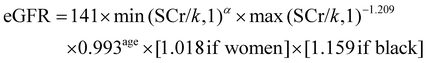Open Access Article
Open Access ArticleThe association between the amount and timing of coffee consumption with chronic kidney disease in diabetic patients†
Yiwei
Tang‡
abc,
Qin
Zhou‡
bc,
Ni
Zhao‡
d,
Fengru
Niu
bc,
Shangying
Li
 bc,
Yingdong
Zuo
bc,
Jiaxin
Huang
e,
Zheng
Wang
bc,
Tianshu
Han
*abc and
Wei
Wei
bc,
Yingdong
Zuo
bc,
Jiaxin
Huang
e,
Zheng
Wang
bc,
Tianshu
Han
*abc and
Wei
Wei
 *bc
*bc
aDepartment of Endocrinology, the Second Affiliated Hospital of Harbin Medical University, Harbin, China. E-mail: snowcalendar@126.com; Tel: +86-451-87502681
bDepartment of Nutrition and Food Hygiene, National Key Discipline, School of Public Health, Harbin Medical University, Harbin, China. E-mail: 102594@hrbmu.edu.cn
cKey Laboratory of Precision Nutrition and Health, Ministry of Education, Harbin Medical University, Harbin, China
dCenter for Interventional Medicine, The Fifth Affiliated Hospital, Sun Yat-sen, University, Zhuhai, Guangdong 519000, China
ePostgraduate Department, the Third Affiliated Hospital of Harbin Medical University (Harbin Medical University Cancer Hospital), Harbin, China
First published on 2nd October 2024
Abstract
Previous studies have suggested that diabetic patients should align their food and nutrient intake with their biological metabolic rhythm. However, the optimal timing of coffee consumption to prevent the development of chronic kidney disease (CKD) in diabetic patients remains unknown. This study aims to examine the association between the amount and timing of coffee consumption and CKD prevalence in diabetic patients. We recruited a nationally representative sample of 8564 diabetes patients from NHANES (National Health and Nutrition Examination Survey) from 2003 to 2018. Coffee intake was assessed using a 24 hour dietary recall and categorized into different time periods throughout the day: dawn-to-forenoon (5:00 a.m. to 8:00 a.m.), forenoon-to-noon (8:00 a.m. to 12:00 p.m.), noon-to-evening (12:00 p.m. to 6:00 p.m.), and evening-to-dawn (6:00 p.m. to 5:00 a.m.). Logistic regression models were used to assess the association between the amount and timing of coffee consumption and the prevalence of CKD in diabetic patients. After adjusting for potential confounders, diabetic patients who had the status of coffee consumption throughout the day had a lower prevalence of CKD compared to those who did not (OR: 0.89, 95% CI: 0.80–0.99). In terms of the timing of coffee consumption, diabetic patients who consumed coffee or had higher levels of coffee consumption from dawn-to-forenoon had a lower incidence risk of CKD (OR: 0.87, 95% CI: 0.77–0.98; OR: 0.83, 95% CI: 0.70–0.98). Conversely, diabetic patients who consumed higher levels of coffee during the noon-to-evening and evening-to-dawn periods had an increased incidence risk of CKD (OR: 1.35, 95% CI: 1.07–1.71 and OR: 1.28, 95% CI: 1.01–1.64, respectively). These observations remained robust across different participant subtypes. Our results indicated that diabetic patients who consumed coffee from dawn-to-forenoon had a lower risk of developing CKD, while those who consumed coffee from noon-to-evening or evening-to-dawn had an increased risk.
Introduction
Diabetes mellitus (DM) is a crucial public health challenge affecting more than 0.5 billion adults worldwide.1 Chronic kidney disease (CKD) is one of the major microvascular complications with approximately 30–40% incidence in DM,1,2 posing a significant threat to diabetes management and economic burden borne by individuals, families, and health systems. It has been reported that diabetic patients with hyperglycemia,3 hypertension,4 and high systematic inflammation status5 are more likely to develop microvascular complication diseases, including CKD. Moreover, diet intervention is identified as a cost-effective strategy to prevent and delay natural diabetes processing;6,7 therefore, an effective diet intervention strategy is paramount to identify in order to prevent CKD development in diabetes.In recent years, chrono-nutrition has been identified as an emerging diet intervention strategy, which emphasizes the importance of food intake time in maintaining organism health.8 Accumulating epidemiological studies have suggested that diabetic patients should synchronize food and nutrient intake with their metabolic biological rhythm, otherwise it may increase the mortality risks of diabetes and cardiovascular disease as well as all-cause mortality.9–13 Coffee is a commonly consumed beverage and possesses abundant bioactive substances, including caffeine, chlorogenic acid, and trigonelline,14 and previous studies have shown that habitual coffee consumption was associated with lower risks of chronic metabolic disease incidence (including hypertension and cardiovascular disease) and all-cause mortality by increasing adiponectin concentrations and decreasing reactive oxygen species and inflammation.14–16 It has also been reported that consuming adequate coffee can improve insulin secretion and antioxidant capacity and reduce inflammatory markers, which would be helpful to reduce the risks of obesity and insulin resistance.17,18 Additionally, numerous studies have documented that moderate regular coffee consumption was inversely associated with both diabetes and CKD incidence.19–21 However, the health impact of coffee intake on CKD development in diabetic patients has been a subject of controversy,22–25 and a major reason lies in ignoring the important role of coffee intake amount and time. A few animal studies have indicated that bioactive substances in coffee can entrain the biological rhythm by regulating various circadian clock genes related with metabolic and inflammatory homeostasis.26,27 Also, due to the disrupted circadian rhythms of blood glucose, blood pressure, and inflammation in diabetes patients,28–31 an optimal intake time of coffee may prevent the natural course of CKD by regulating the physiological rhythm process.32,33 However, limited studies have examined the association between timing of coffee consumption and incidence risk of CKD in diabetes.
In this study, we hypothesized that aligning the time of coffee consumption with biological clocks can prevent CKD incidence among diabetic patients, while misalignment may have the opposite effect. To examine this hypothesis and provide a more effective diet practice, this study aims to investigate the association of coffee consumption in different time periods with CKD prevalence among diabetic patients in a nationally representative sample of U.S. adults.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a research program aimed at evaluating the health and nutritional status of representative population in the U.S.34 Briefly, it collected data through structured interviews, telephone follow-ups, health screenings, and laboratory analyses. Before data collection, the National Center for Health Statistics Research Ethics Review Board gave its approval and obtained written informed consent from all participants. The detailed Research Ethics Committee information and Informed Consent information can be obtained from the following websites (https://www.cdc.gov/nchs/nhanes/irba98.htm; https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm).This study enrolled 9571 adults (aged 18 years or older) with DM who participated in NHANES from 2003 to 2018. DM was defined by meeting any of the following criteria: fasting blood glucose > 7.0 mmol L−1, or random blood glucose/two-hour OGTT blood glucose > 11.1 mmol L−1, glycosylated hemoglobin (HbA1c) > 6.5%, self-reported diagnosis, medication for hyperglycemia. After excluding participants with missing data on weight (n = 169), CKD (n = 708), and hyperlipidemia (n = 1), a total of 8564 diabetic patients (4480 men and 4084 women) were included in the analysis (Fig. 1).
 | ||
| Fig. 1 Flow chart of the selection process of study participants from NHANES 2003–2018. CKD, chronic kidney disease. | ||
Exposure assessment
Data on each type of food item was collected through two cycles of 24-hour dietary recall interviews,35 initially conducted in person and followed by a second telephone recall (3 to 10 days later). The in-person interviews were held privately at NHANES mobile examination centers, utilizing a computer-assisted dietary interview system administered by NHANES interviewers. Coffee intake was estimated using the U.S. Department of Agriculture's Food and Nutrient Database for Dietary Studies (Agricultural Research Service, 2023).36 Detailed information on the timing of coffee intake was recorded in dietary interview questionnaires. Using this data, we calculated the amount of coffee intake throughout the day, and during different time periods: dawn-to-forenoon (5:00 a.m. to 8:00 a.m.), forenoon-to-noon (8:00 a.m. to 12:00 p.m.), noon-to-evening (12:00 p.m. to 6:00 p.m.), and evening-to-dawn (6:00 p.m. to 5:00 a.m.).Outcome assessment
The outcome variable was the prevalence of CKD, defined as glomerular filtration rate (eGFR) < 60 mL per min per 1.73 m2 and/or urinary albumin/creatinine ratio (ACR) > 30 mg g−1.37,38We adopted the CKD-EPI (chronic kidney disease epidemiology collaboration) formula to estimate eGFR.37–39 The formula is shown as follows:
Covariates assessment
This study included the following covariates: age (years), sex (men/women), race (non-Hispanic white or other), education (less than college/college graduate or above), smoking status (current/previous/none), drinking status (current/previous/none), regular exercise habits (yes/no), body mass index (BMI, kg m−2), annual poverty-income ratio (ratio of household income to the poverty line), frequency of coffee intake throughout the day (no/yes), hyperlipidemia (total cholesterol > 200 mg dL−1, or triglycerides > 200 mg dL−1, or high-density lipoproteins < 40 mg dL−1, or low-density lipoproteins > 130 mg dL−1, or current use of cholesterol-lowering medications), and hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or current use of medication to lower high blood pressure).Statistical analysis
Baseline characteristics including sociodemographic information, lifestyle behaviors, and disease status were expressed as mean ± standard deviation (SD) or percentage (n, %). General linear models and chi-square tests were utilized to compare the differences. To examine the association between the amount and timing of coffee consumption and CKD prevalence, we employed two sets of binary logistic regression models. In set 1, participants were grouped by coffee consumption status, with non-coffee drinkers as the reference group. In set 2, participants were categorized into tertiles according to amount of coffee consumption, with those in the lowest tertile serving as the reference group. Logistic regression analyses were also conducted for specific time periods: dawn-to-forenoon, forenoon-to-noon, noon-to-evening, and evening-to-dawn. Participants who did not consume coffee during each time frame were considered as the reference group in set 1, while those in the lowest tertile of coffee intake during the respective period served as the reference group in set 2.Additionally, the adjustments in logistic regression analyses included age, sex, race, education level, smoking and drinking habits, regular exercise, BMI, poverty-income ratio, and prevalence of hyperlipidemia and hypertension. The frequency of coffee intake throughout the day was also controlled. Statistical analyses were performed using R 4.3.1, with the significance set at p < 0.05.
Subgroup analyses and sensitivity analysis
To evaluate the reliability of our findings, we conducted three subgroup analyses and two sensitivity analyses. The first subgroup analysis examined potential sex-specific differences in our results. The second subgroup analysis categorized participants into three groups based on their BMI levels including BMI < 25 kg m−2, 25 ≤ BMI < 30 kg m−2 and BMI ≥ 30 kg m−2. The third subgroup analysis excluded individuals consuming coffee multiple times a day and re-evaluated these associations.In the first sensitive analysis, we repeated the main analyses among non-diabetic participants (n = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553) to assess whether our observations were specific to individuals with diabetes. In the second sensitivity analysis, we excluded participants whose consumption was within mean ± 3 SD to test the robustness of the main results.
553) to assess whether our observations were specific to individuals with diabetes. In the second sensitivity analysis, we excluded participants whose consumption was within mean ± 3 SD to test the robustness of the main results.
Results
The baseline characteristics of studying variables among diabetic patients with varying coffee consumption habits
A total of 8564 diabetic patients were involved, with an average age of 61.88 years, including 4480 (52.93%) men and 4084 (48.25%) women. The baseline characteristics in terms of coffee intake at different time periods across the day are presented in Table 1. In the whole population, per capita coffee consumption was 2.83 g kg−1, and the prevalence of CKD was 41.59%. There were 3331 (38.90%) participants who did not consume coffee across the day, 1510 (17.63%) of them consumed coffee during dawn-to-forenoon, 2367 (27.64%) of them consumed during forenoon-to-noon, 710 (8.29%) of them consumed during noon-to-evening, and 646 (7.54%) consumed during evening-to-dawn.| Characteristics | Total | Not drink coffee | Dawn to forenoon | Forenoon to noon | Noon to evening | Evening to dawn | P-value |
|---|---|---|---|---|---|---|---|
| N = 8564 | N = 3331(38.90%) | N = 1510(17.63%) | N = 2367(27.64%) | N = 710(8.29%) | N = 646(7.54%) | ||
| Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as percentage (n,%). BMI, body mass index and CKD, chronic kidney disease. | |||||||
| Age, years | 61.88 ± 13.67 | 57.95 ± 14.96 | 64.25 ± 11.58 | 64.43 ± 12.23 | 65.97 ± 12.95 | 62.73 ± 11.93 | <0.001 |
| Sex, n(%) | <0.001 | ||||||
| Men | 4480(52) | 1642(49) | 888(59) | 1195(50) | 372(52) | 383(59) | |
| Women | 4084(48) | 1689(51) | 622(41) | 1172(50) | 338(48) | 263(41) | |
| Race | <0.001 | ||||||
| Non-hispanic white, n(%) | 3198(37) | 1040(31) | 712(47) | 902(38) | 314(44) | 230(36) | |
| Others | 5366(63) | 2291(69) | 798(53) | 1465(62) | 396(56) | 416(64) | |
| Education | <0.001 | ||||||
| Not college diploma | 7247(85) | 2814(84) | 1272(84) | 2003(85) | 596(84) | 562(87) | |
| College graduate or above, n(%) | 1307(15) | 513(15) | 235(16) | 362(15) | 113(16) | 84(13) | |
| Smoke | <0.001 | ||||||
| Never smoking, n(%) | 4198(49) | 1949(59) | 583(39) | 1096(46) | 297(42) | 273(42) | |
| Ever smoking, n(%) | 4366(51) | 1382(41) | 927(61) | 1271(54) | 413(58) | 373(58) | |
| Alcohol | <0.001 | ||||||
| Never drinking, n(%) | 2310(27) | 1160(35) | 296(20) | 537(23) | 176(25) | 141(22) | |
| Ever drinking, n(%) | 6254(73) | 2171(65) | 1214(80) | 1830(77) | 534(75) | 505(78) | |
| Exercise regularly, n(%) | 1643(19) | 736(22) | 273(18) | 396(17) | 130(18) | 108(17) | <0.001 |
| BMI, kg m−2 | 31.93 ± 7.42 | 32.86 ± 8.16 | 31.30 ± 6.76 | 31.42 ± 6.88 | 30.85 ± 7.07 | 31.59 ± 6.71 | <0.001 |
| Poverty income ratio | 2.93 ± 2.43 | 2.89 ± 2.49 | 2.94 ± 2.30 | 3.02 ± 2.45 | 2.85 ± 2.40 | 2.82 ± 2.43 | 0.002 |
| Hyperlipidemia, n(%) | 7448(87) | 2828(85) | 1317(87) | 2125(90) | 620(87) | 558(86) | <0.001 |
| Hypertension, n(%) | 6133(72) | 2309(69) | 1112(74) | 1762(74) | 508(72) | 442(68) | <0.001 |
| Coffee intake, g kg−1 | 2.83(3.86) | 0.00(0.00) | 5.35(4.46) | 4.59(4.01) | 3.75(2.63) | 4.08(3.77) | <0.001 |
The dawn-to-forenoon group had the highest coffee consumption and the lowest prevalence of CKD; the noon-to-evening group had the lowest coffee consumption and the highest prevalence of CKD (P < 0.05). Compared to participants who did not consume coffee across the day, participants who consumed coffee during dawn-to-forenoon and noon-to-evening were older, had higher education level, while they had lower BMI level and regular exercise habits proportion but a higher history of smoking and alcohol use (P < 0.05).
The association of coffee consumption (status, total daily amount, and timing) with the prevalence of CKD
The association of coffee consumption status and amount within a day or during different time periods across a day with CKD prevalence is presented in Table 2. As indicated by ORs and 95% CI, after adjustment for potential confounders, compared to diabetic patients without coffee consumption throughout the day, those who consumed coffee experienced a lower incidence risk of CKD (OR: 0.89, 95% CI: 0.80–0.99). Furthermore, we observed that this effect may only be confirmed within certain ranges of 2.621 g kg−1 (2.055, 3.123) per day (OR: 0.88, 95% CI: 0.77–0.99).| Characteristics | Events/N | Med (Q1, Q3) g kg−1 | OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 was adjusted for age, sex, race and education; model 2 is model 1 with additional adjustments for smoking, drinking, physical activity, BMI, poverty-income ratio and multiple times of drinking coffee; model 3 is model 2 with additional adjustments for hypertension and hyperlipidemia. CKD, chronic kidney disease. | |||||||||
| All day coffee intake | |||||||||
| Coffee intake status | |||||||||
| No | 1331/3331 | 0.000(0.000, 0.000) | Ref | Ref | Ref | ||||
| Yes | 2231/5233 | 3.664(2.621, 5.331) | 0.89(0.81, 0.97) | 0.01 | 0.90(0.81, 1.01) | 0.07 | 0.89(0.80, 0.99) | 0.04 | |
| Coffee consumed amount | |||||||||
| T1 | 1331/3331 | 0.000(0.000, 0.000) | Ref | 0.01 | Ref | 0.07 | Ref | 0.05 | |
| T2 | 1130/2617 | 2.621(2.055, 3.123) | 0.89(0.80, 0.99) | 0.90(0.79, 1.02) | 0.88(0.77, 0.99) | ||||
| T3 | 1101/2616 | 5.332(4.337, 7.336) | 0.88(0.79,0.98) | 0.91(0.81, 1.03) | 0.90(0.80, 1.02) | ||||
| Dawn-to-forenoon period | |||||||||
| Coffee consumed status | |||||||||
| No | 2949/7054 | 0.000(0.000, 0.000) | Ref | Ref | Ref | ||||
| Yes | 613/1510 | 4.110(2.891, 6.308) | 0.87(0.77, 0.98) | 0.02 | 0.88(0.78, 0.99) | 0.03 | 0.87(0.77,0.98) | 0.02 | |
| Coffee consumed amount | |||||||||
| T1 | 2949/7054 | 0.000(0.000, 0.000) | Ref | 0.007 | Ref | 0.008 | Ref | 0.006 | |
| T2 | 318/755 | 2.891(2.264, 3.438) | 0.92(0.79, 1.08) | 0.93(0.79, 1.09) | 0.90(0.77, 1.06) | ||||
| T3 | 295/755 | 6.309(4.852, 8.954) | 0.81(0.69, 0.95) | 0.83(0.70, 0.98) | 0.83(0.70, 0.98) | ||||
| Forenoon-to-noon period | |||||||||
| Coffee consumed status | |||||||||
| No | 2553/6197 | 0.000(0.000, 0.000) | Ref | Ref | Ref | ||||
| Yes | 1009/2367 | 3.665(2.622, 5.325) | 0.94(0.85, 1.03) | 0.19 | 0.95(0.86, 1.05) | 0.35 | 0.94(0.85, 1.04) | 0.22 | |
| Coffee consumed amount | |||||||||
| T1 | 2553/6197 | 0.000(0.000, 0.000) | Ref | 0.30 | Ref | 0.47 | Ref | 0.32 | |
| T2 | 499/1184 | 2.622(2.097, 3.122) | 0.90(0.79, 1.03) | 0.91(0.79, 1.04) | 0.89(0.78, 1.02) | ||||
| T3 | 510/1183 | 5.331(4.347, 7.109) | 0.97(0.85, 1.10) | 1.00(0.87, 1.14) | 0.98(0.86, 1.13) | ||||
| Noon-to-evening period | |||||||||
| Coffee consumed status | |||||||||
| No | 3229/7854 | 0.000(0.000, 0.000) | Ref | Ref | Ref | ||||
| Yes | 333/710 | 3.291(2.332, 4.477) | 1.05(0.89, 1.23) | 0.56 | 1.09(0.92, 1.29) | 0.32 | 1.11(0.93,1.32) | 0.26 | |
| Coffee consumed amount | |||||||||
| T1 | 3229/7854 | 0.000(0.000, 0.000) | Ref | 0.17 | Ref | 0.05 | Ref | 0.03 | |
| T2 | 154/355 | 2.330(1.722, 2.786) | 0.88(0.70, 1.10) | 0.89(0.70, 1.12) | 0.90(0.71, 1.14) | ||||
| T3 | 179/355 | 4.477(3.824, 5.916) | 1.27(1.01, 1.57) | 1.33(1.05, 1.67) | 1.35(1.07, 1.71) | ||||
| Evening-to-dawn period | |||||||||
| Coffee consumed status | |||||||||
| No | 3286/7918 | 0.000(0.000, 0.000) | Ref | Ref | Ref | ||||
| Yes | 276/646 | 3.254(2.368, 4.455) | 1.04(0.88, 1.23) | 0.66 | 1.08(0.90, 1.29) | 0.39 | 1.11(0.92, 1.33) | 0.28 | |
| Coffee consumed amount | |||||||||
| T1 | 3286/7918 | 0.000(0.000, 0.000) | Ref | 0.39 | Ref | 0.16 | Ref | 0.11 | |
| T2 | 132/323 | 2.365(1.815, 2.817) | 0.92(0.73, 1.17) | 0.95(0.74, 1.21) | 0.95(0.74, 1.22) | ||||
| T3 | 144/323 | 4.455(3.756, 6.030) | 1.17(0.93, 1.48) | 1.23(0.97, 1.57) | 1.28(1.00, 1.64) | ||||
As for the timing, compared to those who did not consume coffee during dawn-to-forenoon, the diabetic patients who consumed during this time period had a lower incidence risk of CKD (OR: 0.87, 95% CI: 0.77–0.98). More specifically, a higher level of coffee consumption during dawn-to-forenoon was associated with a decreased incidence risk of CKD (OR: 0.83, 95% CI: 0.70–0.98). Meanwhile, compared to those in the lowest tertile of coffee consumption during noon-to-evening, diabetic patients in the highest tertile had a higher incidence risk of CKD (OR: 1.35, 95% CI: 1.07–1.71). Also, compared to those in the lowest tertile of coffee consumption during evening-to-dawn, the diabetic patients in the highest tertile represented an increased incidence risk of CKD (OR: 1.28, 95% CI: 1.01–1.64).
Subgroup analyses
The first subgroup analysis showed that coffee consumption (status and higher amount) during dawn-to-forenoon in men was associated with a lower CKD prevalence (ORstatus: 0.83, 95% CI: 0.71, 0.98; ORamount: 0.76, 95% CI: 0.61, 0.94), whereas the positive association between coffee consumption (amount) during evening-to-dawn and the prevalence of CKD was observed in women (OR: 1.57, 95% CI: 1.06, 2.32, Fig. 2). In the second subgroup analysis, diabetic patients were stratified by BMI levels (Fig. 3). Among diabetic patients whose BMI levels were < 25 kg m−2, we did not observe any association between coffee consumption (status and amount) and the prevalence of CKD. For participants whose BMI were ≥ 25 kg m−2 and < 30 kg m−2, compared to those in the lowest tertile of coffee consumption throughout the day, diabetic patients in the highest tertile had a lower incidence risk of CKD (OR: 0.74, 95% CI: 0.59–0.94). Meanwhile, compared to participants who did not consume coffee during dawn-to-forenoon, those who consumed coffee during this time period had a lower incidence risk of CKD (OR: 0.73, 95% CI: 0.59–0.92). More specifically, compared to those in the lowest tertile of coffee consumption during this time period, diabetic patients in the highest tertile had a reduced incidence risk of CKD (OR: 0.62, 95% CI: 0.45–0.85). For participants whose BMI was ≥ 30 kg m−2, those that consumed higher coffee during noon-to-evening were more likely to have CKD (OR: 1.51, 95% CI: 01.09, 2.08). Additionally, the third subgroup analysis suggested that the coffee consumed status throughout the day was associated with a lower prevalence of CKD among diabetic patients (OR: 0.89, 95% CI: 0.80–0.996) (Fig. 4). Also, a higher level of coffee consumption throughout the day was associated with a lower prevalence of CKD (OR: 0.85, 95% CI: 0.74–0.97). Also, a marginal negative association between amount of coffee consumption during dawn-to-forenoon and the prevalence of CKD with a P < 0.05 was observed.Sensitivity analysis
In the first sensitivity analysis, our results showed that among non-diabetic participants, the association of coffee consumed status throughout the day and during dawn-to-forenoon with a decreased CKD prevalence were still observed (ORthroughout the day: 0.86, 95% CI: 0.78–0.93, ORdawn to forenoon: 0.82, 95% CI: 0.75–0.90), and both showed a dose–response relationship (ORthroughout the day: 0.76, 95% CI: 0.69–0.83, ORdawn to forenoon: 0.70, 95% CI: 0.62–0.80). However, participants who consumed higher levels of coffee from evening-to-dawn had a lower incidence risk of CKD (OR: 0.74, 95% CI: 0.59–0.91), which contrasts with the results observed in diabetic patients (ESI Figure 1†). In the second sensitivity analysis, the associations between coffee consumption during different time periods and CKD prevalence remained consistent with the main model (ESI Figure 2†).Discussion
This study is the first to examine the association between the timing of coffee consumption and CKD development in diabetic patients, providing a new dietary strategy to prevent the process of diabetes-induced microangiopathy. We observed that among diabetic patients, consuming coffee throughout the day was associated with a lower prevalence of CKD. Specifically, coffee consumption within the range of 2.621 g kg−1 may have a beneficial effect in preventing CKD. However, the health effect of coffee consumption varied depending on the daily intake timing. Diabetic patients who consumed coffee from Dawn to forenoon showed a decreased risk of CKD, with a negative dose–response relationship observed. In contrast, those who consumed coffee during the periods from noon to evening or from evening to Dawn exhibited a positive dose–response relationship with CKD risk.It has been reported that moderate regular coffee intake was inversely associated with both CKD and diabetes incidence;20,21 however, some studies have found non-significant associations or even a positive relationship of coffee consumption with renal function and CKD risk.16,22,40 In terms of the relationship between coffee intake and CKD prevalence among diabetic patients, conflicting results still exist. In this study, we observed that diabetic patients who consumed coffee throughout the day had an 11% lower risk of developing CKD, compared to those who did not consume coffee. Additionally, we found that diabetic patients who consumed an adequate amount of coffee (2.621 g per kg per day) were more likely to have a lower incidence risk of CKD. Prior research also suggested that diabetic patients consuming over 2 cups of coffee have a lower incidence risk of CKD,41 which supported the findings in our study. Moreover, coffee contains over 1000 chemical compounds, which play an important role in preventing metabolic diseases by decreasing the inflammation and maintaining glycolipid metabolism.14 These properties might be the potential reason why adequate coffee consumption has a positive impact on the prevention of CKD development among diabetic patients.
Recognizing the importance of meal timing in maintaining overall health, we further investigated whether the timing of coffee consumption played an important role in the prevention of CKD in diabetes. One of our key findings was the time-specific health effect of coffee consumption during the period from dawn-to-evening on CKD development among diabetic patients, independent of total daily coffee intake and other traditional nutritional factors. This association was also significant among non-diabetic individuals. It is well-documented that insulin sensitivity, insulin secretion, and glucose tolerance exhibit biological rhythms, peaking in the forenoon and gradually decreasing throughout the day.42,43 Coffee intake during the period from dawn-to-forenoon may align with biological rhythms related to glucose metabolism, potentially aiding in glucose control. In diabetic patients, peripheral insulin sensitivity tends to be reduced, delayed, or lost,44 and coffee, which temporarily raises glucose levels, consumed during this time period may stimulate peripheral insulin sensitivity, helping to establish serum glucose fluctuations similar to the status in healthy individuals. Additionally, metabolic activity peaks during the forenoon42,45 and coffee has been identified as a key factor in promoting metabolic processes by facilitating fatty acid oxidation,46,47 thereby improving glucose and lipid metabolism. This suggests that coffee intake during this period may also enhance metabolic capability, synergizing with metabolic rhythms to offer beneficial effects.
Another significant finding in our study revealed an inverse association between coffee intake during the periods from noon-to-evening or from evening-to-dawn and the prevalence of CKD among diabetic patients. Previous research has shown that caffeine, a key component of coffee, can disrupt the human circadian rhythm and lengthen the circadian period of molecular oscillations, primarily through an adenosine receptor/cyclic adenosine monophosphate (AMP)-dependent mechanism.48 Therefore, consuming coffee at inappropriate times may worsen impaired glucose tolerance in diabetic patients, potentially heightening the adverse effects linked to CKD development. Also, consuming coffee during the above periods will lower insulin sensitivity and glucose tolerance in peripheral tissues,49,50 potentially exacerbating the risk of CKD among diabetic individuals.
Moreover, consuming coffee from evening-to-dawn may disrupt sleep quality and duration by suppressing the secretion of melatonin,51,52 a hormone essential for regulating the sleep–wake cycle. High-quality sleep at night is associated with lower risks of diabetes and proteinuria,53,54 which is a major risk factor for CKD incidence.55,56 Disrupted sleep patterns, marked by short-term sleep deprivation, can hinder glucose control by lowering insulin sensitivity, glucose tolerance, and islet cell function. It also disrupts the rhythm of hypothalamic-pituitary–related endocrine hormones, elevating glucotropic hormone levels.57 Additionally, diabetic patients experiencing insomnia or staying up late may experience heightened levels of norepinephrine, leading to vasoconstriction and high blood pressure.58 Under normal conditions, blood pressure follows a distinct circadian rhythm, being lower during sleep compared to wakefulness.59,60 Nocturnal hypertension, characterized by elevated blood pressure during sleep, is a significant risk factor for CKD incidence.61,62 Also, temporary coffee consumption can induce symptoms such as increased heart rate and blood pressure.63,64 Thus, consuming coffee in the evening may exacerbate the risk of CKD among diabetic individuals by compromising the sleep quality and raising the blood pressure.
Additionally, we observed a non-significant association between coffee consumption from dawn-to-morning and CKD prevalence in diabetic patients with a BMI < 25 kg m−2 or ≥30 kg m−2. Obesity is a significant risk factor for CKD due to increased inflammation, insulin resistance, and impaired glucose tolerance,65,66 which may obscure the health effects of coffee consumption timing. Conversely, diabetic patients with a lower BMI may have insufficient energy intake, medication use, or endocrine disorders,67,68 which may also obscure the relationship between coffee consumption timing and CKD prevalence. Furthermore, we observed inconsistent results regarding the effect of time-specific coffee consumption on CKD development between different genders or between different times of coffee drinkers. These inconsistencies may be due to metabolic differences between sexes and between multi-time coffee drinkers and one-time drinkers among diabetic patients. Therefore, these issues warrant further investigation in future research.
Strengths and limitations
This study has several strengths. Firstly, it is the first to investigate the health implications of coffee consumption timing in a nationally representative sample of diabetic patients in the U.S. Secondly, our findings are robust, remaining consistent even after adjusting for various traditional confounding factors that could influence disease progression in diabetic patients.However, this study has certain limitations. Firstly, reliance on self-reported 24-hour dietary recall, although widely used and considered the most valid instrument for capturing dietary information in NHANES, is prone to measurement errors due to day-to-day variations in food intake. Secondly, despite our efforts to control numerous potential confounding variables, the observational nature of the study implies the possibility of unmeasured confounders. Thirdly, the study did not differentiate between the specific types of coffee consumed. Future research should explore these associations with a focus on different types of coffee (caffeinated, decaffeinated, instant) to provide a more comprehensive understanding. Lastly, the inability to distinguish between different types of diabetes is a limitation. While previous studies suggest that the majority of diabetic patients in NHANES have type 2 diabetes,69 further investigations differentiating between type 1 and type 2 diabetes are also warranted for more comprehensive evidence. Fifth, our results are primarily analyzed based on an American population; therefore, it is necessary to re-analyze these associations in other racial populations.
Clinical implications
Coffee, as a widely consumed beverage among adults, has garnered considerable attention due to its health benefits. In healthy populations, moderate regular coffee consumption has been shown to effectively reduce the risk of DM and CKD. However, little research has investigated the relationship between the timing of coffee consumption and the incidence risk of CKD among diabetic patients. Our study, for the first time, suggests that the timing of coffee consumption may impact the prevalence of CKD among diabetic patients. Recognizing the crucial role of nutritional therapy in managing diabetes, healthcare professionals should consider integrating insights on the timing of coffee consumption into nutritional guidelines for diabetes. This could offer valuable guidance to diabetic patients, enhancing their overall health.In conclusion, our study highlights the time-specific impact of coffee consumption on CKD prevention among diabetic patients. Consuming coffee from Dawn to forenoon is linked to a reduced incidence risk of CKD, whereas consuming coffee from noon-to-evening or from evening-to-dawn is associated with an increased incidence risk of CKD among diabetic patients.
Transparency statement
Wei Wei affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Health and Nutrition Examination Survey.Author contributions
Wei Wei, and Tianshu Han conceived the study design. Statistical analysis: Qin Zhou, Yingdong Zuo, and Zheng Wang. Repeated-Statistical analysis: Yiwei Tang, Fengru Niu, Shangying Li, and Zheng Wang. Writing-original draft: Wei Wei and Tianshu Han. Writing-review & editing: Yiwei Tang, and Ni Zhao. All authors provided critical revisions of the draft and approved the submitted draft. Validation: Jiaxin Huang. Supervision: Wei Wei and Tianshu Han.Conflicts of interest
The authors did not have any competing interest to declare.Acknowledgements
This research was supported funds from the National Natural Science Foundation (82373561 to Tianshu Han & 82204016 to Wei Wei). The authors thank the participants and staff of the National Health and Nutrition Examination Survey 2003 to 2018 for their valuable contributions.References
- D. J. Magliano, E. J. Boyko and I. D. F. D. A. T. E. S. committee, in Idf diabetes atlas, International Diabetes Federation © International Diabetes Federation, 2021., Brussels, 2021.
- R. Z. Alicic, M. T. Rooney and K. R. Tuttle, Diabetic Kidney Disease: Challenges, Progress, and Possibilities, Clin. J. Am. Soc. Nephrol., 2017, 12, 2032–2045 CrossRef CAS PubMed.
- V. Lyssenko and A. Vaag, Genetics of diabetes-associated microvascular complications, Diabetologia, 2023, 66, 1601–1613 CrossRef PubMed.
- L. Lin, W. Tan, X. Pan, E. Tian, Z. Wu and J. Yang, Metabolic Syndrome-Related Kidney Injury: A Review and Update, Front. Endocrinol., 2022, 13, 904001 CrossRef PubMed.
- P. Düsing, A. Zietzer, P. R. Goody, M. R. Hosen, C. Kurts, G. Nickenig and F. Jansen, Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches, J. Mol. Med., 2021, 99, 335–348 CrossRef PubMed.
- Endotext, ed. K. R. Feingold, B. Anawalt, M. R. Blackman, A. Boyce, G. Chrousos, E. Corpas, W. W. de Herder, K. Dhatariya, K. Dungan, J. Hofland, S. Kalra, G. Kaltsas, N. Kapoor, C. Koch, P. Kopp, M. Korbonits, C. S. Kovacs, W. Kuohung, B. Laferrère, M. Levy, E. A. McGee, R. McLachlan, M. New, J. Purnell, R. Sahay, A. S. Shah, F. Singer, M. A. Sperling, C. A. Stratakis, D. L. Trence and D. P. Wilson, MDText.com, Inc. Copyright © 2000–2024, MDText.com, Inc., South Dartmouth (MA), 2000 Search PubMed.
- L. Alkhatib, L. A. Velez Diaz, S. Varma, A. Chowdhary, P. Bapat, H. Pan, G. Kukreja, P. Palabindela, S. A. Selvam and K. Kalra, Lifestyle Modifications and Nutritional and Therapeutic Interventions in Delaying the Progression of Chronic Kidney Disease: A Review, Cureus, 2023, 15, e34572 Search PubMed.
- M. Franzago, E. Alessandrelli, S. Notarangelo, L. Stuppia and E. Vitacolonna, Chrono-Nutrition: Circadian Rhythm and Personalized Nutrition, Int. J. Mol. Sci., 2023, 24, 2571 CrossRef CAS PubMed.
- M. Takahashi and Y. Tahara, Timing of Food/Nutrient Intake and Its Health Benefits, J. Nutr. Sci. Vitaminol., 2022, 68, S2–S4 CrossRef CAS PubMed.
- S. Almoosawi, S. Vingeliene, F. Gachon, T. Voortman, L. Palla, J. D. Johnston, R. M. Van Dam, C. Darimont and L. G. Karagounis, Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health, Adv. Nutr., 2019, 10, 30–42 CrossRef PubMed.
- A. Flanagan, D. A. Bechtold, G. K. Pot and J. D. Johnston, Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns, J. Neurochem., 2021, 157, 53–72 CrossRef CAS PubMed.
- W. Jiang, Q. Song, J. Zhang, Y. Chen, H. Jiang, Y. Long, Y. Li, T. Han, H. Sun and W. Wei, The Association of Consumption Time for Food With Cardiovascular Disease and All-Cause Mortality Among Diabetic Patients, J. Clin. Endocrinol. Metab., 2022, 107, e3066–e3075 CrossRef PubMed.
- T. Han, J. Gao, L. Wang, C. Li, L. Qi, C. Sun and Y. Li, The Association of Energy and Macronutrient Intake at Dinner Versus Breakfast With Disease-Specific and All-Cause Mortality Among People With Diabetes: The U.S. National Health and Nutrition Examination Survey, 2003–2014, Diabetes Care, 2020, 43, 1442–1448 CrossRef CAS PubMed.
- S. Surma, A. Sahebkar and M. Banach, Coffee or tea: Anti-inflammatory properties in the context of atherosclerotic cardiovascular disease prevention, Pharmacol. Res., 2023, 187, 106596 CrossRef CAS PubMed.
- S. Surma and S. Oparil, Coffee and Arterial Hypertension, Curr. Hypertens. Rep., 2021, 23, 38 CrossRef CAS PubMed.
- S. Surma and M. Banach, Coffee and caffeine consumption and overall mortality. Pleasure with restrictions-where do we really stand in 2022?, Nutrition, 2022, 102, 111747 CrossRef PubMed.
- D. Hang, A. S. Kværner, W. Ma, Y. Hu, F. K. Tabung, H. Nan, Z. Hu, H. Shen, L. A. Mucci, A. T. Chan, E. L. Giovannucci and M. Song, Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals, Am. J. Clin. Nutr., 2019, 109, 635–647 CrossRef PubMed.
- P. V. Dludla, I. Cirilli, F. Marcheggiani, S. Silvestri, P. Orlando, N. Muvhulawa, M. T. Moetlediwa, B. B. Nkambule, S. E. Mazibuko-Mbeje, N. Hlengwa, S. Hanser, D. Ndwandwe, J. L. Marnewick, A. K. Basson and L. Tiano, Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease, Molecules, 2023, 28, 6440 CrossRef CAS PubMed.
- S. N. Bhupathiraju, A. Pan, J. E. Manson, W. C. Willett, R. M. van Dam and F. B. Hu, Changes in coffee intake and subsequent risk of type 2 diabetes: three large cohorts of US men and women, Diabetologia, 2014, 57, 1346–1354 CrossRef CAS PubMed.
- M. Carlström and S. C. Larsson, Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis, Nutr. Rev., 2018, 76, 395–417 CrossRef PubMed.
- S. Surma and F. Kokot, Influence of chronic coffee consumption on the risk of kidney and other organ diseases. Review of the literature and clinical studies, Renal Dis. Transplantat. Forum, 2022, 15, 1–18 Search PubMed.
- K. Wijarnpreecha, C. Thongprayoon, N. Thamcharoen, P. Panjawatanan and W. Cheungpasitporn, Association of coffee consumption and chronic kidney disease: A meta-analysis, Int. J. Clin. Pract., 2017, 71, 1–6 Search PubMed.
- J. H. Jhee, K. H. Nam, S. Y. An, M. U. Cha, M. Lee, S. Park, H. Kim, H. R. Yun, Y. K. Kee, J. T. Park, T. I. Chang, E. W. Kang, T. H. Yoo, S. W. Kang and S. H. Han, Effects of Coffee Intake on Incident Chronic Kidney Disease: A Community-Based Prospective Cohort Study, Am. J. Med., 2018, 131, 1482–1490 CrossRef CAS PubMed.
- E. A. Hu, E. Selvin, M. E. Grams, L. M. Steffen, J. Coresh and C. M. Rebholz, Coffee Consumption and Incident Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study, Am. J. Kidney Dis., 2018, 72, 214–222 CrossRef PubMed.
- M. Mazidi, D. P. Mikhailidis, A. Dehghan, J. Jóźwiak, A. Covic, N. Sattar and M. Banach, The association between coffee and caffeine consumption and renal function: insight from individual-level data, Mendelian randomization, and meta-analysis, Arch. Med. Sci., 2022, 18, 900–911 CAS.
- U. Albrecht, The circadian clock, reward, and memory, Front. Mol. Neurosci., 2011, 4, 41 Search PubMed.
- T. M. Burke, R. R. Markwald, A. W. McHill, E. D. Chinoy, J. A. Snider, S. C. Bessman, C. M. Jung, J. S. O'Neill and K. P. Wright Jr, Effects of caffeine on the human circadian clock in vivo and in vitro, Sci. Transl. Med., 2015, 7, 305ra146 Search PubMed.
- X. Peng, R. Fan, L. Xie, X. Shi, K. Dong, S. Zhang, J. Tao, W. Xu, D. Ma, J. Chen and Y. Yang, A Growing Link between Circadian Rhythms, Type 2 Diabetes Mellitus and Alzheimer’s Disease, Int. J. Mol. Sci., 2022, 23, 504 CrossRef CAS PubMed.
- I. C. Mason, J. Qian, G. K. Adler and F. Scheer, Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes, Diabetologia, 2020, 63, 462–472 CrossRef CAS PubMed.
- E. Matteucci and O. Giampietro, Circadian rhythm of blood pressure in diabetes mellitus: evidence, mechanisms and implications, Curr. Diabetes Rev., 2012, 8, 355–361 CrossRef PubMed.
- R. Tarquini and G. Mazzoccoli, Clock Genes, Metabolism, and Cardiovascular Risk, Heart Fail. Clin., 2017, 13, 645–655 CrossRef PubMed.
- C. J. Henry, B. Kaur and R. Y. C. Quek, Chrononutrition in the management of diabetes, Nutr. Diabetes, 2020, 10, 6 CrossRef PubMed.
- H. Oike, K. Oishi and M. Kobori, Nutrients, Clock Genes, and Chrononutrition, Curr. Nutr. Rep., 2014, 3, 204–212 CrossRef CAS PubMed.
- J. He, Z. Zhu, J. D. Bundy, K. S. Dorans, J. Chen and L. L. Hamm, Trends in Cardiovascular Risk Factors in US Adults by Race and Ethnicity and Socioeconomic Status, 1999–2018, J. Am. Med. Assoc., 2021, 326, 1286–1298 CrossRef PubMed.
- F. Zhang, J. Liu, C. D. Rehm, P. Wilde, J. R. Mande and D. Mozaffarian, Trends and Disparities in Diet Quality Among US Adults by Supplemental Nutrition Assistance Program Participation Status, JAMA Netw. Open, 2018, 1, e180237 CrossRef PubMed.
- N. K. Fukagawa, K. McKillop, P. R. Pehrsson, A. Moshfegh, J. Harnly and J. Finley, USDA's FoodData Central: what is it and why is it needed today?, Am. J. Clin. Nutr., 2022, 115, 619–624 CrossRef PubMed.
- S. Lu, K. Robyak and Y. Zhu, The CKD-EPI 2021 Equation and Other Creatinine-Based Race-Independent eGFR Equations in Chronic Kidney Disease Diagnosis and Staging, J. Appl. Lab. Med., 2023, 8, 952–961 CrossRef PubMed.
- W. Jiang, T. Han, W. Duan, Q. Dong, W. Hou, H. Wu, Y. Wang, Z. Jiang, X. Pei, Y. Chen, Y. Li and C. Sun, Prenatal famine exposure and estimated glomerular filtration rate across consecutive generations: association and epigenetic mediation in a population-based cohort study in Suihua China, Aging, 2020, 12, 12206–12221 CrossRef CAS PubMed.
- S. Ahmed, L. Jafri and A. H. Khan, Evaluation of ‘CKD-EPI Pakistan’ Equation for estimated Glomerular Filtration Rate (eGFR): AComparison of eGFR Prediction Equations in Pakistani Population, J. Coll. Physicians Surg. Pak., 2017, 27, 414–418 Search PubMed.
- Y. C. Hsu, P. H. Lee, C. C. Lei, Y. H. Shih and C. L. Lin, Analgesic use, parents’ clan, and coffee intake are three independent risk factors of chronic kidney disease in middle and elderly-aged population: a community-based study, Renal Fail., 2014, 36, 361–366 CrossRef CAS PubMed.
- A. C. van Westing, C. Ochoa-Rosales, A. C. van der Burgh, L. Chaker, J. M. Geleijnse, E. J. Hoorn and T. Voortman, Association of habitual coffee consumption and kidney function: A prospective analysis in the Rotterdam Study, Clin. Nutr., 2023, 42, 83–92 CrossRef CAS PubMed.
- D. J. Stenvers, F. Scheer, P. Schrauwen, S. E. la Fleur and A. Kalsbeek, Circadian clocks and insulin resistance, Nat. Rev. Endocrinol., 2019, 15, 75–89 CrossRef PubMed.
- D. Jakubowicz, J. Wainstein, S. Tsameret and Z. Landau, Role of High Energy Breakfast “Big Breakfast Diet” in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes, Nutrients, 2021, 13, 1558 CrossRef CAS PubMed.
- F. A. Scheer, M. F. Hilton, C. S. Mantzoros and S. A. Shea, Adverse metabolic and cardiovascular consequences of circadian misalignment, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4453–4458 CrossRef CAS PubMed.
- C. J. Morris, J. N. Yang, J. I. Garcia, S. Myers, I. Bozzi, W. Wang, O. M. Buxton, S. A. Shea and F. A. Scheer, Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E2225–E2234 CrossRef CAS PubMed.
- W. Sakamoto, H. Isomura, K. Fujie, K. Takahashi, K. Nakao and H. Izumi, Relationship of coffee consumption with risk factors of atherosclerosis in rats, Ann. Nutr. Metab., 2005, 49, 149–154 CrossRef CAS PubMed.
- E. Güneş and E. Şensoy, Is Turkish coffee protects Drosophila melanogaster on cadmium acetate toxicity by promoting antioxidant enzymes?, Chemosphere, 2022, 296, 133972 CrossRef PubMed.
- C. Olivares-Yañez, M. P. Alessandri, L. Salas and L. F. Larrondo, Methylxanthines Modulate Circadian Period Length Independently of the Action of Phosphodiesterase, Microbiol. Spectrum, 2023, 11, e0372722 CrossRef PubMed.
- S. Khalili-Moghadam, M. Hedayati, M. Golzarand and P. Mirmiran, Effects of green coffee aqueous extract supplementation on glycemic indices, lipid profile, CRP, and malondialdehyde in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial, Front. Nutr., 2023, 10, 1241844 CrossRef PubMed.
- S. Lestari, T. Sunaryo and R. I. Arvianto, Preparation of decaffeinated coffee extract: study of the effectiveness of decaffeinated coffee extract toward lowering blood sugar in type 2 diabetes mellitus patients, Med. J. Malays., 2023, 78, 515–518 CAS.
- E. C. Jansen, K. Corcoran, W. Perng, G. L. Dunietz, A. Cantoral, L. Zhou, M. M. Téllez-Rojo and K. E. Peterson, Relationships of beverage consumption and actigraphy-assessed sleep parameters among urban-dwelling youth from Mexico, Public Health. Nutr., 2021, 25, 1–10 Search PubMed.
- J. Park, J. W. Han, J. R. Lee, S. Byun, S. W. Suh, T. Kim, I. Y. Yoon and K. W. Kim, Lifetime coffee consumption, pineal gland volume, and sleep quality in late life, Sleep, 2018, 41, 1–8 CrossRef PubMed.
- L. Wang, H. Tian, H. Wang, X. Mao, J. Luo, Q. He, P. Wen, H. Cao, L. Fang, Y. Zhou, J. Yang and L. Jiang, Disrupting circadian control of autophagy induces podocyte injury and proteinuria, Kidney Int., 2024, 105, 1020–1034 CrossRef CAS PubMed.
- E. Zamarrón, A. Jaureguizar, A. García-Sánchez, T. Díaz-Cambriles, A. Alonso-Fernández, V. Lores, O. Mediano, F. Troncoso-Acevedo, S. Cabello-Pelegrín, E. Morales-Ruíz, M. T. Ramírez-Prieto, M. I. Valiente-Díaz, T. Gómez-García, R. Casitas, E. Martínez-Cerón, R. Galera, C. Cubillos-Zapata and F. García-Río, Continuous Positive Airway Pressure Effect on Albuminuria Progression in Patients with Obstructive Sleep Apnea and Diabetic Kidney Disease: A Randomized Clinical Trial, Am. J. Respir. Crit. Care Med., 2023, 207, 757–767 CrossRef PubMed.
- H. S. Lee, H. I. Lim, T. J. Moon, S. Y. Lee and J. H. Lee, Trajectories of atherosclerotic cardiovascular disease risk scores as a predictor for incident chronic kidney disease, BMC Nephrol., 2024, 25, 141 CrossRef CAS PubMed.
- B. Demirelli, B. Boztepe, E. G. Şenol, B. Boynueğri, Y. D. Bildacı, G. Gümrükçü, M. Canbakan and M. B. Öğütmen, Non-diabetic nephropathy in diabetic patients: incidence, HbA1c variability and other predictive factors, and implications, Int. Urol. Nephrol., 2024, 56, 3091–3100 CrossRef CAS PubMed.
- Y. Rosenblum, M. Pereira, O. Stange, F. D. Weber, L. Bovy, S. Tzioridou, E. Lancini, D. A. Neville, N. Klein, T. de Wolff, M. Stritzke, I. Kersten, M. Uhr, J. Claassen, A. Steiger, M. M. Verbeek and M. Dresler, Divergent Associations of Slow-Wave Sleep versus Rapid Eye Movement Sleep with Plasma Amyloid-Beta, Ann. Neurol., 2024, 96, 46–60 CrossRef CAS PubMed.
- J. Fernandez-Mendoza, A. N. Vgontzas, D. Liao, M. L. Shaffer, A. Vela-Bueno, M. Basta and E. O. Bixler, Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort, Hypertension, 2012, 60, 929–935 CrossRef CAS PubMed.
- R. C. Hermida, D. E. Ayala, J. R. Fernández, F. Portaluppi, F. Fabbian and M. H. Smolensky, Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications, Am. J. Hypertens., 2011, 24, 383–391 CrossRef CAS PubMed.
- Y. Xu, C. Gong, J. Liao, Z. Ge, Y. Tan, Y. Jiang, M. Liu, W. Zhong, X. Zhang, N. Dong and X. Shen, Absence of fluctuation and inverted circadian rhythm of blood pressure increase the risk of cognitive dysfunction in cerebral small vessel disease patients, BMC Neurol., 2023, 23, 73 CrossRef PubMed.
- Q. Wang, Y. Wang, J. Wang, L. Zhang and M. H. Zhao, Nocturnal Systolic Hypertension and Adverse Prognosis in Patients with CKD, Clin. J. Am. Soc. Nephrol., 2021, 16, 356–364 CrossRef CAS PubMed.
- M. L. Guzman-Limon, S. Jiang, D. Ng, J. T. Flynn, B. Warady, S. L. Furth and J. A. Samuels, Nocturnal Hypertension in Children With Chronic Kidney Disease Is Common and Associated With Progression to Kidney Replacement Therapy, Hypertension, 2022, 79, 2288–2297 CrossRef CAS PubMed.
- R. M. Brothers, K. M. Christmas, J. C. Patik and P. S. Bhella, Heart rate, blood pressure and repolarization effects of an energy drink as compared to coffee, Clin. Physiol. Funct. Imaging, 2017, 37, 675–681 CrossRef CAS PubMed.
- M. K. Alhabeeb, M. M. Alazzmi, M. S. Alrashidi and N. S. Al-Sowayan, Effect of Caffeinated and Decaffeinated Coffee on Blood Pressure and Heart Rate of Healthy Individuals, Pak. J. Biol. Sci, 2022, 25, 337–344 CrossRef CAS PubMed.
- N. J. McGraw, E. S. Krul, E. Grunz-Borgmann and A. R. Parrish, Soy-based renoprotection, World J. Nephrol., 2016, 5, 233–257 CrossRef PubMed.
- G. M. Parra-Bracamonte, F. E. Parra-Bracamonte, N. Lopez-Villalobos and A. L. Lara-Rivera, Chronic kidney disease is a very significant comorbidity for high risk of death in patients with COVID-19 in Mexico, Nephrology, 2021, 26, 248–251 CrossRef CAS PubMed.
- B. Biondi, G. J. Kahaly and R. P. Robertson, Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders, Endocr. Rev., 2019, 40, 789–824 CrossRef PubMed.
- N. Matovu, F. K. Matovu, W. Sseguya and F. Tushemerirwe, Association of dietary intake and BMI among newly diagnosed type 2 diabetes patients attending diabetic clinics in Kampala, BMC Nutr., 2017, 3, 21 CrossRef PubMed.
- S. A. Berkowitz, J. B. Meigs and D. J. Wexler, Age at type 2 diabetes onset and glycaemic control: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2010, Diabetologia, 2013, 56, 2593–2600 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02777a |
| ‡ These authors contributed to equally. |
| This journal is © The Royal Society of Chemistry 2024 |