Open Access Article
Open Access ArticleProduction of nanochitins via a shell biorefinery process for self-assembly applications as photonic films and Pickering emulsions†
Xuhai
Zhu‡
 a,
Fuyan
Peng‡
ab,
Hui
Li
c,
Rongjun
Lin
ab,
Rui
Lu
a,
Fuyan
Peng‡
ab,
Hui
Li
c,
Rongjun
Lin
ab,
Rui
Lu
 a and
Fang
Lu
a and
Fang
Lu
 *a
*a
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning 110623, China. E-mail: lufang@dicp.ac.cn; Tel: +86-0411-8437-9846
bHenan Institute of Advanced Technology, Zhengzhou University, Zhengzhou, Henan 450052, China
cZhengzhou Tobacco Research Institute, China National Tobacco Corporation, Zhengzhou, Henan 450001, China
First published on 5th October 2024
Abstract
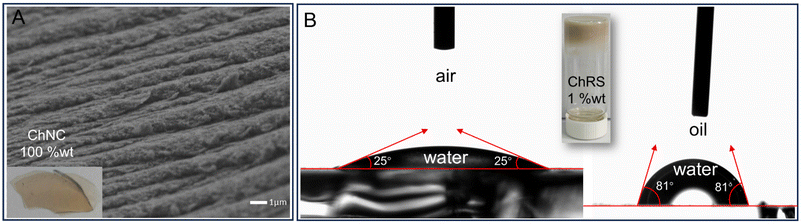
The concept of sustainable development is driving shell biorefineries to develop a cost-effective and environmentally benign approach to transform chitin into high-value products. This study describes a green, simple, and flexible shell biorefinery process for producing two kinds of nanochitins (NCh), chitin solid residues (ChSRs) and chitin nanocrystals (ChNCs), by using p-toluenesulfonic acid-assisted hydrothermal treatment. The ChSR is a bundled nanocrystal with a long length ranging from 100 nm to 450 nm and a width ranging from 10 nm to 30 nm. It has an excellent interfacial behavior between the water and oil phases due to its amphiphilic nature; thus, a foodstuff-related emulsion has been successfully fabricated from soybean oil and a ChSR suspension without the addition of any synthetic surfactants. The further hydrolysed ChNC with a length and width range of 25–225 nm and 15–30 nm, respectively, and a high zeta potential of 41 mV exists as an individual nanocrystal. Owing to the abundance of positively charged groups distributed on its surface, ChNC enables self-assembly to a highly ordered hierarchical structure. Hence, ChNCs show promising results in fabricating large-area photonic films, e.g. for application in bright and vivid automobile painting. Altogether, we provide a novel approach to produce NCh materials from crustacean shell waste and bridge the gap from process engineering to high-value product development.
1 Introduction
Crustacean shell waste is one of a few potential sources of biomass energy, but not the only one; there are other alternatives, such as wood, agricultural straw, poultry and livestock excrement, kitchen waste, etc.1 Large amounts of crustacean waste are generated annually, especially the shells from seafood (e.g., shrimps, crabs, and lobsters).2 Therefore, utilizing shell biomass components for high-value chemicals/materials can be economically profitable and essential in addressing UN sustainability goals.3 The main goal of the shell biorefinery approach is to maximize technical and, therefore, economic outcomes, considering high-value products from all biomass components. The shells are composed of three dominant components, i.e. 20–40% of protein, 20–50% of CaCO3, and 15–40% of chitin.4 Proteins have nutrient value and can be processed as fertilizers or animal feed, while biological organism-originated CaCO3 is suitable for application in the biomedical field.5 Chitin, poly(β-(1 → 4)-N-acetyl-D-glucosamine), is the second most abundant biopolymer after cellulose, primarily used in its highly degraded and deacetylated form, known as chitosan.6 However, chitosan production with abundant waste and low profits can be seen as a limitation to the sustainable use of chitin, which justifies the consideration of new strategies with better environmental and economic prospects.7,8Taking advantage of the novel characteristics of biomaterials at the nanoscale, simple top-down strategies have been applied to isolate their building blocks. In this context, studies on the preparation, characterization, and applications of chitin nanoparticles, referred to as “nanochitin (NCh)”, have been extensively conducted.8–10 Similar to producing nanocellulose from native celluloses, methods such as acid hydrolysis, pretreatments combined with mechanical disintegration, and chemical oxidations are generally employed to produce NCh from purified chitin.10–13 The main challenges that hinder the industrialization of these technologies are three aspects. The first obstacle is the highly tunable processing conditions of the commonly used acid hydrolysis on chitin, which requires significant time and resources to learn the connections between processing conditions and output versatility regarding the NCh structure, yield, and physicochemical properties. To make the manufacture of NCh efficient and more profitable, kinetics-related processing parameters, like reaction temperature, time, and acid concentration, must be optimized to ensure that the properties of the extracted NCh are ideal for the intended end product.14 The second one is chemical recovery and NCh product treatment, which often involve a multistage recovery. Generally, strong acids (sulfuric acid, hydrochloric acid, or nitric acid) or oxidation reagents (TEMPO, NaClO, H2O2) are utilized to catalyse the liberation of NCh from chitin.10–14 However, the non-recyclability of these catalysts increases the production cost and harms the environment. Even though the production of thermally stable and functional nanocellulose by concentrated organic acid hydrolysis has been reported,15 crustacean waste is different in composition from lignocellulosic waste; thus, using easily recoverable and reusable organic acids is a desirable technique for shell biorefineries. The last one is the manufacture of NCh directly from crustacean shells, which involves several cumbersome steps, including the intensive use of strong mineral acids to decompose CaCO3 and NaOH to peel off protein, followed by using NaClO2 to bleach and purify resultant chitins, and finally, isolation of chitin's building blocks at the nanoscale.11,16 This extra chitin fractionation and purification necessitates expenditure on corrosion-resistant equipment and wastewater treatment; therefore, for further commercial applications, it would be desirable to improve the cost-effectiveness of the process and prepare NCh directly from the crustacean biomass. An acidic ionic liquid was recently made for in situ production of individual chitin nanowhiskers with low yield straight from shrimp shells.17 In addition, various types of solid organic acids such as p-toluenesulfonic acid (PTSA), oxalic acid, maleic acid, malonic acid, tartaric acid, and citric acid were introduced to the hydrolysis treatment on biopolymers.15,18,19 Inspired by the pressure cooker used to tenderize meat protein, a new fractionation method was recently reported to extract high-value chitin from shrimp shells using acidic hydrothermal treatment.16 Based on these backgrounds, we are interested in evaluating the feasibility of solid organic acids as acid catalysts for NCh prepared straight from crustacean biomass by hydrothermal treatment.
The present work describes a green, simple, and flexible shell biorefinery process for producing NCh by solid organic acid-assisted hydrothermal treatment. The effect of reaction conditions, including solid organic acid types, temperature, time, and the molar ratio of acid to chitin, on the hydrolysis efficiency of purified chitin is first studied to identify the optimal hydrolysis conditions. Then, we proceed with the hydrolysis of crustacean shell waste. It is envisaged that a high quantity of NCh can be produced from crustacean shells in one pot by using hot water for deproteinization, solid organic acids for demineralization, and deliberation of chitin microfibrils. Furthermore, the recyclability and reusability of solid organic acids are evaluated. The morphology, chemical structure, and properties of the obtained NCh products are also characterized. Finally, the applications associated with the assembly behavior of the resultant NCh products are investigated. The biobased nanoparticles can generate complex structures via self-assembly guided by forces acting at multiple scales, of which functions and applications are determined by the nature of the particles and the assembly process.8,9,14 Depending on the processing conditions of chitin hydrolysis, it is possible to adjust the characteristics of the resultant NCh particles for final applications associated with assembled structures.8,9,14 It is, therefore, desirable to consider the process–property–application relationships involved in NCh for material design and development.
Overall, the main objectives of this work are (1) to study the effect of process conditions on the yield, structure, and physicochemical properties of the NCh products to evaluate the ability to engineer precursors with desirable characteristics; (2) to produce NCh products directly from the raw shell biomass and elucidate their properties; (3) to evaluate the application potential for the assembly behavior of NCh products.
2 Methods
2.1 Preparation of NCh from purified chitin
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min, and the supernatant was removed by decantation. The resultant water-insoluble fraction was re-suspended in water, followed by dialysis against deionized water until a fairly steady pH was reached. Once again, the suspension was centrifuged at 22
000g for 5 min, and the supernatant was removed by decantation. The resultant water-insoluble fraction was re-suspended in water, followed by dialysis against deionized water until a fairly steady pH was reached. Once again, the suspension was centrifuged at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min to obtain two types of NCh products as separate streams: chitin nanocrystals (ChNCs) and chitin solid residues (ChSRs). ChNC formed a stable suspension in the supernatant, whereas ChSR was obtained as a residue. Volumes and weights of each fraction were recorded, and samples were stored at 4 °C until further use. ChNC and ChSR yields were determined using the gravimetric method of oven-drying, where samples were dried at 105 °C until constant weight (eqn (1)):
000g for 5 min to obtain two types of NCh products as separate streams: chitin nanocrystals (ChNCs) and chitin solid residues (ChSRs). ChNC formed a stable suspension in the supernatant, whereas ChSR was obtained as a residue. Volumes and weights of each fraction were recorded, and samples were stored at 4 °C until further use. ChNC and ChSR yields were determined using the gravimetric method of oven-drying, where samples were dried at 105 °C until constant weight (eqn (1)): | (1) |
An actual experiment followed the optimized conditions to verify the predicted production yield. The experimental operations were the same as in the above paragraph. After the hydrolysis reaction, the water-soluble fraction collected from the product's suspension by centrifugation was stored for the subsequent acid recovery and reusing experiment. Some of the ChNC and ChSR products were freeze-dried for analyses, respectively, and the rest were stored at 4 °C before further use.
 | (2) |
The reproducibility and reusability of R-PTSA were evaluated under the optimal conditions at which the maximum yields of ChNC and ChSR were collected. The corresponding ChNC and ChSR production yields were calculated. Some of the ChNC products were freeze-dried for analysis.
2.2 Preparation of NCh from crustacean biomass waste
Before hydrolysis, all shell samples were milled to pass through a 60-mesh screen in a Wiley mill to ensure particle size uniformity and increase surface area. Considering the variation in the composition and density of shell structure in these crustacean biomass wastes, they were subjected to hydrolysis following a procedure modified from the above optimal conditions. This process can realize the demineralization, deproteinization, and NCh production in one pot.In a typical experiment for crab shells, 1.0 g of shell powder, 1.8 g of PTSA, 17.2 g of deionized water, and a stirrer bar were placed in a Teflon-lined autoclave with 50 mL inner volume. The mixture was capped and demineralized at room temperature with constant stirring for 12 h. During this procedure, a large amount of foam can be observed. Afterward, an additional 1.8 g of PTSA was added, and the autoclave was sealed and filled with 0.5 atm N2. The reaction was stirred and subjected to 120 °C heating. To ensure that proteins can be extracted sufficiently by the acid-catalyzed hydrothermal treatment and that chitin fiber thus released can be hydrolysed into the NCh product, the reaction time was extended to 12 h. After the reaction, the product mixture was separated by using a centrifuge (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 5 min). The solid residue was washed thoroughly with water by repeated centrifugation, followed by dialysis against deionized water until a fairly steady pH was reached. Finally, ultrasound treatment was conducted to disperse the NCh particles derived from crab shells (NChcrab). The NCh products from crawfish (NChcrawfish) and shrimp (NChshrimp) shells followed the same general protocol as the NChcrab, the only difference being the added amount of PTSA. In the production of NChcrawfish, 0.9 g and 1.8 g of PTSA were added in the demineralization and acid-catalyzed hydrothermal steps, respectively. In contrast, a lower amount of PTSA (0.9 g) was used in the acid-catalyzed hydrothermal reaction to produce NChshrimp. The remaining steps of the preparation protocol are the same as those for the NChcrab. The overall mass yield of NCh from biomass was calculated based on the chitin available in biomass, and some NCh products were freeze-dried for analysis.
000g, 5 min). The solid residue was washed thoroughly with water by repeated centrifugation, followed by dialysis against deionized water until a fairly steady pH was reached. Finally, ultrasound treatment was conducted to disperse the NCh particles derived from crab shells (NChcrab). The NCh products from crawfish (NChcrawfish) and shrimp (NChshrimp) shells followed the same general protocol as the NChcrab, the only difference being the added amount of PTSA. In the production of NChcrawfish, 0.9 g and 1.8 g of PTSA were added in the demineralization and acid-catalyzed hydrothermal steps, respectively. In contrast, a lower amount of PTSA (0.9 g) was used in the acid-catalyzed hydrothermal reaction to produce NChshrimp. The remaining steps of the preparation protocol are the same as those for the NChcrab. The overall mass yield of NCh from biomass was calculated based on the chitin available in biomass, and some NCh products were freeze-dried for analysis.
2.3 Application of NCh products
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w to obtain ChNC/PVA suspensions after sonication. The mixture suspension was vigorously stirred overnight at room temperature and then transferred to a tank of paint spray gun. The ChNC/PVA composite films were obtained by spraying the resulting suspension on the 201-type stainless-steel plates with different sizes. Each film's characteristic color can be observed after drying for 30 min at room temperature.
1 w/w to obtain ChNC/PVA suspensions after sonication. The mixture suspension was vigorously stirred overnight at room temperature and then transferred to a tank of paint spray gun. The ChNC/PVA composite films were obtained by spraying the resulting suspension on the 201-type stainless-steel plates with different sizes. Each film's characteristic color can be observed after drying for 30 min at room temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 6
3, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 5
4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 4
5, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) with a high-speed shear homogenizer (T-118 Ultra-Turrax, IKA, Germany) at a shear rate of 20
6) with a high-speed shear homogenizer (T-118 Ultra-Turrax, IKA, Germany) at a shear rate of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min. Optical photographs of emulsions were taken after storage at room temperature for 7 days. During this period, the emulsion separation was quantified based on the Emulsion Index (EI, eqn (3)):
000 rpm for 3 min. Optical photographs of emulsions were taken after storage at room temperature for 7 days. During this period, the emulsion separation was quantified based on the Emulsion Index (EI, eqn (3)):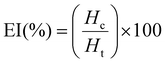 | (3) |
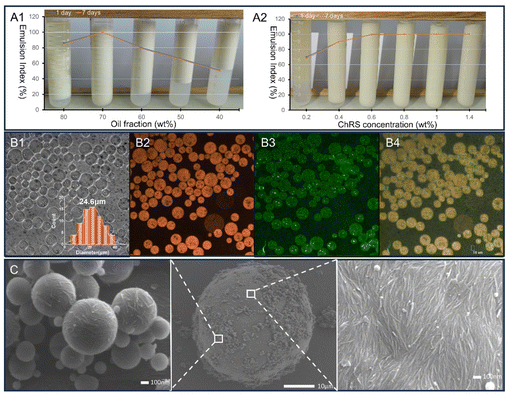
To simultaneously visualize ChSR and soybean oil, emulsification was done using soybean oil dyed with Nile red and ChSR stained with fluorescein isothiocyanate (FITC). Nile red solution (1 mg mL−1 in isopropyl alcohol) was mixed with soybean oil at a mass ratio of 1/25, which was thoroughly stirred overnight at room temperature; FITC solution (1 mg mL−1 in isopropyl alcohol) was stirred with ChSR at a mass ratio of 1/25 overnight at room temperature. The oil-to-water ratio was set as 7/3, and the 1.0 wt% of stained ChSR was used, wherein the mixture was emulsified following the above procedures. The labelled emulsions were stored at 4 °C before characterization.
3 Results and discussion
3.1 General
Fig. 1 illustrates the entire experimental process flow for producing NCh products with minimum chitin loss by organic acid-assisted hydrothermal treatment followed by centrifuge fractionation. In this work, crystalline solid-type organic acids were applied to hydrolyse the purified chitin to gently destroy the chemical bonds of the feed chitin under mild conditions and enhance the yield of NCh under mild acid hydrolysis conditions. The hydrolysis reaction was carried out using acids over a range of molar ratio of acid to N-acetylglucosamine of chitin (n(acid)/n(GlcNAc) = 0.6–1.2), hydrolysis temperature (100–130 °C), and time (2–6 h). At the end of the hydrolysis reaction, double-stage centrifuge treatment was adopted to treat the NCh suspension. The acid collected from the first-stage centrifuge was further extracted and crystallized at low temperature for acid recovery and reused. The resultant NChs were dialyzed to remove the residual acid, followed by the second-stage centrifuge, which was fractionated into two types of products based on their interface stability. They are named ChNC and ChSR, respectively, and the corresponding structure, property, and potential application are evaluated below. The benefits of this novel method include the following:(1) Green and sustainable hydrolysis with fully recyclable crystalline solid-type organic acid.
(2) Simultaneous production of two types of NCh products to minimize chitin loss.
(3) The process's high flexibility allows for the engineering of the resulting products and bridges the gap from process engineering to specific high-value applications.
 | ||
| Fig. 1 Schematic illustration of the preparation of two types of nanochitin products by a recyclable solid organic acid assisted hydrothermal treatment. | ||
3.2 Optimization of acid hydrolysis production
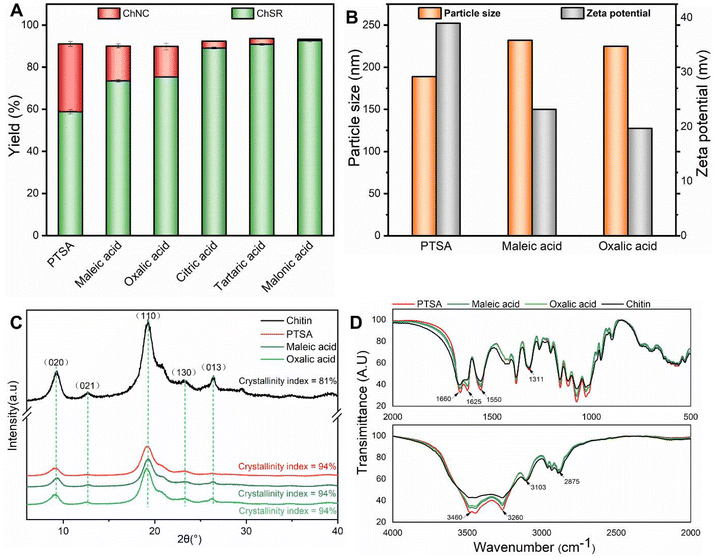
As presented in Fig. 2C, X-ray diffraction (XRD) patterns of the purified chitin and the corresponding NCh products exhibit identical four typical characteristic diffraction peaks of the α-chitin structure, located at 2θ = 9°, 13°, 19°, 21° and 26.6°, which corresponded to the (020), (021), (110), (130), and (013) reflection planes, respectively.22 It indicates that hydrolysis of all solid acids keeps the type of the original chitin crystal structure intact. The enhancement of the ChNC products’ crystallinity index compared with the original chitin is attributed to the removal of the amorphous region of chitin. Notable, the crystallinity of all CHNC products by different types of acid hydrolysis processes is identical.
The FT-IR spectra of the ChNC products from different acid hydrolysis processes are compared in Fig. 2D. The characteristic peaks of purified chitin are observed at around 3460 cm−1 (–OH stretching vibration), 3260 and 3103 cm−1 (–NH2 stretching), 2875 cm−1 (–CH2 and –CH3 stretching), 1660 and 1625 cm−1 (amide I band), 1550 cm−1 (amide II bands), and 1311 cm−1 (amide III bands).23 The same characteristic bands corresponding to chitin are observed in these ChNC products, suggesting the elementary structure of chitin can be maintained for all hydrolysis treatments. However, the resonance of –OH and –NH2 in the ChNC obtained by PTSA is stronger than those two ChNC products obtained from maleic acid and oxalic acid. It indicates that PTSA is the most efficient in the cleavage of glycosidic and amide bonds among the organic acids investigated here.
Therefore, based on the above results of the yields, stability and structure of NCh products, PTSA is thus chosen for the catalytic hydrolysis of chitin to the NCh.
To better visualize the statistically significant factors derived from the statistical analysis, three-dimensional (3D) response surface plots and the corresponding contour plots are given in Fig. 4. These plots depict the combined effects of two factors on yields of NCh products, while another factor is kept constant at their medium levels. Under the same variation range, the response surface for ChNC yields show a convex shape, while that for ChSR yields show a concave shape. It can be explained by the fact that the ChNC is sequentially converted from ChSR by hydrolysis rather than directly obtained by one-step hydrolysis.
Fig. 4A and B show the interaction effects of acid molar ratio and reaction temperature on the yields of ChSR and ChNC, respectively. The shape of the contour plots is elliptical rather than circular, indicating that the mutual interactions between variables are significant in the chitin hydrolysis process. Generally, the loosely tangled amorphous region is more readily hydrolyzed than the compact ordered crystalline regions in chitin.24 Consequently, most of the amorphous regions in chitin are degraded in the initial hydrolysis period, leaving the micro-sized chitin crystalline particles (ChNC) with a maximum amount. As shown in Fig. 4A, at any level of reaction temperature, the increase of acid molar ratio from 0.6 to 1.2 can slightly reduce the amount of ChSR. Accordingly, as shown in Fig. 4B, the ChNC yield from the hydrolysis of ChSR is only enhanced by around 12%. It indicates that independently increasing the acid concentration is limited in promoting the hydrolysis of the crystalline region to a smaller size. Meanwhile, the reaction temperature plays a crucial role in initiating and improving the chemical degradation of the chitin chain (from the microscale to the nanoscale). In the region of acid molar ratio of 0.6 (Fig. 4A), the amount of ChSR significantly decreases from 93% to 47% with the increased reaction temperature from 100 °C to 130 °C. As a result, in Fig. 4B, the ChNC yield is progressively increased from 3% to 45% at the reaction temperature of 100–130 °C. Similar trends are also observed at the high acid molar ratio (1.2) level for both ChSR and ChNC yields. On the one hand, the improved yield can be attributed to the energy barrier for breaking the hydrogen bonding system in chitin, which is lowered with increasing temperature. Consequently, the hydrolysing catalyst quickly penetrates and spreads evenly inside the individual crystallite segments, making the hydrolysis process more kinetically favorable.25 On the other hand, the hydrolysis reaction rate is directly related to the temperature based on the thermal equilibrium theory:26 the higher the temperature, the more the energy that is delivered to the reaction medium, resulting in easier cleavage of covalent bonds inside the chitin fragments (ChSR) and hence more chitin crystalline particles (ChNC) in nano-size are produced. Recent studies have supported this statement on preparing cellulose nanocrystals by using ionic liquids and Ni(NO3)2 catalysts.26,27 The interaction effects of reaction temperature and time on ChSR and ChNC yields are displayed in Fig. 4C and D, respectively. It is observed from Fig. 4C that the yield of ChSR decreases with the increase of reaction temperature at a constant reaction time, especially when the reaction time is within the range of 2–6 h; the increase of reaction temperature from 100 °C to 130 °C leads to the reduction of ChSR content (96–47%). In contrast, the yield of ChNC shows an increasing trend with the increase of temperature, as shown in Fig. 4D; e.g., at the low level of reaction time (2 h), the increase of reaction temperature from 100 °C to 130 °C leads to an increase of ChNC yield (1–44%). Also, the effects of reaction time show that the ChSR yield decreases from 96% to 80% with the reaction time increased from 2 h to 6 h when the hydrolysis temperature is 100 °C (Fig. 4C). An opposite trend can be observed in Fig. 4D, in which the ChNC yield gradually increases from 2% to 18%. It is noted that the contour plots for both ChSR and ChNC yields are elliptical, implying that the interactive effect of reaction temperature and time on yields is significant. High temperature is conducive to the diffusion of hydrolysing catalysts inside the chitin substrate, and the effect is accumulated with the progressive increment of contact time. Consequently, the surface area of treated chitin is increased due to the physical swelling phenomenon and the disintegration of the glycosidic linkage of chitin fibres initiated until its maximum level. An increment in temperature results in an enhanced hydrolysis rate and shortens the reaction time to achieve a high yield of ChNC. However, excessive heat energy causes the color of solid products to deepen and even over-degrades solid products to the water-soluble by-products. The simultaneous dependence of ChSR and ChNC yields on the time vs. acid molar ratio is shown in Fig. 4E and F, respectively. Their corresponding contour plots are close to circular, indicating the relatively mild interactive effects of the two independent variables on the yield. Based on the above results, our hydrolytic reaction to effectively produce high crystalline NCh should be restricted to a shorter time and be more manageable under normal atmospheric pressure conditions.
Finally, the optimal conditions to obtain the maximum yield of ChNC and ChSR are predicted as follows: n(acid)/n(GlcNAc) = 1.2, reaction temperature = 130 °C, reaction time = 6 h. Under the optimal conditions, as shown in Table 1, the experimental yield of ChNC is 39%, and that of ChSR is 54%, which is not significantly different from the predicted values of 41% and 53% at a 95% confidence interval, respectively. In the hydrodynamic diameter distribution pattern measured by dynamic light scattering, the obtained ChSR has an average diameter of about 169 nm with a relatively broad size distribution (PDI = 0.8). In contrast, the ChNC, with an average diameter of about 50 nm, was further degraded from ChSR. Moreover, the ChNC particles have a uniform architecture with a PDI value of 0.3. In addition, the zeta-potential results indicate that ChNC has better colloidal stability than ChSR. It further confirms that chitin chains deacetylated to form cationic NChs as the hydrolysis progressed. Therefore, the model helps to predict the optimal acid hydrolysis process parameters for producing different types of NChs.
| Predicted yield (%) | Experimental yield (%) | Z-average size (nm) | PDI | Zeta potential (mV) | |
|---|---|---|---|---|---|
| ChSR | 53 | 54 | 169 | 0.8 | +35 |
| ChNC | 41 | 39 | 50 | 0.3 | +41 |
| Total | 94 | 93 | — | — | — |
3.3 Verification of NCh production from crustacean shell waste
Next, we performed scale-up production of NChs from three species of crustacean biomass in one-pot modified from the above optimal conditions (as shown in Fig. 5A). The results in Fig. 5B illustrate that different biomass have different composition. Their chitin contents are close to each other, whereas the mineral content is the highest in the crab and the protein content is the highest in the shrimp. The CaCO3 minerals of crustacean samples are well exposed and accessible at the surface due to the milling procedure. This is desirable as it facilitates the demineralization by PTSA with the formation of CO2. After foam evolution ceased, additional PTSA was added to start the deproteinization and chitin hydrolysis reaction with heating and stirring. It is generally accepted that protons and hydroxide generated in hydrothermal water can promote acid- and base-catalysed hydrolysis of proteins into peptides, amino acids and ammonia.28–30 Thus, we consider subcritical water sufficient to decompose proteins from these crustacean samples and leave the chitin to be hydrolysed into nanoparticles under PTSA catalysis. In Fig. 5B, the overall mass yield of NChs is 59% from crab (abbreviated as NChcrab) and 96% from crawfish based on the chitin available in biomass. The over-hydrolysis of chitin in crabs can be attributed to a high amount of easily degradable CaCO3 and a low amount of inert protein under acidic conditions. For the same reason, over 100% of NChs is obtained from shrimp due to the high amount of protein. Therefore, the production of NChs with our system is more effective for crustacean biomass, which is rich in minerals than protein. Also, all the NChs carrying a positive charge (zeta-potential = +24–27 mV) straight from Crustacean biomass could be colloidally stable in water. Fig. 5C shows the hydrodynamic diameter distribution results of the NCh. The average size was 299, 453, and 536 nm for NChcrab, NChcrawfish, and NChshrimp, respectively. Moreover, the chitins in the crab shell were sufficiently hydrolyzed into the NChcrab with a PDI value of 0.4. In contrast, the chitins in the shrimp shell were insufficiently hydrolyzed into the NChshrimp, resulting in an uneven particle distribution (PDI = 0.8). Therefore, these tests suggest that the crab shell is the best crustacean biomass waste for scalable production of NCh.3.4 Characterization of the NCh products
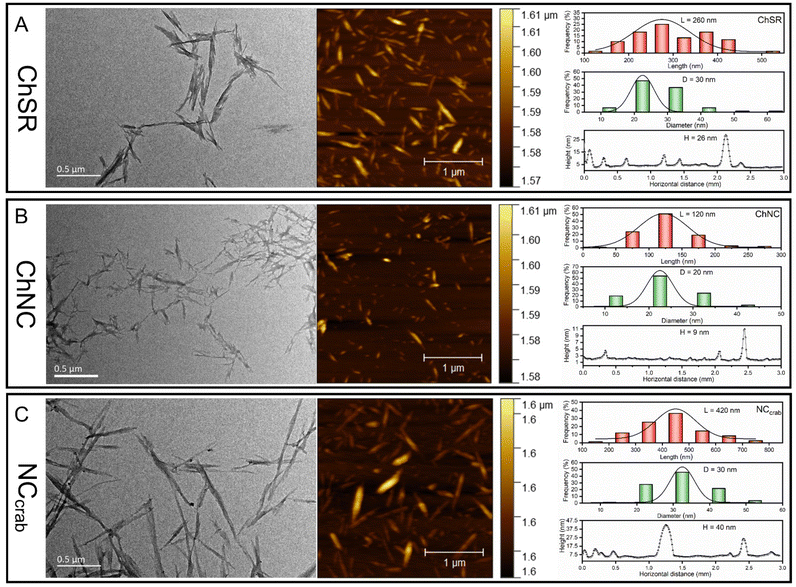 | ||
| Fig. 6 Morphology of the nanochitin products obtained under the optimal conditions: TEM and AFM images and size distributions of ChSR (A), ChNC (B) and NChcrab (C). | ||
The XRD patterns of three types of NCh products are shown in Fig. 7A. The NChcrab exhibits a similar crystal structure and crystallinity index compared with the other two kinds of NCh products from purified chitin. The crystallinity of ChNC (84%) is slightly lower than that of ChSR (89%), which can be attributed to the partial destruction of the crystalline structure and results in more disordered regions in chitin nanofibrils caused by further hydrolysis.31 As a result, the increased effective surface area of chitin improves the accessibility of PTSA reagents to the amide bonds and led to a high charge content.
 | ||
| Fig. 7 XRD patterns (A), FT-IR spectra (B), 13C solid-NMR spectra (C), and TG and DTG curves (D) of NCh products. | ||
The FT-IR spectroscopy was used to investigate the chitin's chemical structure evolution during acid hydrolysis (Fig. 7B). However, since the difference between spectra of ChSR and ChNC are subtle, their structural difference is hardly distinguished. In addition, the NChcrab with a typical chitin's infrared structure spectrum indicates that the extraction and preparation of NCh from crustacean biomass waste can be realized in one-pot by our hydrolysis system.
The solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance (13C CP/MAS-NMR) technique was thus employed to get more detailed information on NCh products (Fig. 7C). Their 13C NMR spectra show eight well-defined resonances of C1–C6 and acetyl, which are ε = 174 ppm for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, ε = 103 ppm for C1, ε = 83 ppm for C4, ε = 75 ppm for C5, ε = 61 ppm for C6, ε = 56 ppm for C2, and ε = 23 ppm for CH3.32 The original chitin and the corresponding ChSR and ChNC products are pure and contain no other impurity, as indicated by the peaks differing from their spectra at a very low level. The degree of acetylation (%DA) was calculated by measuring relative integrals of a methyl group (CH3) compared to the carbon integrals of the polysaccharidic backbone (Fig. S4–S6†).33 The %DA was 99% for original chitin and gradually decreases as the hydrolysis progress. Specifically, the ChSR product exhibits 96%DA, just slightly lower than that for chitin, whereas the %DA of the ChNC product was found to be 90% (Fig. 7C). These results confirm that the breaking of the NH–C(O) bond occurs during the PTSA hydrolysis and that the ChNC is the further deacetylated product of ChSR.
O bond, ε = 103 ppm for C1, ε = 83 ppm for C4, ε = 75 ppm for C5, ε = 61 ppm for C6, ε = 56 ppm for C2, and ε = 23 ppm for CH3.32 The original chitin and the corresponding ChSR and ChNC products are pure and contain no other impurity, as indicated by the peaks differing from their spectra at a very low level. The degree of acetylation (%DA) was calculated by measuring relative integrals of a methyl group (CH3) compared to the carbon integrals of the polysaccharidic backbone (Fig. S4–S6†).33 The %DA was 99% for original chitin and gradually decreases as the hydrolysis progress. Specifically, the ChSR product exhibits 96%DA, just slightly lower than that for chitin, whereas the %DA of the ChNC product was found to be 90% (Fig. 7C). These results confirm that the breaking of the NH–C(O) bond occurs during the PTSA hydrolysis and that the ChNC is the further deacetylated product of ChSR.
The thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of chitin and NCh products hydrolyzed by PTSA are presented in Fig. 7D. All samples show three distinctive steps of thermal degradation in the TG curves. In the first stage, a weight loss rate of ca. 2–8% happens below 100 °C due to the removal of adsorbed water and volatile components upon heating.34 In the second stage, a sharp weight loss rate is observed from 200 to 400 °C, mainly caused by the chitin degradation due to the depolymerization, dehydration, or decomposition of the glycosyl unit, followed by the formation of charred residue.35 Both the starting decomposition temperature (Tonset) and mid-point of the decomposition temperature (T0.5) of the samples are higher in the order of ChNC < NChcrab < ChSR < chitin, as shown in the TG and DTG curves. In contrast with the original purified chitin, the decrease in the thermal degradation of the corresponding NCh products is due to the conversion of chitins to nano-elements, benefiting the amounts of chitin crystallite surfaces and the heat transfer rate.36,37 The thermal stability of nano-scale chitin particles also decreased, as reported in the previous studies.34,37,38 The difference in thermal stability among NCh products appeared to relate with the purity, particle size, crystallinity, and DA%. The NMR spectra of NCh products show that the ChNC and ChSR are pure, whereas the NChcrab directly extracted from crustacean waste contains protein-related impurities (see Fig. S7 in the ESI†). Considering that the impurity derivatives in the NChcrab could significantly affect its thermal stability, we will not give further comparison discussion on NChcrab. For the clean NCh products originating from purified chitin, it is reasonable that the obtained ChNC with a smaller size and lower crystallinity by further PTSA hydrolysis shows a decrease of thermal stability compared with that of obtained ChSR. Also, the reported negative relationship between thermal stability and degree of deacetylation for chitin/chitosan nanofibers agrees with our results in Fig. 7C and D.39 In the last stage of 400–800 °C, the slight weight loss is ascribed to the further depolymerization of residual char into volatile products.33 The final amount of char residues at 800 °C for NCh products (30%) is noticeably larger than that for chitin (15%). A reasonable explanation for this can be referred to the research on similar nanomaterials, like cellulose nanocrystals.40,41 The number of free-end chains on the surface of chitin increases significantly as its size reduces to a nanometer scale. These end chains quickly decompose at lower temperatures, consequently facilitating the increase of char residue.
3.5 Application examples of NCh
The nanocelluloses and nanochitins are examples of some of the most promising biobased nanoparticles. The assembly of nanocelluloses has been the subject of widespread studies, which shows great potential application in composite molding, controlled photonics, delivery systems, and catalyst preparation.45–48 However, the single type of functional group distribution on the surface of nanocelluloses does not have natural advantages in their assembly; thus, introducing charged or hydrophobic groups on the surface of nanocelluloses is necessary to realize their assembly with efficient and precise control. For instance, a stable aqueous suspension of cellulose nanocrystals (CNCs) with thixotropic gelling properties and a shear birefringence phenomenon was obtained by grafting the ammonium-containing groups onto the CNC surface;49 amphiphilic CNCs were produced to be used as a stabilizer in soybean oil/water emulsions by introducing lipophilic groups. Compared with the unmodified CNCs, an apparent effect of the lipophilic groups in the amphiphilic CNCs was observed as the emulsion droplet sizes decreased by a decade.50 Notably, the NCh itself bears a positively charged and amphiphilic nature after the acid hydrolysis treatment since surface protonated amines structurally originate from the deacetylation of acetylglucosamine, and the rest of acetyls expose the hydrophobic planes.9,51 Therefore, we envision that these NCh materials with positive charge or amphiphilic nature will open a sustainable avenue in the fabrication of photonic film or stable Pickering emulsion.4 Conclusions
A simple, environmentally friendly, and flexible shell biorefinery process for integrated utilization of all NCh products in high-value applications has been suggested. It consists of a PTSA-assisted-hydrothermal treatment followed by centrifugal fractionation of NChs from the resulting suspension, thus resulting in ChNC and ChSR products. Under the optimum conditions predicted by the response surface methodology (molar ratio of PTSA to GlcMAc units in chitin of 1.2, 130 °C, 6 h), a ChNC production yield of 39% with simultaneous 54% recovery of ChSR product to achieve minimum chitin loss was obtained. A high quantity of NChs was also produced under the modified optimal conditions directly from crustacean shells by using hot water for deproteinization, PTSA for demineralization, and deliberation of chitin microfibrils. The ChSR product was bundled nanocrystals with a long length ranging from 100 nm to 450 nm and a width mainly ranging from 10 nm to 30 nm. Due to the ChSR particle's amphiphilic nature, it had an excellent interfacial behavior between water and oil phases. In contrast, the further hydrolyzed individual nanocrystal products (ChNC) with a length and width range of 25–225 nm and 15–30 nm, respectively and a high zeta-potential of 41 mV showed a self-assembly behavior to form a highly ordered hierarchical architecture, owing to abundant –NH2 groups distributed on its surface. Accordingly, examples of high-value applications of ChNC products in fabricating large-area photonic films and ChSR products in stabilizing Pickering emulsion were last reported.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 22108273 to X. Z.) and the Youth Innovation Fund of Dalian Institute of Chemical Physics (DICP I202132 to R. L.). We gratefully acknowledge the help from Prof. Guang Chu at Southeast University for the discussion on the structural color.References
- Biomass explained-U.S. Energy Information Administration (EIA), https://www.eia.gov, retrieved 2024-05-24.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860–871 RSC.
- L. Carlsen and R. Bruggemann, Int. J. Sustain. Dev. World Ecol., 2021, 29, 219–229 CrossRef.
- X. Chen, H. Yang and N. Yan, Chem. – Eur. J., 2016, 22, 13402–13421 CrossRef CAS PubMed.
- N. Yan and X. Chen, Nature, 2015, 524, 155–157 CrossRef CAS PubMed.
- H. Sashiwa and S. Aiba, Polym. Sci., 2004, 29, 887–908 CAS.
- T. Philibert, B. Lee and N. Fabien, Appl. Biochem. Biotechnol., 2017, 181, 1314–1337 CrossRef CAS PubMed.
- L. Bai, L. Liu, M. Esquivel, B. L. Tardy, S. Huan, X. Niu, S. Liu, G. Yang, Y. Fan and O. J. Rojas, Chem. Rev., 2022, 122, 11604–11674 CrossRef CAS PubMed.
- L. Bai, T. Kamarainen, W. Xiang, J. Majoinen, J. Seitsone, R. Grande, S. Huan, L. Liu, Y. Fan and O. J. Rojas, ACS Nano, 2020, 14, 6921–6930 CrossRef CAS PubMed.
- Y. Fan, T. Saito and A. Isogai, Biomacromolecules, 2008, 9, 192–198 CrossRef CAS PubMed.
- L. Liu, L. Bai, A. Tripathi, J. Yu, Z. Wang, M. Borghei, Y. Fan and O. J. Rojas, ACS Nano, 2019, 13, 2927–2935 CrossRef CAS PubMed.
- Y. Fan, T. Saito and A. Isogai, Biomacromolecules, 2008, 9, 1919–1923 CrossRef CAS PubMed.
- K. G. Nair and A. Dufresne, Biomacromolecules, 2003, 4, 657–665 CrossRef CAS PubMed.
- A. Narkevicius, L. M. Steiner, R. M. Parker, Y. Ogawa, B. FrkaPetesic and S. Vignolini, Biomacromolecules, 2019, 20, 2830–2838 CrossRef CAS PubMed.
- L. Chen, J. Y. Zhu, C. Baez, P. Kitin and T. Elder, Green Chem., 2016, 18, 3835–3843 RSC.
- H. Yang, G. Gözaydın, R. R. Nasaruddin, J. R. G. Har, X. Chen, X. Wang and N. Yan, ACS Sustainable Chem. Eng., 2019, 7, 5532–5542 CrossRef.
- J. L. Shamshina and N. Abidi, ACS Sustainable Chem. Eng., 2022, 10, 11846–11855 CrossRef.
- J. Jiang, Y. Zhu and F. Jiang, Carbohydr. Polym., 2021, 267, 118188 CrossRef PubMed.
- W. Yang, J. Wang, L. Jiao, Y. Song, C. Li and C. Hu, Green Chem., 2022, 24, 1362–1372 RSC.
- D. D. Perrin, B. Dempsey and E. P. Serjeant, pKa prediction for organic acids and bases, 1981 Search PubMed.
- Y. Fan, H. Fukuzumi, T. Saito and A. Isogai, Int. J. Biol. Macromol., 2012, 50, 69–76 CrossRef PubMed.
- W. T. Wang, J. Zhu, X. L. Wang, Y. Huang and Y. Z. Wang, J. Macromol. Sci., Part B: Phys., 2010, 49, 528–541 CrossRef CAS.
- S. Cao, W. Gu, W. Ou-Yang, D. Chen, B. Yang, L. Lai, Y. Wu, Y. Liu, J. Zhu, W. Chen, Z. Gai, X. Hou, Y. Ma and Y. An, Carbohydr. Polym., 2019, 213, 304–310 CrossRef CAS PubMed.
- J. D. Goodrich and W. T. Winter, Biomacromolecules, 2007, 8, 252–257 CrossRef CAS PubMed.
- C. W. Shuai, Y. H. Peng, L. Y. Xing, C. P. Chen, Z. M. Xin and H. Y. Fei, Carbohydr. Polym., 2011, 83, 1804–1811 CrossRef.
- M. Yahya, Y. W. Chen, H. V. Lee, C. C. Hock and W. H. W. Hassan, J. Polym. Environ., 2019, 27, 678–702 CrossRef CAS.
- X. Y. Tan, S. B. A. Hamid and C. W. Lai, Biomass Bioenergy, 2015, 81, 584–591 CrossRef CAS.
- O. Tavakoli and H. Yoshida, Green Chem., 2006, 8, 100–106 RSC.
- T. M. Aida, M. Oshima and R. L. Smith Jr, ACS Sustainable Chem. Eng., 2017, 5, 7709–7715 CrossRef CAS.
- I. Marcet, C. Álvarez, B. Paredes and M. Díaz, Waste Manage., 2016, 49, 364–371 CrossRef CAS PubMed.
- P. R. Chang, R. Jian, J. Yu and X. Ma, Carbohydr. Polym., 2010, 80, 420–425 CrossRef.
- J. L. Shamshina, R. S. Stein and N. Abidi, Green Chem., 2021, 23, 9646–9657 RSC.
- R. M. Abdel-Rahman, R. Hrdina, A. M. Abdel-Mohsen, M. M. Fouda, A. Y. Soliman, F. K. Mohamed and T. D. Pinto, Int. J. Biol. Macromol., 2015, 80, 107–120 CrossRef PubMed.
- A. A. Oun and J. W. Rhim, Carbohydr. Polym., 2017, 175, 712–720 CrossRef PubMed.
- S. Shankar, J. P. Reddy, J. W. Rhim and H. Y. Kim, Carbohydr. Polym., 2015, 117, 468–475 CrossRef PubMed.
- F. Jiang and Y. L. Hsieh, Carbohydr. Polym., 2013, 95, 32–40 CrossRef PubMed.
- Y. Fan, H. Fukuzumi, T. Saito and A. Isogai, Int. J. Biol. Macromol., 2012, 50, 69–76 CrossRef CAS PubMed.
- J. L. Shamshina and N. Abidi, ACS Sustainable Chem. Eng., 2022, 10, 11846–11855 CrossRef CAS.
- Y. S. Nam, W. H. Park, D. Ihm and S. M. Hudson, Carbohydr. Polym., 2010, 80, 291–295 CrossRef CAS.
- N. Wang, E. Ding and R. Cheng, Polymer, 2007, 48, 3486–3493 CrossRef CAS.
- Q. Liu, N. Chen, X. Yin, L. Long, X. Hou, J. Zhao and X. Yuan, Int. J. Biol. Macromol., 2021, 181, 621–630 CrossRef CAS PubMed.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2022, 7, 21–41 CrossRef.
- J. Tian, J. Chen, P. Wang, J. Guo, W. Zhu, M. R. Khan, Y. Jin, J. Song and O. J. Rojas, Green Chem., 2023, 25, 3671–3679 RSC.
- Y. Zhu, S. Huan, L. Bai, A. Ketola, X. Shi, X. Zhang, J. A. Ketoja and O. J. Rojas, ACS Appl. Mater. Interfaces, 2020, 12, 11240 CrossRef CAS PubMed.
- K. Zhang and H. Liimatainen, Small, 2018, 14, 1801937 CrossRef PubMed.
- G. Chu, G. Vasilyev, D. Qu, S. Deng, L. Bai, O. J. Rojas and E. Zussman, Langmuir, 2020, 36, 979–985 CrossRef CAS PubMed.
- E. Kontturi, P. Laaksonen, M. B. Linder, Nonappa, A. H. Gröschel, O. J. Rojas and O. Ikkala, Adv. Mater., 2018, 30, 1703779 CrossRef PubMed.
- J. Meng, Y. Liu, X. Shi, W. Chen, X. Zhang and H. Yu, Sci. China Mater., 2020, 64, 621–630 CrossRef.
- M. Hasani, E. D. Cranston, G. Westman and D. G. Gray, Soft Matter, 2008, 4, 2238–2244 RSC.
- M. Visanko, H. Liimatainen, J. A. Sirviö, J. P. Heiskanen, J. Niinimäki and O. Hormi, Biomacromolecules, 2014, 15, 2769 CrossRef PubMed.
- J. Li, J. F. Revol and R. Marchessault, J. Appl. Polym. Sci., 1997, 65, 373–380 CrossRef.
- Z. Zhang, C. Wang, Q. Wang, Y. Zhao and L. Shang, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2204113119 CrossRef.
- W. Liu, C. Zhang, R. Alessandri, B. T. Diroll, Y. Li, H. Liang, X. Fan, K. Wang, H. Cho, Y. Liu, Y. Dai, Q. Su, N. Li, S. Li, S. Wai, Q. Li, S. Shao, L. Wang, J. Xu, X. Zhang, D. V. Talapin, J. J. Pablo and S. Wang, Nat. Mater., 2023, 22, 737–745 CrossRef PubMed.
- H. Hu, X. Zhang, W. Liu, Q. Hou and Y. Wang, Chem. Eng. J., 2023, 474, 145980 CrossRef CAS.
- S. Matsumura, S. Kajiyama, T. Nishimura and T. Kato, Small, 2015, 11, 5127–5133 CrossRef CAS PubMed.
- C. C. Thong, D. C. L. Teo and C. K. Ng, Constr. Build. Mater., 2016, 107, 172–180 CrossRef CAS.
- D. N. Chigrin and C. M. S. Torres, Opt. Spectrosc., 2001, 91, 484–489 CrossRef CAS.
- L. Bai, S. Huan, Y. Zhu, G. Chu, D. J. Clements and O. J. Rojas, Annu. Rev. Food Sci. Technol., 2021, 12, 383–406 CrossRef CAS PubMed.
- L. Bai, S. Huan, O. J. Rojas and D. J. Clements, J. Agric. Food Chem., 2021, 69, 8944–8963 CrossRef CAS PubMed.
- Y. Huang, H. Liu, S. Liu and S. Li, J. Agric. Food Chem., 2020, 68, 14620–14631 CrossRef CAS PubMed.
- Y. Zhang, Z. Chen, W. Bian, L. Feng, Z. Wu, P. Wang, X. Zeng and T. Wu, Food Chem., 2015, 183, 115–121 CrossRef CAS PubMed.
- L. Bai, S. Huan, W. Xiang, L. Liu, Y. Yang, R. W. N. Nugroho, Y. Fan and O. J. Rojas, ACS Sustainable Chem. Eng., 2019, 7, 6497–6511 CrossRef CAS PubMed.
- S. Huan, Y. Zhu, W. Xu, D. J. Clements, L. Bai and O. J. Rojas, ACS Appl. Mater. Interfaces, 2021, 13, 12581–12593 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc02680b |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |