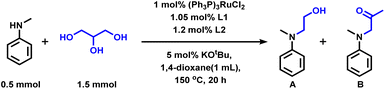Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization of bio-renewable glycerol by catalytic amination reactions
Sandeep
Kumawat†
 a,
Sunidhi
Singh†
a,
Tarun
Bhatt
a,
Anjali
Maurya
a,
Sivakumar
Vaidyanathan
a,
Sunidhi
Singh†
a,
Tarun
Bhatt
a,
Anjali
Maurya
a,
Sivakumar
Vaidyanathan
 a,
Kishore
Natte
*a and
Rajenahally V.
Jagadeesh
a,
Kishore
Natte
*a and
Rajenahally V.
Jagadeesh
 *bc
*bc
aDepartment of Chemistry, Indian Institute of Technology Hyderabad, Kandi, Sangareddy 502 285, Telangana, India. E-mail: kishore.natte@chy.iith.ac.in; kishorenatte@gmail.com
bLeibniz-Institut für Katalyse e.V, Albert-Einstein-Straße 29A, Rostock 18059, Germany. E-mail: jagadeesh.rajenahally@catalysis.de
cNanotechnology Centre, Centre of Energy and Environmental Technologies, VŠB-Technical University of Ostrava, Ostrava-Poruba, Czech Republic
First published on 2nd February 2024
Abstract
Production of value-added chemicals from renewable feedstocks is an attractive platform to alleviate the shortage of petroleum resources and to minimize CO2 emissions. Among different renewable feedstocks, glycerol is an organic triol molecule that is produced from triglycerides of plant and animal sources. This alcohol is produced as the main by-product in a large quantity of about 10% (w/w) in biodiesel production. In addition to its applications in food industries, and medicinal and personal care products, the utilization of glycerol as a key raw material in organic synthesis has garnered significant attention. As an example, selected technologies for the conversion of glycerol into other essential chemicals such as acrylic acid, 1,2-propanediol, glycerol carbonate, epichlorohydrin, and solketal have been well developed. In recent years, glycerol has also been used as the key starting material for the synthesis of amines in laboratories. In general amines are important fine and bulk chemicals used as central precursors and intermediates for advanced chemicals and many life science molecules including pharmaceuticals and agrochemicals. In the last five years, some key advancements have been made using glycerol in carbon–nitrogen bond-forming processes to prepare different kinds of amine in the presence of suitable catalytic systems. Based on the advantages of glycerol including its derived molecules as feedstocks and the importance of amines, we believe it is worthwhile to write a review summarizing the recent reports on glycerol amination and related reactions. In this regard, this review article discusses the latest developments in catalytic amination reactions using glycerol and its tangible applications in modern organic synthesis. We believe that this review will be interesting and beneficial to scientists working in the areas of catalytic utilization of glycerol aminations and valorization of renewable feedstocks.
 Sunidhi Singh | Sunidhi Singh joined the Indian Institute of Technology Hyderabad as a research scholar in August 2022 and worked on organic fluorophores for OLED applications. |
1. Introduction
Glycerol is one of the important renewable feedstocks obtained from triglycerides of plant and animal sources.1 Glycerol is commonly known as glycerine or propane-1,2,3-triol, and has a wide range of applications in the food, personal care, cosmetics, and pharmaceutical industries.1–10 Interestingly, glycerol is a less toxic, edible, and biodegradable alcohol and hence its valorization significantly benefits chemical synthesis without creating any environmental hazards.1–10 Today, >4 million tons of glycerol are obtained mainly as the by-product from biodiesel production as well as soap manufacture by the saponification and hydrolysis of triglycerides, which are esters of fatty acids and glycerol itself (Fig. 1A). In addition, it is also produced from the petrochemical-based feedstock propene (Fig. 1A).11 It should be noted that during the trans-esterification process in biodiesel production, about 1 ton of glycerol is generated for every 9 tons of biodiesel.12 The worldwide glycerol market size was estimated at USD 2.4 billion in 2020 and is anticipated to expand at an annual growth rate (CAGR) of 6.4% from 2021 to 2027 (Fig. 1B).13 In this regard, the substantial surplus accumulation of crude glycerol has been a significant problem to the biofuel sector and may impact on the refined glycerol market. Hence, to make the glycerol biorefinery more viable and profitable, sustainable and efficient approaches for the conversion of glycerol into industrial chemicals are highly desired,14 which is also suggested as one of the important tasks of techno–economic analysis.15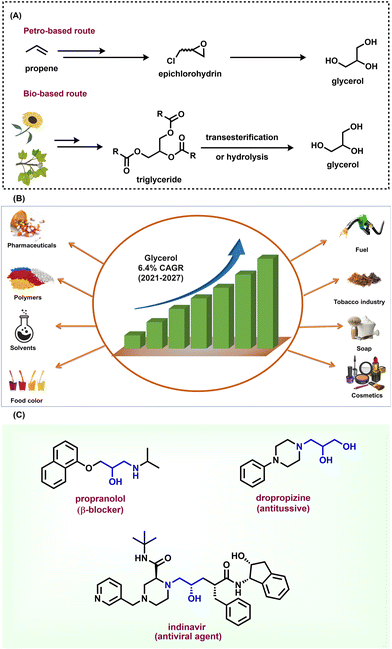 | ||
| Fig. 1 (A) Preparation of glycerol from the fossil-based route and bio-derived feedstock. (B) Glycerol market and applications. (C) Glycerol unit in pharmaceutical synthesis. | ||
Owing to the high presence of the hydroxyl (–OH) moiety in glycerol and its renewable nature, a variety of value-added chemicals can be manufactured from this alcohol, compared with petrochemical-derived feedstocks. Remarkably, glycerol also serves as a recyclable solvent in cross-coupling organic reactions.16 Thus, the valorization of glycerol is of prime importance in producing other products, which is an emerging research area in chemical synthesis.17–22 However, it should be noted that for achieving optimal efficiency, the purity of glycerol presents various challenges and also influences the reaction conversion as well as selectivity. The impurities (ash, non-glycerol organic matter, etc.) present in crude glycerol promote unwanted side reactions and impede the catalyst performance and overall reaction process.23 Despite these facts, over the past decade, noticeable research has been devoted to the use of glycerol in the synthesis and energy sectors, for example, in hydrogen generation, methanol production, as fuel additives including the production of bulk chemicals such as acrylic acid, 1,2-propanediol, glycerol carbonate, epichlorohydrin, and solketal and others.1–10,20,24–27 To valorize glycerol both chemical and biological processes such as dehydration, oxidation, hydrogenolysis, etherification, esterification, and amination processes have been applied.1–10 In the literature, it has been reported that glycerol dehydration mainly produces both acrolein and hydroxyacetone. Nevertheless, the selectivity of these products generally depends on the acid-based properties of the catalyst. Another important reaction is the carboxylation of glycerol, which gives glycerol carbonate in the presence of heterogeneous catalysts. This transformation represents many pathways and therefore makes this conversion more difficult.28 Next, the oxidation of glycerol can be conducted with any of the three hydroxyl units of the component. Many products (e.g., formic acid, glycolic acid, and glyceraldehyde) can be prepared by reacting glycerol with various oxidizing agents.28 The etherification of glycerol produces polyglycerols in the presence of solid catalysts. Interestingly, the use of acid catalysts leads to faster reactions as well as higher conversion, but very little selectivity. Conversely, by employing basic catalysts, the reaction will be slower, but with higher selectivity. Glycerol also reacts with oleic acid, acetic acid, or lauric acid and produces mono-, di-, and tri-glycerides in the presence of metal and mixed metal oxides.28 The hydrogenolysis of glycerol happens when any hydrogen source reacts with glycerol and typically produces a variety of products. Two of the most important ones are 1,3-propylene glycol and 1,4-propylene glycol.28 Nonetheless, all these aforementioned chemical routes typically lead to various side reactions and are not highly selective. In order to develop robust chemical processes starting from glycerol, developments must be achieved to increase conversion, selectivity, and yield under milder and environmentally friendly conditions. Based on these aspects, a number of research and review articles are published on the conversion of glycerol to value-added products.1–10,20
Among the synthetic applications of glycerol, its utilization as a feedstock to produce amines is of paramount importance because amines are highly privileged compounds, which serve as ever-required chemicals in academic laboratories and industries as well as life science activities.29,30 Since glycerol is the smallest polyhydroxylated compound, it could be easy to use as a model compound in theoretical and experimental investigations for the in-depth understanding of glycerol amination reactions under different catalytic reaction conditions. Apart from glycerol, bio-polyols like erythritol, xylitol, sorbitol, and mannitol have 4, 5, and 6 carbons and hydroxyl groups, respectively, which are also suitable for catalytic amination reactions.31 But the selective cleavage of carbon–carbon or carbon–oxygen bonds present in polyols is more challenging with respect to dehydrogenation, dehydration and other side reactions. Therefore, these bio-based feedstocks are less investigated than glycerol for catalytic amination reactions.
Typically, amines are produced by the hydrogenation of nitro compounds,32 hydrogenation of nitriles,33 reductive amination of carbonyl compounds,34 amination of alcohols/phenols,35 and hydroamination of olefins.36 In recent years, the use of polyols, particularly glycerol, has drawn significant attention in amination reactions to produce functionalized amines. As an example, a few potential pharmaceutical agents such as propranolol (β-blocker), indinavir (antiviral agent), and dropropizine (antitussive) comprise a glycerol unit, indicating the synthetic utility of glycerol as a potential building block in API synthesis (Fig. 1C).37–39 In recent years a lot of important efforts have been made on the catalytic amination of glycerol to prepare various amines by employing both homogeneous and heterogeneous catalysis.5,18,40–44 Despite interesting research articles on the amination of glycerol having been published, until now no related review has existed on the utilization of this alcohol for the synthesis of amines. Obviously, such a review article on the catalytic synthesis of amines involving glycerol as the feedstock will attract the attention of readers and researchers working in both academia and industry. Consequently, in this review, we comprehensively discuss these reports published in the last five years on the valorization of glycerol by catalytic aminations.
2. Reaction mechanisms
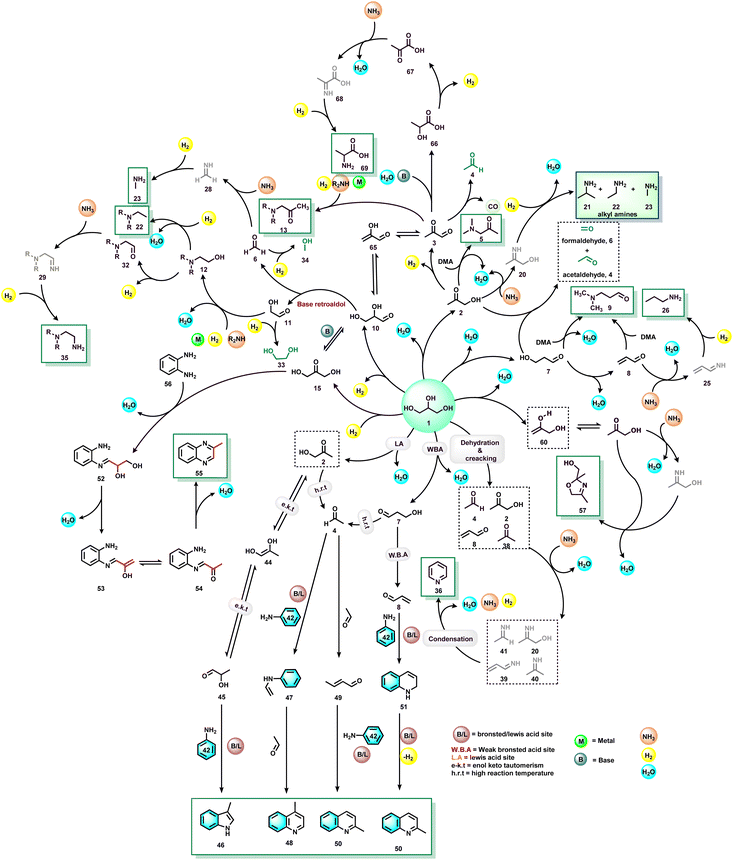
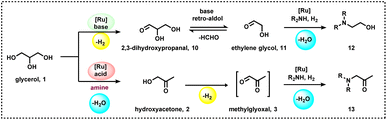
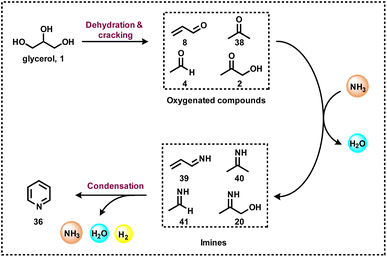
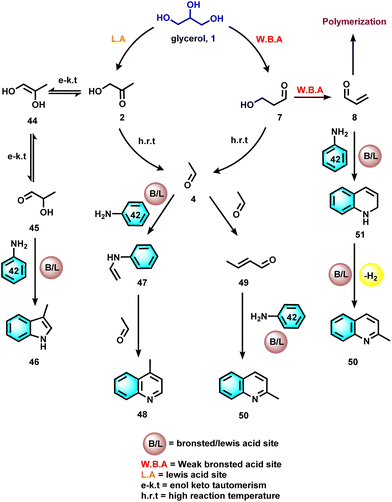
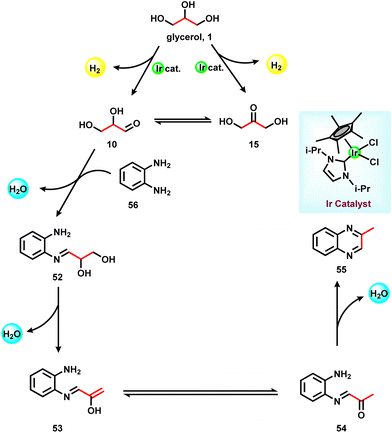
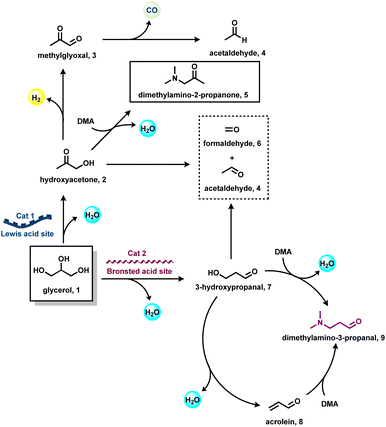
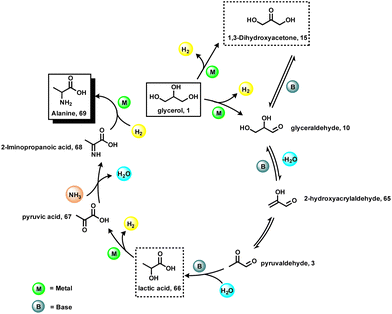
The investigation of reaction mechanisms is very important to improve chemical reactions as well as explore substitute reaction pathways. As shown in Fig. 2, glycerol (1) undergoes dehydrogenation, dehydration, and cracking in the presence of either Lewis or Brønsted acid or transition metal-based catalysts and produces hydroxyacetone (2), 3-hydroxypropanal (7), acrolein (8), acetaldehyde (4), acetone (38), dihydroxyacetone (15), glyceraldehyde (10), and prop-2-ene-1,2-diol (60) as products. With respect to glycerol amination, the chemical compounds 7 and 2 directly react with dimethylamine (DMA) to give the corresponding products 9 and 5 with water as a by-product.41 A number of possible reactions can be seen after the formation of the abovementioned intermediates (2, 7, 8, 4, 38, 15, 10, 60). The first possibility is that the intermediate 2 reacts with ammonia to give intermediate 20via dehydration, which after hydrogenation produces the corresponding alkylamines (21, 22, 23).63 Intermediate 7 reacts with intermediate 2 to give formaldehyde (6) and acetaldehyde (4). In addition, intermediate 7 can also undergo a dehydration reaction to provide acrolein (8) first, which further reacts with dimethylamine (DMA) and ammonia and produces the corresponding amines 9 and 26 respectively. The dehydration reaction of intermediates (2, 4, 8, 38) with ammonia forms imine intermediates (20, 39, 40, 41)69 which, followed by condensation and cyclization, leads to the formation of pyridine (36).69 In the same way, intermediate 60 reacts with ammonia to provide an imine intermediate and in the next step this imine intermediate further reacts with intermediate 60 to produce the oxazoline derivative (57).42 Similarly, intermediate 15 reacts with an aromatic diamine such as 1,2-phenylenediamine (56) and generates the imine intermediate (52), which then undergoes dehydration to give other intermediates 53 and 54. Next, intermediates 53 and 54 undergo dehydration followed by cyclization and give 2-methylquinoxazoline (55).74 On the other hand, the reductive amination of 7 and 2 gives the respective primary amines (21, 22, 23, 26, 35).63 In the same fashion, intermediates 7 and 2 react with aromatic amine 42 and provide the corresponding heterocyclic compounds 46, 48 and 50. Next, the base-catalysed dehydration of methylglyoxal (3) gives lactic acid (66) which undergoes dehydrogenation followed by amination and hydrogenation and finally leads to the formation of alanine (69) by a borrowing-hydrogen methodology.45 The major intermediates of glycerol oxidation are dihydroxyacetone, hydroxypyruvic acid, formic acid and glycolic acid while the hydrogenolysis of glycerol produces 1,2-propanediol and ethylene glycol as major products. Dehydration of glycerol majorly produces acrolein as a key intermediate.453. Glycerol as a building block for the synthesis of aminoketones, amino alcohols, N-formamides, and N-methyl amines
3.1 Synthesis of aminoketones and amino alcohols
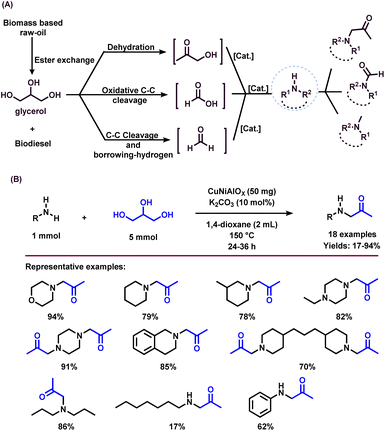
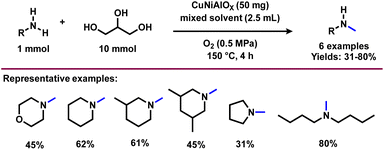
The presence of hydroxyl units in glycerol increases the potency of its reactivity with amines to provide high-value products. Among oxygenated amines, β-amino alcohols, and prochiral aminoketones are important scaffolds present in many bioactive molecules.46,47 The synthesis of these special kinds of amine starting from glycerol is of scientific interest and considered to be the sustainable methodology. In 2015, Katryniok and co-workers reported the direct amination of glycerol with dimethylamine in the presence of a phosphomolybdic acid catalyst (Cs2.5H0.5PMo12O40) and achieved (dimethylamino)acetone in 33% yield.43 In the following year, Brückner, Cui, and Shi et al. demonstrated the remarkable efficiency of the CuNiAlOX catalyst for the synthesis of prochiral aminoketones by the reaction of glycerol and amines (Scheme 1).40 The authors prepared a series of Ni–Cu alloy catalysts by a co-precipitation method, in which collective solutions of Cu(NO3)2, Ni(NO3)2, and Al(NO3)3 were precipitated with Na2CO3. Among various tested catalysts for the reaction of morpholine with glycerol, CuNiAlOX has shown the best catalytic performance in combination with 10% K2CO3, and the desired N-acetonyl morpholine was obtained with 99% selectivity. Under the standard conditions (1 mmol amine, 5 mmol glycerol, 50 mg CuNiAlOX, 10 mol% K2CO3, 2 ml 1,4-dioxane, Ar atmosphere, 150 °C, 24–36 h), various cyclic secondary amines, and piperidine and piperazine derivatives were reacted smoothly with glycerol and gave the corresponding N-acetonyl amines in 78–94% yields (Scheme 1). Remarkably, N,N-diacetonyl products could also be produced from dicyclic amines. Dipropyl amine, dibutyl amine, dioctyl amine, and dibenzyl amine also reacted well with glycerol and obtained the respective products in 70–86% yields. However, n-heptyl amine and aniline resulted in a low to moderate yield of the desired products (17–62% yields), indicating that this catalyst is less active for the reaction of primary amines with glycerol.40 Furthermore, CuNiAlOX was easily recycled and reused up to 3 runs without any significant loss of activity or selectivity.Ding and co-workers applied various types of heteropolyacid/Zr-MCM-41 catalytic system for the amination of glycerol with dimethylamine to produce dimethylamino-3-propanal.41 The authors found that H6P2W18O62/Zr-MCM-41 (Cat 2) displayed higher catalytic stability and selectivity than that of H3PW12O40/Zr-MCM-41 (Cat 1). The higher activity of Cat 2 is attributed to its higher surface area and pore volume as well as the fact it possesses more Brønsted acidic sites and a higher B/(B + L) ratio compared with Cat 1. Obviously, the presence of more Brønsted acid sites favoured the amination of glycerol.41 A plausible reaction pathway for the amination of glycerol (1) with dimethylamine (DMA) is shown in Scheme 2. The authors proposed two distinct dehydration pathways based on different types of acidic site. In the presence of Brønsted acid sites (Cat 2), glycerol dehydrogenates to 3-hydroxypropanal (7), which either can react directly with dimethylamine (DMA) to produce dimethylamino-3-propanal (9) or can easily convert to acrolein (8). The generated acrolein (8) subsequently reacts with dimethylamine (DMA) and results in the formation of dimethylamino-3-propanal (9). 3-Hydroxypropanal (7) can also be decomposed to formaldehyde (6) and acetaldehyde (4). On the Lewis acid sites, (Cat 1), the formation of hydroxyacetone (acetol, 2) takes place via the dehydration and deprotonation of the protonated intermediate. Next, hydroxyacetone (2) gets utilized in three pathways: the first one is the reaction of hydroxyacetone (2) with dimethylamine (DMA) to produce dimethylamino-2-propanone (5), the second one is the formation of methylglyoxal (3) via the dehydrogenation of hydroxyacetone (2), which undergoes decarbonylation to produces acetaldehyde (4), and the last one is the decomposition of hydroxyacetone (2) into formaldehyde (6) and acetaldehyde (4) (Scheme 2).41
 | ||
| Scheme 2 Plausible reaction pathway of the H6P2W18O62/Zr-MCM-41 catalyzed amination of glycerol with dimethylamine. | ||
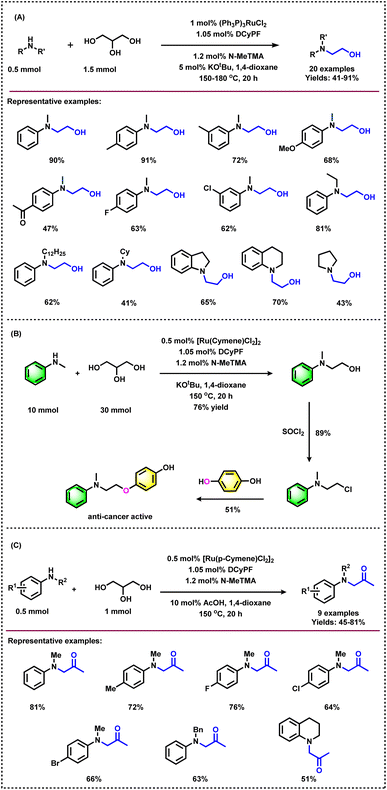
In 2020, the group of Li demonstrated an interesting methodology for the acid–base tuned selective and switchable N-hydroxyethylation and N-acetonylation of amines employing glycerol in the presence of a Ru(II) complex.48 The stability of the active ruthenium species with glycerol coordination is essential for the selective carbon–carbon or carbon–oxygen bond cleavage of glycerol under base/acid conditions. Initially, the authors reacted N-methylaniline with glycerol applying Ru-complexes as catalysts. Various organic ligands were tested with 1 mol% RuCl2(PPh3)3 in the presence of catalytic amounts of potassium t-butoxide under an elevated temperature (Table 1, entry 10). The combination of bidentate phosphine ligand 1,1′-bis(di-cyclohexylphosphino)ferrocene (DCyPF) and thiophene-based nitrogen ligand N-MeTMA (N-methyl-(2-thienylmethyl)amine) was found to be the best ligand system for this Ru-based amination methodology to achieve 94% of the corresponding N-hydroxyethylated product (A). Under the optimized reaction conditions (0.5 mmol amine, 1.5 mmol glycerol, 1 mol% RuCl2(PPh3)3, 1.05 mol% DCyPF, 1.2 mol% N-MeTMA, 5 mol% KOtBu, 1 mL 1,4 dioxane, 150 oC, 20 h), various N-alkylanilines bearing electron-donating and withdrawing groups including halogen-substituted ones and benzo-fused cyclic motifs were reacted with glycerol and obtained the corresponding products in 41–91% yields (Scheme 3A). In addition, secondary aliphatic amines were also tested for the catalytic amination of glycerol to obtain the desired products in 43–60% yields (Scheme 3A). However, phenylamine and benzylamine did not give the targeted products.48 Besides, the authors successfully applied this Ru-based methodology for the synthesis of an anti-cancer drug molecule, showing the robustness of this protocol and possible applications of glycerol in the pharmaceutical industry (Scheme 3B).
| Entry | L1 | L2 | Yield, A (%) | Yield, B (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol N-methylaniline, 1 mL 1,4-dioxane, 150 °C, 20 h. Conversions and yields were determined by GC using n-docecane as an internal standard. b [Ru(p-cymene)Cl2]2 was used. c No base. d 10 mol% of AcOH was used instead of tBuOK. Abbreviation: Pica = 2-picolyamine; N-MePica = 2-[(methylamino)methyl]pyridine; Hafa = 2-furfurylamine; TMA = 2-thiophenemethylamine; TEA = 2-thiopheneethylamine; N-MeTMA = N-methyl-(2-thienylmethyl)amine. | ||||
| 1 | — | — | n.d. | Trace |
| 2 | tol-BINAP | Pica | 12 | Trace |
| 3 | tol-BINAP | — | Trace | n.d. |
| 4 | — | Pica | n.d. | Trace |
| 5 | DCyPF | Pica | 74 | Trace |
| 6 | DCyPF | N-MePica | 80 | Trace |
| 7 | DCyPF | Hafa | 62 | Trace |
| 8 | DCyPF | TMA | 85 | Trace |
| 9 | DCyPF | TEA | 87 | Trace |
| 10 | DCyPF | N-MeTMA | 94 | Trace |
| 11b | DCyPF | N-MeTMA | 94 | Trace |
| 12b,c | DCyPF | N-MeTMA | Trace | 40 |
| 13b,d | DCyPF | N-MeTMA | n.d. | 81 |
Interestingly, by adding 20 mol% of acetic acid as a co-catalyst, the selectivity of Ru catalyst was switched to yield N-acetonylated product (B) in 81% yield (Table 1, entry 13). Based on this result, the authors extended the utilization of glycerol and reacted it with different amines under acidic conditions to afford N-acetonylation products in moderate to good yields (45–81%) (Scheme 3C).48a In the proposed reaction pathway presented in (Scheme 4), ethylene glycol (11) is produced from glycerol (1) through a retro-aldol reaction. In situ NMR experiments with labelled 13C3-glycerol and the detection of ethylene glycol (11) and hydroxyacetone (2) confirmed the involvement of a hydrogen-borrowing pathway and retro-aldol carbon–carbon bond cleavage steps (Scheme 4). The thienyl amine ligand inhibits the formation of nonlabile Ru–O interaction between the catalyst and glycerol due to the hemilabile S–Ru attachment.48a
Apart from the utilization of glycerol as an alkylating agent, biomass-derived carbohydrates also serve as excellent sources of C2 alkylating reagents of arylamines to generate β-amino alcohols in the presence of homogeneous ruthenium complexes under acidic conditions.48b
3.2. Synthesis of formamides
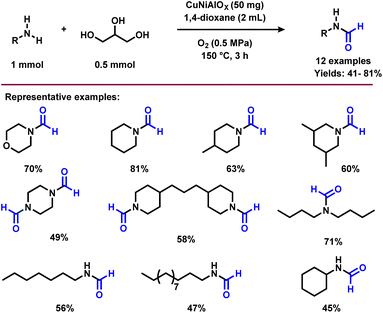
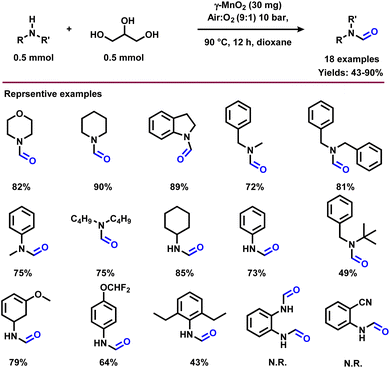
The N-formylation of amines plays an important role in synthetic organic chemistry for the preparation of formamides, which represent an interesting class feedstock and intermediates for the manufacture of pharmaceuticals, herbicides and pesticides.49 In addition, this reaction is applied to protect the amine group in a straightforward manner under mild conditions.50 In addition to general methodologies, in recent years, glycerol has been used as a carbonyl source for the synthesis of formamides. Since, formic acid can be generated via the oxidative carbon–carbon cleavage of glycerol,51 the resulting formic acid would rapidly react with amine to produce N-formyl amine. In 2016, Shi and co-workers reported the synthesis of N-formylamine by reacting glycerol and amines using a CuNiAlOX catalyst in the presence of 10% K2CO3 and molecular oxygen.40 Under the reaction conditions (1 mmol amine, 0.5 mmol glycerol, 50 mg CuNiAlOX, 2 mL 1,4-dioxane, 0.5 MPa O2, 150 °C, and 3 h), cyclic and linear secondary amines including aliphatic primary amines were successfully transformed to the corresponding N-formylamines in moderate to good yields (41–81%) (Scheme 5).40 A few years later, the same research group accomplished the N-formylation of amines to valuable formamides utilizing C2–C3 bio-derived platform compounds such as glycolic acid, 1,3-dihydroxyacetone, and glyceraldehyde in the presence of H2O2 and a Cu-containing zeolite 5A catalyst.52Recently, Bhanage and co-workers explored the application of various C2- and C3-feedstocks such as glycerol, 1,3-dihydroxyacetone, ethylene glycol, glycolic acid, and glyoxal for the selective synthesis of N-formamides using TBHP as oxidant.53 Both 1,3-dihydroxyacetone and glyoxal exhibited the best carbon efficiency and resulted in N-formamide in 100% selectivity.53 Subsequently, the same group reported a crystalline MnO2 nanocatalyst for the preparation of various N-formamides under aerobic conditions using glycerol.54 The selectivity of the reaction can be tuned using mixed crystalline MnO2 nanomaterials and their surface Mn(III)/Mn(IV) species ratios. In the presence of α- and γ-MnO2 the N-formamide product with high selectivity was obtained, while β- and δ-MnO2 gave the mixture of N-formamide and N-methylamine in equal ratio. Under the optimized conditions (0.5 mmol amine, 0.5 mmol glycerol, 30 mg γ-MnO2, 10 bar air![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 (9
O2 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 5 mL dioxane, 90 °C, 12 h), various primary and secondary amines were selectively formylated with glycerol and provided the desired products in good to high yields (43–90%) (Scheme 6). On the other hand, o-phenylenediamine and o-amino benzonitrile failed to give the desired products. This protocol is also amenable for the gram-scale synthesis of N-formylmorpholine. Recycling and reusability of the γ-MnO2 catalyst was demonstrated for 7 times and it was found that the activity of the catalyst in each cycle was slightly dropped due to Mn leaching (Fig. 3).54
1), 5 mL dioxane, 90 °C, 12 h), various primary and secondary amines were selectively formylated with glycerol and provided the desired products in good to high yields (43–90%) (Scheme 6). On the other hand, o-phenylenediamine and o-amino benzonitrile failed to give the desired products. This protocol is also amenable for the gram-scale synthesis of N-formylmorpholine. Recycling and reusability of the γ-MnO2 catalyst was demonstrated for 7 times and it was found that the activity of the catalyst in each cycle was slightly dropped due to Mn leaching (Fig. 3).54
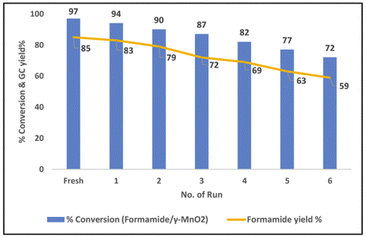 | ||
| Fig. 3 Recyclability of γ-MnO2 catalyst for N-formylation. This figure has been adapted from ref. 54 with permission from Elsevier, copyright 2022. | ||
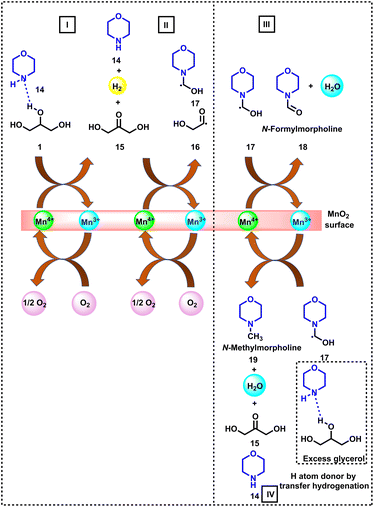
As shown in Fig. 4, the first step of this N-formylation reaction involves the oxidation of glycerol (1) to dihydroxyacetone (15) in the presence of morpholine (14) and MnO2 catalyst. The homolytic cleavage at the carbonyl site of the dihydroxyacetone (15) leads to the generation of radicals (16 and 17), which traps the morpholine moiety and forms activated species. By molecular oxygen the Mn(III) site is re-oxidized to Mn(IV) at a lower rate. The presence of the Mn(IV) site on the surface leads to higher selectivity of the catalyst towards N-formamide (18), whereas a mixture of Mn(III) and Mn(IV) species is responsible for the formation of a mixture of formamide and N-methylamine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio).
1 ratio).
3.3. Synthesis of N-methylamines
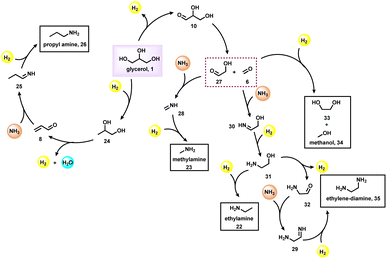
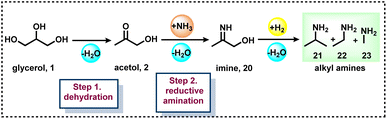
N-Methylamines represent important fine and bulk chemicals, which play a vital role in the field of organic synthesis in drug discovery, and life science applications.55–61 The conventional process for the production of N-methyl amines mainly uses the Eschweiler–Clarke reaction. Over the last ten years, dimethylcarbonate, CO2, methanol, and formic acid are employed as efficient C1 sources for the preparation of N-methylamines.62 Comparatively, glycerol is less explored for the synthesis of N-methylamines. Inspired by the synthesis of aminoketones and N-formamides using glycerol, Shi et al.40 performed the N-methylation of amines using glycerol as a methylation source and using a CuNiAlOX catalyst in the presence of 10% K2CO3 and oxygen. Under standard conditions, cyclic and linear secondary amines reacted smoothly with glycerol and gave the corresponding N-methylamines in low to high yields (31–80%) (Scheme 7).Very interestingly, Jin and Chaudhari et al.63 presented a challenging amination of glycerol (1) with ammonia to produce alkyl amines such as isopropylamine (21), ethylamine (22) and N-methylamine (23) using Ru/C (Scheme 8). To find out the best catalyst, different noble metal-based catalysts such as Ru/C, Pd/C, Rh/C and Pt/C were tested, and among these the Ru-system is found to be the optimal one. It has been observed that glycerol conversion was achieved up to 96% in 48 h of reaction in the presence of Ru/C (Table 2). With respect to the mechanism of this Ru-catalyzed amination process (Fig. 5), firstly, dehydrogenation of glycerol (1) occurs to give propane-1,2-diol (24), which then undergoes dehydration to give acrolein (8). Next, acrolein reacts with amine and generates imine (25), which on reduction provides propylamine (26). The dehydrogenation of glycerol also leads to the formation of glyceraldehyde (10) which upon cleavage gives formaldehyde (6) and glycolaldehyde (27). These intermediates proceed with three possible reaction sequences. The first possibility is to form ethylene glycol (33) and methanol (34) by simple hydrogenation. The second pathway reaction with ammonia forms imine (28), followed by hydrogenation leading to methyl amine (23). The third possibility is reaction with ammonia followed by hydrogenation to produce 2-hydroxyethylamine (31), which can be either hydrogenated or dehydrogenated to form ethylamine (22) and 2-aminoacetaldehyde (32) respectively. The 2-aminoacetaldehyde (32) can produce ethylenediamine (35) by undergoing a reaction with ammonia followed by hydrogenation.
| Experimental conditions: 200 °C, 100 bar H2 pressure, 48 h. Abbreviation: methylamine (MA), ethylamine (EA), propylamine (PA), ethylene-diamine (EDA), 2,6-dimethylpiperazine (2,6-DMP), piperazine (PZ), 2-methyl piperazine (2-MP), 1,2-propanediol (1,2-PDO), methanol (MeOH), ethylene glycol (EG). | ||||
|---|---|---|---|---|
| # | 1 | 2 | 3 | 4 |
| Catalyst | Pd/C | Rh/C | Pt/C | Ru/C |
| Conversion (%) | 16.3 | 32.1 | 55.8 | 96.2 |
| Selectivity (%) | ||||
| MA | 3.9 | 1.5 | 11.6 | 18.6 |
| EA | 0 | 0 | 3.6 | 14.4 |
| PA | 0 | 3.5 | 3.0 | 15.6 |
| EDA | 5.8 | 1.9 | 3.8 | 2.8 |
| 2,6-DMP | 51.0 | 50.1 | 29.3 | 2.9 |
| PZ | 3.7 | 7.4 | 2.8 | 2.2 |
| 2-MP | 4.5 | 3.7 | 4.5 | 3.2 |
| 1,2-PDO | 1.0 | 2.8 | 0.5 | 1.5 |
| MeOH | 4.8 | 1.9 | 3.8 | 15.1 |
| EG | 2.1 | 1.0 | 0.6 | 2.4 |
| Unknowns | 23.3 | 26.2 | 38.5 | 21.4 |
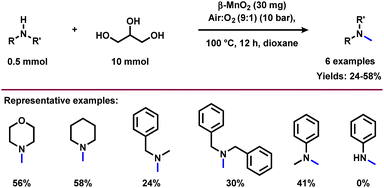
Quite recently, Bhanage and co-workers prepared N-methylamines employing β-MnO2 as a catalyst in the presence of a higher amount of glycerol.54 Under optimized conditions (0.5 mmol amine, 10 mmol glycerol, 30 mg β-MnO2, 10 bar air![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 (9
O2 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 °C, 5 mL dioxane, 12 h), aliphatic secondary amines reacted well with glycerol and obtained the corresponding N-methylated products in average yields (24–58%) (Scheme 9). Primary amine such as aniline failed to deliver the corresponding N-methylamine product under standard conditions. Catalyst recycling experiments showed that the activity of catalysts is significantly decreased in each recycle (Fig. 6). The decrease in the catalytic activity of β-MnO2 was observed due to the Mn leaching. The catalyst β-MnO2 is active in the oxidation state of Mn(III) or Mn(IV) but the excess of glycerol concentration reduces the Mn(III) or Mn(IV) active surface into a Mn(II) surface in the last step of the reaction and leads to the higher Mn leaching content in the case of β-MnO2 which does disturb the redox cycle necessary for a good conversion and resulted in a decrease in the conversion of amines.
1), 100 °C, 5 mL dioxane, 12 h), aliphatic secondary amines reacted well with glycerol and obtained the corresponding N-methylated products in average yields (24–58%) (Scheme 9). Primary amine such as aniline failed to deliver the corresponding N-methylamine product under standard conditions. Catalyst recycling experiments showed that the activity of catalysts is significantly decreased in each recycle (Fig. 6). The decrease in the catalytic activity of β-MnO2 was observed due to the Mn leaching. The catalyst β-MnO2 is active in the oxidation state of Mn(III) or Mn(IV) but the excess of glycerol concentration reduces the Mn(III) or Mn(IV) active surface into a Mn(II) surface in the last step of the reaction and leads to the higher Mn leaching content in the case of β-MnO2 which does disturb the redox cycle necessary for a good conversion and resulted in a decrease in the conversion of amines.
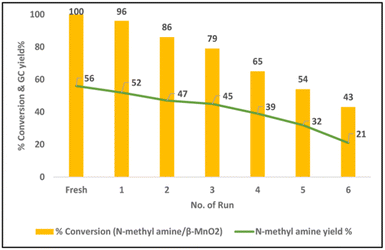 | ||
| Fig. 6 Recyclability of β-MnO2 catalyst for N-methylation. This figure has been adapted from ref. 54 with permission from Elsevier, copyright 2022. | ||
For the synthesis of N-methylamine products, MnO2 was found to be a suitable oxidation catalyst. In the redox cycle of Mn(III)/Mn(IV), these reaction conditions follow a Mars–van Krevelen mechanism for the oxidation of glycerol. The first step involves the oxidation of substrate at the MnO2 surface to form partially reduced MnOx species and the second is the re-oxidation of the MnOx species in the presence of molecular oxygen. The presence of oxidative conditions is important in order to convert Mn(III) into Mn(IV), which is necessary to obtain the catalytic cycle.54
4. Glycerol as a building block for the synthesis of heterocycles
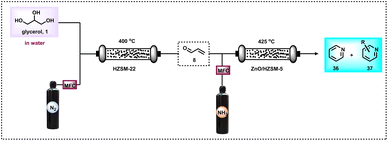
Heterocyclic compounds are important in the fields of organic synthesis, medical chemistry, and biological chemistry.64 Particularly N-heterocycles find increasing interest in drug discovery because heterocyclic motifs play vital roles in the activity of these life science molecules.65a In this respect, the formation of N-heterocycles through the dehydrogenative condensation of alcohols and 1,2-amino alcohols for the selective and consecutive formations of C–C and C–N bonds is very attractive from the view point of sustainability.65b,c The group of Kempe developed excellent sustainable protocols for the synthesis of five- and six-membered nitrogen-based heterocycles.65b,c On the other hand, pyridine-based structural motifs are widely present in many pharmaceuticals, agrochemicals, and biomolecules. Due to their profound effect on pharmacological activities, pyridine and their derivatives are used in drug discovery. In fact, there are about 100 FDA-approved drugs in 2020–2022 that contain either pyridine or dihydropyridine moieties.66 Thus, due to the importance of these N-heterocycles, there is an increasing demand for their production, especially starting from more abundant and renewable feedstocks. Interestingly, pyridine and methyl-substituted pyridines can be prepared from glycerol in a two-step reaction (Scheme 10).67 In a continuous flow process, at 400 °C, glycerol (1) was converted to acrolein (8) using HZSM-22 zeolite. On further reaction with ammonia at 425 °C over zinc oxide the supported HZSM-5 zeolite produced pyridine (36) and methylpyridine (37) in a steady combined yield of 61–65% (Scheme 10).67 Notably, 2-methyl pyrazine can also be produced in a continuous process through the dehydrocyclization of glycerol by reacting with a stoichiometric amount of ethylenediamine over a ZnO–ZnCr2O4 catalyst.68Recently, Wang and Li et al.,69 adapted the DFT M06-2X method for the synthesis of pyridine (36) by reacting glycerol (1) with ammonia at 550 °C. Based on glycerol conversion and mechanistic investigations, it was observed that the dehydration of glycerol produced acrolein (8), acetaldehyde (4), and acetone (38) as well as acetol (2). These oxygenated compounds, particularly acrolein (8) and acetaldehyde (4), react with gaseous ammonia to form imine (20, 39–41), which undergoes sequential reactions such as Michael addition, a Diels–Alder reaction, deammonization, and a dehydrogenation process to form pyridine 36 (Scheme 11).69
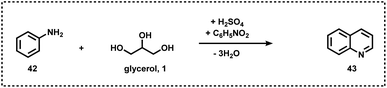
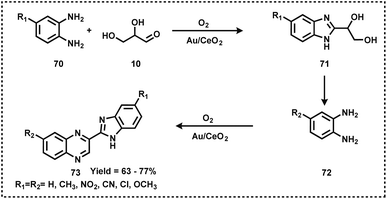
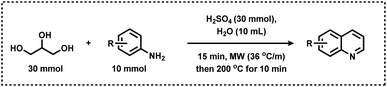
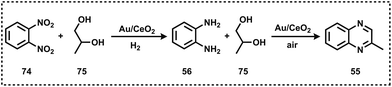
Like pyridine, quinoline-based scaffolds are often found in several biologically active molecules. For the synthesis of quinolines, Skraup synthesis (Scheme 12) represents one of the general methodologies in which glycerol reacts with aniline in the presence of sulphuric acid and an oxidant (nitrobenzene or As2O5).70 In 2013, Corma and Iborra together synthesized benzimidazoylquinoxaline in good yields via a one-pot oxidative coupling of glycerol or glyceraldehydes with 1,2-phenylenediamines in a two-step reaction, employing Au/CeO2 as the heterogeneous catalyst under base-free and mild reaction conditions, and air as the oxidant (Scheme 13).71 Interestingly, 1,2-phenylenediamines bearing electron-withdrawing groups improved the selectivity of the targeted benzimidazoylquinoxaline products. The Au/CeO2 catalyst could be easily recovered and re-used with a slight loss of activity, thereby maintaining high selectivity.71
In 2014, Len and colleagues performed a classical Skraup reaction in neat water under microwave irradiation conditions and synthesized 5-, 6-, 7-, and 8-substituted quinolines employing glycerol and anilines as starting materials (Scheme 14).72 This protocol is also amenable for the synthesis of phenanthrolines by treating nitroaryl derivatives with glycerol.72
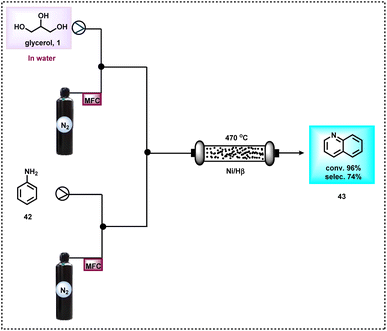
After three years, the group of Chao reported the vapour-phase synthesis of quinoline (43) by reacting glycerol (1) with aniline (42) over Ni/mesoporous beta zeolite (Ni/Hβ-At) as a catalyst at 470 °C and produced quinoline in 71.4% yield (Scheme 15).73 Key to this success is the mesoporous structure and the acidic sites of the catalyst. Mesopores promote the transport of bulky products and the weak Brønsted acid sites favour the dehydration of glycerol to acrolein, as well as the existence of a Lewis acid site accelerating the formation of quinoline. The catalyst was reused only up to 3 cycles due to the deposition of coke.73 A plausible reaction mechanism was also proposed for this Ni-catalyzed synthesis of quinoline (Scheme 16). Firstly, glycerol upon dehydration produces 3-hydroxypropanal (7), acrolein (8), acetol (2), and acetaldehyde (4) which were detected by mass spectral analysis. Next, these obtained intermediates react with aniline and gave different N-heterocycles. For example, the reaction of acetaldehyde (4) and aniline (42) produced 2- or 4-methylquinoline (50 and 48 respectively). 3-Methylindole (46) was generated from the reaction of aniline (42) and acetol (2) (Scheme 16).
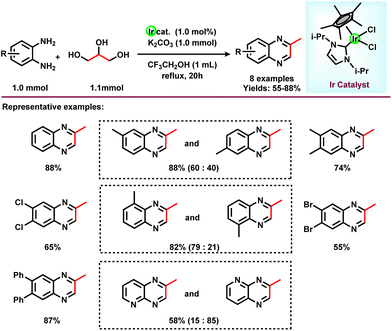
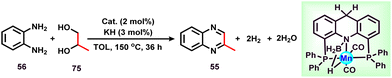
In 2015, Zhang and co-workers reported the synthesis of 2-methyl quinoxalines in satisfactory yields in the presence of a Ru catalyst.44 Very recently, the group of Fujita synthesized 2-methylquinoxaline derivatives by reacting 1,2-phenylenediamine with glycerol in the presence of an N-heterocyclic carbene-based Ir-catalyst.74 Various NHC-based Ir-catalysts have been screened out and it was found that the Ir-complex with an N-isopropyl group containing the NHC ligand had optimal results in the presence of K2CO3 in CF3CH2OH solvent. Under optimized conditions (1.1 mmol glycerol, 1 mmol 1,2-phenylenediamine, 1.0 mol% Ir-catalyst, 1 mmol K2CO3, and 1 mL CF3CH2OH, 20 h), different substituted 1,2-phenylenediamines were reacted with glycerol and gave a 92% yield of the corresponding 2-methylquinoxazoline (Scheme 17). Methyl-substituted 1,2-phenylenediamine gives isomers of dimethylquinoxaline in good yield. Diamine with a pyridine ring skeleton also gives the corresponding quinoxaline in moderate yield (Scheme 17).
As shown in Scheme 18, the glycerol undergoes dehydrogenation and provides an equilibrium mixture of glyceraldehyde (10) and dihydroxyacetone (15) in presence of the NHC-based Ir-catalyst. Owing to the high reactivity of glyceraldehyde, it immediately reacts with 1,2-phenylenediamine (56) and generates an intermediate (52), which undergoes dehydration to give the enol derivative (53) of 2-aminophenyliminopropan-2-one (54). Finally this derivative proceeds with intramolecular dehydration to give 2-methylquinoxazoline (55) (Scheme 18).74
In addition to the importance of bioactive molecules, oxazoline-based compounds are often used as organic ligands in asymmetric catalysis.75 Hence, the use of renewable glycerol for the preparation of oxazoline and its derived compounds is highly desired. In this regard, in 2018, Rode and co-workers reported a simple and environmentally benign approach for the catalytic synthesis of an oxazoline-based compound (57) from glycerol and ammonia.42 The authors have used two catalytic systems to produce oxazoline derivatives in different pathways. Acidic Cu–Zr catalyzed the reaction in two steps in which glycerol converts to acetol 2 (78% yield) followed by amination with ammonia to an oxazoline-based compound with 95% yield (Scheme 19). However, Ru/C catalyzed the amination of glycerol in a one-pot reaction to produce oxazoline derivative (57) with 95% selectivity (Scheme 19). In addition, the Ru/C catalyst was found to be highly active and showed an excellent performance in up to four reaction cycles.
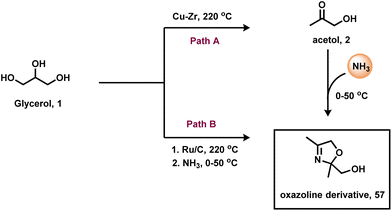 | ||
| Scheme 19 Cu and Ru-catalyzed amination of glycerol with ammonia to produce the oxazoline-based compound. | ||
The authors proposed a plausible mechanistic pathway for the amination of glycerol to an oxazoline derivative (Scheme 20). Initially, acid-catalysed dehydration of glycerol takes place (58–59), in which the active sites of the metal play a crucial role in the activation of glycerol (59). Next, the abstraction of primary –OH of glycerol by Ru (Ru–O) and simultaneous proton abstraction of the adjacent carbon by the bridging oxygen atom of the Ru–O leads to the formation of enol (60) which upon tautomerization is converted into keto (61) by abstraction of the proton of hydroxy group of the enol by Ru(0) to give acetol (61). The bifunctionality of the catalyst was observed because RuO2 and RuO3 act as the acid promoter for the activation of the carbonyl group whereas Ru is an active site for ammonia dissociation. NH3 dissociatively adsorbed on the catalyst surface and a simultaneous lone pair of N attacks on the acetol formed a tetrahedral intermediate (62) which is reversibly converted to carbonyl amine and then to 2-imino-1-propanol (63). The imine formation mainly requires acid sites for the elimination of water. In the last step, the formed 2-imino-1-propanol undergoes condensation (64) with a second molecule of acetol which upon cyclization leads to the formation of oxazoline derivative (57).42 Very recently, Yu and Xiao together reported a robust protocol for the synthesis of 2-methylquinoxalines directly from glycerol and o-phenylenediamine in the presence of Pt/CeO2 catalyst at 140 °C.76 The authors also tested other bio polyols to produce high-value 2-methylquinoxalines.
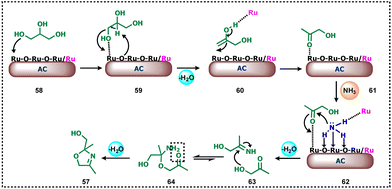 | ||
| Scheme 20 Plausible mechanistic pathway for the amination of glycerol to the oxazoline-based compound using heterogeneous Ru-catalyst. | ||
5. Glycerol as a building block for the synthesis of amino acids
Valorization of glycerol by catalytic amination with ammonia is considered to be an economically and sustainably viable methodology that gives access to valuable organonitrogen compounds. However, this process is very challenging and hence fewer efforts have been made on this amination. As an example of the amination of glycerol, the synthesis of amino acids is an important process due to the importance of the later products.77 In this respect, recently, the group of Yan reported a one-step protocol for the transformation of crude glycerol into 43% alanine over a Ru1Ni7/MgO catalyst in a batch reactor.45 Initially, glycerol transforms into lactic acid as the key intermediate, which then was aminated with ammonia to produce alanine in the presence of a multifunctional catalytic system. The authors evaluated several catalysts such as Ru1M10/MgO, Ru/MgO, Ni/MgO, and RANEY® Ni for the amination of glycerol and found that Ru-based ones exhibited better activity and selectivity compared with Ni-catalysts (Fig. 7a). However, with these systems satisfactory results were not achieved. Then different supported bimetallic materials containing Ru and other metals were prepared and tested for the reaction. Among these, Ru–Ni bimetallic ones showed a better performance and finally Ru1Ni10/MgO was found to be the optimal catalyst (Fig. 7a and b). Varying the ratio of Ru and Ni revealed that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio of these metals’ composition is required to create the best catalytic material (Fig. 7c). Applying the optimal catalyst, Ru1Ni10/MgO, a 45% yield of alanine was achieved at 220 °C for 3.5 h.
10 ratio of these metals’ composition is required to create the best catalytic material (Fig. 7c). Applying the optimal catalyst, Ru1Ni10/MgO, a 45% yield of alanine was achieved at 220 °C for 3.5 h.
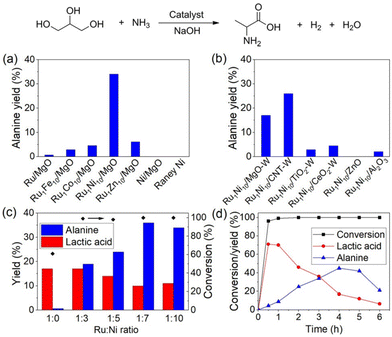 | ||
Fig. 7 Catalytic conversion of glycerol into alanine in a batch reactor over (a) Ru1M10/MgO, Ru/MgO, Ni/MgO and RANEY® Ni catalysts, (b) Ru1Ni10 on different supports (W indicates catalysts prepared by the wet-impregnation method), (c) MgO supported Ru1NiX catalyst with varied Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio, and (d) conversion/yield profile over Ru1Ni7/MgO catalyst as a function of time. Reaction conditions: 100 mg glycerol, 200 mg NaOH, 67 mg catalysts or 30 mg RANEY® Ni (Ru Ni ratio, and (d) conversion/yield profile over Ru1Ni7/MgO catalyst as a function of time. Reaction conditions: 100 mg glycerol, 200 mg NaOH, 67 mg catalysts or 30 mg RANEY® Ni (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate molar ratio = 0.012), 2 mL NH3H2O (25 wt%), 10 bar H2, 220 °C, 4 h. Catalysts were reduced at 500 °C for (a–c) and 600 °C for (d). This figure has been adapted from ref. 45 with permission from Wiley, copyright 2019. substrate molar ratio = 0.012), 2 mL NH3H2O (25 wt%), 10 bar H2, 220 °C, 4 h. Catalysts were reduced at 500 °C for (a–c) and 600 °C for (d). This figure has been adapted from ref. 45 with permission from Wiley, copyright 2019. | ||
The authors have made an interesting experiment by recording the 13C NMR spectrum of the product mixture using glycerol–13C3 as the starting material. The 13C NMR of the product mixture was simple throughout the complete reaction time of either 1 or 4 hours (Fig. 8). During the course of this amination of glycerol, lactic acid and alanine were identified as the major products. In addition, small amounts of 13C formic acid, formamide and 13C2 glyceric acid were also observed. Based on these results, the authors proposed a plausible reaction pathway, which is presented in (Fig. 9). First, lactic acid (66) is produced from glycerol (1) via sequential dehydrogenation, dehydration, and rearrangement reactions. Then the –OH group of the lactic acid (66) reacts with NH3 to form alanine (69) by a borrowing-hydrogen methodology. The other C1 and C2 side products were formed by the hydrogenolysis of glycerol. In this amination process, the catalyst and base cooperatively work by a tandem reaction sequence involving dehydrogenation, hydrogenation, and hydrogenolysis as well as dehydration and a Cannizzaro reaction. The base also facilitates the metal-catalyzed dehydrogenation of glycerol by deprotonation and most likely displaces lactic acid/alanine from the catalyst surface as sodium lactate/sodium alaninate, which accounts for the proportional rise in lactic acid and alanine yields with respect to the amount of NaOH (Fig. 9).
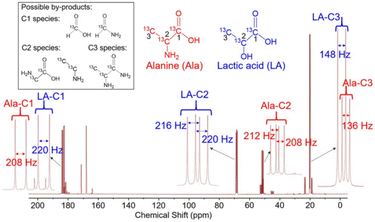 | ||
| Fig. 8 13C NMR spectra of alanine and lactic acid produced by the Ru1Ni10/MgO catalyzed amination of glycerol with ammonia. This figure has been adapted from ref. 45 with permission from Wiley, copyright 2019. | ||
 | ||
| Fig. 9 Proposed reaction mechanism for the synthesis of alanine from glycerol and ammonia in the presence of Ru1Ni10/MgO. | ||
6. Amination of glycerol-derived molecules
The main glycerol-derived molecules are hydroxyacetone, glyceraldehyde, lactic acid, and others. They display different functional groups that were used in hydrogen-borrowing reactions.6.1 Amination of hydroxyacetone
In 2020, Domine and co-workers introduced a novel catalytic pathway employing bio-derived acetol for the synthesis of 2-methyl piperazine (2-MP) through reductive cyclo-amination with ethylenediamine as the nitrogen source under moderate conditions.78 Their approach utilized heterogeneous catalysts based on Pd nanoparticles supported on metallic oxides and mixed oxides and extensively characterized by ICP analysis, XRD, HR-TEM, and NH3-TPD. Notably, the most promising results were achieved with Pd/TiO2–Al2O3 and Pd/ZrO2–Al2O3, with the former yielding over 80% of 2-MP. The Pd/TiO2–Al2O3 catalyst demonstrated the successful activation of the imine group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) owing to a higher concentration of unsaturated Pd sites within its nanoparticles. Additionally, it maintained an appropriate acidity level, allowing the effective and selective execution of the reductive cyclo-amination reaction, even with lower catalyst loadings (≈5 wt%). The reductive cyclo-amination reactions were conducted in a 6 ml “batch” type micro-reactor equipped with a sampling probe. The catalysts Pd/TiO2 and Pd/ZrO2 have shown excellent stability for three consecutive runs, while a decrease of 10% was noticed by the authors when using Pd/Al2O3 for the third time in a row (Fig. 10). Furthermore, the authors found that Pd/MgO has a basic character and exhibited higher deactivation due to organic matter deposition during the reaction, revealing an elevated level of polymerization reactions on its surface.78 Recently, Ducrot et al. have reported the biocatalytic reductive amination of α-hydroxy ketones by native amine dehydrogenases to access short chiral alkyl amines and amino alcohols.79
N) owing to a higher concentration of unsaturated Pd sites within its nanoparticles. Additionally, it maintained an appropriate acidity level, allowing the effective and selective execution of the reductive cyclo-amination reaction, even with lower catalyst loadings (≈5 wt%). The reductive cyclo-amination reactions were conducted in a 6 ml “batch” type micro-reactor equipped with a sampling probe. The catalysts Pd/TiO2 and Pd/ZrO2 have shown excellent stability for three consecutive runs, while a decrease of 10% was noticed by the authors when using Pd/Al2O3 for the third time in a row (Fig. 10). Furthermore, the authors found that Pd/MgO has a basic character and exhibited higher deactivation due to organic matter deposition during the reaction, revealing an elevated level of polymerization reactions on its surface.78 Recently, Ducrot et al. have reported the biocatalytic reductive amination of α-hydroxy ketones by native amine dehydrogenases to access short chiral alkyl amines and amino alcohols.79
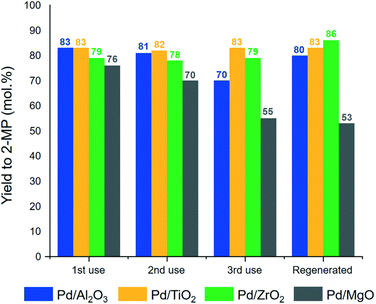 | ||
| Fig. 10 Catalyst re-usability and regeneration for Pd supported on simple oxide materials. This figure has been adapted from ref. 79 with permission from the Royal Society of Chemistry 2020. | ||
6.2. Amination of glycerol-derived propane-1,2-diol
Propane-1,2-diol can be obtained via the deoxygenation of glycerol.80 In the recent years, the exploitation of glycerol-derived molecules has attracted significant attention in the context of developing eco-friendly routes to a myriad of important synthetic organic transformations.81 In the past Corma and Iborra together developed efficient methodologies for the synthesis of quinoxalines (76) from glycols (75) and 1,2-dinitrobenzene (74) derivatives using supported gold nanoparticles as catalysts using reusable Au/CeO2 as the catalyst (Scheme 21). The kinetic experiments revealed that the reaction-controlling step is the oxidation of diol to an α-hydroxycarbonyl compound.82In 2018, the group of Milstein reported a manganese pincer complex for the synthesis of 2-methyl quinoxaline (55) in 80% isolated yield by the dehydrogenative coupling of propane-1,2-diol (75) and ortho phenylenediamine 56 (Scheme 22).83
In 2019, Kundu and co-workers reported a novel and robust method for the synthesis of 2-methyl quinoxaline (55) in an aqueous environment employing propane-1,2-diol (75) and benzene-1,2-diamine (56) under atmospheric conditions.84 Subsequently, the same group also reported a N-doped carbon-supported cobalt catalyst for the synthesis of 2-methyl quinoxaline (55) in 93% yield.85 Interestingly, the combination of NiBr2/1,10-phenanthroline also worked well for the preparation of 2-methyl quinoxaline (55) in excellent yield.86
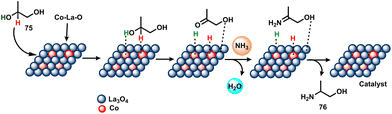
In 2019, Yue and co-workers reported the selective catalytic amination of 1,2-propanediol (75) with NH3 over nano-Co/La3O4 and achieved a 68% conversion and 89% selectivity of 2-amino-1-propanol (76) under optimal conditions (Scheme 23).87 The authors also revealed that the presence of basic lanthanum oxide was essential to facilitate the selective catalytic reaction.
7. Summary and outlook
The use of bio-renewable glycerol for the synthesis of valuable amines is a topic of academic and industrial interest since the availability of glycerol has greatly increased. In this review, the catalytic valorization of glycerol has been discussed to produce various amine-based products. Catalytic amination of glycerol has flourished into broadly applicable methodologies that give straightforward access to a variety of amines, which serve as key intermediates in both the chemical and pharmaceutical industries. Starting from glycerol as a key building block, by applying different catalyst systems a series of functionalized amines such as aminoketones, amino-alcohols, amino acids, N-formamides, and N-methyl amines as well as pyridine- quinoline-, and oxazoline-based heterocycles were prepared. In addition to the synthesis, mechanisms of catalytic amination of glycerol were discussed wherever possible. This review clearly witnessed that in the last five years, glycerol has emerged as a versatile feedstock for the renewable synthesis of value-added amine-based products. However, the amination of glycerol is still at an early stage and chemists have to look forward to the development of more general and selective catalytic protocols with a broad substrate scope and wide functional group tolerance under mild reaction conditions.Despite its notable advantages, there are still challenges in the reaction of glycerol that need to be addressed. One most important challenge is its efficient and highly selective conversion to produce the desired compounds in high yields. Due to the difficulty in the reactivity of glycerol, its application in organic synthesis is less explored. To accomplish these challenges, the development of potential and sustainable catalytic methodologies is the key factor. In particular, the design of highly active and selective catalysts that should convert glycerol in mild conditions is of prime importance. Next, to understand and to solve the complexity of the reaction of glycerol, tools for mechanistic and kinetic investigations need to be strengthened. Glycerol is an ‘ideal’ feedstock and its utilization capacity needs to be increased to produce both fine and bulk chemicals as well as for the preparation of pharmaceuticals and agrochemicals.
Presently, the majority of industrially important amines are produced from fossil-based feedstocks. Owing to the excess availability of glycerol in a form that is comparatively low cost, its conversion into the possible organonitrogen chemicals will not only mitigate the carbon footprint but also bring incentives to the chemical industries and have the potential to become a renewable alternative to fossil fuels. Recent achievements in the catalytic amination of glycerol and its derived molecules to produce amine-based functional compounds will likely support the further advancements of this research area. Therefore, a collaboration between academia and industry is critical to achieve this goal.
We sincerely hope that this review will be useful to the scientific community working in the area of valorization of glycerol and biomass-based feedstocks as well as organic synthesis, amines, heterocycles, and catalysis.
Author contributions
Conceptualization: K. N., and R.V.J.; methodology: K. N., and R.V.J.; data curation: K. N., S.V., S.K., S.S., T.B., A.M., and R.V.J.; formal analysis: K. N., S.K., T.B., S.S., A.M., and R.V.J.; funding acquisition: K.N.; supervision: K.N., S.V., and R.V.J.; writing – original draft preparation: K.N., and R.V.J.; All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
K. N. acknowledges the Seed Grant (SG-128) from IIT Hyderabad for financial support. S. K. acknowledges the UGC-JRF fellowship. T. B. acknowledges PMRF. R. V. J. thanks the European Union under the REFRESH – Research Excellence for Region Sustainability and High-tech Industries project number CZ.10.03.01/00/22_003/0000048 via the Operational Programme Just Transition for financial support.References
- H. W. Tan, A. R. Abdul Aziz and M. K. Aroua, Renewable Sustainable Energy Rev., 2013, 27, 118–127 CrossRef CAS.
- Y. Wang, Y. Xiao and G. Xiao, Chin. J. Chem. Eng., 2019, 27, 1536–1542 CrossRef CAS.
- M. R. Pagliaro and M. Rossi, The Future of Glycerol, ed. J. H. Clark and G. A. Kraus, Green Chem. Series, Royal Society of Chemistry, Cambridge, UK, 2nd edn, 2010, ISBN 978-1-84973-046-4 Search PubMed.
- M. V. Semkiv, J. Ruchala, K. V. Dmytruk and A. A. Sibirny, Trends Biotechnol., 2020, 38, 907–916 CrossRef CAS PubMed.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. D. Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS PubMed.
- M. Simões, S. Baranton and C. Coutanceau, ChemSusChem, 2012, 5, 2106–2124 CrossRef PubMed.
- M. Pagliaro, The Netherlands, Glycerol: The Renewable Platform Chemical, Else, Amsterdam, 1st edn, 2017, ISBN 978-0-12-812205-1 Search PubMed.
- C. P. Mota, B. P. Lima and A. L. De Glycerol, A Versatile Renewable Feedstock for the Chemical Industry, Springer International Publishing, Cham, Switzerland, 2017; ISBN 978-3-319-59375-3 Search PubMed.
- B. Liu and F. Gao, Catalysis, 2018, 8, 44 Search PubMed.
- P. U. Okoye and B. H. Hameed, Renewable Sustainable Energy Rev., 2016, 53, 558–574 CrossRef CAS.
- M. Anitha, S. K. Kamarudin and N. T. Kofli, Chem. Eng. J., 2016, 295, 119–130 CrossRef CAS.
- M. Hájek and F. Skopal, Bioresour. Technol., 2010, 101, 3242–3245 CrossRef PubMed.
- https://www.grandviewresearch.com/industry-analysis/glycerol-market# .
- C. R. Chilakamarry, A. M. M. Sakinah, A. W. Zularisam and A. Pandey, Syst. Microbiol. Biomanuf., 2021, 1, 378–396 CrossRef CAS.
- S. C. D'Angelo, A. Dall'Ara, C. Mondelli, J. Pérez-Ramírez and S. Papadokonstantakis, ACS Sustainable Chem. Eng., 2018, 6, 16563–16572 CrossRef.
- V. G. Ricordi, C. S. Freitas, G. Perin, E. J. Lenardão, R. G. Jacob, L. Savegnago and D. Alves, Green Chem., 2012, 14, 1030–1034 RSC.
- S. M. Gade, V. B. Saptal and B. M. Bhanage, Catal. Commun., 2022, 172, 106542 CrossRef CAS.
- R. Gérardy, D. P. Debecker, J. Estager, P. Luis and J.-C. M. Monbaliu, Chem. Rev., 2020, 120, 7219–7347 CrossRef PubMed.
- P. Prete, D. Cespi, F. Passarini, C. Capacchione, A. Proto and R. Cucciniello, Curr. Opin. Green Sustainable Chem., 2022, 35, 100624 CrossRef CAS.
- A. Abdullah, A. Zuhairi Abdullah, M. Ahmed, J. Khan, M. Shahadat, K. Umar and M. A. Alim, J. Cleaner Prod., 2022, 341, 130876 CrossRef CAS.
- R. S. Varma and C. Len, Curr. Opin. Green Sustainable Chem., 2019, 15, 83–90 CrossRef.
- C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- T. Attarbachi, M. D. Kingsley and V. Spallina, Fuel, 2023, 340, 127485 CrossRef CAS.
- (a) L. R. Smith, M. Douthwaite, K. Mugford, N. F. Dummer, D. J. Willock, G. J. Hutchings and S. H. Taylor, Energies, 2022, 15, 6250 CrossRef CAS; (b) R. Mane, Y. Jeon and C. Rode, Green Chem., 2022, 24, 6751–6781 RSC; (c) R. Gérardy, D. P. Debecker, J. Estager, P. Luis and J.-C. M. Monbaliu, Chem. Rev., 2020, 120(15), 7219–7347 CrossRef PubMed.
- K. Wang, Z. Yang, Y. Ma, W. Zhao, J. Sun, T. Lu and H. He, Biofuels, Bioprod. Biorefin., 2022, 16, 1428–1454 CrossRef CAS.
- A. A. F. d. Costa, A. d. N. de Oliveira, R. Esposito, C. Len, R. Luque, R. C. R. Noronha, G. N. d. Rocha Filho and L. A. S. d. Nascimento, Catalysts, 2022, 12, 570 CrossRef CAS.
- I. Zahid, M. Ayoub, B. B. Abdullah, A. Mukhtar, S. Saqib, S. Rafiq, S. Ullah, A. G. Al-Sehemi, S. Farrukh and M. Danish, ChemBioEng Rev., 2021, 8, 227–238 CrossRef CAS.
- P. Koranian, Q. Huang, A. K. Dalai and R. Sammynaiken, Catalysts, 2022, 12, 897 CrossRef CAS.
- K. Murugesan, T. Senthamarai, V. G. Chandrashekhar, K. Natte, P. C. J. Kamer, M. Beller and R. V. Jagadeesh, Chem. Soc. Rev., 2020, 49, 6273–6328 RSC.
- F. Poovan, V. G. Chandrashekhar, K. Natte and R. V. Jagadeesh, Catal. Sci. Technol., 2022, 12, 6623–6649 RSC.
- L. Jia, X. Wang, X. Gao, M. Makha, X.-C. Wang and Y. Li, Green Synth. Catal., 2022, 3, 259–264 CrossRef CAS.
- R. V. Jagadeesh, A.-E. Surkus, H. Junge, M.-M. Pohl, J. Radnik, J. Rabeah, H. Huan, V. Schünemann, A. Brückner and M. Beller, Science, 2013, 342, 1073–1076 CrossRef CAS PubMed.
- V. G. Chandrashekhar, T. Senthamarai, R. G. Kadam, O. Malina, J. Kašlík, R. Zbořil, M. B. Gawande, R. V. Jagadeesh and M. Beller, Nat. Catal., 2022, 5, 20–29 CrossRef CAS.
- R. V. Jagadeesh, K. Murugesan, A. S. Alshammari, H. Neumann, M.-M. Pohl, J. Radnik and M. Beller, Science, 2017, 358, 326–332 CrossRef CAS PubMed.
- K. Chen, Q.-K. Kang, Y. Li, W.-Q. Wu, H. Zhu and H. Shi, J. Am. Chem. Soc., 2022, 144, 1144–1151 CrossRef CAS PubMed.
- T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed.
- V. A. Tran, N. H. T. Tran, L. G. Bach, T. D. Nguyen, T. T. Nguyen, T. T. Nguyen, T. A. N. Nguyen, T. K. Vo, T.-T. T. Vo and V. T. Le, J. Chem., 2020, 2020, 9597426 Search PubMed.
- A. K. Ghosh, G. Bilcer and G. Schiltz, Synthesis, 2001, 2001, 2203–2229 CrossRef PubMed.
- https://patents.google.com/patent/FR2634765A1/en .
- X. Dai, J. Rabeah, H. Yuan, A. Brückner, X. Cui and F. Shi, ChemSusChem, 2016, 9, 3133–3138 CrossRef CAS PubMed.
- J. Ding, M. Cui, T. Ma, R. Shao, W. Xu and P. Wang, Mol. Catal., 2018, 457, 51–58 CrossRef CAS.
- R. Pandya, R. Mane and C. V. Rode, Catal. Sci. Technol., 2018, 8, 2954–2965 RSC.
- M. Safariamin, S. Paul, K. Moonen, D. Ulrichts, F. Dumeignil and B. Katryniok, Catal. Sci. Technol., 2016, 6, 2129–2135 RSC.
- F. Xie, M. Zhang, H. Jiang, M. Chen, W. Lv, A. Zheng and X. Jian, Green Chem., 2015, 17, 279–284 RSC.
- Y. Wang, S. Furukawa, S. Song, Q. He, H. Asakura and N. Yan, Angew. Chem., Int. Ed., 2020, 59, 2289–2293 CrossRef CAS PubMed.
- P. F. Jackson, D. C. Cole, B. S. Slusher, S. L. Stetz, L. E. Ross, B. A. Donzanti and D. A. Trainor, J. Med. Chem., 1996, 39, 619–622 CrossRef CAS PubMed.
- P. C. Meltzer, D. Butler, J. R. Deschamps and B. K. Madras, J. Med. Chem., 2006, 49, 1420–1432 CrossRef CAS PubMed.
- (a) Z. Xin, L. Jia, Y. Huang, C.-X. Du and Y. Li, ChemSusChem, 2020, 13, 2007–2011 CrossRef CAS PubMed; (b) L. Jia, M. Makha, C.-X. Du, Z.-J. Quan, X.-C. Wang and Y. Li, Green Chem., 2019, 21, 3127–3132 RSC.
- C. Li, M. Wang, X. Lu, L. Zhang, J. Jiang and L. Zhang, ACS Sustainable Chem. Eng., 2020, 8, 4353–4361 CrossRef CAS.
- F. Casti, R. Mocci and A. Porcheddu, Beilstein J. Org. Chem., 2022, 18, 1210–1216 CrossRef CAS PubMed.
- E. Farnetti and C. Crotti, Catal. Commun., 2016, 84, 1–4 CrossRef CAS.
- X. Dai, X. Wang, J. Rabeah, C. Kreyenschulte, A. Brückner and F. Shi, Chem. – Eur. J., 2021, 27, 16889–16895 CrossRef CAS PubMed.
- T. A. Gokhale, S. C. Gulhane and B. M. Bhanage, Eur. J. Org. Chem., 2023, e202200997 CrossRef CAS.
- T. A. Gokhale, V. V. Phatake and B. M. Bhanage, Mol. Catal., 2022, 533, 112771 CrossRef CAS.
- V. Goyal, N. Sarki, A. Narani, G. Naik, K. Natte and R. V. Jagadeesh, Coord. Chem. Rev., 2023, 474, 214827 CrossRef CAS.
- V. Goyal, T. Bhatt, C. Dewangan, A. Narani, G. Naik, E. Balaraman, K. Natte and R. V. Jagadeesh, J. Org. Chem., 2023, 88, 2245–2259 CrossRef CAS PubMed.
- G. Naik, N. Sarki, V. Goyal, A. Narani and K. Natte, Asian J. Org. Chem., 2022, 11, e202200270 CrossRef CAS.
- K. Natte, H. Neumann, R. V. Jagadeesh and M. Beller, Nat. Commun., 2017, 8, 1344 CrossRef PubMed.
- V. Goyal, J. Gahtori, A. Narani, P. Gupta, A. Bordoloi and K. Natte, J. Org. Chem., 2019, 84, 15389–15398 CrossRef CAS PubMed.
- N. Sarki, V. Goyal, N. K. Tyagi, Puttaswamy, A. Narani, A. Ray and K. Natte, ChemCatChem, 2021, 13, 1722–1729 CrossRef CAS.
- V. Goyal, G. Naik, A. Narani, K. Natte and R. V. Jagadeesh, Tetrahedron, 2021, 98, 132414 CrossRef CAS.
- S. Moulay, Curr. Org. Chem., 2019, 23, 1695–1737 CrossRef CAS.
- F. Du, X. Jin, W. Yan, M. Zhao, P. S. Thapa and R. V. Chaudhari, Catal. Today, 2018, 302, 227–232 CrossRef CAS.
- J. Jampilek, Molecules, 2019, 24, 3839 CrossRef CAS PubMed.
- (a) M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247–44311 RSC; (b) S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed; (c) D. Forberg, J. Obenauf, M. Friedrich, S.-M. Hühne, W. Mader, G. Motz and R. Kempe, Catal. Sci. Technol., 2014, 4, 4188–4192 RSC.
- https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Pharmaceuticals%20By%20Retail%20Sales%202020V3.pdf, https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Pharmaceuticals%202021V2.pdf and https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/NjardarsonGroup2022Top200PosterV5.pdf.
- C.-W. Luo, C. Huang, A. Li, W.-J. Yi, X.-Y. Feng, Z.-J. Xu and Z.-S. Chao, Ind. Eng. Chem. Res., 2016, 55, 893–911 CrossRef CAS.
- A. Venugopal, R. Sarkari, C. Anjaneyulu, V. Krishna, M. K. Kumar, N. Narender and A. H. Padmasri, Appl. Catal., A, 2014, 469, 398–409 CrossRef CAS.
- D. Jiang, S. Wang, H. Li, L. Xu, X. Hu, B. Barati and A. Zheng, ACS Sustainable Chem. Eng., 2021, 9, 3095–3103 CrossRef CAS.
- R. H. Manske, Chem. Rev., 1942, 30, 113–144 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra and S. Martínez-Silvestre, ChemCatChem, 2013, 5, 3866–3874 CrossRef CAS.
- H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel and C. Len, RSC Adv., 2014, 4, 21456–21464 RSC.
- A. Li, C. Huang, C.-W. Luo, W.-J. Yi and Z.-S. Chao, RSC Adv., 2017, 7, 9551–9561 RSC.
- T. Tanaka, A. Enomoto, S. Furukawa and K.-i. Fujita, Catalysts, 2021, 11, 1200 CrossRef CAS.
- G. C. Hargaden and P. J. Guiry, Chem. Rev., 2009, 109, 2505–2550 CrossRef CAS PubMed.
- F. Yu and F.-S. Xiao, ACS Sustainable Chem. Eng., 2023, 11, 9372–9381 CrossRef CAS.
- W. Deng, Y. Wang, S. Zhang, K. M. Gupta, M. J. Hülsey, H. Asakura, L. Liu, Y. Han, E. M. Karp, G. T. Beckham, P. J. Dyson, J. Jiang, T. Tanaka, Y. Wang and N. Yan, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5093–5098 CrossRef CAS PubMed.
- J. Mazarío, Z. Raad, P. Concepción, C. Cerdá-Moreno and M. E. Domine, Catal. Sci. Technol., 2020, 10, 8049–8063 RSC.
- L. Ducrot, M. Bennett, A. A. Caparco, J. A. Champion, A. S. Bommarius, A. Zaparucha, G. Grogan and C. Vergne-Vaxelaire, Front. Catal., 2021, 1, 781284 CrossRef.
- M. Główka and T. Krawczyk, ACS Sustainable Chem. Eng., 2023, 11, 7274–7287 CrossRef.
- S. Hameury, H. Bensalem and K. De Oliveira Vigier, Catalysts, 2022, 12, 1306 CrossRef CAS.
- M. J. Climent, A. Corma, J. C. Hernández, A. B. Hungría, S. Iborra and S. Martínez-Silvestre, J. Catal., 2012, 292, 118–129 CrossRef CAS.
- P. Daw, A. Kumar, N. A. Espinosa-Jalapa, Y. Diskin-Posner, Y. Ben-David and D. Milstein, ACS Catal., 2018, 8, 7734–7741 CrossRef CAS PubMed.
- K. Chakrabarti, M. Maji and S. Kundu, Green Chem., 2019, 21, 1999–2004 RSC.
- D. Panja, B. Paul, B. Balasubramaniam, R. K. Gupta and S. Kundu, Catal. Commun., 2020, 137, 105927 CrossRef CAS.
- S. Shee, D. Panja and S. Kundu, J. Org. Chem., 2020, 85, 2775–2784 CrossRef CAS PubMed.
- C.-J. Yue, K. Di, L.-P. Gu, Z.-W. Zhang and L.-L. Ding, Mol. Catal., 2019, 477, 110539 CrossRef.
Footnote |
| † Authors have equally contributed. |
| This journal is © The Royal Society of Chemistry 2024 |