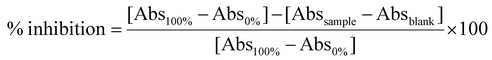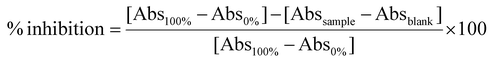Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potential bioaccessibility and bioavailability of polyphenols and functional properties of tiger nut beverage and its by-product during in vitro digestion†
Paula
Llorens‡
a,
Manuela Flavia
Chiacchio‡
b,
Silvia
Tagliamonte
b,
Ana
Juan-García
a,
Noelia
Pallarés
a,
Juan Carlos
Moltó
a,
Paola
Vitaglione
 *b and
Cristina
Juan
a
*b and
Cristina
Juan
a
aLaboratory of Food Chemistry and Toxicology, Faculty of Pharmacy, University of Valencia, Avda. Vicent Andrés Estellés, s/n, 46100 Burjassot, Spain
bDepartment of Agricultural Sciences, University of Naples Federico II, 80055 Portici, Italy. E-mail: paola.vitaglione@unina.it
First published on 5th July 2024
Abstract
“Horchata de chufa” is a beverage produced from tiger nut tubers, which yields a high amount of by-product. This study explored the functional properties of the Spanish tiger nut beverage (TNB) and its by-product (TNBP) together with the bioaccessibility and bioavailability of polyphenols in vitro. TNB and TNBP were characterized for polyphenols via LC/MS/MS and underwent in vitro digestion (INFOGEST). The total antioxidant capacity (TAC) of all bioaccessible fractions and digestion residues was assessed. Intestinal bioaccessible fractions were tested for the ability to inhibit the activity of digestive enzymes (α-amylase, α-glucosidase, and lipase) and the content of polyphenols, whose bioavailability was assessed in a Caco-2 cell model. Thirteen polyphenols were quantified and found to be more abundant in TNB (603 ± 1.4 μg g−1 DW) than in TNBP (187 ± 1.0 μg g−1 DW). Polyphenol bioaccessibility was higher for TNBP than that for TNB (57% vs. 27%), and despite a similar TAC of the intestinal bioaccessible fractions (10.2 ± 0.1 μmoL vs. 9.2 ± 0.03 μmoL eq. Trolox per g DW for TNB and TNBP, respectively), the different patterns of polyphenols released upon digestion suggested the higher ability of TNBP fraction to inhibit α-glucosidase and lipase. TNBP digestion residue showed higher TAC than TNB. Moreover, TNB polyphenols exhibited over 80% bioavailability, whereas TNBP polyphenols’ bioavailability ranged from 62% to 84%. Overall, the findings demonstrated that TNBP maintains a high nutritional value, thus suggesting its possible reuse in innovative, healthy, and sustainable foods.
1. Introduction
Tiger nuts (Cyperus esculentus L.) belong to the Cyperaceae family and are small and sweet tubers that grow in the roots of a perennial grass-like plant that is commonly found in Mediterranean countries. Most of the tiger nuts produced in Valencia (Spain) bear the esteemed protected designation of origin (PDO) “Chufa de Valencia” since 1995.1Tiger nut has high nutritional quality, containing 23.7% lipids (mainly monounsaturated triglycerides), 42.5% carbohydrates, 6.1% proteins, and 7.4% fiber, alongside minerals, such as iron, magnesium, potassium and phosphorus; vitamins C and E;2,3 and polyphenols in the range of 806.7–1150.7 μM GAE g−1.4 This overall composition has been considered responsible for the health benefits associated with the tuber consumption, supporting their use in the prevention of intestinal, coronary heart, and metabolic diseases.5
Tiger nuts are generally consumed fresh or as “Horchata de Chufa”, a beverage made from the tubers and very popular in Spain as a plant-based milk alternative for people with animal-milk allergies and lactose intolerance.3
In Spain, 40 to 50 million liters of “horchata” is manufactured every year,6,7 resulting in the generation of substantial liquid and solid by-products.8 Notably, the solid by-product accounts for 60% of the raw material by weight9 and has historically been discarded or utilized as animal feed.4 The valorization of this by-product represents not only an overlooked opportunity but also an urgent necessity in the pursuit of more sustainable practices. The incorporation of this by-product into the food chain could yield significant environmental and economic benefits, facilitating a shift towards more sustainable production and diet, in line with the key objectives of the United Nations 2030 Agenda.10 This transformation underscores the critical importance of food upcycling and reuse, transforming what was once considered waste into a valuable resource, thereby aligning with the growing awareness around waste food reduction.
From a nutritional perspective, the tiger nut beverage contains carbohydrates (17%), of which 3.1% is starch, lipids (2.7%), proteins (1.2%), and dietary fiber (0.1%)11 beside phenolic compounds and other bioactive compounds.12 On the other hand, the tiger nut solid by-product is composed of 60% dietary fiber,13 lipids and antioxidant compounds as polyphenols in the range of 186.5–222.6 mg GAE per 100 g.14
Previous studies explored the possibility of reusing tiger nut by-product in the food chain. Specifically, some studies focused on the incorporation of the byproduct in gluten-free bread and butter,15 biscuits,16 and fiber-enriched meat burgers.17
The characterization of polyphenols present in the tiger nut beverage as well as the potential nutritional and functional effects of both the beverage and the solid tiger nut by-product upon consumption, are still under investigation.
This study aimed to explore the bioaccessibility and bioavailability of polyphenols from the tiger nut beverage (TNB) and by-product (TNBP) during in vitro digestion and in Caco-2 cells, respectively, along with their potential functional properties in the gastrointestinal tract, such as the total antioxidant capacity (TAC), and the inhibitory activity on digestive enzymes α-amylase, α-glucosidase, and lipase.
2. Materials and methods
2.1 Chemicals
ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)), potassium persulfate, ethanol, methanol, water, formic acid, acetonitrile, HCl 12 M, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), NaOH 6 M, 2,3-dimercapto-1-propanol tributyrate, starch azure, para-nitrophenyl-α-D-glucopyranoside, 5-5′-dithio-bis (2-nitrobenzoic acid), Tris[hydroxymethyl]aminomethane, calcium chloride, sodium phosphate monobasic dihydrate, lipase from porcine pancreas (Type II 100–500 U mg−1), α-amylase from porcine pancreas (type VI-B ≥ 10 U mg−1), α-glucosidase from Saccharomyces cerevisiae (type I ≥ 10 U mg−1), pancreatin from porcine pancreas (4× USP), and pepsin from porcine gastric mucosa (≥250 U mg−1), caffeic acid, trans-ferulic acid, protocatechuic acid, p-coumaric acid, 4-hydroxybenzoic acid, luteolin, and vanillic acid were all purchased from Sigma-Aldrich, Milan, Italy. Glacial acetic acid (96%) and cellulose powder from spruce were purchased from VWR Chemicals (Pennsylvania, USA) and Fluka (Steinheim, GE), respectively.2.2 Tiger nut beverage preparation and by-product collection
Tiger nut flour (PDO “Chufa de Valencia”) Món Orxata S.L (Valencia, Spain) was bought in a local market. TNB was obtained according to the instructions reported in the tiger nut flour package. In detail, 50 g of tiger nut flour was weighed, added to 225 mL of water and mixed using a kitchen blender (Braun, Germany) for 2 min. Then, the mixture was filtered to collect the liquid fraction, i.e., the tiger nut beverage (TNB) and the solid residue fraction, i.e., the tiger nut by-product (TNBP). Prior to the analyses, the samples were kept at −30 °C and freeze-dried by Heto LyoLab 3000 (Fisher Scientific, UK). The sample was digested and analyzed according to the planned study (Fig. 1).2.3 Simulated gastrointestinal digestion in vitro
The simulated gastrointestinal digestion of TNB and TNBP was performed in vitro using the INFOGEST method with slight modifications.18Simulated fluids: salivary (SSF), gastric (SGF) and intestinal (SIF), were used and prepared as detailed by Brodkorb et al.18 These fluids were used in each simulated digested phases: simulated salivary phase (SSP), gastric (SGP) and intestinal (SIP). Briefly, 1.75 mL of simulated salivary fluid (SSF) stock solution (pH 7.0), 0.25 mL of amylase solution (1500 U mL−1), and 12.5 μL of 0.3 M CaCl2 were added to 2.5 g of freeze-dried sample and distilled water was added for the adjustment to a final volume of 5 mL. The resulting mixture was incubated for a period of 2 min at 37 °C in a thermostatic shaking water bath (160 rpm). The sample from oral digesta was centrifuged at 4 °C for 15 min at 4000 rpm and the supernatant, which is considered the bioaccessible fraction (BF), was collected and separated from the pellet. Subsequently, the SGP was started by adding to the SSP content 3.75 mL of simulated gastric fluid (SGF) stock solution (pH 3.0), 0.8 mL of pepsin solution 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1 and 2.5 μL 0.3 M CaCl2. After reaching a pH of 3.0, adjusting with 1 M HCl, the volume was made up to 10 mL with water. The mixture was incubated in a thermostatic shaking water bath at 37 °C for 2 h at 130 rpm. As before, the sample was centrifuged and separated from the pellet. Finally, the gastric content (10 mL) was mixed with 5.5 mL of simulated intestinal fluid (SIF) stock solution (pH 7), 2.5 mL of pancreatin solution 800 U mL−1, 20 μL of 0.3 M CaCl2 and bile solution 1.25 mL (160 mM) to initiate the last step of digestion. The pH of the mixture was adjusted to 7.0 with 1 M NaOH and water was added. The SIP was incubated in a thermostatic shaking bath for 2 h at 37 °C (100 rpm) and then centrifuged at 4 °C for 15 min at 4000 rpm. One mL aliquots of the collected supernatants (corresponding to the soluble BF) and pellets (corresponding to the insoluble, non-bioaccessible fractions) at the end of SSP, SGP, and SIP were freeze-dried prior to the following analyses: TAC, bioaccessibility and bioavailability of polyphenols (only the intestinal BF), and inhibition activity against digestive enzymes (only on the intestinal BF), TAC on the insoluble fraction (pellet, non-bioaccessible fraction).
000 U mL−1 and 2.5 μL 0.3 M CaCl2. After reaching a pH of 3.0, adjusting with 1 M HCl, the volume was made up to 10 mL with water. The mixture was incubated in a thermostatic shaking water bath at 37 °C for 2 h at 130 rpm. As before, the sample was centrifuged and separated from the pellet. Finally, the gastric content (10 mL) was mixed with 5.5 mL of simulated intestinal fluid (SIF) stock solution (pH 7), 2.5 mL of pancreatin solution 800 U mL−1, 20 μL of 0.3 M CaCl2 and bile solution 1.25 mL (160 mM) to initiate the last step of digestion. The pH of the mixture was adjusted to 7.0 with 1 M NaOH and water was added. The SIP was incubated in a thermostatic shaking bath for 2 h at 37 °C (100 rpm) and then centrifuged at 4 °C for 15 min at 4000 rpm. One mL aliquots of the collected supernatants (corresponding to the soluble BF) and pellets (corresponding to the insoluble, non-bioaccessible fractions) at the end of SSP, SGP, and SIP were freeze-dried prior to the following analyses: TAC, bioaccessibility and bioavailability of polyphenols (only the intestinal BF), and inhibition activity against digestive enzymes (only on the intestinal BF), TAC on the insoluble fraction (pellet, non-bioaccessible fraction).
2.4 Polyphenol identification and quantification
Polyphenol characterization was carried out in the extracts obtained from TNB and TNBP (before and after digestion, i.e., bioaccessible fractions) (section 2.5.2) and from the compartments (apical and basolateral) collected over the bioavailability study in Caco-2 cells (section 2.7.2).Polyphenols were identified and quantified by high performance liquid chromatography coupled to ultraviolet/visible detector (HPLC-UV/VIS) and were further confirmed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS).
The analysis by HPLC-UV/VIS was performed using an HPLC SHIMADZU equipped with UV/VIS SPD-20A detector (Prominence, USA) and according to the method proposed by Chiacchio et al.19 For the chromatographic separation, a Prodigy ODS3 100 Å column (250 mm × 4.6 mm, particle size 5 μm) (Phenomenex, CA, USA) was used and the eluents were solvent A, consisting of HPLC grade water with 0.2% (v/v) formic acid, and solvent B, a mixture of acetonitrile/methanol (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v). The gradient was programmed as follows: 20% B (2 min), 30% B (8 min), 40% B (18 min), 50% B (26 min), 90% B (34 min), 90% B (37 min), 20% B (39 min), 20% B (43 min). The injection volume was 20 μL with a flow rate of 1 mL min−1. The analyses were conducted in triplicate. Table S1† shows the retention times, the limit of detection (LOD) and the limit of quantification (LOQ) of all the polyphenols identified by HPLC-UV.
40 v/v). The gradient was programmed as follows: 20% B (2 min), 30% B (8 min), 40% B (18 min), 50% B (26 min), 90% B (34 min), 90% B (37 min), 20% B (39 min), 20% B (43 min). The injection volume was 20 μL with a flow rate of 1 mL min−1. The analyses were conducted in triplicate. Table S1† shows the retention times, the limit of detection (LOD) and the limit of quantification (LOQ) of all the polyphenols identified by HPLC-UV.
Protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, p-coumaric acid, trans-ferulic acid, luteolin, trans-cinnamic, acid caffeic acid hexoside, ferulic acid acyl-b-D-glucoside, 3-hydroxybenzoic acid, an epicatechin derivative (flavan-3-ol) and ethyl vanillin were identified by the corresponding standard compounds and confirmed by LC-MS/MS. Specifically, chromatographic conditions were the same as described above and an API 3000 Triple Quadrupole mass spectrometer (Applied Biosystem Sciex) with a TurboIonSpray as a source was used; the analysis was in Multiple Reaction Monitoring (MRM) and in negative ion mode. The setting conditions for the analysis were the same as reported in the method above: drying gas (air) was heated to 400 °C, capillary voltage (IS) was set to 4000 V, nebulizer gas (air) 12 (arbitrary units), curtain gas (N2) 14 (arbitrary units), collision gas (N2) 4 (arbitrary units). The acquisition parameters such as collision energy (CE), declustering potential (DP), and the collision cell exit potential (CXP) are provided in Table S2.†
The bioaccessibility (%) of each polyphenol was determined using the following equation:
CS stands for the concentration in the extracted intestinal supernatant and CP for the concentration in the phenolic extract of the non-digested product.
2.5 Total antioxidant capacity (TAC)
The direct TAC of TNBP samples before digestion and the insoluble fraction (pellet) obtained upon in vitro digestion were analyzed by using the Quencher methodology.20The soluble TAC of TNB and TNBP samples before digestion and the bioaccessible fractions (supernatants) obtained upon in vitro digestion was determined by using the ABTS radical scavenging methodology.21
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) was added. The oral, gastric and intestinal freeze-dried BF samples were resuspended in 1 mL of methanol/water solution (70
30 v/v) was added. The oral, gastric and intestinal freeze-dried BF samples were resuspended in 1 mL of methanol/water solution (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v). After vortexing for 1 min, the samples were centrifuged for 10 min at 14
30 v/v). After vortexing for 1 min, the samples were centrifuged for 10 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm and 4 °C. The reaction was initiated by adding 1 mL of ABTS˙+ solution (7 mM) to 100 μL of the previously diluted phenolic extract. After 2.5 min, the absorbance was measured at 734 nm. The same reaction was carried out for the blank, where the 100 μL of the sample extract was substituted by methanol/water solution (70
800 rpm and 4 °C. The reaction was initiated by adding 1 mL of ABTS˙+ solution (7 mM) to 100 μL of the previously diluted phenolic extract. After 2.5 min, the absorbance was measured at 734 nm. The same reaction was carried out for the blank, where the 100 μL of the sample extract was substituted by methanol/water solution (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v). Each reaction was performed in triplicate, and the results were expressed as μmol eq. Trolox per g of dry sample, using a Trolox calibration curve.
30 v/v). Each reaction was performed in triplicate, and the results were expressed as μmol eq. Trolox per g of dry sample, using a Trolox calibration curve.
2.6 Inhibition of digestive enzymes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 10 min. To evaluate the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the buffer Tris-HCl 0.1 M. Likewise, to evaluate the minimum activity of the enzyme (0%), the sample and the enzyme were substituted with Tris-HCl 0.1 M. For each sample, a blank was prepared, in which the buffer was added instead of the enzyme. The absorbance of the resulting supernatants was measured at 595 nm with a UV-VIS spectrophotometer (PG Instruments, UK). Each determination was conducted in triplicate, and the results were expressed as % inhibition using the following formula:
800 rpm for 10 min. To evaluate the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the buffer Tris-HCl 0.1 M. Likewise, to evaluate the minimum activity of the enzyme (0%), the sample and the enzyme were substituted with Tris-HCl 0.1 M. For each sample, a blank was prepared, in which the buffer was added instead of the enzyme. The absorbance of the resulting supernatants was measured at 595 nm with a UV-VIS spectrophotometer (PG Instruments, UK). Each determination was conducted in triplicate, and the results were expressed as % inhibition using the following formula:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 10 min and the absorbance of supernatants was measured at 412 nm with a UV-VIS spectrophotometer (PG Instruments, UK). To assess the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the Tris-HCl buffer 0.1 M. To evaluate the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the buffer Tris-HCl 0.1 M. Likewise, to evaluate the minimum activity of the enzyme (0%), the sample and the enzyme were substituted with Tris-HCl 0.1 M. For each sample, a blank was prepared, to which the buffer was added in place of the enzyme. Each determination was conducted in three replicates, and the results were expressed as % inhibition using the following formula:
800 rpm for 10 min and the absorbance of supernatants was measured at 412 nm with a UV-VIS spectrophotometer (PG Instruments, UK). To assess the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the Tris-HCl buffer 0.1 M. To evaluate the maximum activity of the enzyme (100%), the same reaction was reproduced by replacing the sample with the buffer Tris-HCl 0.1 M. Likewise, to evaluate the minimum activity of the enzyme (0%), the sample and the enzyme were substituted with Tris-HCl 0.1 M. For each sample, a blank was prepared, to which the buffer was added in place of the enzyme. Each determination was conducted in three replicates, and the results were expressed as % inhibition using the following formula:2.7 In vitro bioavailability of polyphenols
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. Simultaneously, the basolateral compartment received an equal volume of fresh HBSS-HEPES solution. Over the course of the experiment, samples from the basolateral compartment were collected at one-, two-, and three-hour intervals. At the four-hour mark, contents from both the apical and basolateral compartments were separately gathered to evaluate the transepithelial transport of polyphenols. All collected samples during the experiment were freeze-dried and resuspended in methanol/water (70
1 volume ratio. Simultaneously, the basolateral compartment received an equal volume of fresh HBSS-HEPES solution. Over the course of the experiment, samples from the basolateral compartment were collected at one-, two-, and three-hour intervals. At the four-hour mark, contents from both the apical and basolateral compartments were separately gathered to evaluate the transepithelial transport of polyphenols. All collected samples during the experiment were freeze-dried and resuspended in methanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) before polyphenol analysis by HPLC UV/VIS, as previously described (section 2.4).
30 v/v) before polyphenol analysis by HPLC UV/VIS, as previously described (section 2.4).
The bioavailability (%) of each polyphenol was determined using the following equation:
CB stands for the phenolic concentration in the basolateral compartment after the time of incubation (1 h, 2 h, 3 h, 4 h), and CS is the phenolic concentration at time 0 h in the apical compartment; this concentration is the extracted intestinal supernatant diluted in HBSS-HEPES solution before the exposure cells.
2.8 Statistical analysis
All the analysis was performed in triplicate, and the results were expressed as mean ± standard deviation (SD). Statistical analysis was performed using statistical software SPSS (version 20.0, SPSS, Inc., Chicago, IL, USA). The differences between samples were assessed by One-way ANOVA and Tukey post hoc test and by independent samples t-test (p < 0.05).3. Results and discussion
3.1 Bioaccessibility of polyphenols from TNB and TNBP
Fig. S2† shows the HPLC chromatograms obtained from the analysis of polyphenols from TNB and TNBP. Thirteen polyphenols were identified and quantified in TNB and TNBP. The concentrations of the polyphenols in TNB and TNBP as well as in the bioaccessible oral, gastric and intestinal fractions collected during in vitro digestion, are reported in Tables 1 and 2.| Polyphenol | TNB | Oral phase | Gastric phase | Intestinal phase | Bioaccessibility (%) |
|---|---|---|---|---|---|
| Data are shown as mean ± SD. Different lowercase letters indicate significant differences between the digestion phases for each polyphenol (in the same row). Different uppercase letters indicate differences in the bioaccessibility between polyphenols (in the same column) assessed by ne-way ANOVA and Tukey post hoc (p < 0.05). | |||||
| Caffeic acid hexoside | 99.6 ± 0.8a | 10.8 ± 0.1d | 22.8.4 ± 0.1c | 33.0 ± 0.1b | 33.2 ± 0.3E |
| Ferulic acid acyl-b-D-glucoside | 50.0 ± 0.1b | 24.3 ± 0.2c | 8.6 ± 0.04d | 59.0 ± 0.4a | 118.1 ± 0.6A |
| Protocatechuic acid | 52.8 ± 0.4a | 6.5 ± 0.002d | 9.1 ± 0.01c | 21.9 ± 0.2b | 41.4 ± 0.6C |
| 3-Hydroxybenzoic acid | 7.2 ± 0.1a | 0.4 ± 0.01b | 0.2 ± 0.001c | 0.07 ± 0.02d | 1.2 ± 0.3J |
| 4-Hydroxybenzoic acid | 2.6 ± 0.02a | 0.2 ± 0.02c | 0.7 ± 0.004b | 0.2 ± 0.03c | 5.6 ± 1.1I |
| Vanillic acid | 30.4 ± 0.004a | 1.8 ± 0.004d | 2.2 ± 0.03c | 4.5 ± 0.1b | 14.9 ± 0.3G |
| p-Coumaric acid | 2.1 ± 0.01a | 0.3 ± 0.01c | 0.2 ± 0.003c | 0.8 ± 0.03b | 39 ± 1.3D |
| trans-Ferulic acid | 57.4 ± 0.4a | 4.4 ± 0.003b | 3.6 ± 0.1c | 4.3 ± 0.1b | 7.5 ± 0.1I |
| Epicatechin derivative | 290.6 ± 1.2a | 21.5 ± 0.1b | 20.9 ± 0.2b | 21.6 ± 0.1b | 7.4 ± 0.01I |
| Luteolin | 4.4 ± 0.1a | 0.3 ± 0.001c | 0.4 ± 0.004c | 1.0 ± 0.01b | 23.2 ± 0.4F |
| trans-Cinnamic acid | 3.1 ± 0.03a | 0.1 ± 0.0001c | 0.1 ± 0.002c | 0.3 ± 0.02b | 9.8 ± 0.7H |
| Ethyl vanillin | 3.0 ± 0.04a | 0.5 ± 0.004d | 0.7 ± 0.01c | 1.3 ± 0.01b | 44.9 ± 0.4B |
| ΣTotal polyphenols | 603.3 ± 1.4 a | 71.2 ± 0.3 c | 69.5 ± 0.4 c | 148.0 ± 0.9 b |
![[x with combining macron]](https://www.rsc.org/images/entities/b_i_char_0078_0304.gif) = 27.4 ± 0.5 = 27.4 ± 0.5
|
| Polyphenol | TNBP | Oral phase | Gastric phase | Intestinal phase | Bioaccessibility (%) |
|---|---|---|---|---|---|
| Data are shown as mean ± SD. Different lowercase letters indicate significant differences between the digested steps for each phenolic compound (in the same row). Different uppercase letters indicate differences in the bioaccessibility between phenolic compounds (in the same column) assessed by one-way ANOVA and Tukey post hoc (p < 0.05). | |||||
| Caffeic acid hexoside | 20.4 ± 0.01b | 7.1 ± 0.1d | 15.2 ± 0.3c | 25.2 ± 0.3a | 123.6 ± 1.3A |
| Ferulic acid acyl-b-D-glucoside | 55.7 ± 0.3a | 4.8 ± 0.04d | 9.5 ± 0.3c | 43.4 ± 1.1b | 77.9 ± 1.8D |
| Protocatechuic acid | 23.8 ± 0.2a | 3.7 ± 0.02d | 6.5 ± 0.01c | 9.9 ± 0.04b | 55.7 ± 0.6E |
| 3-Hydroxybenzoic acid | 1.0 ± 0.01a | 0.6 ± 0.01c | 0.8 ± 0.02b | 0.6 ± 0.04c | 56.5 ± 4.1E |
| 4-Hydroxybenzoic acid | 0.4 ± 0.02a | 0.2 ± 0.01b | 0.4 ± 0.002a | 0.4 ± 0.01a | 99.8 ± 3.2B |
| Vanillic acid | 12.5 ± 0.3a | 1.5 ± 0.01c | 1.8 ± 0.01c | 3.6 ± 0.06b | 28.7 ± 0.6G |
| p-Coumaric acid | 0.7 ± 0.002a | 0.2 ± 0.002d | 0.4 ± 0.01c | 0.6 ± 0.1b | 87.1 ± 6.4C |
| trans-Ferulic acid | 17.4 ± 0.1a | 2.9 ± 0.01c | 2.6 ± 0.02d | 5.7 ± 0.2b | 33.0 ± 0.9F,G |
| Epicatechin derivative | 47.6 ± 0.1a | 10.8 ± 0.2c | 13.3 ± 0.02b | 13.6 ± 0.5b | 28.6 ± 1.0G |
| Luteolin | 4.8 ± 0.02a | 0.3 ± 0.003d | 0.4 ± 0.0001c | 0.8 ± 0.01b | 16.6 ± 0.3H |
| trans-Cinnamic acid | 0.7 ± 0.01a | 0.1 ± 0.003c | 0.07 ± 0.002d | 0.3 ± 0.003b | 38.1 ± 0.6F |
| Ethyl vanillin | 1.8 ± 0.01a | 0.1 ± 0.002b | 0.1 ± 0.0004b | 0.08 ± 0.003c | 4.6 ± 0.1I |
| ΣTotal polyphenols | 186.9 ± 1.0 a | 32.5 ± 0.2 d | 50.8 ± 0.6 c | 107.6 ± 1.4 b |
![[x with combining macron]](https://www.rsc.org/images/entities/b_i_char_0078_0304.gif) = 57.5 ± 0.6 = 57.5 ± 0.6
|
Results showed that total polyphenols in TNB tripled those in TNBP; however, in both food matrices, epicatechin derivative was the most abundant polyphenol, followed by caffeic acid hexoside in TNB and ferulic acid acyl-b-D-glucoside in TNBP.
Previously, Hernández-Olivas et al.25 assessed the total phenolic compounds (TPC) in nut-derived products, reporting the highest value in the beverage. In that study, the concentration of TPC, measured by the Folin–Ciocalteu method, was 7.7 mg GAE g−1 DW, thus being higher than that found in the present study using a chromatographic analysis (0.6 ± 0.001 mg g−1 DW). Both the different varieties of the tiger nut and the analytical methods used across the studies accounted for the different results; indeed, the Folin–Ciocalteu method yields TPC values higher than the sum of the individual phenolic compounds assessed by the chromatographic method.26 Regarding the polyphenols in TNBP, the findings of this study disagreed with previous studies both for the overall content and the profile. The total content of polyphenols found in the present study was 3-fold higher than in a previous study.9 Moreover, previous studies reported ferulic acid and sinapinic acid as the predominant polyphenols in the tiger nuts,27 vanillic acid, vanillin and trans-cinnamic acid as the most abundant in tiger nut oils,28 and p-coumaric, quercetin and cinnamic acids as prominent polyphenols in the brown variety of TNB fermented with kefir grains.29
The bioaccessibility of polyphenols from TNB and TNBP was also calculated and shown in Tables 1 and 2.
By enzyme digestion, about 27% and 57% of total polyphenols present in TNB and TNBP, respectively, became bioaccessible, i.e., they may possibly be absorbed and/or exert their functional properties (antioxidant or anti-inflammatory) within the intestinal milieu.30 Interestingly, the bioaccessibility of most polyphenols from TNB did not change across the oral and gastric phases and was significantly higher during intestinal digestion.
Conversely, the bioaccessibility of polyphenols from TNBP increased over the three phases of gastrointestinal digestion. These findings suggest that a higher amount of polyphenols in TNB is in the free form, and the salivary step of the digestion is sufficient to dissolve it in the bioaccessible fraction, whereas polyphenols in TNBP are likely bound to dietary fibers or proteins and need the gastric enzymes and pH condition to get free.30–34
Regarding the mean bioaccessibility of individual polyphenols from TNB, it ranged between 1.2% and 118.1%, with ferulic acid acyl-b-D-glucoside and ethyl vanillin showing the highest bioaccessibility. The bioaccessibility of polyphenols from TNBP ranged between 4.6% and 123.6%, and caffeic acid hexoside and 4-hydroxybenzoic acid were the most bioaccessible. The high bioaccessibility of some polyphenols may be explained by the activity of gastrointestinal enzymes on matrix-bound polyphenols, as shown for 4-hydroxibenzoic acid and catechin-3-O-glucoside.35,36 Moreover, gastrointestinal conditions may favorably influence the stability of some polyphenols, as previously reported by Cantele et al.36 for glycosylated polyphenols that showed improved stability in the gastrointestinal tract, thus resulting in higher bioaccessibility. These findings were in line with total polyphenol bioaccessibility from other beverages (Helal et al.;37 Cantele et al.;36 Kcokaplan et al.38). The low bioaccessibility of epicatechin derivative compared to the other polyphenols (7% and 29% from TNB and TNBP, respectively) agreed with findings from previous studies (Cantele et al.36) and could be likely attributed to the instability of flavan-3-ols in the gastrointestinal tract conditions.39
3.2 Radical scavenging assay
Table 3 shows the results of the soluble TAC (by ABTS assay) of TNB and all the bioaccessible fractions collected over the digestion of TNB and TNBP, as well as the direct TAC (quencher methodology) of TNBP and all the pellets obtained at each digestion phase (insoluble fractions).| Sample | ABTS | QUENCHER |
|---|---|---|
| Data are shown as mean ± SD. Different letters in lowercase indicate differences between the samples (in the same column) for ABTS assay assessed by one-way ANOVA and Tukey post hoc (p < 0.05). | ||
| TNB | 12.5 ± 0.32a | |
| TNB oral | 1.9 ± 0.04d | |
| TNB gastric | 0.8 ± 0.04f | |
| TNB intestinal | 10.2 ± 0.12b | |
| TNBP | 21.9 ± 0.2b | |
| TNBP oral | 1.1 ± 0.09e | 18.4 ± 0.2c |
| TNBP gastric | 1.3 ± 0.01e | 12.3 ± 0.3d |
| TNBP intestinal | 9.2 ± 0.03c | 34.5 ± 0.3a |
TNB exhibited the highest TAC, whereas the lowest TAC was that of the BF collected after the gastric digestion of TNB, slightly reduced compared to the oral fraction; on the contrary, the gastric BF of TNBP was increased compared to the relative oral fraction. TAC of the intestinal BFs from both TNB and TNBP were significantly higher than in the previous digestion phase.
The changes in TAC during digestion reflected the fluctuation of polyphenols released from the matrix both in terms of quantity and quality since different polyphenols might have different abilities to react with free radicals in the ABTS assay.
Similar to the TAC of BFs, that of insoluble fractions increased during the digestion and the intestinal residue of TNBP digestion exerted an antioxidant activity higher than TNBP before digestion. Altogether, these results show that the consumption of TNBP has the potential to quench free radicals, eventually forming in the gastrointestinal lumen, creating a reducing environment in the gastrointestinal tract, which is a condition promoting health.20
3.3. Digestive enzyme inhibition
For the first time, the inhibitory activities of TNB and TNBP intestinal digests against the digestive enzymes α-amylase, α-glucosidase, and lipase were determined. The potential inhibitory effect of each intestinal digest at a concentration of 18 mg mL−1 is reported in Fig. 2.The results showed that TNBP intestinal digest possessed a significantly stronger ability to inhibit α-glucosidase activity than TNB (88 ± 0.3% vs. 85 ± 1.1%, respectively). This result was irrespective of the content of polyphenols that were, in fact, lower in TNBP digest than TNB but was possibly influenced by their quality.
Specifically, lower amounts of glycosidic phenolic compounds (i.e., caffeic and ferulic glycosides) in TNBP digesta might have enhanced the inhibitory property of this material due to a lower steric hindrance of hydroxylated phenols compared to glycosylated ones, which determines better interactions with the α-glucosidase.40
Conversely, lipase was inhibited to a greater extent by TNB (77%) than TNBP (59%), probably due to the synergistic effect between the polyphenols, such as ferulic acid and p-coumaric acid on lipase, as previously reported for grape seed extract and fermented oat.41,42
The lack of effect on α-amylase activity could be explained by the higher amount of certain types of phenolic acids in TNB, which hardly inhibit amylase activity (i.e., vanillic acid) or by the co-existence of other nutrients like polysaccharides and starch, which react with polyphenols reducing their ability to inhibit the enzyme.43
Mounting evidence supports the potential of polyphenols from food by-products to inhibit α-amylase, α-glucosidase, and lipase in the prevention of the risk of metabolic diseases. The findings of the present study suggest that TNBP also has a potential activity to influence carbohydrate and lipid metabolism by delivering polyphenols in the gastrointestinal tract, thus possibly aiding blood glucose and energy intake control.
3.4. Bioavailability of polyphenols from TNB and TNBP
In this study, the bioavailability of various polyphenols present in TNB and TNBP was assessed by differentiated Caco-2 cells. The polyphenols identified were more bioavailable from TNB, showing values above 80%. In contrast, the bioavailability of polyphenols from TNBP ranged from 61.8% to 83.6%. trans-Cinnamic acid and the epicatechin derivative were the most bioavailable polyphenols from TNB and TNBP (Fig. 3).The stability and absorption of polyphenols like epicatechin derivatives and ferulic acid derivatives in the gastrointestinal tract have been widely studied, with results indicating that these compounds can undergo various transformations affecting their bioavailability.44
Kern et al.45 studied the high capacity of human intestinal epithelium to metabolize dietary hydroxycinnamates through various phase I and phase II reactions in Caco-2 cells, which aligns with the findings of this study, as trans-cinnamic acid from TNB and TNBP may undergo similar metabolic pathways, contributing to the bioavailability. The bioavailability of epicatechin derivatives observed in this study is consistent with the results of Achour et al.,46 which assessed the bioavailability of rosemary infusion polyphenols, including epicatechin, in Caco-2 cells.
4. Conclusions
This study demonstrated for the first time that both TNB and TNBP contain polyphenols that may be bioaccessible, bioavailable and able to contribute to the control of oxidative stress in the gastrointestinal tract as well as energy intake by influencing digestive enzyme activity. Furthermore, the findings suggest that TNBP may act as a carrier of polyphenols in the lower gut and likely maintain a reducing environment in the colon in vivo, possibly also influencing the gut microbiome. Altogether, the findings of this study shed light on the potential functional properties of TNB and TNBP upon consumption and demonstrated that TNBP is still a product with high nutritional value. The findings support the implementation of future in vivo studies to evaluate whether the properties of TNBP here demonstrated in vitro are confirmed in humans upon consumption. Such a confirmation is needed to reuse TNBP (actually used for livestock feeding) in the human food chain as a functional ingredient in innovative and sustainable foods designed to provide metabolic benefits to consumers through the delivery of polyphenols in the gastrointestinal tract.Author contributions
Paula Llorens: formal analysis, visualization, validation, writing – original draft; Manuela Flavia Chiacchio: formal analysis, visualization, validation, writing – original draft; Silvia Tagliamonte: writing – review & editing, supervision; Ana Juan-García: formal analysis, supervision, writing – review & editing; Noelia Pallarés: formal analysis; Juan Carlos Moltó: supervision; Paola Vitaglione: conceptualization, methodology, supervision, writing – review & editing; Cristina Juan: conceptualization, supervision, writing – review & editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Conselleria d'Educació, Universitats I Ocupació from Generalitat Valenciana project CIAICO2022/199. Spanish Ministry of Science and Innovation PID2020-115871RB-100. This research has been supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, Project title “ON Foods – Research and innovation network on food and nutrition Sustainability, Safety and Security - Working ON Foods”.References
- Ficha disposición, https://dogv.gva.es/portal/ficha_disposicion_pc.jsp?sig=005850/2010&L=1. (Accessed 02 April 2024).
- R. Zamora-Ros and P. Cervera, Tablas de composición de alimentos del CESNID = taules de composició d'aliments Del CESNID, McGraw-Hill-Interamericana, Madrid, 2010 Search PubMed.
- Y. Yu, X. Lu, T. Zhang, C. Zhao, S. Guan, Y. Pu and F. Gao, Tiger nut (Cyperus esculentus L.): nutrition, processing, function and applications, Foods, 2022, 11, 601 CrossRef CAS.
- I. Ogunlade, A. A. Bilikis and G. A. Aluko Olanrewaju, Chemical compositions, antioxidant capacity of tigernut (Cyperus esculentus) and potential health benefits, Eur. Sci. J., 2015, 1857–7431 Search PubMed.
- A. Adejuyitan, Tigernut processing: its food uses and health benefits, Am. J. Food Technol., 2011, 6, 197–201 CrossRef.
- E. Sánchez-Zapata, J. Fernández-López and J. A. Pérez-Alvarez, Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties and food applications, Compr. Rev. Food Sci. Food Saf., 2012, 11, 366–377 CrossRef.
- Consejo Regulador D.O. Chufa de Valencia., Chufa de Valencia, https://www.chufadevalencia.org/, (Accessed 02 April 2024).
- E. Roselló-Soto, M. M. Poojary, F. J. Barba, J. M. Lorenzo, J. Mañes and J. C. Moltó, Tiger nut and its by-products valorization: From extraction of oil and valuable compounds to development of new healthy products, Innovative Food Sci. Emerging Technol., 2018, 45, 306–312 CrossRef.
- M. del Razola-Díaz, A. M. Gómez-Caravaca, E. J. Guerra-Hernández, B. Garcia-Villanova and V. Verardo, New Advances in the Phenolic Composition of Tiger Nut (Cyperus esculentus L.) by-Products, Foods, 2022, 11, 343 CrossRef PubMed.
- O. Makanjuola, T. Arowosola and C. Du, The utilization of food waste: Challenges and opportunities, J. Food Chem. Nanotechnol., 2020, 6, 182–188 CrossRef.
- A. M. López-Sobaler and R. M. Ortega, La Composición de los Alimentos. Herramienta básica para la valoración nutricional, Gac. Sanit., 2014, 28, 69–71 CrossRef.
- I. Codina-Torrella, B. Guamis and A. J. Trujillo, Characterization and comparison of tiger nuts (Cyperus esculentus L.) from different geographical origi, Ind. Crops Prod., 2015, 65, 406–414 CrossRef CAS.
- E. Sánchez-Zapata, E. Fuentes-Zaragoza, J. Fernández-López, E. Sendra, E. Sayas, C. Navarro and J. A. Pérez-álvarez, Preparation of dietary fiber powder from tiger nut (Cyperus esculentus) milk (“Horchata”) byproducts and its physicochemical properties, J. Agric. Food Chem., 2009, 57, 7719–7725 CrossRef.
- E. Roselló-Soto, F. Barba, P. Putnik, D. B. Kovačević, J. Lorenzo and Y. Cantavella-Ferrero, Enhancing Bioactive Antioxidants’ Extraction from “Horchata de Chufa” By-Products, Foods, 2018, 7, 161 CrossRef.
- N. Aguilar, E. Albanell, B. Miñarro, B. Guamis and M. Capellas, Effect of tiger nut-derived products in gluten-free batter and bread, Food Sci. Technol. Int., 2014, 21, 323–331 CrossRef PubMed.
- M. E. Shenawy, A. M. Hussien and M. T. Fouad, Production of Biscuits from Mixture of Tiger Nut Flour, Milk Permeate and Soft Wheat Flour, Asian J. Agric. Food Sci., 2020, 18, 11–21 CrossRef.
- E. Sánchez-Zapata, C. M. Muñoz, E. Fuentes, J. Fernández-López, E. Sendra, E. Sayas, C. Navarro and J. A. Pérez-Alvarez, Effect of tiger nut fibre on quality characteristics of pork burger, Meat Sci., 2010, 85, 70–76 CrossRef.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 CrossRef CAS.
- M. F. Chiacchio, S. Tagliamonte, A. Visconti, R. Ferracane, A. Mustafa and P. Vitaglione, Baobab-fruit shell and fibrous filaments are sources of antioxidant dietary fibers, Molecules, 2022, 27, 5563 CrossRef CAS PubMed.
- A. Serpen, E. Capuano, V. Fogliano and V. Gökmen, A New Procedure To Measure the Antioxidant Activity of Insoluble Food Components, J. Agric. Food Chem., 2007, 55, 7676–7681 CrossRef CAS PubMed.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radicals Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- A. Colantuono, R. Ferracane and P. Vitaglione, In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies, Food Funct., 2016, 7, 4247–4258 RSC.
- A. Colantuono, P. Vitaglione, R. Ferracane, O. H. Campanella and B. R. Hamaker, Development and functional characterization of new antioxidant dietary fibers from pomegranate, olive and artichoke by-products, Food Res. Int., 2017, 101, 155–164 CrossRef.
- L. Pollini, A. Juan-García, F. Blasi, J. Mañes, L. Cossignani and C. Juan, Assessing bioaccessibility and bioavailability in vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS, Food Biosci., 2022, 48, 101799 CrossRef.
- E. Hernández-Olivas, A. Asensio-Grau, J. Calvo-Lerma, J. García-Hernández, A. Heredia and A. Andrés, Content and bioaccessibility of bioactive compounds with potential benefits for macular health in tiger nut products, Food Biosci., 2022, 49, 101879 CrossRef.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion, Food Chem., 2013, 136, 206–212 CrossRef.
- A. K. Oladele, J. Adebowale and O. P. Bamidele, Phenolic Profile and Antioxidant Activity of Brown and Yellow Varieties of Tigernut (Cyperus esculentus L.), Niger. Food J., 2017, 35, 51–59 Search PubMed.
- O. Ezeh, K. Niranjan and M. H. Gordon, Effect of enzyme pre-treatments on bioactive compounds in extracted tiger nut oil and sugars in residual meals, J. Am. Oil Chem. Soc., 2016, 93, 1541–1549 CrossRef CAS PubMed.
- G. Satir, The effects of fermentation with water kefir grains on two varieties of tigernut (Cyperus esculentus L.) milk, LWT, 2022, 171, 114164 CrossRef CAS.
- B. Ed Nignpense, N. Francis, C. Blanchard and A. B. Santhakumar, Bioaccessibility and bioactivity of cereal polyphenols: A review, Foods, 2021, 10, 1595 CrossRef CAS PubMed.
- A. Baublis, E. A. Decker and F. M. Clydesdale, Food Chem., 2000, 68, 1–6 CrossRef CAS.
- C. M. Liyana-Pathirana and F. Shahidi, Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions, J. Agric. Food Chem., 2005, 53, 2433–2440 CrossRef PubMed.
- F. Saura-Calixto, J. Serrano and I. Goñi, Intake and bioaccessibility of total polyphenols in a whole diet, Food Chem., 2007, 101, 492–501 CrossRef.
- O. Djaoudene, I. Mansinhos, S. Gonçalves, M. J. Jara-Palacios, M. Bachir bey and A. Romano, Phenolic profile, antioxidant activity and enzyme inhibitory capacities of fruit and seed extracts from different Algerian cultivars of date (Phoenix dactylifera L.) were affected by in vitro simulated gastrointestinal digestion, S. Afr. J. Bot., 2021, 137, 133–148 CrossRef.
- K. Wojtunik-Kulesza, A. Oniszczuk, T. Oniszczuk, M. Combrzyński, D. Nowakowska and A. Matwijczuk, Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review, Nutrients, 2020, 12(5), 1401 CrossRef CAS PubMed.
- C. Cantele, O. Rojo-Poveda, M. Bertolino, D. Ghirardello, V. Cardenia, L. Barbosa-Pereira and G. Zeppa, In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell, Foods, 2020, 9, 715 CrossRef CAS.
- A. Helal, D. Tagliazucchi, E. Verzelloni and A. Conte, Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion, J. Funct. Foods, 2014, 7, 506–516 CrossRef CAS.
- Z. B. Kocakaplan, G. Ozkan, S. Kamiloglu and E. Capanoglu, Valorization of pineapple (Ananas comosus) by-products in milk coffee beverage: Influence on bioaccessibility of phenolic compounds, Plant Foods Hum. Nutr., 2024, 1–8 Search PubMed.
- T. M. Mendes, Y. Murayama, N. Yamaguchi, G. R. Sampaio, L. C. Fontes, E. A. Torres, H. Tamura and L. Yonekura, Guaraná (Paullinia cupana) catechins and procyanidins: Gastrointestinal/colonic bioaccessibility, Caco-2 cell permeability and the impact of macronutrients, J. Funct. Foods, 2019, 55, 352–361 CrossRef CAS.
- F. Hua, P. Zhou, H.-Y. Wu, G.-X. Chu, Z.-W. Xie and G.-H. Bao, Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu'an GuaPian tea: molecular docking and interaction mechanism, Food Funct., 2018, 9, 4173–4183 RSC.
- A. Moreno, N. Ilic, A. Poulev, D. L. Brasaemle, S. K. Fried and I. Raskin, Inhibitory effects of grape seed extract on lipases, Nutrition, 2003, 19, 876–879 CrossRef.
- S. Cai, O. Wang, M. Wang, J. He, Y. Wang, D. Zhang, F. Zhou and B. Ji, In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented oats (Avena sativa L.) and synergistic effect of three phenolic acids, J. Agric. Food Chem., 2012, 60, 7245–7251 CrossRef CAS PubMed.
- L. Sun, F. J. Warren and M. J. Gidley, Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase, Trends Food Sci. Technol., 2019, 91, 262–273 CrossRef CAS.
- G. Williamson and C. Manach, Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies, AJCN, 2005, 81, 243S–255S CAS.
- S. M. Kern, R. N. Bennett, P. W. Needs, F. A. Mellon, P. A. Kroon and M.-T. Garcia-Conesa, Characterization of Metabolites of Hydroxycinnamates in the in Vitro Model of Human Small Intestinal Epithelium Caco-2 Cells, J. Agric. Food Chem., 2003, 51, 7884–7891 CrossRef CAS PubMed.
- M. Achour, S. Saguem, B. Sarriá, L. Bravo and R. Mateos, Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems, J. Sci. Food Agric., 2018, 98, 3741–3751 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01537a |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |