 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid phase peptide synthesis using side-chain unprotected arginine and histidine with Oxyma Pure/TBEC in green solvents†
Tommaso
Fantoni
 a,
Andrea
Orlandin
b,
Ilaria
Di Stefano
a,
Marco
Macis
b,
Alessandra
Tolomelli
a,
Andrea
Orlandin
b,
Ilaria
Di Stefano
a,
Marco
Macis
b,
Alessandra
Tolomelli
 a,
Antonio
Ricci
*b,
Walter
Cabri
a,
Antonio
Ricci
*b,
Walter
Cabri
 *a and
Lucia
Ferrazzano
*a and
Lucia
Ferrazzano
 a
a
aProject for Green Innovation Lab, Department of Chemistry, University of Bologna, via Gobetti 85, 40129-Bologna, Italy. E-mail: walter.cabri@unibo.it
bR&D, Fresenius Kabi, via San Leonardo, Villadose(RO), Italy
First published on 18th September 2024
Abstract
The elimination of side-chain orthogonal protective groups of arginine and histidine is critical to improve solid phase peptide synthesis (SPPS) sustainability through an increase in the peptide atom economy (AE) and decrease in impurities generated during the final cleavage step. The combination of Oxyma Pure and tertbutyl ethyl carbodiimide (TBEC) in the correct ratio allowed the use of side-chain free arginine and histidine in green solvents. Etelcalcetide and vasopressin intermediates as well as critical key fragments of liraglutide and semaglutide were successfully synthetized via SPPS using optimized conditions. In addition, the Oxyma Pure/TBEC protocol in NBP/DMC was successfully applied to a sequence containing side-chain unprotected arginine, histidine, tryptophan and tyrosine.
Introduction
According to the evaluation of the Green Chemistry Institute Pharmaceutical Roundtable (GCIPR), oligopeptide synthesis is one of the critical topics that requires further improvements and investigation in terms of sustainability.1 Since its discovery by Merrifield,2 solid-phase peptide synthesis (SPPS) has been one of the most important and consolidated technologies for the production of oligopeptides because it can be easily applied to the sequence of unnatural amino acids and side-chains.3,4 In fact, these modifications resulted in the critical design of drugs with the correct pharmaceutical profile in terms of half-life and/or oral absorption. 9-Fluorenylmethoxycarbonyl (Fmoc) SPPS technology plays a central role in peptide production, and it is used not only for full sequential synthesis but also for hybrid approaches. In fact, peptide fragments produced through SPPS can be linked with LPPS5 or enzymatic couplings to afford long peptide sequences.6 The GLP-1 blockbuster tirzepatide is a 39-mer peptide used for the treatment of diabetes and includes two 2-aminoisobutyric acid (Aib) residues at positions 2 and 13 and a lipophilic sidechain on lysine 20. This homologous peptide is produced by employing a hybrid approach using Fmoc-SPPS technology for the preparation of fragments and solution phase technology for the final connection.7Academic and industrial scientists recently investigated several aspects of SPPS to increase sustainability by focusing on green solvents, safe coupling additives, microwaves, and continuous purification technologies.3,8,9 The main goal has been to obtain high-quality peptides with low process mass intensity (PMI). Upstream solvent volume minimization can be achieved by decreasing resin washings by applying percolation10 and in-line analysis to use only the exact amount of solvent necessary to eliminate contaminants.11,12
Even without considering the resin or tag present in SPPS or LPPS, the atom economy and the ideality factor (<50%) of oligopeptide synthesis are very low because of the presence of amino protecting groups necessary for iterative coupling/deprotection sequences.13,14 The outcome is more worse if Fmoc-amino acid couplings require orthogonal protective groups for sensitive side-chain functions, in particular for arginine and histidine residues. The arginine guanidinium moiety can be protected using various protective groups to facilitate peptide synthesis, and 2,2,4,6,7-pentamethyl-dihydro-benzofuran-5-sulfonyl (Pbf) is one of the most popular groups. The effect is a consistent drop in the atom economy (AE) from 36% to 24% only for a single Fmoc/Pbf arginine incorporation into the sequence (Fig. 1).
Similarly, histidine imidazole is generally protected as trityl (Trt); consequently, AE decreases from 36% to 22%.
In addition to the AE, these protective groups affect downstream purification. In fact, the deprotection of guanidinium and histidine is combined with the cleavage of oligopeptides from the resin performed in the presence of trifluoroacetic acid and scavengers.
In the final step of the process, cleavage from the resin, the formation of highly reactive carbocation fragments occurs, stemming from orthogonal protective groups, such as tertbutyloxycarbonyl (Boc), benzyloxycarbonyl (Cbz), and Trt, or sulfonylating/sulfonating agents derived from Pbf. These fragments can react with sensitive amino acids, such as tryptophan, cysteine, tyrosine, serine or threonine, leading to the formation of by-products worsening the purity profile of the crude peptides.15 The downstream purification process is generally the most time-consuming part of the entire process. The formation of impurities coming from side chain protection fragments can significantly impact not only the overall yield but also the productivity of downstream chromatography. As the number of impurities increases, the chromatographic separation becomes more challenging and time-consuming. Some recent papers described interesting new coupling reagents16 or high-temperature rapid synthesis.17 However, none of these protocols can avoid the use of side chain orthogonal protective groups for arginine and histidine in the iterative synthesis of polypeptides.
On the contrary, Yang et al. recently reported the use of side chain unprotected arginine, histidine and tyrosine for SPPS couplings in toxic dimethylformamide (DMF).18 To achieve efficient synthetic protocols, two coupling agents had to be introduced, namely Oxyma Pure 3a/DIC 5 for Fmoc-Arg(HCl)-OH 1a and PyBOP for Fmoc-His-OH 2a. Therefore, to simplify the synthetic approach and reach a minimal-protection SPPS strategy (MP-SPPS) using 3a/5 for all couplings, the authors reintroduced Fmoc-His(Mtt)-OH.
To set up a complete sustainable MP-SPPS of Arg and His-rich peptide sequences, in this paper, we reported results on the identification of a simple and robust protocol that avoids the use of orthogonal protecting groups for arginine and histidine in binary green solvent mixtures based on the use of 1-tert-butyl-3-ethylcarbodiimide (TBEC) 29 and OxymaPure 3a as an industrial coupling mixture (Fig. 2). The outcome of this protocol is an increase in the AE by eliminating useless protective groups and a decrease in the PMI of the downstream coming from the simplification of the final chromatographic purification. For these considerations, the overall sustainability of the newly developed SPPS protocol matches with Warner–Anastas principles of Green Chemistry, summarized in Table 1.19
| Principle | Target |
|---|---|
| 1. Prevention | Waste prevention/PMI reduction |
| 2. Atom economy | Elimination of protective groups |
| 5. Safer solvents & auxiliaries | Binary green solvent mixtures |
| 8. Reduce derivatives | Fewer impurities |
Results and discussion
Unprotected sidechain arginine in SPPS
Yang et al. reported that the coupling reaction with the Fmoc-Arg-(HCl)-OH 1a proceeded smoothly when carried out in a 1/1/2 ratio between 1a, 3a and 5 in DMF.18 In fact, a further addition of 5 was critical to obtain good conversions in overall 2 h reaction time.To better understand the effect of the unprotected guanidinium moiety on the reaction outcome, we studied the interactions between Fmoc-Arg(HCl)-OH 1a and 3a at different ratios using NMR (Scheme 1). From the 1H-NMR spectra acquired in DMF-d7 (Fig. 3), the position of signals related to the mobile proton of Fmoc-Arg(HCl)-OH 1a completely changed with the addition of one equivalent of 3a (see Fig. S3 in ESI†), suggesting acid/base interaction of the guanidinium with the Oxyma moiety. Interestingly, the formation of an almost 50/50 mixture of unreacted 5 and diisopropyl urea (DIU) 10 was observed by adding 1 equivalent of 5 to 1/1 solution of 1a and 3a (Fig. 3a).
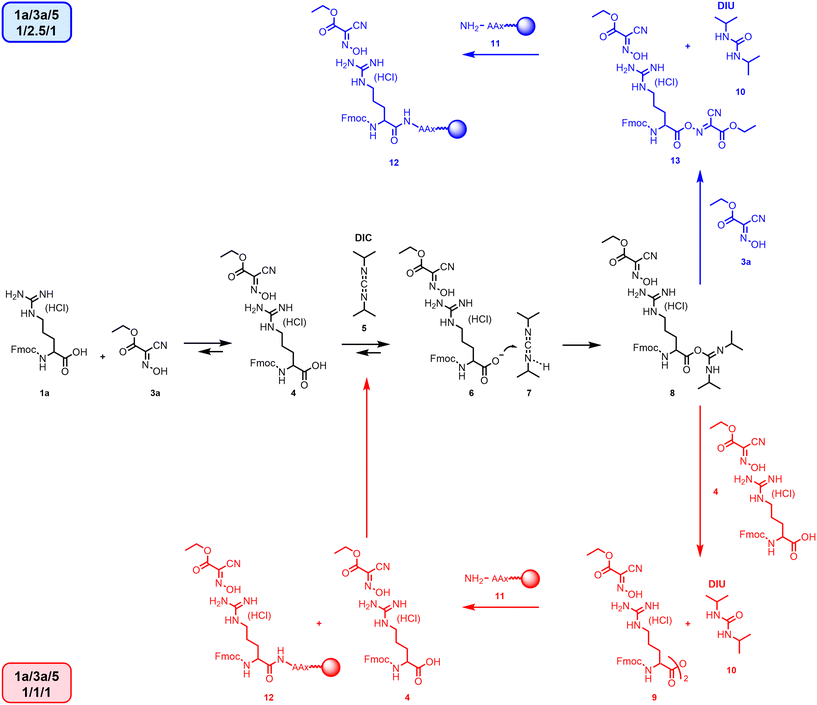 | ||
| Scheme 1 Fmoc-Arg(HCl)-OH activation. Route in red: 1a/3a/5, ratio 1/1/1, see Fig. 3a; route blue 1a/3a/5, ratio 1/2.5/1, see Fig. 3b. | ||
 | ||
| Fig. 3 1H NMR in DMF-d7 spectra to highlight DIC 5 fate during 1a and 1b activation using different ratios of Oxima pure 3a and DIC 5. | ||
In Scheme 1, a mechanism of the amino acid activation compatible with what was observed in the NMR spectra has been depicted. Once the adduct between the amino acid and the carbodiimide 8 is formed, it can react with oxime 3a to generate the activated specie 13 or it can react with another molecule of the amino acid to form anhydride 9. According to the NMR data, with a 1/1/1 ratio between 1a/3a/5, the reaction does not proceed via the formation of oxyma-activated ester 13 but through the formation of anhydride 9. The interaction between the guanidinium group and oxyma 4, which is consequently no longer available for the formation of the activated ester moiety, decreases the reaction rate of formation of 13 in favour of the attack of 1a on O-acyl urea 8, which produces 9 and the simultaneous release of DIU 10. A similar ratio was observed in the absence of 3a (Fig. 3a red trace). The coupling via anhydride favours the formation of impurities coming from amino acid racemization.20,21 In addition, Yang and coworkers achieved complete conversion by adding more 5.18 It is worth noting that the simple addition of 2.5 instead of 1.0 equivalents of 3a restored the target reaction mechanism generating the activated ester 13 (Fig. 3b; Scheme 1 pathway blue) and 5 was completely transformed into urea 10. On the contrary, the side chain-protected arginine Fmoc-Arg(Pbf)-OH 1b reacted with one equivalent of 3a and 5 generating 100% of DIU 10 (Fig. 3c; Scheme 1 pathway red). The formation of 13vs.8 is determined only by stoichiometry of oxyma 3a, and it is independent of the solvent or the concentration of the reaction (see Fig. S23–S38†).
The 1H NMR spectra of Fmoc-Arg (HCl)-OH 1a in the presence of other coupling agents with different pKa values, such as hydroxybenzotriazole (HOBt) 3b, 1-hydroxy-7-azabenzotriazole (HOAt) 3c, and 5-(hydroxyimino)1,3-dimethylpyrimidine-2,4,6-(1H,3H,5H)-trione (oxyma B) 3d, showed a similar interaction with the guanidino moiety, and after the addition of 5, the formation of DIU 10 is always around 50% after 1 h (see ESI†). Therefore, there is no relationship with the activating agent pKa (see Table 2).22
| Entry | Coupling agent (equivalents) | Solvent | pKa20 |
15/14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|
| a 14/1a/additive/5 in a 1/2/X/2 with respect to 14 was dissolved in the solvent and added to the CTC resin at room temperature after 5 min of activation. b Determined via HPLC after cleavage and precipitation. c The reaction was stopped after 1.5 h. | ||||
| 1 | 3a (2) | DMF | 4.6 | >99/1 |
| 2 | 3a (5) | DMF | 4.6 | >99/1 |
| 3 | 3a (5) | NMP | 4.6 | >99/1 |
| 4 | 3b (5) | DMF | 4.6 | >99/1 |
| 5 | 3c (5) | DMF | 3.3 | >99/1 |
| 6 | 3d (5) | DMF | 8.2 | 87/13 |
| 7 | 3a (5) | DMSO/AcOEt 1/9 | 4.6 | 83/17 |
| 8 | 3a (5) | NOP/DMC 8/2 | 4.6 | 97/3c |
| 9 | 3a (5) | NBP/DMC 8/2 | 4.6 | >99/1 |
| 10 | 3a (5) | NBP/AcOEt 8/2 | 4.6 | >99/1 |
In Table 2, we compared the use of different coupling reagents with different pKa values using Fmoc-Arg(HCl)-OH 1a and the tripeptide NH2-Gly-Phe-Leu-OH loaded onto a chlorotrityl chloride (CTC) resin. The coupling was completed within 1 h independently from the amount of 3a (see entries 1 and 2). However, based on the NMR studies, we know that without an excess of 3a, the reactions proceed through the formation of anhydride 9. The kinetics of route blue and red in Scheme 1 were comparable. Similar data have been obtained for N-methyl pyrrolidone (NMP) with 3a (entry 3) and with other additives in DMF 3b and 3c (entries 4 and 5), while 3d was less efficient (entry 6), thus proving the general validity of the protocol. Considering 3b and 3c safety issues,23 and the fact that 3d is affected by side reactions,24 we carried out all the additional studies using 3a. The reaction protocol was extended to green solvent binary mixtures selected from among the most popular ones (entries 7–10). The reaction in DMSO/AcOEt (1/9 ratio) was slow (entry 7),25 and in N-octyl pyrrolidinone (NOP)/dimethyl carbonate (DMC) (8/2 ratio),26 amino acid 1a was poorly soluble, in which the dissolution took around 40 min. However, after 1.5 h, the reaction was almost completed anyway (entry 8).
However, mixtures of N-butyl pyrrolidinone (NBP), a solvent introduced by John Lopez et al. as a green alternative to DMF and NMP,27 with 20% AcOEt or DMC to decrease the viscosity and increase solubility, afforded results similar to those obtained in DMF in terms of solubility and reaction rate (compare entry 2 and with entries 9 and 10). Moving to a sequence where the arginine was the penultimate amino acid (see Table 3), we observed that after Fmoc removal, the coupling using Fmoc-Ala-OH 16 worked perfectly using the standard 1/1/1 ratio among aa/3a/5 for the pre-activation of the terminal amino acid. The presence of the unprotected guanidinium moiety on the resin did not affect the subsequent coupling, which was completed in 1 h in any solvent combination tested (see Table 3).
Protocol extension: synthesis of vasopressin and etelcalcetide
The green minimal-protection SPPS strategy developed for single amino acid incorporation was then applied to the synthesis of linear vasopressin (H-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2) 18 and etelcalcetide-1 (D-H-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2) 19. The final step generating the Cys–Cys disulphide bridge in both compounds was not performed because the target was to evaluate the SPPS iterative coupling procedure (see Scheme 2). These drugs have been chosen for their therapeutic relevance, chemical complexity and different classifications according to Lau and Dunn.28In particular, vasopressin 19 is a native nonapeptide produced in the hypothalamus that increases blood pressure in adults with vasodilatory shock (e.g., post-cardiotomy or sepsis) who remain hypotensive despite fluids and catecholamines.29 The market value in 2023 was almost 890M$ and is expected to grow up to 2B$ by 2030.30 Etelcalcetide 21 is a heterologous decapeptide with 5 nonproteinogenic amino acids that is indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adults, 18 years of age and older,31 and represents a synthetic challenge because of the presence of four arginine residues in D configuration. During the SPPS of linear vasopressin 18 in DMF using an excess of 3a only for the introduction of Fmoc-Arg(HCl)-OH 1a, the formation of a variety of impurities was detected. In particular, misincorporation of Asn, Gln or both was observed (Table 4, entry 1, Fig. S70†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Peptide | Solvent | Base | Purityb (%) | Des (%) | Des Arg (%) | 23 (%) |
|---|---|---|---|---|---|---|---|
| a Reactions were carried out using 1a/3a/5 in a 2/2/2 ratio at room temperature, and the reaction was treated with piperidine after 1.5 h, followed by cleavage and precipitation. b Determined using HPLC. c Synthesis carried out using 1a/3a/5 in a 2/2/2 ratio for all the couplings. d After the first coupling, washing was carried out after deprotection with a 0.5 mol concentration of 3a. | |||||||
| 1c | 18 | DMF | PIP | 86.4 | 10.3 | — | 4.3 |
| 2 | 18 | DMF | PIP | 94.8 | 1.1 | — | 4.1 |
| 3 | 18 | NBP/DMC 8/2 | PIP | 92.5 | 2.6 | — | 4.9 |
| 4 | 18 | NBP/DMC 8/2 | DEAPA | 93.2 | 2.7 | — | 4.1 |
| 5 | 20 | DMF | PIP | 94.8 | 5.2 | 5.2 | — |
| 6 | 20 | NBP/DMC 8/2 | PIP | 93.0 | 7 | 7 | — |
Although the guanidinium moiety has pKa of 13.7,32 which is higher than that of piperidine (pKa 11.1),33 the multiple treatments with an excess of base in the iterative coupling/deprotection sequence determined the progressive elimination of the HCl. Nevertheless, the salification of the guanidinium group was crucial to prevent interference during peptide elongation, and the problem was eliminated by adding an excess of Oxyma Pure 3a. Using a ratio of 2/5/2 among aa/3a/5 for all the couplings after the introduction of arginine, the interference of the guanidino was suppressed, both in DMF and NBP/DMC, (Table 4 entries 2 and 3). The use of piperidine for Fmoc removal affected the vasopressin quality, generating high quantities of aspartimide 23 (Scheme 3). Compound 23 is a source of several impurities, such as isomers of deamidate products 24 and 25, and amides 26 and 27. The use of dimethylaminopropyl amine (DEAPA) instead of piperidine (PIP) allowed for a slight decrease in the formation of 23 (entry 5).34 The formation of aspartimide impurities was not affected by the presence of unprotected arginine in the growing peptide sequence.
 | ||
| Scheme 3 Aspartimide 23 as an intermediate for the formation of deamidate 24/25 and amide impurity 26/27. | ||
Interestingly, unprotected tyrosine was used successfully as a penultimate amino acid.18 The only protective group necessary for the synthesis of vasopressin was the one on sensitive cysteine.
The synthesis of etelcalcetide-1 was successfully carried out using the aa/3a/5 in a 2/5/2 ratio for the first arginine. The presence of three consecutive arginine residues at positions 3, 4, and 5 increases the difficulty of the coupling process. After the first insertion of 1a, and piperidine deprotection, the salification of the guanidinium moieties was achieved by introducing 3a in the second and third washing of the resin. The target was also to monitor washing efficiency for the piperidine removal according to Pedersen et al.12 and to protonate the sequence of arginines present in the growing peptide bound to the resin. This approach prevented solubility issues owing to the large excess of Oxyma Pure 3a in the small solvent volume necessary for coupling. The amount of des-arginine impurity in the crude peptide synthetized in DMF or NBP/DMC 8/2 was almost equivalent (see entries 5 and 6).
The introduction of three consecutive arginines proved to be problematic. Specifically, the formation of des-arginine was detected after the introduction of arginines 4 and 5.
Histidine incorporation into SPPS sequences
The most efficient protocol for the synthesis of Fmoc-His-OH 2a with an unprotected side chain is the use of benzotriazol-1-yl-oxytripyrrolidino-phosphonium hexafluorophosphate (PyBOP) as the coupling additive.18 Yang found that the combination Fmoc-His(Trt)-OH 2b/3a/5 promoted a 4% histidine racemization, while with the use of Fmoc-His-OH, 2a/3a/5 generated a high quantity of 30 containing the D-His in about 2.5% amount. Unfortunately, 5 is incompatible with the use of 2a because it reacts with the imidazolyl moiety. For example, 28 with 5 generates 30 (Scheme 4). The use of PyBOP allowed for the elimination of 5 by avoiding the formation of 30.With the aim of defining a common valid protocol for 2a and 1a, we decided to explore the use of a more hindered activating agent, 1-tbutyl-3-ethyl carbodiimide (TBEC) 29 (see Table 5). As expected, we observed the formation of impurity 30 using 5, entries 1 and 2. On the contrary, TBEC 29 efficiently promoted coupling, and compound 31 was not detected using standard equivalents of the carbodiimides in DMF or NBP/DMC 8/2 (entries 3 and 4). Impurity 31 was observed using only 10 equivalents of 29 with respect to 2a and 3a (entry 5).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Solvent | 3a equiv. | 5 equiv. |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
29 equiv. |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
L/D-His 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|---|---|---|
| a All reactions were carried out using 2a/carbodiimide/3a in a 2/X/2 molar ratio at room temperature. The CTC resin was used. b Determined using HPLC. | |||||||
| 1 | DMF | 2 | 2 | 13 | — | — | 99.4/0.6 |
| 2 | DMF | 5 | 2 | 16 | 99.1/0.9 | ||
| 3 | DMF | 2 | — | — | 2 | nd | 99.4/0.6 |
| 4 | NBP/DMC 8/2 | 2 | — | — | 2 | nd | 99.4/0.6 |
| 5 | NBP/DMC 8/2 | 2 | — | — | 20 | 1.3 | 99.7/0.3 |
The racemization level observed in the coupling using 29 was consistently maintained at <1%, showing a comparable performance with respect to 5. Moving to other carbodiimides,35 dicyclohexylcarbodiimide (DCC) behaves like TBEC 29 (see Fig. S85 and S86†) but, as expected, forms poorly soluble dicyclohexylurea, making its use impractical in SPPS. Meanwhile, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), similar to DIC 5, reacted with the histidine side chains, forming the corresponding adduct (see Fig. S83 and S84†). Therefore, TBEC 29 remains the preferred carbodiimide.
Protocol extension: synthesis of liraglutide and semaglutide N-terminal fragments
This protocol was then tested by carrying out the SPPS of the last four amino acid sequences of the N-terminal of liraglutide and semaglutide that have in common the presence of a histidine as an N-terminal amino acid. Additionally, in these sequences, no traces of 29 adducts on the histidine side chain 31 were detected, as illustrated in Table 6. The use of 29 instead of 5 in green solvent mixtures can be routinely introduced in SPPS. In fact, in addition to eliminating side chain protective groups for arginine, histidine and tyrosine, according to Albericio and de la Torre, 29 can suppress the formation of HCN,36 while Pawlas and coworkers showed that it minimizes racemization, urea precipitation, and radical-induced side reactions.35The iterative process using the 3a/29 mixture for the coupling resulted in a general protocol that was successfully applied to linear vasopressin (see Fig. S95†), provided that when the coupling was carried out with 1a, 2.5 equivalents of 3a were added. The results were identical to those presented in Table 4. The protocol was also applied successfully to the SPPS of sequence 33, which contains tyrosine, histidine and arginine, and 34, which contains an unprotected tryptophan in NBP/DMC 8/2.
No orthogonal side chain protective groups were used, and the two oligopeptides 33 and 34 were isolated with a >97 and >99 purity, respectively (Fig. 4).
 | ||
| Fig. 4 Oligopeptide 33 bearing free tyrosine, histidine and arginine and 34 bearing free arginine, tryptophan and histidine. | ||
Conclusions
The final procedure using Oxyma Pure 3a and TBEC 29 in green solvent mixtures can be employed for iterative synthesis with side chain-unprotected arginine, histidine, and tyrosine, thereby consistently increasing the atom economy of the process. Only the coupling of unprotected arginine 1a required an excess of 3a to prevent the formation of anhydride 9.This protocol was consistent and versatile, and it was successfully applied to the synthesis of linear vasopressin 18 and terminal GLP-1 fragments in green solvent mixtures, increasing the AE of the process. The three consecutive arginines (3, 4, 5) in etelcalcetide-1 20 are usefully introduced by fine tuning the reaction conditions using 3a/5. The suppression of impurities coming from side-chain protective group fragments generated during the cleavage step can facilitate the final purification step by decreasing the overall PMI. Interestingly, the protocol was successfully applied to side chain unprotected Fmoc-tyrosine and Fmoc-tryptophan.
The Oxyma Pure/TBEC protocols are optimized for large-scale incorporation of Fmoc-Arg(HCl)-OH 1a and Fmoc-His-OH 2a in SPPS using industrial-grade reagents, which ensure cost-effectiveness and high efficiency in any solvent comprising green solvents, where side chain unprotected amino acids are soluble.
Experimental procedures
General methods
Unless otherwise specified, all solvents and reagents were obtained from commercial suppliers in the best grade and used without further purification. Specifically, Fmoc amino acids and resins were supplied by Iris Biotech, Alfa Aesar, Merck or Fluorochem. Coupling additives were purchased from Merck or Novabiochem. Piperidine and DEAPA were supplied by Merck or TCI (purity >99%). DMF, NOP, NBP, DMC, and HPLC-quality acetonitrile (CH3CN) were purchased from Merck. Milli-Q water was used for the RP-HPLC analyses. Automated solid-phase peptide syntheses were carried out manually. SPPS at 40 °C was performed using a Minichiller 300 from Huber. The 1H NMR and 13C NMR spectra were recorded using an INOVA 600 MHz instrument with a 5 mm probe. All chemical shifts were quoted relative to the deuterated solvent signals. Unless otherwise specified, HPLC-MS analyses were performed using an Agilent 1260 Infinity II system coupled to an electrospray ionization mass spectrometer (positive-ion mode, m/z = 100–3000 amu, and fragmentor 30 V) using columns Phenomenex Luna C18 5 μm, 250 × 4.6 mm, or Infinity Lab Poroshell C18 2.7 μm, 150 × 3.0 mm; temperature: 35 °C; injection volume: 10 μL; UV: 210 nm; elution phases: H2O + 0.08% TFA (mobile phase A) and CH3CN + 0.08% TFA (mobile phase B) in gradient mode, flow: 1.0 mL min−1. Chemstation software was used for data processing.NMR studies
1a/1b (0.1 mmol, 1.0 eq.) and 3 (0.1–0.25 mmol, 1.0–2.5 eq.) were dissolved in a glass vial with 0.35–1.1 mL of the selected solvent (see chapter 1 ESI†). After 5 minutes, DIC (0.1 mmol, 1.0 eq.) was added and the reaction was stirred at room temperature. After 1–3 h, the 1H NMR spectrum was acquired.Terminal arginine coupling additive efficiency
The syntheses were carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Gly-Phe-Leu-CTC resin (200 mg, loading 0.7 mmol g−1). After swelling of the resin in 2 mL of the selected solvent, Fmoc-L-Arg(HCl)-OH (2.0 eq., 120.1 mg, 0.28 mmol) and the coupling additive (2.0–5.0 eq., 0.28–0.70 mmol) were diluted in 1 mL of the selected solvent and preactivated by DIC (2.0 eq., 43.5 μL, 0.28 mmol) for 5 min, added to the resin and stirred at room temperature. After 90 min, the Fmoc protective group was removed by treating the peptide resin with a 20% piperidine solution in the solvent (2 × 1 mL, 15 min each), and the resin was washed with the solvent (3 × 1 mL). After the Fmoc-cleavage of the N-terminal alpha-amino group, the peptide resin was washed with the selected solvent (3 × 1 mL) and DCM (3 × 1 mL). The dry peptide resin was suspended in 2 mL of the mixture TFA/H2O (95/5 v/v) and stirred for 2 h. The resin was filtered off, and diisopropylether (10 mL) cooled to 4 °C was added to the solution. The peptide was filtered and dried in vacuo to obtain crude H-Arg-Gly-Phe-Leu-OH, and the ratio 14/15 was monitored by HPLC-MS (Analytical method 1, see Table S1†).Coupling efficiency with arginine as a penultimate amino acid
The synthesis of 15 was carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Gly-Phe-Leu-CTC resin (200 mg, loading 0.7 mmol g−1). After the Fmoc removal by treating the peptide resin with a 20% piperidine solution in the selected solvent (2 × 1 mL, 15 min each), the resin was washed with the solvent (3 × 1 mL), and Fmoc-L-Ala-OH (2.0 eq., 87.0 mg, 0.28 mmol) and Oxyma Pure (2.0 eq., 0.28 mmol) were diluted in 1 mL of the selected solvent, preactivated by DIC (2.0 eq., 43.5 μL, 0.28 mmol) for 5 min, added to the resin and stirred at room temperature. After 90 min, the Fmoc protective group was removed by treating the peptide resin with a 20% piperidine solution in the solvent (2 × 2 mL, 15 min each), and the resin was washed with the selected solvent (3 × 1 mL). After the Fmoc-cleavage of the N-terminal alpha-amino group, the peptide resin was washed with the selected solvent (3 × 2 mL) and DCM (3 × 2 mL). The dry peptide resin was suspended in 2 mL of the mixture TFA/H2O (95/5 v/v) and stirred for 2 h. The resin was filtered off, and diisopropylether (10 mL) cooled to 4 °C was added to the solution. The peptide was filtered and dried in vacuo to obtain crude H-Ala Arg-Gly-Phe-Leu-OH, and the ratio 15/17 was monitored by HPLC-MS (Analytical method 1, see Table S2†).SPPS of linear vasopressin 18
Linear vasopressin syntheses were carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Fmoc-Gly-Rink Amide resin (loading 0.5 mmol g−1). After the swelling of the resin in 3.0 mL of the selected solvent, the Fmoc protective group was removed by 20% of base (PIP or DEAPA) in the selected solvent (2 times × 1.5 mL, 15 min each); then, the resin was washed with the solvent (3 times × 1.5 mL, 2 min each). Fmoc-Arg(HCl)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn-OH, Fmoc-Gln-OH, Fmoc-Phe-OH and Fmoc-Tyr-OH (2.0 eq., 0.30 mmol) were pre-activated by Oxyma Pure (2.0–5.0 eq., 0.30–0.75 mmol) and DIC (2.0 eq., 46.4 μL, 0.30 mmol) for 5 minutes and coupled to the resin. After each coupling step, the Fmoc protective group was removed by treating the peptide resin with 20% of base (PIP or DEAPA) in the selected solvent (2 times × 1.5 mL, 15 min each), and the resin was washed with the selected solvent (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the peptide resin was further washed with DCM (3 times × 1.5 mL, 2 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off, washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude linear vasopressin 18, which was directly analyzed using HPLC-MS (Analytical method 2, see Table S4†).SPPS of etelcalcetide-1 20
Etelcalcetide-1 20 syntheses were carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of rink amide MBH resin (loading 0.7 mmol g−1). After the swelling of the resin in 3.0 mL of the selected solvent, the Fmoc protective group was removed by 20% piperidine solution in the selected solvent (2 times × 1.5 mL, 15 min each); then, the resin was washed with the selected solvent (3 times × 1.5 mL, 2 min each). After the loading of the first Fmoc-Arg(HCl)-OH (2.0 eq., 180.1 mg, 0.42 mmol), the resin was washed with the selected solvent (3 times × 1.5 mL, 2 min each), and the capping of the resin was performed with a 1/1/1 solution of AcOH/DIC/Oxyma Pure (5.0 eq., 1.05 mmol) for 1 h. The resin was then washed again with the selected solvent (3 times × 1.5 mL, 2 min each). Fmoc-D-Ala-OH, Fmoc-D-Arg(HCl)-OH and Fmoc-D-Cys(Trt)-OH (2.0 eq., 0.42 mmol) were pre-activated by Oxyma Pure (2.0–5.0 eq., 0.42–1.05 mmol) and DIC (2.0 eq., 65.0 μL, 0.42 mmol) for 5 minutes and coupled to the resin. After each coupling step, the Fmoc protective group was removed by treating the peptide resin with 20% piperidine solution in the selected solvent (2 times × 1.5 mL, 15 min each), and the resin was washed with the selected solvent (2 times × 1.5 mL, 2 min each) and with a 0.5 M solution of Oxyma Pure in the selected solvent (2 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the last amino acid was acetylated after treating the resin with a solution of AcOH/DIC/Oxyma Pure 1/1/1 (5.0 eq., 1.05 mmol) for 1 h. The peptide resin was then washed with the selected solvent (3 times × 1.5 mL, 2 min each), with DCM (3 times × 1.5 mL, 2 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude etelcalcetide-1 20, which was directly analyzed using HPLC-MS (Analytical method 3, see Table S5†).DIC versus TBEC as a coupling reagent with unprotected histidine
The syntheses were carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Gly-Phe-Leu-CTC resin (200 mg, loading 0.7 mmol g−1). After swelling of the resin in 2 mL of the selected solvent, Fmoc-L-His-OH (2.0 eq., 105.6 mg, 0.28 mmol) and the Oxyma Pure (2.0–5.0 eq., 0.28–0.70 mmol) were diluted in 1 mL of the selected solvent and preactivated by the carbodiimide (2.0 eq., 0.28 mmol) for 15 min at 0 °C, added to the resin and stirred at room temperature. After 2 h, the Fmoc protective group was removed by treating the peptide resin with a 20% piperidine solution in the solvent (2 × 1 mL, 15 min each), and the resin was washed with the selected solvent (3 × 1 mL) and DCM (3 × 1 mL). The dry peptide resin was suspended in 2 mL of the mixture TFA/H2O (95/5 v/v) and stirred for 2 h. The resin was filtered off, and diisopropylether (10 mL) cooled to 4 °C was added to the solution. The peptide was filtered and dried in vacuo to obtain crude H-His-Gly-Phe-Leu-OH, and the ratio 14/32 was monitored by HPLC-MS (Analytical method 1, see Table S6†).SPPS of liraglutide and semaglutide residues
Liraglutide and semaglutide syntheses were carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Fmoc-Gly-MBH resin (loading 0.5 mmol g−1). After the swelling of the resin in 3.0 mL of NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8
2, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Aib-OH and Fmoc-His-OH (2.0 eq., 0.30 mmol) were pre-activated by Oxyma Pure (2.0 eq., 42.8 mg, 0.30 mmol) and TBEC (2.0 eq., 46.4 μL, 0.30 mmol) for 5 minutes and coupled to the resin for 2 h. After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8
2 (3 times × 1.5 mL, 2 min each). Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Aib-OH and Fmoc-His-OH (2.0 eq., 0.30 mmol) were pre-activated by Oxyma Pure (2.0 eq., 42.8 mg, 0.30 mmol) and TBEC (2.0 eq., 46.4 μL, 0.30 mmol) for 5 minutes and coupled to the resin for 2 h. After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). After the coupling of the last amino acid, the peptide resin was washed with NBP/DMC 8
2 (3 times × 1.5 mL, 2 min each). After the coupling of the last amino acid, the peptide resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain a crude product directly analysed by HPLC-MS (Analytical method 4, see Table S7†).
2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain a crude product directly analysed by HPLC-MS (Analytical method 4, see Table S7†).
SPPS of H-Tyr-His-Ala-Arg-Gly-Phe-Leu-OH 33
The synthesis of 33 was carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Fmoc-Gly-Phe-Leu-CTC resin (loading 0.7 mmol g−1). After the swelling of the resin in 3.0 mL of NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Fmoc-Arg(HCl)-OH, Fmoc-Ala-OH, Fmoc-His-OH and Fmoc-Tyr-OH (2.0 eq., 0.42 mmol) were pre-activated by Oxyma Pure (5.0 eq., 1.05 mmol) and TBEC (2.0 eq., 65.0 μL, 0.42 mmol) for 5 minutes and coupled to the resin for 2 h (Fmoc-His-OH was pre-activated for 15 min at 0 °C). After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8
2, Fmoc-Arg(HCl)-OH, Fmoc-Ala-OH, Fmoc-His-OH and Fmoc-Tyr-OH (2.0 eq., 0.42 mmol) were pre-activated by Oxyma Pure (5.0 eq., 1.05 mmol) and TBEC (2.0 eq., 65.0 μL, 0.42 mmol) for 5 minutes and coupled to the resin for 2 h (Fmoc-His-OH was pre-activated for 15 min at 0 °C). After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the resin was washed with NBP/DMC 8
2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dried peptide resin was suspended in 3 mL of the TFA/H2O (95/5 v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude 33, which was directly analyzed using HPLC-MS (Analytical method 1, see chapter 8 of ESI†).
2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dried peptide resin was suspended in 3 mL of the TFA/H2O (95/5 v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude 33, which was directly analyzed using HPLC-MS (Analytical method 1, see chapter 8 of ESI†).
SPPS of H-His-Trp-Arg-Gly-Phe-Leu-OH 34
The synthesis of 34 was carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Fmoc-Gly-Phe-Leu-CTC resin (loading 0.7 mmol g−1). After the swelling of the resin in 3.0 mL of NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Fmoc-Arg(HCl)-OH, Fmoc-Trp-OH and Fmoc-His-OH (2.0 eq., 0.42 mmol) were pre-activated by Oxyma Pure (5.0 eq., 1.05 mmol) and TBEC (2.0 eq., 65.0 μL, 0.42 mmol) for 5 minutes and coupled to the resin for 2 h (Fmoc-His-OH was pre-activated for 15 min at 0 °C). After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8
2, Fmoc-Arg(HCl)-OH, Fmoc-Trp-OH and Fmoc-His-OH (2.0 eq., 0.42 mmol) were pre-activated by Oxyma Pure (5.0 eq., 1.05 mmol) and TBEC (2.0 eq., 65.0 μL, 0.42 mmol) for 5 minutes and coupled to the resin for 2 h (Fmoc-His-OH was pre-activated for 15 min at 0 °C). After each coupling step, the Fmoc protective group was removed by 20% piperidine solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the resin was washed with NBP/DMC 8
2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 h. The dry peptide resin was suspended in 3 mL of the TFA/H2O (95/5 v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude 34, which was directly analyzed using HPLC-MS (Analytical method 1, see chapter 9 of ESI†).
2 (3 times × 1.5 mL, 2 min each) and DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 h. The dry peptide resin was suspended in 3 mL of the TFA/H2O (95/5 v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude 34, which was directly analyzed using HPLC-MS (Analytical method 1, see chapter 9 of ESI†).
SPPS of linear vasopressin 18 with TBEC
Linear vasopressin 18 synthesis was carried out at room temperature in glass syringes fitted with a polyethylene porous disc and connected to a vacuum source to remove excess reagents and solvents using 0.3 g of preloaded Fmoc-Gly-Rink Amide resin (loading 0.5 mmol g−1). After the swelling of the resin in 3.0 mL of NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the Fmoc protective group was removed by 20% DEAPA solution in NBP/DMC 8
2, the Fmoc protective group was removed by 20% DEAPA solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). Fmoc-Arg(HCl)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn-OH, Fmoc-Gln-OH, Fmoc-Phe-OH and Fmoc-Tyr-OH (2.0 eq., 0.30 mmol) were pre-activated by Oxyma Pure (5.0 eq., 107.1 mg, 0.75 mmol) and TBEC (2.0 eq., 46.4 μL, 0.30 mmol) for 5 minutes and coupled to the resin for 2 h. After each coupling step, the Fmoc protective group was removed by treating the peptide resin with 20% DEAPA solution in NBP/DMC 8
2 (3 times × 1.5 mL, 2 min each). Fmoc-Arg(HCl)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn-OH, Fmoc-Gln-OH, Fmoc-Phe-OH and Fmoc-Tyr-OH (2.0 eq., 0.30 mmol) were pre-activated by Oxyma Pure (5.0 eq., 107.1 mg, 0.75 mmol) and TBEC (2.0 eq., 46.4 μL, 0.30 mmol) for 5 minutes and coupled to the resin for 2 h. After each coupling step, the Fmoc protective group was removed by treating the peptide resin with 20% DEAPA solution in NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8
2 (2 times × 1.5 mL, 15 min each); then, the resin was washed with NBP/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the peptide resin was further washed with DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude linear vasopressin 18, which was directly analyzed using HPLC-MS (Analytical method 2, see chapter 10 of ESI†).
2 (3 times × 1.5 mL, 2 min each). After the Fmoc cleavage of the N-terminal amino group, the peptide resin was further washed with DCM (3 times × 1.5 mL, 21 min each) and dried under a vacuum for 12 hours. The dry peptide resin was suspended in 3 mL of the TFA/TIS/H2O (95/2.5/2.5 v/v/v) mixture and stirred for 2 h. The resin was filtered off and washed with TFA (1 time × 1 mL, 1 min), and diisopropylether (10 mL) cooled to 4 °C was added to the solution dropwise. The peptide was filtered and dried in vacuo to obtain crude linear vasopressin 18, which was directly analyzed using HPLC-MS (Analytical method 2, see chapter 10 of ESI†).
Author contributions
T. F., A. O. equally contributed to the research. A. T., W. C. and A. R. designed research. T. F., A. O., I. D., performer the experiments, L. F., M. M. analysed the data and all authors contributed to writing the manuscript.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- (a) A. I. Kekessie, K. Wegner, I. Martinez, M. E. Kopach, T. D. White, J. K. Tom, M. N. Kenworthy, F. Gallou, J. Lopez, S. G. Koenig, P. R. Payne, S. Eissler, B. Arumugam, C. Li, S. Mukherjee, A. Isidro-Llobet, O. Ludemann-Hombourger, P. Richardson, J. Kittelmann, D. S. Pedersen and L. J. van den Bos, J. Org. Chem., 2024, 89, 4261 CrossRef PubMed; (b) A. Isidro-Llobet, M. N. Kenworthy, S. Mukherjee, M. E. Kopach, K. Wegner, F. Gallou, A. G. Smith and F. Roschangar, J. Org. Chem., 2019, 84, 4615 CrossRef CAS PubMed.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149 CrossRef CAS.
- L. Ferrazzano, M. Catani, A. Cavazzini, G. Martelli, D. Corbisiero, P. Cantelmi, T. Fantoni, A. Mattellone, C. De Luca, S. Felletti, W. Cabri and A. Tolomelli, Green Chem., 2022, 24, 975 RSC.
- J. Pawlas and J. Rasmussen, in Sustainability in ‘Tides chemistry, RCS, London, 2024, ch. 4, in the press Search PubMed.
- A. Sharma, A. Kumar, B. G. de la Torre and F. Albericio, Chem. Rev., 2022, 122, 13516 CrossRef CAS.
- T. Nuijens, A. Toplak, M. Schmidt, A. Ricci and W. Cabri, Front. Chem., 2019, 7, 829 CrossRef CAS.
- M. O. Frederick, R. A. Boyse, T. M. Braden, J. R. Calvin, B. M. Campbell, S. M. Changi, S. R. Coffin, C. Condon, O. Gowran, J. McClary Groh, S. R. Groskreutz, Z. D. Harms, A. A. Humenik, N. J. Kallman, N. D. Klitzing, M. E. Kopach, J. K. Kretsinger, G. R. Lambertus, J. T. Lampert, L. M. Maguire, H. A. Moynihan, N. S. Mullane, J. D. Murphy, M. E. O'Mahony, R. N. Richey, K. D. Seibert, R. D. Spencer, M. A. Strege, N. Tandogan, F. L. Torres Torres, S. V. Tsukanov and H. Xia, Org. Process Res. Dev., 2021, 25, 1628 CrossRef CAS.
- A. Isidro-Llobet, M. N. Kenworthy, S. Mukherjee, M. E. Kopach, K. Wegner, F. Gallou, A. G. Smith and F. Roschangar, J. Org. Chem., 2019, 84, 4615 CrossRef CAS.
- (a) C. De Luca, G. Lievore, D. Bozza, A. Buratti, A. Cavazzini, A. Ricci, M. Macis, W. Cabri, S. Felletti and M. Catani, Molecules, 2021, 26, 4688 CrossRef CAS; (b) C. De Luca, S. Felletti, G. Lievore, A. Buratti, S. Vogg, M. Morbidelli, A. Cavazzini, M. Catani, M. Macis, A. Ricci and A. W. Cabri, J. Chromatogr., A, 2020, 1625, 461304 CrossRef.
- R. Ravetti Duran and O. Ludemann-Hombourger, Chem. Today, 2021, 39, 42 Search PubMed.
- B. G. de la Torre, S. Ramkisson, F. Albericio and J. Lopez, Org. Process Res. Dev., 2021, 25, 1047 CrossRef.
- S. Lindbæk Broman, M. Rosenberg, F. Wojcik, A. H. Hansen, P. H. G. Egelund, J. Malmstrøm and D. S. Pedersen, Org. Process Res. Dev., 2024, 28, 666 CrossRef.
- A. Tolomelli and W. Cabri, Sustainability in Tides Chemistry, RSC, London, 2024, ch. 2, in the press Search PubMed.
- A. Isidro-Llobet, M. Alvarez and F. Albericio, Chem. Rev., 2009, 109, 2455 CrossRef CAS PubMed.
- (a) Side reactions in Peptide Synthesis, ed. Yi Yang, Elsevier, 2015 Search PubMed; (b) A. J. Wolf and A. Ricci, Sustainability in Tides Chemistry, RSC, London, 2024, ch. 8, in the press Search PubMed.
- (a) T. Liu, Z. Peng, M. Lai, L. Hu and J. Zhao, J. Am. Chem. Soc., 2024, 146, 4270 CrossRef CAS PubMed; (b) Z. Wang, X. Wang, P. Wang and J. Zhao, J. Am. Chem. Soc., 2021, 143, 10374 CrossRef CAS PubMed; (c) Y. Li, J. Li, G. Bao, C. Yu, Y. Liu, Z. He, P. Wang, W. Ma, J. Xie, W. Sun and R. Wang, Org. Lett., 2022, 24, 1169 CrossRef CAS PubMed; (d) T. Tatsumi, K. Sasamoto, T. Matsumoto, R. Hirano, K. Oikawa, M. Nakano, M. Yoshida, K. Oisaki and M. Kanai, Commun. Chem., 2023, 6, 231 CrossRef CAS PubMed.
- K. E. Ruhl, M. J. Di Maso, H. B. Rose, D. M. Schultz, F. Lévesque, S. T. Grosser, S. M. Silverman, S. Li, N. Sciammetta and U. F. Mansoor, Org. Process Res. Dev., 2024, 28, 2896 CrossRef CAS.
- Y. Yang, L. Hansen and P. Ryberg, Org. Process Res. Dev., 2022, 26, 1520 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, pp. 30 Search PubMed.
- W. König and R. Geiger, Chem. Ber., 1970, 103, 2024 CrossRef PubMed.
- F. Albericio and A. El-Faham, Org. Process Res. Dev., 2018, 22, 760 CrossRef CAS.
- S. R. Manne, A. Sharma, A. Sazonovas, A. El-Faham, B. G. de la Torre and F. Albericio, ACS Omega, 2022, 7, 6007 CrossRef CAS PubMed.
- K. D. Wehrstedt, P. A. Wandrey and D. Heitkamp, J. Hazard. Mater., 2015, A126, 1 Search PubMed.
- A. Orlandin, I. Guryanov, L. Ferrazzano, B. Biondi, F. Biscaglia, C. Storti, M. Rancan, F. Formaggio, A. Ricci and W. Cabri, Molecules, 2022, 27, 4235 CrossRef CAS PubMed.
- P. H. G. Egelund, S. Jadhav, V. Martin, H. J. Castro, F. Richner, S. Thordal Le Quement, F. Dettner, C. Lechner, R. Schoenleber and D. S. Pedersen, Green Chem., 2021, 23, 3312 RSC.
- G. Martelli, P. Cantelmi, A. Tolomelli, D. Corbisiero, A. Mattellone, A. Ricci, T. Fantoni, W. Cabri, F. Vacondio, F. Ferlenghi, M. Mor and L. Ferrazzano, Green Chem., 2021, 23, 4095 RSC.
- J. Lopez, S. Pletscher, A. Aemissegger, C. Bucher and F. Gallou, Org. Process Res. Dev., 2018, 22, 494 CrossRef CAS.
- J. L. Lau and M. K. Dunn, Bioorg. Med. Chem., 2018, 26, 2700 CrossRef CAS PubMed.
- VASOSTRICT® (vasopressin injection) 2024, US prescribing information by PAR Pharmaceuticals.
- See: https://www.fortunebusinessinsights.com/vasopressin-for-vasoplegic-shock-market-104065.
- FIRAZYR® (Icatibant injection) 2024 US prescribing information by Takeda.
- C. A. Fitch, G. Platzer, M. Okon, B. E. Garcia-Moreno and L. P. McIntosh, Protein Sci., 2015, 24, 752 CrossRef CAS PubMed.
- H. K. Hall Jr., J. Am. Chem. Soc., 1957, 79, 5441 CrossRef.
- G. Martelli, P. Cantelmi, C. Palladino, A. Mattellone, D. Corbisiero, T. Fantoni, A. Tolomelli, M. Macis, A. Ricci, W. Cabri and L. Ferrazzano, Green Chem., 2021, 23, 8096 RSC.
- J. Pawlas, J.-H. Choi, C. von Bargen, S. Maibom-Thomsen, J. H. Rasmussen and O. Ludemann-Hombourger, Org. Process Res. Dev., 2023, 27, 1348 CrossRef CAS.
- S. R. Manne, O. Luna, G. A. Acosta, M. Royo, A. El-Faham, G. Orosz, B. G. de la Torre and F. Albericio, Org. Lett., 2021, 23, 6900 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc03209h |
| This journal is © The Royal Society of Chemistry 2024 |







