Open Access Article
Open Access ArticleIn vitro gastrointestinal digestion of marine oil emulsions and liposomal solutions: fate of LC-PUFAs upon lipolysis
Sawsan
Amara
*a,
Maureen
Gerlei
b,
Carole
Jeandel
b,
Moulay
Sahaka
c,
Frédéric
Carrière
 c and
Michel
Linder
c and
Michel
Linder
 *b
*b
aLipolytech: Zone Luminy Biotech, 163 avenue de Luminy, 13288 Marseille Cedex 09, France. E-mail: sawsan.amara@lipolytech.com
bUniversité de Lorraine, LIBio, F-54000 Nancy, France. E-mail: maureen.gerlei@univ-lorraine.fr; carole.jeandel@univ-lorraine.fr; michel.linder@univ-lorraine.fr
cAix Marseille Univ, CNRS, UMR7281 Bioénergétique et Ingénierie des Protéines, 31 Chemin Joseph Aiguier, 13009 Marseille, France. E-mail: sahakamoulay@gmail.com; carriere@imm.cnrs.fr
First published on 30th October 2024
Abstract
The bioaccessibility and bioavailability of dietary fatty acids depend on the lipid to which they are esterified, the organisation of theses lipids in water and their recognition by lipolytic enzymes. In this work, we studied the release of marine long-chain polyunsaturated fatty acids (LC-PUFA), depending on their presentation either in the form of phospholipids (PL) or triacylglycerol (TAG). Two formulations based on marine PL or TAG extracted from salmon heads (Salmo salar) were prepared. Lipolysis was first tested in vitro by using individual gastrointestinal lipases and phospholipases to identify the enzymes involved in the digestion. Second, the lipolysis of the prepared formulations by a combination of enzymes was tested under in vitro conditions mimicking the physiological conditions found in the GI tract, both in the stomach and in the upper small intestine, in order to evaluate digestibility of TAG and LC-PUFA-containing liposomes. The in vitro results showed that TAG emulsion was hydrolyzed by porcine pancreatic extracts (PPE) and pure pancreatic lipase (PPL) with its cofactor, colipase, and to a lesser extent by pancreatic-lipase-related protein 2 (PLRP2) and a gastric extract (RGE) containing gastric lipase while no hydrolysis was observed with purified pancreatic phospholipase A2 (PLA2) and carboxyl ester hydrolase (CEH). The PL substrate was found to be hydrolysed by PLA2, PPE and PLRP2. Their phospholipase activities on liposomes formulation was dependent on the presence of bile salts. Using a two-step in vitro digestion model, we measured the kinetics of fatty acid release from TAG and PL during the gastric and intestinal phases of digestion. The highest overall lipolysis level was obtained with liposomes (around 75%) during the intestinal phase while they were preserved during the gastric phase. The overall lipolysis level of TAG emulsion was lower (around 33%), while it started already in the gastric phase. In conclusion, liposomes appear as a better delivery system for intestinal absorption of LC-PUFA than TAG. In addition, their resistance to lipolysis under gastric condition can protect LC-PUFA and provide a gastric stable delivery system for other molecules.
1. Introduction
The global production of seafood and aquaculture, as reported by the FAO (2024),1 exceeded 223 million tons in 2022. With seafood consumption continuing to grow (from 9.9 kg in the sixties to 20.6 kg in 2022, with an estimated consumption of 21.3 kg per capita in 2032), it is necessary to maximize the valorization of by-products, which represent 40 to 60% w/w of the resource,2 in a circular economy approach.3 For marine oils by-products, classical extraction methods with conventional or green solvents can be used4,5 complemented by unconventional processes such as microwave-assisted,6 ultra-high pressure,7 electric field,8,9 or supercritical carbon dioxide10,11 extractions. Moreover, the enzymatic processing of lipid extraction from fish tissues by proteases has long been utilized in the valorization of by-products such as fish and squid eggs, krill, heads, skin, backbones, and cartilages.12–15 These lipids represent a reservoir of long-chain polyunsaturated fatty acids (LC-PUFA), including docosahexaenoic acid (DHA; C22:6n-3), eicosapentaenoic acid (EPA; C20:5n-3), and docosapentaenoic acid (DPA; C22:5n-3). Global health authorities widely recommend consuming fish 2 times per week or 500 mg LC-PUFAs per day.16 Indeed, they play important roles in promoting health and are recognized for their ability to mitigate risk factors linked to the onset of diverse disorders, including cardiovascular and neurological diseases, inflammation, hypertension and various types of cancer.17 Studies comparing the compositions of oil fish by-products (head, skin) highlight that the neutral lipid fraction is rich in saturated and monounsaturated fatty acids, while the polar lipids are rich in LC-PUFAs.3 These are generally esterified predominantly at the sn-2 position in marine triacylglycerols (TAG) and PL and are mainly found in phospholipids.17,18 These amphipathic molecules have been widely used to prepare liposomal formulations of topical, oral, and parenteral drug delivery systems, enhancing membrane permeability and the bioavailability of active compounds.5 They found various in vivo and in vitro applications19 and have the dual functionality of delivering LC-PUFAs present in the liposome bilayer and lipophilic or hydrophilic molecules of interest, or even both simultaneously.19 Although liposomes are classical delivery systems,19,20 their use as oral delivery systems is limited by their instability in the gastrointestinal (GI) tract.21 Their physical stability can be affected by GI factors like the low pH of the gastric juice, the presence of bile salts that might dissolve the liposome phospholipids and the enzymatic hydrolysis resulting in their breakage and release of the loaded molecules in the GI.22 Recently, more concern was given to the liposome behaviour through the gastrointestinal digestion and its stability towards the digestive fluids. In these studies, the stability of liposomes was investigated at the physical–chemical level, using technics that focus on the liposome integrity with the analysis of microstructure by atomic force microscopy (AFM), size and ζ-potential by dynamic light scattering (DLS) and microelectrophoresis, and the release of bioactive molecules.23,24 However, no study on the fate of marine PL upon digestion in the GI tract have been reported.The main goal of the present study was to explore the bioaccessibility of marine LC-PUFA depending on their presentation either in the form of phospholipids (PL) or triacylglycerols (TAG) extracted from salmon heads. A TAG emulsion and a liposomal solution both rich in LC-PUFAs were submitted to digestion by various lipases either tested individually or present in an in vitro digestion model including gastric and intestinal steps and relevant enzymes.25 This study provides information on the fate of LC-PUFAs, such as DHA and EPA, depending on the formulation, as well as on the preservation of vector integrity during digestion.
2. Materials and methods
2.1 Material
Fresh salmon (Salmo salar) heads were obtained from a local plant and stored at −20 °C. They were thawed at 4 °C overnight before use.A controlled enzymatic hydrolysis of salmon heads was performed using Alcalase® 2.4 L and the pH-stat technique as previously described by Gbogouri et al.12 The different hydrolysates, oil, and the pellet containing the heaviest protein fraction were separated by centrifugation at 9000 rpm for 20 min at 4 °C using a Beckman Coulter® Rotor J-10 centrifuge (Beckman Coulter, Brea, CA, USA). Salmon phospholipids were purified through acetone precipitation with the purpose to remove proteins and residual TAGs as described by Lu et al.26
Sodium taurodeoxycholate (NaTDC; T0557), MES hydrate, chloroform, methanol and acetone were obtained from Sigma-Aldrich. Butylated hydroxytoluene (BHT), copper acetate, PLC Silica gel 60 (20 × 20 cm, 2 mm) and TLC Silica gel 60 (10 × 20 cm, 0.2 mm) plates, n-heptane, hexane, diethyl ether, formic acid, ortho-phosphoric acid, sodium chloride (NaCl), calcium chloride (CaCl2), boron trifluoride-methanol solution (BF3) and ammoniac were purchased from Merck.
Lipid standards including triolein (TO), diolein (DO), monoolein (MO), oleic acid (OA), dioleylphosphatidylcholine (PC), dioleylphosphatidylethanolamine (PE), dioleylphosphatidylinosiltol (PI) and dioleylphosphatidylserine (PS) were obtained from Avanti Polar Lipids INC (Alabaster, Alabama, USA). Individual fatty acids were identified using Standard mixtures (PUFA1 from a marine source and PUFA2 from a vegetable source; Supelco, Sigma-Aldrich, Bellefonte, PA, USA).
2.2 Enzyme sources
Alcalase® 2.4 L (EC.3.4.21.14a) was supllied by Novozymes A/S (Bagsvaerd, Danemark). Porcine pancreatic extract (PPE, 8× USP – P7545) and phospholipase A2 (PLA2) from bovine pancreas (≥20 units per mg – P8913) were supplied from Merck-Sigma-Aldrich. Rabbit gastric extract (RGE15, 15 U mg−1), purified porcine pancreatic lipase (PPL, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 U mg−1), Guinea pig pancreatic lipase related protein 2 (GPLRP2, 2700 U mg−1) and human carboxyl ester hydrolase (CEH, 300 U mg−1) were from Lipolytech (Marseille, France).
200 U mg−1), Guinea pig pancreatic lipase related protein 2 (GPLRP2, 2700 U mg−1) and human carboxyl ester hydrolase (CEH, 300 U mg−1) were from Lipolytech (Marseille, France).
2.3 Lipid formulations preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) was prepared by mixing 3 g of fish oil in 100 mL of either 10 mM MES buffer (for pH 5.5 and 6.0) or 10 mM Tris-HCl (for pH 8.0), 150 mM NaCl, 10 mM CaCl2 and 4 mM NaTDC at different pH values (pH 5.5 for incubation with gastric enzymes and pH 6.0 or 8.0 for pancreatic enzymes). These solutions contain salts that are classically used for the assay of digestive lipase.27 150 mM NaCl is a physiological concentration. 10 mM CaCl2 is a high concentration that allows providing calcium in large excess as a cofactor for some lipolytic enzymes like phospholipases. Finally, 4 mM NaTDC corresponds to a supramicellar concentration of a bile salt well represented in human duodenal contents.28 It is lower than the concentration (10 mM) recommended in standardized conditions for in vitro digestion assays (Brodkorb, et al. (2019)),25 but it still fits with concentrations found in the small intestine.28 The bile salt NaTDC was used as the emulsifier because we did not want to use compounds that could be substrates for lipolytic enzymes (lecithin, partial acylglycerols) or interfere with lipolysis (surfactant like Twen 8029 and proteins30). NaTDC was selected as bile salt because it can be used in a large pH range and does not precipitate at acidic pH. The mixture was subsequently subjected to ultrasonic treatment for 4 min (Sonicator Vibra-Cell 75115 with the parameters set at 40% intensity; 1 s on and 1 s off).
v) was prepared by mixing 3 g of fish oil in 100 mL of either 10 mM MES buffer (for pH 5.5 and 6.0) or 10 mM Tris-HCl (for pH 8.0), 150 mM NaCl, 10 mM CaCl2 and 4 mM NaTDC at different pH values (pH 5.5 for incubation with gastric enzymes and pH 6.0 or 8.0 for pancreatic enzymes). These solutions contain salts that are classically used for the assay of digestive lipase.27 150 mM NaCl is a physiological concentration. 10 mM CaCl2 is a high concentration that allows providing calcium in large excess as a cofactor for some lipolytic enzymes like phospholipases. Finally, 4 mM NaTDC corresponds to a supramicellar concentration of a bile salt well represented in human duodenal contents.28 It is lower than the concentration (10 mM) recommended in standardized conditions for in vitro digestion assays (Brodkorb, et al. (2019)),25 but it still fits with concentrations found in the small intestine.28 The bile salt NaTDC was used as the emulsifier because we did not want to use compounds that could be substrates for lipolytic enzymes (lecithin, partial acylglycerols) or interfere with lipolysis (surfactant like Twen 8029 and proteins30). NaTDC was selected as bile salt because it can be used in a large pH range and does not precipitate at acidic pH. The mixture was subsequently subjected to ultrasonic treatment for 4 min (Sonicator Vibra-Cell 75115 with the parameters set at 40% intensity; 1 s on and 1 s off).
A characterization of the emulsion was made by DLS analysis for particle size determination and gas chromatography (GC) for fatty acid composition.
A characterization of the liposomes was made by DLS analysis for particle size determination and GC for fatty acid composition. TEM studies on similar liposomes showing their morphology have been reported previously.31,32
2.4 Dynamic light scattering analysis of TAG emulsion and PL liposomes
DLS analysis of TAG – NaTDC emulsion, and PL liposome ± NaTDC dispersions were carried out using a Zetasizer Nano S (Malvern Instruments) at 37 °C. Each measurement was performed in triplicate and consisted in 10–15 runs of 10 s at a scattering angle of 173. The determination of the hydrodynamic diameter (DH) was based on the Einstein-Stokes relation to obtain the intensity-averaged size distribution. A viscosity of 0.736 cP and a refractive index of 1.3335 (at 37 °C) were used for the dispersion medium, while a value of 1.49 was used as an approximation of the refractive index for lipids.332.5 Hydrolysis of TAG and PL formulations by individual enzymes
1 mL of substrate (TAG emulsion or PL liposome dispersions with or without bile salts), in a 15 mL glass tube with screw-cap, was first incubated in a water bath at 37 °C for 3–5 minutes to stabilize the temperature at 37 °C. Subsequently, 20 μg mL−1 enzymes (or enzyme extract) were added, and the mixture incubated for 60 minutes at 37 °C and under low rotative stirring (Stuart rotator SB-3 Cole-Parmer Bibby scientific UK). After 1 hour of incubation, the reaction was stopped by adding 200 μL of a 1 N HCl solution and samples were freeze-dried (CHRIST, Beta 1–8 LD plus, Grosseron – France) for lipid extraction. The experiments were performed in triplicates for each enzyme (PLA2, PPE, PPL + colipase, GPLRP2, CEH and RGE) and pH tested.For each substrate, a blank without enzyme was also incubated under the same conditions.
2.6 Two-step in vitro digestion
A two-step static in vitro digestion model including relevant gastric and a duodenal phases was adapted from the standardized INFOGEST 2.0 protocol25 with some changes regarding the pH values and the use of purified bile salts instead of complete bile. Fifteen mL of substrate dispersion (emulsion in 10 mM MES, 50 mM NaCl, 0.15 mM CaCl2 and, 4 mM NaTDC or liposomes in 10 mM MES, 50 mM NaCl, 0.15 mM CaCl2) were first mechanically stirred in a temperature-controlled (37 °C) reaction vessel of a pH-stat apparatus (Titrando 902, Metrohm, France) and the pH endpoint was adjusted to 5.5. The gastric phase starts by adding 3 mL of a freshly prepared rabbit gastric extract (RGE15; 1.3% w/w GL) solution to the reaction vessel to obtain a final concentration of 60 U ml−1 gastric lipase (using tributyrin as substrate34) and 2000 U ml−1 pepsin. The pH was kept constant at 5.5 during 30 min reproducing the conditions (lipase concentration and pH) found in the stomach at half-gastric emptying of a test meal,35via the automated titration of free fatty acids (FFAs) with 0.1 M NaOH using the pH-stat device.At t = 30 min, a PPE/bile salts solution was added to the digestion mixture at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v and the pH endpoint was shifted to 6.25 and kept constant for 60 min reproducing the conditions (lipase concentration and pH) found in the upper small intestine at half-gastric emptying of a test meal.35 After adding the PPE/bile salt solution to the reaction vessel, the final pancreatic lipase concentration was 2000 U mL−1 (using tributyrin as substrate34), the final bile salt concentration was 10 mM, CaCl2 0.6 mM and the gastric phase was diluted 2-fold. One mL samples were collected at various times (0, 15, 29, 35, 40, 45, 60 and 90 min), immediately acidified with 200 μL 1 N NaOH for the extraction of residual substrate and lipolysis products and subsequent analysis by TLC, TLC-FID and GC-FID.
1 v/v and the pH endpoint was shifted to 6.25 and kept constant for 60 min reproducing the conditions (lipase concentration and pH) found in the upper small intestine at half-gastric emptying of a test meal.35 After adding the PPE/bile salt solution to the reaction vessel, the final pancreatic lipase concentration was 2000 U mL−1 (using tributyrin as substrate34), the final bile salt concentration was 10 mM, CaCl2 0.6 mM and the gastric phase was diluted 2-fold. One mL samples were collected at various times (0, 15, 29, 35, 40, 45, 60 and 90 min), immediately acidified with 200 μL 1 N NaOH for the extraction of residual substrate and lipolysis products and subsequent analysis by TLC, TLC-FID and GC-FID.
2.7 Lipid extraction and analysis by thin-layer chromatography
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), 1 mM BHT in a 15 mL glass tube with a screw-cap. After phase separation, the lower organic phase was collected using a Pasteur pipette, transferred into a 8 mL vial with a screw-cap and the vial was kept at −20 °C until TLC analysis were performed. BHT was added to prevent lipid oxidation upon storage and when the organic solvent was evaporated for further analysis.
1 v/v), 1 mM BHT in a 15 mL glass tube with a screw-cap. After phase separation, the lower organic phase was collected using a Pasteur pipette, transferred into a 8 mL vial with a screw-cap and the vial was kept at −20 °C until TLC analysis were performed. BHT was added to prevent lipid oxidation upon storage and when the organic solvent was evaporated for further analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v/v) for the migration of neutral lipids or chloroform/methanol/H2O (65
1 v/v/v) for the migration of neutral lipids or chloroform/methanol/H2O (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v/v) for the migration of polar lipids. Following chromatography, the plates were dried at room temperature (air-conditioning at 22 °C) for 5 min and then sprayed with a cupric acetate/ortho-phosphoric acid solution, prepared by mixing a saturated aqueous solution of cupric acetate with 85% ortho-phosphoric acid in a 1-to-1 volume ratio. The plates were then placed in an oven to ensure heating at 180 °C for 5 min.
4 v/v/v) for the migration of polar lipids. Following chromatography, the plates were dried at room temperature (air-conditioning at 22 °C) for 5 min and then sprayed with a cupric acetate/ortho-phosphoric acid solution, prepared by mixing a saturated aqueous solution of cupric acetate with 85% ortho-phosphoric acid in a 1-to-1 volume ratio. The plates were then placed in an oven to ensure heating at 180 °C for 5 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 v/v/v) for the migration of neutral lipids or chloroform/methanol/ammoniac (65
0.2 v/v/v) for the migration of neutral lipids or chloroform/methanol/ammoniac (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v/v) for the migration of polar lipids. The Chromarods™ were then dried at 100 °C for 1 min (Rod dryer TK8, Iatron Laboratories) and transferred to the Iatroscan MK5 to be scanned using FID for detection and quantification of the compounds separated on the silica. The amounts of each individual lipolysis product were estimated at each time point from the calibration curves established with pure lipid standards. TAG emulsion lipolysis level was estimated from the quantification of residual TAG, FFA, DAG and MAG by TLC-FID. Mass amounts were converted into μmoles using mean molecular masses of 895 g mol−1 for TAG, 627.3 g mol−1, for DAG, 359.7 g mol−1 for MAG and 285.7 g mol−1 for FFA. PL liposomes lipolysis level was estimated from the quantification of residual PC, PE and FFA by TLC-FID. Mass amounts were converted into μmoles using mean molecular masses of 802.4 g mol−1 for PC, 759.5 g mol−1 for PE and 290.7 g mol−1 for FFA.
5 v/v/v) for the migration of polar lipids. The Chromarods™ were then dried at 100 °C for 1 min (Rod dryer TK8, Iatron Laboratories) and transferred to the Iatroscan MK5 to be scanned using FID for detection and quantification of the compounds separated on the silica. The amounts of each individual lipolysis product were estimated at each time point from the calibration curves established with pure lipid standards. TAG emulsion lipolysis level was estimated from the quantification of residual TAG, FFA, DAG and MAG by TLC-FID. Mass amounts were converted into μmoles using mean molecular masses of 895 g mol−1 for TAG, 627.3 g mol−1, for DAG, 359.7 g mol−1 for MAG and 285.7 g mol−1 for FFA. PL liposomes lipolysis level was estimated from the quantification of residual PC, PE and FFA by TLC-FID. Mass amounts were converted into μmoles using mean molecular masses of 802.4 g mol−1 for PC, 759.5 g mol−1 for PE and 290.7 g mol−1 for FFA.
These molecular masses were estimated from the fatty acid composition of the oil and the PL used for making the TAG emulsions and the liposomes, respectively (Table 1). Lipolysis levels were expressed as FFA% versus total fatty acids esterified in the TAG emulsions or PL liposomes. The time-course variations in the amounts of each molecular specie was plotted as a function of time.
| Fatty acid | % of total fatty acids | |
|---|---|---|
| TAG | PL | |
| a ALA: α-linoleic acid; ARA: arachidonic acid; EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid. | ||
| Saturated FA | ||
| C14:0 | 4.11 ± 0.04 | 2.45 ± 0.02 |
| C15:0 | 0.30 ± 0.01 | 0.81 ± 0.03 |
| C16:0 | 12.48 ± 0.06 | 21.22 ± 0.09 |
| C17:0 | 0.21 ± 0.01 | 0.67 ± 0.01 |
| C18:0 | 2.78 ± 0.02 | 8.64 ± 0.03 |
| C20:0 | 0.11 ± 0.11 | 0.17 ± 0.12 |
| C22:0 | 4.83 ± 0.01 | 1.83 ± 0.02 |
| Σ Saturated FA | 24.82 ± 0.19 | 35.79 ± 0.33 |
| Monounsaturated FA | ||
| C16:1n-7 | 4.28 ± 0.04 | 2.24 ± 0.03 |
| C18:1n-9 | 31.88 ± 0.07 | 17.19 ± 0.14 |
| C18:1n-7 | 2.95 ± 0.01 | 2.79 ± 0.02 |
| C20:1n-9 | 1.16 ± 0.01 | 0.38 ± 0.27 |
| C22:1n-9 | 1.17 ± 0.01 | 0.55 ± 0.01 |
| Polyunsaturated FA | ||
| C18:2n-6 | 10.94 ± 0.08 | 3.51 ± 0.03 |
| C18:3n-3 (ALA)a | 5.71 ± 0.24 | 2.54 ± 0.02 |
| C18:4n-3 | 3.57 ± 0.15 | 1.29 ± 0.01 |
| C20:4n-6 (ARA)a | 0.14 ± 0.14 | 2.73 ± 0.02 |
| C22:4n-6 | <0.1 | 2.71 ± 0.06 |
| C20:5n-3 (EPA)a | 5.03 ± 0.05 | 6.12 ± 0.02 |
| C22:5n-3 (DPA)a | 1.89 ± 0.02 | 2.18 ± 0.01 |
| C22:6n-3 (DHA)a | 6.46 ± 0.07 | 19.99 ± 0.01 |
| Σ Unsaturated FA | 75.18 ± 1.47 | 64.21 ± 0.18 |
| Σ n-3 FA | 22.66 ± 0.53 | 32.03 ± 0.07 |
2.8 Fatty acid composition determination by gas chromatography (GC)
Fatty acids present in the initial substrate (TAG or PL at 10 mg mL−1) were derivatized into fatty acid methyl esters (FAME) according to the Smith and Morisson method,36 using 2 mL of BF3 in methanol. The mixtures were heated at 100 °C for 1 h. After cooling, the FAME were extracted with 2 mL of heptane. Samples were shaken, and the upper heptane layer was removed and stored at −20 °C before GC analysis.For the characterization of FFAs released upon the hydrolysis of the TAG emulsion or PL liposomes, 500 μL of each total lipid extract were applied as 10 cm bands onto preparative TLC plates, with a 2 mm silica layer. Following elution and lipids separation, the plates were dried at room temperature for 10 min, the bands corresponding to the various lipid fractions were located using a staining with iodine restricted to the edge of the plate/band. Each band was then scraped separately from the TLC plate and transferred into a 15 mL glass tube equipped with a Teflon-lined screw cap and containing 2 mL of a chloroform/methanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After mixing vigorously, and centrifugation at 5000 rpm (Thermofisher Scientific laboratory centrifuge 75007214, Germany) for 10 min, the organic phase was transferred into another tube, the extraction of lipids from silica was repeated twice using 2 mL of acetone and the pooled organic phases were taken to dryness under nitrogen stream. All samples were then derivatized into FAME using methanol-BF3. After extraction with heptane, the volume of each FAME sample was then reduced to 50 μL under a stream of nitrogen for concentration in a vial equipped with 250 μL pulled point conical glass insert (Agilent Technologies, Germany).
1, v/v). After mixing vigorously, and centrifugation at 5000 rpm (Thermofisher Scientific laboratory centrifuge 75007214, Germany) for 10 min, the organic phase was transferred into another tube, the extraction of lipids from silica was repeated twice using 2 mL of acetone and the pooled organic phases were taken to dryness under nitrogen stream. All samples were then derivatized into FAME using methanol-BF3. After extraction with heptane, the volume of each FAME sample was then reduced to 50 μL under a stream of nitrogen for concentration in a vial equipped with 250 μL pulled point conical glass insert (Agilent Technologies, Germany).
The FAME were subsequently analysed by GC on a Shimadzu GC-2010 equipped with a flame-ionization detector (FID) and a SPTM2380 Supelco capillary column (60 m; 0.2 mm internal diameter × 0.25 μm film thickness (Bellefonte, PA, USA)). The oven temperature was set at 200 °C. The detector and injector were at a temperature of 250 °C and nitrogen was used as a carrier gas with a flow rate of 0.79 mL min−1. The oven temperature program used during the run was 120 °C for 2 minutes and then climb to 180 °C for 2 minutes followed by a rise to 220 °C for 25 minutes. The identification of fatty acids was carried out by co-injection of standard compounds (C23 as internal standard and PUFA 1, PUFA2).
3. Results and discussion
3.1. Lipid classes and fatty acid composition determination
3.1.2.1. Fatty acid composition of TAG. The GC analysis results showed that oleic acid (C18:1) was the main fatty acid found in fish oil TAG in which it represents 34.83% w/w of total FA, followed by palmitic (C16:0) and linoleic (C18:2) acids (12.48% and 10.94% of total FA, respectively) (Table 1). As expected, the fish oil showed a higher level of unsaturated fatty acid (75.18% of total FA) compared to saturated FA (24.82% of total FA) with a high level of omega 3 fatty acids accounting for 22.66% of total fatty acids, with EPA (C20:5n-3) and DHA (C22:6n-3) representing 11.49% of total FA.
3.1.2.2. Fatty acid composition of salmon PL. The GC analysis of the FAME from purified salmon PL showed that palmitic acid (C16:0), DHA (C22:6n-3) and oleic acid (C18:1) were the main fatty acids representing 21.22%, 19.99% and 19.98% of total FA, respectively (Table 1). Similarly, to the oil fish TAG, the PL showed a higher level of unsaturated fatty acids (64.21% of total FA) compared to saturated FA (35.79% of total FA). However, the omega 3 fatty acids level (32.03%) was higher in PL than TAG, with a higher EPA (C20:5n-3) and DHA (C22:6n-3) levels (26.11% of total FAs), i.e. more than the double of their proportion in TAG.
3.2. Characterization of TAG and PL dispersions by DLS
The TAG emulsion and liposomes were prepared by dispersing the purified marine TAG or PL in buffer followed by the ultrasonic treatment. For the TAG emulsion, the presence of the bile salt NaTDC, was necessary to make the oil in water dispersion, while liposome dispersion was performed in buffer with or without NaTDC.The particle size distribution of TAG emulsion and liposomes obtained under these conditions were characterized by DLS. For TAG emulsion, average particle sizes of 154.5 ± 1.2 nm, 138.3 ± 1.5 nm and 163.7 ± 1.3 were determined at pH 5.5, 6.0 and 8.0, respectively (Table 2). Liposome dispersions were found to organize in particles of similar size in the absence of bile salt, but with a higher polydispersity than the TAG emulsion. Their average size was found to be slightly affected by the presence of bile salts (Table 2), with a shift from 143–153 nm in absence of bile salts to a smaller average particle size ranging from 108 to 112 nm in presence of bile salts. This suggests that phospholipids organized in smaller size vesicles in the presence of bile salts, or that some phospholipids formed smaller mixed micelles with bile salts, leading to a lower average particle size. The increase in polydispersity (Table 2) supports this hypothesis. The dispersion average size of both TAG emulsion and PL liposomes was found to be slightly affected by the pH (Table 2).
| Emulsion | Liposomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mM NaTDC | 10 mM NaTDC | ||||||||
| pH | 5.5 | 6.0 | 8.0 | 5.5 | 6.0 | 8.0 | 5.5 | 6.0 | 8.0 |
| Size (nm) | 154.5 ± 1.2 | 138.3 ± 1.5 | 163.7 ± 1.3 | 150.8 ± 19.2 | 153.5 ± 12.8 | 143.9 ± 6.2 | 108.4 ± 4.2 | 104.9 ± 9.6 | 112.7 ± 12.7 |
| Pdi | 0.08 | 0.07 | 0.04 | 0.25 | 0.25 | 0.28 | 0.39 | 0.39 | 0.39 |
3.3. In vitro digestion of marine TAG emulsion and PL liposome by individual gastrointestinal lipases
Preliminary lipolysis experiments with various gastrointestinal lipases were performed at different pH, to determine which lipolytic enzymes could act on each substrate formulation and contribute to the whole digestion process. Lipolysis products were then analysed by TLC.With PPE and in absence of bile salts (Fig. 2A and B, lane 8), several bands corresponding to PE and PC totally disappeared but no band corresponding to FFA were detected. As previously proposed regarding the lipolysis of TAG and the absence of a detectable FFA band, these results suggest a preferential release of saturated FA by PPE, which are preferentially esterified in the sn-1 position of the phospholipids. This hypothesis is further supported by the analysis of the FFA released upon digestion (see section 3.4.2. and Fig. 5) and it is consistent with the presence of the PLA1 activity in the PPE.45 In the case of PLA2, specifically releasing FA from the sn-2 position, FFA should be enriched in LC-PUFA and thus well stained using the copper acetate-phosphoric acid reagent preferentially acting on double bounds. However, with PPE, an intense band that migrates just below the front appears on the TLC plate (Fig. 2A and B, lane 8). Since phospholipids and their lipolysis products are polar and migrate in the lower part of the plate, it seems that some apolar compounds are either generated during the reaction or, more probably, are originating from PPE. This hypothesis remains to be confirmed but it is known that PPE contains some residual fat (TAG and lipolysis products) from the pancreas.45 Interestingly, GPLRP2 was found to hydrolyse liposomes since bands corresponding to FFA appeared and bands corresponding to PC and PE substrates disappeared (Fig. 2, line 11). This finding is unexpected since GPLRP2 poorly interacts with PC liposomes and preferentially acts on mixed PC-NaTDC micelles.46,47 Nevertheless, it has been shown that GPLRP2 can act on heterogenous biomimetic membranes48 and, as indicated before, the presence of negatively charged phospholipids in marine PL might favour the interaction of GPLRP2 with the liposome membrane. However, and as expected, the activity of GPLRP2 was found to be higher in presence of bile salts (Fig. 2C and D, line 11) and at pH 8.0 (Fig. 2B and D, line 11). This finding is consistent with the substrate preference of PLRP2 for micellar substrates.41,48,49 Interestingly, DLS analysis indicated that the liposomes structure was not drastically disturbed by bile salts, according to their particle size that was only slightly reduced on average. These findings suggest that either PLRP2 is able to interact with these liposomes and hydrolyse their phospholipids or the activity observed results from the action of GPLRP2 on PL-bile salts mixed micelles co-existing with liposomes.
Regarding CEH, no action of this enzyme on liposomes was observed whatever the bile salt and pH conditions tested (Fig. 2, line 10), although it is known to display some phospholipase A1 activity.49 Similarly, no activity was observed with both PPL and RGE, what is consistent with their lipase activity and high specificity for TAG substrates.
3.4. Two-step in vitro digestion of TAG and PL substrates under simulated physiological conditions
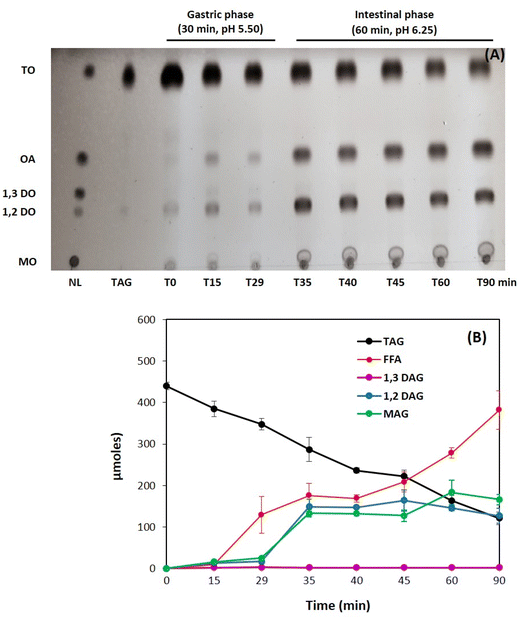
The experimental conditions adopted for the in vitro digestion25,50 were based on in vivo studies and parameters measured at 50% meal gastric emptying.34,35,51 The chosen pH values, 5.50 for the gastric phase and 6.25 for the intestinal phase, differ from those recommended by the INFOGEST static in vitro digestion model. However, they better fit with the optimum activity of digestive lipases. For the gastric phase, rabbit gastric extract (RGE) was chosen as the source of gastric enzymes because it contains pepsin and gastric lipase and the activity of rabbit gastric lipase has a similar range of activity as the human gastric lipase (HGL).52 Porcine Pancreatic Extract (PPE) was used as the source of pancreatic enzymes for the intestinal phase of in vitro digestion. PPE consists of a mixture of digestive enzymes produced by the exocrine cells of the porcine pancreas and contains trypsin, chymotrypsin, α-amylase, and all the lipolytic enzymes required for lipid digestion including pancreatic lipase and its cofactor colipase, pancreatic phospholipase A2, PLRP2 and CEH/BSSL.45 PPE is commonly used for in vitro digestion studies as a substitute of human pancreatic enzymes.25,50When pancreatic enzymes were added at 30 min to initiate the intestinal phase of digestion, a clear jump in the release of fatty acids, 1,2-DAG and MAG was observed while TAG decreased at a higher rate (Fig. 3A and B). During the first 10 min of the intestinal phase, TAG decreased by around 46% as determined by TLC-FID quantification (Fig. 3B). It is worth noticing that the levels of 1,3-DAG did not increase during the lipolysis experiment, suggesting that either they were not formed or were rapidly hydrolyzed and converted in MAG.53 Both 1,2(2,3)-DAG and MAG were found to accumulate and reached a plateau after 35 min. Since FFA still increased while TAG decreased, especially after 45 min (Fig. 3A and B), these results suggest that some MAG are further converted into glycerol (not measured). At the end of the duodenal phase of digestion (t = 90 min), the hydrolysis level reached 32.8 ± 1.2% (Fig. 4) whereas lipolysis levels of emulsions usually exceed 50% after 90 min under these conditions.29,54 The level of absorbable fatty acids is around 46% if we consider the sum of FFA and MAG versus total fatty acids.
In these experiments NaTDC was used as the unique bile salt source in the intestinal phase of the in vitro digestion, whereas bovine bile was recommended in the standardized INFOGEST protocol (Ref Brodkord). Our choice was justified by the presence of endogenous PC and PE in bovine bile which can count up to 22% (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w).55 Thus, these phospholipids might have interfered with the digestion of the PC and PE present in the liposome substrate. The aim of this study being a comparative study of liposome digestibility vs. emulsion, it was necessary to keep the same experimental conditions during in vitro digestion. The use of NaTDC as bile salts (and no other emulsifier) might explain the lower salmon oil TAG lipolysis compared to other oils and emulsions.29
w).55 Thus, these phospholipids might have interfered with the digestion of the PC and PE present in the liposome substrate. The aim of this study being a comparative study of liposome digestibility vs. emulsion, it was necessary to keep the same experimental conditions during in vitro digestion. The use of NaTDC as bile salts (and no other emulsifier) might explain the lower salmon oil TAG lipolysis compared to other oils and emulsions.29
The composition of the fatty acids released upon digestion of emulsion was determined by GC after purification from silica plate. The results showed that C14:0 was released at the end of digestion (67.11 ± 5.93% of total FFA), followed by the long chain FFA C18:0 (13.27 ± 2.08% of total FFA) (Fig. 5). These results suggest that these FAs are present at the sn-1 or sn-3 positions of the TAG substrate which are preferentially hydrolyzed by pancreatic lipase. These findings are in accordance with previous studies that have showed that LC-PUFA are mainly esterified at the sn-2 position of TAG.56 Knowing the contents of C14:0 (4.11 ± 0.04% w/w) and C18:0 (2.78 ± 0.02% w/w) in TAG (Table 1) and the fact that they are not the main fatty acids, their high levels in FFA is constituent with a low lipolysis level of TAG with a preferential release of saturated FAs at the sn-1 or sn-3 positions of the TAG. Polyunsaturated fatty acids C16:1 and C18:2n-6 were also released at the end of digestion (Fig. 5), but at lower levels (2.97 ± 0.34% and 4.07 ± 0.52%, respectively). The total polyunsaturated FFA released at the end of the in vitro digestion of TAG emulsion is calculated to be around 11% of total FFAs released (Fig. 5).
Thus, we have shown that liposomes are resistant to gastric conditions and are largely digested when they reached the upper small intestine conditions. With such a high level of phospholipid hydrolysis, the probability that liposome structure still exists is very low. Therefore, these liposomes appear as a perfect vehicle for delivering hydrophilic molecules in the small intestine while preserving them from gastric environment (Fig. 7).
The composition of the FFA liberated upon digestion of PL liposomes was determined by GC after purification from silica plate. The results showed that C16:0 was the main FFA released at the end of digestion (55.53 ± 0.23% of total FFA released), which is consistent with its high proportion in PL fatty acids (21.22 ± 0.09% w/w; Table 1) followed by C14:0 (29.20 ± 6.62 of total FFA released) and C18:0 (6.58 ± 1.72% of total FFA released) (Fig. 5). Since these FA are mainly present at the sn-1 position of the fish PL substrate,58 the results indicate that the phospholipid hydrolysis occurring during the intestinal phase involves at least a phospholipase A1 activity. Therefore, PLRP2 and not pancreatic PLA2 seems to be the most important enzyme from PPE involved. Indeed, PLRP2 is a phospholipase A159 and the phospholipase activity of GPLRP2 on PL liposomes was shown here (Fig. 2 line 11).
In contrast to the digestion of TAG, significant levels of C18:3n-3 and C22:5n-3 were released from liposomes phospholipids (Fig. 5), in accordance with the PLA2 activity present in the PPE (Fig. 3, lane 7) and the distribution of these FA at the sn-2 position of marine PL.3,18 Nevertheless, the total polyunsaturated FFA released at the end of the in vitro digestion of liposomes (around 9% of total FFA released) was similar to that calculated for TAG emulsion (around 11% of total FFA released (Fig. 5)). No EPA and DHA were observed. Since these fatty acids are mainly located at the sn-2 position,58 this results suggest the low phospholipase A2 activity level in the PPE. Besides, the low phospholipase A2 activity obtained in these conditions (intestinal phase contains 10 mM NaTDC and performed at pH 6.25) fits with the individual activity of PLA2 on PL liposomes in presence of bile salts and at pH 6.0 compared to same results obtained in absence of bile salts and at pH 8.0 (Fig. 2 line 7).
We have seen that, in the case of TAG, the absence of EPA and DHA may simply results from a low lipolysis level and the preservation of the ester bond at the sn-2 position of TAG where most LC-PUFA are located. Aursand et al.56 used 13C NMR to study positional distribution of fatty acids in TAG in salmon oil and found the majority of DHA in the sn-2 position while EPA was more randomly distributed on all three positions. In the case of phospholipids, LC-PUFA can remain esterified at the sn-2 position of lysophospholipids after the removal of the fatty acid at the sn-1 position. Lysophospholipids were however not quantified in this study nor their FA analysed.
Previous studies have shown that EPA and DHA present at the sn-2 positions are more readily absorbed compared to the structured lipids in which these fatty acids are in one of the other positions in the molecule.18 It has been also shown that lysophospholipids with DHA at the sn-2 position were preferentially absorbed and more efficiently transported to the brain by the acetyl, docosahexaenoyl-glycerophosphocholine (AceDoPC).60
To conclude, it seems that the digestion of PL liposomes is in part insured by the phospholipase A1 activity contained in pancreatic extracts PPE. One explanation could be that in the presence of several phospholipases provided by pancreatic extracts, phospholipase A1 (PLRP2) acts first on PL and generates 2-lysophospholipids which was slightly hydrolyzed by phospholipase A2. The 2-lysophospholipid would be absorbed as such in the intestine.
4. Conclusion
The objective of this study was to compare the lipid digestion of oil-in-water emulsion and liposomal solutions formulated from neutral lipids and phospholipids, extracted from salmon heads (Salmo salar). The lipolytic enzymes involved in this digestion were initially identified by testing individual enzymes and the specificity of their action on each of the formulations was defined. Subsequently, an in vitro gastrointestinal digestion of these formulations rich in LC-PUFAs (EPA and DHA) was conducted using a two-step in vitro digestion model to study the stability of these carriers. The FFA analysis showed that DHA and EPA were absent among the FA released from both formulations used. This supports the fact that LC-PUFAs are predominantly esterified at the sn-2 position of TAG and phospholipids, in accordance with the literature. Fatty acids esterified at the sn-2 position are less hydrolyzable by lipases (as is the case for emulsions) or by enzymes with phospholipase A1 activity such as GPLRP2, and to lower extent CEH, in the case of liposomes. Marine-origin nanoliposomes are naturally richer in LC-PUFAs than TAG and they are not submitted to lipolysis in the stomach. This will allow increased protection of vectorized biomolecules, such as EPA and DHA, as well as the much more assimilable 2-lysophospholipid form at the intestinal barrier, as described in the literature.Conflicts of interest
The authors have declared no conflict of interests.References
- The State of World Fisheries and Aquaculture 2024, FAO, 2024, Blue Transformation in action, Rome, 2024, DOI:10.4060/cd0683e.
- A. Hanachi, A. Bianchi, C. J. F. Kahn, E. Velot, E. Arab-Tehrany, C. Cakir-Kiefer and M. Linder, Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays, Mar. Drugs, 2022, 20, 249 CrossRef CAS PubMed.
- M. K. Ahmmed, A. Carne, S. Bunga, H. (Sabrina) Tian and A. E.-D. A. Bekhit, Lipidomic signature of Pacific lean fish species head and skin using gas chromatography and nuclear magnetic resonance spectroscopy, Food Chem., 2021, 365, 130637 CrossRef CAS PubMed.
- D. Xie, J. Jin, J. Sun, L. Liang, X. Wang, W. Zhang, X. Wang and Q. Jin, Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components, Food Chem., 2017, 233, 434–441 CrossRef CAS PubMed.
- M. Haq, S. Suraiya, S. Ahmed and B.-S. Chun, Phospholipids from marine source: Extractions and forthcoming industrial applications, J. Funct. Foods, 2021, 80, 104448 CrossRef CAS.
- B. De La Fuente, J. Pinela, F. Mandim, S. A. Heleno, I. C. F. R. Ferreira, F. J. Barba, H. Berrada, C. Caleja and L. Barros, Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction, Food Chem., 2022, 386, 132778 CrossRef CAS PubMed.
- Y. Zhang, Q. Sun, S. Liu, S. Wei, Q. Xia, H. Ji, C. Deng and J. Hao, Extraction of fish oil from fish heads using ultra-high pressure pre-treatment prior to enzymatic hydrolysis, Innovative Food Sci. Emerging Technol., 2021, 70, 102670 CrossRef CAS.
- Y. Guo, W.-C. Huang, Y. Wu, X. Qi and X. Mao, Application of a low-voltage direct-current electric field for lipid extraction from squid viscera, J. Cleaner Prod., 2018, 205, 610–618 CrossRef CAS.
- Y. Sui, W.-C. Huang, Y. Wu, X. Qi and X. Mao, Lipid extraction from Greenland halibut (Reinhardtius hippoglossoides) by-product in low-voltage DC electric field and its mechanism, J. Cleaner Prod., 2021, 283, 124673 CrossRef CAS.
- M. Haq and B.-S. Chun, Characterization of phospholipids extracted from Atlantic salmon by-product using supercritical CO2 with ethanol as co-solvent, J. Cleaner Prod., 2018, 178, 186–195 CrossRef CAS.
- S. Ahmadkelayeh and K. Hawboldt, Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction, Trends Food Sci. Technol., 2020, 103, 94–108 CrossRef CAS.
- G. A. Gbogouri, M. Linder, J. Fanni and M. Parmentier, Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes, Eur. J. Lipid Sci. Technol., 2006, 108, 766–775 CrossRef CAS.
- Q. Wang, C. Xue, Z. Li and J. Xu, Analysis of DHA-rich phospholipids from egg of squid Sthenoteuthis oualaniensis, J. Food Compos. Anal., 2008, 21, 356–359 CrossRef CAS.
- N. Rubio-Rodríguez, S. M. De Diego, S. Beltrán, I. Jaime, M. T. Sanz and J. Rovira, Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods, J. Food Eng., 2012, 109, 238–248 CrossRef.
- M. K. Ahmmed, A. Carne, H. (Sabrina) Tian and A. E.-D. A. Bekhit, Use of fungal and bacterial protease preparations to enhance extraction of lipid from fish roe: Effect on lipidomic profile of extracted oil, Food Chem.: X, 2022, 16, 100499 CAS.
- C. Tullberg, G. Vegarud and I. Undeland, Oxidation of marine oils during in vitro gastrointestinal digestion with human digestive fluids – Role of oil origin, added tocopherols and lipolytic activity, Food Chem., 2019, 270, 527–537 CrossRef CAS PubMed.
- N. Sun, J. Chen, D. Wang and S. Lin, Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities, Trends Food Sci. Technol., 2018, 80, 199–211 CrossRef CAS.
- E. Falch, T. R. Størseth and M. Aursand, Multi-component analysis of marine lipids in fish gonads with emphasis on phospholipids using high resolution NMR spectroscopy, Chem. Phys. Lipids, 2006, 144, 4–16 CrossRef CAS PubMed.
- S. Khorasani, M. Danaei and M. R. Mozafari, Nanoliposome technology for the food and nutraceutical industries, Trends Food Sci. Technol., 2018, 79, 106–115 CrossRef CAS.
- J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications, Nano Lett., 2010, 10, 3223–3230 CrossRef CAS PubMed.
- D. Pasarin, A.-I. Ghizdareanu, C. E. Enascuta, C. B. Matei, C. Bilbie, L. Paraschiv-Palada and P.-A. Veres, Coating Materials to Increase the Stability of Liposomes, Polymers, 2023, 15, 782 CrossRef CAS PubMed.
- H. He, Y. Lu, J. Qi, Q. Zhu, Z. Chen and W. Wu, Adapting liposomes for oral drug delivery, Acta Pharm. Sin. B, 2019, 9, 36–48 CrossRef PubMed.
- T. Cui, A. Jia, M. Yao, M. Zhang, C. Sun, Y. Shi, X. Liu, J. Sun and C. Liu, Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated, Molecules, 2021, 26, 4144 CrossRef CAS PubMed.
- W. Zhou, C. Cheng, L. Ma, L. Zou, W. Liu, R. Li, Y. Cao, Y. Liu, R. Ruan and J. Li, The Formation of Chitosan-Coated Rhamnolipid Liposomes Containing Curcumin: Stability and In Vitro Digestion, Molecules, 2021, 26, 560 CrossRef CAS PubMed.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 CrossRef CAS PubMed.
- F. S. H. Lu, N. S. Nielsen, C. P. Baron, B. W. K. Diehl and C. Jacobsen, J. Agric. Food Chem., 2012, 12388–12396 CrossRef CAS PubMed.
- M. M. L. Grundy, E. Abrahamse, A. Almgren, M. Alminger, A. Andres, R. M. C. Ariëns, S. Bastiaan-Net, C. Bourlieu-Lacanal, A. Brodkorb, M. R. Bronze, I. Comi, L. Couëdelo, D. Dupont, A. Durand, S. N. El, T. Grauwet, C. Heerup, A. Heredia, M. R. Infantes Garcia, C. Jungnickel, I. E. Kłosowska-Chomiczewska, M. Létisse, A. Macierzanka, A. R. Mackie, D. J. McClements, O. Menard, A. Meynier, M.-C. Michalski, A.-I. Mulet-Cabero, A. Mullertz, F. M. Payeras Perelló, I. Peinado, M. Robert, S. Secouard, A. T. Serra, S. D. Silva, G. Thomassen, C. Tullberg, I. Undeland, C. Vaysse, G. E. Vegarud, S. H. E. Verkempinck, M. Viau, M. Zahir, R. Zhang and F. Carrière, INFOGEST inter-laboratory recommendations for assaying gastric and pancreatic lipases activities prior to in vitro digestion studies, J. Funct. Foods, 2021, 82, 104497 CrossRef CAS.
- L. Humbert, D. Rainteau, N. Tuvignon, C. Wolf, P. Seksik, R. Laugier and F. Carrière, Postprandial bile acid levels in intestine and plasma reveal altered biliary circulation in chronic pancreatitis patients, J. Lipid Res., 2018, 59, 2202–2213 CrossRef CAS PubMed.
- L. Couëdelo, S. Amara, M. Lecomte, E. Meugnier, J. Monteil, L. Fonseca, G. Pineau, M. Cansell, F. Carrière, M. C. Michalski and C. Vaysse, Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis, Food Funct., 2015, 6, 1726–1735 RSC.
- L. Sams, S. Amara, P. Mansuelle, R. Puppo, R. Lebrun, J. Paume, J. Giallo and F. Carrière, Characterization of pepsin from rabbit gastric extract, its action on β-casein and the effects of lipids on proteolysis, Food Funct., 2018, 9, 5975–5988 RSC.
- S. Latifi, A. Tamayol, R. Habibey, R. Sabzevari, C. Kahn, D. Geny, E. Eftekharpour, N. Annabi, A. Blau, M. Linder and E. Arab-Tehrany, Natural lecithin promotes neural network complexity and activity, Sci. Rep., 2016, 6, 25777 CrossRef CAS PubMed.
- E. Passeri, P. Bun, K. Elkhoury, M. Linder, C. Malaplate, F. T. Yen and E. Arab-Tehrany, Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons, Pharmaceutics, 2022, 14, 2172 CrossRef CAS PubMed.
- M. Gagoś, R. Koper and W. I. Gruszecki, Spectrophotometric analysis of organisation of dipalmitoylphosphatidylcholine bilayers containing the polyene antibiotic amphotericin B, Biochim. Biophys. Acta, Biomembr., 2001, 1511, 90–98 CrossRef PubMed.
- F. Carrière, C. Renou, V. Lopez, J. De Caro, F. Ferrato, H. Lengsfeld, A. De Caro, R. Laugier and R. Verger, The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals, Gastroenterology, 2000, 119, 949–960 CrossRef PubMed.
- S. Amara, C. Bourlieu, L. Humbert, D. Rainteau and F. Carrière, Variations in gastrointestinal lipases, pH and bile acid levels with food intake, age and diseases: Possible impact on oral lipid-based drug delivery systems, Adv. Drug Delivery Rev., 2019, 142, 3–15 CrossRef CAS PubMed.
- W. R. Morrison and L. M. Smith, Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol, J. Lipid Res., 1964, 5, 600–608 CrossRef CAS.
- M. El Alaoui, L. Soulère, A. Noiriel, Y. Queneau and A. Abousalham, α-Eleostearic acid-containing triglycerides for a continuous assay to determine lipase sn-1 and sn-3 regio-preference, Chem. Phys. Lipids, 2017, 206, 43–52 CrossRef CAS PubMed.
- E. Rogalska, C. Cudrey, F. Ferrato and R. Verger, Stereoselective hydrolysis of triglycerides by animal and microbial lipases, Chirality, 1993, 5, 24–30 CrossRef CAS PubMed.
- E. Rogalska, S. Ransac and R. Verger, Stereoselectivity of lipases. II. Stereoselective hydrolysis of triglycerides by gastric and pancreatic lipases, J. Biol. Chem., 1990, 265, 20271–20276 CrossRef CAS PubMed.
- J.-C. B. N'Goma, S. Amara, K. Dridi, V. Jannin and F. Carrière, Understanding the lipid-digestion processes in the GI tract before designing lipid-based drug-delivery systems, Ther. Delivery, 2012, 3, 105–124 CrossRef PubMed.
- S. Amara, D. Lafont, B. Fiorentino, P. Boullanger, F. Carrière and A. De Caro, Continuous measurement of galactolipid hydrolysis by pancreatic lipolytic enzymes using the pH-stat technique and a medium chain monogalactosyl diglyceride as substrate, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2009, 1791, 983–990 CrossRef CAS PubMed.
- J. A. F. Op Den Kamp, M. Th. Kauerz and L. L. M. Van Deenen, Action of pancreatic phospholipase A2 on phosphatidylcholine bilayers in different physical states, Biochim. Biophys. Acta, Biomembr., 1975, 406, 169–177 CrossRef CAS PubMed.
- R. Dua, S.-K. Wu and W. Cho, A Structure-Function Study of Bovine Pancreatic Phospholipase A2 Using Polymerized Mixed Liposomes, J. Biol. Chem., 1995, 270, 263–268 CrossRef CAS PubMed.
- J. J. Volwerk, P. C. Jost, G. H. De Haas and O. H. Griffith, Activation of porcine pancreatic phospholipase A2 by the presence of negative charges at the lipid-water interface, Biochemistry, 1986, 25, 1726–1733 CrossRef CAS PubMed.
- A. Salhi, S. Amara, P. Mansuelle, R. Puppo, R. Lebrun, B. Gontero, A. Aloulou and F. Carrière, Characterization of all the lipolytic activities in pancreatin and comparison with porcine and human pancreatic juices, Biochimie, 2020, 169, 106–120 CrossRef CAS PubMed.
- E. Mateos-Diaz, J.-C. Bakala N'Goma, D. Byrne, S. Robert, F. Carrière and H. Gaussier, IR spectroscopy analysis of pancreatic lipase-related protein 2 interaction with phospholipids: 1. Discriminative recognition of mixed micelles versus liposomes, Chem. Phys. Lipids, 2018, 211, 52–65 CrossRef CAS PubMed.
- E. Mateos-Diaz, P. Sutto-Ortiz, M. Sahaka, J. A. Rodriguez and F. Carrière, IR spectroscopy analysis of pancreatic lipase-related protein 2 interaction with phospholipids: 3. Monitoring DPPC lipolysis in mixed micelles, Chem. Phys. Lipids, 2018, 211, 77–85 CrossRef CAS PubMed.
- J. Kergomard, F. Carrière, G. Paboeuf, L. Chonchon, N. Barouh, V. Vié and C. Bourlieu, Interfacial adsorption and activity of pancreatic lipase-related protein 2 onto heterogeneous plant lipid model membranes, Biochimie, 2023, 215, 12–23 CrossRef CAS PubMed.
- S. Amara, D. Lafont, G. Parsiegla, V. Point, A. Chabannes, A. Rousset and F. Carrière, The galactolipase activity of some microbial lipases and pancreatic enzymes, Eur. J. Lipid Sci. Technol., 2013, 115, 442–451 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Ménard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food – an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- L. Sams, J. Paume, J. Giallo and F. Carrière, Relevant pH and lipase for in vitro models of gastric digestion, Food Funct., 2016, 7, 30–45 RSC.
- L. Sams, S. Amara, P. Mansuelle, R. Puppo, R. Lebrun, J. Paume, J. Giallo and F. Carrière, Characterization of pepsin from rabbit gastric extract, its action on β-casein and the effects of lipids on proteolysis, Food Funct., 2018, 9, 5975–5988 RSC.
- J.-C. Bakala-N'Goma, L. Couëdelo, C. Vaysse, M. Letisse, V. Pierre, A. Géloen, M.-C. Michalski, M. Lagarde, J.-D. Leao and F. Carrière, The digestion of diacylglycerol isomers by gastric and pancreatic lipases and its impact on the metabolic pathways for TAG re-synthesis in enterocytes, Biochimie, 2022, 203, 106–117 CrossRef PubMed.
- C. Vors, P. Capolino, C. Guérin, E. Meugnier, S. Pesenti, M.-A. Chauvin, J. Monteil, N. Peretti, M. Cansell, F. Carrière and M.-C. Michalski, Coupling in vitro gastrointestinal lipolysis and Caco-2 cell cultures for testing the absorption of different food emulsions, Food Funct., 2012, 3, 537 RSC.
- A. Esteller, Physiology of bile secretion, World J. Gastroenterol., 2008, 14, 5641 CrossRef CAS PubMed.
- M. Aursand, L. Jørgensen and H. Grasdalen, Positional distribution of ω-3 fatty acids in marine lipid triacylglycerols by high resolution 13C nuclear magnetic resonance spectroscopy., J. Am. Oil Chem. Soc., 1995, 72, 293–297 CrossRef CAS.
- N. A. Mazer, G. B. Benedek and M. C. Carey, Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions, Biochemistry, 1980, 19, 601–615 CrossRef CAS PubMed.
- R. G. Ackman, Fish lipids. Part 1, 1980 Search PubMed.
- M. El Alaoui, L. Soulère, A. Noiriel, F. Popowycz, A. Khatib, Y. Queneau and A. Abousalham, A continuous spectrophotometric assay that distinguishes between phospholipase A1 and A2 activities, J. Lipid Res., 2016, 57, 1589–1597 CrossRef CAS PubMed.
- M. Lagarde, M. Hachem, N. Bernoud-Hubac, M. Picq, E. Véricel and M. Guichardant, Biological properties of a DHA-containing structured phospholipid (AceDoPC) to target the brain, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2015, 92, 63–65 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |