Open Access Article
Open Access ArticleAchieving high productivity of 2-pyrone-4,6-dicarboxylic acid from aqueous aromatic streams with Novosphingobium aromaticivorans†
Bumkyu
Kim
 abc,
Jose M.
Perez
abc,
Jose M.
Perez
 abc,
Steven D.
Karlen
abc,
Steven D.
Karlen
 bc,
Jason
Coplien
bc,
Timothy J.
Donohue
bc,
Jason
Coplien
bc,
Timothy J.
Donohue
 bcd and
Daniel R.
Noguera
bcd and
Daniel R.
Noguera
 *abc
*abc
aDepartment of Civil and Environmental Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA. E-mail: noguera@engr.wisc.edu
bWisconsin Energy Institute, University of Wisconsin-Madison, Madison, WI 53726, USA
cGreat Lakes Bioenergy Research Center, University of Wisconsin-Madison, WI 53726, USA
dDepartment of Bacteriology, University of Wisconsin-Madison, WI 53706, USA
First published on 20th June 2024
Abstract
Enhancing the production of biochemicals from lignocellulosic biomass is one potential way to decrease society's dependence on fossil fuels. Aromatic compounds obtained from plant biomass can be used as substrates for microbial production of dicarboxylic acids such as 2-pyrone-4,6-dicarboxylic acid (PDC) and cis,cis-muconic acid, which are building blocks for the manufacturing of polymer-based fibers and materials. In this study, we used an engineered strain of the bacterium Novosphingobium aromaticivorans to investigate how to increase PDC productivity in flow-through bioreactors receiving aqueous solutions of aromatics. At the best operational conditions tested, we achieved stable PDC production rates of 0.77 gPDC L−1 h−1 with p-hydroxybenzoic acid, 1.93 gPDC L−1 h−1 with syringic acid, and 1.53 gPDC L−1 h−1 with the products from alkaline pretreated poplar biomass. PDC titers in these reactors ranged from 7.7 to 15 g L−1 (42 to 80 mM) and were limited by aromatic solubility in the case of syringic acid, or by accumulation of protocatechuic acid from p-hydroxybenzoic acid when high aromatic loading rates were used. The use of high aromatic loading rates, hollow-fiber membranes to concentrate the microbial cells, and NH4OH for pH control were factors that contributed to this study achieving the highest PDC productivities reported to date. Overall, our findings demonstrate strategies that can be used to increase bioreactor productivity when aromatic substrates are delivered in aqueous form. These findings may also provide useful insight for production of other biochemicals from aromatic streams using N. aromaticivorans or other microbial chassis.
Introduction
The development of biotechnologies for sustainable and economic use of renewable resources is one way to reduce society's dependence on fossil fuels.3–5 The most abundant organic renewable resource on Earth is lignocellulosic biomass, making it an attractive raw material for the production of fuels, chemicals, and materials. Depending on the biomass crops, lignocellulose is composed of 10–25% lignin, 20–30% hemicellulose, and 40–50% cellulose.6,7 To date, the leading lignocellulose application is the conversion of the carbohydrates in cellulose and hemicellulose to biofuels.9 However, lignin is the largest renewable reservoir of aromatic compounds in nature, and thus, there is a growing interest in developing technologies for its use as a source of high-value products, which could both enhance the economic viability of lignocellulosic biorefineries and help ameliorate society's dependence on petrochemicals.10,11 We are interested in developing sustainable and cost-competitive green biotechnologies for conversion of lignin into valuable chemicals.Different chemical approaches to generate aromatic monomers and low-molecular-weight oligomers from lignocellulosic biomass have been proposed.9,12,13 The heterogeneous chemical composition of lignin14,15 results in mixtures of aromatic products whose composition depends on the type of biomass and depolymerization methods used.17 Depending on the methods and post-processing steps, the resulting aromatics streams will be in aqueous solution,18,19 dissolved in an organic solvent,21 or concentrated in lignin oils.22 Microbial funneling of these aromatic mixtures into a single product is a strategy that could potentially be agnostic to the type of biomass or the depolymerization method used.8,21,23
Although some strains have been engineered to produce specific chemicals from aromatics,2 there is still a knowledge gap on culture conditions required to produce the high yields, titers, and production rates needed for these bioprocesses to be sustainable and gain industrial acceptance.19,24,25 To obtain high yields, the microbial catalysts need to be capable of metabolizing as many of the aromatics found in depolymerized lignin as possible. In recent years, this has been a productive research area, with rapid discovery of microbial pathways for metabolism of plant-derived aromatic monomers26–30 and dimers.31–35 Obtaining high product titer requires the use of feed streams containing high concentration of aromatics and microbes that efficiently metabolize these substrates, that are tolerant to product accumulation, and that can tolerate the presence of potentially inhibitory substances. However, aromatic concentrations in these feed streams are often limited by the aqueous solubility of aromatics at circumneutral pH. Demonstrations of high product titers are typically performed in fed-batch bioreactors,1,2,8,19,20 whereas demonstrations of high production rates are less common.20
We are studying Novosphingobium aromaticivorans as a potential microbial chassis for cost-effective microbial funneling of biomass aromatics.8,21,30,36N. aromaticivorans is known for its natural ability to degrade a diverse range of aromatic compounds, including most phenolics derived from plant biomass,8,21,26 which is a critical characteristic needed to achieve high product yields from biomass.37 Recent work has elucidated metabolic pathways and enzymes involved in the degradation of multiple aromatic compounds by N. aromaticivorans,26,30 enabling the engineering of strains that funnel diverse aromatic compounds into extracellular products such as 2-pyrone-4,6-dicarboxylic acid (PDC)8,30 and cis,cis-muconic acid (ccMA).38
In this work, we test the hypothesis that high productivity with N. aromaticivorans could be achieved by creating high-density cultures with flow-through membrane bioreactors (MBR).39 Using PDC as a product, we show that high productivity was achieved from p-hydroxybenzoic acid (pHBA) and syringic acid in experiments using synthetic media, and from aromatics derived from the alkaline pretreatment of poplar biomass. We found that product titers were limited by aromatic solubility when syringic acid was used, and by the accumulation of protocatechuate when pHBA or alkaline pretreated poplar were used. These results revealed that there is a trade-off between maximizing high productivity or maximizing product titer in the MBR system. We also systematically evaluated factors affecting microbial growth during bioreactor operation and show that the base used for pH control can impact the productivity of the microbial culture by reducing accumulation of inhibitory salts.
Results
Continuous PDC production in flow-through MBR system
We evaluated the use of a flow-through MBR system (Fig. S1†) for continuous production of PDC by an engineered N. aromaticivorans strain (strain 12444ΔligIΔdesCD from Perez et al.8). Hollow fiber membranes were used to separate microbial cells from the reactor effluent and return them to the bioreactor, as an operational strategy to achieve high-density cultures. For initial experiments with synthetic medium, pHBA was chosen as the aromatic substrate that can be transformed to PDC, and glucose was used as an auxiliary non-aromatic substrate that provides the carbon and energy needed for cell growth when most carbon in the aromatic substrate is used to make PDC.8 During flow-through operation, PDC production results in acidification of the medium, so neutral pH was maintained by the automated addition of sodium hydroxide (NaOH). Air was continually supplied to provide oxygen needed for growth and for aromatic metabolism.Each MBR experiment was started in batch mode using standard mineral base (SMB) medium amended with 40 mM (7.2 g L−1) glucose as the sole carbon source. This batch mode was maintained for about 20 h, until all the initial glucose was consumed. Next, the system was switched to flow-through mode and fed with SMB medium containing 1 mM (0.138 g L−1) pHBA and 40 mM glucose for approximately one hour to expose the cells to the aromatic substrate, inducing the expression of genes related to aromatic metabolism. Then, the flow-through mode was continued, and the media switched to one containing approximately equimolar concentrations of aromatic substrate and glucose. The MBR experiments were typically conducted for about 5 days (120 hours).
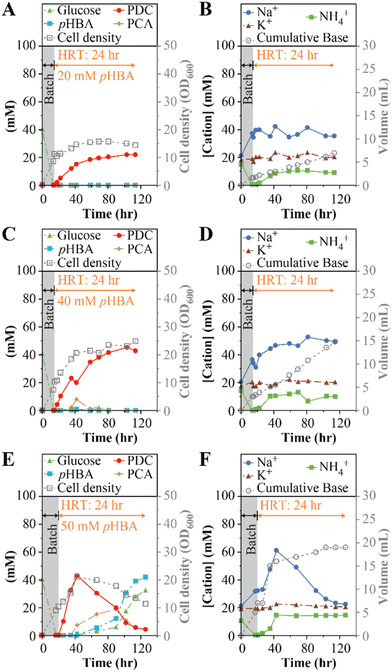
In the first experiment (Table 1), we continuously fed the bioreactor with SMB medium containing 20 mM (2.8 g L−1) pHBA and 20 mM (3.6 g L−1) glucose at a flow rate of 8.33 mL h−1. With a 200 mL working volume, this flow rate corresponds to a hydraulic retention time (HRT) of 24 h. We monitored accumulation of cells as well as the extracellular concentrations of substrates, intermediates of aromatic metabolism, and the product PDC (Fig. 1A). We also monitored the concentrations of Na+, K+, and NH4+ (Fig. 1B), cations that may accumulate depending on the base selected for pH control. Under these operational conditions, the system reached a steady PDC concentration of 21 ± 1 mM (3.8 ± 0.2 g L−1), without observable accumulation of substrates (pHBA or glucose) or detectable intermediates of aromatic metabolism in the medium, indicating 100% molar yield of PDC from pHBA. The microbial culture reached an optical density (600 nm, OD600) of 15 ± 1 (Fig. 1A), equivalent to a dry cell weight (dcw) concentration of 9.5 gdcw L−1. At steady state, the bioreactor reached a PDC production rate of 0.16 gPDC L−1 h−1. The concentrations of the cations Na+, K+, and NH4+ in the feed were 20, 20, and 14 mM respectively, and in the effluent, K+ remained at 20 ± 2 mM (Fig. 1B), whereas Na+ averaged 36 ± 5 mM. The NH4+ present in SMB was consumed during the batch period, but then stabilized at 10 ± 1 mM in the effluent (Fig. 1B). The difference between the influent and effluent NH4+ concentrations reflect NH4+ utilization to fulfill nitrogen requirements for cell growth during flow-through MBR operation.
| No. | Aromatica | Aromatic concentration, mM (g L−1) | Glucose concentration, mM (g L−1) | HRT (h) | Base | PDC titer mM (g L−1) | PDC production rate (g L−1 h−1) |
|---|---|---|---|---|---|---|---|
| a Abbreviations: pHBA, p-hydroxybenzoic acid; SA, syringic acid; APL, alkaline pretreatment liquor; eAPL, APL concentrated by extraction. b ns: a stable condition was not reached. c Experiments were in a single run with progressive HRT reductions. d The main aromatic (99.5%) in the alkaline pretreated liquor (APL) was pHBA (16 mM). e Two batches of eAPL were used; eAPL1 was used with 24-hour HRT and eAPL2 was used with 16-hour, 6-hour, and 4-hour HRTs. | |||||||
| 1 | pHBA | 20 (2.8) | 20 (3.6) | 24 | NaOH | 21 (3.8) | 0.16 |
| 2 | pHBA | 40 (5.5) | 40 (7.2) | 24 | NaOH | 41 (7.5) | 0.31 |
| 3 | pHBA | 50 (6.9) | 50 (9.0) | 24 | NaOH | nsb | ns |
| 4 | pHBA | 80 (11.0) | 80 (14.4) | 24 | NH4OH | 80 (15.0) | 0.63 |
| 5 | pHBA | 100 (13.8) | 100 (18.0) | 24 | NH4OH | ns | ns |
| 6 | pHBA | 50 (6.9) | 50 (9.0) | 12 | NH4OH | 50 (9.2) | 0.77 |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
SA | 43 (8.5) | 50 (9.0) | 24 | NH4OH | 42 (7.7) | 0.32 |
7b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
SA | 43 (8.5) | 50 (9.0) | 12 | NH4OH | 42 (7.7) | 0.64 |
7c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
SA | 43 (8.7) | 50 (9.0) | 4 | NH4OH | 42 (7.7) | 1.93 |
| 8 | APLd | 16 (2.2) | 20 (3.6) | 24 | NH4OH | ns | ns |
9a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
eAPL1e | 17 (2.3) | 50 (9.0) | 24 | NH4OH | 17 (3.1) | 0.13 |
9b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
eAPL2e | 50 (6.9) | 50 (9.0) | 12 | NH4OH | 50 (9.2) | 0.77 |
9c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
eAPL2e | 50 (6.9) | 50 (9.0) | 6 | NH4OH | 50 (9.2) | 1.53 |
9d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
eAPL2e | 50 (6.9) | 50 (9.0) | 4 | NH4OH | ns | ns |
Effect of increasing aromatic concentrations on MBR system performance
Having achieved stable bioreactor operation at 20 mM pHBA, we tested the MBR system with higher concentrations of organic substrates (experiments 2 and 3, Table 1). In separate experiments, pHBA and glucose concentrations were set to 40 mM (5.5 g L−1 and 7.2 g L−1, respectively) and 50 mM (6.9 g L−1 and 9.0 g L−1, respectively).At the 40 mM substrate concentrations, the productivity of the MBR system increased, with PDC reaching 41 ± 2 mM (7.5 ± 0.4 g L−1) near the end of the run (Fig. 1C). With no residual pHBA, this bioreactor achieved 100% molar yield of PDC from pHBA. However, a transient accumulation of as much as 7.8 mM (1.2 g L−1) of the pathway intermediate protocatechuic acid (PCA) was observed 38 h into the experiment (Fig. 1C). With higher glucose than in the first experiment (Fig. 1), the culture reached higher cell densities, with optical density of 22 ± 2 (Fig. 1C), or 17 ± 1 gdcw L−1. At the end of this experiment, the bioreactor achieved a production rate of 0.31 gPDC L−1 h−1. The effluent cation concentrations followed a similar trend as in the prior experiment (Fig. 1D), but the Na+ concentrations were 35% higher, consistent with the need for more NaOH to control pH at higher PDC titer (Fig. 1D).
The MBR system failed to reach stable PDC production when fed 50 mM pHBA and 50 mM glucose (Fig. 1E). In this case, the rate of PDC accumulation was faster, but we observed higher accumulation of PCA, reaching its highest concentration of 18 mM (2.8 g L−1) 91 h into the experiment. Concomitant with the increase in PCA, incomplete consumption of both glucose and pHBA occurred (Fig. 1E). At the end of the experiment, pHBA and glucose concentrations were increasing, suggesting significant inhibition of microbial metabolism in the bioreactor. Consistent with the inhibition of microbial metabolism, we found that the rate of base addition for pH control decreased (Fig. 1F), and that the effluent NH4+ concentration equaled the influent concentration.
Identifying factors impacting MBR system performance
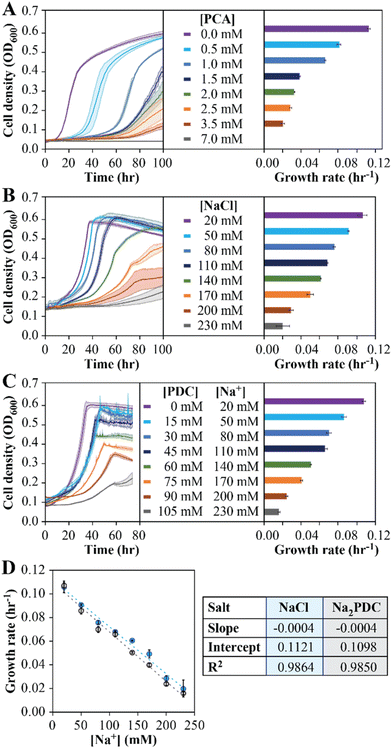
To investigate the reason for the inability to reach a stable PDC production rate when the MBR system was fed 50 mM pHBA and 50 mM glucose, we used batch culture experiments. First, to evaluate the potential inhibitory effect of PCA accumulation, batch growth tests with increasing PCA concentrations were performed (Fig. 2A). The analysis showed a decrease in growth rates as the PCA concentration in the medium was increased, with 7 mM (1.1 g L−1) added PCA completely inhibiting growth in the batch experiments (Fig. 2A). We conclude from these experiments that PCA accumulation has a negative impact on N. aromaticivorans growth, but the inhibitory concentrations in the batch experiments do not correlate well with inhibitory concentrations in the MBR experiments.Second, to evaluate whether Na+ accumulation affected microbial growth, batch experiments with increasing NaCl addition were performed (Fig. 2B). In these experiments, the growth rate decreased with increasing Na+ concentration, showing an 81% decrease in growth rate at the highest Na+ condition tested (230 mM), but less than a 15% decrease at the range of concentrations of this cation that were observed in the MBR effluents (40 to 60 mM).
Third, the impact of product accumulation was also evaluated in batch tests with media containing glucose and increasing concentrations of PDC (Fig. 2C). Since addition of PDC to the medium also requires the addition of a base to keep neutral pH, we used NaOH for this purpose, which resulted in experiments in which medium Na+ concentrations increased as more PDC was added. For example, when we tested initial PDC concentrations ranging from 0 to 105 mM, this resulted in Na+ concentrations ranging from 20 to 230 mM (Fig. 2C). We observed an 85% decrease in the growth rate when the initial PDC concentration was 100 mM, but only a 39% decrease at 45 mM PDC (Fig. 2C), which approximates the maximum PDC concentrations seen during successful operation of the flow-through MBR system (Fig. 1C). To deconvolute the impact of PDC from Na+ on microbial activity, we compared the growth rates of cultures supplemented with NaCl (Fig. 2B) and those supplemented with PDC (Fig. 2C) at the same Na+ concentrations. Using a linear model to represent the effect of Na+ in growth rate (Fig. 2D), we observed slight differences in the linear regression coefficients, which were not statistically different at the 95% confidence level (p > 0.05). We conclude from this analysis that PDC, at the concentrations tested, has a negligible impact on microbial growth rates.
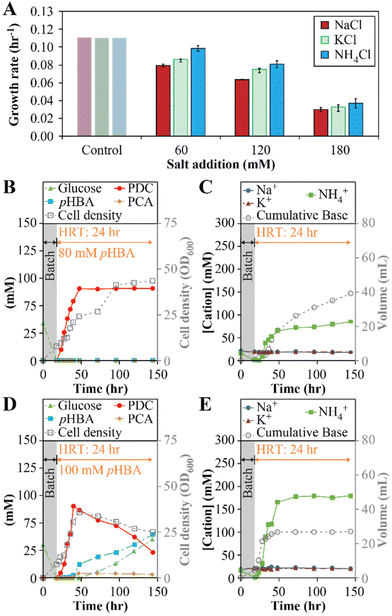
The above results predict that, in addition to PCA accumulation, some decrease in growth rate can be attributed to the increase in Na+ concentrations due to the use of NaOH for pH control. To test this hypothesis, we investigated whether replacing the base used for pH control could influence PDC production by changing the type of cation that accumulates. For this, we conducted growth batch tests with NaCl, KCl, and NH4Cl at concentrations of 60, 120, and 180 mM, to reflect concentrations of Na+, K+, or NH4+ that could be potentially observed in flow-through MBR experiments (Fig. 3A). At the three concentrations tested, small but significant differences in growth rates were observed in cultures exposed to the three cations (one-way ANOVA, P < 10−3), with the higher growth rates observed in the presence of NH4Cl and the lowest growth rates observed when NaCl was added (Fig. 3E). The results lead us to propose that using NH4OH instead of NaOH for pH control might improve performance of the flow-through MBR system.
To test this hypothesis, we analyzed flow-through MBR performance using NH4OH as the base employed for pH control (experiments 4 and 5, Table 1). In one experiment, we used a feed containing 80 mM (11.0 g L−1) pHBA and 80 mM (14.4 g L−1) glucose (Fig. 3B and C), concentrations higher than those that allowed stable operation with NaOH as the base (Fig. 1). At this condition, the MBR system reached stable operation with 100% molar yield of PDC from pHBA and higher cell density (OD600 = 42 ± 1, Fig. 3B) than in experiments at lower substrate concentrations (OD600 = 22 ± 2, Fig. 1C). In addition, there was no observable accumulation of PCA, glucose, or pHBA in the bioreactor effluent. By the third day of operation, the MBR was producing effluent containing 80 ± 0.1 mM PDC (15 ± 0.02 gPDC L−1) at a production rate of 0.63 gPDC L−1 h−1. We also tested MBR performance at 100 mM (13.8 g L−1) pHBA and 100 mM (18.0 g L−1) glucose. In this case, we found that PCA accumulated and observed incomplete utilization of pHBA and glucose, indicating a new limit for the MBR performance at this condition (Fig. 3D and E).
Increasing PDC productivity by using a shorter HRT
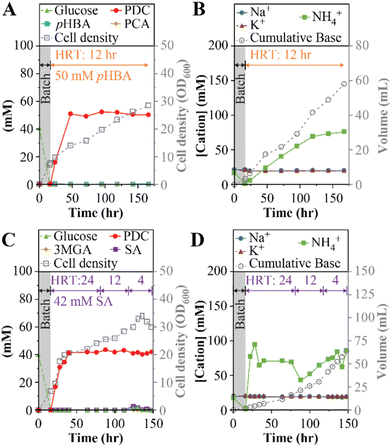
The previous MBR experiments using a 24-hour HRT identified limitations to the strategy of improving PDC productivity by increasing the concentration of aromatics in the influent or changing the base used for pH control. Thus, we tested whether PDC production rates could be improved by reducing the HRT to 12-hour, using NH4Cl for pH control, and 50 mM each of pHBA and glucose in the feed (experiment 6, Table 1). Under these conditions, steady PDC production was achieved (Fig. 4A and B). With a titer of 50 ± 1 mM PDC (9.2 ± 0.2 gPDC L−1), the production rate was 0.77 gPDC L−1 h−1, which is a 24% increase compared to the best result observed using a 24-hour HRT (experiment 4, Table 1). This experiment confirmed that productivity can be improved by increasing the aromatic loading rate into the reactor while maintaining stable PDC titers.PDC productivity with syringic acid
We performed experiments with syringic acid to evaluate whether the MBR conditions that increased PDC productivity with pHBA also allowed high productivity of PDC from a syringyl compound (Fig. 4C and D).In this test (experiments 7a to 7c, Table 1), the influent contained 50 mM (9.0 g L−1) glucose and 43 mM (8.5 g L−1) syringic acid, the maximum concentration of this aromatic that was soluble at pH 7, and NH4OH for pH control. The reactor was initially operated with a 24-hour HRT (Fig. 4C and D), similar to the initial runs with pHBA. To investigate whether PDC productivity could be increased by increasing the aromatic loading rates, the HRT was subsequently decreased to 12 h and 4 h during the same reactor run. Under these conditions, steady PDC production was achieved and maintained at 42 ± 1 mM PDC (7.7 ± 0.2 gPDC L−1) for all HRT conditions (Fig. 4C). The corresponding production rates were 0.32 gPDC L−1 h−1, 0.64 gPDC L−1 h−1, and 1.93 gPDC L−1 h−1, at the 24-hour, 12-hour, and 4-hour HRTs, respectively (Table 1). Thus, this experiment confirmed that it was possible to achieve high PDC productivity with syringic acid by increasing the aromatic loading rates even when limited by the aqueous solubility of syringic acid.
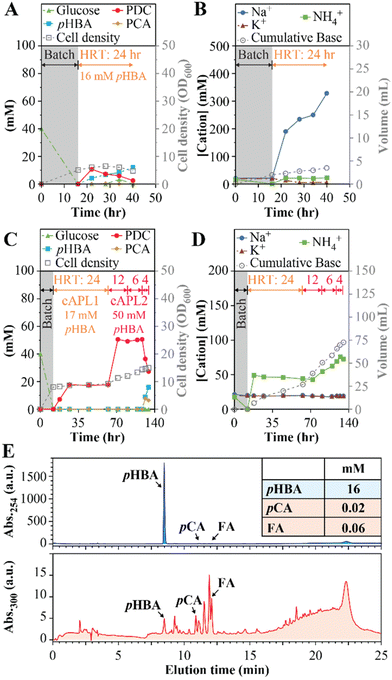
PDC productivity with aromatics derived from poplar biomass
We also investigated whether similar conditions could be used to produce PDC from aromatics derived from poplar via alkaline pretreatment. Alkaline pretreatment liquors (APL) result from incubating biomass at high pH and high temperature (e.g., 50–130 °C). This process releases monoaromatic compounds that are attached to the cell wall via ester linkages,19,36 and can also extract other aromatic compounds found in plant biomass.36 Given the negative impact of Na+ accumulation on MBR performance, we used APL-producing conditions with less NaOH than commonly used during alkaline pretreatment (i.e., 0.5 M NaOH instead of 1.0 M NaOH or higher19) and tested the resulting APL in a MBR operated with 24-hour HRT and using NH4OH for pH control (experiment 8, Table 1). Analysis of this APL showed it contained 16.0 ± 0.2 mM pHBA and minor concentrations of other aromatics (0.02 mM p-coumaric acid and 0.06 mM ferulic acid).For these experiments (Fig. 5A and B), the APL was amended with 20 mM glucose to reach close to equimolar amounts of aromatics and glucose, consistent with other experiments in this study. A nitrogen source was not added because NH4OH was used for pH control. As with other MBR experiments, this experiment started in batch mode using SMB medium containing 40 mM glucose (Fig. 5A) and then switched to flow-through operation after the glucose was consumed. In flow-through mode, the PDC concentration increased to 11 mM (2.1 g L−1), which represents ∼69% conversion of the pHBA in the APL to PDC, but steady PDC production could not be maintained and pHBA concentrations increased (Fig. 5B). The observed rapid increase in Na+ concentration (Fig. 5B) potentially caused the inhibition of PDC production, although the presence of other inhibitory compounds in the APL cannot be ruled out.
To lower the Na+ concentration, we tested the effect of concentrating the aromatics in APL by liquid–liquid extraction and reconstituting them in controlled media (see Materials and methods). Two different solutions of extracted APL were produced (eAPL1 and eAPL2) and used in another MBR experiment (experiments 9a to 9d in Table 1 and Fig. 5C, D). The pHBA concentrations in eAPL1 and eAPL2 were 17.0 ± 0.2 mM and 49.7 ± 0.3 mM, respectively. For the MBR experiments, these eAPLs were adjusted to neutral pH with NH4OH and amended with glucose (50 mM) and phosphate buffer. After the initial batch period with SMB medium, flow-through mode was initiated with eAPL1 using 24-hour HRT and NH4OH for pH control (experiment 9a, Table 1). With this eAPL1, steady PDC production was achieved (Fig. 5C), resulting in a PDC titer of 17.3 ± 0.3 mM PDC (3.2 ± 0.1 gPDC L−1), a production rate of 0.13 gPDC L−1 h−1, and with Na+ and K+ at ∼20 mM and NH4+ stabilizing at ∼50 mM (Fig. 5D). Next, the feed was switched to eAPL2, which had a higher concentration of pHBA (49.7 ± 0.3 mM). With eAPL2 we tested the MBR performance using 12-hour, 6-hour, and 4-hour HRTs (Fig. 5C and D). At 12-hour HRT, we observed the MBR reaching a steady PDC titer of 50 ± 1 mM (9.2 ± 0.2 gPDC L−1), a condition that was maintained after the HRT was decreased to 6-hour, resulting in PDC production rates of 0.77 gPDC L−1 h−1 and 1.53 gPDC L−1 h−1, at 12-hour and 6-hour HRT, respectively. Upon a shift to 4-hour HRT, stable PDC production was no longer achievable and PCA and pHBA accumulated in the bioreactor effluent, showing a limit on stable productivity with concentrated APL.
Discussion
Microbial funneling is an emergent strategy for biomass aromatic valorization that relies on the microbial transformation of phenolic mixtures into one or a limited number of products.23 For this strategy to be industrially attractive and environmentally sustainable, it is necessary to develop microbial culturing techniques capable of delivering high production rates, titers, and yields within a lignocellulosic biorefinery.10The major monoaromatic phenolics derived from plant biomass can be classified according to the number of methoxy groups in the aromatic ring as syringyl (S-type; two methoxy groups), guaiacyl (G-type; one methoxy group) and p-hydroxyphenyl aromatics (H-type; no methoxy groups).40,41 Microbes such as N. aromaticivorans, can naturally metabolize all three types of aromatics,8 whereas others cannot naturally metabolize syringyl aromatics,2 although advances are being made to engineer Pseudomonas and other bacterial strains with this capability.42 Obtaining high product yields from lignocellulosic biomass will ultimately depend on the ability of the engineered microbial strains to metabolize all major types of aromatics found in lignin.
In this study, we demonstrated the use of a MBR to generate high-density cultures of the engineered N. aromaticivorans 12444ΔligIΔdesCD strain,8 which reached production rates as high as 1.93 gPDC L−1 h−1 with syringic acid. This is ∼14% greater than the highest PDC productivity from aromatics reported to date,20 albeit with a titer lower than other reports.2,20 This is also the first report of a S-type aromatic converted to PDC with a high production rate. We selected PDC as the product of microbial funneling because of its promising use as a building block for the manufacturing of products from renewable materials,1,16 and because it is a product for which some productivity metrics have been reported with N. aromaticivorans and other microbial chassis (Table 2). We also report high PDC productivity using a poplar APL to illustrate the ability of a N. aromaticivorans MBR to generate this compound from aromatics derived from lignocellulosic biomass. Below we discuss the findings from this study in the context of other microbial funneling strategies investigated to achieve high PDC productivity.
| Microbial chassis | Reactor typea | Aromatic substrateb (g L−1) | Non-aromatic substratec (g L−1) | Production rate (g L−1 h−1) | Titer (g L−1) | Yieldd (mol per mol) | OD600 | Ref. |
|---|---|---|---|---|---|---|---|---|
| nr: not reported.a Reactor type: FB: fed-batch, MBR: continuous flow membrane bioreactor.b Aromatic substrates: PCA: protocatechuic acid, VA: vanillic acid, VN: vanillin, pCA: p-coumaric acid, pHBA: p-hydroxybenzoic acid, SA: syringic acid, eAPL2: aromatics extracted from poplar biomass by alkaline pretreatment containing mostly pHBA.c Non-aromatic substrates: G: glucose, Gly: glycerol.d Yield expressed in mol PDC per mol aromatic substrate. Assuming 1 mol aromatic substrate yields 1 mol PDC, the theoretical yields from the different aromatic substrates are: 1.22 gPDC gPCA−1, 1.12 gPDC gVA−1, 1.15 gPCA gpCA−1, 0.97 gPDC gFA−1, 1.36 gPDC gpHBA−1, 0.95 gPDC gSA−1. | ||||||||
| P. putida PpY1100 | FB | PCA (100) | G (18) | 0.31 | 11 | 0.99 | 14 | Otsuka et al. 2006![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| P. putida KT2440 | FB | pHBA (120) | G (100) | 0.20 | 58 | 0.81 | 12 | Johnson et al. 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| N. aromaticivorans DSM12444 | FB | VA (38), VN (5) | G (99) | 0.12 | 4.9 | 0.56 | nr | Perez et al. 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| P. putida KT2440 | FB | pCA (98.4) | Gly (10) | 0.21 | 22.7 | 1.00 | 6 | Lee et al. 2022![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| G (2.5) | ||||||||
| P. putida PpY1100 | FB | VA (220) | G (230) | 1.69 | 99.9 | 0.99 | 70 | Otsuka et al. 2023![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| N. aromaticivorans DSM12444 | MBR | pHBA (6.9) | G (9) | 0.77 | 9.2 | 1.00 | 30 | This study |
| N. aromaticivorans DSM12444 | MBR | SA (8.3) | G (9) | 1.93 | 7.7 | 1.00 | 34 | This study |
| N. aromaticivorans DSM12444 | MBR | eAPL2 (6.9) | G (9) | 1.53 | 9.2 | 1.00 | 15 | This study |
PDC productivity using different microbial chassis
In the first reported microbial strain capable of extracellular accumulation of PDC, P. putida PpY1100, a microorganism described as unable to naturally degrade aromatic compounds,1 was engineered by heterologous expression of a protocatechuate 4,5-dioxygenase (LigAB) and a dehydrogenase (LigC) from Sphingobium SYK-6, to produce PDC from PCA by extradiol cleavage of the aromatic ring.1 Because PCA is an intermediate in the degradation pathway of G- and H-type aromatics, engineered strains of P. putida PpY1100 have been reported to produce high yields of PDC from these aromatics but it is unable to transform S-type aromatics.43 In other studies, P. putida KT2440, a strain reported to naturally degrade PCA and catechol via intradiol cleavage of the aromatic ring has been engineered to produce a variety of aromatic products via pathways that have PCA or catechol as intermediates.2 Similar to P. putida PpY1100, when ligAB and ligC are heterologously expressed in this organism, it enables the production of PDC from G- and H-type aromatics,16 but not S-aromatics.In contrast, N. aromaticivorans DSM12444 naturally grows on S-, G-, and H-type aromatics by extradiol cleavage of the aromatic ring.26 The engineering of strains to accumulate PDC from phenolics was accomplished by interrupting the metabolic pathways downstream of PDC without the need for expression of heterologous enzymes (Fig. S2†).8,30 Consequently, the engineered PDC-producing strains have high PDC yields from all three major types of aromatics found in lignocellulosic biomass.8,30
Reports of microbial production of PDC at high titers or with high production rates have used either G- or H-type aromatic substrates (Table 2). The initial experiments with P. putida PpY110035 yielded 11 g L−1 PDC with a production rate of 0.31 gPDC L−1 h−1 and a yield of 0.99 molPDC per molPCA, fed-batch experiments with a concentrated PCA solution (100 g L−1).1 The highest PDC titers reported thus far have been 58 g L−1 with P. putida KT2440,2 99.9 g L−1 with P. putida PpY1100,20 and 4.9 g L−1 with N. aromaticivorans DSM12444.8
While fed-batch operation can deliver high product titers, they are limited by the concentration of the aromatic in the feeding stream, which is slowly added to the reactor to prevent substrates from accumulating to inhibitory levels.19 Because of the feeding strategy, this mode of operation has modest production rates (Table 2), unless high-density cultures are used.20 Moreover, the addition of the aromatic-containing feed to a batch culture also increases the volume of the batch, thus diluting the product and imposing a titer limit that is well below the concentration of the aromatic in the feed stream (Table 2). While aqueous solutions with 100 g L−1 aromatics or greater, at circumneutral pH, can be prepared with aromatics such as pHBA2 or PCA,1 this is not possible with less soluble aromatics such as syringic acid, for which we observed an aqueous solubility of 8.3 g L−1 at circumneutral pH. Otsuka et al.20 reported using vanillic acid solutions of up to 220 g L−1 in an ammonia-based solution and being able to obtain a titer of ∼100 g L−1, the highest PDC titer reported to date. They also reported that attempting to increase the titer by feeding the culture with vanillic acid in powdered form immediately suppressed microbial activity.20
Given the limited solubility of many biomass-derived aromatics in aqueous solutions, we investigated whether it would be possible to operate flow-through bioreactors that achieve high PDC production rates even though the titers are limited by aromatic solubility. We reasoned that high productivity could be achieved by culturing N. aromaticivorans in conditions that led to high density, and for this purpose, we used hollow fiber membranes to filter the reactor effluent and keep the active cells in the reactor while producing a PDC-containing clear effluent. In these MBR systems, the maximum product titer is limited to the molar concentration of the aromatic substrate in the influent stream. As predicted, we achieved 100% conversion of the aromatic to PDC with pHBA or syringic acid as substrates (Table 1). Although our PDC titers were lower compared to some reported in fed-batch reactors with pHBA or p-coumaric acid, the production rates were between 3 and 10 times higher than previously reported (Table 2).
In addition, only the fed-batch experiment of Otsuka et al.20 with P. putida achieved comparative production rates to the rates we obtained (Table 2). We propose that three factors contributed to the high production rate observed in this previous study. First, they used a vanillic acid feed stream that is over two times more concentrated than used in other studies. This concentration of aromatics would be difficult to achieve with many plant-derived aromatic streams unless a solvent is used to increase the solubility of the aromatics fed into the reactor, and in that case, it would be necessary to ensure the compatibility of the solvent with the microbial chassis used. Second, they created a high-density culture (OD600 ∼ 70 to 80)20 before adding the aromatic that was at least two times greater than the densities we achieved with the MBR system (OD600 ∼ 15 to 40) and about 5-fold higher than densities in other published studies using fed-batch cultures (Table 2). Achieving these high densities in a fed-batch reactor, without the help of membranes to retain the microbial cells during flow-through operation, as we did in our study, also required the use of higher concentrations of the non-aromatic substrate to support microbial metabolism (230 g L−1 glucose, compared to the 3.6 to 18 g L−1 glucose we used in the MBR system). Third, the authors changed the pH-control base from NaOH to NH4OH, which is the same change we implemented to increase productivity in our flow-through MBR experiments. While we made this change based on NH4+ having less toxicity to N. aromaticivorans than comparable levels of Na+ (Fig. 3A), their motivation was to prevent formation of insoluble Na-PDC salts, which reduced soluble PDC titers at their high titer goal of 100 g L−1. In any case, PDC productivity in the Otsuka et al.20 and in this study was improved by preventing accumulation of Na+ in the medium.
PDC production from plant-derived aromatic streams
Although PDC production from biomass aromatic streams has been demonstrated,8,21,36 there are no reports of high PDC productivity with such solutions. Depending on the methods and post-processing steps used, biomass-derived aromatic-rich streams could be in aqueous solution,19,36 dissolved in an organic solvent,21 or concentrated as oils.22 Thus, it is reasonable to predict that performance of a PDC producing bioreactor will depend on the mode and effectiveness of delivery of the biomass-derived aromatics to the bioreactor. Alkaline pretreatment of biomass has been used to generate aqueous streams containing aromatics36 but they often have aromatic concentrations below what is needed to obtain high productivity and titers in fed-batch reactors. Furthermore, alkaline conditions in these pretreatments are typically created using molar concentrations of NaOH, and the results of this and previous studies20 indicate high Na+ concentrations would not be compatible with high productivity of PDC. Catalytic fractionation of lignin results in monoaromatics and small oligomers that are dissolved in an organic solvent, and recovering most, or all, of the solvent, produces streams with high aromatic concentrations. The efficient delivery of these highly concentrated aromatics to a bioreactor is still limited by the solubility of the aromatics in the aqueous environment needed for the microbial process to occur. Thus, to attain high productivities from lignocellulosic biomass aromatics in fed-batch reactors, further research is needed to develop strategies for the effective delivery of the aromatics to the microbial culture.In previous research,36 we have used APLs from poplar, sorghum, and Arabidopsis to demonstrate PDC production from plant-derived aromatics with N. aromaticivorans strains, but in those studies, the liquors had a limited concentration of aromatics (less than 15 mM), and the Na+ concentration was kept at levels shown to be non-inhibitory in this study (∼70 mM). In this study, to create an APL with high aromatic concentration that could be used for high PDC productivity in the MBR system, we modified the procedure (see Materials and methods) and obtained a poplar aqueous solution that had 16 mM pHBA as the major aromatic and 500 mM Na+. PDC production from this APL by N. aromaticivorans 12444ΔligIΔdesCD could not be maintained, presumably because of high concentrations of Na+ or other unidentified inhibitors that had a negative impact on microbial growth or aromatic metabolism. To overcome this bottleneck, we processed the APL by liquid–liquid extraction, a step that allowed us to prepare a solution (eAPL2) with a pHBA concentration of 50 mM (6.9 g L−1) and a Na+ concentration of 20 mM. We found that a N. aromaticivorans MBR fed eAPL2 reached a productivity of 1.53 gPDC L−1 h−1 with a titer of 9.2 gPDC L−1, demonstrating the feasibility of using biomass-derived aromatics for PDC production at high rates, albeit requiring an additional extraction step.
Implications of PDC production from aqueous streams using MBR systems
This study demonstrated that attaining high PDC productivity from aromatics can be accomplished by creating high-density N. aromaticivorans 12444ΔligIΔdesCD cultures using a flow-through MBR system. This is an important advancement in the development of green chemistry approaches for production of PDC from a renewable resource such as lignocellulosic biomass since higher production rates translate to smaller process footprints. One trade-off of PDC production with flow-through MBR is that product titers will not be as high when compared to fed-batch reactors that are operated to achieve the highest possible titers. A disadvantage of lower titers is in the downstream separation of the product from the culture broth, as product concentration influences the solvent volume needed for efficient product extraction and larger volumes of residual wastewater may need to be processed. The optimal outcome would be high titers and high production rates, as demonstrated by Otsuka et al.20 However, the high cell densities required to achieve this high productivity was considered to be a physical upper limit of cell densities in their experiments, and therefore, not likely to be suitable for an effective and stable industrial process.The N. aromaticivorans cell densities achieved in the MBR system studied here were less than half of the densities reported by Otsuka et al.20 and the reactors achieved similar production rates. Microbial growth in these systems is dependent on the amount of non-aromatic substrate used, and therefore, being able to operate a high productivity process with lower cell density has the additional advantage that a lower amount of non-aromatic substrate is required (Table 2). With less non-aromatic substrate used, the amount of waste in the form of microbial cells may also be reduced. In this regard, further analysis of the green chemistry potential of these biotechnologies would need to consider the waste treatment processes needed to manage microbial cell waste. One possibility is to create value-added benefits from these materials by treating them in an anaerobic digester to recover energy in the form of biogas. Furthermore, strategies to gaining additional value from the microbial cells could be realized by engineering microbial strains capable of accumulating additional industrially relevant chemicals inside the cells, as has been recently reported for the concurrent production of extracellular PDC and intracellular carotenoids.44
A potential challenge with MBR systems is the potential fouling of the membranes, which can add operational costs by requiring additional chemicals for cleaning or more frequent membrane replacement.39 In our experiments with N. aromaticivorans, membrane fouling was not observed, and we were able to operate the high-density bioreactors without the need for membrane cleaning. Thus, this research demonstrates the compatibility of N. aromaticivorans with MBR operation, although additional research is needed to evaluate whether this compatibility remains when using aromatic streams that are more complex than the poplar APL or the synthetic media used here. Furthermore, the compatibility of MBR with other microbial chassis remains to be demonstrated.
A critical condition to enable industrially relevant high-rate microbial production of PDC and other products from plant aromatics is the delivery of feed streams to the bioreactors. This highlights the need for further research to create biomass streams with high aromatic concentrations and low concentrations of Na+ and other potential inhibitors of microbial activity. In addition, strategies are needed for high-rate delivery of these feed streams to a bioreactor, in a way that is compatible with the microbial process. Achieving these outcomes would contribute to advancing the economic and environmental sustainability of these biotechnologies.
In sum, by combining the native metabolic capabilities of N. aromaticivorans with the ability of an MBR to produce high-density cultures we describe a strategy that achieved high PDC production rates using either pure aromatics or aromatics derived from lignocellulosic biomass. A technoeconomic analysis is needed to weigh the added costs of concentrating the biomass aromatics versus the savings in capital cost due to smaller bioreactors operating at high rates with shorter HTRs. In this regard, MBR systems have an advantage of producing clear effluents, so separation of the culture media from the cell catalyst is integrated into the strategy. Furthermore, life cycle assessments are needed to evaluate the environmental impact of the proposed processes for PDC production, which employ aromatics obtained from renewable resources. Such analyses would be useful to identify critical points that could be addressed in the future to improve the technology from a green chemistry perspective. Finally, additional studies are also needed to see if the lessons learned from PDC production by N. aromaticivorans are relevant to generating other extracellular and intracellular products that may be produced from biomass aromatics by this and other microbial catalysts.
Materials and methods
Bacterial strains and culture inoculation
All experiments used a strain of N. aromaticivorans DSM12444 engineered to produce PDC by deletion of the ligI and desCD genes (strain 12444ΔligIΔdesCD from Perez et al.8). For cell growth from frozen stocks, cultures were incubated overnight, at 30 °C, on 10 mL of standard mineral base (SMB) medium containing 22 mM (4 g L−1) glucose. The cultures were shaken at 200 rpm. After overnight incubation, 2 mL of the culture was centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The resulting pellet was resuspended with 2 mL SMB medium without glucose. This cell resuspension was used to inoculate MBR experiments (2 mL inoculum) and to prepare microplate batch tests (80 μL aliquots).
000 rpm for 5 min. The resulting pellet was resuspended with 2 mL SMB medium without glucose. This cell resuspension was used to inoculate MBR experiments (2 mL inoculum) and to prepare microplate batch tests (80 μL aliquots).
Standard mineral base (SMB) medium
The SMB medium contains 10 mM Na2HPO4, 20 mM KH2PO4, 1 g L−1 (NH4)2SO4, and 20 mL of Hunter's vitamin-free concentrated base.32 Hunter's vitamin-free concentrated base was prepared by adding the following chemicals to 1 L of distilled water:45 10 mg nitrilotriacetic acid, 14.45 mg MgSO4, 3.34 mg CaCl2·2H2O, 9.25 mg (NH4)6Mo7O24·4H2O, 99 mg FeSO4·4H2O, 55 mg nicotinic acid, 24 mg thiamin·HCl, 0.5 mg biotin, and 50 ml of Metals “44” solution. Metals “44” includes per 100 ml: 250 mg ethylenediaminetetraacetic acid, 1095 mg ZnSO4·7H2O, 500 mg FeSO4·7H2O, 154 mg MnSO4·H2O, 39.2 mg CuSO4·5H2O, 24.8 mg of Co(NO3)2·6H2O, and 17.7 mg Na2B4O7·10H2O.MBR system configuration
The MBR studies were conducted in a Multifors 2 Parallel bioreactor system (INFORS-HT, Bottmingen, Switzerland). The reactor vessel had a capacity of 240 mL, and all experiments used a 200 mL working volume, except one experiment having a 133 mL working volume (experiments 7c, Table 1). The culture was continually mixed by a stirrer with a speed between 250 and 320 rpm. The system included pumps for adding an aqueous stream, air delivery, acid and base input for pH control, recirculation of the culture through the membrane module, and bioreactor effluent. It was also equipped with sensors for pH, DO, and temperature.A hollow-fiber membrane module (Repligen D04-P20U-05-S, Waltham, MA, USA) was connected to the reactor via a recirculation loop. One pump was used to control the flow rate through the membrane in the recirculation loop, and a second pump controlled the flow rate of the filtered effluent. The membrane had a pore size of 0.2 μm and a surface area of 290 cm2. The recirculation loop was operated at discrete time intervals around 20 min, controlled by a time controller (Chrontrol XT-4S, Chrontrol, San Diego, CA, USA). When the recirculation system was active, the flow rate entering the recirculation loop was 10 times the influent flow rate, and to maintain a constant volume in the bioreactor, the flow rate of the filtered effluent was set to produce a volume of effluent equal to the volume fed when the system was off.
Reactor operation was controlled using the bioprocess platform software Eve (INFORS HT, Bottmingen, Switzerland). This software managed the feed, air supply, acid and base pumps, as well as pH, DO, and temperature sensors. The experiments were performed at 25 °C and pH 7. Continuous aeration was supplied at a flow rate of 1 L min−1.
Batch cultures
A microplate reader (Infinite M1000, Tecan, Switzerland) was used for batch cultures using 24-well CytoOne multiple well plates (USA Scientific, Ocala, FL, USA) to compare growth rates under different conditions. Cultures were incubated at 25 °C for 62 h, with hourly recording of optical density (OD600). All cultures used 1 mL of SMB medium modified to contain indicated concentrations of the tested chemicals, 12 mM (2.16 g L−1) glucose, pH 7, and 5 μL of pre-grown culture as inoculum. The chemicals tested included PCA at concentrations from 0 to 7 mM, NaCl at concentrations from 20 to 230 mM, PDC at concentrations from 0 to 105 mM, and NaCl, KCl, or NH4Cl at concentrations between 0 and 180 mM. Growth rates for each batch culture were determined using linear regression analysis of the log of growth vs. time.Preparation of alkaline pretreatment liquor (APL)
Wiley milled (5 mm) ground poplar NM6 (Poplus nigra L. × P. maximowiczii A. Henry) was used. In each of three 1 L plastic bottles, 100 g of milled poplar was mixed with 900 mL of 500 mM NaOH solution (10 wt% solids loading) and heated in an oven for 3 h at 90 °C. Then, the mixture was cooled down to room temperature, the solids were removed, and the liquid collected using a Buchner funnel and vacuum with a Whatman 6 qualitative filter paper (18.5 cm diameter and 3 μm pore size), followed by filtration with a 0.2 μm polysulfonic acid (PES) filter. The filtrate, referred to here on as alkaline pretreatment liquor (APL) had 500 mM Na+ and for MBR experiments, it was charged with 20 mM glucose (3.6 g L−1) and acidified to pH 8 with 72% H2SO4.Preparation of low-Na+ eAPL1 and eAPL2
A fresh batch of APL was produced as described and then the pH was adjusted with HCl to be less than 1, to aid in the extraction of carboxylic acids. The aromatics were then extracted from the APL using a series of 5–10 liquid–liquid extractions with ethyl acetate, until the pHBA remaining in the aqueous phase was below the threshold of detection by HPLC analysis. The organic fractions were combined and the solvent removed by evaporation to yield the dried extracted aromatics. The dried aromatics were dissolved in distilled water, with the volume of water chosen based on the desired final aromatic concentration. That is, eAPL1 contained 17 ± 0.2 mM pHBA, which is similar to the concentration of the originally prepared APL, and eAPL2 contained 49.7 ± 0.3 mM pHBA, aiming for a concentration comparable to the syringic acid experiment (experiment 7, Table 1). For MBR experiments, each eAPL solution was pH balanced with NH4OH, amended with glucose to reach 50 mM, with phosphate buffer to reach 10 mM Na2HPO4 and 20 mM KH2PO4, and with 20 mL L−1 of Hunter's vitamin-free concentrated base.Analytical methods
Extracellular pHBA, PCA and PDC were measured via liquid chromatography-mass spectrometry (LC-MS) using methods described previously.8 Glucose was measured with a Biochemistry Analyzer (YSI 2900, Xylem, USA). Na+ and K+ were measured in a Varian Vista-MPX Axial ICP-OES (Agilent Technologies, Santa Clara, California, USA) using liquid argon, wavelengths for Na+ of 588.995 nm and K+ of 766.491 nm, and a standard curve from 0–150 mg L−1. Samples were diluted 10 times to provide data within the range of the standard curve. The sum of ammonium and ammonia was measured colorimetrically in a plate reader (Infinite M1000, Tecan, Switzerland). A standard solution was prepared following Standard Method 4500-NH3.46 Cell densities were measured spectroscopically on a Biospec-Mini (Shimadzu, Japan) based on absorbance at 600 nm (OD600).PDC extraction
PDC was purified from MBR effluents for batch experiments with varying initial PDC concentrations. First, the pH of the effluent was dropped to less than 1 using HCl. Second, a series of liquid–liquid extractions with ethyl acetate were performed until the PDC remaining dissolved in the aqueous phase was below the threshold of detection by HPLC analysis. Then, the PDC-containing ethyl acetate fraction was used in additional liquid-liquid extractions with distilled water to increase PDC purity. After solvent evaporation, PDC was recovered as a white solid.Conclusions
In summary, our study shows that the optimized MBR system with N. aromaticivorans can achieve the highest productivity of PDC reported. Membrane separation increases cell density in the reactor, boosting the rate of PDC production. Also, our study identified that the accumulation of PCA was a key inhibiting factor that limited PDC productivity. In addition, replacing the base used for pH control, from NaOH for NH4OH, allowed to build up higher PDC titers and to subsequently increase flow rates without PCA accumulation, thus contributing to achieving high PDC production rates. Our work highlights that the optimized MBR system can also be applied to aromatics produced from lignocellulosic biomass. Future studies will address the application of the system with other feedstocks, aromatic feed sources, and other microbially produced chemicals derived from aromatics feed streams.Author contributions
B. Kim: conceptualization, investigation, data curation, visualization, methodology, writing – original draft, writing – review and editing. J. M. Perez: conceptualization, investigation, writing – review and editing. S. D. Karlen: investigation, visualization, data curation, methodology, writing – review and editing. J. Coplien: investigation, methodology, data curation. T. J. Donohue: conceptualization, investigation, writing – review and editing, funding acquisition. D. R. Noguera: conceptualization, investigation, supervision, writing – original draft, writing – review and editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Biological and Environmental Research Program under Award Number DE-SC0018409. We thank Jackie Bastyr-Cooper for help with analytical methods.References
- Y. Otsuka, M. Nakamura, K. Shigehara, K. Sugimura, E. Masai, S. Ohara and Y. Katayama, Efficient production of 2-pyrone 4,6-dicarboxylic acid as a novel polymer-based material from protocatechuate by microbial function, Appl. Microbiol. Biotechnol., 2006, 71, 608–614 CrossRef CAS PubMed.
- C. W. Johnson, D. Salvachua, N. A. Rorrer, B. A. Black, D. R. Vardon, P. C. St John, N. S. Cleveland, G. Dominick, J. R. Elmore, N. Grundl, P. Khanna, C. R. Martinez, W. E. Michener, D. J. Peterson, K. J. Ramirez, P. Singh, T. A. VanderWall, A. N. Wilson, X. N. Yi, M. J. Biddy, Y. J. Bomble, A. M. Guss and G. T. Beckham, Innovative chemicals and materials from bacterial aromatic catabolic pathways, Joule, 2019, 3, 1523–1537 CrossRef CAS.
- J. Chow, R. J. Kopp and P. R. Portney, Energy resources and global development, Science, 2003, 302, 1528–1531 CrossRef PubMed.
- F. Martins, C. Felgueiras, M. Smitkova and N. Caetano, Analysis of fossil fuel energy consumption and environmental impacts in European countries, Energies, 2019, 12, 1–11 Search PubMed.
- S. Pacala and R. Socolow, Stabilization wedges: Solving the climate problem for the next 50 years with current technologies, Science, 2004, 305, 968–972 CrossRef CAS PubMed.
- V. Menon and M. Rao, Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept, Prog. Energy Combust. Sci., 2012, 38, 522–550 CrossRef CAS.
- D. V. Sahayaraj, A. Lusi, A. J. Kohler, H. Bateni, H. Radhakrishnan, A. Saraeian, B. H. Shanks, X. L. Bai and J. P. Tessonnier, An effective strategy to produce highly amenable cellulose and enhance lignin upgrading to aromatic and olefinic hydrocarbons, Energy Environ. Sci., 2023, 16, 97–112 RSC.
- J. M. Perez, W. S. Kontur, M. Alherech, J. Coplien, S. D. Karlen, S. S. Stahl, T. J. Donohue and D. R. Noguera, Funneling aromatic products of chemically depolymerized lignin into 2-pyrone-4-6-dicarboxylic acid with Novosphingobium aromaticivorans, Green Chem., 2019, 21, 1340–1350 RSC.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph, R. Rinaldi, Y. Roman-Leshkov, J. S. M. Samec, B. F. Sels and F. Wang, Guidelines for performing lignin-first biorefining, Energy Environ. Sci., 2021, 14, 262–292 RSC.
- D. R. Vardon, M. A. Franden, C. W. Johnson, E. M. Karp, M. T. Guarnieri, J. G. Linger, M. J. Salm, T. J. Strathmann and G. T. Beckham, Adipic acid production from lignin, Energy Environ. Sci., 2015, 8, 617–628 RSC.
- R. Mori, Replacing all petroleum-based chemical products with natural biomass-based chemical products: a tutorial review, RSC Sustainability, 2023, 1, 179–212 RSC.
- S. Van den Bosch, W. Schutyser, R. Vanholme, T. Driessen, S. F. Koelewijn, T. Renders, B. De Meester, W. J. J. Huijgen, W. Dehaen, C. M. Courtin, B. Lagrain, W. Boerjan and B. F. Sels, Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps, Energy Environ. Sci., 2015, 8, 1748–1763 RSC.
- L. Xu, S. J. Zhang, C. Zhong, B. Z. Li and Y. J. Yuan, Alkali-based pretreatment-facilitated lignin valorization: A review, Ind. Eng. Chem. Res., 2020, 59, 16923–16938 CrossRef CAS.
- Z. H. Liu, B. Z. Li, J. S. Yuan and Y. J. Yuan, Creative biological lignin conversion routes toward lignin valorization, Trends Biotechnol., 2022, 40, 1550–1566 CrossRef CAS PubMed.
- R. Vanholme, K. Morreel, C. Darrah, P. Oyarce, J. H. Grabber, J. Ralph and W. Boerjan, Metabolic engineering of novel lignin in biomass crops, New Phytol., 2012, 196, 978–1000 CrossRef CAS PubMed.
- S. Lee, Y. J. Jung, S. J. Park, M. H. Ryu, J. E. Kim, H. M. Song, K. H. Kang, B. K. Song, B. H. Sung, Y. H. Kim, H. T. Kim and J. C. Joo, Microbial production of 2-pyrone-4,6-dicarboxylic acid from lignin derivatives in an engineered Pseudomonas putida and its application for the synthesis of bio-based polyester, Bioresour. Technol., 2022, 352, 1–8 CrossRef PubMed.
- W. Schutyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- A. Rodriguez, D. Salvachúa, R. Katahira, B. A. Black, N. S. Cleveland, M. Reed, H. Smith, E. E. K. Baidoo, J. D. Keasling, B. A. Simmons, G. T. Beckham and J. M. Gladden, Base-catalyzed depolymerization of solid lignin-rich streams enables microbial conversion, ACS Sustainable Chem. Eng., 2017, 5, 8171–8180 CrossRef CAS.
- D. Salvachúa, C. W. Johnson, C. A. Singer, H. Rohrer, D. J. Peterson, B. A. Black, A. Knapp and G. T. Beckham, Bioprocess development for muconic acid production from aromatic compounds and lignin, Green Chem., 2018, 20, 5007–5019 RSC.
- Y. Otsuka, T. Araki, Y. Suzuki, M. Nakamura, N. Kamimura and E. Masai, High-level production of 2-pyrone-4,6-dicarboxylic acid from vanillic acid as a lignin-related aromatic compound by metabolically engineered fermentation to realize industrial valorization processes of lignin, Bioresour. Technol., 2023, 377, 1–9 CrossRef PubMed.
- J. M. Perez, C. Sener, S. Misra, G. E. Umana, J. Coplien, D. Haak, Y. D. Li, C. T. Maravelias, S. D. Karlen, J. Ralph, T. J. Donohue and D. R. Noguera, Integrating lignin depolymerization with microbial funneling processes using agronomically relevant feedstocks, Green Chem., 2022, 24, 2795–2811 RSC.
- E. M. Anderson, R. Katahira, M. Reed, M. G. Resch, E. M. Karp, G. T. Beckham and Y. Román-Leshkov, Reductive catalytic fractionation of corn stover lignin, ACS Sustainable Chem. Eng., 2016, 4, 6940–6950 CrossRef CAS.
- G. T. Beckham, C. W. Johnson, E. M. Karp, D. Salvachua and D. R. Vardon, Opportunities and challenges in biological lignin valorization, Curr. Opin. Biotechnol., 2016, 42, 40–53 CrossRef CAS PubMed.
- A. J. Borchert, W. R. Henson and G. T. Beckham, Challenges and opportunities in biological funneling of heterogeneous and toxic substrates beyond lignin, Curr. Opin. Biotechnol., 2022, 73, 1–13 CrossRef CAS PubMed.
- J. Nielsen and J. D. Keasling, Engineering cellular metabolism, Cell, 2016, 164, 1185–1197 CrossRef CAS PubMed.
- J. H. Cecil, D. C. Garcia, R. J. Giannone and J. K. Michener, Rapid, parallel identification of catabolism pathways of lignin-derived aromatic compounds in Novosphingobium aromaticivorans, Appl. Environ. Microbiol., 2018, 84, 1–13 CrossRef PubMed.
- M. M. Fetherolf, D. J. Levy-Booth, L. E. Navas, J. Liu, J. C. Grigg, A. Wilson, R. Katahira, G. T. Beckham, W. W. Mohn and L. D. Eltis, Characterization of alkylguaiacol-degrading cytochromes P450 for the biocatalytic valorization of lignin, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 25771–25778 CrossRef CAS PubMed.
- E. Masai, Y. Katayama and M. Fukuda, Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds, Biosci., Biotechnol., Biochem., 2007, 71, 1–15 CrossRef CAS PubMed.
- E. Masai, Y. Yamamoto, T. Inoue, K. Takamura, H. Hara, D. Kasai, Y. Katayama and M. Fukuda, Characterization of ligV essential for catabolism of vanillin by Sphingomonas paucimobilis SYK-6, Biosci., Biotechnol., Biochem., 2007, 71, 2487–2492 CrossRef CAS PubMed.
- J. M. Perez, W. S. Kontur, C. Gehl, D. M. Gille, Y. J. Ma, A. V. Niles, G. Umana, T. J. Donohue and D. R. Noguera, Redundancy in aromatic O-demethylation and ring-opening reactions in Novosphingobium aromaticivorans and their impact in the metabolism of plant-derived phenolics, Appl. Environ. Microbiol., 2021, 87, 1–23 CrossRef PubMed.
- N. Kamimura, Y. Hirose, R. Masuba, R. Kato, K. Takahashi, Y. Higuchi, S. Hishiyama and E. Masai, LsdD has a critical role in the dehydrodiconiferyl alcohol catabolism among eight lignostilbene α,β-dioxygenase isozymes in Sphingobium, sp. strain SYK-6, Int. Biodeterior. Biodegrad., 2021, 159, 1–7 Search PubMed.
- W. S. Kontur, C. A. Bingman, C. N. Olmsted, D. R. Wassarman, A. Ulbrich, D. L. Gall, R. W. Smith, L. M. Yusko, B. G. Fox, D. R. Noguera, J. J. Coon and T. J. Donohue, Novosphingobium aromaticivorans uses a Nu-class glutathione S-transferase as a glutathione lyase in breaking the β-aryl ether bond of lignin, J. Biol. Chem., 2018, 293, 4955–4968 CrossRef CAS PubMed.
- W. S. Kontur, C. N. Olmsted, L. M. Yusko, A. V. Niles, K. A. Walters, E. T. Beebe, K. A. Vander Meulen, S. D. Karlen, D. L. Gall, D. R. Noguera and T. J. Donohue, A heterodimeric glutathione S-transferase that stereospecifically breaks lignin's β(R)-aryl ether bond reveals the diversity of bacterial β-etherases, J. Biol. Chem., 2019, 294, 1877–1890 CrossRef CAS PubMed.
- G. N. Presley, A. Z. Werner, R. Katahira, D. C. Garcia, S. J. Haugen, K. J. Ramirez, R. J. Giannone, G. T. Beckham and J. K. Michener, Pathway discovery and engineering for cleavage of a β-1 lignin-derived biaryl compound, Metab. Eng., 2021, 65, 1–10 CrossRef CAS PubMed.
- K. Takahashi, K. Miyake, S. Hishiyama, N. Kamimura and E. Masai, Two novel decarboxylase genes play a key role in the stereospecific catabolism of dehydrodiconiferyl alcohol in Sphingobium sp. strain SYK-6, Environ. Microbiol., 2018, 20, 1739–1750 CrossRef CAS PubMed.
- G. E. Umana, J. M. Perez, F. Unda, C. Y. Lin, C. Sener, S. D. Karlen, S. D. Mansfield, A. Eudes, J. Ralph, T. J. Donohue and D. R. Noguera, Biological funneling of phenolics from transgenic plants engineered to express the bacterial 3-dehydroshikimate dehydratase (qsuB) gene, Front. Chem. Eng., 2022, 4, 1–12 Search PubMed.
- C. C. Azubuike, M. N. Allemann and J. K. Michener, Microbial assimilation of lignin-derived aromatic compounds and conversion to value-added products, Curr. Opin. Microbiol., 2022, 65, 64–72 CrossRef CAS PubMed.
- A. C. Vilbert, W. S. Kontur, D. Gille, D. R. Noguera and T. J. Donohue, Engineering Novosphingobium aromaticivorans to produce cis,cis-muconic acid from biomass aromatics, Appl. Environ. Microbiol., 2023, 90, 1–22 Search PubMed.
- S. Judd, The status of membrane bioreactor technology, Trends Biotechnol., 2008, 26, 109–116 CrossRef CAS PubMed.
- J. Ralph, K. Lundquist, G. Brunow, F. Lu, H. Kim, P. F. Schatz, J. M. Marita, R. D. Hatfield, S. A. Ralph, J. H. Christensen and W. Boerjan, Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids, Phytochem. Rev., 2004, 3, 29–60 CrossRef CAS.
- D. L. Gall, J. Ralph, T. J. Donohue and D. R. Noguera, Biochemical transformation of lignin for deriving valued commodities from lignocellulose, Curr. Opin. Biotechnol., 2017, 45, 120–126 CrossRef CAS PubMed.
- S. Notonier, A. Z. Werner, E. Kuatsjah, L. Dumalo, P. E. Abraham, E. A. Hatmaker, C. B. Hoyt, A. Amore, K. J. Ramirez, S. P. Woodworth, D. M. Klingeman, R. J. Giannone, A. M. Guss, R. L. Hettich, L. D. Eltis, C. W. Johnson and G. T. Beckham, Metabolism of syringyl lignin-derived compounds in Pseudomonas putida enables convergent production of 2-pyrone-4,6-dicarboxylic acid, Metab. Eng., 2021, 65, 111–122 CrossRef CAS PubMed.
- Y. Suzuki, Y. Okamura-Abe, Y. Otsuka, T. Araki, M. Nojiri, N. Kamimura, E. Masai and M. Nakamura, Integrated process development for grass biomass utilization through enzymatic saccharification and upgrading hydroxycinnamic acids via microbial funneling, Bioresour. Technol., 2022, 363, 1–9 CrossRef PubMed.
- B. W. Hall, W. S. Kontur, J. C. Neri, D. M. Gille, D. R. Noguera and T. J. Donohue, Production of carotenoids from aromatics and pretreated lignocellulosic biomass by Novosphingobium aromaticivorans, Appl. Environ. Microbiol., 2023, 89, 1–16 CrossRef CAS PubMed.
- G. Cohenbazire, W. R. Sistrom and R. Y. Stanier, Kinetic studies of pigment synthesis by non-sulfur purple bacteria, J. Cell. Comp. Physiol., 1957, 49, 25–68 CrossRef CAS PubMed.
- American Public Health Association, American Water Works Association and Water Environment Federation, Standard Methods for the Examination of Water and Wastewater, 23rd edn, APHA Press, 2017 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc01975j |
| This journal is © The Royal Society of Chemistry 2024 |