Synthesis of the dibenzo[b,d]azepine skeleton via a catalyst-free ring expansion domino reaction†
Tao
Guo
 a,
Penghua
Hu
a,
Jiaxin
Li
a,
Yujia
Zhou
a,
Panke
Zhang
a,
Penghua
Hu
a,
Jiaxin
Li
a,
Yujia
Zhou
a,
Panke
Zhang
 *b,
Yunhui
Zhao
*b,
Yunhui
Zhao
 *c and
Congjun
Zhu
*c and
Congjun
Zhu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou, Henan 450001, PR China
bGreen Catalysis Center, College of Chemistry, Henan Advanced Institute of Technology, Zhengzhou University, Zhengzhou 450001, PR China
cSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, PR China
First published on 21st February 2024
Abstract
In this article, a hitherto unreported catalyst-free ring expansion reaction of tetrahydroisoquinolines with o-alkynylarylaldehydes for the construction of the dibenzo[b,d]azepine skeleton is described. Using air as a “green” oxidant, some important biologically active dibenzo[b,d]azepines were produced with favorable functional group tolerance and a wide substrate scope. The synthetic potential was further confirmed by facile scale-up reactions and the synthesis of isoindoline derivatives.
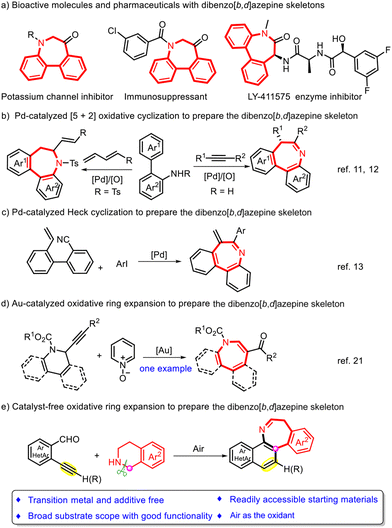
Introduction
Dibenzoazepines, a particularly valuable class of seven-membered N-heterocycles, frequently occur in many bioactive molecules, pharmaceuticals, and natural products.1–3 Among these compounds, dibenzo[b,d]azepines have attracted most attention since they exhibit an extensive range of important bioactivities, serving as, e.g., potassium channel inhibitors, enzyme inhibitors, immunosuppressants, and anticancer agents (Scheme 1a).4–7 Due to the high benefit of dibenzo[b,d]azepine derivatives, various strategies have been developed over the last decades to synthesize such compounds.8–10 In 2015 and 2017, Luan and co-workers reported a Pd-mediated [5 + 2] oxidative cyclization of biphenyl-2-amines with alkynes and dienes to prepare the dibenzo[b,d]azepine skeleton (Scheme 1b).11,12 In 2022, Zhu and co-workers disclosed a different approach based on the Pd-catalyzed Heck reaction of 2-isocyano-2′-vinyl-1,1′-biphenyl with various aryl iodides to obtain the same skeleton (Scheme 1c).13 In recent years, ring expansions emerged as some of the most attractive chemical conversions from an atom- and step-economic perspective to synthesize a versatile range of heterocyclic cores.14,15 To date, many remarkable advancements using transition metals (e.g., Ir, Rh, and Pd) have been achieved in this field,16,17 and the most recent work has been concentrated on strained-ring systems such as three- and four-membered rings (e.g. cyclopropanes, cyclobutanes, aziridines, and oxiranes).18–20 The ring expansion of six-membered rings has been less explored and only one example has been reported for the synthesis of the dibenzo[b,d]azepine skeleton (Scheme 1d).21 Despite these achievements, the development of straightforward and efficient methods for constructing the dibenzo[b,d]azepine skeleton from readily accessible substrates without the use of transition metals or additives is still in its early stages. Therefore, we investigated a hitherto unreported catalyst-free ring expansion reaction of tetrahydroisoquinolines22–25 for the construction of the dibenzo[b,d]azepine skeleton.Results and discussion
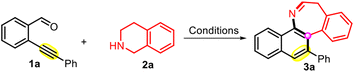
Recently, our group completed the syntheses of a series of heterocyclic compounds.26–30 Due to our ongoing research interest in this area, reaction screening was conducted with 2-(phenylethynyl)benzaldehyde 1a and 1,2,3,4-tetrahydroisoquinoline (THIQ) 2a as the model substrates to identify the suitable reaction conditions, and the results are provided in Table 1. First, the product yields were determined for various solvents under an air atmosphere after a reaction time of 4 h. Toluene exhibited the best performance with an 81% yield of the anticipated product 3a, while the application of the other investigated solvents resulted in significantly lower yields or no reaction (entries 1–10). Control experiments revealed that no desired product was formed when the reaction was performed under N2, implying that the presence of an oxidant was essential for this transformation (entry 11). Then, apart from air, various oxidants were screened. When the reaction was performed under an O2 atmosphere, the obtained yield (entry 12) was similar to that observed under an air atmosphere (entry 9), indicating that the use of pure O2 was not required for the reaction to proceed. The addition of other oxidants such as KIO3, di-tert-butyl peroxide (DTBP), and MnO2 did not further benefit the reaction (entries 13–15). Furthermore, we attempted to increase the yield of the reaction using various additives including bases (NaHCO3 and K2CO3), Lewis acid (AlCl3), iodine source (n-Bu4NI), and metal catalysts ((Ph3P)2PdCl2, NiCl2, and Cu(OAc)2), but none of these investigated additives exhibited the desired effect (entries 16–22). Subsequent examination of the effect of temperature revealed that inferior yields of 3a were obtained when the temperature was reduced to rt, 80 °C, 90 °C or increased to 110 °C (entries 23–26). The effect of reaction time was also investigated, but no improvement was achieved when performing the reaction for 8 h instead of 4 h (entry 27). Therefore, the parameters used in entry 9 of Table 1 were identified as the optimized conditions.| Entrya | Oxidant (equiv.) | Additive (1 equiv.) | Solvent (2 mL) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.4 mmol, 2 equiv.), 2a (0.2 mmol), solvent (1 mL), air atmosphere, 4 h. b Isolated yield. c Under N2. d Reaction time: 8 h. | |||||
| 1 | Air | — | 1,4-Dioxane | 100 | 0 |
| 2 | Air | — | DMSO | 100 | 0 |
| 3 | Air | — | DMF | 100 | 48 |
| 4 | Air | — | NMP | 100 | 0 |
| 5 | Air | — | DME | 100 | 0 |
| 6 | Air | — | MeCN | 80 | 0 |
| 7 | Air | — | Dichloroethane | 80 | 0 |
| 8 | Air | — | Xylenes | 100 | 67 |
| 9 | Air | — | Toluene | 100 | 81 |
| 10 | Air | — | THF | Reflux | 0 |
| 11c | None | — | Toluene | 100 | 0 |
| 12 | O2 | — | Toluene | 100 | 78 |
| 13 | KIO3 (2) | — | Toluene | 100 | 65 |
| 14 | DTBP (2) | — | Toluene | 100 | 73 |
| 15 | MnO2 (2) | — | Toluene | 100 | 46 |
| 16 | Air | NaHCO3 | Toluene | 100 | 58 |
| 17 | Air | K2CO3 | Toluene | 100 | 0 |
| 18 | Air | AlCl3 | Toluene | 100 | Trace |
| 19 | Air | n-Bu4NI | Toluene | 100 | 38 |
| 20 | Air | (Ph3P)2PdCl2 | Toluene | 100 | 71 |
| 21 | Air | NiCl2 | Toluene | 100 | 32 |
| 22 | Air | Cu(OAc)2 | Toluene | 100 | 69 |
| 23 | Air | — | Toluene | rt | 0 |
| 24 | Air | — | Toluene | 80 | 35 |
| 25 | Air | — | Toluene | 90 | 71 |
| 26 | Air | — | Toluene | 110 | 63 |
| 27d | Air | — | Toluene | 100 | 76 |
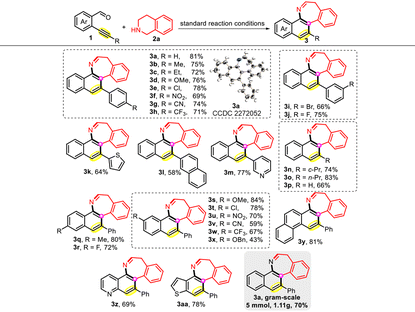
Based on the above-established optimal conditions, the scope and generality of this reaction were evaluated by examining various functionalized o-alkynylarylaldehydes, and the results are listed in Table 2. Generally, the electronic effect on the aromatic ring directly bonded to alkyne had no significant impact on reactivity. Substrates with various functional groups containing electron-donating or electron-withdrawing groups (Me, Et, OMe, Cl, Br, NO2, CN, and CF3) could easily undergo the ring expansion reaction to form the corresponding tricyclic compounds 3b–3j in good yields. The stability of halogen substituents provides the possibility for downstream manipulations such as transition metal-mediated coupling reactions. The configuration of 3a was verified by single-crystal X-ray diffraction analysis (CCDC 2272052†). The reaction was also viable with heteroaryl and polycyclic substituents on the triple bond, delivering the compounds 3k–3m in 58–77% yields.
Notably, 2-formylphenylacetylene and aliphatic terminal alkynes bearing cyclopropyl and n-propyl groups also appeared to be suitable reactants, providing the corresponding products 3n–3p in good yields. Subsequently, the influence of substituents on the benzaldehyde core was investigated. A range of substrates with various substituents at the 4- and 5-positions of the benzaldehyde moiety were compatible under the standard reaction conditions, delivering the target products in acceptable yields. The synthesis of polycyclic arene-/heteroarene-fused azepine derivatives from the corresponding 1-(phenylethynyl)-2-naphthaldehyde (3y), 2-(phenylethynyl)-3-pyridylaldehyde (3z), and 2-(phenylethynyl)-3-thenaldehyde (3aa) could also be achieved. To assess the scalability of this ring expansion reaction, a scale-up reaction was conducted, and we found that the protocol could be readily scaled up from 0.2 to 5.0 mmol with minimal yield reduction (3a, 70% vs. 81%).
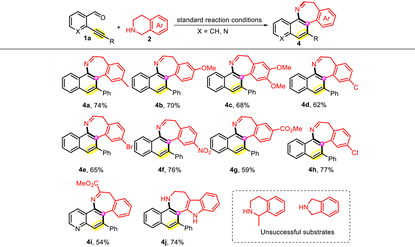
Then, the scope of the THIQ substrate was examined (Table 3). The introduction of diverse substituents, such as Me, OMe, Cl, Br, NO2, CO2Me, and CN, at the 3-, 6-, and 7-positions of the benzene ring was more compatible with this transformation, and the expected compounds 4a–4i were obtained in good yields (54–77%). In particular, when tetrahydro-β-carboline was employed, the unoxidized azepine derivative 4j was produced instead. Finally, the reaction of 1-methyl-THIQ and isoindoline with 1a was investigated; however, the desired product failed to generate under optimal conditions.
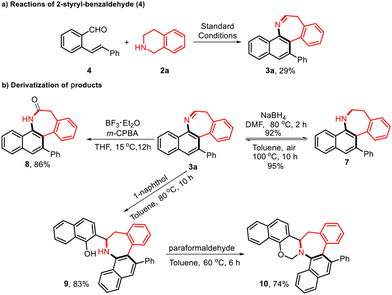
Encouraged by the successful results, we wondered whether 2-styrylbenzaldehyde (4) (Table 4) and 2-cyanobenzaldehyde (5) (ESI Fig. S3†) would undergo similar efficient ring expansion reactions to provide other valuable azepine derivatives under the same conditions. 3a could also be obtained when 4 was employed instead of 1a. However, the reaction of 5 with THIQ compounds produced a series of 3-amino-1-isoindoline derivatives (6a–6d) (see the ESI for details, Fig. S3†). To demonstrate the efficacy of this method, further transformations to dibenzo[b,d]azepine products were pursued. 3a could be converted into 7 and 8 in 92% and 86% isolated yields, respectively. Furthermore, the reaction of 1-naphthol with 3a resulted in the bifunctional aminonaphthol 9 in 83% yield. The ring closure of 9 with a solution of HCHO as the cyclizing agent afforded a new oxazine derivative 10 in 74% yield.
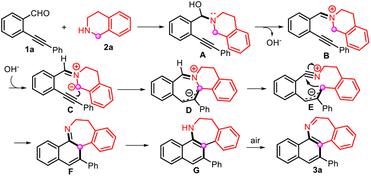
Based on previous reports31–33 and our control experiments (see the ESI for details, Fig. S1 and S2†), a feasible mechanism of the ring expansion reaction is outlined in Scheme 2. Initially, the addition of THIQ (2a) to o-alkynylarylaldehyde (1a) to form N,O-acetal species A is followed by the elimination of the hydroxide ion to afford iminium B. Then, B is deprotonated and subsequent electrocyclic ring closure generates intermediate D. The ring opening of D yields the allyl anion species E. Finally, the nucleophilic addition of the allyl anion species to the nitrile function, followed by aromatization and oxidation by air, furnishes the target product 3a.
Conclusions
In conclusion, a novel approach towards biologically important dibenzo[b,d]azepines through metal- and additive-free ring expansion was developed. Remarkably, employing air as a “green” oxidant is an attractive reaction protocol for application in organic synthesis and pharmaceutical chemistry. The synthetic potential and practicality were proved by good functional group tolerance, successful scale-up experiments, and the possible expansion toward the synthesis of isoindoline derivatives. Further efforts are in progress to utilize the reaction and study the potential pharmaceutically active compounds in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge support from the National Natural Science Foundation of China (22202060), the Project of Youth Backbone Teachers of Henan University of Technology (21420072), the Science and Technology Cooperation Foundation of Zhengzhou City (21ZZXTCX16), the Innovative Funds Plan of Henan University of Technology (2021ZKCJ08), the Funding plan of key scientific research projects in colleges and universities of Henan Province (23A150029), the Doctoral Scientific Research Start-up Foundation from Henan University of Technology (2021BS080), and the Natural Science Foundation of Hunan Provincial (No. 2020JJ4028). The authors are also thankful to Xiaodi Yang, Shanghai University of Traditional Chinese Medicine, for her assistance with X-ray crystallography.References
- T. Hu, Z. Ye, K. Zhu, K. Xu, Y. Wu and F. Zhang, Org. Lett., 2020, 22, 505–509 CrossRef CAS PubMed.
- D. Tsvelikhovsky and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14048–14051 CrossRef CAS PubMed.
- Y. Yu, L. Ma, J. Xia, L. Xin, L. Zhu and X. Huang, Angew. Chem., Int. Ed., 2020, 59, 18261–18266 CrossRef CAS PubMed.
- S. Pegoraro, M. Lang, T. Dreker, J. Kraus, S. Hamm, C. Meere, J. Feurle, S. Tasler, S. Prütting, Z. Kuras, V. Visan and S. Grissmer, Bioorg. Med. Chem. Lett., 2009, 19, 2299–2304 CrossRef CAS PubMed.
- R. Rajagopalan, A. Bandyopadhyaya, D. R. Rajagopalan and P. Rajagopalan, Bioorg. Med. Chem. Lett., 2014, 24, 576–579 CrossRef CAS PubMed.
- G. T. Wong, D. Manfra, F. M. Poulet, Q. Zhang, H. Josien, T. Bara, L. Engstrom, M. Pinzon-Ortiz, J. S. Fine, H.-J. J. Lee, L. Zhang, G. A. Higgins and E. M. Parker, J. Biol. Chem., 2004, 279, 12876–12882 CrossRef CAS.
- B.-J. Zhang, M.-F. Bao, C.-X. Zeng, X.-H. Zhong, L. Ni, Y. Zeng and X.-H. Cai, Org. Lett., 2014, 16, 6400–6403 CrossRef CAS PubMed.
- F. Ling, Z. Xie, J. Chen, C. Ai, H. Shen, Z. Wang, X. Yi and W. Zhong, Adv. Synth. Catal., 2019, 361, 3094–3101 CrossRef CAS.
- V. Narbonne, P. Retailleau, G. Maestri and M. Malacria, Org. Lett., 2014, 16, 628–631 CrossRef CAS PubMed.
- P. Rodríguez-Salamanca, R. Martín-de la Calle, V. Rodríguez, P. Merino, R. Fernández, J. M. Lassaletta and V. Hornillos, Chem. Sci., 2021, 12, 15291–15297 RSC.
- L. Bai, Y. Wang, Y. Ge, J. Liu and X. Luan, Org. Lett., 2017, 19, 1734–1737 CrossRef CAS PubMed.
- Z. Zuo, J. Liu, J. Nan, L. Fan, W. Sun, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2015, 54, 15385–15389 CrossRef CAS PubMed.
- W. Hu, X. Wang, Y. Peng, S. Luo, J. Zhao and Q. Zhu, Org. Lett., 2022, 24, 3642–3646 CrossRef CAS PubMed.
- G. Li, M. N. Lavagnino, S. Z. Ali, S. Hu and A. T. Radosevich, J. Am. Chem. Soc., 2023, 145, 41–46 CrossRef CAS.
- G. Masson, D. Gomez Pardo and J. Cossy, Eur. J. Org. Chem., 2020, 7103–7118 CrossRef CAS.
- Z. C. Chen, Z. Chen, Z. H. Yang, L. Guo, W. Du and Y. C. Chen, Angew. Chem., 2019, 131, 15163–15167 CrossRef.
- J.-J. Feng, T.-Y. Lin, C.-Z. Zhu, H. Wang, H.-H. Wu and J. Zhang, J. Am. Chem. Soc., 2016, 138, 2178–2181 CrossRef CAS PubMed.
- B. Biletskyi, P. Colonna, K. Masson, J.-L. Parrain, L. Commeiras and G. Chouraqui, Chem. Soc. Rev., 2021, 50, 7513–7538 RSC.
- D. J. Mack and J. T. Njardarson, ACS Catal., 2013, 3, 272–286 CrossRef CAS.
- T. Sarkar, K. Talukdar, B. K. Das, T. A. Shah, B. Debnath and T. Punniyamurthy, Org. Biomol. Chem., 2021, 19, 3776–3790 RSC.
- M. Chen, Y. Chen, N. Sun, J. Zhao, Y. Liu and Y. Li, Angew. Chem., Int. Ed., 2015, 54, 1200–1204 CrossRef CAS PubMed.
- P. S. Gao, X. J. Weng, Z. H. Wang, C. Zheng, B. Sun, Z. H. Chen, S. L. You and T. S. Mei, Angew. Chem., Int. Ed., 2020, 59, 15254–15259 CrossRef CAS.
- P. Jha, S. Husen and R. Kumar, Green Chem., 2021, 23, 2950–2955 RSC.
- D. Jing, C. Lu, Z. Chen, S. Jin, L. Xie, Z. Meng, Z. Su and K. Zheng, Angew. Chem., 2019, 131, 14808–14814 CrossRef.
- W. Lin, T. Cao, W. Fan, Y. Han, J. Kuang, H. Luo, B. Miao, X. Tang, Q. Yu, W. Yuan, J. Zhang, C. Zhu and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 277–281 CrossRef CAS PubMed.
- T. Guo, L. Bi, L. Shen, Q. Wei, C. Zhu, P. Zhang and Y. Zhao, Org. Biomol. Chem., 2023, 21, 127–131 RSC.
- T. Guo, L. Bi, M. Zhang, C.-J. Zhu, L.-B. Yuan and Y.-H. Zhao, J. Org. Chem., 2022, 87, 16907–16912 CrossRef CAS.
- T. Guo, P.-H. Hu, Y. Liu, P. Zhang, Y. Zhao and C. Zhu, Chem. Commun., 2023, 59, 12455–12458 RSC.
- T. Guo, X.-N. Wei, Y. Liu, P.-K. Zhang and Y.-H. Zhao, Org. Chem. Front., 2019, 6, 1414–1422 RSC.
- T. Guo, X.-N. Wei, M. Zhang, Y. Liu, L.-M. Zhu and Y.-H. Zhao, Chem. Commun., 2020, 56, 5751–5754 RSC.
- C. L. Jarvis, M. T. Richers, M. Breugst, K. N. Houk and D. Seidel, Org. Lett., 2014, 16, 3556–3559 CrossRef CAS PubMed.
- M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, J. Am. Chem. Soc., 2014, 136, 6123–6135 CrossRef CAS.
- F. Xie, Q.-H. Chen, R. Xie, H.-F. Jiang and M. Zhang, ACS Catal., 2018, 8, 5869–5874 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2272052. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3gc04626e |
| This journal is © The Royal Society of Chemistry 2024 |