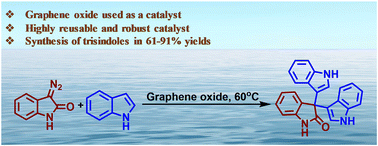
The insertion or transfer of carbene using diazo compounds as a precursor is being used widely to synthesize/functionalize different classes of organic compounds. Different types of catalysts (e.g., transition metals, enzymes, Lewis acids, Brønsted acids) have significant roles in this context. Nevertheless, there is a high demand for “green”, stable, cost-effective, and reusable catalysts for carbene insertion/transfer reactions. Herein, we report (for the first time) the application of carbon material (i.e., graphene oxide) as a catalyst in the carbene-transfer reaction using 3-diazo oxindole as a carbene precursor reacting with indoles to furnish biologically important 3,3′,3′′-trisindoles. The generality and robustness of the developed protocol was shown by employing different types of substitutions at the 3-diazo oxindole and indole which, in turn, provided 3,3′,3′′-trisindoles derivatives in good-to-excellent yield (61–91%). We revealed the utility of the developed protocol by synthesizing different biologically active molecules on the gram-scale.