The neurotoxicity of acrylamide in ultra-processed foods: interventions of polysaccharides through the microbiota–gut–brain axis
Chen
Cai
a,
Zheyi
Song
b,
Xinrui
Xu
a,
Xin
Yang
a,
Siyu
Wei
a,
Fang
Chen
a,
Xu
Dong
c,
Xin
Zhang
 *b and
Yuchen
Zhu
*b and
Yuchen
Zhu
 *a
*a
aCollege of Food Science and Nutritional Engineering, National Engineering Research Centre for Fruits and Vegetables Processing, Key Laboratory of Storage and Processing of Fruits and Vegetables, Ministry of Agriculture, Engineering Research Centre for Fruits and Vegetables Processing, Ministry of Education, China Agricultural University, Beijing 100083, P.R. China. E-mail: zhuyuchen19880710@sina.com
bDepartment of Food Science and Engineering, Ningbo University, Ningbo 315211, P.R. China. E-mail: zhangxin@nbu.edu.cn
cDepartment of Gynaecology, Beilun People's Hospital, Ningbo 315800, P.R. China
First published on 18th November 2024
Abstract
Ultra-processed foods (UPFs) have become popular in recent years, however, the detrimental effects of their excessive consumption have also become evident. Acrylamide (AA), a processing hazard present in UPFs, can further aggravate the harmful effects of UPFs. AA can cause significant damage to both the intestinal barrier and gut microbiota, thereby affecting the nervous system through the microbiota–gut–brain (MGB) axis. Natural polysaccharides have demonstrated the capacity to significantly alleviate the oxidative stress and inflammatory response associated with AA exposure. In addition, they exhibit neuroprotective properties that may be mediated through the MGB axis. This paper reviews literature on the presence of AA in certain UPFs and its potential to inflict serious harm on the human gut microbiota and brain. Moreover, the possibility of utilizing polysaccharides as a preventative measure against AA-induced neurotoxicity was also proposed. These findings provide new insights into the safety risks associated with the overconsumption of UPFs and highlight the potential of polysaccharides to counteract the neurodegeneration induced by AA.
Introduction
The rapid development of the food industry has introduced more and more diversity in food types and processing methods.1 Due to the accelerated lifestyle of modern people, many consumers prefer to eat convenient and highly processed food instead of freshly prepared foods in order to save time. As a result, traditional dietary patterns have gradually shifted, with ultra-processed foods (UPFs) gaining increasing popularity. Previous studies have established that consuming UPFs elevates the risk of chronic diseases such as cardiovascular diseases (CVDs),2,3 obesity,4 diabetes,5 and dementia.6 Process contaminants found in UPFs, such as heterocyclic aromatic amines (HAAs), acrylamide (AA), advanced glycation end-products (AGEs), trans fatty acids, and furans, have been identified as significant contributors to the health risks associated with these products, as these process contaminants can disrupt the endocrine system and gut microbiota.2,6AA is a key processing contaminant produced in UPFs, known for its neurotoxic effects.7 It is formed when carbohydrate-rich foods are heated at high temperatures.8 Although AA can also be produced in some simply processed foods, UPFs were reported to contain high levels of AA as they tend to be heated for a long time at high temperatures. For example, the AA content in both French Fries and potato chips can be up to 100 times higher than in boiled potatoes.9 Upon ingestion, AA interacts with the intestinal tract during digestion and absorption, and then enters the human body, resulting in toxicity.10 Exposure to AA induces oxidative stress, inflammation, apoptosis, and DNA damage.11 Various neurodegenerative diseases, as well as brain pathology, such as Parkinson's disease and Alzheimer's disease, are associated with oxidative stress and inflammation.12
The gut microbiota, a complex microbial community, significantly influences various aspects of host physiology.13 It has been demonstrated that gut microbiota and its metabolites maintain gastrointestinal (GI) functions due to their capacity to affect intestinal permeability and mucosal immune function.14 Short-chain fatty acids (SCFAs), one of the important metabolites of gut microbiota, play a key role in maintaining the intestinal barrier and regulating intestinal inflammation.15 The intricate microbiota in the mammalian intestinal tract plays an important role in immunity and defence when pathogenic bacteria invade the host's intestinal tract.16 For example, Lactobacillus can mediate the production of reactive oxygen species (ROS) via regulating the redox state of host cells, thereby reducing oxidative stress.17 Besides, the gut microbiota and its metabolites can affect the central nervous system (CNS) and regulate brain function through the microbiota–gut–brain (MGB) axis,18 thus affecting mood and behaviour, including depression, anxiety, circadian rhythm disorders, etc.19–21 The bidirectional communication between the GI and the brain is essential for maintaining balance in the body and regulating the nervous system, and provides a potential pathway for the gut microbiota and its metabolites to enter the brain.22
Long-term intake of UPFs will damage the gut microbiota, leading to metabolic disorders, oxidative stress, and inflammation, thus increasing the risk of brain diseases.23 One of the major reasons for these damages may be that some UPFs contain AA. AA stimulates the inflammatory response in the brain by disrupting the intestinal barrier integrity and increasing the levels of circulating lipopolysaccharides (LPS), interleukin (IL)-1β, and TNF-α (tumor necrosis factor-α). This further aggravates the harm caused by excessive consumption of UPFs and causes significant damage to both the human gut microbiota and nervous system through the MGB axis.24 Studies have shown that AA can cause neurobehavioural disorders, including cognitive and motor impairments, in zebrafish through the MGB axis.25
Polysaccharides, natural active macromolecules composed of various monosaccharides connected by glycosidic chains, are widely found in nature.26 Polysaccharides have a variety of biological activities, such as immune regulation, anti-inflammation, anti-oxidation, anti-cancer, and hypoglycemia, which play an important role in maintaining human health.27 Further studies have shown that the biological activity of polysaccharides is closely related to its regulation of gut microbiota, so the interaction between polysaccharides and gut microbiota has become a research hotspot.21,28Ganoderma atrum polysaccharides,29Cyclocarya paliurus polysaccharides,30 and kiwifruit polysaccharides31 show regulatory effects on intestinal microbial ecology, and then improve brain damage through the bidirectional communication between the gut and brain.
The consumption of UPFs has been linked to a variety of potential health issues, with AA being a notable concern due to its presence in these products. AA is known to cause significant damage to the gut microbiota and detrimental effects on the nervous system through the MGB axis. Polysaccharides, as a natural ingredient, can promote the growth and reproduction of beneficial gut microbiota. The mechanism by which they regulate gut microbiota-mediated microbial metabolism to alleviate or treat neurodegenerative diseases is gaining recognition.32 This review synthesizes recent relevant literature to elucidate the intervention mechanism and structure–activity relationship of polysaccharides on AA-induced neurotoxicity from multiple aspects, such as biological activity and the MGB axis. It provides new insights into the safety risks associated with excessive consumption of UPFs and lays the foundation for natural polysaccharides to be used to counteract AA-induced neurodegeneration.
The current development of UPFs
The concept of UPFs was first proposed by researchers in 2009, in observation of the link between global trends in obesity and obesity-related chronic diseases and the increasing consumption of convenient/pre-prepared foods.33 NOVA classification divides foods into four groups according to the degree and purpose of modern food industrial processing: unprocessed or minimally processed foods, such as fresh fruits and vegetables, pasteurized milk, and frozen meat (group 1), processed culinary ingredients, such as sugar, honey, salt, and butter (group 2), processed foods, such as fruits in syrup and vegetables in brine (group 3) and ultra-processed foods (group 4).34UPFs are increasingly favoured by consumers, especially young people, due to their palatability and convenience. UPFs, such as sweet drinks, biscuits, cakes, potato chips, and fried chicken, are consumed by children and teenagers in countries with different income levels.35–37 It is reported that UPF consumption has increased sharply around the world. According to the data from the National Health and Nutrition Examination Survey (NHANES), the proportion of total calorie intake from UPFs among young Americans between the ages of 2–19 has risen from 61.4% to 67.0% over the past 20 years.38 Similar increases in the consumption of UPFs have been observed in South Korea (2010–2018: from 23.1% to 26.1%),39 Canada (2004–2015: from 47.8% to 45.7%),4 and other countries.35,36,40 UPFs are gradually replacing minimally processed foods and freshly prepared foods. UPFs accounted for over half of the total energy intake in developed countries.41 However, most UPFs are nutritionally imbalanced, containing more so-called limiting nutrients (added sugars, sodium, and saturated and trans-fats), while being relatively deficient in promoting nutrients (fibres and micronutrients).42 Furthermore, excessive processing at high temperatures leads to significant accumulation of process contaminants, such as AA,43 AGEs,44 and HAAs,45 which further endanger consumer health.
The safety of UPFs
The consumption of UPFs has been correlated with a heightened risk of developing chronic metabolic diseases.4 The emergence of these health issues is multifactorial, and excessive intake of fat and sugars in UPFs is a significant contributor. Furthermore, exposure to processing-induced contaminants in UPFs is also a crucial element in the pathogenesis of these diseases.46 Among these contaminants, AA is particularly concerning as it can not only disrupt the gut microbiota but also cause neurotoxicity.2The production and prevention of AA
AA is primarily formed through the Maillard reaction, which involves the reaction between glucose and asparagine.47 The process begins with the reaction of the amino residues (–NH2) of asparagine and the carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of glucose, resulting in the formation of the corresponding N-glycosyl conjugation and its corresponding Schiff base. The Schiff base may facilitate decarboxylation, either through the Schiff betaine or through the oxazolidine-5-one intermediate, leading to the formation of the azomethine ylide. This intermediate then undergoes tautomerization to yield the decarboxylated Amadori product. Subsequently, AA may be formed either via the β-elimination of the decarboxylated Amadori compound, or via deamination of 3-aminopropionamide, which is regarded as an important precursor of AA.48 Alternatively, the Schiff base can undergo rearrangement to form the Amadori compound, which subsequently generates carbonyl compounds. Previous studies have proved that these carbonyl compounds can also react with asparagine to yield AA.49 In addition, AA can be produced through the acrolein pathway. When fats or oils, especially unsaturated oils, are subjected to excessively high temperatures, acrolein is generated.50 In the presence of asparagine, acrolein is oxidized to form acrylic acid, which then reacts with ammonia to produce AA.51
O) of glucose, resulting in the formation of the corresponding N-glycosyl conjugation and its corresponding Schiff base. The Schiff base may facilitate decarboxylation, either through the Schiff betaine or through the oxazolidine-5-one intermediate, leading to the formation of the azomethine ylide. This intermediate then undergoes tautomerization to yield the decarboxylated Amadori product. Subsequently, AA may be formed either via the β-elimination of the decarboxylated Amadori compound, or via deamination of 3-aminopropionamide, which is regarded as an important precursor of AA.48 Alternatively, the Schiff base can undergo rearrangement to form the Amadori compound, which subsequently generates carbonyl compounds. Previous studies have proved that these carbonyl compounds can also react with asparagine to yield AA.49 In addition, AA can be produced through the acrolein pathway. When fats or oils, especially unsaturated oils, are subjected to excessively high temperatures, acrolein is generated.50 In the presence of asparagine, acrolein is oxidized to form acrylic acid, which then reacts with ammonia to produce AA.51
According to European Commission regulations, the residue of AA in potato chips should be under 750 μg kg−1, while for roasted coffee, the limit is set between 400–850 μg kg−1.52 However, a survey by the European Food Safety Authority (EFSA) showed that the average concentration of AA in fried potato products was 1000 μg kg−1, and alarmingly, in coffee, it reached as high as 4500 μg kg−1.53 These levels are considerably higher than the recommended by the European Commission.
Numerous strategies have been proposed to inhibit the formation of AA, including modifications to food processing techniques and the incorporation of specific inhibitors. For instance, Zhong et al.54 found that a pre-treatment freezing process at 10 °C effectively reduces AA concentration in potato chips and chips, with minimal impact on the product's appearance. Vacuum frying has been shown to produce French Fries with a low AA content while maintaining quality characteristics.55 Antunes-Rohling et al.56 demonstrated that a 30-minute water pre-treatment for potatoes, followed by high-intensity ultrasound, led to a 90% decrease in AA concentration. Besides, the addition of amino acids, antioxidants, enzymes, salts, and vitamins can reduce the AA content in food.57,58 Asparaginase can catalyse the hydrolysis of asparagine to ammonia and aspartic acid, thereby reducing the concentration of the precursor necessary for the Maillard reaction and inhibiting the production of AA.59 Treating potato chips and bread with 300 U asparaginase resulted in reductions of 85% and 78% in asparagine content, respectively, while the AA content in potato chips and baked bread decreased by 94% and 86%, respectively.60
The harm of AA
AA is classified as a Class 2A carcinogen by the International Agency for Research on Cancer.61 Upon entering the body, it is distributed to various tissues, such as the heart, kidney, and brain.47 The genotoxicity, neurotoxicity, reproductive toxicity, and potential carcinogenicity of AA were demonstrated in animal experiments.62–64 Moreover, exposure to AA is associated with the onset of CVDs, coronary heart disease, and neurodegenerative diseases, which are also closely related to the consumption of UPFs.65,66Long-term exposure to low concentrations of AA, particularly through food intake, poses potential risks to human health.67 Such exposure may result in neurotoxic effects, including difficulties in learning, memory, cognition, and numbness in the hands and feet.62,67 Increasing evidence indicates that the inflammatory response, oxidative stress, and apoptosis of neurocytes significantly contributes to neurodegenerative diseases.68,69 The levels of ROS, 8-hydroxy-2-deoxyguanosine, and malondialdehyde (MDA) increased, while glutathione (GSH) content decreased in mice administered with 5–50 mg kg−1 AA, leading to oxidative stress.70 Inflammation-induced immune system disorders are related to the increase of several inflammatory cytokines caused by AA, including IL-1α, IL-1β, IL6, and TNF-α peripheral tissues.71 Zhao et al.72 found increased TNF-α, IL-6, and IL-1β levels in the primary microglia during the latter stages of AA exposure, further confirming the involvement of the immune-inflammatory response in AA-induced neurotoxicity. In addition, AA can induce apoptosis in various neurocytes, such as human neuroblastoma SH-SY5Y cells and astrocytoma U1240-MG cells.73 Therefore, AA may damage brain homeostasis and induce neurotoxicity by inducing nerve cell apoptosis.
The effect of AA on nerves through gut microbiota
The effect mechanism of AA on gut microbiota
Apart from inflammatory reactions and oxidative stress, AA can cause other obvious destructive effects on intestinal tissue.29,79 A prior investigation revealed that exposure to AA destroyed the structural integrity of ileal villi in rats, resulting in obvious atrophy and deformation, and this causes significant damage to the intestinal epithelial cells.79 Intestinal epithelial cells play a crucial role in defending against intestinal pathogens and maintaining intestinal transport and barrier functions. Similarly, studies showed that AA changes the morphology and histology of the small intestinal wall, reducing the proliferation of the intestinal wall, muscular and submucosal thickness, villus length, crypt depth, crypt number, and intestinal absorption surface. On the contrary, there was an increase in the apoptosis rate, enterocyte number, the level of hemoglobin adducts, the staining intensity of epithelial cells, the thickness of villus epithelial, and the width of crypt.75 Therefore, AA has a negative effect on the tissue structure, regeneration, and innervation of the small intestinal wall, and indirectly affects the absorption function of the small intestinal mucosa.
On the other hand, AA can also change the metabolism of gut microbiota, which in turn influences host metabolism by regulating metabolites, such as bile acids (BAs) and SCFAs.84 BAs can promote the absorption and transport of fat in the intestinal tract, thereby regulating intestinal incretin secretion, hepatic gluconeogenesis, glycogen synthesis, energy expenditure, inflammation, and gut microbiota configuration.85 AA exposure significantly reduced the content of microbial genes involved in the secondary biosynthesis of BAs. Compared with the control group, the expression levels of genes involved in BA synthesis, reabsorption, and signal transduction were significantly decreased in AA-treated rats.79 SCFAs, such as butyric acid, propionic acid, and acetate, are by-products of intestinal microbial fermentation. They serve not only as the energy source for intestinal epithelial cells but also play a crucial role in reducing inflammation and improving the integrity of the intestinal barrier.15 SCFAs can inhibit the NF-κB (nuclear factor κB) pathway and reduce the production of proinflammatory cytokines.86 However, Bacteroides, Escherichia_Shigella, Dubosiella, and Alloprevotella, which are related to the synthesis of SCFAs, were negatively affected by AA. This resulted in a significant reduction in SCFAs levels and promoted inflammation.87
The effect of AA on the nervous system
In the neural pathway, the autonomic nervous system (ANS), combined with the activity of the enteric nervous system (ENS) and the regulation of the CNS, is responsible for maintaining physiological balance and responding to endocrine, motor, autonomic, and behavioural regions. ANS, connected with neuroendocrine and neuroendocrine signals, can induce changes in the regulation of the CNS in the intestinal tract (the top-down effect).14 ENS, a neural network located at the interface between the microbiota and the host, is positioned to respond either directly, or indirectly, to the microbiota and its metabolites. In the context of gut–brain signals, ENS communicates with CNS by separating neurons from the intestine to the sympathetic ganglion, and sensory information is transmitted through external primary afferent neurons that follow the afferent routes of the spinal cord and vagus nerve.93 Microbiota–host interaction at the intestinal level leads to the release of cytokines, chemokines, neurotransmitters, neuropeptides, endocrine messengers, and microbial by-products, which can infiltrate the blood and lymphatic system, or affect the neural information carried by the vagus nerve and spinal cord afferent neurons, thus constantly communicating with the brain, and regulate the brain and behaviour.91
The immune regulation of microbiota is becoming a major way to coordinate MGB communication.94 Gut microbiota has the function of regulating the maturation of resident immune cells in the CNS. Therefore, the gut microbiota and immune system are associated with the etiology or manifestation of neurodevelopmental, mental, and neurodegenerative diseases, such as autism spectrum disorders, depression, and Alzheimer's disease.13 The interaction between microorganisms and local immune cells may lead to functional changes other than GI, such as altering the release of cytokines into the systemic circulation or regulating immune cells in other parts of the body, including the brain. In addition to the effects of microorganisms on peripheral immune cells, the gut microbiota has also been reported to regulate the development, maturation, and function of microglia, which are resident immune cells in the brain.95 In turn, the inflammatory response in the brain caused by neurodegenerative diseases has been shown to alter the composition of the gut microbiota consequently aggravating the neuroimmune response and exacerbating the pathology development.92
Besides, endocrine pathways are also important for the MGB axis. Enteroendocrine cells (EECs) can secrete a variety of signal molecules, and they are essential for maintaining gut homeostasis. Enteroendocrine L cells, one of the major EECs, secrete glucagon-like peptide-1 (GLP-1) and peptide YY in a postprandial state, and the receptors of these peptides are locally expressed in the gut enteric neurons and vagal afferents, as well as CNS.96 Enterochromaffin cells (ECCs) produce the majority of 5-hydroxytryptamine (5-HT) in the body from dietary tryptophan.91 5-HT activates a variety of receptor families on the internal and external afferent nerve fibers of the GI tract and mediates many GI functions, including intestinal peristalsis, electrolyte secretion, pain, and inflammation.97 Besides, 5-HT released by ECCs may affect gut–brain signals by regulating vagus nerve afferent activity97 and inflammation in the gut.98 Microbial products and metabolites, such as secondary BAs and SCFAs, regulate the secretion of neuropeptides (such as GLP-1) and neuromodulators (such as hormones and 5-HT) through EECs and ECCs. The gut microbiota also interacts with the neuroendocrine signalling pathway mediated by the hypothalamus-pituitary-adrenal (HPA) axis. The activation of the HPA axis induced by stress affects GI function and then changes the composition of gut microbiota.95
The MGB axis is a bidirectional communication system that integrates the neural, immune, and endocrine pathways between the intestinal tract and the brain. These three pathways are highly complex and interrelated. For example, stimulating immune cells in the intestinal tract can lead to the local and systemic release of inflammatory cytokines and affect the permeability of the intestinal tract and brain, which is conducive to the entry of microbial by-products into the portal circulation and intestinal parenchyma, resulting in the excitability of local nerve endings, and eventually modify the brain homeostasis.95
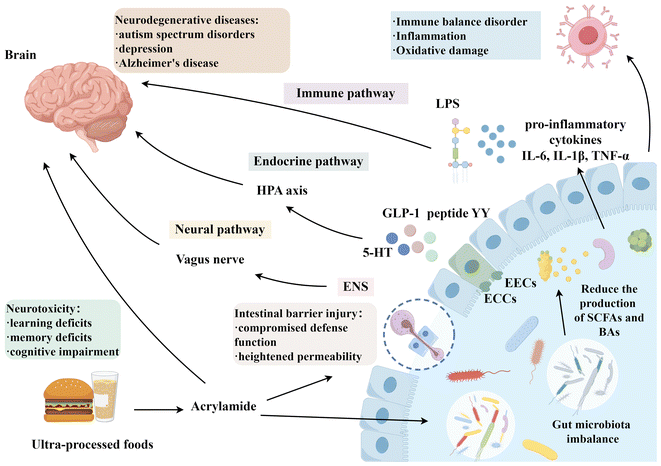 | ||
| Fig. 1 Nerve injury induced by AA through MGB axis. AA can harm the intestinal tissue and the gut microbiota, leading to the occurrence of neurodegenerative diseases through the MGB axis. | ||
Research has demonstrated that AA induces Parkinson-like neurobehavioural abnormalities in adult zebrafish through the MGB axis. This is mainly attributed to the alteration in antioxidant levels in the intestine and brain, such as increased oxidative stress and changes in the nuclear factor E2-related factor-2 (Nrf2) signal pathway, a key pathway that regulates the balance of intracellular oxidation and anti-oxidation.108 Additionally, there are modifications in the level of inflammatory mediators, specifically in the NF-κB signalling pathway, which contribute to neurobehavioural disorders.25 Meanwhile, studies have shown that AA treatment can cause circadian rhythm disorder and inhibit the expression of circadian rhythm-related proteins, especially TJ proteins occludin and claudin-1in the mouse intestine.24,100 In general, these findings suggest that AA-induced cognitive and memory decline may be due to defects in the MGB axis, and the underlying neurotoxic mechanism via the MGB axis homeostasis requires further investigation.
Intervention effects of polysaccharides on AA related neurotoxicity
Natural polysaccharides are a therapeutic agent for neurotoxicity through oxidative stress, inflammatory response, and intestinal barrier damage. Due to the lack of polysaccharide hydrolase in organisms, many polysaccharides cannot be digested and absorbed directly after entering the body, and the gut microbiota can play an effective role in intermediate transformation.109 It has been shown that some polysaccharides can improve the disease related to the gut microbiota as prebiotics, then further improve brain injury through the MGB axis (Table 1).32,110 In addition, they can also play a neuroprotective role through their biological activities, including antioxidation, anti-inflammation, and immunomodulatory activities (Fig. 2). Therefore, polysaccharides have broad application prospects in the treatment of neurotoxicity caused by AA.| Polysaccharide | Neurodegenerative disease | Model | Results | Potential mechanisms | Ref. |
|---|---|---|---|---|---|
| Dendrobium officinale polysaccharide | Depression-like symptoms | Mouse perimenopausal depression model | 1. Campilobacterota, Desulfobacterota and Cyanobacteria ↓ | Regulation of gut microbiota-neuroinflammation | 133 |
| 2. Bacteroidota↑ | |||||
| 3. Lachnospiraceae and Helicobacter ↓ | |||||
| 4. Lactobacillus and Muribaculaceae ↑ | |||||
| 5. IL-1β, IL-6 and TNF-α ↓ | |||||
| 6. Corticotropin-releasing hormone, adrenocorticotropic hormone and corticosterone levels ↓ | |||||
| Ganoderma lucidum polysaccharides | Depression | Chronic social defeat stress depression animal model | 1. IL-1β and TNF-α ↓ | Inflammation regulation; regulation of neuroimmune system | 134 |
| 2. IL-10 and BDNF ↑ | |||||
| 3. Activation of microglia and proliferation of astrocytes ↓ | |||||
| 4. GluA1 S845 phosphorylation, GluA1 and GluA2 expression ↑ | |||||
| 5. Dectin-1 expression ↑ | |||||
| Inulin | Alzheimer's disease | Asymptomatic apolipoprotein ε4 allele transgenic mice | 1. Prevotella and Lactobacillus ↑ | Regulation of gut microbiota; suppression of brain inflammation; increase in metabolism | 135 |
| 2. Escherichia, Turicibacter and Akkermansia ↓ | |||||
| 3. SCFAs, tryptophan-derived metabolites, BAs, glycolytic metabolites and scyllo-inositol ↑ | |||||
| 4. Brain inflammation ↓ | |||||
| Flammulina velutipes polysaccharides | Learning and memory impairment | Cognitive impairment mice induced by scopolamine | 1. The ratio of F/B ↓ | Regulation of gut microbiota; suppression of neuroinflammation | 136 |
| 2. Clostridia and Bacilli ↓ | |||||
| 3. Bacteroidia, Erysipelotrichia and Actinobacteria ↑ | |||||
| 4. E. fergusonii ↓ | |||||
| 5. IL-1β, TNF-α and IL-6 ↓ | |||||
| 6. IL-10 ↑ | |||||
| Polymannuronic acid in the brown seaweed polysaccharides | Parkinson's disease | 1-Methyl-4-phenyl-1,2,3,6tetrahydropyridine induced Parkinson's disease mice model | 1. Striatal homovanillic acid, 5-HT, 5-hydroxyindole acetic acid and γ-aminobutyric acid ↑ | Suppression of inflammation; improvement in the integrity of the intestinal barrier and blood–brain barrier; regulation of gut microbiota | 137 |
| 2. SCFAs, including acetic acid and propionic acid and butyric acid ↑ | |||||
| 3. TNF-α and IL-6 ↓ | |||||
| 4. ZO-1 and occluding mRNA expressions ↑ | |||||
| 5. Turicibacter and Lactobacillus↓ | |||||
| 6. Clostridiales, Coprococcus and Ruminococcus ↑ | |||||
| 7. MAPK signalling pathway ↓ | |||||
| Polygonatum sibiricum polysaccharides | Alzheimer's disease | 5xFAD mice | 1. Synaptic density ↑ | Gut microbiota remodeling; Inflammation regulation; repair of intestinal barrier; prevention of intestinal Amyloid-β deposition | 138 |
| 2. Amyloid-β1–40 and amyloid-β1–42 ↓ | |||||
| 3. Microglial M2 phenotype transition and microglial recruitment around the 2. amyloid-β plaques ↑ | |||||
| 4. Helicobacter mastomyrinus and Helicobacter typhlonius↓ | |||||
| 5. Akkermansia muciniphila ↑ | |||||
| 6. TNF-α and IL-6 ↓ | |||||
| 7. The mRNA levels of occludin and ZO-1 ↑ |
Structural characterization of polysaccharides
Polysaccharides are prebiotics and are widely found in nature. With the advantages of being non-toxic, stable, and easy to obtain, natural polysaccharides have attracted significant attention in various fields.111 Based on their composition, polysaccharides can be divided into homopolysaccharides and heteropolysaccharides. Homopolysaccharides consist of a single type of monosaccharide, while heteropolysaccharides are composed of various monosaccharides. Polysaccharides have been reported to have health benefits such as antioxidant, anti-inflammatory, intestinal protective, and neuroprotective activities.32 These biological activities of polysaccharides are intricately linked to their primary structure, which encompasses molecular weights (MWs), monosaccharide compositions, and glycosidic bond types.112 Studies have shown that most polysaccharides with intestinal barrier protection capabilities exhibit high MWs. This is because high-MWs polysaccharides have elevated viscosity and can form a hydrophilic gel layer like the intestinal mucosa layer, facilitating cross-linking with membrane receptors to maintain the integrity of the intestinal barrier structure.113 For example, the polysaccharides from Ganoderma atrum with an MW of 1013.0 kDa have been shown to mend the intestinal barrier function damage induced by AA in mice through the TLR4/MyD88/NF-κB pathway.106The monosaccharide composition and glycosidic bond type influence the affinity of polysaccharides with targets in vivo. It is generally accepted that a more complex monosaccharide composition correlates with enhanced biological activity.114 Most polysaccharides with intestinal barrier protection functions are heteropolysaccharides, primarily composed of galactose (Gal), mannose (Man), arabinose (Ara), xylose (Xyl), rhamnose (Rha) and other monosaccharides.113 In addition, polysaccharides containing Man and Rha have been shown to exhibit tumor suppression and antioxidant activities, respectively.115,116 However, the specific rules and mechanisms have not yet been clarified and need further exploration.
Additionally, it is generally believed that polysaccharides with β-configuration have a higher activity.117 The majority of polysaccharides that modulate the gut microbiota are characterized by (1 → 3) glycosidic linkages. For example, Hericium erinaceus polysaccharides, primarily consisting of β-(1 → 3)-D-glucose, have been shown to augment both the diversity and richness of rat gut microbiota, as well as optimize the ratio of acetic acid and butyric acid.118
Regulation effect of polysaccharides on gut microbiota
A study on the effects of compound polysaccharides (Lycium barbarum polysaccharides, Poria cocos polysaccharides and Lentinan, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on gut microbiota in rats showed that, according to 16S rRNA analysis, the relative abundance of four bacterial genera (Bifidobacterium, Lactobacillus, Allobaculum and Oligella) in the compound polysaccharides treatment group was significantly higher than that in the control group, while the relative abundance of one bacterial genus (Enterococcus) decreased significantly. At the same time, compound polysaccharides treatment promoted the functional maturity of the intestinal bacterial community, which was characterized by an increase in basic metabolism (amino acid metabolism and energy metabolism), SCFAs related metabolism and nucleotide metabolism.128 It is worth noting that intestinal microorganisms, including Lactobacillus fermentum, Lactobacillus acidophilus, Bifidobacterium lactis, and so forth, are of great significance in improving cognitive impairment.110 For example, it has been suggested that Lactobacillus fermentum can have beneficial effects on cognitive function, physiological behaviour and immune response in aged mice.129 Many studies have also shown that polysaccharides can have a beneficial effect on the nervous system by regulating the gut microbiota. Fucoidan increased the relative abundances of Prevotellaceae, Rikenellaceae, Prevotella, Barnesiella, and Alistipes and decreased the relative abundance of Verrucomicrobiaceae, to ameliorate depression.130 Ginkgo biloba leaves polysaccharides131 and inulin132 upregulated the relative abundances of lactic acid bacteria (especially Lactobacillus reuteri) and Bifidobacterium, thereby ameliorating nerve injury via modulating the MGB axis. Thus, polysaccharides have great potential in the treatment of AA-induced cognitive impairment through improving gut microbiota.
1) on gut microbiota in rats showed that, according to 16S rRNA analysis, the relative abundance of four bacterial genera (Bifidobacterium, Lactobacillus, Allobaculum and Oligella) in the compound polysaccharides treatment group was significantly higher than that in the control group, while the relative abundance of one bacterial genus (Enterococcus) decreased significantly. At the same time, compound polysaccharides treatment promoted the functional maturity of the intestinal bacterial community, which was characterized by an increase in basic metabolism (amino acid metabolism and energy metabolism), SCFAs related metabolism and nucleotide metabolism.128 It is worth noting that intestinal microorganisms, including Lactobacillus fermentum, Lactobacillus acidophilus, Bifidobacterium lactis, and so forth, are of great significance in improving cognitive impairment.110 For example, it has been suggested that Lactobacillus fermentum can have beneficial effects on cognitive function, physiological behaviour and immune response in aged mice.129 Many studies have also shown that polysaccharides can have a beneficial effect on the nervous system by regulating the gut microbiota. Fucoidan increased the relative abundances of Prevotellaceae, Rikenellaceae, Prevotella, Barnesiella, and Alistipes and decreased the relative abundance of Verrucomicrobiaceae, to ameliorate depression.130 Ginkgo biloba leaves polysaccharides131 and inulin132 upregulated the relative abundances of lactic acid bacteria (especially Lactobacillus reuteri) and Bifidobacterium, thereby ameliorating nerve injury via modulating the MGB axis. Thus, polysaccharides have great potential in the treatment of AA-induced cognitive impairment through improving gut microbiota.
In addition, polysaccharides not only directly influence the richness and diversity of gut microbiota, but also significantly affect microbial metabolites, such as SCFAs and BAs. It has been indicated that Ganoderma lucidum polysaccharides could increase the level of SCFAs (acetate and butyrate in particular), improve the intestinal barrier function, reduce the LPS level, and regulate the microbiota to increase the production of beneficial bacteria. Specifically, some SCFAs-producing bacteria and potential probiotics such as Allobaculum, Bifidobacterium, and Christensenellaceae_R-7_group increased due to the Ganoderma lucidum polysaccharides in serum and adipose tissue.139 The polysaccharides derived from Xiaoyaosan have been shown to modulate the composition and abundance of gut microbiota, restore the diversity of SCFAs-producing bacteria, and increase the content of SCFAs, thereby playing an antidepressant role in rats.140 Besides, polysaccharides also have a significant regulatory effect on BA metabolism. For instance, Grifola frondosa polysaccharides significantly enhanced the expression of cholesterol 7α-hydroxylase (CYP7A1), facilitating the biotransformation of BAs, and activated the bile salt export pump (BSEP), contributing to the synthesis and absorption of BAs in the liver.141 A sulfated polysaccharide from Gracilaria Lemaneiformis has been found to promote the excretion of total BAs in mouse feces by regulating gut microbiota, hereby ameliorating the disorders related to BAs metabolism.142 In conclusion, because of their probiotic properties, polysaccharides are involved in maintaining or restoring microecological balance, so they have been increasingly developed as health food adjuvants. They are also becoming a promising way to alleviate or prevent diseases associated with gut microbiota.143
Mitigating the effect of polysaccharides on neurotoxicity
As mentioned earlier, AA has the potential to induce neurotoxic effects in humans, primarily through the mechanisms of oxidative stress and inflammation. Obviously, polysaccharides play a significant role by improving the above oxidative stress and inflammatory response in the treatment of many diseases associated with gut microbiota. Therefore, they are expected to alleviate AA-induced neurotoxicity through the MGB axis, due to their biological activities and beneficial effects on gut microbiota.In vivo studies showed that some polysaccharides have beneficial antioxidant effects by decreasing the level of lipid peroxidation and increasing the activity of antioxidant enzymes.144,145 A study revealed that Ganoderma lucidum polysaccharides mitigated exercise-induced oxidative stress by increasing the activity of antioxidant enzymes: superoxide dismutase (from 110–170 U mg−1 protein), catalase (from 1.58–1.95 U mg−1 protein), and glutathione peroxidase (from 6.0–15.0 U mg−1 protein).146Astragalus polysaccharides can also alleviate 6-hydroxydopamine-induced neurotoxicity by reducing oxidative stress, regulating the apoptosis pathway and cholinergic system.147 Silent information regulator 1 (Sirt1) can regulate the expression of antioxidant molecules through various mechanisms, and Sirt1 is closely related to the stability and activity of Nrf2, which may be involved in the oxidative stress regulation mediated by Nrf2.148 In vitro experiments have shown that a polysaccharide (AFP-2) derived from the flowers of Apios americana Medik activated the intracellular antioxidant system through the Sirt1/Nrf2 signalling pathway. This activation effectively mitigates ROS accumulation and ameliorates mitochondrial dysfunction. Further studies have shown that AFP-2 can induce autophagy, which was found to be involved in the antioxidative process of AFP-2 in rat pheochromocytoma line 12 (PC12) cells.108
Polysaccharides possess potential anti-neuroinflammatory effects, effectively targeting the inhibition of excessive microglial activation, reduction of pro-inflammatory factors, and modulation of related signalling pathways.149 Some polysaccharides can reduce the levels of inflammatory cytokines IL-1β, IL-6, IL-8, and IL-17, increase the level of anti-inflammatory cytokines IL-10, and thus play an anti-inflammatory role.150 For example, Fu et al.151 studied the structure of polysaccharides from the leaves of Chinese aconitum (Aconitum Carmichaelii) and proved that these polysaccharides have anti-inflammatory effects on intestinal epithelial cell inflammation induced by LPS. Furthermore, Ganoderma lucidum polysaccharides can also attenuate the LPS-mediated inflammatory response by inhibiting NF-κB or the mitogen activated protein kinases (MAPK) signalling pathway (the main signal pathway that controls the macrophage immune response), reducing the release of above pro-inflammatory cytokines and improving resistance to neurodegenerative diseases.110 Similarly, Zhong et al.152 studied the neuroprotective and anti-neuroinflammatory effects of Acorus tatarinowii polysaccharides by using the BV2 microglia model, which is induced by LPS. The mechanism of action involves inhibiting the overactivation of the LPS-induced pro-inflammatory BV2 microglia, blocking the TLR4-mediated phosphoinositide 3-kinase/protein kinase B and MyD88/NF-κB signalling pathways, thereby reducing the levels of inflammatory mediators and cytokines. Additionally, Acorus tatarinowii polysaccharides have been shown to protect cortical and hippocampal primary neurons from neurotoxic damage. Additionally, current biochemical and clinical studies have shown that some polysaccharides are effective immunomodulators. The immunomodulatory activity is related to their effects on effector cells, such as macrophages, B and T lymphocytes, natural killer cells and dendritic cells.153 For instance, Bupleurum polysaccharides could inhibit the overexpression of pro-inflammatory mediators (TNF-α, IL-1β, and IL-6) in LPS-induced macrophages, which confer benefits on autoimmune inflammatory diseases.154 Studies have shown that Ganoderma lucidum polysaccharides produced great protection of the spleen and thymus, which are both important organs closely related to immune function. They were also effective in promoting hematopoiesis and increasing the levels of IgA in serum.155 In addition, polysaccharides from Anemarrhena asphodeloides Bunge (AAP70-1) have been shown to protect the SH-SY5Y cell from CoCl2-induced apoptosis. The results suggest that AAP70-1 has potential as a therapeutic agent for CNS diseases or as an immunomodulatory agent.156 Because of the existence of bidirectional communication between the nervous and immune systems, polysaccharides can be used as an immunomodulator to promote the development of the nervous system.13
In summary, numerous studies have demonstrated that polysaccharides can positively impact the intestinal barrier through their various biological activities. Specifically, these polysaccharides can decrease lipid peroxidation, reduce ROS levels in the intestine, and inhibit oxidative stress through multiple pathways. They can also exhibit anti-inflammatory properties by reducing pro-inflammatory factors in the intestine and inhibiting the overactivation of microglia. Additionally, they can protect the CNS by modulating immunity and reducing cell apoptosis. Therefore, it is speculated that polysaccharides have great potential in improving the neurotoxicity caused by AA.
Conclusions
AA, produced during the processing of many UPFs, can cause severe neurotoxicity in humans. AA damages the intestinal tissue by injuring the intestinal barrier and changing the composition and abundance of the gut microbiota, resulting in neurotoxicity through the MGB axis. Therefore, it is advisable to reduce the intake of UPFs and develop healthy eating habits. Polysaccharides play a key role in improving the intestinal barrier and microbiota and because of their extensive biological activities, they have great potential in the treatment of neurotoxicity caused by AA. Current research on the neuroprotective effects of polysaccharides predominantly focuses on animal and cell models. Future clinical trials are necessary to validate the therapeutic effects of these polysaccharides on patients with neurodegenerative diseases. In addition, most experiments utilize crude polysaccharides, which may cause antagonistic effects between different components, thereby interfering with the research. Thus, additional purification experiments and structural analyses are needed to obtain polysaccharides of higher purity and better activity. Given the pivotal role of the gut microbiota and the brain in the host, future studies could concentrate on polysaccharide-based foods aimed at promoting gut health. In addition, the structure–activity relationships of natural polysaccharides also warrant further investigation to better comprehend how various structural characteristics influence gut microbiota and the MGB axis.In daily diets, individuals can modulate gut microbiota and reduce the harm of AA in UPFs by consuming more polysaccharide-rich foods. Additionally, the development of more microbiota-oriented foods may emerge as a new trend, and medicines or functional foods with natural polysaccharides as primary ingredients may find extensive clinical applications in treating neurodegenerative diseases.
Author contributions
Chen Cai: investigation, writing–original draft. Zheyi Song: writing–review and editing. Xinrui Xu: writing–review and editing. Xin Yang: writing–original draft. Siyu Wei: conceptualization, investigation. Fang Chen: conceptualization, investigation. Xu Dong: supervision, writing–review and editing. Xin Zhang: supervision, writing–review and editing. Yuchen Zhu: writing–review and editing, funding acquisition, supervision.Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 32072333) and the Young Elite Scientists Sponsorship Program by CAST (No. 2020QNRC001).References
- A. Aguirre, M. T. Borneo, S. El Khori and R. Borneo, Food Res. Int., 2019, 115, 535–540 CrossRef PubMed.
- R. J. Ostfeld and K. E. Allen, J. Am. Coll. Cardiol., 2021, 77, 1532–1534 CrossRef PubMed.
- F. Juul, G. Vaidean, Y. Lin, A. L. Deierlein and N. Parekh, J. Am. Coll. Cardiol., 2021, 77, 1520–1531 CrossRef PubMed.
- M. Nardocci, B. S. Leclerc, M. L. Louzada, C. A. Monteiro, M. Batal and J. C. Moubarac, Can. J. Public Health, 2019, 110, 4–14 CrossRef PubMed.
- R. B. Levy, F. Rauber, K. Chang, M. Louzada, C. A. Monteiro, C. Millett and E. P. Vamos, Clin. Nutr., 2021, 40, 3608–3614 CrossRef PubMed.
- Y. Zhang and E. L. Giovannucci, Crit. Rev. Food Sci. Nutr., 2023, 63, 10836–10848 CrossRef PubMed.
- B. Y. Wu, X. Y. Chai, A. M. He, Z. Huang, S. Chen, P. F. Rao, L. J. Ke and L. W. Xiang, Food Chem. Toxicol., 2021, 154, 112315 CrossRef PubMed.
- M. A. Adimas, B. D. Abera, Z. T. Adimas, H. W. Woldemariam and M. A. Delele, Heliyon, 2024, 10, e30258 CrossRef PubMed.
- V. Paul, R. Ezekiel and R. Pandey, Acta Physiol. Plant., 2016, 38, 276 CrossRef.
- B. Zödl, D. Schmid, G. Wassler, C. Gundacker, V. Leibetseder, T. Thalhammer and C. Ekmekcioglu, Toxicology, 2007, 232, 99–108 CrossRef PubMed.
- E. Sengul, V. Gelen, S. Yildirim, S. Tekin and Y. Dag, Biol. Trace Elem. Res., 2020, 199, 173–184 CrossRef PubMed.
- M. Zhao, L. Deng, X. Lu, L. Fan, Y. Zhu and L. Zhao, Environ. Sci. Pollut. Res., 2022, 29, 41151–41167 CrossRef PubMed.
- T. C. Fung, C. A. Olson and E. Y. Hsiao, Nat. Neurosci., 2017, 20, 145–155 CrossRef PubMed.
- E. A. Mayer, K. Tillisch and A. Gupta, J. Clin. Invest., 2015, 125, 926–938 CrossRef PubMed.
- B. M. Tian, J. H. Zhao, M. Zhang, Z. F. Chen, Q. Y. Ma, H. C. Liu, C. X. Nie, Z. Q. Zhang, W. An and J. X. Li, Mol. Nutr. Food Res., 2021, 65, 2000745 CrossRef PubMed.
- M. Kreuzer and W. D. Hardt, Annu. Rev. Microbiol., 2020, 74, 787–813 CrossRef PubMed.
- Y. L. Hu, D. W. Chen, P. Zheng, J. Yu, J. He, X. B. Mao and B. Yu, BioMed Res. Int., 2019, 2019, 5403761 Search PubMed.
- A. H. Al-Khafaji, S. D. Jepsen, K. R. Christensen and L. K. Vigsnæs, J. Funct. Foods, 2020, 74, 104176 CrossRef.
- Y. Y. Cheng, Y. Wang, W. Zhang, J. B. Yin, J. C. Dong and J. T. Liu, Medicine, 2022, 101, e28910 CrossRef PubMed.
- D. Song, C. S. Yang, X. Zhang and Y. Wang, Crit. Rev. Food Sci. Nutr., 2020, 61, 139–148 CrossRef PubMed.
- X. Z. Wang, L. Cheng, Y. A. Liu, R. L. Zhang, Z. F. Wu, P. F. Weng, P. Zhang and X. Zhang, Front. Microbiol., 2022, 13, 807076 CrossRef.
- J. F. Cryan and S. M. O'Mahony, Neurogastroenterol. Motil., 2011, 23, 187–192 CrossRef PubMed.
- M. Zinöcker and I. Lindseth, Nutrients, 2018, 10, 365 CrossRef PubMed.
- X. Tan, J. Ye, W. Liu, B. Zhao, X. Shi, C. Zhang, Z. Liu and X. Liu, Arch. Toxicol., 2018, 93, 467–486 CrossRef PubMed.
- A. Singh, Parkinsonism Relat. Disord., 2020, 79, E21–E21 CrossRef.
- G. M. Shashidhar, P. Giridhar and B. Manohar, RSC Adv., 2015, 5, 16050–16066 RSC.
- L. Yuan, Z. C. Zhong and Y. Liu, Int. J. Biol. Macromol., 2020, 154, 1400–1407 CrossRef PubMed.
- C. García-Montero, O. Fraile-Martínez, A. M. Gómez-Lahoz, L. Pekarek, A. J. Castellanos, F. Noguerales-Fraguas, S. Coca, L. G. Guijarro, N. García-Honduvilla, A. Asúnsolo, L. Sanchez-Trujillo, G. Lahera, J. Bujan, J. Monserrat, M. Álvarez-Mon, M. A. Álvarez-Mon and M. A. Ortega, Nutrients, 2021, 13, 699 CrossRef.
- Y. Yang, L. Zhang, G. Jiang, A. Lei, Q. Yu, J. Xie and Y. Chen, Food Funct., 2019, 10, 5863–5872 RSC.
- Y. Yao, L. J. Yan, H. Chen, N. Wu, W. B. Wang and D. S. Wang, Phytomedicine, 2020, 77, 153268 CrossRef.
- M. Chen, X. Chen, K. Wang, L. Cai, N. Liu, D. Zhou, W. Jia, P. Gong, N. Liu and Y. Sun, Front. Nutr., 2023, 10, 1080825 CrossRef.
- Y. X. Guo, X. F. Chen, P. Gong, Z. X. Li, Y. P. Wu, J. Zhang, J. T. Wang, W. B. Yao, W. J. Yang and F. X. Chen, Phytomedicine, 2023, 109, 154566 CrossRef.
- C. A. Monteiro, Public Health Nutr., 2009, 12, 729–731 CrossRef.
- C. A. Monteiro, J. C. Moubarac, R. B. Levy, D. S. Canella, M. Louzada and G. Cannon, Public Health Nutr., 2018, 21, 18–26 CrossRef PubMed.
- F. Lauria, M. Dello Russo, A. Formisano, S. De Henauw, A. Hebestreit, M. Hunsberger, V. Krogh, T. Intemann, L. Lissner, D. Molnar, L. A. Moreno, L. A. Reisch, M. Tornaritis, T. Veidebaum, G. Williams, A. Siani, P. Russo and I. F. Consortium, Nutr. Metab. Cardiovasc. Dis., 2021, 31, 3031–3043 CrossRef.
- V. Magalhaes, M. Severo, D. Correia, D. Torres, R. Costa de Miranda, F. Rauber, R. Levy, S. Rodrigues and C. Lopes, J. Nutr. Sci., 2021, 10, e89 CrossRef PubMed.
- E. Ruggiero, S. Esposito, S. Costanzo, A. Di Castelnuovo, C. Cerletti, M. B. Donati, G. de Gaetano, L. Iacoviello, M. Bonaccio and I. S. Investigators, Public Health Nutr., 2021, 24, 6258–6271 CrossRef.
- L. Wang, E. Martínez Steele, M. Du, J. L. Pomeranz, L. E. O'Connor, K. A. Herrick, H. Luo, X. Zhang, D. Mozaffarian and F. F. Zhang, J. Am. Med. Assoc., 2021, 326, 519–530 CrossRef PubMed.
- J. S. Shim, S. Y. Shim, H. J. Cha, J. Kim and H. C. Kim, Nutrients, 2021, 13, 1120 CrossRef PubMed.
- P. V. L. Moreira, L. Hyseni, J.-C. Moubarac, A. P. B. Martins, L. G. Baraldi, S. Capewell, M. O'Flaherty and M. Guzman-Castillo, Public Health Nutr., 2017, 21, 181–188 CrossRef.
- M. J. Gibney, Curr. Dev. Nutr., 2019, 3, nzy077 CrossRef PubMed.
- D. Martini, J. Godos, M. Bonaccio, P. Vitaglione and G. Grosso, Nutrients, 2021, 13, 3390 CrossRef PubMed.
- B. P. Riboldi, Á. M. Vinhas and J. D. Moreira, Food Chem., 2014, 157, 310–322 CrossRef.
- F. Juul, G. Vaidean and N. Parekh, Adv. Nutr., 2021, 12, 1673–1680 CrossRef PubMed.
- E. Oz, Food Chem., 2021, 352, 129378 CrossRef.
- A. Fardet and E. Rock, Trends Food Sci. Technol., 2019, 93, 174–184 CrossRef.
- V. Matoso, P. Bargi-Souza, F. Ivanski, M. A. Romano and R. M. Romano, Food Chem., 2019, 283, 422–430 CrossRef PubMed.
- Z. L. Li, C. Y. Zhao and C. W. Cao, Molecules, 2023, 28, 3476 CrossRef PubMed.
- A. Hamzalioglu and V. Gökmen, Food Chem., 2020, 318, 126467 CrossRef PubMed.
- D. Jiang, S. Wang, H. P. Li, L. J. Xu, X. Hu, B. Barati and A. Q. Zheng, ACS Sustainable Chem. Eng., 2021, 9, 3095–3103 CrossRef.
- I. Govindaraju, M. Sana, I. Chakraborty, M. H. Rahman, R. Biswas and N. Mazumder, Foods, 2024, 13, 556 CrossRef.
- E. Commission, Off. J. Eur. Communities, 2017, 304, 24–44 Search PubMed.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), EFSA J., 2015, 13, 4140 Search PubMed.
- S. P. Zhong, Z. He, M. N. Chaudhary, Y. X. Mu, K. Zhou, A. Yang, L. Y. Luo, L. Zeng and W. Luo, LWT – Food Sci. Technol., 2024, 200, 116174 CrossRef.
- E. Devseren, D. Okut, M. Koç, Ö. Ocak, H. Karatas and F. Kaymak-Ertekin, Acta Chim. Slov., 2021, 68, 25–36 CrossRef.
- A. Antunes-Rohling, S. Ciudad-Hidalgo, J. Mir-Bel, J. Raso, G. Cebrián and I. Álvarez, Innovative Food Sci. Emerging Technol., 2018, 49, 158–169 CrossRef.
- L. Rifai and F. A. Saleh, Int. J. Toxicol., 2020, 39, 93–102 CrossRef PubMed.
- T. P. Rajesh, V. A. Basheer, A. S. B. Packirisamy, S. N. Ravi and S. Vallinayagam, Top. Catal., 2024, 67, 300–312 CrossRef.
- R. Y. Jia, X. Wan, X. Geng, D. M. Xue, Z. X. Xie and C. R. Chen, Microorganisms, 2021, 9, 1659 CrossRef CAS PubMed.
- A. K. Meghavarnam and S. Janakiraman, LWT – Food Sci. Technol., 2018, 89, 32–37 CrossRef.
- IARC, Acrylamide, Lyon, France, 1994 Search PubMed.
- Y. Liu, X. Zhang, D. D. Yan, Y. Q. Wang, N. Wang, Y. F. Liu, A. J. Tan, X. Y. Chen and H. Yan, Toxicol. Appl. Pharmacol., 2020, 393, 114949 CrossRef PubMed.
- H. Mojska, I. Gielecinska, J. Winiarek and W. Sawicki, Toxics, 2021, 9, 298 CrossRef.
- J. S. Park, P. Samanta, S. Lee, J. Lee, J. W. Cho, H. S. Chun, S. Yoon and W. K. Kim, Int. J. Mol. Sci., 2021, 22, 3518 CrossRef.
- B. Srour, L. K. Fezeu, E. Kesse-Guyot, B. Allès, C. Méjean, R. M. Andrianasolo, E. Chazelas, M. Deschasaux, S. Hercberg, P. Galan, C. A. Monteiro, C. Julia and M. Touvier, BMJ [Br. Med. J.], 2019, 365, l1451 Search PubMed.
- Y. Zhang, M. M. Huang, P. Zhuang, J. J. Jiao, X. Y. Chen, J. Wang and Y. N. Wu, Environ. Int., 2018, 117, 154–163 CrossRef PubMed.
- S. Lee, H. R. Park, J. Y. Lee, J. H. Cho, H. M. Song, A. H. Kim, W. Lee, Y. Lee, S. C. Chang, H. S. Kim and J. Lee, J. Toxicol. Environ. Health, Part A, 2018, 81, 254–265 CrossRef PubMed.
- B. Newland, P. Wolff, D. Z. Zhou, W. Wang, H. Zhang, A. Rosser, W. X. Wang and C. Werner, Biomater. Sci., 2016, 4, 400–404 RSC.
- M. Zhao, B. Zhang and L. Deng, Front. Nutr., 2022, 9, 859189 CrossRef PubMed.
- M. Y. Zhao, L. L. Deng, X. X. Lu, L. Q. Fan, Y. Zhu and L. M. Zhao, Environ. Sci. Pollut. Res., 2022, 29, 41151–41167 CrossRef PubMed.
- J. G. Wang, H. H. Dou and Q. Y. Liang, Eur. J. Haematol., 2024 DOI:10.1111/ejh.14310.
- M. Y. Zhao, F. S. L. Wang, X. S. Hu, F. Chen and H. M. Chan, Food Chem. Toxicol., 2017, 106, 25–35 CrossRef PubMed.
- J. G. Lee, Y. S. Wang and C. C. Chou, Toxicol. In Vitro, 2014, 28, 562–570 CrossRef PubMed.
- E. Altinoz, Y. Turkoz and N. Vardi, Bratislava Med. J., 2015, 116, 252–258 CrossRef PubMed.
- P. Dobrowolski, P. Huet, P. Karlsson, S. Eriksson, E. Tomaszewska, A. Gawron and S. G. Pierzynowski, Nutrition, 2012, 28, 428–435 CrossRef PubMed.
- I. Ghorbel, A. Elwej, K. Jamoussi, T. Boudawara, N. G. Kamoun and N. Zeghal, Food Funct., 2015, 6, 1126–1135 RSC.
- S. Gedik, M. E. Erdemli, M. Gul, B. Yigitcan, H. Gozukara Bag, Z. Aksungur and E. Altinoz, Biotech. Histochem., 2018, 93, 267–276 CrossRef PubMed.
- L. Pastorelli, C. De Salvo, J. R. Mercado, M. Vecchi and T. T. Pizarro, Front. Immunol., 2013, 4, 280 Search PubMed.
- Z. Yue, Y. Chen, Q. Dong, D. Li, M. Guo, L. Zhang, Y. Shi, H. Wu, L. Li and Z. Sun, Food Res. Int., 2022, 157, 111405 CrossRef PubMed.
- Z. Wang, H. Liu, J. Liu, X. Ren, G. Song, X. Xia and N. Qin, Foods, 2021, 10, 2990 CrossRef PubMed.
- P. J. Turnbaugh, M. Hamady, T. Yatsunenko, B. L. Cantarel, A. Duncan, R. E. Ley, M. L. Sogin, W. J. Jones, B. A. Roe, J. P. Affourtit, M. Egholm, B. Henrissat, A. C. Heath, R. Knight and J. I. Gordon, Nature, 2009, 457, U480–U487 CrossRef PubMed.
- S. H. Guo, M. Nighot, R. Al-Sadi, T. Alhmoud, P. Nighot and T. Y. Ma, J. Immunol., 2015, 195, 4999–5010 CrossRef PubMed.
- B. Nan, C. Y. Yang, L. Li, H. Q. Ye, H. Y. Yan, M. H. Wang and Y. Yuan, Food Chem. Toxicol., 2021, 148, 111937 CrossRef CAS PubMed.
- K. A. Krautkramer, J. Fan and F. Bäckhed, Nat. Rev. Microbiol., 2021, 19, 77–94 CrossRef CAS PubMed.
- H. Shapiro, A. A. Kolodziejczyk, D. Halstuch and E. Elinav, J. Exp. Med., 2018, 215, 383–396 CrossRef CAS.
- J. S. Park, E. J. Lee, J. C. Lee, W. K. Kim and H. S. Kim, Int. Immunopharmacol., 2007, 7, 70–77 CrossRef CAS PubMed.
- Y. Yuan, L. Lu, N. Bo, Y. Chaoyue and Y. Haiyang, J. Agric. Food Chem., 2021, 69, 12837–12852 CrossRef.
- T. R. Sampson and S. K. Mazmanian, Cell Host Microbe, 2015, 17, 565–576 CrossRef PubMed.
- E. M. M. Quigley, Curr. Neurol. Neurosci. Rep., 2017, 17, 94 CrossRef.
- K. G. Margolis, J. F. Cryan and E. A. Mayer, Gastroenterology, 2021, 160, 1486–1501 CrossRef.
- J. F. Cryan, K. J. O'Riordan, C. S. M. Cowan, K. V. Sandhu, T. F. S. Bastiaanssen, M. Boehme, M. G. Codagnone, S. Cussotto, C. Fulling, A. V. Golubeva, K. E. Guzzetta, M. Jaggar, C. M. Long-Smith, J. M. Lyte, J. A. Martin, A. Molinero-Perez, G. Moloney, E. Morelli, E. Morillas, R. O'Connor, J. S. Cruz-Pereira, V. L. Peterson, K. Rea, N. L. Ritz, E. Sherwin, S. Spichak, E. M. Teichman, M. van de Wouw, A. P. Ventura-Silva, S. E. Wallace-Fitzsimons, N. Hyland, G. Clarke and T. G. Dinan, Physiol. Rev., 2019, 99, 1877–2013 CrossRef.
- Z. Song, R. Song, Y. Liu, Z. Wu and X. Zhang, Food Res. Int., 2023, 167, 112730 CrossRef.
- J. B. Furness, Nat. Rev. Gastroenterol. Hepatol., 2012, 9, 286–294 CrossRef.
- J. Cryan, Psychoneuroendocrinology, 2021, 131, S35–S35 CrossRef.
- G. Agirman and E. Y. Hsiao, Cell, 2021, 184, 2524–2525 CrossRef.
- A. D. Silva and S. R. Bloom, Gut Liver, 2012, 6, 10–20 CrossRef.
- G. M. Mawe and J. M. Hoffman, Nat. Rev. Gastroenterol. Hepatol., 2013, 10, 473–486 CrossRef CAS.
- M. S. Shajib, A. Baranov and W. I. Khan, ACS Chem. Neurosci., 2017, 8, 920–931 CrossRef PubMed.
- J. Liang, H. L. Li, J. Q. Chen, L. He, X. H. Du, L. Zhou, Q. P. Xiong, X. P. Lai, Y. Q. Yang, S. Huang and S. Z. Hou, Pharmacol. Res., 2019, 148, 104417 CrossRef.
- K. Oh-oka, H. Kono, K. Ishimaru, K. Miyake, T. Kubota, H. Ogawa, K. Okumura, S. Shibata and A. Nakao, PLoS One, 2014, 9, e98016 CrossRef.
- J. Guo, P. Lin, X. Zhao, J. Zhang, X. Wei, Q. Wang and C. Wang, Neuroscience, 2014, 263, 1–14 CrossRef PubMed.
- C. Hyman, M. Hofer, Y. A. Barde, M. Juhasz, G. D. Yancopoulos, S. P. Squinto and R. M. Lindsay, Nature, 1991, 350, 230–232 CrossRef.
- A. H. Nagahara, D. A. Merrill, G. Coppola, S. Tsukada, B. E. Schroeder, G. M. Shaked, L. Wang, A. Blesch, A. Kim, J. M. Conner, E. Rockenstein, M. V. Chao, E. H. Koo, D. Geschwind, E. Masliah, A. A. Chiba and M. H. Tuszynski, Nat. Med., 2009, 15, 331–337 CrossRef.
- L. P. Zhao, Nat. Rev. Microbiol., 2013, 11, 639–647 CrossRef.
- K. U. Saikh, Immunol. Res., 2021, 69, 117–128 CrossRef PubMed.
- D. Su, A. T. Lei, C. C. Nie and Y. Chen, Food Chem. Toxicol., 2023, 171, 113548 CrossRef.
- X. Z. Zhang, Y. L. Wan, J. G. Feng, M. H. Li and Z. Q. Jiang, Mol. Med. Rep., 2021, 23, 230 CrossRef PubMed.
- Q. Chu, M. Chen, D. X. Song, X. X. Li, Y. Y. Yang, Z. H. Zheng, Y. L. Li, Y. Y. Liu, L. S. Yu, Z. Hua and X. D. Zheng, Int. J. Biol. Macromol., 2019, 123, 1115–1124 CrossRef PubMed.
- X. Xie, Y. Wu, H. Xie, H. Wang, X. Zhang, J. Yu, S. Zhu, J. Zhao, L. Sui and S. Li, Front. Pharmacol., 2022, 13, 790136 CrossRef.
- Y. Sun, C. T. Ho, Y. Zhang, M. Hong and X. Zhang, J. Tradit. Complementary Med., 2023, 13, 128–134 CrossRef.
- M. Yin, Y. Zhang and H. Li, Front. Immunol., 2019, 10, 145 CrossRef PubMed.
- Y. X. Guo, X. F. Chen and P. Gong, Int. J. Biol. Macromol., 2021, 183, 1753–1773 CrossRef.
- J. Y. Huo, Z. Y. Wu, W. Z. Sun, Z. H. Wang, J. H. Wu, M. Q. Huang, B. W. Wang and B. G. Sun, J. Agric. Food Chem., 2022, 70, 711–735 CrossRef.
- B. Wang, L. L. Yan, S. C. Guo, L. Wen, M. L. Yu, L. Feng and X. B. Jia, Front. Nutr., 2022, 9, 908175 CrossRef.
- Y. Saito, M. Kinoshita, A. Yamada, S. Kawano, H. S. Liu, S. Kamimura, M. Nakagawa, S. Nagasawa, T. Taguchi, S. Yamada and H. Moritake, Cancer Sci., 2021, 112, 4944–4956 CrossRef.
- R. Sorourian, A. E. Khajehrahimi, M. Tadayoni, M. H. Azizi and M. Hojjati, Int. J. Biol. Macromol., 2020, 160, 758–768 CrossRef.
- B. Du, M. Meenu, H. Z. Liu and B. J. Xu, Int. J. Mol. Sci., 2019, 20, 4032 CrossRef PubMed.
- S. Shao, D. D. Wang, W. Zheng, X. Y. Li, H. Zhang, D. Q. Zhao and M. X. Wang, Int. Immunopharmacol., 2019, 71, 411–422 CrossRef.
- Q. Song, Y. Wang, L. Huang, M. Shen, Y. Yu, Q. Yu, Y. Chen and J. Xie, Food Res. Int., 2021, 140, 109858 CrossRef PubMed.
- C. Guo, D. Guo, L. Fang, T. Sang, J. Wu, C. Guo, Y. Wang, Y. Wang, C. Chen, J. Chen, R. Chen and X. Wang, Carbohydr. Polym., 2021, 267, 118231 CrossRef PubMed.
- S. Z. Xie, B. Liu, H. Y. Ye, Q. M. Li, L. H. Pan, X. Q. Zha, J. Liu, J. Duan and J. P. Luo, Carbohydr. Polym., 2019, 206, 149–162 CrossRef PubMed.
- H. Tremlett, K. C. Bauer, S. Appel-Cresswell, B. B. Finlay and E. Waubant, Ann. Neurol., 2017, 81, 369–382 CrossRef.
- H. J. Flint, E. A. Bayer, M. T. Rincon, R. Lamed and B. A. White, Nat. Rev. Microbiol., 2008, 6, 121–131 CrossRef PubMed.
- Q. Fang, J. Hu, Q. Nie and S. Nie, Trends Food Sci. Technol., 2019, 92, 65–70 CrossRef.
- Q. Shang, H. Jiang, C. Cai, J. Hao, G. Li and G. Yu, Carbohydr. Polym., 2018, 179, 173–185 CrossRef PubMed.
- N. M. Koropatkin, E. A. Cameron and E. C. Martens, Nat. Rev. Microbiol., 2012, 10, 323–335 CrossRef PubMed.
- N. Zmora, J. Suez and E. Elinav, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 35–56 CrossRef.
- M. Wang, Z. Xie, L. Li, Y. Chen, Y. Li, Y. Wang, B. Lu, S. Zhang, F. Ma, C. Ma, L. Lin and Q. Liao, Food Funct., 2019, 10, 2658–2675 RSC.
- M. R. Park, M. Shin, D. Mun, S. Y. Jeong, D. Y. Jeong, M. Song, G. Ko, T. Unno, Y. Kim and S. Oh, Sci. Rep., 2020, 10, 21701 CrossRef CAS PubMed.
- M. L. Xue, X. Y. Teng, H. Liang, J. L. Zhao, Y. S. Jiang, X. Qiu, Z. Zhang, Z. Q. Pei, N. Zhang and Y. M. Qin, J. Funct. Foods, 2021, 86, 104726 CrossRef.
- P. Chen, M. F. Hei, L. L. Kong, Y. Y. Liu, Y. Yang, H. B. Mu, X. Y. Zhang, S. T. Zhao and J. Y. Duan, Food Funct., 2019, 10, 8161–8171 RSC.
- L. Guo, P. L. Xiao, X. X. Zhang, Y. Yang, M. Yang, T. Wang, H. X. Lu, H. Y. Tian, H. Wang and J. Liu, Food Funct., 2021, 12, 1156–1175 RSC.
- Q. P. Zhang, J. Cheng, Q. Liu, G. H. Xu, C. F. Li and L. T. Yi, J. Funct. Foods, 2022, 88, 104912 CrossRef.
- H. R. Li, Y. H. Xiao, H. Li, Y. Jia, S. L. Luo, D. D. Zhang, L. Zhang, P. Wu, C. J. Xiao, W. J. Kan, J. Du and H. K. Bao, Brain Res. Bull., 2021, 171, 16–24 CrossRef.
- J. D. Hoffman, L. M. Yanckello, G. Chlipala, T. C. Hammond, S. D. McCulloch, I. Parikh, S. Sun, J. M. Morganti, S. J. Green and A. L. Lin, PLoS One, 2019, 14, e0221828 CrossRef.
- A. X. Su, W. J. Yang, L. Y. Zhao, F. Pei, B. Yuan, L. Zhong, G. X. Ma and Q. H. Hu, Food Funct., 2018, 9, 1424–1432 RSC.
- X. L. Dong, X. Wang, F. Liu, X. Liu, Z. R. Du, R. W. Li, C. H. Xue, K. H. Wong, W. T. Wong, Q. Zhao and Q. J. Tang, Int. J. Biol. Macromol., 2020, 164, 994–1005 CrossRef.
- S. L. Luo, X. Zhang, S. Huang, X. P. Feng, X. J. Zhang and D. X. Xiang, Int. J. Biol. Macromol., 2022, 213, 404–415 CrossRef.
- T. Sang, C. Guo, D. Guo, J. Wu, Y. Wang, Y. Wang, J. Chen, C. Chen, K. Wu, K. Na, K. Li, L. Fang, C. Guo and X. Wang, Carbohydr. Polym., 2021, 256, 117594 CrossRef PubMed.
- L. L. Xiong, Y. N. Wu, Q. L. Shu and W. Xiong, J. Appl. Microbiol., 2023, 134, lxad052 CrossRef.
- W. L. Guo, J. C. Deng, Y. Y. Pan, J. X. Xu, J. L. Hong, F. F. Shi, G. L. Liu, M. Qian, W. D. Bai, W. Zhang, B. Liu, Y. Y. Zhang, P. J. Luo, L. Ni, P. F. Rao and X. C. Lv, Int. J. Biol. Macromol., 2020, 153, 1231–1240 CrossRef.
- S. M. Huang, D. R. Pang, X. Li, L. J. You, Z. G. Zhao, P. C. K. Cheung, M. W. Zhang and D. Liu, Food Funct., 2019, 10, 3224–3236 RSC.
- R. T. Wu, L. F. Wang, Y. F. Yao, T. Sang, Q. L. Wu, W. W. Fu, M. Wan and W. J. Li, Biomed. Pharmacother., 2022, 155, 113681 CrossRef.
- D. Cör, Z. Knez and M. K. Hrncic, Molecules, 2018, 23, 649 CrossRef PubMed.
- Y. Liu, Y. Y. Sun and G. L. Huang, Int. J. Biol. Macromol., 2018, 111, 780–786 CrossRef PubMed.
- Z. H. Zhao, X. W. Zheng and F. Fang, Saudi J. Biol. Sci., 2014, 21, 119–123 CrossRef PubMed.
- H. Li, R. Shi, F. Ding, H. Wang, W. Han, F. Ma, M. Hu, C. W. Ma and Z. Huang, Oxid. Med. Cell. Longevity, 2016, 2016, 1–10 CrossRef PubMed.
- J. B. Pi, Q. Zhang, J. Q. Fu, C. G. Woods, Y. Y. Hou, B. E. Corkey, S. Collins and M. E. Andersen, Toxicol. Appl. Pharmacol., 2010, 244, 77–83 CrossRef PubMed.
- X. L. Xu, S. Li, R. Zhang and W. D. Le, Neural Regener. Res., 2022, 17, 1907–1912 CrossRef PubMed.
- S. Zhang, M. Zhang, W. Li, L. Ma, X. Liu, Q. Ding, W. Yu, T. Yu, C. Ding and W. Liu, Int. J. Biol. Macromol., 2023, 253, 126799 CrossRef PubMed.
- Y. P. Fu, C. Y. Li, X. Peng, Y. F. Zou, F. Rise, B. S. Paulsen, H. Wangensteen and K. T. Inngjerdingen, Carbohydr. Polym., 2022, 291, 119655 CrossRef PubMed.
- J. Zhong, X. Qiu, Q. Yu, H. Y. Chen and C. Y. Yan, Int. J. Biol. Macromol., 2020, 163, 464–475 CrossRef PubMed.
- S. L. Zhang, G. B. Pang, C. Chen, J. Z. Qin, H. Yu, Y. M. Liu, X. H. Zhang, Z. T. Song, J. Zhao, F. J. Wang, Y. Y. Wang and L. W. Zhang, Carbohydr. Polym., 2019, 205, 192–202 CrossRef PubMed.
- X. Q. Cheng, H. Li, X. L. Yue, J. Y. Xie, Y. Y. Zhang, H. Y. Di and D. F. Chen, J. Ethnopharmacol., 2010, 130, 363–368 CrossRef PubMed.
- J. Li, F. Gu, C. Cai, M. Hu, L. Fan, J. Hao and G. Yu, Int. J. Biol. Macromol., 2019, 143, 806–813 CrossRef PubMed.
- S. J. Zhang, Q. Zhang, L. J. An, J. J. Zhang, Z. G. Li, J. Zhang, Y. H. Li, M. Tuerhong, Y. Ohizumi, J. Jin, J. Xu and Y. Q. Guo, Carbohydr. Polym., 2020, 229, 115477 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |

