 Open Access Article
Open Access ArticleTowards more sustainable cooking practices to increase the bioaccessibility of colourless and provitamin A carotenoids in cooked carrots†
Ana M.
Benítez-González
 ,
Carla M.
Stinco
,
Carla M.
Stinco
 *,
Francisco J.
Rodríguez-Pulido
*,
Francisco J.
Rodríguez-Pulido
 ,
Isabel M.
Vicario
,
Isabel M.
Vicario
 and
Antonio J.
Meléndez-Martínez
and
Antonio J.
Meléndez-Martínez

Food Colour and Quality Laboratory, Facultad de Farmacia, Universidad de Sevilla, 41012 Sevilla, Spain. E-mail: cstinco@us.es; abenitez@us.es; rpulido@us.es; vicario@us.es; ajmelendez@us.es; Tel: +34 954557017
First published on 9th August 2024
Abstract
The effect of different cooking methods (boiling, baking, steaming and microwaving) on the colour and texture of carrots, as well as on the bioaccessibility of carotenoids, was investigated in order to identify the more “sustainable cooking” methods. Cooking resulted in statistically significant increases in total carotenoid bioaccessibility, both with intensity and duration of treatments. In particular, significant increases in carotenoid bioaccessible content (CBC) were observed, ranging from 6.03-fold (microwave) to 8.90-fold (baking) for the most intense cooking conditions tested. Although the relative concentration of the colourless carotenoids (phytoene and phytofluene) in raw carrots is lower than that of provitamins A α- and β-carotene, the bioaccessible content of the colourless ones is much higher. From an energy consumption standpoint and considering samples with the same tenderness, the highest CBC values per kWh decreased in the order microwaving > baking > water cooking > steaming. Our findings are important to help combat vitamin A deficiency since increases of up to ∼40-fold and ∼70-fold in the CBCs of the vitamin A precursors α- and β-carotene, respectively, were observed. These results provide a basis for defining “sustainable cooking” as “cooking practices that optimize intensity, duration and other parameters leading to a more efficient use of energy to maximize the bioavailability of nutrients and other beneficial food components (such as bioactives) while ensuring food appeal and safety”.
1. Introduction
Carotenoids are widespread dietary compounds responsible for the colour of many foods. They contribute to health promotion, either as precursors of vitamin A or as compounds associated with reduced incidence of cancers, coronary heart disease and other degenerative conditions.1 They also provide cosmetic benefits, hence they are of great interest in agro-food, health promotion and cosmetics.2Industrial and culinary practices have been long known to decrease the carotenoid content of foods and produce carotenoid derivatives. Evidence has accumulated pointing out that they can also increase their release (either during extraction with solvents, cooking or even digestion) depending on the conditions used. This is attributed to a large extent to changes caused by the rupture of cell structures (e.g. cell walls, plastids or carotenoid-protein associations).3–6 Size reduction of the carotenoid-containing matrix as a result of industrial processing is known to affect bioaccessibility of carotenoids.7–9 It is important to control the conditions of the industrial or domestic processing (intensity, time) because if they are too severe the losses of carotenoids cannot be compensated by their increased release from the matrix. On the other hand, overcooking results in unnecessary energy use optimizing energy use in culinary practices can contribute to adopting more sustainable foods.The effect of different domestic cooking methodologies (boiling, frying, microwaving, steaming, baking) on the stability of phytochemicals has been reviewed6 concluding that processing time, high temperature and size reduction are factors that usually reduce the levels of carotenoids. The heating of vegetables is known to cause changes in texture due to the breakdown of structures (membranes, cell walls) and modifications in pectin.10 Thus, culinary treatments usually lead to softening/disruption effects in the food matrix that increase the release of compounds, including carotenoids. Some studies reported increased levels of carotenoids in cooked samples, which can be seen as contradictory as thermal and other treatments used in cooking are known to degrade this compounds.11 Such apparent increases are often attributed to a higher release owed to the breakdown and softening processes mentioned before6 which are also thought to affect the bioaccessibility of phytochemicals in general. The water and soluble solids losses caused by cooking can also account to a large extent to this, although they are not always taken into account.11Information about how different common cooking practices affect carotenoid amount/bioaccessibility is important for practical purposes, for instance for nutritionist and dieticians. From a scientific standpoint, it is desirable to establish objective parameters that allow to compare the equivalence of treatments more meaningfully by taking into account the degree of matrix change. Parameters such as texture could be used to this end. Some authors studied mashed carrots12 or potatoes13 and defined hardness as the force required to compress the sample until it reaches a predefined percentage of its original thickness.
Besides, the carotenoid bioaccessible content in relation to the energy expenditure has not been reported before, so this parameter could be useful to pinpoint cooking methods that are not only more beneficial nutritionally, but also more sustainable energetically. Information about these methods that optimize energy and bioaccessibility of food components needs to be efficiently communicated to consumers as their progressive adoption can contribute considerably to health promotion and sustainability.
This study evaluates the effect of boiling, steaming, microwaving, and baking on the bioaccessible content of distinct groups of carotenoids. On one hand, the coloured carotenoids lutein, α-carotene and β-carotene, of which the latter two are provitamin A carotenoids. Vitamin A deficiency is a major nutritional problem in the world accounting for a large proportion of child and maternal mortality in developing countries. The study of the effect of cooking practices on the bioaccessibility of provitamin A carotenoids appears as an important research topic to complement those intended to increase the provitamin A carotenoid levels of staple foods (sweet potato, maize, etc.), a matter that has been the subject of much investigation.14 The colourless carotenoids phytoene and phytofluene, have markedly different chemical structures as compared to the rest of the major food carotenoids and are eliciting increased interest as they may intervene in health-promoting biological actions.2 We aimed at using a parameter that allows for the comparison of cooking methods in terms of matrix softening, considering that carrots may vary in texture from crunchy to unstructured tissue depending on the conditions used. “Hardness” is a parameter already used in food texture analysis. It is defined as the maximum slope reached when representing the force (N) versus the distance (mm) shifted by the probe this measurement is expressed in units of N mm−1.15
This parameter appears to be very useful not only because it is related to the release of carotenoids and other food components during digestion, but also for consumers’ preferences and energy expenditure during cooking. The results of this study may be important in promoting news studies on fine-tuning of cooking conditions to improve carotenoid bioavailability, thus contributing to the adoption of more sustainable practices to promote health through diet.
The bioaccessibility of carrot carotenoids have been the subject of previous studies. For instance, Lemmens et al.16 observed that β-carotene in vitro bioaccessibility increased with increasing processing temperature and time until steady-state conditions were reached after prolonged heating. Palmero et al.17 demonstrated that the chromoplast structure is a determinant in the bioaccessibility of carotenoids from carrots since it acts as a barrier to the release of provitamin carotenoids compared to other carotenoids in carrots. The aim of this work is to build on previous studies by highlighting the importance of considering little explored aspects, specifically (1) the carotenoid bioaccessible content (CBC) rather than the bioaccessibility in percentage; (2) the need of studying the long ignored colourless carotenoids phytoene and phytofluene, which are major dietary carotenoids increasingly recognised for their possible health and cosmetic benefits; (3) the energy consumption during cooking in relation to changes in carrot hardness and CBC. Additionally, a definition of “sustainable cooking” considering the concept of achieving higher bioaccessible contents with lower energy consumption is proposed.
2. Materials and methods
2.1. Chemicals
Methanol (MeOH), methyl tert-butyl ether (MTBE) and ethyl acetate were HPLC-grade and were supplied by Merck (Merck, Darmstadt, Germany) and Panreac (Barcelona, Spain). Ultrapure water (NANOpure Dlamond™ system, Arnsted Inc., Dubuque, IO) was used for the RRLC (Rapid Resolution Liquid Chromatography) analyses. Enzymes and all other reagents used to prepare simulated oral fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were purchased from Sigma-Aldrich (St Louis, MO, USA). Standards for carotenoids were from Sigma-Aldrich (purity >95%). Colourless carotenoids (PT and PF) were isolated from appropriate sources following standard procedures.182.2. Preparation of samples
10 kg of freshly harvested carrots (Daucus carota cv ‘Nantesa’) of a single lot were purchased on July from a local producer (Ecofrutas, Seville, Spain) and divided into five batches (2 kg each). Immediately, carrots were washed with distilled water and peeled with a manual peeler, removing inedible parts. To obtain the maximum reproducibility, samples were carefully sliced with the same thickness and size into discs (20 ± 5 mm diameter, 10 mm thick) with an electrical food slicer model fino1 (Ritter, Germany). One batch of whole carrots was kept in the fridge at 7 °C to be used as a control until the processing of the cooked samples was completed. The remaining four batches were submitted to different domestic cooking practices: boiling, steaming, microwave cooking and baking.2.3. Domestic cooking treatments
• WC: carrot slices/water ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (W/V); temperatures: 80 °C and 100 °C; times: 10, 15 and 20 min.
5 (W/V); temperatures: 80 °C and 100 °C; times: 10, 15 and 20 min.
• ST: 500 ml of water; temperture: 100 °C under atmospheric pressure; times: 5, 10, 15 and 20 min.
2.4. Colour analysis
Colour was evaluated using a hand–held colorimeter (Model CR-410, Konica Minolta Sensing, Inc., Japan). The instrument was calibrated to standard black and white plates before analysis.The CIELAB parameters (L*, a*, and b*) were obtained directly from the apparatus using the CIE 10° standard observer and the illuminant D65. Chroma ( ) and hue (hab) are calculated from a* and b*. To evaluate the effect of the cooking treatments on the colour, the colorimetric parameters were recorded before and after each treatment. For this purpose, six slices of carrots were analysed. The individual differences in L*, a* and b* values of each treatment respect to the uncooked samples were evaluated using colour differences (
) and hue (hab) are calculated from a* and b*. To evaluate the effect of the cooking treatments on the colour, the colorimetric parameters were recorded before and after each treatment. For this purpose, six slices of carrots were analysed. The individual differences in L*, a* and b* values of each treatment respect to the uncooked samples were evaluated using colour differences ( ), which are calculated as the Euclidean distance between two points in the three-dimensional space defined by L*, a*and b*:19
), which are calculated as the Euclidean distance between two points in the three-dimensional space defined by L*, a*and b*:19
 | (1) |
2.5. Texture
The compression tests were performed using a TAXT Plus Texture Analyzer (Stable Micro System, Godalming, UK) equipped with an HDP/90 platform. Weight calibration of equipment was carried out using a 5 kg load cell, and previous calibrations of force and distance were performed with a 30 kg (294 N) load cell. The compression test was performed using a 20 mm cylindrical aluminium probe. The test parameters were: speed pre-test, 1.0 mm s−1; speed test, 1.0 mm s−1; speed post-test, 10 mm s−1; distance, 10.0 mm; activation force, 0.05 N. The compression test ended when the sample reached 30% of the initial thickness or when the load cell reached its maximum, whichever occurred first. Ten replicates were carried out for each cooking condition. In texture analysis in food science, hardness is defined as the mechanical property relating to the force required to break an object. In fragile samples, it is usually expressed as the maximum force (N) applied by the equipment to break the object. In samples that do not have a critical breaking point or where the loss of cohesion is gradual, hardness is usually defined as the resistance of a material to an applied force, and is measured in units of force per distance, such as Newtons per millimeter (N mm−1). In this case, where there was a large difference in the consistency of the carrot samples, this magnitude has been chosen for the measurement of texture.20,212.6. Simulated in vitro digestion method
An in vitro static gastrointestinal model simulating digestion, following the INFOGEST 2.0 protocol by Brodkorb et al.22 was used. All parameters such as electrolytes (composition of simulated fluids), enzymes, bile, dilution, incubation time, pH and temperature (similar to the physiological conditions), N2 (to avoid oxidation) were the same as those described in this protocol. INFOGEST 2.0 protocol recommends diluting all food 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) with SSF to achieve a swallow able bolus with a paste-like consistency. For this, approximately 2.5 g of sample were added to 50 mL Falcon tubes and 2.5 mL of SSF and then were homogenized, to simulate the mastication, using a T25 Ultra-Turrax (IKA Works, Wilmington, NC, USA) at 24
1 (wt/wt) with SSF to achieve a swallow able bolus with a paste-like consistency. For this, approximately 2.5 g of sample were added to 50 mL Falcon tubes and 2.5 mL of SSF and then were homogenized, to simulate the mastication, using a T25 Ultra-Turrax (IKA Works, Wilmington, NC, USA) at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min.
000 rpm for 5 min.
Once completed the digestion process, the methodology described by Rodrigues et al.23 was used to isolate the micelle-containing fraction. The samples were centrifuged at 3900g for 20 min at 4 °C using an Allegra X-12R centrifuge (Beckman Coulter, USA). Supernatants were filtered through 0.22 μm nylon membrane (Agilent Technologies, USA) and the micellar fractions thus recovered were stored at −20 °C in a nitrogen atmosphere until carotenoid analyses. The samples were carried out in quadruplicate.
2.7. Carotenoids analyses
For extraction, 0.2–0.3 g undigested samples (a triplicate) were vigorously homogenized with 2 mL of water, and then with 5 mL of diethyl ether using a T25 UltraTurrax (IKA Works, Wilmington, NC, USA) at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The mixture was centrifuged at 3900g at 4 °C for 5 min. After recovering the coloured fraction, the residue was submitted to the same extraction process until colour exhaustion. The coloured fractions were combined and concentrated to dryness in a rotary evaporator (Eppendorf Concentrator Plus, Hamburg, Germany).
000 rpm for 5 min. The mixture was centrifuged at 3900g at 4 °C for 5 min. After recovering the coloured fraction, the residue was submitted to the same extraction process until colour exhaustion. The coloured fractions were combined and concentrated to dryness in a rotary evaporator (Eppendorf Concentrator Plus, Hamburg, Germany).
For the digested samples, the micellar fraction (10–12 mL) was homogenized with 5 mL of diethyl ether and 0.5 g of NaCl using a T25 UltraTurrax at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and centrifuged at 3900g at 4 °C for 5 min. The supernatant was recovered, and the residue was extracted using the conditions described above until colour exhaustion. All supernatants were pooled and concentrated to dryness in a rotary evaporator at a temperature below 30 °C. All dried extracts (undigested and digested) were kept dry at −20 °C under a nitrogen atmosphere until RRLC analyses.
000 rpm for 5 min and centrifuged at 3900g at 4 °C for 5 min. The supernatant was recovered, and the residue was extracted using the conditions described above until colour exhaustion. All supernatants were pooled and concentrated to dryness in a rotary evaporator at a temperature below 30 °C. All dried extracts (undigested and digested) were kept dry at −20 °C under a nitrogen atmosphere until RRLC analyses.
Before the injection, dry extracts were redissolved in ethyl acetate, centrifuged at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min at 4 °C and poured into amber glass vials. The determination of carotenoids was performed by RRLC on an Agilent 1260 chromatograph (Agilent Technologies, Palo Alto, CA; USA) equipped with a diode-array detector (DAD) and a C30 YMC column (3 μm, 250 × 4.6 mm) (YMC, Wilmington, NC) kept at 20 °C. The chromatographic conditions (linear gradient, flow rate, DAD conditions, etc.) were established following the validated method by Stinco et al.24
000g for 5 min at 4 °C and poured into amber glass vials. The determination of carotenoids was performed by RRLC on an Agilent 1260 chromatograph (Agilent Technologies, Palo Alto, CA; USA) equipped with a diode-array detector (DAD) and a C30 YMC column (3 μm, 250 × 4.6 mm) (YMC, Wilmington, NC) kept at 20 °C. The chromatographic conditions (linear gradient, flow rate, DAD conditions, etc.) were established following the validated method by Stinco et al.24
The carotenoids were identified based on their retention time and UV-vis spectra, and by comparison with our data library and standards when available. The tentative identification of Z (cis) isomers was performed by comparing their chromatographic and spectroscopic features with those of a standard mixture of CARs obtained by iodine-catalysed isomerization and by comparison with data reported by other authors.25–28 The quantification was achieved by external calibration, using curves obtained with standard solutions of carotenoids.24 The total carotenoid content of samples was calculated as the sum of the individual concentration of compounds, expressed as mg of carotenoids per 100 g of carrot serving.
2.8. Carotenoid bioaccessible content (CBC)
Carotenoid bioaccessible content (CBC) refers to the amount of the compound that is incorporated into micelles and is bioaccessible, expressed as mg of carotenoids per 100 g of carrot serving. That is, it is an estimation of the amount of carotenoid that is potentially absorbable and therefore bioavailable from a certain food amount. This parameter has been preferred over bioaccessibility (usually expressed as percentage) as CBC provides a more meaningful information about the potential bioavailability.2.9. Statistical analysis
Results were expressed as mean and standard deviation of three independent determinations. Homoscedasticity criteria were evaluated by the Levene tests. Since the result did not show significance the homoscedasticity hypothesis of variance was accepted. One-way analysis of variance (ANOVA) was used to compare the means. Statistical analysis was performed with Statistica v.8.0 software. Differences were considered significant at (p < 0.05) using Tukeýs multiple comparison procedure for each treatment compared to the control. The texture analysis data were exported with the software Exponent 6.1.4.0 (Stable Micro System, Godalming, UK) and processed with MATLAB R2020 (The Mathworks Inc., Natick, USA). The ‘Curve Fitting Toolbox’ included in MATLAB R2020 was also used to build the fitting equations within every treatment.Among the different regression methods, polynomial at different degrees, exponential, logarithmic models were used. The goodness of fit of each of the models was assessed by the coefficient of determination between the measured data and the predicted data after fitting, as well as by comparing the root mean square error in each case.
3. Results and discussion
3.1. Texture
Table 1 shows the hardness defined as the maximum slope reached when representing the force (N) versus the distance shifted (mm) by the probe in this measurement. As expected, the hardness of the samples significantly decreased with increasing intensity and duration of treatments. The maximum softening was found for ST (100 °C) after 20 minutes (36 ± 9 N mm−1) but a more pronounced reduction in hardness in a shorter time was observed for the sample treated with MW at 750 W (100% potency) (a decrease of 98 units in 3 minutes). The cooking treatment with the lowest influence on carrot texture was WC at 80 °C, only after 15 minutes were statistically significant differences (p < 0.05) relative to the raw sample observed. The relationship between the decrease in hardness and time of treatment was not linear. In the case of WC (100 °C), the samples lost most of their consistency after 15 minutes. For MW-treated samples, a major decrease in consistency occurred between minutes 2 and 3. It was also noticeable that different times of MW heating (1, 2 and 3 minutes) induced significant differences in texture while the two different MW intensities (600 and 750 W) did not (p > 0.05).| Treatment | Time (min) | Hardness (N mm−1) | Q (J) |
|---|---|---|---|
| Different letters indicate statistically significant differences at p < 0.05, including Raw data in each treatment. Nomenclature used for cooking process: ST (100 °C), steaming at 100 °C; WC (80 °C), water cooking at 80 °C; WC (100 °C), water cooking at 100 °C; BK (180 °C), baking at 180 °C; BK (210 °C), baking at 210 °C; MW (80%), microwaving at 600 W; MW (100%), microwaving at 750 W. | |||
| Raw | 0 | 148 ± 22a | 0 |
| ST (100 °C) | 5 | 138 ± 12ab | 967.5 ± 3.7a |
| ST (100 °C) | 10 | 63 ± 12c | 1377.3 ± 2.4b |
| ST (100 °C) | 15 | 45 ± 13d | 1445.9 ± 8.4c |
| ST (100 °C) | 20 | 36 ± 9d | 1560.7 ± 6.0d |
| WC (80 °C) | 10 | 137 ± 14ab | 1174.8 ± 3.7a |
| WC (80 °C) | 15 | 126 ± 10b | 1252.1 ± 7.3b |
| WC (80 °C) | 20 | 133 ± 13b | 1363.7 ± 6.0c |
| WC (100 °C) | 10 | 109 ± 8c | 1398.8 ± 6.0d |
| WC (100 °C) | 15 | 63 ± 11d | 1406.8 ± 6.0e |
| WC (100 °C) | 20 | 52 ± 8d | 1526.4 ± 3.7f |
| BK (180 °C) | 10 | 115 ± 12b | 1157.3 ± 2.4a |
| BK (180 °C) | 15 | 106 ± 14b | 1274.5 ± 8.6b |
| BK (180 °C) | 20 | 82 ± 22c | 1386.1 ± 3.7c |
| BK (210 °C) | 10 | 104 ± 12b | 1400.4 ± 6.0c |
| BK (210 °C) | 15 | 79 ± 12c | 1481.7 ± 5.0d |
| BK (210 °C) | 20 | 61 ± 10d | 1705.0 ± 10.4e |
| MW (80%) | 1 | 120 ± 26b | 1160.4 ± 8.4a |
| MW (80%) | 2 | 102 ± 20c | 1330.3 ± 6.0b |
| MW (80%) | 3 | 58 ± 11d | 1424.3 ± 10.0c |
| MW (100%) | 1 | 128 ± 25b | 1268.1 ± 7.3d |
| MW (100%) | 2 | 102 ± 18c | 1449.1 ± 9.6e |
| MW (100%) | 3 | 50 ± 12d | 1476.2 ± 7.3f |
In this context, in addition to the intensity and duration of the treatments applied, other aspects such as the heat absorbed by the carrot could be important in explaining the change in texture.
To this end, an estimate of the heat absorbed by the sample was made for each of the conditions tested. This parameter was calculated using the equation
| Q = m × Cp × ΔT | (2) |
The results obtained (Table 1) show that the higher the heat absorbed by the sample, the greater the changes in hardness. In other words, both hardness and heat absorbed increased with the intensity and duration of the treatments applied.
3.2. Carotenoid contents
Table 2 shows individual and total carotenoid contents. A total of 11 carotenoids bioavailable in humans were identified, belonging to three groups: (1) colourless carotenoids, namely (15Z)-phytoene (15Z-PT) and three phytofluene isomers (PF-1, PF-2 and PF-3); (2) provitamin A carotenoids, specifically (all-E)-α-carotene (ACAR), (9Z)-α-carotene (9Z-ACAR), (all-E)-β-carotene (BCAR) and three Z-isomers (9Z, 13Z and 15Z-BCAR); (3) (all-E)-lutein (LUT).| Treatment | Time | Carotenoids | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15Z-PT | PF-1 | PF-2 | PF-3 | Total PF | LUT | ACAR | 9Z-ACAR | 15Z-BCAR | 13Z-BCAR | BCAR | 9Z-BCAR | Total Z-BCARs | Total CARs | ||
| Nomenclature used for cooking process: Raw, raw carrots; ST (100 °C), steaming at 100 °C; WC (80 °C), water cooking at 80 °C; WC (100 °C), water cooking at 100 °C; BK (180 °C), baking at 180 °C; BK (210 °C), baking at 210 °C; MW (80%), microwaving at 600 W; MW (100%), microwaving at 750 W. Nomenclature used for carotenoids: 15Z-PT, 15Z phytoene; PF, phytofluene; Total PF, total Z phytofluene isomers; LUT, lutein; ACAR, α-carotene; 9Z-ACAR, 9Z α-carotene; 15Z-BCAR, 15Z β-carotene; 13Z-BCAR, 13Z β-carotene BCAR, β-carotene; 9Z-BCAR, 9Z β-carotene; Total Z-BCARs, total Z β-carotene isomers; Total CARs, total carontenoids content. Different letters within the same column indicate statistically significant differences (p < 0.05) for each treatment compared to control. Number of technical replicates, n = 3. | |||||||||||||||
| Raw | 0 | 1.260 ± 0.106a | 0.614 ± 0.036a | 1.055 ± 0.098a | 0.032 ± 0.002a | 1.702 ± 0.134a | 0.471 ± 0.056a | 13.037 ± 0.403a | 0.069 ± 0.007a | 0.061 ± 0.008a | 0.085 ± 0.009a | 12.504 ± 0.917a | 0.110 ± 0.010a | 0.255 ± 0.016a | 29.298 ± 1.276a |
| ST (100 °C) | 5 | 3.069 ± 0.074b | 1.212 ± 0.102b | 2.580 ± 0.184c | 0.056 ± 0.005b | 3.848 ± 0.215bc | 0.530 ± 0.034a | 25.311 ± 1.144bc | 0.175 ± 0.015a | 0.069 ± 0.009a | 0.170 ± 0.017b | 22.339 ± 0.938b | 0.197 ± 0.011b | 0.435 ± 0.035b | 55.707 ± 1.207bc |
| 10 | 2.781 ± 0.144c | 1.785 ± 0.150c | 2.088 ± 0.094b | 0.074 ± 0.008bc | 3.947 ± 0.243cd | 0.824 ± 0.029b | 28.536 ± 2.334c | 0.227 ± 0.004b | 0.249 ± 0.019b | 0.408 ± 0.016c | 20.967 ± 1.726b | 0.312 ± 0.014c | 0.969 ± 0.045c | 58.249 ± 3.807c | |
| 15 | 3.184 ± 0.068b | 1.851 ± 0.132c | 2.288 ± 0.113bc | 0.075 ± 0.007bc | 4.214 ± 0.120cd | 0.910 ± 0.096b | 24.190 ± 1.879b | 0.231 ± 0.013c | 0.368 ± 0.027c | 0.569 ± 0.022d | 19.645 ± 0.687bc | 0.325 ± 0.034c | 1.263 ± 0.032d | 53.637 ± 2.267bc | |
| 20 | 3.328 ± 0.101b | 1.924 ± 0.036c | 2.352 ± 0.125bc | 0.079 ± 0.011c | 4.356 ± 0.166cd | 0.964 ± 0.111b | 22.730 ± 1.570b | 0.237 ± 0.020d | 0.432 ± 0.022d | 0.681 ± 0.042e | 16.295 ± 1.873c | 0.400 ± 0.025d | 1.514 ± 0.062e | 49.349 ± 3.364b | |
| WC (80 °C) | 10 | 2.526 ± 0.157d | 1.308 ± 0.039c | 1.911 ± 0.081c | 0.103 ± 0.002c | 3.322 ± 0.059d | 0.697 ± 0.075b | 21.869 ± 1.129b | 0.129 ± 0.005b | 0.073 ± 0.006ab | 0.284 ± 0.005b | 20.487 ± 1.161c | 0.113 ± 0.036a | 0.470 ± 0.034b | 49.501 ± 2.212c |
| 15 | 2.373 ± 0.170cd | 1.285 ± 0.116c | 1.681 ± 0.101bc | 0.101 ± 0.006bc | 3.067 ± 0.109cd | 0.691 ± 0.044b | 21.373 ± 1.646b | 0.182 ± 0.011c | 0.090 ± 0.007ab | 0.387 ± 0.028c | 18.506 ± 1.507bc | 0.174 ± 0.006b | 0.651 ± 0.035c | 46.843 ± 2.764bc | |
| 20 | 2.334 ± 0.158cd | 1.177 ± 0.102c | 1.593 ± 0.133b | 0.089 ± 0.008bc | 2.859 ± 0.112c | 0.612 ± 0.050ab | 21.186 ± 1.127b | 0.199 ± 0.015c | 0.102 ± 0.015bc | 0.439 ± 0.017cd | 18.247 ± 1.044bc | 0.210 ± 0.020bc | 0.751 ± 0.053cd | 46.188 ± 0.703bc | |
| WC (100 °C) | 10 | 2.021 ± 0.080bc | 1.103 ± 0.050bc | 1.669 ± 0.027bc | 0.088 ± 0.017bc | 2.860 ± 0.028b | 0.585 ± 0.050ab | 21.786 ± 1.059b | 0.243 ± 0.004d | 0.132 ± 0.016c | 0.494 ± 0.021de | 20.515 ± 0.724c | 0.222 ± 0.006bc | 0.848 ± 0.019d | 48.860 ± 1.621c |
| 15 | 1.806 ± 0.100b | 0.889 ± 0.050b | 1.484 ± 0.022b | 0.087 ± 0.002b | 2.461 ± 0.026b | 0.466 ± 0.036a | 21.915 ± 1.772b | 0.272 ± 0.009de | 0.202 ± 0.008d | 0.566 ± 0.033e | 17.728 ± 1.040abc | 0.220 ± 0.012bc | 0.989 ± 0.053e | 45.636 ± 2.730c | |
| 20 | 2.017 ± 0.115bc | 1.181 ± 0.077c | 1.614 ± 0.091b | 0.090 ± 0.005bc | 2.885 ± 0.156b | 0.547 ± 0.061ab | 18.669 ± 1.602b | 0.285 ± 0.022e | 0.206 ± 0.023d | 0.572 ± 0.058e | 16.600 ± 0.547b | 0.246 ± 0.016c | 1.024 ± 0.089f | 42.027 ± 2.309b | |
| BK (180 °C) | 10 | 2.405 ± 0.139b | 1.632 ± 0.070b | 1.642 ± 0.089b | 0.075 ± 0.002b | 3.348 ± 0.072b | 0.790 ± 0.071d | 26.661 ± 0.805b | 0.126 ± 0.015b | 0.069 ± 0.006a | 0.123 ± 0.009a | 22.896 ± 1.138b | 0.114 ± 0.014a | 0.306 ± 0.012a | 56.533 ± 1.768b |
| 15 | 2.377 ± 0.218b | 1.682 ± 0.094b | 1.994 ± 0.117cd | 0.074 ± 0.002b | 3.750 ± 0.208bc | 0.778 ± 0.056cd | 31.513 ± 2.719bcd | 0.190 ± 0.023cd | 0.095 ± 0.011a | 0.213 ± 0.016b | 22.118 ± 1.094b | 0.181 ± 0.023b | 0.489 ± 0.046b | 61.214 ± 2.097bc | |
| 20 | 2.336 ± 0.070b | 1.650 ± 0.061b | 1.928 ± 0.090bcd | 0.074 ± 0.011b | 3.652 ± 0.049bc | 0.580 ± 0.032ab | 33.884 ± 2.590cd | 0.238 ± 0.010e | 0.146 ± 0.012b | 0.274 ± 0.018b | 21.098 ± 1.731b | 0.231 ± 0.013c | 0.652 ± 0.040c | 62.439 ± 1.756bc | |
| BK (210 °C) | 10 | 3.007 ± 0.215c | 2.012 ± 0.175c | 1.823 ± 0.073bc | 0.072 ± 0.008b | 3.908 ± 0.181cd | 0.648 ± 0.053bc | 27.633 ± 2.756bc | 0.186 ± 0.015c | 0.169 ± 0.009b | 0.295 ± 0.012b | 21.768 ± 0.788b | 0.259 ± 0.009c | 0.722 ± 0.017c | 57.872 ± 3.940b |
| 15 | 3.258 ± 0.186cd | 2.047 ± 0.014c | 2.209 ± 0.145de | 0.075 ± 0.001b | 4.329 ± 0.156de | 0.652 ± 0.074bc | 34.871 ± 1.883d | 0.231 ± 0.016de | 0.244 ± 0.013c | 0.443 ± 0.027c | 23.261 ± 1.443b | 0.275 ± 0.012c | 0.962 ± 0.027d | 67.564 ± 2.869cd | |
| 20 | 3.770 ± 0.227d | 2.249 ± 0.143c | 2.355 ± 0.155e | 0.077 ± 0.003b | 4.682 ± 0.286e | 0.705 ± 0.023bcd | 36.604 ± 2.364d | 0.263 ± 0.010e | 0.372 ± 0.024d | 0.870 ± 0.061d | 24.326 ± 1.737b | 0.355 ± 0.026d | 1.597 ± 0.056e | 71.947 ± 3.653d | |
| MW (80%) | 1 | 2.462 ± 0.137c | 1.245 ± 0.128c | 1.685 ± 0.009c | 0.119 ± 0.007b | 3.050 ± 0.127c | 0.574 ± 0.042b | 25.157 ± 1.675b | 0.189 ± 0.009b | 0.085 ± 0.010ab | 0.271 ± 0.017b | 19.132 ± 1.253c | 0.158 ± 0.018b | 0.514 ± 0.041b | 51.078 ± 3.040c |
| 2 | 1.964 ± 0.073b | 1.146 ± 0.109bc | 1.362 ± 0.119b | 0.104 ± 0.004b | 2.611 ± 0.176b | 0.486 ± 0.015ab | 21.795 ± 1.445b | 0.198 ± 0.017bc | 0.109 ± 0.009bc | 0.380 ± 0.028c | 17.038 ± 1.264bc | 0.172 ± 0.023b | 0.661 ± 0.056c | 44.753 ± 2.572bc | |
| 3 | 1.854 ± 0.114b | 1.000 ± 0.099b | 1.344 ± 0.064b | 0.070 ± 0.007c | 2.414 ± 0.048b | 0.437 ± 0.048a | 20.471 ± 1.455b | 0.198 ± 0.018bc | 0.124 ± 0.011c | 0.460 ± 0.024d | 14.836 ± 1.104ab | 0.178 ± 0.017b | 0.763 ± 0.043cd | 40.972 ± 1.742b | |
| MW (100%) | 1 | 1.830 ± 0.168b | 1.145 ± 0.081bc | 1.335 ± 0.076b | 0.065 ± 0.006c | 2.544 ± 0.112b | 0.496 ± 0.038ab | 22.731 ± 2.418b | 0.246 ± 0.005cd | 0.117 ± 0.010c | 0.505 ± 0.022d | 17.832 ± 0.769c | 0.182 ± 0.006b | 0.804 ± 0.029d | 46.483 ± 3.387bc |
| 2 | 1.8.20 ± 0.107b | 1.091 ± 0.052bc | 1.310 ± 0.059b | 0.064 ± 0.005c | 2.465 ± 0.075b | 0.419 ± 0.015a | 21.069 ± 2.169b | 0.269 ± 0.040d | 0.159 ± 0.010d | 0.787 ± 0.017e | 16.787 ± 1.063bc | 0.186 ± 0.008b | 1.133 ± 0.025e | 43.961 ± 3.027b | |
| 3 | 1.920 ± 0.105b | 1.100 ± 0.064bc | 1.185 ± 0.085ab | 0.044 ± 0.009a | 2.330 ± 0.013b | 0.420 ± 0.003a | 21.893 ± 1.682b | 0.274 ± 0.018d | 0.169 ± 0.008d | 0.962 ± 0.030f | 14.779 ± 0.296ab | 0.202 ± 0.022b | 1.333 ± 0.057f | 42.948 ± 1.994b | |
The apparent total carotenoid content in raw carrots (29.298 ± 1.276 mg Cars per 100 g carrot serving) was significantly lower than in all cooked samples. However, the behaviour was not consistent either across carotenoids or cooking practices, observing that the total carotenoid content decreased significantly when increasing the time/temperature conditions (ST or WC) or power/time for the MW in all cooking treatments, except for BK with the inverse tendency.
Similar results were observed for colourless carotenoids PT and PF isomers with lower content in raw carrots than in cooked samples. For the different treatments and conditions, the same trend described previously for total carotenoids was also observed. Colourless carotenoid content remained constant or slightly decreased at the lowest MW and WC treatment conditions, while increases of PF isomers 1 and 3 were observed for ST. Conversely, baking at 210 °C for 20 min resulted in an increase of 300% of 15Z-PT and 240–360% of PF isomers, compared to raw carrot. Likewise, Miglio et al.29 reported an increase in PT and PF concentrations after three cooking treatments (boiled, steamed and dried), especially in the case of frying.
Provitamin A carotenoids content (ACAR, BCAR and Z-isomers) in carrot slices also increased significantly in processed samples. Concerning ACAR, increasing the time or temperature of treatment in the ST and WC treatments significantly decreased the content of this compound, while for BK at any time/temperature conditions the content significantly increased by 27% and 32.5% for 10 min time at 180 °C and 210 °C, respectively. Besides, the content of 9Z-ACAR increased after all treatments. Similar behaviour was observed in the different BCAR isomers: 9Z, 13Z and 15Z with increased contents related to longer time or/and higher intensity (temperature/power) treatments. Increased levels of Z carotenoid isomers resulting from heating are expected as carotenoids, due to their highly unsaturated structure, are prone to geometrical isomerization, which is favoured by heating.11 If the thermal treatment is long enough an equilibrium state in which the proportion of geometrical isomers remains stable can be reached.30 In this respect, Lemmens et al.31 reported heat-induced changes in the levels of geometrical isomers of BCAR until an equilibrium state is reached after prolonged heating.
In contrast to ACAR, BCAR content, showed no significant differences in the BK treatment, while for the ST (100 °C), WC (100 °C) and MW (both intensities) treatments decreases were observed. Other authors reported similar results. Bureau et al.32 studied several treatments and only the microwave cooking induced a clear degradation of BCAR. Considering the results from the present study, MW at 750 W and WC at 100 °C were the treatment that most negatively affected BCAR and ACAR contents, respectively.
Finally, lutein content was differently affected by the different cooking processes and conditions. While ST at 100 °C for 20 min increased its levels by ∼2-fold, WC did not affect it significantly in general. On the contrary, MW led to decreases in LUT levels independently of the intensity used. Surprisingly, baking at 180 °C resulted in a decrease in lutein.
Apparent increases such as the ones observed in this study for total and individually carotenoids, are usually attributed to an enhanced extractability caused by matrix changes induced by some treatments. However, although there can be a certain extractability enhancement due to matrix softening (especially in hard ones such as raw carrots), the most probable cause is the concentration effects due to partial dehydration of foods during cooking and the loss of soluble solids, effects that result in a concentration of carotenoids, as discussed in a reference guide for carotenoid analysis.11
3.3. Colour
Fig. 1 shows the colour changes produced by the different conditions evaluated as these led to important changes in carotenoid levels. As expected, overall, the magnitude of colour changes increased with the intensity and duration of the treatments. The cooking processes that showed the greatest colour difference between cooked and raw sample was MW at 750 W for 3 min (17.9 CIELAB units), followed by WC at 100 °C for 20 min (16.2 CIELAB units) (Fig. 1). The lowest colour differences were observed for the samples steamed at 100 °C for 5 min (5.6 CIELAB units) followed by that water-cooked at 80 °C for 10 min (6.2 CIELAB units). These results are in agreement with those reported by Vervoort et al.,33 who observed that all the processing conditions tested (mild thermal pasteurization, mild high pressure (HP) pasteurization, severe thermal pasteurization, severe HP pasteurization, thermal sterilization and HP sterilization) caused noticeable changes in colour relative raw carrots, with HP sterilization preserving the original colour the most. Taking into account that colour differences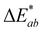 up to 3 CIELAB units are noticeable by the human eyes,33,34 it could be affirmed that all domestic cooking processes assayed in the different conditions tested produced colour changes perceptible to the human eye.
up to 3 CIELAB units are noticeable by the human eyes,33,34 it could be affirmed that all domestic cooking processes assayed in the different conditions tested produced colour changes perceptible to the human eye.
3.4. Carotenoid bioaccessible content (CBC)
Table 3 summarizes the CBC values considering 100 g servings, an estimation of the amount of carotenoids theoretically incorporated into micelles and potentially absorbable from such servings. The carotenoids in the micellar fractions included those found in the undigested samples, some Z isomers of lutein, α- and β-carotene. Considering the total carotenoid contents, it can be observed that cooking can lead to significant increases in CBC values. Thus, increases of up to 6.04-fold (MW), 6.97-fold (WC), 7.98-fold (ST) and 8.91-fold (BK) were observed for the most intense conditions.| Treatment | Time | Carotenoids | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15Z-PT | PF-1 | PF-2 | PF-3 | Total PF | 15Z-LUT | 13Z-LUT | Total Z-LUT | LUT | ACAR | 9Z-ACAR | 15Z-BCAR | 13Z-BCAR | BCAR | 9Z-BCAR | Total Z-BCARs | Total CARs | ||
| Nomenclature used for cooking process: Raw, raw carrots; ST (100 °C), steaming at 100 °C; WC (80 °C), water cooking at 80 °C; WC (100 °C), water cooking at 100 °C; BK (180 °C), baking at 180 °C; BK (210 °C), baking at 210 °C; MW (80%), microwaving at 600 W; MW (100%), microwaving at 750 W. Nomenclature used for carotenoids: 15Z-PT, 15Z phytoene; PF, phytofluene; Total PF, total Z phytofluene isomers; 15Z-LUT, 15Z lutein; 13Z-LUT, 13Z lutein; Total Z-LUT, total Z lutein isomers; LUT, lutein; ACAR, α-carotene; 9Z-ACAR, 9Z α-carotene; 15Z-BCAR, 15Z β-carotene; 13Z-BCAR, 13Z β-carotene BCAR, β-carotene; 9Z-BCAR, 9Z β-carotene; Total Z-BCARs, total Z β-carotene isomers; Total CARs, total carontenoids content. Different letters within the same column indicate statistically significant differences (p < 0.05) for each treatment compared to control. Number of technical replicates, n = 4. | ||||||||||||||||||
| Raw | 0 | 0.375 ± 0.035a | 0.068 ± 0.009a | 0.170 ± 0.015a | 0.008 ± 0.001a | 0.246 ± 0.009a | 0.005 ± 0.001a | 0.004 ± 0.001a | 0.008 ± 0.001a | 0.090 ± 0.007a | 0.036 ± 0.006a | 0.001 ± 0.001a | 0.001 ± 0.001a | 0.001 ± 0.001a | 0.015 ± 0.002a | 0.001 ± 0.001a | 0.002 ± 0.0002a | 0.780 ± 0.044a |
| ST (100 °C) | 5 | 0.398 ± 0.038a | 0.202 ± 0.020a | 0.098 ± 0.008a | 0.007 ± 0.001a | 0.307 ± 0.023a | 0.004 ± 0.001a | 0.002 ± 0.001a | 0.005 ± 0.002a | 0.064 ± 0.002a | 0.119 ± 0.018a | 0.001 ± 0.001a | 0.007 ± 0.001b | 0.006 ± 0.001a | 0.067 ± 0.004a | 0.002 ± 0.001a | 0.015 ± 0.001ab | 0.980 ± 0.071a |
| 10 | 0.357 ± 0.034a | 0.158 ± 0.013a | 0.313 ± 0.018b | 0.009 ± 0.002a | 0.480 ± 0.032c | 0.027 ± 0.001b | 0.015 ± 0.001b | 0.042 ± 0.002c | 0.407 ± 0.023b | 0.352 ± 0.035b | 0.003 ± 0.001a | 0.010 ± 0.002b | 0.052 ± 0.010a | 0.294 ± 0.033b | 0.010 ± 0.002a | 0.072 ± 0.011b | 2.006 ± 0.161b | |
| 15 | 1.010 ± 0.095b | 0.660 ± 0.068b | 0.679 ± 0.061c | 0.021 ± 0.004b | 1.359 ± 0.052d | 0.044 ± 0.002c | 0.205 ± 0.001c | 0.069 ± 0.003d | 0.556 ± 0.015c | 1.220 ± 0.043c | 0.012 ± 0.001b | 0.054 ± 0.003c | 0.252 ± 0.037b | 0.960 ± 0.046c | 0.038 ± 0.008b | 0.344 ± 0.043c | 5.556 ± 0.232c | |
| 20 | 1.132 ± 0.097b | 0.797 ± 0.091b | 0.720 ± 0.031c | 0.024 ± 0.002b | 1.541 ± 0.062e | 0.047 ± 0.002c | 0.027 ± 0.003c | 0.074 ± 0.005d | 0.578 ± 0.048c | 1.366 ± 0.110c | 0.102 ± 0.003b | 0.058 ± 0.002c | 0.320 ± 0.031c | 1.071 ± 0.075c | 0.054 ± 0.008c | 0.432 ± 0.038d | 6.227 ± 0.326d | |
| WC (80 °C) | 10 | 0.602 ± 0.065b | 0.197 ± 0.004b | 0.377 ± 0.029b | 0.022 ± 0.004b | 0.596 ± 0.034b | 0.014 ± 0.002bc | 0.010 ± 0.002bc | 0.024 ± 0.004bc | 0.238 ± 0.023b | 0.200 ± 0.033b | 0.002 ± 0.001a | 0.004 ± 0.001b | 0.007 ± 0.002ab | 0.120 ± 0.016a | 0.006 ± 0.001ab | 0.017 ± 0.003b | 1.806 ± 0.176b |
| 15 | 0.58 ± 0.029b | 0.175 ± 0.020b | 0.318 ± 0.013b | 0.024 ± 0.001b | 0.517 ± 0.024b | 0.011 ± 0.002b | 0.006 ± 0.001ab | 0.017 ± 0.003b | 0.250 ± 0.030b | 0.218 ± 0.010b | 0.002 ± 0.001a | 0.005 ± 0.001b | 0.011 ± 0.001b | 0.13 ± 0.012a | 0.008 ± 0.003ab | 0.024 ± 0.003bc | 1.853 ± 0.065b | |
| 20 | 0.621 ± 0.031b | 0.154 ± 0.020b | 0.413 ± 0.063b | 0.026 ± 0.003b | 0.592 ± 0.081b | 0.011 ± 0.001b | 0.007 ± 0.001abc | 0.018 ± 0.003b | 0.249 ± 0.018b | 0.217 ± 0.020b | 0.003 ± 0.001ab | 0.004 ± 0.001b | 0.021 ± 0.002c | 0.135 ± 0.014a | 0.011 ± 0.004b | 0.036 ± 0.007c | 1.891 ± 0.159b | |
| WC (100 °C) | 10 | 0.910 ± 0.059c | 0.160 ± 0.015b | 0.918 ± 0.018c | 0.048 ± 0.007c | 1.126 ± 0.010c | 0.016 ± 0.002c | 0.011 ± 0.001c | 0.027 ± 0.002c | 0.333 ± 0.006c | 0.427 ± 0.059c | 0.005 ± 0.001b | 0.011 ± 0.001c | 0.030 ± 0.003d | 0.367 ± 0.055b | 0.013 ± 0.001b | 0.054 ± 0.002d | 3.261 ± 0.156c |
| 15 | 0.947 ± 0.052c | 0.184 ± 0.021b | 0.980 ± 0.036c | 0.054 ± 0.004c | 1.218 ± 0.040c | 0.023 ± 0.002d | 0.015 ± 0.002d | 0.038 ± 0.004d | 0.434 ± 0.061d | 0.690 ± 0.060d | 0.009 ± 0.001c | 0.013 ± 0.001c | 0.038 ± 0.004e | 0.566 ± 0.047c | 0.021 ± 0.004c | 0.072 ± 0.005e | 3.979 ± 0.229d | |
| 20 | 1.201 ± 0.078d | 0.267 ± 0.034c | 1.160 ± 0.061d | 0.071 ± 0.002d | 1.498 ± 0.068d | 0.027 ± 0.001d | 0.016 ± 0.001d | 0.044 ± 0.002d | 0.448 ± 0.021d | 1.124 ± 0.120e | 0.014 ± 0.002d | 0.024 ± 0.002d | 0.055 ± 0.005f | 0.977 ± 0.088d | 0.040 ± 0.001d | 0.118 ± 0.008f | 5.436 ± 0.342e | |
| BK (180 °C) | 10 | 0.648 ± 0.072b | 0.279 ± 0.034b | 0.384 ± 0.040b | 0.018 ± 0.003b | 0.681 ± 0.018b | 0.012 ± 0.001a | 0.007 ± 0.001ab | 0.019 ± 0.002ab | 0.284 ± 0.016b | 0.301 ± 0.043b | 0.003 ± 0.001ab | 0.007 ± 0.002ab | 0.026 ± 0.001ab | 0.202 ± 0.023b | 0.010 ± 0.001ab | 0.043 ± 0.004ab | 2.183 ± 0.132b |
| 15 | 0.703 ± 0.069b | 0.384 ± 0.021bc | 0.452 ± 0.043bc | 0.017 ± 0.002b | 0.854 ± 0.050b | 0.020 ± 0.003b | 0.011 ± 0.002b | 0.031 ± 0.004bc | 0.313 ± 0.046bc | 0.337 ± 0.037b | 0.004 ± 0.001ab | 0.010 ± 0.001ab | 0.028 ± 0.003b | 0.258 ± 0.020bc | 0.020 ± 0.003bc | 0.059 ± 0.004b | 2.562 ± 0.205b | |
| 20 | 0.846 ± 0.062b | 0.599 ± 0.073d | 0.538 ± 0.048c | 0.030 ± 0.003d | 1.167 ± 0.118b | 0.023 ± 0.001b | 0.014 ± 0.001bc | 0.037 ± 0.001c | 0.382 ± 0.022c | 0.575 ± 0.019c | 0.011 ± 0.001cd | 0.021 ± 0.002c | 0.090 ± 0.001c | 0.353 ± 0.017cd | 0.033 ± 0.002d | 0.144 ± 0.004c | 3.526 ± 0.106c | |
| BK (210 °C) | 10 | 0.784 ± 0.064b | 0.425 ± 0.025c | 0.433 ± 0.042bc | 0.022 ± 0.001bc | 0.880 ± 0.056c | 0.021 ± 0.002b | 0.012 ± 0.002b | 0.033 ± 0.004bc | 0.368 ± 0.048bc | 0.676 ± 0.067c | 0.007 ± 0.001bc | 0.017 ± 0.002bc | 0.071 ± 0.005c | 0.405 ± 0.047d | 0.018 ± 0.001b | 0.106 ± 0.007c | 3.276 ± 0.294c |
| 15 | 1.559 ± 0.101c | 0.962 ± 0.089e | 0.708 ± 0.053d | 0.028 ± 0.001cd | 1.698 ± 0.137d | 0.038 ± 0.002c | 0.020 ± 0.001cd | 0.058 ± 0.002d | 0.522 ± 0.032d | 1.098 ± 0.103d | 0.013 ± 0.003d | 0.059 ± 0.006d | 0.230 ± 0.011d | 0.721 ± 0.071e | 0.032 ± 0.010cd | 0.321 ± 0.022d | 6.023 ± 0.354d | |
| 20 | 1.695 ± 0.090c | 1.077 ± 0.051e | 0.737 ± 0.012d | 0.037 ± 0.002e | 1.851 ± 0.056d | 0.049 ± 0.006d | 0.027 ± 0.007d | 0.076 ± 0.013e | 0.611 ± 0.012e | 1.266 ± 0.053e | 0.021 ± 0.002e | 0.094 ± 0.010e | 0.409 ± 0.022e | 0.858 ± 0.027f | 0.040 ± 0.060d | 0.543 ± 0.032e | 6.947 ± 0.060e | |
| MW (80%) | 1 | 0.594 ± 0.033b | 0.290 ± 0.030c | 0.236 ± 0.026a | 0.020 ± 0.002bc | 0.545 ± 0.002c | 0.012 ± 0.004b | 0.006 ± 0.002ab | 0.018 ± 0.002bc | 0.135 ± 0.016a | 0.205 ± 0.023b | 0.003 ± 0.001ab | 0.007 ± 0.001b | 0.005 ± 0.001a | 0.123 ± 0.009b | 0.006 ± 0.002a | 0.019 ± 0.003a | 1.659 ± 0.063b |
| 2 | 0.790 ± 0.036c | 0.454 ± 0.028d | 0.419 ± 0.016bc | 0.025 ± 0.003cd | 0.899 ± 0.045d | 0.015 ± 0.001bc | 0.010 ± 0.001cd | 0.025 ± 0.002cd | 0.342 ± 0.014cd | 0.470 ± 0.060d | 0.005 ± 0.001b | 0.011 ± 0.001bc | 0.038 ± 0.008b | 0.352 ± 0.038c | 0.019 ± 0.003bc | 0.068 ± 0.009b | 2.966 ± 0.090d | |
| 3 | 0.911 ± 0.058d | 0.543 ± 0.048e | 0.466 ± 0.038c | 0.030 ± 0.001d | 1.038 ± 0.064e | 0.023 ± 0.003d | 0.013 ± 0.002d | 0.036 ± 0.005e | 0.378 ± 0.025d | 0.772 ± 0.062e | 0.008 ± 0.001c | 0.034 ± 0.004e | 0.127 ± 0.013d | 0.543 ± 0.059d | 0.031 ± 0.008c | 0.192 ± 0.014b | 3.898 ± 0.061e | |
| MW (100%) | 1 | 0.324 ± 0.027a | 0.201 ± 0.021b | 0.178 ± 0.015a | 0.016 ± 0.001b | 0.395 ± 0.032c | 0.009 ± 0.002ab | 0.006 ± 0.001ab | 0.014 ± 0.003ab | 0.246 ± 0.030b | 0.332 ± 0.034bc | 0.003 ± 0.001b | 0.014 ± 0.002cd | 0.081 ± 0.008c | 0.157 ± 0.018b | 0.007 ± 0.002ab | 0.102 ± 0.010c | 1.575 ± 0.145b |
| 2 | 0.606 ± 0.039b | 0.462 ± 0.026d | 0.366 ± 0.033b | 0.023 ± 0.003c | 0.852 ± 0.062d | 0.011 ± 0.003ab | 0.007 ± 0.001bc | 0.019 ± 0.002bc | 0.297 ± 0.022bc | 0.455 ± 0.052cd | 0.005 ± 0.001b | 0.016 ± 0.002cd | 0.035 ± 0.002b | 0.308 ± 0.020c | 0.019 ± 0.003bc | 0.069 ± 0.003d | 2.622 ± 0.167c | |
| 3 | 1.195 ± 0.059e | 0.610 ± 0.015f | 0.660 ± 0.025d | 0.030 ± 0.003d | 1.300 ± 0.040f | 0.021 ± 0.003cd | 0.012 ± 0.002d | 0.033 ± 0.005de | 0.374 ± 0.006d | 0.944 ± 0.051f | 0.010 ± 0.001c | 0.019 ± 0.001d | 0.071 ± 0.005c | 0.687 ± 0.067e | 0.049 ± 0.006d | 0.139 ± 0.010e | 4.706 ± 0.177f | |
Regarding the correlations between texture and CBC of total carotenoids, more aggressive treatments resulted in higher values of total carotenoids (coloured and uncoloured). In all cases, this correlation is significant, and even in treatments such as MW, WC and ST, R-squared values between 0.8 and 0.9 were obtained (ESI Table 2†), but in no case could this correlation be used to predict CBC of total carotenoids from texture. In any case, these correlations were only observed when categorised into treatments, not across the range of all carrot samples.
The major carotenoids in decreasing order of CBC from raw carrots were 15Z-PT (∼50% of total CBC), PF-2, LUT, PF-1, ACAR and BCAR (Table 3). It can be readily observed that colourless carotenoids account for most of the bioaccessibility from raw carrots and that the contribution of the major carotenoids in this matrix (ACAR and BCAR) is the lowest. Considering 15Z-PT, the increases in CBC relative to raw carrots were, in decreasing order, 4.52-fold (BK), 3.20-fold (WC), 3.19-fold (MW) and 3.02-fold (ST). PF-1 and PF-2 were by far the predominant PF isomers detected in the micelles. The effect of cooking in their CBCs was noticeable. Increases relative to raw carrots of up 15.84-fold (BK), 11.72-fold (ST), 8.97-fold (MW), and 3.93-fold (WC) were observed for PF-1. Increases in relation to raw carrots of up 6.82-fold (WC), 4.34-fold (BK), 4.24-fold (ST) and 3.88-fold (MW) were observed for PF-2. The positive effect of different processes in the CBC of colourless carotenoids in citrus juices has been demonstrated recently. Mapelli-Brahm et al.8 reported that PT and PF in ultra-frozen juice were more bioaccessible when thawed at room temperature or in the microwave oven, compared to fresh juice. Likewise, juices homogenized at 150 MPa (ref. 9) and pulp homogenized at 150 MPa and posteriorly reconstituted and pasteurized at 85 °C (ref. 35) showed significant increases in bioaccessible colourless carotenoid content compared to fresh juices. However, to the authors’ knowledge, there is no similar information related to colourless carotenoids in carrots and domestic cooking treatments.
The maximum increases in the CBC of ACAR were up to 37.94-fold (ST) and 26.22-fold (MW), and for BCAR they ranged from 71.40-fold (ST) to 45.8-fold (MW) relative to raw carrots. Thus, the very low bioaccessibility of these provitamin A carotenoids in raw carrots can be increased considerably due to the thermal treatments tested.36 The heat treatments resulted in isomerisation of both carotenoids, and some Z isomers (9Z-ACAR, 9Z-BCAR, 13Z-BCAR, 15Z-BCAR) were readily detected in the micellar fraction. With few exceptions, their CBCs increased with the duration and intensity of treatments. The CBC of the isomer identified as 13Z-BCAR were in some cases (intense steaming and baking) remarkable. Aherne et al.37 found that the micellar fractions from cooked carrots (boiled, pureed) generally contained significantly higher levels of 13Z-BCAR and 15Z-BCAR and higher but not significant contents of 9Z-BCAR. BCAR is known to aggregate in carrots to form crystals, which are thought to make their release during digestion more difficult.17,38
Concerning LUT, in general, the CBC increased with the intensity and duration of the treatments, like the other carotenoids. Increases in the CBC of LUT up to 6.79-fold (BK), 6.42-fold (ST), 4.98-fold (WC), and 4.16-fold (MW), relative to raw carrots, were observed. Similarly, to what was commented for phytoene and phytofluene, despite the levels of LUT being much lower than those of ACAR and BCAR in the matrices, its bioaccessibility was higher (ranging between ∼13 and ∼90%) and therefore the CBC of lutein was comparable to that of the provitamin A carotenoids. Two Z isomers of lutein (13Z and 15Z) were found in the CBC, whose levels also increased with the severity of the treatments.
3.5. Carotenoid bioaccessible content in equivalent cooking treatments
As discussed above, several studies have concluded that cooking treatments have a positive effect on the bioaccessibility of carotenoids. However, to facilitate a meaningful comparison across thermal treatments it is necessary to establish parameters that serve to somehow assess the effect of structural changes in the matrix. The evidence obtained in this work points out that each of the treatments assayed has a different effect on the structure of the matrix resulting in differences in hardness (Table 1). To find out equivalent experimental conditions that allow a reasonable comparison of treatments concerning bioaccessibility we tested different linear and non-linear models of the texture of the samples versus time for each treatment and temperature. Finally, the one with the highest coefficient of determination (R2) and the lowest value of the root mean square error (RMSE) was selected. The equation that best fitted the curves was of exponential type (eqn (2)).39 Equations obtained from each cooking process studied are included in ESI Table 1.†| hardness = ae−b·time | (3) |
Once the coefficients a and b that yielded the best fit were calculated, the different curves of hardness versus time were compared. As expected, each treatment and temperature affected the speed of texture change. According to the objectives of the study, it did not make sense to compare samples with the same treatment time but to compare samples with a similar texture. Therefore, an optimal texture level for consumption was chosen and, for each treatment and temperature, the processing time leading to equivalent levels of hardness was obtained.
From the texture equations obtained, it was determined that the treatment conditions leading to hardness levels that could be considered equivalent were:
Group A: ST (100 °C, 5 min)/WC (100 °C, 10 min)/BK (210 °C, 15 min)/MW (100%, 2 min).
Group B: ST (100 °C, 10 min)/WC (100 °C, 15 min)/MW (100%, 3 min).
Fig. 2 shows the bioaccessible content of the different carotenoids for the two groups of equivalent cooking treatments. Considering group A, the highest CBC for the provitamin A carotenoids, LUT, 15Z-PT and PF-1 were observed for baking at 210 °C. The highest CBC for PF-2 and PF-3 were obtained in carrots cooked in water at 100 °C. Regarding group B, the highest CBC for PT, PF-1 and provitamin A carotenoids were found in the microwave-cooked carrots. For PF-2 and PF-3, the treatment that provided the highest CBC was WC at 100 °C (Fig. 2) and LUT showed no significant differences among the three treatments.
The results obtained showed that in the equivalent treatment group A, the highest total carotenoid CBC was observed in the BK at 210 °C, while in the equivalent treatment group B, it was observed in the MW at 750 W (Fig. 2).
If the Q obtained for each sample (Table 1) in each of the equivalent treatments of both groups are compared, it can be observed that the conditions leading to higher CBC of total carotenoids correspond to the conditions with the highest Q. It could be argued that higher thermal energy inputs facilitate to a greater extent the breakdown of cell walls and plant tissue structures, resulting in a softer texture and allowing a more efficient release of carotenoids and other cell components.
3.6. Implications of equivalent cooking treatments about sustainability
It is important to bear in mind that hardness is related not only to the bioavailability of components but also to consumers’ preferences and energy expenditure during cooking. For this reason, the approximate energy expenditure of the different equivalent treatments was estimated. For group A, the energy consumptions were in increasing order: 0.025 kW h (MW 100%, 2 min); 0.133 kW h (ST 100 °C, 5 min); 0.267 kW h (WC 100 °C, 10 min) and 0.695 kW h (BK 210 °C, 15 min). That is, in this group the energy consumption of MW was 5.32-, 10.68- and 27.80-fold lower relative to ST, WC and BK, respectively. Dividing CBC for total carotenoids by the energy consumption, it was observed that the highest CBC values per kW h (104.88 CBC per kW h) corresponded to MW 100%, 2 min, and the lowest (7.37 CBC per kW h) corresponded to ST 100 °C, 5 min (ESI Fig. 1†). Considering provitamin A carotenoids the highest CBC values per kW h (33.60 CBC per kW h) corresponded to MW 100%, 2 min, and the lowest (1.52 CBC per kW h) corresponded to ST 100 °C, 5 min.For group 2, the consumption was: 0.038 kW h (MW 100%, 3 min); 0.267 kW h (ST 100 °C, 10 min) and 0.400 kW h (WC 100 °C, 15 min). That is, in this group the energy consumption of MW was 7.03- and 10.53-fold lower relative to ST, and WC, respectively. It was observed that the highest CBC values for total carotenoids per kW h (123.84 CBC per kW h) corresponded to MW 100%, 3 min and the lowest (7.51 CBC per kW h) corresponded to ST 100 °C, 10 min. Considering provitamin A carotenoids the highest CBC values per kW h (46.89 CBC per kW h) corresponded to MW 100%, 3 min, and the lowest (2.70 CBC per kW h) corresponded to ST 100 °C, 10 min.
The results serve to illustrate that the cooking of carrots affects sensory (colour, hardness) and nutritional (carotenoid bioavailability, vitamin A bioavailability) quality. On the one hand, the milder conditions (those consuming less energy) lead to lower loss of hardness and colour changes. On the other hand, more drastic conditions lead to important colour and hardness changes but significantly and markedly improved carotenoid/vitamin A bioaccessibility. The results of this study can contribute to fostering new studies aiming at fine-tuning cooking conditions to help make more informed decisions about cooking practices and effects on colour, softness, carotenoid bioavailability and energy consumption. In this sense, apart from optimizing food production practices and making changes in dietary habits as well as reducing food waste as highlighted by the EAT-Lancet Commission,40 optimizing cooking conditions appears as another important strategy to make better use of resources. This has led us to define the concept of “sustainable cooking” as “cooking practices that optimize intensity, duration and other parameters leading to a more efficient use of energy to maximize the bioavailability of nutrients and other beneficial food components (such as bioactive) while ensuring food appeal and safety”.
4. Conclusions
Colour and texture are two important parameters influencing consumer's preferences in purchasing and/or degree of cooking. All the treatments applied led to colour differences perceptible to the human eye. Overall, the greatest and lowest colour differences were observed for MW at 750 W and WC at 80 °C, respectively. The maximum tenderness was found for the sample steamed at 100 °C for 20 minutes. The cooking treatment with the lowest influence on carrot texture was WC at 80 °C. Thus, it can be inferred that WC under these conditions is the treatment that affects these two organoleptic properties the least.CBC helps estimate the amount of carotenoid that is potentially available from a food serving. It can be argued that CBC is the result of different phenomena, including inherent carotenoid solubility in micelles, softening due to the cooking practices, enhanced extractability relative to the raw sample or the different susceptibilities that carotenoids with different structures and differently organized in plastids have to the different conditions of the treatments. Cooking led to noticeably significant (p < 0.05) increases in CBC values considering total carotenoids, ranging from 6.03-fold (MW) to 8.91-fold (BK) for the most intense conditions tested. It can be concluded that despite the considerably higher contents of ACAR and BCAR in the carrot matrices, the colourless carotenoids have the highest CBC. These results highlight the importance of conducting bioaccessibility studies in addition to compositional studies to obtain more meaningful information in the context of health promotion through diet. The highest increases in CBC relative to raw carrots were observed for the provitamin A carotenoids. Increases in the CBC of ACAR and BCAR up to 37.9-fold and 71.4-fold (ST) were observed. Baking was the process leading to the highest potential bioavailability of total carotenoids, whereas steaming led to a slightly higher potential bioavailability of provitamin A carotenoids. Despite there being ample evidence that processing in the industry or in the kitchen can favour the bioavailability of carotenoids and other health-promoting compounds in different foods, consumers are largely unaware of it, so the industry could educate on this additional benefit of processing technologies. Indeed, information about processing and cooking practices that optimize energy and bioavailability of food components needs to be efficiently communicated to consumers as their progressive adoption can make a remarkable contribution to health promotion and sustainability. Considering samples with the same level of tenderness, the highest CBC values per kW h corresponded to microwave cooking, indicating that the heating produced at much shorter times compared to the other practices tested was the most efficient from an energetic point of view to increase CBC. This adds to the versatility and convenience of microwaves in the kitchen and can lead to more scientific research on the topic to contribute to the production of healthier and more sustainable foods. From a nutritional point of view, fruits and vegetables are considered important as sources of fibre, minerals, vitamins and bioactives. At least 5 servings per day are being recommended to promote health through the diet. Increasing the bioavailability of these compounds through cooking (and also through other treatments in the field, the industry, the markets or in households) might be important to obtain appropriate levels for health promotion with fewer servings, which may contribute to the saving of resources and therefore to sustainability. This appears as a research topic that deserves further attention and multidisciplinary approaches.
Author contributions
Benítez-González, A. M.: formal analysis, investigation, writing – original draft; Stinco, C. M: formal analysis, investigation, writing – original draft; Rodríguez-Pulido, F. J.: formal analysis, writing – original draft; Vicario, I. M.: writing – review & editing; Meléndez-Martínez, A. J.: conceptualization. Investigation, writing – review & editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by project P18-RT-1847-BIOLESS funded by the European Union and Junta de Andalucía through European Regional Development Fund ERDF (Fondo Europeo de Desarrollo Regional FEDER). Authors thank the quality assistance from the technical staff of the Service of Biology (SGI, Universidad de Sevilla). CMS, AMBG and AJMM are members of the Spanish Carotenoid Network (CaRed), grant RED2022-134577-T, funded by MCIN/AEI/10.13039/501100011033. The authors would like to thank Professor Alberto Romero García Departament of Chemical Engineering, Universidad de Sevilla for his help in clarifying and estimating the concept of thermal impact.References
- M. Eggersdorfer and A. Wyss, Carotenoids in Human Nutrition and Health, Arch. Biochem. Biophys., 2018, 652, 18–26 CrossRef.
- A. J. Meléndez-Martínez, C. M. Stinco and P. Mapelli-Brahm, Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene, Nutrients, 2019, 11(5), 1093 CrossRef.
- F. J. Barba, L. R. B. Mariutti, N. Bragagnolo, A. Z. Mercadante, G. V. Barbosa-Cánovas and V. Orlien, Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing, Trends Food Sci. Technol., 2017, 67, 195–206 CrossRef.
- A. J. Meléndez-Martínez, A. Pérez-Gálvez, M. Roca, R. Estévez-Santiago, B. Olmedilla-Alonso, A. Z. Mercadante and J. de J. Ornelas-Paz, Biodisponibilidad de Carotenoides, Factores Que La Determinan y Métodos de Estimación, in Carotenoides En Agroalimentación Y Salud, ed. A. J. Meléndez-Martínez, Editorial Terracota, Ciudad de México, México, 2017, pp. 574–608 Search PubMed.
- B. V. Neves, L. M. D. S. Mesquita, D. C. Murador, A. I. do P. Cheberle, A. R. C. Braga, A. Z. Mercadante and V. V. de Rosso, Improvement of Bioactive Compound Levels, Antioxidant Activity, and Bioaccessibility of Carotenoids from Pereskia Aculeata after Different Cooking Techniques, ACS Food Sci. Technol., 2021, 1, 1285–1293 CrossRef.
- M. Palermo, N. Pellegrini and V. Fogliano, The Effect of Cooking on the Phytochemical Content of Vegetables, J. Sci. Food Agric., 2014, 94, 1057–1070 CrossRef CAS PubMed.
- P. Mapelli-Brahm, C. Desmarchelier, M. Margier, E. Reboul, A. J. Meléndez-Martínez and P. Borel, Phytoene and Phytofluene Isolated from a Tomato Extract Are Readily Incorporated in Mixed Micelles and Absorbed by Caco-2 Cells, as Compared to Lycopene, and SR-BI Is Involved in Their Cellular Uptake, Mol. Nutr. Food Res., 2018, 62, 1–9 CrossRef PubMed.
- P. Mapelli-Brahm, C. M. Stinco, M. J. Rodrigo, L. Zacarías and A. J. Meléndez-Martínez, Impact of Thermal Treatments on the Bioaccessibility of Phytoene and Phytofluene in Relation to Changes in the Microstructure and Size of Orange Juice Particles, J. Funct. Foods, 2018, 46, 38–47 CrossRef CAS.
- E. Sentandreu, C. M. Stinco, I. M. Vicario, P. Mapelli-Brahm, J. L. Navarro and A. J. Meléndez-Martínez, High-Pressure Homogenization as Compared to Pasteurization as a Sustainable Approach to Obtain Mandarin Juices with Improved Bioaccessibility of Carotenoids and Flavonoids, J. Clean. Prod., 2020, 262, 121325 CrossRef CAS.
- M. Aamir, M. Ovissipour, S. S. Sablani and B. Rasco, Predicting the Quality of Pasteurized Vegetables Using Kinetic Models: A Review, Int. J. Food Sci., 2013, 2013, 1–29 CrossRef.
- D. Rodríguez-Amaya and M. Kimura, HarvestPlus Handbook for Carotenoid Analysis, in HarvestPlus Technical Monographs, 2004, pp. 1–63 Search PubMed.
- M. Sharma, E. Kristo, M. Corredig and L. Duizer, Effect of Hydrocolloid Type on Texture of Pureed Carrots: Rheological and Sensory Measures, Food Hydrocolloids, 2017, 63, 478–487 CrossRef CAS.
- W. Canet, M. D. Alvarez, C. Fernández and M. E. Tortosa, The Effect of Sample Temperature on Instrumental and Sensory Properties of Mashed Potato Products, Int. J. Food Sci. Technol., 2005, 40, 481–493 CrossRef.
- G. Giuliano, Provitamin A Biofortification of Crop Plants: A Gold Rush with Many Miners, Curr. Opin. Biotechnol., 2017, 44, 169–180 CrossRef PubMed.
- M. J. Cejudo-Bastante, F. J. F. J. Rodríguez-Pulido, F. J. Heredia and M. L. González-Miret, Assessment of Sensory and Texture Profiles of Grape Seeds at Real Maturity Stages Using Image Analysis, Foods, 2021, 10(5), 1098 CrossRef PubMed.
- L. Lemmens, I. J. P. Colle, S. Van Buggenhout, A. M. Van Loey and M. E. Hendrickx, Quantifying the Influence of Thermal Process Parameters on in Vitro β-Carotene Bioaccessibility: A Case Study on Carrots, J. Agric. Food Chem., 2011, 59, 3162–3167 CrossRef.
- P. Palmero, L. Lemmens, A. Ribas-Agustí, C. Sosa, K. Met, J. de Dieu Umutoni, M. Hendrickx and A. Van Loey, Novel Targeted Approach to Better Understand How Natural Structural Barriers Govern Carotenoid in Vitro Bioaccessibility in Vegetable-Based Systems, Food Chem., 2013, 141, 2036–2043 CrossRef CAS.
- P. Mapelli-Brahm, J. Corte-Real, A. J. Meléndez-Martínez and T. Bohn, Bioaccessibility of Phytoene and Phytofluene Is Superior to Other Carotenoids from Selected Fruit and Vegetable Juices, Food Chem., 2017, 229, 304–311 CrossRef CAS.
- CIE, Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms, CIE Central Bureau, Publ. No. 15 (E-1.3.1), Viena, Austria, 1978 Search PubMed.
- D. Ghorbel, N. Ben Bettaïeb, F. Ghrib, M. Ben Slema and H. Attia, Textural Properties of Commercial Processed Cheese Spreads: Instrumental and Sensory Evaluations, Int. J. Food Prop., 2016, 19, 1513–1521 CrossRef.
- S. Montes-Lora, F. J. Rodríguez-Pulido, M. J. Cejudo-Bastante and F. J. Heredia, Implications of the Red Beet Ripening on the Colour and Betalain Composition Relationships, Plant Foods Hum. Nutr., 2018, 73, 216–221 CrossRef PubMed.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion, Nat. Protoc., 2019, 14, 991–1014 CrossRef.
- D. B. Rodrigues, C. Chitchumroonchokchai, L. R. B. Mariutti, A. Z. Mercadante and M. L. Failla, Comparison of Two Static in Vitro Digestion Methods for Screening the Bioaccessibility of Carotenoids in Fruits, Vegetables, and Animal Products, J. Agric. Food Chem., 2017, 65, 11220–11228 CrossRef.
- C. M. Stinco, A. M. Benítez-González, A. J. Meléndez-Martínez, D. Hernanz and I. M. Vicario, Simultaneous Determination of Dietary Isoprenoids (Carotenoids, Chlorophylls and Tocopherols) in Human Faeces by Rapid Resolution Liquid Chromatography, J. Chromatogr. A, 2019, 1583, 63–72 CrossRef.
- A. Meléndez-Martínez, C. M. Stinco, C. Liu and X.-D. Wang, A Simple HPLC Method for the Comprehensive Analysis of Cis/Trans (Z/E) Geometrical Isomers of Carotenoids for Nutritional Studies, Food Chem., 2013, 138, 1341–1350 CrossRef.
- P. Gupta, Y. Sreelakshmi and R. Sharma, A Rapid and Sensitive Method for Determination of Carotenoids in Plant Tissues by High Performance Liquid Chromatography, Plant Methods, 2015, 11, 5 CrossRef CAS PubMed.
- J. Corte-Real, M. Bertucci, C. Soukoulis, C. Desmarchelier, P. Borel, E. Richling, L. Hoffmann and T. Bohn, Negative Effects of Divalent Mineral Cations on the Bioaccessibility of Carotenoids from Plant Food Matrices and Related Physical Properties of Gastro-Intestinal Fluids, Food Funct., 2017, 8, 1008–1019 RSC.
- V. Bohm, N. L. Puspitasari-Nienaber, M. G. Ferruzzi, S. J. Schwartz, V. Böhm, N. L. Puspitasari-Nienaber, M. G. Ferruzzi and S. J. Schwartz, Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of Alpha-Carotene, β-Carotene, Lycopene, and Zeaxanthin, J. Agric. Food Chem., 2002, 50, 221–226 CrossRef.
- C. Miglio, E. Chiavaro, A. Visconti, V. Fogliano and N. Pellegrini, Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables, J. Agric. Food Chem., 2008, 56, 139–147 CrossRef CAS.
- A. J. Meléndez-Martínez, M. Paulino, C. M. Stinco, P. Mapelli-Brahm and X.-D. Wang, Study of the Time-Course of Cis/Trans (Z/E) Isomerization of Lycopene, Phytoene, and Phytofluene from Tomato, J. Agric. Food Chem., 2014, 62, 12399–12406 CrossRef.
- L. Lemmens, K. De Vleeschouwer, K. R. N. Moelants, I. J. P. Colle, A. M. Van Loey and M. E. Hendrickx, B-Carotene Isomerization Kinetics during Thermal Treatments of Carrot Puree, J. Agric. Food Chem., 2010, 58, 6816–6824 CrossRef CAS PubMed.
- S. Bureau, S. Mouhoubi, L. Touloumet, C. Garcia, F. Moreau, V. Bédouet and C. M. G. C. Renard, Are Folates, Carotenoids and Vitamin C Affected by Cooking? Four Domestic Procedures Are Compared on a Large Diversity of Frozen Vegetables, LWT – Food Sci. Technol., 2015, 64, 735–741 CrossRef CAS.
- L. Vervoort, I. Van Der Plancken, T. Grauwet, P. Verlinde, A. Matser, M. Hendrickx and A. Van Loey, Thermal versus High Pressure Processing of Carrots: A Comparative Pilot-Scale Study on Equivalent Basis, Innovative Food Sci. Emerging Technol., 2012, 15, 1–13 CrossRef.
- R. Fernández-Vázquez, C. M. Stinco, D. Hernanz, F. J. Heredia and I. M. Vicario, Colour Training and Colour Differences Thresholds in Orange Juice, Food Qual. Prefer., 2013, 30, 320–327 CrossRef.
- C. M. Stinco, E. Sentandreu, P. Mapelli-Brahm, J. L. Navarro, I. M. Vicario and A. J. Meléndez-Martínez, Influence of High Pressure Homogenization and Pasteurization on the in Vitro Bioaccessibility of Carotenoids and Flavonoids in Orange Juice, Food Chem., 2020, 331, 127259 CrossRef PubMed.
- A. J. Meléndez-Martínez, An Overview of Carotenoids, Apocarotenoids and Vitamin A in Agro–Food, Nutrition, Health and Disease, Mol. Nutr. Food Res., 2019, 63, 1801045 CrossRef.
- S. A. Aherne, T. Daly, M. A. Jiwan, L. O'Sullivan and N. M. O'Brien, Bioavailability of β-Carotene Isomers from Raw and Cooked Carrots Using an in Vitro Digestion Model Coupled with a Human Intestinal Caco-2 Cell Model, Food Res. Int., 2010, 43, 1449–1454 CrossRef CAS.
- R. M. Schweiggert, D. Mezger, F. Schimpf, C. B. Steingass and R. Carle, Influence of Chromoplast Morphology on Carotenoid Bioaccessibility of Carrot, Mango, Papaya, and Tomato, Food Chem., 2012, 135, 2736–2742 CrossRef CAS PubMed.
- A. C. Miano, V. D. Sabadoti and P. E. D. Augusto, Enhancing the Hydration Process of Common Beans by Ultrasound and High Temperatures: Impact on Cooking and Thermodynamic Properties, J. Food Eng., 2018, 225, 53–61 CrossRef CAS.
- W. Willett, J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, D. Tilman, F. DeClerck, A. Wood, M. Jonell, M. Clark, L. J. Gordon, J. Fanzo, C. Hawkes, R. Zurayk, J. A. Rivera, W. De Vries, L. Majele Sibanda, A. Afshin, A. Chaudhary, M. Herrero, R. Agustina, F. Branca, A. Lartey, S. Fan, B. Crona, E. Fox, V. Bignet, M. Troell, T. Lindahl, S. Singh, S. E. Cornell, K. Srinath Reddy, S. Narain, S. Nishtar and C. J. L. Murray, Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems, Lancet, 2019, 393, 447–492 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02752c |
| This journal is © The Royal Society of Chemistry 2024 |


