Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalyst screening for dehydration of primary alcohols from renewable feedstocks under formation of alkenes at energy-saving mild reaction conditions†
Adil
Allahverdiyev
,
Jianing
Yang
and
Harald
Gröger
 *
*
Chair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, Universitätsstr. 25, 33615 Bielefeld, Germany. E-mail: harald.groeger@uni-bielefeld.de
First published on 21st March 2024
Abstract
Among current challenges for the chemical industry is the shift of the raw material basis from fossil feedstocks to renewable sources, which is also of relevance for the field of the industrial product class of alkenes with a chain length of C6 or more. A process concept based on CO2 and renewable energy is the conversion of 1-hexanol, being accessable from such renewable sources, to hexene sources. In this contribution, the dehydration of 1-hexanol catalyzed by Lewis acids such as metal triflates is presented. The prioritized catalysts have been also applied for the dehydration of C7–C12 primary alcohols. Hf(IV) and Ti(IV) triflates have shown the highest conversions in comparison to 13 other metal triflates, leading to high alkene yields of more than 70%. Furthermore, this study revealed a process running at energy-saving conditions and the so far lowest reaction temperatures for a chemocatalytic dehydration of primary alcohols being in the range of 140–180 °C only.
1 Introduction
The change of raw materials from fossil feedstocks to renewable raw materials is a key challenge for the chemical industry and of high urgency.1 Nearly 80% of the energy consumption are accounted by nonrenewable resources,2 yet these sources are destined to run out.3 Therefore, renewable resources such as cellulose from biomass or directly CO2 from air represent attractive feedstocks for the future.4 Today the major class of required industrial organic compounds in terms of mass are alkenes.5 While ethylene and propylene are the compounds with the largest production volumes and numerous applications for plastics, higher homologues such as hexene are of utmost importance as well and serve as indispensable intermediates for manufacturing a broad range of diverse specialty chemicals. A typical production route to hexenes is through crude oil refining processes. An alternative approach, which enables the use of renewable raw materials, is based on the dehydration of 1-hexanol. Since recently, 1-hexanol has been produced on technical scale by means of the Siemens–Evonik process starting from carbon dioxide and water by combining artificial photosynthesis with microbial fermentation.6 Otherwise, the formation of such hydrocarbons starting from syngas is typically done by, e.g., Fischer–Tropsch synthesis.7 Though, Fischer–Tropsch-synthesis is accompanied by challenges, such as high temperatures,8 low catalyst stability9 and low activity as well as selectivity of the catalyst.8,10However, while 1-hexanol as starting material originates from a “green” source and on the first glance this simple-looking reaction of 1-alkanol dehydration appears like “standard” textbook chemistry, surprisingly this reaction is far from being well studied. Typically, the reaction conditions are very harsh with extremely high temperatures being needed, which makes such processes very energy intensive, thus leading to an unfavored carbon footprint. For example, the dehydration of primary alcohols by boric acid requires the use of a stoichiometric amount of catalyst under rather harsh conditions (350 °C).11 In addition, isomerization of the alkenes takes place under these conditions. For example, if the reaction undergoes an E1 elimination, a carbocation is formed which can then yield an internal alkene through Wagner–Meerwein-rearrangement.12
Thus, in order to make use of the advantages of such a potentially environmentally friendly access to hexenes, dehydration conditions at much lower temperatures are needed. In fact, however, just a very few examples are known to proceed at lower temperature and energy-saving milder conditions.
Typically, Brønsted acid-based catalysts such as sulfuric acid or solid catalysts like zeolites,13 alumina14 or zirconia15 are used for the dehydration reaction. However, Brønsted acids are associated with limitations, such as low selectivity, low functional group tolerance and corrosiveness.16 Additionally, the use of Brønsted acids requires neutralization of the acid residues which in turn increases the waste.4
Preliminary work utilizing metal-type Lewis acid catalysts has been reported by Laali et al., who noted within their studies on dehydration of a range of alcohols with Cu(OTf)2 that also a primary alcohol can be dehydrated, which was exemplified by one experiment with 1-hexanol as substrate.17 In this experiment the resulting hexenes were obtained in a total yield of 38% by means of an in situ-removal of the alkenes via distillation, but an experimental protocol with the detailed temperature for dehydration of this primary alcohol substrate was not provided. Recently, within an intensive study of the dehydration of metal triflates on secondary alcohols reported by Repo et al., the authors also described two experiments on the dehydration of 1-octanol.18 These reactions run at a temperature of 180 °C. In the presence of the metal triflates Fe(OTf)3 and Hf(OTf)4 as catalysts, the resulting octenes were obtained in total yields of 2% and 65%, respectively. These initial attempts and proof-of-concepts17,18 illustrate the promising potential of metal triflates as catalysts for this purpose of primary alcohol dehydration although temperatures of at least 180 °C are needed. A further very recent study by Vorholt et al. demonstrated that reaction temperatures below 200 °C, can be achieved also with Brønsted acids.19 In detail, the Vorholt group used phosphoric acid in stoichiometric amount and succeeded in dehydrating 1-octanol at 190 °C mainly to the ether but also in lower amounts to the alkenes.
The high industrial interest in efficient dehydration strategies is also reflected by numerous patent applications filed in this field. Most of these patent applications focus on the use of heterogeneous catalysts as reusability of catalysts plays a major role and reactions are most of the time carried out in the gas phase, thus enabling high space–time yields.
The most common dehydration catalysts are those based on alumina. While Ziehe et al. described the dehydration of C4–C14 alcohols at a very high reaction temperature of 280–320 °C and with a feed of 2 mL min−1,20 a further patent application reported the dehydration and etherification of fatty alcohols such as NACOL® 16-99. At an, however, again very high reaction temperature of 260 °C and by means of 10 kg h−1 NACOL®, the etherification is mentioned to proceed with almost quantitative yields.21 In a more recent example from 2022, Koo et al. describes the use of barium oxide modified alumina supports for the dehydration of 1-octanol, which is conducted at a reaction temperature of 300–400 °C and resulted in yields of >60% and selectivities of >50%.22 The reported substrate range comprises C4–C20 alcohols.
The whole concept of our study towards a sustainable production of hexenes with minimized energy demand is based on the combination of an initial Siemens–Evonik process,6 which converts carbon dioxide with sunlight and by means of water-splitting through artificial photosynthesis into syngas, followed by microbial conversion to 1-hexanol and a subsequent metal triflate-catalyzed dehydration. The overall process concept is shown in Scheme 1. The work is part of the ongoing EU-Japan-funded research project being entitled 4AirCRAFT, aiming on the development of aviation fuels from renewable sources.23 Until 2040 at least 30% of total transportation fuels should be replaced by renewable fuel sources.24 In the work described in this contribution, we explored the catalyst potential of metal triflates for such a low temperature and energy-saving dehydration of primary alcohols with a focus on 1-hexanol. In particular our goal was to identify the lowest possible reaction temperature for still conducting the dehydration with reasonable reaction rate, thus minimizing energy demand of this endothermic reaction. We further aimed on getting insight into the reaction mechanism and a detailed catalyst-activity relationship.
 | ||
| Scheme 1 Overall process concept for producing hexenes from CO2, water and renewable energy such as sunlight and wind in a chemoenzymatic cascade with metal triflate dehydration as a key step. | ||
2 General process concept and reaction set-up
Since the dehydration of primary alcohols is thermodynamically unfavored, very harsh conditions are typically needed. To reduce the needed energy, the equilibrium is shifted towards the desired alkene products by distillative in situ-removal of these easily vaporizable products.In principle, starting from the primary alcohol, first either an ether or alkene is formed whereby the ether formation is favored since it is slightly exothermic.25,26 On the other hand, the dehydration towards the alkene is endothermic and unfavored.27,28 However, either the ether or alcohol can be converted under formation of the alkene. The activation energy for the C–O-cleavage of the ether under alkene formation is lower compared to the one for the alcohol, which results in the overall mechanism described in more detail below in chapter 7.29 Thus, in accordance with literature we hypothesize that at first, in a condensation reaction under release of water, the ether is formed, which is then subsequently cleaved to furnish the corresponding alkene and alcohol. In order to achieve this "formal" dehydration under moderate temperatures, the reactions were carried out under distillation and, thus, in situ-removal of the formed alkene fraction.
To avoid any distillation of the substrate 1-hexanol, the reactions were first carried out under reduced temperature at 150 °C for 1.5 h as the model substrate 1-hexanol has a boiling point of 157 °C. Afterwards, the oil bath was heated to 180 °C.
However, in situ-measurements showed that the reaction already takes place at 150 °C, which, to best of our knowledge, is the lowest reaction temperature ever reported for a chemocatalytic dehydration of a primary aliphatic alcohol. The reactions were carried out at a reaction time of 6–22 h. Typically, the end of the reaction was recognised by the fact that no further product was distilled. The real reaction set-up (B) and the schematically drawn one (A) are shown in Fig. 1.
3 Dehydration by Brønsted acids
In classic textbook chemistry, elimination of alcohols under formation of alkenes via dehydration typically is exemplified by catalysis with strong Brønsted acids. This approach has been studied intensively. However, very high temperatures are typically needed and mostly in literature reaction temperatures around or above 200 °C are mentioned when it comes to the dehydration of primary alcohols. In order to get a “benchmark” for our work using metal triflates as catalysts, we became interested to evaluate these typical Brønsted catalysts with respect to their potential to conduct this challenging transformation of converting 1-hexanol as a model substrate for a primary alcohol to hexene. Such a dehydration of 1-hexanol was investigated by means of various typical Brønsted acids, e.g., phosphoric acid and sulfuric acid. In order to shift the equilibrium to the product side, these endothermic reactions were performed, as described above in chapter 2, under in situ-removal of the hexene products by means of distillation. In a typical set-up the dehydration was conducted on a 40 mmol scale of 1-hexanol. To avoid distillation of the substrate, the reactions were first performed at 150 °C which was afterwards heated to 180 °C oil bath temperature.It is noteworthy that in case of phosphoric acid (pKa = 2.1) no conversion to either the ether or corresponding alkenes was observed. On the first glance this result appears to be in contrast to the dehydration study done by Vorholt et al. who obtained a formation of alkenes from primary alcohols with phosphoric acid as a catalyst.19 However, higher reaction temperatures (190–200 °C) as well as much higher catalyst loadings (1 eq.) were used in their work.19 Likewise, no conversion was achieved when acetic acid (pKa = 4.8) or trifluoracetic acid (pKa = 0.23) were used as further acidic catalysts.
In the presence of the much stronger acids tosylic acid (pKa = −2.8) or sulfuric acid (pKa = −3) at least the ether was formed and isolated in 96% and 87% yield, respectively (Scheme 2). This improved reactivity found when using TsOH or sulfuric acid can be rationalized by a higher pKa, which facilitates the elimination of water from 1-hexanol and, thus contributes to a better conversion. By means of 1H-NMR and 13C-NMR spectroscopy, however, only the formation of the linear ether di-n-hexyl ether was identified. In neither case, the alkene was formed.
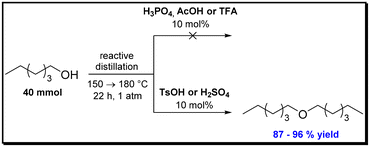 | ||
| Scheme 2 Attempts for dehydration of 1-hexanol by several Brønsted acids showing that only with TsOH and sulfuric acid ether formation was observed. In neither case, the alkenes were formed. | ||
4 Dehydration by Lewis acids
Since the dehydration of 1-hexanol by Brønsted acids only lead to the formation of the ether within the desired “lower temperature window” the dehydration under such temperature conditions was investigated with Lewis acids. The literature-known dehydration under similar conditions, which was reported by Laali et al. based on the use of Cu(OTf)2, served as a starting point for our study.17 However, under the originally described conditions no conversion of the primary alcohol 1-hexanol towards the alkenes nor to the ether was observed. Therefore, the dehydration process was then optimized by slowly increasing the temperature to a still relatively moderate temperature in order to enable formation of the alkenes. Since the boiling point of 1-hexanol is at 157 °C, the temperature was first set to 150 °C, followed by an increase to 180 °C to initiate the dehydration without distillation of the substrate. To our surprise, under such conditions now the desired alkene fraction was formed with an already high crude yield of 81% and an alkene purity of 90%, thus corresponding to an alkene yield of 73% (Scheme 3). | ||
| Scheme 3 Dehydration of 1-hexanol by Cu(OTf)2 at 150–180 °C oil bath temperature. Alkenes were formed whereas 2-hexene was the major product. In traces the ether was detected. | ||
When the reaction was repeated using a two-necked flask equipped with a thermometer (Fig. 2), a temperature of 150 °C was recorded when the distillation of the product occurred. Also in this case, a similar alkene yield was obtained. As far as these authors are aware, this is the lowest reaction temperature described so far for the dehydration of primary alcohols in the presence of a chemocatalyst.
 | ||
| Fig. 2 Set-up for the determination of the temperature inside the flask during the dehydration of 1-hexanol. | ||
In the next step, the catalyst loading was reduced to 2 mol% while the reaction time was prolonged to 22 h. However, under these conditions the alkene yield was reduced to 64%.
In order to investigate the influence of the anion component of the metal catalysts, the dehydration was carried out with CuCl2 (10 mol%). Conditions remained identical to the ones of the previous experiments. Interestingly, when CuCl2 was used, no alkene formation was observed, whereas the ether was formed in a low yield of 19%. As a side-product with a yield of 4% hexyl chloride was formed. Thus, these results suggest that the counter anion plays an essential role in the catalytic activity. The use of triflate anion results in an increased Lewis acidity of the metal, which leads to improved catalytic activity.
5 Computational design
Brønsted acid- vs. Lewis acid-catalyzed alcohol dehydration
We became interested to rationalize the interesting finding that even “classic” strong Brønsted acids do not lead to the formation of the desired hexenes when applying the envisaged moderate and energy-saving temperature range of 150–180 °C while in case of Cu(OTf)2 such a transformation proceeds efficiently. To identify the reason for these findings, we calculated the free Gibbs energies for the dehydration step as an indication about the catalytic suitability of Cu(OTf)2 for the desired elimination reaction compared to the one when utilizing strong Brønsted acids (exemplified for sulfuric acid).In detail, by means of density functional theory (DFT), we calculated the transition state for the dehydration of ethanol as a structurally very simple primary alcohol and compared the catalytic efficiency when using H2SO4 as Brønsted acid and Cu(OTf)2 as Lewis acid for β-elimination of 1-ethanol as a model reaction in the gas phase (Fig. 3).
It is noteworthy that we found a strong difference in the activation of ethanol when using the Brønsted acid H2SO4 and the Lewis acid Cu(OTf)2 as catalysts for this dehydration. The free Gibbs energy for the transition state in the direct dehydration with H2SO4 was calculated to be 43.14 kcal mol−1, thus being much less favourable than the one with Cu(OTf)2, which was 30.48 kcal mol−1. These calculations are in good agreement with the experimental observation that activation of 1-hexanol and the dehydration reaction proceeds under energy-saving conditions with a temperature range around 150–180 °C only in case of Cu(OTf)2 but not with H2SO4. Thus, as a next step, based on this result, various metal triflates were investigated to achieve the best-performing catalyst.
6 Catalyst screening for the dehydration of 1-hexanol
However, when using Cu(OTf)2 a decomposition of the catalyst was observed. Thus, in order to find more active metal triflates as catalysts, which additionally avoid catalyst decomposition, 14 further metal triflates were investigated. Since different reaction rates were obtained with these catalysts when an in situ-removal of the product was done, the reaction time was adjusted within a range of 6–22 h depending on the metal component. Only seven of the screened metal triflates, which are Hf(IV), Ti(IV), Al(III), Fe(III), Sc(III), Cu(II) and Ag(I), showed moderate to high alkene formation. For Sc(III) only a low percentage of alkene was formed. The main product was the ether.Interestingly, for all other triflates based on lanthanoid metals no conversion was observed, neither to the ether nor to the alkenes. For the other studied transition metal triflates, either the dehydration to the alkene or ether as main products was observed. The triflates with Hf(IV) and Ti(IV) gave the highest activities in terms of dehydration to the alkene. For Al(III) triflate, the alkenes were formed within a moderate reaction time of 12 h. For the triflates based on the non-coordinating and weak Lewis acidic alkali metals Na(I) and K(I), as expected, no conversion was observed. This indicates that the dehydration is strongly dependent on the coordination to a metal ion serving as a Lewis acid catalyst.
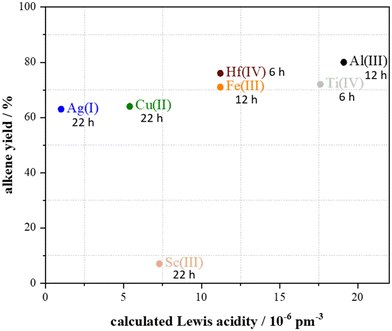
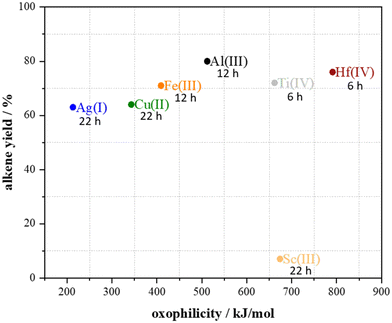
To find out the reasons for the observed differences in alkene yields when using the 15 metal triflates, the obtained yields were plotted against the Lewis acidity and oxophilicity of the metals (Fig. 4 and 5). In previous work of Repo et al., who investigated the dehydration of the secondary alcohol 2-octanol, for secondary alcohols a dependency on the oxophilicity was found while the Lewis acidity did not did not influence the reaction as strongly (Table 1).18
| Entry | Metal triflate | Time/h | Oxophilicity/kJ mol−1 | Lewis acidity/10−6 ppm | Main product | Alkene yield/% |
|---|---|---|---|---|---|---|
| 1 | Hf(IV) | 6 | 791 | 11.2 | Alkene | 76 |
| 2 | Ti(IV) | 6 | 662 | 17.6 | Alkene | 72 |
| 3 | Al(III) | 12 | 512 | 19.1 | Alkene | 80 |
| 4 | Fe(III) | 12 | 409 | 11.2 | Alkene | 71 |
| 5 | Sc(III) | 22 | 674 | 7.3 | Ether | 7 |
| 6 | La(III) | 22 | 799 | 2.7 | — | 0 |
| 7 | Dy(III) | 22 | 611 | 1.3 | — | 0 |
| 8 | Er(III) | 22 | 611 | 4.1 | — | 0 |
| 9 | Yb(III) | 22 | 380 | 4.3 | — | 0 |
| 10 | Y(III) | 22 | 715 | 4.6 | — | 0 |
| 11 | Cu(II) | 22 | 343 | 5.1 | Alkene | 64 |
| 12 | Mn(II) | 22 | 402 | 6.6 | — | 0 |
| 13 | Ag(I) | 22 | 213 | 1 | Alkene | 63 |
| 14 | Na(I) | 22 | 257 | 0.94 | — | 0 |
| 15 | K(I) | 22 | 239 | 0.38 | — | 0 |
Since only for seven out of the 15 investigated metal triflates formation of alkene was observed, the alkene yields of these catalysts in the dehydration of 1-hexanol were plotted against their Lewis acidity and oxophilicity (Fig. 4 and 5). Typical yields in the range of 60–80% were obtained but the reaction time strongly differs depending on the type of metal ion. Since the product needs to be removed from the reaction to shift the equilibrium to the product side by distillation, only the alkene yields were plotted. A plot of the conversion was not possible by means of the described synthetic method. Similar to the previously reported dehydration of secondary alcohols, no correlation between the Lewis acidity and alkene yield was observed (Fig. 4).18 However, for the dehydration of the primary alcohol 1-hexanol, there seems to be a correlation between the oxophilicity and alkene yield. Except for Sc(III) triflate, the higher the oxophilicity of the respective metal in the metal triflate is, the higher the yields are and the shorter the reaction time becomes (Fig. 4).
Since Hf(IV) and Ti(IV) showed the highest activities for the dehydration of 1-hexanol (taken into account the obtained yield and the needed reaction time), further experiments were conducted with these two catalysts to also dehydrate C7–C12 primary alcohols in order to gain insight into the substrate scope. In addition, Cu(OTf)2 was included as a further catalyst in this study since reasonable activities were obtained before and since this catalyst is also economically attractive. In this case, however, the catalyst loading of Cu(OTf)2 was increased to 10 mol% to have a similar reaction time compared to Hf(OTf)4 and Ti(OTf)4, which are more reactive and have been used with a catalyst loading of 2 mol%. The resulting results are summarized in Table 2.
| Entry | Substrate | C-number | Pressure | Alkene yield/% | ||
|---|---|---|---|---|---|---|
| Cu(II)a | Ti(IV)b | Hf(IV)c | ||||
| a 10 mol% Cu(OTf)2. b 2 mol% Hf(OTf)4. c 2 mol% Ti(OTf)4. d not determined. | ||||||
| 1 | 1-Hexanol | 6 | 1 atm | 73 | 72 | 75 |
| 2 | 1-Heptanol | 7 | 1 atm | 71 | 74 | 84 |
| 3 | 1-Octanol | 8 | 1 atm | 57 | n.d.d | 57 |
| 4 | 1-Octanol | 8 | 550–600 mbar | 74 | 62 | 69 |
| 5 | 1-Nonanol | 9 | 1 atm | 26 | n.d.d | 9 |
| 6 | 1-Nonanol | 9 | 350–400 mbar | 61 | 50 | 62 |
| 7 | 1-Decanol | 10 | 250–300 mbar | 64 | 52 | 70 |
| 8 | 1-Undecanol | 11 | 180–200 mbar | 54 | 28 | 44 |
| 9 | 1-Dodecanol | 12 | 100–120 mbar | 52 | 22 | 32 |
For the resulting C8–C12 alkene products, a reduced pressure was used for the in situ-product removal in order to avoid any decomposition at the boiling point of the produced alkenes at ambient pressure. For example, in the dehydration of 1-octanol under reduced pressure the alkene yield was increased from 57% to 69% when Hf(OTf)4 was used as a catalyst. A much higher difference in alkene yield has been observed in case of 1-nonanal with an increase of the yield from 9 at 1 atm to 62% under reduced pressure (350–400 mbar). For C6–C8 alkenes, comparable yields were found, and with Hf(OTf)4 as a catalyst the highest yields were observed. With increasing number of carbon atoms of the primary alcohol, the yield decreased drastically, which can be rationalized by either an increase of the activation energies, a more difficult in situ-product removal by distillation or a lowered solubility of the catalyst in the alcohol component.
7 Investigating the mechanism of the dehydration catalyzed by metal triflates
An experimental study with different hexanols, which underlines the need for a dramatically higher activation to dehydrate primary alcohols in comparison to secondary and tertiary alcohols is shown in Fig. 6 and ESI Table 6.† Accordingly, lower reaction temperatures as well as shorter reaction times can be applied in the latter cases compared to the dehydration of 1-hexanol. These findings are in accordance with the well-known elimination tendency of alcohols following the sequence tertiary > secondary > primary alcohols. In addition, dehydration of secondary and tertiary alcohols is in literature described as a direct elimination reaction with the formation of a more stable carbocation as an intermediate (E1) and therefore requiring less energy.31 | ||
| Fig. 6 Order of reactivity for the dehydration of primary, secondary and tertiary alcohols using Hf(OTf)4. | ||
However, still the question of the mechanism for the dehydration of primary alcohols, e.g., 1-hexanol as our substrate of choice, remains open. For primary alcohols, the E1-mechanism can be ruled out as the formation of primary carbocations is less likely,32 and, thus, other mechanisms have to be taken into consideration.
In general, two different mechanisms are presented in literature for the dehydration of alkanols under formation of alkenes, which in principle could also serve as an explanation for the studied dehydration of 1-hexanol. First, a direct dehydration of 1-hexanol could give the corresponding hexene with water as a by-product. Second, alternatively one 1-hexanol molecule is attacked by a second one, thus forming di-n-hexyl ether under removal of water, and subsequently then the ether is cleaved under formation of the desired hexene and the substrate molecule 1-hexanol. These two proposed mechanisms are shown in Scheme 4. To investigate which of the two mechanisms is responsible for the dehydration of primary alcohols catalyzed by these metal triflates at temperatures of 150–180 °C, various experiments were done. Since in the dehydration of alcohols either directly the alkenes or at first the ether can be formed it was investigated if the ether can be directly dehydrated by the metal triflates towards the alkenes. In addition, we studied if another intermediate, namely 1-hexyltriflate is formed and, under these conditions, can undergo elimination to the alkenes. In order to evaluate if di-n-hexyl ether could serve as an intermediate, this compound was treated under identical conditions as in the dehydration of 1-hexanol. Since this ether represents a dimer of the alcohol, double as much of the amount of the catalyst was used to have comparable catalyst loading related to the amount of hexenes, which could be formed. In the publication of Repo et al. the dehydration of the ether was already described but it was postulated that 1 eq. of water was needed to initiate the reaction.18 Therefore, we also investigated the impact of water.
In our experiments we found that in each case, with Cu(OTf)2 as well as Hf(OTf)4, the ether was cleaved to the alkenes, respectively. Furthermore, similar yields as for the dehydration of 1-hexanol were obtained when di-n-hexyl ether was used as a substrate. In our study, water (0.5 eq.) as an additive did not influence the reaction much, since in both cases the alkene was formed, and quite similar yields were obtained.
In addition we synthesized 1-hexyltriflate following the reaction procedure of Cibulka et al.33 to investigate if 1-hexyl triflate is an intermediate in the dehydration of 1-hexanol. In detail, 1-hexyltriflate was synthesized by using 1-hexanol with triflic anhydride and pyridine as a base in 88% yield. In the next step, the elimination to the alkenes starting from 1-hexyl triflate was tested under various conditions: (a) without any catalyst, (b) with Cu(OTf)2 as a catalyst and (c) using EtOH as a solvent. In each case, however, the alkenes were not formed. In case without the catalyst and Cu(OTf)2 only decompositions products were found while in case of EtOH as a solvent the non-symmetric ether, namely ethyl hexyl ether, was formed, which is a plausible product since the triflate group is an excellent leaving group. Yet, the alkenes were not formed also in this case. Thus, it can be concluded that 1-hexyl triflate is not an intermediate in the dehydration reaction.
Since 1-hexyl triflate was ruled out as a possible intermediate the dehydration of 1-hexanol is expected to follow one of the two pathways shown in Scheme 4: (a) direct dehydration or (b) indirect dehydration. In (a) 1-hexanol is dehydrated to the alkenes without the formation of an intermediate while in (b) first the ether is formed, which in a second step is converted to the corresponding alkenes (Scheme 4). When carrying out experiments at reaction temperatures below 150 °C, we observed that even with the catalytically very active metal triflates no hexenes are formed from 1-hexanol, but di-n-hexyl ether. In addition, ether formation is known to be thermodynamically favored and, thus, exothermic according to literature while the dehydration is an endothermic step.25–29 These findings support the mechanism according to hypothesis (b). However, it would have to be shown that the ether can be cleaved under the applied temperature range of 150–180 °C. Thus, we conducted studies using di-n-hexyl ether as a substrate in the presence of Cu(OTf)2 and Hf(OTf)4 as catalysts to proof that the ether could be cleaved under such conditions, thus leading to the hexene products. For comparison, we also conducted this study in parallel using 1-hexanol as a substrate. The results, which are shown in Table 3, show that also when using the ether as a substrate, the desired hexene products are formed in yields being comparable to the ones obtained when using 1-hexanol as a substrate. In contrast to the often proposed direct dehydration according to Scheme 4, route (a),18,22 our findings suggest that the reaction mechanism proceeds through a two-step process, which consists of an initial, exothermic formation of the di-n-hexyl ether from 1-hexanol, followed by a decomposition of di-n-hexyl ether under formation of the hexene products (Scheme 4, route (b)). Thus, even if the overall process represents a “formal” dehydration, according to such a reaction mechanism no direct elimination of water, as described in many articles and textbook chemistry, occurs. Accordingly, not the primary alcohol serves as the “real” substrate in the elimination key step, which is furnishing the alkene product, but the in situ-formed ether, which was described in previous work18 as a side-product. Our proposed mechanism for the formation of 1-hexene is also supported by various literature reports. Based on the findings of, e.g., Busca et al.34a and Yu et al.34b it can be expected that route (b) is the preferred route at the applied reaction temperature of 150–180 °C, as it was shown that at milder temperatures route (b) is preferred, while at higher temperatures route (a) is favored. In contrast, high temperature dehydration methods, e.g., the one reported by Koo et al.22 running at 300–400 °C, are more likely to proceed via route (a).
| Entry | Catalyst | Catalyst loading/mol% | Substrate | Substrate loading/mmol | Alkene yield/% |
|---|---|---|---|---|---|
| a 0.5 eq. of water was used to enhance the reaction. | |||||
| 1 | Cu(II) | 10 | 1-Hexanol | 40 | 73 |
| 2 | 20 | Di-n-hexyl ether | 22 | 68 | |
| 3 | 20 | Di-n-hexyl ether | 22 | 71a | |
| 4 | Hf(IV) | 2 | 1-Hexanol | 40 | 75 |
| 5 | 4 | Di-n-hexyl ether | 22 | 66 | |
| 6 | 4 | Di-n-hexyl ether | 22 | 67a | |
8 Reusability of Hf(OTf)4
Taking into account the price differences of (at least most) catalysts and commodity chemicals, in general recycling and reusability of the catalyst is crucial for an industrial application in this field. Accordingly, also in our work on the metal triflate-catalyzed dehydration process we became interested in the recyclability of the catalyst. An elegant method was reported by Cullen et al. already in 2009.35 By simple aqueous extraction and subsequent removal of water under reduced pressure and elevated temperatures, the catalyst was recycled while still maintaining its activity for three cycles.35However, as the product is continuously removed in our case, we envisioned that the extraction step can be fully avoided by ensuring reusability simply by adding fresh substrate to the reactor, which is at the same time the distillation flask. As Hf(OTf)4 showed the highest yields, this catalyst was chosen for our reusability study. Reaction conditions were identical to previous reaction where only one cycle was performed. In the recycling study, then additional substrate was added after completion of the previous reaction cycle in order to start the next reaction cycle (Scheme 5).
We were pleased to find that high alkene yields of in average 79% were obtained in each cycle (Fig. 7). The slight increase in some of the cycles can be rationalized by distillation of some of the product from the previous cycle, which remained in the distillation flask.
 | ||
| Fig. 7 Reusability of Hf(OTf)4 (2 mol%) for the dehydration of 1-hexanol. In red the crude yield is shown while in green the alkene yield. | ||
By means of this in situ-product removal by distillation as the only work-up step, an effective and efficient way to reuse the catalyst without the need of further unit operation steps was found. Thus, this process concepts also fully avoids the use of solvents for extraction and, thus, effectively contributes to minimizing waste production.
Besides the distillation fraction containing the desired hexene products, the crude mixture of the reaction flask was also analyzed as in average 85% crude yield is obtained and therefore 15% remains in the flask. Interestingly, neither 1-hexanol nor di-n-hexyl ether were found in the remaining crude mixture. Only hydrocarbons were found according to NMR and GC measurements. Main products included hexene and its oligomers whereby the C12 dimers were the main components. The accumulation of such C12 dimers can be rationalized by their high boiling point and represents an interesting finding because such C12 compounds are also of interest in terms of utilization as jet-fuels or intermediates thereof.
9 Sustainability metrics
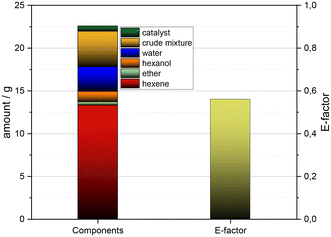
After having been able to demonstrate the recyclability of the catalyst, which is a key criteria for an economic process, next we became interested in an initial evaluation of this process in terms of sustainability metrics data. Ahead of the results from such calculations it should be added that the complete avoidance of any solvent utilization and the straightforward work-up by simple distillation of the product during the reaction as an in situ-product removal represent advantages also in terms of sustainability.Conducting the process under neat conditions and the avoidance of any solvent utilization, neither at the stage of the reaction nor at the stage of the work-up, substantially contributes to the very attractive E-factor, which has been calculated to be 0.57, thus being in a very low range (Fig. 8). It should be added that this E-factor calculation is based on the recycling experiment with five reaction cycles under recycling of the catalyst Hf(OTf)4. Besides the E-factor, also the PMI turned out to be in a low range and in detail, a PMI-value of 1.51 was calculated.
Besides such quantitative sustainability metrics data, three further facts should be added which makes this process attractive from the perspective of sustainability: first, the dehydration of 1-hexanol only gives water as an environmentally friendly by-product. Second, even the remaining 15% of mass, which is related to non-hexene fractions and consists of a large fraction of C12 dimers, are of potential interest for industrial applications (Fig. 8). Third, the starting material is obtained in a process being highly attractive also from the perspective of sustainability. In detail, the starting material 1-hexanol has been recently reported to be produced from CO2 and water within the Siemens–Evonik-process that combines an artificial photosynthesis using solar energy with a microbial fermentation step.6 Thus, the starting material is accessible from renewable carbon sources as well as green energy and consequently avoids the utilization of fossil feedstocks.
10. Computational details
All computations were completed by utilizing ORCA 5.0.3 software.36 The geometries of all minima and transition states were optimized and characterized at the PBE0 D3BJ def2-TZVP def2/J level of theory in the gas phase.37,38 This method has showed good results for thermochemical approach and is suggested as an efficient tool for the DFT-calculations.37–39 All reported free energies were performed with entropy corrections at 298.15 K and 1 atm. The transition states were obtained by full geometry optimization, verified by vibrational frequency (imaginary mode), and subsequently validated with Intrinsic Reaction Coordinate (IRC) calculations. The alkyl chain of alcohol was simplified in the calculations. Avogardo40 and CYLview41 as software were applied for the visualization of 3D-optimized structures.11 Conclusion
This study describes the use of specific metal triflates as catalysts for the dehydration of primary alcohols, exemplified for 1-hexanol, under formation of the hexene products. This route turned out as a highly efficient way to dehydrate primary alcohols under relatively mild conditions with a reaction temperature of 150–180 °C in case of 1-hexanol. Within the catalyst screening, 15 different metal triflates were screened for the dehydration of 1-hexanol, and among them seven metal triflates have yielded the alkenes. Ti(IV) and Hf(IV) triflates have shown the best properties with respect to high alkene yields, which exceeded 70%. By plotting the Lewis acidity and oxophilicity against the alkene yield it was shown the Lewis acidity is not influencing the reaction while the oxophilicity did. Furthermore, the substrate scope was investigated with the prioritized Cu(OTf)2, Ti(OTf)4 and Hf(OTf)4 catalysts for the dehydration of C6–C12 primary alcohols, whereby a yield of 69–84% was obtained for C6–C8. In case of longer carbon chains, however, the alkene yield decreased with prolonged chain length. In addition, the reaction mechanism was studied, and experimental data indicates that the dehydration proceeds through a two-step process with an initial formation of di-n-hexyl ether and subsequent cleavage under formation of hexene products. Due to the superior catalytic activity of Hf(OTf)4, this catalyst was chosen for reusability studies where at least five cycles were performed with no loss of activity. Lastly, several sustainability metrics were calculated whereby an E-factor as small as 0.56 was obtained for Hf(OTf)4. Comparable to previous studies, which run at very high reaction temperatures in the range of 260–400 °C in the gas phase and in the presence of fixed-bed reactors,20–22 in our process the dehydration is performed in liquid phase with a homogeneous catalyst at a dramatically decreased reaction temperature of 150–180 °C in combination with an in situ-product removal through distillation.Current research activities are focusing on process intensification and scale-up of this process technology. A further task of future work will center on the design of further improved catalysts. Among various conceivable concepts, synergistic effects of combined Brønsted and Lewis acids properties in such catalysts will be explored (as suggested by one of the reviewers of this article).
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge financial support from the European Union (EU) within the Horizon 2020 research and innovation programme for the funded project “4AirCRAFT” (grant agreement 101022633). We gratefully acknowledge the computing time provided to them on the high-performance computers [noctua 1] at the NHR Center PC2, which are funded by the Federal Ministry of Education and Research of Germany and the state governments participating on the basis of the resolutions of the GWK for the national high-performance computing at universities (https://www.nhr-verein.de/unsere-partner). The authors are grateful for having received the suggestion for the development of further catalysts by combining Brønsted and Lewis acids functionalities in synergistic manner, which was made by one of the reviewers of this article and will be subject of future work. The authors also thank Tibor Schumski and James Kamtsikis for technical assistance.References
- T. Marzi, V. Knappertsbusch, A. Grevé, G. Deerberg, C. Doetsch and E. Weidner, Chem. Ing. Tech., 2018, 90, 1374–1383 CrossRef CAS.
- (a) D. X. Martínez-Vargas, L. Sandoval-Rangel, O. Campuzano-Calderon, M. Romero-Flores, F. J. Lozano, K. D. P. Nigam, A. Mendoza and A. Montesinos-Castellanos, Ind. Eng. Chem. Res., 2019, 58, 15872–15901 CrossRef; (b) S. H. Mohr, J. Wang, G. Ellem, J. Ward and D. Giurco, Fuel, 2015, 141, 120–135 CrossRef CAS.
- G. Nicoletti, N. Arcuri, G. Nicoletti and R. Bruno, Energy Convers. Manage., 2015, 89, 205–213 CrossRef.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS.
- H.-J. A. K. Weissermel, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 3rd edn., 2008 Search PubMed.
- T. Haas, R. Krause, R. Weber, M. Demler and G. Schmid, Nat. Catal., 2018, 1, 32–39 CrossRef CAS.
- (a) B. Kamm, Angew. Chem., Int. Ed., 2007, 46, 5056–5058 CrossRef CAS PubMed; (b) E. W. Kuipers, I. H. Vinkenburg and H. Oosterbeek, J. Catal., 1995, 152, 137–146 CrossRef CAS.
- Q. Zhang, J. Kang and Y. Wang, ChemCatChem, 2010, 2, 1030–1058 CrossRef CAS.
- E. de Smit and B. M. Weckhuysen, Chem. Soc. Rev., 2008, 37, 2758–2781 RSC.
- Y. Chen, J. Wei, M. S. Duyar, V. V. Ordomsky, A. Y. Khodakov and J. Liu, Chem. Soc. Rev., 2021, 50, 2337–2366 RSC.
- W. Brandenberg and A. Galat, J. Am. Chem. Soc., 1950, 72, 3275–3276 CrossRef CAS.
- S. Moulay, Chem. Educ. Res. Pract., 2002, 3, 33–64 RSC.
- (a) W. Moser, J. Catal., 1989, 117, 19–32 CrossRef CAS; (b) H. Xin, X. Li, Y. Fang, X. Yi, W. Hu, Y. Chu, F. Zhang, A. Zheng, H. Zhang and X. Li, J. Catal., 2014, 312, 204–215 CrossRef CAS.
- (a) H. Pines and J. Manassen, in The Mechanism of Dehydration of Alcohols over Alumina Catalysts, ed. H. Pines and J. Manassen, Elsevier, 1966, vol. 16, pp. 49–93 Search PubMed; (b) H. Knozinger, J. Catal., 1972, 24, 57–68 CrossRef; (c) J. Lee, E. J. Jang and J. H. Kwak, J. Catal., 2017, 345, 135–148 CrossRef CAS.
- G. Larsen, E. Lotero, L. M. Petkovic and D. S. Shobe, J. Catal., 1997, 169, 67–75 CrossRef CAS.
- T. J. Korstanje, E. F. de Waard, J. T. B. H. Jastrzebski and R. J. M. Klein Gebbink, ACS Catal., 2012, 2, 2173–2181 CrossRef CAS.
- K. Laali, R. J. Gerzina, C. M. Flajnik, C. M. Geric and A. M. Dombroski, Helv. Chim. Acta, 1987, 70, 607–611 CrossRef CAS.
- J. Keskiväli, A. Parviainen, K. Lagerblom and T. Repo, RSC Adv., 2018, 8, 15111–15118 RSC.
- J. T. Vossen, A. J. Vorholt and W. Leitner, ACS Sustainable Chem. Eng., 2022, 10, 5922–5931 CrossRef CAS.
- H. Ziehe, J. Schimanski, A. Brasch and E.-O. Tönsen, WO2004078336A2, 2004.
- H. Ziehe, T. Gross and A. Weitze, DE102008005721C5, 2009.
- K. Y. Koo, U. Jung, Y. Kim, H. B. Im, D. Chun, M. H. Youn, H. Jeong, G. B. Rhim, J. Park and D. Lee, US20220032272A1, 2022.
- 4AirCRAFT, https://4aircraft-project.eu, access: 2023.
- R. W. R. Zwart, H. Boerrigter and A. van der Drift, Energy Fuels, 2006, 20, 2192–2197 CrossRef CAS.
- D. M. Newitt and G. Semerano, Proc. R. Soc. London, Ser. A, 1936, 157, 348–358 CAS.
- Dimethyl Ether, ed. A. C. Dimian, C. S. Bildea and A. A. Kiss, Elsevier, 2019 Search PubMed.
- T. L. Lohr, Z. Li and T. J. Marks, Acc. Chem. Res., 2016, 49, 824–834 CrossRef CAS PubMed.
- C. K. Narula, Z. Li, E. M. Casbeer, R. A. Geiger, M. Moses-Debusk, M. Keller, M. V. Buchanan and B. H. Davison, Sci. Rep., 2015, 5, 16039 CrossRef CAS PubMed.
- Z. Li, R. S. Assary, A. C. Atesin, L. A. Curtiss and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 104–107 CrossRef CAS PubMed.
- N. A. Lange, Lange's Handbook of Chemistry, McGraw-Hill, New York, 15th edn., 1999 Search PubMed.
- C. Bockisch, E. D. Lorance, H. E. Hartnett, E. L. Shock and I. R. Gould, ACS Earth Space Chem., 2018, 2, 821–832 CrossRef CAS.
- T. Hansen, P. Vermeeren, F. M. Bickelhaupt and T. A. Hamlin, Chem. Commun., 2022, 58, 12050–12053 RSC.
- T. Hartman, J. Šturala and R. Cibulka, Adv. Synth. Catal., 2015, 357, 3573–3586 CrossRef CAS.
- (a) T. K. Phung and G. Busca, Chem. Eng. J., 2015, 272, 92–101 CrossRef CAS; (b) M. Zhang and Y. Yu, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 CrossRef CAS.
- D. B. G. Williams and A. Cullen, J. Org. Chem., 2009, 74, 9509–9512 CrossRef CAS PubMed.
- (a) S. Grimme, J. G. Brandenburg, C. Bannwarth and A. Hansen, J. Chem. Phys., 2015, 143, 54107 CrossRef PubMed; (b) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS; (c) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed; (d) F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- (a) D. Coskun, S. V. Jerome and R. A. Friesner, J. Chem. Theory Comput., 2016, 12, 1121–1128 CrossRef CAS PubMed; (b) J. M. Del Campo, J. L. Gázquez, S. B. Trickey and A. Vela, J. Chem. Phys., 2012, 136, 104108 CrossRef PubMed.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 17 CAS.
- C.-Y. Legault, CYLview, 1.0b, Université de Sherbrooke, 2009, https://www.cylview.org Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc01038h |
| This journal is © The Royal Society of Chemistry 2024 |