Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unveiling the anti-inflammatory potential of 11β,13-dihydrolactucin for application in inflammatory bowel disease management†
Melanie S.
Matos
 ab,
María Ángeles
Ávila-Gálvez
ac,
Antonio
González-Sarrías
ab,
María Ángeles
Ávila-Gálvez
ac,
Antonio
González-Sarrías
 d,
Nuno-Valério
Silva
c,
Carolina Lage
Crespo
d,
Nuno-Valério
Silva
c,
Carolina Lage
Crespo
 c,
António
Jacinto
c,
António
Jacinto
 ce,
Ana Teresa
Serra
ab,
Ana A.
Matias
a and
Cláudia
Nunes dos Santos
ce,
Ana Teresa
Serra
ab,
Ana A.
Matias
a and
Cláudia
Nunes dos Santos
 *abce
*abce
aInstituto de Biologia Experimental e Tecnológica (iBET), 2780-157 Oeiras, Portugal. E-mail: claudia.nunes.santos@nms.unl.pt
bInstituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa (ITQB-NOVA), 2780-157 Oeiras, Portugal
cNOVA Medical School, Faculdade de Ciências Médicas, NMS|FCM, Universidade Nova de Lisboa, 1169-056 Lisboa, Portugal
dLaboratory of Food & Health, Research Group on Quality, Safety and Bioactivity of Plant Foods, CEBAS-CSIC, 30100 Campus de Espinardo, Murcia, Spain
eNOVA Institute for Medical Systems Biology, NIMSB, Universidade Nova de Lisboa, 1099-085 Lisboa, Portugal
First published on 20th August 2024
Abstract
Management of inflammatory bowel disease (IBD) poses significant challenges, and there is a need for innovative therapeutic approaches. This study investigates the anti-inflammatory properties of the dietary sesquiterpene lactone (SL) 11β,13-dihydrolactucin, which can be found in chicory, in three distinct complementary models of intestinal inflammation (two cell models and a zebrafish model), offering comprehensive insights into its potential application for IBD treatment alternatives. In a triple cell co-culture composed of Caco-2, HT29-MTX-E12, and Raji B, 11β,13-dihydrolactucin demonstrated remarkable anti-inflammatory activity at several levels of the cellular inflammatory response. Notably, 11β,13-dihydrolactucin prevented the activation of critical signalling pathways associated with inflammation, namely NF-κB and MAPK p38. This SL also decreased the release of the neutrophil-recruiting chemokine IL-8. Additionally, the compound reduced the gene expression of IL-6 and TNF-α, as well as the gene and protein expression of the inflammatory inducible enzymes iNOS and COX-2. In a myofibroblast-like human cell model, 11β,13-dihydrolactucin decreased the release of the cytokine TNF-α and the COX-2-derived inflammation mediator PGE2. Finally, in a zebrafish model of gut inflammation, 11β,13-dihydrolactucin effectively reduced neutrophil infiltration, further supporting its anti-inflammatory efficacy in a physiological context. Collectively, our findings highlight the promising anti-inflammatory potential of 11β,13-dihydrolactucin across various facets of intestinal inflammation, providing a foundation for the consideration of chicory as a promising candidate for incorporation in food or nutraceutical products for the potential prevention of IBD.
Introduction
Chronic inflammation, generally characterized by the sustained release of pro-inflammatory mediators, has been implicated in the pathogenesis of several diseases.1 Inflammatory Bowel Disease (IBD) is an example of such and is characterized by chronic, self-destructive inflammation of the gastrointestinal tract. Crohn's disease (CD) and ulcerative colitis (UC) are the two main manifestations of IBD, leading to a substantially impaired quality of life, due to clinical relapses, recurrent need for surgery, and increased risk of developing colon cancer.2 It is estimated that IBD affected nearly 5 million people globally in 2019, and the worldwide incidence and prevalence have been rapidly increasing.3 The disease was first more prevalent in Western Europe and North America but, in the past decades, the incidence has grown in countries from Asia, Eastern Europe, and Africa, in line with emerging alterations in dietary habits and industrialization.3,4 IBD can arise in all ages, and there seems to be a gender distinction in terms of susceptibility, with females being more likely to develop CD, while males have a higher likelihood of being diagnosed with UC.5Current therapeutic approaches, such as immunosuppressants, present many drawbacks, including risk of relapse or failure to induce remission, as well as side effects and high costs.6 Consequently, there is a pressing need to identify novel, effective compounds that can mitigate intestinal inflammation while minimizing side effects. The exact cause of IBD remains elusive, but it is believed to stem from a complex interplay of factors, encompassing genetic predisposition, microbiota alterations, and environmental triggers that can precipitate abnormal immune reactions.6 The association between dietary patterns and the pathogenesis of IBD has been extensively documented. This recognition has spurred the exploration of diverse dietary interventions in both animal models and human subjects, aimed at assessing the influence of different diet constituents on the course of IBD.4,7–10 Given the recognized role of diet in mitigating non-communicable diseases, considerable attention has been directed toward the potential benefits of various plant-derived foods in preventing conditions such as IBD. Indeed, there are several reports on the potential of natural extracts to decrease intestinal inflammation. For instance, extracts from cactus pear, or blackcurrant and bilberry, were shown to decrease inflammation biomarkers in differentiated Caco-2 cells,11,12 while an olive leaf extract has been reported to reduce intestinal inflammation in a mice model of colitis.13 In this regard, dietary plant-derived bioactive compounds, such as sesquiterpene lactones (SLs), have been attracting attention due to their potential to modulate inflammation.14
SLs constitute a diverse group of lipophilic terpenoids mainly found in the Asteraceae family. These compounds are consumed through our diet, sourced from plants such as chicory (Cichorium intybus L.), and exhibit promising health-promoting properties.14–16 The usage of chicory is popular for culinary and industrial applications. The leaves are mainly used for salads, and the roots are commercially exploited to produce inulin, which is used as a sugar substitute and dietary fibre to promote gut health.15 Inulin processing comprises the removal of terpenes, including sesquiterpene lactones, due to their bitter taste. However, due to the inherent value of these discarded SLs, there is a compelling opportunity to harness the full potential of chicory as a dietary source of numerous compounds with health benefits. SLs are known to combat inflammation by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and nuclear factor of activated T-cells (NFAT) pathways, both of which have a central role in the expression of genes involved in inflammation, including the pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumour necrosis factor α (TNF-α).17 There are also reports demonstrating the ability of SLs to prevent the expression of inflammatory enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), as well as the phosphorylation of mitogen-activated protein kinase (MAPK) proteins, namely p38.17,18
Although there are not many reports on chicory SLs, we recently reported that the guaianolide 11β,13-dihydrolactucin, a lactucin derivative present in chicory, significantly reduced inflammation in a yeast reporter system based on the activity of Crz-1, the yeast orthologue of the human NFAT.19 Due to the promising results obtained in our previous research, we decided to further explore the anti-inflammatory potential of 11β,13-dihydrolactucin, thus embarking on the quest to identify novel therapeutic agents for the treatment of IBD. For this purpose, we performed an initial evaluation of the ability of the four major chicory SLs to prevent IL-8 release in a triple cell co-culture of the inflamed intestinal mucosa, and after confirming the anti-inflammatory potential of 11β,13-dihydrolactucin, the impact of this SL on the release and expression of key inflammatory players was further analysed, using three physiologically relevant models of the inflamed intestinal mucosa: (1) a triple cell co-culture composed of absorptive enterocytes, mucus-secreting goblet cells, and antigen-uptake facilitator microfold cells; (2) a myofibroblast-like human cell line; and (3) a zebrafish in vivo physiological model of gut inflammation, triggered by a high-cholesterol diet. These complementary models provide the means to seek information regarding the different stages of the intestinal inflammatory response and will contribute to elucidating the mechanisms of action of 11β,13-dihydrolactucin in IBD.
Experimental section
Chemicals
Parthenolide and costunolide were acquired from Sigma-Aldrich (SML0417, P0667) (Gillingham, UK). Lactucin, lactucopicrin, 11β,13-dihydrolactucin, and 11β,13-dihydrolactucopicrin were acquired from Extrasynthese (3809, 3813, 3810, 3811) (Genay Cedex, France). BMS-345541 was purchased from Abcam (ab144822) (Cambridge, UK).Cell culture
Human colon carcinoma Caco-2 cells (DSMZ, Braunschweig, Germany), mucus-secreting HT29-MTX-E12 subclone (ECACC, Dublin, Ireland), and human Burkitt's lymphoma Raji B cells (ECACC, Oxford, UK) were routinely grown separately in high glucose, high pyruvate, Dulbecco's modified Eagle's medium (DMEM) (Gibco, Life Technologies, Grand Island, NY, USA), supplemented with 10% (v/v) of heat-inactivated fetal bovine serum (FBS) (Biowest, Nuaillé, France), 100 units per mL penicillin, 100 μg mL−1 streptomycin (Gibco, Life Technologies, Grand Island, NY, USA), and 10 mM nonessential amino acids (Gibco, Life Technologies, Paisley, UK). These three cell lines were cultured in a humidified atmosphere at 37 °C with 5% CO2. The myofibroblast-like human cell line (CCD-18Co) was obtained from the American Type Culture Collection (ATCC, Rockville, USA) and cultured as described elsewhere.20Caco-2 cells were used between passages 13 and 47, HT29-MTX-E12 between passages 55 and 75, Raji B between passages 11 and 36, and CCD-18Co cells were from passages 27 to 32.
Caco-2:HT29-MTX-E12:Raji B triple cell co-culture
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, and at a total density of 1.0 × 105 cell per cm2. After a 14-day co-culture period, Raji B cells were introduced into the basolateral compartment at a concentration of 4.0 × 104 cell per mL, and this triple co-culture system was maintained for 7 days. To ensure cell growth and viability, the culture medium was renewed thrice weekly throughout the entire culture period. This approach for the development of a model of the inflamed intestinal mucosa using an already established triple co-culture22,23 was adapted from prior studies utilizing only Caco-2 cells,11 and its validity was confirmed through various methodologies, including LDH release and resazurin metabolization evaluation, TEER monitoring, fluorescein permeability assessment, and determination of IL-8 release.21
1, respectively, and at a total density of 1.0 × 105 cell per cm2. After a 14-day co-culture period, Raji B cells were introduced into the basolateral compartment at a concentration of 4.0 × 104 cell per mL, and this triple co-culture system was maintained for 7 days. To ensure cell growth and viability, the culture medium was renewed thrice weekly throughout the entire culture period. This approach for the development of a model of the inflamed intestinal mucosa using an already established triple co-culture22,23 was adapted from prior studies utilizing only Caco-2 cells,11 and its validity was confirmed through various methodologies, including LDH release and resazurin metabolization evaluation, TEER monitoring, fluorescein permeability assessment, and determination of IL-8 release.21
To ensure the establishment and integrity of the monolayer, trans-Epithelial Electrical Resistance (TEER) was monitored using an EVOM voltmeter (WPI, Berlin, Germany) throughout the entire culture duration and before each experiment. Only monolayers exhibiting values of at least 400 Ω cm2 after a 21-day culture period were selected for the subsequent inflammation assays.
Primarily, the anti-inflammatory potential of four chicory SLs (lactucin, lactucopicrin, 11β,13-dihydrolactucin, and 11β,13-dihydrolactucopicrin), along with their precursor costunolide, and the established anti-inflammatory parthenolide, applied in the apical side of the model, was assessed in the intestinal cell triple co-culture, in terms of IL-8 release determination by ELISA. These SLs were tested at a concentration of 10 μM, a non-cytotoxic concentration as determined elsewhere.19 As an anti-inflammatory positive control targeting the NF-κB pathway, the effect of 5 μM BMS 345541 was also assessed. Then, 11β,13-dihydrolactucin was selected for further assessment of other inflammation biomarkers by western blot and RT-PCR.
For the activation of signalling pathways, a 15-minute timepoint was selected after an incubation period screening aiming at determining the timepoint in which the phosphorylation levels of NF-κB subunit p65 and MAPK p38 were highest (ESI Fig. S1†).
For IL6 (IL-6), TNF (TNF-α), IL1B (IL-1β), NOS2 (iNOS), and PTGS2 (COX-2) gene expression, the cells were co-incubated with the pro-inflammatory stimulus and 11β,13-dihydrolactucin or BMS 345541 for 3 hours, an incubation period shown to induce a significant increase in the gene expression of these inflammation-related biomarkers (ESI Fig. S2†).
Following gene expression, 12 hours was the selected timepoint for evaluating the impact of 11β,13-dihydrolactucin on the protein expression levels of the inflammatory inducible enzymes iNOS and COX-2. The 12-hour timepoint was selected following the testing of a range of incubation periods carried out to determine the moment in which the protein expression of iNOS and COX-2 was highest (ESI Fig. S3†).
The levels of IL-8 were determined using the Human IL-8 (CXCL8) standard TMB ELISA development kit (Peprotech, NJ, USA) following the manufacturer's guidelines. The results were quantified by measuring absorbance at 450 and 620 nm in a microplate spectrophotometer (EPOCH 2, Biotek Instruments, Wonooski, VT, USA). For analysis, each cell media sample was diluted with PBS to fit within the IL-8 calibration curve (ranging from 0 to 200 pg mL−1) and tested in at least two technical replicates for each biological replicate (n ≥ 3).
The cDNA obtained in the previous step, by reverse transcription of the extracted RNA, was amplified in a LightCycler® 480 System (Roche Diagnostics, IN, USA), using the LightCycler® 480 SYBR Green I Master mix (Roche Diagnostics, IN, USA), following the manufacturer's instructions. The PCR protocol consisted of an initial 10-minute pre-incubation at 95 °C, followed by 40 amplification cycles comprised of 10-second denaturation at 95 °C, 20-second annealing at a primer-dependent temperature, and 30-second extension at 72 °C. KiCqStart™ SYBR® Green Primers (Sigma-Aldrich, MO, USA) were employed: FH1_TNF/BH1_TNF for TNF; FH2_IL1B/BH2_IL1B for IL1B; FH1_IL6/BH1_IL6 for IL6; FH1_NOS2/BH1_NOS2 for NOS2; and FH1_PTGS2/BH1_PTGS2 for PTGS2.
The annealing temperatures used in the PCR amplification protocol were 58 °C for GAPDH, TNF, IL1B, and IL6; and 55 °C for GAPDH, NOS2, and PTGS2. The specificity of the amplification was confirmed by performing a melting curve.
Gene expression levels were normalized to the expression of the housekeeping gene GAPDH for each condition, and the fold change relative to the control was calculated based on the comparative cycle threshold (Ct) using the Pfaffl method,24 which considers primer efficiency values (E). Data are presented as the mean ± standard deviation (SD) from at least three independent biological replicates. The following equation was used:
| Fold change = E−ΔΔCt |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in RIPA). Protein quantification was then accomplished using the Micro BCA Protein Assay Kit (Thermo Scientific, IL, USA), following the guidelines specified by the manufacturer. Protein extracts were diluted in PBS to fit within the bovine serum albumin (BSA) calibration curve (ranging from 0 to 20 μg mL−1) and tested in at least two technical replicates for each biological replicate (n ≥ 3).
100 in RIPA). Protein quantification was then accomplished using the Micro BCA Protein Assay Kit (Thermo Scientific, IL, USA), following the guidelines specified by the manufacturer. Protein extracts were diluted in PBS to fit within the bovine serum albumin (BSA) calibration curve (ranging from 0 to 20 μg mL−1) and tested in at least two technical replicates for each biological replicate (n ≥ 3).
Samples were separated by SDS-PAGE and proteins were transferred onto nitrocellulose membranes, as previously described.16 Rabbit anti-iNOS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), rabbit anti-p65 and rabbit anti-phospho-p65 (1
1000), rabbit anti-p65 and rabbit anti-phospho-p65 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), rabbit anti-p38 and rabbit anti-phospho-p38 (1
1000), rabbit anti-p38 and rabbit anti-phospho-p38 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) (E2M9F, D14E12, 93H1, D13E1, D3F9, Cell Signalling Technology, Inc., MA, USA), and rabbit anti-COX-2 (1
1000) (E2M9F, D14E12, 93H1, D13E1, D3F9, Cell Signalling Technology, Inc., MA, USA), and rabbit anti-COX-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) (EP1978Y, Abcam, Cambridge, UK) were used as primary antibodies for the proteins of interest. Mouse anti-β-actin (1
1000) (EP1978Y, Abcam, Cambridge, UK) were used as primary antibodies for the proteins of interest. Mouse anti-β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) (8H10D10, Cell Signalling Technology, Inc., MA, USA) was employed as a primary antibody for the loading control in all cases. Species-specific HRP-conjugated secondary antibodies, namely sheep anti-rabbit (1
1000) (8H10D10, Cell Signalling Technology, Inc., MA, USA) was employed as a primary antibody for the loading control in all cases. Species-specific HRP-conjugated secondary antibodies, namely sheep anti-rabbit (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) and horse anti-mouse (1
2000) and horse anti-mouse (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000) (7074 and 7076, Cell Signalling Technology, Inc., MA, USA), were then applied. The ensuing signal was detected using the Clarity Western-enhanced chemiluminescence (ECL) (Bio-Rad Laboratories, Inc., CA, USA) on an iBright FL1500 transilluminator (Invitrogen, Thermo Fisher Scientific, MA, USA). Protein levels for all proteins under study were normalized to the loading control in the same membrane. For NF-κB p65 and MAPK p38, results are presented as the ratio between the normalized forms of phosphorylated and non-phosphorylated protein. Data are presented as the mean ± standard deviation (SD) from at least three independent biological replicates.
3000) (7074 and 7076, Cell Signalling Technology, Inc., MA, USA), were then applied. The ensuing signal was detected using the Clarity Western-enhanced chemiluminescence (ECL) (Bio-Rad Laboratories, Inc., CA, USA) on an iBright FL1500 transilluminator (Invitrogen, Thermo Fisher Scientific, MA, USA). Protein levels for all proteins under study were normalized to the loading control in the same membrane. For NF-κB p65 and MAPK p38, results are presented as the ratio between the normalized forms of phosphorylated and non-phosphorylated protein. Data are presented as the mean ± standard deviation (SD) from at least three independent biological replicates.
IL-1β-treated human colon-derived CCD-18Co myofibroblast cells
Zebrafish intestinal inflammation model
The in vivo experiments were carried out in a zebrafish model, as described by Silva et al.25 In brief, transgenic zebrafish embryos bearing fluorescently labelled neutrophils were raised with E3 medium supplemented with 0.03% methylene blue in Petri dishes, at a density not exceeding 50 embryos per dish. Larvae with homogeneous fluorescent reporter expression, spontaneous inflammation, and suitable development were sorted and maintained in a container with E3 medium (supplemented with 0.03% methylene blue) for six days at 28 °C.Larvae were transferred to 12-well microplates containing E3 medium without methylene blue (12–15 larvae per well) and fed with either a 10% w/w cholesterol-enriched diet (HCD) as the inflammatory insult or a control feed (SPE) three times over a 24-hour period. To avoid food deposition, media is replaced before the second feeding time. Handling was made carefully with a glass Pasteur pipette to avoid wounding or larvae damage. To allow for intestine emptying, larvae were then kept in E3 medium for 15 hours without feeding. Larvae mortality after this procedure, at the end of the whole assay period, was minor or none, which allowed us to have at least circa 12 larvae to image and obtain statistical power. Following these steps, larvae were anesthetized, fixed on 4% paraformaldehyde, and imaged in an automated motorized inverted microscope (Zeiss Axio Observer) using a 10× objective (NA 0.3) and a mercury lamp. Z-Stack acquisition was performed in 49 focal planes (149 μm range; 3 μm z step) to allow for full-depth visualization of the gut. Neutrophils were imaged in the mCherry channel (λex/λem = 585 nm/610 nm), and brightfield images were taken to detect the whole area of the gut. The neutrophilic inflammation index (NII) was calculated as described by Silva et al.25 and corresponds to the area fraction occupied by neutrophils in the defined area of the intestine.
The animal study was reviewed and approved by Animal User and Ethical Committees at Centro de Estudos de Doenças Crónicas (CEDOC) – NOVA Medical School, following the guidelines from the Portuguese National Authority for Animal Health (DGAV) (approval reference 0421/000/000/2021).
Statistical analysis
All results for the anti-inflammatory effects in vitro are the averages of at least three independent biological replicates and are reported as mean ± standard deviation (SD). Statistical analyses of the results were conducted using GraphPad Prism 10.0.2 software (GraphPad Software, San Diego, CA, USA). To determine whether the means were significantly different (α ≤ 0.05), the results were compared through either a one-way analysis of variance (ANOVA), followed by the Dunnet test for multiple comparisons, or through individual Student t-tests, followed by the Holm-Šídák test for correction of multiple comparisons.Results from in vivo experiments in zebrafish were statistically analysed by the Kruskal–Wallis test (one-way ANOVA on ranks) for multiple comparisons.
Results
Anti-inflammatory effects of 11β,13-dihydrolactucin and other chicory SLs in an intestinal triple cell co-culture
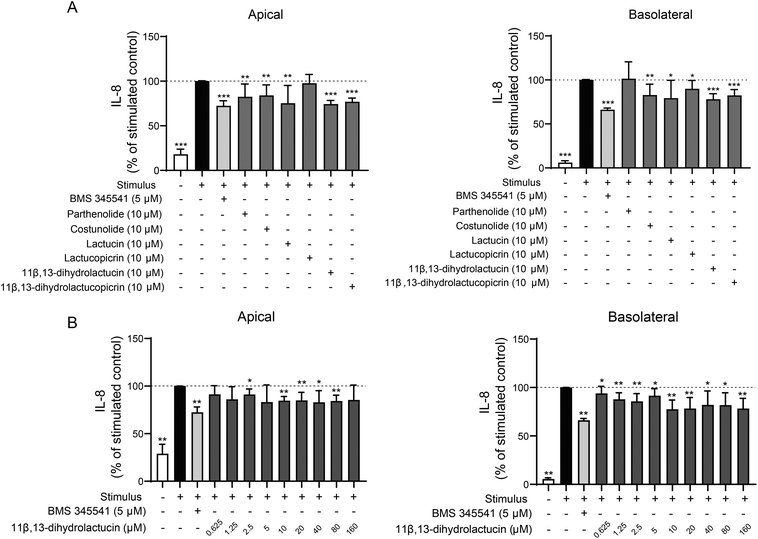
An initial anti-inflammatory screening based on the release of IL-8 was carried out for four major chicory SLs (lactucin, lactucopicrin, 11β,13-dihydrolactucin, and 11β,13-dihydrolactucopicrin), along with their precursor costunolide and the already validated anti-inflammatory SL, parthenolide.26Following the same rationale as in our previous publication,19 a non-cytotoxic concentration (10 μM) of each pure SL was tested in the triple co-culture model of the inflamed intestinal mucosa. For each condition, the SL was added to the apical side for 48 hours in co-incubation with the inflammatory insult, as previously described.21 Chicory SLs lactucin, lactucopicrin, 11β,13-dihydrolactucin, and 11β,13-dihydrolactucopicrin, delivered to the apical side, displayed an ability to decrease IL-8 release to some extent, either on the apical or on the basolateral side of the cell monolayer (Fig. 1A). However, the two latter were the most promising compounds, having caused a decrease in IL-8 release of 20–25% in both the apical and basolateral sides of the mucosa. The effect of these chicory SL dihydro-derivatives seemed even more pronounced than that of the SLs costunolide and parthenolide. In fact, the prevention of IL-8 release caused by 11β,13-dihydrolactucin and 11β,13-dihydrolactucopicrin came close to the results obtained for the positive control BMS 345541 (5 μM), with the overall best results being displayed by 11β,13-dihydrolactucin.
11β,13-Dihydrolactucin had previously demonstrated the most marked anti-inflammatory effect in a yeast reporter system based on the activity of Crz1, the yeast orthologue of the human NFAT,19 an effect that has been confirmed in the human triple co-culture model of the inflamed intestinal mucosa presented in this study. Based on these data, this SL was selected for further evaluation. Accordingly, a dose–response assay was carried out for 11β,13-dihydrolactucin, between 0.625 and 20 μM (Fig. 1B). Results show that the reduction of IL-8 release, in both apical and basolateral cell media, is not potentiated further with increasing concentration higher than 10 μM of the compound. Considering these results, 10 μM was the concentration chosen for additional assessment of the anti-inflammatory effects of 11β,13-dihydrolactucin.
To understand if 11β,13-dihydrolactucin specifically hampers the inflammatory response induced by the mediators that composed the cocktail individually, these were tested independently in the co-culture to better understand which mediator's inflammatory response is most significantly affected by 11β,13-dihydrolactucin (Fig. 2). In particular, the effect of this chicory SL, delivered in the apical side, on the release of IL-8 into both apical and basolateral cell media was assessed in cells stimulated with the pro-inflammatory cocktail, consisting of 10 μg mL−1 LPS on the apical compartment, and 25 ng mL−1 IL-1β and 50 ng mL−1 TNF-α on the basolateral compartment, or with each of these inflammatory mediators individually.
When the cells were stimulated with the pro-inflammatory cocktail, 11β,13-dihydrolactucin led to a significant decrease in IL-8 release to both apical and basolateral compartments (Fig. 2A). For cells stimulated with IL-1β alone on the basolateral compartment, the IL-8-decreasing effect was much more pronounced in the basolateral side of the cell monolayer (Fig. 2B). When TNF-α was used as the sole inflammation inducer on the basolateral compartment (Fig. 2C), 11β,13-dihydrolactucin had a significant anti-inflammatory result in both sides of the intestinal mucosa, though the percentage of IL-8 decrease was more marked on the apical side. Finally, for inflammation induced solely by LPS on the apical compartment (Fig. 2D), 11β,13-dihydrolactucin was able to reduce the percentage of IL-8 release to the apical supernatant but failed to do so for the basolateral compartment.
IL-6, TNF-α, and IL-1β are also key players in the cellular inflammatory response. As such, gene expression levels of these cytokines were also assessed after treatment with 10 μM of 11β,13-dihydrolactucin in co-incubation with the pro-inflammatory stimulus for 3 hours (Fig. 3). The effects of 11β,13-dihydrolactucin varied according to the cytokine. Similarly to BMS 345541, 11β,13-dihydrolactucin caused a strong reduction (65%) of IL-6 gene expression levels to values below the ones displayed by the non-inflamed control (Fig. 3A). In the case of TNF-α, both compounds led to a gene expression decrease exceeding 60% when compared to the stimulated control (Fig. 3B). On the other hand, and despite the apparent reduction tendency, 11β,13-dihydrolactucin failed to induce a significant gene expression decrease of IL-1β (Fig. 3C).
To further check the anti-inflammatory potential of 11β,13-dihydrolactucin, the gene and protein expression of two enzymes induced upon inflammation (iNOS and COX-2) were analysed (Fig. 4). The reduced gene expression caused by 11β,13-dihydrolactucin was significant in the case of iNOS (51%) (Fig. 4A). Concerning COX-2 (Fig. 4B), 11β,13-dihydrolactucin led to a decreased gene expression to approximately 33% of the inflamed control. Concomitantly with the reduction in gene expression induced by 11β,13-dihydrolactucin, there was also a significant reduction in the protein expression of iNOS and COX-2 in the presence of the inflammatory stimulus (Fig. 4C and D), to an extent similar to that achieved by the positive control BMS 345541 in both cases. In particular, when compared to the stimulated control, 11β,13-dihydrolactucin led to a reduction of 66% of iNOS protein expression, and 47% of COX-2 protein expression.
To try to unravel the mechanisms of action by which 11β,13-dihydrolactucin exerts its anti-inflammatory effects, the impact of this chicory SL was evaluated in two relevant signalling pathways enrolled in the inflammatory response, the NF-κB and MAPKs pathways (Fig. 5). The phosphorylation status of the p65 subunit of NF-κB and the MAPK p38 were evaluated after co-incubation of cells with the test compound and the inflammatory stimulus for 15 minutes (Fig. 5A and B). However, the effect observed was very limited, 11β,13-dihydrolactucin was unable to prevent phosphorylation of either NF-κB p65 or MAPK p38. BMS 345541 did not prevent NF-κB p65 phosphorylation but caused a slight decrease in the phosphorylation levels of MAPK p38 (Fig. 5B). It is possible that cells need to be exposed to BMS 345541 for longer periods than 15 minutes, to allow for an anti-inflammatory effect. Indeed, we only found literature reports assessing the effect of BMS 345541 for incubation periods of at least 1 hour before inflammatory stimulation.27–29 To ascertain whether the reduced time of exposure to compounds could be limiting their effects we decided to test a prolonged exposure period, in which the cells were pre-incubated with the test compounds for 4 hours prior to a 15-minute inflammatory induction (Fig. 5C and D). This pre-incubation approach led to a significant decrease in the phosphorylation levels of both NF-κB p65 (≈30%) and MAPK p38 (≈20%) by 11β,13-dihydrolactucin.
Effect of 11β,13-dihydrolactucin in an IL-1β-induced intestinal myofibroblast-like human cell line
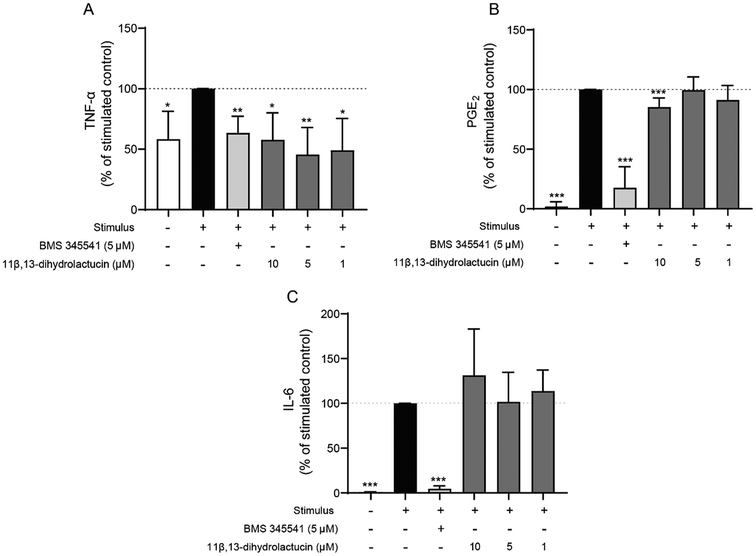
Given the important role played by colonic fibroblasts in intestinal inflammation, the anti-inflammatory potential of 11β,13-dihydrolactucin was also assessed in a human myofibroblast-like cell line isolated from healthy colon tissue (Fig. 6). In this experiment, the human cell line CCD-18Co was stimulated with IL-1β for 18 hours and then the levels of the pro-inflammatory cytokines TNF-α and IL-6 were measured in the cell media, along with the levels of prostaglandin E2 (PGE2), a major inflammatory molecule resulting from COX-2 activity. The levels of TNF-α released to the cell media were diminished by more than 40% upon treatment with non-toxic doses of 11β,13-dihydrolactucin (1–10 μM) (Fig. 6A), similar to what was observed for BMS 345541 (5 μM). The release of PGE2 was also decreased by the studied SL at the highest dose tested (10 μM) (Fig. 6B). Conversely, 11β,13-dihydrolactucin was unable to interfere with IL-6 levels in any of the tested concentrations (Fig. 6C).Anti-inflammatory effect of 11β,13-dihydrolactucin in a zebrafish model of intestinal inflammation
The anti-inflammatory potential of 11β,13-dihydrolactucin was ultimately validated in vivo in a whole organism setting, using a transgenic zebrafish model with a fluorescent reporter expression system in neutrophils.25 In this assay, an inflammatory phenotype was induced by a high-cholesterol diet (HCD), and the ability of 11β,13-dihydrolactucin to prevent neutrophil infiltration into the gut in zebrafish larvae was measured. Concentrations of 25 μM and 100 μM were chosen for testing 11β,13-dihydrolactucin in zebrafish. The decision to include 25 μM is supported by the common use of this concentration in zebrafish drug screens,30 while the higher concentration of 100 μM proves valuable for assessing natural compounds with low toxicity and potentially lower activity. Results show that 11β,13-dihydrolactucin can significantly decrease neutrophil infiltration in the gut at a concentration as low as 25 μM, with no further intensification of such effect with a higher concentration (100 μM) (Fig. 7).Discussion
In this study, by using different and complementary models of the inflamed intestinal mucosa, and assessing the effect on gene and protein expression, as well as the impact on cell signalling pathways, culminating with a whole organism response, we attempted to elucidate the anti-inflammatory mechanisms of action of 11β,13-dihydrolactucin. This study confirms our previous results revealing 11β,13-dihydrolactucin as an anti-inflammatory molecule, as was shown in a yeast reporter system based on the activity of Crz1, the yeast orthologue of the human NFAT.19In a physiologically representative human triple co-culture cell model, composed of absorptive enterocytes (Caco-2), mucus-secreting goblet cells (HT29-MTX-E12), and antigen-uptake facilitator microfold (M)-cells (Raji B-induced),21 11β,13-dihydrolactucin had a noteworthy effect on the gene and protein expression levels of several pro-inflammatory cytokines, as well as inflammatory inducible enzymes, relevant for the pathobiology of IBD.
IL-8 is a pro-inflammatory chemokine capable of attracting neutrophils into the site of inflammation and is commonly used as an inflammation biomarker.31 The percentage increase of IL-8 levels for the inflamed control in the basolateral compartment (representing the systemic circulation) of the cell model is notably higher than that observed in the apical compartment (representing the intestinal lumen) when inflammation was induced with either the pro-inflammatory cocktail (IL-1β, TNF-α, and LPS), IL-1β, or TNF-α. Similarly, in a study on a Caco-2:HT29-MTX-E12 co-culture in Transwell®, the inflammatory response in terms of IL-8 release tended to be stronger in the basolateral compartment.32 There are also studies on human epithelial cells showing that the secretome of polarized cells can significantly differ on each side of the epithelium, both qualitatively and quantitatively, thus influencing the behaviour and response of mucosal immune cells.33–35 In one of these studies, the release of IL-8 occurred mainly in the basolateral side of the monolayer of pulmonary epithelial cells, a result that has been associated with the systemic role of this chemokine in the recruitment of neutrophils.35 These observations may be a result of the placement and concentration of the stimulus, which is intimately connected with the presence of different membrane receptors on each side of polarized epithelial cells.
In this study, the treatment of cells with 11β,13-dihydrolactucin, in the apical side to mimic a dietary exposure, in the presence of the pro-inflammatory cocktail, was able to cause a significant reduction of IL-8 release into both apical and basolateral cell media, suggesting that 11β,13-dihydrolactucin influences intestinal inflammation not only locally, but also systemically. Notably, 11β,13-dihydrolactucin did not show a dose-dependent effect, since the prevention of IL-8 release was not further incremented for concentrations of 11β,13-dihydrolactucin above 10 μM.
The impact of 11β,13-dihydrolactucin on IL-8 release in the human triple co-culture after stimulation with the individual inflammation mediators that compose the cocktail was also assessed, to elucidate which mediator's inflammatory response is mostly affected by this chicory SL. IL-1β and TNF-α were applied on the basolateral side since these two cytokines have been detected in the systemic circulation in subjects with IBD.36,37 LPS, an inflammation mediator present in bacterial cell walls, was added to the apical side. The results were notably different depending on the inflammatory stimulus used. When compared to the non-stimulated control, the cocktail containing all three inflammation mediators caused the highest response in terms of IL-8 release, followed by inflammation induced by IL-1β, TNF-α, and finally LPS.
When the inflammation was induced by incubation with IL-1β on the basolateral side of the co-culture, 11β,13-dihydrolactucin strongly inhibited the IL-8 release into the basolateral cell media, to similar extent as the positive control BMS 345541. However, neither of these compounds had a remarkable effect on the apical supernatant. The effect of 11β,13-dihydrolactucin on the basolateral environment of the mucosa, having significantly decreased IL-8 release, might translate into a lower neutrophil recruitment. Notably, the imbalance of IL-1β levels has been associated with IBD, being responsible for intestinal barrier dysfunction, due to apoptosis of epithelial cells and interference with tight and adherens junction proteins,38,39 thereby facilitating the passage of biomolecules and immune cells involved in the inflammatory response.40 In fact, we reported that the pro-inflammatory cocktail causes a TEER decrease in the human triple co-culture,21 which may be a direct consequence of IL-1β. The fact that 11β,13-dihydrolactucin was able to counteract the inflammatory response caused by IL-1β may signify that this SL could potentially be able to prevent the phenotypic consequences of this cytokine.
Following TNF-α-induced inflammation, 11β,13-dihydrolactucin significantly decreased IL-8 release into both apical and basolateral sides. This result suggests that 11β,13-dihydrolactucin may be able to lessen the resulting phenotype of TNF-α-induced responses, such as the activation of macrophages and effector T cells, and regulation of numerous inflammatory pathways including the expression of COX-2 and iNOS.41,42 TNF-α can also activate several cellular signalling pathways culminating in the activation of transcription factors, including NF-κB.43
In the case of LPS stimulation, 11β,13-dihydrolactucin had a more local anti-inflammatory effect, having been able to cause a significant decrease in IL-8 release only in the apical side of the mucosa. This could be explained by the need for epithelial cells to directly activate mucosal immune cells, to neutralize bacteria present in the intestinal lumen. Indeed, LPS from Escherichia coli can lead to a pro-inflammatory response in intestinal epithelial cells.44
Concerning other pro-inflammatory cytokines, 11β,13-dihydrolactucin reduced the gene expression of IL-6, TNF-α, and IL-1β, when cells were treated with the SL in co-incubation with the pro-inflammatory cocktail. The expression of IL-6 is a result of the activity of several possible transcription factors, among which the NF-κB.43 Since the effect of 11β,13-dihydrolactucin on IL-6 gene expression was comparable to that of the IKK-1/-2 inhibitor BMS 345541, one might infer that the SL may also interfere with the NF-κB pathway. Moreover, IL-6 is a pleiotropic cytokine involved in cellular defense against pathogens and tissue damage, and it is also associated with chronic intestinal inflammation through complexation with the soluble form of its receptor (sIL6R) leading to resistance of mucosal T-cells to apoptosis.45,46
In the case of TNF-α, one of the most significant cytokines in the pathogenesis of IBD,37 the gene expression was remarkably increased in the human triple co-culture upon stimulation with the pro-inflammatory cocktail. Following this increase in gene expression, increased levels of this pro-inflammatory cytokine in intestinal epithelial cells are expected and could be triggering an augmented inflammatory response, thus potentially initiating a positive feedback loop.47 This phenomenon would elucidate the notable surge in gene expression observed (50-fold increase in comparison to the non-inflamed control). 11β,13-Dihydrolactucin caused a marked decrease in the TNF-α gene expression levels, achieving a similar result to the one obtained for BMS 345541, the positive control.
As for IL-1β gene expression, the pro-inflammatory cocktail caused a 10-fold increase relative to the non-inflamed control, probably resulting in an increased expression of the cytokine itself. Similarly to TNF-α, and considering the presence of the IL-1 receptor in epithelial cells,48 these increased levels of the cytokine might have led to a positive feedback loop, further increasing its expression. Despite the promising effect of 11β,13-dihydrolactucin on other inflammation biomarkers, the SL did not have a significant effect on the gene expression of IL-1β, as opposed to BMS 345541, suggesting that the two compounds have different mechanisms of action. BMS 345541 is an inhibitor of the NF-κB pathway, but there are other transcription factors involved in the expression of IL-β,49 upon which 11β,13-dihydrolactucin may not have a strong inhibitory effect.
The co-incubation of either BMS 345541 or 11β,13-dihydrolactucin with the pro-inflammatory stimulus for 15 minutes did not have a promising outcome when it comes to NF-κB and MAPK signalling modulation. p65 is the main player in the canonical activation of the NF-κB pathway. During this process, p65 undergoes phosphorylation, a transformation commonly measured experimentally to monitor the pathway activation.50 The positive control (inhibitor of IKK-1/-2) failed to prevent NF-κB p65 subunit phosphorylation, whereas the treatment with 11β,13-dihydrolactucin seemed to increase this phosphorylation even further when compared with the untreated control. Due to the very short incubation period and the co-stimulation condition, the rapid activation of the NF-κB pathway, one of the initial events in the cellular inflammatory response, may have occurred swiftly and overwhelmed the potential anti-inflammatory activity of the compounds. Conversely, when the human triple co-culture was incubated with the compounds for 4 hours prior to a 15-minute inflammation induction, both positive control and SL prevented NF-κB p65 phosphorylation. It has also been stated in the literature that SLs can inhibit the phosphorylation of IκB, which may be contributing to the prevention of NF-κB phosphorylation and translocation into the nucleus, as well as its binding to the DNA.17 These results may explain the ability of 11β,13-dihydrolactucin to decrease the gene expression of the cytokines IL-6 and TNF-α, and the release of the chemokine IL-8.
p38 is a stress-activated MAPK that is involved in IBD, playing an important role in the regulation of pro-inflammatory cytokines and inducible enzymes.51,52 In the 15-minute co-incubation, BMS 345541 apparently prevented p38 phosphorylation to some extent, but 11β,13-dihydrolactucin had no effect under the tested conditions. When the incubation period with the SL was increased from 15 minutes to 4 hours, its anti-inflammatory effect on the MAPK pathway was also improved, similar to what was observed for the NF-κB pathway, resulting in a modest but significant decrease in p38 phosphorylation levels. This modest decrease may be linked to the lack of effect observed for 11β,13-dihydrolactucin in terms of the gene expression of IL-1β, a cytokine that could result from the activation of the MAPK p38 pathway.49,53
These results suggest that 11β,13-dihydrolactucin could be effective in a prophylactic approach, potentially preventing the onset of intestinal inflammation in individuals susceptible to the development of IBD. This preventive approach would align with the dietary intake of 11β,13-dihydrolactucin through chicory, for health-promoting purposes.
Furthermore, the impact of 11β,13-dihydrolactucin on the inducible enzymes iNOS and COX-2 was assessed in terms of gene and protein expression. In this study, the pro-inflammatory cocktail promoted a significant increase in the gene expression of iNOS, which translated into an increased protein expression of the enzyme. The effect of 11β,13-dihydrolactucin on the inhibition of the gene and protein expression of iNOS was significant. Nitric oxide (NO), the lipophilic and highly diffusible product of iNOS, plays a role in the regulation of cellular signalling pathways, participating in the activation and regulation of immune cell function.17 Hence, the ability of 11β,13-dihydrolactucin to prevent the expression of this inducible enzyme contributes to counteracting the inflammatory effects of its product NO.
Conversely, when it comes to COX-2, the inflammatory stimulus produced a much higher response in the gene expression than in the protein levels of the enzyme. This is not a surprising outcome, since many regulation steps at the post-transcriptional, translational, and post-translational levels can take place, contributing to this lack of correlation.54 Nonetheless, 11β,13-dihydrolactucin led to a significant decrease of COX-2 gene and protein expression, to levels identical to those of the non-inflamed control, or even below the control in the case of protein expression. COX-2 is mainly responsible for the production of lipid mediators of inflammation, including prostaglandins such as PGE2, from arachidonic acid.55 Notably, a link between iNOS and COX-2 in the context of chronic inflammation has been discussed, since the products of these enzymes, NO and PGE2 respectively, can cause mutual activation.56 Additionally, the products of these enzymes are also involved in the expression of inflammatory cytokines like IL-6, IL-8, and TNF-α.56 Considering that NF-κB plays a key role in transcription regulation of both iNOS and COX-2,57 the ability of 11β,13-dihydrolactucin to prevent the phosphorylation and activation of NF-κB p65 may partly explain the capacity of this SL to decrease the expression of the two inflammatory inducible enzymes. Since these inflammatory events are all woven together, the anti-inflammatory effect of 11β,13-dihydrolactucin on one inflammation biomarker may be influencing the biological outcomes of other interconnected inflammation mediators, which in turn may result in an overall improvement of the inflammatory phenotype.
On another note, since intestinal myofibroblasts are key players in the inflammatory phenotype of IBD, due to the excessive production of extracellular matrix as a result of chronic inflammation, leading to fibrosis,58,59 a cell model of inflammation with human intestinal myofibroblast-like cells was also used. After stimulation of these cells with IL-1β, the levels of PGE2 and IL-6 were markedly increased. Indeed, intestinal myofibroblasts play a crucial role in the renewal of intestinal epithelial cells, and in the maintenance of epithelial homeostasis, through the production of several cytokines, among which IL-6.58 Moreover, COX-2 production is upregulated in these cells, leading to high amounts of PGE2.54 Conversely, the levels of TNF-α were more modestly, but still significantly, increased. 11β,13-Dihydrolactucin was only able to produce a significant decreasing effect on TNF-α and PGE2. The prevention of PGE2 release by 11β,13-dihydrolactucin is in line with what was observed in the human triple co-culture, where the SL could decrease the gene and protein expression of COX-2, the enzyme responsible for the production of PGE2.
Similar results to those obtained for 11β,13-dihydrolactucin in the cell models studied herein have been reported for another SL belonging to the guaianolide subclass in brain immune cells (microglia) stimulated with LPS.60 In the referred study, Sun et al.60 described an anti-inflammatory effect of micheliolide, where the SL decreased the gene and protein expression of the inducible enzymes iNOS and COX-2, and the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β, along with a decrease in the activation of the MAPK and NF-κB pathways.
Finally, a zebrafish model of intestinal inflammation25 was employed to validate the in vivo anti-inflammatory potential of 11β,13-dihydrolactucin. Acute intestinal inflammation was induced by an HCD, which is a physiological and targeted trigger. It has been reported that the HCD causes infiltration of neutrophils into the intestines of zebrafish larvae, recapitulating the first stages of intestinal inflammation.61,62 In the present study, 11β,13-dihydrolactucin significantly inhibited the neutrophilic infiltration caused by the HCD, reaching levels close to those of the non-inflamed control. This result is consistent with those obtained in the human triple co-culture model, in which the SL decreased the release of IL-8, the most relevant chemokine responsible for neutrophil recruitment. In the zebrafish animal model, we observed that an increased concentration of 11β,13-dihydrolactucin does not necessarily lead to an improved anti-inflammatory effect.
Other SLs, belonging to the SL subclass of heliangolides (lychnopholide, eremantholide C, and goyazensolide), were also reported to prevent neutrophil migration to the inflamed site in a mouse model of acute gout.63 Another study in a mouse model of colitis also showed the potential of the guaianolide micheliolide to prevent neutrophil infiltration.64 However, to the best of our knowledge, there are still no published studies concerning the ability of 11β,13-dihydrolactucin, the guaianolide studied herein, to prevent neutrophil infiltration in vivo.
Overall, our results show that one of the anti-inflammatory mechanisms of 11β,13-dihydrolactucin is based on the prevention of neutrophil migration to the site of inflammation, most probably through the modulation of IL-8 release. 11β,13-Dihydrolactucin was also able to modulate the NF-κB and MAPK p38 signalling pathways, leading to a lower expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, as well as the inducible inflammatory enzymes iNOS and COX-2. These findings hold significant clinical relevance in the context of IBD. By modulating key signaling pathways, and reducing the expression of cytokines and inflammatory enzymes, while inhibiting neutrophil migration, 11β,13-dihydrolactucin demonstrates potential as a therapeutic agent for managing inflammation associated with IBD, potentially offering patients relief from symptoms.
Further preclinical research in more complex in vivo models of intestinal inflammation will allow for the exploration of the full therapeutic potential of 11β,13-dihydrolactucin in the context of intestinal inflammation. The effect of long-term dietary exposure to 11β,13-dihydrolactucin as a prophylactic, adjuvant, or therapeutic approach to IBD treatment could be assessed, along with the impact of such exposure on individuals with different risk factors or severity of the disease.
Conclusions
In our study, we have shown that 11β,13-dihydrolactucin, a guaianolide sesquiterpene lactone present in chicory, exhibits great potential as a natural anti-inflammatory molecule with the ability to influence several stages of the intestinal cellular inflammatory response. In vitro, 11β,13-dihydrolactucin can effectively modulate crucial inflammatory signalling by inhibiting the phosphorylation of MAPK p38 and NF-κB p65, while reducing the release and expression of IL-8, IL-6, TNF-α, IL-1β, and PGE2. This naturally occurring SL was also found to downregulate both the gene and protein expression of iNOS and COX-2, inducible enzymes with significant roles in the inflammatory process. The promising anti-inflammatory potential of 11β,13-dihydrolactucin was validated in vivo, through the prevention of neutrophil infiltration into the inflamed gut of zebrafish larvae.In sum, our study indicates that this dietary sesquiterpene lactone, naturally occurring in chicory, may help alleviate colonic inflammation. As such, the incorporation of chicory products into the diet could be further explored for their potential in preventing inflammatory bowel disease (IBD).
Author contributions
M.S.M., M.A.A.G., and C.N.S. designed the study; M.S.M., M.A.A.G., and N.V.S. performed the experiments and analysed the data. M.S.M., M.A.A.G., A.G.S., C.N.S., C.L.C., and A.T.S. drafted the manuscript. A.J. and A.A.M. provided resources and revised the work. All authors reviewed the manuscript and agreed to the final version.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement no. 760891 (CHIC). iNOVA4Health Research Unit (UIDB/04462/2020 and UIDP/04462/2020), which is cofunded by Fundação para a Ciência e Tecnologia (FCT)/Ministério da Ciência e do Ensino Superior (MCES) through National Funds and by FEDER under the PT2020 Partnership Agreement is acknowledged. Zebrafish were reproduced and maintained by the CEDOC Fish Facility, supported by Congento LISBOA-01-0145-FEDER-022170, co-financed by FCT/MCES and Lisboa2020, under the PORTUGAL2020 agreement (European Regional Development Fund). The authors also acknowledge the support from the Associate Laboratories LS4FUTURE (LA/P/0087/2020). C. L. C. and A. J. acknowledge FCT/MCES for the funding grant PTDC/BTM-SAL/29377/2017. M. S. M. and A. T. S. also acknowledge the financial support from FCT/MCTES for their individual grants SFRH/BD/145551/2019 and CEECIND/04801/2017, respectively. A. J. and C. N. S. acknowledge the European Commission (grant number 101060346). A. G. S. acknowledges the grant 22030/PI/22 funded by the Programa Regional de Fomento de la Investigación Científica y Técnica (Plan de Actuación 2022) de la Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia (Murcia, Spain). The authors would like to acknowledge the support from the CEDOC Fish and Microscopy facilities.References
- R. Medzhitov, Origin and physiological roles of inflammation, Nature, 2008, 454, 428–435 CrossRef CAS PubMed.
- M. W. M. D. Lutgens, F. P. Vleggaar, M. E. I. Schipper, P. C. F. Stokkers, C. J. Van Der Woude, D. W. Hommes, D. J. De Jong, G. Dijkstra, A. A. Van Bodegraven and B. Oldenburg, et al., High frequency of early colorectal cancer in inflammatory bowel disease, Gut, 2008, 57(9), 1246–1251 CrossRef CAS PubMed.
- C. J. Li, Y. K. Wang, S. M. Zhang, M. D. Ren and S. X. He, Global burden of inflammatory bowel disease 1990–2019: A systematic examination of the disease burden and twenty-year forecast, World J. Gastroenterol., 2023, 29(42), 5751–5767 CrossRef PubMed.
- Y. A. Ghouri, V. Tahan and B. Shen, Secondary causes of inflammatory bowel diseases, World J. Gastroenterol., 2020, 26(28), 3998–4017 CrossRef CAS PubMed.
- S. C. Shah, H. Khalili, C. Gower-Rosseau, O. Olen and E. I. Benchimol, et al., Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries, Gastroenterology, 2018, 155(4), 1079–1089 CrossRef PubMed.
- M. Coskun, S. Vermeire and O. H. Nielsen, Novel Targeted Therapies for Inflammatory Bowel Disease, Trends Pharmacol. Sci., 2017, 38(2), 127–142 CrossRef CAS PubMed.
- A. R. Bourgonje, L. A. Bolte, L. L. C. Vranckx, L. M. Spekhorst and R. Gacesa, et al., Long-Term Dietary Patterns Are Reflected in the Plasma Inflammatory Proteome of Patients with Inflammatory Bowel Disease, Nutrients, 2022, 14(12), 2522 CrossRef CAS PubMed.
- A. Otley, A. S. Day and M. Zachos, Nutritional management of inflammatory bowel disease, in Pediatric Inflammatory Bowel Disease, 2023, vol. 27, pp. 333–356 Search PubMed.
- T. Yamamoto and T. Shimoyama, Nutrition and diet in inflammatory bowel disease, Curr. Opin. Gastroenterol., 2023, 39(2), 110–114 CrossRef CAS PubMed.
- J. Yan, L. Wang, Y. Gu, H. Hou, T. Liu, Y. Ding and H. Cao, Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges, Nutrients, 2022, 14(19), 4003 CrossRef CAS PubMed.
- A. Matias, S. L. Nunes, J. Poejo, E. Mecha, A. T. Serra, P. J. A. Madeira, M. R. Bronze and C. M. M. Duarte, Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice, Food Funct., 2014, 5(12), 3269–3280 RSC.
- A. Speciale, R. Bashllari, C. Muscarà, M. S. Molonia, A. Saija, S. Saha, P. J. Wilde and F. Cimino, Anti-Inflammatory Activity of an In Vitro Digested Anthocyanin-Rich Extract on Intestinal Epithelial Cells Exposed to TNF-α, Molecules, 2022, 27(17), 5368 CrossRef CAS PubMed.
- T. Vezza, F. Algieri, A. Rodríguez-Nogales, J. Garrido-Mesa and M. P. Utrilla, et al., Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation, Mol. Nutr. Food Res., 2017, 61(10), 1601066 CrossRef PubMed.
- M. S. Matos, J. D. Anastácio and C. N. Dos Santos, Sesquiterpene lactones: Promising natural compounds to fight inflammation, Pharmaceutics, 2021, 13(7), 991 CrossRef CAS PubMed.
- K. Janda, I. Gutowska, M. Geszke-Moritz and K. Jakubczyk, The common cichory (Cichorium intybus L.) as a source of extracts with health-promoting properties—a review, Molecules, 2021, 26(6), 1814 CrossRef CAS PubMed.
- M.Á. Ávila-Gálvez, C. Rafael-Pita, N. Fernández, J. Baixinho, J. D. Anastácio, K. Cankar, D. Bosch and C. Nunes dos Santos, Targeting proteases involved in the viral replication of SARS-CoV-2 by sesquiterpene lactones from chicory (Cichorium intybus L.), Food Funct., 2022, 13(17), 8977–8988 RSC.
- N. Arizmendi, S. B. Alam, K. Azyat, D. Makeiff, A. D. Befus and M. Kulka, The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation, Molecules, 2022, 27(8), 2450 CrossRef CAS PubMed.
- E. Novianti, G. Katsuura, N. Kawamura, A. Asakawa and A. Inui, Atractylenolide-III suppresses lipopolysaccharide-induced inflammation via downregulation of toll-like receptor 4 in mouse microglia, Heliyon, 2021, 7(10), e08269 CrossRef CAS PubMed.
- M. S. Matos, J. D. Anastácio, J. W. Allwood, D. Carregosa, D. Marques, J. Sungurtas, G. J. McDougall, R. Menezes, A. A. Matias and D. Stewart, et al., Assessing the intestinal permeability and anti-inflammatory potential of sesquiterpene lactones from chicory, Nutrients, 2020, 12(11), 3547 CrossRef CAS PubMed.
- J. A. Giménez-Bastida, M.Á. Ávila-Gálvez, J. C. Espín and A. González-Sarrías, The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells, Food Chem. Toxicol., 2020, 139, 111260 CrossRef PubMed.
- K. Cankar, J. C. Hakkert, R. Sevenier, C. Papastolopoulou and B. Schipper, et al., Lactucin Synthase Inactivation Boosts the Accumulation of Anti-inflammatory 8-Deoxylactucin and Its Derivatives in Chicory (Cichorium intybus L.), J. Agric. Food Chem., 2023, 71, 6061–6072 CAS.
- F. Antunes, F. Andrade, F. Araújo, D. Ferreira and B. Sarmento, Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs, Eur. J. Pharm. Biopharm., 2013, 83(3), 427–435 CrossRef CAS PubMed.
- I. Lozoya-Agullo, F. Araújo, I. González-Álvarez, M. Merino-Sanjuán, M. González-Álvarez, M. Bermejo and B. Sarmento, Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture, Mol. Pharm., 2017, 14, 1264–1270 CrossRef CAS PubMed.
- M. W. Pfaffl, A new mathematical model for relative quantification in real-time RT–PCR, Nucleic Acids Res., 2001, 29(9), e45 CrossRef CAS PubMed.
- N. V. Silva, D. Carregosa, C. Gonçalves, O. V. Vieira, C. Nunes dos Santos, A. Jacinto and C. L. Crespo, A Dietary Cholesterol-Based Intestinal Inflammation Assay for Improving Drug-Discovery on Inflammatory Bowel Diseases, Front. Cell Dev. Biol., 2021, 9, 674749 CrossRef PubMed.
- V. B. Mathema, Y. S. Koh, B. C. Thakuri and M. Sillanpää, Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities, Inflammation, 2012, 35(2), 560–565 CrossRef CAS PubMed.
- N. Lampiasi and G. Montana, An in vitro inflammation model to study the Nrf2 and NF-κB crosstalk in presence of ferulic acid as modulator, Immunobiology, 2018, 223(4–5), 349–355 CrossRef CAS PubMed.
- M. Giacomarra, M. La Torre and G. Montana, Effects of Inhibition of IKK Kinase Phosphorylation On the Cellular Defence System and HSP90 Activity, Inflammation, 2024, 47, 74–83 CrossRef CAS PubMed.
- J. R. Burke, M. A. Pattoli, K. R. Gregor, P. J. Brassil and J. F. MacMaster, et al., BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks NF-κB-dependent transcription in mice, J. Biol. Chem., 2003, 278(3), 1450–1456 CrossRef CAS PubMed.
- A. J. Rennekamp and R. T. Peterson, 15 years of zebrafish chemical screening, Curr. Opin. Chem. Biol., 2015, 24, 58–70 CrossRef CAS PubMed.
- T. Yoshimura, Discovery of IL-8/CXCL8 (the story from Frederick), Front. Immunol., 2015, 6, 278 Search PubMed.
- A. A. M. Kämpfer, M. Busch, V. Büttner, G. Bredeck, B. Stahlmecke, B. Hellack, I. Masson, A. Sofranko, C. Albrecht and R. P. F. Schins, Model Complexity as Determining Factor for In Vitro Nanosafety Studies: Effects of Silver and Titanium Dioxide Nanomaterials in Intestinal Models, Small, 2021, 17(15), 2004223 CrossRef PubMed.
- W. Skronska-Wasek, S. Durlanik, J. P. Garnett and S. Pflanz, Polarized cytokine release from airway epithelium differentially influences macrophage phenotype, Mol. Immunol., 2021, 132, 142–149 CrossRef CAS PubMed.
- L. Terheyden, J. Roider and A. Klettner, Basolateral activation with TLR agonists induces polarized cytokine release and reduces barrier function in RPE in vitro, Graefe's Arch. Clin. Exp. Ophthalmol., 2021, 259(2), 413–424 CrossRef CAS PubMed.
- B. A. Calvert, E. J. Quiroz, Z. Lorenzana, N. Doan and S. Kim, et al., Neutrophilic inflammation promotes SARS-CoV-2 infectivity and augments the inflammatory responses in airway epithelial cells, Front. Immunol., 2023, 14, 1112870 CrossRef PubMed.
- S. De Santis, D. Kunde, V. Galleggiante, M. Liso and L. Scandiffio, et al., TNFα deficiency results in increased IL-1β in an early onset of spontaneous murine colitis, Cell Death Dis., 2017, 8, e2993 CrossRef CAS PubMed.
- R. F. Souza, M. A. F. Caetano, H. I. R. Magalhães and P. Castelucci, Study of tumor necrosis factor receptor in the inflammatory bowel disease, World J. Gastroenterol., 2023, 29(18), 2733–2746 CrossRef CAS PubMed.
- R. H. Dosh, N. Jordan-Mahy, C. Sammon and C. Le Maitre, Interleukin 1 is a key driver of inflammatory bowel disease-demonstration in a murine IL-1Ra knockout model, Oncotarget, 2019, 10(37), 3559–3575 CrossRef PubMed.
- M. Rawat, M. Nighot, R. Al-Sadi, Y. Gupta, D. Viszwapriya, G. Yochum, W. Koltun and T. Y. Ma, IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA, Gastroenterology, 2020, 159(4), 1375–1389 CrossRef CAS PubMed.
- R. Bednarek, In Vitro Methods for Measuring the Permeability of Cell Monolayers, Methods Protoc., 2022, 5(1), 17 CrossRef CAS PubMed.
- B. Gareb, A. T. Otten, H. W. Frijlink, G. Dijkstra and J. G. W. Kosterink, Review: Local tumor necrosis factor-α inhibition in inflammatory bowel disease, Pharmaceutics, 2020, 12(6), 539 CrossRef CAS PubMed.
- L. Peyrin-Biroulet, W. J. Sandborn, R. Panaccione, E. Domènech, L. Pouillon, B. Siegmund, S. Danese and S. Ghosh, Tumour necrosis factor inhibitors in inflammatory bowel disease: the story continues, Ther. Adv. Gastroenterol., 2021, 14, 17562848211059954 CAS.
- T. Liu, L. Y. Zhang, D. Joo and S. C. Sun, NF-kappa B signalling in inflammation, Signal Transduction Targeted Ther., 2017, 2, 17023 CrossRef PubMed.
- H. B. Thapa, P. Kohl, F. G. Zingl, D. Fleischhacker, H. Wolinski, T. A. Kufer and S. Schild, Characterization of the Inflammatory Response Evoked by Bacterial Membrane Vesicles in Intestinal Cells Reveals an RIPK2-Dependent Activation by Enterotoxigenic Escherichia coli Vesicles, Microbiol. Spectrum, 2023, 11(4), e0111523 CrossRef PubMed.
- J. Mudter and M. F. Neurath, IL-6 signalling in inflammatory bowel disease: Pathophysiological role and clinical relevance, Inflamm. Bowel Dis., 2007, 13(8), 1016–1023 CrossRef PubMed.
- S. Schreiber, K. Aden, J. P. Bernardes, C. Conrad and F. Tran, et al., Therapeutic Interleukin-6 Trans-signalling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease, Gastroenterology, 2021, 160(7), 2354–2366 CrossRef CAS PubMed.
- D. I. Jang, A. H. Lee, H. Y. Shin, H. R. Song, J. H. Park, T. B. Kang, S. R. Lee and S. H. Yang, The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics, Int. J. Mol. Sci., 2021, 22(5), 2719 CrossRef CAS PubMed.
- M. Coccia, O. J. Harrison, C. Schiering, M. J. Asquith, B. Becher, F. Powrie and K. J. Maloy, IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4 + Th17 cells, J. Exp. Med., 2012, 209(9), 1595–1609 CrossRef CAS PubMed.
- J. Roman, J. D. Ritzenthaler, M. J. Fenton, S. Roser and W. Schuyler, Transcriptional regulation of the human interleukin 1β gene by fibronectin: Role of protein kinase C and activator protein 1 (AP-1), Cytokine, 2000, 12(11), 1581–1596 CrossRef CAS PubMed.
- M. S. Hayden and S. Ghosh, Shared principles in NF-κB signaling, Cell, 2008, 132(3), 344–362 CrossRef CAS PubMed.
- M. Coskun, J. Olsen, J. B. Seidelin and O. H. Nielsen, MAP kinases in inflammatory bowel disease, Clin. Chim. Acta, 2011, 412(7–8), 513–520 CrossRef CAS PubMed.
- G. H. Waetzig, D. Seegert, P. Rosenstiel, S. Nikolaus and S. Schreiber, p38 Mitogen-Activated Protein Kinase Is Activated and Linked to TNF-α Signalling in Inflammatory Bowel Disease, J. Immunol., 2002, 168(10), 5342–5351 CrossRef CAS PubMed.
- R. Nagesh, K. M. Kiran Kumar, M. Naveen Kumar, R. H. Patil and S. C. Sharma, Stress activated p38 MAPK regulates cell cycle via AP-1 factors in areca extract exposed human lung epithelial cells, Cytotechnology, 2019, 71(2), 507–520 CrossRef CAS PubMed.
- D. Wang, Discrepancy between mRNA and protein abundance: Insight from information retrieval process in computers, Comput. Biol. Chem., 2008, 32(6), 462–468 CrossRef CAS PubMed.
- L. S. Simon, Role and regulation of cyclooxygenase-2 during inflammation, Am. J. Med., 1999, 106(5B), 37S–42S CrossRef CAS PubMed.
- D. Basudhar, G. Bharadwaj, V. Somasundaram, R. Y. S. Cheng, L. A. Ridnour, M. Fujita, S. J. Lockett, S. K. Anderson, D. W. McVicar and D. A. Wink, Understanding the tumour micro-environment communication network from an NOS2/COX2 perspective, Br. J. Pharmacol., 2019, 176(2), 155–176 CrossRef CAS PubMed.
- P. P. Tak and G. S. Firestein, NF-κB in defense and disease NF-κB: a key role in inflammatory diseases, J. Clin. Invest., 2001, 107(1), 7–11 CrossRef CAS PubMed.
- M. C. Barnhoorn, S. K. Hakuno, R. S. Bruckner, G. Rogler, L. J. A. C. Hawinkels and M. Scharl, Stromal cells in the pathogenesis of inflammatory bowel disease, J. Crohn's Colitis, 2020, 14(7), 995–1009 CrossRef CAS PubMed.
- J. M. Park, J. Kim, Y. J. Lee, S. U. Bae and H. W. Lee, Inflammatory bowel disease-associated intestinal fibrosis, J. Pathol. Transl. Med., 2023, 57(1), 60–66 CrossRef PubMed.
- Z. Sun, G. Li, T. Tong and J. Chen, Micheliolide suppresses LPS-induced neuroinflammatory responses, PLoS One, 2017, 12(10), e0186592 CrossRef PubMed.
- O. Wéra, P. Lancellotti and C. Oury, The dual role of neutrophils in inflammatory bowel diseases, J. Clin. Med., 2016, 5(12), 118 CrossRef PubMed.
- F. Progatzy, N. J. Sangha, N. Yoshida, M. McBrien and J. Cheung, et al., Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation, Nat. Commun., 2014, 23(5), 5864 CrossRef PubMed.
- A. C. F. P. F. Bernardes, R. C. Matosinhos, M. C. de Paula Michel Araújo, C. H. Barros, R. D. de Oliveira Aguiar Soares, D. C. Costa, D. Sachs and D. A. Saúde-Guimarães, Sesquiterpene lactones from Lychnophora species: Antinociceptive, anti-inflammatory, and antioxidant pathways to treat acute gout, J. Ethnopharmacol., 2021, 269, 113738 CrossRef CAS PubMed.
- E. Viennois, B. Xiao, S. Ayyadurai, L. Wang, P. G. Wang, Q. Zhang, Y. Chen and D. Merlin, Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer, Lab. Invest., 2014, 94(9), 950–965 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01446d |
| This journal is © The Royal Society of Chemistry 2024 |