Open Access Article
Open Access ArticleLactose utilisation to furan carboxylates: a unique source for platform molecules†
Joseph
Install
 a,
Anže
Zupanc
a,
Anže
Zupanc
 a,
Mikko
Nikunen
b,
Janne
Jänis
a,
Mikko
Nikunen
b,
Janne
Jänis
 b and
Timo
Repo
b and
Timo
Repo
 *a
*a
aDepartment of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, P.O. Box 55, 00014 Finland. E-mail: timo.repo@helsinki.fi
bDepartment of Chemistry, University of Eastern Finalnd, P.O Box 111, FI-80101 Joesnsuu, Finland
First published on 20th December 2023
Abstract
A highly efficient utilisation of lactose, a widely produced side-stream product to produce furancarboxylates in two steps, is presented. Firstly an enhanced nitric acid oxidation of lactose with Fe(NO3)3 to achieve a 75% yield of mucic acid was developed. Mucic acid was then quantitatively dehydrated to produce dibutyl-2,5-furandicarboxylate and butyl-2-furancarboxylate (66.6% and 33.1% respectively) using phosphotungstic acid catalyst in 1-butanol as a reaction solvent. The overall process presents and excellent carbon utilisation efficiency of 49.9 mol%.
Biorefinery as a feasible and sustainable source of fine, bulk, and pharmaceutical chemicals is becoming increasingly attractive. The biomass-based product market is estimated to reach 50 billion euros by 2030.1 Aside from the economics, it also holds a key piece in the circular economy concept, whereby our by-products are transferred back into other useable products. The predominant biomass sources in research laboratories and pilot and full-scale plant activities are lignocellulosic biomass,2,3 with cellulose offering crystalline glucose post fractionation4 for short-chain organic acids following thermo-catalytic conversion as an example.5 Aside from cellulose, there is also lignin as a source for phenolics and many routes have been developed.6,7
In the EU by 2031 milk production is expected to be around 162 million tonnes per year,8 and globally the production is continuing to grow. Processes that produce lactose include crude extraction from milk to produce lactose-reduced milk products, the demand for which is growing. Additionally, a substantial amount of lactose is discarded during the cheese fermentation process in whey. It is estimated 9–10 L of whey is produced per 1 kg of cheese during production9 and 75% of the whey powder is estimated to be comprised of lactose. Intuitively, as the demand for these products continues to grow so does the side-stream production. Beside milk products, other dairy product streams such as Greek yoghurt production, which produces acidic whey as a lactose-rich side-stream, are increasing globally.10
Lactose is a disaccharide composed of units of glucose and galactose, both hexose C6 sugars differing only in the orientation of the hydroxyl groups within their backbones. They are held together with a β-glycosidic bond, which unlike sucrose cannot be hydrolysed in weak organic acids; however, it is readily cleaved in the presence of strong mineral acids.11 During this research we looked for distinctive differences in reactivities of the two monosaccharides and found the oxidation to aldaric acids provides significant deviation between glucose and galactose. Oxidation of glucose yields glucaric acid12 once the aldehyde and the terminal alcohol have been oxidised to carboxylic acid groups and requires a considerably strong and selective system.13 Galactose, upon full oxidation yields galactaric acid, commonly known as mucic acid. It is obtained using simpler oxidation systems such as nitric acid oxidation14 in the protonated form, and is virtually insoluble in all common solvents (Fig. 1). This provides many practical advantages for purification and separation between glucaric and mucic acid yielding the selective separation of lactose disaccharides.
 | ||
| Fig. 1 Comparison of current bio-based process routes to FDCA and the process presented in this work (created with https://Biorender.com). | ||
Mucic acid has gained much attention in recent advances15 and product routes to afford desirable adipic acid16–18 providing much promise for future plant-scale application. Biomass-derived adipic acid derivatives and renewable carboxylic acids are highly desireable.19–21 However, here we have focused on 2,5-furandicarboxylic acid (FDCA) which together with ethylene glycol form polyethylenefuranoate (PEF). It is achievable in a biobased process and has beneficial properties comparable to polyethylene terephthalate (PET).22 These include a greater oxygen and carbon dioxide barrier as well as a greater degradation potential.
Current industrial applications of FDCA synthesis from biomass sources are accelerating; this is generally achieved through glucose dehydration into HMF and oxidation to FDCA.23–25 The extraction and purification of glucose from the raw biomass sources, although well established, must be considered in the life-cycle assessment of the overall process,26 whereas through fewer steps FDCA can be obtained from alternative waste or side-streams through aldaric acids.27,28
Alternative sources for mucic acid have been evaluated including orange peel, through the extraction of pectin as a source of glucuronic acid, and this can then be oxidised to obtain glucaric acid, following dehydration similar to FDCA. This route has many advantages and N. Van Strien et al.29 have demonstrated efficient sulfonic acid-based dehydrations; however the feedstock and its global availability does not suffice with the global demand of the polymer industry for mass-produced PET.30 Therefore, multiple easily accessible routes from renewable sources are needed for this conversion, and we present one here.
In this work we report two separate systems for full lactose valorisation. The first, using diluted nitric acid with modifications to directly hydrolyse and oxidise lactose to form mucic acid. From the oxidation products we also present heteropolyacids as an efficient catalyst for the dehydration of mucic acid to FDCA and its esters and the formation of side products namely 2-furancarboxylic acid, paving the way for mechanistic understanding.
Oxidation of lactose to mucic acid and glucose oxidation products
Nitric acid oxidation of lactose is a common test for its presence in food products and yields insoluble mucic acid which verifies the presence of lactose.31 Considering that lactose is comprised of galactose which post oxidation yields mucic acid and glucose and would yield glucaric acid under similar conditions; however it will remain in the acid solution and require extraction from the mixture. We aimed to improve the simple nitric acid procedure32 although already with a 35% HNO3 solution stirred at 80 °C for 24 h we obtained an adequate 50.3% yield of mucic acid directly from lactose. The procedure is very convenient as the mucic acid precipitates out of the solution and therefore requires no further isolation techniques. We subsequently screened more conventional industrial oxidation procedures for an alternative route for mucic acid production from lactose,33,34 which proved to be troublesome, with many poor abilities to hydrolyse and oxidize in one system. For example, Pt/C with pressurised O2 under acidic conditions failed to yield mucic acid from lactose.34 We appreciate that there are drawbacks with HNO3 oxidations35 and aimed to mitigate these as much as possible during this work, which we describe below. It has been demonstrated that lactose can be a viable source of mucic acid for other conversions and is not limited to furan carboxylate production.Expanding further on the nitric acid oxidation of lactose and utilising ESI FT-ICR for the in-depth analysis of the resulting oxidation mixture (ESI3 & 4†), we gained insights into the oxidation process which helped understand the further developments of this reaction. As depicted in Fig. 2 we propose the oxidation based on the known oxidation routes of lactose, and from our observations, to mucic and glucaric acid. Two feasible pathways are proposed; the first (a) through the known oxidation pathway of lactose to lactobionic acid, confirmed by MS, which revealed the existence of a diacid-disaccharide from the calculated DBE values, this could be due to an additional oxidation of lactobionic acid. Then acid hydrolysis and further oxidation of monosaccharide units furnished the final oxidation products. The other alternative (b) is the initial acid-catalysed hydrolysis to glucose and galactose, followed by oxidation of the aldehyde group, the easier oxidation compared to the preceding terminal hydroxy group oxidation which requires a stronger oxidation potential for this to occur. Both are feasible and yield insoluble mucic acid.
The observation of monocarboxylic acid monosaccharides (gluconic and galactonic acid) led us also to believe that the nitric acid system is more than adequate for the hydrolysis of lactose, and the full oxidation to aldaric acids was not fully efficient. To enhance this, we screened multiple additives for the system with known oxidation loops with HNO3 with varying successes such as radical initiators like TEMPO and metal salts. Oxidation of carbohydrates has been reported with TEMPO36 as well as with other systems,37 for the production of uronic acids which were observed in ESI FT-ICR (ESI4†) but did not increase the overall conversion to mucic acid. The most promising result is the addition of Fe(NO3)3 with Fe in its +3 oxidation state, enhancing the isolated yield of mucic acid to 74.9% with full conversion of lactose in 24 h. Na(NO2), K(NO3) and Mn(NO3)2 were studied to verify this and it was found that Fe opposed the nitrate addition which enhanced the yield and the results are summarised in Table 1. As reported in monosaccharide oxidations,38 nitrates were used as oxidation additives and Fe(NO3)3 played a role in this reaction.39 In addition, Fe3+ itself had a significant oxidation power in the reaction. This resulted in a simple, effective and cheap oxidation process of lactose to mucic acid utilising Fe(NO3)3 (5 mol%) as an additive in dilute nitric acid (35%).
Esterification of mucic acid to mucate esters
At a glance, the structure of mucic acid suggests it would be adequately soluble in solution to enable further processing; however, its solubility is poor, achieving only 3 g L−1. This problem is overcome by acid esterification which significantly increases the solubility to allow more efficient further processing. We achieved this using p-toluene sulfonic acid, achieving yields of >94% for methyl and butyl esters (Fig. 4). Although it represents another step in the processing, it is necessary to achieve an overall high utilisation efficiency.Dehydration of mucic acid and mucate esters to FDCA
Previous studies of mucic acid dehydration have used 1-butanol as the reaction medium and esterifying alcohol and therefore obtained dibutyl-2,5-furandicarboxylate as the final product. This has advantages as 1-butanol is immiscible with water and has a higher boiling point than smaller alcohols. We expanded the scope of alcohol to examine the sensitives of the reaction and to aim ultimately to obtain the desirable product dimethyl-2,5-furandicarboxylate (2b, Fig. 5) and understand further the effect of ester/alcohol solvent selection.Similar to HMF40,41 conversions, the dehydration of alkyl mucates requires Brønsted acidity. However, due to the presence of two carboxylic acid groups in proximity to an aromatic system, the Brønsted acidity also promotes unfavourable decarboxylation. This leads to 2-furancarboxylates (3b, 3c) or 2-furancarboxylic acid (3a) as a side product. Therefore, the acidity needs to be adjusted carefully. We observed this during the initial screening with solid acid zeolites, where the zeolites with an Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 30 performed well to give higher difuran carboxylate yields (Table SI3†) compared to a higher Si
Al ratio of 30 performed well to give higher difuran carboxylate yields (Table SI3†) compared to a higher Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio which is synonymous with the increased Brønsted acidity which promoted decarboxylation and then gave 3b as the major product. With zeolite beta (Si
Al ratio which is synonymous with the increased Brønsted acidity which promoted decarboxylation and then gave 3b as the major product. With zeolite beta (Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al 30
Al 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) we achieved a maximum of 55% yield for 2b and 3b at 220 °C for 3 h (30% and 25% respectively) and concluded that even with a suitable mix of Lewis and Brønsted acid sites, zeolites lacked the ability to provide high yields of 2b in methanol.
1) we achieved a maximum of 55% yield for 2b and 3b at 220 °C for 3 h (30% and 25% respectively) and concluded that even with a suitable mix of Lewis and Brønsted acid sites, zeolites lacked the ability to provide high yields of 2b in methanol.
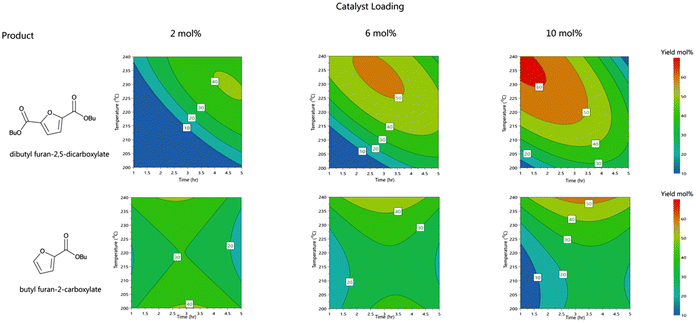
Later we employed a heteropoly acid phosphotungstic acid (HPW) as it has strong Brønsted acidity comparable to zeolites but is also hygroscopic in nature allowing for the abstraction of the produced water in the reaction mixture. Combined with the immiscibility of 1-butanol with water the pairing of HPW in 1-butanol as a reaction solvent provided efficient dehydration conditions. We observed the influence of the reaction time and temperature on the selectivity between the di and mono ester product; a longer reaction time allowed for more decarboxylation and a hence greater proportion of the monocarboxylate was formed. However, this was also dependent on the reaction temperature. For efficient visualisation of this, we employed a design of experiment (DOE) d-optimal design to evaluate the effect of temperature, time and catalytic loading on the product distribution and the overall conversion to carboxylates (2c and 3c). A highly reproducible and descriptive model of the dehydration of mucic acid to furancarboxylates was obtained, represented by high R2, model validity and reproducibility (summarised in SIX). The model can be visualised in the contour plots in Fig. 3.
As can be observed from Fig. 3 and predicted plots (SI16), a maximum yield of 62.1% of 2c is predicted under the following conditions: 240 °C, 1 h and with 10 mol% of HPW. We verified this with subsequent trials and achieved a maximum yield of 63.6% for 2c with a yield of 33.1% for 3c. With an overall dehydration yield of 96.8% this shows excellent carbon utilisation of the process. Compared with glucose conversions into HMF, which have been shown to produce yields of >60% (ref. 40 and 42–44) and considering only one, valuable side-product (3c) is formed and this conversion is appealing. Further benefit is clear considering the further oxidation required for FDCA from HMF.
We monitored reducing the catalytic loading of HPW. Although it is an easily recyclable material, a higher loading is undesirable, we achieved this by the addition of H2SO4 (0.4 mmol) to replace lost Brønsted acid sites from the reduction of HPW. This showed the possibility to still obtain a high yield of 2c with reduced loading, although without HPW the major product was 3c with increased decarboxylation of 2c, but still quantitative conversion was achieved. This highlights the importance of Brønsted acidity as well as Lewis acidity in controlling the selectivity between the products (Table 2).
| Entries | HPW (mol%) | H2SO4 (mmol) | Yield 2c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (mol%) a (mol%) |
Yield 3c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (mol%) a (mol%) |
|---|---|---|---|---|
| Reaction conditions: 0.4 mmol dibutyl mucate ester, 15 mL 1-butanol, catalyst as described in the table, added to a sealed reactor charged with 10 bar N2, and stirred (600 rpm) at 240 °C for 1 h.a Analytical yield. | ||||
| 1 | 10 | 0 | 63.7 | 33.1 |
| 2 | 2 | 0.4 | 57.9 | 39.1 |
| 3 | 0 | 0.4 | 42.5 | 51.8 |
Conclusion
The lactose utilisation process outlined in the presented results depicts a simple, efficient, and tunable route to high-value platform chemicals which are potential feedstocks for PEF polymer production. Using commercially available and industrially appealing reagents the much-overlooked by-product lactose can be transformed in as few as 3 steps. The modified nitric acid oxidation of lactose provides high yields of 74.9% of mucic acid (improved from 50.3% in diluted nitric acid) as a solid precipitate from the solution with excellent purity without additional purification techniques. We attempted to mitigate the environmental impact of the process with dilution and the inclusion of additives; however, there is scope to improve the green potential further. We provide insights into the dehydration of aldaric acids, namely mucic acid and the imperative selection of reaction temperature and time to minimise the decarboxylation of di-2,5-furandicarboxyilic acids and carbonates to the mono-functionalised counterparts. The temperature requirements of this reaction are high but from the statistical model provided it shows a significant drop in the yield once the temperature is decreased; however the reaction time is short which reduces the energy demand for heating to high temperatures such as these. Within an 1-hour reaction time at 240 °C we achieved a yield of 63.6% dibutyl-2,5-furandicarboxylate with a side-stream of 33.1% of butyl-2-furanmonocarboxylate from mucate esters, representing a near-quantitative utilisation of the starting material. In comparison with the existing routes from cellulosic materials which require pre-fractionation,2 more reaction steps4,5,23,24,26,42 and challenges in separation, the presented route towards lactose to furan carboxylates presents a unique straightforward alternative approach. We hope the presented procedures can provide an outlook for alternative side-stream sources to be integrated to the biopolymer and biorefinery industry.Conflicts of interest
No conflicts to declare.Acknowledgements
J. I. is grateful for financial support from Business Finland 43486/31/2020. A. Z. would like to thank the Magnus Ehrnrooth Foundation for financial support.References
- N. Singh, R. R. Singhania, P. S. Nigam, C. Di Dong, A. K. Patel and M. Puri, Bioresour. Technol., 2022, 344, 126415 CrossRef PubMed.
- Y. Wang, J. Sun, B. He and M. Feng, Green Chem. Eng., 2020, 1, 94–108 CrossRef.
- W. Li, Y. Shen, H. Liu, X. Huang, B. Xu, C. Zhong and S. Jia, Green Chem. Eng., 2023, 4, 160–172 CrossRef.
- A. Brandt-Talbot, F. J. V. Gschwend, P. S. Fennell, T. M. Lammens, B. Tan, J. Weale and J. P. Hallett, Green Chem., 2017, 19, 3078–3102 RSC.
- J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- M. A. Hossain, T. Saelee, S. Tulaphol, M. S. Rahaman, T. K. Phung, T. Maihom, P. Praserthdam, S. Praserthdam, D. J. Yelle and N. Sathitsuksanoh, ChemCatChem, 2022, 14, 1–9 CrossRef.
- X. Zhang, Q. Zhang, J. Long, Y. Xu, T. Wang, L. Ma and Y. Li, BioResources, 2014, 9, 3347–3360 Search PubMed.
- R. Bhat, J. D. Pasquale, F. I. Bánkuti, T. T. d. Silva Siqueira, P. Shine and M. D. Murphy, Sustainability, 2022, 14, 4193 CrossRef.
- T. Sar, S. Harirchi, M. Ramezani, G. Bulkan, M. Y. Akbas, A. Pandey and M. J. Taherzadeh, Sci. Total Environ., 2022, 810, 152253 CrossRef CAS PubMed.
- M. J. Lindsay, M. S. Molitor, T. B. Goculdas, J. Zhao, J. R. Featherman, M. Li, J. B. Miller, S. Avraamidou, S. A. Rankin, J. A. Dumesic and G. W. Huber, Green Chem., 2022, 24, 8538–8551 RSC.
- M. G. Gänzle, G. Haase and P. Jelen, Int. Dairy J., 2008, 18, 685–694 CrossRef.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351–1390 RSC.
- N. Merbouh, J. F. Thaburet, M. Ibert, F. Marsais and J. M. Bobbitt, Carbohydr. Res., 2001, 336, 75–78 CrossRef CAS PubMed.
- W. W. Pigman, B. L. Browning, W. H. McPherson, C. R. Calkins and R. L. Leaf, J. Am. Chem. Soc., 1949, 71, 2200–2204 CrossRef CAS.
- G. Leonardi, J. Li, G. I. C. Righetti, A. M. Truscello, C. Gambarotti, G. Terraneo, A. Citterio and R. Sebastiano, Eur. J. Org. Chem., 2020, 2020, 241–251 CrossRef CAS.
- B. Hočevar, A. Prašnikar, M. Huš, M. Grilc and B. Likozar, Angew. Chem., Int. Ed., 2021, 60, 1244–1253 CrossRef PubMed.
- W. Deng, L. Yan, B. Wang, Q. Zhang, H. Song, S. Wang, Q. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 4712–4719 CrossRef CAS PubMed.
- J. Rios, J. Lebeau, T. Yang, S. Li and M. D. Lynch, Green Chem., 2021, 23, 3172–3190 RSC.
- R. S. Atapalkar, P. R. Athawale, D. Srinivasa Reddy and A. A. Kulkarni, Green Chem., 2021, 23, 2391–2396 RSC.
- B. M. Cochran, Synlett, 2016, 245–248 CAS.
- M. Lang and H. Li, ChemSusChem, 2022, 15, e202101531 CrossRef CAS PubMed.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- C. Van Nguyen, Y. Te Liao, T. C. Kang, J. E. Chen, T. Yoshikawa, Y. Nakasaka, T. Masuda and K. C. W. Wu, Green Chem., 2016, 18, 5957–5961 RSC.
- M. Sajid, X. Zhao and D. Liu, Green Chem., 2018, 20, 5427–5453 RSC.
- M. M. Cajnko, U. Novak, M. Grilc and B. Likozar, Biotechnol. Biofuels, 2020, 13, 1–11 CrossRef PubMed.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Green Chem., 2021, 23, 3154–3171 RSC.
- Y. Taguchi, A. Oishi and H. Iida, Chem. Lett., 2008, 37, 50–51 CrossRef CAS.
- J. Lewkowski, Pol. J. Chem., 2001, 75, 1943–1946 CAS.
- N. Van Strien, S. Rautiainen, M. Asikainen, D. A. Thomas, J. Linnekoski, K. Niemelä and A. Harlin, Green Chem., 2020, 22, 8271–8277 RSC.
- M. Ortiz-Sanchez, J. C. Solarte-Toro, J. A. González-Aguirre, K. E. Peltonen, P. Richard and C. A. Cardona Alzate, Biochem. Eng. J., 2020, 161, 107680 CrossRef CAS.
- E. O. Whittier, Chem. Rev., 1925, 2(1), 85–125 CrossRef CAS.
- G. T. Austin, Ind. Eng. Chem. Prod. Res. Dev., 1969, 8(4), 424–426 CrossRef CAS.
- S. Sadula and B. Saha, ChemSusChem, 2018, 11, 2124–2129 CrossRef CAS PubMed.
- J. Lee, B. Saha and D. G. Vlachos, Green Chem., 2016, 18, 3815–3822 RSC.
- R. A. F. Tomás, J. C. M. Bordado and J. F. P. Gomes, Chem. Rev., 2013, 113, 7421–7469 CrossRef PubMed.
- N. J. Davis and S. L. Flitsch, Tetrahedron Lett., 1993, 34, 1181–1184 CrossRef CAS.
- E. V. Murzina, A. V. Tokarev, K. Kordás, H. Karhu, J. P. Mikkola and D. Y. Murzin, Catal. Today, 2008, 131, 385–392 CrossRef CAS.
- C. A. Carpenter, K. I. Hardcastle and D. E. Kiely, Carbohydr. Res., 2013, 376, 29–36 CrossRef CAS PubMed.
- T. N. Smith, K. Hash, C. L. Davey, H. Mills, H. Williams and D. E. Kiely, Carbohydr. Res., 2012, 350, 6–13 CrossRef CAS PubMed.
- H. Chang, I. Bajaj, A. H. Motagamwala, A. Somasundaram, G. W. Huber, C. T. Maravelias and J. A. Dumesic, Green Chem., 2021, 23, 3277–3288 RSC.
- Z. Li, K. Su, J. Ren, D. Yang, B. Cheng, C. K. Kim and X. Yao, Green Chem., 2018, 20, 863–872 RSC.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- P. Wrigstedt, J. Keskiväli, M. Leskelä and T. Repo, ChemCatChem, 2015, 7, 501–507 CrossRef CAS.
- P. Wrigstedt, J. Keskiväli, J. E. Perea-Buceta and T. Repo, ChemCatChem, 2017, 9, 4244–4255 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04317g |
| This journal is © The Royal Society of Chemistry 2024 |