Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of consumption of the food additive carrageenan on the gut microbiota and the intestinal homeostasis of mice†
Alicia
Bellanco
a,
Judith
Félix
 b,
Estefanía
Díaz Del Cerro
b,
M. Carmen
Martínez Cuesta
b,
Estefanía
Díaz Del Cerro
b,
M. Carmen
Martínez Cuesta
 a,
Mónica
De la Fuente
b and
Teresa
Requena
a,
Mónica
De la Fuente
b and
Teresa
Requena
 *a
*a
aDepartment of Food Biotechnology and Microbiology, Instituto de Investigación en Ciencias de la Alimentación CIAL-CSIC, Madrid, Spain. E-mail: t.requena@csic.es
bDepartment of Genetics, Physiology and Microbiology (Animal Physiology Unit), Faculty of Biological Sciences, Complutense University of Madrid, 28040 Madrid, Spain
First published on 23rd May 2024
Abstract
The safety of the carrageenan (CGN) consumption as a food additive is under debate, with negative effects being associated with the products of hydrolysis of CGN. Moreover, there is an increasing need to integrate gut microbiome analysis in the scientific risk assessment of food additives. The objective of this study was to test the effects of CGN consumption on the gut microbiota and the intestinal homeostasis of young male and female mice. Female and male ICR-CD1 mice (8 weeks old) orally received 540 mg kg−1 day−1 of CGN, representing the maximum-level exposure assessment scenario surveyed for children, over the course of two weeks. Fecal material and peritoneal immune cells were analyzed to determine changes in the fecal microbiota, based on the analysis of bacterial 16S rRNA gene amplicon sequences and short-chain fatty acid (SCFA) concentrations, and some immune functions and redox parameters of peritoneal leukocytes. Non-significant microbiota taxonomical changes associated with CGN intake were found in the mouse stools, resulting the housing time in an increase in bacterial groups belonging to the Bacteroidota phylum. The PICRUSt2 functional predictions showed an overall increase in functional clusters of orthologous genes (COGs) involved in carbohydrate transport and metabolism. A significant increase in the cytotoxicity of fecal supernatants was observed in CGN-fed mice, which correlated with worsening of immune functions and oxidative parameters. The altered immunity and oxidative stress observed in young mice after the consumption of CGN, along with the fecal cytotoxicity shown towards intestinal epithelial cells, may be associated with the gut microbiota’s capacity to degrade CGN. The characterization of the gut microbiota’s ability to hydrolyze CGN should be included in the risk assessment of this food additive.
Introduction
Food additives are substances intentionally added during the technological preparation of processed foods in order to improve their safety, taste, texture or appearance, among other things. Carrageenans (CGNs) are widely used due to convenient physicochemical properties, such as gelling, thickening and stabilizing properties, and protein reactivity.1 CGNs are linear sulfated polygalactans that, depending on their structural characteristics and sulfation patterns, are divided into several types.2 For example, kappa- (κ-), iota- (ι-), and lambda- (λ-)CGN have one, two, and three sulfate groups in the disaccharide repeating units, respectively.3 Particularly, κ-CGN is commonly used for industrial purposes in the production of dairy products, juice drinks, ice cream, sugar products, meat products, beer, etc., and has the EU additive code E407.4For the characterization of CGNs, they are frequently evaluated in vitro for beneficial properties such as antioxidant, anti-inflammatory, or antithrombotic activities;5 however, they are applied as a model in vivo to induce impairing effects such as paw edema, intestinal inflammation or colon ulcerative lesions.6 This disparity in scientific outcomes has been attributed to molecular weight (Mw) differences between the assayed compounds, differentiating between the high Mw of the food additive (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–800
000–800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and the low Mw of the products of hydrolysis of CGN, degraded CGN or polygeenan (10
000 Da) and the low Mw of the products of hydrolysis of CGN, degraded CGN or polygeenan (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da). The latter is not authorized as a food additive4 and is classified as a potential human carcinogen by the International Agency for Research on Cancer.7
000 Da). The latter is not authorized as a food additive4 and is classified as a potential human carcinogen by the International Agency for Research on Cancer.7
CGN hydrolysis during passage through the gastrointestinal tract has been barely described; in addition, due to protein reactivity, CGN may attenuate protein digestibility by pepsin.8 Likewise, little is known about the ability of the gut microbiota to hydrolyze CGN and, in such a case, the safe threshold limit of the low-molecular-weight products. Bacteria able to hydrolyze CGN into sulfated oligosaccharides have been isolated mainly from marine environments,9,10 but no conclusive information exists about CGN-degrading bacteria present in the human intestine. In addition to changes in polysaccharide molecular weight, the sulfated CGN products can be degraded by bacterial sulfatases and sulfate reductases to produce hydrogen sulfide, which has been implicated in inflammatory bowel diseases.11
The increasing knowledge of the interactions between the gut microbiome and dietary components has motivated food safety authorities, such as the EFSA, to explore integrating gut microbiome analysis into the regulatory scientific risk assessment of food additives and xenobiotics, among other things.12,13 In recent work using a dynamic gut microbiota simulator (BFBL Gut Model,14), we have observed a dose-dependent response in the permeabilizing effect of supernatants from CGN-fed microbiota when incubated with intestinal epithelium Caco-2 line cells, but no effect with an equal amount of CGN in the incubation medium.15 When performing in vivo studies, the intestinal homeostasis can be assessed as it is achieved via adequate communication among the gut microbiota, immune cells, and epithelium. In mice, the functional capacity and redox state of peritoneal leukocytes are good markers of intestinal homeostasis, but also of the general homeostatic response and the health condition of the animals.16–18 There are scarce studies on the effects of CGN on the function and oxidative stress of leukocytes, and these have shown contradictory results.19–21 Moreover, the effects of CGN ingestion on several relevant immune functions and redox parameters of peritoneal leukocytes from mice are unknown. Therefore, the objective of this study was to test the in vivo effects of feeding CGN, at exposure values assessed for children,4 on the gut microbiota and the intestinal homeostasis of young male and female mice.
Materials and methods
Animals and experimental design
Young (8 weeks old) ICR-CD1 female (FC8w) and male (MC8w) mice were housed (five per cage and separated by sex) and maintained with ad libitum access to food (A04 diet, Panlab S.L., Barcelona, Spain) and tap water under light (12/12 h reversed light/dark cycle; lights off at 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 A.M.), and controlled temperature (22 ± 2 °C) and humidity (50–60%).
00 A.M.), and controlled temperature (22 ± 2 °C) and humidity (50–60%).
The animals (n = 40) were distributed into control and experimental groups (10 per group and sex). The mice orally received 540 mg kg−1 day−1 of κ-carrageenan resuspended in 200 μL of PBS for 15 days in the experimental groups (FCGN10w and MCGN10w) and PBS in the control groups (FC10w and MC10w). The administered CGN dose was selected at the maximum-level exposure assessment scenario surveyed for children.4 Body weight was monitored before and after the two-week experimental period.
The protocol was approved by the Experimental Animal Committee of the Complutense University of Madrid and Community of Madrid (PROEX. 224.0/21). The animals were treated in accordance with the guidelines of Royal Decree 118/2021 of 23 February 2021 (BOE no. 47) on the protection of animals used for experimentation and other scientific purposes.
Faecal analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min), and the pellet used for genomic DNA purification. DNA was extracted using an E.Z.N.A. Stool DNA Kit (Omega Bio-tek) and quantified using a NanoDrop 1000 UV/VIS Spectrophotometer (Thermo Fisher). DNA samples were analyzed via amplicon-based metagenomic sequencing of the 16S rDNA V3–V4 region, performed by Novogen (Cambridge, UK) on an Illumina platform, to generate 250 bp paired-end reads. Bacterial taxonomy was assigned to the obtained ASVs (Amplicon Sequence Variants) by using the QIIME2 software. The absolute abundance of ASVs was normalized using a standard of the sequence number corresponding to the sample with the least sequences. Subsequent analyses of alpha diversity and beta diversity were all performed based on the normalized data. PICRUSt2 (V2.3.0) was used to predict the metagenomic functions according to 16S rRNA gene sequencing data based on Cluster of Orthologous Genes (COG), Enzyme Commission (EC), and Pathway (PWY) databases. The raw generated sequences were deposited in the Digital.CSIC database (https://doi.org/10.20350/digitalCSIC/16174).
000g, 10 min), and the pellet used for genomic DNA purification. DNA was extracted using an E.Z.N.A. Stool DNA Kit (Omega Bio-tek) and quantified using a NanoDrop 1000 UV/VIS Spectrophotometer (Thermo Fisher). DNA samples were analyzed via amplicon-based metagenomic sequencing of the 16S rDNA V3–V4 region, performed by Novogen (Cambridge, UK) on an Illumina platform, to generate 250 bp paired-end reads. Bacterial taxonomy was assigned to the obtained ASVs (Amplicon Sequence Variants) by using the QIIME2 software. The absolute abundance of ASVs was normalized using a standard of the sequence number corresponding to the sample with the least sequences. Subsequent analyses of alpha diversity and beta diversity were all performed based on the normalized data. PICRUSt2 (V2.3.0) was used to predict the metagenomic functions according to 16S rRNA gene sequencing data based on Cluster of Orthologous Genes (COG), Enzyme Commission (EC), and Pathway (PWY) databases. The raw generated sequences were deposited in the Digital.CSIC database (https://doi.org/10.20350/digitalCSIC/16174).
Analysis of immune function parameters
The immune analyses were carried out with peritoneal leukocytes that were obtained as previously described.25 The peritoneal suspensions were adjusted with Hanks’ solution (Sigma-Aldrich) to a specific concentration of macrophages, lymphocytes or total leukocytes, which were identified and quantified using Neubauer chambers (Blaubrand). Cellular viability (higher than 95 ± 1%) was checked using trypan blue (Sigma-Aldrich).The natural killer (NK) cell cytotoxicity was evaluated via the lysis of murine YAC-1 lymphoma cells (105 mL−1) induced by peritoneal leukocytes (106 mL−1) by quantifying the lactate dehydrogenase released into the medium (CytoTox 96, Promega). The results were expressed as the percentage of tumor cells killed (% lysis), as previously described.17
The proliferative capacities of lymphocytes, basal and stimulated by the mitogens concanavalin A (ConA) (1 μg mL−1; Sigma-Aldrich) and lipopolysaccharide (LPS) (1 μg mL−1, Escherichia coli 055:B5; Sigma-Aldrich), were evaluated in 106 leukocytes per mL incubated with 3H-thymidine (0.5 μCi, MP Biomedicals) for 24 h.25 The 3H-thymidine uptake by the lymphocytes was quantified in a liquid scintillation beta counter (LKB) and the results were expressed in counts per minute (cpm). The percentage of stimulation was expressed as the % mitogen-stimulated lymphoproliferation in relation to the basal cpm.
Macrophage phagocytosis was evaluated based on their capacity to take in latex beads (1% in PBS).17 The number of latex beads ingested per 100 macrophages (phagocytic index) and the percentage of macrophages that ingested at least one latex bead (phagocytic efficacy) were determined.
Evaluation of oxidative stress parameters
The oxidative parameters were assayed with mouse peritoneal leukocytes adjusted to 106 cells per mL in Hanks’ solution. The glutathione content (reduced, GSH, and oxidized, GSSG) of sonicated leukocyte suspensions (106 mL−1) was assayed using o-phthalaldehyde at pH 8 and pH 12, respectively, as previously described.16 The protein content of the samples was determined following the BCA Protein Assay Kit protocol (Sigma-Aldrich). The results were expressed as nmol per mg protein. The GSSG/GSH ratio was calculated for each sample.The glutathione reductase (GR) activity of the leukocyte suspension (106 cells per ml) was measured with 80 mM GSSG (Sigma-Aldrich) as the substrate, as previously described.26 For the glutathione peroxidase (GPx) activity, the leukocytes were tested with cumene hydroperoxide as the substrate (cumene-OOH; Sigma-Aldrich).26 The enzymatic activities are expressed in mU of GR or GPx activity per mg protein.
Statistical analyses
Results were expressed as mean ± standard error of the mean (SEM) or median and interquartile range (IQR). One-way analysis of variance (ANOVA) and Tukey's multiple comparison tests (when samples were normally distributed) or Klustal–Wallis analysis were performed to determine differences between treatment groups, employing SPSS Statistics for Windows, version 29.0 (IBM Corporation). Student’s t-test (when samples were normally distributed) or Mann–Whitney U analysis was performed for comparison between controls and CGN-fed mice under the same conditions. The significance level of the statistical tests was set to p < 0.05. Pearson's correlation (r, *p < 0.05, **p < 0.01) was used to analyse the correlation between cytotoxicity and immune and oxidative functions. Heatmap plots were obtained applying the ClustVis webtool (https://biit.cs.ut.ee/clustvis/, accessed on 15 January 2024).27Results
Housing and animal weight
During the experimental period (two weeks), the mice of the control groups (male and female) increased in weight with respect to the initial value (Table 1), in accordance with the normal pattern of weight gain of the ICR mice.28 The administration of CGN (540 mg kg−1 day−1) during the two weeks, however, limited weight gain in both female (F) and male (M) groups, resulting in a non-significant weight increase in the CGN-fed mice during the two weeks of treatment.| Group | N | Weight (g) | Shannon | Simpson |
|---|---|---|---|---|
| *, significant differences (p < 0.05) between the start (8w) and after two weeks (10w) of treatment (C or CGN), using Student’s t-test analysis. | ||||
| FC8w | 18 | 29.52 (0.62) | 6.39 (0.12) | 0.963 (0.005) |
| FC10w | 9 | 31.30* (0.63) | 5.90* (0.16) | 0.941* (0.010) |
| FCGN10w | 9 | 29.23 (0.53) | 5.92* (0.07) | 0.947* (0.003) |
| MC8w | 19 | 34.63 (0.47) | 6.79 (0.08) | 0.976 (0.002) |
| MC10w | 8 | 36.58* (0.55) | 5.81* (0.20) | 0.945* (0.011) |
| MCGN10w | 8 | 35.64 (0.46) | 6.10* (0.27) | 0.942* (0.013) |
Gut microbiota composition and metabolism
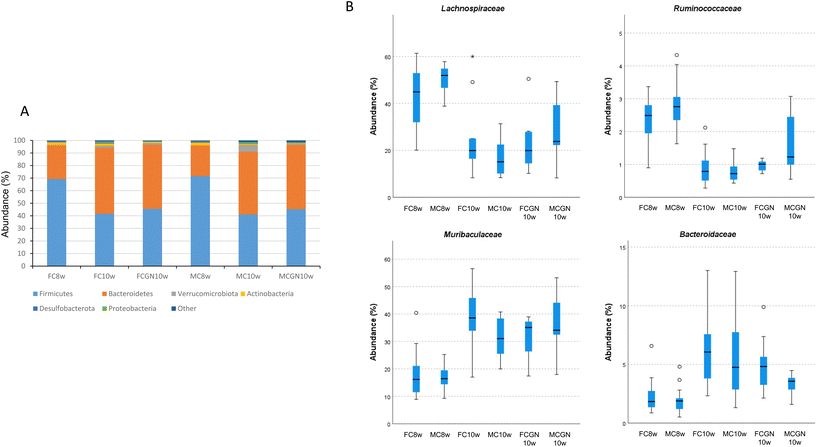
The microbiota composition was obtained from the ASV-based analysis of the 16S rRNA gene amplicon sequences obtained from the mouse faecal DNA. The results indicated a decrease in the estimated bacterial richness (Shannon index) of the microbiome and its evenness (Simpson index) during the 2-week experimental period, which was similar for female and male mice in both the control and the CGN groups (Table 1).The microbial changes observed during the 2-week experimental period can be attributed to the decrease in the relative abundance (%; mean ± SEM) of populations of the phyla Bacillota (syn. Firmicutes), 70.30 ± 1.29 vs. 43.35 ± 1.85, and Actinomycetota (syn. Actinobacteria), 2.16 ± 0.23 vs. 0.96 ± 0.14, and the corresponding increase in the relative abundances in the phyla Bacteroidota (syn. Bacteroidetes), 25.61 ± 1.32 vs. 50.78 ± 1.68, Verrucomicrobiota (syn. Verrucomicrobia), 0.24 ± 0.15 vs. 2.45 ± 0.63, and Pseudomonadota (syn. Proteobacteria), 0.18 ± 0.04 vs. 0.74 ± 0.15. The changes were observed in both male and female mice and were not related to the CGN intake (Fig. 1A). The mentioned changes could be attributed to decreases in the families Lachnospiraceae and Ruminococcaceae and increases in Muribaculaceae and Bacteroidaceae (Fig. 1B).
Table 2 shows the bacterial genera representing at least 0.1% relative abundance in any of the tested mice. Most of the differences were observed after the two weeks of the experimental period, with only the increase in Alloprevotella being associated with CGN intake in both the female and male CGN10w mouse groups. However, the observed CGN effect on this genus’ abundance cannot provide enough information to infer a positive29 or negative effect.30Bacteroides was the genus showing the highest increase during the experimental period in both F and M mouse groups, whereas the Eubacterium xylanophilum group showed the most marked decrease during the assayed time (Table 2). Differences between the F and M groups were only observed in the relative abundances of Lactobacillus and Bifidobacterium.
| FC8w | FC10w | FCGN10w | MC8w | MC10w | MCGN10w | |
|---|---|---|---|---|---|---|
| *, significant differences (p < 0.05) between the start (8w) and after two weeks (10w) of treatment (C or CGN). ‡, significant differences between F and M under the same conditions, using the Mann–Whitney analysis. | ||||||
| Lactobacillus | 8.93 (5.35–11.05) | 3.79 (2.33–4.78) | 10.56 (3.73–12.31) | 1.75‡ (1.14–3.66) | 3.08 (2.55–8.53) | 2.60‡ (1.67–4.99) |
| Turicibacter | 0.08 (0.04–0.13) | 0.13 (0.09–0.37) | 0.56 (0.36–1.59) | 0.07 (0.04–0.26) | 0.42 (0.33–0.70) | 0.07 (0.02–0.10) |
| Alloprevotella | 3.97 (2.26–5.49) | 3.08 (1.77–9.55) | 12.13* (5.95–14.99) | 1.87 (1.54–3.14) | 4.40 (1.32–6.11) | 6.46* (2.95–8.91) |
| Eubacterium xylanophilum group | 2.78 (1.29–3.99) | 0.96* (0.63–1.71) | 0.69* (0.51–0.83) | 4.45 (3.26–5.85) | 0.28* (0.13–0.48) | 0.91* (0.44–1.70) |
| Akkermansia | 0.05 (0.02–0.15) | 0.11 (0.04–1.81) | 0.16 (0.06–0.80) | 0.02 (0.01–0.10) | 2.26* (0.19–8.52) | 0.07 (0.01–0.12) |
| Bacteroides | 1.83 (1.35–2.74) | 6.08* (3.82–7.60) | 4.84* (3.33–5.64) | 1.89 (1.20–2.12) | 4.76* (2.94–6.66) | 3.58* (2.87–3.87) |
| Bifidobacterium | 0.00 (0.00–0.01) | 0.10* (0.04–0.45) | 0.15* (0.05–0.31) | 0.01 (0.00–0.02) | 0.00‡ (0.00–0.01) | 0.00‡ (0.00–0.00) |
| Alistipes | 1.01 (0.73–1.41) | 0.72 (0.50–1.44) | 0.86 (0.71–1.31) | 0.74 (0.63–1.30) | 1.48 (0.62–2.88) | 1.42 (1.31–1.92) |
| Romboutsia | 0.00 (0.00–0.00) | 0.04* (0.01–0.07) | 0.07* (0.05–0.13) | 0.01 (0.00–0.05) | 0.18 (0.10–0.33) | 0.06 (0.02–0.10) |
| Enterorhabdus | 1.65 (1.30–2.66) | 0.79* (0.54–1.07) | 0.52* (0.44–0.64) | 1.67 (1.29–2.40) | 0.61* (0.42–0.68) | 0.39* (0.26–0.40) |
| Odoribacter | 0.57 (0.31–1.11) | 0.63 (0.55–1.18) | 0.65 (0.43–1.20) | 0.56 (0.33–1.59) | 0.44 (0.33–2.17) | 0.92 (0.77–1.38) |
| Lachnoclostridium | 0.88 (0.69–1.24) | 0.40* (0.23–0.61) | 0.44* (0.32–0.47) | 0.76 (0.61–1.05) | 0.23* (0.18–0.35) | 0.27* (0.26–0.44) |
| Eubacterium coprostanoligenes group | 1.36 (0.94–1.94) | 0.53* (0.49–0.58) | 0.85* (0.70–0.90) | 1.66 (0.90–1.88) | 1.00 (0.60–1.62) | 1.04 (0.37–1.76) |
| Ruminococcus | 0.72 (0.56–0.97) | 0.15* (0.08–0.41) | 0.44* (0.35–0.55) | 0.85 (0.62–1.29) | 0.30* (0.22–0.46) | 0.44 (0.28–1.11) |
| Blautia | 0.52 (0.37–0.66) | 0.15 (0.13–0.58) | 0.41 (0.24–0.80) | 0.45 (0.29–0.62) | 0.16 (0.06–0.25) | 0.31 (0.21–0.57) |
| Roseburia | 0.39 (0.21–0.67) | 0.31 (0.18–0.73) | 0.20 (0.18–0.37) | 0.69 (0.41–1.15) | 0.39 (0.17–1.02) | 0.28 (0.16–0.63) |
| Erysipelatoclostridium | 0.12 (0.08–0.23) | 0.12 (0.05–0.30) | 0.13 (0.10–0.17) | 0.16 (0.13–0.37) | 0.10 (0.06–0.22) | 0.07 (0.05–0.10) |
| Muribaculum | 1.01 (0.68–1.50) | 2.14 (1.68–2.40) | 1.34 (1.12–2.02) | 1.01 (0.72–1.21) | 1.26 (0.94–1.51) | 1.10 (1.00–1.32) |
| Monoglobus | 0.27 (0.19–0.33) | 0.15* (0.04–0.30) | 0.09* (0.07–0.13) | 0.41 (0.27–0.65) | 0.07* (0.04–0.08) | 0.13* (0.10–0.19) |
| Oscillibacter | 0.57 (0.30–0.68) | 0.22* (0.20–0.29) | 0.27* (0.25–0.32) | 0.68 (0.58–0.94) | 0.20* (0.14–0.26) | 0.42* (0.27–0.46) |
| Parabacteroides | 0.04 (0.03–0.07) | 0.10* (0.07–0.12) | 0.17* (0.09–0.24) | 0.03 (0.02–0.05) | 0.14* (0.06–0.22) | 0.23* (0.13–0.26) |
| Colidextribacter | 0.38 (0.28–0.58) | 0.13* (0.10–0.18) | 0.20* (0.14–0.23) | 0.51 (0.41–0.61) | 0.12* (0.09–0.17) | 0.24* (0.21–0.26) |
| Parasutterella | 0.06 (0.04–0.09) | 0.33* (0.13–0.90) | 0.21* (0.16–0.42) | 0.04 (0.03–0.07) | 0.10* (0.05–0.29) | 0.05 (0.04–0.19) |
| Anaerotruncus | 0.14 (0.08–0.19) | 0.09* (0.05–0.09) | 0.05* (0.04–0.06) | 0.17 (0.13–0.28) | 0.05* (0.04–0.09) | 0.10* (0.05–0.16) |
| Butyricicoccus | 0.13 (0.11–0.15) | 0.08* (0.03–0.09) | 0.07* (0.06–0.10) | 0.16 (0.13–0.24) | 0.03* (0.02–0.06) | 0.07* (0.05–0.08) |
The PICRUSt2 functional predictions based on 16S rDNA amplicons showed an overall clustering of samples according to age (ESI: Fig. S1, COGs; Fig. S2, Pathway; Fig. S3, EC†). The predicted functions of COGs related to carbohydrate transport and metabolism were examined in more detail (Fig. 2). Shifts in functional COGs of the gut microbiota involved in carbohydrate transport and metabolism during the experimental period indicated an increase in function related to complex glycan hydrolysis, such as arabinogalactan, glucan and polygalacturan-related enzyme activities, among others. Conversely, a decrease in bacterial polysaccharide transport systems and galactosidase and glucosidase activities could coincide with the changes in microbial composition.
Table 3 shows changes in the microbial metabolism, assessed as the concentration of SCFAs and ammonium. The results indicated a decrease in succinic acid with time, independently of the CGN intake, and a trend of increasing concentration of acetic and propionic acids, being only significantly higher in the M10w groups.
| Group | Succinate | Lactate | Acetate | Propionate | Butyrate | Ammonium |
|---|---|---|---|---|---|---|
| *, significant differences (p < 0.05) between the start (8w) and after two weeks (10w) of treatment (C or CGN), using Student’s t-test analysis. | ||||||
| FC8w | 13.16 (1.95) | 59.26 (10.64) | 15.61 (1.81) | 2.34 (0.32) | 3.78 (0.77) | 0.78 (0.09) |
| FC10w | 6.66* (1.98) | 47.79 (13.53) | 19.22 (2.85) | 3.25 (0.75) | 2.99 (0.69) | 0.46 (0.08) |
| FCGN10w | 5.92* (0.63) | 53.99 (13.71) | 22.68 (2.79) | 4.46 (1.18) | 2.03 (0.32) | 0.96 (0.38) |
| MC8w | 12.70 (0.91) | 45.09 (4.89) | 12.78 (1.68) | 2.81 (0.32) | 2.69 (0.19) | 0.71 (0.07) |
| MC10w | 5.18* (0.96) | 65.45 (10.90) | 26.78 (3.24) | 6.45* (1.53) | 2.09 (0.26) | 1.05 (0.35) |
| MCGN10w | 4.04* (0.59) | 79.54 (11.70) | 22.23 (1.86) | 6.94* (1.56) | 2.48 (0.36) | 0.77 (0.19) |
Cytotoxicity and physiological effects
In addition to intestinal microbiota changes associated with CGN intake, we studied possible physiological effects caused by CGN feeding during the two weeks of the experimental period. In previous studies, we observed that hydrolyzed CGN caused cytotoxicity towards Caco-2 cells at the IC50 value of 100 μg mL−1 (Bellanco et al., unpublished results). The results shown in Table 4 indicate a significant increase in toxicity of the supernatants from the peptone-washed fecal material of the mice from the CGN groups. The possible CGN-permeabilization effect in the gut was correlated with changes in some immune function parameters (Fig. S4†), such as the proliferative capacity of lymphocytes stimulated by concanavalin A (Pearson 0.561***, p < 0.001) and the macrophage phagocytic efficiency (Pearson 0.515**, p < 0.01). Other parameters that significantly worsened during the CGN intake were the NK cell cytotoxicity, the macrophage phagocytic index and the GR activity (Table 4).| Group | Cytotoxicity (% cell viability) | NK (%) | ConA (%) | LPS (%) | PE | PI | GR |
|---|---|---|---|---|---|---|---|
| *, significant differences (p < 0.05) between the mouse control and the mice fed CGN under the same conditions, using Student’s t-test analysis. | |||||||
| FC10w | 56.74 (6.58) | 45.16 (2.90) | 114.99 (2.60) | 107.18 (3.39) | 83.75 (1.00) | 576.12 (33.94) | 9.25 (2.44) |
| FCGN10w | 7.46* (2.57) | 35.72* (1.65) | 104.70* (1.22) | 101.29 (2.68) | 76.62* (1.29) | 340.67* (43.88) | 1.94* (0.53) |
| MC10w | 65.69 (6.59) | 45.14 (2.87) | 118.38 (2.84) | 108.21 (3.16) | 84.25 (0.84) | 579.14 (59.29) | 3.36‡ (0.69) |
| MCGN10w | 17.46* (5.82) | 35.70* (3.92) | 94.67* (3.02) | 89.75* (6.11) | 78.86* (1.03) | 390.37* (26.27) | 1.01* (0.52) |
Discussion
Carrageenan (CGN) is one of the authorized food additives that encounter a substantial number of controversial opinions in the scientific literature. Favorable considerations are based on its worldwide approved use in foods by regulatory authorities,31 and the long-term consumption habit in Asia of macroalgaes and their commercial application for CGN extraction,32 which is based on its convenient technological properties as a gelling, thickening, and stabilizing agent.1 In addition, there are a number of in vitro studies that indicate potential health benefits of CGN, such as antioxidant, anticoagulant and antiviral activities, among others.5 On the other hand, CGN has been used in animal models to induce inflammation.33 Moreover, it has been demonstrated to contribute to earlier relapse in patients with ulcerative colitis in remission34 and to alter the gastrointestinal degradation of proteins, reducing protein and peptide bioaccessibility.8A scoping review on the gut effects of the food additive CGN35 and other recent reports36,37 state, as hallmark conclusions, the importance of addressing the interaction of the additive with the gut microbiome. Our results show that adverse effects, such as reduced animal weight and lowered immune functions (Tables 1 and S1†), were observed in spite of non-significant microbiota taxonomical changes associated with CGN intake (Table 2). The results show a significant effect of the housing time on the microbiota (Tables 1 and 2, Fig. 1), characterized by a decrease in the Shannon (microbial richness) and Simpson (microbial evenness) taxonomic indices, a lower relative abundance of Bacillota and an increase in bacterial groups belonging to the Bacteroidota phylum. Such a swift change has been observed in this type of mouse strain after weaning38 and it has been associated with the adaptation to changes in solid diets.39
Regarding the adverse effects of CGN observed in our study (Tables 1, 4 and S1†), an extensive body of evidence explains the damage as being related to CGN degradation to low molecular weight (average 20 to 30 kDa) compounds,25,27,30 which have demonstrated mainly inflammatory effects along with decreasing barrier function and/or increasing permeability.35 In previous studies,15 we have observed that the same CGN brand supplied to the mice, evaluated at values as high as 3 mg mL−1, did not have any impact either on Caco-2 cell viability or on the epithelial monolayer integrity; however, hydrolysed CGN (heated at 121 °C for 15 min at pH < 2) decreased cellular viability and increased epithelial permeability at an IC50 value of 0.1 mg mL−1. The results in Table 4, showing that the fecal content of mice fed CGN increased the cytotoxicity towards Caco-2, would indicate some degree of CGN hydrolysis through intestinal transit and/or degradation by the gut microbiota. Considering that the formation of fragments with Mw lower than 50 kDa from CGN, using an artificial stomach, resulted in about 3% hydrolysis,40 additional CGN degradation by the gut microbiota could trigger intestinal barrier damage. The increased abundance of ASVs assigned to Bacteroidota, in particular to the genus Bacteroides, in 10w mice (Fig. 1, Table 2) would provide the mouse microbiota with an increased potential utilization of complex carbohydrates, such as arabinogalactans, glucans, xylose, polygalactans and betagalactans, among others (Fig. 2). Actually, gut microbiotas with a marked abundance of Bacteroides have been described to be effective at utilizing galactans, including CGN.41–43
The cytotoxicity and permeabilizing effect of degraded CGN in the gut mucosal barrier could be associated with the physiological damage observed in the mice, such as delayed growth (Table 4). Body weight loss after intragastric administration of CGN has also been shown in BALB/c mice.44 In addition, in male and female mice, the ingestion of CGN caused lower values in relevant immune functions associated with health condition, such as natural killer antitumor activity, lymphoproliferative response to the mitogen ConA, and phagocytosis capacity (Tables 4 and S1†). These results agree with some previous studies in which immune suppressant effects of CGN in vivo have been observed.21 Considering that degraded CGN is used as an experimental inflammation model, similar to the dextran sulfate sodium (DSS) colitis model,45 the potential hydrolysis of CGN by the gut microbiota could have favored the damage caused by low-Mw CGN products to the intestinal barrier permeability.35 The sulfate groups from the polygalactan could facilitate disturbing the intestinal mucus layer by making it more permeable to gut microbiota that reach the epithelial cells and trigger an inflammatory reaction.46 Thus, a correlation between mouse fecal cytotoxicity and altered leukocyte function parameters, such as the proliferative capacity of lymphocytes stimulated by ConA and the macrophage phagocytic efficiency, was detected in the present study (Fig. S4†). In addition, taking into account that inflammation and oxidation are two processes that occur together,47 and that oxidative stress is the basis of deteriorated function of immune cells,48 the lower antioxidant activity observed in leukocytes from mice after CGN ingestion could explain their worse immune function.
In conclusion, the altered immunity and oxidative stress observed in young mice after the consumption of CGN, along with the cytotoxicity shown towards intestinal epithelial cells incubated with their fecal supernatants, may be associated with the gut microbiota’s capacity to hydrolyze the CGN, although non-significant bacterial taxonomic changes have been observed. The identification of microbiota species that hydrolyze sulfated polysaccharides is still limited, as is the knowledge of the long-term effects of current scenarios of increasing food-additive consumption, which is bound to have an impact on human health.
Author contributions
MDLF, MCMC and TR conceived and designed the experiments, acquired funding and supervised all experimental works. AB, JF and EDDC performed experiments. AB, JF, MDLF and TR carried out data analyses. AB, MDLF and TR drafted the manuscript. All authors have made critical revisions to the manuscript and read and approved the final manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research is part of the grants from the Spanish State Research Agency PID2019-106071RB-I00, PID2022-136874OB-C31 and PID2022-136874OB-C32 (funded by MCIN/AEI/10.13039/501100011033). The authors are indebted to E.F. Sáez Martínez for technical support. We acknowledge the CSIC Open Access Publication Support (URICI).References
- T. Udo, G. Mummaleti, A. Mohan, R. K. Singh and F. Kong, Current and emerging applications of carrageenan in the food industry, Food Res. Int., 2023, 173, 113369 CrossRef CAS PubMed.
- L. Hilliou, Structure–elastic properties relationships in gelling carrageenans, Polymers, 2021, 13, 4120 CrossRef CAS PubMed.
- S. H. Knutsen, D. E. Myslabodski, B. Larsen and A. I. Usov, A modified system of nomenclature for red algal galactans, Bot. Mar., 1994, 37, 163–170 CAS.
- M. Younes, P. Aggett, F. Aguilar, R. Crebelli, M. Filipič, M. J. Frutos, P. Galtier, D. Gott, U. Gundert-Remy, G. G. Kuhnle, C. Lambré, J. Leblanc, I. T. Lillegaard, P. Moldeus, A. Mortensen, A. Oskarsson, I. Stankovic, I. Waalkens-Berendsen, R. A. Woutersen, M. Wright, L. Brimer, O. Lindtner, P. Mosesso, A. Christodoulidou, S. Ioannidou, F. Lodi and B. Dusemund, Re–evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives, EFSA J., 2018, 16, e05238 Search PubMed.
- B. Pradhan and J.-S. Ki, Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease, Int. J. Biol. Macromol., 2023, 238, 124085 CrossRef CAS PubMed.
- J. Watt and R. Marcus, Carrageenan-induced ulceration of the large intestine in the guinea pig, Gut, 1971, 12, 164–171 CrossRef CAS PubMed.
- IARC, Evaluation of the carcinogenic risks to humans, Geneva, 1987. (Accessed March 19, 2024, at https://monographs.iarc.who.int/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf) Search PubMed.
- L. Fahoum, A. Moscovici, S. David, R. Shaoul, G. Rozen, E. G. Meyron-Holtz and U. Lesmes, Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function, Mol. Nutr. Food Res., 2017, 61, 1600545 CrossRef PubMed.
- E. Ficko-Blean, A. Préchoux, F. Thomas, T. Rochat, R. Larocque, Y. Zhu, M. Stam, S. Génicot, M. Jam, A. Calteau, B. Viart, D. Ropartz, D. Pérez-Pascual, G. Correc, M. Matard-Mann, K. A. Stubbs, H. Rogniaux, A. Jeudy, T. Barbeyron, C. Médigue, M. Czjzek, D. Vallenet, M. J. McBride, E. Duchaud and G. Michel, Carrageenan catabolism is encoded by a complex regulon in marine heterotrophic bacteria, Nat. Commun., 2017, 8, 1685 CrossRef PubMed.
- J. Jung, S. S. Bae, D. Chung and K. Baek, Tamlana carrageenivorans sp. nov., a carrageenan-degrading bacterium isolated from seawater, Int. J. Syst. Evol. Microbiol., 2019, 69, 1355–1360 CrossRef CAS PubMed.
- N. Stummer, R. G. Feichtinger, D. Weghuber, B. Kofler and A. M. Schneider, Role of hydrogen sulfide in inflammatory bowel disease, Antioxidants, 2023, 12, 1570 CrossRef CAS PubMed.
- C. Merten, R. Schoonjans, D. Di Gioia, C. Peláez, Y. Sanz, D. Maurici and T. Robinson, Editorial: Exploring the need to include microbiomes into EFSA's scientific assessments, EFSA J., 2020, 18, e18061 Search PubMed.
- A. Ampatzoglou, A. Gruszecka-Kosowska and M. Aguilera-Gómez, Microbiota analysis for risk assessment of xenobiotics: toxicomicrobiomics, incorporating the gut microbiome in the risk assessment of xenobiotics and identifying beneficial components for One Health, EFSA J., 2022, 20, e200915 CAS.
- T. Requena, Y. Song, C. Peláez and M. C. Martínez-Cuesta, Modulation and metabolism of obesity-associated microbiota in adynamic simulator of the human gut microbiota, LWT–Food Sci. Technol., 2021, 141, 110921 CrossRef CAS.
- A. Bellanco, T. Requena, E. Sáez, C. Peláez and M. C. Martínez-Cuesta, Carrageenan intake affects the gut microbiota composition and the epithelial permeability, EFSA ONE Conference 2022. (Accessed March 19, 2024, at https://EfsaBynderCom/Web/4248ae8eaf381a14/e-Posters-Many-Ways/?MediaId=680383CB-D9BA-485C-A952DF115941C14F2022).
- I. Martínez de Toda, C. Vida, A. Garrido and M. De la Fuente, Redox parameters as markers of the rate of aging and predictors of life span, J. Gerontol., Ser. A, 2020, 75, 613–620 CrossRef PubMed.
- I. Martínez de Toda, I. Maté, C. Vida, J. Cruces and M. De la Fuente, Immune function parameters as markers of biological age and predictors of longevity, Aging, 2016, 8, 3110–3119 CrossRef PubMed.
- J. Félix, I. Martínez de Toda, E. Díaz-Del Cerro, F. Gil-Agudo and M. De la Fuente, The immunity and redox clocks in mice, markers of lifespan, Sci. Rep., 2024, 14, 1703 CrossRef PubMed.
- T. Abe, H. Kawamura, S. Kawabe, H. Watanabe, F. Gejyo and T. Abo, Liver injury due to sequential activation of natural killer cells and natural killer T cells by carrageenan, J. Hepatol., 2002, 36, 614–623 CrossRef CAS PubMed.
- Y. Shu, X. Liu, X. Ma, J. Gao, W. He, X. Cao and J. Chen, Immune response mechanism of mouse monocytes/macrophages treated with κ-carrageenan polysaccharide, Environ. Toxicol. Pharmacol., 2017, 53, 191–198 CrossRef CAS PubMed.
- E. Cicinskas, M. A. Begun, V. V. Vikhareva, Y. A. Karetin and A. A. Kalitnik, Immunological effects of Chondrus armatus carrageenans and their low molecular weight degradation products, J. Biomed. Mater. Res., Part A, 2021, 109, 1136–1146 CrossRef CAS PubMed.
- M. L. Sanz, N. Polemis, V. Morales, N. Corzo, A. Drakoularakou, G. R. Gibson and R. A. Rastall, In vitro investigation into the potential prebiotic activity of honey oligosaccharides, J. Agric. Food Chem., 2005, 53, 2914–2921 CrossRef CAS PubMed.
- E.-H. Doo, C. Chassard, C. Schwab and C. Lacroix, Effect of dietary nucleosides and yeast extracts on composition and metabolic activity of infant gut microbiota in PolyFermS colonic fermentation models, FEMS Microbiol. Ecol., 2017, 93, fix088 CrossRef PubMed.
- I. Bustos, T. García-Cayuela, B. Hernández-Ledesma, C. Peláez, T. Requena and M. C. Martínez-Cuesta, Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells, J. Agric. Food Chem., 2012, 60, 9082–9088 CrossRef CAS PubMed.
- N. Guayerbas, M. Puerto, V. Víctor, J. Miquel and M. De la Fuente, Leukocyte function and life span in a murine model of premature immunosenescence, Exp. Gerontol., 2002, 37, 249–256 CrossRef CAS PubMed.
- C. Alvarado, P. Álvarez, L. Jiménez and M. De la Fuente, Oxidative stress in leukocytes from young prematurely aging mice is reversed by supplementation with biscuits rich in antioxidants, Dev. Comp. Immunol., 2006, 30, 1168–1180 CrossRef CAS PubMed.
- T. Metsalu and J. Vilo, ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap, Nucleic Acids Res., 2015, 43, W566–W570 CrossRef CAS PubMed.
- H.-J. Shin, Y. M. Cho, H. J. Shin, H. D. Kim, K. M. Choi, M. G. Kim, H. D. Shin and M.-W. Chung, Comparison of commonly used ICR stocks and the characterization of Korl:ICR, Lab. Anim. Res., 2017, 33, 8–14 CrossRef PubMed.
- D. Pietrucci, A. Teofani, M. Milanesi, B. Fosso, L. Putignani, F. Messina, G. Pesole, A. Desideri and G. Chillemi, Machine learning data analysis highlights the role of parasutterella and alloprevotella in autism spectrum disorders, Biomedicines, 2022, 10, 2028 CrossRef PubMed.
- A. Esberg, L. Johansson, I. Johansson and S. R. Dahlqvist, oral microbiota identifies patients in early onset rheumatoid arthritis, Microorganisms, 2021, 9, 1657 CrossRef CAS PubMed.
- H. J. Bixler, The carrageenan controversy, J. Appl. Phycol., 2017, 29, 2201–2207 CrossRef CAS.
- M. D. Torres, N. Flórez-Fernández and H. Domínguez, Integral utilization of red seaweed for bioactive production, Mar. Drugs, 2019, 17, 314 CrossRef CAS PubMed.
- J. K. Tobacman, Review of harmful gastrointestinal effects of carrageenan in animal experiments, Environ. Health Perspect., 2001, 109, 983–994 CrossRef CAS PubMed.
- S. Bhattacharyya, T. Shumard, H. Xie, A. Dodda, K. A. Varady, L. Feferman, A. G. Halline, J. L. Goldstein, S. B. Hanauer and J. K. Tobacman, A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity, Nutr. Healthy Aging, 2017, 4, 181–192 CAS.
- M. Tahiri, C. Johnsrud and I.-L. Steffensen, Evidence and hypotheses on adverse effects of the food additives carrageenan (E 407)/processed Eucheuma seaweed (E 407a) and carboxymethylcellulose (E 466) on the intestines: a scoping review, Crit. Rev. Toxicol., 2023, 53, 521–571 CrossRef CAS PubMed.
- S. David, C. Shani Levi, L. Fahoum, Y. Ungar, E. G. Meyron-Holtz, A. Shpigelman and U. Lesmes, Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods?, Food Funct., 2018, 9, 1344–1352 RSC.
- F. Liu, P. Hou, H. Zhang, Q. Tang, C. Xue and R. W. Li, Food–grade carrageenans and their implications in health and disease, Compr. Rev. Food Sci. Food Saf., 2021, 20, 3918–3936 CrossRef CAS PubMed.
- A. L. Russell, Z. L. McAdams, E. Donovan, N. Seilhamer, M. Siegrist, C. L. Franklin and A. C. Ericsson, The contribution of maternal oral, vaginal, and gut microbiota to the developing offspring gut, Sci. Rep., 2023, 13, 13660 CrossRef CAS PubMed.
- P. D. Schloss, A. M. Schubert, J. P. Zackular, K. D. Iverson, V. B. Young and J. F. Petrosino, Stabilization of the murine gut microbiome following weaning, Gut Microbes, 2012, 3, 383–393 Search PubMed.
- I. Capron, M. Yvon and G. Muller, In vitro gastric stability of carrageenan, Food Hydrocolloids, 1996, 10, 239–244 Search PubMed.
- S. Li, J. Hu, H. Yao, F. Geng and S. Nie, Interaction between four galactans with different structural characteristics and gut microbiota, Crit. Rev. Food Sci. Nutr., 2023, 63, 3653–3663 CrossRef CAS PubMed.
- M. Li, Q. Shang, G. Li, X. Wang and G. Yu, Degradation of marine algae-derived carbohydrates by Bacteroidetes isolated from human gut microbiota, Mar. Drugs, 2017, 15, 92 Search PubMed.
- T. Fu, L. Zhou, Z. Fu, B. Zhang, Q. Li, L. Pan, C. Zhou, Q. Zhao, Q. Shang and G. Yu, Enterotype-specific effect of human gut microbiota on the fermentation of marine algae oligosaccharides: A preliminary proof-of-concept in vitro study, Polymers, 2022, 14, 770 CrossRef CAS PubMed.
- W. Wu, F. Wang, X. Gao, T. Niu, X. Zhu, X. Yan and H. Chen, Synergistic effect of κ-carrageenan on oxazolone-induced inflammation in BALB/c mice, BMC Gastroenterol., 2016, 16, 41 Search PubMed.
- I. Okayasu, S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki and R. Nakaya, A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice, Gastroenterology, 1990, 98, 694–702 CrossRef CAS PubMed.
- M. E. V. Johansson, J. K. Gustafsson, K. E. Sjöberg, J. Petersson, L. Holm, H. Sjövall and G. C. Hansson, Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model, PLoS One, 2010, 5, e12238 Search PubMed.
- M. De la Fuente, Oxidation and inflammation in the immune and nervous systems, a link between aging and anxiety in Handbook of Immunosenescence, ed. T. Fulop, C. Franceschi, K. Hirokawa and G. Pawelec, Springer International Publishing, Cham, 2018, pp. 1–31 Search PubMed.
- M. Fuente and J. Miquel, An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging, Curr. Pharm. Des., 2009, 15, 3003–3026 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01418a |
| This journal is © The Royal Society of Chemistry 2024 |