representation of bacterial behavior in both surfaces. (a ii) Comparing bacterial growth in hydrophilic and superhydrophobic surfaces. (a iii) Illustration of the bacterial repellent mechanism exhibited by the superhydrophobic surface.60,61 Copyright 2021, Frontiers; copyright 2020, Multidisciplinary Digital Publishing Institute. (b) Graphical representation of the lotus effect (b i) Cassie model, (b ii) petal effect and (b iii) transition from the Cassie to Wenzel model with forces affecting the interfaces.64 Copyright 2023, American Chemical Society. | ||
When bacteria like P. aeruginosa or P. claripennis cling to the nanopillars of cicada wings, the adhesive layer is divided into two distinct areas: one where it is in intimate contact with the pillar and the other where it is dangling between pillars. This happens because the preponderance of bacterial cells is micron-sized, whereas the textured surfaces are in the nano-sized range. In the spaces stretched between the pillars, the surface area of the region of direct pillar contact rises, thrusting the cell membrane and causing membrane rupture (Fig. 7a–c). As a result, this model predicts that cell death is highly dependent on how rigid the bacterial cell membranes are. When referred to less stiff Gram-negative bacteria strains, rigid Gram-positive bacteria strains may resist nanopatterned surfaces of cicada wings.77
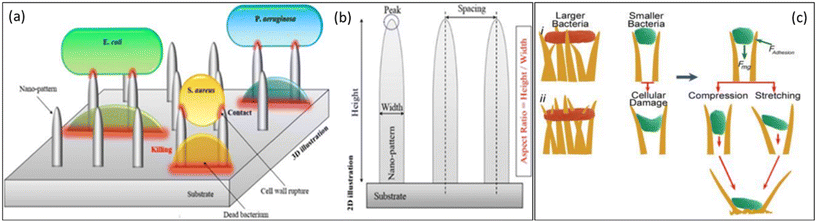 | ||
| Fig. 7 (a) Schematic representation of the bactericidal mechanism of NPs based on direct contact and cell wall rupture. (b) Illustration of the main geometrical parameters of representative 2D NPs.77 Copyright 2021, Elsevier. (c) Proposed approaches of interaction of the nanotipped hairs of gecko skin against bacteria. (i) Bacteria can be observed on the tips of cell wall penetrations. (ii) Small bacteria interact with the side edges of hairs, causing cell damage due to adhesion (Fadhesion) and gravity (Fmg).78 Copyright 2020, Elsevier. | ||
Although bacteria come in many shapes, they are predominantly only a few micrometers large. Bacteria have been divided into Gram-negative and Gram-positive groups based on the structural amenities of their broad categorization of cell walls. Gram-negative species' cytoplasmic and outer membranes typically have a very thin layer of cell fence surrounding them, whereas Gram-positive bacteria have a comparatively thicker cell wall. The peptidoglycan layer is primarily responsible for this variability in cell wall thickness because the cytoplasmic membrane, periplasmic space, and outer membrane (all of which are present in Gram-negative bacteria) are all extremely thin and collectively are predicted to be thicker than 1 nm.78,79 According to reports, peptidoglycan demonstrates nonlinear viscoelastic characteristics and stress-stiffening behavior. The study examined the adhesion behavior of two species of Gram-positive cocci, Planococcus maritimus and S. aureus, and the Gram-positive, rod-shaped bacterium Bacillus subtilis on cicada wing surfaces to explore the predictions of the proposed model and ascertain the importance of the mechanism. It is widely known that Gram-positive bacteria strive to be more rigid than their rod-shaped counterparts. Study research performed comparative attachment tests to determine whether Gram-positive cells react similarly to the Gram-negative Pseudomonas aeruginosa. The outcomes of this study showed that the nanopillar structures on the wing surface did not affect any of the three species tested (B. subtilis, Planococcus maritimus, and S. aureus). The model predicts that the layer stiffness has an inverse relationship with the effective interaction parameter, proportional to the attraction between the bacterial layer and the wing surface. Therefore, for stiff cells to sufficiently expand to the point of rupture, there seems to be a stronger interaction with the surface. Comparative higher rigidity can be a potential cause for the resistance to the action of cicada wings. This is conclusive evidence demonstrating that the membrane's mechanical characteristics are the major determinants of bacteria's susceptibility to the action of the wing surface (i.e., the rigidity and initial stretching).80
This analysis shows firm physical damage to microbes adhering to nano surfaces that had not been observed before. The cell wall, composed of a cross-linked peptidoglycan network, is exceptionally resistive to mechanical forces, like those that result from adhesion. These models could discern the bacterial deformation profile on nanopillars by aligning the free energy of adherence with the bacteria's kinetic strain. The strain on the bacterial cell wall was then estimated using the equilibrium shape of the bacteria. This investigation of the wing topography of a cicada revealed that nanopillar arrays caused by enhanced bacterial adhesion can produce significant cell wall strains that can cause the mechanical breakdown of the cell wall. The model has been further evidenced by experimental studies that divulged that cicada wings have much lower bactericidal efficacy against Gram-positive cells like Bacillus subtilis and Staphylococcus aureus than against Gram-negative cells like E. coli and Pseudomonas fluorescens. Gram-positive bacteria benefit from thicker cell walls as they retain more elastic energy, reducing the strain on nanopillars and producing a weaker bactericidal effect.82
Ivanova's team augmented their research after attempting to make this pivotal discovery by carrying out comparable studies on the wings of three different species of dragonflies, including D. bipunctata, A. multipunctata, and H. papuensis. They intriguingly revealed that the surfaces of all three dragonfly wings exhibit bactericidal properties against a diverse range of bacterial species, in contrast to cicada wings, which only showed efficiency against Gram-negative bacteria. Their research revealed that these dragonflies have nanopillar-like features on their wings that set them apart from cicada wings in terms of height, smaller diameter and spacing, and a larger degree of randomization in the dimensions. The other key factor that impacts a bacterium's susceptibility to the physicomechanical bactericidal action of nanopillar surfaces is cell wall rigidity. The peptidoglycan covering and an internal turgor pressure interact to keep bacteria in their required form.83
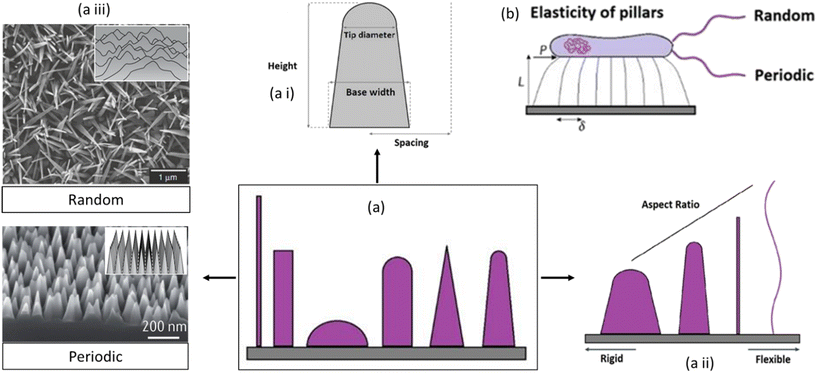 | ||
| Fig. 8 Schematic representation of nanosurface key parameters affecting the mechano-bactericidal activity of nanopillars. Representation of (a) different nanopatterns, (a i) tip diameter, base diameter, and height, (a ii) with increased aspect ratio from rigid to flexible, (a iii) the different nanopatterns can be either periodic or random arrays of surface features, and (b) amount of lateral tip deflection of flexible nanopillars.84,85 Copyright 2021, Elsevier; copyright 2020, Nature. | ||
A central key element is the height of nanostructures. Recently, it has been demonstrated that nanopillar patterns with short, blunt features and elevated pillar density (for example, height of 200 nm or less and spacing of 100 nm or less), befitting the nano-topography of a cicada wing, manifested higher levels of mechanobactericidal behavior than patterns containing more widely spaced, more prominent features (for example, height greater than 300 nm and spacing greater than 300 nm). Further research, however, reported that sharper pillars amplified the bactericidal prowess of nanopatterned surfaces. Since the overall area of the bacterial cell membrane resulting from adsorption onto the nanopillars increases proportionally with density, a higher pillar density will contribute to more stretching of the cell membrane. The susceptibility to stretching-induced membrane rupture will depend on the relationship between the membrane's attraction to the nanopillar surface and its stiffness.85,86
A characteristic interspacing of less than 300 nm is prevalent in most mechanobactericidal nanopatterns reported in the literature. However, if the inflexible pillars are arranged too closely together, there won't be sufficient space for the cell membrane to transit between them, which would culminate in the “bed of nails” effect. On the contrary, if the pillars are too short and sparse, the cell wall will stop expanding when it reaches the substrate between the pillars, minimizing the pattern's ability to kill bacteria. The bactericidal efficacy is reduced by spacings greater than the bacterial size, even for nanostructures with high aspect ratios. Instead of settling on the nanopillar tips, bacteria congregate between the structures. Similarly, the bacterial size should not transcend the nanopillar diameter. Nanostructures with a wide diameter and tight packing can be bacterial growing sites.86
Furthermore, a greater aspect ratio (the ratio of feature width to feature height) of the nanofeatures can increase pillar flexibility, which has been demonstrated to correspond to greater cell membrane stretching owing to the pillar's deflection during bacterium adsorption. This flexibility might be crucial for particular applications or interactions, possibly in the context of biological systems. Theoretical studies of pillar elasticity have shown that in contrast to relatively rigid substrates with a smaller aspect ratio, flexible substrates with flexible pillars are required to stimulate lateral stretching that may expose the organism to undue stress. These factors include deflection, deformation force, and the release of mechanical energies that cause retraction. Several material properties are critical in determining the activity of antibacterial nanopatterned surfaces: surface charge, topography, free energy, elastic modulus, etc. Surface charge affects the electrostatic interaction between the bacterial cell membrane and the surface. Opposite charges can disrupt membrane integrity, leading to bacterial death. Nanostructures provide more surface area in topographical interference, allowing better contact between the surface and bacterial cells and amplifying their effectiveness. Surface free energy influences biofilm formation, which is critical for preventing bacterial colonization and persistence. Lower surface free energy can prevent initial bacterial adhesion, hindering colonization and subsequent bacterial growth. Surface flexibility or stiffness affects how the surface interacts with bacterial cells. Stiffer surfaces may hinder bacterial attachment, while more flexible surfaces aid in disrupting bacterial structures.85
Combining these factors allows the creation of surfaces optimized for targeting specific bacteria or preventing bacterial colonization. The increased aspect ratio of the surface nanostructures may promote the improved bactericidal activity of pillar arrays toward bacterial cells on their attachment to the surface (Fig. 8).86 When bacteria adhere to these flexible pillars, the pillars deflect, causing stretching of the cell membrane. The deflection of the pillars during bacterium adsorption seems to be a critical factor in promoting cell membrane stretching. The overall implication is that the design of nanofeatures with a specific aspect ratio can influence the mechanical properties of the pillars and, consequently, affect the interaction with biological entities such as bacteria.87
In a recent study by Valiei et al., the researchers explored the significance of external forces, whether intentionally designed or unintentionally occurring, in inducing rapid cell death on nanopillar surfaces. The investigation aimed to determine the potential role of external mechanical forces in nanopillar-mediated bacterial killing efficiency on various hydrophilic nanopillar surfaces. The study revealed that bacteria on multiple hydrophilic surfaces, termed “mechano-bactericidal,” remained viable unless exposed to a moving air–liquid interface, leading to substantial cell death. The observed bactericidal activity was contingent on the movement of an air–liquid interface, and the researchers hypothesized that the considerable normal forces generated by the capillary action of fluids during this movement might be responsible for rupturing bacteria on the nanopillars. Notably, the described external normal forces exhibited the ability to kill bacteria within seconds, presenting a potentially practical and efficient mechanism compared to other proposed methods.88
Along with this evolvement, diverse researchers have posited alternate mechanisms to contribute to their observations. Even if all of these identified schemes attribute bacterial death to physical forces, they differ in their theories about the source and strength of these constraints. Jenkins et al. investigated the bactericidal activity of titanium dioxide against S. aureus, E. coli, and K. pneumoniae bacteria, which asserts oxidative stress as a potential bactericidal driving mechanism. The evidence for this mechanism was retrieved through electron microscopy analysis, which showed that nanopillars sparsely penetrated and distorted cell membranes and intermittently caused morphological alterations. Viability tests proceeded to reveal a decline in the population of living bacteria, which suggested that a physiological impact instead of a mechanical element might be involved in the process that causes the internal damage through the release of free radicals.89
Many industrial, agricultural, and medical applications for bactericidal nanostructured surfaces are connected with fluid flow. Ship hulls, water or petroleum fuel pipes, liquid storage tanks, and food and beverage packaging are examples of industrial and agricultural applications. The accumulation of bacterial colonies on solid surfaces causes tremendous issues in many industries, costing billions of dollars in economic damages and human lives. Biomimicking nanostructured surfaces has shown a promising future in bacterial colonization and associated concerns. Among the medicinal uses for antibacterial nanostructured surfaces are bone implants, vascular stents, and catheters. Knowing that biological materials come in direct contact with the inner or external environment of the human body, it is crucial to equip them with antibacterial qualities. The antibacterial surface protects the biological substance and minimizes functional impairment when operating on the human body. Such biocide-free, physic mechanical strategies may provide many materials encountered in food processing, packaging, and food preparation environments with long-term biofilm mitigation capabilities. Thus, reproducing such natural nanopatterns on food-contact surfaces is an intriguing method for minimizing contamination, increasing productivity, and alleviating operating costs.90
The real-world application of mechano-bactericidal surfaces, particularly in healthcare- associated infections (HAIs), demands careful consideration of various factors, and durability emerges as a pivotal aspect. Ensuring the longevity and efficacy of these surfaces requires assessment in critical areas, including their ability to withstand repeated touching, resistance to rigorous cleaning procedures, sustained bactericidal effectiveness over time, compatibility with existing hospital materials, avert thrombus formation if in contact with blood, and be economical and easy to manufacture on an immense scale, impact on human health, and compliance with regulatory standards. Successfully navigating these considerations is essential for successfully integrating mechano-bactericidal surfaces in healthcare settings to combat HAIs. The concern of whether an antibacterial nanopattern is detrimental to mammalian cells has often been raised; subsequently, eukaryotic cells can adapt to deformational strain exerted by nanopatterned surfaces by perforating the surface features. Due to the enormous physical size of eukaryotic cells and their elastic membrane, they can inhabit nanostructured surfaces successfully. Surface nano topography has also been observed to impact eukaryotic cell adhesion and migration. Cells can also be strategically aligned using certain nanopatterns. Black silicon surfaces, for example, have been shown to prevent bacterial biofilm formation while facilitating the attachment of eukaryotic cells on pre-infected surfaces.91
The efficacious deployment of this technology in such industries will increase the quality of life by limiting economic and health hazards. However, the prevalence of infections linked with medical devices continues to rise due to the complex structure of bacterial biofilms, antibiotic resistance, and the inadequate capacity of monofunctional antibacterial materials to prevent bacterial colonization on the medical surface. As a result, many contemporary methods are centered on developing innovative antibacterial surfaces with dual antimicrobial efficacy. These surfaces are based on combining two components into one unit that can eliminate attached bacteria (antibiotics, peptides) and resist or release bacterial adherence (hydrophilic polymers, antiadhesive, topography, bioinspired surfaces, and so on). These hybrid antibacterial surfaces inhibit bacterial growth and may possess additional functionalities, such as self-cleaning, resistance to biofilm formation, or compatibility with specific environments. Materials incorporating several antibacterial processes are predicted to synergize and enhance combined defense against medical device infections.91,92
5. Summary and outlook
Instead of viral infections, future pandemics could arise from other hazardous pathogens, such as bacteria associated with poor food and surface contamination. Both humans and animals can contract infections from bacteria that have developed multi-drug resistance and novel mutations. They might spread infections that are more challenging to treat than those brought on by non-resistant bacteria. Antibiotic resistance will inevitably result in high mortality, expensive medical infrastructure, and hospitalization. Diverse microorganisms can grow under stable conditions, which may produce biofilms. Biofilm is a persistent source of harmful microorganisms that cause severe infectious diseases. The sustainability and feasibility of the antimicrobial model to successfully constrain bacterial adhesion and biofilm development have been proven by existing studies on the bactericidal capability of a biomimetic surface. Nature has perfected the art of creating antibacterial and antibiofouling surfaces over millions of years of evolution. Studying these surfaces at the micro and nanoscale can unlock the secrets behind their effectiveness, paving the way for the development of next-generation antibacterial solutions with minimal side effects. Nature's examples serve as a guiding light in addressing challenges like antibiotic resistance, biocompatibility, lifespan, efficiency, and more. In this way, different texturing on the surfaces of plants, insect wings, lizards, and sharks protects them from bacterial infection and colonization. There are numerous synthetic analogs with comparable antibacterial properties.Depending on the underlying mechanisms, antibacterial surfaces can resist or repress bacteria when they come into direct contact with each other. The ability of bacteria to perceive mechanical cues from surfaces, such as surface texture, is well recognized. At the micro level, hydrodynamics influences bacteria, while physicochemical forces and cell membrane deformation impact features at the nanoscale level. The evolution of surface-attachment mechanisms for micro-patterned surfaces and the removal of attached bacteria through self-cleaning have significantly contributed to developing novel anti-biofilm techniques. The review presents bioinspired materials with remarkable antibacterial or anti-adhesion characteristics. The attributes of nanotexturing need to be examined to determine how the surface interacts with and disrupts the cell through nano-stretching. The study reveals that the characteristics of the bacterial cell, including the composition of the cell wall, thickness of the peptidoglycan layer, outer membrane, and a few crucial nano-parameters, determine the rate of killing by a given surface. The articulation of surface topography has led to the recognition that antibacterial activities are not just constrained to chemical interactions and that nanopatterns with particular geometries (Fig. 9) can also inhibit or eliminate bacteria. Even though the bactericidal effects of nanopatterned surfaces would be restricted to the bacteria in direct contact with them and, unlike chemical-based methods, the nanopatterns are unable to sterilize the surrounding environment, their numerous noteworthy advantages still mandate further research to comprehend and optimize their antibacterial functions. These patterned surfaces are sustainable antibacterial surfaces for a variety of uses because they don't show toxicity, do not even make microbes resistant, and are not even metabolically consumed. The design of a nanostructured surface and the quantification of the distinctive aspects of surface morphology contribute to enabling a viable solution to confer bacterial resistance to different biomedical applications. It is equally crucial that antimicrobial surfaces in actual clinical settings be used carefully to consider both biocompatibility and durability to reduce the potential of adverse effects caused by inherent properties.
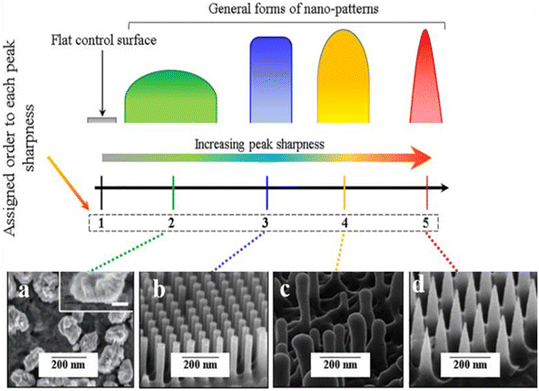 | ||
| Fig. 9 Illustrations of different forms of the peak sharpness and their assigned orders in this study compared to the flat control surface and examples of the corresponding shapes in the literature (a) nano-nuggets (magnified SEM image is given in the inset, scale bar: 50nm), (b) nanopillars, (c) nanopillar (of dragonfly wing) and (d) nano-spikes.78 Copyright 2021, Elsevier. | ||
Conflicts of interest
■■■■Acknowledgements
This work was supported by SERB (Science & Technology Research Board)—a statutory body under the Department of Science & Technology, Government of India, under the Research grant of the Ramanujan Fellow Award (File number: SB/S2/RJN-013/2018), and by J&K Science, Technology & Innovation Council, Department of Science & Technology.References
- J. L. Vincent, J. Rello, J. Marshall, E. Silva, A. Anzueto, C. D. Martin, R. Moreno, J. Lipman, C. Gomersall, Y. Sakr and K. Reinhart, International Study of the Prevalence and Outcomes of Infection in Intensive Care Units, JAMA, J. Am. Med. Assoc., 2009, 302(21), 2323–2329, DOI:10.1001/jama.2009.1754.
- A. Kramer, I. Schwebke and G. Kampf, How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review, BMC Infect. Dis., 2006, 6(1), 130, DOI:10.1186/1471-2334-6-130.
- A. Rynda-Apple, K. M. Robinson and J. F. Alcorn, Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease, Infect. Immun., 2015, 83(10), 3764–3770, DOI:10.1128/IAI.00298-15.
- D. Hu, H. Li, B. Wang, Z. Ye, W. Lei, F. Jia, Q. Jin, K. F. Ren and J. Ji, Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus Aureus Biofilm, ACS Nano, 2017, 11(9), 9330–9339, DOI:10.1021/acsnano.7b04731.
- B. Allegranzi, S. B. Nejad, C. Combescure, W. Graafmans, H. Attar, L. Donaldson and D. Pittet, Burden of Endemic Health-Care-Associated Infection in Developing Countries: Systematic Review and Meta-Analysis, Lancet, 2011, 377(9761), 228–241, DOI:10.1016/S0140-6736(10)61458-4.
- H. Getachew, A. Derbie and D. Mekonnen, Surfaces and Air Bacteriology of Selected Wards at a Referral Hospital, Northwest Ethiopia: A Cross-Sectional Study, Int. J. Microbiol., 2018, 2018, 1–7, DOI:10.1155/2018/6413179.
- H. Lei, Y. Li, S. Xiao, X. Yang, C. Lin, S. L. Norris, D. Wei, Z. Hu and S. Ji, Logistic Growth of a Surface Contamination Network and Its Role in Disease Spread, Sci. Rep., 2017, 7(1), 14826, DOI:10.1038/s41598-017-13840-z.
- A. M. Wilson, M. H. Weir, S. F. Bloomfield, E. A. Scott and K. A. Reynolds, Modeling COVID-19 Infection Risks for a Single Hand-to-Fomite Scenario and Potential Risk Reductions Offered by Surface Disinfection, Am. J. Infect. Control, 2021, 49(6), 846–848, DOI:10.1016/j.ajic.2020.11.013.
- H. Gibson, J. H. Taylor, K. E. Hall and J. T. Holah, Effectiveness of Cleaning Techniques Used in the Food Industry in Terms of the Removal of Bacterial Biofilms, J. Appl. Microbiol., 1999, 87(1), 41–48, DOI:10.1046/j.1365-2672.1999.00790.x.
- E. H. Ahmed, H. A. M. Hassan, N. M. El-Sherbiny and A. M. A. Soliman, Bacteriological Monitoring of Inanimate Surfaces and Equipment in Some Referral Hospitals in Assiut City, Egypt, Int. J. Microbiol., 2019, 2019, 1–9, DOI:10.1155/2019/5907507.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Bacterial Biofilms: From the Natural Environment to Infectious Diseases, Nat. Rev. Microbiol., 2004, 2(2), 95–108, DOI:10.1038/nrmicro821.
- C. Pal, M. D. Maciá, A. Oliver, I. Schachar and A. Buckling, Coevolution with Viruses Drives the Evolution of Bacterial Mutation Rates, Nature, 2007, 450(7172), 1079–1081, DOI:10.1038/nature06350.
- N. Shafran, I. Shafran, H. Ben-Zvi, S. Sofer, L. Sheena, I. Krause, A. Shlomai, E. Goldberg and E. H. Sklan, Secondary Bacterial Infection in COVID-19 Patients Is a Stronger Predictor for Death Compared to Influenza Patients, Sci. Rep., 2021, 11(1), 12703, DOI:10.1038/s41598-021-92220-0.
- D. Campoccia, L. Montanaro and C. R. Arciola, A Review of the Biomaterials Technologies for Infection-Resistant Surfaces, Biomaterials, 2013, 34(34), 8533–8554, DOI:10.1016/j.biomaterials.2013.07.089.
- H. C. Flemming and J. Wingender, The Biofilm Matrix, Nat. Rev. Microbiol., 2010, 8(9), 623–633, DOI:10.1038/nrmicro2415.
- C. Berne, A. Ducret, G. G. Hardy and Y. V. Brun, Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria, Microbiol. Spectrum, 2015, 3(4), 163–199, DOI:10.1128/microbiolspec.mb-0018-2015.
- A. M. Lazdunski, I. Ventre and J. N. Sturgis, Regulatory Circuits and Communication in Gram-Negative Bacteria, Nat. Rev. Microbiol., 2004, 2(7), 581–592, DOI:10.1038/nrmicro924.
- R. A. Gittens, T. McLachlan, R. Olivares-Navarrete, Y. Cai, S. Berner, R. Tannenbaum, Z. Schwartz, K. H. Sandhage and B. D. Boyan, The Effects of Combined Micron-/Submicron-Scale Surface Roughness and Nanoscale Features on Cell Proliferation and Differentiation, Biomaterials, 2011, 32(13), 3395–3403, DOI:10.1016/j.biomaterials.2011.01.029.
- J. A. Lichter, K. J. Van Vlietpa and M. F. Rubner, Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform, Macromolecules, 2009, 42(22), 8573–8586, DOI:10.1021/ma901356s.
- T. Bjarnsholt, O. Ciofu, S. Molin, M. Givskov and N. Høiby, Applying Insights from Biofilm Biology to Drug Development-Can a New Approach Be Developed?, Nat. Rev. Drug Discovery, 2013, 12(10), 791–808, DOI:10.1038/nrd4000.
- M. Liu, M. T. Li, S. Xu, H. Yang and H. B. Sun, Bioinspired Superhydrophobic Surfaces via Laser-Structuring, Front. Chem., 2020, 8, 835, DOI:10.3389/fchem.2020.00835.
- E. P. Ivanova, J. Hasan, H. K. Webb, V. K. Truong, G. S. Watson, J. A. Watson, V. A. Baulin, S. Pogodin, J. Y. Wang, M. J. Tobin, C. Löbbe and R. J. Crawford, Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas Aeruginosa Cells by Cicada Wings, Small, 2012, 8(16), 2489–2494, DOI:10.1002/smll.201200528.
- M. Sun, G. S. Watson, Y. Zheng, J. A. Watson and A. Liang, Wetting Properties on Nanostructured Surfaces of Cicada Wings, J. Exp. Biol., 2009, 212(19), 3148–3155, DOI:10.1242/jeb.033373.
- S. Pogodin, J. Hasan, V. A. Baulin, H. K. Webb, V. K. Truong, T. H. Phong Nguyen, V. Boshkovikj, C. J. Fluke, G. S. Watson, J. A. Watson, R. J. Crawford and E. P. Ivanova, Biophysical Model of Bacterial Cell Interactions with Nanopatterned Cicada Wing Surfaces, Biophys. J., 2013, 104(4), 835–840, DOI:10.1016/j.bpj.2012.12.046.
- J. Román-Kustas, J. B. Hoffman, D. Alonso, J. H. Reed, A. E. Gonsalves, J. Oh, S. Hong, K. D. Jo, C. E. Dana, M. Alleyne, N. Miljkovic and D. M. Cropek, Analysis of Cicada Wing Surface Constituents by Comprehensive Multidimensional Gas Chromatography for Species Differentiation, Microchem. J., 2020, 158, 105089, DOI:10.1016/j.microc.2020.105089.
- D. P. Linklater, S. Juodkazis and E. P. Ivanova, Nanofabrication of Mechano-Bactericidal Surfaces, Nanoscale, 2017, 9(43), 16564–16585, 10.1039/c7nr05881k.
- S. Dehghani, M. Mashreghi, A. H. N. Nezhad, J. Karimi, S. Hosseinpour and A. Davoodi, Exploring Mechano-Bactericidal Nature of Psalmocharias Cicadas Wings: An Analytical Nanotopology Investigation Based on Atomic Force Microscopy Characterization, Surf. Interfaces, 2021, 26, 101407, DOI:10.1016/j.surfin.2021.101407.
- S. H. T. Nguyen, H. K. Webb, J. Hasan, M. J. Tobin, R. J. Crawford and E. P. Ivanova, Dual Role of Outer Epicuticular Lipids in Determining the Wettability of Dragonfly Wings, Colloids Surf., B, 2013, 106, 126–134, DOI:10.1016/j.colsurfb.2013.01.042.
- D. E. Mainwaring, S. H. Nguyen, H. Webb, T. Jakubov, M. Tobin, R. N. Lamb, H.-F. Wu, R. Marchant, R. J. Crawford and E. P. Ivanova, The Nature of Inherent Bactericidal Activity: Insights from the Nanotopology of Three Species of Dragonfly, Nanoscale, 2016, 8(12), 6527–6534, 10.1039/C5NR08542J.
- C. D. Bandara, S. Singh, I. O. Afara, A. Wolff, T. Tesfamichael and K. Ostrikov, Oloyede, Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia Coli, ACS Appl. Mater. Interfaces, 2017, 9(8), 6746–6760, DOI:10.1021/acsami.6b13666.
- S. Cheeseman, S. Owen, V. K. Truong, D. Meyer, S. H. Ng, J. Vongsvivut, D. Linklater, M. J. Tobin, M. Werner, V. A. Baulin, P. Luque, R. Marchant, S. Juodkazis, R. J. Crawford and E. P. Ivanova, Pillars of Life: Is There a Relationship between Lifestyle Factors and the Surface Characteristics of Dragonfly Wings?, ACS Omega, 2018, 3(6), 6039–6046, DOI:10.1021/acsomega.8b00776.
- G. D. Bixler, A. Theiss, B. Bhushan and S. C. Lee, Antifouling Properties of Microstructured Surfaces Bio-Inspired by Rice Leaves and Butterfly Wings, J. Colloid Interface Sci., 2014, 419, 114–133 CrossRef CAS PubMed.
- Z. Han, et al., Long-term durability of superhydrophobic properties of butterfly wing scales after continuous contact with water, Colloids Surf., A, 2017, 518, 139–144 CrossRef CAS.
- Y. Fang and G. Sun, Complex Wettability and Self-Cleaning Performance of Butterfly Wing Surface, Appl. Mech. Mater., 2015, 723, 943–947, DOI:10.4028/www.scientific.net/amm.723.943.
- G. D. Bixler and B. Bhushan, Fluid Drag Reduction and Efficient Self-Cleaning with Rice Leaf and Butterfly Wing Bioinspired Surfaces, Nanoscale, 2013, 5(17), 7685–7710, 10.1039/c3nr01710a.
- G. D. Bixler and B. Bhushan, Fluid Drag Reduction with Sharkskin Riblet Inspired Microstructured Surfaces, Adv. Funct. Mater., 2013, 23(36), 4507–4528, DOI:10.1002/adfm.201203683.
- X. Pu, G. J. Li and Y. H. Liu, Progress and Perspective of Studies on Biomimetic Shark Skin Drag Reduction, ChemBioEng Rev., 2016, 3(1), 26–40, DOI:10.1002/cben.201500011.
- D. Y. Zhao, Z. P. Huang, M. J. Wang, T. Wang and Y. Jin, Vacuum Casting Replication of Micro-Riblets on Shark Skin for Drag-Reducing Applications, J. Mater. Process. Technol., 2012, 212(1), 198–202, DOI:10.1016/j.jmatprotec.2011.09.002.
- X. Pu, G. Li and H. Huang, Preparation, Anti-Biofouling and Drag-Reduction Properties of a Biomimetic Shark Skin Surface, Biol. Open, 2016, 5(4), 389–396, DOI:10.1242/bio.016899.
- G. Xiao, Y. Zhang, Y. He and S. He, Optimization of Belt Grinding Stepover for Biomimetic Micro-Riblets Surface on Titanium Alloy Blades, Int. J. Adv. Des. Manuf. Technol., 2020, 110(5–6), 1503–1513, DOI:10.1007/s00170-020-05935-1.
- W. R. Hansen and K. Autumn, Evidence for self-cleaning in gecko setae, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(2), 385–389 CrossRef CAS PubMed.
- X. Li, G. S. Cheung, G. S. Watson, J. A. Watson, S. Lin, L. Schwarzkopf and D. W. Green, The Nanotipped Hairs of Gecko Skin and Biotemplated Replicas Impair and/or Kill Pathogenic Bacteria with High Efficiency, Nanoscale, 2016, 8(45), 18860–18869, 10.1039/c6nr05046h.
- D. W. Green, K. K.-H. Lee, J. A. Watson, H.-Y. Kim, K.-S. Yoon, E.-J. Kim, J.-M. Lee, G. S. Watson and H.-S. Jung, High Quality Bioreplication of Intricate Nanostructures from a Fragile Gecko Skin Surface with Bactericidal Properties, Sci. Rep., 2017, 7(1), 41023, DOI:10.1038/srep41023.
- G. S. Watson, L. Schwarzkopf, B. W. Cribb, S. Myhra, M. Gellender and J. A. Watson, Removal Mechanisms of Dew via Self-Propulsion off the Gecko Skin, J. R. Soc., Interface, 2015, 12(105), 20141396, DOI:10.1098/rsif.2014.1396.
- J. Ma, Y. Sun, K. Gleichauf, J. Lou and Q. Li, Nanostructure on Taro Leaves Resists Fouling by Colloids and Bacteria under Submerged Conditions, Langmuir, 2011, 27(16), 10035–10040, DOI:10.1021/la2010024.
- M. Kumar and R. Bhardwaj, Wetting Characteristics of Colocasia Esculenta(Taro) Leaf and a Bioinspired Surface Thereof, Sci. Rep., 2020, 10(1), 935, DOI:10.1038/s41598-020-57410-2.
- H. J. Ensikat, P. Ditsche-Kuru, C. Neinhuis and W. Barthlott, Superhydrophobicity in Perfection: The Outstanding Properties of the Lotus Leaf, Beilstein J. Nanotechnol., 2011, 2(1), 152–161, DOI:10.3762/bjnano.2.19.
- Y. Y. Yan, N. Gao and W. Barthlott, Mimicking Natural Superhydrophobic Surfaces and Grasping the Wetting Process: A Review on Recent Progress in Preparing Superhydrophobic Surfaces, Adv. Colloid Interface Sci., 2011, 169(2), 80–105, DOI:10.1016/j.cis.2011.08.005.
- M. Konar, B. Roy and T. Govindaraju, Molecular Architectonics-Guided Fabrication of Superhydrophobic and Self-Cleaning Materials, Adv. Mater. Interfaces, 2020, 7(11), 2000246, DOI:10.1002/admi.202000246.
- L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Petal Effect: A Superhydrophobic State with High Adhesive Force, Langmuir, 2008, 24(8), 4114–4119, DOI:10.1021/la703821h.
- S. Nishimoto and B. Bhushan, Bioinspired Self-Cleaning Surfaces with Superhydrophobicity, Superoleophobicity, and Superhydrophilicity, RSC Adv., 2013, 3(3), 671–690, 10.1039/c2ra21260a.
- D. Ebert and B. Bhushan, Wear-Resistant Rose Petal-Effect Surfaces with Superhydrophobicity and High Droplet Adhesion Using Hydrophobic and Hydrophilic Nanoparticles, J. Colloid Interface Sci., 2012, 384(1), 182–188, DOI:10.1016/j.jcis.2012.06.070.
- Y. Zheng, C. Zhang, J. Wang, Y. Liu, C. Shen and J. Yang, Robust Adhesion of Droplets via Heterogeneous Dynamic Petal Effects, J. Colloid Interface Sci., 2019, 557, 737–745, DOI:10.1016/j.jcis.2019.09.070.
- T. Darmanin and F. Guittard, Superhydrophobic and Superoleophobic Properties in Nature, Mater. Today, 2015, 18(5), 273–285, DOI:10.1016/j.mattod.2015.01.001.
- S. Dai, Y. Zhu, Y. Gu and Z. Du, Biomimetic Fabrication and Photoelectric Properties of Superhydrophobic ZnO Nanostructures on Flexible PDMS Substrates Replicated from Rose Petal, Appl. Phys. A: Mater. Sci. Process., 2019, 125(2), 138, DOI:10.1007/s00339-019-2438-7.
- Y. Cheng, G. Feng and C. I. Moraru, Micro-and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review, Front. Microbiol., 2019, 10, 410243, DOI:10.3389/fmicb.2019.00191.
- X. Yang, W. Zhang, X. Qin, M. Cui, Y. Guo, T. Wang, K. Wang, Z. Shi, C. Zhang, W. Li and Z. Wang, Recent Progress on Bioinspired Antibacterial Surfaces for Biomedical Application, Biomimetics, 2022, 7(3), 88, DOI:10.3390/biomimetics7030088.
- G. Mohd, I. M. Bhat, I. Kakroo, A. Balachandran, R. Tabasum, K. Majid, M. F. Wani, U. Manna, G. Ghodake and S. Lone, Azolla Pinnata: Sustainable Floating Oil Cleaner of Water Bodies, ACS Omega, 2024, 9(11), 12725–12733, DOI:10.1021/acsomega.3c08417.
- W. Barthlott and C. Neinhuis, Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces, Planta, 1997, 202(1), 1–8, DOI:10.1007/s004250050096.
- S. Zheng, M. Bawazir, A. Dhall, H. E. Kim, L. He, J. Heo and G. Hwang, Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion, Front. Bioeng. Biotechnol., 2021, 9, 643722, DOI:10.3389/fbioe.2021.643722.
- J. Liu, X. Zhang, R. Wang, F. Long and L. Liu, A Stable and Indurative Superhydrophobic Film with Excellent Anti-Bioadhesive Performance for 6061 Al Protection, Materials, 2020, 13(23), 1–17, DOI:10.3390/ma13235564.
- T. Koishi, K. Yasuoka, S. Fujikawa, T. Ebisuzaki and C. Z. Xiao, Coexistence and Transition between Cassie and Wenzel State on Pillared Hydrophobic Surface, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(21), 8435–8440, DOI:10.1073/pnas.0902027106.
- X. Zhang, L. Wang and E. Levänen, Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion, RSC Adv., 2013, 3(30), 12003–12020, 10.1039/c3ra40497h.
- A. Balachandran, H. Parayilkalapurackal, S. Rajpoot and S. Lone, Bioinspired Green Fabricating Design of Multidimensional Surfaces for Atmospheric Water Harvesting, ACS Appl. Bio Mater., 2023, 6(1), 44–63 CrossRef CAS PubMed.
- A. Balachandran, I. M. Bhat, R. Tabasum, G. Mohd, M. F. Wani, K. Majid, M. Wahid and S. Lone, Transfer-Printed Environmental-Friendly Anisotropic Filter with Laser- Controlled Micropores for Efficient Oil/Water Separation, ACS Appl. Polym. Mater., 2023, 5(3), 2272–2281 CrossRef CAS.
- J. Ju, K. Xiao, X. Yao, H. Bai and L. Jiang, Bioinspired Conical Copper Wire with Gradient Wettability for Continuous and Efficient Fog Collection, Adv. Mater., 2013, 25(41), 5937–5942, DOI:10.1002/adma.201301876.
- A. Riveiro, A. L. B. Maçon, J. del Val, R. Comesaña and J. Pou, Laser Surface Texturing of Polymers for Biomedical Applications, Front. Phys., 2018, 6, 16, DOI:10.3389/fphy.2018.00016.
- B. J. Zhang, J. Park, K. J. Kim and H. Yoon, Biologically Inspired Tunable Hydrophilic/Hydrophobic Surfaces: A Copper Oxide Self-Assembly Multitier Approach, Bioinspiration Biomimetics, 2012, 7(3), 036011, DOI:10.1088/1748-3182/7/3/036011.
- L. Peng, L. Chang, X. Liu, J. Lin, H. Liu, B. Han and S. Wang, Antibacterial Property of a Polyethylene Glycol-Grafted Dental Material, ACS Appl. Mater. Interfaces, 2017, 9(21), 17688–17692, DOI:10.1021/acsami.7b05284.
- L. B. Boinovich, V. V. Kaminsky, A. G. Domantovsky, K. A. Emelyanenko, V. Aleshkin, E. R. Zulkarneev, I. A. Kiseleva and A. M. Emelyanenko, Bactericidal activity of superhydrophobic and superhydrophilic copper in bacterial dispersions, Langmuir, 2019, 35(7), 2832–2841 CrossRef CAS PubMed.
- J. Bruzaud, J. Tarrade, E. Celia, T. Darmanin, E. T. de Givenchy, F. Guittard, J.-M. Herry, M. Guilbaud and M.-N. Bellon-Fontaine, The design of superhydrophobic stainless steel surfaces by controlling nanostructures: A key parameter to reduce the implantation of pathogenic bacteria, Mater. Sci. Eng., C, 2017, 73, 40–47 CrossRef CAS PubMed.
- T. Sun, H. Tan, D. Han, Q. Fu and L. Jiang, No Platelet Can Adhere-Largely Improved Blood Compatibility on Nanostructured Superhydrophobic Surfaces, Small, 2005, 1(10), 959–963, DOI:10.1002/smll.200500095.
- M. Beeby, J. C. Gumbart, B. Roux and G. J. Jensen, Architecture and Assembly of the Gram-Positive Cell Wall, Mol. Microbiol., 2013, 88(4), 664–672, DOI:10.1111/mmi.12203.
- X. Li and T. Chen, Enhancement and Suppression Effects of a Nanopatterned Surface on Bacterial Adhesion, Phys. Rev. E, 2016, 93(5), 052419, DOI:10.1103/PhysRevE.93.052419.
- X. Li, Bactericidal Mechanism of Nanopatterned Surfaces, Phys. Chem. Chem. Phys., 2015, 18(2), 1311–1316, 10.1039/c5cp05646b.
- T. Liu, Q. Cui, Q. Wu, X. Li, K. Song, D. Ge and S. Guan, Mechanism Study of Bacteria Killed on Nanostructures, J. Phys. Chem. B, 2019, 123(41), 8686–8696, DOI:10.1021/acs.jpcb.9b07732.
- E. Maleki, M. J. Mirzaali, M. Guagliano and S. Bagherifard, Analyzing the mechano- bactericidal effect of nanopatterned surfaces on different bacteria species, Surf. Coat. Technol., 2021, 408, 126782 CrossRef CAS.
- C. Echeverria, M. D. T. Torres, M. Fernández-García, C. de la Fuente-Nunez and A. Muñoz-Bonilla, Physical Methods for Controlling Bacterial Colonization on Polymer Surfaces, Biotechnol. Adv., 2020, 43, 107586, DOI:10.1016/j.biotechadv.2020.107586.
- C. M. Bhadra, M. Werner, V. A. Baulin, V. K. Truong, M. Kobaisi, S. H. Nguyen, A. Balcytis, S. Juodkazis, J. Y. Wang, D. E. Mainwaring, R. J. Crawford and E. P. Ivanova, Subtle Variations in Surface Properties of Black Silicon Surfaces Influence the Degree of Bactericidal Efficiency, Nano-Micro Lett., 2018, 10(2), 36, DOI:10.1007/s40820-017-0186-9.
- Y. Deng, M. Sun and J. W. Shaevitz, Measuring Peptidoglycan Elasticity and Stress- Stiffening of Live Bacterial Cells, Biophys. J., 2011, 100(3), 514a–515a, DOI:10.1016/j.bpj.2010.12.3012.
- M. Akbari Edgahi, S. M. Naghib, A. Emamian, H. Ramezanpour, F. Haghiralsadat and D. Tofighi, A Practical Review over Surface Modification, Nanopatterns, Emerging Materials, Drug Delivery Systems, and Their Biophysiochemical Properties for Dental Implants: Recent Progresses and Advances, Nanotechnol. Rev., 2022, 11(1), 637–679, DOI:10.1515/ntrev-2022-0037.
- D. P. Linklater, M. De Volder, V. A. Baulin, M. Werner, S. Jessl, M. Golozar, L. Maggini, S. Rubanov, E. Hanssen, S. Juodkazis and E. P. Ivanova, High Aspect Ratio Nanostructures Kill Bacteria via Storage and Release of Mechanical Energy, ACS Nano, 2018, 12(7), 6657–6667, DOI:10.1021/acsnano.8b01665.
- E. P. Ivanova, D. P. Linklater, M. Werner, V. A. Baulin, X. M. Xu, N. Vrancken, S. Rubanov, E. Hanssen, J. Wandiyanto, V. K. Truong, A. Elbourne, S. Maclaughlin, S. Juodkazis and R. J. Crawford, The Multi-Faceted Mechano-Bactericidal Mechanism of Nanostructured Surfaces, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(23), 12598–12605, DOI:10.1073/pnas.1916680117.
- J. Jenkins, M. I. Ishak, M. Eales, A. Gholinia, S. Kulkarni, T. F. Keller, P. W. May, A. H. Nobbs and B. Su, Resolving physical interactions between bacteria and nanotopographies with focused ion beam scanning electron microscopy, iScience, 2021, 24(7), 102818, DOI:10.1016/j.isci.2021.102818.
- J. Jenkins, J. Mantell, C. Neal, A. Gholinia, P. Verkade, A. H. Nobbs and B. Su, Antibacterial Effects of Nanopillar Surfaces Are Mediated by Cell Impedance, Penetration and Induction of Oxidative Stress, Nat. Commun., 2020, 11(1), 1626, DOI:10.1038/s41467-020-15471-x.
- M. Resnik, M. Benčina, E. Levičnik, N. Rawat, A. Iglič and I. Junkar, Strategies for Improving Antimicrobial Properties of Stainless Steel, Materials, 2020, 13(13), 2944, DOI:10.3390/ma13132944.
- E. C. F. Baldo, F. A. P. Anjolette, E. C. Arantes and M. A. Baldo, Toad Poison and Drug Discovery, in Toxins and Drug Discovery, 2017, p. 373 Search PubMed.
- C. D. Bandara, G. Ballerin, M. Leppänen, T. Tesfamichael, K. K. Ostrikov and C. B. Whitchurch, Resolving Bio-Nano Interactions of E. Coli Bacteria-Dragonfly Wing Interface with Helium Ion and 3D-Structured Illumination Microscopy to Understand Bacterial Death on Nanotopography, ACS Biomater. Sci. Eng., 2020, 6(7), 3925–3932, DOI:10.1021/acsbiomaterials.9b01973.
- A. Valiei, N. Lin, J. F. Bryche, G. McKay, M. Canva, P. G. Charette, D. Nguyen, C. Moraes and N. Tufenkji, Hydrophilic Mechano-Bactericidal Nanopillars Require External Forces to Rapidly Kill Bacteria, Nano Lett., 2020, 20(8), 5720–5727, DOI:10.1021/acs.nanolett.0c01343.
- X. M. Yang, J. W. Hou, Y. Tian, J. Y. Zhao, Q. Q. Sun and S. B. Zhou, Antibacterial Surfaces: Strategies and Applications, Sci. China: Technol. Sci., 2022, 65(5), 1000–1010, DOI:10.1007/s11431-021-1962-x.
- C. D. W. Wilkinson, M. Riehle, M. Wood, J. Gallagher and A. S. G. Curtis, The Use of Materials Patterned on a Nano- and Micro-Metric Scale in Cellular Engineering, Mater. Sci. Eng. Carbon, 2002, 19(1–2), 263–269, DOI:10.1016/S0928-4931(01)00396-4.
- M. K. Chug and E. J. Brisbois, Recent Developments in Multifunctional Antimicrobial Surfaces and Applications toward Advanced Nitric Oxide-Based Biomaterials, ACS Mater. Au, 2022, 2(5), 525–551, DOI:10.1021/acsmaterialsau.2c00040.
| This journal is © The Royal Society of Chemistry 2024 |