Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cocoa flavanols rescue stress-induced declines in endothelial function after a high-fat meal, but do not affect cerebral oxygenation during stress in young, healthy adults
Rosalind
Baynham
 a,
Jet J. C. S.
Veldhuijzen van Zanten
a and
Catarina
Rendeiro
*ab
a,
Jet J. C. S.
Veldhuijzen van Zanten
a and
Catarina
Rendeiro
*ab
aSchool of Sport, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham B15 2TT, UK. E-mail: c.rendeiro@bham.ac.uk
bCentre for Human Brain Health, University of Birmingham, Birmingham, UK
First published on 18th November 2024
Abstract
Food choices during stressful periods often worsen, which can influence the impact of stress on vascular health. For instance, fat consumption impairs the recovery of endothelial function following mental stress, while flavanols have been shown to enhance recovery. This randomised, counterbalanced, double-blinded, crossover, postprandial intervention study examined whether flavanols consumed in combination with fat can mitigate the negative impact of fat on stress-induced impairments in endothelial function. Twenty-three young, healthy males and females ingested a high-fat meal (56.5 g fat) with high-flavanol (150 mg (−)-epicatechin) or low-flavanol (<6 mg (−)-epicatechin) cocoa 1.5 hours before an 8-minute mental stress task. The primary outcome, brachial flow-mediated dilatation (FMD), was assessed at pre-intervention baseline and 30 and 90 minutes post-stress. Pre-frontal cortical oxygenation was assessed post-meal at rest and during stress. Forearm blood flow (FBF), blood pressure (BP), cardiovascular activity, common carotid artery (CCA) diameter and blood flow and mood were assessed before, during and/or after stress. FMD was impaired at 30 and 90 minutes post-stress after the low-flavanol cocoa. High-flavanol cocoa attenuated FMD impairments at 30 minutes and improved FMD at 90 minutes post-stress. Mental stress induced similar increases in cortical oxygenation, FBF, BP, cardiovascular activity, and disruptions to mood, in both conditions. CCA diameter increased and CCA retrograde blood flow decreased post-stress, with no difference between conditions. In summary, flavanols can counteract declines in endothelial function induced by consuming fat in the context of stress, but do not impact cerebral oxygenation. These findings can have important implications for flavanol-rich dietary choices to protect the vasculature from stress.
1. Introduction
Stress is increasingly prevalent, with 17.1 million working days lost to work-related stress in the last year,1 and currently the most substantial increase in anxiety is being experienced by young adults (18–25 year olds).2 Episodes of acute mental stress have been implicated as a trigger for myocardial infarction and sudden cardiac death.3–5 Acute mental stress can also trigger stroke.6Temporary impairments in vascular function have been implicated as a mechanism linking stress to poor cardiovascular health.7 For example, individuals who experience stress-induced myocardial ischemia also have attenuated peripheral vasodilatory responses8 and increased vascular resistance during stress.9,10 Importantly, mental stress continues to impact the vasculature following a stressful event, as shown by transient declines in endothelial function (as measured by brachial flow-mediated dilatation; FMD) from 15 to 90 minutes following stress in young, healthy adults.11–14 This can be of clinical significance, given that a reduction in FMD translates to an increase in cardiovascular disease (CVD) risk.7 The impact of stress on the cerebral vasculature is less understood,15 but impairments in FMD are also associated with increased risk of vascular events, including stroke.16 Mechanisms underpinning stress-induced impairments in vascular function may include reduced nitric oxide (NO) bioavailability,17 driven by increases in cortico-releasing hormone (CRH), cortisol, inflammatory cytokines,11 and oxidative stress markers.18
Interestingly, stress can also negatively influence health through changes in eating behaviour.19,20 During stressful periods young adults are likely to overeat and consume more unhealthy foods (i.e., high-fat) and fewer fruits and vegetables.21–25 For example, 38% of adults report to have overeaten or eaten unhealthy foods in the previous month due to stress, and half of these adults report this shift in food choices at least once a week.26 Increased cortisol reactivity27 and maladaptive effects on brain regions responsible for decision making and emotion regulation have been suggested as likely mechanisms.28 Importantly, stress-induced negative shifts in eating behaviour may contribute to increased weight gain: stress has been identified as an independent risk factor for obesity.29,30 Weight gain may be accelerated as fat oxidation slows down during stressful periods,31 and overweight individuals are more prone to overeating during stressful periods.32 Notably, obesity is a risk factor in the development of CVD.33 Therefore, stress can not only directly impact vascular function acutely, but also indirectly contribute to poorer vascular health, through chronic unhealthier food choices.
Our group has recently shown that acute saturated fat consumption impairs the recovery of endothelial function following mental stress in young healthy adults, with FMD remaining significantly impaired (reduction of 1.15% FMD) in the high-fat condition in comparison with the low-fat control.34 We further demonstrated that acute saturated fat consumption attenuates cerebral oxygenation in the prefrontal cortex during mental stress.35 Both fat consumption and stress exposure have been shown to stimulate the vasoconstrictor endothelin-1 (ET-1), reactive oxygen species (ROS) and inflammatory markers,36–38 which are known to reduce endothelium-derived NO39 and likely underpin the stress and fat-induced impairments in vascular function. As such, acute unhealthy food choices (such as foods high in saturated fats) during stressful periods can exacerbate the negative impact of stress on vascular health. Therefore, it is important to find dietary strategies that may be able to counteract the negative impact of stress and fat in the vasculature.
Our group has previously shown that an intervention rich in flavonoids, a group of small molecules present in most fruits and vegetables, can be protective for the vasculature in the context of a stressful episode. Specifically, it was demonstrated that acute cocoa flavanol intake improved vasodilatory responses during stress and attenuated the impairment in brachial FMD following stress in young healthy adults in a fasted state.40 Similarly, previous research has shown cocoa flavanols to improve brachial FMD within 1–3 hours of intake.41–44 Cocoa flavanols are thought to exert their protective vascular effect by increasing NO bioavailability and reducing ET-1.44–47 Furthermore, cocoa flavanols have been shown to improve cerebral oxygenation responses to hypercapnia48 and hypoxia,49 but their effect on the brain vasculature during stress remains unknown. As such, adding a flavonoid-rich food to a high-fat snack during stress might be an effective strategy to, at least partially, reduce the negative impact of poor food choices on the human vasculature.
The current study aimed to investigate whether high-flavanol cocoa (HFC), consumed in combination with a high-fat meal (HFM), can mitigate the negative impact of fat on stress-induced impairments in endothelial function, as measured by brachial FMD. Furthermore, we aimed to investigate whether HFC can restore cortical oxygenation during mental stress following fat consumption. We hypothesised that HFC will attenuate the stress-induced decline in brachial FMD and improve cortical oxygenation during stress following fat consumption.
2. Methods
2.1 Participants
Twenty-three participants (11 male, 12 female) were recruited via email and poster advertisements. Participants were between 18 and 45 years old. Exclusion criteria were: (i) smokers, (ii) consumption of >21 units of alcohol per week, (iii) acute illness/infection, (iv) history of cardiovascular, respiratory, metabolic, liver, inflammatory diseases, or blood-clotting disorders, (v) all allergies or food intolerances, (vi) weight-reducing dietary regimen or dietary supplements, and (vii) long-term medication or antibiotics in the previous 3 months. Participants were awarded course credit marks when applicable. Ethical approval was obtained from the University of Birmingham Science, Technology, Engineering, and Mathematics ethics committee (ERN17_1755E), and all participants gave written informed consent prior to participation in the study.2.2 Study design
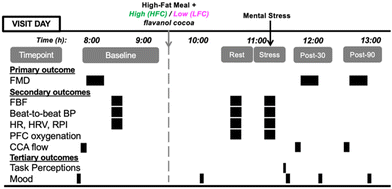
The study used a randomised, counterbalanced, double-blinded, cross-over, postprandial intervention design (Fig. 1). Participants visited the laboratory twice, at least a week apart for males and approximately one month apart for females. Females were tested during the same phase of the menstrual cycle (early follicular, days 1–5 of menstruation) to control for the influence of menstrual hormones.50,51 Participants were asked to refrain from food for 12 hours and from alcohol, caffeine, flavonoid-rich foods, and vigorous exercise 24 hours before each testing session. Participants were contacted 24 hours before and reminded of these pre-visit requirements, which were then assessed verbally using a 24-hour food recall questionnaire as well as asking if vigorous exercise had been undertaken, alcohol and caffeine had been consumed, and whether they had been fasted for 12 hours. Each session commenced at approximately 8:00. First, mood was assessed and then, habitual dietary intake was recorded (visit 1 only). Following this, participants rested in a supine position for 20 minutes before pre-intervention (baseline) measurements were taken: (i) common carotid artery (CCA) blood flow, (ii) brachial FMD, (iii) forearm blood flow (FBF), (iv) cardiovascular activity (beat-to-beat blood pressure [BP], heart rate [HR], heart rate variability [HRV] and R-wave to pulse interval [RPI]). Following these assessments, participants consumed a high-fat meal (HFM) with either a high-flavanol cocoa (HFC) intervention or a low-flavanol cocoa (LFC) intervention. Participants then rested for 1.5 hours during which they completed a mood questionnaire and lifestyle questionnaires (data not reported, session 1 only) and had the option to complete their own work or watch a nature documentary. Subsequently, FBF, cardiovascular activity and prefrontal cortex (PFC) tissue oxygenation (measured by Near-Infrared Spectroscopy, NIRS) were assessed during an 8-minute rest (rest) and during an 8-minute mental stress – Paced-Auditory-Serial-Addition-Task (PASAT) (stress). During each 8-minute assessment, FBF was measured during minutes 2, 4, 6, and 8. BP, HR, RPI, HRV and PFC oxygenation were analysed during all 8 minutes. CCA blood flow, brachial FMD and mood were measured 30 minutes and 90 minutes following stress. Both sessions lasted 5 hours and participants were debriefed following completion of both visits (Fig. 2). | ||
| Fig. 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram for postprandial intervention study. | ||
2.3 Habitual dietary intake
Habitual dietary intake was assessed using the validated European Prospective Investigation into Diet and Cancer (EPIC) Norfolk Food Frequency Questionnaire (FFQ).52 The questionnaire consists of 131 different food items for participants to select the frequency of consumption on a 9-point scale (never or less than once per month, 1–3 per month, once a week, 2–4 per week, 5–6 per week, once a day, 2–3 per day, 4–5 per day, and 6+ per day) to estimate usual dietary intake over the previous 12 months. The FFQ EPIC Tool for Analysis (FETA) was used to calculate nutrient data.53 In order to calculate flavonoid intake, the FLAVIOLA food composition database was inputted into the FETA software, which allows the estimation of flavonoids and its subclasses.54 The following nutrients are reported in this study: energy (kcal), fat (g), saturated fat (g), carbohydrate (g), sugars (g), fibre (g), protein (g), total flavonoids (mg), and portions of fruit and vegetables (calculated as 1 portion corresponding to 80 g, NHS guidelines), to give a general view of habitual dietary intake.2.4 High-fat meal intervention
The HFM was prepared just before consumption, and all fresh ingredients were bought within 24 hours of each testing session. The composition of the HFM is presented in Table 1, and contained 56.5 g fat, as used previously.34 All ingredients were purchased from Tesco, and the meal consisted of 2 butter croissants (67 g each) with 10 g salted butter, 1.5 slices of cheddar cheese (37.5 g total) and 250 ml whole milk. Participants were asked to consume the meal within 20 minutes. 4 participants did not finish the meal, but no adverse side effects were reported.| Meal type | High-fat meal (HFM) |
|---|---|
| The HFM consisted of 2 butter croissants with 10 g of salted butter, 1.5 slices of cheddar cheese and 250 ml of whole milk. | |
| Nutrient composition: | |
| Energy (kcal) | 891.00 |
| Fat (g) | 56.50 |
| Saturated fat (g) | 35.10 |
| Carbohydrate (g) | 65.00 |
| Sugars (g) | 20.20 |
| Fibre (g) | 2.40 |
| Protein (g) | 29.85 |
| Salt (g) | 2.00 |
2.5 High- and low-flavanol interventions
Cocoa flavanol beverages were prepared by dissolving 12 g cocoa powder into 250 ml of whole milk (from the HFM). The cocoa powders are commercially available (Barry Callebaut, Zurich, Switzerland): the low-flavanol powder was a fat-reduced alkalized cocoa powder (commercial name: 10/12 DDP Royal Dutch) delivering <6.0 mg (−)-epicatechin and 5.6 mg of total flavanols per serving; and the high-flavanol cocoa powder was a non-alkalized fat-reduced powder (‘Natural Acticoa’), delivering 150.0 mg (−)-epicatechin powder and 695.0 mg total flavanols per serving, as used in previous research40,48 (Table 2). Both interventions were matched for all other micro- and macro-nutrients, including caffeine and theobromine. Cocoa powder levels for flavanol monomers, procyanidin and methylxanthines (caffeine, theobromine) were measured by high-performance liquid chromatography (HPLC) as described previously.55,56 The dose of flavanol monomers used in the present study is in line with previous studies, shown to be safe and effective in modifying human endothelial function in young healthy adults.43,44,57 The cocoa powder sachets were labelled with an alphanumeric identifier, and were stored at −20 °C. Intervention beverages were identical in texture, consistency and taste, and were presented in an opaque container with a black opaque straw to ensure double-blindness. All participants finished both intervention beverages. The unblinding of the interventions was performed only after all data analyses were completed.| High-flavanol cocoa (HFC) | Low-flavanol cocoa (LFC) | |
|---|---|---|
| Total flavanols (mg) | 695.00 | 5.60 |
| (−)-Epicatechin (mg) | 150.00 | <6.00 |
| (−) and (+)-Catechin (mg) | 85.44 | <6.00 |
| Procyanidins (mg) | 459.60 | ND |
| Theobromine (mg) | 262.80 | 278.40 |
| Caffeine (mg) | 27.60 | 22.20 |
| Fat (g) | 1.68 | 1.32 |
| Carbohydrates (g) | 2.70 | 1.24 |
| Protein (g) | 2.69 | 2.66 |
| Fibre (g) | 1.82 | 4.02 |
| Energy (kcal) | 41.40 | 36.60 |
2.6 Mental stress task
The mental stress task used was the 8-minute PASAT, shown to have good test–retest reliability and to induce a physiological response.58–60 The PASAT requires participants to add two sequentially presented single-digit numbers (1–9), adding the number presented to the previous number they heard. The delivery of the numbers became quicker, with time intervals reducing every 2 minutes; from a 2.8 second interval to 2.4 seconds, 2.0 seconds, and finally 1.6 seconds. Participants were filmed and asked to watch themselves on a screen, which they were told would be evaluated by 2 independent body language assessors. An experimenter marked the participants’ responses, whilst sounding a loud aversive buzzer at standard intervals once every 10 answers: either following an incorrect response or at the end of the 10-number block. The participants were told they were in direct competition with other participants and lost points for each incorrect answer. These elements of social evaluation, punishment, and competition have been used previously40 and have been shown to enhance the provocativeness of the task.61 Immediately following the PASAT, an experimenter asked the participant to verbally rate how difficult, stressful, competitive, and enjoyable they found the task, and to what extent they were trying to perform well, scored on a 7-point scale ranging from 0 ‘not at all’ to 6 ‘extremely’. Following both visits, participants were informed about the deception in the body language assessment and competition.2.7 Mood ratings
Mood was assessed on a 5-point scale using items that assessed positive affect, negative affect, energy, and fatigue. The positive affect scale included items that represent activated (happy, cheerful) and deactivated (calm) pleasure. The negative affect scale included items that represent activated (tense, stressed) and deactivated (sad, angry) displeasure, as detailed previously.62 Physical feeling states were represented by the assessment of energy and fatigue (tired). These nine constructs happy, cheerful, calm, tense, stressed, sad, angry, energetic, and tired were rated on a 5-point scale (1 = not at all, 5 = extremely), and correspond to how participants felt at that moment.2.8 Cardiovascular activity
2.9 Forearm blood flow
FBF was measured using venous occlusion plethysmography. A mercury-in-silastic strain gauge was connected to a plethysmograph (ECG, Hokanson; Jacksonville, WA, USA), producing an output voltage with frequency 0–25 Hz. The plethysmograph signal was digitised at 100 Hz with 16-bit resolution, via a Power1401 (CED) connected to a computer programmed in Spike2, as previously described by Paine et al. (2013).58 One congestion cuff was placed around the wrist (TMC7, Hokanson), and inflated for 1 minute to supra-systolic blood pressure (>220 mmHg). Another congestion cuff was placed around the brachial region of the upper arm (SC12, Hokanson), and inflated for 5 seconds to above venous pressure (40 mmHg), every 15 seconds providing 3 blood flow measurements each minute. Blood flow analysis and calibration were undertaken offline using Spike2 (CED). Each increase in limb circumference was identified as a slope, which were averaged to yield a mean blood flow per minute.58 Forearm vascular conductance (FVC) was calculated by dividing FBF by MAP per minute of assessment.2.10 Prefrontal cortical haemodynamics
NIRS (NIRO-200NX, Hamamatsu Photonics KK, Japan) was used to assess prefrontal cortical haemodynamics. The NIRS device measures changes in chromophore concentrations of oxyhaemoglobin (O2Hb) and deoxyhaemoglobin (HHb), providing depth-resolved measures of tissue oxygen saturation (total oxygenation index, TOI) and tissue haemoglobin content (normalised tissue haemoglobin index, nTHI).66 Probes were positioned over the left and right pre-frontal sites and secured to the head with a black headband. Probes were enclosed in light-shielding rubber housing that maintained emitter-to-detector optode spacing (4 cm), and signals were acquired at sample interval 0.2 s (5 Hz). NIRS was assessed during 8 minutes of rest and 8 minutes of stress. Measures of TOI, nTHI, O2Hb and HHb were averaged to provide 1 value for each minute of rest and stress. Minutes 2, 4, 6 and 8 of stress are reported, in line with the minutes by which peripheral vasodilation (FBF) was assessed.2.11 Flow-mediated dilatation
FMD was used to assess endothelial function of the brachial artery. A 15–4 MHz (15L4 Smart MarK™; Terason, Burlington, MA, USA) transducer was attached to a Terason Duplex Doppler System (Usmart 3300 NexGen Ultrasound; Terason). This has wall-tracking and automatic edge-detection software (Cardiovascular Suite, Quipu; Pisa, Italy), which allows for continuous measurement of diameter and blood velocity throughout the FMD assessment. Following 20 minutes of supine rest, the brachial artery was imaged longitudinally, 5–10 cm proximal to the antecubital fossa. A brachial cuff was placed around the forearm and, following a 1-minute baseline, this was inflated to 220 mmHg for 5 minutes, to cause ischaemia. Subsequently, the rapid cuff deflation caused reactive hyperaemia, and the image was recorded continuously for 5 minutes post-pressure release. This is in accordance with established guidelines.67 All file images were analysed by a trained researcher, blinded to condition and measurement details. Peak diameter was defined as the largest diameter obtained after occlusion was released. The FMD response was calculated as the relative diastolic diameter change between baseline and peak diameter. Resting arterial diameter was also estimated based on a time-average across the first minute of recording. All measurements were undertaken by the same trained researcher, who demonstrated excellent reproducibility in brachial FMD (coefficient of variation: intra-day 5.49%, inter-day 10.87%). Allometrically scaled FMD was calculated in accordance with published guidelines.68 The slope of the regression between the logarithmically transformed baseline diameter and peak diameter was 0.94.2.12 Common carotid artery diameter and blood flow
Duplex ultrasound was used to assess CCA diameter and blood flow. A 15–4 MHz (15L4 Smart MarK™; Terason, Burlington, MA, USA) transducer was attached to a Terason Duplex Ultrasound System (Usmart 3300 NexGen Ultrasound; Terason). This was combined with wall-tracking and automatic edge-detection software (Cardiovascular Suite, Quipu; Pisa, Italy), which allows for continuous measurement of diameter and blood velocity.50 Following 10 minutes of supine rest, the participant was asked to turn their head and neck slightly to the left side. Then, a two-minute recording of the right CCA was obtained. All file images were analysed by a trained researcher, blinded to condition and measurement details. Analysis allows estimation of resting arterial diameter and calculation of arterial blood flow based on a time-average across two minutes of the recording.50 All measurements were undertaken by the same trained researcher, with suitable reproducibility of CCA diameter (coefficient of variation: intra-day 1.93%, inter-day 2.37%) and shear rate (coefficient of variation: intra-day 11.79%, inter-day 12.27%).2.13 Statistical analysis
All statistical analyses were conducted using IBM SPSS software (version 29). The cardiovascular and FBF measurements during pre-intervention baseline, rest, and stress were averaged separately to provide a mean pre-intervention baseline, rest, and stress value for each outcome. Pre-intervention baseline measures (FMD, FBF, HR, SBP, DBP), task perceptions and PASAT scores were compared using a 2-condition (HFM + HFC, HFM + LFC) repeated measures analysis of variance (ANOVA). Separate 2-condition (HFM + HFC, HFM + LFC) by 5-time (baseline, rest, stress, post-30, post-90) repeated measures ANOVAs were used to assess mood ratings. A series of 2-condition (HFM + HFC, HFM + LFC) by 3-time (baseline, rest, stress) repeated measures ANOVAs was conducted to analyse the cardiovascular and FBF variables. A mixed effects model was used for SBP, DBP, and FVC analysis to account for missing data due to finapress malfunction. NIRS variables at rest and during stress (8 minutes averaged) were compared using separate one-sample t-tests for both conditions, which is most appropriate given that the resting values were 0. NIRS variables were further analysed using a two-way repeated measures ANOVA with condition (HFM + HFC, HFM + LFC) and time (stress 2, stress 4, stress 6, stress 8) as within-subject factors. FMD (including resting arterial diameter) and CCA variables were analysed using a 2-condition (HFM + HFC, HFM + LFC) by 3-time (baseline, post-30, post-90) repeated measures ANOVA. Where appropriate, pairwise comparisons using Bonferroni correction were conducted to investigate significant effects in more detail. A Linear Mixed Model was used to analyse allometrically scaled FMD. Given the lack of disparity in SD following allometric correction, 95% confidence intervals have been reported in text for this allometrically scaled FMD. All values reported in text, tables, and graphs are mean ± SD. All analyses were also conducted with sex as a between-subject variable. As there were no significant condition × sex, time × sex, or condition × time × sex interaction effects, these results are not reported. Four participants did not finish the meal. All statistical tests were repeated excluding these 4 participants. The results were similar to the analyses with the full sample; therefore, as an intention-to-treat analysis, it was decided to include all participants to maximise power. For all analyses, significance was set at α < 0.05. Sample size was estimated based on previous data from our laboratory on flavanol-induced changes in brachial FMD,40 denoting a sample size of 11 participants was required to detect an interaction effect of the cocoa intervention on FMD post-stress, with power at 99% and alpha at 0.05.69 This sample size should also be sufficient to detect the effect of cocoa flavanols on cortical oxygenation, shown with n = 18 by Gratton et al. (2020).483. Results
3.1 Participant characteristics
Participant characteristics are presented in Table 3. Participants were aged 19 to 35 years old, with a healthy body mass index (BMI) and identified as white European ethnicity (n = 20), Asian ethnicity (n = 1) or black African ethnicity (n = 2). Pre-intervention baseline FMD, FBF, HR, BP, brachial and CCA diameter were similar in both conditions (Table 3).| Participant characteristics | HFM + LFC | HFM + HFC | p value |
|---|---|---|---|
| BMI: body mass index, CCA: common carotid artery, DBP: diastolic blood pressure, FBF: forearm blood flow, FMD: flow-mediated dilatation, HFC: high-flavanol cocoa, HFM: high-fat meal, HR: heart rate, LFC: low-flavanol cocoa, SBP: systolic blood pressure. Separate 2-condition (HFM + HFC, HFM + LFC) repeated measures ANOVAs were used to assess pre-intervention baseline measures. | |||
| n | 23 (M:11, F:12) | ||
| Age (years) | 21.57 ± 4.11 | ||
| BMI (kg m−2) | 22.31 ± 2.58 | ||
| FMD (%) | 6.93 ± 2.58 | 6.55 ± 2.73 | 0.065 |
| FBF (mm/100 ml min−1) | 2.63 ± 0.87 | 2.58 ± 0.75 | 0.823 |
| HR (bpm) | 58.29 ± 8.57 | 57.45 ± 9.78 | 0.533 |
| SBP (mmHg) | 113.38 ± 15.40 | 110.53 ± 10.78 | 0.371 |
| DBP (mmHg) | 52.17 ± 6.86 | 50.25 ± 4.82 | 0.419 |
| Brachial diameter (mm) | 3.60 ± 0.54 | 3.61 ± 0.55 | 0.561 |
| CCA diameter (mm) | 6.54 ± 0.39 | 6.52 ± 0.38 | 0.656 |
3.2 Habitual dietary intake
Table 4 displays participants’ estimated daily intake of key nutrients, as well as the percentage of participants exceeding or not meeting daily recommendations, as suggested by the National Health Service (NHS).70 The average daily intake of fat was 66.76 ± 19.99 g (43.48% exceeding the recommended daily intake) and of saturated fat was 25.18 ± 7.72 g (47.83% exceeding the recommended daily intake). 75% of females exceeded the female saturated fat recommendation (20 g), whilst only 18% of males exceeded their saturated fat recommendation (30 g). The average intake of fruit and vegetables was 4.76 ± 2.55 portions per day, with females consuming over 1 extra portion a day compared with males. 65% of all participants did not meet the suggested recommendation of 5 portions a day. 100% of participants did not meet the suggested recommendations for fibre intake and exceeded the recommended sugar intake.| Nutrients | Sample average | % of participants over/under recommended daily intake |
|---|---|---|
| Recommendations – fat: <70 g per day, saturated fat: <30 g per day (male)/<20 g per day (female), sugar: <30 g per day, fibre: >30 g per day, fruit & vegetables: >5 portions per day.a 1 portion = 80 g. (NHS guidelines). N/A: not available. | ||
| Energy (kcal) | 1726.10 ± 494.46 | N/A |
| Fat (g) | 66.76 ± 19.99 | 43.48% over |
| Saturated fat (g) | 25.18 ± 7.72 | 47.83% over |
| Carbohydrate (g) | 195.14 ± 67.86 | N/A |
| Sugars (g) | 90.01 ± 39.19 | 100.00% over |
| Fibre (g) | 12.84 ± 5.88 | 100.00% under |
| Protein (g) | 84.11 ± 30.82 | N/A |
| Total flavonoids (mg) | 239.55 ± 195.88 | N/A |
| Portions of fruit & vegetablesa | 4.76 ± 2.55 | 65.22% under |
3.3 Mental stress task ratings
Separate two-condition (HFM + HFC, HFM + LFC) ANOVAs revealed no significant difference in task performance (PASAT score) or task perceptions between flavanol conditions. Participants perceived the task as similarly difficult, stressful, competitive, enjoyable, and tried to perform well to the same extent after both conditions (Table 5).| Task ratings | HFM + LFC | HFM + HFC | p value |
|---|---|---|---|
| PASAT score (/228) | 133 ± 39 | 134 ± 30 | 0.869 |
| Perceived difficulty (0–6) | 4.83 ± 0.65 | 4.78 ± 0.80 | 0.814 |
| Perceived stressfulness (0–6) | 4.96 ± 0.77 | 5.04 ± 0.83 | 0.628 |
| Perceived competitiveness (0–6) | 4.48 ± 0.85 | 4.35 ± 1.07 | 0.601 |
| Perceived enjoyment (0–6) | 1.95 ± 1.51 | 1.65 ± 1.43 | 0.186 |
| Perception of trying to perform well (0–6) | 5.30 ± 0.70 | 5.35 ± 0.83 | 0.770 |
3.4 Mood ratings
Two-condition (HFM + HFC, HFM + LFC) × 5-time (baseline, rest, stress, post-30, post-90) ANOVAs revealed a significant decrease in happiness, calmness, and cheerful (p's < 0.001) and increase in stress, tension and anger (p's < 0.001) immediately after stress compared with all other time points. Participants also reported to feel calmer at 30 and 90 minutes post-stress compared with baseline. There was a significant time effect for tiredness (p = 0.005) and energy (p < 0.001), whereby participants felt more energetic and less tired at pre-stress rest compared with baseline, and less energetic 30 minutes post-stress compared with rest and stress, and less energetic and more tired 90 minutes post-stress compared with rest and stress. There was a significant time (p = 0.018) and condition × time interaction effect (p = 0.013) for sadness, but post-hoc analyses revealed no significances between time points. Post-hoc analyses for main effects are reported in Table 6.| HFM + LFC | HFM + HFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Rest | Stress | Post-30 | Post-90 | Baseline | Rest | Stress | Post-30 | Post-90 | |
| * Significantly different compared with all other time points, # Significantly different to baseline, @ Significantly different to rest and stress. Mood rating scored from 1–5. Separate 2-condition (HFM + HFC, HFM + LFC) by 5-time (baseline, rest, stress, post-30, post-90) repeated measures ANOVAs were used to assess mood ratings. HFC: high-flavanol cocoa, HFM: high-fat meal, LFC: low-flavanol cocoa. | ||||||||||
| Happy | 3.57 ± 0.73 | 3.74 ± 0.62 | 2.26 ± 0.81* | 3.39 ± 0.66 | 3.57 ± 0.66 | 3.48 ± 0.67 | 3.57 ± 0.66 | 2.26 ± 0.86* | 3.48 ± 0.67 | 3.61 ± 0.58 |
| Stressed | 1.57 ± 0.84 | 1.26 ± 0.45 | 4.09 ± 0.56* | 1.22 ± 0.42 | 1.22 ± 0.42 | 1.48 ± 0.51 | 1.26 ± 0.45 | 3.91 ± 0.85* | 1.35 ± 0.57 | 1.30 ± 0.56 |
| Energetic | 2.35 ± 1.03 | 3.04 ± 0.93# | 2.87 ± 1.14 | 2.26 ± 0.86@ | 2.17 ± 0.83@ | 2.43 ± 0.73 | 3.00 ± 1.04# | 3.00 ± 1.00 | 2.17 ± 0.94@ | 2.22 ± 0.80@ |
| Cheerful | 3.17 ± 0.83 | 3.48 ± 0.90 | 2.39 ± 1.03* | 3.17 ± 0.72 | 3.35 ± 0.71 | 3.26 ± 0.75 | 3.43 ± 0.79 | 2.17 ± 1.07* | 3.22 ± 0.74 | 3.39 ± 0.78 |
| Tense | 1.52 ± 0.73 | 1.35 ± 0.57 | 3.74 ± 0.86* | 1.39 ± 0.78 | 1.26 ± 0.54 | 1.43 ± 0.73 | 1.22 ± 0.42 | 3.74 ± 0.86* | 1.17 ± 0.39 | 1.22 ± 0.42 |
| Tired | 2.91 ± 0.90 | 2.35 ± 0.83# | 2.22 ± 1.09 | 2.70 ± 1.15 | 2.91 ± 0.85@ | 2.87 ± 1.06 | 2.39 ± 0.99# | 2.30 ± 1.02 | 2.87 ± 1.14 | 3.17 ± 0.94@ |
| Sad | 1.30 ± 0.56 | 1.13 ± 0.34 | 1.22 ± 0.42 | 1.13 ± 0.34 | 1.04 ± 0.21 | 1.09 ± 0.29 | 1.00 ± 0.00 | 1.48 ± 0.85 | 1.00 ± 0.00 | 1.04 ± 0.21 |
| Calm | 3.35 ± 0.83 | 3.57 ± 0.99 | 1.83 ± 0.78* | 3.91 ± 0.85# | 3.87 ± 0.87# | 3.26 ± 0.69 | 3.65 ± 0.78 | 1.65 ± 0.78* | 3.70 ± 0.88# | 3.70 ± 0.97# |
| Angry | 1.04 ± 0.21 | 1.00 ± 0.00 | 2.30 ± 0.93* | 1.04 ± 0.21 | 1.00 ± 0.00 | 1.04 ± 0.21 | 1.00 ± 0.00 | 2.43 ± 0.90* | 1.00 ± 0.00 | 1.00 ± 0.00 |
3.5 Cardiovascular activity during acute mental stress
Separate 2-condition (HFM + HFC, HFM + LFC) × 3-time (baseline, rest, stress) ANOVAs revealed an overall time effect for HR, RPI, HRV, SBP and DBP (all p's < 0.001) (Fig. 3). Post-hoc analyses revealed that HR was significantly higher during rest and stress compared with baseline (p's < 0.001) and significantly higher during stress compared with rest (p < 0.001). RPI and HRV were significantly lower during rest and stress compared with baseline (all p's < 0.001), and lower during stress compared with rest (p's < 0.001). SBP was significantly higher during rest and stress compared with baseline (p's < 0.001) and significantly higher during stress compared with rest (p < 0.001). DBP was significantly higher during stress compared with baseline (p < 0.001) and rest (p < 0.001), with no difference between baseline and rest (p = 0.497).There were no significant condition or condition × time interaction effects for HR (condition: p = 0.160, interaction: p = 0.210), RPI (condition: p = 0.051, interaction: p = 0.050), HRV (condition: p = 0.968, interaction: p = 0.192), SBP (condition: p = 0.854, interaction: p = 0.204) or DBP (condition: p = 0.367, interaction: p = 0.224).
3.6 Forearm blood flow during acute mental stress
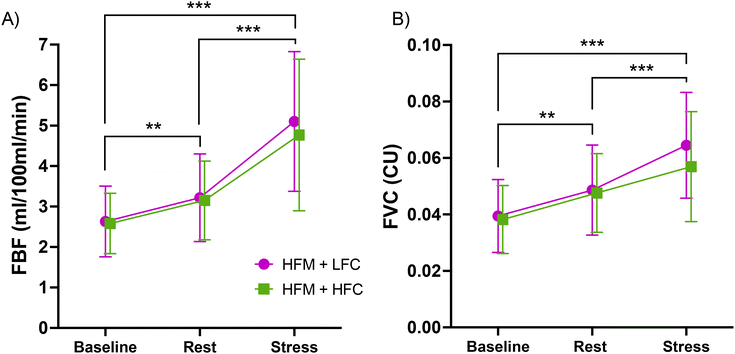
A 2-condition × 3-time ANOVA revealed an overall time effect for FBF and FVC (p's < 0.001) (Fig. 4). Post-hoc analyses revealed that FBF and FVC were significantly higher during stress compared with both baseline (p's < 0.001) and rest (p's < 0.001), and FBF and FVC were significantly higher at rest compared with baseline (FBF: p = 0.002, FVC: p = 0.001). There were no condition nor condition × time interaction effects for FBF (condition: p = 0.486, interaction: p = 0.576) or FVC (condition: p = 0.351, interaction: p = 0.439).3.7 Cerebral oxygenation during acute mental stress
One-sample t-tests revealed that left and right PFC TOI were significantly greater during stress compared with rest in both conditions (left: HFC p < 0.001, LFC p = 0.001; right: HFC p < 0.001, LFC p = 0.002). One-sample t-tests revealed that left nTHI was significantly higher during stress compared with rest in the HFC condition (p = 0.024) but not the LFC condition (p = 0.736). There were no significant differences in right nTHI during stress compared with rest in both conditions (HFC: p = 0.938, LFC: p = 0.384). One-sample t-tests revealed that left and right O2Hb and HHb were significantly different during stress compared with rest in both conditions (all p < 0.001) (Fig. 5).Separate 2-condition (HFC, LFC) × 4-time (stress 2, stress 4, stress 6, stress 8) ANOVAs revealed an overall time effect for right TOI (p = 0.008), right HHb (p = 0.015) and left HHb (p = 0.005). There were no significant time effects for left TOI (p = 0.119), left nTHI (p = 0.218), right nTHI (p = 0.058), left O2Hb (p = 0.666) or right O2Hb (p = 0.256). There were no significant condition (L-TOI: p = 0.254, R-TOI: p = 0.229, L-nTHI: p = 0.346, R-nTHI: p = 0.451, L-O2Hb: p = 0.619, R-O2Hb: p = 0.255, L-HHb: p = 0.314, R-HHb: p = 0.674) effects for any outcome measure. There was a significant condition × time interaction for right O2Hb (p = 0.018) but no other condition × time interaction (L-TOI: p = 0.324, R-TOI: p = 0.064, L-nTHI: p = 0.283, R-nTHI: p = 0.517, L-O2Hb: p = 0.118, R-O2Hb: p = 0.055, L-HHb: p = 0.083, R-HHb: p = 0.400) effects. Post-hoc analyses for the time effects are reflected in Fig. 5.
3.8 Flow-mediated dilatation following acute mental stress
Brachial FMD following mental stress is reported in Fig. 6. A 2-condition × 3-time ANOVA revealed a significant condition effect for brachial FMD (p = 0.002). Post-hoc analyses showed that FMD was significantly higher in the HFM + HFC condition compared with the HFM + LFC condition. Furthermore, there was a significant condition × time interaction effect for brachial FMD (p < 0.001). Further exploration of this interaction effect revealed that FMD was significantly higher 30 minutes (p = 0.006) and 90 minutes (p < 0.001) post-stress in the HFC condition compared with the LFC condition. Examination of the time effects in both conditions separately showed that in the HFC condition, there was no significant difference in FMD at 30 minutes post-stress compared with baseline (p = 0.383), yet FMD significantly increased at 90 minutes post-stress compared with baseline (p = 0.004). However, in the LFC condition FMD was significantly lower at 30 minutes post stress (p = 0.003) and 90 minutes post-stress (p = 0.017) compared with baseline. In summary, in the LFC condition, FMD was significantly impaired at 30 and 90 minutes post-stress, yet in the HFC condition, FMD was unchanged at 30 minutes post-stress and increased at 90 minutes post-stress. There was no significant time effect for brachial FMD (p = 0.373).Allometrically scaled FMD is also reported in Fig. 6. A 2 × 3 Linear Mixed Model revealed a significant condition (p < 0.001) and condition × time interaction (p < 0.001) effect but no significant time effect (p = 0.325). Post-hoc analyses showed that FMD was significantly higher in the HFM + HFC condition compared with the HFM + LFC condition. Examination of the time effects in both conditions separately showed that in the HFC condition, there was no significant difference in FMD at 30 minutes post-stress [95%CI: 6.08, 8.44] compared with baseline [95%CI: 5.34, 7.68] (p = 0.105). Yet, FMD was significantly higher at 90 minutes post-stress [95%CI: 7.14, 9.53] compared with baseline (p < 0.001) and 30 minutes post-stress (p = 0.023). In the LFC condition FMD was significantly lower at 30 minutes post-stress [95%CI: 4.50, 6.82] compared with baseline [95%CI: 5.65, 8.00] (p = 0.014). There was no significant difference in FMD between 90 minutes post-stress [95%CI: 4.71, 7.04] and baseline (p = 0.050), and between 30 minutes post-stress and 90 minutes post-stress (p = 0.616). In summary, in the LFC condition FMD was significantly impaired at 30 minutes post-stress, yet in the HFC condition FMD was unchanged at 30 minutes post-stress but significantly increased at 90 minutes post-stress.
Brachial arterial diameter, anterograde blood flow and retrograde blood flow are reported in Table 7. There was a significant time effect for arterial diameter (p = 0.002), with a significantly higher diameter at 30 minutes (p = 0.008) and 90 minutes (p = 0.010) post-stress compared with baseline. There was also a significant condition × time interaction for arterial diameter (p = 0.019), whereby diameter was significantly greater in the LFC condition compared with the HFC condition at 90 minutes post-stress (p = 0.011). Similarly, in the LFC condition diameter significantly increased following stress, yet was unchanged following stress in the HFC condition. There was no condition effect for brachial artery diameter (p = 0.091). There was no significant condition (p = 0.830), time (p = 0.633) or condition × time interaction (p = 0.824) effect for anterograde blood flow. There was a significant time effect for retrograde blood flow (p < 0.001), whereby retrograde blood flow was significantly greater at 30 minutes (p < 0.001) and 90 minutes (p = 0.006) post-stress compared with baseline, and was significantly greater at 30 minutes post-stress compared with 90 minutes post-stress (p = 0.002). There was no condition (p = 0.635) or condition × time interaction (p = 0.968) effect for retrograde blood flow.
| Timepoint | HFM + HFC | HFM + LFC | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-30 | Post-90 | Baseline | Post-30 | Post-90 | |
| * Significantly different to baseline, $ significantly different to HFM + HFC. ^ Significantly different to post-30. DBP: diastolic blood pressure, HFC: high-flavanol cocoa, HFM: high-fat meal, LFC: low-flavanol cocoa, SBP: systolic blood pressure. | ||||||
| Brachial SBP (mmHg) | 116.81 ± 8.72 | 121.00 ± 9.97** | 120.14 ± 6.98** | 118.54 ± 9.90 | 120.83 ± 10.61** | 122.36 ± 10.81** |
| Brachial DBP (mmHg) | 64.97 ± 5.44 | 63.03 ± 5.01** | 62.82 ± 5.99* | 67.04 ± 6.50 | 63.77 ± 4.51** | 63.86 ± 6.02* |
| Brachial diameter (mm) | 3.61 ± 0.55 | 3.63 ± 0.53** | 3.63 ± 0.51* | 3.60 ± 0.54 | 3.68 ± 0.55** | 3.72 ± 0.55*$ |
| Brachial anterograde blood flow (ml min−1) | 91.06 ± 42.25 | 94.23 ± 61.20 | 95.62 ± 55.46 | 87.29 ± 56.81 | 95.59 ± 57.47 | 94.11 ± 48.80 |
| Brachial retrograde blood flow (ml min−1) | −7.66 ± 9.23 | −17.15 ± 14.79*** | −12.17 ± 10.30**/^^ | −7.26 ± 7.22 | −16.11 ± 9.65*** | −11.67 ± 8.23**/^^ |
Brachial blood pressure is presented in Table 7. A 2 × 3 ANOVA revealed a significant time effect for SBP (p < 0.001) and DBP (p = 0.002), whereby SBP was higher and DBP was lower at 30 and 90 minutes post-stress compared with baseline. There were no significant condition (SBP: p = 0.273, DBP: p = 0.211) or condition × time interaction (SBP: p = 0.152, DBP: p = 0.582) effects for blood pressure.
3.9 Common carotid artery blood flow following acute mental stress
CCA diameter and blood flow are reported in Table 8. A 2-condition × 3-time ANOVA revealed a significant time effect in CCA diameter (p < 0.001), with a significantly greater diameter at 30 and 90 minutes post-stress compared with baseline (p's < 0.001), and a significantly greater diameter at 90 minutes post-stress compared with 30 minutes post-stress (p < 0.001). There was no significant condition (p = 0.056) or condition × time interaction (p = 0.163) for CCA diameter. There was no significant condition (p = 0.655), time (p = 0.740) or condition × time interaction (p = 0.153) for CCA anterograde blood flow. There was a significant time effect for CCA retrograde blood flow (p < 0.001), whereby retrograde blood flow was significantly less at 30 and 90 minutes post-stress compared with baseline (p's < 0.001). There was no significant condition (p = 0.779) or condition × time interaction (p = 0.324) effect for retrograde blood flow.| Timepoint | HFM + HFC | HFM + LFC | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-30 | Post-90 | Baseline | Post-30 | Post-90 | |
| * Significantly different to baseline, ^ Significantly different to post-30. CCA: common carotid artery. | ||||||
| CCA diameter (mm) | 6.52 ± 0.38 | 6.66 ± 0.39*** | 6.74 ± 0.36***/^^^ | 6.54 ± 0.39 | 6.74 ± 0.37*** | 6.85 ± 0.41***/^^^ |
| CCA anterograde flow (ml min−1) | 578.33 ± 155.19 | 585.32 ± 165.72 | 534.97 ± 195.97 | 546.13 ± 149.58 | 552.74 ± 134.96 | 567.59 ± 131.13 |
| CCA retrograde flow (ml min−1) | −5.95 ± 4.76 | −1.44 ± 1.56*** | −2.10 ± 3.52*** | −4.92 ± 4.81 | −3.10 ± 4.48*** | −2.11 ± 3.15*** |
4. Discussion
This study aimed to investigate whether cocoa flavanols can be used as a dietary strategy to protect endothelial function and improve brain oxygenation in the context of mental stress following an acute high-fat meal. To our knowledge, this is the first study to show that high-flavanol cocoa can attenuate the stress-induced decline in brachial FMD following a high-fat meal. On the other hand, flavanol intake did not improve cortical oxygenation during stress. Both brachial and carotid artery diameter increased following stress and fat intake, with a greater increase in the brachial artery following low-flavanol cocoa compared with high-flavanol cocoa. Retrograde blood flow increased post-stress and fat intake in the brachial artery and decreased in the carotid artery, yet these were unaffected by the flavanol intervention. As predicted and shown previously, mental stress induced changes in HR, HRV, RPI, BP and FBF, but these were not affected by flavanol intake. Importantly, perceptions of the stress task and task performance were not significantly different between conditions, suggesting a consistent stress experience across interventions.The stress-induced decline in brachial FMD at 30 minutes post-stress (1.29%) is in line with previous work, showing a 1–3% impairment in endothelial function following stress in healthy adults.12–14,71,72 Furthermore, the fat-induced delay in endothelial recovery at 90 minutes post-stress (FMD remains 1.11% lower than baseline) is in line with our previous work showing FMD to remain impaired by 1.16% 90 minutes following stress, when a high-fat meal had been consumed.34
Cocoa flavanols were effective at preventing the decline in endothelial function post-stress following fat consumption, with brachial FMD being significantly higher following high-flavanol cocoa compared with low-flavanol cocoa at both 30 and 90 minutes post-stress. Given the significant difference in brachial artery diameter at 90 minutes post-stress between flavanol conditions, allometrically scaled FMD was also estimated, confirming the significant differences in FMD between high versus low flavanol interventions even when diameter changes are corrected for. More specifically, cocoa flavanols attenuated the 1.29% decline in FMD at 30 minutes post-stress and improved FMD by 1.37% at 90 minutes post-stress (compared with the 1.16% decline below baseline following low-flavanol cocoa). This is of clinical significance given that a 1% decline in FMD has been associated with a 9–13% increased risk of future cardiovascular events.7,73 We have previously reported these differences at 30–90 minutes post-stress in participants in a fasted state: the high-flavanol cocoa attenuated the stress-induced decline in brachial FMD by approx. 1.36%.40 In agreement with our data, previous research has shown that both cocoa flavanols74 and citrus flavanones75 attenuate fat-induced impairments in FMD, yet do not completely eliminate the negative effect of fat. However, this is the first study to investigate fat intake prior to mental stress, and to show that cocoa flavanols can mitigate the combined impact of fat consumption and stress. The mechanisms by which flavanol ingestion improve vascular function are thought to be NO-related,44 with evidence of (−)-epicatechin enhancing endothelial NO synthase activation through activation of signalling pathways such as P13K, Akt and PKA. Flavanols have also been shown to downregulate the bioavailability of ET-1,47,76 reduce interleukin-6 (IL-6) production and reactive oxygen species (ROS).77 These mechanisms likely drive the improvements in FMD following stress and fat consumption. Whilst there is some evidence that chronic flavanol intake can reduce triglyceride concentration and improve biochemical parameters of lipid metabolism,78,79 the effect of acute flavanol ingestion on lipid bioavailability is unclear.80 In summary, our data on peripheral vascular function suggest that when food choices during stress target high-fat foods, the addition of a high-flavanol food can be effective at preventing the negative compounded effect of stress and fat on endothelial function.
The present study showed a greater brachial artery retrograde blood flow at 30 and 90 minutes post-stress, with the greatest retrograde flow at 30 minutes post-stress, which is in line with the largest stress-induced reduction in brachial FMD observed.12 This is in agreement with our previous work, showing increases in retrograde blood flow post-stress.34 An increased retrograde blood flow response to stress has been shown previously,81 and likely results in elevated ET-1, expression of adhesion molecules, ROS-producing enzymes and decreased NO production.82 In line with that, increased retrograde shear rate has been associated with a reduction in endothelial function,82 which is in agreement with our observations in the current study. Importantly, the flavanol intervention does not seem to affect retrograde flow, suggesting that dietary flavanols improve FMD independently of retrograde blood flow.
In regard to the cerebral vasculature, we have observed an increase in PFC tissue oxygenation during stress, which is in line with previous research evidencing elevated CBF during similar arithmetic tasks.15,35,83 Stress-induced increases in cardiac output (driven by increased HR, Fig. 3A) and BP (Fig. 3D and E) have been shown to contribute to elevated cerebral perfusion84–86 as well as neurovascular coupling mechanisms driven by increases in neural activation during the cognitively demanding mental arithmetic task.87 Critically, we have shown recently that acute fat consumption during mental stress reduces PFC tissue oxygenation.35 We hypothesised that cocoa flavanols would counteract the fat-induced reduction in PFC tissue oxygenation during stress. However, contrary to their effect in the periphery, cocoa flavanols did not improve cortical oxygenation during mental stress. Previous research has shown cocoa flavanols to improve cerebral oxygenation during hypercapnia.48 Timing of ingestion may influence the effect of flavanols, as Gratton and colleagues assessed cerebral oxygenation 2 hours following flavanol consumption.48 In the present study, we investigated oxygenation 1.5 hours post-flavanol ingestion, given that flavanol metabolites are shown to peak in the blood at 1–2 hours post-intake,41,88 but flavanol absorption may be delayed due to the concomitant intake of a fatty meal. It is also plausible that the effect of flavanol consumption on the brain during stress is smaller than on the periphery, possibly due to the greater need to tightly control and regulate brain blood flow (which can increase maximally by <one fold) compared with peripheral blood flow (which can increase 5–10 fold).89 Retrospective power calculations reveal that using the observed medium effect size in TOI (0.26, ηp2 = 0.065) and with power set to 0.9 and alpha at 0.05, 40 participants are required to appropriately power our study to detect changes in cortical oxygenation due to flavanol intake. Therefore, future research should investigate the impact of flavanol and fat intake on the cerebrovasculature in larger samples and investigate whether these effects are replicable in other areas of the brain, for example by utilising fMRI, in addition to NIRS and ultrasound. Overall, the present study suggests that the potential protective effect of flavonoids during mental stress seems to be smaller (or inexistent) in the brain compared with the periphery.
Our previous work has also shown that alongside reduced PFC tissue oxygenation during mental stress, fat intake also induced a disturbance in mood.35 Importantly, high-flavanol cocoa did not influence mood parameters in the present study. Whilst there is some evidence to suggest polyphenols can reduce negative mood and improve positive mood,90 this might not be the case in the context of saturated fat intake and mental stress.
In agreement with observations during stress, there were no differences in carotid artery blood flow and diameter between high and low-flavanol cocoa interventions. The postprandial increase in carotid artery diameter was in line with our previous findings.35 We also detected a postprandial increase in brachial artery diameter at these time points (Table 7), in line with previous studies.91,92 There is evidence that fat consumption induces peripheral vasodilation (FBF), likely mediated by changes in insulin and triglycerides.91 However, in the present study, flavanol consumption seems to mask this effect, shown by a reduced brachial artery diameter following high-flavanol cocoa compared with low-flavanol cocoa at 90 minutes post-stress (p = 0.011). Interestingly, this condition effect at 90 minutes post-stress does not quite reach significance in the carotid artery (p = 0.056). This is possibly due to being underpowered, given the large effect size detected (ηp2 = 0.156, f2 = 0.43). Future research should utilise larger sample sizes and additional assessments of the internal carotid artery to confirm these observations.
In line with previous research, the present study showed an increase in cardiovascular, BP and peripheral vasodilatory responses during stress.40,58 There was no difference in the FBF response to stress between conditions. Previous studies have reported increases in FBF following cocoa flavanols at rest93 and during stress,40 in a fasted state. As previously mentioned, fat consumption likely delayed the absorption of flavanol metabolites, reducing the magnitude of the NO-dependent increase in vasodilation at the time of stress.94 Similarly, flavanol consumption had no effect on cardiovascular responses during stress, in agreement with our previous results in a fasted state.40
The habitual diet of the participants in the current study appears to be similar to the diet of the UK population. For example, whilst 100% of participants exceeded the daily recommended sugar value compared with 61% of British citizens,95 43% exceeded the recommended saturated fat value compared with 75% of UK adults.96 Furthermore, 35% of participants consumed at least 5 portions of fruit and vegetables, in line with the UK average of 28%. However, the present sample consumed on average 1 extra portion per day compared with UK adults (4.8 portions per day vs. 3.7 portions per day),97 yet their flavonoid intake (239 mg per day) was more in line with participants with lower flavonoid intake (mean quintile 1: 174 mg per day, mean quintile 2: 321 mg per day) in a large cross-sectional study.98 Therefore, our findings have relevance for the general population. However, it is possible that flavonoid-induced protection of endothelial function may be particularly beneficial for populations with poorer habitual diet. Therefore, future research should target these populations, such as those with lower socioeconomic status, low income or education, certain ethnic minority groups, smokers, and physically inactive individuals.99 Furthermore, the gut microbiome may also play a role in the effect of fat and/or flavonoids on vascular responses to stress. For example, both stress and high-fat diets are associated with alterations in gut integrity, which can enhance systemic inflammation and contribute to the development of CVD.100,101 Thus, a potential value of flavonoids in relation to CVD, and during periods of stress and high-fat feeding, is following their transformation through gut microbiota metabolism.102 Therefore, future work should assess the concentration of flavonoids in circulation, as well as the role played by intestinal permeability in the interaction between stress, fat and/or flavonoid intake, and vascular function.
4.1 Limitations
One limitation of the present study is that the high-fat meal was not tailored to individual metabolic rate. However, tailoring fat consumption to metabolic rate is not very relevant to everyday life, and intervening with one consistent dose of fat likely results in higher variability in responses between participants. Similarly, it has been established that 50 g of fat is sufficient to impact endothelial function,103 which is in line with the dose used in the current study. Secondly, previous evidence has shown that polyphenol microbial-derived metabolites can be detected in the blood for up to 48 hours,104 yet we only restricted polyphenol intake for 24 hours prior to each visit. As there is a large reduction in urinary polyphenol metabolites from 24 to 48 hours, most metabolites are likely to be excreted within the first 24 hours.105 Furthermore, our sample size was moderate. However, a robust crossover design was employed and post-hoc power analyses revealed that a sample of 23 participants, power at 90% and alpha at 0.05, allowed the detection of a medium size interaction effect (0.31) for our primary outcome measure brachial FMD.69 Nevertheless, more participants are likely required to detect the effect of flavanol-rich cocoa on cortical oxygenation, and so future research should continue this investigation in a larger sample. Finally, the potential mechanisms discussed are speculative, and thus a more in-depth investigation of mechanisms of action underlying these responses should be the focus of future work.5. Conclusions
In summary, this study demonstrates that flavonoid-rich foods have the potential to acutely protect endothelial function against poor food choices, such as high-fat snacks, during episodes of stress in young healthy adults. It further suggests that such protection does not extend to the cerebral vasculature. However, our data indicate that the size of the flavanol effect in the brain is smaller, and a larger sample is needed to clarify the protective effects in the brain during stress. Given the prognostic value of FMD for future risk of CVD,7 these findings are clinically relevant, particularly given the documented prevalence of stress and the trend towards increased consumption of high-fat foods during periods of heightened stress. This work has relevance for application in everyday diet, as the administered dose of flavanols could be achieved through consumption of, for instance, 2 cups of green tea, 5.5 tbsp of unprocessed cocoa or 300 g of berries.106 As such, our data have important implications for future acute dietary recommendations to protect the vasculature during stressful periods.Author contributions
Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, software, validation, and visualization, R. B., J. V. v. Z. and C. R., writing – original draft, R. B., project administration, supervision, writing – review & editing, J. V. v. Z. and C. R.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article are available at the University of Birmingham Repository https://edata.bham.ac.uk/1182/.Acknowledgements
The authors would like to thank Barry Callebaut (Leen Allegaert) for providing the high and low-flavanol cocoa interventions. The authors would like to thank Samuel Weaver and David McIntyre for their support with data analyses, and Emily Waterfield, Ella Clarke, and Benjamin Drury for their help with data collection. The authors declare financial support was received for the research of this article, funded by the Economic and Social Research Council (ESRC), Grant Number: 2388587. The graphical abstract has been designed using resources from Flaticon.com.References
- Health and Safety Executive, Work-related stress, depression or anxiety statistics in Great Britain, https://www.hse.gov.uk/stress/standards/downloads.htm, (accessed 25 March, 2024).
- R. D. Goodwin, A. H. Weinberger, J. H. Kim, M. Wu and S. Galea, Trends in anxiety among adults in the United States, 2008-2018: Rapid increases among young adults, J. Psychiatr. Res., 2020, 130, 441–446 CrossRef PubMed.
- D. Carroll, S. Ebrahim, K. Tilling, J. MacLeod and G. D. Smith, Admissions for myocardial infarction and World Cup football: database survey, Br. Med. J., 2002, 325, 1439–1442 CrossRef PubMed.
- M. Bergovec, S. Mihatov, H. Prpic, S. Rogan, V. Batarelo and V. Sjerobabski, Acute Myocardial-Infarction among civilians in Zagreb City Area, Lancet, 1992, 339, 303–303 CrossRef.
- J. Leor and R. A. Kloner, The Northridge earthquake as a trigger for acute myocardial infarction, Am. J. Cardiol., 1996, 77, 1230–1232 CrossRef PubMed.
- M. Prasad, P. Khanna, V. K. Katyal and R. Verma, Acute Psychological Stress is a Trigger for Stroke: A Case-Crossover Study, J. Stroke Cerebrovasc. Dis., 2020, 29, 104799 CrossRef PubMed.
- Y. Inaba, J. A. Chen and S. R. Bergmann, Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis, Int. J. Cardiovasc. Imaging, 2010, 26, 631–640 CrossRef PubMed.
- M. M. Burg, B. Graeber, A. Vashist, D. Collins, C. Earley, J. Liu, R. Lampert and R. Soufer, Noninvasive Detection of Risk for Emotion Provoked Myocardial Ischemia, Psychosom. Med., 2009, 71, 14–20 CrossRef PubMed.
- A. D. Goldberg, L. C. Becker, R. Bonsall, J. D. Cohen, M. W. Ketterer, P. G. Kaufman, D. S. Krantz, K. C. Light, R. P. McMahon, T. Noreuil, C. J. Pepine, J. Raczynski, P. H. Stone, D. Strother, H. Taylor and D. S. Sheps, Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI), Circulation, 1996, 94, 2402–2409 CrossRef PubMed.
- D. Jain, S. M. Shaker, M. Burg, F. J. T. Wackers, R. Soufer and B. L. Zaret, Effects of Mental Stress on Left Ventricular and Peripheral Vascular Performance in Patients With Coronary Artery Disease, J. Am. Coll. Cardiol., 1998, 31, 1314–1322 CrossRef PubMed.
- V. J. Poitras and K. E. Pyke, The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance, Int. J. Psychophysiol., 2013, 88, 124–135 CrossRef PubMed.
- L. Ghiadoni, A. E. Donald, M. Cropley, M. J. Millen, G. Oakley, M. Taylor, G. O’Connor, J. Betteridge, N. Klein, A. Steptoe and J. E. Deanfield, Mental Stress induces Transient endothelial Dysfunction in humans, Circulation, 2000, 2473–2478 CrossRef PubMed.
- L. Lind, K. Johansson and J. Hall, The effects of mental stress and the cold pressure test on flow-mediated vasodilation, Blood Pressure, 2002, 11, 22–27 CrossRef PubMed.
- L. E. Spieker, D. Hürlimann, F. Ruschitzka, R. Corti, F. Enseleit, S. Shaw, D. Hayoz, J. E. Deanfield, T. F. Lüscher and G. Noll, Mental Stress Induces Prolonged Endothelial Dysfunction via Endothelin-A Receptors, Circulation, 2002, 105, 2817–2820 CrossRef PubMed.
- L. N. Shoemaker, L. C. Wilson, S. J. E. Lucas, L. Machado and J. D. Cotter, Cerebrovascular regulation is not blunted during mental stress, Exp. Physiol., 2019, 104, 1678–1687 CrossRef PubMed.
- D. Santos-García, M. Blanco, J. Serena, M. Rodríguez-Yáñez, R. Leira and J. Castillo, Impaired brachial flow-mediated dilation is a predictor of a new-onset vascular event after stroke, Cerebrovasc. Dis., 2011, 32, 155–162 CrossRef.
- N. Toda and M. Nakanishi-Toda, How mental stress affects endothelial function, Pflugers Arch., 2011, 462, 779–794 CrossRef.
- A. J. Wadley, J. J. C. S. Veldhuijzen van Zanten, N. J. Paine, M. T. Drayson and S. Aldred, Underlying inflammation has no impact on the oxidative stress response to acute mental stress, Brain, Behav., Immun., 2014, 40, 182–190 CrossRef PubMed.
- D. Hill, M. Conner, F. Clancy, R. Moss, S. Wilding, M. Bristow and D. B. O'Connor, Stress and eating behaviours in healthy adults: a systematic review and meta-analysis, Health Psychol. Rev., 2021, 1–25, DOI:10.1080/17437199.2021.1923406.
- D. B. O'Connor, J. F. Thayer and K. Vedhara, Stress and Health: A Review of Psychobiological Processes, Annu. Rev. Psychol., 2021, 72, 663–688 CrossRef.
- E. Newman, D. B. O'Connor and M. Conner, Daily hassles and eating behaviour: The role of cortisol reactivity status, Psychoneuroendocrinology, 2006, 32, 125–132 CrossRef.
- C. J. Roberts, I. C. Campbell and N. Troop, Increases in Weight during Chronic Stress are Partially Associated with a Switch in Food Choice towards Increased Carbohydrate and Saturated Fat Intake: Stress Food Choice Weight Increase, eur. Eat. Disord. Rev., 2014, 22, 77–82 CrossRef.
- G. Oliver and J. Wardle, Perceived Effects of Stress on Food Choice, Physiol. Behav., 1999, 66, 511–515 CrossRef PubMed.
- D. A. Zellner, S. Loaiza, Z. Gonzalez, J. Pita, J. Morales, D. Pecora and A. Wolf, Food selection changes under stress, Physiol. Behav., 2006, 87, 789–793 CrossRef PubMed.
- C. K. Gardiner, S. L. Hagerty and A. D. Bryan, Stress and number of servings of fruit and vegetables consumed: Buffering effects of monetary incentives, J. Health Psychol., 2021, 26, 1757–1763 CrossRef PubMed.
- Amerian Psychological Association, stress and eating, https://www.apa.org/news/press/releases/stress/2013/eating, (accessed 22 February, 2024).
- E. Pool, S. Delplanque, G. Coppin and D. Sander, Is comfort food really comforting? Mechanisms underlying stress-induced eating, Food Res. Int., 2015, 76, 207–215 CrossRef.
- B. S. McEwen and P. J. Gianaros, Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease, Ann. N. Y. Acad. Sci., 2010, 1186, 190–222 CrossRef PubMed.
- C. J. Moore and S. A. Cunningham, Social Position, Psychological Stress, and Obesity: A Systematic Review, J. Acad. Nutr. Diet., 2012, 112, 518–526 CrossRef PubMed.
- J. Wardle, Y. Chida, E. L. Gibson, K. L. Whitaker and A. Steptoe, Stress and Adiposity: A Meta-Analysis of Longitudinal Studies, Obesity, 2011, 19, 771–778 CrossRef.
- J. K. Kiecolt-Glaser, D. L. Habash, C. P. Fagundes, R. Andridge, J. Peng, W. B. Malarkey and M. A. Belury, Daily Stressors, Past Depression, and Metabolic Responses to High-Fat Meals: A Novel Path to Obesity, Biol. Psychiatry, 2015, 77, 653–660 CrossRef.
- E. W. Cotter and N. R. Kelly, Stress-related eating, mindfulness, and obesity, Health Psychol., 2018, 37, 516–525 Search PubMed.
- T. M. Powell-Wiley, P. Poirier, L. E. Burke, J.-P. Després, P. Gordon-Larsen, C. J. Lavie, S. A. Lear, C. E. Ndumele, I. J. Neeland, P. Sanders, M.-P. St-Onge and n. null, Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association, Circulation, 2021, 143, e984–e1010 CrossRef PubMed.
- R. Baynham, S. R. C. Weaver, C. Rendeiro and J. J. C. S. Veldhuijzen van Zanten, Fat intake impairs the recovery of endothelial function following mental stress in young healthy adults, Front. Nutr., 2023, 10, 1275708 CrossRef.
- R. Baynham, S. J. E. Lucas, S. R. C. Weaver, J. J. C. S. Veldhuijzen van Zanten and C. Rendeiro, Fat Consumption Attenuates Cortical Oxygenation during Mental Stress in Young Healthy Adults, Nutrients, 2023, 15, 3969 CrossRef PubMed.
- W. C. Tsai, Y. H. Li, C. C. Lin, T. H. Chao and J. H. Chen, Effects of oxidative stress on endothelial function after a high-fat meal, Clin. Sci., 2004, 106, 315–319 CrossRef PubMed.
- J.-H. Bae, E. Bassenge, K.-B. Kim, Y.-N. Kim, K.-S. Kim, H.-J. Lee, K.-C. Moon, M.-S. Lee, K.-Y. Park and M. Schwemmer, Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress, Atherosclerosis, 2001, 155, 517–523 CrossRef PubMed.
- H. O. Steinberg, M. Tarshoby, R. Monestel, G. Hook, J. Cronin, A. Johnson, B. Bayazeed and A. D. Baron, Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation, J. Clin. Invest., 1997, 100, 1230–1239 CrossRef.
- A. W. C. Man, H. Li and N. Xia, Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function, Oxid. Med. Cell. Longevity, 2020, 2020, 1496462 Search PubMed.
- R. Baynham, J. J. C. S. Veldhuijzen van Zanten, P. W. Johns, Q. S. Pham and C. Rendeiro, Cocoa flavanols improve vascular responses to acute mental stress in young healthy adults, Nutrients, 2021, 13(4), 1103 CrossRef PubMed.
- K. D. Monahan, R. P. Feehan, A. R. Kunselman, A. G. Preston, D. L. Miller and M. E. J. Lott, Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults, J. Appl. Physiol., 2011, 111, 1568–1574 CrossRef PubMed.
- A. Rodriguez-Mateos, M. Hezel, H. Aydin, M. Kelm, J. O. Lundberg, E. Weitzberg, J. P. E. Spencer and C. Heiss, Interactions between cocoa flavanols and inorganic nitrate: Additive effects on endothelial function at achievable dietary amounts, Free Radicals Biol. Med., 2015, 80, 121–128 CrossRef PubMed.
- R. Sansone, J. I. Ottaviani, A. Rodriguez-Mateos, Y. Heinen, D. Noske, J. P. Spencer, A. Crozier, M. W. Merx, M. Kelm, H. Schroeter and C. Heiss, Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: randomized, double-masked controlled studies, Am. J. Clin. Nutr., 2017, 105, 352–360 CrossRef PubMed.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029 CrossRef.
- A. Moreno-Ulloa, D. Romero-Perez, F. Villarreal, G. Ceballos and I. Ramirez-Sanchez, Cell membrane mediated (-)-epicatechin effects on upstream endothelial cell signaling: evidence for a surface receptor, Bioorg. Med. Chem. Lett., 2014, 24, 2749–2752 CrossRef PubMed.
- I. Ramirez-Sanchez, L. Maya, G. Ceballos and F. Villarreal, (-)-Epicatechin Activation of Endothelial Cell Endothelial Nitric Oxide Synthase, Nitric Oxide, and Related Signaling Pathways, Hypertension, 2010, 55, 1398–U1198 CrossRef PubMed.
- W. M. Loke, J. M. Hodgson, J. M. Proudfoot, A. J. McKinley, I. B. Puddey and K. D. Croft, Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men, Am. J. Clin. Nutr., 2008, 88, 1018–1025 CrossRef PubMed.
- G. Gratton, S. R. Weaver, C. V. Burley, K. A. Low, E. L. Maclin, P. W. Johns, Q. S. Pham, S. J. E. Lucas, M. Fabiani and C. Rendeiro, Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults, Sci. Rep., 2020, 10, 19409 CrossRef PubMed.
- P. M. Bloomfield, J. P. Fisher, D. M. Shaw and N. Gant, Cocoa flavanols protect cognitive function, cerebral oxygenation, and mental fatigue during severe hypoxia, J. Appl. Physiol., 2023, 135, 475–484 CrossRef PubMed.
- K. N. Thomas, N. C. Lewis, B. G. Hill and P. N. Ainslie, Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2015, 309, R707–R720 CrossRef.
- D. H. J. Thijssen, M. A. Black, K. E. Pyke, J. Padilla, G. Atkinson, R. A. Harris, B. Parker, M. E. Widlansky, M. E. Tschakovsky and D. J. Green, Assessment of flow-mediated dilation in humans: a methodological and physiological guideline, Am. J. Physiol.: Heart Circ. Physiol., 2011, 300, H2–H12 CrossRef PubMed.
- S. A. Bingham, A. A. Welch, A. McTaggart, A. A. Mulligan, S. A. Runswick, R. Luben, S. Oakes, K. T. Khaw, N. Wareham and N. E. Day, Nutritional methods in the European Prospective Investigation of Cancer in Norfolk, Public Health Nutr., 2001, 4, 847–858 CrossRef PubMed.
- A. A. Mulligan, R. N. Luben, A. Bhaniani, D. J. Parry-Smith, L. O'Connor, A. P. Khawaja, N. G. Forouhi and K. T. Khaw, A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability, BMJ Open, 2014, 4, e004503 CrossRef.
- A. Vogiatzoglou, A. A. Mulligan, A. Bhaniani, M. A. H. Lentjes, A. McTaggart, R. N. Luben, C. Heiss, M. Kelm, M. W. Merx, J. P. E. Spencer, H. Schroeter, K. T. Khaw and G. G. C. Kuhnle, Associations between flavan-3-ol intake and CVD risk in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk), Free Radicals Biol. Med., 2015, 84, 1–10 CrossRef PubMed.
- F. A. Alsolmei, H. Li, S. L. Pereira, P. Krishnan, P. W. Johns and R. A. Siddiqui, Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells, Nutrients, 2019, 11(5), 1107 CrossRef.
- R. J. Robbins, J. Leonczak, J. Li, J. C. Johnson, T. Collins, C. Kwik-Uribe and H. H. Schmitz, Determination of flavanol and procyanidin (by degree of polymerization 1–10) content of chocolate, cocoa liquors, powder(s), and cocoa flavanol extracts by normal phase high-performance liquid chromatography: collaborative study, J. AOAC Int., 2012, 95, 1153–1160 CrossRef PubMed.
- C. Heiss, A. Dejam, P. Kleinbongard, T. Schewe, H. Sies and M. Kelm, Vascular effects of cocoa rich in flavan-3-ols, JAMA, J. Am. Med. Assoc., 2003, 290, 1030–1031 CrossRef PubMed.
- N. J. Paine, C. Ring, S. Aldred, J. A. Bosch, A. J. Wadley and J. J. Veldhuijzen van Zanten, Eccentric-exercise induced inflammation attenuates the vascular responses to mental stress, Brain, Behav., Immun., 2013, 30, 133–142 CrossRef.
- A. T. Ginty, P. J. Gianaros, S. W. Derbyshire, A. C. Phillips and D. Carroll, Blunted cardiac stress reactivity relates to neural hypoactivation, Psychophysiology, 2013, 50, 219–229 CrossRef PubMed.
- J. J. C. S. Veldhuijzen van Zanten, C. Ring, D. Carroll and G. D. Kitas, Increased C reactive protein in response to acute stress in patients with rheumatoid arthritis, Ann. Rheum. Dis., 2005, 64, 1299 CrossRef.
- J. J. C. S. Veldhuijzen van Zanten, D. De Boer, L. K. Harrison, C. Ring, D. Carroll, G. Willemsen and E. J. C. De Geus, Competitiveness and hemodynamic reactions to competition, Psychophysiology, 2002, 39, 759–766 CrossRef.
- Y. Liao, C.-P. Chou, J. Huh, A. Leventhal and G. Dunton, Examining acute bi-directional relationships between affect, physical feeling states, and physical activity in free-living situations using electronic ecological momentary assessment, J. Behav. Med., 2017, 40, 445–457 CrossRef.
- J. D. Lane, L. Greenstadt, D. Shapiro and E. Rubinstein, Pulse transit time and blood pressure: an intensive analysis, Psychophysiology, 1983, 20, 45–49 CrossRef.
- D. de Boer, C. Ring, A. C. Curlett, M. Ridley and D. Carroll, Mental stress-induced hemoconcentration and its recovery: a controlled study of time course and mechanisms, Psychophysiology, 2007, 44, 161–169 CrossRef PubMed.
- D. B. Newlin, Relationships of pulse transmission times to pre-ejection period and blood pressure, Psychophysiology, 1981, 18, 316–321 CrossRef PubMed.
- D. J. Davies, Z. Su, M. T. Clancy, S. J. Lucas, H. Dehghani, A. Logan and A. Belli, Near-Infrared Spectroscopy in the Monitoring of Adult Traumatic Brain Injury: A Review, J. Neurotrauma, 2015, 32, 933–941 CrossRef.
- D. H. J. Thijssen, R. M. Bruno, A. van Mil, S. M. Holder, F. Faita, A. Greyling, P. L. Zock, S. Taddei, J. E. Deanfield, T. Luscher, D. J. Green and L. Ghiadoni, Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans, Eur. Heart J., 2019, 40, 2534–2547 CrossRef PubMed.
- G. Atkinson and A. M. Batterham, The percentage flow-mediated dilation index: A large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine, Vasc. Med., 2013, 18, 354–365 Search PubMed.
- F. Faul, E. Erdfelder, A.-G. Lang and A. Buchner, G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences, Behav. Res. Methods, 2007, 39, 175–191 Search PubMed.
- National Health Service, Eat Well, NHS choices, https://www.nhs.uk/live-well/eat-well/ (accessed 17 September, 2024).
- A. J. M. Broadley, A. Korszun, E. Abdelaal, V. Moskvina, C. J. H. Jones, G. B. Nash, C. Ray, J. Deanfield and M. P. Frenneaux, Inhibition of cortisol production with metyrapone prevents mental stress-induced endothelial dysfunction and baroreflex impairment, J. Am. Coll. Cardiol., 2005, 46, 344–350 CrossRef PubMed.
- Z. Jambrik, L. Sebastiani, E. Picano, B. Ghelarducci and E. L. Santarcangelo, Hypnotic modulation of flow-mediated endothelial response to mental stress, Int. J. Psychophysiol., 2005, 55, 221–227 CrossRef PubMed.
- D. J. Green, H. Jones, D. Thijssen, N. T. Cable and G. Atkinson, Flow-Mediated Dilation and Cardiovascular Event Prediction, Hypertension, 2011, 57, 363–369 CrossRef PubMed.
- S. Westphal and C. Luley, Flavanol-rich cocoa ameliorates lipemia-induced endothelial dysfunction, Heart Vessels, 2011, 26, 511–515 CrossRef PubMed.
- C. Rendeiro, H. Dong, C. Saunders, L. Harkness, M. Blaze, Y. Hou, R. L. Belanger, V. Altieri, M. A. Nunez, K. G. Jackson, G. Corona, J. A. Lovegrove and J. P. Spencer, Flavanone-rich citrus beverages counteract the transient decline in postprandial endothelial function in humans: a randomised, controlled, double-masked, cross-over intervention study, Br. J. Nutr., 2016, 116, 1999–2010 CrossRef PubMed.
- M. Gomez-Guzman, R. Jimenez, M. Sanchez, M. J. Zarzuelo, P. Galindo, A. M. Quintela, R. Lopez-Sepulveda, M. Romero, J. Tamargo, F. Vargas, F. Perez-Vizcaino and J. Duarte, Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension, Free Radicals Biol. Med., 2012, 52, 70–79 CrossRef PubMed.
- J. Wang, D. Y. Zhao, S. Tiano, A. Esteban-Fernandez, B. Yuan, C. Smith, J. Brathwaite, Z. Jlayer, Q. L. Wu, J. E. Simon, K. J. Trageser and G. M. Pasinetti, Prophylactic effect of flavanol rich preparation metabolites in promoting resilience to a mouse model of social stress, Transl. Psychiatry, 2020, 10(1), 183 CrossRef PubMed.
- G. Gutiérrez-Salmeán, E. Meaney, M. A. Lanaspa, C. Cicerchi, R. J. Johnson, S. Dugar, P. Taub, I. Ramírez-Sánchez, F. Villarreal, G. Schreiner and G. Ceballos, A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects, Int. J. Cardiol., 2016, 223, 500–506 CrossRef PubMed.
- A. Leyva-Soto, R. A. Chavez-Santoscoy, L. R. Lara-Jacobo, A. V. Chavez-Santoscoy and L. N. Gonzalez-Cobian, Daily Consumption of Chocolate Rich in Flavonoids Decreases Cellular Genotoxicity and Improves Biochemical Parameters of Lipid and Glucose Metabolism, Molecules, 2018, 23(9), 2220 CrossRef PubMed.
- J. Rynarzewski, L. Dicks, B. F. Zimmermann, B. Stoffel-Wagner, N. Ludwig, H. P. Helfrich and S. Ellinger, Impact of a Usual Serving Size of Flavanol-Rich Cocoa Powder Ingested with a Diabetic-Suitable Meal on Postprandial Cardiometabolic Parameters in Type 2 Diabetics-A Randomized, Placebo-Controlled, Double-Blind Crossover Study, Nutrients, 2019, 11(2), 417 CrossRef PubMed.
- H. N. M. Rocha, G. F. Teixeira, G. M. S. Batista, A. S. Storch, V. P. Garcia, J. Mentzinger, E. A. C. Gomes, M. O. Campos, A. C. L. Nóbrega and N. G. Rocha, AT1R blocker prevents mental stress induced retrograde blood flow in overweight/obese men, Physiol. Rep., 2023, 11, e15566 CrossRef PubMed.
- D. H. J. Thijssen, E. A. Dawson, T. M. Tinken, N. T. Cable and D. J. Green, Retrograde Flow and Shear Rate Acutely Impair Endothelial Function in Humans, Hypertension, 2009, 53, 986–992 CrossRef CAS PubMed.
- Y. Nagasawa, M. Ishida, Y. Komuro, S. Ushioda, L. Hu and K. Sakatani, in Oxygen Transport to Tissue XLI, ed. P.-D. Ryu, J. C. LaManna, D. K. Harrison and S.-S. Lee, Springer International Publishing, Cham, 2020, pp. 99–104. DOI:10.1007/978-3-030-34461-0_14.
- C. K. Willie, Y.-C. Tzeng, J. A. Fisher and P. N. Ainslie, Integrative regulation of human brain blood flow, J. Physiol., 2014, 592, 841–859 CrossRef.
- S. Ogoh, M. Brothers, Q. Barnes, W. L. Eubank, M. N. Hawkins, S. Purkayastha, A. O-Yurvati and P. B. Raven, The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise, J. Physiol., 2005, 569, 697–704 CrossRef PubMed.
- R. C. Brindle, A. T. Ginty, A. C. Whittaker, D. Carroll and S. J. E. Lucas, Assessment of the cerebral pressure-flow relationship using psychological stress to manipulate blood pressure, Psychophysiology, 2018, 55, e13265 CrossRef PubMed.
- D. Wang, H. Rao, G. Wetmore, P. Furlan, M. Korczykowski, D. Dinges and J. Detre, Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17804–17809 CrossRef PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79, 727–747 CrossRef PubMed.
- J. L. Koep, C. E. Taylor, J. S. Coombes, B. Bond, P. N. Ainslie and T. G. Bailey, Autonomic control of cerebral blood flow: fundamental comparisons between peripheral and cerebrovascular circulations in humans, J. Physiol., 2022, 600, 15–39 CrossRef PubMed.
- D. J. Lamport and C. M. Williams, Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses, Brain Plast., 2021, 6, 139–153 Search PubMed.
- O. T. Raitakari, N. Lai, K. Griffiths, R. McCredie, D. Sullivan and D. S. Celermajer, Enhanced peripheral vasodilation in humans after a fatty meal, J. Am. Coll. Cardiol., 2000, 36, 417–422 CrossRef PubMed.
- N. Gokce, Impaired flow-mediated dilation following a high fat meal is attributable to a change in basal tone, Circulation, 1998, 98, 242 CrossRef PubMed.
- C. Heiss, R. Sansone, H. Karimi, M. Krabbe, D. Schuler, A. Rodriguez-Mateos, T. Kraemer, M. M. Cortese-Krott, G. G. Kuhnle, J. P. Spencer, H. Schroeter, M. W. Merx, M. Kelm and E. U. t. F. P. Flaviola Consortium, Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial, Age, 2015, 37, 9794 CrossRef.
- N. M. Dietz, J. M. Rivera, S. E. Eggener, R. T. Fix, D. O. Warner and M. J. Joyner, Nitric-Oxide Contributes to the Rise in Forearm Blood-Flow During Mental Stress in Humans, J. Physiol., 1994, 480, 361–368 CrossRef CAS PubMed.
- F. Rauber, M. L. d. C. Louzada, E. Martinez Steele, L. F. M. Rezende, C. Millet, C. A. Monteiro and R. B. Levy, Ultra-processed foods and excessive free sugar intake in the UK: a nationally representative cross-sectional study, BMJ Open, 2019, 9, e027546 CrossRef.
- Scientific Advisory Committee on Nutrition, Saturated Fats and health – GOV.UK, Scientific Advisory Committee on Nutrition: reports and position statements, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/814995/SACN_report_on_saturated_fat_and_health.pdf (accessed 01 August, 2023).
- National Health Service, Statistics on Obesity, Physical Activity and Diet, England, https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020/part-6-diet-copy (accessed 01 August, 2023).
- N. P. Bondonno, F. Dalgaard, K. Murray, R. J. Davey, C. P. Bondonno, A. Cassidy, J. R. Lewis, C. Kyrø, G. Gislason, A. Scalbert, A. Tjønneland and J. M. Hodgson, Higher Habitual Flavonoid Intakes Are Associated with a Lower Incidence of Diabetes, J. Nutr., 2021, 151, 3533–3542 Search PubMed.
- F. Arjvand, M. Moeeni, B. Najafi and S. Nosratnejad, Factors Affecting Inequality in the Quality Diets: A Scoping Review, Value Health Reg. Issues, 2023, 37, 105–112 Search PubMed.
- C. V. Lewis and W. R. Taylor, Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease, Am. J. Physiol.: Heart Circ. Physiol., 2020, 319, H1227–h1233 CrossRef PubMed.
- D. La Torre, L. Van Oudenhove, T. Vanuytsel and K. Verbeke, Psychosocial stress-induced intestinal permeability in healthy humans: What is the evidence?, Neurobiol. Stress, 2023, 27, 100579 CrossRef.
- Q. Li, B. Gao, B. Siqin, Q. He, R. Zhang, X. Meng, N. Zhang, N. Zhang and M. Li, Gut Microbiota: A Novel Regulator of Cardiovascular Disease and Key Factor in the Therapeutic Effects of Flavonoids, Front. Pharmacol., 2021, 12, 651926 CrossRef PubMed.
- K. G. Jackson, C. K. Armah and A. M. Minihane, Meal fatty acids and postprandial vascular reactivity, Biochem. Soc. Trans., 2007, 35, 451–453 CrossRef PubMed.
- A. Rodriguez-Mateos, D. Vauzour, C. G. Krueger, D. Shanmuganayagam, J. Reed, L. Calani, P. Mena, D. Del Rio and A. Crozier, Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update, Arch. Toxicol., 2014, 88, 1803–1853 CrossRef.
- G. Borges, J. I. Ottaviani, J. J. J. van der Hooft, H. Schroeter and A. Crozier, Absorption, metabolism, distribution and excretion of (-)-epicatechin: A review of recent findings, Mol. Aspects Med., 2018, 61, 18–30 CrossRef PubMed.
- S. Bhagwat, D. B. Haytowitz, S. I. Wasswa-Kintu and J. M. Holden, USDA develops a database for flavonoids to assess dietary intakes, in 36th National Nutrient Databank Conference, ed. P. Stumbo and S. McNutt, Elsevier Science Bv, Amsterdam, 2013, vol. 2, pp. 81–86 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |