Aliphatic amines from waste polyolefins by tandem pyrolysis, hydroformylation, and reductive amination†
Houqian
Li
 a,
Amy A.
Cuthbertson
a,
Amy A.
Cuthbertson
 b,
Ahmad Amer
Alamer
a,
Victor S.
Cecon
b,
Ahmad Amer
Alamer
a,
Victor S.
Cecon
 c,
Harish
Radhakrishnan
d,
Jiayang
Wu
a,
Greg W.
Curtzwiler
c,
Harish
Radhakrishnan
d,
Jiayang
Wu
a,
Greg W.
Curtzwiler
 c,
Keith L.
Vorst
c,
Xianglan
Bai
c,
Keith L.
Vorst
c,
Xianglan
Bai
 d,
Clark R.
Landis
e,
Gregg T.
Beckham
d,
Clark R.
Landis
e,
Gregg T.
Beckham
 b and
George W.
Huber
b and
George W.
Huber
 *a
*a
aDepartment of Chemical and Biological Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA. E-mail: gwhuber@wisc.edu
bRenewable Resources and Enabling Sciences Center, National Renewable Energy Laboratory, Golden, CO 80401, USA
cPolymer and Food Protection Consortium, Department of Food Science and Human Nutrition, Iowa State University, Ames, IA 50011, USA
dDepartment of Mechanical Engineering, Iowa State University, Ames, IA 50011, USA
eDepartment of Chemistry, University of Wisconsin-Madison, Madison, WI 53706, USA
First published on 16th April 2024
Abstract
Pyrolysis of waste plastics can produce a product mixture with a high concentration of olefins (>50 wt%). The olefins, as building blocks in the petroleum industry, are potential precursors for valuable commodity chemicals with higher values (>$2000 per ton). In this work, we produce aldehydes by hydroformylation of the olefins present in pyrolysis oil from colored post-consumer recycled high-density polyethylene (PCR-HDPE). The obtained aldehydes in the oil are then converted into aliphatic amines via reductive amination with a Ru/C catalyst. The aminated oil was characterized by multiple analytical chemistry techniques including elemental analysis, nuclear magnetic resonance spectroscopy, high-resolution liquid chromatography-mass spectrometry, and gas chromatography-mass spectrometry with a Polyarc flame ionization detector. The concentration of metals in the PCR-HDPE and oil changes during the tandem processes, showing limited effects of these elements (e.g., Al, Ca, Fe, Mg, Ti, Zn) on hydroformylation and reductive amination. Additionally, we demonstrated that reductive amination of aldehydes with varied carbon numbers and branching properties can be achieved in the presence of a complex mixture, including paraffins and aromatics. The results indicate that waste plastics have the potential to serve as a renewable source for mono and di aliphatic amines, thereby diminishing reliance on fossil feedstocks as the current primary amine source.
Introduction
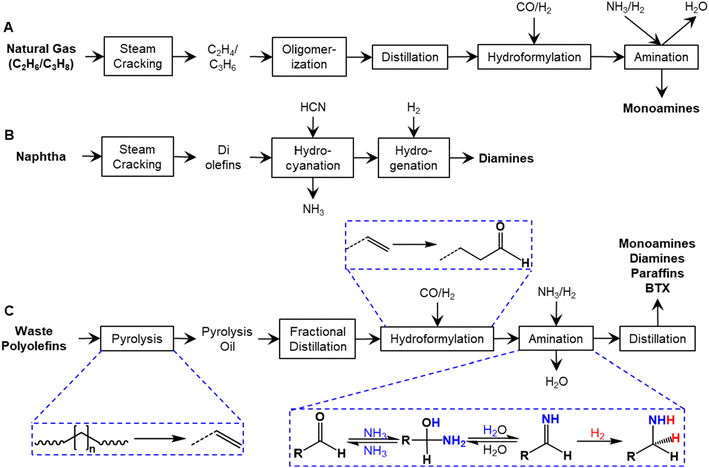
Plastic waste can be used as a feedstock to produce a range of chemicals, and at present multiple companies are building pyrolysis plants to produce a waste plastics-derived pyrolysis oil on a scale ranging from 5–120 kilotons per year.1–3 With polyolefin-rich feedstocks, waste plastics-derived pyrolysis oils are typically a mixture of paraffins, aromatics, and olefins, ranging in composition from C5 to C25+ compounds depending on the pyrolysis conditions.4,5 The most abundant hydrocarbons are olefins and diolefins with concentrations that can be up to 60 wt%.5 These olefins are typically a mixture of long-chain (>C5) olefins, which require further processing. Steam cracking or catalytic conversion of the pyrolysis oil generates ethylene, propylene, and aromatic species that could be integrated into the petroleum industry.6–8 We have recently reported that hydroformylation can be utilized to convert olefins in pyrolysis oil to aldehydes, which can further be hydrogenated into alcohols.5 The aldehydes, in principle, could also be converted to amines via reductive amination.Aliphatic amines are used as solvents, pesticides, feed additives, rubber processing chemicals, and water treatment chemicals, with solvents and agrochemicals comprising approximately 50% of the market.9 In 2021, the prices of these aliphatic amines exceeded $2000 per metric ton, with diamines being approximately 1.5 times more expensive than monoamines. Monoamines (e.g., n-butylamines, C12–C16 primary amines) are mainly produced from natural gas by five separate reactions: steam cracking, oligomerization, distillation, hydroformylation, and amination as shown in Fig. 1A.10 Diamines (e.g., 1,6-hexanediamine) are typically produced from naphtha by steam cracking, hydrocyanation (which requires hydrogen cyanide, HCN), and hydrogenation (Fig. 1B).11
As an alternative pathway to manufacture amines and diamines, here we propose a route to generate both of these chemical classes via tandem hydroformylation and reductive amination of the olefins in plastic pyrolysis oil, as illustrated in Fig. 1C, with ammonia as the nitrogen source. Aliphatic amines are targeted products, as only aliphatic aldehydes are produced from plastic oil hydroformylation. We have also selected reaction conditions and a catalyst that favor the formation of aliphatic amines. Additionally, it has recently been reported that aromatic amines can be produced from waste plastics.12 Reductive amination reactions are typically performed at temperatures ranging from 80 °C to 120 °C with pressurized H2 (40–70 bar) in the presence of ammonia.13–15 Imine is initially formed and then reduced to amine, resulting in water as a byproduct (Fig. 1C).14 Reductive amination is catalyzed by a range of metal catalysts on metal oxides or carbon supports.14,15 For example, Lercher and coworkers reported that primary amines are the main products from butyraldehyde over ruthenium and rhodium catalysts while secondary amines are produced with palladium and platinum catalysts.16 Hydroformylated pyrolysis oil consists of paraffins, aromatics, aldehydes, dialdehydes, and some residual Co catalyst, and other impurities from plastic waste (e.g., Ca, Fe, Si, Pb, Mg, Al).17 Considering the complexity of the oil, it is unknown whether the same reductive amination catalytic system will work for the hydroformylated pyrolysis oil. It is also challenging to analyze the complex product composition.
This work demonstrates the catalytic chemistry for the production of primary amines and diamines from post-consumer recycled high-density polyethylene (PCR-HDPE). We used both model compounds (n-octanal and octanedial) and plastic-derived pyrolysis oil to find appropriate reaction conditions and catalysts for the reductive amination of hydroformylated pyrolysis oil. The aminated oil was rigorously analyzed using a combination of CHN(O)S elemental analysis, inductively coupled plasma mass spectrometry (ICP-MS), 13C-nuclear magnetic resonance (NMR) spectroscopy, high-resolution liquid chromatography-mass spectrometry (HRLC-MS), and gas chromatography-mass spectrometry with a Polyarc flame ionization detection (GC-MS/Polyarc-FID). The majority of the amines produced from the PCR-HDPE are C5–C18 monoamines, where near-quantitative reductive amination was achieved using a batch reactor with an ammonia/methanol solution and 5 wt% Ru/C under H2. Metals in the PCR-HDPE, pyrolysis oil, and upgraded oil vary after each step, although significant effects of these metals on hydroformylation and reductive amination were not observed.
Experimental and materials
Ammonia methanol solution (499145, 7 N in methanol), Co(NO3)2·6H2O (239267), 5 wt% Pd/C (205680), 5 wt% Pt/C (205931), 5 wt% Ru/C (206180), 5 wt% Rh/C (643149), n-octanal (8.06901), and 1,5-hexadiene (128554) were purchased from Sigma Aldrich and used as purchased. The 5 wt% metal catalysts were separately reduced under H2 flow (70 mL min−1, 99.99%, Air Gas) at 400 °C (ramping rate: 1 °C min−1) for 3 h in a home-built three-neck glass fluidized bed reactor. The catalysts were then stored in a glove box after reduction to avoid oxidation. Pyrolysis of the PCR-HDPE and the hydroformylation of the olefins in the obtained pyrolysis oil were described in our previous work.5 Note that the PCR-HDPE (MW: 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920, MN: 27
920, MN: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292) used in this work was obtained from an operational material recovery facility.
292) used in this work was obtained from an operational material recovery facility.
Catalytic reductive amination was performed in a 75 mL stainless steel Parr batch reactor equipped with a Teflon high-pressure liner (Parr, 2920HC2HA liner, 75 mL 5050 PTFE). The catalyst was loaded to the batch reactor in a glove box, sealed, transferred to a Parr 5000 Multi Reactor Heater and Stirrer System, and purged three times with H2. Into the reactor, 0.45 g of n-octanal and 6.5 g of ammonia methanol solution were then injected separately at ambient pressure by a syringe. The dialdehyde was obtained from hydroformylation of 1,5-hexadiene.5 The amination of the oil generated from waste plastics was performed with the same experimental procedure. The hydroformylated pyrolysis oil was injected first followed by the ammonia methanol solution. The reactor was purged another two times with H2 and heated up to 90 °C under continuous stirring (800 rpm). The system was then pressurized by H2 (40 bar) to initiate the reaction. In a typical experiment, 10.00 g of ammonia methanol solution, 1.42 g of hydroformylated pyrolysis oil, and 0.22 g of 5 wt% Ru/C were used.
After the reaction is complete, the reactor was cooled in ice water for quenching, followed by centrifuging the mixture to separate the solid catalyst from the liquid solution. Methanol was removed via rotary evaporation. The liquid solution (∼30 mL) was placed in a 100 mL pear-shaped vessel connected to a Buchi rotary evaporator which was operated at 35 °C and 350 mbar vacuum for 8 hours. The oil obtained after rotary evaporation is referred to as aminated oil.
Elemental composition in the waste plastics derived oils were determined using a Vario MICRO cube (Elementar, Hanau, Germany) elemental analyzer with acetanilide as a standard reference material. Carbon, hydrogen, nitrogen and sulfur contents were measured and oxygen content was calculated by the mass difference. The tests were triplicated and the average values were reported.
ICP-MS was performed using an Agilent 8900 ICP-QQQ to analyze the minerals in the oil before and after each step (i.e., pyrolysis, hydroformylation, and amination) with a previously established method.18 Karl Fischer (KF) titration was performed with a Mettler Toledo V20 Volumetric KF Titrator to measure the amount of water in the final product. In a typical experiment, 1 g of aminated oil was used for KF titration. The density of the aminated oil was measured by weighing 5 mL of aminated oil using a volumetric flask.
All NMR experiments were conducted on a Bruker Avance-500 with a DCH cryoprobe at University of Wisconsin-Madison Chemistry NMR Facility. The sample was dispersed in CDCl3 (sample/CDCl3 = 1/7 mass ratio), sonicated, and transferred to an NMR sample tube. The parameters for NMR experiments were:
(1) 1H NMR (zg30): 32 scans, delay of 1 s, 90-degree pulse width of 9.6 μs
(2) 13C NMR (zgig): 80 scans, delay of 20 s, 90-degree pulse width of 10.35 μs
(3) HSQC (hsqcedetgp): the acquire size was [1024, 400] with 2 scans per increment, delay of 2 s.
For analysis by HRLC-MS, samples were diluted 1000× into methanol prior to injection. The analysis was performed on a Waters Acquity liquid chromatography system (UPLC H-class, Waters Corporation) using a Phenomenex Kinetex 1.7 μm EVO C18 100 mm × 2.1 mm column (Phenomenex) at a constant flow rate of 0.5 mL min−1, an injection volume of 10 μL, and column oven temperature of 25 °C. The mobile phase solvents consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. A gradient program was used to separate the analytes of interest: (A) = 99% and (B) = 1% at time t = 0; (A) = 5% and (B) = 95% at t = 35 min; (A) = 99% and (B) = 1% at t = 35.01 min; (A) = 99% and (B) = 1% at t = 40 min.
Compounds eluting from the column were ionized by electrospray positive-ion mode ionization using 3 kV spray voltage on a Waters Synapt G2-Si mass spectrometer (Waters Corporation). The desolvation temperature was set to 450 °C, while the source temperature was set to 140 °C. Ions were scanned between a m/z range of 50–1200 in resolution mode and data were collected in profile. Nitrogen was used for the desolvation gas, sweep gas, and nebulizer gas, and set at 1000 L h−1. Mass calibration was performed prior to each data set, and additionally as needed if the LockMass drifted more than ±30 ppm mass accuracy. The LockMass reference mass calibration solution consisted of leucine-enkephalin at 100 ng mL−1 in water/acetonitrile 75/20 (v/v) with 0.1% formic acid and was infused at 5 μL min−1 with data collected at intervals of 0.5 min.
Data processing was conducted using Progenesis QI (QIP) (Waters Corporation), with parameters set for peak picking at instrumental responses >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and retention times were ignored before 1.4 minutes and after 36.0 min. Elemental composition assignment parameters included only C (0–100), H (0–200), N (0–3), O (0–3). Precursor tolerance was set to 20 ppm, and isotope similarity >75%. If mass accuracy exceeded 5 ppm in QIP, the mass peak was manually centered and recalculated for mass accuracy. The parameters used to center data included the minimum peak width at half height with 3 channels and 80% centroid top. Assignment preference was given for the lowest mass error and [M + H] ions.
000 and retention times were ignored before 1.4 minutes and after 36.0 min. Elemental composition assignment parameters included only C (0–100), H (0–200), N (0–3), O (0–3). Precursor tolerance was set to 20 ppm, and isotope similarity >75%. If mass accuracy exceeded 5 ppm in QIP, the mass peak was manually centered and recalculated for mass accuracy. The parameters used to center data included the minimum peak width at half height with 3 channels and 80% centroid top. Assignment preference was given for the lowest mass error and [M + H] ions.
Semi-quantification of primary amines was conducted using an Agilent 7890 gas chromatograph 5975C mass spectrometer (Agilent Technologies) equipped with a Polyarc (Activated Research Company) flame ionization detector (GC-MS/Polyarc-FID) using an Agilent CP-Sil 8 CB for amines 30 m × 0.25 mm × 0.25 μm column (Agilent Technologies). First, aminated oil was diluted 10× and 100× in dichloromethane, then 2.5 μL was injected into a 250 °C inlet at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split ratio with a split flow of 14.18 mL min−1. The oven program was as follows: 40 °C for 1 min, 5 °C min−1 to 300 °C, hold for 10 minutes for a total run of 63 min. The mass spectrometer detector transfer line temperature was 310 °C, the source was 230 °C at 70 eV, and the quadrupole was 150 °C. The MS was run in scan mode from m/z 30–600. The FID detector was set to 315 °C with H2 flow at 30 mL min−1, air flow at 350 mL min−1, and the makeup flow at 20 mL min−1. To target primary amines, m/z 30 was extracted. The standard calibration for 7-heptanamine in dichloromethane was used as a reference calibration for semi-quantification of all amines using the Polyarc-FID signal. It should be noted that this method has not been evaluated for limits of detection across a broad range of carbon numbers.
1 split ratio with a split flow of 14.18 mL min−1. The oven program was as follows: 40 °C for 1 min, 5 °C min−1 to 300 °C, hold for 10 minutes for a total run of 63 min. The mass spectrometer detector transfer line temperature was 310 °C, the source was 230 °C at 70 eV, and the quadrupole was 150 °C. The MS was run in scan mode from m/z 30–600. The FID detector was set to 315 °C with H2 flow at 30 mL min−1, air flow at 350 mL min−1, and the makeup flow at 20 mL min−1. To target primary amines, m/z 30 was extracted. The standard calibration for 7-heptanamine in dichloromethane was used as a reference calibration for semi-quantification of all amines using the Polyarc-FID signal. It should be noted that this method has not been evaluated for limits of detection across a broad range of carbon numbers.
Quantification of methanol after reductive amination and evaporation of excess solvent was conducted on an Agilent 7890A GC equipped with a 5975C MS using an Agilent DB-WAX 30 m × 0.25 mm × 0.25 μm column (Agilent Technologies). Samples were diluted 5000× into dichloromethane and 1 μL was injected splitless onto a 250 °C inlet. The oven program temperature was 35 °C held for 8 min. The mass spectrometer detector transfer line temperature was 260 °C, the source was 230 °C at 70 eV, and the quadrupole was 150 °C. The MS was run in scan mode with a m/z range of 29–200.
Results
Reductive amination of model compounds and plastic pyrolysis oil
We first studied amination with n-octanal with a ammonia methanol solution at 90 °C and 40 bar of H2. The activity for amination of n-octanal conversion was measured over a variety of catalysts including (all 5 wt% metal) Pt/C, Pd/C, Rh/C, and Ru/C (Table 1). The disappearance of the formyl group in NMR spectra after reductive amination indicates complete conversion of n-octanal. Based on quantitative 13C NMR results of the octanal after reductive amination, the production of n-octylamine was only obtained with Rh/C and Ru/C, while no n-octylamine was generated with Pt/C and Pd/C. The selectivity in the reductive amination is determined by the rates of competitive reaction of the key intermediate, Schiff base. Rh and Ru primarily catalyze the hydrogenolysis of geminal diamines, generated from Schiff base, to yield primary amines. Metals with strong hydrogenation capabilities like platinum and palladium favor the hydrogenation of Schiff bases to produce secondary amines.16 Complete amination of octyl dialdehydes occurred with Ru/C (Fig. S2†) demonstrating that this catalyst is able to aminate dialdehydes as well.| Activity | |
|---|---|
| Catalyst | Yield to n-octylamine (%) |
| 5 wt% Pt/C | 0 |
| 5 wt% Pd/C | 0 |
| 5 wt% Rh/C | 100 |
| 5 wt% Ru/C | 100 |
We then used these same conditions for reductive amination of a hydroformylated pyrolysis oil generated from colored PCR-HDPE. However, it is worth noting that certain reaction conditions, such as contact time and temperature, can be optimized to further improve performance. PCR-HDPE was pyrolyzed in a fluidized bed reactor where sand was used as a heat carrier in the reactor generating plastic pyrolysis oil with a high concentration of olefins (∼60 wt%).5 The olefins were then reacted into aldehydes via hydroformylation catalyzed by a commercial Co2(CO)8 catalyst in a batch reactor. Appropriate catalyst, reaction conditions, and contact time are necessary to prevent the formation of alcohols from aldehydes in the hydroformylation step.19 The Co2(CO)8 catalyst was removed from the mixture before further upgrading (e.g., reductive amination to amines) by oxidizing the homogeneous cobalt catalyst to cobalt salt that could be dissolved in the aqueous phase. The hydroformylated pyrolysis oil containing cobalt catalyst was mixed with a solution of the same volume containing acetic acid (0.2 g mL−1) and cobalt nitrate (0.02 g mL−1). The mixture was stirred at 700 rpm until the color of the oil turned light yellow, indicating the removal of dark red Co complex dissolved in the oil, and the time depended on the amount of mixture (e.g., 10 hours for 10 g hydroformylated pyrolysis oil). Further details regarding the production of hydroformylated pyrolysis oil from PCR-HDPE can be found in our previous work.5
Characterization of the aminated oil
Aminated oil obtained from reductive amination of hydroformylated pyrolysis oil was analyzed by multiple techniques. First, the methanol introduced during reductive amination was removed by rotary evaporation, then elemental analysis was conducted by ICP-MS and CHN(O)S (Tables 2 and 3). After pyrolysis, a reduction in metal impurities in liquid phase, such as Al, Cd, Ca, Fe, Pb, Ti, and Zn, was observed. After hydroformylation, the concentrations of Ca, Co, Fe, and Mg increased. The Co was likely residue from the Co catalyst that was used from hydroformylation while Ca, Fe, and Mg were possibly introduced during the Co catalyst removal steps. Subsequently, an increase in Al, Ni, and Zn concentrations, coupled with a decrease in Co and Fe concentrations, was observed after reductive amination. After hydroformylation, 11.5 wt% of oxygen and, after amination, 7.8 wt% of nitrogen were observed, demonstrating the insertion of formyl groups in the hydroformylation step and the addition of nitrogen in the following reductive amination step. The methanol in the aminated oil after rotary evaporation was quantified using GC-MS and is estimated to be approximately 234 g L−1. The density of the aminated oil was measured to be 0.79 g mL−1, indicating that it still contains 30 wt% methanol after rotary evaporation. Water is a byproduct of the amination reaction, and the water content of the aminated oil was 9 wt% using a KF titration method (Table S1†).| Sample | ICP-MS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Sb | Cd | Ca | Co | Cr | Cu | Fe | Pb | Mg | Ni | Sn | Ti | Zn | ||||||||
| ppm | %a | ppm | ppm | %a | ppm | %a | ppm | %a | ppm | ppm | ppm | %a | ppm | ppm | %a | ppm | ppm | ppm | ppm | %a | |
| a Represents the percentage of the metal concentration normalized by the concentration of the metal in PCR-HDPE. b Represents values between limit of quantification (LOQ) and limit of detection (LOD). | |||||||||||||||||||||
| PCR-HDPE | 285 | 100 | <LOD | 125 | 100 | 2206 | 100 | 906 | 100 | 84 | <LOD | 252 | 100 | 251 | 65 | 100 | <LOD | 97 | 97 | 100 | |
| Pyrolysis oil | 84 | 29.47 | <LOD | 12 | 9.60 | 25 | 1.13 | <LOD | n/a | <LOD | <LOD | 12 | 4.76 | <LOD | n/a | <LOD | <LOD | <LOD | <LOD | n/a | |
| Hydroformylated pyrolysis oil | 113 | 39.65 | <LOD | <LOD | n/a | 618 | 28.01 | 565 | 62.36 | <LOD | <LOD | 118 | 46.83 | <LOD | 14 | 21.54 | <LOD | <LOD | <LOD | 2 | 2.06 |
| Aminated oil without MeOH & H2O | 181 | 63.51 | <LOD | <LOD | n/a | 645 | 29.24 | 21 | 2.32 | <LOD | <LOD | 29 | 11.51 | <LOD | 19 | 29.23 | 14 | <LOD | <LOD | 35 | 36.08 |
| LOD | 6.0 | 2.11 | 10.0 | 0.1 | 0.08 | 0.1 | 0.01 | 1.0 | 0.11 | 2.0 | 1.0 | 0.4 | 0.16 | 4.0 | 1.0 | 1.54 | 1.0 | 16.0 | 0.5 | 0.5 | 0.52 |
| LOQ | 21.0 | 7.37 | 33.0 | 0.3 | 0.24 | 0.4 | 0.02 | 3.0 | 0.33 | 5.0 | 4.0 | 1.2 | 0.48 | 13.0 | 3.0 | 4.62 | 4.0 | 55.0 | 1.6 | 1.8 | 1.86 |
| Sample | CHN(O)S analysis | |||
|---|---|---|---|---|
| C | H | N | O | |
| % | % | % | % | |
| PCR-HDPE | 84.6 | 14.7 | 0.3 | n/a |
| Pyrolysis oil | 86.5 | 12.8 | 0.7 | 0.0 |
| Hydroformylated pyrolysis oil | 76.6 | 11.8 | 0.1 | 11.5 |
| Aminated oil without MeOH & H2O | 81.0 | 11.1 | 7.8 | 0.1 |
13C-NMR spectroscopy was used to analyze the hydroformylated pyrolysis oil before and after reductive amination as shown in Fig. 2. In Fig. 2B, peaks in the range of 190–210 ppm are attributed to the carbon in formyl groups and the peaks in the range of 40–48 ppm are in the carbon immediately adjacent to the formyl groups.20 In Fig. 2C, the peaks assigned to formyl groups disappeared, however, the 40–48 ppm peaks are still present and are assigned to a carbon atom bonded to an –NH2 group.20 The NMR results show complete conversion of the aldehydes after the amination step. The plastic oil comprises more than a thousand chemical species, which results in multiple peaks in the 13C-NMR spectrum. The different types of amines (1° to 3° amines) in the oil were identified using the Hinsberg test,21 a well-established method for distinguishing between primary, secondary, and tertiary amines. Products formed by Hinsberg reagents (benzene sulfonyl chloride) exhibit varied solubility in water. This test shows that the dominant amines in the mixtures are primary amines (Fig. S4†).
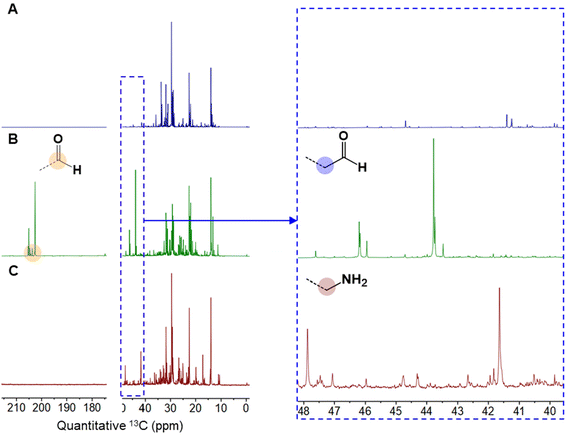 | ||
| Fig. 2 13C NMR of (A) pyrolysis oil, (B) hydroformylated pyrolysis oil, and (C) the aminated oil. In all cases, the blue box on the left is shown magnified on the right. The full spectra can be found in Fig. S3.† | ||
HRLC-MS was used to analyze the amines in the aminated oil (Fig. 3). Despite a high dilution factor, and conservative molecular formula matching parameters, 441 unique organonitrogen products were identified with assigned molecular formulas with mass accuracies <5 ppm (Table S2†) between C10–C41. The major product series, with 274 unique compounds, have molecular formulas assignments of CxHyN and appear to be primary monoamines with varying degrees of branching. The branching is evidenced by multiple compounds with the same molecular formula at different retention times. We also detected several minor series of nitrogenous compounds with molecular formulas including CxHyNO, CxHyNO3, and CxHyN2O2. Their total count is 157, with 124 of them being the dominant species with the molecular formula CxHyNO which are likely oxime or hydrocylamine generated during the reductive amination step.22
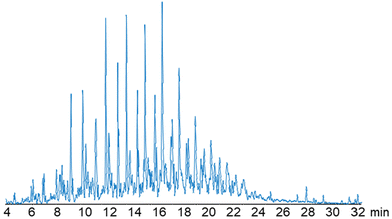 | ||
| Fig. 3 HRLC-MS chromatogram of aminated oil at 1000× dilution. The aminated oil had 441 assigned molecular formulae containing nitrogen between C10–C41, which can be found in Table S2.† Of those 441 formulae, 274 were assigned CxHyN, 124 were CxHyNO, and 43 were a mix of CxHyNO2, CxHyNO3, CxHyN2O, CxHyN2O2, CxHyN2O3, and CxHyN3. | ||
GC-MS/Polyarc-FID was used to semi-quantify primary amines and investigate the carbon distribution from C5–C18 (Fig. 4). The chromatographic information including retention time, library matching, and reference mass spectra can be found in Table S3 and Fig. S5.† GC-MS/Polyarc-FID results indicate that a large amount of products are formed in the C5–C10 range and then decrease in concentration as the carbon number increases. HRLC-MS is less sensitive for lower molecular weight compounds (i.e., <C8), but shows a similar trend of decreasing instrumental response as carbon number increases. The nitrogenous compounds containing oxygen were not well detected on the GC-MS/Polyarc-FID indicating that their concentration is likely below the detection limit estimated to be approximately 2 wt% in the aminated oil in the carbon range that is detectable by that method (C4–C30). Instrumental sensitivity to specific compounds has not been evaluated fully. Parrafins, aromatics, and alcohols were also observed by GC-MS, but they were not quantified.
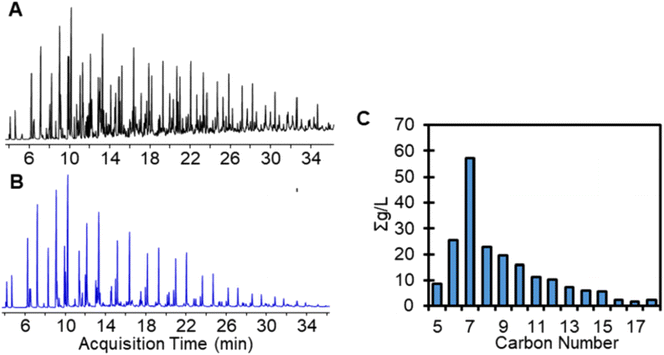 | ||
| Fig. 4 (A) Total-ion chromatogram of the 10× dilution of aminated oil, (B) extracted ion m/z 30, highlighting the amines quantified, and (C) carbon distribution of C5–C18 primary amines in the aminated oil as measured by GC-MS/Polyarc-FID. The retention times, library matches, and estimated carbon number of compounds that were quantified can be found in Table S3.† | ||
Discussion
Currently, the primary source for the production of aliphatic amines is fossil feedstocks (Fig. 1). In this work, we demonstrate that polyolefins derived from the pyrolysis of waste plastics can be utilized as feedstocks for the production of amines through tandem pyrolysis, hydroformylation, and reductive amination. The olefins in the pyrolysis oil can undergo hydroformylation to produce aldehydes,5 which can subsequently react through reductive amination to obtain aminated oil containing paraffins, aromatics, monoamines, and diamines. The remaining discussion focuses on the composition and market analysis of the aminated oil obtained from PCR-HDPE.Elements in the aminated oil
The drastic decreased content of Al, Ca, Fe, Ti, and Zn (Table 2) after pyrolysis suggests that most of the impurities were left in solids in the reactor during pyrolysis. These metals were likely adsorbed onto the sand that remained in the fluidized bed reactor after pyrolysis. The Co content increased after hydroformylation because of the Co2(CO)8 catalyst. A 10 wt% of Co2(CO)8 (containing 3.4 wt% Co) was used, indicating that the Co content in the hydroformylated pyrolysis oil was reduced from 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm to 565 ppm by oxidizing the Co complex to Co salts dissolved in aqueous phase.5 The relatively high concentration of Co does not affect the reductive amination under the reaction conditions used in this work. Hydroformylation and the subsequent Co catalyst removal steps likely introduce metal impurities (e.g., from Co2(CO)8, Co(NO3)2·6H2O, water, or acetic acid) which results in increased content of certain metals (Table 2, e.g., Ca, Fe, Mg). Reductive amination, conversely, could remove Co and Fe but possibly introduces Al, Ni, and Zn. In addition, it has been reported that formation of metal nitride on the catalyst surface hinders the reductive amination which could be prevented by feeding excess hydrogen.23,24 Negligible deactivation in reductive amination has been observed over Ru, Co, and Ni catalysts, while deactivation does occur with Ag and Cu catalysts due to the accumulation of carbonaceous deposits on the catalyst.14
000 ppm to 565 ppm by oxidizing the Co complex to Co salts dissolved in aqueous phase.5 The relatively high concentration of Co does not affect the reductive amination under the reaction conditions used in this work. Hydroformylation and the subsequent Co catalyst removal steps likely introduce metal impurities (e.g., from Co2(CO)8, Co(NO3)2·6H2O, water, or acetic acid) which results in increased content of certain metals (Table 2, e.g., Ca, Fe, Mg). Reductive amination, conversely, could remove Co and Fe but possibly introduces Al, Ni, and Zn. In addition, it has been reported that formation of metal nitride on the catalyst surface hinders the reductive amination which could be prevented by feeding excess hydrogen.23,24 Negligible deactivation in reductive amination has been observed over Ru, Co, and Ni catalysts, while deactivation does occur with Ag and Cu catalysts due to the accumulation of carbonaceous deposits on the catalyst.14
As shown in Fig. 1C water is a byproduct of the amination step. The oxygen in the water originates from aldehydes, while two hydrogen atoms are derived from ammonia. Based on the NMR spectrum of aminated oil after rotary evaporation, the area of the peaks assigned to carbon atoms bonded to hydroxyl groups in the oil are negligible compared to the that of carbon in methanol (Fig. S3†) suggesting that the majority of oxygen in the mixture is attributed to methanol. The production of water via reductive amination can thus be calculated based on the N content. The elemental analysis of aminated oil, excluding the contribution of methanol, reveals that the aminated oil contains 75 wt% C, 10 wt% H, 7 wt% N, and 8 wt% O. The presence of 7 wt% of N suggests that an 8 wt% yield of O is generated from reductive amination of aldehydes in the hydroformylated pyrolysis oil, as observed in the O content of the aminated oil after excluding methanol (Table S4†). This observation is also consistent with the water concentration (9 wt%) measured by KF titration.
Solvent in the reductive amination
The methanol solvent can be removed from the aminated oil. However, rotary evaporating at higher temperatures or lower vacuum would result in evaporation of amines or hydrocarbons in the aminated oil. Rotary evaporation decreased the methanol content from 90 wt% to 30 wt% as measured by GC-MS. It presents a challenge to fully eliminate the methanol without also removing paraffins, aromatics, and amines from the aminated oil at the laboratory scale. However, achieving methanol-free aminated oil is feasible on a larger scale, considering the higher volatility of methanol compared to other species present in the aminated oil (Table S5†). Note that an ammonia-methanol solution was utilized in this work to eliminate the need for an ammonia cylinder, thereby simplifying the reductive amination reactor system. Methanol has been identified as the most suitable solvent for the reductive amination of ketones,25 although solvent-free amination, which have been reported at the lab scale, would be ideal for industrial applications.14Aliphatic amines in the aminated oil
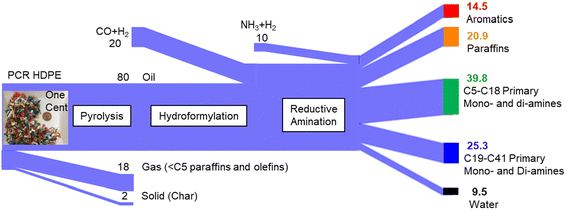
HRLC-MS and GC-MS/Polyarc-FID results also provided evidence that the majority of amines present are primary monoamines. Other minor product series were observed with HRLC-MS. It should be noted that the sensitivity carbon range between the HRLC-MS and the GC-MS/Polyarc-FID varies due to different chromatographic separations and ionization techniques, where HRLC-MS will have much higher detection limits on longer amine carbon chains vs. GC-MS/Polyarc FID, which will have higher detection limits on lower amine carbon chains. GC-MS/Polyarc-FID was able to semi-quantify C5–C18 amines for a total of 197 g L−1 in the mixture, although up to C36 primary amines were observed using HRLC-MS. We speculate that some of the amines semi-quantified by GC-MS belong to the diamine class because the carbon distribution follows the trend of theoretical carbon distribution of the sum of amines (Fig. S6†) based on the mono and diolefins in the pyrolysis oil. The concentration of aromatics and paraffins was determined by their percentage in the pyrolysis oil, given their inert behavior during the tandem processes. Nitrogenous compounds containing oxygen and alcohols were also detected, but at a low concentration, based on the CHN(O)S results (Table 3).The Sankey diagram for the production of aliphatic amines from waste plastics, post-methanol removal, is shown in Fig. 5. One hundred kg of plastic waste produce 14.5 kg of aromatics and 20.9 kg of paraffins in the overall process.5 These hydrocarbons today sell for $600–800 per ton and could be used to produce gasoline, diesel, and plastics.26 Their yield can be manipulated by adjusting parameters or utilizing catalysts in the pyrolysis step. The olefins generated from pyrolysis can be hydroformylated to aldehydes achieving a near complete conversion (>99%).5 Production of amines from aldehydes is accompanied with the generation of water (9.5 kg per 100 kg waster plastics). A high yield (40% from PCR-HDPE) of primary mono and diamines from waste polyolefins was achieved via the proposed route with a carbon distribution ranging from C5 to C18 (Fig. 4). The remaining 25% of the product yield are mainly C18–C41 mono and diamines. Although performing GC-MS/Polyarc-FID analysis of these high molecular aliphatic amines is challenging, based on the Hinsberg test (Fig. S4†), we speculate that the dominant amines are primary amines. Additionally, this cut likely also contains nitrogenous compounds with the presence of oxygen; however, their concentration is relatively low (e.g., total concentration < 2 wt%).
The most valuable chemicals produced in this route are mono and diamines. C6 to C20 monoamines can be used to make agricultural chemicals, nylon plasticizers, oil or paint additives.27 As for diamines, their main application is to make crop protection agents or polymers like nylon and polyurethane.28,29 It is estimated that approximately 40 kg of C5–C18 mono and diamines can be obtained from 100 kg of waste polyolefins. We speculate that more than 10 kg (30% of the theoretical diamine yield) of diamines are produced from 100 kg of polyolefin waste and these amines are priced between $2000–3500 per ton.28,29 Based on the input–output analysis (Fig. 5) and the price difference between feedstocks and products (from 2021),30,31 over $1200 per ton of potential profit can be obtained from this route (details in Table S6†). This analysis only includes the value of the products minus the cost of the feedstocks and does not include any processing or separation costs. In 2020, the global capacity for alkylamines reached approximately 3 million metric tons; however, the production was around 2 million metric tons.22 Considering the boiling point differences between species with the same carbon number (e.g., decane at 174 °C, 1-aminodecane at 220 °C, and 1,10-diaminodecane at 299 °C), a high-purity (e.g., >90%) chemical product with an identical carbon number could potentially be obtained through the sequential processes of fractional distillation to obtain plastic oil with shorter carbon range, hydroformylation, reductive amination, and distillation, as we proposed previously.5 The main application of aliphatic amines is for solvents and agrochemicals which will require the separation of the aminated oil from the paraffins. We estimate the capital investment for separation, at a scale of 100 kiloton per year to be $5 million (Table S7†). By converting waste plastics into valuable aliphatic amines, the developed process potentially offers desirable energy efficiency and mitigates secondary pollution. This process minimizes reliance on fossil resources while utilizes existing hydroformylation and reductive amination facilities.
Conclusions
Aliphatic amines can be produced from waste polyolefins by a combination of pyrolysis, hydroformylation, and reductive amination. Near-quantitative amination of aldehydes in hydroformylated pyrolysis oil to amines can be achieved by using a 5 wt% Ru/C catalyst at 90 °C and 40 bar H2 with an ammonia methanol solution. The formation of primary C5–C18 mono and diamines from PCR-HDPE was evidenced by a combination of NMR spectroscopy, GC, and elemental measurements. Although most of the metals from the plastics are removed in the pyrolysis step, hydroformylation of the olefins in the pyrolysis oil followed by reductive amination of the aldehydes can be conducted in the presence of low concentrations (e.g., <200 ppm) of varied metals. The presence of paraffins or aromatics in the oil has limited effects on the cascade production of amine mixtures from mono/diolefins. Economic analysis indicates that products with prices more than five times that of the feedstocks can be obtained using the approach outlined in this work.Author contributions
H. Li: conceptualization; data curation; formal analysis; investigation; methodology; visualization; roles/writing – original draft; writing – review & editing. A. A. Cuthbertson: investigation; methodology; roles/writing – review & editing. A. A. Alamer: data curation; writing – review & editing. V. S. Cecon: data curation; writing – review & editing. H. Radhakrishnan: data curation; writing – review & editing. J. Wu: data curation. G. W. Curtzwiler: roles/writing – review & editing. K. L. Vorst: conceptualization. X. Bai: roles/writing – review & editing. C. R. Landis: conceptualization. G. T. Beckham: roles/writing – review & editing. G. W. Huber: conceptualization; supervision; funding acquisition; project administration; resources; writing – review & editing.Conflicts of interest
Professor G. W. Huber has an ownership interest in Anellotech, which is commercializing the PlasTCat plastic pyrolysis technology.Acknowledgements
We would like to acknowledge William Michener at NREL for running the amine oil samples by HRLC-MS. This material is based upon work supported by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office under Award Number DEEE0009285. This work was authored in part by Alliance for Sustainable Energy, LLC, the manager and operator of the National Renewable Energy Laboratory for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding was provided by the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office.References
- M. T. Dow, Dow and Mura Technology plan to locate Europe's largest advanced recycling facility at Dow's site in Böhlen, Germany, https://www.prnewswire.com/news-releases/dow-and-mura-technology-plan-to-locate-europes-largest-advanced-recycling-facility-at-dows-site-in-bohlen-germany-301624125.html, accessed Oct 15, 2023.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown and G. W. Huber, Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges, Green Chem., 2022, 24, 8899–9002 RSC.
- LyondellBasell to Build Industrial-scale Advanced Recycling Plant in Germany, https://www.prnewswire.com/news-releases/lyondellbasell-to-build-industrial-scale-advanced-recycling-plant-in-germany-301992900.html, accessed Feb 22, 2024.
- D. Zhao, X. Wang, J. B. Miller and G. W. Huber, The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals, ChemSusChem, 2020, 13(7), 1764–1774 CrossRef CAS PubMed.
- H. Li, J. Wu, Z. Jiang, J. Ma, V. M. Zavala, C. R. Landis, M. Mavrikakis and G. W. Huber, Hydroformylation of Pyrolysis Oils to Aldehydes and Alcohols from Polyolefin Waste, Science, 2023, 381(6658), 660–666 CrossRef CAS PubMed.
- S. Dong, H. Li, I. K. Bloede, A. J. Al Abdulghani, E. A. Lebrón-Rodríguez, G. W. Huber and I. Hermans, Catalytic conversion of model compounds of plastic pyrolysis oil over ZSM-5, Appl. Catal., B, 2023, 324, 122219 CrossRef CAS.
- M. Kusenberg, A. Eschenbacher, M. R. Djokic, A. Zayoud, K. Ragaert, S. De Meester and K. M. Van Geem, Opportunities and Challenges for the Application of Post-Consumer Plastic Waste Pyrolysis Oils as Steam Cracker Feedstocks: to Decontaminate or not to Decontaminate?, Waste Manage., 2022, 138, 83–115 CrossRef CAS PubMed.
- J. Gancedo, H. Li, J. S. Walz, L. Faba, S. Ordoñez and G. W. Huber, Investigation into the shape selectivity of zeolites for conversion of polyolefin plastic pyrolysis oil model compound, Appl. Catal., A, 2024, 669, 119484 CrossRef CAS.
- S. P. Global, Alkylamines, 2021.
- A. Ricci, Modern amination methods, John Wiley & Sons, 2008 Search PubMed.
- Y. Zhu, L. Gao, L. Wen and B. Zong, A review of adiponitrile industrial production processes and associated atom economies, Chin. Sci. Bull., 2015, 60, 1488–1501 CrossRef.
- P. T. Nguyen, G. Gözaydın, J. Ma, B. Yao, Q. He and N. Yan, Direct amination of poly(p-phenylene oxide) to substituted anilines over bimetallic Pd–Ru catalysts, Green Chem., 2024, 26, 3949–3957 RSC.
- T. Gross, A. M. Seayad, M. Ahmad and M. Beller, Synthesis of primary amines: first homogeneously catalyzed reductive amination with ammonia, Org. Lett., 2002, 4(12), 2055–2058 CrossRef CAS PubMed.
- T. Irrgang and R. Kempe, Transition-metal-catalyzed reductive amination employing hydrogen, Chem. Rev., 2020, 120(17), 9583–9674 CrossRef CAS PubMed.
- J. Gallardo-Donaire, M. Ernst, O. Trapp and T. Schaub, Direct synthesis of primary amines via ruthenium-catalysed amination of ketones with ammonia and hydrogen, Adv. Synth. Catal., 2016, 358(3), 358–363 CrossRef CAS.
- J. Bódis, L. Lefferts, T. Müller, R. Pestman and J. Lercher, Activity and selectivity control in reductive amination of butyraldehyde over noble metal catalysts, Catal. Lett., 2005, 104, 23–28 CrossRef.
- K. M. Van Geem, Plastic waste recycling is gaining momentum, Science, 2023, 381(6658), 607–608 CrossRef CAS PubMed.
- M. L. Kneer, J. Lazarcik and M. Ginder-Vogel, Investigation of ICP-MS/MS for total sulfur quantification in freshwater dissolved organic matter, J. Environ. Qual., 2021, 50(6), 1476–1485 CrossRef CAS PubMed.
- F. Hebrard and P. Kalck, Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle, Chem. Rev., 2009, 109(9), 4272–4282 CrossRef CAS PubMed.
- D. Banfi and L. Patiny, www.nmrdb.org: Resurrecting and processing NMR spectra on-line, Chimia, 2008, 62(4), 280–280 CrossRef CAS.
- O. Hinsberg, Ueber die bildung von säureestern und säureamiden bei gegenwart von wasser und alkali, Ber. Dtsch. Chem. Ges., 1890, 23(2), 2962–2965 CrossRef.
- R. W. Layer, The Chemistry of Imines, Chem. Rev., 1963, 63(5), 489–510 CrossRef CAS.
- A. Rausch, E. Van Steen and F. Roessner, New aspects for heterogeneous cobalt-catalyzed hydroamination of ethanol, J. Catal., 2008, 253(1), 111–118 CrossRef CAS.
- K. Kim, Y. Choi, H. Lee and J. W. Lee, Y2O3-Inserted Co-Pd/zeolite catalysts for reductive amination of polypropylene glycol, Appl. Catal., A, 2018, 568, 114–122 CrossRef CAS.
- S. Song, Y. Wang and N. Yan, A remarkable solvent effect on reductive amination of ketones, Mol. Catal., 2018, 454, 87–93 CrossRef CAS.
- EIA Petroleum & Other Liquids Spot Prices, https://www.eia.gov/dnav/pet/pet_pri_spt_s1_d.htm, accessed Oct 02, 2022.
- BASF Overview of Aliphatic Monoamines, https://chemicals.basf.com/global/en/Intermediates/Product_groups/Aliphatic_Monoamines.html, accessed Oct 02, 2023.
- I. Markit, Hexamethylenediamine/Adiponitrile Chemical Economics Handbook, https://www.spglobal.com/commodityinsights/en/ci/products/hexamethylenediamine-adiponitrile-chemical-economics-handbook.html, accessed Oct 02, 2023.
- BASF Octamethylendiamine, https://products.basf.com/global/en/ci/octamethylenediamine.html, accessed Oct 02, 2023.
- RecyclingMarkets, RecyclingMarkets.net, https://www.recyclingmarkets.net, accessed Feb 02, 2022.
- U.S. Geological Survey, M. C. S. Nitrogen, (Fixed) – Ammonia, https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-nitrogen.pdf, accessed Oct 09, 2023.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc01013b |
| This journal is © The Royal Society of Chemistry 2024 |