Open Access Article
Open Access ArticleRed raspberry (Rubus idaeus) preserves intestinal barrier integrity and reduces oxidative stress in Caco-2 cells exposed to a proinflammatory stimulus†
Mirko
Marino
 a,
Marco
Rendine
a,
Marco
Rendine
 a,
Samuele
Venturi
a,
Marisa
Porrini
a,
Claudio
Gardana
a,
Dorothy
Klimis-Zacas
b,
Patrizia
Riso
a and
Cristian
Del Bo’
a,
Samuele
Venturi
a,
Marisa
Porrini
a,
Claudio
Gardana
a,
Dorothy
Klimis-Zacas
b,
Patrizia
Riso
a and
Cristian
Del Bo’
 *a
*a
aUniversità degli Studi di Milano, DeFENS – Department of Food, Environmental and Nutritional Sciences, Via Celoria 2, 20133 Milano, Italy. E-mail: cristian.delbo@unimi.it
bSchool of Food and Agriculture, University of Maine, Orono, Maine, USA
First published on 10th June 2024
Abstract
Growing evidence showed the capacity of (poly)phenols to exert a protective role on intestinal health. Nevertheless, the existing findings are still heterogeneous and the underlying mechanisms remain unclear. This study investigated the potential benefits of a red raspberry (Rubus idaeus) powder on the integrity of the intestinal barrier, focusing on its ability to mitigate the effects of tumor necrosis factor-α (TNF-α)-induced intestinal permeability. Human colorectal adenocarcinoma cells (i.e., Caco-2 cells) were used as a model to assess the impact of red raspberry on intestinal permeability, tight junction expression, and oxidative stress. The Caco-2 cells were differentiated into polarized monolayers and treated with interferon-γ (IFN-γ) (10 ng mL−1) for 24 hours, followed by exposure to TNF-α (10 ng mL−1) in the presence or absence of red raspberry extract (1–5 mg mL−1). The integrity of the intestinal monolayer was evaluated using transepithelial electrical resistance (TEER) and fluorescein isothiocyanate–dextran (FITC-D) efflux assay. Markers of intestinal permeability (claudin-1, occludin, and zonula occludens-1 (ZO-1)) and oxidative stress (8-hydroxy-2-deoxyguanosine (8-OHdG) and protein carbonyl) were assessed using ELISA kits. Treatment with red raspberry resulted in a significant counteraction of TEER value loss (41%; p < 0.01) and a notable reduction in the efflux of FITC-D (−2.5 times; p < 0.01). Additionally, red raspberry attenuated the levels of 8-OHdG (−48.8%; p < 0.01), mitigating the detrimental effects induced by TNF-α. Moreover, red raspberry positively influenced the expression of the integral membrane protein claudin-1 (+18%; p < 0.01), an essential component of tight junctions. These findings contribute to the growing understanding of the beneficial effects of red raspberry in the context of the intestinal barrier. The effect of red raspberry against TNF-α-induced intestinal permeability observed in our in vitro model suggests, for the first time, its potential as a dietary strategy to promote gastrointestinal health.
1. Introduction
The gastrointestinal tract serves as a vital functional barrier, safeguarding the inner environment from the transmission of harmful substances. The integrity of the intestinal barrier (IB) relies on the intricate interplay of various components, including microorganisms, chemicals like mucins and antimicrobial peptides, immune cells, and primarily intestinal epithelial cells.1 Achieving selective permeability within the IB involves both transcellular (passage through transmembrane proteins) and paracellular (passage through spaces between adjacent cells) mechanisms. The paracellular route's selectivity is established through junction proteins, comprising gap junctions, adherens junctions, tight junctions, and desmosomes.2 While gap junctions enable electrochemical connections between cells, adherens junctions, tight junctions and desmosomes primarily facilitate intracellular adhesion. Furthermore, gap junctions play a crucial role in regulating the passage of ions and small molecules between adjacent cells.3 Tight junction proteins, such as claudins, occludin, and tricellulin, situated between the apical and basolateral membrane, play a pivotal role in controlling intestinal epithelial cells permeability through the paracellular route.4 Adherens junctions, located immediately below the tight junctions, consist of two transmembrane complexes (nectin–afadin and cadherin–catenin), closely anchored to the actin cytoskeleton. Additionally, gap junctions associate directly with tight junctions through interactions with intracellular scaffold proteins, like zonula occludens.5 Furthermore, desmosomes serve as intercellular junctions, further stabilizing interactions between cells.6 The integrity of intracellular junctions is meticulously regulated by various signaling pathways.7 However, physiological and pathological factors can disrupt intracellular junction organization, leading to IB impairment and increased IP, a condition referred to as leaky gut syndrome.8,9 Proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), have been shown to directly reduce zonula occludens-1 protein levels through a mechanism involving nuclear factor κ-B (NF-κB) signaling.10 The compromised integrity of intercellular junctions allows improper paracellular passage of food components and bacterial components, such as lipopolysaccharide, from the gut lumen to reach immune cells in the lamina propria. The last event triggers a robust immune response characterized by the production of pro-inflammatory cytokines, resulting in impaired IB, increased intestinal permeability (IP), and intestinal and systemic inflammation.11,12Red raspberries (RB) are among the most popular berries globally and have garnered significant interest in the last decade due to their potential health benefits attributed to their content of vitamins, minerals, and bioactive compounds.13 Notably, RB are rich in two primary classes of (poly)phenols (PP), namely anthocyanins responsible for their red color, and ellagitannins.14 The total anthocyanins content of RB, determined among different harvest seasons and disparate cultivars, ranged from 12.4 to 113 mg per 100 g of fresh weight (FW) with the major anthocyanins constituted by cyanidin-3-sophoroside, cyanidin 3-O-glucosyl-rutinoside and cyanidin-3-glucoside.15–17 Regarding ellagitannins, lambertianin C and sanguiin H-6 are the principal compounds detected in RB with a mean of 38 and 55 mg per 100 g FW, respectively.16 However, it is widely known that these compounds have poor bioavailability and are not absorbed per se. On the contrary, they are mainly subjected to the action of the gut microbiota which results in the production of different phenolic metabolites.18 In this context, some of the beneficial effects of these compounds, both parent and metabolites, can be attributed to their permanence in the lumen of the gut suggesting intestinal cells as potential targets for their biological activity. Numerous studies have demonstrated that RB consumption can reduce oxidative stress,19 inflammation,20,21 dyslipidemia and hyperglycemia,22 and positively modulate the gut microbiota,23 attributed to their PP content. Moreover, emerging evidence suggests potential beneficial effects of RB PP on gut health, as an anthocyanin-rich fraction from RB has been shown to reduce intestinal inflammation and reverse morphological alterations in the gut.20,24 Additionally, RB-enriched diets have shown promise in improving IB function in a model of alcohol-induced IB dysfunction through inhibiting the NF-κB/MLCK pathway and upregulating tight junction proteins, such as zonula occludens 1, occludin, claudin-1, and claudin-4.25
However, limited evidence exists regarding RB's ability to modulate IB and its permeability. Hence, the present study aims to investigate the effects of a RB powder in a Caco-2 intestinal model, targeting to unravel the molecular mechanisms regulating IP.
2. Materials and methods
2.1 Chemicals, cells and reagents
Human Caucasian colon adenocarcinoma (Caco-2) cells (Cat. no 09042001-1VL) were from the European Collection of Authenticated Cell Cultures (ECACC) and purchased from Sigma-Aldrich (St Louis, MO, USA). Minimum Essential Medium (MEM; Cat. no. 51411C-1000 mL), penicillin–streptomycin solution (Cat. no. P4333–100 mL), MEM non-essential amino acid solution (100×) (Cat. no. M7145-100 mL), trypsin-EDTA (Cat no. T4049-100 mL), phosphate saline buffer (PBS; Cat no. 806544-500 mL), interferon-γ human (IFN-γ; Cat no. I17001-100UG), trypan blue (Cat no. T8154-100 mL), potassium chloride, sodium chloride, fluorescein-5-isothiocyanate dextran (FITCd; Cat no. 46944-100 mg), and Triton x100 were provided by Sigma-Aldrich. MEM phenol red free (Cat no. 51200-038), sodium pyruvate (Cat no. 11360-070), and fetal bovine serum (FBS; Cat no. A47668-01) were provided by Thermo Fisher Scientific (Waltham, MA, USA). TNF-α (Cat no. rcyc-htnfa) was provided by InvivoGen (San Diego, CA, USA). Millicell® tissue culture plate well inserts (Cat. no. PIHP01250), ethylenediaminetetraacetic acid disodium salt dihydrate (DSEDTA; Cat no. E5134-500 g), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Cat no. M2128-500 mm), dimethyl sulfoxide, SDS (Cat no. 822050) and ethanol were purchased from Merck (Darmstadt, Germany). Tris-(hydroxymethyl)-aminomethane (Tris; Cat no. 33742) was provided by Honeywell International Inc. (Monza, MB, Italy). ProteinSafeTM Protease Inhibitor Cocktail (100×) (Cat no. DI111-02) was provided by CliniSciences (Guidonia Montecelio, RM, Italy). Standards of cyanidin (Cy)-, delphinidin (D)-, petunidin (Pt)-, peonidin (Pe)-, malvidin (Mv)- and their 3-O-glucoside (glc), Cy-, Pet-, Peo-, Mv-3-O-galactoside (gal) and Cy-arabinoside (Cy-ara) were purchased from Polyphenols Laboratory (Sandnes, Norway). Acetonitrile, and phosphoric acid were from VWR (Radnor, PA, USA). Water was from a Milli-Q apparatus (Millipore, Milford, MA). Red Raspberry (RB; Rubus ideaus) freeze-dried powder was provided by FutureCeuticals (Momence, Ill., USA) and stored at −20 °C until use.2.2 Preparation of the red raspberry stock solution
The raspberry freeze-dried powder (100 mg) was dissolved in MEM (1 mL), vortexing for 30 seconds and stored overnight at 4 °C, to produce the extract. The RB solution was centrifugated at 865 rcf (3000 rpm) for 10 minutes and at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249 rcf (13
249 rcf (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 1 minute twice (rotor Cat. No. RA24-2 86 mm). Successively the extract was filtered by sterilized filters (diameter 0.2 μm) to provide a stock solution at 100 mg mL−1. Subsequently, the stock solution was diluted in MEM to test concentrations of 1, 5 and 10 mg mL−1.
000 rpm) for 1 minute twice (rotor Cat. No. RA24-2 86 mm). Successively the extract was filtered by sterilized filters (diameter 0.2 μm) to provide a stock solution at 100 mg mL−1. Subsequently, the stock solution was diluted in MEM to test concentrations of 1, 5 and 10 mg mL−1.
2.3 Chemical characterization of the red raspberry stock solution
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (75
acetic acid (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.5
24.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 v/v/v). The mixture was vortexed for 30 s, sonicated for 10 min, and then centrifuged at 1650g for 5 min at 20 °C. The supernatant was collected, and the residue was subjected to another extraction with 4 mL of the same acetone
0.5 v/v/v). The mixture was vortexed for 30 s, sonicated for 10 min, and then centrifuged at 1650g for 5 min at 20 °C. The supernatant was collected, and the residue was subjected to another extraction with 4 mL of the same acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid solution. This was followed by vortexing, sonication, and centrifugation as described before. The two supernatants were combined and diluted to a final volume of 10 mL with water for subsequent DMAC assays.
acetic acid solution. This was followed by vortexing, sonication, and centrifugation as described before. The two supernatants were combined and diluted to a final volume of 10 mL with water for subsequent DMAC assays.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2% trifluoroacetic acid (TFA) solution (20
2% trifluoroacetic acid (TFA) solution (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) and subjected to sonication for 10 min. The resulting suspension was then centrifuged at 1650g for 10 min, and the supernatant was collected. The residue was subjected to three additional extractions with a methanol
80, v/v) and subjected to sonication for 10 min. The resulting suspension was then centrifuged at 1650g for 10 min, and the supernatant was collected. The residue was subjected to three additional extractions with a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2% TFA solution (20
2% TFA solution (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) until the color disappeared. The combined supernatants were adjusted to a final volume of 25 mL with an aqueous 2% TFA solution and stored at −20 °C. The total anthocyanin (ACNs) content was determined spectrophotometrically using the method described by Lee et al.33 while the chromatographic analysis was performed according to Spinardi et al.34
80, v/v) until the color disappeared. The combined supernatants were adjusted to a final volume of 25 mL with an aqueous 2% TFA solution and stored at −20 °C. The total anthocyanin (ACNs) content was determined spectrophotometrically using the method described by Lee et al.33 while the chromatographic analysis was performed according to Spinardi et al.34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution (20
water solution (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) and sonicated for 10 min. Afterward, the suspension was centrifuged at 1650g for 10 min, and the resulting supernatant was collected. The residue was then extracted with 10 mL of a methanol
80, v/v) and sonicated for 10 min. Afterward, the suspension was centrifuged at 1650g for 10 min, and the resulting supernatant was collected. The residue was then extracted with 10 mL of a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution (20
water solution (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) and subjected to another centrifugation at 1650g for 10 min. The supernatants were mixed, and the final volume adjusted to 25 mL with water. The solution was stored at −20 °C. The ellagitannin chromatographic analysis was conducted using an UHPLC model Vanquish Flex (Thermo) coupled to a Vanquish HL PDA (Thermo) and an HR-MS Orbitrap mod. Exactive (Thermo) equipped with a HESI-II probe for electrospray ionization (ESI) and a collision cell (HCD).
80, v/v) and subjected to another centrifugation at 1650g for 10 min. The supernatants were mixed, and the final volume adjusted to 25 mL with water. The solution was stored at −20 °C. The ellagitannin chromatographic analysis was conducted using an UHPLC model Vanquish Flex (Thermo) coupled to a Vanquish HL PDA (Thermo) and an HR-MS Orbitrap mod. Exactive (Thermo) equipped with a HESI-II probe for electrospray ionization (ESI) and a collision cell (HCD).
2.4 Caco-2 Cell culture
Minimum essential medium (MEM) supplemented with 10% (v/v) fetal bovine serum, 1 mM sodium pyruvate, 1% (v/v) of 100× non-essential amino acids, and antibiotics (50 U per mL penicillin, 50 μg per mL streptomycin) was used to culture Caco-2 cells. The cell culture was maintained at 37 °C and 5% CO2. The medium was changed every 2–3 days during cell growth and differentiation. Cells were subcultured at 80% confluence with trypsin/EDTA (0.05%). Cells were used between passages 3 and 15.2.5 Cell viability assay
The cytotoxicity of the extracts on Caco-2 cell culture was evaluated using the Trypan Blue exclusion assay and the MTT assay. Caco-2 cells were seeded in 12-well plates (4 × 105 cells per well). Cell cultures with 80% confluence grade were treated with RB 1 mg mL−1, RB 5 mg mL−1, RB 10 mg mL−1, and TNF-α (10 ng mL−1) for 24 h. Cell culture medium was used as a negative control (NC), while 0.1% TRITON X as a positive control. Subsequently, cells were detached with trypsin/EDTA (0.05%), resuspended, and analysed with Trypan exclusion assay using a TC20TM automated cell counter and dedicated dual-chamber cell counting slides (BIORAD, Segrate, Milan, Italy). Three independent experiments were performed, in every experiment each condition was tested in triplicate. Moreover, the cytotoxicity was evaluated through an MTT assay.35 Cells were seeded in 96-well plates (5 × 103 cells per well). After 24 h the cells were treated with the RB 1, 5 and 10 mg mL−1, TNF-α (10 ng mL−1), NC and TRITON X. The MTT solution, made in the cell culture complete medium (1 mg mL−1), was filter-sterilized (0.2 μm), and 100 μl was added to each well. Cells with MTT solution were incubated at 37 °C for 4 h avoiding light. Subsequently, the MTT solution was removed, and 200 μl DMSO was added to each well to solubilize formazan crystals. The plate was incubated in the dark on a diametral shaker for 20 min at room temperature. The absorbance from each well was measured at a wavelength of 570 nm using a microplate reader (mod. F200, TECAN, Milan Italy). Three independent experiments were executed in which each condition was tested in six replicates.2.6 Transepithelial electrical resistance (TEER) measurement
The transepithelial electrical resistance (TEER) was performed to evaluate the tight junction integrity of the Caco-2 monolayer.36 Cells were seeded at a density of 2 × 104 cells per well on Transwell® 24-well permeable media (12 mm, 0.4 μm pore polyester membranes), a well without cells was used as a blank. Caco-2 cells spontaneously differentiated into polarized monolayers after 18–21 days on Transwell inserts. The medium in the lower and upper chambers, respectively 0.6 and 0.4 mL, was replaced every 2–3 days. After differentiation, the cells were pre-treated with interferon-γ (IFN-γ; 10 ng mL−1) for 24 h. IFN-γ was added to the medium in the lower compartment, in order to promote the translocation of the tumor necrosis factor-α (TNF-α) receptors outside the cell membrane.36 Subsequently, cells were exposed to TNF-α (10 ng mL−1) introduced into the lower chamber medium, either with or without the presence of extracts (RB 1 and 5 mg mL−1) in the upper chamber. This 24-hour exposure to TNF-α led to an augmentation in IP as a result of the inflammatory stimulation. Cell culture medium was used as a negative control, while the treatment with TNF-α only was the positive control. The experiments were performed with medium without serum and phenol red-free. TEER was measured before and after the treatment with IFN-γ/TNF-α with extracts, and after the replacement of fresh medium. TEER was estimated through a Millicell-ERS Resistance System (Millipore, Bedford, MA, USA), which includes a dual-electrode volt-ohm-meter, by the following equation: TEER = (Rm − Ri) × A, where Rm is the transmembrane resistance, Ri is the intrinsic resistance of cell-free media, and A is the membrane surface area in cm2. Cell monolayers with TEER values ranging between 350 and 450 Ω cm2 were used for the experiments. Three independent experiments were performed in which each condition was tested in triplicate.2.7 Transport of fluorescein isothiocyanate–dextran (FITC-dextran)
The IP was also determined through the FITC-D efflux assay, which allows to evaluate the paracellular transport of a nonpolar fluorescent molecule detectable by spectrophotometer.36 After the evaluation of TEER, the medium in the upper chamber was replaced with fresh medium, and FITC-dextran (1 mg mL−1) was added. Cells were incubated at 37 °C in the dark, and after 4 h, 50 μl of the medium in the lower chamber was collected in a 96-well black plate. The quantification of FITC-D was performed using a fluorescence plate reader (mod. F200 Infinite, TECAN, Milan, Italy), with the excitation and emission wavelength at 488 and 520 nm, respectively. Analyses were conducted in triplicate, and the results were derived from three independent experiments.2.8 Assessment of intestinal permeability and oxidative stress markers by enzyme-linked immunosorbent assay (ELISA)
To evaluate markers of IP and oxidative stress, cells were seeded in a 6-well plate at a density of 3 × 105 cells per well. The cells differentiated into polarized monolayers after 8–10 days. The same treatment phases described above were followed. Cell supernatant was collected to evaluate protein carbonyl (PC) and 8-hydroxy-2-deoxyguanosine (8-OHdG), while cell lysate was collected to measure claudin-1 (CLN-1), zonula occludens-1 (ZO-1) and occludin (OCLN). After RB and TNF-α treatments, cell supernatant was collected, centrifuged at 216 rcf for 10 min at 4 °C, and stored at −80 °C until analysis. Protein extraction was done following the kit's protocol. Briefly, cells were placed on ice, washed with cold PBS, and incubated for 1 min with lysis buffer (100 mM Tris, 150 mM NaCl, 1 mM EDTA Na, 1% SDS, 1% TRITON-X 100, 1% proteases inhibitor). The cells were detached, incubated on ice for 30 min, and vortexed every 10 min. The cell extract was centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249 rcf for 10 min at 4 °C. The supernatant was stored at −80 °C until analysis. ELISA kits were used to quantify the markers levels of CLN-1, ZO-1, OCLN, PC and 8-OHdG. Cat No. were MBS3800570, MBS2605490, MBS451733, MBS2602535, MBS267161, respectively (MyBioSource, San Diego, CA, USA). Three independent experiments were performed in which each condition was tested in duplicate.
249 rcf for 10 min at 4 °C. The supernatant was stored at −80 °C until analysis. ELISA kits were used to quantify the markers levels of CLN-1, ZO-1, OCLN, PC and 8-OHdG. Cat No. were MBS3800570, MBS2605490, MBS451733, MBS2602535, MBS267161, respectively (MyBioSource, San Diego, CA, USA). Three independent experiments were performed in which each condition was tested in duplicate.
2.9 Statistical analysis
Statistical analysis was executed through GraphPad software (GraphPad Software Boston, MA, USA). The effect of the RB extract on Caco-2 cell monolayer integrity, tight junction proteins and oxidative stress markers was assessed through one-way analysis of variance (ANOVA). Post hoc analysis of the differences between treatments was conducted using the least significant difference (LSD) test with a significance level set at p ≤ 0.05. Data derived from three independent experiments in which each condition was tested in triplicate for TEER evaluation and FITC-D efflux assay, and in duplicate for tight junctions and oxidative stress markers. Results are reported as mean ± standard deviation.3. Results
3.1 Nutritional composition of red raspberry stock solution
The RB stock solution was characterized for both the nutritional and non-nutritional components, and Table 1 shows the qualitative and quantitative composition. The characterization of the RB powder has been published in a previous work.26| Chemical composition | Stock solution (mg mL−1) |
|---|---|
| a As gallic acid equivalent. b Cyanidin-glucoside. c As procyanidin A2 equivalent. d Quantified on the punicalagin calibration curve and the amount corrected by the MW ratio (MW analyte/MW punicalagin). | |
| Protein | 3.9 ± 0.5 |
| Sugars | 31.1 ± 0.3 |
| Fructose | 11.4 ± 0.4 |
| Glucose | 9.7 ± 0.3 |
| Sucrose | 11.1 ± 0.2 |
| Total (poly)phenolsa | 3.2 ± 0.2 |
| Anthocyaninsb | 0.51 ± 0.05 |
| Proanthocyaninsc | 0.68 ± 0.03 |
| Ellagitanninsd | 0.59 ± 0.03 |
The sugar content of red raspberries stock solution is nearly evenly distributed among fructose, glucose, and sucrose, with a total sum of approximately 3.5 mg mL−1. As for the phenolic content, anthocyanins, proanthocyanins, and ellagitannins were the main representing approximately 60% of the total phenols. Anthocyanins and ellagitannins have been identified by co-chromatography, online UV-Vis spectra, accurate mass and fragment ions obtained by collision-induced dissociation.26 Cyanidin-3-O-sophoroside and cyanidin-3-O-glucoside were the main ACNs constituting approximately 77 and 18% of total ACNs, respectively. Regarding ellagitannins, the main was sanguiin H-6, sanguiin H-10 and its isomers and lambertianin C, respectively, and whose total amount in the stock solution was on average 60 μg mL−1.
3.2 Effect of red raspberry on cell viability
Fig. 1(A and B) reports the effect of the RB extract on cell toxicity as evaluated by the Trypan Blue exclusion assay (1A) and the MTT assay (1B). The results obtained from both assays agreed. It is worth mentioning that the positive control (TX-100) showed a decrease of less than 10% in cell viability values. On the other hand, when TNF-α (10 ng mL−1), RB1, and RB5 were used as the treatment, cell viability values remained above 90%, except for RB10 that significantly reduced the vitality (p < 0.05) compared to the negative control. ESI Fig. 1(A and B)† displays the effects of RB1, RB5, and RB10 on cell viability when tested alone.3.3 Effect of red raspberry on TNFα-induced permeabilization
Fig. 2(A and B) displays the results about the impact of RB extracts on IP, which was assessed using TEER measurements (Fig. 2A) and FITC assay (Fig. 2B). The inflammatory response triggered by TNF-α (10 ng mL−1) led to a significant decrease in TEER (−42.5%; p < 0.0001) compared to the negative control. However, the administration of RB1 and RB5 effectively prevented the reduction in TEER caused by the inflammatory stimulus (p < 0.001), maintaining TEER values similar to the negative control (Fig. 2A). The transport of FITC across the Caco-2 cell monolayer was significantly increased by TNF-α, reaching levels 2.65 times higher than in the absence of the inflammatory stimulus (Fig. 2B) (p < 0.0001). Treatment with RB1 and RB5 successfully inhibited the increase in paracellular transport (p < 0.0001) in the presence of TNF-α, resulting in values comparable to the negative control. ESI Fig. 2(A and B)† illustrates the impact of RB1 and RB10 on intestinal permeabilization when used without TNF-α.3.4 Effect of red raspberry on tight junction proteins
Fig. 3(A–C) illustrates the levels of claudin-1 (A), occludin (B), and ZO-1 (C) following different treatments. As shown in Fig. 3A, the inflammatory stimulus resulted in an 18.9% decrease in claudin-1 production compared to the negative control (p < 0.01). The incubation with both RB1 and RB5 mitigated the impairment caused by TNF-α, as it maintained claudin-1 levels comparable to the control (p > 0.05). In terms of occludin (Fig. 3B) and ZO-1 (Fig. 3C), both TNF-α and RB had no significant impact on the protein levels (p > 0.05). ESI Fig. 3(A–C)† presents the effects of RB1 and RB10 on tight junction proteins in the absence of an induced inflammatory condition.3.5 Effect of red raspberry on oxidative stress markers
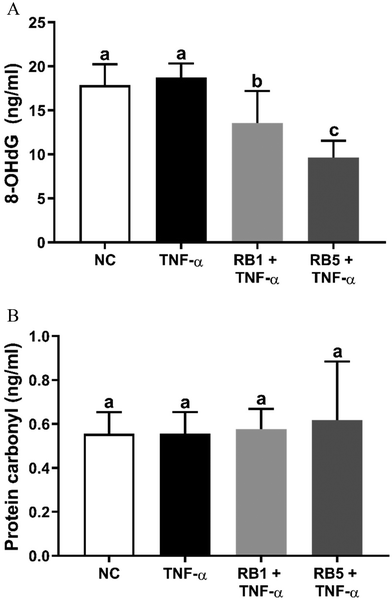
The results regarding the levels of 8-OHdG and protein carbonyl, as markers of oxidative stress, are presented in Fig. 4A and B, respectively. Despite the use of a pro-inflammatory stimulus to induce oxidative stress, no significant changes were observed in the levels of both biomarkers following TNF-α administration. However, treatment with RB1 and RB5 resulted in a significant reduction in 8-OHdG levels (−22.5 and −48.8%, respectively; p < 0.05, Fig. 4A) compared to the control. Notably, RB5 showed a more pronounced effect compared to RB1. Regarding protein carbonyl (Fig. 4B), neither TNF-α stimulation nor RB administration had an impact on their amount (p > 0.05).The impact of RB1 and RB10 on oxidative stress markers under non-inflammatory conditions is depicted in ESI Fig. 4(A and B).†
4. Discussion
The present study investigated, for the first time, the potential of red raspberry in preventing TNF-α-induced cell barrier permeability in the Caco-2 cell line. Previous research has demonstrated that TNF-α negatively affects tight junction integrity, leading to increased paracellular passage, as indicated by TEER and FITC assays.36 We documented that TNF-α induced impairments in the intestinal barrier by reducing TEER and increasing FITC passage. However, treatments with RB counteracted the inflammation by preserving the integrity of the Caco-2 monolayer. This beneficial effect was evident at both solutions tested (1 and 5 mg mL−1 extract).Our findings align with those of other studies examining PP from different berries, such as blueberries, which have demonstrated beneficial effects on IB integrity using the same Caco-2 cellular model and assay.36–40 We have recently reported the capacity of a wild blueberry powder to preserve IB integrity and permeability as documented by an increase in TEER and a decrease in FITC particularly correlated to a reduction of the levels of 8-OHdG and the preservation of the integral membrane protein claudin-1.39 Although the different powders showed similar results, the RB powder demonstrated the capacity to counteract the intestinal barrier disruption also at the lower concentration. This could be explained by the different bioactive profile; specifically, the RB powder is characterized by a higher total PP content and a higher amount of ellagitannins compared to the blueberry powder tested in our previous work. Cremonini et al.36 studied the capacity of several anthocyanin-rich extracts (between 103 and 963 μmol g−1) and single compounds (i.e., O-glucosides of cyanidin, and delphinidin) to prevent a reduction of TEER subsequently to an inflammatory stimulus in a similar Caco-2 model. The authors observed no correlation between TEER values and the total anthocyanins. On the contrary, a positive correlation was evidenced for cyanidins and delphinidins suggesting that the beneficial effect of extracts depends mainly on the chemical structure of individual compounds. Although these results are similar to those obtained in our study, we observed substantial differences in the methods used. Indeed, Cremonini et al.36 treated intestinal monolayers with extracts or single compounds for 30 min before the addition of TNF-α (5 ng mL−1 for 6 h), while in our study we tested RB solution for 24 h in conjunction with the inflammatory challenge (TNF-α,10 ng mL−1). Other studies have evaluated the efficacy of PP and/or PP-rich extracts on IP.36–38 Most of the evidence suggests a role of PP in improving the IB as evidenced by TEER and FITC. Particularly, the best positive modulation seems to be mediated by (-)-epicatechin,41 kaempferol,36 and naringenin.42 From a molecular perspective, tight junctions play a pivotal role in modulating IP. In our study, TNF-α stimulation resulted in reduced claudin-1 levels. However, treatment with RB 1 and 5 mg mL−1 mitigated these deleterious effects, maintaining claudin-1 protein levels comparable to the untreated control. In contrast, neither TNF-α nor RB had a significant effect on protein levels of occludin and ZO-1. These results partially differ from other studies using similar phenolic compounds. Hering et al.43 investigated the effect of ellagitannins (ellagic acid 150 μM) on tight junction protein levels in a Caco-2 monolayer without exposing cells to any inflammatory challenge. The authors reported no effects on claudin-1. However, in our study we observed the effect of RB on claudin-1 in mitigating TNF-α-induced IB impairments, possibly suggesting that claudin-1 modulation occurs when IB integrity is compromised. Alternatively, the modulation of claudin-1 could depend on other compounds present in the ellagitannin fraction of RB, such as sanguiin H-6, sanguiin H-10 and lambertianin C which are the most abundant. In fact, in accordance to our study, although using an in vivo model, Wu et al.44 observed an increase in the expression of claudin-1 following the intervention with fermented raspberry juice. In particular, the raspberry juice was rich in ellagic acid and was supplemented for 30 days in healthy mice. In another study, Remenyik et al.45 tested the effects of an anthocyanins-rich sour cherry extract (85 μM) on tight junctions in Caco-2 cells exposed to an inflammatory challenge (TNF-α 50 ng mL−1 for 24 h). Authors reported that treatment with the extract resulted in up-regulation of ZO-1 protein levels and down-regulation of occludin protein levels. In line with our observation, the authors observed that occludin was not affected by TNF-α. On the other hand, Taladrid et al.46 showed the inability of a PP-rich extract derived from the intestinal digestion of grape pomace to regulate ZO-1 and occludin mRNA levels. However, in the same study, the extract effectively determined a reduction of FITC paracellular transport through the Caco-2 cells monolayer. These data indicate how the regulation of IP is complex and involves intricate molecular networks and distinct proteins that differently modulate the IB. Furthermore, in a recent study, the effects of different PP-rich extracts on intestinal integrity and oxidative stress in Caco-2 cells were investigated.38 The results showed that some extracts (tested at 10 and 100 ppm) were able to increase gene expression of ZO-1 (i.e., ginger essential oil, tea tree oil, grape seed extract, green tea extract, thyme essential oil) and occludin (i.e., grape seed extract, green tea extract, thyme essential oil), while other extracts were ineffective (i.e., olive extract, chestnut extract, and capsicum oleoresin) or either decreased mRNA levels of occludin (i.e., pomegranate extract). Additionally, in the same study, the positive modulation of tight junction gene expression was complemented by an improvement in TEER values. Different sources of variability can explain diverse findings between studies. For example, different foods from which bioactive compounds are derived (such as blueberry, cherry extract, grape pomace, and botanicals). Another explanation of conflicting results can be provided by disparate mechanisms of action through which bioactive compounds exert their role. In accordance with data from literature, the characterization of our RB powder26 evidenced the abundance of some ellagitannins such as sanguiin H-6, sanguiin H-10 and lambertianin C as well as some anthocyanins, such as cyanidin-3-O-sophoroside and cyanidin-3-O-glucoside.16 In the current study, the modulation of claudin-1 could be attributed to different compounds, which most likely may act synergistically rather than as single compounds. In this context, Valdez et al.47 reported the effectiveness of an Aronia berry extract (5 mg mL−1) to prevent IB dysfunctions in a Caco-2 cell model, while no effect has been evidenced by individual PP and their metabolites. In the same study, but in contrast with our findings, authors reported an increase in claudin-1 gene expression following the treatment with an inflammatory cocktail. However, it's important to note that authors investigated claudin-1 mRNA levels which do not necessarily correspond to protein levels.
Our findings are in line with evidence from animal studies. In a study conducted in mice, Cremonini et al.48 demonstrated the ability of ACN supplementation (40 mg per kg BW), provided for 14 days, to prevent claudin-1 reduction induced by a high-fat diet, which it had previously shown to aberrate tight junctions proteins in the ileum resulting in IB dysfunctions. The dose of ACNs used by the authors corresponded to a human equivalent intake of approximately 227 mg of ACNs per day. This calculation results in a human equivalent dose for ACN of 3.24 mg kg−1, which equates to a 227 mg dose of ACN for a 70 kg person.49 Additionally, using Caco-2 cells monolayer, the authors documented the ACN efficacy (0.1–5 μM) to counteract oxidative damage induced by a 6 h inflammatory stimulus (TNF-α 5 ng mL−1), which also matched with an improvement in FITC and TEER rates. In addition, two animal studies investigated specifically the effect of RB in mitigating IB impairments. Zogona et al.25 investigated the efficacy of a standard diet supplemented with low (2% w/w), medium (4% w/w), or high doses (8% w/w) of RB in reversing EtOH-induced IB dysfunction in C57BL/6J mice model. Authors evidenced that RB supplement, in a dose-dependent manner, effectively ameliorated IB of mice exposed to EtOH by increasing mRNA and protein levels of tight junctions (ZO-1, occludin, claudin-1, claudin-4, and E-cadherin) and these results were strictly related to the inhibition of NF-κB/MLCK pathway. Moreover, RB supplemented-diet was also able to improve the EtOH-mediated oxidative stress in the liver decreasing malondialdehyde and inflammatory markers (TNF-α, IL-1β, and IL-6) and increasing glutathione, glutathione peroxidase, and catalase levels. Another study evaluated the effects of a standard diet enriched with RB (5% DW) in a mouse model of dextran sulfate sodium-induced acute colitis for six weeks.20,21 Results showed that RB was able to improve the IB integrity in the colon by modulating tight junctions’ assembly (enhanced claudin-3 and ZO-1 protein levels), resulting in a reduction of colitis symptoms, inflammation, and mucosal damage. The modulation of tight junctions, in the context of IB dysfunction, exerted by PP and/or PP-rich sources have been evaluated in different studies.20,45,48 The results observed, as the ones obtained in this study, are very heterogeneous due to many confounding variables, such as methodology of the study, variety of foods, botanicals, extracts, and/or single compounds, the amounts, time of treatments, and the model to induce IB impairments. A more complete understanding of the mechanisms of action related to these bioactive compounds needs further studies, which consider a broader picture of markers and molecular pathways involved in the maintenance of IB integrity.
Inflammation as well as oxidative stress can play a pivotal role in the onset of IP. Inflammation determines an increase in the production of reactive oxygen species (ROS) leading to structural damage to proteins, lipids, and DNA oxidation. Protein carbonyls (PC) are one of the main products of protein oxidation, whose formation is normally derived from oxidative deamination of alkaline amino acids (e.g., arginine, proline, and lysine). Since protein carbonylation is an irreversible process, the main biological consequence is the loss of protein architecture. This can result in many different alterations of cellular physiology (e.g., communication, signaling transduction, and functions) at the level of any cell type including intestinal epithelial cells with a deleterious consequence on IB integrity and permeability.50 From this perspective, several studies evaluated the capacity of berries or PP to reduce oxidative stress. For example, Ershad et al.51 used differentiating Caco-2 cells in a model of induced oxidative stress, through a 24 h exposure of cells with mixed micelles of fatty acids and bile acids, to evaluate the potential protective effects of an ACN-rich bilberry extract. Results indicated that bilberry extract (1.25–20 μM) significantly mitigated ROS generation and mitochondrial superoxide generation. Furthermore, these findings were consistent with a recovery of TEER value evidenced in the Caco-2 monolayer. However, in the context of oxidative stress, there is poor evidence regarding the role of berry-PP on the production of PC and the available results failed to demonstrate a beneficial effect. Our data seem in line with these observations. Indeed, neither the inflammatory stimulus (TNF-α) nor the administration of RB was capable of influencing PC amount. These results may be attributed to the inability of TNF-α to influence PC level in the Caco-2 cell line. Consistent with this, a prior study demonstrated the inefficacy of a well-known toxic stimulus, cobalt ferrite, to induce an increase in PC in the same cellular model.52 Conversely, in the same study, treatment with cobalt ferrite nanoparticles was shown to increase DNA damage as well as markers of oxidative insult, including malondialdehyde and 8-OHdG levels.52 A more appropriate oxidative challenge to observe a significant change in PC level could be represented by hydrogen peroxide. For instance, Kim et al.53 successfully induced a 4.5-fold increase in PC level after stimulating Caco-2 cells with hydrogen peroxide.
In addition to protein oxidation, another important ROS-mediated damage is the oxidation of DNA. In this regard, recent studies have shown that increased levels of DNA oxidation at the level of the intestinal epithelium can result in tight junction alterations and consequent impaired IB functionality.54 In the current study, we also assessed the effects of RB on 8-OHdG levels, which is a recognized biomarker of oxidative DNA damage and strictly related to IB impairments. Michielan et al.12 reported that high levels of 8-OHdG were found in patients with evident alteration of IB and permeability, such as those with inflammatory bowel diseases (IBDs). Dissimilarly from what was noted for PC, RB administration was effective in reducing the levels of 8-OHdG. A reduction in oxidative damage at the level of the IB could protect cells against apoptosis and preserve functional structures (e.g., villi and crypt), as reported by Cai et al.55 So, the reduction of 8-OHdG found may explain, at least in part, the improvement of IB integrity and permeability observed. These findings align with those of a human study in which the 4-week consumption of Vaccinium meridionale resulted in a significant reduction of the oxidative marker 8-OHdG in subjects with metabolic syndrome. The presence of a high amount of anthocyanins in this berry could be associated with its antioxidant activity.56
The abundant presence of phenolic compounds in raspberries confers upon them remarkable antioxidant capabilities, primarily attributed to their radical-scavenging activity. Among these constituents, anthocyanins seem to exert a superior antioxidative efficacy in contrast to ellagitannins.57 Recent ex vivo fermentation studies conducted with raspberries have shed light on the profound impact of their phenolic catabolites on the Nrf2-ARE pathway, a pivotal regulator in oxidative stress responses. This modulation elicited a significant downregulation in the generation of ROS and reactive nitrogen species (RNS), thereby mitigating oxidative stress. Notably, anthocyanins exhibited a noteworthy ability to attenuate inflammation by suppressing the expression levels of inflammatory mediators, including inducible nitric oxide synthase, IL-1β, IL-6, and cyclooxygenase-2. The anti-inflammatory properties of these phenolic compounds stem from their dual action, interfacing with oxidative stress signaling pathways and inhibiting proinflammatory cascades. This intricate interplay likely underpins the observed reductions in oxidative stress and inflammation associated with raspberry consumption, underscoring their potential as therapeutic agents targeting these pathways.57 Moreover, studies, such as that conducted by Sławińska et al.58 underscore the protective role of cyanidin-3-O-rutinoside, a principal anthocyanin in raspberries, against oxidative DNA damage by mitigating free radical activity. Regarding the potential implication of anthocyanin in our results, Chen et al.59 showed that a raspberry extract rich in cyanidin 3-O-glucoside was able to counteract H2O2-induced oxidative stress in HepG2 cells, mediated through the activation of the Keap1/Nrf2 signaling axis.
However, the results observed in our study might be mediated by an alternative category of phenolic compounds, specifically ellagitannins and their derivatives. Notably, raspberries are abundant in ellagic acid and sanguiin H-6, both known for their significant antioxidant activity. This suggests that fractions rich in ellagitannins containing sanguiin H-6, derived from R. idaeus fruits, may exhibit more robust antioxidative capabilities compared to fractions containing anthocyanins.60 The study conducted by Iglesias and colleagues61 delved into the underlying mechanisms through which ellagic acid intervenes in the inhibition of TNFα-induced inflammation, oxidative stress, and the compromise of barrier integrity. Employing Caco-2 cells differentiated into an intestinal epithelial cell monolayer, the study subjected them to TNF-α (10 ng mL−1) in the presence of varying concentrations of ellagic acid. The research highlighted ellagic acid's predominant mode of action, primarily mediated via the inhibition of NF-κB and ERK1/2 pathways. This intervention effectively disrupted the cycle involving inflammation, oxidative stress, and the activation of redox-sensitive pathways such as NF-κB and ERK1/2, consequently preventing intestinal permeabilization.61 The protective effect of raspberry on oxidative DNA damage was demonstrated also by Aiyer et al.62 Interestingly, the authors showed that the treatment with ellagic acid was the most effective in reducing the levels of 8-OHdG induced by H2O2/CuCl2, potentially through mechanisms involving DNA repair.62 Thus, the ellagic acid abundant in raspberry extract may represent a potential phenolic compound involved in the beneficial effect observed in our study. Isolating and identifying the individual compounds within the powder accountable for enhancing intestinal barrier integrity will provide a more precise and interpretable assessment compared to the utilization of the entire powder mixture.
Limitations of the present study are as follows. It is worth noting that while existing research supports the notion that the caco-2 cell line exhibits the capacity to metabolize phenolic compounds,63–65 we did not characterize the cell culture medium post-incubation/treatment for the content of phenolic compounds and potential metabolites. This may represent a limit in our understanding of the metabolic activity of Caco-2 cells, potentially precluding an in-depth examination of the formation of metabolites that could elicit synergistic effects with their parent compounds. However, it is relevant to highlight that studies have shown a significant presence of these compounds in ileal fluid following raspberry consumption in healthy individuals.27,66 This underscores the importance of considering the role of these compounds beyond absorption in the small intestine, as they transit to the lower intestine where they could exert part of their beneficial effect. Another limitation of our study is the incomplete exploration of inflammatory biomarkers and related pathways, such as NF-κB or Nrf2. These pathways are important for understanding the modulation of tight junction protein and intestinal permeability. Future research should aim to comprehensively analyse these pathways to gain a more detailed understanding of the impact of these compounds on inflammatory processes and intestinal barrier function. Moreover, while our study provides valuable insights, limitations related to the cell model should be noted. The use of Caco-2 cells, while common, may not fully replicate the complexity of the human intestinal epithelium. Additionally, the two-dimensional culture system lacks certain physiological aspects present in vivo, such as mucus-secreting goblet cells and immune cell interactions. Variability in cell differentiation status and the simplified inflammatory model using TNF-α, further underscore the need for caution in interpreting results. These limitations highlight the necessity for future studies to explore more representative models to further elucidate the effects of dietary compounds on intestinal health.
5. Conclusions
This study sheds light on the promising role of red raspberry as a natural and accessible dietary intervention to support intestinal health. The results reveal the capacity of RB to protect the integrity of the intestinal barrier and mitigate the negative effects induced by inflammation in Caco-2 cells. By deepening our understanding of the beneficial impacts of red raspberry consumption, this research opens new avenues for further studies exploring mechanisms of action in greater detail and the identification of effective compounds could enhance future potential applications in managing intestinal disorders and inflammatory conditions.Abbreviations
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide |
| 8-OHdG | 8-Hydroxy 2-deoxyguanosine |
| ACNs | Anthocyanins |
| CLN-1 | Claudin-1 |
| FITC-D | Fluorescein isothiocyanate-dextran |
| GAE | Gallic acid equivalents |
| IFN-γ | Interferon γ |
| IB | Intestinal barrier |
| IP | Intestinal permeability |
| NF-κB | Nuclear factor κ-B |
| OCLN | Occludin |
| PP | (Poly)phenols |
| RB | Raspberry |
| RP | Raspberry powder |
| TFA | Trifluoroacetic acid |
| TEER | Transepithelial electrical resistance |
| TNF-α | Tumor necrosis factor α |
| ZO-1 | Zonula occludens-1 |
Authorship contribution statement
Conceptualization, C.D.B.; methodology, C.D.B. and M.M.; software, M.M.; formal analysis, M.M. and C.G.; investigation, M.M., S.V. and M.R.; resources, C.D.B.; data curation, M.M. and M.R.; writing—original draft preparation, M.M. and M.R.; writing—review and editing, C.D.B., P.R., D.K.Z. and M.P.; supervision, C.D.B., P.R., and M.P.; funding acquisition, C.D.B. All authors have read and agreed to the published version of the manuscript.The study was conducted in the Cell Culture laboratories of the Department of Food, Environmental and Nutritional Sciences (DeFENS) (University of Milan, Italy).
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by a contribution of the “Piano di Sostegno Alla Ricerca-Linea 2”, azione A-grant numbers PSR2021_CDELB and PSR2022_MMARI.References
- M. Gieryńska, L. Szulc-Dąbrowska, J. Struzik, M. B. Mielcarska and K. P. Gregorczyk-Zboroch, Animals, 2022, 12, 145 CrossRef PubMed.
- M. Rübsam, J. A. Broussard, S. A. Wickström, O. Nekrasova, K. J. Green and C. M. Niessen, Cold Spring Harbor Perspect. Biol., 2018, 10, a029207 CrossRef PubMed.
- G. Meşe, G. Richard and T. W. White, J. Invest. Dermatol., 2007, 127, 2516–2524 CrossRef PubMed.
- D. Günzel and M. Fromm, Compr. Physiol., 2012, 2, 1819–1852 Search PubMed.
- M. Itoh, A. Nagafuchi, S. Moroi and S. Tsukita, J. Cell Biol., 1997, 138, 181–192 CrossRef CAS PubMed.
- D. Garrod and M. Chidgey, Biochim. Biophys. Acta, 2008, 1778, 572–587 CrossRef CAS PubMed.
- D. Ye, I. Ma and T. Y. Ma, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, 290, G496–G504 CrossRef CAS PubMed.
- M. Vancamelbeke and S. Vermeire, Expert Rev. Gastroenterol. Hepatol., 2017, 11, 821–834 CrossRef CAS PubMed.
- R. S. Aleman, M. Moncada and K. J. Aryana, Molecules, 2023, 28, 619 CrossRef CAS PubMed.
- T. Y. Ma, M. A. Boivin, D. Ye, A. Pedram and H. M. Said, Am. J. Physiol.: Gastrointest. Liver Physiol., 2005, 288, G422–G430 CrossRef CAS PubMed.
- J. M. Bates, J. Akerlund, E. Mittge and K. Guillemin, Cell Host Microbe, 2007, 2, 371–382 CrossRef CAS PubMed.
- A. Michielan and R. D’Incà, Mediators Inflammation, 2015, 2015, 628157 Search PubMed.
- M. Tosun, S. Ercisli, H. Karlidag and M. Sengul, J. Food Sci., 2009, 74, C575–C579 CrossRef CAS PubMed.
- J.-Y. Chen, J. Du, M.-L. Li and C.-M. Li, LWT–Food Sci. Technol., 2020, 128, 109448 CrossRef CAS.
- E. Sariburun, S. Sahin, C. Demir, C. Türkben and V. Uylaşer, J. Food Sci., 2010, 75, C328–C335 CrossRef CAS PubMed.
- S. P. Mazur, A. Nes, A.-B. Wold, S. F. Remberg and K. Aaby, Food Chem., 2014, 160, 233–240 CrossRef CAS PubMed.
- W. Mullen, J. McGinn, M. E. J. Lean, M. R. MacLean, P. Gardner, G. G. Duthie, T. Yokota and A. Crozier, J. Agric. Food Chem., 2002, 50, 5191–5196 CrossRef CAS PubMed.
- I. A. Ludwig, P. Mena, L. Calani, G. Borges, G. Pereira-Caro, L. Bresciani, D. Del Rio, M. E. J. Lean and A. Crozier, Free Radicals Biol. Med., 2015, 89, 758–769 CrossRef CAS PubMed.
- L. Zhang, J. Li, S. Hogan, H. Chung, G. E. Welbaum and K. Zhou, Food Chem., 2010, 119, 592–599 CrossRef CAS.
- S. Bibi, M. Du and M.-J. Zhu, J. Nutr., 2018, 148, 667–674 CrossRef PubMed.
- S. Bibi, Y. Kang, M. Du and M.-J. Zhu, J. Nutr. Biochem., 2018, 51, 40–46 CrossRef CAS PubMed.
- N. Piña-Contreras, A. G. Martínez-Moreno, J. D. P. Ramírez-Anaya, A. C. Espinoza-Gallardo and E. H. M. Valdés, J. Med. Food, 2022, 25, 121–129 CrossRef PubMed.
- D. Plamada and D. C. Vodnar, Nutrients, 2021, 14, 137 CrossRef PubMed.
- L. Li, L. Wang, Z. Wu, L. Yao, Y. Wu, L. Huang, K. Liu, X. Zhou and D. Gou, Sci. Rep., 2014, 4, 6234 CrossRef CAS PubMed.
- D. Zogona, A. W.-S. Zongo, A. E. Elkhedir, M. Salah, M. Tao, R. Li, T. Wu and X. Xu, Food Funct., 2023, 14, 1209–1226 RSC.
- M. Marino, C. Gardana, M. Rendine, D. Klimis-Zacas, P. Riso, M. Porrini and C. Del Bo’, Foods, 2024, 13, 1051 CrossRef CAS PubMed.
- R. González-Barrio, G. Borges, W. Mullen and A. Crozier, J. Agric. Food Chem., 2010, 58, 3933–3939 CrossRef PubMed.
- S. Kamiloglu, E. Capanoglu, C. Grootaert and J. Van Camp, Int. J. Mol. Sci., 2015, 16, 21555–21574 CrossRef CAS PubMed.
- C. Gardana, C. Del Bo’, M. C. Quicazán, A. R. Corrrea and P. Simonetti, J. Food Compost. Anal., 2018, 73, 29–38 CrossRef CAS.
- AOAC , Official method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24.
- V. L. Singleton and J. A. Rossi Jr., Am. J. Enol. Vitic., 1965, 16, 144–158 CrossRef CAS.
- C. Gardana and P. Simonetti, Molecules, 2019, 24, 1504 CrossRef CAS PubMed.
- J. Lee, R. W. Durst and R. E. Wrolstad, J. AOAC Int., 2005, 88, 1269–1278 CrossRef CAS PubMed.
- A. Spinardi, G. Cola, C. S. Gardana and I. Mignani, Front. Plant Sci., 2019, 10, 1045 CrossRef PubMed.
- N. F. Abo El-Magd, P. O. Barbosa, J. Nick, V. Covalero, G. Grignetti and G. Bermano, Nutrition, 2022, 93, 111424 CrossRef CAS PubMed.
- E. Cremonini, A. Mastaloudis, S. N. Hester, S. V. Verstraeten, M. Anderson, S. M. Wood, A. L. Waterhouse, C. G. Fraga and P. I. Oteiza, Food Funct., 2017, 8, 2915–2923 RSC.
- T. Suzuki, S. Tanabe and H. Hara, J. Nutr., 2011, 141, 87–94 CrossRef CAS PubMed.
- S. Noda, S. Tanabe and T. Suzuki, J. Agric. Food Chem., 2012, 60, 4628–4633 CrossRef CAS PubMed.
- A. Toschi, A. Piva and E. Grilli, Animals, 2022, 12, 2188 CrossRef PubMed.
- M. Marino, S. Venturi, M. Rendine, M. Porrini, C. Gardana, D. Klimis-Zacas, C. Del Bo’ and P. Riso, Food Funct., 2023, 14, 7387–7399 RSC.
- E. Cremonini, Z. Wang, A. Bettaieb, A. M. Adamo, E. Daveri, D. A. Mills, K. M. Kalanetra, F. G. Haj, S. Karakas and P. I. Oteiza, Redox Biol., 2018, 14, 588–599 CrossRef CAS PubMed.
- S. Noda, S. Tanabe and T. Suzuki, Mol. Nutr. Food Res., 2013, 57, 2019–2028 CrossRef CAS PubMed.
- N. A. Hering, J. Luettig, B. Jebautzke, J. D. Schulzke and R. Rosenthal, Front. Pharmacol., 2021, 12, 610164 CrossRef CAS PubMed.
- T. Wu, X. Chu, Y. Cheng, S. Tang, D. Zogona, S. Pan and X. Xu, Foods, 2021, 10, 3055 CrossRef CAS PubMed.
- J. Remenyik, A. Biró, Á. Klusóczki, K. Z. Juhász, T. Szendi-Szatmári, Á. Kenesei, E. Szőllősi, G. Vasvári, L. Stündl, F. Fenyvesi, J. Váradi and A. Markovics, Int. J. Mol. Sci., 2022, 23, 9036 CrossRef CAS PubMed.
- D. Taladrid, D. González de Llano, I. Zorraquín-Peña, A. Tamargo, M. Silva, N. Molinero, M. V. Moreno-Arribas and B. Bartolomé, Nutrients, 2021, 13, 2467 CrossRef CAS PubMed.
- J. C. Valdez, J. Cho and B. W. Bolling, Arch. Biochem. Biophys., 2020, 688, 108409 CrossRef CAS PubMed.
- E. Cremonini, E. Daveri, A. Mastaloudis, A. M. Adamo, D. Mills, K. Kalanetra, S. N. Hester, S. M. Wood, C. G. Fraga and P. I. Oteiza, Redox Biol., 2019, 26, 101269 CrossRef CAS PubMed.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, FASEB J., 2008, 22, 659–661 CrossRef CAS PubMed.
- Y. Wang, Y. Chen, X. Zhang, Y. Lu and H. Chen, J. Funct. Foods, 2020, 75, 104248 CrossRef CAS.
- M. Ershad, M. K. Shigenaga and B. Bandy, Food Funct., 2021, 12, 2950–2961 RSC.
- M. Abudayyak, T. Altincekic Gurkaynak and G. Özhan, Biol. Trace Elem. Res., 2017, 175, 458–465 CrossRef CAS PubMed.
- L. Kim, Y. Kim, O. Kwon and J. Y. Kim, Food Sci. Biotechnol., 2017, 26, 1085–1091 CrossRef CAS PubMed.
- A. Watari, M. Hasegawa, K. Yagi and M. Kondoh, PLoS One, 2016, 11, e0145631 CrossRef PubMed.
- X. Cai, F. Yang, L. Zhu, Y. Xia, Q. Wu, H. Xue and Y. Lu, Molecules, 2019, 24, 3027 CrossRef CAS PubMed.
- J. Espinosa-Moncada, C. Marín-Echeverri, Y. Galvis-Pérez, G. Ciro-Gómez, J. C. Aristizábal, C. N. Blesso, M. L. Fernandez and J. Barona-Acevedo, Nutrients, 2018, 10, 1639 CrossRef PubMed.
- A. V. Lopez-Corona, I. Valencia-Espinosa, F. A. González-Sánchez, A. L. Sánchez-López, L. E. Garcia-Amezquita and R. Garcia-Varela, Antioxidants, 2022, 11, 1192 CrossRef CAS PubMed.
- N. Sławińska, K. Prochoń and B. Olas, Nutrients, 2023, 15, 1422 CrossRef PubMed.
- L. Chen, K. Li, Q. Liu, J. L. Quiles, R. Filosa, M. A. Kamal, F. Wang, G. Kai, X. Zou, H. Teng and J. Xiao, Food Chem. Toxicol., 2019, 133, 110781 CrossRef CAS PubMed.
- M. Krauze-Baranowska, D. Głód, M. Kula, M. Majdan, R. Hałasa, A. Matkowski, W. Kozłowska and A. Kawiak, BMC Complementary Altern. Med., 2014, 14 DOI:10.1186/1472-6882-14-480.
- D. E. Iglesias, E. Cremonini, C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2020, 152, 776–786 CrossRef CAS PubMed.
- H. S. Aiyer, M. V. Vadhanam, R. Stoyanova, G. D. Caprio, M. L. Clapper and R. C. Gupta, Int. J. Mol. Sci., 2008, 9, 327–341 CrossRef PubMed.
- S. S. Ekbatan, M. M. Iskandar, L. Sleno, K. Sabally, J. Khairallah, S. Prakash and S. Kubow, Foods, 2018, 7, 8 CrossRef PubMed.
- X. Zhang, J. Song, X. Shi, S. Miao, Y. Li and A. Wen, Sci. World J., 2013, 2013, 382350 Search PubMed.
- Y. Yao, F. Xu, X. Ju, Z. Li and L. Wang, J. Agric. Food Chem., 2020, 68, 4205–4214 CrossRef CAS PubMed.
- R. González-Barrio, C. A. Edwards and A. Crozier, Drug Metab. Dispos., 2011, 39, 1680–1688 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01050g |
| This journal is © The Royal Society of Chemistry 2024 |