Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbonic anhydrase assisted acidogenic fermentation of forest residues for low carbon hydrogen and volatile fatty acid production: enhanced in situ CO2 reduction and microbiological analysis
Omprakash
Sarkar
 *a,
Io
Antonopoulou
*a,
Io
Antonopoulou
 *a,
Charilaos
Xiros
b,
Ylva
Bruce
b,
Sarra
Souadkia
b,
Ulrika
Rova
a,
Paul
Christakopoulos
*a,
Charilaos
Xiros
b,
Ylva
Bruce
b,
Sarra
Souadkia
b,
Ulrika
Rova
a,
Paul
Christakopoulos
 a and
Leonidas
Matsakas
a and
Leonidas
Matsakas
 *a
*a
aBiochemical Process Engineering, Division of Chemical Engineering, Department of Civil, Environmental and Natural Resources Engineering, Luleå University of Technology, SE-97187 Luleå, Sweden. E-mail: omprakash.sarkar@ltu.se; io.antonopoulou@ltu.se; leonidas.matsakas@ltu.se
bRISE Processum AB, SE-891 22, Örnsköldsvik, Sweden
First published on 5th April 2024
Abstract
Carbonic anhydrase (CA) is considered an efficient enzyme for fermentation systems exhibiting a wide range of applications, enhancing both the efficacy and output of the fermentation process. The present study aimed to evaluate the production of acidogenic biohydrogen (bioH2) and volatile fatty acids (VFA) using forest residues as a renewable feedstock. Specifically, the study examined the integration of CA derived from Desulfovibrio vulgaris into the acidogenic fermentation (AF) process. The experimental procedure involved a cascade design conducted in two distinct phases. In phase I, the concentration of CA in the AF was systematically optimized, with glucose serving as the substrate. In phase II, three influential parameters (pH, pressurization with in situ generated gas and organic load) were evaluated on AF in association with optimized CA concentration from phase I. In phase II, glucose was replaced with renewable sugars obtained from forest residues after steam explosion pretreatment followed by enzymatic saccharification. The incorporation of CA in AF was found to be beneficial in steering acidogenic metabolites. Alkaline conditions (pH 8) promoted bioH2, yielding 210.9 mLH2 gCOD−1, while introducing CA further increased output to 266.6 mLH2 gCOD−1. This enzymatic intervention improved the production of bioH2 conversion efficiency (HCE) from 45.3% to 57.2%. Pressurizing the system accelerated VFA production with complete utilization of in situ produced H2 + CO2 compared to non-pressurized systems. Particularly, caproic acid production was improved under pressurized conditions which was accomplished by the targeted enrichment of chain-elongating bacteria in the mixed culture. The microbial diversity analysis showed the dominance of Firmicutes suggesting a significant degree of adaptation to the experimental contexts, leading to an enhanced production of acidogenic metabolites.
1. Introduction
Currently, there has been an increasing emphasis on addressing climate change and ensuring energy security, thus the need for renewable sources to contribute to the world's increased energy supply. Among the various renewable sources, forest waste/residues have drawn attention to the rising worldwide demand for energy and the need to cut greenhouse gas (GHG) emissions.1 Forest residues are considered renewable, unlike fossil fuels, which deplete long-term carbon storage; second, forest biomass recycles carbon, rather than releasing it into the atmosphere.Due to its geographical positioning and climatic conditions, Sweden exhibits favorable characteristics for a growing number of forests, which encompass around 60% of the nation's land area. The yearly growth of the forest amounts to around 100 MCM (million cubic meter) of standing volume, and the amount of timber harvested is between 50–80 MCM of standing volume annually during the last decades.2,3 The process of forest cutting results in the production of a significant quantity of byproducts referred to as residues, which are typically regarded as waste materials.4 Logging residues may constitute a substantial environmental concern owing to their potential to undergo decomposition and subsequently emit methane, a potent greenhouse gas. In addition, they possess the potential to provide a fire risk and may serve as a magnet for various forms of pests and illnesses.2
Forest residues are considered a valuable resource due to their inherent advantages and characteristics demonstrating the capacity to produce a wide array of products including biofuels, renewable chemicals and other value-added.5,6 While energy production from forest logging leftovers is an efficient method for mitigating environmental impact.7,8 It is pertinent to highlight that the utilization of forest biomass residues, possesses the capacity to function as a feasible and environmentally sustainable energy resource for the country.7 Consequently, several governmental initiatives have been implemented in Sweden with the aim of fostering the use of forest residuals. Furthermore, several regional and municipal policies have been implemented to encourage the use of residual forest biomass.3,9,10
Hydrogen (H2), being a clean-burning fuel, holds immense potential as an energy carrier in the pursuit of industrial decarbonization including steelmaking and cement production.
Acidogenic fermentation (AF), an intermediate stage of anaerobic digestion (AD) and is a highly versatile platform that facilitates the conversion of organic waste/wastewater into a diverse range of value-added products, such as biohydrogen and volatile fatty acids (VFAs).11 AF process holds immense significance in the realm of bioenergy and bio-based chemicals production, as it facilitates the retrieval of valuable resources from waste streams, thereby aiding in waste minimization and fostering environmental sustainability.12,13 AF is a complex interaction of microorganisms that facilitates the breakdown of organic matter in anaerobic conditions, thereby producing hydrogen gas along with volatile fatty acids (VFAs).13,14 VFAs are a class of organic acids that exhibit a diverse array of industrial applications. Acetic, propionic acid and butyric acid are the major VFAs produced.15 Harnessing the potential of wood biomass/residues as a feedstock for AF holds immense potential in advancing sustainability and driving the growth of a circular bioeconomy.
The complex structural of wood biomass makes it difficult to use as a substrate for microbes to access and degrade the sugars contained in the cellulose and hemicellulose. Besides its main role, lignin provides an important barrier to microbial decomposition. To render wood biomass amenable to microbial activities, pretreatment is necessary to facilitate the decomposition of its intricate structure and enhance the accessibility of sugars to microbes. Steam explosion (SE) is a physicochemical pretreatment method that is a highly effective for lignocellulosic biomass pretreatment.16,17
The generation of CO2 is an integral part of the acidogenic/acetogenic/methanogenic process. Metabolic pathways for various products include decarboxylation steps, leading to decreased product yield and quick release of CO2 into the environment. The acidogenic process results in the production of 2 moles of CO2 for every two moles of acetate and butyrate produced through the corresponding pathways.11,18 Similarly, over 40% of carbon is emitted as CO2 during bio-methanation process (CH3COOH → CH4 + CO2). Employing carbonic anhydrase in the fermentation system for in situ CO2 capture offers a novel opportunity to transform it into renewable chemicals and low carbon fuels along with reduction of CO2 emissions into the environment.19,20 Moreover, utilizing biogenic CO2 is favored to guarantee that the products stay carbon neutral and help in achieving climate neutrality. For instance, Europe produces 69.7 Mt of CO2 annually from the upgrading and combustion of biogas, bioethanol, and other chemicals produced through fermentation processes.21
CO2 capture has proven to be significant in enhancing the efficacy of various fermentation procedures, including ethanol, lactic, succinic acid, and biogas production.22,23 For example, the CO2 generated during the ethanol fermentation process was effectively captured using a packed column filled with NaOH, KOH, or NH4OH, facilitating the absorption of CO2. The resulted carbonate solution serves a dual purpose: firstly, it aids in maintaining the desired pH levels, and secondly, it acts as a valuable source of CO2 for a succinic acid fermentation process.22 CAs, which are ubiquitous enzymes are known to be highly prevalent among bacteria, archaea, and eukaryotes, wherein they are postulated to fulfill a multitude of roles.24,25 CA serve as catalysts for the reversible hydration reaction of carbon dioxide (CO2) into bicarbonate, accelerating the limiting step of CO2 solubilization in aqueous media.26,27 As such, CA has the potential to mitigate CO2 levels in bioconversion processes resulting in elevated levels of CO2 uptake by the microbes or enzymes and providing a viable carbon supply for the production of high-added value products, such as biofuels and chemicals.28–31 In a study pertaining to ethanol fermentation, the use of CA in situ during fermentation was found to enhance the production of ethanol by 20%.28
In the present study, we evaluated the effect of supplementation of CA derived from Desulfovibrio vulgaris to the acidogenic fermentation process towards biohydrogen and volatile fatty acids production from forest residue hydrolysates derived through stem explosion pretreatment. Specifically, this study provides an insight into the intricate mechanisms of AF towards biohydrogen and volatile fatty acids in the presence of CA, investigating its potential role in regulating the pH levels, while concurrently utilizing the acidogenic CO2 within the reactor supporting its mitigation. Furthermore, microbial diversity study was conducted to provide and insight into the alterations occurring during AF of FR derived sugars with respect to experimental variations implemented.
2. Materials and methods
2.1. Acidogenic mixed consortia and substrate
The inoculum used in this study is obtained from a biogas production plant located in Luleå, Sweden. Prior to being used as inoculum, the sludge underwent filtration through nylon mesh to remove stones and other solid objects. Filtered sludge was given heat shock pretreatment at 80–90 °C for 1 hour. The pretreated sludge was then enriched using a nutrient media consisting of glucose: 3 gCOD L−1, CaCl2: 5 mg L−1, CoCl2: 25 mg L−1, CuCl2: 10.5 mg L−1, FeCl3: 25 mg L−1, K2HPO4: 0.25 g L−1, KH2PO4: 0.25 g L−1, MgCl2: 0.3 g L−1, MnCl2: 15 mg L−1, NH4Cl: 0.5 g L−1, and ZnCl2: 11.5 mg L−1.2.2. Enzyme production
Enzyme expression was done according to Sjöblom et al. (2020).32 The nucleotide sequence of the thermostable CA variant, DvCA8.0, derived from Desulfovibrio vulgaris and combined with a polyhistidine tag (6xHis-tag), was precisely integrated within the NdeI and XhoI restriction sites of the pET22b(+) vector.33 The gene synthesis and cloning were executed by GenScript, (Piscataway, New Jersey, USA). The vector underwent a subsequent transformation process, resulting in its integration into Escherichia coli BL21(DE3). Following this transformation, stock cultures were diligently preserved for future use. To synthesize CA, a volume of 500 μL of a cultured population of genetically modified E. coli BL21 (DE3) cells was introduced into a 50 mL solution of Luria–Bertani preculture medium, supplemented with 100 μg mL−1 of the antibiotic ampicillin. This mixture was then subjected to an overnight incubation period at a temperature of 37 °C, with continuous agitation at a rate of 180 rpm. A 1% v/v pre-culture was introduced into an auto-inducing lactose medium (specifically ZYP-5052, devoid of trace elements) supplemented with 100 μg mL−1 of ampicillin. After 24 h of cultivation at 37 °C and 180 rpm, the cultures were harvested. Since no CA activity was observed, the harvested culture was centrifuged at 8000 rpm for 5 minutes, and the obtained supernatant was discarded. The 1 L culture cells were resuspended in 200 mL in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v solution of Tris-HCl 0.1 M pH 8.0 and NaOH 0.2 M. After that, they underwent three cycles of lysis at 700 bar pressure in a laboratory homogenizer (SPX, Crawley, UK). Using a pressurized filtration system (Sterlitech, Kent, WA, USA), the cell debris was removed by centrifugation at 8000 rpm for 5 minutes. The recovered supernatant, which is the entire cell lysate, was filtered to a particle size of 0.2 μm. Lastly, a tangential flow filtering system (Millipore, Burlington, MA, USA; MWCO 10 kDa) was used to concentrate the lysate 30 times. CA expression was confirmed by using a E. coli culture having a non-cloned pET22b(+) vector as negative control. The CA activity was determined by the Wilbur–Anderson assay. The activity of the lysate was determined as an equivalent of 143 WAU per μL CA.
1 v/v solution of Tris-HCl 0.1 M pH 8.0 and NaOH 0.2 M. After that, they underwent three cycles of lysis at 700 bar pressure in a laboratory homogenizer (SPX, Crawley, UK). Using a pressurized filtration system (Sterlitech, Kent, WA, USA), the cell debris was removed by centrifugation at 8000 rpm for 5 minutes. The recovered supernatant, which is the entire cell lysate, was filtered to a particle size of 0.2 μm. Lastly, a tangential flow filtering system (Millipore, Burlington, MA, USA; MWCO 10 kDa) was used to concentrate the lysate 30 times. CA expression was confirmed by using a E. coli culture having a non-cloned pET22b(+) vector as negative control. The CA activity was determined by the Wilbur–Anderson assay. The activity of the lysate was determined as an equivalent of 143 WAU per μL CA.
2.3. Pretreatment of forest residues
Forestry residues (FR; residues gathered from tree thinning or logging, such as tops and branches) was collected from Swedish forest. The untreated FR composition is presented in Table 1. Steam explosion (SE) pretreatment of FR was performed evaluating the influence of sulphur dioxide (SO2) and sulfuric acid (H2SO4). For treatments employing H2SO4, residence time, reaction temperature, and acid quantity were optimized aiming towards maximizing the sugars release and minimizing the inhibitors generated. In detail, different concentrations of H2SO4 (1 to 5% w/w), varying temperatures (170 to 215 °C), and different pretreatment times (2 to 15 minutes) were examined. The most promising results were obtained with 1% H2SO4 at either 195 °C for 5 minutes or at 215 °C for 10 minutes. Treatment with SO2 took place at 205 °C, (∼18 bar) and 7 min of reaction time. Table 1 displays the composition of the solid fraction resulting from the SE pretreatment of FR (the liquid portion was not used in this study) (Table 1). The solid materials obtained from the SO2 pretreatment method were used for acidogenic fermentation.| Treatment temperature | Hemicellulose (g kg−1 of dry material) | Cellulose (g kg−1 of dry material) | Lignin (Klason; g kg−1 of dry material) | Yield (% w/w) | |
|---|---|---|---|---|---|
| H2SO4 | 195 °C | 35.55 | 285.3 | 567 | 44.9 |
| 215 °C | 7.3 | 249.3 | 666 | 43.8 | |
| SO2 | 205 °C | 23.1 | 279.9 | 585 | 33.4 |
| Untreated forest residue | — | 145.1 | 224.1 | 384 | — |
2.4. Acidogenesis: feedstock and fermentation
The feedstock was prepared in two steps. In step I, the solid fraction obtained from the steam explosion process was separated using filter paper (liquid portion was not used in this study). In step II, the separated solid fraction was employed for enzymatic hydrolysis. Enzymatic hydrolysis was carried out using cellulase, enzyme blend (Cellic® CTec2, Sigma-Aldrich; 10 FPU per g solid biomass) in sodium citrate buffer at 50 °C for 30 h.34 Slurry obtained from enzymatic hydrolysis was further centrifuged at 2000 rpm to separate leftover solids particles, the liquid portion containing reducing sugars (composed of glucose, xylose, arabinose, galactose, and mannose) was evaluated as acidogenic fermentation substrate/medium for biohydrogen and volatile fatty acids in serum bottles (200 mL) as reactors. The composition of reducing sugar is provided in section 3.1. The pretreated filtered sludge was used as inoculum with an inoculum-to-substrate ratio of 1 for VS. The study was conducted in two phases (Fig. 1). During phase I, the concentration of CA in the acidogenic fermentation was optimized. Three different concentrations of enzyme were added: 15 μL mLfeed−1 (R1), 7.5 μL mLfeed−1 (R2), and 3.76 μL mLfeed−1 (R3). Furthermore, R4 (only substrate without CA) and R5 (without substrate and CA) were used as control experiments. The substrate used phase I was glucose at an organic load of 12 gCOD L−1. In phase II, experiments were conducted in a cascade where initially the best enzyme concentration (from phase I) was selected for its evaluation on acidogenic fermentation using sugars hydrolysate as fermentation substrate (derived from enzymatic hydrolysis of solids derived from steam explosion of forest residues). Here in phase II, in presence of CA, initially the impact of different pH (R6: pH 6, R7: pH 7 and R8: pH 8) and the influence of in situ headspace pressure (in situ generated H2 + CO2 from organics fermentation) at pH 6 (R9), pH 7 (R10) and pH 8 (R11) were studied. The optimal identified conditions (best pH + in situ pressure/non-pressurization) were then applied at higher organic load (R12: 20 gCOD L−1, R13: 30 gCOD L−1 and R14: 40 gCOD L−1). Reactors (R6–14) were operated with CA were compared with its respective control that didn't contain enzyme (R15–R23). In both the phases, nutrient solution (composed of nutrients as indicated in section 2.1 expect glucose) was added along with fermentation media.2.5. Analytical methods
The chance in pH during fermentation was monitored using a pH meter (pHenomenal-pH1100L; VWR, Stockholm, Sweden). VFAs in the fermentation medium were analyzed on a high-performance liquid chromatography system (PerkinElmer, Waltham, MA, USA) equipped with a pump (410 LC), a refractive index detector (RID-6A), and an Aminex HPX-87H column (300 × 7.8 mm; Bio-Rad, Hercules, CA, USA). The column was maintained at 65 °C. The mobile phase was 5 mM H2SO4 eluted at a flow rate of 0.6 mL min−1. VFAs were quantified using calibration curves derived from commercially available standards (10 mM, VFA mix; Sigma-Aldrich). The headspace pressure was measured using Greisinger GMH 3111 pressure gauge equipped with Greisinger GMSD 10 BR Relative pressure gauge GMSD 10 BR. The chemical composition of the biogas was assessed on an Agilent 990 micro-GC instrument, equipped with a COX column (COX UM1MX0.8MMID BF, CP-PORABOND Q, 1MX 0.25 mm × 3 μm; Agilent Technologies, Santa Clara, CA, USA). The column was maintained at 110 °C and the injector at 80 °C. Hydrogen energy was calculated considering its LHV value (120 MJ kg−1). An ANOVA was conducted to evaluate the differences between pairs of means for the CA enzyme addition on fermentation process (Table 2). The statistical analysis (Tukey's test) was performed with OriginPro 2021 (Academic edition, version 9.8.0.200).| Comparison between reactors operated with varied concentration of CA enzyme | Mean difference | q value | Probability | Significancea,b,c |
|---|---|---|---|---|
| a Significance value equal 1 indicates the difference of the mean is significant at α = 0.05. b Significance value equal 0 indicates the difference of the mean is not significant at α = 0.05. c Significance value equal −1 indicates the difference of the mean is not tested. | ||||
| R2–R1 | 44.41 | 56.41 | 0 | 1 |
| R3–R1 | 104.31 | 132.5 | 0 | 1 |
| R3–R2 | 59.9 | 76.08 | 0 | 1 |
| R4–R1 | 39.28 | 49.89 | 0 | 1 |
| R4–R2 | −5.13 | 6.52 | 0.007 | 1 |
| R4–R3 | −65.03 | 82.6 | 0 | 1 |
2.6. DNA extraction
Upon completion of the experiment, on day 3, 1–2 g of biomass was collected from the reactor and preserved at −20 °C. The biomass sample was rinsed with phosphate-buffered saline solution and then subjected to centrifugation at a speed of 8000 rpm for 15 minutes. The DNA was isolated using the FastDNA spin kit for soil (MP Biomedicals, Irvine, CA, USA) according to the manufacturer's protocol. Subsequently, the DNA concentration was determined using the Qubit dsDNA HS/BR assay kit (Thermo Fisher Scientific, Waltham, MA, USA).2.7. Library preparation, DNA sequencing, and bioinformatics processing
Amplicon libraries were prepared according to a standard protocol to target the variable region 4 (abV4C) of the 16S rRNA gene in bacteria and archaea.35 In summary, a total of 10 nanograms of extracted DNA was used to perform polymerase chain reaction (PCR) amplification of the abV4C amplicons in both bacterial and archaeal samples. Each PCR reaction had a volume of 25 μL, consisting of 12.5 μL of PCRBIO Ultra mix and 400 nM concentrations of both forward and reverse primers. The forward primer [515FB] 5′-GTGYCAGCMGCCGCGGTAA-3′ and reverse primer [806RB] 5′-GGACTACNVGGGTWTCTAAT-3′ were developed according to the instructions provided in the Illumina Technical Manual.35 Primer tails facilitate the linkage of Illumina Nextera adaptors for sequencing in a following PCR. The amplicon libraries were purified following the standard CleanNGS SPRI beads protocol (CleanNA, NL) with a bead to sample ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The DNA was eluted in 25 μL of nuclease free water from Qiagen in Germany. The DNA concentration was determined using the Qubit dsDNA HS Assay kit (Thermo Fisher Scientific, USA). Conducting gel electrophoresis with the Tapestation 2200 and D1000/High sensitivity D1000 screentapes (Agilent, USA) was essential in verifying the size and purity of a portion of the amplicon libraries. A detailed overview of the methods employed in DNA sequencing, library preparation, and bioinformatics processing has been previously described.36
5. The DNA was eluted in 25 μL of nuclease free water from Qiagen in Germany. The DNA concentration was determined using the Qubit dsDNA HS Assay kit (Thermo Fisher Scientific, USA). Conducting gel electrophoresis with the Tapestation 2200 and D1000/High sensitivity D1000 screentapes (Agilent, USA) was essential in verifying the size and purity of a portion of the amplicon libraries. A detailed overview of the methods employed in DNA sequencing, library preparation, and bioinformatics processing has been previously described.36
3. Results and discussion
3.1. Pretreatment of forest residues
Two different catalysts, i.e. H2SO4 and SO2, were tested for the pretreatment of forest residuals (FR) which contained mainly tops and branches form the trees (mixture of softwood and hardwood). SO2 pretreated solids were used for the subsequent biogas experiments. As shown in Table 1, the SE of forest residues with SO2 at 205 °C resulted to production of pretreated solids containing 279.9 g kgsolid−1 cellulose, 23.1 g kgsolid−1 hemicellulose and 585 g kgsolid−1 lignin. Further, the steam exploded FR (solid fraction) was subjected to enzyme hydrolysis (10 FPU per gsolids). Enzyme hydrolysis resulted in reducing sugars (53.8 g L−1) composed of glucose (48.3 g L−1) followed by xylose (3.1 g L−1), arabinose (0.6 g L−1), galactose (0.4 g L−1) and mannose (1.4 g L−1). This reducing sugar was employed as a renewable substrate in the second phase of the study.3.2. Biogas production
The experiment consisted of two distinct phases. During phase I, glucose was used as the substrate for the acidogenic fermentation (AF) to determine the optimal concentration of CA. Fig. 2 depicts biogas output with respect to varied concentration of CA (R1: 3.76 μL mLfeed−1, R2: 7.5 μL mLfeed−1, R3:15 μL mLfeed−1) at initial pH 7. The biogas production was found to be the highest in R3, with a cumulative gas volume of 430 mL gCOD−1 followed by R4 (410 mL gCOD−1; no CA addition), R2 (390 mL gCOD−1) and R1 (385 mL gCOD−1). Interestingly, the reactor (R5) that was operated without substrate but supplemented with enzyme (CA: 15 μL mLfeed−1) failed to demonstrate biogas production, thereby suggesting that the enzyme introduced did not serve as a substrate for the mixed culture fermentation. The optimization process was examined using one-way ANOVA. The analysis of variance (ANOVA) showed that the effects of CA on the fermentation process are statistically significant, indicating that the mean difference between the selected CA concentrations was significantly greater with R3 (Table 2). Phase II of the AF experiments included substituting glucose with renewable sugars derived from forest residuals, and the optimal concentration of CA (15 L mLfeed−1) was selected based on its excellent acidogenic performance towards increased production of gases observed in phase I. | ||
| Fig. 2 (a) Biogas production and (b) biohydrogen production recorded during fermentation of glucose and sugars from forest residuals under various experimental conditions. | ||
In phase II, during evaluating effect of three different pH, the highest biogas production was notice at pH 8 (R8: 430 mL gCOD−1) compared to pH 7 (R7: 410 mL gCOD−1) and pH 6 (R6: 350 mL gCOD−1). These findings highlight the importance of alkaline conditions and the efficiency of pretreatment method adopted for lignocellulosic biomass in delivering sugars for fermentation. The fermentation microenvironment, specifically in an alkaline redox microenvironment, exhibited greater potential for enhanced biohydrogen production when compared to its corresponding neutral and acidic conditions. In contrast to an acidic pH, an alkaline redox microenvironment elicits an enhanced hydrolytic effect on carbohydrates and proteins. This is achieved through the ionization of charged groups, such as carboxylic groups, thereby promoting the accessibility of soluble and readily assimilable substrates for fermentation.18,37 Typically, this phenomenon occurs during the initial hour of the fermentation process. In the context of an elevated alkaline pH, it is noteworthy that a significant proportion of carbohydrates exhibit the capacity for anaerobic biodegradation. Furthermore, alkaline redox conditions serve to impede the proliferation of hydrogenoclastic methanogens, thereby preserving the molecular hydrogen (H2) in its original form and preventing its conversion into terminal methanogenic byproducts, such as CH4. Presence of carbonic anhydrase enzyme along with alkaline pH conditions significantly contributes to the buffering of these biological systems.18,37 In contrast, reactors R15 (310 mL gCOD−1), R16 (384 mL gCOD−1), and R17 (398 mL gCOD−1) operated without CA as control setup for R6, R7 and R8 exhibited a reduced biogas production. In addition, headspace in situ pressurization (4.8–5.1 bar) did not improve biogas output, as this was declined to 415 mL gCOD−1 (R11; pH 8), 385 mL gCOD−1 (R10; pH 7) and 350 mL gCOD−1 (R9; pH 6). Absence of CA for in situ pressurized reactors (R9–11) considerably lowered the biogas values to 310 mL gCOD−1 (R18; pH 6), 340 mL gCOD−1 (R19; pH 7) and 375 mL gCOD−1 (R20; pH 8). The pressure within the headspace has the potential to affect the partial pressure of H2 in the system.38,39 The concept of partial pressure refers to the pressure exerted by an individual gas within a mixture of gases.40 Within a reactor, gases produced by microbes including H2 during fermentation processes. The partial pressure of H2 is a significant determinant influencing the microbial metabolites and its profile through various biochemical reactions. Under pressurized condition the transfer of H+ ions and electrons play a crucial role in facilitating the normal progression of the reactions. Furthermore, the decrease in the partial pressure of H2 leads to a decrease in the Gibbs free energy of reactions involving H2 as a product.40,41 This change in energy favors the production of H2, particularly in reactions like homoacetogenesis. However, it is important to note that this reduction in partial pressure can be detrimental to hydrogen consumption when it is lowered.39,40,42 Pressurized system may result in the saturation of CO2 in the medium of the reactors, potentially impacting pH levels and causing changes to microbial activity leading to a shift in the metabolic pathway.43 Nevertheless, the presence of carbonic anhydrase in the reactor can effectively mitigate this issue by utilizing in situ accumulated CO2 within the reactor. This suggest regulation of headspace pressure that could be one of the strategies to control the metabolic pathways towards the target product.20,39,44 Combining best pH (8), and pressurized condition along with CA enzyme (15 μL mLfeed−1), the influence of organic load (OL) was examined which showed an increase in biogas production up to 30 gCOD L−1, slightly declined at 50 gCOD L−1. Through the implementation of CA enzyme-enhanced fermentation, the greater biogas production occurred at a concentration of 20 gCOD L−1 (R12: 435 mL gCOD−1), and 30 gCOD L−1 (R13: 423 mL gCOD−1) and declined at 40 gCOD L−1 (R14: 397 mL gCOD−1). Conversely, in the absence of CA enzyme, the biogas production was notably lower even under identical experimental conditions, with volumes of 365 mL gCOD−1 (R21: 20 gCOD L−1), 378 mL gCOD−1 (R22: 30 gCOD L−1) and 366 mL gCOD−1 (R23: 40 gCOD L−1). This marked difference in biogas production clearly demonstrates the significant role played by CA enzyme during the fermentation process.
The documented bioH2 production in phase II is impressive and can be compared with glucose fermentation in phase I, emphasizing the effective adaptation of mixed culture to the lignocellulosic hydrolysate under implemented experimental conditions. Table 3 presents the bioH2 and other acidogenic metabolites derived from various lignocellulosic based feedstocks (Table 3). Using lignocellulosic feedstocks to produce bioH2 without costly and energy-intensive purification and neutralization appears promising. The addition of CA was beneficial in promoting the acidogenic process. Enhanced bioH2 production with CA addition can be ascribed to its ability to regulate pH and improve redox equilibrium. Acidogenic fermentation is often associated with the redox equilibrium within the microbial population, and CA was found to potentially regulate the pH and CO2 levels. No significant increase in bioH2 generation was found when the OL was raised to 30 g L−1 and 40 g L−1, in comparison to the levels of 10 g L−1 and 20 g L−1. This might be due to the volumetric nature of the CA enzyme addition (15 μL mLfeed−1), as the introduction of more organic load led to an elevated generation of CO2 in the reactor. However, this rise in CO2 production was inadequate to compensate for the lower enzyme quantity.
| Feedstock/biomass | Reactor type | Pretreatment/hydrolysis | Acidogenic metabolites (VFA/hydrogen yield) | Ref. |
|---|---|---|---|---|
| Birch sawdust | 2 L glass bottle reactor; AMPTS-II | Organosolv fractionation | 121.4 mL gVS−1 | 75 |
| VFAs 20.44 g L−1 | ||||
| Duckweed | 125 mL serum bottle | Acid, alkaline, and thermal hydrolyses. | 169.3 mLH2 gbiomass−1 | 76 |
| Rice straw | 100 mL serum bottles | Fungus Gymnopus contrarius J2 | 5.71 mmol gbiomass−1 | 77 |
| Rice crop wastes | 20 L anaerobic bioreactor | Untreated | 40 mL H2 gVS−1 | 78 |
| Corn stover | 500 mL glass reactor | Enzymatic hydrolysis: Ctec2 | 36.1 mLH2 gbiomass−1 | 79 |
| Pine tree wood | 100 mL serum bottles | Dilute acid pretreatment | 1.28–1.36 molH2 molsugar−1 | 80 |
| Waste paper | 250 mL serum bottle | Acid hydrolyzed | 15.31–139.97 mLH2 gsugar−1 | 81 |
| Grass waste | 150 mL glass bottles | Ionizing radiation + acid pretreatment | 68 mL ggrass added−1 | 82 |
| Forest residues | 200 mL glass bottles | Organosolv fractionation + enzyme hydrolysis | 266.6 mLH2 gCOD−1 | This study |
| 0.8 gVFA gCOD−1 |
3.3. Influence of CA on volatile fatty acids production
Volatile fatty acids (VFAs) are the byproduct of the metabolic activity of acidogenic/acetogenic bacteria, which occurs through the oxidative transformation of sugars and various organic compounds during the fermentation process (eqn (1) & (2)).47| C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2 | (1) |
| C6H12O6 → CH3CH2CH2COOH + 2CO2 + 2H2 | (2) |
VFAs that cogenerate during hydrogen fermentation serve as an indicator to determine the progress of the process. Organic waste/wastewater fermentation based VFAs have the potential to fuel sustainability, maximize resources, and used as green platform chemicals in different industries. In this study, the presence of CA in the fermentation medium had an influence on both the concentration and composition of the VFAs. In the phase I, it was found that the net VFA production from glucose was greater in the reactor dosed with a higher enzyme load (0.95 gCOD L−1: R3) compared to the control (0.76 gCOD L−1: R5) and other two enzyme loads (0.9 gCOD: R2 and 0.88 gCOD L−1: R1). In phase II, to develop more knowledge of the CA role in the AF process, the results of all the reactors operated with CA (R6–R14) were compared against the efficiency of all the reactors run without CA (R15–R23). In the first experiments evaluating redox conditions, it was seen that a pH 8 enhanced VFA production. Specifically, R8, supplemented with CA yielded 0.9 gCOD L−1, while R17, which lacked enzyme, only achieved 0.78 gCOD L−1. In the next step, the reactors were subjected to pressurization using in situ generated H2 + CO2, which along with the addition of CA, the production level raised to 0.95 gCOD L−1 (R10, pH 7), which was 1.28 times more than that of the control reactor (R19, pH 7).
Finally, the combined effect of optimized parameters including in situ pressurization, alkaline pH 8 and CA were employed to assess the impact of organic load on acidogenic metabolites. The results indicated that the reactor fermenting 20 gCOD L−1 (R12) was superior producing VFA of 0.94 gCOD L−1, in comparison to the reactors operating even at higher loadings of 30 gCOD L−1 (R13) and 40 gCOD L−1 (R14). In contrast, in absence of CA, the production value exhibited a significant decrease ranging 0.67–0.84 gCOD L−1 in R21–R23 (control to R12–R13). Overall, the net VFA production values with the reactors supported by CA (R6–R14) exhibited 1.28–1.12 higher compared to its control reactors (R15–R23) (Table 4).
| Reactors | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | R22 | R23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 (%) | 45 | 55 | 64 | 51 | 0 | 53 | 57 | 62 | 51 | 55 | 64 | 56.76 | 60 | 64 | 50 | 51 | 53 | 44 | 45 | 48 | 52 | 53 | 57 |
| CO2 (%) | 55 | 45 | 36 | 49 | 0 | 47 | 43 | 38 | 49 | 45 | 36 | 43.24 | 40 | 51.72 | 50 | 49 | 47 | 56 | 55 | 52 | 45.95 | 45.62 | 52.90 |
| Lactic acid (gCOD h−1) | 0.006 | 0.006 | 0.008 | 0.009 | 0 | 0.013 | 0.007 | 0.005 | 0.031 | 0.026 | 0.020 | 0.039 | 0.006 | 0.007 | 0.012 | 0.003 | 0.001 | 0.001 | 0.002 | 0.001 | 0.010 | 0.015 | 0.016 |
| Ethanol (gCOD h−1) | 0.001 | 0.001 | 0.002 | 0.001 | 0 | 0.023 | 0.022 | 0.020 | 0.023 | 0.016 | 0.018 | 0.027 | 0.015 | 0.006 | 0.019 | 0.014 | 0.012 | 0.031 | 0.023 | 0.006 | 0.001 | 0.022 | 0.013 |
| H2 energy (kJ gCOD−1) | 1.73 | 2.15 | 2.75 | 2.09 | 0.00 | 1.86 | 2.34 | 2.67 | 1.79 | 2.12 | 2.66 | 2.47 | 2.54 | 2.54 | 1.55 | 1.96 | 2.11 | 1.36 | 1.53 | 1.80 | 1.90 | 2.01 | 2.09 |
| pH | 5.13 | 5.62 | 5.8 | 5.1 | 6.89 | 5.26 | 6.57 | 6.14 | 5.11 | 6.21 | 6.34 | 6.35 | 6.21 | 6.23 | 4.87 | 5.26 | 5.47 | 4.79 | 5.19 | 6.45 | 6.25 | 6.24 | 6.37 |
It is interesting that in phase I, the synthesis of acetic acid (0.21 gCOD) + butyric acid (0.43 gCOD) represented 84.55% of the net volatile fatty acids (VFAs) in R4. The concentrations of these specific fatty acids with R4 were somewhat higher compared to the values observed in the reactors supplemented with CA (R1–R3). In contrast, earlier observations indicated that the reactor with CA (R1, R2, and R3) had a higher net VFA production in comparison to R4. This striking difference in the distribution of individual fatty acids can be ascribed to the transformation of SCFAs into MCFAs such as caproic acid within the reactor. Caproic acids is a chain elongated fatty acids which formed through reverse β-oxidation (RBO) pathway where SCFA (acetic and butyric acid) bio-convert to longer carbon chain (caproic acid) in presence of an electron donor. The production of caproic acid through glucose fermentation, aided by CA, resulted in a significant increase in production. Specifically, the production values of 0.15 gCOD (R3) followed by 0.13 gCOD (R2) and 0.12 gCOD (R1) represented a 2.21, 1.93 and 1.83-fold increase, respectively, compared to the R4 (0.068 gCOD) operated without CA. This increase in caproic acid production may be ascribed to the utilization of in situ CO2 along with organic substrate for the synthesis of SCFA, which are then transformed into MCFA. Other than RBO, MCFA yield is determined by chain elongation cyclic reactions is fatty acid synthesis. Both cyclic routes need reduced compounds as electron donors such as sugar alcohols, sugars, lactate, ethanol, methanol, and H2, with ethanol being the most popular for high-rate chain elongation.49 Caproic acid, exhibits significant potential in various domains, including the production of biofuels and biochemicals. To enhance the productivity of caproic acid, the microbial process needs proper investigations and regulation considering the influential factors.36,50
In phase II, the metabolic route was altered with respect to substrate and fermentation pH (6, 7 and 8) impacting the pattern and distribution of SCFA and MCFA. While acetic and butyric acid production was minimal across all reactors, caproic acid production was significantly influenced. Upon examining the distribution pattern, it was found that SCFA (HAc + HBu) biosynthesis was favored at acidic (pH 6: 81.59%, R6) and neutral (pH 7: 78.98%, R7) compared to alkaline environment (pH 8: 77.1%, R8), on the other hand, caproic acid biosynthesis was favored by initial pH 8 (0.17 gCOD, R8) and pH 7 (0.14 gCOD, R7) compared to acidic pH 6 (0.12 gCOD, R6).
Further, the alkaline pH was found to be favorable in enhancing acetate and butyrate production when the reactors R9, R10, and R11 were pressurized. The recorded acetate production was 0.20 gCOD (R11, pH 8) > 0.19 gCOD (R10, pH 7) > 0.18 gCOD (R9, pH 6) whereas butyrate production was 0.57 gCOD (pH 8, R11) > 0.56 gCOD (pH 7, R10) > 0.53 gCOD (pH 6, R9). Alkaline pH promotes hydrolysis of substrate and provide excellent buffering capabilities.51,52 On the other side, the catalytic activity of the CA is found to be more efficient in alkaline microenvironment.53 Additionally, CA regulates pH and buffering in biological processes. Specifically, at alkaline pH, this enzyme expedites the swift conversion between CO2 and bicarbonate ions, contributing to the stabilization of pH.54,55 Additionally, the reactors R18, R19, and R20, employed as control equivalents to R9, 10, and R11, exhibited a comparable pattern marked by elevated levels of acetate and butyrate production under alkaline conditions. However, it is important to highlight that the fatty acids in the control reactors remained accumulated, whereas in R9–R11 with CA, the accumulated fatty acids were slightly consumed indicating its transformation.
The synergistic application of pH 7, CA and pressurized system have been observed to exhibit a promising approach in steering the biosynthesis of caproic acid. The observed outcome from this combination delivered a yield of 0.31 gCOD, in contrast to the pH 6 (0.23 gCOD) and pH 8 (0.22 gCOD). Pressurized systems are advantageous since H2 in the headspace serve as an electron donor along with ethanol and lactic acid for chain elongation which was further supported by its complete consumption in the reactor.39,56 Although pressurized, the yields noticed from the reactors that were operated without CA was 0.097 gCOD (pH 6), 0.104 gCOD (pH 7), and 0.089 gCOD (pH 8) which was 59.5%, 67% and 59,6% lower, respectively compared to the reactors dosed with CA. Furthermore, the partial pressure can influence the formation of H2 in the reactor. The presence of CA can potentially influence the process by reducing H2 partial pressure that hinder the process, promoting its production through shifting the reaction. The preferential accumulation of acetate and butyrate at elevated concentrations in the fermentation medium during H2 fermentation can be ascribed to the pathways that have been and discussed earlier. Moreover, various other metabolites, including propionate was found at lower levels which is an indication towards sustained H2 production because propionic acid pathway can lead with significant consumption of H2. Increasing the headspace pressure with in situ produced H2 + CO2 has been found a way to potentially change the acid product spectrum.
In the final step of phase II, examining the impact of organic load in conjunction with CA and pressurization, it was found that 20 gCOD L−1 was superior to 30 gCOD L−1 and 40 gCOD L−1, resulting in the production of 0.94 gCOD (R12), 0.76 gCOD (R13), and 0.79 gCOD (R14) respectively. In contrast, the formation of MCFA without CA was extremely poor and restricted to 0.19 gCOD (20 gCOD L−1), 0.14 gCOD (30 gCOD L−1), and 0.11 gCOD (40 gCOD L−1) in R21, R22 and R23 respectively which was 1.46, 2.72, and 2.68 times lower than its respective CA dosed reactors (R12, R13 and R14). It was expected that the yields of SCFA and MCFA would be greater at higher organic loads. The same pattern appeared in the case of bioH2, where no significant rise was noticed. This suggests that the dosage of CA in the fermenting medium was inadequate, resulting in a limited solubilization of in situ CO2 in the reactor. Consequently, this disruption of the redox equilibrium within the system occurred.
3.4. Inorganic (IC) and organic carbon (OC) utilization
An efficient utilization of organic and inorganic carbon plays a crucial role in determining the efficiency of acidogenic/acetogenic towards biohydrogen and VFA production signifying the importance in the context of biomass hydrogen fermentation. The addition of CA significantly influenced the consumption of both the organic substrate (sugars from forest residuals) and the inorganic carbon (CO2). Among all the 23 reactors operated in phase I and II, the highest carbon utilization efficiency above 85% was showed by the reactors dosed with CA (Fig. 4). In phase I, the complete utilization of the substrate in R3 can be attributed to the simpler substrate fermentation by mixed culture accompanied by CA addition converting 1.23 gCOD of organic along with 154.8 mL of CO2 to microbial metabolites. In contrast, the reactor without CA (R4) achieved only 74.54% of substrate utilization, transforming only organic part (0.97 gCOD) to SCFA/MCFA leaving 200.9 mL of CO2 unused. In the second part of the experiment, the reactors with CA operated under alkaline conditions and pressurization (R8, R10, R11 and R12) exhibited >85% substrate utilization. R12 exhibited the highest substrate utilization efficiency of 91.54%, followed by R8 (89.90%), R10 (89.22%), and R11 (88.39%) with simultaneous utilization of CO2. These reactors successfully transformed substrate of 1.19 gCOD, 1.17 gCOD, 1.16 gCOD, and 1.15 gCOD, respectively, into metabolites. The control reactors (R15–R23), which were run in parallel with the specified configuration without enzymes, demonstrated that the absence of CA may have an influence on the formation of metabolites inside the reactor, leading to a decrease in the efficiency of product generation. Reactors supplemented with CA used almost all the in situ CO2 they produced, whereas lack of CA in R13–R23 used just 12–37%. These results suggest that presence of CA not only helped to enhance organic substrate transformation to metabolites but also assisted in the in situ CO2 utilization. The utilization of CO2 through the addition of CA within the reactor presents a promising avenue for effectively managing and harnessing its potential while mitigating its environmental impact. This approach holds the potential to not only minimize the adverse effects of CO2 but also create additional value in the process. The utilization of CA in fermentation system is anticipated to result in a favorable response in terms of promoting the proliferation and metabolic functionality of bacteria such as homoacetogens that exhibit the capability utilizing CO2 and H2.27 CA can potentially impact a specific group of microorganisms, steering them towards the efficient utilization of CO2 by providing a conveniently accessible carbon substrate. This substrate is particularly advantageous for bacteria that rely on CO2 as their primary source of carbon. Additionally, the use of CA in the mitigation of CO2 particularly during fermentation process exhibits considerable potential as a technology to enhance the efficacy of the processes along with controlling the release of GHG into the atmosphere. | ||
| Fig. 4 Substrate utilization efficiency exhibited by individual reactors fermenting glucose and sugars from forest residuals during phase I and phase II studies. | ||
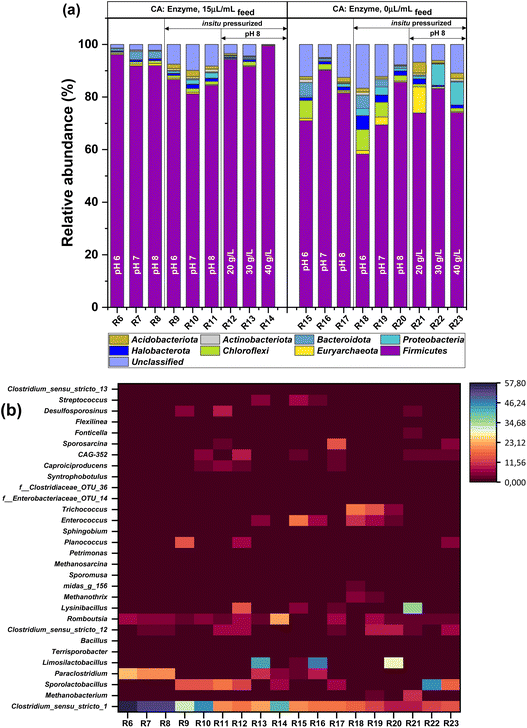
3.5. Microbiological analysis
Microbiome investigations were conducted to assess the microbial composition with respect to the progression of the acidogenic metabolites by a mixed culture, both in the presence and absence of CA. Fig. 6 and 7 presents a comprehensive depiction of the relative abundance of various microbial species that constitute the acidogens enrichment at the end of the experiment. The microbial community structure of all 18 reactors (R6–R23) operated during phase II was assessed by comparing the reactors with (R6–R14) and without CA (R15–R23). The Venn diagram illustrates greater levels of genetic variation and difference among the reactors operated at different experimental conditions. Fig. 6a shows the comparative analysis utilizing the 6 samples obtained from the reactors, which were subjected to varying pH conditions, demonstrated that among the 475 operational taxonomic units (OTUs), a total of 5 OTUs (1.05%) were found to be present in all six groups, regardless of the presence or absence of the enzyme in the reactor. This observation suggests that these OTUs might exhibit a species-specific nature. The R6, followed by R7, R8, R15, R16, and R17, exhibited a total of 71, 70, 67, 69, 66, and 74 unique OTUs respectively. The reactors with (R9–R11) and those without CA (R18–R20) operated under pressurized conditions shared 12 OTUs, however CA-dosed reactors possessed 65 (R9), 68 (R10), and 63 (R11) unique OTUs, while control to these reactors displayed 73 (R18), 100 (R19), and 68 (R20) (Fig. 6b). During OL operation, all the six reactors exhibited a shared set of 9 OTUs among them. The reactors, with CA, displayed unique OTUs of 60 (R12), 67 (R13), and 60 (R14). In contrast, without CA operation exhibited unique OTUs of 65 (R21), 72 (R22), and 69 (R23) respectively (Fig. 6).Another hydrogen-producing genus that dominated reactors was Paraclostridium which is an obligate anaerobe that can efficiently produce hydrogen from wastewater sludge and glucose by dark fermentation.59,61 The documented significant production of hydrogen particularly with R6, R7, and R8 can be connected to the concurrent proliferation of Paraclostridium in conjunction with Clostridium sensu stricto. The prevalence of Paraclostridium, was seen in the reactors operated with CA was higher at pH 6 (23.8%, R6) in comparison to pH 7 (20.9%, R7) and pH 8 (19.4%, R8). Conversely, in the absence of enzymes, these percentages significantly decreased to a range of 3–8% in R15, R16 and R17. Paraclostridium, an obligate anaerobe, exhibits the ability to undergo sporulation. The utilization of organic carbon by Paraclostridium sp. result with an efficient hydrogen production. The addition of CA did not stimulate a growth of Paraclostridium in pressurized reactors, as its proliferation was restricted to a range of 4–10%. In contrast, the operation without CA under pressurized conditions resulted in the total elimination of Paraclostridium. The suppressed development growth of Paraclostridium under pressurization condition indicated that the pressure could have impacts on its growth. However, the observed developed headspace pressure (3–4 bar) was found to be beneficial for other genera, including Clostridiumsensu stricto 1, Clostridium sensu stricto 12, Romboutsia, and Caproiciproducens. These pressure-adapted genera are especially known for their ability to produce biohydrogen and volatile fatty acids.
Clostridium sensu stricto 1, Clostridium sensu stricto 12, Romboutsia, Paraclostridium, and Caproiciproducens were the genera associated with biohydrogen and VFAs production. The Clostridium butyricum, a bacterium belonging to the genera Clostridium sensu stricto 1 (Firmicutes), exhibited a strong association with the implemented experimental conditions. The Firmicutes bacterial populations exhibited dominance in terms of hydrogen production, with the related species of the Clostridium genus occupying the prominent population position in the reactor, thereby resulting in enhanced efficiency of hydrogen production. The primary metabolic pathways associated with hydrogen production encompass the acetate and butyrate pathway. The experimental findings show a strong positive connection between acetate and butyrate biosynthesis with hydrogen cogeneration. Clostridium has garnered interest for hydrogen fermentation. Mixed culture inoculum investigations related most Clostridium genus to high hydrogen production from a variety of organic substrates.62 This study contained more than four genera of Clostridium. One of the most common fermentative H2 producer is Clostridium butyrium, which makes up to 17–27.65% of all bacteria in the reactor dosed with CA operated under in situ pressure.
Romboutsia, a member of the family Peptostreptococcaceae within the phylum Firmicutes has the capacity to metabolize carbohydrates, resulting in the production of acetate, formate, ethanol, and lactic acid.58,63 The observed increased prevalence of Romboutsia, in conjunction with other genera, exhibited a consistent pattern with the elevated VFA and bioH2 in the reactors. The abundance of Romboutsia in during pH optimization was 5.2 ± 0.84% (R6–R8), while without CA it was a slightly lower fraction (2.72 + 1.72%), highlighting the significance of CA in in situ buffering and promoting favorable condition for microbial growth. Further, growth of Romboutsia was marginally decreased particularly under pressurization indicating that developed in situ pressure was unfavorable on its propagation regardless of the presence or absence of CA. However, moving towards increase in organic load proved to be beneficial as it notably facilitated the proliferation of the bioH2 and VFA producer Romboutsia, especially in the reactors with CA (10.23%, R12–R14) over without CA (2.86%, R21–R23).
The biosynthesis of caproic acid can be attributed to the enrichment of chain elongator bacteria in the mixed culture. Caproiciproducens belongs to the family Ruminococcaceae under firmicutes known for caproic acid along with molecular hydrogen and other VFAs production.64,65 Chain elongation driven by lactate occurs in a few species of Caproiciproducens. During lactate oxidation, the yielded pyruvate undergoes subsequent oxidation, resulting in the formation of acetyl-CoA and CO2 with simultaneous electrons in the form of reduced ferredoxin. The acetyl-CoA, which is obtained through a process, serves as substrate for the biosynthesis of butyrate and caproate via a reverse β-oxidation pathway.66 The reactors R11and R12 exhibited a presence of Caproiciproducens (>3%), particularly when dosed with CA and subjected to in situ pressurization. These reactors showed a lactic acid consumption at the rate of 0.02 gCOD h−1 (R11), and 0.039 gCOD h−1 (R12) leading to its complete utilization within 48-hour. On the other side, the proliferation of Caproiciproducens was much lower (<3%) in the other reactors. Despite being below 3% in these reactors, the production of caproic acid showed significant efficacy, potentially attributable to the other chain elongators and their synergistic interactions within the reactors. The batch experiments demonstrated the production of lactic acid and ethanol, as well as the substantial consumption and transformation of these molecules into short-chain fatty acids (SCFAs) and medium-chain fatty acids (MCFAs). These findings indicate the presence of a beneficial synergy in the mixed culture, despite the presence of lactic acid bacteria (LAB), which ultimately enhanced the yield of the desired products.
Few setups in this study (particularly when the reactors were pressurized; R9, R10, R11, R12, R22 and R23) showed the dominance of LAB. The contribution of LAB, specifically Sporolactobacillus, in bioH2 production remains an area that requires further elucidation. Previous studies showed that LAB can have a negative impact on bioH2 production due to substrate competition or with release of bacteriocins. For example, Cieciura-Włoch et al. (2020) observed instability in the bioH2 production attributing towards the prevalence of the Lactobacillaceae family accounting for 59% of the total microbial population in the reactor.67 As per the findings of Park et al., there was a sudden termination in bioH2 production due to accumulation of lactic acid.68 In the study conducted by Chatellard et al. (2016), it was observed that the presence of various carbohydrates had distinct effects on the growth of mixed bacterial communities. Specifically, hexoses were found to promote the proliferation of LAB, while pentoses were observed to stimulate the growth of BioH2 producing bacteria.69 Forest residues hydrolysate used as substrate in this study, containing of good fraction of glucose which theoretically provides equal conditions for the proliferation of both lactic acid and hydrogen producing bacterial species. Nevertheless, other research has shown that LAB have a superior growth rate and enhanced substrate absorption capabilities compared to Clostridia, hence granting them a competitive advantage over other fermentative bacterial species.70,71
In this study, lactic acid synthesis was observed on day one, but its complete utilization was demonstrated as well by day 3, indicating its transformation to other metabolites such as chain elongated butyric or caproic acid. This finding aligns with previous studies investigating microbial diversity derived from hydrogen-producing bioreactors where Esquivel-Elizondo et al. (2017) postulated that Lactobacillaceae are the primary producers of butyrate in the reactor.72 Likewise, other studies have made similar assumptions on volatile fatty acids production.36,58,71 In a study conducted by De Leeuw (2019), it was observed that the presence of Caproiciproducens ASV2 in the reactor, alongside Lactobacillus rhamnosus ASV4, during the initial high level of total VFAs production resulted in a higher production of butyric and caproic acids, while the production of acetic acid was lower. This outcome is likely attributed to a chain elongation process facilitated by Caproiciproducens, utilising lactic acid generated by lactobacilli as an electron donor.73 External electron donors, such as lactic acid or ethanol, in the media promote the development of chain elongators. However, lactic acid and ethanol are also byproducts of fermentation. The findings of our study clearly show that the complete utilization of these electron donors within the reactor serves as the primary source of electrons for the chain elongation process. On contrary, studies that have documented the presence of LAB with H2 production indicating the potential role of LAB in providing supplementary substrates to hydrogen-producing bacteria, either in the form of lactate or through the direct conversion of lactate into hydrogen.
The typical chain elongator Clostridium kluyveri, is known for caproic acid production from acetic and butyric acid and is a member of the genus Clostridium sensu stricto 12, which also included homoacetogens. Genera Clostridium sensu stricto 12 had the second-highest growth after Clostridium sensu stricto 1. Reactors supplemented with enzyme showed the good association between Clostridium sensu stricto 12 and consumption of ethanol, H2 and in situ CO2. Particularly the abundancy of Clostridium sensu stricto 12 was higher with the reactor operated under in situ pressurization. The application of pressurization potentially elicited an augmented uptake of carbon dioxide in the presence of an enzyme, thereby promoting the proliferation of the microorganism Clostridium sensu stricto 12. Furthermore, the application of pressurization conditions promotes the utilization of H2 and CO2 by homoacetogens.74 Similar to the pattern of lactic acid consumption, ethanol was found to be consumed at a rate ranging from 0.001 to 0.031 gCOD h−1 until it was completely consumed. The production and consumption of ethanol was slightly higher with the reactors operated at pH 6 (0.023–0.031 gCOD h−1) compared to pH 7 (0.022–0.023 gCOD h−1) and pH 8 (0.013–0.014 gCOD h−1). The increased fraction of two genera Clostridium sensu stricto 12 and Caproiciproducens in the mixed culture was responsible for the production of hexanoic acid through chain elongation process. The observed shifts in microbial communities and the selective enrichment of certain bacterial species during mixed culture fermentation highlight the importance of experimental conditions shaping the structure and adaptability of the community towards biohydrogen and VFAs from wood biomass hydrolysate.
Exploring bio-based chemicals and fuels as a sustainable alternative to fossil-based production is a widely explored method for ensuring fuel and chemical supply while also reducing CO2 emissions. Even though the CO2 produced from fermentation processes is classified as biogenic since the carbon is sourced from sustainable resources and is part of a natural biogenic carbon cycle. It is not recommended to overlook CO2 emissions from fermentation processes because of their substantial impact on greenhouse gas emissions. Implementing the traditional fermentation process in conjunction with the CA enzyme-based method can be a viable option contributing to reducing carbon emissions in a roundabout yet efficient manner, transforming CO2 into biobased fuels and chemicals. Employing an appropriate treatment such as stem explosion fractionization, forest residues can serve as an excellent feedstock for biobased products. This study particularly focused on examining the effects of CA on mixed culture fermentation of stem exploded forest resides optimizing various influential factors. However further research in carbonic anhydrase-based fermentation at different scales are necessary to gain greater insight of the process and regulate the system to enhance the recovery of metabolites.
4. Conclusion
Forest residues exhibit great potential as a renewable feedstock for the production of biobased fuels and chemicals. In this study, we have successfully demonstrated the potential of carbonic anhydrase derived from Desulfovibrio vulgaris on the acidogenic process across various experimental settings. The addition of CA not only enhanced the production of acidogenic metabolites such as biohydrogen and volatile fatty acids, but also contributed to the mitigation of in situ CO2. The individual and intricate interactions of the chosen factors (pH, organic load, and in situ gas pressurization) were observed to greatly enhance the acidogenic product outcome compared to their individual effects in the presence of CA. The abundance of biohydrogen and VFA-producing clostridium genera and its synergistic interaction with other bacterial community in the mixed culture, indicated the crucial role of CA in regulating the process efficiency, leading to the development of an environment conducive to the efficient synthesis of acidogenic/acetogenic metabolites. Overall, this strategy of utilizing produced acidogenic CO2 within a system is in line with the principles of circular economy, waste reduction, and sustainable resource management. It helps maximize the benefits of bioprocesses, such as anaerobic digestion, while minimizing environmental impacts and contributing to a more sustainable and environmentally friendly approach to huge quantities of forest residuals utilization for bioenergy and other value-added products biosynthesis.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Authors would like to thank Bio4Energy for financial support (B4E3-FM-1-10).References
- F. Schipfer, in https://task40.ieabioenergy.com/roles-of-bioenergy-technologies-in-energy-system-pathways-towards-a-wb2-sdg-world/, 2019.
- R. Lundmark, The supply of forest-based biomass for the energy sector: the case of Sweden, International Institute for Applied Systems Analysis, IIASA, 2003 Search PubMed.
- A. Kumar, S. Adamopoulos, D. Jones and S. O. Amiandamhen, Waste Biomass Valorization, 2021, 12, 65–80 CrossRef.
- R. Kurt, Biofuels, Bioprod. Biorefin., 2020, 14, 315–325 CrossRef CAS.
- S. K. Bhatia, S. S. Jagtap, A. A. Bedekar, R. K. Bhatia, K. Rajendran, A. Pugazhendhi, C. V. Rao, A. E. Atabani, G. Kumar and Y.-H. Yang, Sci. Total Environ., 2021, 765, 144429 CrossRef CAS PubMed.
- R. Hu, J. Zhan, Y. Zhao, X. Xu, G. Luo, J. Fan, J. H. Clark and S. Zhang, Green Chem., 2023, 25, 8970–9000 RSC.
- E. Commission, J. R. Centre, A. Camia, N. Robert, R. Jonsson, R. Pilli, S. García-Condado, R. López-Lozano, M. Velde, T. Ronzon, P. Gurría, R. M′barek, S. Tamosiunas, G. Fiore, R. Araujo, N. Hoepffner, L. Marelli and J. Giuntoli, Biomass production, supply, uses and flows in the European Union – First results from an integrated assessment, Publications Office, 2018 Search PubMed.
- J. Giuntoli, A. Agostini, S. Caserini, E. Lugato, D. Baxter and L. Marelli, Biomass Bioenergy, 2016, 89, 146–158 CrossRef CAS.
- L. Di Lucia and K. Ericsson, Energy Res. Soc. Sci., 2014, 4, 10–20 CrossRef.
- A. Millot, A. Krook-Riekkola and N. Maïzi, Energy Policy, 2020, 139, 111358 CrossRef CAS.
- R. Moscoviz, E. Trably, N. Bernet and H. Carrère, Green Chem., 2018, 20, 3159–3179 RSC.
- O. Sarkar, R. Katakojwala and S. V. Mohan, Green Chem., 2021, 23, 561–574 RSC.
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Green Chem., 2022, 24, 6373–6405 RSC.
- G. A. Martinez, S. Rebecchi, D. Decorti, J. M. B. Domingos, A. Natolino, D. Del Rio, L. Bertin, C. Da Porto and F. Fava, Green Chem., 2016, 18, 261–270 RSC.
- E. V. Fufachev, B. M. Weckhuysen and P. C. A. Bruijnincx, Green Chem., 2020, 22, 3229–3238 RSC.
- D. Carpenter, T. L. Westover, S. Czernik and W. Jablonski, Green Chem., 2014, 16, 384–406 RSC.
- G. Tofani, E. Jasiukaitytė-Grojzdek, M. Grilc and B. Likozar, Green Chem., 2024, 26, 186–201 RSC.
- O. Sarkar, A. N. N. Kumar, S. Dahiya, K. V. V. Krishna, D. K. D. K. Yeruva and S. V. V. Mohan, RSC Adv., 2016, 6, 18641–18653 RSC.
- R. R. Bommareddy, Y. Wang, N. Pearcy, M. Hayes, E. Lester, N. P. Minton and A. V. Conradie, iScience, 2020, 23(6) DOI:10.1016/j.isci.2020.101218.
- O. Sarkar, U. Rova, P. Christakopoulos and L. Matsakas, Sustainable Energy Fuels, 2022, 6(3), 778–790 RSC.
- V. Rodin, J. Lindorfer, H. Boehm and L. Vieira, J. CO2 Util., 2020, 41, 101219 CrossRef CAS.
- N. P. Nghiem and G. E. Senske, Appl. Biochem. Biotechnol., 2015, 175, 2104–2113 CrossRef CAS PubMed.
- P. L. Fosbøl, J. Gaspar, B. Jacobsen, J. Glibstrup, A. Gladis, K. M. Diaz, K. Thomsen, J. M. Woodley and N. von Solms, Energy Procedia, 2017, 114, 1434–1443 CrossRef.
- C. T. Supuran, A. Di Fiore and G. De Simone, Expert Opin. Emerging Drugs, 2008, 13, 383–392 CrossRef CAS PubMed.
- P. Lozano and E. García-Verdugo, Green Chem., 2023, 25, 7041–7057 RSC.
- J. G. Ferry, Bioorg. Med. Chem., 2013, 21, 1392–1395 CrossRef CAS PubMed.
- Z. Zhang, B. Lian, W. Hou, M. Chen, X. Li and Y. Li, Afr. J. Microbiol. Res., 2011, 5, 106–112 CAS.
- J. Qian and P. Zheng, Biochem. Eng. J., 2023, 191, 108809 CrossRef CAS.
- M. Sangeetha, A. Sivarajan, M. Radhakrishnan, N. Siddharthan and R. Balagurunathan, Arch. Microbiol., 2022, 204, 270 CrossRef CAS PubMed.
- E. Sapountzaki, U. Rova, P. Christakopoulos and I. Antonopoulou, ChemSusChem, 2023, 16, e202202312 CrossRef CAS PubMed.
- I. Antonopoulou, U. Rova and P. Christakopoulos, in Multienzymatic Assemblies: Methods and Protocols, Springer, 2022, pp. 317–344 Search PubMed.
- M. Sjöblom, I. Antonopoulou, I. G. Jiménez, A. de Oliveira Maciel, S. G. Khokarale, J.-P. Mikkola, U. Rova and P. Christakopoulos, ACS Sustainable Chem. Eng., 2020, 8, 13672–13682 CrossRef.
- O. Alvizo, L. J. Nguyen, C. K. Savile, J. A. Bresson, S. L. Lakhapatri, E. O. P. Solis, R. J. Fox, J. M. Broering, M. R. Benoit and S. A. Zimmerman, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16436–16441 CrossRef CAS PubMed.
- M. Monção, K. Hrůzová, U. Rova, L. Matsakas and P. Christakopoulos, Molecules, 2021, 26 Search PubMed.
- Illumina, 2014. 16S Metagenomics Studies with the MiSeq ® System, pp. 1–4.
- O. Sarkar, U. Rova, P. Christakopoulos and L. Matsakas, Eng. Life Sci., 2022, 22(10), 650–661 CrossRef CAS PubMed.
- S. Dahiya, O. Sarkar, Y. V. V. Swamy and S. Venkata Mohan, Bioresour. Technol., 2015, 182, 103–113 CrossRef CAS PubMed.
- L. Li, Y. Li, R. Yasser Farouk and Y. Wang, Bioresour. Technol., 2019, 289, 121684 CrossRef CAS PubMed.
- O. Sarkar, S. K. Butti and S. Venkata Mohan, Energy, 2017, 118, 425–434 CrossRef CAS.
- B. H. Yan, A. Selvam and J. W. C. Wong, Bioresour. Technol., 2017, 245, 1000–1007 CrossRef CAS PubMed.
- B. H. Yan, A. Selvam, S. Y. Xu and J. W. C. Wong, Bioresour. Technol., 2014, 159, 249–257 CrossRef CAS PubMed.
- K.-S. Lee, T.-S. Tseng, Y.-W. Liu and Y.-D. Hsiao, Int. J. Hydrogen Energy, 2012, 37, 15556–15562 CrossRef CAS.
- J. Huang, Y. Zhao, C. Lei, Y. Liu and Y. Wang, Bioresour. Technol., 2021, 329, 124830 CrossRef CAS PubMed.
- E. A. Cazier, E. Trably, J.-P. Steyer and R. Escudié, Bioresour. Technol., 2015, 190, 106–113 CrossRef CAS PubMed.
- G. Kyazze, N. Martinez-Perez, R. Dinsdale, G. C. Premier, F. R. Hawkes, A. J. Guwy and D. L. Hawkes, Biotechnol. Bioeng., 2006, 93, 971–979 CrossRef CAS PubMed.
- S. Srikanth and S. Venkata Mohan, Int. J. Hydrogen Energy, 2014, 39, 10028–10040 CrossRef CAS.
- S. Dahiya, S. Chatterjee, O. Sarkar and S. V. Mohan, Bioresour. Technol., 2021, 321, 124354 CrossRef CAS PubMed.
- H. J. Van Lingen, C. M. Plugge, J. G. Fadel, E. Kebreab, A. Bannink and J. Dijkstra, PLoS One, 2016, 11, e0161362 CrossRef PubMed.
- X. Shi, L. Wu, W. Wei and B.-J. Ni, Crit. Rev. Environ. Sci. Technol., 2022, 52, 3787–3812 CrossRef CAS.
- P. Candry and R. Ganigué, Curr. Opin. Biotechnol., 2021, 67, 99–110 CrossRef CAS PubMed.
- O. Sarkar, U. Rova, P. Christakopoulos and L. Matsakas, Bioresour. Technol., 2021, 319, 124233 CrossRef CAS PubMed.
- K. Khatami, M. Atasoy, M. Ludtke, C. Baresel, Ö. Eyice, Z. Cetecioglu, E. L. Emerson and P. J. Weimer, Chemosphere, 2021, 101, 129981 CrossRef PubMed.
- I. Bertini, C. Luchinat and A. Scozzafava, Inorg. Chim. Acta, 1980, 46, 85–89 CrossRef CAS.
- A. de Oliveira Maciel, P. Christakopoulos, U. Rova and I. Antonopoulou, Chemosphere, 2022, 299, 134419 CrossRef CAS PubMed.
- Y. Wang, M. Li, Z. Zhao and W. Liu, J. Mol. Catal. B: Enzym., 2015, 116, 89–94 CrossRef CAS.
- F. C. F. Baleeiro, S. Kleinsteuber and H. Sträuber, Front. Bioeng. Biotechnol., 2021, 9, 1–15 CrossRef.
- G. Yang, Y. Yin and J. Wang, Int. J. Hydrogen Energy, 2019, 44, 13147–13156 CrossRef CAS.
- O. Sarkar, L. Matsakas, U. Rova and P. Christakopoulos, iScience, 2023, 26(4), 106519 CrossRef CAS PubMed.
- G. Yang and J. Wang, Int. J. Hydrogen Energy, 2019, 44, 25542–25550 CrossRef CAS.
- G. Kumar, P. Bakonyi, P. Sivagurunathan, N. Nemestóthy, K. Bélafi-Bakó and C.-Y. Lin, Biofuel Res. J., 2015, 2, 209–2014 CrossRef CAS.
- Y. Hu, Y. Shen and J. Wang, J. Cleaner Prod., 2020, 268, 122190 CrossRef CAS.
- I. Valdez-Vazquez, L. G. Castillo-Rubio, M. Pérez-Rangel, A. Sepúlveda-Gálvez and A. Vargas, Ind. Crops Prod., 2019, 137, 105–111 CrossRef CAS.
- J. Gerritsen, A. Umanets, I. Staneva, B. Hornung, J. Ritari, L. Paulin, G. T. Rijkers, W. M. de Vos and H. Smidt, Int. J. Syst. Evol. Microbiol., 2018, 68, 3479–3486 CrossRef CAS PubMed.
- B.-C. Kim, B. Seung Jeon, S. Kim, H. Kim, Y. Um and B.-I. Sang, Int. J. Syst. Evol. Microbiol., 2015, 65, 4902–4908 CrossRef CAS PubMed.
- F. R. Bengelsdorf, A. Poehlein, R. Daniel and P. Dürre, Microbiol. Resour. Announce., 2019, 8, e00346–e00319 Search PubMed.
- C. M. Spirito, H. Richter, K. Rabaey, A. J. M. M. Stams and L. T. Angenent, Curr. Opin. Biotechnol., 2014, 27, 115–122 CrossRef CAS PubMed.
- W. Cieciura-Włoch, S. Borowski and A. Otlewska, Renewable Energy, 2020, 153, 1226–1237 CrossRef.
- J.-H. Park, S.-H. Lee, H.-J. Ju, S.-H. Kim, J.-J. Yoon and H.-D. Park, Renewable Energy, 2016, 86, 889–894 CrossRef CAS.
- L. Chatellard, E. Trably and H. Carrère, Bioresour. Technol., 2016, 221, 541–549 CrossRef CAS PubMed.
- J. L. Rombouts, E. M. M. Kranendonk, A. Regueira, D. G. Weissbrodt, R. Kleerebezem and M. C. M. van Loosdrecht, Biotechnol. Bioeng., 2020, 117, 1281–1293 CrossRef CAS PubMed.
- O. García-Depraect, R. Castro-Muñoz, R. Muñoz, E. R. Rene, E. León-Becerril, I. Valdez-Vazquez, G. Kumar, L. C. Reyes-Alvarado, L. J. Martínez-Mendoza and J. Carrillo-Reyes, Bioresour. Technol., 2021, 324, 124595 CrossRef PubMed.
- S. Esquivel-Elizondo, Z. E. Ilhan, E. I. Garcia-Peña and R. Krajmalnik-Brown, mSystems, 2017, 2, 10–1128 CrossRef PubMed.
- K. D. De Leeuw, C. J. N. Buisman and D. P. B. T. B. Strik, Environ. Sci. Technol., 2019, 53, 7704–7713 CrossRef CAS PubMed.
- F. C. F. Baleeiro, L. Varchmin, S. Kleinsteuber, H. Sträuber and A. Neumann, Biotechnol. Biofuels Bioprod., 2023, 16, 26 CrossRef CAS PubMed.
- O. Sarkar, U. Rova, P. Christakopoulos and L. Matsakas, Bioresour. Technol., 2022, 344, 126164 CrossRef CAS PubMed.
- D. Mu, H. Liu, W. Lin, P. Shukla and J. Luo, Bioresour. Technol., 2020, 302, 122879 CrossRef CAS PubMed.
- T. Sheng, L. Zhao, L. Gao, W. Liu, G. Wu, J. Wu and A. Wang, RSC Adv., 2018, 8, 22924–22930 RSC.
- A. Sattar, C. Arslan, C. Ji, S. Sattar, M. Umair, S. Sattar and M. Z. Bakht, Int. J. Hydrogen Energy, 2016, 41, 11050–11061 CrossRef CAS.
- T. Zhang, D. Jiang, H. Zhang, Y. Jing, N. Tahir, Y. Zhang and Q. Zhang, Int. J. Hydrogen Energy, 2020, 45, 3807–3814 CrossRef CAS.
- R. R. Gonzales, P. Sivagurunathan and S.-H. Kim, Int. J. Hydrogen Energy, 2016, 41, 21678–21684 CrossRef CAS.
- S. Eker and M. Sarp, Int. J. Hydrogen Energy, 2017, 42, 2562–2568 CrossRef CAS.
- G. Yang and J. Wang, Bioresour. Technol., 2018, 255, 7–15 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |