Open Access Article
Open Access ArticleAcute supplementation with a curcuminoid-based formulation fails to enhance resting or exercise-induced NRF2 activity in males and females†
Josh
Thorley
 a,
Abrar
Alhebshi
a,
Abrar
Alhebshi
 ab,
Ana
Rodriguez-Mateos
ab,
Ana
Rodriguez-Mateos
 c,
Zicheng
Zhang
c,
Stephen J.
Bailey
c,
Zicheng
Zhang
c,
Stephen J.
Bailey
 a,
Neil R. W.
Martin
a,
Neil R. W.
Martin
 a,
Nicolette C.
Bishop
a,
Nicolette C.
Bishop
 a and
Tom
Clifford
a and
Tom
Clifford
 *a
*a
aSchool of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, LE11 3TU, UK. E-mail: t.clifford@lboro.ac.uk
bDepartment of Clinical Nutrition, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia
cDepartment of Nutritional Sciences, School of Life Course and Population Sciences, King's College London, London, UK
First published on 7th October 2024
Abstract
Purpose: Exercise and (poly)phenols may activate nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor that coordinates antioxidant synthesis. The purpose of this study was to determine whether curcuminoid supplementation augments resting and exercise-induced NRF2 activity. Methods: In a double-blinded, randomised, between-subjects design, 14 males and 12 females performed plyometric exercise (100 drop jumps, 50 squat jumps) following 4 d supplementation with a curcuminoid-based formulation (CUR + EX; n = 13; ∼200 mg d−1 curcuminoids) or a placebo (PLA + EX; n = 13). NRF2/DNA binding in peripheral blood mononuclear cells, plasma glutathione peroxidase (GPX), and plasma cytokines (interleukin-6 [IL-6], tumour necrosis factor-alpha [TNF-α]) were measured pre-, post-, 1, 2 h post-exercise. Curcuminoid metabolites were measured 0, 1, 2 h post-administration of a single bolus. Results: Total area under the curve for total curcuminoid metabolites was greater in CUR + EX (p < 0.01), with bioavailability peaking at 2 h post administration (CUR + EX: [0 h] 80.9 ± 117 nM [1 h] 76.6 ± 178.5 nM [2 h] 301.1 ± 584.7 nM; PLA + EX: [0 h] 10.4 ± 1.6 [1 h] 8.5 ± 2.6 [2 h] 10.6 ± 2.1). NRF2 activity did not increase in PLA + EX (p = 0.78) or CUR + EX (p = 0.76); however, curcuminoid metabolite concentrations did positively predict NRF2/DNA binding (R2 = 0.39; p = 0.02). Exercise increased IL-6 (p = 0.03) but TNF-α was unresponsive (p = 0.97) and lower across PLA + EX (p = 0.03). GPX activity was higher in CUR + EX (p < 0.01) but not in PLA + EX (p = 0.94). Conclusion: Supplementation with a curcuminoid-based formulation failed to augment resting or exercise-induced NRF2/DNA binding; however, higher concentrations of curcuminoid metabolites predicted NRF2/DNA binding response, suggesting effects may be dependent on bioavailability.
Introduction
Exercise induces biochemical, metabolic, and circulatory perturbations which, over time, promote training adaptations that improve physical performance and protect against homeostatic challenge.1 One such biochemical process that transiently increases in response to strenuous exercise is the production of reactive oxygen species (ROS), such as superoxide (O2˙−) by NADPH oxidases (NOXs).2 Exercise-induced ROS may contribute to various training adaptations through redox signalling. One adaptation ascribed to ROS is a bolstered antioxidant defence system, driven by their activation of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2), a critical regulator of endogenous antioxidant and anti-inflammatory defence pathways.3,4In humans, strenuous aerobic exercise increases NRF2 activity in skeletal muscle5–7 and in peripheral blood mononuclear cells (PBMCs),8–10 purportedly through the inhibition of kelch like ECH associated protein 1 (KEAP1), the negative regulator of NRF2. These changes can be driven by ROS-induced oxidation or alkylation of KEAP1 cysteine (Cys) thiol residues.3 Ubiquitination allows nascent NRF2 to translocate to the nucleus where it binds to antioxidant response elements (ARE) on DNA to initiate the transcription of antioxidants, such as glutathione peroxidase (GPX).11 In addition, NRF2 is thought to moderate immune responses by competitively inhibiting the transcription factor nuclear factor-κB (NF-κB), thereby attenuating the production of cytokines with pro-inflammatory properties including interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α).12,13 Previously, we reported that plyometric exercise elevates NRF2 activity in peripheral blood mononuclear cells (PBMCs) compared to a resting, non-exercise group.14 Repeated exercise-induced NRF2 activation is thought to strengthen cell defences, protecting against future perturbations to redox balance and pro-inflammatory milieu after exercise training.15,16 Thus, enhancing NRF2 activity could elicit widespread health benefits, especially in diseases characterized by aberrant immune or redox systems.
Given the Cys-rich structure of KEAP1, ROS are not considered the only molecules to repress KEAP1 through Cys modification; rather, several of KEAP1 Cys thiols are sensitive to modification by electrophiles via Michael addition reactions.17 Curcuminoids, such as curcumin, demethoxycurcumin, and bisdemethoxycurcumin, are naturally occurring (poly)phenols that are isolated from the rhizome of Curcuma longa (turmeric); these molecules are reported to possess an α,β-unsaturated carbonyl moiety that demonstrates electrophilic properties in vitro.18 Therefore, it is postulated that this electrophile endows curcuminoids with their supposed antioxidant and anti-inflammatory effects in vivo.19,20
Several rodent and cell culture models suggest curcuminoid administration can activate NRF2.21–25 Although the precise mechanisms of activation are not fully elucidated, it has been demonstrated that curcuminoids can activate NRF2 either canonically after KEAP1 Cys151 modification by its α,β-unsaturated carbonyl moiety,21 or alternatively, non-canonically by activating AMP-activated protein kinases (AMPK) and p62.24 Despite the apparent health benefits of curcuminoids in humans, curcuminoid bioavailability is low, driven by its poor solubility and absorption from the gut, plus rapid metabolism and systemic elimination.26 Collectively, these factors are thought to impair curcuminoids ability to reach and modulate cellular targets. Nonetheless, two studies have previously investigated the effects of curcumin on NRF2 activity in humans, utilizing a single bolus (4 g) and chronic supplementation (15 d; 500 mg d−1), and both have shown to increase NRF2 expression in healthy individuals.27,28
There is evidence in animals that combining curcuminoid supplementation with exercise could augment NRF2 activation. Indeed, feeding curcumin (100 mg kg−1) to Wistar rats increased NRF2 activity following treadmill running to a greater extent than running alone.25 However, the combined effects of curcumin and exercise on NRF2 signalling is yet to be examined in humans. Therefore, the aim of this study was to examine whether supplementing with a curcumin-based formulation for 4 d increased the systemic concentration of curcuminoid metabolites in young, healthy individuals, and if so, whether these molecules augmented resting or exercise-induced NRF2 activity in PBMCs. Further, a secondary aim was to measure changes in NRF2-regulated GPX activity, and the cytokines IL-6 and TNF-α which are known to be negatively regulated by NRF2. It was hypothesised that curcuminoid supplementation would increase systemic curcuminoid metabolite concentration, exercise would increase NRF2 activity, and that supplementation with curcuminoids would amplify NRF2 activity post-exercise.
Methods
Participants
Sample size was based on a simulation-based power analysis for our primary outcome, changes in NRF2/ARE binding activity, using the ANOVA_power app.29 At the time of conceptualization for this study, no current study, to our knowledge, had performed this analysis in humans, so we estimated a mean and SD using data from a previous study using the same assay in animals.30 Our analysis is more conservative given the different study designs and suggested that with a difference in means and SD of 0.01 units and 0.0075, with 11 participants per group, we had ≥80% power to detect a time and interaction effect (effect size of ≥0.22 (partial eta squared)). A total of 30 participants were initially recruited for this study; of these, 4 withdrew due to various reasons (i.e., time constraints, unwillingness to undertake standardizations, fear of needles). Therefore, 26 participants (n = 13 per group; mean [SD]: age: 25 [6] years, height: 171.0 [8.7] cm, weight: 68.9 [10.9] kg) completed the study.This cohort included 14 males and 12 females, of which 8 females were eumenorrheic and 4 females were monophasic oral contraceptive (mOCP) users. Females using mOCP reported taking their respective contraception for a minimum of 2 years as prescribed (28 d cycle; 7 d break after every 21 d pill consumption period). Eumenorrheic female participants reported having regular cycles without using any form of hormonal contraceptives for at least 5 months. Each of the 4 mOCP users reported taking different contraceptive brands; these were Desogestiel®, Rigevidon®, Dianette® and Cerelle®. Female participants menstrual cycle phase was estimated with a subjective questionnaire.33 For female participants classified as eumenorrheic, the second visit took place during the early follicular phase (d 1–10 after first bleed) of their menstrual cycle to ensure oestrogen concentrations remained stable, due to this hormone's possible effect on oxidative stress.32 For the female participants who reported taking mOCP, the second visit took place during the first 10 d of pill withdrawal.
All participants completed a health screening survey to assess their eligibility. Individuals with a past or present history of cardiovascular or metabolic disease, musculoskeletal injury, food allergy, or those currently taking medication were not included in the study. Throughout the experimental procedures, participants were advised not to use proposed recovery aids (e.g., compression garments, foam rollers) or consume any dietary supplements. Additionally, exercise outside of the experimental procedures was prohibited from 48 h before the second visit until the conclusion of the study.
Experimental design
This study employed a double-blinded, placebo-controlled, between-subjects design and was pre-registered on the Open Science Framework (osf.io/5fpvn). Ethical approval was granted by the Loughborough University Research Committee and all procedures were conducted in accordance with the Declaration of Helsinki. Participants attended the laboratory on two occasions. During the first visit, anthropometric measurements were collected, and participants were given an opportunity to practice the exercise intervention with visual and verbal guidance. Participants were then given 3 opportunities to achieve their maximal counter movement jump (CMJ) and squat jump (SJ) height. Thereafter, participants were randomly allocated to either a curcuminoid and exercise (CUR + EX; n = 13; ♂ = 6 ♀ = 7) or placebo and exercise (PLA + EX; n = 13; ♂ = 8 ♀ = 5) group using minimization randomisation based on their sex and maximal CMJ height. In both groups, participants were allocated 8 capsules that were sealed in an opaque envelope by an investigator not involved with data collection.Participants were instructed to consume 2 capsules of their respective supplements for 3 d prior to, and on the morning of the second visit (4 d in total). As this study formed part of a wider investigation examining the effects of a high (poly)phenol diet at the same time-points, all participants were also provided with low (poly)phenol control breakfasts (energy intake of meal provided in Table S1†). Participants consumed 1000 mg d−1 of the experimental supplement (CurcuWIN®, OmniActive Health Technologies Ltd, Mumbai, India), or 1000 mg d−1 of inulin (Blackburn Distributions, Burnley, UK) immediately after their control meal. Each 1000 mg dose of the experimental supplement contained 20–28% (∼200 mg) of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin), as well as a combination of hydrophilic carriers (63–75%), cellulosic derivatives (10–40%), and natural antioxidants (1–3%), which reportedly increase the absorption and bioavailability of curcuminoids compared to other commercial unformulated curcuminoid products.31 Participants completed an adverse effects questionnaire 24 h prior to administration of their final experimental capsule to monitor tolerability of the supplement dose.
During the second laboratory visit, participants arrived at 09:00 am in a hydrated state after an overnight fast (8–10 h) and a resting blood sample was collected. They then consumed a final dose of their allocated supplement and control meal. After 30 min of seated rest participants completed the exercise intervention. Further blood samples were collected immediately post-, 1 h, and 2 h post-exercise.
Exercise protocol
This study employed a plyometric-type exercise protocol which consisted of performing 100 drop jumps from a 0.6 m steel box, followed by 50 squat jumps. This protocol is a more metabolically challenging adaptation to the eccentric-heavy exercise employed in our previous work, which was shown to augment NRF2 activity in PBMCs.14 Briefly, participants performed 100 drop jumps from a 60 cm box, with each jump interspaced by a 5 s rest period, and every 20 jumps with a 1 min rest. Upon completion of the drop jumps, 50 consecutive SJs were performed with 5 s between each jump. In both jumping protocols, exercise intensity was standardized; participants were instructed perform each jump to a height that was within 20% of their maximal effort CMJ and SJ height which was recorded during familiarisation. This was assessed by participants landing on a contact jump mat (JumpMat™, FSL Scoreboards, Cookstown, Northern Ireland) which immediately provided jump height data for direct comparison to max scores.Dietary standardisations
In the 24 h before the second visit, participants recorded their dietary intake through a weighed food diary. Total energy, carbohydrate, fat, protein, omega-3 fatty acids, and vitamin C, D, and E intakes were evaluated using online dietary analysis software (Nutritics Education v5.81, Nutritics, Dublin, Ireland). Throughout the study, participants were instructed to maintain their regular diet and not increase their intake (poly)phenol rich foods to enhance the ecological validity of the study. A list of these foods was provided to participants before the initiation of the supplementation period (list provided in Fig. S1†).Blood sampling and analysis
At pre-, post-, 1 h and 2 h post-exercise, venous blood was drawn into Vacuette containers treated with ethylenediaminetetraacetic acid (EDTA) and lithium heparin (LH) (Vacuette, Greiner Bio-One, Austria). EDTA blood then centrifuged at 1500g for 10 min at 4 °C. Plasma was subsequently pipetted into cryovials and stored at −80 °C for later analysis. To collect PBMCs, 10 mL of LH blood was mixed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with 1× phosphate-buffered saline (PBS) (Thermo Fisher Scientific, Loughborough, UK). The mixture was then carefully layered onto 15 mL Ficoll® Paque PLUS (Merck, Darmstadt, Germany) and centrifuged without the brakes (400g, 20 °C, 35 min). The buffy coat interphase, which contains PBMCs, was collected and washed twice in PBS at 300g for 10 min at 20 °C. After washing, the supernatant was removed, and the pellet was reconstituted in RPMI 1640 Complete Medium (Merck, Darmstadt, Germany). The PBMCs were aliquoted at a concentration of 9 × 106 cells per mL in cryopreservation media consisting of 50% RPMI 1640, 40% fetal bovine serum, and 10% dimethyl sulfoxide (Merck, Darmstadt, Germany). Subsequently, PBMCs were frozen at a rate of −1 °C min−1 to −80 °C using a Mr. Frosty™ freezing container (Thermo Fisher Scientific, Loughborough, UK). Nuclear proteins were later fractionated from PBMCs using a commercial extraction kit (nuclear extraction kit, cat. no. 40010, Active Motif, Waterloo, Belgium) according to manufacturer's instructions. Protein content of nuclear fractions was measured using a commercial bovine serum albumin assay (Prostain™ Protein Quantification Kit, cat. no. 15001, Active Motif, Waterloo, Belgium).
1 ratio with 1× phosphate-buffered saline (PBS) (Thermo Fisher Scientific, Loughborough, UK). The mixture was then carefully layered onto 15 mL Ficoll® Paque PLUS (Merck, Darmstadt, Germany) and centrifuged without the brakes (400g, 20 °C, 35 min). The buffy coat interphase, which contains PBMCs, was collected and washed twice in PBS at 300g for 10 min at 20 °C. After washing, the supernatant was removed, and the pellet was reconstituted in RPMI 1640 Complete Medium (Merck, Darmstadt, Germany). The PBMCs were aliquoted at a concentration of 9 × 106 cells per mL in cryopreservation media consisting of 50% RPMI 1640, 40% fetal bovine serum, and 10% dimethyl sulfoxide (Merck, Darmstadt, Germany). Subsequently, PBMCs were frozen at a rate of −1 °C min−1 to −80 °C using a Mr. Frosty™ freezing container (Thermo Fisher Scientific, Loughborough, UK). Nuclear proteins were later fractionated from PBMCs using a commercial extraction kit (nuclear extraction kit, cat. no. 40010, Active Motif, Waterloo, Belgium) according to manufacturer's instructions. Protein content of nuclear fractions was measured using a commercial bovine serum albumin assay (Prostain™ Protein Quantification Kit, cat. no. 15001, Active Motif, Waterloo, Belgium).
NRF2/ARE binding
NRF2/ARE binding was performed ex vivo using a commercially available human NRF2 activity assay (cat. no. TFEH-NRF2-1, RayBiotech, Georgia, United States) following the manufacturer's guidelines. For the initiation of binding, nuclear proteins were allowed to incubate overnight in wells containing immobilized oligonucleotides that possessed ARE consensus binding sites (5′-GTCACAGTACTCAGCAGAATCTG-3′). After appropriate washing steps, wells were treated with anti-NRF2 antibodies, followed by incubation with HRP-conjugated secondary antibodies. The absorbance was then measured at 450 nm using a Varioskan™ LUX multimode microplate reader (Thermo Fisher Scientific, Loughborough, UK). It is noteworthy that, as per our pre-registration, our initial intention was to also investigate NF-κB DNA binding. However, due to technical issues with the assay and limited sample for further analysis, this aspect of the study was not completed.Curcuminoid metabolite analysis
Curcuminoid metabolites were extracted from plasma samples using a micro-solid phase extraction (μ-SPE) method previously reported.32 Briefly, digested samples were thawed on ice and centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min at 4 °C. Supernatant was collected and acidified with 4% phosphoric acid (85% HPLC grade, Yorlab, Fluka, York, UK) in the ratio of 1
000g for 15 min at 4 °C. Supernatant was collected and acidified with 4% phosphoric acid (85% HPLC grade, Yorlab, Fluka, York, UK) in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) and vortexed thoroughly. The acidified samples were loaded onto the Oasis hydrophilic–lipophilic balanced (HLB) reversed-phase sorbent μ-SPE 96-well cartridge plate (Waters, Eschborn, Germany) using a semi-automated positive pressure 96 processor (Waters, Eschborn, Germany). The plate then was washed with 200 μL HPLC grade water and 200 μL 0.2% acetic acid. Targeted compounds were eluted with 30 μL methanol containing 0.1% formic acid and 10 nM ammonium formate for 3 times into a 96 deep-well collection plate. An additional 35 μL of HPLC water with 5 μL of internal standards (0.25 mg mL−1 taxifolin) were added in the collection plate, making a 130 μL final volume. Finally, the collection plate was covered with an adhesive seal to prevent samples from evaporating and mixed on a plate mixer for 3 min. Samples in the collection plate were finally transferred into the HPLC vials and stored in −70 °C before the analysis.
1 (v/v) and vortexed thoroughly. The acidified samples were loaded onto the Oasis hydrophilic–lipophilic balanced (HLB) reversed-phase sorbent μ-SPE 96-well cartridge plate (Waters, Eschborn, Germany) using a semi-automated positive pressure 96 processor (Waters, Eschborn, Germany). The plate then was washed with 200 μL HPLC grade water and 200 μL 0.2% acetic acid. Targeted compounds were eluted with 30 μL methanol containing 0.1% formic acid and 10 nM ammonium formate for 3 times into a 96 deep-well collection plate. An additional 35 μL of HPLC water with 5 μL of internal standards (0.25 mg mL−1 taxifolin) were added in the collection plate, making a 130 μL final volume. Finally, the collection plate was covered with an adhesive seal to prevent samples from evaporating and mixed on a plate mixer for 3 min. Samples in the collection plate were finally transferred into the HPLC vials and stored in −70 °C before the analysis.
The quantification of curcuminoids in the samples and standard mixes was achieved with a UPLC system (Vanquish, Thermo Fisher Scientific, Runcorn, UK) equipped with electrospray ionisation and triple quadrupole mass spectrometry (Vantage, Thermo Fisher Scientific, Runcorn, UK). 5 μL of sample or standard mixes were injected into the system and passed through a Raptor Biphenyl reversed phase column 2.1 × 50 mm, 1.8 μm (Restek, Bellefonte, USA) coupled with a compatible guard cartridge 5 × 2.1 mm, 2.7 μm (Restek, Bellefonte, USA) with the column temperature of 30 °C. The mobile phases were HPLC grade water and HPLC grade acetonitrile that both were acidified with 0.1% LC-MS grade formic acid (v/v), as solvent A and B, respectively. The gradients were as followed: 0–1 min, 1% B; 1–4 min, 1–12% B; 4–8 min, 12% B; 8–11 min, 12–15% B; 11–11.5 min, 15–30% B; 11.5–12 min, 30–99% B; 12–14 min, 99% B; 14–14.1 min, 99–1% B; and 14.1–16 min, 1% B, with a flow rate of 0.35 mL min−1. The MS analysis was performed under the following conditions: collision gas pressure 1.0 mTorr, capillary temperature 270 °C, vaporizer temperature 350 °C, sheath gas pressure 49 arb., aux gas pressure 10 arb., ion sweep gas pressure 0.0 arb., spray voltage 3000 °C. All the compounds were analysed in negative ion mode. Five HPLC grade standards were used for the identification and quantification of curcuminoids in the samples. All the standards were mixed as the master calibration mix and 11 dilutions were made for plotting the calibration curve. Taxifolin (0.25 mg mL−1) was fortified into all samples and calibration mixes in the same concentration as the internal standard. The final MRM analysis method was generated with the confirmed retention time of the compounds, 2 min RT window, 0.70 FWHM Q1 peak width, and 3.0 seconds cycle time.
The peak integration was conducted with the TraceFinder 5.0 Software (Thermo Fisher Scientific, Runcorn, UK) and data calculation was performed on Microsoft Excel. The identification of the compounds was according to the RT and the reference ion ratios. Linear calibration curve of the standards was made by their area ratios in the calibration mixes of the same batch. The quantification of the samples was conducted according to the corresponding linear calibration curves. The area ratios of the target compounds to internal standard taxifolin were used in the quantification to balance the variations in device performance during the batch run. Chromatograms for each individual curcuminoid can be found in ESI Fig. 1–3.† Total curcuminoid metabolite concentration was calculated by summarising the concentrations of all individual metabolites (curcumin, bisdemethoxycurcumin, curcumin glucureonide, curcumin sulfate, demethoxycurcumin). Total curcuminoid metabolite concentration at ‘post-supplementation’ reflects the accumulation of metabolites after 3 d of 1000 mg d−1 CurcuWIN (∼200 mg d−1 of curcuminoids) supplementation, whilst levels at 1 and 2 h post-administration reflect levels after administration of an acute bolus.
Glutathione peroxidase activity
GPX activity was measured in plasma using a commercially available assay (cat. no. 703102, Cayman Chemical, Michigan, USA) according to manufacturer's instructions. One unit of GPX activity is defined as the number of enzymes causing the formation of 1 nmol of NAPDH to NADP+ per min (nmol min−1 mL−1).Cytokine analysis
EDTA plasma samples were analysed for TNF-α and IL-6 concentrations using SimplePlex™ Ella microfluidic cartridges (ProteinSimple, Bio-Techne, Oxford, UK). Values were read in duplicate, with the intra-assay CV 3.6% for TNF-α and 2.9% for IL-6.Statistical analysis
Statistical analysis was performed using jamovi v2.3.26. Prior to analysis, data were checked for normality by inspecting histograms and QQ plots of the residuals. All variables were normally distributed aside from IL-6 and total curcuminoid metabolite concentrations; thus, this data was log10 transformed, which successfully reduced skewness. Linear mixed models (2 [supplement: CUR + EX vs. PLA + EX] × 4 [time: pre-, post-, 1 h-, 2 h post-exercise]) (LMM; gamlj v2.6.6) were performed on antioxidant, cytokine and NRF2 activity. A 2 [supplement: CUR + EX vs. PLA + EX] × 3 [time: 0 h, 1 h, 2 h post-administration] LMM was performed on total and individual curcuminoid metabolite concentration. Any significant main effects from the LMM were followed up with Holm–Bonferroni corrected post hoc tests. Effect sizes for LMM analysis were calculated using partial eta squared (ηp2: small: 0.01, medium: 0.05, large 0.14).33 Total area under the curve (tAUC), where possible, was calculated using an automated spreadsheet;34 this was to provide insight into the total antioxidative and inflammatory response to exercise between groups, and to represent the total quantity of curcuminoid in the bloodstream over the duration. tAUC could not be calculated for participants with first and/or last datapoint missing; thus, these participants were excluded from tAUC analysis.As an exploratory aim, a simple linear regression model was performed to determine whether average (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) ) total curcuminoid metabolite concentrations (covariate) predicted downstream responses (dependant variable) in the CUR + EX group. For NRF2/ARE binding/curcuminoid metabolite regression analysis, cooks distance was examined since a perceived outlier was present. Cooks distance was <1 for this model, and even after removing this outlier from analysis, statistical significance (p = 0.047) was still evident.
) total curcuminoid metabolite concentrations (covariate) predicted downstream responses (dependant variable) in the CUR + EX group. For NRF2/ARE binding/curcuminoid metabolite regression analysis, cooks distance was examined since a perceived outlier was present. Cooks distance was <1 for this model, and even after removing this outlier from analysis, statistical significance (p = 0.047) was still evident.
Independent samples t-tests were performed to evaluate group differences in physical characteristics, mean and maximal CMJ height, 24 h energy intake, and were used to calculate pre-post exercise/supplement differences in tAUC for blood variables. Statistical significance was set at p < 0.05 prior to analysis. Figures were produced using GraphPad Prism (v9.4.1, Boston, USA).
Results
Physical characteristics and total energy, macronutrient, and micronutrient intakes are summarised in Table 1. Regarding the monitoring of exercise intensity, 7/13 participants in the CUR + EX group achieved a mean CMJ height that was within 20% of their pre-recorded maximal CMJ height. In the PLA + EX group, 10/13 participants achieved this. Examination of food diaries revealed that all participants reported adherence to the dietary restrictions. No side effects were reported following curcuminoid supplementation; however, after placebo supplementation, the following side effects were reported (headache: mild [n = 2] moderate [n = 1]; stomach upset: moderate [n = 1]; diarrhoea: mild [n = 1]; skin rash: mild [n = 1]); 4/6 of these symptoms were, however, reported by the same participant.| CUR + EX (n = 13) | PLA + EX (n = 13) | p | |
|---|---|---|---|
| Values are mean ± SD. CMJ = counter movement jump. SJ = squat jump. Statistical significance set at p < 0.05. | |||
| Physical characteristics | |||
| Sex (M/F) | 6/7 | 8/5 | — |
| Age (years) | 26 ± 6 | 23 ± 4 | 0.26 |
| Height (cm) | 168.1 ± 7.7 | 179.5 ± 6.8 | 0.08 |
| Body mass (kg) | 66.0 ± 7.9 | 79.7 ± 8.3 | 0.18 |
| Maximal CMJ height (cm) | 28.9 ± 6.2 | 28.5 ± 8.6 | 0.74 |
| Mean CMJ height across exercise (cm) | 25.4 ± 2.2 | 26.2 ± 2.9 | 0.44 |
| Maximal SJ height (cm) | 27.8 ± 8.9 | 29.1 ± 5.4 | 0.66 |
| Mean SJ height across exercise (cm) | 20.2 ± 7.1 | 22.8 ± 7.5 | 0.37 |
| 24 h energy intake | |||
| Energy (kcal) | 2378 ± 426 | 2222 ± 517 | 0.65 |
| Protein (g) | 119 ± 25 | 113 ± 31 | 0.63 |
| Fat (g) | 89 ± 22 | 88 ± 25 | 0.90 |
| Carbohydrate (g) | 274 ± 62 | 242 ± 74 | 0.69 |
| Omega-3 fatty acid (n-3) (g) | 1.2 ± 1.2 | 0.8 ± 0.8 | 0.23 |
| Vitamin C (mg) | 73.2 ± 81.7 | 37.1 ± 22.0 | 0.72 |
| Vitamin D (μg) | 2.5 ± 2.5 | 3.6 ± 3.8 | 0.81 |
| Vitamin E (mg) | 7.9 ± 5.9 | 6.2 ± 4.3 | 0.53 |
Total and individual curcuminoid metabolite concentrations
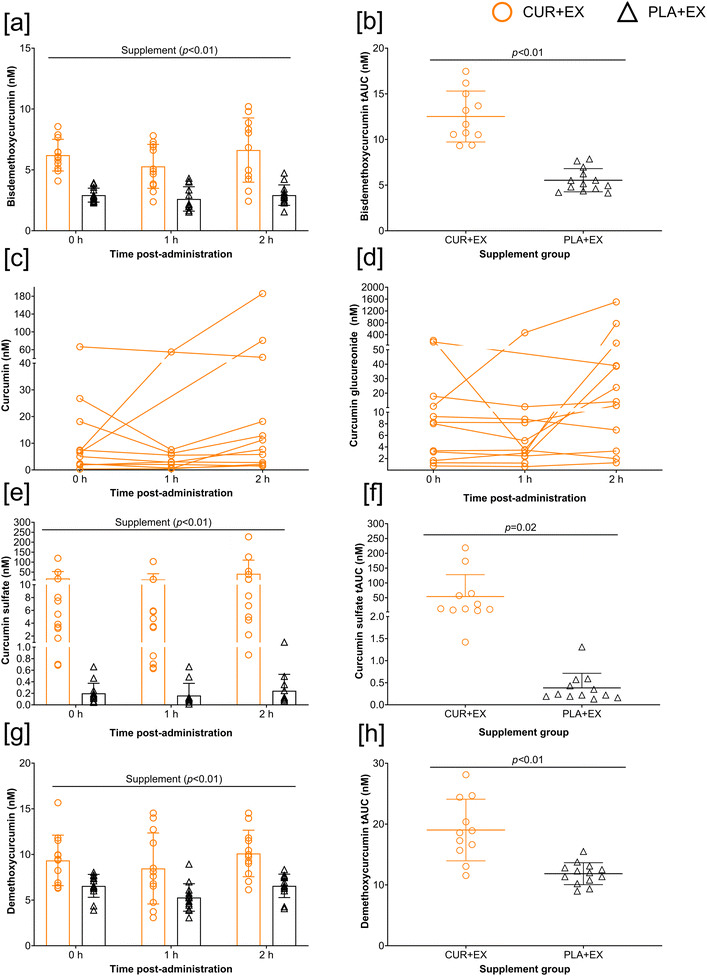
Total curcuminoid metabolite concentrations were significantly greater in the CUR + EX vs. PLA + EX group (supplement: p < 0.01; ηp2 = 0.56) (Fig. 1[a]). Total curcuminoid bioavailability increased after administration of the acute bolus (time: p = 0.01; ηp2 = 0.12); bioavailability at 2 h post-acute bolus was greater than that measured at 1 h (p < 0.01). No time × supplement effect was observed (p = 0.17; ηp2 = 0.06). Total curcuminoid metabolite tAUC response was greater in CUR + EX vs. PLA + EX group (p < 0.01) (Fig. 1[b]). 3/78 data points were missing for this analysis. Fig. 1[c] depicts individual responses of total curcuminoid metabolites measured in the CUR + EX group, whilst Fig. 1[d] presents the individual responses measured in the PLA + EX group, prior to log10 transformation to better represent transient changes in total curcuminoid bioavailability following supplementation, but prior to administration of a single bolus (0 h) and after acute administration (1 h, 2 h).Regarding the individual responses of curcuminoid metabolites, bisdemethoxycurcumin was greater in the CUR + EX vs. PLA + EX group (supplement p < 0.01; ηp2 = 0.60) but no time (p = 0.14; ηp2 = 0.07) or time × supplement (p = 0.49; ηp2 = 0.02) effect was observed (Fig. 2[a]). Bisdemethoxycurcumin tAUC response was greater in CUR + EX vs. PLA + EX (p < 0.01) (Fig. 2[b]).
For curcumin, 5/39 samples were detectable in the PLA + EX group whilst 34/39 samples were detectable in the CUR + EX group; thus, due to insufficient statistical power, LMM and tAUC analysis was not performed for curcumin, however individual responses in the CUR + EX group are presented in Fig. 2[c].
For curcumin glucureonide, 19/39 samples were detectable in the PLA + EX group whilst 36/39 were detectable in the CUR + EX group; thus, due to insufficient statistical power, LMM and tAUC analysis was not performed for curcumin glucureonide, however individual responses in the CUR + EX group are presented in Fig. 2[d].
Curcumin sulfate was greater in the CUR + EX vs. PLA + EX group (supplement p < 0.01; ηp2 = 0.15) but no time (p = 0.27; ηp2 = 0.05) or time × supplement (p = 0.31; ηp2 = 0.04) effect was observed (Fig. 2[e]). Curcumin sulfate tAUC response was greater in CUR + EX vs. PLA + EX (p = 0.02) (Fig. 2[f]).
Demethoxycurcumin was greater in the CUR + EX vs. PLA + EX group (supplement p < 0.01; ηp2 = 0.39) but no time (p = 0.07; ηp2 = 0.09) or time × supplement (p = 0.86; ηp2 < 0.01) effect was observed (Fig. 2[g]). Demethoxycurcumin tAUC response was greater in CUR + EX vs. PLA + EX (p < 0.01) (Fig. 2[h]).
NRF2/ARE binding
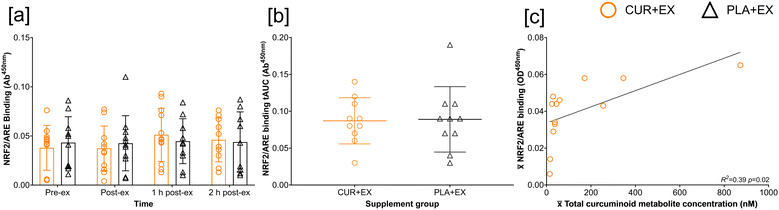
Due to technical difficulties, >75% of data was missing for 3 participants ([CUR + EX] n = 1 [PLA + EX] n = 2), thus, these participants were excluded from analysis. Exercise did not increase NRF2/ARE binding (time p = 0.69; ηp2 = 0.02), and this response was not augmented by the supplementation of curcuminoids (supplement p = 0.94; ηp2 < 0.01). No time × supplement (p = 0.84; ηp2 < 0.01) effect was found for NRF2/ARE binding (Fig. 3[a]). No group differences were reported for NRF2 tAUC response (p = 0.91) (Fig. 3[b]). 13/104 data points were missing for this analysis. Linear regression revealed that![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) curcuminoid metabolite concentration predicted NRF2/ARE binding response (R2 = 0.39; p = 0.02) (Fig. 3[c]).
curcuminoid metabolite concentration predicted NRF2/ARE binding response (R2 = 0.39; p = 0.02) (Fig. 3[c]).
Cytokines
Exercise increased IL-6 (time p = 0.03; ηp2 = 0.11); IL-6 was greater at 1 h post vs. pre-exercise (p = 0.02) (Fig. 4[a]). No supplement (p = 0.82; ηp2 < 0.01) or time × supplement (p = 0.96; ηp2 < 0.01) effect was observed for IL-6. No group differences were found for IL-6 tAUC response (p = 0.87) (Fig. 4[c]).In the CUR + EX group, 1 participant was missing >75% data for TNF-α, thus, they were removed from analysis. TNF-α did not change in response to exercise (time p = 0.97; ηp2 < 0.01), however, TNF-α was higher in the CUR + EX vs. PLA + EX group (supplement p = 0.02; ηp2 = 0.06) (Fig. 4[b]). No time × supplement (p = 0.80; ηp2 = 0.01) effect was reported for TNF-α. No group differences were reported for TNF-α tAUC response (p = 0.40) (Fig. 4[d]). 11/208 data points were missing for this analysis. ![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) curcuminoid metabolite concentrations did not predict either
curcuminoid metabolite concentrations did not predict either ![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) IL-6 or
IL-6 or ![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) TNF-α concentration ([IL-6] R2 = 0.05, p = 0.47 [TNF-α] R2 = 0.15, p = 0.19) (Fig. 4[e] and [f]).
TNF-α concentration ([IL-6] R2 = 0.05, p = 0.47 [TNF-α] R2 = 0.15, p = 0.19) (Fig. 4[e] and [f]).
Glutathione peroxidase activity
GPX activity was not influenced by exercise (time p = 0.94; ηp2 < 0.01) but was significantly greater in the CUR + EX group (supplement p < 0.01; ηp2 = 0.11) (Fig. 5[a]). No time × supplement effect was found for GPX activity (p = 0.86; ηp2 < 0.01). There was a significant difference in tAUC between groups (p < 0.01) (Fig. 5[b]). 7/104 data points were missing for this analysis.![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) curcuminoid metabolite concentrations did not predict
curcuminoid metabolite concentrations did not predict ![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) GPX activity (R2 = 0.13, p = 0.22) (Fig. 5[c]).
GPX activity (R2 = 0.13, p = 0.22) (Fig. 5[c]).
Discussion
This study aimed to evaluate whether supplementing with a curcumin-based formulation for 4 d would augment resting or exercise-induced NRF2 activity in PBMCs. The key findings from this study were: (1) supplementing with curcuminoids for 3 days, followed by administration of an acute bolus (1000 mg CurcuWIN; ∼200 mg curcuminoids) on the day of exercise increased total curcuminoid metabolite concentration vs. placebo supplementation; (2) exercise did not increase NRF2 activity in PBMCs, and the addition of curcuminoid supplementation did not augment this response, although higher systemic curcuminoid concentrations was a significant predictor of NRF2/ARE binding; (3) exercise increased IL-6 but not TNF-α concentrations, but TNF-α was higher in the CUR + EX group; (4) GPX activity was significantly greater following curcuminoid supplementation. Collectively, these findings suggest that strenuous plyometric exercise did not activate NRF2, and curcuminoids did not significantly augment the exercise-induced NRF2 response.Plyometric-type exercise did not increase NRF2/ARE binding in this study. We have reported similar null results after a less-intensive plyometric exercise protocol,35 but conversely increased NRF2/ARE binding after a less-intensive protocol.14 The discrepancy in these findings is likely mediated by two important factors; firstly, NRF2/ARE binding emits a highly variable inter-individual response, as shown here and across previous experiments.14,30,35 Ultimately reducing the statistical power of this measure. Secondly, in the study which reported elevated NRF2 activity after plyometric exercise,14 NRF2/ARE binding was compared to responses measured in a non-exercise, control group to account for circadian oscillations in NRF2 activity. Not employing a control group in this present study means that these oscillations were not accounted and potentially restricted the observation of small effects, therefore this acknowledged as a limitation of this study.
Curcuminoids, (poly)phenols ubiquitously found in the widely used medicinal spice turmeric, purportedly possesses anti-inflammatory and antioxidative properties.19,20 However, research has indicated that curcuminoids have limited bioavailability in humans after oral consumption, which may limit its bioactivity in vivo.36 However, it has been suggested that improved curcuminoid formulations with a hydrophilic carrier, cellulosic derivatives, and natural antioxidants can enhance their lipophilicity and adsorption. Indeed, such a formulation was previously shown to improve curcuminoid bioavailability by 46-fold compared to a 95% curcumin powder.31 In line with these findings, our results confirm the bioavailability of curcuminoids in this formulation, as plasma concentrations of all the measurable curcumin metabolites (curcumin, bisdemethoxycurcumin, curcumin glucureonide, curcumin sulfate, and demethoxycurcumin) were greater than that measured in the placebo group. Going forward, similar studies examining the biological effects of curcuminoids should utilise formulations like employed here to maximise the bioavailability and subsequent downstream measurable effects of curcuminoids.
Despite the increase in curcuminoid metabolites, the supplementation did not augment resting and exercise-induced NRF2 activity. This contrasts with some previous work demonstrating that curcumin activates NRF2. However, most studies showing that curcuminoids activates NRF2 are in vitro studies with large doses,21,22,24 utilise rodent models,25,37 or in other tissues (i.e., skeletal and cardiac muscle). The few existing studies in humans have led to equivocal findings. Cheng et al.28 and Yang et al.27 reported that supplementing with 4 g (single bolus) and 500 mg (for 15 d) of curcumin elevated NRF2 mRNA expression in whole blood and lymphocytes isolated from healthy humans and type-II diabetics, respectively. In contrast, a lower dose of curcumin (320 mg d−1) supplemented for 8 weeks did not alter NRF2/ARE binding in patients with nondiabetic or diabetic chronic kidney disease.38 The discrepancy in the findings from this study and those of Jiménez-Osorio et al.38 compared to those reporting a positive effect of curcuminoids on NRF2 could stem from differences in the analytical methods used to measure NRF2 activity. Whilst this study and Jiménez-Osorio et al.38 quantified the binding of nuclear-bound NRF2 to synthetic ARE oligonucleotides, the aforesaid studies utilized PCR techniques to assess changes in NRF2 mRNA expression. Despite the popularity of using PCR to delineate changes in protein abundance, there is not a strong correlation between mRNA expression and protein expression;39 thus, NRF2/ARE binding, which mimics the pathway NRF2 follows to trigger protein expression, may be a more valid indication of physiologically relevant changes to NRF2 activity by proposed activators such as curcuminoids.
Another possibility for the lack of NRF2 activation by curcuminoids could be attributed to the tissue type we employed. PBMCs were chosen since NRF2 is widely expressed and commonly assayed in these cells, and they offer an accessible alternative to measuring transcriptional changes in skeletal muscle.40,41 However, akin to our previous work with green tea (poly)phenols,42 it appears the electrophilic (and possibly pro-oxidative) properties of (poly)phenols have limited effect on NRF2/ARE binding in humans PBMCs. It has been reported that other redox-sensitive signalling pathways in human PBMCs pretreated ex vivo with curcuminoids are modulated,43,44 suggesting that PBMCs are likely inducible to curcuminoids electrophilic α,β-unsaturated carbonyl moiety, albeit exogenously. Thus, curcuminoids inability to activate NRF2 in human PBMCs could be due to other factors, including the large inter-individual differences in (poly)phenol absorption that exists after ingestion.45,46 One intriguing finding of this study was that total curcuminoid metabolite concentrations predicted NRF2/ARE binding response; this suggests that higher systemic curcuminoid concentrations may be required to significantly activate NRF2. The dose used in this present study was based on two factors; firstly, there is scant research in humans examining curcuminoids on NRF2 activity, but our dose was higher than that provided by Yang et al.27 who observed increased NRF2 expression following supplementation with 500 mg of a commercially available turmeric powder. Secondly, the ecological validity of the results; our aim was to use a typical curcuminoid dose that was recommended in commercial products, such as that found in CurcuWIN®. We therefore believe that our data has real-world translatability to consumers seeking to purchase curcuminoid products. However, future research may wish to explore whether a greater dose could elicit more favourable effects.
Whether or not exercise alongside curcuminoid supplementation increased NRF2 activity in other tissues is unknown. Interestingly however, plasma GPX activity was greater in the CUR + EX group, and since GPX expression is regulated by NRF2, this could infer that NRF2 was activated by curcuminoids, but in other tissues aside from PBMCs. The heightened activity in this group could be due to the pro-oxidant activities most (poly)phenols can exert under certain conditions, such as in the presence of transition metals, which can lead to H2O2 production.47,48 Because GPX functions to catalyse H2O2 into water,49 an elevated presence of GPX activity may be indicative of heightened H2O2 generated by curcuminoids. Heightened H2O2 by curcuminoids could be interpreted as a positive outcome, since H2O2 is the primary ROS governing redox signalling, and this may infer how curcuminoids impart their antioxidative effects; conversely, it could be viewed as a negative outcome, as extreme levels of H2O2 which could accompany large doses of curcuminoids, could contribute to oxidative distress. Nonetheless, this is merely speculation since we were unable to quantify H2O2 production. As no pre-supplementation blood sample was collected, it is also unclear whether the heightened GPX activity was due to the curcuminoids or random variation at baseline.
This study also measured the cytokines TNF-α and IL-6, which play pivotal roles in coordinating immune responses to stressors such as exercise,50 and are negatively regulated by NRF2 via competitive inhibition of NF-κB.12,13 In this study, one notable finding was that TNF-α concentrations were higher in the CUR + EX group; this was despite previous research suggesting that curcuminoids downregulated stress-induced TNF-α expression both in vitro51,52 and in vivo animal models.53–55 As such, it is uncertain why TNF-α was greater in the curcuminoid group. It was speculated that this could be due to outliers in the data, since 2 participants in the CUR + EX group had basal and exercise-induced TNF-α concentrations exceeding 16 pg mL−1, concentrations 2–3-fold greater than the group mean. However, even after excluding these participants from statistical analysis, a supplement effect was still present (p = 0.03). As differences in resting TNF-α concentrations ranged from 0.18 to 20.4 pg mL−1 in the CUR + EX group, it cannot be ruled out that these group differences were due to random variation, given the large inter-individual differences in TNF-α concentrations. These individual differences could be influenced by underlying inflammation,56 or genetic polymorphisms in the promotor region of TNF-α;57 however, the former seems unlikely as no such group differences were evident for IL-6. Regardless, if curcuminoids did increase circulating TNF-α, then this may have paradoxical implications. Firstly, it may be beneficial in immune surveillance by modulating an appropriate inflammatory response to infection and injury. Conversely, a heightened pro-inflammatory immune response may induce damage to tissues. Future research in larger homogeneous samples is needed however to examine the impact of curcuminoids on these NRF2 regulated cytokines, particularly TNF-α.58
Strengths and limitations
A strength of this study is the characterisation of plasma curcuminoid metabolites. To our knowledge this is the first study to report the bioavailability of curcuminoid supplementation alongside NRF2/DNA binding in humans. This allowed us to examine whether systemic curcuminoid concentrations could explain any changes in NRF2/DINA binding and other gene targets. Furthermore, this was the first study to examine such responses in females. Our study included both mOCP users and eumenorrheic females; however, given that only 4 of the females recruited were mOCP users, we did not have a sufficient sample size to analyse any potential differences.There were several limitations to acknowledge; (1) to reduce participant burden, a pre-supplementation blood sample was not collected, but this could have helped to determine whether the differences in plasma GPX and TNF-α were due to the curcuminoid supplementation or random variation; (2) to retain higher statistical power we did not recruit a non-exercise control group, but this may have helped us detect small changes in exercise-induced NRF2/ARE binding; (3) due to the large variability in NRF2/ARE binding, the sample size for this study likely needed to be larger; however, the sample size for this study was based on the only available data using this assay;30 (4) in line with our findings from the correlation analysis between total curcuminoid bioavailability and NRF2/ARE binding response, this study could have used a greater dosage of curcuminoids as this may have elicited greater biological effects; however, as previously discussed, this may not have been ecologically valid; (5) ideally, this study would have measured NRF2 downstream targets from the cytoplasmic fraction isolated from PBMCs. We were unable to perform this, as all the sample was used for the NRF2/ARE binding assay. In addition, it would have been useful to collect additional samples to perform epigenetic analysis on NRF2 and downstream targets; this is worth exploring in future studies.
Conclusion
Strenuous plyometric exercise, with and without curcuminoid supplementation, does not increase NRF2/ARE binding in PBMCs from healthy males and females. Furthermore, curcuminoids did not influence IL-6 but may have augmented plasma GPX activity and TNF-α. However, total curcuminoid metabolite concentrations positively predicted NRF2/ARE binding response, suggesting higher systemic curcuminoid concentrations may be required to modify NRF2 activity in vivo. This could be achieved with higher doses, but curcuminoid bioavailability showed high inter-individual bioavailability, suggesting effects are likely highly independent.Author contributions
Conceptualization (T. C., J. T., S. J. B., N. M., N. C. B.). Collection, analysis, or interpretation of data (T. C., A. A., A. R. M., Z. Z., J. T.). Writing – original draft preparation (T. C., J. T.). Writing – review and editing (T. C., J. T., S. J. B., N. M., A. R. M., N. C. B.). All authors have read and agreed to the published version of the manuscript.Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts of interest to declare.References
- L. L. Ji, C. Kang and Y. Zhang, Exercise-induced hormesis and skeletal muscle health, Free Radicals Biol. Med., 2016, 98, 113–122 CrossRef PubMed.
- S. K. Powers, R. Deminice, M. Ozdemir, T. Yoshihara, M. P. Bomkamp and H. Hyatt, Exercise-induced oxidative stress: Friend or foe?, J. Sport Health Sci., 2020, 9, 415–425 CrossRef PubMed.
- A. J. Done and T. Traustadóttir, Nrf2 mediates redox adaptations to exercise, Redox Biol., 2016, 10, 191 CrossRef.
- A. T. Dinkova-Kostova, W. D. Holtzclaw and T. W. Kensler, The role of Keap1 in cellular protective responses, Chem. Res. Toxicol., 2005, 18, 1779–1791 Search PubMed.
- H. A. Scott, J. R. Latham, R. Callister, J. J. Pretto, K. Baines, N. Saltos, J. W. Upham and L. G. Wood, Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma, Ann. Allergy, Asthma, Immunol., 2015, 114, 470–479 Search PubMed.
- C. Ballmann, G. McGinnis, B. Peters, D. Slivka, J. Cuddy, W. Hailes, C. Dumke, B. Ruby and J. Quindry, Exercise-induced oxidative stress and hypoxic exercise recovery, Eur. J. Appl. Physiol., 2014, 114, 725–733 CrossRef PubMed.
- V. Galvan-Alvarez, M. Martin-Rincon, A. Gallego-Selles, M. Martínez Canton, N. Der HamedChaman, M. Gelabert-Rebato, M. Perez-Valera, E. García-Gonzalez, A. Santana, H. C. Holmberg, R. Boushel, J. Hallén and J. A. L. Calbet, Determinants of the maximal functional reserve during repeated supramaximal exercise by humans: The roles of Nrf2/Keap1, antioxidant proteins, muscle phenotype and oxygenation, Redox Biol., 2023, 66, 102859 CrossRef CAS PubMed.
- A. J. Done, M. J. Newell and T. Traustadóttir, Effect of exercise intensity on Nrf2 signalling in young men, Free Radical Res., 2017, 51, 646–655 CrossRef CAS PubMed.
- A. J. Done, M. J. Gage, N. C. Nieto and T. Traustadóttir, Exercise-induced Nrf2-signaling is impaired in aging, Free Radicals Biol. Med., 2016, 96, 130–138 CrossRef CAS.
- E. L. Ostrom and T. Traustadóttir, Aerobic exercise training partially reverses the impairment of Nrf2 activation in older humans, Free Radicals Biol. Med., 2020, 160, 418–432 CrossRef CAS PubMed.
- C. J. Harvey, R. K. Thimmulappa, A. Singh, D. J. Blake, G. Ling, N. Wakabayashi, J. Fujii, A. Myers and S. Biswal, Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress, Free Radicals Biol. Med., 2009, 46, 443 CrossRef CAS PubMed.
- E. H. Kobayashi, T. Suzuki, R. Funayama, T. Nagashima, M. Hayashi, H. Sekine, N. Tanaka, T. Moriguchi, H. Motohashi, K. Nakayama and M. Yamamoto, Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription, Nat. Commun., 2016, 7, 11624 CrossRef CAS.
- R. K. Thimmulappa, H. Lee, T. Rangasamy, S. P. Reddy, M. Yamamoto, T. W. Kensler and S. Biswal, Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis, J. Clin. Invest., 2006, 116, 984–995 CrossRef CAS PubMed.
- J. Thorley, C. Thomas, S. J. Bailey, N. R. W. Martin, N. C. Bishop and T. Clifford, Mechanically demanding eccentric exercise increases nuclear factor erythroid 2-related factor 2 activity in human peripheral blood mononuclear cells, J. Sports Sci., 2023, 41(12) DOI:10.1080/02640414.2023.2263713.
- M. C. Gomez-Cabrera, E. Domenech and J. Viña, Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training, Free Radicals Biol. Med., 2008, 44, 126–131 CrossRef PubMed.
- G. Parise, S. M. Phillips, J. J. Kaczor and M. A. Tarnopolsky, Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults, Free Radicals Biol. Med., 2005, 39, 289–295 CrossRef.
- A. T. Dinkova-Kostova, R. V. Kostov and P. Canning, Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants, Arch. Biochem. Biophys., 2017, 617, 84–93 CrossRef.
- R. J. Mayer, P. W. A. Allihn, N. Hampel, P. Mayer, S. A. Sieber and A. R. Ofial, Electrophilic reactivities of cyclic enones and α,β-unsaturated lactones, Chem. Sci., 2021, 12, 4850–4865 RSC.
- I. Silva de Sá, A. P. Peron, F. V. Leimann, G. N. Bressan, B. N. Krum, R. Fachinetto, J. Pinela, R. C. Calhelha, M. F. Barreiro, I. C. F. R. Ferreira, O. H. Gonçalves and R. P. Ineu, In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions, Food Chem. Toxicol., 2019, 125, 29–37 CrossRef.
- Y. Panahi, M. S. Hosseini, N. Khalili, E. Naimi, L. E. Simental-Mendía, M. Majeed and A. Sahebkar, Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial, Biomed. Pharmacother., 2016, 82, 578–582 CrossRef.
- J. W. Shin, K. S. Chun, D. H. Kim, S. J. Kim, S. H. Kim, N. C. Cho, H. K. Na and Y. J. Surh, Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification, Biochem. Pharmacol., 2020, 173, 113820 CrossRef.
- E. Balogun, M. Hoque, P. Gong, E. Killeen, C. J. Green, R. Foresti, J. Alam and R. Motterlini, Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element, Biochem. J., 2003, 371, 887 CrossRef.
- J. S. Kim, J. M. Oh, H. Choi, S. W. Kim, S. W. Kim, B. G. Kim, J. H. Cho, J. Lee and D. C. Lee, Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter, BMC Complementary Med. Ther., 2020, 20, 101 CrossRef.
- J. Y. Park, H. Y. Sohn, Y. H. Koh and C. Jo, Curcumin activates Nrf2 through PKCδ-mediated p62 phosphorylation at Ser351, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- K. Sahin, R. Pala, M. Tuzcu, O. Ozdemir, C. Orhan, N. Sahin and V. Juturu, Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: An in vivo model, J. Inflammation Res., 2016, 9, 147–154 CrossRef.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Bioavailability of curcumin: Problems and promises, Mol. Pharm., 2007, 4, 807–818 CrossRef.
- H. Yang, W. Xu, Z. Zhou, J. Liu, X. Li, L. Chen, J. Weng and Z. Yu, Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies, Exp. Clin. Endocrinol. Diabetes, 2015, 123, 360–367 CrossRef.
- D. Cheng, W. Li, L. Wang, T. Lin, G. Poiani, A. Wassef, R. Hudlikar, P. Ondar, L. Brunetti and A. N. Kong, Pharmacokinetics, pharmacodynamics and PKPD modeling of curcumin in regulating antioxidant and epigenetic gene expression in human healthy volunteers, Mol. Pharm., 2019, 16, 1881 CrossRef PubMed.
- D. Lakens and A. R. Caldwell, Simulation-Based Power Analysis for Factorial Analysis of Variance Designs, Adv. Methods Pract. Psychol. Sci., 2021, 4(1) DOI:10.1177/2515245920951503.
- E. L. Ostrom, A. P. Valencia, D. J. Marcinek and T. Traustadóttir, High intensity muscle stimulation activates a systemic Nrf2-mediated redox stress response, Free Radicals Biol. Med., 2021, 172, 82 CrossRef.
- R. Jäger, R. P. Lowery, A. V. Calvanese, J. M. Joy, M. Purpura and J. M. Wilson, Comparative absorption of curcumin formulations, Nutr. J., 2014, 13, 1–8 CrossRef PubMed.
- M. Domínguez-Fernández, Y. Xu, P. Young Tie Yang, W. Alotaibi, R. Gibson, W. L. Hall, L. Barron, I. A. Ludwig, C. Cid and A. Rodriguez-Mateos, Quantitative Assessment of Dietary (Poly)phenol Intake: A High-Throughput Targeted Metabolomics Method for Blood and Urine Samples, J. Agric. Food Chem., 2021, 69, 537–554 CrossRef.
- J. Cohen, Statistical Power Analysis for the Social Sciences, Lawrence Erlbaum Associates, Hillsdale, New Jersey, 2nd edn, 1988 Search PubMed.
- B. J. Narang, G. Atkinson, J. T. Gonzalez and J. A. Betts, A Tool to Explore Discrete-Time Data: The Time Series Response Analyser, Int. J. Sport Nutr. Exercise Metab., 2020, 30, 374–381 Search PubMed.
- J. Thorley, C. Thomas, N. Thon, H. Nuttall, N. R. W. Martin, N. Bishop, S. J. Bailey and T. Clifford, Combined effects of green tea supplementation and eccentric exercise on nuclear factor erythroid 2-related factor 2 activity, Eur. J. Appl. Physiol., 2024, 124, 245–256 CrossRef.
- W. Liu, Y. Zhai, X. Heng, F. Y. Che, W. Chen, D. Sun and G. Zhai, Oral bioavailability of curcumin: problems and advancements, J. Drug Targeting, 2016, 24, 694–702 CrossRef PubMed.
- C. Zeng, P. Zhong, Y. Zhao, K. Kanchana, Y. Zhang, Z. A. Khan, S. Chakrabarti, L. Wu, J. Wang and G. Liang, Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo, J. Mol. Cell Cardiol., 2015, 79, 1–12 CrossRef.
- A. S. Jiménez-Osorio, W. R. García-Niño, S. González-Reyes, A. E. Álvarez-Mejía, S. Guerra-León, J. Salazar-Segovia, I. Falcón, H. Montes de Oca-Solano, M. Madero and J. Pedraza-Chaverri, The Effect of Dietary Supplementation With Curcumin on Redox Status and Nrf2 Activation in Patients With Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study, J. Renal Nutr., 2016, 26, 237–244 CrossRef.
- D. Greenbaum, C. Colangelo, K. Williams and M. Gerstein, Comparing protein abundance and mRNA expression levels on a genomic scale, Genome Biol., 2003, 4, 117 CrossRef.
- I. Rudkowska, C. Raymond, A. Ponton, H. Jacques, C. Lavigne, B. J. Holub, A. Marette and M. C. Vohl, Validation of the use of peripheral blood mononuclear cells as surrogate model for skeletal muscle tissue in nutrigenomic studies, OMICS, 2011, 15, 1–7 CrossRef PubMed.
- L. Neilson, J. Quinn and N. Gray, Peripheral Blood NRF2 Expression as a Biomarker in Human Health and Disease, Antioxidants, 2020, 10, 1–12 CrossRef.
- J. Thorley, C. Thomas, N. Thon, H. Nuttall, N. R. W. Martin, N. Bishop, S. J. Bailey and T. Clifford, Combined effects of green tea supplementation and eccentric exercise on nuclear factor erythroid 2-related factor 2 activity, Eur. J. Appl. Physiol., 2023, 124(1) DOI:10.1007/S00421-023-05271-8.
- W. Shang, L. J. Zhao, X. L. Dong, Z. M. Zhao, J. Li, B. B. Zhang and H. Cai, Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways, Mol. Med. Rep., 2016, 14, 3620 CrossRef PubMed.
- S. Vadhan-Raj, D. M. Weber, M. Wang, S. A. Giralt, S. K. Thomas, R. Alexanian, X. Zhou, P. Patel, C. E. Bueso-Ramos, R. A. Newman and B. B. Aggarwal, Curcumin Downregulates NF-κB and Related Genes in Patients with Multiple Myeloma: Results of a Phase I/II Study, Blood, 2007, 110, 1177–1177 CrossRef.
- C. Liu, S. Boeren and I. M. C. M. Rietjens, Intra- and Inter-individual Differences in the Human Intestinal Microbial Conversion of (−)-Epicatechin and Bioactivity of Its Major Colonic Metabolite 5-(3′,4′-Dihydroxy-Phenyl)-γ-Valerolactone in Regulating Nrf2-Mediated Gene Expression, Front. Nutr., 2022, 9, 910785 CrossRef PubMed.
- A. Kerimi, N. U. Kraut, J. A. da Encarnacao and G. Williamson, The gut microbiome drives inter- and intra-individual differences in metabolism of bioactive small molecules, Sci. Rep., 2020, 10(1), 1–12 CrossRef.
- D. Procházková, I. Boušová and N. Wilhelmová, Antioxidant and prooxidant properties of flavonoids, Fitoterapia, 2011, 82, 513–523 CrossRef.
- S. Eghbaliferiz and M. Iranshahi, Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals, Phytother. Res., 2016, 30, 1379–1391 CrossRef CAS.
- R. Brigelius-Flohé and M. Maiorino, Glutathione peroxidases, Biochim. Biophys. Acta, 2013, 1830, 3289–3303 CrossRef.
- S. Docherty, R. Harley, J. J. McAuley, L. A. N. Crowe, C. Pedret, P. D. Kirwan, S. Siebert and N. L. Millar, The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review, BMC Sports Sci., Med. Rehabil., 2022, 14(5), 1–14 Search PubMed.
- S. C. Gupta, S. Prasad, J. H. Kim, S. Patchva, L. J. Webb, I. K. Priyadarsini and B. B. Aggarwal, Multitargeting by curcumin as revealed by molecular interaction studies, Nat. Prod. Rep., 2011, 28, 1937 RSC.
- C. Zhao, J. Yang, Y. Wang, D. Liang, X. Yang, X. Li, J. Wu, X. Wu, S. Yang, X. Li and G. Liang, Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages, Bioorg. Med. Chem., 2010, 18, 2388–2393 CrossRef.
- V. O. Gutierres, C. M. Pinheiro, R. P. Assis, R. C. Vendramini, M. T. Pepato and I. L. Brunetti, Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes, Br. J. Nutr., 2012, 108, 440–448 CrossRef PubMed.
- M. A. El-Moselhy, A. Taye, S. S. Sharkawi, S. F. I. El-Sisi and A. F. Ahmed, The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids, Food Chem. Toxicol., 2011, 49, 1129–1140 CrossRef PubMed.
- C. Billerey-Larmonier, J. K. Uno, N. Larmonier, A. J. Midura, B. Timmermann, F. K. Ghishan and P. R. Kiela, Protective Effects of Dietary Curcumin in Mouse Model of Chemically Induced Colitis Are Strain Dependent, Inflammatory Bowel Dis., 2008, 14, 780 CrossRef.
- C. Popa, M. G. Netea, P. L. C. M. Van Riel, J. W. M. Van Der Meer and A. F. H. Stalenhoef, The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk, J. Lipid Res., 2007, 48, 751–762 CrossRef PubMed.
- A. J. P. Smith and S. E. Humphries, Cytokine and cytokine receptor gene polymorphisms and their functionality, Cytokine Growth Factor Rev., 2009, 20, 43–59 CrossRef CAS PubMed.
- S. Sorichter, M. Martin, P. Julius, A. Schwirtz, M. Huonker, W. Luttmann, S. Walterspacher and A. Berg, Effects of unaccustomed and accustomed exercise on the immune response in runners, Med. Sci. Sports Exercise, 2006, 38, 1739–1745 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02681k |
| This journal is © The Royal Society of Chemistry 2024 |