Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lactiplantibacillus plantarum HEAL9 attenuates cognitive impairment and progression of Alzheimer's disease and related bowel symptoms in SAMP8 mice by modulating microbiota-gut-inflammasome-brain axis†
C.
Di Salvo‡
 a,
V.
D'Antongiovanni‡
b,
L.
Benvenuti
a,
A.
d'Amati
c,
C.
Ippolito
b,
C.
Segnani
b,
C.
Pierucci
b,
G.
Bellini
d,
T.
Annese
ce,
D.
Virgintino
c,
R.
Colucci
f,
L.
Antonioli
a,
M.
Fornai
a,
M.
Errede
*c,
N.
Bernardini
b and
C.
Pellegrini
a,
V.
D'Antongiovanni‡
b,
L.
Benvenuti
a,
A.
d'Amati
c,
C.
Ippolito
b,
C.
Segnani
b,
C.
Pierucci
b,
G.
Bellini
d,
T.
Annese
ce,
D.
Virgintino
c,
R.
Colucci
f,
L.
Antonioli
a,
M.
Fornai
a,
M.
Errede
*c,
N.
Bernardini
b and
C.
Pellegrini
 *b
*b
aUnit of Pharmacology and Pharmacovigilance, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
bUnit of Histology and Medical Embryology, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy. E-mail: carolina.pellegrini87@gmail.com
cHuman Anatomy and Histology Unit, Department of Basic Medical Sciences, Neuroscience, and Sensory Organs, University of Bari School of Medicine, Bari, Italy. E-mail: mariella.errede@uniba.it
dUnit of Neurology, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
eDepartment of Medicine and Surgery, University LUM Giuseppe Degennaro, Casamassima, Bari, Italy
fDepartment of Pharmaceutical and Pharmacological Sciences, University of Padova, Padova, Italy
First published on 20th September 2024
Abstract
Background: Growing evidence highlights the relevance of the microbiota-gut-brain axis in Alzheimer's disease (AD). AD patients display gut dysbiosis, altered intestinal barrier and enteric inflammation that, besides bowel symptoms, can contribute to brain pathology. In this context, the modulation of gut microbiota is emerging as a therapeutical option to halt or slow down central pathology. Herein, we examined the effects of Lactiplantibacillus plantarum HEAL9 in a spontaneous mouse model of AD. Methods: Senescence-accelerated mouse prone 8 (SAMP8) mice and control SAMR1 mice were treated orally with HEAL9 1 × 109 CFU per mouse per day or placebo for two months to evaluate the effects of the probiotic during the earliest stages of AD, before the development of brain pathology. Cognitive impairment, in vivo and in vitro colonic motility, astrocyte and microglia reactive response, brain and colonic amyloid-β1–42 (Aβ1–42) levels, and inflammasome components activation (NLRP3, ASC, caspase-1 and interleukin-1β) were assessed. In addition, gut barrier alterations [circulating lipopolysaccharide-binding protein (LBP) levels] and acidic mucus were evaluated. Results: HEAL9 administration significantly attenuated cognitive impairment and counteracted colonic dysmotility in SAMP8 mice. Moreover, HEAL9 decreased astrogliosis and microgliosis, Aβ1–42 accumulation and inflammasome activation in colon and brain and normalized plasma LBP levels and colonic acidic mucus content. Conclusion: HEAL9 intake alleviated cognitive decline and normalized colonic motility in the prodromal phases of AD via the modulation of microbiota-gut-inflammasome-brain signalling. Thus, dietary supplementation with HEAL9 could be considered as a suitable therapeutical option for the treatment of AD and related intestinal symptoms in the early stages of the disease.
Introduction
Age-related cognitive disorders, including mild cognitive impairment (MCI), senile dementia and Alzheimer's disease (AD), have become a prevalent health concern in the elderly population. MCI, an intermediate condition between age-related cognitive decline and dementia, represents the prodromal stage before the development of AD.1,2 AD is strongly associated with progressive and irreversible cognitive impairment, with loss of memory and language capacities.3 In terms of histopathological features, AD is characterized by hallmarks of neurodegeneration, such as the accumulation of parenchymal β-amyloid plaques and intracellular neurofibrillary tangles, as well as an increase in inflammatory responses within the central nervous system (CNS).4,5At present, there are no disease-targeted medications, and patients must rely solely on symptomatic treatments.6
Over the past decade, the microbiota-gut-brain (MGB) axis has emerged as one of the most promising therapeutic targets for the treatment of neurological disorders, including MCI and AD.7 As a result of the gut barrier breakdown, pathogenic bacterial products can translocate into the blood stream and spread to the brain, where they affect central circuits.8 In addition, bacterial products can directly activate systemic or local immune/inflammatory cells, which, in turn, promote neurogenic/inflammatory responses, including activation of NLRP3 inflammasome multiprotein complex, regarded as the bacteria-immune sentinel involved in modulating systemic and central neuroinflammation in AD.8–11 One such feature of MCI and AD patients is gut dysbiosis, impaired intestinal barrier and enteric inflammation along with gut dysfunctions.12–15 A recent clinical study reported that AD patients with less frequent bowel movements displayed worse cognitive function, equivalent to three years more of chronological cognitive aging.16 In this setting, the manipulation of gut microbiota through the administration of pre- and probiotics has been found to counteract pathological, behavioural, and cognitive alterations in both MCI and AD animal models and patients.17–20 Interestingly, recent evidence has reported beneficial effects exerted by Lactobacillus species and their metabolites in brain disorders.21–23 For instance, Lactiplantibacillus plantarum OLL2712, showed protective effects on memory functions in older adults with early memory deficits24 and Lactiplantibacillus plantarum HEAL9 decreased inflammatory markers linked to acute stress in chronically stressed individuals.25
Based on these premises, the present study aimed to evaluate the putative effects of Lactiplantibacillus plantarum HEAL9 in alleviating cognitive decline and intestinal symptoms in a mouse model of spontaneous AD, as well as to characterize the underlying molecular mechanisms.
Materials and methods
Animal model of MCI
The study was carried out on SAMP8 (Senescence accelerated mice P8) male mice and their control strain SAMR1 (Senescence-Accelerated Mouse-Resistant 1), all weighing 20–25 g and aged 2 months old at the start of the study, purchased from ENVIGO S.r.l (San Pietro al Natisone, UD, Italy). The choice of using male SAMP8 and SAMR1 mice has been established according to previous studies in which female SAMP8 mice showed less robust memory changes than male SAMP8. Indeed, most of studies have been carried out in male SAMP8 mice.26–28Animals were housed, three in a cage, in rooms with constant temperature (22 ± 1 °C) and humidity (50–60%) on a 12-hour light cycle. They were fed with a standard laboratory chow (3.1 kcal g−1, 18% fat, 24% protein, 58% carbohydrates) (Altromin International, Germany; SD, TD. 2018) and tap water ad libitum. Starting from 4 months of age, animals were treated daily for two months with Lactiplantibacillus plantarum HEAL9 1 × 109 CFU per mouse per day or placebo by oral gavage. Treatment groups were arranged as follows:
(1) SAMR1, fed with standard diet and treated daily with placebo by oral gavage for two months (n = 6);
(2) SAMR1, fed with standard diet and treated with Lactiplantibacillus plantarum HEAL9 1 × 109 CFU per mouse per day by oral gavage for two months (n = 6);
(3) SAMP8, fed with standard diet and treated daily with placebo by oral gavage for two months (n = 6);
(4) SAMP8, fed with standard diet and treated with Lactiplantibacillus plantarum HEAL9 1 × 109 CFU per mouse per day by oral gavage for two months (n = 6);
Lyophilized Lactiplantibacillus plantarum HEAL9 was provided by Probi AB, Lund, Sweden and then dissolved in 0.3% carboxymethyl cellulose. The treatment time was established in accordance with the main pathophysiological and clinical features of SAMP8 to evaluate the effects of HEAL9 during the development of the first symptoms related to cognitive impairment at the age of 6 months. The feeding behaviour (frequency and amount) was assessed until the day before sacrifice. During the last week of treatment, the Morris Water Maze (MWM) test was performed. At the end of MWM test, mice were euthanized. Their care and handling were in accordance with the provisions of the European Community Council Directive 210/63/UE, recognized and adopted by the Italian Government. The experiments were approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health on February 11th, 2022 (Authorization no. 115/2022-PR).
Morris water maze (MWM) test
The MWM uses a round pool (90 cm in diameter and 60 cm in height) filled with opaque water (26 ± 1 °C temperature). The pool is divided into four quadrants of equal area and a circular platform (10 cm in diameter and 30 cm in height) is placed in the centre of one quadrant.The MWM test consists of 3 different phases: the visible-platform acquisition training, the hidden-platform training, and the probe trial.
In the acquisition training, the escape latency, described as the time required to reach the platform once the mouse is put inside the pool, is assessed for each animal. Each animal is subjected to sessions of four trials every day for 2 days.
In the hidden-platform training, performed by submerging the platform 1.5 cm below the surface of the water, escape latency is evaluated over the next 5 days. Each animal is subjected to sessions of four trials every day. On the eighth day, the platform is removed from the tank for the probe trial.
The number of target crossings, the number of entries into the target quadrant where the platform was placed, and the swimming speed and swim distance were evaluated during 60 s. Data were expressed as raw values [escape latency (s), number of target crossings (n), number of entries into the target quadrant (n), swimming speed (cm s−1) and swim distance (cm)].
Evaluation of in vivo colonic transit
Faecal output was recorded from 9:00 AM to 10:00 AM during the last week of treatment. Each animal was removed from its home cage and placed in a clean plastic cage without food or water for 1 h. Stools were collected immediately after expulsion and placed in sealed tubes; then the stools were counted and weighed (total weight), dried overnight at 65 °C and weighed again to estimate the dry weight.Evaluation of ex vivo colonic motility
The contractile activity of colonic muscle preparations was recorded as previously described.29 Following euthanasia, the abdomen was immediately opened, the colon was removed and placed in Krebs solution. Segments of the colon were opened along the mesenteric insertion and cut along the longitudinal axis into strips of approximately 3 mm in width and 8 mm in length. The preparations were set up in organ baths (n = 4 strips for each animal) containing Krebs solution at 37 °C, bubbled with 95% O2 + 5% CO2 and connected to isometric transducers (constant load = 0.5 g). Krebs solution was composed as follows (mM): NaCl 113, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25 and glucose 11.5 (pH 7.4 ± 0.1). The mechanical activity was recorded by BIOPAC MP150 (Biomedica Mangoni, Pisa, Italy). Each preparation was allowed to equilibrate for at least 30 min, with intervening washings at 10 min intervals. A pair of coaxial platinum electrodes was positioned at 10 mm from the longitudinal axis of each preparation to deliver electrical stimulation by a BM-ST6 stimulator (Biomedica Mangoni, Pisa, Italy). Electrical stimuli (ES) were applied: 10 s single trains consisting of square wave pulses (0.5 ms, 30 mA). The tension developed by each preparation was normalized by the wet tissue weight and expressed as grams per gram of wet tissue (g per g tissue).Data for each animal (n = 6 per group) are obtained by the mean of the tension developed by the 4 strips.
Preliminary experiments allowed to select the frequency of 10 Hz and the concentration of 1 μM of Carbachol (CCh) and 1 μM of substance P (SP).
In the first series of experiments, electrically induced contractions were recorded from colonic preparations maintained in standard Krebs solution.
In the second series, colonic specimens were maintained in Krebs solution containing Nω-nitroL-arginine methylester (L-NAME, nitric oxide synthase inhibitor, 100 μM), guanethidine (adrenergic blocker 10 μM), N-acetyl-l-tryptophan 3,5-bis(trifluoromethyl) benzylester (L-732138, neurokinin NK1 receptor antagonist, 10 μM), 5-fluoro-3-[2-[4-methoxy-4-[[(R)-phenylsulphinyl]methyl]-1-piperidinyl]ethyl]-1H-indole (GR159897, NK2 receptor antagonist, 1 μM) and (R)-[[(2-phenyl-4-quinolinyl)carbonyl]amino]-methyl ester benzeneacetic acid (SB218795, NK3 receptor antagonist, 1 μM) and contractions were elicited by ES in order to examine the patterns driven by excitatory cholinergic nerves.
The third series of experiments was designed to study the neurogenic NK1-mediated contribution to muscle contraction. For this purpose, colonic tissues were maintained in Krebs solution containing L-NAME, guanethidine, atropine sulphate (muscarinic receptor antagonist, 1 μM), GR159897 and SB218795 and electrically evoked tachykininergic contractions were recorded.
In the last set of experiments, colonic cholinergic and tachykininergic NK1-mediated contractions were evoked by direct pharmacological activation of muscarinic receptors and NK1 receptors located on smooth muscle cells. For this purpose, colonic preparations were maintained in Krebs solution containing tetrodotoxin (TTX, 1 μM) and stimulated with CCh (1 μM) or exogenous SP (1 μM), respectively.
Evaluation of amyloid β1–42 (Aβ1–42) accumulation in colon, brain and plasma
Aβ1–42 levels in the colon, brain and plasma were measured by an ELISA kit (KMB3441, Invitrogen) as previously described.26,30,31 For this purpose, colon distal portion and cerebral cortex samples (30 mg), stored previously at −80 °C, were weighed, thawed and homogenized in 0.4 mL of 5 M guanidine-HCl/50 mM Tris (pH 8.0 at room temperature) for 3–4 h. Samples were then diluted ten-fold with cold phosphate-buffered saline (PBS) with 1× protease inhibitor cocktail (Sigma) with a serine protease inhibitor 1 mM (AEBSF, Sigma), since serine proteases can rapidly degrade Aβ peptides. Then samples (n = 6 per group) were centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min at 4 °C; after that, supernatants were transferred into clean microcentrifuge tubes and kept on ice. Tissue supernatants (100 μl) and aliquots of plasma (100 μl) were then used for the assay. Aβ levels were expressed as ρg per mg of tissue (for colon and brain) and ρg per ml of plasma.
000g for 20 min at 4 °C; after that, supernatants were transferred into clean microcentrifuge tubes and kept on ice. Tissue supernatants (100 μl) and aliquots of plasma (100 μl) were then used for the assay. Aβ levels were expressed as ρg per mg of tissue (for colon and brain) and ρg per ml of plasma.
Immunofluorescence analysis of GFAP and Iba-1 in brain
Whole brains were removed and post-fixed by immersion in 2% paraformaldehyde (PFA) and 0.2% glutaraldehyde in PBS fixative solution for 4 h at 4 °C, then washed in PBS overnight (ON) at 4 °C. Using a vibrating microtome (Leica Microsystems; Milton Keynes, UK), 30 μm sagittal sections, evenly spaced at 200 μm intervals, were cut from each hemisphere. The sections of cerebral cortex and subcortical white matter were stored in 0.02% PFA in PBS at 4 °C as free-floating sections. After permeabilization with 0.5% Triton X-100 in PBS, free-floating sections were incubated with single antibody anti-GFAP (Rat IgG, Invitrogen) or anti-Iba1 (Gt IgG, Abcam) at 4 °C ON, appropriate fluorophore-conjugated secondary antibody (Gt anti-Rt IgG, Alexa Fluor 568, Invitrogen, cat. No A11077) for 45 min at room temperature, and counterstained with TO-PRO3™ (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10k in PBS; Invitrogen). The sections were collected on polylysine slides (Menzel-Glaser) and coverslip with Vectashield mounting medium (Vector Laboratories), and finally sealed with varnish. Negative controls were prepared by omitting the primary antibody and mismatching the secondary antibody. Sections were examined under a Leica fluorescence microscope (Leica Microsystems). Images of cerebral cortex and subcortical white matter sections immunolabeled for GFAP were taken at 20× magnification. Quantification of fluorescent-labeled area was performed at 20× magnification on the mice (n = 6 for each experimental group) samples (1 section/sample, 5 randomly selected fields/section) using Cell F as image analysis software (Olympus Italia; Rozzano, Italy). Data plotting and statistical analysis were performed on GraphPad.
10k in PBS; Invitrogen). The sections were collected on polylysine slides (Menzel-Glaser) and coverslip with Vectashield mounting medium (Vector Laboratories), and finally sealed with varnish. Negative controls were prepared by omitting the primary antibody and mismatching the secondary antibody. Sections were examined under a Leica fluorescence microscope (Leica Microsystems). Images of cerebral cortex and subcortical white matter sections immunolabeled for GFAP were taken at 20× magnification. Quantification of fluorescent-labeled area was performed at 20× magnification on the mice (n = 6 for each experimental group) samples (1 section/sample, 5 randomly selected fields/section) using Cell F as image analysis software (Olympus Italia; Rozzano, Italy). Data plotting and statistical analysis were performed on GraphPad.
Evaluation of lipopolysaccharide-binding protein (LBP) levels in plasma
Lipopolysaccharide-binding protein (LPB) level in plasma was measured by ELISA kit (ab213876, Abcam), as previously described.32,33 For the procedure, blood samples were centrifuged for 5 minutes at 4000g at 2–8 °C and, after the centrifugation, supernatants were collected. Aliquots (100 μl) were used for the assay. LBP levels were expressed as ρg per mL of plasma.Histochemical evaluation of mucus layer in colon
Sections from formalin-fixed full-thickness colonic samples (distal portion) were processed for histochemical Alcian Blue (AB) technique, to detect acid mucins blue-stained, as previously described.34 The histochemical staining was carried out blinded by two histologists and was observed using a Leica DMRB light microscope (object 20×), equipped with a DFC480 digital camera (Leica Microsystems, Mannheim, Germany). Positive areas were estimated by the image analysis system L.A.S. software v.4 and quantified as fold changes vs. control value (SAMR1 + placebo).Evaluation of NLRP3 inflammasome signalling activation in colon and brain
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, A3854, Sigma Aldrich), nucleotide-binding oligomerization domain leucine rich repeat and pyrin domain containing protein 3 (NLRP3) (diluted 1
5000, A3854, Sigma Aldrich), nucleotide-binding oligomerization domain leucine rich repeat and pyrin domain containing protein 3 (NLRP3) (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab214185, Abcam), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (diluted 1
1000, ab214185, Abcam), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, #67824S, Cell Signaling), and caspase-1 (diluted 1
1000, #67824S, Cell Signaling), and caspase-1 (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab1872, Abcam) were used. Secondary antibodies were obtained from Abcam (anti-mouse ab97040 and anti-rabbit ab6721). Protein bands were detected with ECL reagents (Clarity-Western ECL Blotting Substrate, Biorad). Densitometry was performed by IBright Analysis software.
1000, ab1872, Abcam) were used. Secondary antibodies were obtained from Abcam (anti-mouse ab97040 and anti-rabbit ab6721). Protein bands were detected with ECL reagents (Clarity-Western ECL Blotting Substrate, Biorad). Densitometry was performed by IBright Analysis software.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min at 4 °C. Aliquots (100 μL) of supernatants were then used for the assay. Tissue IL-1β levels were expressed as ρg per mg of tissue.
000g for 5 min at 4 °C. Aliquots (100 μL) of supernatants were then used for the assay. Tissue IL-1β levels were expressed as ρg per mg of tissue.
Statistical analysis
The statistical analysis of data complies with the requirements of good laboratory practices (GLP). The statistical analysis was undertaken only for studies where each group size was at least n = 6 (see ESI† for sample size calculation). The results are presented as mean ± standard error of the mean (S.E.M.). All the group sizes were designed to be homogeneous. n refers to the number of mice, and statistical analysis was carried out using these independent values. The significance of differences was evaluated by two-way or one-way ANOVA followed by post hoc analysis with Dunnet test or Tukey test where appropriate. Post hoc tests were conducted only if F in ANOVA (or equivalent) achieved a statistical significance lower than 0.05 and there was no significant variance inhomogeneity.p values <0.05 were considered significantly different. All statistical procedures were performed by commercial software (GraphPad Prism, version 9.0, RRID: SCR_002798, GraphPad Software Inc., San Diego, CA, USA). Data on histochemical and immunofluorescence analysis in colonic and brain tissues were quantitatively estimated by two blind histologists (C. S. and C. I. and A. d’. A. and M. E., respectively).
Results
HEAL9 alleviates cognitive impairment in SAMP8 mice
To investigate the effect of HEAL9 on cognitive and behavioural functions, MWM test was performed.During training test, SAMP8 mice treated with placebo displayed an increased escape latency time, starting from the first day of the test (+65%), as compared with SAMR1 mice, statically significant on the fifth day (Fig. 1A). Daily treatment with HEAL9 decreased escape latency time in SAMP8 mice from the third day of the MWM test (−33%), as compared with SAMP8 mice treated with placebo (statically significant the last day of the training test) (Fig. 1A).
During the probe trial, the number of target crossings, entries into the target quadrant, swimming speed and total swimming distance significantly decreased in SAMP8 mice treated with placebo, as compared with SAMR1 mice (Fig. 1B–E). Supplementation with HEAL9 increased all these parameters, as compared with SAMP8 mice treated with placebo (Fig. 1B–E), highlighting the amelioration of the impaired cognitive and motor abilities in SAMP8 mice.
Overall, HEAL9 treatment counteracted the cognitive decline in SAMP8 animals during the phase of MCI, before the development of AD.
HEAL9 improves colonic motility through the modulation of enteric excitatory cholinergic pathway
MCI and AD patients are often characterized by gastrointestinal (GI) dysmotility with a serious impact on daily living and quality of life.40,41In this set of experiments, the effect of HEAL9 in improving SAMP8 mice colonic dysmotility was examined, investigating the viability to modulate gut microbiota as a therapeutical tool to treat GI dysfunctions in the early phases of AD.
SAMP8 mice treated with placebo displayed a significant decrease in stool frequency, as compared with control animals (SAMR1 mice) (Fig. 2A). Treatment with HEAL9 significantly increased faecal output over 1 hour, as compared with SAMP8 placebo mice (Fig. 2A), demonstrating the capacity of HEAL9 to improve the impaired colonic transit. During the observation period, no significant differences in frequency and amount of food intake (around 4 g per day per mouse) were observed in SAMP8 and SAMR1 mice, neither in probiotic nor placebo. With the aim to demonstrate if the impairment in colonic transit could be related to alterations in the enteric neurotransmissions, ex vivo colonic motor pathways were assessed.
SAMP8 mice displayed a significant reduction in total electrically evoked colonic contractions, as compared with SAMR1 controls (Fig. 2B), confirming several lines of evidence describing the impairment of colonic motility in both SAMP8 mice and MCI/early AD patients.26 Treatment with HEAL9 significantly normalized colonic motor activity, as compared with placebo treated SAMP8 animals (Fig. 2B).
Thereafter, cholinergic and tachykininergic motor pathways in colonic muscle preparations were tested. SAMP8 animals showed a significant reduction both in atropine-sensitive cholinergic contractions and in SP-mediated contractions as compared with SAMR1 mice (Fig. 2C and E), demonstrating an impaired overall colonic propulsive motility, probably due to the various neuronal plastic rearrangements already described in AD patients.42,43 The reduced cholinergic and tachykininergic activities recorded in SAMP8 mice were significantly counteracted by HEAL9 administration, indicating that probiotic treatment improves colonic motility likely by promoting enteric excitatory neurotransmissions (Fig. 2C and E).
Subsequently, with the aim to verify whether the decrease in cholinergic and tachykininergic colonic contractions resulted from changes in the density of muscarinic or NK1 tachykininergic receptors on smooth muscle cells, myogenic colonic contractions were examined, through direct stimulation of muscarinic and NK1 receptors with CCh and exogenous substance P, respectively, in the presence of tetrodotoxin. In this condition, the stimulation by CCh or SP of colonic preparations from SAMP8 or SAMR1 mice treated with placebo or HEAL9 elicited contractions of similar magnitude (Fig. 2D and F), suggesting no alterations in CCh and SP-induced myogenic contractions.
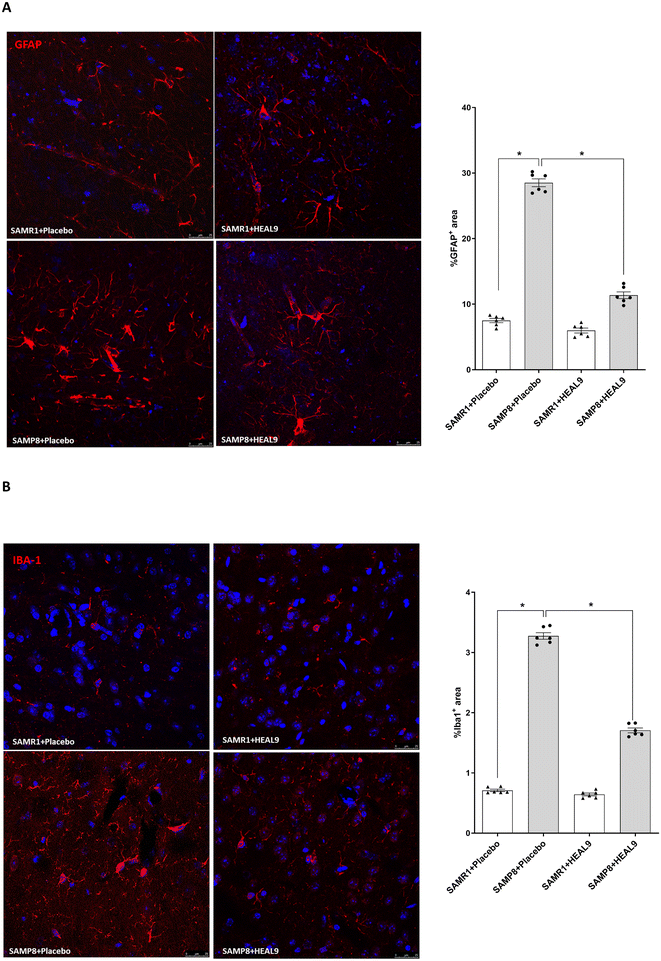
HEAL9 counteracts reactive astrogliosis and microgliosis in brain
Astrocytes and microglial cells represent critical actors of neurogenic/inflammatory responses in the CNS, as their activation (known as “astrogliosis” and “microgliosis”) plays a crucial role in several brain pathologies, including AD.44,45 Indeed, previous papers conducted in SAMP8 mice have evaluated astrogliosis and microgliosis in cerebral cortex sections,46,47 since these cell types are ubiquitously distributed in the whole brain.48,49Therefore, we examined the effect of HEAL9 on astrocytes and microglia activation in cerebral cortex and subcortical white matter.
Astrocytes derived from SAMP8 mice treated with placebo appeared hyper-ramified with more branched processes as compared to SAMR1 mice (Fig. 3A). HEAL9 treatment decreased the morphological complexity of GFAP-positive astrocytes (Fig. 3A). In addition, the quantification of %GFAP + area (Fig. 3A) confirmed the ability of HEAL9 to counteract reactive astrogliosis in SAMP8 mice.
Microglia cells from SAMP8 mice treated with placebo showed hypertrophic cell bodies and numerous short thorny processes, together with cells that completely lack processes and appear hyporamified/amoeboid, as a sign of microgliosis, as compared with SAMR1 mice in which the labelling for Iba1 revealed the morphology of surveillant microglia with a faint staining (Fig. 3B).
Following treatment with HEAL9, microglia cells showed a morphology and cell body size similar to what was observed in SAMR1 mice (Fig. 3B). In addition, the quantification of Iba1 + area (Fig. 3B) confirmed that HEAL9 treatment reduced reactive microgliosis in SAMP8 mice.
HEAL9 decreases colonic and central accumulation of Aβ1–42 in colon and brain
Aβ1–42 deposition represents a hallmark of AD;50 among the different types of Aβ1–42 polymers produced by the aberrant amyloidogenic pathway in the AD state,50,51 Aβ1–42, even if less abundant, represents the most neurotoxic, and the more aggregation-prone.52 The accumulation of Aβ1–42 has been documented both in brain and in peripheral tissues, including the gut, in patients with confirmed AD.53,54 Accordingly, the putative role of HEAL9 in counteracting Aβ1–42 accumulation was assessed.Aβ1–42 levels were significantly increased in the distal colon, cerebral cortex and plasma of placebo-treated SAMP8 mice as compared with SAMR1 (Fig. 4A–C). HEAL9 treatment did not exert any effects on Aβ1–42 plasmatic levels, whereas significantly decreased Aβ1–42 in both colonic and brain tissues from SAMP8 animals (Fig. 4A–C), suggesting that HEAL9 counteracts intestinal and central Aβ1–42 accumulation.
HEAL9 normalizes gut barrier permeability and mucus layer
AD patients display gut barrier alterations, which could contribute to the translocation of bacteria and their products, into the intestinal mucosa contributing to bowel motor dysfunctions and, subsequently into bloodstream, promoting systemic and central immune/inflammatory responses.12,15,55Herein, the effect of HEAL9 on preserving the integrity and the organization of intestinal epithelial barrier (IEB) was assessed. First, we evaluated the circulating levels of LBP, which is regarded as an indirect index of intestinal permeability.56 LBP plasma levels were significantly increased in SAMP8 mice, as compared with SAMR1, suggesting an impairment of IEB permeability during MCI and in the early phases of AD (Fig. 5A). Treatment with HEAL9 was able to restore LBP levels in SAMP8 mice, as compared with SAMP8 animals treated with placebo (Fig. 5A).
The luminal surface of the GI tract is covered with a mucus layer which acts as a protective barrier; moreover, mucus production by goblet cells plays a pivotal role in maintaining enteric barrier integrity.57 Of note, mucins were found altered both in patients with digestive and extra-digestive disease.56 In particular, several studies reported an increase in acidic mucin expression in colonic specimens from patients with inflammatory bowel diseases and Parkinson's disease (PD) who also displayed an impairment of the intestinal mucosal barrier.56 In particular, the increase in acidic mucin observed in PD patients could decrease the protective function of the mucus layer towards pathogen translocation as well as alter the repair processes of the epithelium, thus contributing to the impairment of the intestinal barrier in PD.56 Therefore, the effect of HEAL9 on acidic mucins content was examined. SAMP8 mice displayed a significant increase in colonic acid mucins, as compared with SAMR1 animals (Fig. 5B). Treatment with HEAL9 significantly reduced acid mucins levels, as compared with SAMP8 treated with placebo (Fig. 5B). Overall, these data indicate HEAL9's ability to normalize gut mucus barrier and permeability.
HEAL9 inhibits NLRP3 inflammasome activation pathway in brain and colon
Central and intestinal inflammation occurs in patients with AD.58 In addition, several studies in animal models of AD demonstrated that brain and colonic inflammation is mainly characterized by NLRP3 inflammasome signalling activation.10,59 Of note, Aβ, found increased in central and enteric tissues from SAMP8 mice, can promote immune/inflammatory pathways, including the activation of NLRP3 inflammasome.26 Thus, we evaluated the effect of HEAL9 on the expression of inflammasome signalling activation, including NLRP3, ASC and caspase-1 expression and IL-1β release in brain and colonic tissues from SAMP8 mice.Our results showed that the expression levels of NLRP3 and pro-caspase-1 (the inactive form of caspase-1) in cerebral cortex and distal colon did not differ among animal groups (Fig. 6A and B). Levels of ASC and cleaved active caspase-1 in brain and colon tissues were, however, significantly increased in SAMP8 mice as compared with SAMR1, indicating the activation of NLRP3 inflammasome pathway. In lines with these results, IL-1β levels in both brain and colonic tissues significantly increased in SAMP8 mice, as compared with SAMR1 (Fig. 6A and B). Treatment with HEAL9 significantly counteracted the increased expression of ASC and active caspase-1 as well as IL-1β levels in brain and colonic tissues observed in SAMP8-placebo mice, indicating that HEAL9 inhibits inflammasome pathway activation (Fig. 6A and B).
Discussion
The MGB axis is emerging as a pivotal pathway that can influence/play a role in neurological disorders, including MCI and AD and, its modulation with pre- and probiotics is gaining attention as a suitable therapeutical approach to prevent, or slow down disease progression.60 Based on this, the aim of the present study was to evaluate the efficacy of Lactiplantibacillus plantarum HEAL9 administration in alleviating cognitive decline and intestinal symptoms in a spontaneous mouse model of cognitive impairment and AD and to characterize the underlying molecular mechanisms. To pursue these aims, we employed a murine model of accelerated senescence, the SAMP8 mouse, regarded as a trustworthy experimental model to study the pathological features of AD.61 SAMP8 mice begin to develop early learning and memory deficits at 4 months of age, MCI at 6 months along with accumulation of Aβ, p-tau and neuroinflammation in the CNS, while the full development of brain pathology occurs from 8 months of age.26 In addition, SAMP8 mice at 6 months of age are characterized by altered intestinal barrier integrity and permeability, enteric inflammation, Aβ accumulation and gut motor dysfunctions.26 In our study, 4 months old SAMP8 mice were treated with HEAL9 for 2 months to evaluate the effects of HEAL9 on the early phases of learning and memory loss up to MCI, a phase that precedes the full development of AD.As a first step, we showed that HEAL9 could counteract cognitive decline and colonic dysmotility in SAMP8 animals. SAMP8 mice treated with HEAL9 displayed an amelioration of cognitive functions in terms of learning and memory capacities, as displayed in the MWM results. HEAL9 also mitigated the impaired locomotor abilities in SAMP8 animals, a motor symptom documented in 15–50% of AD patients.62 These results are in line with several studies performed in both animals and MCI/AD patients showing that oral intake of a single or a mixture of probiotics ameliorated spatial memory, anxiety-like and exploratory behaviors, as well as mental state and memory, respectively.20,63–65 Moreover, HEAL9 was recently shown to improve cognitive performance, especially memory and learning functions in moderately stressed but otherwise healthy adults.23 This indicates the potential benefit of the HEAL9 single strain probiotic as a treatment or even a preventative action in both diseased and healthy populations. In the present study HEAL9 also improved colonic transit in SAMP8 mice, through a normalization of enteric cholinergic and tachykininergic neurogenic contractions, suggesting that HEAL9 can influence enteric nervous system (ENS) activity and neurochemistry. These findings are in accordance with other studies showing that enteric bacteria and their metabolites, such as butyrate, can influence ENS neurochemistry. For instance, butyrate has been found to increase the proportion of myenteric cholinergic neurons in rats.66,67 However, the effect of HEAL9 on the enteric neuronal coding remodelling remains to be clarified and it represents the continuation of our research on this topic.
Subsequently, since Aβ accumulation in AD has been found to contribute to neuronal damage and inflammation in central and peripheral tissues, including the gut,68 the effect of HEAL9 in counteracting Aβ accumulation in the cerebral cortex and distal colon was assessed. HEAL9 consumption significantly decreased central and enteric Aβ levels in SAMP8 mice, thus providing the relevant finding that HEAL9 can counteract the accumulation of the main pathological protein in age-related disorders. These described findings are consistent with another study in which Bifidobacterium breve MCC1274 supplementation significantly decreased hippocampal Aβ production and Aβ plaque load in AD mice.69
Of interest, AD is associated with the activation of inflammatory cells in the CNS, including astrocytes and microglia, that contribute to neuroinflammatory and neurodegenerative processes.70,71 In this study, SAMP8 mice displayed astrocyte and microglial activation reactivity in the cerebral cortex, as documented by immunofluorescence analysis of GFAP and Iba-1. Supplementation with HEAL9 attenuated astrogliosis and microgliosis, suggesting that the beneficial effects of HEAL9 on cognitive decline could result from its ability to decrease Aβ levels and to attenuate inflammatory processes.
Of note, alterations in enteric barrier integrity and permeability, documented previously in MCI and AD patients, can facilitate the translocation of bacteria into the intestinal mucosa and then trigger systemic and central neurogenic and inflammatory responses.12 In addition, in a previous study we demonstrated that SAMP8 mice are characterized by an altered gut barrier, confirming the suitability of this model to investigate the role of MGB in brain disorders.26 Therefore, we chose to examine the effects of HEAL9 in restoring intestinal barrier permeability and integrity, by evaluating circulating LBP levels and acidic mucins, respectively.56 We found that HEAL9 treatment was associated with a decrease in LBP levels and a normalization of the acidic components of mucins, suggesting that HEAL9 treatment can prevent enteric barrier breakdown in early AD, thus decreasing pathogen translocation into intestinal mucosa, and subsequent induction of intestinal and central immune/inflammatory responses.8,12 In support of this view, we found that HEAL9 significantly decreased central and enteric inflammation as documented by the decrease in brain and colonic IL-1β levels. In line with our results, several studies have also shown that modulation of the gut microbiota with probiotics can counteract the impaired gut barrier and enteric and central inflammation using the SAMP8 model.65,72 In a recent publication involving healthy individuals with moderate stress, intake of HEAL9 for 12 weeks was found to maintain certain proinflammatory parameters at a lower level, as compared to the placebo group that showed significantly increased proinflammatory levels during the study.23 The strengthening of the intestinal wall, and the lower levels of proinflammatory markers in this trial support the findings in the randomized controlled trial in healthy human individuals.
Of interest, Aβ accumulation and increased IL-1β levels in both brain and colonic tissues from SAMP8 mice suggest the involvement of NLRP3 inflammasome signalling in the onset of enteric and central inflammation.73 Indeed, several studies in both animals and AD patients demonstrated an overactivation of NLRP3 inflammasome signalling in central and peripheral tissues.59,74 In addition, pioneering evidence suggests a dynamic interplay between the gut microbiota and NLRP3 inflammasome, currently referred to the “microbiota-gut-inflammasome-brain axis”. In this context, enteric bacteria can promote NLRP3 activation that, in turn, shape peripheral and central neurogenic/inflammatory responses, thus contributing to CNS neuroinflammation and neurodegeneration.10,75 In parallel, enteric, and central Aβ accumulation can also promote the overactivation of NLRP3, potentially altering gut microbiota composition, thus creating a vicious cycle that could further contribute to the ongoing neuroinflammatory and neurodegenerative processes.10 Therefore, in the final phase of the present study, we evaluated the effects of HEAL9 on the expression of inflammasome signalling components, including NLRP3, ASC, and caspase-1 in brain and colonic tissues from SAMP8 mice. AD-mice showed a significant increase in both ASC and cleaved-caspase-1 levels, confirming the involvement of NLRP3 inflammasome activation in neuroinflammatory processes.
In this context, HEAL9 intake significantly decreased ASC and cleaved-caspase-1 expression, while no changes in NLRP3 expression were detected. These findings support the hypothesis that HEAL9 counteracts the activation of the canonical inflammasome activation pathway,76 as demonstrated by the decrease in caspase-1 active form and IL-1β levels. These results provide the first evidence that HEAL9 counteracts cognitive decline via inhibition of inflammasome signalling activation in brain and gut tissues.
The molecular mechanisms through which HEAL9 inhibits inflammasome activation is not still elucidated but it could possibly be ascribed to increase in levels of butyrate. Indeed, butyrate has been found to exert anti-inflammatory activity via NLRP3 inhibition,77,78 to cross the blood brain barrier, to maintain its integrity, to decrease central neuroinflammation, and to counteract neuronal cell death in different animal models.77–81 Accordingly, human and pre-clinical studies have reported that Lactiplantibacillus plantarum assumption enriched the SCFAs-producers bacterial strains, with consequent increase in SCFAs, especially butyrate.65,82–84
However, the mechanisms underlying the inhibitory effects of HEAL9 on inflammasome signalling need to be further investigated.
In conclusion, intake of HEAL9 alleviated cognitive decline, normalized colonic motility, decreased astrogliosis and microgliosis, counteracted central and enteric Aβ protein accumulation, and prevented gut barrier impairment. Decreased gut and brain inflammation in SAMP8 mice in the prodromal phases of AD was also seen, proposed to be modulated by the microbiota-gut-inflammasome-brain axis. Based on these findings, we propose that HEAL9 could represent a suitable therapeutical option for alleviating MCI and AD, as well as related intestinal symptoms in the early stage of disease pathology. HEAL9 may also be relevant for other brain disorders characterized by intestinal symptoms, gut dysbiosis, enteric barrier alterations or activation of central and peripheral inflammasome pathways such as PD, depression, and autism.12
Author contributions
N. B. and C. P. contributed to the conception and design of the study. C. D. S. and V. D. wrote the original draft. C. D. S., L. B., A. D. A., C. I., C. S., C. P., G. B. performed the research and analyzed the data. V. D., N. B., R. C., T. A., M. E. and C. P. interpreted the data and prepared figures. L. A., M. F., N. B., and C. P. edited the manuscript. All authors read and approved the final manuscript.Ethics approval and consent to participate
All animals care and procedures were in accordance with the provisions of the European Community Council Directive 210/63/UE and approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health (authorization no. 115/2022-PR).Abbreviations
| MGB | Microbiota-gut-brain axis |
| AD | Alzheimer's disease |
| MCI | Mild cognitive impairment |
| PD | Parkinson's disease |
| CNS | Central nervous system |
| ENS | Enteric nervous system |
| IEB | Intestinal epithelial barrier |
| SAMP8 | Senescence-accelerated mouse prone 8 |
| SAMR1 | Senescence-accelerated mouse-resistant 1 |
| NLRP3 | Nucleotide-binding oligomerization domain leucine rich repeat and pyrin domain containing protein 3 |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| IL-1β | Interleukin-1β |
| Aβ1–42 | Amyloid-β |
| LBP | Lipopolysaccharide-binding protein |
| MWM | Morris water maze |
| ES | Electrical stimuli |
| SP | Substance P |
| L-NAME | Nω-NitroL-arginine methylester |
| TTX | Tetrodotoxin |
| CCh | Carbachol |
| PFA | Paraformaldehyde |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Gunilla Önning and Caroline Montelius, Probi AB, for fruitful discussions about the results obtained. We also thank Probi AB for financial support and for providing L. plantarum HEAL9 that was used in the study. This work was supported by a research grant from Probi AB, Lund, Sweden, which had no direct involvement in study design, data acquisition, data interpretation, or the decision to submit this work for publication, and by a research grant from MUR “Brain penetrant and gut-directed NLRP3 inhibitors in protection of physiological barriers and treatment of Alzheimer's disease (INFLA-BAD)” – 20229WP2JJ”.References
- C. A. Lane, J. Hardy and J. M. Schott, Alzheimer's disease, Eur. J. Neurol., 2018, 25, 59–70 CrossRef CAS PubMed.
- P. Scheltens, B. De Strooper, M. Kivipelto, H. Holstege, G. Chételat, C. E. Teunissen, J. Cummings and W. M. van der Flier, Alzheimer's disease, Lancet, 2021, 397, 1577–1590 CrossRef CAS PubMed.
- S. Khan, K. H. Barve and M. S. Kumar, Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer's Disease, Curr. Neuropharmacol., 2020, 18, 1106–1125 CrossRef CAS PubMed.
- G. Gallardo and D. M. Holtzman, Amyloid-β and Tau at the Crossroads of Alzheimer's Disease, Adv. Exp. Med. Biol., 2019, 1184, 187–203 CrossRef CAS PubMed.
- F. Leng and P. Edison, Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?, Nat. Rev. Neurol., 2021, 17, 157–172 CrossRef PubMed.
- R. Briggs, S. P. Kennelly and D. O'Neill, Drug treatments in Alzheimer's disease, Clin. Med., 2016, 16, 247–253 CrossRef PubMed.
- K. Socała, U. Doboszewska, A. Szopa, A. Serefko, M. Włodarczyk, A. Zielińska, E. Poleszak, J. Fichna and P. Wlaź, The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders, Pharmacol. Res., 2021, 172 DOI:10.1016/J.PHRS.2021.105840.
- C. Pellegrini, L. Antonioli, R. Colucci, C. Blandizzi and M. Fornai, Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases?, Acta Neuropathol., 2018, 136, 345–361 CrossRef CAS PubMed.
- M. Saresella, F. La Rosa, F. Piancone, M. Zoppis, I. Marventano, E. Calabrese, V. Rainone, R. Nemni, R. Mancuso and M. Clerici, The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease, Mol. Neurodegener., 2016, 11 DOI:10.1186/S13024-016-0088-1.
- C. Pellegrini, L. Antonioli, V. Calderone, R. Colucci, M. Fornai and C. Blandizzi, Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications?, Prog Neurobiol., 2020, 191 DOI:10.1016/J.PNEUROBIO.2020.101806.
- M. T. Heneka, R. M. McManus and E. Latz, Author Correction: Inflammasome signalling in brain function and neurodegenerative disease, Nat. Rev. Neurosci., 2019, 20, 187 CrossRef CAS PubMed.
- C. Pellegrini, M. Fornai, V. D'Antongiovanni, L. Antonioli, N. Bernardini and P. Derkinderen, The intestinal barrier in disorders of the central nervous system, Lancet Gastroenterol. Hepatol., 2023, 8, 66–80 CrossRef PubMed.
- N. M. Vogt, R. L. Kerby, K. A. Dill-McFarland, S. J. Harding, A. P. Merluzzi, S. C. Johnson, C. M. Carlsson, S. Asthana, H. Zetterberg, K. Blennow, B. B. Bendlin and F. E. Rey, Gut microbiome alterations in Alzheimer’s disease, Sci. Rep., 2017, 7 DOI:10.1038/S41598-017-13601-Y.
- M. Duan, F. Liu, H. Fu, S. Lu and T. Wang, Preoperative Microbiomes and Intestinal Barrier Function Can Differentiate Prodromal Alzheimer’s Disease From Normal Neurocognition in Elderly Patients Scheduled to Undergo Orthopedic Surgery, Front. Cell. Infect. Microbiol., 2021, 11 DOI:10.3389/FCIMB.2021.592842.
- X. Wang, Y. Niu, C. X. Yue, S. Fu and R. Wang, Increased ileal bile acid binding protein and galectin-9 are associated with mild cognitive impairment and Alzheimer's disease, J. Psychiatr. Res., 2019, 119, 102–106 CrossRef PubMed.
- T. Nakase, Y. Tatewaki, B. Thyreau, T. Mutoh, N. Tomita, S. Yamamoto, Y. Takano, M. Muranaka and Y. Taki, Impact of constipation on progression of Alzheimer's disease: A retrospective study, CNS Neurosci. Ther., 2022, 28, 1964–1973 CrossRef PubMed.
- Z. Rezaei Asl, G. Sepehri and M. Salami, Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease, Behav. Brain Res., 2019, 376 DOI:10.1016/J.BBR.2019.112183.
- L. Bonfili, V. Cecarini, M. Cuccioloni, M. Angeletti, S. Berardi, S. Scarpona, G. Rossi and A. M. Eleuteri, SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model, Mol. Neurobiol., 2018, 55, 7987–8000 CrossRef CAS PubMed.
- F. Leblhuber, K. Steiner, B. Schuetz, D. Fuchs and J. M. Gostner, Probiotic Supplementation in Patients with Alzheimer's Dementia - An Explorative Intervention Study, Curr. Alzheimer Res., 2018, 15, 1106–1113 CrossRef CAS PubMed.
- E. Akbari, Z. Asemi, R. D. Kakhaki, F. Bahmani, E. Kouchaki, O. R. Tamtaji, G. A. Hamidi and M. Salami, Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial, Front. Aging Neurosci., 2016, 8 DOI:10.3389/FNAGI.2016.00256.
- Z. Rezaeiasl, M. Salami and G. Sepehri, The Effects of Probiotic Lactobacillus and Bifidobacterium Strains on Memory and Learning Behavior, Long-Term Potentiation (LTP), and Some Biochemical Parameters in β-Amyloid-Induced Rat's Model of Alzheimer's Disease, Prev. Nutr. Food Sci., 2019, 24, 265–273 CrossRef CAS PubMed.
- L. Wang, Z. Zhao, L. Zhao, Y. Zhao, G. Yang, C. Wang, L. Gao, C. Niu and S. Li, Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson's Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder, J. Agric. Food Chem., 2022, 70, 1163–1173 CrossRef CAS PubMed.
- G. Önning, C. Montelius, M. Hillman and N. Larsson, Intake of Lactiplantibacillus plantarum HEAL9 Improves Cognition in Moderately Stressed Subjects: A Randomized Controlled Study, Nutrients, 2023, 15 DOI:10.3390/NU15153466.
- K. Sakurai, T. Toshimitsu, E. Okada, S. Anzai, I. Shiraishi, N. Inamura, S. Kobayashi, T. Sashihara and T. Hisatsune, Effects of Lactiplantibacillus plantarum OLL2712 on Memory Function in Older Adults with Declining Memory: A Randomized Placebo-Controlled Trial, Nutrients, 2022, 14 DOI:10.3390/NU14204300.
- G. Önning, M. Hillman, M. Hedin, C. Montelius, J. Eriksson, S. Ahrné and P. Jönsson, Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double blind, placebo-controlled study, Physiol. Behav., 2020, 15 DOI:10.1016/J.PHYSBEH.2020.113083.
- C. Pellegrini, S. Daniele, L. Antonioli, L. Benvenuti, V. D′Antongiovanni, R. Piccarducci, D. Pietrobono, V. Citi, E. Piragine, L. Flori, C. Ippolito, C. Segnani, P. Palazon-Riquelme, G. Lopez-Castejon, A. Martelli, R. Colucci, N. Bernardini, M. L. Trincavelli, V. Calderone, C. Martini, C. Blandizzi and M. Fornai, Prodromal Intestinal Events in Alzheimer’s Disease (AD): Colonic Dysmotility and Inflammation Are Associated with Enteric AD-Related Protein Deposition, Int. J. Mol. Sci., 2020, 21 DOI:10.3390/IJMS21103523.
- J. E. Morley, H. J. Armbrecht, S. A. Farr and V. B. Kumar, The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer's disease, Biochim. Biophys. Acta, 2012, 1822, 650–656 CrossRef CAS PubMed.
- J. F. Flood, S. A. Farr, F. E. Kaiser and J. E. Morley, Age-related impairment in learning but not memory in SAMP8 female mice, Pharmacol., Biochem. Behav., 1995, 50, 661–664 CrossRef CAS PubMed.
- C. Pellegrini, M. Fornai, L. Benvenuti, R. Colucci, V. Caputi, P. Palazon-Riquelme, M. C. Giron, A. Nericcio, F. Garelli, V. D'Antongiovanni, C. Segnani, C. Ippolito, M. Nannipieri, G. Lopez-Castejon, P. Pelegrin, G. Haskó, N. Bernardini, C. Blandizzi and L. Antonioli, NLRP3 at the crossroads between immune/inflammatory responses and enteric neuroplastic remodelling in a mouse model of diet-induced obesity, Br. J. Pharmacol., 2021, 178, 3924–3942 CrossRef CAS PubMed.
- Y. F. Xian, C. Qu, Y. Liu, S. P. Ip, Q. J. Yuan, W. Yang and Z. X. Lin, Magnolol Ameliorates Behavioral Impairments and Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease, Oxid. Med. Cell. Longevity, 2020 DOI:10.1155/2020/5920476.
- V. D’Antongiovanni, C. Pellegrini, L. Antonioli, L. Benvenuti, C. Di Salvo, L. Flori, R. Piccarducci, S. Daniele, A. Martelli, V. Calderone, C. Martini and M. Fornai, Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer’s Disease, Front. Pharmacol., 2021, 12 DOI:10.3389/FPHAR.2021.748021.
- V. D’Antongiovanni, M. Fornai, L. Benvenuti, C. Di Salvo, C. Pellegrini, F. Cappelli, S. Masi and L. Antonioli, Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity, Metabolites, 2023, 13 DOI:10.3390/METABO13030410.
- L. Benvenuti, V. D’Antongiovanni, C. Pellegrini, M. Fornai, N. Bernardini, C. Ippolito, C. Segnani, C. Di Salvo, R. Colucci, A. Martelli, L. Flori, V. Calderone, G. Carta, E. Ghelardi, M. Calvigioni, A. Panattoni, R. Coppolecchia, A. Arini and L. Antonioli, Dietary Supplementation with the Probiotic SF68 Reinforces Intestinal Epithelial Barrier in Obese Mice by Improving Butyrate Bioavailability, Mol. Nutr. Food Res., 2023, 67 DOI:10.1002/MNFR.202200442.
- V. D'Antongiovanni, C. Segnani, C. Ippolito, L. Antonioli, R. Colucci, M. Fornai, N. Bernardini and C. Pellegrini, Pathological Remodeling of the Gut Barrier as a Prodromal Event of High-Fat Diet-Induced Obesity, Lab. Invest., 2023, 103, 100194 CrossRef PubMed.
- V. D'Antongiovanni, C. Pellegrini, L. Benvenuti, M. Fornai, C. Di Salvo, G. Natale, L. Ryskalin, L. Bertani, E. Lucarini, L. Di Cesare Mannelli, C. Ghelardini, Z. H. Nemeth, G. Haskó and L. Antonioli, Anti-inflammatory Effects of Novel P2X4 Receptor Antagonists, NC-2600 and NP-1815-PX, in a Murine Model of Colitis, Inflammation, 2022, 45, 1829–1847 CrossRef PubMed.
- C. Pellegrini, V. D’Antongiovanni, F. Miraglia, L. Rota, L. Benvenuti, C. Di Salvo, G. Testa, S. Capsoni, G. Carta, L. Antonioli, A. Cattaneo, C. Blandizzi, E. Colla and M. Fornai, Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology, NPJ Parkinsons Dis., 2022, 8 DOI:10.1038/S41531-021-00263-X.
- C. Pellegrini, M. Fornai, R. Colucci, L. Benvenuti, V. D’Antongiovanni, G. Natale, F. Fulceri, M. Giorgis, E. Marini, S. Gastaldi, M. Bertinaria, C. Blandizzi and L. Antonioli, A Comparative Study on the Efficacy of NLRP3 Inflammasome Signaling Inhibitors in a Pre-clinical Model of Bowel Inflammation, Front. Pharmacol., 2018, 9 DOI:10.3389/FPHAR.2018.01405.
- C. Pellegrini, M. Fornai, R. Colucci, E. Tirotta, F. Blandini, G. Levandis, S. Cerri, C. Segnani, C. Ippolito, N. Bernardini, K. Cseri, C. Blandizzi, G. Haskó and L. Antonioli, Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration, J. Neuroinflammation, 2016, 13 DOI:10.1186/S12974-016-0608-5.
- V. D’Antongiovanni, L. Antonioli, L. Benvenuti, C. Pellegrini, C. Di Salvo, M. Calvigioni, A. Panattoni, L. Ryskalin, G. Natale, S. Banni, G. Carta, E. Ghelardi and M. Fornai, Use of Saccharomyces boulardii CNCM I-745 as therapeutic strategy for prevention of nonsteroidal anti-inflammatory drug-induced intestinal injury, Br. J. Pharmacol., 2023, 180 DOI:10.1111/BPH.16200.
- T. Zhang, Y. R. Han, J. Y. Wang, D. Hou, H. Deng, Y. L. Deng and Z. Song, Comparative Epidemiological Investigation of Alzheimer’s Disease and Colorectal Cancer: The Possible Role of Gastrointestinal Conditions in the Pathogenesis of AD, Front. Aging Neurosci., 2018, 10 DOI:10.3389/FNAGI.2018.00176.
- C. L. Chen, T. M. Liang, H. H. Chen, Y. Y. Lee, Y. C. Chuang and N. C. Chen, Constipation and Its Associated Factors among Patients with Dementia, Int. J. Environ. Res. Public Health, 2020, 17, 1–11 Search PubMed.
- D. Mercerón-Martínez, C. Ibaceta-González, C. Salazar, W. Almaguer-Melian, J. A. Bergado-Rosado and A. G. Palacios, Alzheimer's Disease, Neural Plasticity, and Functional Recovery, J. Alzheimer's Dis., 2021, 82, S37–S50 Search PubMed.
- H. Hampel, M. M. Mesulam, A. C. Cuello, M. R. Farlow, E. Giacobini, G. T. Grossberg, A. S. Khachaturian, A. Vergallo, E. Cavedo, P. J. Snyder and Z. S. Khachaturian, The cholinergic system in the pathophysiology and treatment of Alzheimer's disease, Brain, 2018, 141, 1917–1933 CrossRef PubMed.
- G. Di Benedetto, C. Burgaletto, C. M. Bellanca, A. Munafò, R. Bernardini and G. Cantarella, Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation, Cells, 2022, 11 DOI:10.3390/CELLS11172728.
- V. D’Antongiovanni, C. Pellegrini, L. Antonioli, C. Ippolito, C. Segnani, L. Benvenuti, A. D’Amati, M. Errede, D. Virgintino, M. Fornai and N. Bernardini, Enteric Glia and Brain Astroglia: Complex Communication in Health and Disease along the Gut-Brain Axis, Neuroscientist, 2024, 30 DOI:10.1177/10738584231163460.
- M. Yi, P. Yu, Q. Lu, H. M. Geller, Z. Yu and H. Chen, KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer's disease, Mol. Cell. Neurosci., 2016, 76, 21–32 CrossRef CAS PubMed.
- F. X. Sureda, J. Gutierrez-Cuesta, M. Romeu, M. Mulero, A. M. Canudas, A. Camins, J. Mallol and M. Pallàs, Changes in oxidative stress parameters and neurodegeneration markers in the brain of the senescence-accelerated mice SAMP-8, Exp. Gerontol., 2006, 41, 360–367 CrossRef CAS PubMed.
- Y. V. Gorina, A. B. Salmina, A. I. Erofeev, E. I. Gerasimov, A. V. Bolshakova, P. M. Balaban, I. B. Bezprozvanny and O. L. Vlasova, Astrocyte Activation Markers, Biochemistry, 2022, 87, 851–870 CAS.
- H. S. Kwon and S. H. Koh, Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes, Transl. Neurodegener., 2020, 9 DOI:10.1186/S40035-020-00221-2.
- B. Liu, J. Liu and J.-S. Shi, SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer's Disease, J. Alzheimer's Dis., 2020, 75, 385–395 CAS.
- G. S. Bloom, Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis, JAMA Neurol., 2014, 71, 505–508 CrossRef PubMed.
- D. A. Butterfield, A. M. Swomley and R. Sultana, Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression, Antioxid. Redox Signal., 2013, 19, 823–835 CrossRef CAS PubMed.
- J. Wang, B. J. Gu, C. L. Masters and Y. J. Wang, A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain, Nat. Rev. Neurol., 2017, 13, 612–623 CrossRef CAS PubMed.
- K. L. Puig, B. M. Lutz, S. A. Urquhart, A. A. Rebel, X. Zhou, G. D. Manocha, M. A. Sens, A. K. Tuteja, N. L. Foster and C. K. Combs, Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system, J. Alzheimer's Dis., 2015, 44, 1263–1278 CAS.
- V. D'Antongiovanni, C. Pellegrini, M. Fornai, R. Colucci, C. Blandizzi, L. Antonioli and N. Bernardini, Intestinal epithelial barrier and neuromuscular compartment in health and disease, World J. Gastroenterol., 2020, 26, 1564–1579 CrossRef PubMed.
- G. Bellini, L. Benvenuti, C. Ippolito, D. Frosini, C. Segnani, F. Rettura, A. Pancetti, L. Bertani, V. D’Antongiovanni, G. Palermo, E. Del Prete, L. Antonioli, V. Nardini, R. Morganti, C. Pellegrini, N. Bernardini, R. Ceravolo, M. Fornai and M. Bellini, Intestinal histomorphological and molecular alterations in patients with Parkinson’s disease, Eur. J. Neurol., 2023, 30 DOI:10.1111/ENE.15607.
- T. Breugelmans, B. Oosterlinck, W. Arras, H. Ceuleers, J. De Man, G. L. Hold, B. Y. De Winter and A. Smet, The role of mucins in gastrointestinal barrier function during health and disease, Lancet Gastroenterol. Hepatol., 2022, 7, 455–471 CrossRef PubMed.
- J. Xie, L. Van Hoecke and R. E. Vandenbroucke, The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology, Front. Immunol., 2021, 12 DOI:10.3389/FIMMU.2021.796867.
- M. T. Heneka, M. P. Kummer, A. Stutz, A. Delekate, S. Schwartz, A. Vieira-Saecker, A. Griep, D. Axt, A. Remus, T. C. Tzeng, E. Gelpi, A. Halle, M. Korte, E. Latz and D. T. Golenbock, NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice, Nature, 2013, 493, 674–678 CrossRef CAS PubMed.
- E. M. M. Quigley, Microbiota-Brain-Gut Axis and Neurodegenerative Diseases, Curr. Neurol. Neurosci. Rep., 2017, 17 DOI:10.1007/S11910-017-0802-6.
- B. Liu, J. Liu and J.-S. Shi, SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer's Disease, J. Alzheimer's Dis., 2020, 75, 385–395 CAS.
- J. Vöglein, K. Paumier, M. Jucker, O. Preische, E. McDade, J. Hassenstab, T. L. Benzinger, J. M. Noble, S. B. Berman, N. R. Graff-Radford, B. Ghetti, M. R. Farlow, J. Chhatwal, S. Salloway, C. Xiong, C. M. Karch, N. Cairns, H. Mori, P. R. Schofield, C. L. Masters, A. Goate, V. Buckles, N. Fox, M. Rossor, P. Chrem, R. Allegri, J. M. Ringman, G. Höglinger, H. Steiner, M. Dieterich, C. Haass, C. Laske, J. C. Morris, R. J. Bateman, A. Danek and J. Levin, Clinical, pathophysiological and genetic features of motor symptoms in autosomal dominant Alzheimer's disease, Brain, 2019, 142, 1429–1440 CrossRef PubMed.
- S. Y. Huang, L. H. Chen, M. F. Wang, C. C. Hsu, C. H. Chan, J. X. Li and H. Y. Huang, Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice, Nutrients, 2018, 10 DOI:10.3390/NU10070894.
- S. W. Lin, Y. S. Tsai, Y. L. Chen, M. F. Wang, C. C. Chen, W. H. Lin and T. J. Fang, Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice, Nutrients, 2021, 13 DOI:10.3390/NU13082860.
- X. Fang, M. Yue, J. Wei, Y. Wang, D. Hong, B. Wang, X. Zhou and T. Chen, Evaluation of the Anti-Aging Effects of a Probiotic Combination Isolated From Centenarians in a SAMP8 Mouse Model, Front. Immunol., 2021, 12 DOI:10.3389/FIMMU.2021.792746.
- Z. H. Geng, Y. Zhu, Q. L. Li, C. Zhao and P. H. Zhou, Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders, Front. Aging Neurosci., 2022, 14 DOI:10.3389/FNAGI.2022.810483.
- R. Soret, J. Chevalier, P. De Coppet, G. Poupeau, P. Derkinderen, J. P. Segain and M. Neunlist, Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats, Gastroenterology, 2010, 138 DOI:10.1053/J.GASTRO.2010.01.053.
- G. K. Gouras, T. T. Olsson and O. Hansson, β-Amyloid peptides and amyloid plaques in Alzheimer's disease, Neurotherapeutics, 2015, 12, 3–11 CrossRef CAS PubMed.
- M. Abdelhamid, C. Zhou, K. Ohno, T. Kuhara, F. Taslima, M. Abdullah, C. G. Jung and M. Michikawa, Probiotic Bifidobacterium breve Prevents Memory Impairment Through the Reduction of Both Amyloid-β Production and Microglia Activation in APP Knock-In Mouse, J. Alzheimer's Dis., 2022, 85, 1555–1571 CAS.
- X. H. Qian, X. L. Liu, S. Di Chen and H. D. Tang, Integrating peripheral blood and brain transcriptomics to identify immunological features associated with Alzheimer’s disease in mild cognitive impairment patients, Front. Immunol., 2022, 13 DOI:10.3389/FIMMU.2022.986346.
- T. A. Pascoal, A. L. Benedet, N. J. Ashton, M. S. Kang, J. Therriault, M. Chamoun, M. Savard, F. Z. Lussier, C. Tissot, T. K. Karikari, J. Ottoy, S. Mathotaarachchi, J. Stevenson, G. Massarweh, M. Schöll, M. J. de Leon, J. P. Soucy, P. Edison, K. Blennow, H. Zetterberg, S. Gauthier and P. Rosa-Neto, Microglial activation and tau propagate jointly across Braak stages, Nat. Med., 2021, 27, 1592–1599 CrossRef CAS PubMed.
- S. Y. Huang, L. H. Chen, M. F. Wang, C. C. Hsu, C. H. Chan, J. X. Li and H. Y. Huang, Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice, Nutrients, 2018, 10 DOI:10.3390/NU10070894.
- C. Pellegrini, M. Fornai, L. Antonioli, C. Blandizzi and V. Calderone, Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases, Int. J. Mol. Sci., 2019, 20 DOI:10.3390/IJMS20122876.
- I. C. Stancu, N. Cremers, H. Vanrusselt, J. Couturier, A. Vanoosthuyse, S. Kessels, C. Lodder, B. Brône, F. Huaux, J. N. Octave, D. Terwel and I. Dewachter, Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo, Acta Neuropathol., 2019, 137, 599–617 CrossRef CAS PubMed.
- G. B. Rogers, D. J. Keating, R. L. Young, M. L. Wong, J. Licinio and S. Wesselingh, From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways, Mol. Psychiatry, 2016, 21, 738–748 CrossRef CAS PubMed.
- C. Pellegrini, L. Antonioli, G. Lopez-Castejon, C. Blandizzi and M. Fornai, Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation, Front. Immunol., 2017, 8 DOI:10.3389/FIMMU.2017.00036.
- X. Pan, X. Fang, F. Wang, H. Li, W. Niu, W. Liang, C. Wu, J. Li, X. Tu, L. L. Pan and J. Sun, Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms, Br. J. Pharmacol., 2019, 176, 4446–4461 CrossRef CAS PubMed.
- X. Li, C. Wang, J. Zhu, Q. Lin, M. Yu, J. Wen, J. Feng and C. Hu, Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway, Oxid. Med. Cell. Longevity, 2022 DOI:10.1155/2022/3745135.
- H. Wei, C. Yu, C. Zhang, Y. Ren, L. Guo, T. Wang, F. Chen, Y. Li, X. Zhang, H. Wang and J. Liu, Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis, Biomed. Pharmacother., 2023, 160 DOI:10.1016/J.BIOPHA.2023.114308.
- Z. Wang, T. Li, M. Du, L. Zhang, L. Xu, H. Song and J. Zhang, β-hydroxybutyrate improves cognitive impairment caused by chronic cerebral hypoperfusion via amelioration of neuroinflammation and blood-brain barrier damage, Brain Res. Bull., 2023, 193, 117–130 CrossRef CAS PubMed.
- H. Li, J. Sun, F. Wang, G. Ding, W. Chen, R. Fang, Y. Yao, M. Pang, Z. Q. Lu and J. Liu, Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice, Brain Res., 2016, 1642, 70–78 CrossRef CAS PubMed.
- Z. Zeng, Z. Huang, W. Yue, S. Nawaz, X. Chen and J. Liu, Lactobacillus plantarum modulate gut microbiota and intestinal immunity in cyclophosphamide-treated mice model, Biomed. Pharmacother., 2023, 169 DOI:10.1016/J.BIOPHA.2023.115812.
- G. Wang, J. Song, Y. Huang, X. Li, H. Wang, Y. Zhang and H. Suo, Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis, Food Funct., 2022, 13, 675–687 RSC.
- Y. He, L. Mei, L. Wang, X. Li, J. Zhao, H. Zhang, W. Chen and G. Wang, Lactiplantibacillus plantarum CCFM1019 attenuate polycystic ovary syndrome through butyrate dependent gut-brain mechanism, Food Funct., 2022, 13, 1380–1392 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02075h |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |