Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Turning Pd-catalysed direct C–H arylation of thiophene derivatives into green : industrial wastewater as an effective reaction medium†
Stefano
Nejrotti
 *a,
Barbara
Centrella
a,
Davide
Gallo
ab,
Claudia
Barolo
*a,
Barbara
Centrella
a,
Davide
Gallo
ab,
Claudia
Barolo
 acd and
Matteo
Bonomo
acd and
Matteo
Bonomo
 *a
*a
aDepartment of Chemistry, NIS and INSTM Reference Centre, Università degli Studi di Torino, via Pietro Giuria 7, 10126 Torino, Italy. E-mail: stefano.nejrotti@unito.it; matteo.bonomo@unito.it
bAhlstrom Italia S.p.A., via Stura 98, 10075 Mathi, TO, Italy
cIstituto di Scienza, Tecnologia e Sostenibilità per lo Sviluppo dei Materiali Ceramici (ISSMC-CNR), via Granarolo 64, 48018 Faenza, RA, Italy
dICxT Interdepartmental Center, Università Degli Studi di Torino, Lungo Dora Siena 100, 10153, Torino, Italy
First published on 29th May 2024
Abstract
The Pd-catalysed direct C–H arylation of thiophene derivatives, appealing for technological application, is performed under green conditions using water as the sole reaction medium. The reaction is independent of the grade of the water, thus enabling the virtuous repurposing of low-purity grade industrial wastewater, allowing one to bring the process to the gram scale and improve its green metrics.
The formation of a new C–C bond between two (hetero)aromatic rings often represents a crucial synthetic step in the assembly of a wide variety of molecular architectures, ranging from active pharmaceutical ingredients (APIs) to technologically relevant compounds. The latter family includes functional molecules and polymers, with applications in the field of materials for advanced applications, such as organic electronics and photovoltaics.1–5 The vast majority of these polyaromatic structures rely on the use of heteroaromatics as building blocks, since they enable a broad modulation of the electronic and optical properties, attaining high-performing materials for the pursued application. In particular, the thiophene ring represents a versatile and largely exploited donor moiety.6–8 Among the synthetic methodologies that enable the construction of extensively conjugated molecular and polymeric scaffolds, a predominant role has historically been covered by “classical” cross-coupling reactions, generally involving an organometallic partner and a halide coupling partner.9 More recently, the catalytic direct C–H arylation strategy has emerged as an attractive alternative, thanks to its intrinsic step- (and atom-) economy:10 in fact, since it does not require the pre-functionalisation of one of the aromatic reagents as organometallic species, it appears to have all the credentials to make synthesis simpler, more straightforward and more sustainable.11–14 However, the claimed advantage in terms of sustainability needs to be assessed from a quantitative point of view before being taken for granted. This point has been brought to attention by Vaccaro et al. in a very recent critical review, in which a green metrics-based approach was applied to compare cross coupling vs. direct C–H arylation strategies in the synthesis of some APIs.15 Interestingly, the results highlighted that C–H arylations are generally superior in terms of environmental and safety profile but suffer from higher E-factor values, i.e. the mass of waste generated per gram of product.16–19 According to the analysed data, this is mainly related to the larger amount of solvents employed both as the reaction medium and throughout the post-reaction treatment. The solvent hence represents a central issue in improving the sustainability of direct C–H coupling synthetic methodologies, not only in terms of the quantity employed, but also for what concerns its chemical nature. Indeed, it is desirable to overcome the use of those solvents which are considered hazardous, both for human health and the environment, according to the rankings and classifications drawn up by several pharmaceutical companies and by the US Food & Drug Administration.20–23 While water and alcohols are considered as recommended solvents, those generally employed for catalytic direct C–H arylation reactions, i.e. N,N-dimethylformamide, N,N-dimethylacetamide, and 1,4-dioxane, fall into the “hazardous” or “problematic” categories. In the search for alternative reaction media for such transformations, some research groups have turned their attention to deep eutectic solvents (DESs), reporting the effective direct arylation of thiophene derivatives for the synthesis of functional compounds to be applied as non-fullerene acceptors in organic solar cells24 or as fluorescent dyes in luminescent solar concentrators.25 In particular, the synthesis of symmetrical aryl-substituted 3,4-ethylenedioxythiophene (EDOT) or thieno[3,4-b]pyrazine (TP) derivatives has been accomplished in the DES composed of choline chloride (ChCl) and glycerol (Gly) in a 1/2 molar ratio (Scheme 1).25 This showcased the potential of combining the step-economy of direct C–H arylation with the sustainability of unconventional solvents such as DESs, attaining remarkable results in terms of green chemistry metrics. DESs are generally regarded as a greener choice because of their presumed low toxicity, good biodegradability, and production from potentially renewable sources.26–29 On this matter, some of us recently reviewed such features for a selection of representative DESs to provide a critical insight into the actual reliability of these eutectic mixtures as “sustainable solvents”.30 There, it was highlighted that the assumption should not be taken lightly, especially from the point of view of the supposed renewability of DES components: indeed, while for some of them (such as Gly), industrial production is currently based on biorenewable sources, for others, such as ChCl, the implementation of fossil-free processes is definitely far from being available.
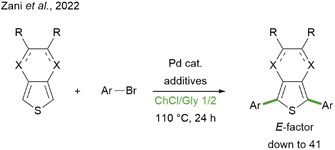 | ||
| Scheme 1 Previous report on the direct C–H arylation of EDOT (X = O, R = H, single bonds) or TP (X = N, R ≠ H, double bonds) with aryl bromides in the ChCl/Gly DES.25 | ||
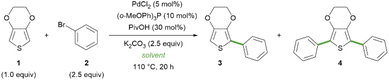
On this ground, with the aim of simplifying and improving the sustainability of the direct C–H arylation of thiophene derivatives, we first tested the Pd-catalysed arylation of EDOT 1 with bromobenzene 2 to afford the mono-arylated product 3 and the di-arylated product 4 in pure Gly instead of the ChCl/Gly eutectic mixture (Table 1). We applied the previously reported catalytic system, consisting of palladium(II) chloride and tris(o-methoxyphenyl)phosphine, in the presence of pivalic acid as an additive and potassium carbonate as a base at 110 °C for 20 hours (Table 1, entry 2).25 Under these conditions, we observed a significant decrease in the conversion of 1, compared to the reported results obtained with ChCl/Gly 1/2, which we were able to successfully reproduce (entry 1). This could be attributed to a specific effect of the presence of ChCl or to the different physicochemical properties of the eutectic mixture, compared to Gly alone: for example, it is known that the formation of the DES reduces the viscosity by altering the H-bond network of glycerol.31 With the aim of reproducing the lower viscosity while at the same time ruling out a specific role of ChCl, we performed the C–H arylation in Gly/H2O mixtures of varying molar ratios (entries 3–6). Remarkably, the conversion into the desired di-arylated product 4 increased with the increasing percentage of water, and it was restored to almost completeness with the Gly/H2O 1/3 mixture (entry 6).
| Entry | Solventa | Relative ratio 1/3/4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|
| a [1] = 0.2 M, i.e. 0.5 mmol of 1 in 2.5 mL of solvent. All mixture compositions are expressed as molar ratios. b Determined by 1H NMR analysis of the crude reaction mixture, after work-up. c Reproducing literature conditions (reported relative ratio: 1/1/98). d 83% yield of 4 after column chromatography. | ||
| 1 | ChCl/Gly 1/2 | 2/0/98c |
| 2 | Gly | 19/19/62 |
| 3 | Gly/H2O 2/1 | 9/14/77 |
| 4 | Gly/H2O 1/1 | 6/8/86 |
| 5 | Gly/H2O 1/2 | 4/6/90 |
| 6 | Gly/H2O 1/3 | 3/4/93 |
| 7 | H2O (type II) | 0/0/100 |
| 8 | H2O (sink) | 0/0/100 |
| 9 | H2O (seawater) | 0/0/100 |
| 10 | H2O (industrial wastewater) | 0/0/100d |
| 11 | Kolliphor-EL 2% in type II H2O41 | 0/0/100 |
Based on these results, we envisaged the opportunity of making the methodology further simpler and less impactful by eliminating Gly and performing the Pd-catalysed C–H arylation in H2O alone as the solvent. Under these conditions, complete conversion into 4 was observed (entry 7). Water is in general a more desirable solvent than any organic compound since it is widely available, non-toxic, and cheap.11,32,33 In the past two decades, advances in the use of water as the reaction medium for the C–H arylation of aromatic scaffolds,34–39 including thiophene derivatives,40,41 have been made possible by the introduction of a surfactant in the reaction environment, thus exploiting micellar catalysis. In line with previously reported methodologies, the synthesis of 4 proved effective in aqueous micellar solution (entry 11); however, the results presented in Table 1 clearly show that the addition of a surfactant is not necessary, as the reaction proceeds smoothly in water alone. Instead, it should be noted that the need for high purity grade water inevitably increases the economic, energetic and subsequently environmental burden of its production, not to mention the possible interference with the production of water for human consumption.42 Moreover, contaminated water, downstream of its use as a reaction medium, must be treated before its ultimate disposal, thus significantly impacting the cost and sustainability. To address these issues, we investigated the robustness of the C–H arylation protocol for different qualities of water by testing tap water from the laboratory sink (entry 8), a sample of seawater collected from the Tyrrhenian Sea (entry 9) and, most importantly, several samples of wastewater coming from the industrial processes of the company Ahlstrom Italia S.p.A. (entry 10). The results show that the reaction is tolerant to the different types of water employed, as full conversion into 4 was observed in all cases. Upon isolation through flash column chromatography, a remarkable 83% yield of 4 was obtained in the case of the reaction run in industrial wastewater (entry 10). Employing wastewater as a medium constitutes a valuable opportunity to repurpose waste, which is entirely a cost, thus mitigating both the economic burden and the consumption of fresh resources.
Once we established the feasibility of the direct C–H arylation of 1 into 4 in industrial wastewater, we turned our attention to another aspect related to sustainability, that is the consumption of energy. Indeed, the methodology involves heating the reaction mixture at a quite elevated temperature, 110 °C, for 20 hours. To this end, we studied the kinetics of the transformation in the same industrial effluent by performing the Pd-catalysed arylation of 1 with 2 at different reaction times. The results, reported in Fig. 1, show that a full conversion into 4 is obtained after just 4 hours. By comparison, the reaction performed in the ChCl/Gly 1/2 DES afforded much lower conversion in the same timeframe, with a relative ratio of 1/3/4 as high as 31/22/47 after 4 hours.
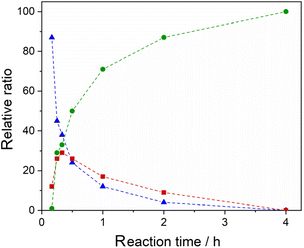 | ||
Fig. 1 Relative ratios, determined by 1H NMR analysis of the crude reaction mixture after work-up, of 1 ( ), 3 ( ), 3 ( ) and 4 ( ) and 4 ( ) at different reaction times. Dashed lines are employed as a guide for the reader. Reaction conditions: see Table 1, entry 10. ) at different reaction times. Dashed lines are employed as a guide for the reader. Reaction conditions: see Table 1, entry 10. | ||
To assess the effective sustainability improvement of a synthetic methodology, it is necessary to analyse it through the lens of quantitative or semi-quantitative parameters, such as green chemistry metrics. As mentioned above, direct C–H arylation reactions generally suffer from impacting values of mass-related metrics.15 Among such metrics, the most common is the E-factor;18,19 however, the reaction mass efficiency (RME) or the generalized mass efficiency (gRME) can be helpful as well. RME and gRME are calculated as the ratio of the product obtained to the mass employed. Still, RME considers the mass of reactants only, while for gRME (also referred to as mass productivity, MP), all the mass involved in the process, including solvents and purification materials, is considered.16,17,43 The use of green metrics represents a method to assess the sustainability of a given chemical process and, notably, may help guide its design as well.17 Having determined the conditions for the effective direct C–H arylation of 1 in industrial wastewater in a reaction time of 4 hours, we aimed at optimising its mass-related metrics while simultaneously verifying its scalability to the gram-scale. To this purpose, we increased the amount of substrate by 20 times, up to a 10 mmol scale, and we modified both the reaction parameters and the work-up and purification procedure, compared to the one applied to the previous part of the study. In particular, the concentration of the reaction was increased, thus decreasing the amount of solvent employed per mmol of substrate, and the catalyst loading was decreased from 5 mol% to 1 mol% (see the ESI† for details). The liquid/liquid extraction work-up (with an organic solvent, i.e. CH2Cl2) was replaced by precipitation and filtration, and the purification was performed by trituration instead of column chromatography (see the ESI† for details). Under the optimised conditions, we obtained the di-arylated product 4 in 74% yield (Table 2). For the whole process, we calculated an E-factor value of 31, lowered by 40% compared to the previously reported methodology performed in DESs (E-factor = 52, Table 2).25 A “classical” cross coupling strategy for the synthesis of 4, involving the bromination of EDOT and the subsequent Suzuki–Miyaura coupling with phenylboronic acid (see the ESI† for details),44,45 is also included in the comparison and shows a considerably higher E-factor value of 121 (Table 2). Moving to the analysis of the other mass-related green metrics, the RME value reveals that the reaction performed in industrial wastewater is apparently slightly less efficient than the one performed in DESs (0.237 vs. 0.287, Table 2). This is a direct reflection of the lower chemical yield of our process (74% vs. 90%), since RME considers the waste generated by the reactants only, which is in the same amount for the two different methodologies. However, when all the materials employed in the reaction and post-reaction treatments are included, the calculated gRME values show an improvement of 63% for the industrial wastewater-based reaction compared to the DES-based one (0.031 vs. 0.019, Table 2). Again, the “classical” bromination–Suzuki coupling strategy outperformed, displaying significantly lower values, in particular for the gRME metric (0.0074, Table 2). The significance of these figures also relies on the fact that, when industrial wastewater is used as a reaction medium, it contributes to the overall sum but formally does not introduce new waste into the production stream since it is already a waste from the previous process. To account for this fact, it could be possible to calculate the E-factor and the gRME by excluding the water from the sum of the waste, obtaining an E-factor value of 22 and a gRME value of 0.043, more than double the literature value of 0.019. It ought to be noted that another commonly used metric is the EcoScale, which assigns a score (out of 100) based on the “penalties” attributed to each non-sustainable aspect of the methodology.46 However, this parameter is not helpful in analysing the difference between the reactions performed in a DES and in water, since both solvents are considered not hazardous and thus obtain the same score on the EcoScale.
| Synthetic methodologya | No. of steps | Yield | Metrics |
|---|---|---|---|
| a See the ESI† for details. b If reaction water is not considered as waste, the E-factor is 22 and gRME is 0.043. c Overall yield and metrics over 2 steps. | |||
| C–H arylation in industrial wastewater (this work) | 1 | 74% | E-Factor 31b |
| RME 0.237 | |||
| gRME 0.031 | |||
| C–H arylation in a DES25 | 1 | 90% | E-Factor 52 |
| RME 0.287 | |||
| gRME 0.019 | |||
| Suzuki–Miyaura cross coupling44,45 | 2 | 55%c | E-Factor 121c |
| RME 0.125 | |||
| gRME 0.0074 | |||
To showcase the potential of this methodology for the assembly of technologically appealing molecular scaffolds, we approached the synthesis of three fluorescent dyes based on the EDOT or the 2,3-dimethylthieno[3,4-b]pyrazine central core, symmetrically decorated with aryl rings (Fig. 2).47,48 The terthienyl derivative 7 has been studied as a monomer for the assembly of narrow bandgap copolymers,49–51 while its diphenyl-substituted analogue 6 has never been synthesised so far. Through modification of the two coupling partners, it is possible to obtain fluorophores emitting in the desired region throughout the whole visible spectrum with large Stokes shift values (see the ESI† for details). The dianthracenyl substituted derivative 5 emits in the blue region (λem = 475 nm), while the replacement of the EDOT core of 4 with the acceptor TP scaffold, such as in compounds 6 and 7, dramatically shifts the optical properties towards emission in the orange (6, λem = 609 nm) and red regions (7, λem = 671 nm). From the synthetic point of view, when moving from liquid reagents 1 and 2 to solid ones, such as 9-bromoanthracene or 2,3-dimethylthieno[3,4-b]pyrazine, a foreseeable problem might come from the insolubility of such reagents in the aqueous reaction medium. Nevertheless, the synthesis proceeded smoothly towards di-arylated derivatives 5, 6 and 7. In particular, high yields of the desired products were obtained when synthesizing the TP-based fluorophores 6 and 7; in contrast, the C–H arylation of TP in a DES was reported to require higher amounts of aryl bromide.25 In all cases, it was possible to recover the product from the reaction mixture through filtration, and the purification was feasible without column chromatography (see the ESI† for details). It ought to be noted that the absence of a chromatographic purification step could represent a concern in terms of residual Pd content in the final product, which should therefore be monitored in view of a possible application of the methodology.52 Moving to the analysis of the green metrics, the calculated E-factor and gRME mainly reflect the amount of solvent employed for the purification of the final products. At the same time, the RME is affected by the chemical yield and the atom economy of the transformation. Notably, the synthesis of 6 afforded a substantially pure crude reaction product, which required minimal purification, resulting in a lower E-factor and a higher gRME value. On the other hand, the direct C–H arylation towards product 5 resulted in a lower yield and a higher amount of impurities, negatively impacting the values of green metrics (Fig. 2). For the synthesis of 7, it was necessary to slightly increase the equivalents of the aryl bromide coupling partner (3.0 equiv. instead of 2.5 equiv., see the ESI† for details) to avoid the presence of a mono-arylated product in the crude reaction product, which would necessarily imply a chromatographic purification step. This is in line with the expected lower reactivity of electron-rich aryl bromides, such as 2-bromothiophene.
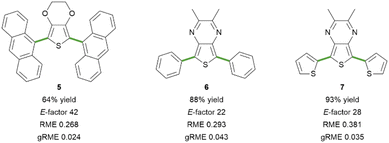 | ||
| Fig. 2 EDOT- and TP-based fluorescent dyes synthesized via direct C–H arylation in industrial wastewater. See the ESI† for details on the synthetic procedures. | ||
Conclusions
In conclusion, in this work we have proposed, for the first time, the use of water (without any organic cosolvent or micellar additive) as the sole reaction medium in the direct C–H arylation of thiophene derivatives, improving sustainability from different points of view. First, the choice of water as the reaction medium allows one to employ a non-toxic medium, with benefits in terms of process safety. The possibility of using low-purity grade water, such as industrial wastewater, without affecting the reaction outcome, opens the way to reducing costs and the consumption of fresh resources. This adds to the fact that water allows one to break free from the reliance on fossil sources involved in the production of conventional organic solvents as well as some DES components. Second, the use of water as a solvent enables a straightforward and advantageous post-reaction treatment, thus positively impacting the mass efficiency of the process, as illustrated by the mass-related green metrics, and directly tackling the acknowledged weakness of C–H arylation reactions. The possibility of increasing the substrate concentration at low catalyst loading allows the successful scale-up to the gram scale and is a promising feature in view of an industrial application. In this regard, the shorter reaction times (up to one sixth, compared to the literature) align in the same direction. As an added value, the methodology has been applied to the synthesis of polyaromatic scaffolds of potential technological interest. Future development of this work could include the application of direct C–H arylation in industrial wastewater to the synthesis of conjugated polymers for optoelectronic or thermoelectric applications.Author contributions
S. N.: conceptualization, investigation, and writing – original draft; B. C.: investigation; D. G.: investigation; C. B.: supervision, funding acquisition, and writing – review & editing; M. B.: conceptualization, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Ahlstrom Italia S.p.A. for providing industrial wastewater aliquots. This research acknowledges support from the project CH4.0 under the MUR program “Dipartimenti di Eccellenza 2023–2027” (CUPD13C22003520001). This study is a result of the research project “nuovi Concetti, mAteriali e tecnologie per l'iNtegrazione del fotoVoltAico negli edifici in uno scenario di generazione diffuSa” (CANVAS), funded by the Italian Ministry of the Environment and the Energy Security through the Research Fund for the Italian Electrical System (type-A call, published on G.U.R.I. no. 192 on 18-08-2022). This research has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 863170 (ARTIBLED). Dr Stefano Bertinetti is acknowledged for ICP-AES analyses.References
- G. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K.-Y. Jen and H.-L. Yip, Chem. Rev., 2022, 122, 14180–14274 CrossRef CAS PubMed.
- T. Kim and K. Lee, Macromol. Rapid Commun., 2015, 36, 943–958 CrossRef CAS PubMed.
- A. Marrocchi, A. Facchetti, D. Lanari, S. Santoro and L. Vaccaro, Chem. Sci., 2016, 7, 6298–6308 RSC.
- A. Facchetti, Chem. Mater., 2011, 23, 733–758 CrossRef CAS.
- Q. Liu, S. E. Bottle and P. Sonar, Adv. Mater., 2020, 32, 1903882 CrossRef CAS PubMed.
- G. Barbarella, M. Melucci and G. Sotgiu, Adv. Mater., 2005, 17, 1581–1593 CrossRef CAS.
- S. Ye, V. Lotocki, H. Xu and D. S. Seferos, Chem. Soc. Rev., 2022, 51, 6442–6474 RSC.
- G. Turkoglu, M. E. Cinar and T. Ozturk, Top. Curr. Chem., 2017, 375, 84 CrossRef PubMed.
- L. Zani, A. Dessì, D. Franchi, M. Calamante, G. Reginato and A. Mordini, Coord. Chem. Rev., 2019, 392, 177–236 CrossRef CAS.
- P. A. Wender, F. C. Bi, G. G. Gamber, F. Gosselin, R. D. Hubbard, M. J. C. Scanio, R. Sun, T. J. Williams and L. Zhang, Pure Appl. Chem., 2002, 74, 25–31 CrossRef CAS.
- G. Albano, A. Punzi, M. A. M. Capozzi and G. M. Farinola, Green Chem., 2022, 24, 1809–1894 RSC.
- B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949–957 CAS.
- L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed.
- I. A. Stepek and K. Itami, ACS Mater. Lett., 2020, 2, 951–974 CrossRef CAS.
- F. Ferlin, G. Brufani, G. Rossini and L. Vaccaro, Green Chem., 2023, 25, 7916–7933 RSC.
- J. Martínez, J. F. Cortés and R. Miranda, Processes, 2022, 10, 1274 CrossRef.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- US Food and Drug Administration, Q3C—Tables and List Guidance for Industry, Washington DC, 2017 Search PubMed.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- A. Punzi, D. I. Coppi, S. Matera, M. A. M. Capozzi, A. Operamolla, R. Ragni, F. Babudri and G. M. Farinola, Org. Lett., 2017, 19, 4754–4757 CrossRef CAS PubMed.
- F. D'Amico, C. Papucci, D. Franchi, G. Reginato, M. Calamante, L. Zani, A. Dessì and A. Mordini, ACS Sustainable Chem. Eng., 2022, 10, 3037–3047 CrossRef.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- S. Nejrotti, A. Antenucci, C. Pontremoli, L. Gontrani, N. Barbero, M. Carbone and M. Bonomo, ACS Omega, 2022, 7, 47449–47461 CrossRef CAS PubMed.
- A. H. Turner and J. D. Holbrey, Phys. Chem. Chem. Phys., 2019, 21, 21782–21789 RSC.
- M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- B. H. Lipshutz and S. Ghorai, Green Chem., 2014, 16, 3660–3679 RSC.
- E. Borrego, A. Caballero and P. J. Pérez, Organometallics, 2022, 41, 3084–3098 CrossRef CAS PubMed.
- G. La Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644–683 RSC.
- T. Nishikata, A. R. Abela and B. H. Lipshutz, Angew. Chem., Int. Ed., 2010, 49, 781–784 CrossRef CAS PubMed.
- Z. Xu, Y. Xu, H. Lu, T. Yang, X. Lin, L. Shao and F. Ren, Tetrahedron, 2015, 71, 2616–2621 CrossRef CAS.
- Z. Xu, T. Yang, X. Lin, J. D. Elliott and F. Ren, Tetrahedron Lett., 2015, 56, 475–477 CrossRef CAS.
- G. N. Vaidya, S. Fiske, H. Verma, S. K. Lokhande and D. Kumar, Green Chem., 2019, 21, 1448–1454 RSC.
- M. H. Daniels, J. R. Armand and K. L. Tan, Org. Lett., 2016, 18, 3310–3313 CrossRef CAS PubMed.
- A. M. Calascibetta, S. Mattiello, A. Sanzone, I. Facchinetti, M. Sassi and L. Beverina, Molecules, 2020, 35, 3717 CrossRef PubMed.
- Y. Wada, L. P. H. Van Beek, N. Wanders and M. F. P. Bierkens, Environ. Res. Lett., 2013, 8, 034036 CrossRef.
- F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC.
- Y. Zhu and M. O. Wolf, J. Am. Chem. Soc., 2000, 122, 10121–10125 CrossRef CAS.
- K. Tsuchiya, T. Sakakura and K. Ogino, Macromolecules, 2011, 44, 5200–5208 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 Search PubMed.
- S. C. Rasmussen, R. L. Schwiderski and M. E. Mulholland, Chem. Commun., 2011, 47, 11394–11410 RSC.
- S. C. Rasmussen, M. E. Mulholland, R. L. Schwiderski and C. A. Larsen, J. Heterocycl. Chem., 2012, 3, 479–493 CrossRef.
- R. L. Schwiderski and S. C. Rasmussen, J. Org. Chem., 2013, 78, 5453–5462 CrossRef CAS PubMed.
- R. L. Schwiderski and S. C. Rasmussen, Synth. Met., 2014, 193, 58–63 CrossRef CAS.
- C. Kitamura, S. Tanaka and Y. Yamashita, J. Chem. Soc., Chem. Commun., 1994, 1585 RSC.
- ICP-AES analyses of compounds 4–7 (see the ESI† for details) revealed a Pd content in the range 1–6 ppm, which is lower than the 10 ppm limit for drugs for oral administration (see the EMA guidelines) and is likely to comply even with the 1 ppm limit for parenteral administration, given that these values refer not to the pure active pharmaceutical ingredient but to the drug formulation.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc01139b |
| This journal is © The Royal Society of Chemistry 2024 |