Open Access Article
Open Access ArticleAn examination of the impact of unmelted, melted, and deconstructed cheese on lipid metabolism: a 6-week randomised trial†
Aileen
O'Connor‡
ab,
Martina
Rooney‡
 ab,
Simone
Dunne
ab,
Nupur
Bhargava
ab,
Caroline
Matthews
ab,
Shuhua
Yang
ab,
Sitong
Zhou
a,
Adam
Cogan
c,
Jeremiah J.
Sheehan
ab,
Simone
Dunne
ab,
Nupur
Bhargava
ab,
Caroline
Matthews
ab,
Shuhua
Yang
ab,
Sitong
Zhou
a,
Adam
Cogan
c,
Jeremiah J.
Sheehan
 c,
André
Brodkorb
c,
Nessa
Noronha
b,
Michael
O'Sullivan
a,
Dolores
O'Riordan
ab,
Emma L.
Feeney
ab and
Eileen R.
Gibney
*ab
c,
André
Brodkorb
c,
Nessa
Noronha
b,
Michael
O'Sullivan
a,
Dolores
O'Riordan
ab,
Emma L.
Feeney
ab and
Eileen R.
Gibney
*ab
aInstitute of Food and Health, School of Agriculture and Food Science, Institute of Food and Health, University College Dublin, Dublin, Ireland. E-mail: eileen.gibney@ucd.ie
bFood for Health Ireland (FHI), University College Dublin, Dublin, Ireland
cTeagasc Food Research Centre, Moorepark, Fermoy P61 C996, Ireland
First published on 15th July 2024
Abstract
Background: Evidence suggests cheese has a favourable or neutral effect on cardiometabolic health, compared to butter. To date, studies have only considered the cheese matrix in its unmelted form, while the effect of melted cheese remains unknown. Objective: To test the effect of 6-week daily consumption of ∼40 g dairy fat, eaten in either as unmelted cheese, melted cheese, or in a fully deconstructed form, on markers of metabolic health in overweight adults aged ≥50 years of age. Design: A 6-week randomised parallel intervention, where 162 participants (43.3% male) received ∼40 g of dairy fat per day, in 1 of 3 treatments: (A) 120 g full-fat Irish grass-fed cheddar cheese, eaten in unmelted form (n 58); (B) 120 g full-fat Irish grass-fed cheddar cheese eaten in melted form (n 53); or (C) the equivalent components; butter (49 g), calcium caseinate powder (30 g), and Ca supplement (CaCO3; 500 mg) (n 51). Results: There was no difference in weight, fasting glucose, or insulin between the groups post-intervention. Melted cheese, compared to unmelted cheese, increased total cholesterol (0.23 ± 0.79 mmol L−1vs. 0.02 ± 0.67 mmol L−1, P = 0.008) and triglyceride concentrations (0.17 ± 0.39 mmol L−1vs. 0.00 ± 0.42 mmol L−1, P = 0.016). Melted cheese increased total cholesterol concentrations by 0.20 ± 0.15 mmol L−1 and triglyceride concentrations by 0.17 ± 0.08 mmol L−1 compared to unmelted cheese. No significant differences were observed between the cheese forms for change in HDL, LDL or VLDL cholesterol. Conclusion: Compared to unmelted cheese, melted cheese was found to increase total cholesterol and triglyceride concentrations in middle-aged, overweight adults with no effect on weight or glycaemic control.
Introduction
Elevated intakes of saturated fatty acids (SFA) can increase low-density lipoprotein cholesterol (LDL) concentrations, considered to be one of the primary risk factors for cardiovascular disease (CVD).1,2 Current healthy eating guidelines do not take into account the evidence that not all SFAs are equivalent and that the health effects of SFA varies considerably depending on the specific type of fatty acid as well as the food source.3,4 For example, within dairy foods, ∼60% of the fats are in the form of SFA, contributing to approximately one fifth of total SFA intakes in the Irish population, thus dairy foods are one of the main contributors to overall SFA intakes.5 Contrary to expectation, evidence from several meta-analyses have demonstrated, that SFA intakes from some dairy sources have been associated with neutral or beneficial health effects.6–10 This is in contrast with other SFA sources such as meat and meat products, where SFA intakes from such foods have been associated with negative health effects, including lipid profiles.6 Furthermore, Feeney et al.,11 highlights how many studies have used the blanket term “dairy”, despite the fact that individual dairy products contain different components and structures, as well as differing amounts of nutrients, including SFA. Some studies that have distinguished between different dairy products have found favourable impacts on blood lipid profiles associated with consumption of some dairy products, particularly cheese. In a number of recent randomised control trials, (RCT) a neutral, or beneficial effect of cheese consumption on markers of metabolic health and CVD risk have been demonstrated.11–14 Previous research conducted by Feeney et al.,11 has both supported and added to this existing evidence. A significant reduction in total and LDL cholesterol of subjects, post-intervention, was observed when SFA was contained within a cheese matrix, in comparison to butter and reduced-fat cheese matrices.11 Of note, previous studies have specified not to heat the cheese prior to consumption and that the effect of heating explicitly has not been tested in a human feeding study to date.11–15While the components and nutrients within the cheese matrix determine the structure of the cheese, there are additional factors that can influence the structural organisation of the cheese.4,16,17 This includes the composition and pre-treatment of cheese milk, manufacturing conditions, maturation, and preparation prior to consumption i.e., cooking, baking, and grilling. Cheese is generally consumed in either an unmelted or heated/melted form. Pizza toppings, lasagne and grilled cheese sandwiches are some examples of how cheese is consumed when melted. These cooking and preparation methods result in various physicochemical changes to the structural components.16,17 Thus, the structural properties of the cheese matrix change significantly during these heating processes and it is thought that heat-induced modification of the cheese structure may impact its digestion behaviour.4,17,17 Using a human gastric simulator, Ye et al.,18 investigated the rate of release of fat globules from heated and unheated whole milk, and found the fat globules in unheated milk were entrapped in the protein matrix, which slowed the release of lipids compared to heated milk, where the lipid globules were distributed more evenly within the protein matrix.18
To date, human intervention studies have used cheese in its unmelted state, although some in vitro studies support the idea that cheese structure can affect lipid digestion.19,20 Increasing the calcium content increases the hardness of cheddar cheese, and in vitro work has demonstrated greater resistance to digestion and slower lipolysis rates in such harder cheeses,19,20 although the mechanism interlinking calcium, the cheese matrix and lipolysis is yet to be elucidated.19–22 Thus, it remains unknown if heat-induced changes, particularly those occasioned by cooking processes such as melting, effects the overall matrix of the cheese in relation to its potential impact on metabolic health and CVD risk in humans.17 In addition, it is important to examine the matrix of the cheese when consumed in a heated or melted state as these processes reflect the cheese consumption habits of a large proportion of the public. Therefore, this study aims to expand and deepen the knowledge of cheese consumption on metabolic health and CVD risk by examining the impact of the state of the cheese matrix (unmelted, melted or deconstructed state) on markers of metabolic health.
Materials and methods
Participants
A total of 252 participants were recruited from Dublin, Ireland, and the surrounding areas between January 2020 and December 2022. Inclusion criteria included participants aged ≥50 years, with a body mass index (BMI) ≥25 kg m−2, no chronic co-morbidities, free from dairy intolerance/allergy and consumed an omnivorous diet. An overweight population was chosen as this is similar to other studies in the area and this is a group that is often advised to avoid consuming cheese owing to the SFA content. Exclusion criteria were being prescribed medications for cholesterol or blood pressure reduction purposes, prescribed or therapeutic diets, or actively trying to lose weight. All participants gave written consent before enrolling in the study. As the study took place during the COVID-19 pandemic, participants recruited during that period completed a COVID-19 screening questionnaire prior to study visits, to ensure the health and safety of both participants and staff. All study procedures were approved by the University College Dublin Human Research Ethics Committee (LS-19-78-Gibney).Study design
The overall study was a parallel-arm design with four diet intervention groups. Participants (n 252) were assigned to one of the four groups: (A) 120 g per day of pasture-fed, full-fat, unmelted cheddar cheese; (B) 120 g per day of pasture-fed, full-fat, melted cheddar cheese; (C) 49 g butter, 30 g calcium caseinate powder, and a 500 mg calcium supplement (CaCO3) per day; and (D) 120 g per day of total mixed ration (TMR), full-fat, unmelted cheddar cheese. Tirlán provided the grass-fed cheese, Kerry Group supplied the butter and Teagasc Moorepark supplied the TMR-fed cheese for the study. Calcium caseinate powder was purchased from Bacarel and Company Ltd. Calcium supplements were purchased from Holland and Barrett. The focus of the current piece of work is to compare groups A, B and C; a separate analysis of group A and group D will be presented elsewhere to compare the effect of grass-fed and TMR-fed cheese on metabolic health. Each diet contained ∼40 g of dairy fat in different matrices over a 6-week period (Table 1). All cheeses were consumed at a maturity of 8–12 months. All intervention diets were matched for energy, fat, casein, and calcium content. A total of 162 participants completed the intervention in groups A, B and C (n 70/43.2% male). A further 33 (n 12/36.4% male) completed group D; comparisons between group A and D will be presented elsewhere as outlined in the trial registry. Groups were allocated to treatment groups using an online randomisation tool (https://www.sealedenvelope.com). The distribution of participants between groups and reasons for dropping out are displayed in Fig. 1. Due to the dissimilar nature of the study foods involved in the three arms here, the participants or the researchers could not be blinded during the study, however, blood biomarker analyses were conducted blind to the intervention. This trial was registered at ISRCTN as ISRCTN11913510.| Group | Intervention | Energy, kcal | Protein, g | Fat, g | Calcium, mg |
|---|---|---|---|---|---|
| A | 120 g Irish Cheddar cheese (unmelted) | 468 | 31.2 | 38.4 | 828 |
| B | 120 g Irish Cheddar cheese (melted) | 468 | 31.2 | 38.4 | 828 |
| C | 49 g butter + 30 g calcium caseinate + CaCO3 supplement (deconstructed) | 476.2 | 26.7 | 39.2 | 817 |
Intervention diets
All intervention diets were matched as closely as possible for total energy, macronutrients, and calcium. During the intervention, participants were asked to limit all other dairy to approximately 50 mL, but no other dietary restrictions were required. Those assigned to Group A were requested to only consume the cheese in an unmelted state over the 6-week intervention, whereas participants assigned to Group B were instructed to consume the cheese in a melted state, after heating in 60 g portions for 30 seconds in an 800 W microwave, or under the grill. In-house testing demonstrated average temperatures in excess of 79 °C for microwave melted cheese and 75 °C for oven grilled cheese. All participants kept a daily compliance log throughout the 6-week period where they recorded exactly how much of the study food they consumed each day.Measurements
A number of anthropometric measurements were collected at baseline (visit 1) and post-intervention (visit 2). Researchers conducting anthropometric measurements were fully trained and followed standard operating procedures in order to reduce inter-operator differences. Body weight, BMI, and body fat percentage was measured on a Tanita scale, (Model BC-420MA), height was measured with a freestanding SECA stadiometer, and waist and hip were measured with a SECA non-stretch measuring tape. Waist measurement was taken at a level point, midway between the lower rib margin and iliac crest, with the tape held taught but not tight, while the participant was standing and looking straight ahead. Blood pressure was measured in the upper left arm using an Omron digital monitor (Model M6 HEM-7360-E), while the participant was seated, at rest with both feet firmly on the floor and the left arm resting on a support so the antecubital fossa is at the level of the heart. Blood pressure was measured in triplicate, with 5 minutes between readings. An average of the three readings was used in the analysis. Fasting blood samples were collected at baseline and post-intervention. Lipid and glucose concentrations were measured by nuclear magnetic resonance (NMR) spectroscopy (LabCorp, Morrisville, NC) using the LipoProfile-4 algorithm. LDL cholesterol was calculated using the Friedewald equation.23 Fasting insulin was analysed in serum samples at University College Dublin using Mercodia Insulin ELISA kits (Uppsala, Sweden). Dietary intakes were also assessed at both time-points via the validated EPIC-Norfolk Food Frequency Questionnaire (FFQ).Statistical analysis
Statistical analysis was performed with SPSS version 29.0.1.0 for Mac (SPSS Inc., Chicago, IL). Descriptive analysis was performed on demographic, anthropometric, dietary, and clinical chemistry data for the total population and separated by intervention group. Data were examined for distribution and nonparametric tests were used, where appropriate. Delta (Δ) values were calculated by subtracting the pre-intervention from the post-intervention values. For baseline values, differences in values were tested via 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA, where appropriate. For Δ values, differences across groups were assessed using general linear models, controlling for baseline values, age, sex, Δ weight, Δ percentage total energy (%TE) from protein and Δ %TE from SFA. Post hoc analysis was conducted using Fisher's least significant difference (LSD) test.The primary study outcome was a comparison in change in blood lipid concentrations from baseline in response to 6-week intervention between (A) unmelted, (B) melted, or (C) deconstructed Irish cheddar cheese. Power calculations were performed, using mean and standard deviation of LDL cholesterol concentrations from our previous study examining the cheese matrix.11 The study was powered to observe a ≥15% difference in LDL cholesterol between groups A and C, assuming α error of probability of 0.05 and power (1-β err prob) of 0.80, with n 45 required per group. Assuming a drop-out rate of 25%, a target of 60 participants per group was set to ensure a minimum of n 45 completing each intervention.
Analysis was performed on both the population that completed the intervention (n 162, Completer approach), and on those who consumed ≥80% of the test foods (n 141, compliant approach). Analysis of those who were compliant is presented here, in line with previous published work11 and with guidelines on the reporting for nutrition intervention studies.24
Results
Compliance
On average, the cohort was 91.9% compliant with the intervention diets. Total compliance (100%) was achieved by 28% of the cohort; n 17 (30%) in those who consumed unmelted cheese, n 14 (26%) in those who consumed melted cheese and n 8 (16%) in the deconstructed cheese group. A total of n 141 were 80% compliant, n 54 (95%) in the unmelted cheese group, n 45 (85%) in the melted cheese group and n 42 (82%) in the deconstructed cheese group.Anthropometry
Participants were aged 58.5 years ± 5.8 (S.D.) on average. There was a slightly lower, but non-significant, percentage of males in group C (37.5%) compared to group A (44.4%) and group B (53.3%). No differences in anthropometry were observed between groups at baseline. After the intervention there was a mean decrease of 2.5 cm ± 3.5 in change (Δ) in waist circumference for group B, which was significantly different to both group A (P = 0.014) and group C (P = 0.033, Table 2). Considering no other differences in anthropometry were observed post intervention, differences may be a result of variance in the measurement.| Unmelted cheese (n 54) | Melted cheese (n 45) | Deconstructed cheese (n 42) | PV1a | PΔb | |
|---|---|---|---|---|---|
| Data presented as mean ± standard deviation, unless otherwise indicated.a PV1, differences across groups for visit 1 calculated with 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA where appropriate.b PΔ, differences across groups for delta values (visit 2–visit 1) calculated with 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA where appropriate.c Chi-square test.d n 139 for body fat percentage.e n 92 for waist circumference.f Significant differences for Δ waist circumference (cm) between groups A and B (P = 0.014) and groups B and C (P = 0.033). | |||||
| Age (years) | 58.0 ± 5.6 | 59.1 ± 6.2 | 58.3 ± 5.6 | 0.622 | — |
| Gender n (%) | |||||
| Male | 24 (44.4) | 24 (53.3) | 15 (35.7) | 0.255c | — |
| Female | 30 (55.6) | 21 (46.7) | 27 (64.3) | ||
| Weight (kg) | 0.200 | 0.650 | |||
| Visit 1 | 82.0 ± 14.0 | 84.3 ± 15.3 | 78.8 ± 13.2 | ||
| Visit 2 | 81.7 ± 14.2 | 83.8 ± 14.7 | 78.7 ± 13.2 | ||
| BMI (kg m −2 ) | 0.575 | 0.664 | |||
| Visit 1 | 28.6 ± 3.5 | 28.6 ± 4.6 | 27.8 ± 4.0 | ||
| Visit 2 | 28.5 ± 3.7 | 28.4 ± 4.5 | 27.7 ± 4.1 | ||
| Body fat (%) | 0.898 | 0.541 | |||
| Visit 1 | 34.2 ± 7.3 | 33.5 ± 9.8 | 33.6 ± 8.1 | ||
| Visit 2 | 33.7 ± 7.1 | 33.2 ± 9.7 | 33.3 ± 7.9 | ||
| Waist circumference (cm) | 0.247 | 0.032f | |||
| Visit 1 | 94.1 ± 12.6 | 97.9 ± 10.6 | 92.1 ± 13.3 | ||
| Visit 2 | 94.1 ± 11.8 | 95.1 ± 11.0 | 91.7 ± 12.8 | ||
Biochemistry
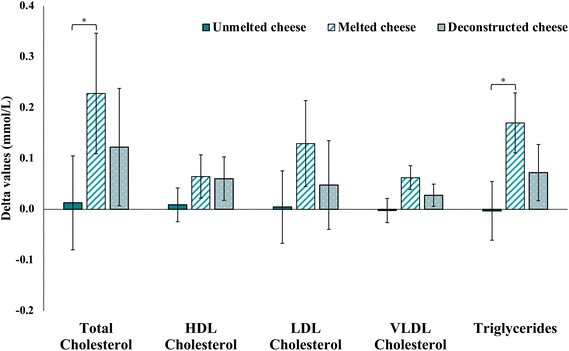
In those who were ≥80% compliant, there were no differences in blood lipid parameters between the groups at baseline (total, HDL, LDL or VLDL cholesterol, or triglyceride concentrations; Table 3). Post-intervention, there were significant differences in Δ total cholesterol (P = 0.027), and triglyceride (P = 0.049) concentrations. Post hoc analyses indicated significant differences in absolute change from pre-post intervention between group A and group B for total cholesterol (0.02 ± 0.67 vs. 0.23 ± 0.79 mmol L−1; P = 0.008), and triglycerides (0.00 ± 0.42 vs. 0.17 ± 0.39 mmol L−1; P = 0.016) respectively. No difference was observed between the groups for HDL, LDL or VLDL cholesterol (Fig. 2).| Unmelted cheese (n 54) | Melted cheese (n 45) | Deconstructed cheese (n 42) | PVa | PΔb | |
|---|---|---|---|---|---|
| Data presented as mean ± standard deviation. Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.a PV1, differences across groups for visit 1 calculated with 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA where appropriate.b PΔ, differences across groups for delta values (visit 2–visit 1) calculated with general linear models controlling for baseline values, sex, age, weight change, change in SFA intake as a % of TE and change in protein as a % of TE. Post hoc analysis was conducted via Fisher's least significant difference (LSD) test.c Significant differences for Δ total cholesterol (mmol L−1) between groups A and B (P = 0.008).d Significant differences for Δ triglycerides (mmol L−1) between groups A and B (P = 0.016). | |||||
| Total cholesterol (mmol L −1 ) | 0.613 | 0.022c | |||
| Visit 1 | 5.70 ± 0.85 | 5.83 ± 0.89 | 5.65 ± 0.94 | ||
| Visit 2 | 5.72 ± 0.91 | 6.07 ± 0.95 | 5.78 ± 0.94 | ||
| HDL cholesterol (nmol L −1 ) | 0.583 | 0.368 | |||
| Visit 1 | 1.63 ± 0.42 | 1.68 ± 0.44 | 1.72 ± 0.47 | ||
| Visit 2 | 1.64 ± 0.46 | 1.74 ± 0.53 | 1.78 ± 0.52 | ||
| LDL cholesterol (mmol L −1 ) | 0.616 | 0.155 | |||
| Visit 1 | 3.50 ± 0.77 | 3.61 ± 0.73 | 3.42 ± 0.76 | ||
| Visit 2 | 3.51 ± 0.80 | 3.72 ± 0.71 | 3.45 ± 0.79 | ||
| VLDL cholesterol (mmol L −1 ) | 0.375 | 0.065 | |||
| Visit 1 | 0.51 ± 0.27 | 0.50 ± 0.40 | 0.46 ± 0.19 | ||
| Visit 2 | 0.51 ± 0.22 | 0.55 ± 0.34 | 0.48 ± 0.20 | ||
| Triglycerides (mmol L −1 ) | 0.674 | 0.049d | |||
| Visit 1 | 1.25 ± 0.62 | 1.20 ± 0.91 | 1.12 ± 0.47 | ||
| Visit 2 | 1.25 ± 0.52 | 1.36 ± 0.75 | 1.20 ± 0.49 | ||
| Glucose (mmol L −1 ) | 0.163 | 0.382 | |||
| Visit 1 | 5.60 ± 0.54 | 5.68 ± 0.79 | 5.41 ± 0.63 | ||
| Visit 2 | 5.47 ± 0.68 | 5.61 ± 0.79 | 5.52 ± 0.77 | ||
| Insulin (mU L −1 ) | 0.528 | 0.513 | |||
| Visit 1 | 7.95 ± 4.48 | 7.86 ± 4.81 | 6.50 ± 2.85 | ||
| Visit 2 | 7.82 ± 4.41 | 7.25 ± 4.70 | 6.75 ± 3.73 | ||
| SBP (mmHg) | 0.923 | 0.415 | |||
| Visit 1 | 133.3 ± 17.9 | 133.1 ± 17.0 | 133.9 ± 16.8 | ||
| Visit 2 | 130.0 ± 18.6 | 130.1 ± 15.3 | 128.0 ± 14.7 | ||
| DBP (mmHg) | 0.618 | 0.412 | |||
| Visit 1 | 87.1 ± 9.6 | 87.5 ± 9.2 | 85.6 ± 9.5 | ||
| Visit 2 | 85.9 ± 10.5 | 85.9 ± 8.6 | 83.4 ± 9.8 | ||
There were no significant differences in changes in the remaining metabolic markers between the groups following the intervention (glucose, insulin, and blood pressure). The results in the ‘Completer’ analyses followed similar, albeit non-significant trends (ESI Tables 1 and 2†).
Diet
There were no differences between the intervention groups at baseline for total energy, or for %TE from protein, fat, SFA and carbohydrate (Table 4). Post-intervention, protein intake was significantly lower in group C (14.5 ± 3.3%TE) compared to both group A (19.2 ± 3.6%TE; P < 0.001) and group B (19.2 ± 4.4%TE; P < 0.001). As the diets were matched for macronutrients (Table 1), differences in background diets may have impacted lipid response results. Post intervention, %TE from SFA was significantly higher in group C (20.5 ± 5.5%TE) compared to group A (17.1 ± 5.6%TE; P = 0.035) and group B (16.5 ± 4.8%TE; P = 0.010). There were no other significant differences in diet throughout the intervention period between the groups for total energy, and %TE from fat or carbohydrate.| Unmelted cheese (n 51) | Melted cheese (n 45) | Deconstructed cheese (n 41) | PVa | PΔb | |
|---|---|---|---|---|---|
| Data presented as mean ± standard deviation. Abbreviations: CHO, carbohydrate; kcal, kilocalories; SFA, saturated fatty acid.a PV1, differences across groups for visit 1 calculated with 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA where appropriate.b PΔ, differences across groups for delta values (visit 2–visit 1) calculated with 1-factor ANOVA or Kruskal–Wallis nonparametric ANOVA where appropriate.c Significant differences in Δ protein intake as a percentage of total energy intake between groups A and C (P < 0.001) and groups B and C (P < 0.001).d Significant differences in Δ SFA intake as a percentage of total energy intake between groups A and C (P = 0.035) and groups B and C (P = 0.010). | |||||
| Energy (kcal day −1 ) | 0.623 | 0.084 | |||
| Visit 1 | 2221 ± 815 | 2085 ± 853 | 2151 ± 818 | ||
| Visit 2 | 1797 ± 636 | 1788 ± 777 | 2182 ± 1270 | ||
| Protein (% of total energy) | 0.839 | <0.001c | |||
| Visit 1 | 17.2 ± 3.1 | 17.4 ± 3.1 | 17.0 ± 3.2 | ||
| Visit 2 | 19.2 ± 3.6 | 19.2 ± 4.4 | 14.5 ± 3.3 | ||
| Fat (% of total energy) | 0.145 | 0.563 | |||
| Visit 1 | 38.6 ± 5.8 | 39.1 ± 6.0 | 41.0 ± 6.0 | ||
| Visit 2 | 41.0 ± 7.8 | 40.7 ± 7.7 | 44.4 ± 7.3 | ||
| SFA (% of total energy) | 0.292 | 0.025d | |||
| Visit 1 | 15.4 ± 3.7 | 15.6 ± 3.3 | 16.5 ± 3.8 | ||
| Visit 2 | 17.1 ± 5.6 | 16.5 ± 4.8 | 20.5 ± 5.5 | ||
| CHO (% of total energy) | 0.193 | 0.176 | |||
| Visit 1 | 43.6 ± 5.9 | 43.7 ± 6.9 | 41.3 ± 7.9 | ||
| Visit 2 | 39.2 ± 11.1 | 40.4 ± 9.6 | 41.1 ± 8.4 | ||
Discussion
Published research has demonstrated a beneficial effect of cheese consumption on markers of metabolic health and CVD risk.6–10,25,26 To date, studies have investigated the effect of unmelted cheese on markers of metabolic health but the effect of melted cheese consumption remains unknown.11,15 The current study aimed to address this gap in the knowledge. In the present study in a cohort of overweight Irish adults, melted cheese consumption was found to significantly increase total cholesterol and triglyceride concentrations compared to unmelted (non-melted) cheese and to individual cheese components, with no effects of melting observed on LDL cholesterol, weight or on markers of glycaemic control.A previous study explored the effect of fat contained within or outside the cheese matrix on metabolic health in a similar cohort of 127 overweight Irish adults (45.7% male), with a mean ± SD age of 60.3 ± 6.8 years.11 In that study, fat contained within the cheese matrix was found to significantly lower cholesterol concentrations, compared to the same amount of dairy fat consumed differently; either as a reduced-fat cheddar cheese plus butter intervention, or as part of a ‘deconstructed’ cheese; namely butter, caseinate powder and calcium supplement intervention.11 The same study also observed significant changes for both total and LDL cholesterol between the full fat cheddar cheese group and the reduced-fat cheddar cheese plus butter group, with no differences in HDL cholesterol or triglyceride concentrations.11 Those results differ from the findings of this current study, where significant changes in total cholesterol and triglyceride, but not LDL cholesterol, concentrations, were found. Investigating if or how such differences influence response to consumption of dairy fat, secondary analysis of the earlier trial demonstrated that individual variation in response is influenced by biochemical factors.27 Those with the highest baseline cholesterol concentrations at baseline showed the greatest reductions in cholesterol by the end of the trial, regardless of dietary intervention group, whereas anthropometry and age did not have an effect.27 Furthermore, the total and LDL cholesterol lowering effects of cheese previously demonstrated11 may be explained by the likelihood of many participants having normal cholesterol concentrations in the present study.27 The lipid responses observed here are clinically meaningful, as reductions in cholesterol and triglyceride concentrations have been shown to lower risk of major vascular event.28 It must also be noted that the current study was conducted during the COVID-19 pandemic, where dietary and lifestyle factors were likely altered. Comprehensive analysis of almost 4000 Irish residents as part of the National COVID-19 Food Study, reports dietary and lifestyle changes in 2020 as Ireland entered lockdown. Of n 1435 participants aged 45–65 years, increased consumption of snacks (35.7%) and treats (41.0%) was reported, while 62.8% reported working from home.29 Furthermore, the trial was conducted over a longer duration and delays in delivery of intervention foods affected randomisation at times throughout the study, although this is unlikely to have affected study results. Notwithstanding, these findings indicate the physical form of cheese has an important effect on blood lipid parameters in middle-aged, overweight adults.
Dairy lipids are composed of 98% triglycerides30 and a notable difference between the current and earlier11 cheese matrix studies is the difference in triglyceride response. When investigating the effect of fat contained within or outside the cheese matrix, no significant response in triglyceride concentration was observed11 whereas in the current study triglyceride concentrations increased significantly in response to melted versus unmelted cheese, further indicating an effect of the cheese form on lipid digestion. Upon heating cheese, fat coalesces and there is the formation of free oil, known as ‘oiling-off’, in addition to contraction of the casein networks.16 The physical deconstruction of the cheese matrix by heat, i.e., the disruption of the protein structure holding the fat globules, and subsequent increase in triglyceride concentrations in the current study, is further evidence of the cheese matrix effect demonstrated in other published studies.11,12,31 Furthermore, textural parameters of cheese, such as hardness, have been shown to affect post-prandial lipaemia, with previous studies reporting differences in the rate of digestion when dairy fat is contained within dairy products with different levels of hardness.32,32 Drouin-Chartier and colleagues found triglyceride response to soft cream cheese was significantly greater after two hours compared to the response to firm cheddar or butter.32 Another post-prandial study in humans reported a significantly greater triglyceride iAUC0–6 h response to sour cream compared to both butter and cheese, although it must be noted that the type of cheese investigated was not identified.33 Interestingly, sour cream also induced a larger HDL cholesterol iAUC0–6 h response compared to cheese, where HDL cholesterol increased after consumption of sour cream but decreased in response to cheese.33 Nonetheless, the findings from Drouin-Chartier et al.,32 and Hansson et al.,33 mirror the triglyceride changes observed in the current study. In unmelted cheese, the lipid droplets are embedded in a semi-solid matrix which must be disintegrated to allow lipolysis during digestion, whereas in a semi-solid or fluid matrices lipid droplets will be rapidly dispersed within the digestive juices,34,35 which may have contributed to the lipid responses observed. In support of this, Guinot et al.,36 found cream cheese to be almost completely disintegrated, whereas cheddar cheese was less than 55% disintegrated after 2 hours in a static gastric digestion model,36 with similar findings from other in vitro studies.37,38 Moreover, milk fat globules are comprised of 98% triglyceride which are encased in the milk fat globule membrane (MFGM), a phospholipid trilayer.30 A study by Rosqvist et al.,39 investigated the effect of 40 g dairy fat per day, in the form of a scone baked with MFGM-rich whipping cream or MGFM-deficient butter oil in healthy, overweight adults.39 After 8-weeks, total and LDL cholesterol concentrations increased in response to the butter intervention, with no change observed in the whipping cream diet group.39 In addition, expression of 19 genes, most of which were associated with lipid metabolism, was suppressed in the MFGM-deficient group, but increased in the MFGM-rich group,39 thus offering some mechanistic insight into the link between MFGM and lipid metabolism, although inhibited intestinal cholesterol absorption has also been suggested.40 Furthermore, increasing temperature has been shown to affect MFGM microstructure. Lipid domains in the MFGM have been shown to change from an ordered to a disordered state with increasing temperature.41,42 Et-Thakafy et al., investigated the effect of temperature on MFGM morphology and physical properties of the lipid domains using confocal microscopy, and found diffusion of lipid domains within the MFGM, coalescence with neighbouring domains and reduction in domain size upon heating to 60 °C.42 These morphological changes in response to increasing temperature may affect the nutritional functions of dairy fat globules, in particular lipid digestion.42 Therefore, it is possible the MFGM in the melted cheese intervention was disrupted by heat and this contributed to the increased triglyceride concentrations observed in the present work.
Understanding the mechanism whereby consumption of dairy fat within the cheese matrix appears to have a less detrimental effect on blood lipid concentrations, and thus CVD risk, is important, to translate these findings for public health. Published evidence does suggest that the physical state of the lipid droplets is an important modulator of digestion and lipolysis, with in vitro digestion of lipids in a liquid state found to be digested more rapidly and to a greater extent than solid lipid particles, as there is increased lipase adsorption to the lipid droplet surfaces and thus increased lipolysis in the liquid state.43 Textural properties of cheese, such as cohesiveness and hardness, have also been negatively correlated with the rate of in vivo digestion,7 potentially due to differences in moisture and fat content, and levels of casein hydration and mineralisation which affect the texture.38 Lamothe et al., studied the in vitro digestion properties of milk, yogurt and cheese and found both proteolysis and release of free fatty acids was lower in the semi-solid cheese matrix, compared to the liquid milk and yogurt matrices.38 In the current study, differences were observed between the unmelted versus melted cheese groups, which may be explained by the physical state of the cheese, and lipids, at the time of consumption. In the melted cheese group, a greater proportion of the lipid is expected to be in liquid form at time of consumption, owing to the ‘oiling-off’ of lipids, although this cannot be confirmed as participants were provided with instructions to heat 60 g of cheese for 30 seconds, based on an 800 W microwave, rather than to achieve a certain temperature. In-house testing demonstrated microwave melting was uniform and homogenous, with development of an immediate liquid fat layer form, whereas oven grilling developed smaller pockets of fat which were slower to form a liquid fat layer. To the best of our knowledge, this is the first human intervention study to consider melted cheese and future work could consider this limitation.
This study has many strengths owing to its study design, nutrient-matched intervention diets and participants were well matched at baseline. Nonetheless, the work also has some limitations. It should be highlighted that the number of participants completing to ≥80% compliance in Group C was lower than the groups consuming whole cheese (Group A, n 54; Group B, n 45; Group C, n 42), which may have meant this group was under-powered to detect differences and is a limitation of the current work and may introduce bias. Results should be interpreted with caution, as while similar trends were observed in the Completer analysis, no significant differences were observed. Results are applicable to middle-aged, overweight, Irish adults and therefore lack generalisability to other populations. Even though all three diets were matched for fat, protein and calcium, the overall protein and SFA intake in Group C was slightly lower, and a non-dairy form of calcium was used in the deconstructed group, all of which may have influenced the current findings. Moreover, sodium and potassium contents of the diets was not considered when matching for nutrients. In the deconstructed cheese group, the compositional elements of the cheese, i.e., butter, caseinate powder and a calcium supplement, may have been eaten on separate eating occasions during the day, therefore limiting, or perhaps negating, any effect the individual elements may contribute to the markers of metabolic health in this group. Future studies incorporating a test diet with multiple elements should include a more in-depth compliance log or questionnaire regarding timing of test food consumption. Furthermore, in order to maintain compliance participants consuming melted cheese were advised to either grill or microwave cheese, and mechanistic work investigating whether these melting methods differentially affect cheese composition, e.g., water loss, is warranted. Given the inability to blind the treatment allocation owing to the nature of the intervention, some bias may have been introduced as the participants were aware of what they were eating, and this may have had an impact on feelings of satiety and subsequent food intake. Furthermore, a per-protocol analysis approach was applied to the analysis, as described in the statistical analysis section, which is recommended in nutritional studies, although it is recognised that this may also introduce bias into the comparison between groups. The Completer analysis followed similar, albeit non-significant trends as the compliant approach and is available in ESI Table 2.† Finally, as faecal samples were collected as part of the study, the effect of cheese form on lipid excretion could be investigated to help further understand the mechanism linking the dairy matrix and cardiovascular health, as there is the possibility lipids in the unmelted cheese matrix may bind with calcium and form calcium soaps, an effect which may not occur in melted or deconstructed cheese.
Current nutritional guidelines recommend limiting SFA intake to <10% of total energy intake,44 or as low as possible45 with an aim to replace SFA with polyunsaturated fatty acids.46 These recommendations fail to take into account that the effect of SFA intake on health varies depending on the food source, and current guidelines may be contributing to less healthful dietary intakes at a population level3,15 particularly with respect to SFA from dairy sources which has been shown to be protective against CVD.6,7,9 The findings of the current study support the call for food-based translations of SFA guidelines,3 as no differences were observed across the groups for body weight or glycaemic control. Furthermore, these findings have important implications for food innovation in the modification of food texture so as to affect subsequent digestion behaviour and thus modulate impacts on blood lipid profiles.
To the best of our knowledge, this is the first study to investigate the impact of melted cheese consumption on markers of metabolic health in humans. The results reported here further indicates that the form of cheddar cheese plays an important role in lipid digestion and subsequent lipid profiles in adults at risk of metabolic disease. Further work in this area is warranted to further understand this mechanism and its link to health outcomes, which may identify new avenues in dairy food innovation.
Author contributions
Conceptualisation, ELF and ERG; data curation and formal analysis, MR, ELF and ERG; investigation, AO'C, SD, NB, CM, SY, SZ; methodology, ELF, ERG, MOS, DOR, JJS, AB and NN; project administration, AO'C, SD, ELF; supervision, ELF and ERG; visualisation and writing of the original draft, MR, AO'C, ELF and ERG. All authors reviewed and edited the final draft.Data availability
Data described in the manuscript, will not be made available, in line with current UCD HREC data storage and retention guidelines, the de-identified archived data are only accessible to study investigators and will not be accessible to others.Conflicts of interest
The authors report no conflict of interest.Acknowledgements
The authors with to thank all volunteers that participated in the trial. This study was supported by Food for Health Ireland, from Enterprise Ireland (TC-2018-0025 to ERG and ELF). The funders had no role in the analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the findings. AO'C, MR, SD, NB, SY, JJS, AB, NN, MOS, DOR, ERG and ELF, have received funding from Food for Health Ireland, a dairy technology centre part-financed by Enterprise Ireland and partly by dairy companies in Ireland. SY's PhD has been funded by the Chinese Academy of Agricultural Sciences (CAAS).References
- R. P. Mensink and World Health Organization, Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis, World Health Organization, 2016 Search PubMed.
- L. Te Morenga and J. M. Montez, Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis, PLoS One, 2017, 12(11), e0186672, DOI:10.1371/JOURNAL.PONE.0186672.
- A. Astrup, H. C. Bertram, J. P. Bonjour, L. C. De Groot, D. Oliveira, M. C. Otto, E. L. Feeney, M. L. Garg, I. Givens, F. J. Kok, R. M. Krauss and B. Lamarche, WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach?, Br. Med. J., 2019, 366, 14137 Search PubMed.
- E. L. Feeney, P. Lamichhane and J. J. Sheehan, The cheese matrix: Understanding the impact of cheese structure on aspects of cardiovascular health – A food science and a human nutrition perspective, Int. J. Dairy Technol., 2021, 74, 656–670 CrossRef CAS.
- E. L. Feeney, A. P. Nugent, B. Mc Nulty, J. Walton, A. Flynn and E. R. Gibney, An overview of the contribution of dairy and cheese intakes to nutrient intakes in the Irish diet: results from the National Adult Nutrition Survey, Br. J. Nutr., 2016, 115, 709–717 CrossRef CAS PubMed.
- M. C. De Oliveira Otto, D. Mozaffarian, D. Kromhout, A. G. Bertoni, C. T. Sibley, D. R. Jacobs and J. A. Nettleton, Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis, Am. J. Clin. Nutr., 2012, 96, 397–404 CrossRef CAS PubMed.
- Z. Chen, M. Ahmed, V. Ha, K. Jefferson, V. Malik, P. A. B. Ribeiro, P. Zuchinali and J. P. Drouin-Chartier, Dairy Product Consumption and Cardiovascular Health: A Systematic Review and Meta-analysis of Prospective Cohort Studies, Adv. Nutr., 2022, 13, 439–454 CrossRef PubMed.
- M. Chen, A. Pan, V. S. Malik and F. B. Hu, Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials, Am. J. Clin. Nutr., 2012, 96, 735–747 CrossRef PubMed.
- M. Kratz, T. Baars and S. Guyenet, The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease, Eur. J. Nutr., 2013, 52, 1–24 CrossRef PubMed.
- P. C. Elwood, J. E. Pickering, D. Ian Givens and J. E. Gallacher, The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence, Lipids, 2010, 45, 925–939 CrossRef CAS PubMed.
- E. L. Feeney, R. Barron, V. Dible, Z. Hamilton, Y. Power, L. Tanner, C. Flynn, P. Bouchier, T. Beresford, N. Noronha and E. R. Gibney, Dairy matrix effects: Response to consumption of dairy fat differs when eaten within the cheese matrix - A randomized controlled trial, Am. J. Clin. Nutr., 2018, 108, 1–8 CrossRef PubMed.
- J. Hjerpsted, E. Leedo and T. Tholstrup, Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content, Am. J. Clin. Nutr., 2011, 94, 1479–1484 CrossRef CAS PubMed.
- P. J. Nestel, A. Chronopulos and M. Cehun, Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects, Eur. J. Clin. Nutr., 2005, 59, 1059–1063 CrossRef CAS PubMed.
- T. Tholstrup, C. E. Høy, L. N. Andersen, R. D. K. Christensen and B. Sandström, Does fat in milk, butter and cheese affect blood lipids and cholesterol differently?, J. Am. Coll. Nutr., 2004, 23, 169–176 CrossRef.
- T. K. Thorning, H. C. Bertram, J. P. Bonjour, L. De Groot, D. Dupont, E. Feeney, R. Ipsen, J. M. Lecerf, A. Mackie, M. C. McKinley, M. C. Michalski, D. Rémond, U. Risérus, S. S. Soedamah-Muthu, T. Tholstrup, C. Weaver, A. Astrup and I. Givens, Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps, Am. J. Clin. Nutr., 2017, 105, 1033–1045 CrossRef CAS.
- T. Guinee, in Advanced Dairy Chemistry: Volume 1B: Proteins: Applied Aspects, ed. P. L. H. McSweeney and J. A. O'Mahony, Springer, New York, 2016, pp. 347–415 Search PubMed.
- P. Lamichhane, A. L. Kelly and J. J. Sheehan, Symposium review: Structure-function relationships in cheese, J. Dairy Sci., 2018, 101, 2692–2709 CrossRef CAS PubMed.
- A. Ye, J. Cui, D. Dalgleish and H. Singh, The formation and breakdown of structured clots from whole milk during gastric digestion, Food Funct., 2016, 7, 4259–4266 RSC.
- E. Ayala-Bribiesca, D. Lussier, M. Chabot, S. L. Turgeon and M. Britten, Effect of calcium enrichment of Cheddar cheese on its structure, in vitro digestion and lipid bioaccessibility, Int. Dairy J., 2016, 53, 1–9 CrossRef CAS.
- S. Lamothe, N. Rémillard, J. Tremblay and M. Britten, Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment, Food Res. Int., 2017, 92, 138–146 CrossRef CAS.
- E. L. Feeney, A. Daly, S. Dunne, V. Dible, R. Barron, S. Seratlic, J. C. Jacquier, M. O'Sullivan, T. Beresford, S. K. Jensen and E. R. Gibney, Effect of reduced-calcium and high-calcium cheddar cheese consumption on the excretion of faecal fat: a 2-week cross-over dietary intervention study, Eur. J. Nutr., 2023, 62, 1755–1765 CrossRef.
- J. B. Hjerpsted, L. O. Dragsted and T. Tholstrup, Cheese intake lowers plasma cholesterol concentrations without increasing bile acid excretion, J. Nutr. Intermed. Metab., 2016, 3, 12–17 CrossRef.
- W. T. Friedewald, R. I. Levy and D. S. Fredrickson, Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge, Clin. Chem., 1972, 18, 499–502 CrossRef.
- R. W. Welch, J. M. Antoine, J. L. Berta, A. Bub, J. De Vries, F. Guarner, O. Hasselwander, H. Hendriks, M. Jäkel, B. V. Koletzko, C. C. Patterson, M. Richelle, M. Skarp, S. Theis, S. Vidry and J. V. Woodside, Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods, Br. J. Nutr., 2011, 106, S3–S15 CrossRef CAS PubMed.
- J. De Goede, J. M. Geleijnse, E. L. Ding and S. S. Soedamah-Muthu, Effect of cheese consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials, Nutr. Rev., 2015, 73, 259–275 CrossRef PubMed.
- R. Pradeilles, T. Norris, L. Sellem and O. Markey, Effect of Isoenergetic Substitution of Cheese with Other Dairy Products on Blood Lipid Markers in the Fasted and Postprandial State: An Updated and Extended Systematic Review and Meta-Analysis of Randomized Controlled Trials in Adults, Adv. Nutr., 2023, 14, 1579–1595 CrossRef CAS.
- A. O’Connor, E. L. Feeney, N. Bhargava, N. Noronha and E. R. Gibney, Determination of factors associated with serum cholesterol response to dairy fat consumption in overweight adults: Secondary analysis from an RCT, Front. Nutr., 2022, 9, 945723 CrossRef PubMed.
- N. A. Marston, R. P. Giugliano, K. Im, M. G. Silverman, M. L. O'Donoghue, S. D. Wiviott, B. A. Ference and M. S. Sabatine, Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials, Circulation, 2019, 140(16), 1308–1317 CrossRef CAS PubMed.
- E. Gibney, S. O'Reilly, A. O'Sullivan, C. Murrin, E. Feeney and C. Timon, National COVID-19 Food Study, University College Dublin, Institute of Food and Health, Dublin, 2019 Search PubMed.
- M. Alothman, S. A. Hogan, D. Hennessy, P. Dillon, K. N. Kilcawley, M. O'Donovan, J. Tobin, M. A. Fenelon and T. F. O'Callaghan, The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk, Foods, 2019, 8, 350 CrossRef CAS.
- T. K. Thorning, F. Raziani, N. T. Bendsen, A. Astrup, T. Tholstrup and A. Raben, Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: A randomized crossover trial, Am. J. Clin. Nutr., 2015, 102, 573–581 CrossRef CAS.
- J. P. Drouin-Chartier, A. J. Tremblay, J. Maltais-Giguère, A. Charest, L. Guinot, L. E. Rioux, S. Labrie, M. Britten, B. Lamarche, S. L. Turgeon and P. Couture, Differential impact of the cheese matrix on the postprandial lipid response: A randomized, crossover, controlled trial, Am. J. Clin. Nutr., 2017, 106, 1358–1365 CrossRef PubMed.
- P. Hansson, K. B. Holven, L. K. L. Øyri, H. K. Brekke, A. S. Biong, G. O. Gjevestad, G. S. Raza, K. H. Herzig, M. Thoresen and S. M. Ulven, Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: A randomized controlled cross-over trial, J. Nutr., 2019, 149, 422–431 CrossRef.
- S. L. Turgeon and G. Brisson, Symposium review: The dairy matrix—Bioaccessibility and bioavailability of nutrients and physiological effects, J. Dairy Sci., 2020, 103, 6727–6736 CrossRef.
- D. J. McClements and Y. Li, Review of in vitro digestion models for rapid screening of emulsion-based systems, Food Funct., 2010, 1, 32–59 RSC.
- L. Guinot, L. E. Rioux, S. Labrie, M. Britten and S. L. Turgeon, Identification of texture parameters influencing commercial cheese matrix disintegration and lipid digestion using an in vitro static digestion model, Food Res. Int., 2019, 121, 269–277 CrossRef CAS.
- X. Fang, L. E. Rioux, S. Labrie and S. L. Turgeon, Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion, Int. Dairy J., 2016, 56, 169–178 CrossRef CAS.
- S. Lamothe, N. Rémillard, J. Tremblay and M. Britten, Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment, Food Res. Int., 2017, 92, 138–146 CrossRef CAS.
- F. Rosqvist, A. Smedman, H. Lindmark-Månsson, M. Paulsson, P. Petrus, S. Straniero, M. Rudling, I. Dahlman and U. Risérus, Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study, Am. J. Clin. Nutr., 2015, 1, 20–30 CrossRef.
- V. Conway, P. Couture, C. Richard, S. F. Gauthier, Y. Pouliot and B. Lamarche, Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women, Nutr., Metab. Cardiovasc. Dis., 2013, 23, 1255–1262 CrossRef CAS.
- J. Wang, Y. Xi, B. Sun, J. Deng and N. Ai, Utilization of low-temperature heating method to improve skim milk production: Microstructure, stability, and constituents of milk fat globule membrane, Food Chem., 2024, 21, 101187 CAS.
- O. Et-Thakafy, F. Guyomarc'h and C. Lopez, Lipid domains in the milk fat globule membrane: Dynamics investigated in situ in milk in relation to temperature and time, Food Chem., 2017, 220, 352–361 CrossRef CAS.
- L. Bonnaire, S. Sandra, T. Helgason, E. A. Decker, J. Weiss and D. J. McClements, Influence of lipid physical state on the in vitro digestibility of emulsified lipids, J. Agric. Food Chem., 2008, 56, 3791–3797 CrossRef CAS.
- Public Health England, Saturated fats and health: SACN report, 2019 Search PubMed.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA), Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol, EFSA J., 2010, 8, 1461–1566 Search PubMed.
- Food and Agriculture Organization of the United Nations, FAO Food Nutr. Pap., 2010, 91, 1–166 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02708f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |