Open Access Article
Open Access ArticleComprehensive assessment on the association of dietary vitamins with all-cause and cardiovascular mortality among individuals with prediabetes: evidence from NHANES 1999–2018†
Wenxuan
Ren‡
a,
Yang
Li‡
b,
Cihang
Lu
a,
Siying
Liu
b,
Ying
Shao
a and
Xiaoguang
Shi
 *a
*a
aDepartment of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, 110001, Liaoning, China. E-mail: xiaoguangshi_cmu@163.com
bDepartment of Endocrinology and Metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang, 110001, Liaoning, China
First published on 12th September 2024
Abstract
Background: Prediabetes has become a global health issue, and currently, the relationship between vitamin levels and mortality in prediabetes remains unclear. This study aims to investigate the association between the levels of eleven vitamins and all-cause and cardiovascular disease (CVD) mortality in prediabetes patients. Methods: This cross-sectional study included 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 prediabetes patients from 10 cycles of the National Health and Nutrition Examination Survey between 1999 and 2018. Mortality and underlying causes of death were determined by linking records from the National Death Index until December 31, 2019. Multivariable Cox proportional hazards regression models were established to assess hazard ratios and 95% confidence intervals for all-cause, CVD, cancer, and other mortalities. Restricted cubic splines were used to visualize non-linear associations between various vitamins and mortality risk. Results: During the follow-up period, 2316/14
634 prediabetes patients from 10 cycles of the National Health and Nutrition Examination Survey between 1999 and 2018. Mortality and underlying causes of death were determined by linking records from the National Death Index until December 31, 2019. Multivariable Cox proportional hazards regression models were established to assess hazard ratios and 95% confidence intervals for all-cause, CVD, cancer, and other mortalities. Restricted cubic splines were used to visualize non-linear associations between various vitamins and mortality risk. Results: During the follow-up period, 2316/14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 prediabetes patients died (12.55%), with 722 deaths (3.68%) attributed to CVD. After multivariable adjustment, vitamin B1, niacin, folate, vitamin C, vitamin E, and vitamin K levels exhibited non-linear associations with all-cause mortality (all p < 0.05). Vitamin B1, niacin, and vitamin E levels showed non-linear associations with CVD mortality (p < 0.05). Vitamin B6 exhibited a linear negative association with all-cause, CVD, and other mortalities (p > 0.05). However, vitamins A and B2 levels were not significantly associated with mortality rates (all p > 0.05). Consistent results were observed in the subgroup analyses after complete adjustment for variables. Conclusions: Higher levels of dietary vitamins B1, B6, niacin, folate, vitamin C, vitamin E, and vitamin K were significantly associated with lower risk of all-cause mortality and CVD mortality in patients with prediabetes. There was no association between vitamin A and B2 levels and all-cause and CVD mortality among individuals with prediabetes. These findings suggest the importance of correcting vitamin deficiencies to prevent mortality in prediabetes patients.
634 prediabetes patients died (12.55%), with 722 deaths (3.68%) attributed to CVD. After multivariable adjustment, vitamin B1, niacin, folate, vitamin C, vitamin E, and vitamin K levels exhibited non-linear associations with all-cause mortality (all p < 0.05). Vitamin B1, niacin, and vitamin E levels showed non-linear associations with CVD mortality (p < 0.05). Vitamin B6 exhibited a linear negative association with all-cause, CVD, and other mortalities (p > 0.05). However, vitamins A and B2 levels were not significantly associated with mortality rates (all p > 0.05). Consistent results were observed in the subgroup analyses after complete adjustment for variables. Conclusions: Higher levels of dietary vitamins B1, B6, niacin, folate, vitamin C, vitamin E, and vitamin K were significantly associated with lower risk of all-cause mortality and CVD mortality in patients with prediabetes. There was no association between vitamin A and B2 levels and all-cause and CVD mortality among individuals with prediabetes. These findings suggest the importance of correcting vitamin deficiencies to prevent mortality in prediabetes patients.
Background
As a global health issue, prediabetes is an intermediate metabolic state between normal glucose metabolism and diabetes that reportedly increases the risk of developing type 2 diabetes to 70%,1,2 with approximately 5–10% of individuals with prediabetes progressing to diabetes each year.3 Notably, a review of the data from the National Health and Nutrition Examination Survey (NHANES) shows that the prevalence of prediabetes is gradually increasing worldwide across all age groups,4 making it a global issue. In 2021, the global prevalence rates of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) were 9.1% (464 million) and 5.8% (298 million), respectively; these are projected to increase to 10.0% (638 million) and 6.5% (414 million), respectively, by 2045.5 Additionally, prediabetes is associated with an increased risk of atrial fibrillation, congestive heart failure, stroke, kidney diseases, peripheral neuropathy, and cancer.6–8 Previous studies have indicated that prediabetes is independently associated with a significantly increased risk of developing atrial fibrillation,6 with 15.7% of individuals with prediabetes experiencing heart failure;7 moreover, it is associated with poorer prognoses in patients with heart failure.9 Furthermore, individuals with prediabetes are at a higher risk of all-cause and cardiovascular disease (CVD) mortality.10 Therefore, early identification of individuals with prediabetes, enhanced monitoring of prediabetes, and effective implementation of diabetes prevention policies and interventions are crucial to prevent or delay the progression of diabetes and improve related diseases, prognoses, and mortality risks in individuals with prediabetes.Controlling vitamin supplementation is among the most cost-effective and crucial treatment strategies. Vitamins are a group of organic compounds essential for maintaining bodily health; they play a significant role in the normal functioning of human physiological functions and support various basic metabolic pathways essential for basic cell functions.12 Certain vitamins have been identified to delay the progression of prediabetes and improve the survival rate of individuals with prediabetes,11 with numerous studies confirming that vitamin D supplementation in individuals with prediabetes can effectively reduce the risk of developing type 2 diabetes and increase the rate of reversion from prediabetes to normal glucose levels.11,13,14 Vitamin D insufficiency is associated with a higher risk of all-cause and CVD mortality in individuals with prediabetes.15,16 However, other results show that vitamin D supplementation does not reduce the risk of developing type 2 diabetes in individuals with prediabetes nor does it reduce insulin resistance.17 Additionally, compared with the general population, individuals with prediabetes often exhibit unique vitamin requirements. For example, adults with prediabetes have a higher need for vitamin C intake.18 However, there is insufficient evidence regarding the effects of vitamin intake from food or supplements on the health and mortality rates of the prediabetes population. Therefore, the true relationship between vitamin D intake and the risk of prediabetes as well as the specific effects of other vitamins on the risk of all-cause mortality and CVD mortality in individuals with prediabetes remain to be explored.
Therefore, this study aimed to explore the relationship between the intake of eleven vitamins and the rates of all-cause and CVD mortality in a large, nationally representative sample of individuals with prediabetes.
Methods
Study population
The NHANES is a large-scale cross-sectional survey conducted by the National Center for Health Statistics (NCHS) and Centers for Disease Control and Prevention. This survey uses a complex, stratified, multistage probability sampling design to collect information on the health and nutritional status of a nationally representative sample of a civilian, non-institutionalized population. Its primary aim is to identify risk factors for diseases and determine the prevalence of major diseases. Since 1999, the NHANES has been continuously collecting and publicly releasing biennial data. The initial survey protocol was approved by the Institutional Review Board of the National Center for Health Statistics, and informed consent was obtained from all participants. The data for this study were drawn from 10 cycles of the NHANES (1999–2018), encompassing 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316 participants. This dataset is available on the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). After excluding participants who were pregnant at baseline (n = 1670), less than 18 years of age (n = 42
316 participants. This dataset is available on the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). After excluding participants who were pregnant at baseline (n = 1670), less than 18 years of age (n = 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112), with incomplete glucose information or a history of diabetes (n = 1851), with incomplete survival follow-up information (n = 135), and who did not meet the criteria for prediabetes (n = 40
112), with incomplete glucose information or a history of diabetes (n = 1851), with incomplete survival follow-up information (n = 135), and who did not meet the criteria for prediabetes (n = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914), 14
914), 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 individuals with prediabetes were included in the final analysis (ESI Fig. 1†).
634 individuals with prediabetes were included in the final analysis (ESI Fig. 1†).
Diagnosis of prediabetes
According to the 2023 American Diabetes Association guidelines 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507
507![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 649, prediabetes is defined as not having diabetes but meeting at least one of the following criteria: fasting plasma glucose (FPG) level of 100–125 mg dL−1 (5.5–6.9 mmol L−1), meeting the criteria for impaired fasting glucose; 2 h post-load plasma glucose (2 h PG) (75 g oral glucose tolerance test) level of 140–199 mg dL−1 (7.8–11.0 mmol L−1), meeting the criteria for IGT; hemoglobin A1c levels between 5.7% and 6.4% (39–47 mmol mol−1); and previously informed by a doctor or other healthcare professional of any of the following: prediabetes, impaired fasting glucose, IGT, or borderline diabetes.
649, prediabetes is defined as not having diabetes but meeting at least one of the following criteria: fasting plasma glucose (FPG) level of 100–125 mg dL−1 (5.5–6.9 mmol L−1), meeting the criteria for impaired fasting glucose; 2 h post-load plasma glucose (2 h PG) (75 g oral glucose tolerance test) level of 140–199 mg dL−1 (7.8–11.0 mmol L−1), meeting the criteria for IGT; hemoglobin A1c levels between 5.7% and 6.4% (39–47 mmol mol−1); and previously informed by a doctor or other healthcare professional of any of the following: prediabetes, impaired fasting glucose, IGT, or borderline diabetes.
Assessment of mortality
All-cause mortality was ascertained using the NHANES publicly available linked mortality files up to December 31, 2019, which were linked to the National Death Index (NDI) using probabilistic matching algorithms conducted by the NCHS. The study end points were all-cause and CVD mortality rates. The causes of death were determined using the International Classification of Diseases, Tenth Revision (ICD-10) (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm). All-cause mortality was defined as death from any cause, including heart diseases (054–068), cancers (00–97), accidents (112–123), cerebrovascular disease (070), diabetes (046), and other causes (010). CVD mortality was defined as death from CVDs including rheumatic heart disease, hypertensive heart and kidney disease, ischemic heart disease, heart failure, and cerebrovascular disease (ICD-10 codes: I00–I09, I11, I13, I20–I51, and I60–I69, respectively). Other causes of death include: influenza and pneumonia (J09–J18), chronic lower respiratory diseases (J40–J47), accidents (unintentional injuries) (V01–X59, Y85–Y86), and diabetes mellitus (E10–E14). The follow-up time for each individual was calculated from the date of enrollment to the date of death or December 31, 2019 (the last update of the NDI database).Covariates
Our study utilized a standardized interview questionnaire to gather information on sex (male or female), age (<60 and ≥60 years), body mass index (BMI, kg m−2), ethnicity, educational level, household income-to-poverty ratio, smoking status, alcohol consumption, Healthy Eating Index (HEI), total energy intake from diet, and self-reported health status. BMI was calculated by dividing weight in kilograms by the square of height in meters and categorized into normal weight (<25 kg m−2), overweight (25–30 kg m−2), and obesity (≥30 kg m−2). Ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other ethnicity. Educational level was stratified as less than high school, high school or equivalent, or college or above. Family income relative to poverty ratio was divided into less than 1.0%, 1.0–3.0%, and more than 3.0%, with higher ratios indicating better economic status. Smoking status was categorized into non-smokers, former smokers (those who had smoked >100 cigarettes in total and had quit smoking), and current smokers (those who had smoked >100 cigarettes in their lifetime). Alcohol intake and total dietary energy intake were determined based on participants’ 24 h dietary recalls. Alcohol drinking status was grouped into non-drinker, low-to-moderate drinker (defined as <2 drinks per day in males and <1 drink per day in females), or heavy drinker (defined as ≥2 drinks per day in males and ≥1 drink per day in females). Dietary quality was assessed using the Healthy Eating Index-2015 (HEI-2015), divided into quarter 1 (0–40.562), quarter 2 (40.562–49.689), quarter 3 (49.689–59.359), and quarter 4 (59.359–97.877); higher scores indicated better diet quality.19 Self-reported health status was categorized as very good to excellent, good, or poor to fair. We also included baseline histories of CVDs, chronic obstructive pulmonary disease (COPD), chronic kidney disease, and cancer obtained through the baseline survey questionnaire.Statistical analysis
All statistical analyses in this study considered the complex, multi-stage, stratified, and clustered sampling design of the NHANES, which includes the oversampling of certain subpopulations. This approach reflects the use of NHANES sample weights, strata, and primary sampling units to provide accurate estimates representative of the United States (US) civilian population.20 Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and CVD mortality associated with vitamin intake levels among individuals with prediabetes. Three Cox regression models were constructed to adjust for various confounding factors. Model 1 was adjusted for sex, age, BMI, ethnicity, educational level, and family income poverty ratio. Model 2 was adjusted for Model 1 variables as well as smoking status, alcohol consumption, HEI, and total energy intake from the diet. Model 3 was adjusted for Models 1 and 2 variables as well as self-reported health status, family history of CVDs, self-reported history of CVD, COPD, chronic kidney disease, and cancer. The study participants were divided into four groups based on the quartiles (Q1–Q4) of vitamin intake. Comparisons of baseline characteristics across quartiles of different vitamin levels were conducted using analysis of variance with the Taylor series linearization method for continuous variables, t-tests for comparisons of continuous variables between two groups, and Rao-Scott chi-squared tests for categorical variables. The incidence rates of all-cause, CVD, cancer-related mortality, and other causes of death during the follow-up period were calculated for each vitamin quartile group. A restricted cubic spline (RCS) model was also employed to visualize the dose–response relationship between vitamin levels and mortality in individuals with prediabetes. Multiple imputations were used for missing covariates.21 We performed several sensitivity analyses to test the robustness of our results. Furthermore, subgroup analyses were performed by sex, age, BMI, ethnicity, educational level, family income poverty ratio, smoking status, alcohol consumption, HEI, total energy intake from diet, self-reported health status, family history of CVD, and self-reported CVD, COPD, chronic kidney disease, and cancer to assess the impact of vitamin levels on all-cause and CVD mortality among individuals with prediabetes. All analyses were performed using R software version 4.1.0. Statistical significance was set at a two-sided p-value of less than 0.05.Results
Baseline characteristics of the study population
This study included 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 individuals with prediabetes. Based on the survival and mortality statistics, the participants were categorized into the survival (n = 12
634 individuals with prediabetes. Based on the survival and mortality statistics, the participants were categorized into the survival (n = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318) and mortality groups (n = 2316). Compared with the survival group, higher mortality was more likely to be associated with older age, non-Hispanic white ethnicity, college education or higher, obesity, higher family income, former smoking status, low-to-moderate alcohol consumption, higher HEI score, higher total energy intake from diet, poorer self-reported health status, and self-reported history of CVD, chronic kidney disease, COPD, and cancer. Additional baseline characteristics of the study participants are summarized in Table 1. ESI Table 1† outlines the relationships between the 11 vitamins, stratified by quartiles, and laboratory measurements of individuals with prediabetes.
318) and mortality groups (n = 2316). Compared with the survival group, higher mortality was more likely to be associated with older age, non-Hispanic white ethnicity, college education or higher, obesity, higher family income, former smoking status, low-to-moderate alcohol consumption, higher HEI score, higher total energy intake from diet, poorer self-reported health status, and self-reported history of CVD, chronic kidney disease, COPD, and cancer. Additional baseline characteristics of the study participants are summarized in Table 1. ESI Table 1† outlines the relationships between the 11 vitamins, stratified by quartiles, and laboratory measurements of individuals with prediabetes.
| Variable | Total (n = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634) 634) |
Survival (n = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318) 318) |
Death (n = 2316) | P value |
|---|---|---|---|---|
| Data are presented as numbers (percentages) unless otherwise stated. All estimates accounted for complex survey designs. | ||||
| Sex, % | 0.14 | |||
| Male | 7726 (52.03) | 6385 (51.76) | 1341 (53.92) | |
| Female | 6908 (47.97) | 5933 (48.24) | 975 (46.08) | |
| Age, years, n (%) | <0.0001 | |||
| <60 | 8628 (65.50) | 8245 (71.33) | 383 (24.93) | |
| ≥60 | 6006 (34.50) | 4073 (28.67) | 1933 (75.07) | |
| BMI, kg m−2, n (%) | <0.0001 | |||
| Normal weight (<25) | 3417 (22.26) | 2716 (21.41) | 701 (28.20) | |
| Overweight (25–30) | 5126 (34.82) | 4284 (34.69) | 842 (35.77) | |
| Obesity (≥30) | 6091 (42.92) | 5318 (43.91) | 773 (36.02) | |
| Race, % | <0.0001 | |||
| Non-Hispanic White | 6187 (66.03) | 4679 (63.87) | 1508 (81.11) | |
| Non-Hispanic Black | 3258 (12.36) | 2854 (12.66) | 404 (10.27) | |
| Mexican American | 2551 (8.55) | 2309 (9.32) | 242 (3.22) | |
| Others | 2638 (13.06) | 2476 (14.16) | 162 (5.40) | |
| Education level, % | <0.0001 | |||
| Less than high school | 4136 (18.62) | 3282 (17.21) | 854(28.39) | |
| High school or equivalent | 3456 (25.49) | 2861 (25.06) | 595 (28.50) | |
| College or above | 7042 (55.90) | 6175 (57.73) | 867 (43.11) | |
| Family income-poverty ratio, % | <0.0001 | |||
| <1.0 | 2974 (14.15) | 2529 (14.07) | 445 (14.72) | |
| 1.0–3.0 | 6331 (37.45) | 5113 (35.79) | 1218 (48.99) | |
| >3.0 | 5329 (48.40) | 4676 (50.14) | 653 (36.28) | |
| Smoking status, % | <0.0001 | |||
| Never | 7660 (50.89) | 6760 (52.90) | 900 (36.87) | |
| Former | 3947 (28.06) | 2992 (26.43) | 955 (39.41) | |
| Current | 3027 (21.05) | 2566 (20.67) | 461 (23.72) | |
| Alcohol drinking, % | <0.0001 | |||
| Never | 5050 (30.19) | 4183 (29.35) | 867 (36.00) | |
| Low to moderate | 7268 (53.76) | 6281 (55.19) | 987 (43.81) | |
| Heavy | 2316 (16.05) | 1854 (15.46) | 462 (20.19) | |
| Healthy eating index score, % | 0.01 | |||
| Quarter 1 | 3533 (25.05) | 3054 (25.61) | 479 (21.16) | |
| Quarter 2 | 3624 (25.30) | 3051 (25.33) | 573 (25.12) | |
| Quarter 3 | 3713 (24.46) | 3090 (24.15) | 623 (26.64) | |
| Quarter 4 | 3764 (25.18) | 3123 (24.91) | 641 (27.08) | |
| Total energy intake from diet, kcal, n (%) | <0.0001 | |||
| [0, 1445.5] | 3593 (20.92) | 2813 (19.67) | 780 (29.61) | |
| [1445.5, 1908] | 3666 (24.85) | 3043 (24.45) | 623 (27.65) | |
| [1908, 2494.5] | 3809 (26.94) | 3266 (27.33) | 543 (24.16) | |
[2494.5, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594] 594] |
3566 (27.29) | 3196 (28.54) | 370 (18.58) | |
| Self reported health, % | <0.0001 | |||
| Very good to excellent | 3523 (19.30) | 2726 (17.53) | 797 (31.67) | |
| Good | 5539 (36.33) | 4750 (36.78) | 789 (33.19) | |
| Poor to fair | 5572 (44.37) | 4842 (45.69) | 730 (35.13) | |
| Family history of cardiovascular disease, % | 0.16 | |||
| No | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 (74.64) 162 (74.64) |
9456 (74.89) | 1706 (72.84) | |
| Yes | 3472 (25.36) | 2862 (25.11) | 610 (27.16) | |
| Cardiovascular disease, % | <0.0001 | |||
| No | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905 (89.64) 905 (89.64) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 284 (92.34) 284 (92.34) |
1621 (70.83) | |
| Yes | 1729 (10.36) | 1034 (7.66) | 695 (29.17) | |
| Chronic obstructive pulmonary diseases, % | <0.0001 | |||
| No | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053 (96.02) 053 (96.02) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 (97.15) 986 (97.15) |
2067 (88.13) | |
| Yes | 581 (3.98) | 332 (2.85) | 249 (11.87) | |
| History of chronic kidney disease, % | <0.0001 | |||
| No | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 (97.61) 235 (97.61) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 033 (97.95) 033 (97.95) |
2202 (95.25) | |
| Yes | 399 (2.39) | 285 (2.05) | 114 (4.75) | |
| Cancer, % | <0.0001 | |||
| No | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 (88.29) 060 (88.29) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 263 (89.99) 263 (89.99) |
1797 (76.40) | |
| Yes | 1574 (11.71) | 1055 (10.01) | 519 (23.60) | |
Associations of vitamin levels with all-cause and CVD mortality
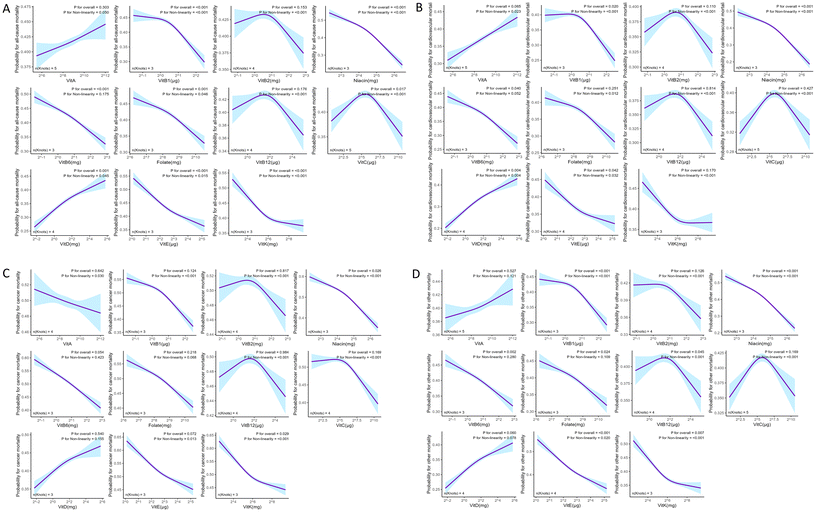
During the follow-up period of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 individuals with prediabetes, 2316 deaths (12.55%) occurred, with 722 (3.68%) attributed to CVD, 577 (3.30%) to cancer, and 1017 (5.57%) to other causes. The relationship between mean vitamin intake levels and mortality in individuals with prediabetes is shown in Table 2. Compared with participants with low vitamin intake levels, higher levels of vitamins B1, niacin, B6, folate, E, and K were significantly associated with reduced mortality rates among individuals with prediabetes. Conversely, higher vitamin D levels were significantly associated with increased prediabetic mortality. No significant associations were observed between vitamins A, B2, B12, or C levels and mortality rates (Table 2). The Kaplan–Meier survival analysis demonstrated significant differences in the mortality rates of individuals with prediabetes among the quartiles of each vitamin level during the follow-up period (ESI Fig. 2†). Table 3 outlines the four Cox regression models used to assess the correlation between the levels of the 11 vitamins and all-cause, CVD, cancer, and other mortalities among individuals with prediabetes. Cox proportional hazards regression models were used to evaluate the non-linear relationship between vitamin levels and mortality among the study population (Fig. 1). In Model 3, the highest quartile of vitamin D level was significantly associated with an increased risk of all-cause mortality (HR:1.44; 95% CI: 1.12, 1.84; p trend = 0.004), whereas the highest quartile of vitamin K level was significantly associated with a decreased risk of all-cause mortality (HR: 0.81; 95% CI:0.66, 0.98; p trend = 0.03). Regarding cancer mortality, the highest quartile of vitamin A (HR: 0.64; 95% CI: 0.44, 0.91) and K (HR: 0.57; 95% CI: 0.38, 0.86) levels were significantly associated with a decreased risk of cancer mortality (p trend = 0.01) (Table 3). The RCS analysis showed that after multivariable adjustment, there was no significant association between vitamin A and B2 levels and all-cause, CVD, cancer, and other mortalities among individuals with prediabetes (all p for overall >0.05). Vitamin B1 levels exhibited a reverse L-shaped relationship with all-cause, CVD, and other mortalities (p for non-linearity <0.05), however, there was no significant association with cancer mortality (p for overall >0.05). Niacin levels showed a significant non-linear negative association with all-cause, CVD, cancer, and other mortalities (p for non-linearity <0.001). Vitamin B6 levels exhibited a negative linear association with all-cause, CVD, and other mortalities (p for non-linearity >0.05), with no significant association with cancer mortality (p for overall >0.05). Folate levels showed a non-linear negative association with all-cause mortality (p for non-linearity <0.05), a negative linear association with other mortalities (p for non-linearity >0.05), and no association with CVD or cancer mortality. Vitamin B12 levels exhibited an inverse U-shaped relationship with other causes of mortality (p for non-linearity = 0.001). Vitamin C levels exhibited an inverse U-shaped relationship with all-cause mortality (p for non-linearity <0.001). Higher levels of vitamin D were non-linearly and positively associated with an increased risk of all-cause and CVD mortality (p for non-linearity <0.05). Vitamin E levels exhibited a non-linear negative association with all-cause, CVD, and other mortalities (p for non-linearity <0.05), with no significant association with cancer mortality (p for overall >0.05). Vitamin K levels exhibited an L-shaped relationship with all-cause, cancer, and other mortalities (p for non-linearity <0.05), with no significant association with CVD mortality (p for overall >0.05) (Fig. 1A–D).
634 individuals with prediabetes, 2316 deaths (12.55%) occurred, with 722 (3.68%) attributed to CVD, 577 (3.30%) to cancer, and 1017 (5.57%) to other causes. The relationship between mean vitamin intake levels and mortality in individuals with prediabetes is shown in Table 2. Compared with participants with low vitamin intake levels, higher levels of vitamins B1, niacin, B6, folate, E, and K were significantly associated with reduced mortality rates among individuals with prediabetes. Conversely, higher vitamin D levels were significantly associated with increased prediabetic mortality. No significant associations were observed between vitamins A, B2, B12, or C levels and mortality rates (Table 2). The Kaplan–Meier survival analysis demonstrated significant differences in the mortality rates of individuals with prediabetes among the quartiles of each vitamin level during the follow-up period (ESI Fig. 2†). Table 3 outlines the four Cox regression models used to assess the correlation between the levels of the 11 vitamins and all-cause, CVD, cancer, and other mortalities among individuals with prediabetes. Cox proportional hazards regression models were used to evaluate the non-linear relationship between vitamin levels and mortality among the study population (Fig. 1). In Model 3, the highest quartile of vitamin D level was significantly associated with an increased risk of all-cause mortality (HR:1.44; 95% CI: 1.12, 1.84; p trend = 0.004), whereas the highest quartile of vitamin K level was significantly associated with a decreased risk of all-cause mortality (HR: 0.81; 95% CI:0.66, 0.98; p trend = 0.03). Regarding cancer mortality, the highest quartile of vitamin A (HR: 0.64; 95% CI: 0.44, 0.91) and K (HR: 0.57; 95% CI: 0.38, 0.86) levels were significantly associated with a decreased risk of cancer mortality (p trend = 0.01) (Table 3). The RCS analysis showed that after multivariable adjustment, there was no significant association between vitamin A and B2 levels and all-cause, CVD, cancer, and other mortalities among individuals with prediabetes (all p for overall >0.05). Vitamin B1 levels exhibited a reverse L-shaped relationship with all-cause, CVD, and other mortalities (p for non-linearity <0.05), however, there was no significant association with cancer mortality (p for overall >0.05). Niacin levels showed a significant non-linear negative association with all-cause, CVD, cancer, and other mortalities (p for non-linearity <0.001). Vitamin B6 levels exhibited a negative linear association with all-cause, CVD, and other mortalities (p for non-linearity >0.05), with no significant association with cancer mortality (p for overall >0.05). Folate levels showed a non-linear negative association with all-cause mortality (p for non-linearity <0.05), a negative linear association with other mortalities (p for non-linearity >0.05), and no association with CVD or cancer mortality. Vitamin B12 levels exhibited an inverse U-shaped relationship with other causes of mortality (p for non-linearity = 0.001). Vitamin C levels exhibited an inverse U-shaped relationship with all-cause mortality (p for non-linearity <0.001). Higher levels of vitamin D were non-linearly and positively associated with an increased risk of all-cause and CVD mortality (p for non-linearity <0.05). Vitamin E levels exhibited a non-linear negative association with all-cause, CVD, and other mortalities (p for non-linearity <0.05), with no significant association with cancer mortality (p for overall >0.05). Vitamin K levels exhibited an L-shaped relationship with all-cause, cancer, and other mortalities (p for non-linearity <0.05), with no significant association with CVD mortality (p for overall >0.05) (Fig. 1A–D).
| Variable | Total | Survival | Death | P value |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Data are presented as HR (95% CI) unless otherwise indicated. Adjusted for sex, age, race, BMI, educational level, smoking status, drinking status, family income poverty ratio, healthy eating index – 2015, total energy intake from the diet, self-reported health status, family history of cardiovascular diseases, self-reported cardiovascular disease, self-reported chronic obstructive pulmonary disease, self-reported chronic kidney disease, self-reported cancer, survey weights of NHANES, and survey cycles. | ||||
| Vitamin A (RAE mcg per d) | 635.01 (607.31, 662.72) | 635.43 (604.28, 666.58) | 632.11 (591.16, 673.07) | 0.9 |
| Vitamin B1 (mg d−1) | 1.61 (1.59, 1.64) | 1.63 (1.60, 1.66) | 1.51 (1.46, 1.56) | <0.0001 |
| Vitamin B2 (mg d−1) | 2.16 (2.12, 2.19) | 2.17 (2.13, 2.21) | 2.09 (2.02, 2.16) | 0.06 |
| Niacin (mg d−1) | 25.11 (24.67, 25.55) | 25.60 (25.14, 26.07) | 21.68 (21.02, 22.33) | <0.0001 |
| Vitamin B6 (mg d−1) | 2.03 (1.98, 2.08) | 2.07 (2.01, 2.12) | 1.80 (1.75, 1.86) | <0.0001 |
| Folate (DFE mcg d−1) | 396.72 (390.47, 402.97) | 400.13 (393.61, 406.64) | 372.99 (359.81, 386.16) | <0.001 |
| Vitamin B12 (mcg d−1) | 5.26 (5.02, 5.49) | 5.29 (5.02, 5.55) | 5.06 (4.73, 5.39) | 0.29 |
| Vitamin C (mg d−1) | 82.74 (80.33, 85.14) | 82.70 (80.04, 85.36) | 83.01 (78.56, 87.46) | 0.91 |
| Vitamin D (mcg d−1) | 4.58 (4.40, 4.76) | 4.53 (4.34, 4.72) | 5.20 (4.76, 5.63) | 0.01 |
| Vitamin E (mg d−1) | 8.26 (8.09, 8.44) | 8.47 (8.29, 8.66) | 6.83 (6.50, 7.15) | <0.0001 |
| Vitamin K (mcg d−1) | 111.04 (103.72, 118.37) | 114.02 (105.96, 122.08) | 87.60 (81.16, 94.04) | <0.0001 |
| Quartiles of vitamin levels | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P trend | |
| Vitamin A | |||||
| Data are presented as HR (95% CI) unless otherwise indicated. Crude model: without adjustment. Model 1: adjusted for sex, age, BMI, ethnicity, educational level, and family income poverty ratio. Model 2: adjusted for model 1 variables as well as smoking status, alcohol drinking status, HEI, and total energy intake from the diet. Model 3: adjusted for model 1 and model 2 variables as well as self-reported health status, family history of cardiovascular diseases, self-reported history of cardiovascular disease, self-reported chronic obstructive pulmonary disease, chronic kidney disease, and cancer. | |||||
| All-cause mortality | |||||
| Number of deaths | 541 (12.26) | 563 (11.85) | 614 (13.69) | 598 (12.35) | |
| Crude model h (95% CI) P-value | 1 | 1.03 (0.87, 1.21) 0.76 | 1.18 (1.00, 1.39) 0.05 | 1.08 (0.92, 1.27) 0.33 | 0.17 |
| Model 1 h (95% CI) P-value | 1 | 0.84 (0.72, 0.99) 0.03 | 0.81 (0.69, 0.95) 0.01 | 0.76 (0.64, 0.91) 0.002 | 0.01 |
| Model 2 h (95% CI) P-value | 1 | 0.92 (0.78, 1.09) 0.32 | 0.92 (0.79, 1.07) 0.28 | 0.89 (0.74, 1.07) 0.21 | 0.27 |
| Model 3 h (95% CI) P-value | 1 | 0.91 (0.77, 1.07) 0.24 | 0.92 (0.79, 1.07) 0.26 | 0.88 (0.73, 1.06) 0.18 | 0.26 |
| CVD mortality | |||||
| Number of deaths | 148 (2.85) | 178 (3.68) | 197 (4.19) | 199 (3.87) | |
| Crude model h (95% CI) P-value | 1 | 1.37 (1.04, 1.80) 0.02 | 1.56 (1.16, 2.08) 0.003 | 1.46 (1.09, 1.95) 0.02 | 0.02 |
| Model 1 h (95% CI) P-value | 1 | 1.09 (0.82,1.46) 0.54 | 1.00 (0.76,1.32) 0.98 | 0.97 (0.72,1.32) 0.87 | 0.67 |
| Model 2 h (95% CI) P-value | 1 | 1.16 (0.86,1.58) 0.34 | 1.14 (0.85,1.52) 0.39 | 1.15 (0.81,1.61) 0.44 | 0.59 |
| Model 3 h (95% CI) P-value | 1 | 1.14 (0.84,1.56) 0.39 | 1.15 (0.86,1.55) 0.35 | 1.15 (0.80,1.64) 0.45 | 0.55 |
| Cancer mortality | |||||
| Number of deaths | 148 (3.78) | 139 (2.98) | 155 (3.61) | 135 (2.92) | |
| Crude model h (95% CI) P-value | 1 | 0.83 (0.62, 1.12) 0.23 | 1.01 (0.77, 1.32) 0.97 | 0.83 (0.59, 1.16) 0.27 | 0.47 |
| Model 1 h (95% CI) P-value | 1 | 0.68 (0.51, 0.92) 0.01 | 0.69 (0.53, 0.91) 0.01 | 0.58 (0.41, 0.82) 0.002 | 0.004 |
| Model 2 h (95% CI) P-value | 1 | 0.74 (0.54, 1.02) 0.07 | 0.76 (0.58, 1.00) 0.05 | 0.64 (0.45, 0.92) 0.01 | 0.02 |
| Model 3 h (95% CI) P-value | 1 | 0.75 (0.55, 1.03) 0.07 | 0.76 (0.58, 1.00) 0.05 | 0.64 (0.44, 0.91) 0.01 | 0.02 |
| Other mortality | |||||
| Number of deaths | 245 (5.63) | 246 (5.20) | 262 (5.89) | 264 (5.56) | |
| Crude model h (95% CI) P-value | 1 | 0.98 (0.76, 1.27) 0.89 | 1.11 (0.87, 1.41) 0.41 | 1.06 (0.84, 1.35) 0.61 | 0.42 |
| Model 1 h (95% CI) P-value | 1 | 0.83 (0.65, 1.06) 0.14 | 0.79 (0.62, 1.02) 0.07 | 0.78 (0.60, 1.01) 0.06 | 0.07 |
| Model 2 h (95% CI) P-value | 1 | 0.92 (0.71, 1.19) 0.52 | 0.91 (0.70, 1.18) 0.48 | 0.93 (0.70, 1.22) 0.58 | 0.62 |
| Model 3 h (95% CI) P-value | 1 | 0.89 (0.69, 1.16) 0.39 | 0.90 (0.69, 1.16) 0.40 | 0.91 (0.69, 1.20) 0.50 | 0.58 |
| Vitamin B1 | |||||
| All-cause mortality | |||||
| Number of deaths | 644 (14.08) | 593 (12.54) | 616 (13.46) | 463 (10.40) | |
| Crude model h (95% CI) P-value | 1 | 0.93 (0.79, 1.08) 0.34 | 0.96 (0.82, 1.13) 0.63 | 0.74 (0.61, 0.89) 0.001 | 0.004 |
| Model 1 h (95% CI) P-value | 1 | 0.93 (0.80, 1.07) 0.30 | 0.90 (0.77, 1.06) 0.20 | 0.83 (0.68, 1.02) 0.08 | 0.08 |
| Model 2 h (95% CI) P-value | 1 | 0.98 (0.84, 1.14) 0.77 | 1.02 (0.87, 1.21) 0.78 | 0.95 (0.77, 1.18) 0.65 | 0.79 |
| Model 3 h (95% CI) P-value | 1 | 1.01 (0.86, 1.18) 0.94 | 1.06 (0.90, 1.25) 0.49 | 0.99 (0.80, 1.24) 0.95 | 0.91 |
| CVD mortality | |||||
| Number of deaths | 195 (3.93) | 188 (3.64) | 204 (4.26) | 135 (2.95) | |
| Crude model h (95% CI) P-value | 1 | 0.96 (0.73, 1.28) 0.80 | 1.09 (0.81, 1.48) 0.56 | 0.75 (0.53, 1.05) 0.10 | 0.19 |
| Model 1 h (95% CI) P-value | 1 | 0.97 (0.74, 1.27) 0.81 | 1.00 (0.74, 1.36) 0.98 | 0.84 (0.60, 1.19) 0.33 | 0.41 |
| Model 2 h (95% CI) P-value | 1 | 1.03 (0.78, 1.36) 0.86 | 1.13 (0.83, 1.53) 0.45 | 0.98 (0.68, 1.41) 0.90 | 0.94 |
| Model 3 h (95% CI) P-value | 1 | 1.09 (0.81, 1.46) 0.57 | 1.20 (0.89, 1.62) 0.24 | 1.08 (0.75, 1.57) 0.68 | 0.54 |
| Cancer mortality | |||||
| Number of deaths | 162 (4.01) | 137 (2.99) | 138 (3.15) | 140 (3.13) | |
| Crude model h (95% CI) P-value | 1 | 0.77 (0.57, 1.05) 0.10 | 0.78 (0.57, 1.08) 0.14 | 0.78 (0.55, 1.11) 0.17 | 0.21 |
| Model 1 h (95% CI) P-value | 1 | 0.72 (0.53, 0.98) 0.04 | 0.67 (0.49, 0.93) 0.02 | 0.78 (0.54, 1.11) 0.17 | 0.18 |
| Model 2 h (95% CI) P-value | 1 | 0.75 (0.55, 1.01) 0.06 | 0.73 (0.53, 1.02) 0.06 | 0.81 (0.56, 1.17) 0.27 | 0.32 |
| Model 3 h (95% CI) P-value | 1 | 0.74 (0.54, 1.01) 0.06 | 0.75 (0.54, 1.03) 0.07 | 0.80 (0.55, 1.16) 0.24 | 0.3 |
| Other mortality | |||||
| Number of deaths | 287 (6.13) | 268 (5.91) | 274 (6.05) | 188 (4.32) | |
| Crude model h (95% CI) P-value | 1 | 1.00 (0.80, 1.25) 0.98 | 0.99 (0.80, 1.23) 0.94 | 0.71 (0.54, 0.93) 0.01 | 0.01 |
| Model 1 h (95% CI) P-value | 1 | 1.04 (0.83, 1.29) 0.74 | 1.00 (0.79, 1.25) 0.97 | 0.86 (0.64, 1.15) 0.31 | 0.29 |
| Model 2 h (95% CI) P-value | 1 | 1.11 (0.88, 1.39) 0.37 | 1.16 (0.91, 1.48) 0.23 | 1.01 (0.73, 1.40) 0.94 | 0.83 |
| Model 3 h (95% CI) P-value | 1 | 1.14 (0.91, 1.44) 0.26 | 1.20 (0.94, 1.54) 0.14 | 1.05 (0.76, 1.46) 0.76 | 0.66 |
| Vitamin B2 | |||||
| All-cause mortality | |||||
| Number of deaths | 588 (12.87) | 603 (13.30) | 582 (12.28) | 543 (11.96) | |
| Crude model h (95% CI) P-value | 1 | 1.07 (0.90, 1.27) 0.43 | 1.02 (0.88, 1.18) 0.80 | 0.91 (0.76, 1.08) 0.28 | 0.16 |
| Model 1 h (95% CI) P-value | 1 | 0.92 (0.79, 1.08) 0.31 | 0.83 (0.71, 0.96) 0.01 | 0.85 (0.70, 1.04) 0.11 | 0.08 |
| Model 2 h (95% CI) P-value | 1 | 0.94 (0.80, 1.10) 0.44 | 0.87 (0.75, 1.01) 0.06 | 0.90 (0.73, 1.10) 0.31 | 0.27 |
| Model 3 h (95% CI) P-value | 1 | 0.97 (0.83, 1.15) 0.75 | 0.88 (0.76, 1.02) 0.08 | 0.94 (0.77, 1.16) 0.57 | 0.44 |
| CVD mortality | |||||
| Number of deaths | 184 (3.45) | 178 (3.83) | 191 (3.82) | 169 (3.61) | |
| Crude model h (95% CI) P-value | 1 | 1.15 (0.90, 1.47) 0.25 | 1.19 (0.92, 1.52) 0.18 | 1.03 (0.76, 1.39) 0.86 | 0.94 |
| Model 1 h (95% CI) P-value | 1 | 0.98 (0.77, 1.24) 0.86 | 0.95 (0.74, 1.23) 0.70 | 0.97 (0.70, 1.34) 0.86 | 0.84 |
| Model 2 h (95% CI) P-value | 1 | 1.00 (0.78, 1.29) 0.97 | 1.04 (0.77, 1.39) 0.82 | 1.10 (0.76, 1.58) 0.62 | 0.59 |
| Model 3 h (95% CI) P-value | 1 | 1.05 (0.81, 1.36) 0.71 | 1.05 (0.78, 1.40) 0.75 | 1.18 (0.82, 1.71) 0.37 | 0.39 |
| Cancer mortality | |||||
| Number of deaths | 141 (3.73) | 159 (3.29) | 120 (2.75) | 157 (3.49) | |
| Crude model h (95% CI) P-value | 1 | 0.91 (0.65, 1.26) 0.57 | 0.78 (0.54, 1.12) 0.19 | 0.91 (0.65, 1.29) 0.60 | 0.55 |
| Model 1 h (95% CI) P-value | 1 | 0.73 (0.52, 1.03) 0.08 | 0.58 (0.41, 0.83) 0.003 | 0.75 (0.53, 1.05) 0.09 | 0.12 |
| Model 2 h (95% CI) P-value | 1 | 0.71 (0.50, 1.02) 0.07 | 0.56 (0.40, 0.78) <0.001 | 0.68 (0.48, 0.97) 0.03 | 0.05 |
| Model 3 h (95% CI) P-value | 1 | 0.72 (0.50, 1.03) 0.07 | 0.56 (0.40, 0.78) <0.001 | 0.68 (0.48, 0.97) 0.03 | 0.05 |
| Other mortality | |||||
| Number of deaths | 263 (5.69) | 266 (6.18) | 271 (5.71) | 217 (4.86) | |
| Crude model h (95% CI) P-value | 1 | 1.13 (0.88, 1.44) 0.35 | 1.07 (0.87, 1.32) 0.50 | 0.83 (0.64, 1.08) 0.17 | 0.1 |
| Model 1 h (95% CI) P-value | 1 | 1.02 (0.81, 1.27) 0.89 | 0.93 (0.75, 1.16) 0.52 | 0.85 (0.63, 1.13) 0.26 | 0.2 |
| Model 2 h (95% CI) P-value | 1 | 1.05 (0.83, 1.33) 0.70 | 1.00 (0.80, 1.26) 0.97 | 0.91 (0.66, 1.27) 0.59 | 0.51 |
| Model 3 h (95% CI) P-value | 1 | 1.10 (0.87, 1.40) 0.42 | 1.00 (0.80, 1.26) 0.98 | 0.96 (0.70, 1.33) 0.80 | 0.62 |
| Niacin | |||||
| All-cause mortality | |||||
| Number of deaths | 768 (16.78) | 646 (14.47) | 529 (11.32) | 373 (8.65) | |
| Crude model h (95% CI) P-value | 1 | 0.91 (0.80, 1.04) 0.18 | 0.76 (0.65, 0.89) <0.001 | 0.58 (0.49, 0.69) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 0.98 (0.86, 1.10) 0.70 | 0.84 (0.72, 0.98) 0.02 | 0.85 (0.71, 1.01) 0.07 | 0.02 |
| Model 2 h (95% CI) P-value | 1 | 1.03 (0.91, 1.18) 0.62 | 0.94 (0.81, 1.11) 0.48 | 0.94 (0.79, 1.12) 0.49 | 0.32 |
| Model 3 h (95% CI) P-value | 1 | 1.04 (0.91, 1.19) 0.55 | 0.95 (0.82, 1.11) 0.55 | 0.94 (0.79, 1.12) 0.51 | 0.35 |
| CVD mortality | |||||
| Number of deaths | 249 (4.95) | 195 (4.16) | 172 (3.34) | 106 (2.57) | |
| Crude model h (95% CI) P-value | 1 | 0.89 (0.68, 1.17) 0.41 | 0.77 (0.57, 1.03) 0.08 | 0.59 (0.41, 0.85) 0.004 | 0.003 |
| Model 1 h (95% CI) P-value | 1 | 0.97 (0.74, 1.26) 0.81 | 0.85 (0.63, 1.16) 0.31 | 0.92 (0.64, 1.31) 0.63 | 0.47 |
| Model 2 h (95% CI) P-value | 1 | 1.01 (0.77, 1.32) 0.94 | 0.97 (0.72, 1.31) 0.83 | 1.06 (0.74, 1.53) 0.75 | 0.86 |
| Model 3 h (95% CI) P-value | 1 | 1.02 (0.78, 1.34) 0.88 | 0.97 (0.72, 1.31) 0.85 | 1.08 (0.75, 1.56) 0.66 | 0.79 |
| Cancer mortality | |||||
| Number of deaths | 189 (4.44) | 145 (3.44) | 131 (3.11) | 112 (2.44) | |
| Crude model h (95% CI) P-value | 1 | 0.81 (0.60, 1.10) 0.18 | 0.79 (0.56, 1.10) 0.16 | 0.61 (0.43, 0.89) 0.01 | 0.01 |
| Model 1 h (95% CI) P-value | 1 | 0.81 (0.61, 1.09) 0.16 | 0.77 (0.56, 1.04) 0.09 | 0.73 (0.51, 1.05) 0.09 | 0.07 |
| Model 2 h (95% CI) P-value | 1 | 0.83 (0.62, 1.12) 0.23 | 0.81 (0.59, 1.11) 0.18 | 0.74 (0.52, 1.04) 0.08 | 0.08 |
| Model 3 h (95% CI) P-value | 1 | 0.83 (0.61, 1.13) 0.23 | 0.82 (0.60, 1.12) 0.22 | 0.74 (0.53, 1.05) 0.09 | 0.09 |
| Other mortality | |||||
| Number of deaths | 330 (7.40) | 306 (6.87) | 226 (4.86) | 155 (3.64) | |
| Crude model h (95% CI) P-value | 1 | 0.98 (0.79, 1.22) 0.87 | 0.74 (0.58, 0.95) 0.02 | 0.56 (0.43, 0.72) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 1.09 (0.89, 1.33) 0.42 | 0.88 (0.68, 1.15) 0.34 | 0.87 (0.65, 1.18) 0.37 | 0.21 |
| Model 2 h (95% CI) P-value | 1 | 1.18 (0.94, 1.48) 0.15 | 1.02 (0.76, 1.37) 0.90 | 1.00 (0.72, 1.38) 1 | 0.81 |
| Model 3 h (95% CI) P-value | 1 | 1.20 (0.95, 1.51) 0.12 | 1.02 (0.76, 1.38) 0.88 | 0.99 (0.72, 1.37) 0.97 | 0.78 |
| Vitamin B6 | |||||
| All-cause mortality | |||||
| Number of deaths | 706 (15.29) | 580 (12.72) | 573 (12.59) | 457 (9.98) | |
| Crude model h (95% CI) P-value | 1 | 0.87 (0.76, 1.00) 0.05 | 0.88 (0.76, 1.03) 0.11 | 0.73 (0.62, 0.85) <0.0001 | <0.001 |
| Model 1 h (95% CI) P-value | 1 | 0.94 (0.83, 1.06) 0.31 | 0.92 (0.79, 1.09) 0.33 | 0.85 (0.71, 1.00) 0.05 | 0.07 |
| Model 2 h (95% CI) P-value | 1 | 1.04 (0.91, 1.19) 0.53 | 1.07 (0.89, 1.27) 0.48 | 1.01 (0.83, 1.23) 0.93 | 0.87 |
| Model 3 h (95% CI) P-value | 1 | 1.07 (0.94, 1.22) 0.29 | 1.12 (0.94, 1.33) 0.20 | 1.04 (0.86, 1.25) 0.72 | 0.63 |
| CVD mortality | |||||
| Number of deaths | 222 (4.36) | 180 (3.70) | 173 (3.60) | 147 (3.16) | |
| Crude model h (95% CI) P-value | 1 | 0.89 (0.70, 1.13) 0.34 | 0.88 (0.67, 1.17) 0.39 | 0.81 (0.60, 1.08) 0.15 | 0.17 |
| Model 1 h (95% CI) P-value | 1 | 0.97 (0.76, 1.22) 0.77 | 0.93 (0.70, 1.23) 0.61 | 0.95 (0.69, 1.32) 0.78 | 0.72 |
| Model 2 h (95% CI) P-value | 1 | 1.06 (0.82, 1.36) 0.66 | 1.05 (0.78, 1.41) 0.74 | 1.16 (0.82, 1.66) 0.40 | 0.44 |
| Model 3 h (95% CI) P-value | 1 | 1.07 (0.84, 1.37) 0.59 | 1.08 (0.81, 1.45) 0.60 | 1.21 (0.85, 1.73) 0.29 | 0.32 |
| Cancer mortality | |||||
| Number of deaths | 187 (4.41) | 126 (2.99) | 139 (3.19) | 125 (2.72) | |
| Crude model h (95% CI) P-value | 1 | 0.71 (0.52, 0.97) 0.03 | 0.77 (0.58, 1.03) 0.08 | 0.69 (0.50, 0.95) 0.02 | 0.04 |
| Model 1 h (95% CI) P-value | 1 | 0.70 (0.51, 0.94) 0.02 | 0.72 (0.54, 0.97) 0.03 | 0.68 (0.49, 0.95) 0.02 | 0.03 |
| Model 2 h (95% CI) P-value | 1 | 0.75 (0.55, 1.01) 0.06 | 0.81 (0.60, 1.09) 0.16 | 0.73 (0.52, 1.03) 0.07 | 0.14 |
| Model 3 h (95% CI) P-value | 1 | 0.74 (0.55, 1.00) 0.05 | 0.83 (0.61, 1.13) 0.24 | 0.73 (0.52, 1.02) 0.07 | 0.15 |
| Other mortality | |||||
| Number of deaths | 297 (6.52) | 274 (6.04) | 261 (5.80) | 185 (4.10) | |
| Crude model h (95% CI) P-value | 1 | 0.97 (0.78, 1.22) 0.82 | 0.96 (0.75, 1.22) 0.71 | 0.70 (0.55, 0.89) 0.004 | 0.01 |
| Model 1 h (95% CI) P-value | 1 | 1.10 (0.90, 1.35) 0.36 | 1.07 (0.83, 1.37) 0.61 | 0.89 (0.69, 1.14) 0.35 | 0.41 |
| Model 2 h (95% CI) P-value | 1 | 1.25 (1.00, 1.57) 0.05 | 1.27 (0.96, 1.67) 0.09 | 1.10 (0.82, 1.48) 0.52 | 0.5 |
| Model 3 h (95% CI) P-value | 1 | 1.33 (1.06, 1.67) 0.01 | 1.37 (1.04, 1.79) 0.02 | 1.14 (0.86, 1.52) 0.35 | 0.34 |
| Folate | |||||
| All-cause mortality | |||||
| Number of deaths | 671 (14.35) | 605 (12.66) | 566 (13.22) | 474 (10.19) | |
| Crude model h (95% CI) P-value | 1 | 0.89 (0.77, 1.02) 0.10 | 0.92 (0.79, 1.08) 0.31 | 0.70 (0.60, 0.83) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 0.88 (0.76, 1.01) 0.07 | 0.93 (0.80, 1.09) 0.36 | 0.80 (0.67, 0.95) 0.01 | 0.03 |
| Model 2 h (95% CI) P-value | 1 | 0.97 (0.84, 1.11) 0.62 | 1.06 (0.91, 1.24) 0.44 | 0.91 (0.76, 1.10) 0.34 | 0.61 |
| Model 3 h (95% CI) P-value | 1 | 0.99 (0.86, 1.13) 0.85 | 1.07 (0.92, 1.25) 0.36 | 0.95 (0.78, 1.15) 0.59 | 0.86 |
| CVD mortality | |||||
| Number of deaths | 202 (4.04) | 182 (3.82) | 187 (4.02) | 151 (2.91) | |
| Crude model h (95% CI) P-value | 1 | 0.95 (0.74, 1.22) 0.70 | 1.00 (0.74, 1.34) 0.99 | 0.71 (0.52, 0.97) 0.03 | 0.07 |
| Model 1 h (95% CI) P-value | 1 | 0.93 (0.72, 1.20) 0.59 | 1.01 (0.77, 1.32) 0.94 | 0.81 (0.59, 1.11) 0.20 | 0.31 |
| Model 2 h (95% CI) P-value | 1 | 1.01 (0.78, 1.32) 0.93 | 1.13 (0.87, 1.47) 0.35 | 0.94 (0.69, 1.30) 0.73 | 0.95 |
| Model 3 h (95% CI) P-value | 1 | 1.05 (0.80, 1.38) 0.74 | 1.20 (0.92, 1.57) 0.18 | 1.03 (0.74, 1.42) 0.87 | 0.63 |
| Cancer mortality | |||||
| Number of deaths | 158 (3.69) | 158 (3.46) | 132 (3.17) | 129 (2.93) | |
| Crude model h (95% CI) P-value | 1 | 0.94 (0.69, 1.27) 0.69 | 0.86 (0.61, 1.20) 0.38 | 0.79 (0.55, 1.13) 0.20 | 0.17 |
| Model 1 h (95% CI) P-value | 1 | 0.88 (0.65, 1.20) 0.43 | 0.80 (0.57, 1.12) 0.19 | 0.80 (0.56, 1.15) 0.24 | 0.2 |
| Model 2 h (95% CI) P-value | 1 | 0.97 (0.71, 1.31) 0.83 | 0.90 (0.63, 1.28) 0.56 | 0.89 (0.60, 1.32) 0.55 | 0.49 |
| Model 3 h (95% CI) P-value | 1 | 0.97 (0.71, 1.33) 0.87 | 0.89 (0.62, 1.28) 0.53 | 0.87 (0.59, 1.30) 0.50 | 0.43 |
| Other mortality | |||||
| Number of deaths | 311 (6.62) | 265 (5.39) | 247 (6.04) | 194 (4.35) | |
| Crude model h (95% CI) P-value | 1 | 0.82 (0.66, 1.02) 0.07 | 0.91 (0.72, 1.16) 0.45 | 0.65 (0.51, 0.83) <0.001 | 0.002 |
| Model 1 h (95% CI) P-value | 1 | 0.84 (0.68, 1.03) 0.10 | 0.97 (0.76, 1.23) 0.80 | 0.78 (0.60, 1.02) 0.07 | 0.18 |
| Model 2 h (95% CI) P-value | 1 | 0.93 (0.75, 1.16) 0.54 | 1.12 (0.87, 1.44) 0.37 | 0.90 (0.67, 1.22) 0.50 | 0.88 |
| Model 3 h (95% CI) P-value | 1 | 0.96 (0.77, 1.19) 0.69 | 1.12 (0.87, 1.43) 0.39 | 0.94 (0.70, 1.28) 0.71 | 0.96 |
| Vitamin B12 | |||||
| All-cause mortality | |||||
| Number of deaths | 555 (11.95) | 591 (13.49) | 625 (13.05) | 545 (11.68) | |
| Crude model h (95% CI) P-value | 1 | 1.11 (0.95, 1.29) 0.20 | 1.10 (0.93, 1.31) 0.28 | 0.96 (0.82, 1.13) 0.60 | 0.52 |
| Model 1 h (95% CI) P-value | 1 | 1.04 (0.89, 1.21) 0.64 | 1.04 (0.86, 1.25) 0.69 | 0.94 (0.79, 1.13) 0.53 | 0.52 |
| Model 2 h (95% CI) P-value | 1 | 1.06 (0.90, 1.24) 0.50 | 1.08 (0.90, 1.30) 0.40 | 1.01 (0.85, 1.20) 0.95 | 0.93 |
| Model 3 h (95% CI) P-value | 1 | 1.07 (0.91, 1.25) 0.41 | 1.05 (0.88, 1.25) 0.58 | 1.01 (0.84, 1.20) 0.94 | 0.96 |
| CVD mortality | |||||
| Number of deaths | 174 (3.51) | 177 (3.89) | 192 (3.67) | 179 (3.65) | |
| Crude model h (95% CI) P-value | 1 | 1.09 (0.78, 1.53) 0.61 | 1.06 (0.81, 1.40) 0.66 | 1.02 (0.76, 1.37) 0.88 | 0.96 |
| Model 1 h (95% CI) P-value | 1 | 0.99 (0.73, 1.35) 0.95 | 0.97 (0.73, 1.28) 0.82 | 0.98 (0.73, 1.31) 0.90 | 0.88 |
| Model 2 h (95% CI) P-value | 1 | 1.01 (0.74, 1.38) 0.94 | 1.02 (0.77, 1.35) 0.87 | 1.08 (0.81, 1.45) 0.60 | 0.6 |
| Model 3 h (95% CI) P-value | 1 | 1.03 (0.75, 1.42) 0.84 | 1.01 (0.76, 1.33) 0.97 | 1.11 (0.82, 1.50) 0.51 | 0.58 |
| Cancer mortality | |||||
| Number of deaths | 134 (3.34) | 159 (3.39) | 141 (3.07) | 143 (3.40) | |
| Crude model h (95% CI) P-value | 1 | 0.99 (0.74, 1.34) 0.96 | 0.92 (0.66, 1.29) 0.64 | 1.00 (0.70, 1.43) 0.99 | 0.91 |
| Model 1 h (95% CI) P-value | 1 | 0.90 (0.66, 1.22) 0.49 | 0.82 (0.59, 1.14) 0.23 | 0.90 (0.64, 1.28) 0.57 | 0.54 |
| Model 2 h (95% CI) P-value | 1 | 0.90 (0.66, 1.22) 0.51 | 0.82 (0.60, 1.14) 0.24 | 0.91 (0.64, 1.30) 0.60 | 0.57 |
| Model 3 h (95% CI) P-value | 1 | 0.90 (0.67, 1.22) 0.51 | 0.82 (0.59, 1.13) 0.22 | 0.90 (0.63, 1.28) 0.56 | 0.53 |
| Other mortality | |||||
| Number of deaths | 247 (5.11) | 255 (6.21) | 292 (6.30) | 223 (4.63) | |
| Crude model h (95% CI) P-value | 1 | 1.19 (0.95, 1.49) 0.13 | 1.24 (0.97, 1.60) 0.09 | 0.89 (0.71, 1.12) 0.31 | 0.35 |
| Model 1 h (95% CI) P-value | 1 | 1.16 (0.91, 1.49) 0.24 | 1.24 (0.95, 1.63) 0.12 | 0.93 (0.71, 1.22) 0.61 | 0.7 |
| Model 2 h (95% CI) P-value | 1 | 1.19 (0.91, 1.54) 0.20 | 1.30 (0.99, 1.72) 0.06 | 1.00 (0.77, 1.31) 0.98 | 0.86 |
| Model 3 h (95% CI) P-value | 1 | 1.20 (0.93, 1.54) 0.16 | 1.25 (0.96, 1.62) 0.10 | 0.99 (0.76, 1.29) 0.96 | 0.98 |
| Vitamin C | |||||
| All-cause mortality | |||||
| Number of deaths | 554 (12.44) | 553 (11.16) | 630 (13.81) | 579 (12.87) | |
| Crude model h (95% CI) P-value | 1 | 0.86 (0.73, 1.02) 0.09 | 1.08 (0.91, 1.28) 0.39 | 0.93 (0.78, 1.11) 0.41 | 0.98 |
| Model 1 h (95% CI) P-value | 1 | 0.75 (0.65, 0.88) <0.001 | 0.79 (0.67, 0.94) 0.01 | 0.76 (0.64, 0.90) 0.002 | 0.01 |
| Model 2 h (95% CI) P-value | 1 | 0.82 (0.71, 0.96) 0.01 | 0.92 (0.78, 1.08) 0.30 | 0.92 (0.77, 1.10) 0.37 | 0.72 |
| Model 3 h (95% CI) P-value | 1 | 0.83 (0.71, 0.96) 0.01 | 0.92 (0.78, 1.08) 0.30 | 0.93 (0.77, 1.12) 0.43 | 0.78 |
| CVD mortality | |||||
| Number of deaths | 158 (3.40) | 160 (3.26) | 216 (4.19) | 188 (3.92) | |
| Crude model h (95% CI) P-value | 1 | 0.92 (0.67, 1.27) 0.62 | 1.20 (0.90, 1.59) 0.22 | 1.03 (0.74, 1.43) 0.87 | 0.49 |
| Model 1 h (95% CI) P-value | 1 | 0.80 (0.59, 1.08) 0.14 | 0.84 (0.63, 1.11) 0.21 | 0.80 (0.57, 1.13) 0.21 | 0.31 |
| Model 2 h (95% CI) P-value | 1 | 0.86 (0.63, 1.16) 0.32 | 0.94 (0.69, 1.29) 0.72 | 0.95 (0.65, 1.41) 0.81 | 0.99 |
| Model 3 h (95% CI) P-value | 1 | 0.84 (0.62, 1.14) 0.27 | 0.93 (0.68, 1.28) 0.67 | 0.98 (0.66, 1.45) 0.91 | 0.91 |
| Cancer mortality | |||||
| Number of deaths | 148 (3.44) | 141 (2.91) | 155 (3.92) | 133 (2.91) | |
| Crude model h (95% CI) P-value | 1 | 0.82 (0.60, 1.11) 0.19 | 1.11 (0.82, 1.49) 0.50 | 0.76 (0.53, 1.10) 0.15 | 0.43 |
| Model 1 h (95% CI) P-value | 1 | 0.69 (0.52, 0.93) 0.02 | 0.81 (0.61, 1.07) 0.13 | 0.61 (0.43, 0.88) 0.01 | 0.03 |
| Model 2 h (95% CI) P-value | 1 | 0.77 (0.58, 1.02) 0.07 | 0.94 (0.72, 1.25) 0.68 | 0.74 (0.52, 1.06) 0.10 | 0.26 |
| Model 3 h (95% CI) P-value | 1 | 0.77 (0.58, 1.02) 0.07 | 0.95 (0.72, 1.25) 0.69 | 0.73 (0.51, 1.05) 0.09 | 0.23 |
| Other mortality | |||||
| Number of deaths | 248 (5.61) | 252 (4.99) | 259 (5.70) | 258 (6.05) | |
| Crude model h (95% CI) P-value | 1 | 0.86 (0.66, 1.11) 0.24 | 0.99 (0.74, 1.31) 0.92 | 0.97 (0.75, 1.25) 0.79 | 0.91 |
| Model 1 h (95% CI) P-value | 1 | 0.77 (0.59, 0.99) 0.04 | 0.76 (0.57, 1.02) 0.07 | 0.82 (0.64, 1.05) 0.11 | 0.17 |
| Model 2 h (95% CI) P-value | 1 | 0.84 (0.65, 1.08) 0.17 | 0.88 (0.65, 1.18) 0.40 | 1.01 (0.78, 1.30) 0.95 | 0.8 |
| Model 3 h (95% CI) P-value | 1 | 0.86 (0.67, 1.11) 0.26 | 0.89 (0.67, 1.19) 0.44 | 1.03 (0.81, 1.33) 0.79 | 0.7 |
| Vitamin D | |||||
| All-cause mortality | |||||
| Number of deaths | 204 (5.55) | 240 (7.67) | 240 (7.72) | 294 (9.40) | |
| Crude model h (95% CI) P-value | 1 | 1.28 (1.01, 1.62) 0.04 | 1.31 (1.01, 1.70) 0.04 | 1.62 (1.28, 2.04) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 1.09 (0.85, 1.40) 0.49 | 0.98 (0.77, 1.24) 0.86 | 1.33 (1.04, 1.71) 0.02 | 0.04 |
| Model 2 h (95% CI) P-value | 1 | 1.16 (0.91, 1.49) 0.23 | 1.12 (0.90, 1.41) 0.30 | 1.49 (1.16, 1.92) 0.002 | 0.003 |
| Model 3 h (95% CI) P-value | 1 | 1.17 (0.92, 1.48) 0.20 | 1.09 (0.88, 1.37) 0.43 | 1.44 (1.12, 1.84) 0.004 | 0.01 |
| CVD mortality | |||||
| Number of deaths | 48 (1.23) | 80 (2.32) | 67 (2.29) | 92 (2.85) | |
| Crude model h (95% CI) P-value | 1 | 1.77 (1.18, 2.67) 0.01 | 1.77 (1.10, 2.86) 0.02 | 2.23 (1.31, 3.78) 0.003 | 0.005 |
| Model 1 h (95% CI) P-value | 1 | 1.48 (0.95, 2.31) 0.09 | 1.24 (0.74, 2.07) 0.42 | 1.77 (1.03, 3.02) 0.04 | 0.07 |
| Model 2 h (95% CI) P-value | 1 | 1.48 (0.95, 2.30) 0.08 | 1.31 (0.79, 2.17) 0.30 | 1.84 (1.05, 3.22) 0.03 | 0.06 |
| Model 3 h (95% CI) P-value | 1 | 1.44 (0.92, 2.26) 0.11 | 1.29 (0.78, 2.15) 0.33 | 1.74 (0.98, 3.09) 0.06 | 0.08 |
| Cancer mortality | |||||
| Number of deaths | 55 (1.63) | 65 (2.25) | 57 (1.97) | 75 (2.41) | |
| Crude model h (95% CI) P-value | 1 | 1.27 (0.77, 2.10) 0.34 | 1.14 (0.65, 2.00) 0.66 | 1.41 (0.87, 2.26) 0.16 | 0.26 |
| Model 1 h (95% CI) P-value | 1 | 1.05 (0.64, 1.72) 0.85 | 0.84 (0.49, 1.44) 0.52 | 1.12 (0.68, 1.84) 0.65 | 0.84 |
| Model 2 h (95% CI) P-value | 1 | 1.09 (0.66, 1.79) 0.73 | 0.94 (0.55, 1.61) 0.82 | 1.19 (0.71, 2.00) 0.50 | 0.64 |
| Model 3 h (95% CI) P-value | 1 | 1.16 (0.71, 1.90) 0.54 | 0.96 (0.56, 1.64) 0.88 | 1.25 (0.75, 2.07) 0.40 | 0.58 |
| Other mortality | |||||
| Number of deaths | 101 (2.69) | 95 (3.10) | 116 (3.46) | 127 (4.14) | |
| Crude model h (95% CI) P-value | 1 | 0.88 (0.81, 0.96) 0.005 | 0.92 (0.86, 0.98) 0.01 | 0.93 (0.86, 1.00) 0.06 | 0.11 |
| Model 1 h (95% CI) P-value | 1 | 0.86 (0.80, 0.94) <0.001 | 0.89 (0.84, 0.94) <0.0001 | 0.92 (0.86, 0.98) 0.02 | 0.03 |
| Model 2 h (95% CI) P-value | 1 | 0.86 (0.79, 0.93) <0.001 | 0.89 (0.84, 0.94) <0.0001 | 0.91 (0.85, 0.98) 0.01 | 0.04 |
| Model 3 h (95% CI) P-value | 1 | 0.86 (0.79, 0.93) <0.001 | 0.88 (0.83, 0.93) <0.0001 | 0.92 (0.85, 0.99) 0.02 | 0.05 |
| Vitamin E | |||||
| All-cause mortality | |||||
| Number of deaths | 786 (17.16) | 643 (14.97) | 508 (11.15) | 379 (8.28) | |
| Crude model h (95% CI) P-value | 1 | 0.94 (0.80, 1.10) 0.45 | 0.75 (0.63, 0.89) <0.001 | 0.64 (0.53, 0.77) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 0.95 (0.81, 1.11) 0.53 | 0.78 (0.66, 0.93) 0.01 | 0.78 (0.65, 0.94) 0.01 | 0.002 |
| Model 2 h (95% CI) P-value | 1 | 1.02 (0.86, 1.21) 0.84 | 0.88 (0.74, 1.06) 0.19 | 0.93 (0.74, 1.16) 0.51 | 0.29 |
| Model 3 h (95% CI) P-value | 1 | 1.06 (0.89, 1.25) 0.53 | 0.91 (0.76, 1.10) 0.34 | 0.94 (0.76, 1.17) 0.61 | 0.35 |
| CVD mortality | |||||
| Number of deaths | 252 (4.77) | 197 (4.16) | 146 (3.22) | 127 (2.87) | |
| Crude model h (95% CI) P-value | 1 | 0.94 (0.74, 1.21) 0.65 | 0.78 (0.61, 1.01) 0.06 | 0.79 (0.58, 1.08) 0.14 | 0.07 |
| Model 1 h (95% CI) P-value | 1 | 0.98 (0.78, 1.22) 0.84 | 0.83 (0.64, 1.08) 0.17 | 1.04 (0.75, 1.43) 0.83 | 0.87 |
| Model 2 h (95% CI) P-value | 1 | 1.06 (0.84, 1.35) 0.60 | 0.98 (0.73, 1.32) 0.91 | 1.33 (0.93, 1.90) 0.12 | 0.22 |
| Model 3 h (95% CI) P-value | 1 | 1.14 (0.91, 1.43) 0.27 | 1.04 (0.77, 1.40) 0.80 | 1.39 (0.98, 1.99) 0.07 | 0.14 |
| Cancer mortality | |||||
| Number of deaths | 190 (4.24) | 155 (4.07) | 137 (2.97) | 95 (2.22) | |
| Crude model h (95% CI) P-value | 1 | 1.03 (0.76, 1.39) 0.85 | 0.80 (0.55, 1.16) 0.24 | 0.69 (0.47, 1.00) 0.05 | 0.02 |
| Model 1 h (95% CI) P-value | 1 | 0.98 (0.73, 1.31) 0.89 | 0.76 (0.53, 1.09) 0.14 | 0.72 (0.51, 1.03) 0.07 | 0.03 |
| Model 2 h (95% CI) P-value | 1 | 1.04 (0.77, 1.39) 0.81 | 0.82 (0.58, 1.18) 0.29 | 0.79 (0.57, 1.12) 0.19 | 0.09 |
| Model 3 h (95% CI) P-value | 1 | 1.04 (0.77, 1.39) 0.80 | 0.83 (0.58, 1.18) 0.30 | 0.78 (0.56, 1.09) 0.15 | 0.07 |
| Other mortality | |||||
| Number of deaths | 344 (8.15) | 291 (6.73) | 225 (4.96) | 157 (3.19) | |
| Crude model h (95% CI) P-value | 1 | 0.89 (0.70, 1.13) 0.35 | 0.70 (0.57, 0.87) 0.001 | 0.52 (0.41, 0.67) <0.0001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 0.92 (0.72, 1.19) 0.53 | 0.77 (0.61, 0.97) 0.02 | 0.68 (0.52, 0.88) 0.004 | 0.002 |
| Model 2 h (95% CI) P-value | 1 | 0.98 (0.75, 1.28) 0.88 | 0.86 (0.66, 1.11) 0.25 | 0.78 (0.57, 1.08) 0.14 | 0.1 |
| Model 3 h (95% CI) P-value | 1 | 1.01 (0.77, 1.32) 0.93 | 0.88 (0.68, 1.15) 0.35 | 0.81 (0.59, 1.11) 0.18 | 0.13 |
| Vitamin K | |||||
| All-cause mortality | |||||
| Number of deaths | 630 (14.93) | 542 (12.65) | 439 (10.29) | 347 (8.08) | |
| Crude model h (95% CI) P-value | 1 | 0.90 (0.77, 1.05) 0.17 | 0.80 (0.67, 0.96) 0.01 | 0.69 (0.58, 0.84) <0.001 | <0.0001 |
| Model 1 h (95% CI) P-value | 1 | 0.94 (0.80, 1.09) 0.40 | 0.81 (0.68, 0.96) 0.02 | 0.71 (0.60, 0.85) <0.001 | <0.0001 |
| Model 2 h (95% CI) P-value | 1 | 0.99 (0.83, 1.17) 0.87 | 0.91 (0.76, 1.08) 0.27 | 0.79 (0.65, 0.96) 0.02 | 0.01 |
| Model 3 h (95% CI) P-value | 1 | 1.01 (0.84, 1.20) 0.95 | 0.92 (0.77, 1.09) 0.33 | 0.81 (0.66, 0.98) 0.03 | 0.01 |
| CVD mortality | |||||
| Number of deaths | 192 (3.99) | 160 (3.43) | 143 (3.41) | 105 (2.36) | |
| Crude model h (95% CI) P-value | 1 | 0.91 (0.69, 1.20) 0.51 | 0.98 (0.72, 1.35) 0.92 | 0.75 (0.54, 1.05) 0.09 | 0.17 |
| Model 1 h (95% CI) P-value | 1 | 0.94 (0.71, 1.26) 0.70 | 0.99 (0.73, 1.35) 0.96 | 0.78 (0.56, 1.09) 0.15 | 0.22 |
| Model 2 h (95% CI) P-value | 1 | 0.98 (0.73, 1.31) 0.88 | 1.09 (0.79, 1.49) 0.61 | 0.89 (0.62, 1.27) 0.51 | 0.71 |
| Model 3 h (95% CI) P-value | 1 | 1.01 (0.74, 1.37) 0.97 | 1.11 (0.79, 1.54) 0.55 | 0.94 (0.66, 1.34) 0.72 | 0.91 |
| Cancer mortality | |||||
| Number of deaths | 159 (4.20) | 124 (3.06) | 108 (2.60) | 90 (1.99) | |
| Crude model h (95% CI) P-value | 1 | 0.77 (0.57, 1.05) 0.09 | 0.71 (0.51, 1.01) 0.05 | 0.60 (0.41, 0.88) 0.01 | 0.01 |
| Model 1 h (95% CI) P-value | 1 | 0.79 (0.57, 1.08) 0.14 | 0.70 (0.49, 0.99) 0.04 | 0.59 (0.40, 0.86) 0.01 | 0.01 |
| Model 2 h (95% CI) P-value | 1 | 0.81 (0.58, 1.13) 0.21 | 0.74 (0.53, 1.04) 0.08 | 0.60 (0.40, 0.89) 0.01 | 0.01 |
| Model 3 h (95% CI) P-value | 1 | 0.79 (0.57, 1.11) 0.18 | 0.74 (0.52, 1.04) 0.08 | 0.57 (0.38, 0.86) 0.01 | 0.01 |
| Other mortality | |||||
| Number of deaths | 279 (6.74) | 258 (6.16) | 188 (4.29) | 152 (3.73) | |
| Crude model h (95% CI) P-value | 1 | 0.97 (0.75, 1.26) 0.82 | 0.74 (0.53, 1.04) 0.08 | 0.72 (0.55, 0.94) 0.01 | 0.01 |
| Model 1 h (95% CI) P-value | 1 | 1.03 (0.79, 1.32) 0.85 | 0.77 (0.56, 1.06) 0.11 | 0.76 (0.59, 0.97) 0.03 | 0.01 |
| Model 2 h (95% CI) P-value | 1 | 1.11 (0.84, 1.46) 0.46 | 0.90 (0.64, 1.25) 0.53 | 0.86 (0.65, 1.15) 0.31 | 0.19 |
| Model 3 h (95% CI) P-value | 1 | 1.14 (0.87, 1.50) 0.33 | 0.92 (0.66, 1.27) 0.60 | 0.89 (0.67, 1.18) 0.41 | 0.23 |
Subgroup and sensitivity analysis
After adjusting for sex, age, ethnicity, BMI, educational level, smoking status, drinking status, family income poverty ratio, HEI-2015, total dietary energy intake, self-reported health status, family history of CVDs, and self-reported CVD, COPD, chronic kidney disease, and cancer, subgroup analyses were performed to assess the association between vitamin levels and mortality (ESI Table 2†).1–11 Among individuals with prediabetes, the association between vitamin B1 levels and CVD mortality was stronger in those with moderate alcohol consumption and HEI Q2 (both p interaction = 0.04). The association between niacin levels and cancer mortality was stronger in individuals with educational levels lower than high school and college or higher (p interaction = 0.04 and 0.002, respectively). The associations between CVD mortality and vitamins B6 (p interaction = 0.01), B12 (p interaction = 0.004), and D (p interaction = 0.01) levels were stronger in males. Meanwhile, the association between vitamin B12 levels and both CVD and cancer mortality was stronger in individuals reporting very good-to-excellent self-reported health status (both p interaction = 0.01). The association between vitamin D levels and all-cause mortality was stronger with higher total dietary energy intake (p interaction = 0.01). Similarly, the association between vitamin D levels and cancer mortality was stronger in individuals with poor-to-fair self-reported health statuses (p interaction = 0.01). The association between vitamin E levels and all-cause mortality was stronger in individuals with educational levels of college or above (p interaction = 0.02), former smokers (p interaction = 0.02), HEI Q4 (p interaction = 0.03), and poor-to-fair self-reported health statuses (p interaction = 0.01). Likewise, the association between vitamin E levels and CVD mortality was stronger in individuals reporting very good-to-excellent self-reported health statuses (p interaction <0.001), whereas such stronger association with cancer mortality was observed among former smokers (p interaction = 0.04). The association between vitamin K levels and all-cause (p interaction = 0.03) and cancer mortality (p interaction = 0.02) was stronger in individuals with an educational level of college or higher. No significant interactions were observed between vitamin levels and the remaining stratified variables (p interaction >0.05). Sensitivity analyses excluding participants who experienced mortality events during the first 3 years of follow-up yielded similar results, with all significant associations observed in the main analysis remaining intact (ESI Table 3†).Discussion
In this study, we explored the relationship between the intake of eleven vitamins and the rates of all-cause and CVD mortality in individuals with prediabetes. We identified both non-linear and linear relationships between different vitamins and mortality rates. Our study indicated that higher levels of vitamins B1, niacin, folate, vitamin C, vitamin E, and vitamin K were significantly associated with lower risks of all-cause and CVD mortality. Conversely, higher vitamin D levels were associated with a higher risk of all-cause and CVD mortality. No significant correlations were observed between the levels of vitamins A, B2, and B12 and mortality rates. Various stratified and sensitivity analyses demonstrated the robustness of our findings. To the best of our knowledge, this is the first study to reveal non-linear associations between vitamins levels and all-cause and CVD mortality among individuals with prediabetes.Previous clinical studies have also explored the relationship between vitamin levels and all-cause mortality, CVD incidence, and mortality in different patient groups and the general population. We observed that higher vitamin B1 levels were associated with reduced mortality rates in individuals with prediabetes. Vitamin B1, also known as thiamine, is an essential micronutrient for cellular metabolism.22 A study based on NHANES data showed that with increasing dietary intake of vitamin B1, the risks of hypertension, heart failure, and CVD mortality gradually decreased,23 consistent with our findings. Additionally, elevated niacin levels are associated with reduced all-cause and CVD mortality in individuals with prediabetes. Several studies based on NHANES data have demonstrated a significantly lower risk of all-cause mortality among patients with cancer,24 non-alcoholic fatty liver disease,25 and diabetes26 with higher dietary niacin intakes. Niacin supplementation has been shown to improve survival rates among cancer patients.24 A cohort study with an average 12-year follow-up of 4573 participants from the Rotterdam Study reported similar results, suggesting that dietary niacin prolongs the lifespan of diabetes patients by upregulating the activity of SIRT1, a gene that protects cells from oxidative stress and aging.27 However, some uncertainty regarding the benefits of niacin intake in individuals with prediabetes remains, necessitating further confirmation.
Folate is a water-soluble vitamin crucial for cell growth and reproduction. Meta-analyses investigating the relationship between folate supplementation and glucose metabolism have suggested its potentially beneficial effects on insulin homeostasis and blood glucose control in both the general population and diabetes patients.28,29 Dietary and supplemental folate intake reportedly reduces the incidence and mortality of CVD in the general population.30–32 Lower serum folate levels are significantly associated with an increased risk of CVD mortality in type 2 diabetes patients.33 However, we found that only all-cause mortality, not CVD mortality, was significantly associated with folate levels in individuals with prediabetes; this inconsistent finding may be attributed to differences in disease models. Overall, the existing data suggest that folate intake may play an important role in nutritional strategies for individuals with prediabetes.
Moreover, the important antioxidants vitamins C and E have been implicated in the risk of mortality in several studies. Low dietary intake or inadequate serum vitamin C levels are associated with an increased mortality risk in patients with type 2 diabetes.34 Adults with prediabetes have a high demand for vitamin C.18 A dose–response meta-analysis demonstrated that higher dietary intake or blood levels of vitamins C and E were associated with a reduced risk of CVD and all-cause mortality,35 consistent with our findings. Furthermore, vitamin E treatment reduced fatal arrhythmias during severe hypoglycemia in diabetic rats.36 Vitamin E deficiency and oxidative stress are associated with prediabetes in healthy individuals.37 Several studies have indicated that low vitamin K levels are associated with an increased risk of all-cause mortality.38,39 Supplementation with vitamin K in women with prediabetes for 14 weeks did not affect insulin resistance; however, it had beneficial effects on glycemic status and insulin sensitivity.40 Existing data suggest that vitamin C, E, and K levels may play important roles in individuals with prediabetes.
The relationship between vitamin D levels and mortality and diabetes is a topic of considerable interest. Several studies have demonstrated that vitamin D supplementation in individuals with prediabetes can effectively reduce the risk of developing type 2 diabetes and increase the rate of reversal to normal blood glucose levels.11,13,14 However, conflicting findings suggest that vitamin D supplementation does not lower the risk of progression from prediabetes to type 2 diabetes or decrease insulin resistance.17 Additionally, there was no causal relationship between vitamin D and type 2 diabetes or prediabetes in the Chinese population.41 Therefore, it remains unclear whether vitamin D can reverse the progression of prediabetes to diabetes. Vitamin D deficiency is reportedly associated with a higher risk of all-cause and CVD mortality in individuals with prediabetes,15,16 which differs from our findings that higher vitamin D levels increase the risk of all-cause and CVD mortality. This discrepancy may be due to our inclusion of total vitamin D levels instead of serum 25-hydroxyvitamin D levels, which were used in the aforementioned studies. Nonetheless, the relationship between various forms of activated vitamin D and the risk of prediabetes warrants further investigation.
We observed no significant association between vitamin A levels and all-cause or CVD mortality. Previous research has shown that vitamin A supplementation does not reduce mortality in the general population,42,43 which is consistent with our findings in individuals with prediabetes. However, a study based on NHANES data suggested a significant association between vitamin A intake from food or supplements and reduced mortality in adult diabetes patients in the US.26 This difference may be attributed to the relatively higher prevalence of vitamin A deficiency among adult diabetes patients in the US, which leads to a more pronounced compensatory effect of vitamin A supplementation. Further studies are needed to confirm the relationship between vitamin A levels and mortality.
Although our study identified several vitamins associated with reduced mortality in adults with prediabetes, a previous randomized clinical trial43 and systematic review42 did not observe any significant benefits of vitamin supplementation in reducing the risk of mortality, cardiovascular events, or cancer events. Given that vitamin deficiencies are common in patients with poorly controlled diabetes, adequate daily intake of vitamins from food is crucial for individuals with prediabetes.
To our knowledge, the present study is the largest investigation of the associations of eleven vitamin levels with all-cause and CVD mortality among individuals with prediabetes, with consideration of a multitude of potential confounding factors. In addition, the present analysis is based on a nationally representative sample of US adults with prediabetes, which facilitates the generalization of the findings. This study also has some limitations. First, its cross-sectional design conducted at a single center, which cannot establish causal relationships between the levels of the 11 vitamins and mortality in individuals with prediabetes. Therefore, prospective cohort studies are required to validate these findings. Second, although we attempted to control for confounding variables through multivariate adjustment and subgroup analysis, residual confounding factors that affect prognosis may still exist.
Conclusions
In a nationally representative sample of U.S. adults with prediabetes, we identified associations between the levels of eleven vitamins and the risk of all-cause and CVD mortality. Future research should explore whether interventions targeting different vitamins can improve the glycemic status in individuals with prediabetes, delay the progression from prediabetes to diabetes, and improve the prognosis of individuals with prediabetes.List of abbreviations
| ADA | American diabetes association |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| CIs | Confidence intervals |
| COPD | Chronic obstructive pulmonary diseases |
| CVD | Cardiovascular disease |
| HbA1c | Hemoglobin A1c |
| HEI | Healthy eating index |
| HRs | Hazard ratios |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| NAFLD | Nonalcoholic fatty liver disease |
| NCHS | National center for health statistic |
| NHANES | National health and nutrition examination survey |
| NDI | National death index |
| RCS | Restricted cubic spline |
| T2D | Type 2 diabetes |
| U.S. | United States |
Author contributions
WXR and YL conducted analysis and drafted the manuscript. XGS is the corresponding author supervising and the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CHL, SYL and YS supervised the study. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.Ethics approval and consent to participate
The original survey protocol was approved by the Institutional Review Board of the National Center of Heath Statistics (https://www.cdc.gov/nchs/nhanes/irba98.htm). All participants signed informed consent forms. The present study was deemed exempt by the Institutional Review Board of our center.Consent for publication
All authors read the manuscript and agreed to its publication.Data availability
The datasets generated and analysed during the current study are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 82170804, 82300933). We thank Editage for the linguistic editing and proofreading of the manuscript. The authors thank the participants and staff of the National Health and Nutrition Examination Survey 1999–2018 for their valuable contributions.References
- A. G. Tabák, C. Herder, W. Rathmann, E. J. Brunner and M. Kivimäki, Prediabetes: a high-risk state for diabetes development, Lancet, 2012, 379(9833), 2279–2290 CrossRef.
- Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria, The DECODE study group, European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe, Lancet, 1999, 354(9179), 617–621 CrossRef.
- H. C. Gerstein, P. Santaguida, P. Raina, K. M. Morrison, C. Balion and D. Hunt, et al., Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies, Diabetes Res. Clin. Pract., 2007, 78(3), 305–312 CrossRef PubMed.
- K. M. Bullard, S. H. Saydah, G. Imperatore, C. C. Cowie, E. W. Gregg and L. S. Geiss, et al., Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010, Diabetes Care, 2013, 36(8), 2286–2293 CrossRef CAS PubMed.
- M. R. Rooney, M. Fang, K. Ogurtsova, B. Ozkan, J. B. Echouffo-Tcheugui and E. J. Boyko, et al., Global Prevalence of Prediabetes, Diabetes Care, 2023, 46(7), 1388–1394 CrossRef CAS.
- J. C. Hsu, Y. Y. Yang, S. L. Chuang, L. Y. Lin and T. H. H. Chen, Prediabetes as a risk factor for new-onset atrial fibrillation: the propensity-score matching cohort analyzed using the Cox regression model coupled with the random survival forest, Cardiovasc. Diabetol., 2023, 22(1), 35 CrossRef CAS PubMed.
- J. Y. Huang, Y. K. Tse, H. L. Li, C. Chen, C. T. Zhao and M. Y. Liu, et al., Prediabetes Is Associated With Increased Risk of Heart Failure Among Patients With Atrial Fibrillation, Diabetes Care, 2023, 46(1), 190–196 CrossRef PubMed.
- Y. Huang, X. Cai, M. Qiu, P. Chen, H. Tang and Y. Hu, et al., Prediabetes and the risk of cancer: a meta-analysis, Diabetologia, 2014, 57(11), 2261–2269 CrossRef CAS PubMed.
- L. Mai, W. Wen, M. Qiu, X. Liu, L. Sun and H. Zheng, et al., Association between prediabetes and adverse outcomes in heart failure, Diabetes, Obes. Metab., 2021, 23(11), 2476–2483 CrossRef PubMed.
- X. Cai, Y. Zhang, M. Li, J. H. Wu, L. Mai and J. Li, et al., Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis, Br. Med. J., 2020, 370, m2297 CrossRef.
- A. G. Pittas, T. Kawahara, R. Jorde, B. Dawson-Hughes, E. M. Vickery and E. Angellotti, et al., Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes : A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials, Ann. Intern. Med., 2023, 176(3), 355–363 CrossRef.
- A. L. Tardy, E. Pouteau, D. Marquez, C. Yilmaz and A. Scholey, Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence, Nutrients, 2020, 12(1), 228 CrossRef CAS.
- Y. Zhang, H. Tan, J. Tang, J. Li, W. Chong and Y. Hai, et al., Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis, Diabetes Care, 2020, 43(7), 1650–1658 CrossRef CAS PubMed.
- R. M. Pojednic, E. M. Trussler, J. D. Navon, S. C. Lemire, E. C. Siu and E. S. Metallinos-Katsaras, Vitamin D deficiency associated with risk of prediabetes among older adults: Data from the National Health and Nutrition Examination Survey (NHANES), 2007–2012, Diabetes/Metab. Res. Rev., 2022, 38(3), e3499 CrossRef CAS.
- A. Jayedi, M. Daneshvar, A. T. Jibril, J. D. Sluyter, M. Waterhouse and B. D. Romero, et al., Serum 25(OH)D Concentration, Vitamin D Supplementation, and Risk of Cardiovascular Disease and Mortality in Patients with Type 2 Diabetes or Prediabetes: a Systematic Review and Dose-Response Meta-Analysis, Am. J. Clin. Nutr., 2023, 118(3), 697–707 CrossRef.
- P. Zhang, D. Guo, B. Xu, C. Huang, S. Yang and W. Wang, et al., Association of Serum 25-Hydroxyvitamin D With Cardiovascular Outcomes and All-Cause Mortality in Individuals With Prediabetes and Diabetes: Results From the UK Biobank Prospective Cohort Study, Diabetes Care, 2022, 45(5), 1219–1229 CrossRef CAS PubMed.
- A. Pieńkowska, J. Janicka, M. Duda, K. Dzwonnik, K. Lip and A. Mędza, et al., Controversial Impact of Vitamin D Supplementation on Reducing Insulin Resistance and Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review, Nutrients, 2023, 15(4), 983 CrossRef.
- R. Wilson, J. Willis, R. Gearry, P. Skidmore, E. Fleming and C. Frampton, et al., Inadequate Vitamin C Status in Prediabetes and Type 2 Diabetes Mellitus: Associations with Glycaemic Control, Obesity, and Smoking, Nutrients, 2017, 9(9), 997 CrossRef.
- S. M. Krebs-Smith, T. E. Pannucci, A. F. Subar, S. I. Kirkpatrick, J. L. Lerman and J. A. Tooze, et al., Update of the Healthy Eating Index: HEI-2015, J. Acad. Nutr. Diet., 2018, 118(9), 1591–1602 CrossRef.
- Z. Wan, J. Guo, A. Pan, C. Chen, L. Liu and G. Liu, Association of Serum 25-Hydroxyvitamin D Concentrations With All-Cause and Cause-Specific Mortality Among Individuals With Diabetes, Diabetes Care, 2021, 44(2), 350–357 CrossRef CAS.
- L. Yu, W. Liu, X. Wang, Z. Ye, Q. Tan and W. Qiu, et al., A review of practical statistical methods used in epidemiological studies to estimate the health effects of multi-pollutant mixture, Environ. Pollut., 2022, 306, 119356 CrossRef CAS.
- S. Manzetti, J. Zhang and D. van der Spoel, Thiamin function, metabolism, uptake, and transport, Biochemistry, 2014, 53(5), 821–835 CrossRef CAS PubMed.
- H. Wen, X. Niu, R. Zhao, Q. Wang, N. Sun and L. Ma, et al., Association of vitamin B1 with cardiovascular diseases, all-cause and cardiovascular mortality in US adults, Front. Nutr., 2023, 10, 1175961 CrossRef PubMed.
- H. Ying, L. Gao, N. Liao, X. Xu, W. Yu and W. Hong, Association between niacin and mortality among patients with cancer in the NHANES retrospective cohort, BMC Cancer, 2022, 22(1), 1173 CrossRef CAS PubMed.
- J. Pan, Y. Zhou, N. Pang and L. Yang, Dietary Niacin Intake and Mortality Among Individuals With Nonalcoholic Fatty Liver Disease, JAMA Network Open, 2024, 7(2), e2354277 CrossRef.
- W. Liu, S. Cao, D. Shi, Z. Ye, L. Yu and R. Liang, et al., Association between dietary vitamin intake and mortality in US adults with diabetes: A prospective cohort study, Diabetes/Metab. Res. Rev., 2024, 40(2), e3729 CrossRef CAS.
- M. C. Zillikens, J. B. J. van Meurs, E. J. G. Sijbrands, F. Rivadeneira, A. Dehghan and J. P. T. M. van Leeuwen, et al., SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin, Free Radical Biol. Med., 2009, 46(6), 836–841 CrossRef CAS.
- J. V. Zhao, C. M. Schooling and J. X. Zhao, The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials, Ann. Epidemiol., 2018, 28(4), 249–257.e1 CrossRef PubMed.
- P. Sudchada, S. Saokaew, S. Sridetch, S. Incampa, S. Jaiyen and W. Khaithong, Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis, Diabetes Res. Clin. Pract., 2012, 98(1), 151–158 CrossRef CAS.
- R. Cui, H. Iso, C. Date, S. Kikuchi and A. Tamakoshi, Japan Collaborative Cohort Study Group, Dietary folate and vitamin b6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study, Stroke, 2010, 41(6), 1285–1289 CrossRef CAS.
- E. Lonn, S. Yusuf, M. J. Arnold, P. Sheridan, J. Pogue and M. Micks, et al., Homocysteine lowering with folic acid and B vitamins in vascular disease, N. Engl. J. Med., 2006, 354(15), 1567–1577 CrossRef CAS.
- Y. Li, T. Huang, Y. Zheng, T. Muka, J. Troup and F. B. Hu, Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials, J. Am. Heart Assoc., 2016, 5(8), e003768 CrossRef PubMed.
- Y. Liu, T. Geng, Z. Wan, Q. Lu, X. Zhang and Z. Qiu, et al., Associations of Serum Folate and Vitamin B12 Levels With Cardiovascular Disease Mortality Among Patients With Type 2 Diabetes, JAMA Network Open, 2022, 5(1), e2146124 CrossRef PubMed.
- H. Sun, J. Karp, K. M. Sun and C. M. Weaver, Decreasing Vitamin C Intake, Low Serum Vitamin C Level and Risk for US Adults with Diabetes, Nutrients, 2022, 14(19), 3902 CrossRef CAS.
- D. Aune, N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta and D. C. Greenwood, et al., Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies, Am. J. Clin. Nutr., 2018, 108(5), 1069–1091 CrossRef.
- C. M. Reno-Bernstein, M. Oxspring, J. Bayles, E. Y. Huang, I. Holiday and S. J. Fisher, Vitamin E treatment in insulin-deficient diabetic rats reduces cardiac arrhythmias and mortality during severe hypoglycemia, Am. J. Physiol. Endocrinol. Metab., 2022, 323(5), E428–E434 CrossRef CAS PubMed.
- G. Rodríguez-Ramírez, L. E. Simental-Mendía, M. d. l. A. Carrera-Gracia and M. A. Quintanar-Escorza, Vitamin E Deficiency and Oxidative Status are Associated with Prediabetes in Apparently Healthy Subjects, Arch. Med. Res., 2017, 48(3), 257–262 CrossRef PubMed.
- C. R. Palmer, J. W. Bellinge, F. Dalgaard, M. Sim, K. Murray and E. Connolly, et al., Association between vitamin K1 intake and mortality in the Danish Diet, Cancer, and Health cohort, Eur. J. Epidemiol., 2021, 36(10), 1005–1014 CrossRef CAS PubMed.
- A. J. van Ballegooijen, J. W. J. Beulens, L. M. Kieneker, M. H. de Borst, R. T. Gansevoort and I. P. Kema, et al., Combined low vitamin D and K status amplifies mortality risk: a prospective study, Eur. J. Nutr., 2021, 60(3), 1645–1654 CrossRef CAS PubMed.
- H. Rasekhi, M. Karandish, M. T. Jalali, M. Mohammad-Shahi, M. Zarei and A. Saki, et al., The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial, Eur. J. Clin. Nutr., 2015, 69(8), 891–895 CrossRef CAS PubMed.
- N. Wang, C. Wang, X. Chen, H. Wan, Y. Chen and C. Chen, et al., Vitamin D, prediabetes and type 2 diabetes: bidirectional Mendelian randomization analysis, Eur. J. Nutr., 2020, 59(4), 1379–1388 CrossRef CAS PubMed.
- E. A. O'Connor, C. V. Evans, I. Ivlev, M. C. Rushkin, R. G. Thomas and A. Martin, et al., Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force, J. Am. Med. Assoc., 2022, 327(23), 2334–2347 CrossRef PubMed.
- H. D. Sesso, P. M. Rist, A. K. Aragaki, S. Rautiainen, L. G. Johnson and G. Friedenberg, et al., Multivitamins in the prevention of cancer and cardiovascular disease: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial, Am. J. Clin. Nutr., 2022, 115(6), 1501–1510 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02893g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |