Precise and accurate isotopic analysis of uranium and thorium in uranium ore concentrates using ICP-MS and their age dating for nuclear forensic analysis
Wei
Wang
 ,
Jiang
Xu
,
Ruiyang
Xi
,
Siqi
Guo
,
Yongyang
Su
,
Jiang
Xu
,
Ruiyang
Xi
,
Siqi
Guo
,
Yongyang
Su
 ,
Sui
Fang
,
Haitao
Zhang
,
Yalong
Wang
,
Jinlong
Fan
,
Lei
Feng
,
Yufeng
Wang
and
Zhiming
Li
*
,
Sui
Fang
,
Haitao
Zhang
,
Yalong
Wang
,
Jinlong
Fan
,
Lei
Feng
,
Yufeng
Wang
and
Zhiming
Li
*
Northwest Institute of Nuclear Technology, P.O. Box 69-14, Xi'an 710024, China. E-mail: lizhiming@nint.ac.cn
First published on 8th December 2023
Abstract
Isotope ratios and age dating of uranium ore concentrates (UOCs) are of great significance in nuclear forensic analysis. This paper presents accurate and precise isotopic analysis of uranium and thorium in 15 different UOCs using two types of ICP-MS instruments. 235U/238U, 234U/238U and 230Th/232Th isotope ratios were determined by Multi-Collector Inductively Coupled-Plasma Mass Spectrometry (MC-ICP-MS), while 230Th/234U and 234U/232Th atom ratios were determined by triple quadruple Inductively Coupled-Plasma Mass Spectrometry (ICP-MS/MS). Results show that the relative uncertainties in the determined values of 235U/238U, 234U/238U and 230Th/234U ratios for CRM124-1 are 0.0014%, 0.02% and 3.2%, respectively. It has been proved that an aliquot containing 1.12 μg of 238U was the minimum sample amount to obtain the representative 235U/238U ratios of the UOC samples for bulk analysis by MC-ICP-MS, especially when the sample of UOCs was inhomogeneous or a mixture of several UOCs. No correlation between the measured 238U/235U and 235U/234U ratios is observed in the UOC samples. It is possible to distinguish a single UOC sample from others by synthesizing 235U/238U ratio, 234U/238U ratio and 230Th/234U ratio simultaneously. The 230Th–234U model ages estimated using a 230Th–234U chronometer lie between 3.5 a and 4435 a. However, it is usually not reliable to determine the age over 100 a of UOCs, mainly because the material was not completely purified from relevant decay products during production. To overcome this problem, an alternative approach for age dating is proposed using multiple measurements of the ratios of 230Th/232Th and 232Th/234U at an interval of about 2 a to 5 a. This work demonstrates that multiple parameters obtained from a combination of multiple analytical techniques, including bulk analysis, imaging and microanalysis techniques, can be used to narrow the range of potential origins for nuclear forensic purpose.
1. Introduction
Nuclear forensic analysis aims at providing information (origin, intended use and last legal owner) on seized and illegal nuclear materials.1–4 Uranium ore concentrate (UOC), often referred to as “yellow cake”, is produced from the mining and milling of uranium ore with uranium isotopic composition close to natural.1,3 As an intermediate front-end product in the nuclear fuel cycle,3 modern UOCs exist in the form of uranium oxide, ammonium diuranate, sodium diuranate, uranyl hydroxide, uranyl peroxide, etc.2 UOC is easily transported and traded as a commodity in the global market. Hence, UOC has become an attractive target for nuclear fuel cycle and nuclear forensic analysis because of its richness of geographical information.In the domain of nuclear forensics,1–5 the signatures of UOCs include uranium isotopic abundances, elemental concentrations, rare earth elemental patterns, physicochemical properties, and the isotope compositions of certain trace elements. Among all these indicators,3,6 uranium isotope ratios are essential signatures, considered to be the major parameters for tracing the source and process conditions.7–10 Recent work has shown that the 238U/235U ratio is not a constant value (=137.88) on the earth and in our solar system because of isotope fractionation.11–13 Accurate and precise measurement of the 238U/235U ratios in geological samples and UOCs is now possible with advanced mass spectrometry. Using the gravimetrically calibrated n(233U)/n(236U) IRMM3636 double spike with internal mass fractionation correction on the Thermal Ionization Mass Spectrometer (TIMS) or Multi-Collector Inductively Coupled Plasma Mass Spectrometer (MC-ICP-MS),7,11,12 a relative standard deviation (RSD) of 0.002% for the 235U/238U isotope ratio and of 0.01% for the 234U/238U isotope ratio can be achieved. The 238U/235U isotope ratios obtained for 40 UOCs from mining facilities around the world are between 137.809 and 137.934,7 while the 235U/234U isotope ratios lie from 83.63 to 164.17. Because 90–95% of the uranium from the ore is concentrated into the uranium ore concentrate, even if minor isotope fractionation occurs during conversion from the ore to UOC,7 this effect would be negligible compared to the variations reported here based on the high uranium yield during the milling process. In other words,6,7 ratios of naturally occurring isotopes (234U, 235U, 238U) can point to or exclude some uranium ore sources and their formation environment.7 Brennecka found that low-temperature uranium deposits are, on an average, isotopically ∼0.4‰ heavier than uranium deposited at high temperatures or by non-redox processes. He suggested that low-temperature redox changes are the major cause of fractionation between 238U and 235U, and that preferential leaching of 234U and fractionation of 238U/235U in uranium ore bodies are not linked. The isotopic variation of U is therefore a potential signature that can be used to trace the origin of uranium ore concentrate.
Since the investigated materials are forensic evidence,14 special attention has to be paid to minimize the required sample amount. In nearly all situations that require chemical identification of an unknown substance,2–5 a wealth of information can be obtained through different methods, among which the isotope ratios recovered are for the bulk of the material. As is known to all,14,15 the sample weighing can influence the measurements of uranium isotope ratios, which mainly involve procedure blank, isobaric interferences, matrix effect and mass fractionation. To the best of our knowledge, the effect of sample amount on the accuracy of uranium isotopic measurement using destructive techniques has not been investigated.
A scenario may be possible that the analyzed uranium ore concentrate is a mixture of two or more UOCs, or the UOC is primarily polluted,16–18 which is similar to the two uranium dioxide fuel pellets used in the fifth Collaborative Materials Exercise (CMX-5). As a fact, it is easier for smugglers and illegal organizations to mix UOCs by physical methods rather than chemical valence exchange,18 which means that maybe the physical structure and isotopic composition are heterogeneous. In most nuclear forensic investigations,2,4,5 optical microscopy is the first method employed to screen the appearance and homogeneity of interdicted items. As uranium is the major constituent of UOC which is easily contaminated,2,16,18 it is necessary to check the spatial distribution of uranium isotopes in solid nuclear materials by precise micro-analytical methods like Laser Ablation Multi-Collector ICP-MS (LA-MC-ICP-MS) and Secondary Ion Mass Spectrometry (SIMS) prior to any destructive method.
The age of a sample in nuclear forensic analysis refers to the time since the last separation of the progeny isotope from the radioactive parent (usually uranium or plutonium).5,19 It is also referred to as the production (or sometimes separation) date. The measured age is referred to as the “model” age which relies on a number of assumptions,2,19 including complete separation of the progeny isotope from the parent at time zero and the system is “closed”, with no gain or loss of parent or relevant progeny isotopes. Uranium age is perhaps the most useful signature for uranium materials.
During the process of mining and milling of uranium ores,5,20 the U-series equilibrium is broken and the uranium concentration of UOC traded on the commercial market should be over 65 wt%,21 resulting in higher 230Th abundance in UOC than in the ore at the clock to “zero”, which means the beginning of the production process. In uranium materials,22–25 the 230Th–234U chronometer is the most widely utilized chronometer due to the relatively high abundance of ingrown 230Th. However,5,20 the calculated age or “model” age of UOC was usually older than the real age because of the incomplete separation of thorium from uranium. There is a viewpoint which lasted for years5 that the age of UOCs determined is less reliable. However,5 the production date measured on the UOC sample seized in Australia is thought to be possible. The age dating of impure UOC using a radiochronometer is still a nuclear forensic challenge. For the first time,20 Varga developed an alternative method for the production date determination of impure UOC samples by the measurement of trace-level 232Th and its daughter nuclide (228Th) and their variation over time. Due to the short-lived daughter 228Th,20 the method is applicable to date materials with the age of less than 30 years.
As we can see, the in-growth of the long-lived daughter isotope 230Th will change the 230Th/234U ratio and the 230Th/232Th ratio in the UOC during and after its production. So the measured 230Th/234U atom ratio and 230Th/232Th isotope ratio imply important information about their production and history. At the same time,12 the 230Th/234U atom ratio and 230Th/232Th isotope ratios could be different from each other, which are of great potential as time related fingerprints of uranium materials. The idea of these hypotheses is newly developed, and20–22 their potential for UOC age dating has not yet been fully investigated.
In most of the papers dedicated to age dating,23–25230Th/234U ratios have been achieved by laboratories that performed 230Th and 234U concentration measurements by isotope dilution mass spectrometry (ID-MS). This method is accurate and precise,22 reaching an uncertainty of about 0.4 a. But the procedure is time consuming because the anion exchange separation procedure is involved. Nowadays, triple-quadrupole inductively coupled plasma-mass spectrometry (ICP-MS/MS) has become an attractive technique for the measurement of long-lived radionuclides.26 The abundance sensitivity of ICP-MS/MS could reach the order of 10−10, which is suitable for measuring 230Th/234U ratios in the UOC solution without the uranium and thorium clean-up procedure. To the best of our knowledge, there are few published literature studies that have mentioned about this application.
In order to support nuclear forensics,2,3,5 it is desirable to increase the variety of UOCs by manufacture, to develop new technologies and to expand the capacity of the databases. We have developed some modern nuclear forensic techniques to determine the major signatures of 15 UOC samples. After direct analysis of 235U/238U ratios by LA-MC-ICP-MS, a series of primary solutions were prepared by dissolving a few grams of UOC sample in PFA bottles. For the 235U/238U isotope ratio and 234U/238U isotope ratio measurement, two aliquots of the primary solution were taken and only one was spiked with IRMM3636. Isotopic measurements were performed on a Thermo Scientific Neptune XT MC-ICP-MS. The certified 233U/236U of IRMM3636 was used for mass-bias correction of 235U/238U. Then the corrected 235U/238U was used as an internal standard ratio to calibrate the mass-bias of 234U/238U. To the best of our knowledge, this paper demonstrates for the first time the standard sample bracketing (SSB) method of ICP-MS/MS based on the CRM124-1 reference material to correct the mass fractionation of 230Th/234U ratios in the primary solution without the anion exchange separation procedure. We also developed a novel method by measuring 230Th/234U and 230Th/232Th for the determination of the production date (age) of UOCs with incomplete separation of their daughter products.
2. Materials and methods
2.1. Sample preparation
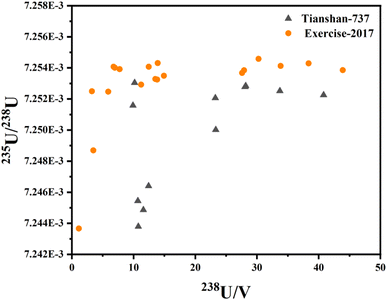
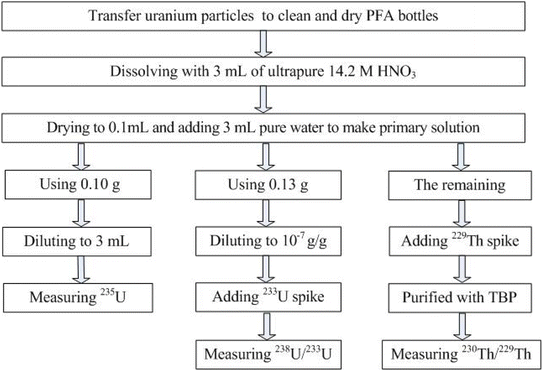
Fifteen UOC samples, obtained from China Institute of Atomic Energy (CIAE) and Beijing Research Institute of Chemical Engineering and Metallurgy (BRICEM), were analyzed in this study. An aliquot of ∼1 g of each UOC sample was taken and transferred into a 15 mL PFA bottle. All the UOCs were dried at 110 °C for twelve hours to achieve stable weight before analysis. For direct analysis of the UOCs using LA-MC-ICP-MS, an aliquot of the dried UOC were efficiently transferred to two low-tack adhesive tapes by a micro-manipulation method. The mounts were scanned by SEM-EDX to map the uraniferous grains, see Fig. 1. The agglomerate can be quickly identified and then located using the optical microscope of the laser ablation system.In order to identify the variation of the 235U/238U ratio relevant to sample size, UOC samples named Exercise-2017 and Tianshan-737 from BRICEM were chosen. The UOC Exercise-2017, produced by mixing UOC from Guangdong Province and UOC from Gansu Province, was used in the Chinese national round-robin laboratory inter-comparison exercises in 2017. Tianshan-737 from the Xinjiang Uygur Autonomous Region of China is simply known as sodium diuranate. Twenty aliquots with different sizes or masses of UOC Exercise-2017 were transferred to twenty 15 mL PFA bottles separately, and dissolved with ultrapure 1 mL 7.5 M HNO3. These solutions were spiked with IRMM3636 after uranium concentrations were determined using an Agilent 8800 ICP-MS/MS. Twelve aliquots of UOC Tianshan-737 were treated in the same way.
For the precise and accurate isotope bulk analysis of UOC samples,19 an aliquot containing about 0.5 g of each UOC solid sample was dissolved with ultrapure 20 mL 7.5 M HNO3 + 0.05 mL 1 M HF in individual clean and dry FEP bottles to obtain a primary solution by total dissolution. No residue was observed with the naked eye in the bottles after 24 hours. Due to the extremely high uranium concentrations associated with these samples, several aliquots were taken and appropriately diluted in 2% HNO3 only after uranium concentrations were determined using ICP-MS/MS. For the 235U/238U isotope ratio and 234U/238U isotope ratio measurement, two aliquots of each primary solution, containing approximately 2 μg of 238U, were taken and only one was spiked with IRMM3636 as the double spike. Another aliquot of each primary solution, containing approximately 8 μg of 238U, was taken and appropriately diluted for the 230Th/234U and 234U/232Th measurement by ICP-MS/MS.
Thorium was purified for 230Th/232Th analysis using an appropriate separation procedure. Each of the 6 mL primary solution was loaded on a single column prepared with TBP resin (TrisKem, TBP-B50-A, 100–150 μm). Every TBP resin column was firstly rinsed with 5 mL 3 M HNO3, under which conditions the uranium and thorium would stay on the bed. Thorium was stripped from the resin with 5 mL of 4 M HCl, and the fraction of thorium was evaporated to near dryness and then redissolved in 5 mL of 2% HNO3 for ICP-MS/MS and MC-ICP-MS measurement. The ICP-MS/MS results show that the 238U concentration of each purified Th solution is less than 2 × 10−8 g g−1.21,30 The 232Th concentration of the most purified Th solution is more than 6 × 10−8 g g−1 and the 232Th blank is less than 3.6 × 10−11 g g−1, which means that the blank of 238U and 232Th has little effect on 230Th/232Th measurement.
2.2. Instruments and reagents
A laser ablation system (UP213 213 nm, New Wave Research, USA) was connected to an MC-ICP-MS (Nu Plasma, UK). 235U and 238U ion signals were both collected using Faraday cups coupled with a 1011 Ω amplifier. A standard sample bracketing (SSB) method based on the combination of LA-MC-ICP-MS and a desolvating nebuliser system (DSN-100, Nu Instruments, UK)10,22,27,28 was established to correct the mass discrimination effect. Mass discrimination correction factors were determined by the isotopic analysis of IRMM187 (Institute for Reference Materials and Measurements, EU) solution introduced via the DSN-100 desolvating nebulizer prior to LA analysis. The optimized parameters for LA-MC-ICP-MS are listed in Table 1.| Operating parameters | |
|---|---|
| New Wave Research ™ laser ablation system | |
| Carrier gas | Ar |
| Carrier gas flow | 622 mL min−1 |
| Laser wavelength | 213 nm |
| Spot size | 30–40 μm |
| Repetition rate | 20 Hz |
| Acquisition time | 30 s |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Nu Instruments ™ DSN-100 | |
| Hot gas flow | 300 mL min−1 |
| Membrane gas flow | 1640 mL min−1 |
| Solution uptake rate | 0.05 mL min−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Nu Instruments ™ Nu plasma MC-ICP-MS | |
| Auxiliary gas flow rate | 980 mL min−1 |
| Cooling gas flow rate | 1350 mL min−1 |
| RF power | 1325 W |
| Quad 1 | 60.0 V |
| Quad 2 | −275.3 V |
Another MC-ICP-MS (Neptune XT, Thermo Fisher Scientific, USA) was used for the measurement of 235U/238U ratio, 234U/238U ratio and 230Th/232Th ratio. The measurement conditions for isotope analysis are listed in Table 2. After each measurement, a washout was performed with 1 M HNO3 for 5 min followed by 0.5 M HNO3 for 5 min to eliminate the memory effect.
| Neptune XT MC-ICP-MS | Operating parameters |
|---|---|
| RF power | 1008 W |
| Sampler cone | Jet |
| Skimmer cone | X |
| Resolution | Low |
| Cool gas | 16.0 L min−1 |
| Auxiliary gas | 0.87 L min−1 |
| Nebulizer gas | 0.97 L min−1 |
| Nebulizer | Micromist PFA, 100 mL min−1 |
| Typical sensitivity | 100 V per μg per mL−1 |
| Integration time | 4.2 s |
| Decelerator voltages | 8343.9 V |
| Suppressor voltages | 9964.2 V |
In order to have a desirable method with similar levels of precision and accuracy to ID-MS, the SBS method based on the Agilent 8800 ICP-MS/MS (Agilent, Japan) was established to correct the mass fractionation of 230Th/234U ratios and 234U/232Th ratios simultaneously. The optimized parameters are summarized in Table 3.
| ICP-MS/MS | Operating parameters |
|---|---|
| RF power | 1500 W |
| Sampling depth | 8 mm |
| Acquisition mode | MS/MS |
| Cell gas | He |
| Cell gas flow rate | 0.7 mL min−1 |
| Omega voltages | −9.8 V |
| Deflect voltages | 13.8 V |
| Plate bias | −66 V |
| Mass pair | Q1 = Q2 |
| Integration time for mass 230 | 30 s |
| Integration time for mass 232, 234, 235 and 238 | 0.3 s |
2.3. Mass spectrometric analysis
In order to determine the 235U/238U ratio precisely and accurately,11–13 the 233U/236U double spike IRMM3636 was added into each UOC sample before sample preparation to correct the mass discrimination effect in mass spectrometric analysis.13 The term mass discrimination is taken here to be the sum of all mass-dependent isotopic effects related to mass-spectrometry, including both the source and detector. The signals of 235U, spiked 236U and 238U are about 0.3 V, 4–6 V and 40 V, respectively. The relative intensity contributed by hydride (238U1H) and peak-tailing of strong 238U at m/z = 239, relative to that of m/z = 238, was about 3.8 × 10−5, resulting in 235U1H/236U c.<3.5 × 10−6, which could be neglected. 233U, 235U, 236U and 238U were collected on Faraday cups coupled to the 1011 Ω amplifier to measure the 235U/233U, 236U/233U and 238U/233U simultaneously.13 Using the exponential law, the 235U/238U ratio can be calculated and corrected with: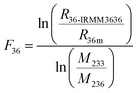 | (1) |
 | (2) |
Another aliquot of each UOC primary solution without IRMM3636 was taken to measure the 234U/235U ratio using the Neptune XT MC-ICP-MS. 235U and 238U were collected on Faraday cups coupled to the 1011 Ω amplifier. A weaker signal of 234U was collected using a Faraday cup in combination with a 1013 Ω amplifier resistor in order to improve the signal to noise ratio. The 234U/238U ratios can be calculated using the following equations:
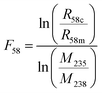 | (3) |
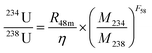 | (4) |
Determination of the 230Th/232Th ratio in UOC was performed using the Neptune XT MC-ICP-MS. Due to the lack of a suitable 230Th/232Th standard reference material,31 a proven technique in different research groups is the use of other elements, which have similar relative atomic masses. Based on the fact that the instrumental mass-discrimination effect is mass dependent, mass discrimination correction was applied to 230Th/232Th using bracketing measurements of the U isotopic composition reference materials. For those UOCs whose 230Th/232Th ratios are higher than 10−4 and the sample mass is sufficient, 230Th and 232Th were collected on Faraday cups coupled to the 1011 Ω amplifier to determine the 230Th/232Th ratio. For those UOCs whose 230Th/232Th ratios are less than 10−4 or the sample size is small, 230Th and 232Th were collected on a secondary electron multiplier (SEM) and a Faraday cup coupled to the 1011 Ω amplifier, respectively. The 230Th/232Th ratio was calculated using the following equation:
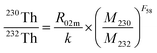 | (5) |
An Agilent 8800 ICP-MS/MS was utilized to measure 230Th/234U ratios and 234U/232Th ratios simultaneously.29 CRM124-1 is used as a matrix-matched 230Th/234U reference standard, whose 230Th/234U value was determined by ID-MS. A laboratory standard, which is made by mixing a 232Th standard (No. 140-051-920, SCP SCIENCE) with CRM124-1 solution, was used to correct the mass fractionation between 234U and 232Th.19 The model ages (t) are calculated using the following expression:
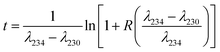 | (6) |
3. Results and discussion
3.1. Spatial distribution of 235U/238U ratios in UOCs determined by LA-MC-ICP-MS
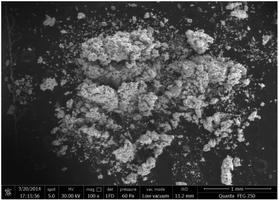
LA-MC-ICP-MS was utilized for in situ analysis of 235U/238U ratios in UOC samples for rapid screening. The method precision was evaluated using CRM124-1 particles, U3O8 particles certificated by New Brunswick Laboratory (NBL). The 235U/238U ratios of CRM124-1 were calculated in twenty independent replicates during the experiment, with an average value of 7.2544 × 10−3 (RSD of 0.04%). The laser beam diameters, the energy densities and the ablation time of each UOC were optimized to guarantee that the 238U signal is stronger than 4 V (1 V = 6.25 × 107 counts per s for a 1011 Ω resistor) and lasts for more than 30 s. It takes about two hours to finish six continuous ablation spots on individual UOC mass, with bracketing solution standard samples for each spot. The 235U/238U ratios in all samples are found to be in the range of 7.194 × 10−3 and 7.275 × 10−3, consistent with the natural abundance ratio reported in the literature.7,8 The precision of 235U/238U ratios of fifteen UOCs measured by LA-MC-ICP-MS was evaluated. The RSD of Exercise-2017 (0.4%) is one order of magnitude higher than those of other UOCs (0.02% to 0.05%), suggesting that 235U/238U of the UOC of Exercise-2017 is inhomogeneous. In order to support the hypothesis, especially when all the scanning electron microscopy (SEM) images are similar (Fig. 1), a zone of the powdered particles sized about 200 μm × 100 μm was chosen and ablated spot by spot with LA-MC-ICP-MS, see in Fig. 2(A). The corrected 235U/238U ratios of the laser ablated spots are shown graphically in Fig. 2(B), whose average value is 7.275 × 10−3. The RSD (=0.2%) is three times larger than that of others, which could be significant evidence for the heterogeneously mixed UOCs.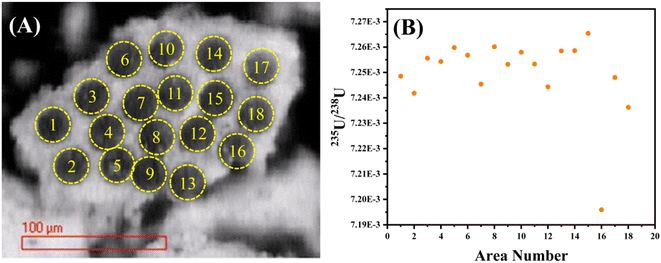 | ||
| Fig. 2 (A) SEM image of the UOC section after laser ablation; (B) spatial distribution of 235U/238U ratios by LA-MC-ICP-MS. | ||
The proposed method by LA-MC-ICP-MS is suitable for rapid measurement of 235U/238U with a precision of a few ten thousandths. The micron-sized analysis, especially isotopic analysis with high precision, has the advantage of rapidly evaluating features of UOCs. It is possible for LA-MC-ICP-MS to distinguish physically homogeneous but chemically inhomogeneous samples,2,17,18 providing a better understanding of the investigated materials.
3.2. The effect of sample amount on the measurement accuracy of 235U/238U
Bulk and in situ analyses are by nature fundamentally different: much less material is available for an in situ analysis. The accuracy and precision of 235U/238U measured by solution MC-ICP-MS may be affected by the total number of atoms present in the analyzed volume, because of the sample nature, the procedure blank, the stability achieved in mass spectrometry, etc. Among the samples of Exercise-2017 with different sampling sizes, the intensity of 238U in one sample is weaker than 0.5 V, leading to poor precision, and this result is abandoned. All “flat top” peaks of uranium isotope signals can be obtained by adjusting the ion beam dispersion appropriately. The 235U/238U values in different PFA bottles with a series of masses of UOC Exercise-2017 and UOC Tianshan-737 are figured with the intensity of 238U, as graphed in Fig. 3. The 235U/238U ratios of both UOCs become more and more precise within counting statistics when the 238U signals are stronger than 28.1 V. The average 235U/238U value of the last four samples of UOC Exercise-2017 is 7.25421 × 10−3 and that of UOC Tianshan-737 is 7.25260 × 10−3, with an RSD of 0.0033% for both UOCs. Hence, the total 238U needed for accurate 235U/238U measurement is more than 1.12 μg, based on the sample volume and instrumental sensitivity, supporting the hypothesis that the variation of uranium isotope ratio was subject to the sample size.3.3. Uranium isotopic measurement of UOCs
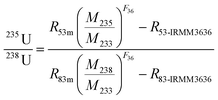
In the process of sample preparation, seven blank samples were bracketed between every two samples. The average concentration of 238U in the blank samples is 4.0 × 10−14 g g−1, which could be neglected compared to the 238U mass of about 2 × 10−6 g in the sample loaded by MC-ICP-MS. In order to evaluate the precision and accuracy of the spike calibration, IRMM184 and CRM124-1 were used to calculate the uncertainties of 235U/238U ratios. As a result, the average 235U/238U values of IRMM184 and CRM124-1 were 7.25252 × 10−3 and 7.25517 × 10−3, respectively, while the RSD of 235U/238U for both IRMM184 and CRM124-1 is 0.0028% (2σ), calculated from nine independent replicates.7,12 The 235U/238U value determined in IRMM184 agrees with the certified value (235U/238U = 7.2523 × 10−3 ± 2.2 × 10−6) within uncertainty, while the 235U/238U ratio determined in CRM124-1 is within analytical uncertainty of that (7.25547 × 10−3) determined by the Triton TIMS. At the same time, the RSD of 234U/238U for both IRMM184 and CRM124-1 is 0.039% (2σ), and the ion registration efficiency (η) between the 1011 Ω amplifier and 1013 Ω amplifier is varied from 0.99935 to 1.00107 according to eqn (4), which should be corrected by bracketing the standard with precise 234U/238U, such as IRMM183 or IRMM184. In conclusion, the precision and accuracy in the analysis of 235U/238U and 234U/238U for natural uranium using the double spike IRMM-3636 was excellent and well-suited to nuclear forensic applications.The developed spike calibration method has been applied for investigation of the 15 UOCs. Every uranium isotope ratio was the average of six runs, displayed in Table 4. The results showed that the 235U/238U ratio observed in the sample lay from 7.24933 × 10−3 to 7.26021 × 10−3, while the 234U/238U ratios lay from 5.0724 × 10−5 to 6.1441 × 10−5. Based on the sample archives, the UOCs analyzed in this work from Inner Mongolia of China and the Xinjiang Uygur Autonomous Region of China were all sandstone deposits, which belong to low-temperature redox uranium deposit type. The 235U/238U ratios in the UOC of Tongliao and UOC of Bayanwula are obviously lower than those of other UOCs, while the 235U/238U ratio in the UOC of Guyuan mined from medium-low temperature hydrothermal uranium deposits is as high as 7.26021 × 10−3, closely matching that of the literature,7 suggesting that low-temperature redox changes are the major cause of fractionation between 238U and 235U. Similarly, there is no evidence of correlation between the measured 238U/235U and 235U/234U ratios in our data from Fig. 4,7 indicating that there is no correlation between the preferential leaching of 234U and fractionation of 238U/235U in uranium ore bodies. In a word, precise and accurate isotopic analysis of 235U/238U and 234U/238U is possible, and the results will provide sufficient information about the type of the U-ore deposit (low-temperature redox deposit vs. high-temperature redox deposit/non-redox) and the uranium ore source to investigate the origin of UOC for nuclear forensics.
| UOC name | Place of origin | Deposit type | 235U/238U | 234U/238U |
|---|---|---|---|---|
| Namibia-2015 | Namibia | — | 7.25358 × 10−3 | 5.6342 × 10−5 |
| Kazakhstan | Kazakhstan | — | 7.25698 × 10−3 | 5.4847 × 10−5 |
| Exercise-2017 | China-“blind sample” | — | 7.25393 × 10−3 | 5.3586 × 10−5 |
| Exercise-2018 | China-“blind sample” | — | 7.25327 × 10−3 | 5.1904 × 10−5 |
| Guyuan | China-Hebei | Meso-epithermal porp-hype-type U–Mo | 7.26021 × 10−3 | 5.3823 × 10−5 |
| Nalinggou | China-Inner Mongolia | Sandstone | 7.25680 × 10−3 | 6.1441 × 10−5 |
| Tongliao | China-Inner Mongolia | Sandstone | 7.25134 × 10−3 | 5.1502 × 10−5 |
| Bayanwula | China-Inner Mongolia | Sandstone | 7.24933 × 10−3 | 6.1374 × 10−5 |
| Jinyuan | China-Guangdong | Granite type | 7.25819 × 10−3 | 5.5493 × 10−5 |
| Qinglong | China-Liaoning | Volcanic type | 7.25586 × 10−3 | 5.6699 × 10−5 |
| Tianshan-735 | China-Xinjiang | Sandstone | 7.25329 × 10−3 | 5.6762 × 10−5 |
| Tianshan-737 | China-Xinjiang | Sandstone | 7.25264 × 10−3 | 5.0724 × 10−5 |
| Tianshan-738 | China-Xinjiang | Sandstone | 7.25563 × 10−3 | 5.5765 × 10−5 |
| Tianshan-739 | China-Xinjiang | Sandstone | 7.25292 × 10−3 | 5.6795 × 10−5 |
| Lantian | China-Gansu | Medium-low temperature metamorphic | 7.25654 × 10−3 | 5.4043 × 10−5 |
3.4. The 230Th/234U ratio and age of UOCs
The model age or model production date of uranium materials is an important signature that has been used to constrain the production history and possible origins of the material,23 and also establish genetic links among different samples of interest in a nuclear forensic investigation. In order to understand the mechanism of the 230Th/234U method, we performed 230Th/234U measurement by the classical ID-MS method and proposed ICP-MS/MS in this paper.At present,19 few reference materials for age dating are certified and available to the scientific community for use in the validation of Th–U age dating methods.19,23,30 The ID-MS method was firstly applied to obtain the concentrations of 238U and 230Th of CRM124, GBW04234 and GBW04238 from the primary solutions, as graphed in Fig. 5. High purity 229Th was milked from 233U bulk solution in the course of the previous work. The 229Th concentration and 233U concentration were measured by ID-MS using a commercially available 232Th standard (No. 140-051-900, SCP SCIENCE) and 235U (IRMM050) as isotope tracers, respectively. Thorium was purified for analysis using a single column prepared with a TBP resin bed on 23 April 2020, with which the sample was loaded and then Th was eluted with 4 mol L−1 HCl. 233U/238U ratios in diluted samples and 230Th/232Th ratios in purified samples are measured using the Neptune XT MC-ICP-MS.
The 230Th/234U ratios of the primary solution and the 230Th–234U model dates are calculated from the analytical results, listed in Table 5. The relative uncertainties of the 230Th/234U ratios of CRM124-4, GBW04234 and GBW04238 are less than 2.7%, 6.1% and 1.8% (2σ), respectively, which mainly come from the experimental uncertainty and the expanded uncertainties of the certificated value. The model date of CRM124, ca.1962, is close to the start of the nuclear era. The model ages of GBW04234 and GBW04238 match well with each other within the measurement uncertainty, concordant with the known last purified time. In addition to these results, the range of production dates of CRM124 and GBW04238 studied here makes these materials well suited for use as informal reference materials for uranium dating techniques with the 230Th–234U chronometers.
| Sample no. | 230Th/234U | n | u Th-230/U-234 (1σ) | Model age/a | u age/a (1σ) |
|---|---|---|---|---|---|
| CRM124-4 | 1.632 × 10−4 | 5 | 2.2 × 10−6 | 57.81 | 0.77 |
| GBW04234 | 6.59 × 10−5 | 4 | 2.0 × 10−6 | 23.32 | 0.71 |
| GBW04238 | 6.496 × 10−5 | 5 | 5.6 × 10−7 | 22.99 | 0.20 |
In order to develop a fast and flexible methodology for the determination of 230Th/234U, ICP-MS/MS was used to measure the 230Th/234U values of the standard samples on 11 August 2021. The results are listed in Table 6. The RSD is less than 3.2% and the atomic fraction percent is less than 6.1%. The standard CRM124-1, GBW04234 and GBW04238 could be used as external standards to correct the mass discrimination of UOCs measured by ICP-MS/MS, whose 230Th/234U reference ratios could be calculated from Table 5 based on the 230Th–234U chronometer.
| No. | 230Th/234U in CRM124-1 | 230Th/234U in GBW04234 | 230Th/234U in GBW04238 |
|---|---|---|---|
| 1 | 1.519 × 10−4 | 6.66 × 10−5 | 7.010 × 10−5 |
| 2 | 1.593 × 10−4 | 6.90 × 10−5 | 6.922 × 10−5 |
| 3 | 1.588 × 10−4 | 7.20 × 10−5 | 6.911 × 10−5 |
| 4 | 1.526 × 10−4 | 7.22 × 10−5 | 6.981 × 10−5 |
| 5 | 1.627 × 10−4 | 7.31 × 10−5 | 7.120 × 10−5 |
| 6 | 1.525 × 10−4 | 7.01 × 10−5 | 6.878 × 10−5 |
| Average | 1.563 × 10−4 | 7.05 × 10−5 | 6.970 × 10−5 |
| μ A (1σ) | 4.2 × 10−6 | 2.2 × 10−6 | 8.0 × 10−7 |
| RSD/% | 2.7 | 3.2 | 1.2 |
| Reference vales | 1.664 × 10−4 | 6.88 × 10−5 | 6.88 × 10−5 |
| RE/% | −6.1 | −2.5 | 1.3 |
The 232Th/234U ratio and 230Th/234U ratio of the 15 UOCs were measured by the SSB method using ICP-MS/MS on 23 February 2022. The intensity of 238U detected by ICP-MS/MS in every portion of the UOC primary solution was around 6 × 109 cps to obtain an intensity of 230Th as strong as possible. Due to the smaller 230Th/234U ratio, the intensities of 230Th for UOC Bayanwula are about 6.3 cps, leading to an RSD of about 11.9% (1σ) for 230Th/234U in six runs. Similarly, the intensities of 230Th for UOC Nalinggou, Exercise-2017, Kazakhstan and Tianshan738 lie from 13.7 cps to 22.8 cps, resulting in an RSD of about 4.6% (1σ) for all the 230Th/234U values in six runs. The model ages calculated with the 230Th–234U radiochronometer are listed in Table 7. These model ages of UOC Nalinggou and Bayanwula are concordant with the sample archive recorded production age (around 2018) within given uncertainties. The experimentally measured values of the 230Th/234U ratio in the UOC ranged from 9.78 × 10−6 to 1.235 × 10−2, with which several UOCs could be distinguished from others by 230Th/234U ratios. It was proved that the atom ratio 230Th/234U is a time correlated fingerprint of UOCs. We can conclude that the model ages less than 100 a are reliable or at least could offer information in the investigation of such incidents, while those larger than 100 a are beyond fact. The possible explanation for the trustworthy model age is that the material was completely purified from relevant decay products during the production process. As a fact, more information is needed to find the relationship between the suitable model age and leaching ways of uranium.
| UOC name | Place of origin | 232Th/234U | 230Th/234U | Model age (a) |
|---|---|---|---|---|
| Namibia-2015 | Namibia | 6.50 × 10−1 | 5.23 × 10−3 | 1.864 × 103 |
| Kazakhstan | Kazakhstan | 1.98 × 10−1 | 5.70 × 10−5 | 2.020 × 101 |
| Exercise-2017 | China-“blind sample” | 2.37 × 10−1 | 3.98 × 10−5 | 1.409 × 101 |
| Exercise-2018 | China-“blind sample” | 6.42 × 10−1 | 1.857 × 10−3 | 6.592 × 102 |
| Guyuan | China-Hebei | 3.41 × 10−1 | 4.567 × 10−4 | 1.618 × 102 |
| Nalinggou | China-Inner Mongolia | 8.29 × 10−3 | 1.804 × 10−5 | 6.39 |
| Tongliao | China-Inner Mongolia | 4.24 × 10−2 | 1.021 × 10−4 | 3.618 × 101 |
| Bayanwula | China-Inner Mongolia | 2.58 × 10−3 | 9.78 × 10−6 | 3.46 |
| Jinyuan | China-Guangdong | 5.29 | 1.235 × 10−2 | 4.435 × 103 |
| Qinglong | China-Liaoning | 5.63 × 10−1 | 3.981 × 10−3 | 1.416 × 103 |
| Tianshan-735 | China-Xinjiang | 8.98 × 10−1 | 2.572 × 10−3 | 9.138 × 102 |
| Tianshan-737 | China-Xinjiang | 1.71 | 2.676 × 10−3 | 9.506 × 102 |
| Tianshan-738 | China-Xinjiang | 2.78 × 10−3 | 2.976 × 10−5 | 1.054 × 101 |
| Tianshan-739 | China-Xinjiang | 3.91 × 10−1 | 1.070 × 10−3 | 3.796 × 102 |
| Lantian | China-Gansu | 2.27 × 101 | 2.557 × 10−3 | 9.082 × 102 |
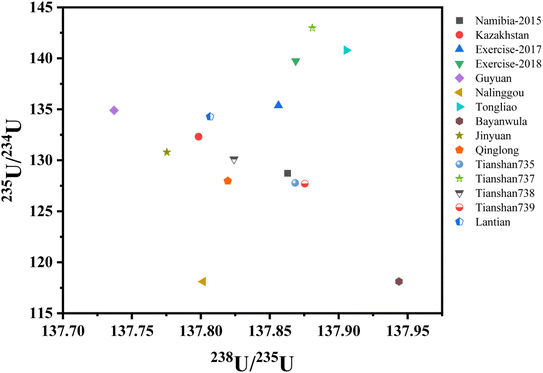
Multivariate and pattern recognition techniques are of great interest in data analysis and database building for nuclear forensic purpose.7,32 Here we showed 235U/238U, 234U/238U and 230Th/234U in 3D space, where every sample is obviously unique from others. The visualization of the data proved that the application of multiple isotopic systems or signatures will be helpful to discriminate the possible sources and have valuable potential in this endeavor (Fig. 6).
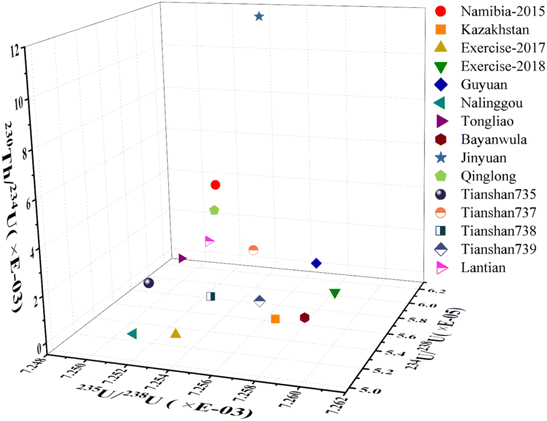 | ||
| Fig. 6 The visualization of 235U/238U, 234U/238U and 230Th/234U ratios for the 15 samples in 3D space. | ||
3.5. Age dating of UOC using 230Th/232Th ratios
Decay of the radioactive nuclides enables the calculation of the production date or age,5,20 which is called the predictive signature of the material. The uranium age can help to verify the declared origin or to identify the possible production facility. Based on the 234U–230Th radioactive chain,20 the number of atoms of 230Th at the analysis time is given by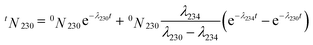 | (7) |
As the time for the ingrowth of these decay products in an anthropogenic material is less than 100 a,19t is very short relative to the long half-lives of 230Th (7.57 × 104 a) and 234U (2.455 × 105 a). Given that λt is a whole variable, we get λ230t ≪ 1 and λ234t ≪ 1. Using Taylor expansion, eqn (7) can be simplified as follows:
| tN230 = (λ2340N234 − λ2300N230)t + 0N230 | (8) |
Because 0N230 is usually unknown, it is necessary to find another way to solve t. As the relative decay ratio of 234U is less than 0.03% during 100 a, tN230 ≈ 0N230.We noticed that the 230Th/234U ingrowth with t can be calculated using the following equation:
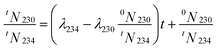 | (9) |
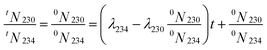 | (10) |
Meanwhile, 230Th/232Th ingrowth with t can be calculated using the following equation:
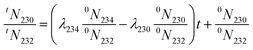 | (11) |
Eqn (11) shows that tN230/tN232 varies linearly with time (t) and that 0N234/0N232 and 0N230/0N232 can be determined from the fitted slope.
According to eqn (9)–(11), measurements of 230Th/234U or 230Th/232Th ratios performed at least three times are needed to solve model age t. In order to check the feasibility of the proposed method, the 230Th/232Th ratios in 15 UOC samples were measured by MC-ICP-MS after the thorium was purified on 14 April 2021. Then the ingrowth of 230Th/234U and 230Th/232Th after 5 years was calculated based on the results, as shown in Table 8. The RSDs for 230Th/232Th at level 10−5 and 10−3 are 0.2% and 0.03%, respectively. The results showed that 230Th/232Th ratios of UOCs differed distinctly from that of UOCs from another source, proving that 230Th/232Th is a promising fingerprint of UOCs. It also suggested that the relative variations of 230Th/234U and 230Th/232Th in 5 years lie from 0.1% to 144%, most of which could be observed with per mil precision. As the relative uncertainty of ID-MS for 230Th/234U is near or larger than 0.3% because of the relative uncertainty of the spike and the measurement, it is very hard to determine a relative variation of 0.5% for 230Th/234U. Conversely, precise and accurate measurements of 230Th/232Th isotope ratios can be easily achieved by MC-ICP-MS, providing an alternative technique to the uranium age determination of UOCs with the residual daughter nuclide after the separation. In addition, it is possible to simulate 230Th/234U and 230Th/232Th at any given time, which is useful to rebuild the histories of uranium materials and to complete the database of UOCs with time.
| UOC name | 232Th/234U | 230Th/234U | 230Th/232Th | ||
|---|---|---|---|---|---|
| Measured at t1 | Calculated to t1+5 | Measured at t2 | Calculated to t2+5 | ||
| Namibia-2015 | 6.50 × 10−1 | 5.23 × 10−3 | 5.25 × 10−3 | 7.469 × 10−3 | 7.491 × 10−3 |
| Kazakhstan | 1.98 × 10−1 | 5.70 × 10−5 | 7.11 × 10−5 | 2.634 × 10−4 | 3.348 × 10−4 |
| Exercise-2017 | 2.37 × 10−1 | 3.98 × 10−5 | 5.39 × 10−5 | 1.557 × 10−4 | 2.154 × 10−4 |
| Exercise-2018 | 6.42 × 10−1 | 1.857 × 10−3 | 1.871 × 10−3 | 2.859 × 10−3 | 2.881 × 10−3 |
| Guyuan | 3.41 × 10−1 | 4.57 × 10−4 | 4.71 × 10−4 | 1.343 × 10−3 | 1.384 × 10−3 |
| Nalinggou | 8.29 × 10−3 | 1.804 × 10−5 | 3.22 × 10−5 | 2.295 × 10−3 | 3.997 × 10−3 |
| Tongliao | 4.24 × 10−2 | 1.021 × 10−4 | 1.163 × 10−4 | 2.528 × 10−3 | 2.861 × 10−3 |
| Bayanwula | 2.58 × 10−3 | 9.78 × 10−6 | 2.39 × 10−5 | 3.926 × 10−3 | 9.407 × 10−3 |
| Jinyuan | 5.29 | 1.235 × 10−2 | 1.236 × 10−2 | 2.025 × 10−3 | 2.028 × 10−3 |
| Qinglong | 5.63 × 10−1 | 3.98 × 10−3 | 4.00 × 10−3 | 6.658 × 10−3 | 6.683 × 10−3 |
| Tianshan-735 | 8.98 × 10−1 | 2.572 × 10−3 | 2.586 × 10−3 | 2.760 × 10−3 | 2.776 × 10−3 |
| Tianshan-737 | 1.71 | 2.676 × 10−3 | 2.690 × 10−3 | 1.520 × 10−3 | 1.528 × 10−3 |
| Tianshan-738 | 2.78 × 10−3 | 2.976 × 10−3 | 4.387 × 10−3 | 1.123 × 10−2 | 1.630 × 10−2 |
| Tianshan-739 | 3.91 × 10−1 | 1.071 × 10−3 | 1.084 × 10−3 | 2.761 × 10−3 | 2.797 × 10−3 |
| Lantian | 2.27 × 101 | 2.557 × 10−3 | 2.577 × 10−3 | 9.174 × 10−5 | 9.235 × 10−5 |
In addition to these results, the developed method in this study may require about ten years to accurately determine 232Th/234U, 230Th/232Th and the model ages using 230Th–234U chronometers. However, it is nowadays the best way to distinguish samples originally from the same place and the same production process, but at different times of production.
4. Conclusions
To categorize UOC samples for nuclear forensic investigation, special attention has been paid to those internal signatures which are always stable and are of high importance to establish links with uranium ores and constrain the production history. In this work, precise and accurate uranium isotope ratios, thorium isotope ratios and uranium age of fifteen UOCs were identified simultaneously. In situ LA-MC-ICP-MS is a rapid method to achieve fast screening of UOCs with high throughput, especially when the sample is physically inhomogeneous or mixed with several UOCs. We suggested that an aliquot containing at least 1.12 μg of 238U was essential to obtain the representative results of the 235U/238U ratio in the UOC sample for bulk analysis by MC-ICP-MS. The 235U/238U ratios and 234U/238U ratios are the most important parameters that may reveal a certain source. The standard sample bracketing (SSB) method of ICP-MS/MS was established to acquire 230Th/234U ratios and to diagnose model age within a week without anion exchange separation, which is a time-consuming procedure in the classical ID-MS method. It is possible to distinguish one UOC sample from others by synthesizing samples with 235U/238U ratios, 234U/238U ratios and 230Th/234U ratios simultaneously. The time related 230Th/234U ratio and 230Th/232Th ratio are potential signatures that can be used to trace the origin of UOCs and exclude the UOCs from the same place and process, which have other similar parameters. Significant variations in 230Th/232Th can be observed during every 2 a to 5 a, which is proposed to be a novel and practicable approach to determine the model age and production date of impure UOC. Therefore, effective nuclear forensic investigations rely on applying multiple analytical techniques and suitable signatures for nuclear forensic science, contributing heavily to the expansion of nuclear forensic capabilities.Author contributions
Wang Wei: conceptualization, methodology, formal analysis, investigation, writing. Xu Jiang: investigation, resources, valuation. Xi Ruiyang: formal analysis, project administration, visualization. Guo Siqi: formal analysis, writing-original draft. Su Yongyang: writing – review & editing. Fang Sui: formal analysis. Zhang Haitao: writing – review. Wang Yalong: formal analysis, validation. Fan Jinlong: methodology. Feng Lei: formal analysis. Wang Yufeng: methodology. Li Zhiming: methodology, project administration, investigation, resources, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2021YFF0700100).References
- A. Donard, A.-C. Pottin, F. Pointurierb and C. Pécheyran, J. Anal. At. Spectrom., 2015, 30, 2420 RSC.
- M. D. Straub, J. Arnold, J. Fessenden and J. L. Kiplinger, Anal. Chem., 2021, 93, 3–22 CrossRef CAS PubMed.
- J. M. Rolisona, M. Drucea, Q. R. Shollenbergera, T. M. Kayzar-Boggsa, R. E. Lindvall and J. Wimpenny, Appl. Geochem., 2019, 103, 97–105 CrossRef.
- M. Wallenius, K. Mayer and I. Ray, Forensic Sci. Int., 2006, 156, 55–62 CrossRef CAS PubMed.
- E. Keegan, M. J. Kristo, K. Toole, R. Kips and E. Young, Anal. Chem., 2016, 88, 1496–1505 CrossRef CAS PubMed.
- T. L. Spano, A. Simonetti, E. Balboni, C. Dorais and P. C. Burns, Appl. Geochem., 2017, 84, 277–285 CrossRef CAS.
- G. A. Brennecka, L. E. Borg, I. D. Hutcheon, M. A. Sharp and A. D. Anbar, Earth Planet. Sci. Lett., 2010, 291, 228–233 CrossRef CAS.
- M. B. Andersen, C. H. Stirling and S. Weyer, Rev. Mineral. Geochem., 2017, 82, 799–850 CrossRef CAS.
- A. Basu, R. A. Sanford, T. M. Johnson, C. C. Lundstrom and F. E. Löffler, Geochi. Cosmochim. Acta, 2014, 136, 100–113 CrossRef CAS.
- N. S. Lloyd, R. R. Parrish, M. S. A. Horstwood and S. R. N. Chenery, J. Anal. At. Spectrom., 2008, 24, 752–758 RSC.
- S. Richter, A. Alonso-Munoz, R. Eykens, U. Jacobsson, H. Kuehn, A. Verbruggen, Y. Aregbe, R. Wellum and E. Keegan, Int. J. Mass Spectrom., 2008, 269, 145–148 CrossRef CAS.
- S. Richter, R. Eykens, H. Kühna, Y. Aregbe, A. Verbruggen and S. Weyer, Int. J. Mass Spectrom., 2010, 295, 94–97 CrossRef CAS.
- D. J. Condon, N. McLean, S. R. Noble and S. A. Bowring, Geochi. Cosmochim. Acta, 2010, 74, 7127–7143 CrossRef CAS.
- Z. Stefánka, R. Katona and Z. Varga, J. Anal. At. Spectrom., 2008, 23, 1030–1033 RSC.
- G. Craig, M. S. A. Horstwood, H. J. Reid and B. L. Sharp, J. Anal. At. Spectrom., 2020, 35, 1011 RSC.
- S. V. Jovanovic, P. K. Weber, A. J. Pidduck, A. M. Gaffney, P. Girard, F. Pointurier, M. Hedberg, A. J. Simons, V. Stebelkov, T. Kell, K. Knight, T. P. Davis, M. Kristo, R. W. Williams, K. C. Treinen, N. J. Montgomery, J. King, A. Wickenden, D. Knight, A.-L. Fauré, A. Hubert, N. Albert, M.-C. Vincent, M. Wallenius, I. A. Elantyev, K. D. Zhizhin, J. M. Schwantes, O. Marsden and F. Taylor, J. Radioanal. Nucl. Chem., 2020, 326, 1853–1866 CrossRef CAS.
- M. Krachler, Z. Varga, A. Nicholl, M. Wallenius and K. Mayer, Microchem. J., 2018, 140, 24–30 CrossRef CAS.
- Z. Varga, M. Wallenius, A. Nicholl and K. Mayer, Spectrochim. Acta, Part B, 2020, 171, 105920 CrossRef CAS.
- A. M. Gaffney, A. Hubert, W. S. Kinman, M. Magara, A. Okubo, F. Pointurier, K. C. Schorzman, R. t. E. Steiner and R. W. Williams, J. Radioanal. Nucl. Chem., 2016, 307, 2055–2060 CrossRef CAS.
- Z. Varga, M. Wallenius, K. Mayer and E. Hrnecek, J. Radioanal. Nucl. Chem., 2011, 290, 485–492 CrossRef CAS.
- J. Xu, W. Wang, R. Y. Xi, Y. Y. Su, W. L. Wang, Z. M. Li, L. H. Zhai, S. Fang, H. T. Zhang, J. L. Fan and Y. F. Wang, Chinese J. Anal. Chem., 2022, 50(2), 310–316 CAS.
- J. S. Becker, C. Pickhardt and H.-J. Dietze, Int. J. Mass Spectrom., 2000, 202, 283–297 CrossRef CAS.
- J. M. Rolison, K. C. Treinen, K. C. McHugh, A. M. Gaffney and R. W. Williams, J. Radioanal. Nucl. Chem., 2017, 314, 2459–2467 CrossRef CAS.
- M. Higginson, C. Gilligan, F. Taylor, D. Knight, P. Kaye, T. Shaw and P. Thompson, J. Radioanal. Nucl. Chem., 2018, 318, 157–164 CrossRef CAS.
- F. Pointurier, A. Hubert and G. Roger, J. Radioanal. Nucl. Chem., 2013, 296, 593–598 CrossRef CAS.
- W. C. Zhang, J. F. Lin, S. Fang, C. Li, X. W. Yi, X. L. Hou, N. Chen, H. T. Zhang, Y. H. Xu, H. J. Dang, W. Wang and J. Xu, Talanta, 2021, 234, 122652 CrossRef CAS PubMed.
- S. F. Boulyga and T. Prohaska, Anal. Bioanal. Chem., 2008, 390, 531–539 CrossRef CAS PubMed.
- S. Kappel, S. F. Boulyga and T. Prohaska, J. Environ. Radioact., 2012, 113, 8–15 CrossRef CAS PubMed.
- S. V. Jovanovic, T. Kell, J. El-Haddad, C. Cochrane, C. Drummond and A. EI-Jaby, J. Radioanal. Nucl. Chem., 2020, 323, 831–838 CrossRef CAS.
- W. Wang, J. Xu, Z. M. Li, X. P. Shen, Y. Y. Su, J. L. Fan, H. T. Zhang, Y. F. Wang, S. Fang, X. L. Yuan, H. Ddeng and W. L. Wang, Morden Applied Physics, 2022, 13(2), 020801 Search PubMed.
- A. Simonetti, L. M. Heaman, R. P. Hartlaub, R. A. Creaser, T. G. MacHattie and C. Böhm, J. Anal. At. Spectrom., 2005, 20, 677–686 RSC.
- J. H. Su, J. Wu and S. D. Hu, Ann. Nucl. Energy, 2019, 126, 43–47 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |