Open Access Article
Open Access ArticleMild aqueous metal catalyzed oxidative conversion of low-density polyethylene to low molecular weight aliphatic carboxylic acids†
Oleg
Davydovich
 a,
Hemant
Choudhary
a,
Hemant
Choudhary
 b,
Daniella V.
Martinez
a,
Jay E.
Salinas
a,
Estevan J.
Martinez
c,
Ryan D.
Davis
b,
Daniella V.
Martinez
a,
Jay E.
Salinas
a,
Estevan J.
Martinez
c,
Ryan D.
Davis
 d,
Nathan R.
Bays
d,
David P.
Schafer
d and
Michael S.
Kent
d,
Nathan R.
Bays
d,
David P.
Schafer
d and
Michael S.
Kent
 *a
*a
aDepartment of Environmental System Biology, Sandia National Laboratories, Albuquerque, New Mexico, 87123, USA. E-mail: mmkent88@gmail.com
bDepartment of Bioresource and Environmental Security, Sandia National Laboratories, Livermore, California, 94551, USA
cDepartment of Organic Materials Science, Sandia National Laboratories, Albuquerque, New Mexico 87123, USA
dDepartment of Materials Reliability, Sandia National Laboratories, Albuquerque, New Mexico 87123, USA
First published on 4th October 2024
Abstract
Aqueous oxidative deconstruction of low-density polyethylene (LDPE) was investigated using homogeneous first-row transition metal catalysts under mild conditions (130–150 °C and ≤100 PSI oxygen pressure). Oxidation of LDPE resulted in high yields of low molecular weight carboxylic acids (up to 75% yield as determined by carbon balance). Aqueous processing is well-suited for biological conversion of the breakdown products.
Commodity plastics have become an integral part of modern society. Due to their low costs and broad applicability, production of plastics continues to grow, approaching 400 million tons (Mt) annually.1 A significant portion of plastics are used for short-term applications and are immediately discarded after use. Recent studies report that only 9% of discarded plastics are recycled globally; the rest end up in landfills, are incinerated, or are discarded into marine environments.2 The amount of plastics in landfills and marine environments is expected to reach 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mt and 150 Mt, respectively, by 2050.3,4 The majority of plastics in these waste streams are polyolefins (POs) due to their versatility, low cost, and appealing chemical and mechanical properties.5 While POs such as high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP) eventually break down in land-based and marine environments, hundreds of years are required to achieve complete degradation.3
000 Mt and 150 Mt, respectively, by 2050.3,4 The majority of plastics in these waste streams are polyolefins (POs) due to their versatility, low cost, and appealing chemical and mechanical properties.5 While POs such as high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP) eventually break down in land-based and marine environments, hundreds of years are required to achieve complete degradation.3
Efforts to mechanically recycle POs are often inefficient, resulting in materials with substantially reduced mechanical properties, and use processes that release volatile organic compounds.4,5 The decline in mechanical perfromance renders the recycled materials useless, leading to their disposal. To advance beyond traditional recycling methods, chemical or biological upcycling of POs has attracted considerable interest for achieving a more circular carbon economy.6–8 The primary goal is to produce high value monomeric feedstocks, waxes, lubricants, or fuels.9–14 Due to their stable C–C backbone bond structure, PO chemical conversion is challenging, typically requiring harsh conditions including high temperatures and pressures, toxic metal catalysts, and caustic chemical species. Upcycling polyolefin films is particularly of interest since film products can damage mechanical recycling equipment and thus are generally unwanted by material recovery facilities.15 Against the backdrop of inefficient mechanical recycling, the demands of harsh conditions for C–C bond cleavage, and pragmatic concerns in industrial processes, we expect that processes for upcycling waste plastics should embrace the principles of green chemistry.16
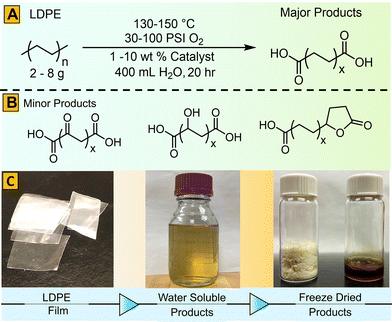
Herein, we report a new method of aqueous metal-catalyzed oxidative deconstruction of LDPE film to produce intermediates suitable for either chemical or biological conversion to value-added products. Earth-abundant and inexpensive first-row transition metal catalysts were utilized to develop a “green” chemical conversion process.17–19 In prior work, partial oxidation of PE was used to generate low molecular weight polymeric or oligomeric waxes or oils for use as emulsifiers, coatings, inks, additives for textiles, and lubricants.10,20–24 More extensive deconstruction to low molecular weight oxidized compounds has been demonstrated using dilute aqueous nitric acid with heat25 or microwave radiation,26 NOx/O2,27 permanganate,20,21,28 aerobic oxidation in acetic acid using metal/bromide29 or Co(II) and Mn(II) catalysts,30 Fenton reaction after sulfonation,31 O2/ozone,32 and thermal oxidative degradation.33,34 In the present work, we sought to convert commercial LDPE film into valuable chemicals or precursors (aliphatic carboxylic acids) using mild, aqueous oxidative conditions that do not rely on high temperatures (>150 °C), flammable organic solvents, or caustic/reactive species like nitric acid.18,19,23,24
Our initial focus was to survey the effects of various factors, including temperature, metal catalyst type, and catalyst concentration on the aqueous oxidative deconstruction of LDPE film (30 μm thick, Goodfellow). This film was specifically chosen since it is composed of high molecular weight LDPE (225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). Higher molecular weight polymers generally require more strenuous deconstruction conditions. Several catalysts were utilized, including KMnO4, CuSO4, CoSO4, and FeSO4 with loadings ranging from 2.5 to 10 weight percent relative to LDPE substrate (Scheme 1). Reactions were carried out in a 1 L T316 stainless steel reactor using a 5 mg mL−1 loading of LDPE at 130 °C and 150 °C, and an initial oxygen pressure of 100 PSI (6.9 bar) over the course of 20 h. Oxidation yielded a high fraction of water-soluble products and small amounts of insoluble materials. Reactions at temperatures at or below the melting point of LDPE (110–120 °C) resulted in greatly decreased yields of soluble products and were not studied further. To facilitate the analysis of products, insoluble material was removed by centrifugation. The remaining solution was mixed with a cation exchange resin to remove metal salts (Table S1†), filtered, and freeze-dried (Scheme 1C).
000 g mol−1). Higher molecular weight polymers generally require more strenuous deconstruction conditions. Several catalysts were utilized, including KMnO4, CuSO4, CoSO4, and FeSO4 with loadings ranging from 2.5 to 10 weight percent relative to LDPE substrate (Scheme 1). Reactions were carried out in a 1 L T316 stainless steel reactor using a 5 mg mL−1 loading of LDPE at 130 °C and 150 °C, and an initial oxygen pressure of 100 PSI (6.9 bar) over the course of 20 h. Oxidation yielded a high fraction of water-soluble products and small amounts of insoluble materials. Reactions at temperatures at or below the melting point of LDPE (110–120 °C) resulted in greatly decreased yields of soluble products and were not studied further. To facilitate the analysis of products, insoluble material was removed by centrifugation. The remaining solution was mixed with a cation exchange resin to remove metal salts (Table S1†), filtered, and freeze-dried (Scheme 1C).
The water-soluble products were first analyzed by elemental analysis to determine the efficiency of each reaction. Fig. 1 and Tables S2 and S3† show the yield for each reaction, quantified as the mole percent of carbon recovered as water-soluble products relative to that in the original LDPE film. Fig. 1 also shows the portion of the carbon in water-insoluble species that did not fully break down and, by difference, the volatile fraction lost during the reaction or upon freeze-drying. Water-insoluble compounds were not characterized by mass spectrometry due to their low abundance and heterogeneity, but size exclusion chromatography (SEC) confirmed they are partially deconstructed (discussed below). The major fraction of the oxidized products was water-soluble and accounted for 24–70 mol% of the carbon relative to the pristine LDPE film. Surprisingly, reactions using the lowest catalyst concentrations (2.5 wt% relative to substrate) at 130 °C demonstrated the highest yields. For those reactions, 51–70 mol% carbon was recovered (Fig. 1) which is comparable to previously reported oxidative deconstruction methods.26,29,30,35 In all cases, except for oxidation using KMnO4, increasing the reaction temperature to 150 °C (at 10 wt% catalyst) resulted in a significant decrease in carbon recovery. This decrease is consistent with Partenheimer's work on metal-catalyzed LDPE autooxidation, which demonstrated a similar reduction in yield when increasing reaction temperature, presumably through over-oxidation resulting in volatile products.29 We note that stainless steel reactors can catalyze LDPE oxidation, thus several control experiments including no catalyst were performed to establish the catalytic activity of the metal salts used in this study. Reactions without catalyst showed a small amount of conversion (23 ± 2.9 mol% of carbon recovered, Fig. S21†).
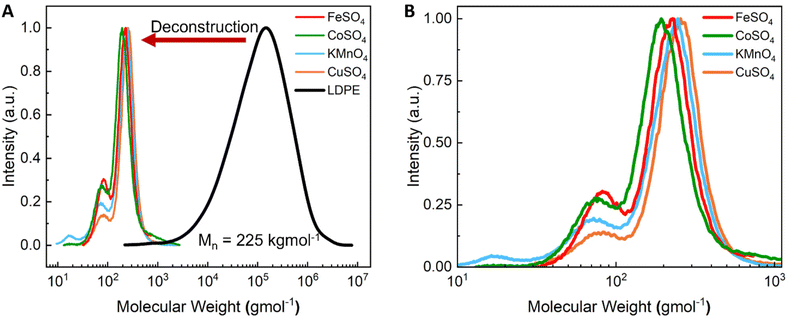
Thermally driven polyethylene autoxidation proceeds through an intricate multistep process that imparts oxygen-containing moieties onto the aliphatic backbone, which can be followed by a C–C cleavage event. Prior studies have reported that metal-catalyzed PO autooxidation (in the presence of Co and Mn catalysts) proceeds via a free radical reaction to generate alkyl radicals (postulated mechanism shown in Fig. S1†).29,36–38 Oxygen reacts with alkyl radicals to generate peroxy radical intermediates. Metal catalyzed homolytic O–O bond cleavage of the peroxide results in the formation of alkoxy radicals which can then undergo C–C bond cleavage via β-scission. Further oxidation results in the formation of carboxylic acid moieties, primarily aliphatic dicarboxylic acids (ADAs) (Scheme 1a); however species containing ketones and γ-lactones may also be formed.30,34 While the present work employs individual metal catalysts rather than combinations of Co and Mn catalysts as in the prior work, we posit that a similar autooxidation mechanism occurs. Given their solubility in water, we surmised that the products were likely comprised of significantly deconstructed fragments with hydrophilic groups such as carboxylic acids and hydroxyls. Aqueous SEC shown in Fig. 2 and Fig. S2–S5† demonstrates LDPE deconstruction into low molecular weight compounds ranging from 100–600 gmol−1. Interestingly, the molecular weight range for the deconstructed products was not strongly impacted by reaction temperature and catalyst concentration (Fig. S2–S5†). The remaining water-insoluble species were characterized by SEC (toluene mobile phase) which indicated partial LDPE deconstruction into compounds with molecular weights ranging from 500–1200 gmol−1 (Fig. S6†). However, these products were generally heterogenous and were not always completely recovered from the reactor. Fourier transform infrared spectroscopy (FTIR) was used to identify the various functional groups of the water-soluble products. FTIR spectra shown in Fig. 3 and Fig. S7–S11† reveal the presence of carboxylic acids (1704 cm−1) and carboxylates (1565 cm−1).39 The peaks shift between the protonated and deprotonated state upon adjusting the pH, as shown in Fig. 3A and Fig. S7–S10.† pH adjustment from 2 to 11 revealed the presence of small amounts of ketone and γ-lactone species (Fig. 3, 1710 cm−1 and 1772 cm−1, respectively).40
The deconstructed products were further characterized using high-performance liquid chromatography mass spectrometry (LCMS) and direct injection electrospray ionization high resolution mass spectrometry (ESI-MS). Both techniques show a complex distribution of water-soluble products. LCMS characterization confirmed ADAs as the major products via spiking with commercial standards (Fig. S12, see ESI† for details). Direct injection ESI-MS spectra (Fig. S13–S16†) showed the total distribution of products for each reaction that range from 100–400 g mol−1. FeSO4 reactions at 130 °C exhibited increased selectivity for lower molecular weight compounds at a reduced catalyst concentration, whereas CuSO4 and CoSO4 reactions showed opposite trends (Fig. S14 and S15†). On the other hand, oxidation with KMnO4 showed minimal changes in product distributions except for reactions with 10 wt% catalyst at 130 °C which demonstrated a narrower distribution of compounds ranging from 150–250 gmol−1 (Fig. S16C and D†). Reactions with Co, Cu, and Fe salts at 150 °C yielded higher amounts of lower molecular weight compounds (Fig. S13D–S15D†). Notably, oxidation with 10 wt% KMnO4 at 150 °C did not significantly alter the distribution relative to reactions at 130 °C (Fig. S16D†).
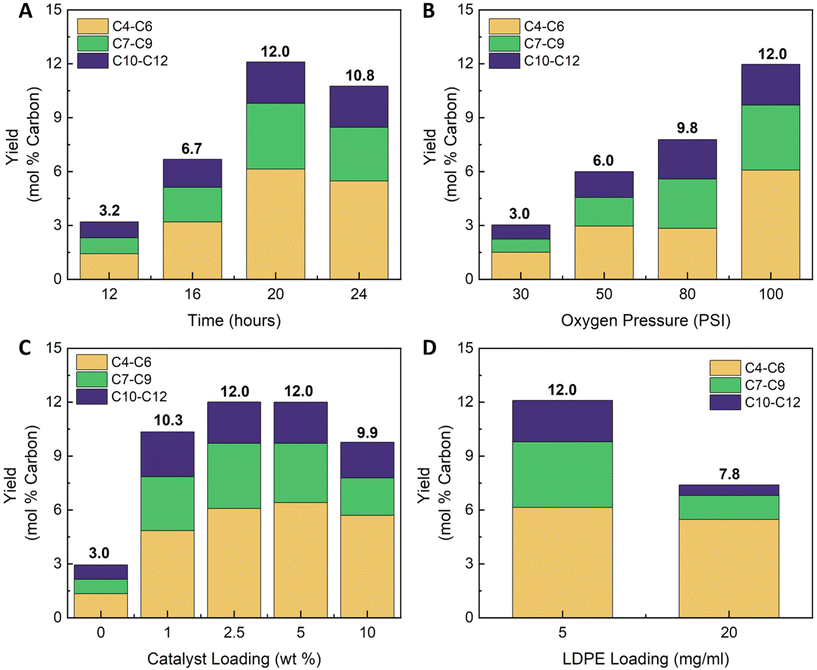
Having surveyed various catalysts for their ability to oxidatively deconstruct LDPE, we further investigated the most efficient method, utilizing CuSO4, to better understand the effects on efficiency and product distribution. Specifically, reaction time, oxygen content (noted as the initial oxygen pressure), catalyst concentration, and substrate loading were varied. Herein, water-soluble compounds were analyzed in the aqueous phase (after filtration and without freeze-drying) and carbon recovery was quantified via total dissolved organic carbon measurements (Table S4†). Fig. S17A† shows the recovered carbon yield over the course of 24 h of oxidation. A noticeable induction period, where no water-soluble products are formed, occurs during the first 6 h (<5 mol% water-soluble carbon), a common phenomenon present in other oxidation reactions in absence of an initiator.34 After this point, the yield of water-soluble products increased and appeared to plateau at 24 h (Fig. S17A,† 75 mol% carbon recovered). Multiple reactions were performed at the same conditions to determine reproducibility (2.5 wt% CuSO4, 100 psi O2, 130 °C, 20 h) yielding an average of 68 ± 3.8 mol% carbon recovered as soluble species, shown in Fig. S21.† LCMS characterization indicates that the major peaks correspond to ADAs. Fig. 4A shows ADA quantities based on their chain lengths. The major fraction of ADAs was consistently comprised of lower molecular weight compounds (C4–C6). ADA yield consistently increased until the 20 h mark, reaching a maximum of 12 mol%. Oxygen loading (initial oxygen pressure) had a marked impact on both the yield and ADA distribution (Fig. S17B† and Fig. 4B). Presumably, this outcome arises from the increased water solubility of oxygen at higher partial pressures of oxygen, thus increasing the amount of reactive oxidizing species present in the solution.41 The total carbon yield peaks when using 80 PSI (5.5 bar) of oxygen (Fig. S17B†), whereas the selectivity for ADAs increased consistently up to 100 PSI oxygen (Fig. 4B). Alternatively, the effect of varying catalyst concentration (1–10 wt%) had a nominal effect on the total carbon yield. The highest selectivity for ADAs was attained when using 2.5 and 5 wt% CuSO4 (Fig. 4C). Raising the catalyst concentration resulted in a decrease in total amount of ADA (Fig. 4C) which may be caused by ADA oxidation and conversion into other compounds (Scheme 1C). Increasing the loading of LDPE to 20 mg ml−1 resulted in a decrease in the total carbon recovered (51 mol%, Table S3†) and ADA yield (7.8 mol%, Fig. 4D). At higher substrate loadings, some of the ADAs may have precipitated which could explain the reduction in yield. Comparing all the reactions together shows that the total yield of ADAs ranged between 6–12 mol% (Fig. 4), yet the total yield of soluble carbon approached 75 mol% (Fig. S17†). The significant disparity between ADA content and overall yield may stem from oxidation reactions that impart other oxygen functional groups onto the aliphatic backbone. The prominence of carboxylic acid groups in the FTIR data (Fig. S18†) suggests that the non-ADA products are comprised of functionalized low molecular weight aliphatic carboxylic acids. Possible compounds include ADAs functionalized with hydroxyl, carbonyl groups, and aliphatic carboxylic acids bearing γ-lactones (Scheme 1B). Further, substantial carboxylic acid-metal complexes in the product mixture may partially explain the lower yield of ADAs compared to total soluble carbon since metal-complexed carboxylic acids could not be identified using LCMS.
As a proof of concept, we assessed the broad applicability of this oxidation process by performing oxidation using FeSO4 on other commercial LDPE products including a Ziploc bag, a recycled LDPE six pack ring, and an alternative packaging film (detailed in the ESI†). SEC data shown in Fig. S19† demonstrates successful deconstruction into low molecular weight fragments. The distribution of products by ESI-MS was consistent with the results described above for Goodfellow LDPE film (Fig. S20†). However, the yield of carbon in the water-soluble products was substantially reduced (<30 mol%) in the case of the six-pack holder and Ziploc bag and very little insoluble material was present (Fig. 5). Together, this suggests that the material was overoxidized, resulting in the release of low molecular weight volatile compounds (i.e., CO2). Overoxidation may arise from the presence of other components in these commercial products that may be more susceptible to the formation of volatile compounds under oxidative conditions (i.e., polypropylene in Ziploc bags). A more in-depth characterization of the various components in each material is required to fully understand the oxidation reaction for these more complex materials.
Conclusions
We developed a mild and efficient method of metal-catalyzed aqueous oxidation to convert LDPE into aliphatic carboxylic acids. Our findings indicate that this deconstruction process results in high carbon recovery (up to 75% carbon recovery) in the form of low molecular weight carboxylic acids as major products. The oxidative process developed in this work serves as an efficient “green” platform to generate valuable feedstocks for upcycling into useful polymers such as polyamides or polyesters. Utilization of aqueous conditions and a biocompatible catalyst (notably FeSO4) will also allow microbial bioconversion without requiring catalyst removal or other post processing steps. For chemical upgrading, removal of catalyst may be required. We note that the majority of catalyst can be removed with pH-based precipitation, a cation exchange resin, or by reactive distillation which is a common industrial practice for purification of carboxylic acids. Moreover, the use of low-cost and earth-abundant catalysts such as Fe and Cu is promising for scale-up, although a full technoeconomic analysis is required to determine the industrial viability of this method. Since this method cleaves C–C bonds in the backbone of LDPE, we expect it to be broadly applicable in the chemical conversion of other POs such as HDPE and PP.Author contributions
O. D. and M. S. K. developed the initial project idea. O. D. ran deconstruction experiments, and characterized deconstructed samples using size exclusion and mass spectrometry. D. M. and J. S. helped with deconstruction experiments and with size exclusion chromatography. E. J. M. performed Fourier transform infrared spectroscopy, R. D. D., D. P. S., and N. R. B. helped develop the guidelines for mass spectrometry experiments. H. C. performed large scale reactions and provided valuable discussions. All authors particiapted in writing and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
O. D., H. C., and M. S. K. are named inventors on a related patent application. There are no other conflicts to declare.Acknowledgements
The authors acknowledge the efforts of Benjamin Juba for ICP-OES measurements. This work was supported by the Laboratory Directed Research Development program at Sandia National Laboratories (Project # 225925). This article has been authored by an employee of National Technology & Engineering Solutions of Sandia, LLC under Contract No. DENA0003525 with the U.S. Department of Energy (DOE). The employee owns all right, title and interest in and to the article and is solely responsible for its contents. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this article or allow others to do so, for United States Government purposes. The DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan https://www.energy.gov/downloads/doepublic-access-plan. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the DOE or the United States Government.References
- Y. Miao, A. von Jouanne and A. Yokochi, Polymers, 2021, 449 CrossRef CAS PubMed.
- A. L. Brooks, S. Wang and J. R. Jambeck, Sci. Adv., 2018, 4, eaat0131 CrossRef PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- Q. Hou, M. Zhen, H. Qian, Y. Nie, X. Bai, T. Xia, M. L. U. Rehman, Q. Li and M. Ju, Cell Rep. Phys. Sci., 2021, 2, 100514 CrossRef CAS.
- D. Jubinville, E. Esmizadeh, S. Saikrishnan, C. Tzoganakis and T. Mekonnen, Sustainable Mater. Technol., 2020, 25, e00188 CrossRef CAS.
- A. H. Westlie, E. Y. Chen, C. M. Holland, S. S. Stahl, M. Doyle, S. R. Trenor and K. M. Knauer, Macromol. Rapid Commun., 2022, 43, e2200492 CrossRef PubMed.
- M. Chu, W. Tu, S. Yang, C. Zhang, Q. Li, Q. Zhang and J. Chen, SusMat, 2022, 2, 161–185 CrossRef CAS.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckham, Nat. Catal., 2021, 4, 539–556 CrossRef CAS.
- P. Patel and A. Tullo, ACS Discovery Report – The future of plastic, C&EN, 2020 Search PubMed.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2020, 8, 1084–1129 RSC.
- J. E. Rorrer, G. T. Beckham and Y. Román-Leshkov, JACS Au, 2021, 1, 8–12 CrossRef CAS PubMed.
- S. Liu, P. A. Kots, B. C. Vance, A. Danielson and D. G. Vlachos, Sci. Adv., 2021, 7, eabf8283 CrossRef CAS PubMed.
- X. Zhen, E. M. Nuwayo, Z. Qikun, S. Mengqi, P. Carlos, P. Venturo, N. A. Rorrer, J. Miscall, B. G. Sumpter and G. Liu, Science, 2023, 381, 666–671 CrossRef PubMed.
- Y. Miao, Y. Zhao, G. I. N. Waterhouse, R. Shi, L.-Z. Wu and T. Zhang, Nat. Commun., 2023, 14, 4242 CrossRef CAS PubMed.
- M. A. Martín-Lara, J. A. Moreno, G. Garcia-Garcia, S. Arjandas and M. Calero, J. Cleaner Prod., 2022, 365, 132625 CrossRef.
- J. García-Serna, R. Piñero-Hernanz and D. Durán-Martín, Catal. Today, 2022, 387, 237–243 CrossRef.
- J. R. Ludwig and C. S. Schindler, Chem, 2017, 2, 313–316 CAS.
- R. A. Singer, S. Monfette, D. Bernhardson, S. Tcyrulnikov, A. K. Hubbell and E. C. Hansen, Org. Process Res. Dev., 2021, 25, 1802–1815 CrossRef CAS.
- M. Bystrzanowska, P. Petkov and M. Tobiszewski, ACS Sustainable Chem. Eng., 2019, 7, 18434–18443 CrossRef CAS.
- J. Zawadiak, A. A. Marek, B. Orlińska and Z. Stec, J. Appl. Polym. Sci., 2010, 118, 1414–1420 CrossRef CAS.
- J. Zawadiak, B. Orlinska and A. A. Marek, J. Appl. Polym. Sci., 2013, 127, 976–981 CrossRef CAS.
- L. Schuster, A. Hettche, W. Liedy, S. Weiss and L. Ehemann, US Pat, 5,064,908, 1991 Search PubMed.
- N. Sharifi-Sanjani and P. Bataille, Polym.-Plast. Technol. Eng., 1999, 38, 1013–1030 CrossRef CAS.
- K. Wang, R. Jia, P. Cheng, L. Shi, X. Wang and L. Huang, Angew. Chem., Int. Ed., 2023, 62, e202301340 CrossRef CAS PubMed.
- L. R. Melby, Am. Chem. Soc., 1978, 11, 50–56 CAS.
- E. Bäckström, K. Odelius and M. Hakkarainen, Ind. Eng. Chem. Res., 2017, 56, 14814–14821 CrossRef.
- A. Pifer and A. Sen, Angew. Chem., Int. Ed., 1998, 37, 3306–3308 CrossRef CAS PubMed.
- M. W. Guzik, T. Nitkiewicz, M. Wojnarowska, M. Sołtysik, S. T. Kenny, R. P. Babu, M. Best and K. E. O'Connor, Waste Manage., 2021, 135, 60–69 CrossRef CAS PubMed.
- W. Partenheimer, Catal. Today, 2003, 81, 117–135 CrossRef CAS.
- K. P. Sullivan, A. Z. Werner, K. Ramirez, L. D. Ellis, J. R. Bussard, B. Black, D. G. Brandnew, F. Bratti, B. L. Buss, X. Dong, S. J. Haugen, M. A. Ingraham, M. O. Konev, W. E. Michener, J. Miscall, I. Pardo, S. P. Woodworth, A. M. Guss, Y. Román-Leshkov, S. S. Stahl and G. T. Beckham, Science, 2022, 379, 207–211 CrossRef PubMed.
- C. Chow, W. Wong, K. Y. Ho, C. Chan and C. Gong, Chem. – Eur. J., 2016, 22, 9513–9518 CrossRef CAS PubMed.
- I. Radecka, V. Irorere, G. Jiang, D. Hill, C. Williams, G. Adamus, M. Kwiecien, A. A. Marek, J. Zawadiak, B. Johnston and M. Kowalczuk, Materials, 2016, 9, 367 CrossRef PubMed.
- A. B. Bjerre and E. Sorensen, Ind. Eng. Chem. Res., 1994, 33, 736–739 CrossRef CAS.
- T. J. Smak, P. de Peinder, J. C. Van der Waal, R. Altink, I. Vollmer and B. M. Weckhuysen, ChemSusChem, 2024, 17, e202301198 CrossRef CAS PubMed.
- J. Y. Yao, Y. W. Wang, T. Muppaneni, R. Shrestha, J. L. Roy and G. D. Figuly, US Pat, 10,519,292B2, 2019 Search PubMed.
- F. Gugumus, Polym. Degrad. Stab., 2002, 76, 329–340 CrossRef CAS.
- F. Gugumus, Polym. Degrad. Stab., 2006, 91, 3416–3428 CrossRef CAS.
- Y. Ahn, G. Roma and X. Colin, Macromol, 2022, 55, 8676–8684 CrossRef CAS.
- T. Balakrishnan, M.-J. Lee, J. Dey and S.-M. Choi, CrystEngComm, 2019, 21, 4063–4071 RSC.
- R. Yang, Y. Liu, J. Yu and K. Wang, Polym. Degrad. Stab., 2006, 91, 1651–1657 CrossRef CAS.
- D. Tromans, Hydrometallurgy, 1998, 49, 327–342 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc02187h |
| This journal is © The Royal Society of Chemistry 2024 |