Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorisation of the industrial hemp residue from essential oil production by recovery of cannabidiol and chemo-enzymatic conversion to cannabielsoin†
Daniele
Fiorito‡
 ,
Davide
Tessaro‡
,
Davide
Tessaro‡
 ,
Fabio
Sangalli
,
Celeste
Nobbio
,
Fabio
Sangalli
,
Celeste
Nobbio
 ,
Mario
Nebuloni
,
Matteo
Vezzini
,
Elisabetta
Brenna
,
Mario
Nebuloni
,
Matteo
Vezzini
,
Elisabetta
Brenna
 and
Fabio
Parmeggiani
and
Fabio
Parmeggiani
 *
*
Department of Chemistry, Materials and Chemical Engineering “G. Natta”, Politecnico di Milano, P.za Leonardo da Vinci, 20133 Milano, Italy. E-mail: fabio.parmeggiani@polimi.it
First published on 20th March 2024
Abstract
The production of essential oils by steam distillation is invariably associated with large amounts of organic waste which is normally disposed of or returned to the fields, although it may still contain some valuable components. In particular, Cannabis sativa essential oil produced by steam distillation of the apical part of industrial hemp plants yields a soaked biomass residue that may contain high-value cannabinoids. From the perspective of sustainable exploitation of agricultural resources, a method to extract cannabidiol (CBD) from such waste was demonstrated and scaled-up, using bioethanol as a renewable bio-based solvent and without requiring chromatographic separation, with an overall yield of 10.1 mg CBD per g waste. The work paves the way to an integrated complete utilisation of industrial hemp byproducts. Furthermore, two alternative lipase-mediated chemo-enzymatic derivatisations have been designed and optimised for the conversion of the recovered CBD into cannabielsoin (CBE), an underexploited cannabinoid with attractive bioactivity. The processes are practical and efficient, with 31–47% isolated yields and green metrics comparing well with the established chemical alternatives.
Introduction
Substances that have long represented a waste, often associated with a disposal expense, are now becoming valuable resources. Organic waste and residual materials from bio-based industries ought to gain increasing importance in the modern economy, and exploiting the potential of such resources requires a systemic change for a transition to a sustainable industrial system.1 The need for a more intelligent use of resources, materials and energy, or for the decoupling of economic growth from environmental impacts is now considered to be critical, as highlighted, for instance, in the UN Sustainable Development Goals (Goal 12: Ensure sustainable consumption and production patterns).2Food waste and crop residues have significant potential as bio-resources since they offer a high level of chemical complexity that should be exploited as much as possible before sending the material to the conventional treatment of these residues, such as animal feeding, composting, anaerobic digestion, incineration or landfill disposal.3 This more far-sighted waste valorisation approach requires the development of new techniques and approaches to recover high-value compounds such as fine chemicals, materials or fuels, before the residue is conventionally processed. In order to meet the increasing societal and environmental pressure, it is essential to boost the implementation of such strategies, moving towards a fully circular and sustainable economy,4 which will decrease our dependence on natural resources, reduce pollution and prevent unnecessary waste.
As an example, hemp (Cannabis sativa) is one of the most ancient and versatile crops, cultivated in many temperate climate areas and appreciated for its strength, durability and resistance to rot. It has been employed for millennia, with documented evidence dating back to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BC, primarily as a source of fibre to produce coarse fabrics, paper and rope, but also for food applications, religious and spiritual practices, and oriental medicine. The plant seeds have been used for producing seed oil (rich in polyunsaturated fatty acids)5 or flour for both human and animal consumption due to their nutritional properties, while the essential oil finds use in aromatherapy and cosmetics, as discussed below.
000 BC, primarily as a source of fibre to produce coarse fabrics, paper and rope, but also for food applications, religious and spiritual practices, and oriental medicine. The plant seeds have been used for producing seed oil (rich in polyunsaturated fatty acids)5 or flour for both human and animal consumption due to their nutritional properties, while the essential oil finds use in aromatherapy and cosmetics, as discussed below.
Currently, interest in hemp is growing because it is seen as a low-cost, sustainable and versatile material suitable as a replacement for fossil-based materials and acoustic and thermal insulators. Its cultivation has proven environmental benefits and, due to its high harvest yield and sustainability, it is an attractive biomass for chemical and energetic valorisation.6 Nowadays, hemp is cultivated in at least 40 countries and global leaders in hemp production are in particular Canada, China, France and Chile, with a total productivity of around 200 × 103 tons per year and an estimated market value of over $6 billion in 2022.7 The global hemp industry has the potential to expand significantly since the consumer demand for eco-friendly and organic products rises. Notably, in the food sector, in the last few decades the hemp-based food market share has grown steadily ($3.9 billion in 2020, expected to double before 2027).8
Among the many applications of industrial hemp, the production of essential oil is quite relevant, due to its uses in aromatherapy, biopesticides, antimicrobials, food additives, cosmetics and even for claimed therapeutic properties.9C. sativa essential oil is predominantly obtained by steam distillation of fresh aerial parts of the plant.10 The extraction is carried out by injection of steam, which crosses plant matter from the bottom up and carries the volatile materials into a condenser. After phase separation in a decanter, the essential oil is collected from the surface while the condensed water (saturated with essential oil) is also a product of commercial value for the cosmetic industry, known as hydrolate (Fig. 1).
The essential oil extraction yield is intrinsically low (0.1–0.4% w/w of the starting material) and, currently, a large amount of soaked biomass depleted of volatiles represents a waste from an environmental perspective: typically, this residue is either partly digested to recover energy or returned to the field (Fig. 1a). Several factors limit its use for biodigestion and composting: a low nitrogen content prevents rapid decomposition11 and its antimicrobial properties are detrimental to many classes of microorganisms. From a circular bioeconomy perspective,12 the best option would be to further exploit the material to extract high-value compounds, for a profitable valorisation. Therefore, the aim of this project was to study the possibility of processing this soaked distillation residue to recover potentially useful components and also to explore chemo-enzymatic conversion of the latter into high-value derivatives (Fig. 1b).
Results and discussion
Recovery of CBD from the hemp essential oil distillation residue
The steam distillation process selectively extracts only the volatile components which are easily removed by steam at atmospheric or near-atmospheric pressure. Indeed, the main constituents of C. sativa essential oil are monoterpenes (α-terpinolene, α- and β-pinene, β-myrcene) and sesquiterpenes (α-caryophyllene, α-bergamotene and caryophyllene oxide),13 which are responsible for the characteristic aroma of the plant.Among different hemp varieties, C. sativa Futura 75 achieved the highest content and yield of essential oil.14 The soaked waste obtained from essential oil distillation of C. sativa Futura 75 was kindly provided by Sacmar s.r.l. (Settimo Milanese, Italy), a company manufacturing natural extracts, flavours and fragrances for food and personal care applications. Under typical environmental conditions, 1 ha of cultivated field affords approximately 20–25 tons of fresh above-ground matter,15 from which around 20–40 kg of essential oil can be obtained. Therefore, the vast majority of the plant matter ends up in the form of the soaked lignocellulosic residue for disposal, containing approximately 50% cellulose, 15% hemicelluloses, 20% pectic substances and 5% lignin.6d
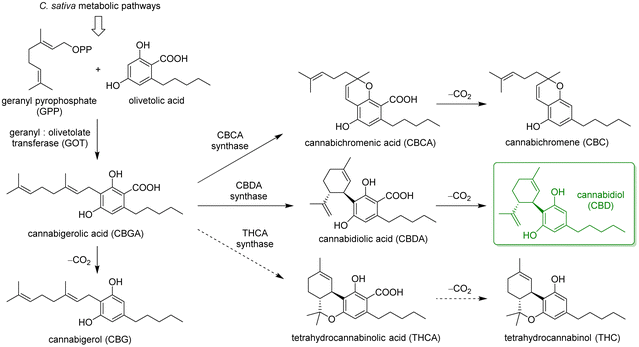
However, among the most precious components of the plant residues are cannabinoids, a class of meroterpenoids, specifically terpenophenolics, mainly synthesized in epidermal trichomes, which are more abundant in female inflorescences. The main biosynthetic pathways leading to the most abundant phytocannabinoids in C. sativa are summarised in Scheme 1. The key step is the enzyme-mediated alkylation of an alkyl resorcinol derivative with a monoterpene unit, leading to the main precursor cannabigerolic acid (CBGA), which is then cyclised by specific enzymes to a variety of terpenophenolic acids, followed by decarboxylation to give the corresponding neutral cannabinoids.16,17 These compounds have raised the interest of medicinal chemists and pharmacologists for many years, due to their ability to interact with multiple receptors, triggering a range of effects on pain perception, sleep, memory and other biological mechanisms.18
In general, the plant shows the highest content of cannabinoids when harvested at full maturity. The precise ratios and amounts of the different cannabinoids in the plant depend on a number of factors, including cultivar, growth conditions, climate and harvest time, as well as its physical state (fresh or dried). Furthermore, the spontaneous non-enzymatic decarboxylation of terpenophenolic acids into neutral cannabinoids can be accelerated by light exposure, heating, or ageing. This is extremely relevant for the distinction between legal varieties (i.e., industrial hemp) and restricted varieties (i.e., marijuana) of C. sativa. According to current regulations, legal hemp plants are characterised by <0.2–0.3% w/w Δ9-tetrahydrocannabinol (THC), which is the major psychotropic cannabinoid in the plant, responsible for its narcotic and recreational properties. Futura 75 belongs to this class, due to the lack of a functional gene encoding for THCA synthase (dashed arrows in Scheme 1).19 The cannabinoid profile of this variety is dominated by CBD (>95%), along with minor amounts of CBG.
Due to their relatively high molar mass, poor volatility and very low solubility in water, only minute amounts of cannabinoids are removed by steam distillation (<1% in the Futura 75 essential oil obtained from the samples used in this study) and the vast majority remains in the soaked waste at the end of the process. Therefore, the first goal of this project was to examine the residual cannabinoids content of the waste and to verify the feasibility of their extraction and concentration.20
Maceration tests were performed using a range of different solvents used in the literature for cannabinoid extractions (methanol, ethanol, chloroform, methanol/chloroform 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and n-heptane). For comparison, a weighed amount of biomass (2 g) in 50 mL of solvent was subjected to magnetic and/or mechanical stirring at room temperature for 2 hours. The solution was then filtered, and the residue was extracted for the second time with the same procedure. The filtrates of the two extractions were then combined, evaporated under reduced pressure and dried. The yields of the extracts obtained from the soaked waste (either wet or dried at 60 °C) are reported in Table 1, expressed as mg extract per g sample. Tests were repeated in duplicate to verify consistency and only minor variations were observed. In order to establish a reference value, also the fresh aerial parts of the same crop were extracted with the same procedure. In terms of the total amount of material extracted, the differences observed were not particularly remarkable, except for the case of n-heptane, which was the least efficient, likely due to its low polarity. The latter was also tested with a Soxhlet apparatus, affording even slightly lower yields. From this dataset, the best solvent appeared to be ethanol, not only because of the highest recovered mass in all cases (although the differences were not statistically significant), but also in terms of sustainability, safety and environmental impact.
1, and n-heptane). For comparison, a weighed amount of biomass (2 g) in 50 mL of solvent was subjected to magnetic and/or mechanical stirring at room temperature for 2 hours. The solution was then filtered, and the residue was extracted for the second time with the same procedure. The filtrates of the two extractions were then combined, evaporated under reduced pressure and dried. The yields of the extracts obtained from the soaked waste (either wet or dried at 60 °C) are reported in Table 1, expressed as mg extract per g sample. Tests were repeated in duplicate to verify consistency and only minor variations were observed. In order to establish a reference value, also the fresh aerial parts of the same crop were extracted with the same procedure. In terms of the total amount of material extracted, the differences observed were not particularly remarkable, except for the case of n-heptane, which was the least efficient, likely due to its low polarity. The latter was also tested with a Soxhlet apparatus, affording even slightly lower yields. From this dataset, the best solvent appeared to be ethanol, not only because of the highest recovered mass in all cases (although the differences were not statistically significant), but also in terms of sustainability, safety and environmental impact.
| Sample | Yield [mg extract per g sample] | |||||
|---|---|---|---|---|---|---|
| MeOH | EtOH | CHCl3 | MeOH/CHCl3 | n-C7 | n-C7 Soxhlet | |
| Wet waste | 18 | 22 | 15 | 16 | 12 | 11 |
| Dried waste | 22 | 33 | 31 | 29 | 23 | 15 |
| Dried aerial parts | 42 | 51 | 45 | 50 | 44 | 25 |
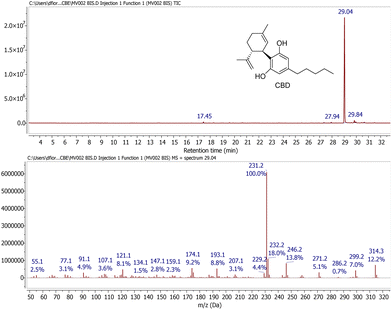
Pleasingly, GC-MS analysis of the extracts obtained by maceration of the waste showed that, of the various possible neutral cannabinoids, only cannabidiol (CBD) was present in significant amounts, constituting the predominant peak in all the samples. A representative GC-MS trace is shown in Fig. 2. It can be inferred that all carboxylated cannabinoid precursors were converted into the corresponding decarboxylated neutral cannabinoids during the steam distillation process. Typically, CBD accounted for >95% of the total volatile components observed by GC-MS. However, this was invariably associated with a consistent amount of polar non-volatile materials (e.g., chlorophylls, flavonoids and pigments) not detected by GC-MS. Therefore, to establish the correct CBD contents of the extraction samples, the compound was purified by column chromatography, which removed completely all the associated impurities. Table 2 shows the yield of pure CBD obtained, expressed as mg CBD per g sample.
| Solvent | Yield [mg CBD per g sample] |
|---|---|
| MeOH | 10.8 |
| EtOH | 12.4 |
| CHCl3 | 10.7 |
| MeOH/CHCl3 | 11.4 |
| n-C7 | 9.8 |
The data confirms that the highest CBD extraction yield is achieved using ethanol, which also offers the additional advantages of greenness and low toxicity. With the aim of minimising the environmental footprint of this process even further, the use of commercial hardware store bioethanol was also considered (1H NMR analysis of the solvent is provided in the ESI, Fig. S1†). This inexpensive and fully renewable option gave identical results to those obtained with laboratory grade ethanol. Furthermore, the results are in line with the reported CBD content of C. sativa Futura 75, quoted by producers in the range 1.5–3.0% w/w.21
As an alternative to column chromatography, which requires relatively large amounts of solvents, the purification of CBD by Kugelrohr distillation was considered: a larger extraction batch using 10 g of dried waste and 2 × 100 mL of bioethanol afforded 0.97 g of crude extract, which, when subjected to vacuum distillation in a Kugelrohr apparatus (120–140 °C, 0.1 mbar), yielded pure CBD. The overall yield was 10.1 mg CBD per g waste, slightly lower than that obtained by chromatography, but the minimal solvent consumption and better scalability make this option considerably more appealing for CBD recovery on an industrial scale. Complete NMR and GC-MS characterisation data of the isolated CBD confirmed the identity of the compound and proved the high purity of the sample. A comparison of the 1H NMR analysis of samples of CBD recovered from waste by chromatography and by vacuum distillation is provided in the ESI (Fig. S2†).
Currently, CBD is a very valuable commercial product with an estimated global market of $6.4 billion in 2022.22 It is a bioactive molecule of wondrous diversity with claimed antioxidant, neuroprotective, immunomodulatory, anti-inflammatory, antipsychotic and anticonvulsant activity.23 It is non-psychoactive and essentially non-toxic; it has been administered to humans since the 1980s,24 and in 2018 it was approved by the FDA under the trade name Epidiolex® for the treatment of two forms of epilepsy in children: Dravet syndrome and Lennox-Gastaut syndrome. Lately, CBD has even been proposed as a potential preventative agent for COVID-19.25 Furthermore, it is also added in small amounts to cosmetics, food products, beverages and over-the-counter supplements. A recent perspective extensively covered the scientific evidence related to CBD uses in medicinal chemistry.26 Thus, the possibility to recover CBD easily and effectively from the hemp essential oil distillation residue seems to be a viable strategy to extract additional value from the waste before its disposal, in line with the mandates of circular economy.
Chemo-enzymatic conversion of CBD to CBE
Inspired by the rich ethnopharmacology of Cannabis extracts and the broad diversity of the bioactivity observed for several natural phytocannabinoids, CBD has been used as a starting point for many medicinal chemistry studies aimed at boosting specific activities.26 A number of examples of bioactive chemically modified CBD derivatives are known in the literature (Fig. 3), such as the hydrogenated–hydroxylated HU-446,27 the ring-fluorinated HUF,28 the quinone HU-331,29 the 2-O′-glucuronide30 and the monoepoxide CBDO.31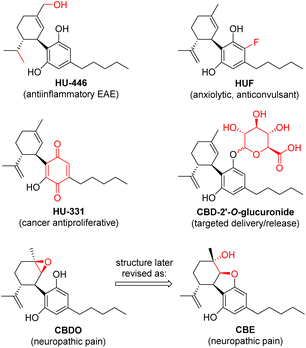 | ||
| Fig. 3 Examples of chemically modified CBD derivatives with specific bioactivity reported in the literature. | ||
Therefore, the other major target of our project was to examine the possibility of developing new chemo-enzymatic strategies to convert the CBD recovered from the hemp distillation residue into other cannabinoids with relevant bioactivity, as added-value products. Our attention first turned to CBD monoepoxidation, inspired by a recent study reporting the potent inhibitory activity of CBDO against the Wnt/β-catenin signalling pathway, implicated in pain sensation in a dose-dependent manner.31 The compound has been studied as an effective neuroprotective agent for the management of neuropathic pain, a debilitating form of treatment-resistant chronic pain caused by damage to the nervous system. In this study, the authors claimed to have obtained CBDO by treatment with Oxone® in acetone at ambient temperature overnight, in 95% yield.
However, in a more recent report,32 an inconsistency of the published NMR data with the structure of CBDO was uncovered, proving that the molecule actually isolated and responsible for the bioactivity was instead cannabielsoin (CBE, Fig. 3).33 This is formed spontaneously after epoxidation, by nucleophilic attack of one of the phenolic oxygens on the less substituted carbon of the epoxide functionality.34 The authors also reported a much lower yield (24%) with the same Oxone® method published in the previous study, and developed a new and improved synthesis of CBE based on the protection of the phenolic groups by silylation with BSTFA at 60 °C, oxidation with mCPBA in an ice bath and deprotection with ring closure with NaOH in methanol at ambient temperature, with an overall yield of 72%.32 Analogously, a similar route from CBD to CBE was published very recently, involving acetylation with Ac2O, chemical epoxidation with mCPBA at low temperature and deprotection/cyclisation with K2CO3 in methanol, with an overall yield of 51%.35
In this context, we envisaged an alternative one-pot single-step approach for the epoxidation of CBD and conversion to CBE under milder conditions, by employing a chemo-enzymatic procedure (Scheme 2). This involves peroxyacetic acid as the epoxidising reagent obtained in situ by lipase-catalysed perhydrolysis of the solvent EtOAc in the presence of hydrogen peroxide. This method for the epoxidation of alkenes was first described by Björkling et al. using octanoic acid to generate the peroxyacid in a water/toluene biphasic mixture as a solvent.36 Commercial immobilized lipase B from Candida antarctica, Novozym® 435, was found to have the highest perhydrolytic activity among other conventional lipases. The use of a biphasic water/EtOAc system avoids the need for an additional carboxylic acid since the peracid is formed directly by perhydrolysis of the organic solvent in the presence of low concentrations of H2O2.37
The reaction was firstly screened at a 50 mg scale in a range of conditions, varying the amount of H2O2 (1.1–4.0 equiv.), enzyme loading (5–10% w/w), temperature (4–30 °C) and reaction time (1–24 h). Complete conversion of CBD to CBE could never be achieved under any of the conditions tested (see ESI, Table S1†), affording a maximum analytical yield of 53%. This could be ascribed mainly to a relatively slow reaction rate, coupled with the formation of multiple polar overoxidation products, likely oligomeric, visible by TLC but not by GC-MS (see ESI, Fig. S3†), which is in line with previously reported observations on direct chemical epoxidations of CBD.32 Attempts to increase the rate of epoxidation also led to faster accumulation of polar by-products, either not affecting or even decreasing the overall yield of CBE. Although similar yields could be obtained in a range of conditions, the reaction appeared most efficient with 1.1 equiv. of peroxide and 5% w/w biocatalyst, at 30 °C for 4 h.
The epoxidation was scaled-up to 200 mg CBD, and the product was isolated and purified by column chromatography, affording pure CBE in 31% overall yield. The NMR characterisation data of the product matches the data provided for CBE, as reassigned by Monroe et al. recently.32,35
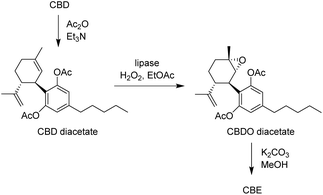
As an alternative, we speculated that the formation of overoxidation products and the related yield loss could be limited by decreasing the electronic activation of the aromatic ring by acylation of the phenolic hydroxyl groups, inspired by the recently published route to CBE involving acetylation, chemical epoxidation and deprotection.35 Therefore, the recovered CBD was firstly acetylated with Ac2O, and then subjected to enzymatic epoxidation to afford CBDO diacetate, followed by hydrolysis of the acetyl groups, leading to pure CBE (Scheme 3). Notably, the epoxidation run under the same conditions described above (1.1 equiv. of H2O2 and 5% w/w biocatalyst, 30 °C) did not show accumulation of large quantities of polar side-products by TLC, even for extended reaction times (up to 24 h). The isolated yield of the epoxidation could thus be increased to 70%, and the overall yield for the 3-step conversion of CBD to CBE was 47%, higher than the direct chemo-enzymatic conversion described above (31%), and very similar to the 3-step chemical method reported (51%).35
Overall, in spite of their similar or lower isolation yields, these chemo-enzymatic approaches for the synthesis of CBE are appealing because of the mild and safe conditions in which the critical oxidation step is run, the absence of wasteful side-products (as opposed to mCPBA epoxidations) or toxic reagents, and the simplicity of operation. A summary of the methods reported in the literature and a comparison with the routes presented in this work is provided in Table 3, highlighting the overall yields, the simplified E-factors (sEFs),38 and other green metrics. From the comparison, it is apparent that the methods presented in this work compare favourably in terms of those parameters with established chemical routes, albeit at the cost of a lower overall yield.
| Method | Ref. | Overall yield [%] | No. of steps | sEFa | Low temp. steps | Atom economy |
|---|---|---|---|---|---|---|
| a Simplified E-factor, calculated according to Roschangar et al.,38 including all waste originated from reagents, co-substrates and catalysts, while not taking into account water, solvents and recyclable materials (see the ESI† for detailed calculations). b 3-Step sequence without purification of the intermediates. | ||||||
| Chemical via silylated CBD | 32 | 72 | 3b | ∼10 | Yes | Low |
| Chemical via CBD diacetate | 35 | 51 | 3 | ∼7 | Yes | Medium |
| Chemo-enzymatic via CBD diacetate | This work | 47 | 3 | ∼7 | No | Medium |
| Chemo-enzymatic one-pot | This work | 31 | 1 | ∼3 | No | High |
Conclusion
The principles of circular economy are bound to become increasingly more central in the future societal, economic and technological development. As a consequence, methods to recover and recycle chemical components from waste biomasses before disposal are highly sought after. One of the most wasteful processes is the steam distillation of essential oils, generating high volumes of spent material per kg of oil.As a specific example, in hemp essential oil production, such a residue constitutes a particularly valuable source because of the presence of cannabinoids, not removed by steam distillation. In this work, the feasibility of the extraction and isolation of CBD from the hemp distillation residue has been demonstrated, using a simple and environmentally friendly maceration with bioethanol followed by vacuum distillation (yield ∼10 g CBD per kg waste). This represents a first step towards the fully circular utilisation of the waste considered. As a general perspective, it would be significantly important to convert also the remaining lignocellulosic residue into fermentable sugar fractions for bioethanol production, solid lignin-derived biofuels, and high-value biomaterials like cellulose nanocrystals or functionalised lignin nanoparticles.39 This would lead to the development of an integrated model for full exploitation of this residual stream, with very little or no waste. Ongoing work is focused in this direction.
Additionally, in this paper, two novel chemo-enzymatic strategies have been proposed and demonstrated for the conversion of the recovered CBD to its structural homolog CBE, a minor cannabinoid with an interesting pharmacological profile. Both routes exploit chemo-enzymatic epoxidation mediated by lipase, leading to pure CBE in 31–47% yield, without the need for low temperatures and hazardous organic peracids in stoichiometric amounts. Further work is ongoing to explore alternative chemo-enzymatic approaches to the valorisation of recovered CBD from the spent hemp distillation residue.
Experimental section
General methods
The C. sativa Futura 75 spent distillation residue has been obtained from Sacmar s.r.l. (Settimo Milanese, Italy). All chemicals and solvents were purchased from Zentek s.r.l. (Milano, Italy) or Merck (Merck Life Sciences s.r.l., Milano, Italy) and used without further purification. All the chromatographic purifications were carried out on a PuriFlash XS-420+ (Interchim) using Purezza-Daily Standard Flash cartridges (Sepachrom, Italy). TLC analyses were performed on Merck Kieselgel 60 F254 plates purchased from Merck. Novozym® 435 (Novozymes) was purchased from Strem Chemicals Inc. (Bischheim, France). 1H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer in CDCl3 solution at room temperature. GC-MS analyses were performed on an Agilent 7890A gas-chromatograph with a 5975C mass detector, using an HP-5MS column (30 m × 0.25 mm × 0.25 μm). Detected substances were identified using GC-MS by comparing the MS data with the National Institute of Standards and Technology (NIST) database, the available standards, or literature data. The following temperature program was employed: 60 °C (1 min)/6 °C min−1/150 °C (1 min)/12 °C min−1/280 °C (5 min).Solvent extraction and purification of CBD
Extraction by dynamic maceration was performed in duplicate on a weighed amount of hemp distillation residue (2 g) with 50 mL of the extraction solvent (MeOH, EtOH, n-heptane, CHCl3, MeOH/CHCl3 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at room temperature for >2 h, under magnetic stirring. The solution was filtered and the residue was extracted again with the same procedure. The filtrates of the two extractions were then combined together and subjected to evaporation under reduced pressure at 40 °C using a rotary evaporator. Yields are reported as mg extract per g sample.
1 v/v) at room temperature for >2 h, under magnetic stirring. The solution was filtered and the residue was extracted again with the same procedure. The filtrates of the two extractions were then combined together and subjected to evaporation under reduced pressure at 40 °C using a rotary evaporator. Yields are reported as mg extract per g sample.
Soxhlet extraction was performed on a weighed amount of hemp distillation residue (10 g) with 250 mL of the extraction solvent (n-heptane, CHCl3, MeOH/CHCl3 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under reflux for >2 h. The solution was filtered and the residue was subjected to evaporation under reduced pressure at 40 °C using a rotary evaporator. Yields are reported as mg extract per g sample.
1 v/v) under reflux for >2 h. The solution was filtered and the residue was subjected to evaporation under reduced pressure at 40 °C using a rotary evaporator. Yields are reported as mg extract per g sample.
The presence of CBD was monitored by TLC using the Fast Blue salt B stain (0.2% w/v in MeOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). TLC separations were performed with an n-hexane/Et2O ratio of 8
1). TLC separations were performed with an n-hexane/Et2O ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; the plates were left to dry and sprayed with Fast Blue salt B solution. CBD was identified by its Rf and colour (Rf = 0.73, colour = orange-red).40 Purification of CBD was carried out either by column chromatography on silica gel (gradient elution from pure n-hexane to n-hexane/EtOAc of 96
2; the plates were left to dry and sprayed with Fast Blue salt B solution. CBD was identified by its Rf and colour (Rf = 0.73, colour = orange-red).40 Purification of CBD was carried out either by column chromatography on silica gel (gradient elution from pure n-hexane to n-hexane/EtOAc of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 over 20 column volumes) or by vacuum distillation in a Kugelrohr apparatus (120–140 °C, 0.1 mbar). Yields are reported as mg CBD per g sample. 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.41,42
4 over 20 column volumes) or by vacuum distillation in a Kugelrohr apparatus (120–140 °C, 0.1 mbar). Yields are reported as mg CBD per g sample. 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.41,42
Characterisation data of cannabidiol (CBD)
1H NMR (CDCl3, 400 MHz): δ 6.24 (2H, br s, 3′ + 5′), 6.00 (1H, br s, 2′-OH), 5.60 (1H, s, 2), 4.77 (1H, br s, 6′-OH), 4.70–4.66 (1H, m, 9-trans), 4.60–4.57 (1H, m, 9-cis), 3.92–3.85 (1H, dm, J = 10.1 Hz, 1), 2.46 (2H, t, J = 7.6 Hz, 1′′), 2.43 (1H, dt, J = 10.6, 3.4 Hz, 6), 2.33–2.20 (1H, m, 4a), 2.16–2.08 (1H, m, 4b), 1.89–1.75 (2H + 3H, m, 5ab + 7), 1.68 (3H, s, 10), 1.58 (2H, quint, 2′′, J = 7.4 Hz), 1.39–1.26 (2H + 2H, m, 3′′ + 4′′), 0.91 (3H, t, 5′′, J = 6.9 Hz). 13C NMR (101 MHz, CDCl3): δ 155.1 (br d, J = 195.6 Hz), 149.3, 143.1, 140.0, 124.3, 113.9, 111.0, 108.9 (br d, J = 166.0 Hz), 46.3, 37.3, 35.6, 31.6, 30.8, 30.5, 28.5, 23.8, 22.7, 20.5, 14.1. GC-MS: tR = 29.0 min, m/z (%) = 314 (M+, 7), 246 (13), 231 (100), 193 (9), 174 (9), 121 (7).One-pot chemo-enzymatic oxidation of CBD to CBE
To a solution of CBD (200 mg, 0.637 mmol) in EtOAc (30 mL, 20 mM), Novozym® 435 (10 mg, 5% w/w) was added, followed by H2O2 (680 μL, 3.5% w/w aq. solution, 1.1 equiv.). The suspension was magnetically stirred in a water bath set at 30 °C, until the highest content of CBE was obtained, as checked by GC-MS (4 h). Enzyme beads were removed by filtration and the reaction mixture was quenched with aq. Na2SO3 solution (1 mL) and aq. sat. NaHCO3 solution (1 mL). Water (5 mL) was added and the mixture was extracted with EtOAc. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The combined organic extracts were washed with brine, dried over Na2SO4, concentrated under reduced pressure, and purified by flash chromatography (SiO2, pet. ether/EtOAc = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 85
1 to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) to afford CBE as a colorless oil (65 mg, 0.197 mmol, 31% yield, 98% purity by GC-MS). Rf = 0.2 (pet. ether/EtOAc = 85
15) to afford CBE as a colorless oil (65 mg, 0.197 mmol, 31% yield, 98% purity by GC-MS). Rf = 0.2 (pet. ether/EtOAc = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.32,35
15). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.32,35
Chemo-enzymatic conversion of CBD to CBE via diacetate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to give CBD diacetate as a colorless oil (290 mg, 0.73 mmol, 77% yield). Rf = 0.4 (pet. ether/EtOAc = 95
5) to give CBD diacetate as a colorless oil (290 mg, 0.73 mmol, 77% yield). Rf = 0.4 (pet. ether/EtOAc = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.35
5). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.35
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 9
5 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give CBDO diacetate as a colorless oil (174 mg, 0.42 mmol, 70% yield). Rf = 0.2 (pet. ether/EtOAc = 95
1) to give CBDO diacetate as a colorless oil (174 mg, 0.42 mmol, 70% yield). Rf = 0.2 (pet. ether/EtOAc = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.35
5). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.35
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 7
2 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to give CBE as a colorless oil (101 mg, 0.30 mmol, 88% yield). Rf = 0.2 (pet. ether/EtOAc = 85
3) to give CBE as a colorless oil (101 mg, 0.30 mmol, 88% yield). Rf = 0.2 (pet. ether/EtOAc = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.32,35
15). 1H NMR, 13C NMR and GC-MS data matched those reported in the literature.32,35
Characterisation data of cannabielsoin (CBE)
1H NMR (400 MHz, CDCl3): δ 6.30 (s, 1H), 6.27 (s, 1H), 5.35 (s, 1H), 5.07 (s, 1H), 5.04 (s, 1H), 4.11 (d, J = 5.9 Hz, 1H), 3.33 (dd, J = 11.1, 5.9 Hz, 1H), 2.49 (t, J = 7.9 Hz, 2H), 1.92–1.85 (m, 1H), 1.83 (s, 3H), 1.77–1.68 (m, 3H), 1.62–1.46 (m, 6H), 1.36–1.25 (m, 4H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 160.1, 153.4, 152.1, 145.0, 116.9, 111.5, 109.8, 103.3, 89.4, 69.3, 48.5, 42.1, 36.2, 34.8, 31.7, 31.0, 28.4, 25.9, 22.7, 22.7, 14.1. GC-MS: tR = 29.5 min, m/z (%) = 330 (M+, 48), 247 (73), 205 (100), 147 (37), 135 (14).Author contributions
Conceptualisation: DT, EB, and FP; data curation: DF, FS, and CN; formal analysis: DF, DT, and FP; investigation: DF, DT, MV, MN, and FP; supervision: DT, EB, and FP; writing – original draft: MV, MN, and FP; writing – review & editing: all authors.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). Sacmar s.r.l. (Dr Ruben Francot) is kindly acknowledged for supplying the C. sativa Futura 75 spent distillation residue used as a starting material.References
- (a) From waste to value: Valorisation pathways for organic waste streams in circular bioeconomies, ed. A. Klitkou, M. Fevolden and M. Capasso, Routledge, London and New York, 2019 Search PubMed; (b) Valorization of agri-food wastes and by-products: Recent trends, innovations and sustainability challenges, ed. R. Bhat, Elsevier, Academic Press, London, 2021 Search PubMed.
- United Nations, Sustainable Development Goals, Goal 12: Ensure sustainable consumption and production patterns, https://sdgs.un.org/goals/goal12, last accessed 05/01/2024.
- O. Schmid, S. Padel and L. Levidow, The Bio-Economy Concept and Knowledge Base in a Public Goods and Farmer Perspective, Bio-Based Appl. Econ., 2012, 1, 47–63 Search PubMed.
- S. Maina, V. Kachrimanidou and A. Koutinas, A roadmap towards a circular and sustainable bioeconomy through waste valorization, Curr. Opin. Green Sustainable Chem., 2017, 8, 18–23 CrossRef.
- B. D. Oomah, M. Busson, D. V. Godfrey and J. C. G. Drover, Characteristics of hemp (Cannabis sativa L.) seed oil, Food Chem., 2002, 76, 33–43 CrossRef CAS.
- (a) G. Crini, E. Lichtfouse, G. Chanet and N. Morin-Crini, Traditional and new applications of hemp, in Sustainable Agriculture Reviews 42: Hemp Production and Applications, ed. G. Crini and E. Lichtfouse, Springer, 2020, pp. 37–87 CrossRef; (b) J. Visković, V. D. Zheljazkov, V. Sikora, J. Noller, D. Latković, C. M. Ocamb and A. Koren, Industrial hemp (Cannabis sativa L.) agronomy and utilization: a review, Agronomy, 2023, 13, 931 CrossRef; (c) G. Kaur and R. Kander, The sustainability of industrial hemp: a literature review of its economic, environmental, and social sustainability, Sustainability, 2023, 15, 6457 CrossRef; (d) M. Saif Ur Rehman, N. Rashid, A. Saif, T. Mahmood and J.-I. Han, Potential of bioenergy production from industrial hemp (Cannabis sativa): Pakistan perspective, Renewable Sustainable Energy Rev., 2013, 18, 154–164 CrossRef.
- (a) C. Schluttenhofer and L. Yuan, Challenges towards revitalizing hemp: A multifaceted crop, Trends Plant Sci., 2017, 22, 917–929 CrossRef CAS PubMed; (b) United Nations, Commodities at a glance – Special issue on industrial hemp, UNCTAD, 2022; (c) Industrial hemp market report by type, source, application, and region, IMARC group, 2023, https://www.imarcgroup.com/industrial-hemp-market, last accessed 02/03/2024.
- S. Okomo Aloo, G. Mwiti, L. Wanjiku Ngugi and D.-H. Oh, Uncovering the secrets of industrial hemp in food and nutrition: The trends, challenges, and new-age perspectives, Crit. Rev. Food Sci. Nutr., 2022, 1–20 CrossRef PubMed.
- B. C. Rodríguez, V. H. Durán-Zuazo, I. F. García-Tejero and B. G. Ruiz, Current and future applications for hemp essential oils: a review, in Current Applications, Approaches, and Potential Perspectives for Hemp, ed. I. F. García-Tejero and V. H. Durán-Zuazo, Elsevier, Academic Press, London, 2023, pp. 365–391 Search PubMed.
- (a) A. el Asbahani, K. Miladi, W. Badri, M. Sala, E. H. A. Addi, H. Casabianca, A. el Mousadik, D. Hartmann, A. Jilale, F. N. R. Renaud and A. Elaissari, Essential oils: From extraction to encapsulation, Int. J. Pharm., 2015, 483, 220–243 CrossRef CAS PubMed; (b) P. Masango, Cleaner production of essential oils by steam distillation, J. Cleaner Prod., 2005, 13, 833–839 CrossRef.
- N. Mahato, M. Sinha, K. Sharma, R. Koteswararao and M. H. Cho, Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes, Foods, 2019, 8, 523 CrossRef CAS PubMed.
- D. A. Teigiserova, L. Hamelin and M. Thomsen, Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy, Sci. Total Environ., 2020, 706, 136033 CrossRef CAS PubMed.
- A. Smeriglio, D. Trombetta, L. Cornara, M. Valussi, V. de Feo and L. Caputo, Characterization and Phytotoxicity Assessment of Essential Oils from Plant Byproducts, Molecules, 2019, 24, 2941 CrossRef CAS PubMed.
- L. Nissen, A. Zatta, I. Stefanini, S. Grandi, B. Sgorbati, B. Biavati and A. Monti, Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.), Fitoterapia, 2010, 81(5), 413–419 CrossRef CAS PubMed.
- P. C. Struik, S. Amaducci, M. J. Bullard, N. C. Stutterheim, G. Venturi and H. T. H. Cromack, Agronomy of fibre hemp (Cannabis sativa L.) in Europe, Ind. Crops Prod., 2000, 11, 107–118 CrossRef.
- (a) M. M. Radwan, S. Chandra, S. Gul and M. A. El Sohly, Cannabinoids, phenolics, terpenes and alkaloids of cannabis, Molecules, 2021, 26, 2774 CrossRef CAS PubMed; (b) I. J. Flores-Sanchez and R. Verpoorte, PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants, Plant Cell Physiol., 2008, 49, 1767–1782 CrossRef CAS PubMed; (c) I. J. Flores-Sanchez and R. Verpoorte, Secondary metabolism in cannabis, Phytochem. Rev., 2008, 7, 615–639 CrossRef CAS.
- L. O. Hanuš, S. M. Meyer, E. Muñoz, O. Taglialatela-Scafati and G. Appendino, Phytocannabinoids: a unified critical inventory, Nat. Prod. Rep., 2016, 33, 1357–1392 RSC.
- (a) Phytocannabinoids: unraveling the complex chemistry and pharmacology of Cannabis sativa, in Progress in the Chemistry of Organic Natural Products, ed. D. Kinghorn, H. Falk, S. Gibbons and K. Kobayashi, Springer, 2017, vol. 103, pp. 1–131 Search PubMed; (b) A. Bilbao and R. Spanagel, Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications, BMC Med., 2022, 20, 259 CrossRef PubMed.
- F. Fulvio, R. Paris, M. Montanari, C. Citti, V. Cilento, L. Bassolino, A. Moschella, I. Alberti, N. Pecchioni, G. Cannazza and G. Mandolino, Analysis of sequence variability and transcriptional profile of cannabinoid synthase genes in Cannabis sativa L. chemotypes with a focus on cannabichromenic acid synthase, Plants, 2021, 10, 1857 CrossRef CAS PubMed.
- A. Sainz Martinez, O. Lanaridi, K. Stagel, H. Halbwirth, M. Schnürch and K. Bica-Schröder, Extraction techniques for bioactive compounds of cannabis, Nat. Prod. Rep., 2023, 40, 676–717 RSC.
- Hemp variety datasheet: C. sativa Futura 75, https://www.konopko.si/files/file/sorte/Datasheet%20Futura%2075%20(FR).pdf, last accessed 05/01/2024.
- Cannabidiol market size, share & trends analysis report by source type, by sales type, by end-use, by region, and segment forecasts, Grand View Research, 2022, https://www.grandviewresearch.com/industry-analysis/cannabidiol-cbd-market, last accessed 05/01/2024.
- (a) E. B. Russo, Taming THC: potential cannabis synergy and phytocannabinoid–terpenoid entourage effects, Br. J. Pharmacol., 2011, 163, 1344–1364 CrossRef CAS PubMed; (b) E. B. Russo, Cannabidiol claims and misconceptions, Trends Pharm. Sci., 2017, 38, 198–201 CrossRef CAS PubMed.
- J. M. Cunha, E. A. Carlini, A. E. Pereira, O. L. Ramos, C. Pimentel, R. Gagliardi, W. L. Sanvito, N. Lander and R. Mechoulam, Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients, Pharmacology, 1980, 21, 175–185 CrossRef CAS PubMed.
- L. C. Nguyen, D. Yang, V. Nicolaescu and T. J. Best, et al., Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses, Sci. Adv., 2022, 8, eabi6110 CrossRef CAS PubMed.
- K. M. Nelson, J. Bisson, G. Singh, J. G. Graham, S. Chen, J. Brient Friesen, J. L. Dahlin, M. Niemitz, M. A. Walters and G. F. Pauli, The essential medicinal chemistry of cannabidiol (CBD), J. Med. Chem., 2020, 63, 12137–12155 CrossRef CAS PubMed.
- E. Kozela, C. Haj, L. Hanuš, M. Chourasia, A. Shurki, A. Juknat, N. Kaushansky, R. Mechoulam and Z. Vogel, HU-446 and HU-465, Derivatives of the Non-psychoactive Cannabinoid Cannabidiol, Decrease the Activation of Encephalitogenic T Cells, Chem. Biol. Drug Des., 2016, 87, 143–153 CrossRef CAS PubMed.
- A. Breuer, C. G. Haj, M. V. Fogaça, F. V. Gomes, N. R. Silva, J. F. Pedrazzi, E. A. D. Bel, J. C. Hallak, J. A. Crippa, A. W. Zuardi, R. Mechoulam and F. S. Guimarães, Fluorinated Cannabidiol Derivatives: Enhancement of Activity in Mice Models Predictive of Anxiolytic, Antidepressant and Antipsychotic Effects, PLoS One, 2016, 11, e0158779 CrossRef PubMed.
- G. Appendino, M. L. Bellido Cabello De Alba and E. Muñoz Blanco, Novel Cannabidiol Quinone Derivatives, 2015, WO2015158381A1.
- J. M. Hardman, R. T. Brooke and B. J. Zipp, Cannabinoid glycosides: In vitro production of a new class of cannabinoids with improved physicochemical properties, bioRxiv, 2017, preprint, DOI:10.1101/104349.
- Y. Nalli, M. S. Dar, N. Bano, J. U. Rasool, A. R. Sarkar, J. Banday, A. Q. Bhat, B. Rafia, R. A. Vishwakarma, M. J. Dar and A. Ali, Analyzing the role of cannabinoids as modulators of Wnt/β-catenin signaling pathway for their use in the management of neuropathic pain, Bioorg. Med. Chem. Lett., 2019, 29, 1043–1046 CrossRef CAS PubMed.
- A. Z. Monroe, W. H. Gordon, J. S. Wood, G. E. Martin, J. B. Morgan and R. T. Williamson, Structural revision of a Wnt/β-catenin modulator and confirmation of cannabielsoin constitution and configuration, Chem. Commun., 2021, 57, 5658–5661 RSC.
- R. Anand, R. Painuli, V. Kumar and P. P. Singh, Chemistry and pharmacological aspects of furanoid cannabinoids and related compounds: Is furanoid cannabinoids open a new dimension towards the non-psychoactive cannabinoids?, Eur. J. Med. Chem., 2024, 268, 116164 CrossRef CAS PubMed.
- D. B. Uliss, R. K. Razdan and H. D. Dalzell, Stereospecific intramolecular epoxide cleavage by phenolate anion. Synthesis of novel and biologically active cannabinoids, J. Am. Chem. Soc., 1974, 96, 7372–7374 CrossRef CAS PubMed.
- D. G. Dennis, S. D. Anand, A. J. Lopez, J. Petrovčič, A. Das and D. Sarlah, Synthesis of the cannabimovone and cannabifuran class of minor phytocannabinoids and their anti-inflammatory activity, J. Org. Chem., 2022, 87, 6075–6086 CrossRef CAS PubMed.
- F. Björkling, S. E. Godtfredsen and O. Kirk, Lipase-mediated formation of peroxycarboxylic acids used in catalytic epoxidation of alkenes, J. Chem. Soc., Chem. Commun., 1990, 19, 1301–1303 RSC.
- (a) E. G. Ankudey, H. F. Olivo and T. L. Peeples, Lipase-mediated epoxidation utilizing urea-hydrogen peroxide in ethyl acetate, Green Chem., 2006, 8, 923–926 RSC; (b) B. Casali, E. Brenna, F. Parmeggiani, F. Tentori and D. Tessaro, Multi-step chemo-enzymatic synthesis of azelaic and pelargonic acids from the soapstock of high-oleic sunflower oil refinement, Green Chem., 2022, 24, 2082–2093 RSC; (c) A. Brandolese, D. H. Lamparelli, M. A. Pericàs and A. W. Kleij, ACS Sustainable Chem. Eng., 2023, 11, 4885–4993 CrossRef CAS PubMed.
- F. Roschangar, R. A. Sheldon and C. H. Senayanake, Overcoming barriers to green chemistry in the pharmaceutical industry – the Green Aspiration Level™ concept, Green Chem., 2015, 17, 752–768 RSC.
- (a) A. Zoghlami and G. Paës, Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis, Front. Chem., 2019, 7, 874 CrossRef CAS PubMed; (b) R. Zhang, H. Gao, Y. Wang, B. He, J. Lu, W. Zhu, L. Peng and Y. Wang, Challenges and perspectives of green-like lignocellulose pretreatments selectable for low-cost biofuels and high-value bioproduction, Bioresour. Technol., 2023, 369, 128315 CrossRef CAS PubMed; (c) Y. Wang, P. Liu, G. Zhang, Q. Yang, J. Lu, T. Xia, L. Peng and Y. Wang, Cascading of engineered bioenergy plants and fungi sustainable for low-cost bioethanol and high-value biomaterials under green-like biomass processing, Renewable Sustainable Energy Rev., 2021, 137, 110586 CrossRef CAS.
- N. Galand, D. Ernouf, F. Montigny, J. Dollet and J. Pothier, Separation and identification of cannabis components by different planar chromatography techniques (TLC, AMD, OPLC), J. Chromatogr. Sci., 2004, 42, 130–134 CAS.
- Y. H. Choi, A. Hazekamp, A. M. G. Peltenburg-Looman, M. Frédérich, C. Erkelens, A. W. M. Lefeber and R. Verpoorte, NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa, Phytochem. Anal., 2004, 15, 345–354 CrossRef CAS PubMed.
- E. Chiurchiù, S. Sampaolesi, P. Allegrini, D. Ciceri, R. Ballini and A. Palmieri, A novel and practical continuous flow chemical synthesis of cannabidiol (CBD) and its CBDV and CBDB analogues, Eur. J. Org. Chem., 2021, 1286–1289 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary figures and tables, details of sEF calculations, and NMR and GC-MS characterisation of compounds. See DOI: https://doi.org/10.1039/d4gc00415a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |