Open Access Article
Open Access ArticleDietary sugar kelp (Saccharina latissima) consumption did not attenuate atherosclerosis in low-density lipoprotein receptor knockout mice†
Hyungryun
Jang
a,
Hayoung
Woo
a,
Olivia
Corvino
a,
Hyunju
Kang
 ab,
Mi-Bo
Kim
a,
Ji-Young
Lee
ab,
Mi-Bo
Kim
a,
Ji-Young
Lee
 a and
Young-Ki
Park
a and
Young-Ki
Park
 *a
*a
aDepartment of Nutritional Sciences, University of Connecticut, Storrs, Connecticut, 27 Manter Rd, Storrs, CT 06269, USA. E-mail: young-ki.park@uconn.edu
bDepartment of Food and Nutrition, Keimyung University, Daegu, South Korea
First published on 31st May 2024
Abstract
We previously demonstrated the beneficial effects of U.S.-grown sugar kelp (Saccharina latissima), a brown seaweed, on reducing serum triglycerides (TG) and total cholesterol (TC) and protecting against inflammation and fibrosis in the adipose tissue of diet-induced obesity mice. In this current study, we aimed to explore whether the dietary consumption of sugar kelp can prevent atherosclerosis using low-density lipoprotein receptor knockout (Ldlr KO) mice fed an atherogenic diet. Eight-week-old male Ldlr KO mice were fed either an atherogenic high-fat/high-cholesterol control (HF/HC) diet or a HF/HC diet supplemented with 6% (w/w) sugar kelp (HF/HC-SK) for 16 weeks. Consumption of sugar kelp significantly increased the body weight gain without altering fat mass and lean mass. Also, there were no significant differences in energy expenditure and physical activities between the groups. The two groups did not show significant differences in serum and hepatic TG and TC levels or the hepatic expression of genes involved in cholesterol and lipid metabolism. Although serum alanine aminotransferase (ALT) activity did not differ significantly between the two groups, there were significant increases in the expression of macrophage markers, including adhesion G protein-coupled receptor E1 and cluster of differentiation 68, as well as tumor necrosis factor alpha in the HF/HC-SK group compared to the HF/HC mice. The consumption of sugar kelp did not elicit a significant effect on the development of aortic lesions. Moreover, lipopolysaccharide-stimulated splenocytes isolated from HF/HC-SK-fed mice showed no significant changes in the mRNA levels of pro-inflammatory genes compared with those from the HF/HC mice. In summary, the consumption of dietary sugar kelp did not elicit anti-atherogenic and hepatoprotective effects in Ldlr KO mice.
1. Introduction
Atherosclerosis, characterized by plaque deposition in arterial walls, increases the risk of cardiovascular diseases.1 High saturated fat and cholesterol content in a Western diet promote dyslipidemia, such as elevated low-density lipoprotein (LDL) cholesterol.2 Also, oxidative stress and endothelial dysfunction lead to the formation of oxidized LDL, lipid-laden foam cells, and arterial wall inflammation.3 Pro-inflammatory cytokines, such as tumor necrosis alpha (TNFα) and interleukin-1 beta (IL-1β), produced by immune cells, further contribute to atherosclerotic plaque development.4 Also, excessive intake of calories and refined carbohydrates in the Western diet worsens the risk of atherosclerosis through insulin resistance and dysregulated lipid metabolism.5The high caloric density of a Western diet is also a crucial factor for metabolic dysfunction-associated steatotic liver disease (MASLD, previously known as nonalcoholic fatty liver disease) pathogenesis.6 MASLD encompasses a spectrum of liver disorders, ranging from simple steatosis to more severe forms of inflammation, hepatocellular injury, and fibrosis.7 Excess energy is stored as triglycerides (TG) in hepatocytes, leading to hepatic steatosis.8 The Western diet can also induce systemic inflammation and oxidative stress, significantly contributing to MASLD progression.9 High intake of added sugars and saturated fats triggers liver inflammation, activating inflammatory signaling pathways and releasing pro-inflammatory cytokines, resulting in liver cell damage and the progression to nonalcoholic steatohepatitis (NASH).7,10
In the United States, there is growing recognition of the health benefits of edible seaweed products in preventing type 2 diabetes, inflammation, and oxidative stress.11–14 Sugar kelp is a brown seaweed cultivated in several regions of the United States. Polyphenols, rich in sugar kelp, have antioxidant, anti-inflammatory, lipid-lowering, and anti-fibrotic properties, which contribute to the prevention of MASLD, cardiovascular diseases, type 2 diabetes, neurodegeneration, and cancer.15 Interestingly, we recently demonstrated that consuming U.S.-grown sugar kelp mitigates obesity-related metabolic disturbances, inflammation, and fibrosis in male diet-induced obesity (DIO) mice.16 Furthermore, fucoidan, a sulfated polysaccharide in sugar kelp,17 has shown potential in attenuating atherosclerosis in LDL receptor knockout (Ldlr KO) mice by inhibiting pro-inflammatory gene expression and reducing reactive oxygen species.18 Therefore, sugar kelp has therapeutic potential for MASLD and cardiovascular diseases.
However, the potential role of whole sugar kelp in the pathogenesis of atherosclerosis and MASLD has not been conducted using an atherosclerosis mice model. In the present study, we used Ldlr KO mice as an in vivo model because they exhibit a plasma lipoprotein profile similar to that of humans.19 This genetic abnormality leads to slower clearance of very low-density lipoprotein (VLDL) and LDL, resulting in elevated cholesterol levels even on a normal chow diet.20 When exposed to a high-fat/high-cholesterol diet, the severity of atherosclerotic lesions and hypercholesterolemia is further exacerbated in Ldlr KO mice.21 Our objective was to investigate the potential preventive effects of whole sugar kelp against atherosclerosis and MASLD in Ldlr KO mice fed an atherogenic high-fat/high-cholesterol diet.
2. Methods
2.1. Sugar kelp powder preparation
Fresh sugar kelp was purchased from a local farm in Connecticut and then thoroughly washed with tap water to remove salt and sand residues. Subsequently, it was freeze-dried using a lyophilizer (FreeZone® 12 liters freeze dry system, Labconco Corp., Kansas City, MO, USA) and then ground into a powder using an IKA A11 basic analytical mill grinder (IKA®, Wilmington, NC, USA). The powdered sugar kelp was stored at −80 °C until it was used for the experimental diet. The composition of the sugar kelp powder was analyzed by Medallion Labs (Minneapolis, MN, USA), which was used to formulate experimental diets shown in Table 1. It contains 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 μg of iodine and 856 mg of sodium per 100 g.
265 μg of iodine and 856 mg of sodium per 100 g.
| Ingredient | HF/HC control | HF/HC-SK |
|---|---|---|
| g kg−1 diet | ||
| a Solka-Floc cellulose. b AIN-93 mineral mix. c Customized mineral mix composed of minerals in sugar kelp. d AIN-93 vitamin mix. | ||
| Cornstarch | 343.2 | 340.8 |
| Casein | 140.0 | 128.4 |
| Sucrose | 100.0 | 100.0 |
| Dextrinized cornstarch | 155.0 | 155.0 |
| Lard | 120.0 | 120.0 |
| Soybean oil | 40.0 | 38.10 |
| Fibera | 50.0 | 15.3 |
| Mineral mixb | 25.6 | 25.6 |
| Customized mineral mixc | 9.4 | 0.0 |
| Vitamin mixd | 10.0 | 10.0 |
| L-Cystine | 1.8 | 1.8 |
| t-Butylhydroquinone | 0.008 | 0.008 |
| Cholesterol | 2.5 | 2.5 |
| Sugar kelp powder | 0.0 | 60.0 |
2.2. Animal care and diet
Male Ldlr KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) at the age of 7 weeks. After a one-week acclimatization period, mice were randomly assigned to a high-fat/high-cholesterol control group (HF/HC; 16% fat, 0.25% cholesterol, w/w, n = 12) or HF/HC containing 6% (w/w) powdered sugar kelp (HF/HC-SK; n = 12). Table 1 shows detailed dietary composition. Both HF/HC control and HF/HC-SK diet had similar energy content and nutrient distribution. The sugar kelp supplementation level (6% w/w) is the equivalent of approximately 25 grams of daily seaweed consumption in humans based on body surface normalization for a 70 kg individual.16 Throughout the study, mice were housed under a 12 hour light/dark cycle, with unrestricted access to food and water. After 16 weeks on the experimental diets, mice were fasted for 6 hours and then anesthetized using ketamine (110 mg kg−1) and xylazine (10 mg kg−1) from Henry Schein Animal Health (Dublin, OH). Blood samples were collected through cardiac puncture, and then mice were euthanized by cervical dislocation. After 30 minutes of incubation at room temperature, serum samples were obtained by centrifuging the blood at 2000g for 10 minutes at 4 °C. The liver was isolated and immediately frozen in liquid nitrogen for gene expression analysis or fixed in 10% formalin for histological analysis. The aorta and thyroid gland samples were also fixed in 10% formalin for Oil Red O and H&E staining, respectively. The spleen was harvested for cell isolation for ex vivo experiments. All serum and frozen liver samples were stored at −80 °C until use. The Institutional Animal Care and Use Committee at the University of Connecticut approved all animal procedures under protocol number A19-033.2.3. Serum analysis
Serum TG and total cholesterol (TC) concentrations were determined using enzymatic methods using the procedures described in our previous work.22 Serum alanine aminotransferase (ALT) activity was measured using a Liquid ALT reagent (Pointe Scientific, Canton, MI, USA) according to the manufacturer's instructions.2.4. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from tissue samples was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription for cDNA synthesis and subsequent qRT-PCR analysis were conducted using the CFX96 Real-Time system (Bio-Rad, Hercules, CA, USA), following the established protocol described in our previous study.232.5. Indirect calorimetry and body composition measurement
To assess metabolic rates, energy expenditure, and physical activity, mice were subjected to the Oxymax Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, OH, USA) as we described.24 The EchoMRI™ system (Echo Medical Systems, LLC., Houston, TX, USA) was used to determine the body composition of mice following the manufacturer's instructions. These measurements were conducted 10 weeks after the mice were on the experimental diets.2.6. Histological evaluations of the liver
Immunofluorescent analysis was performed to detect hepatic adhesion G protein-coupled receptor 1 (ADGRE1), also known as F4/80, following our established protocol.25 For imaging, a Nikon A1R Spectral Confocal microscope (Nikon, Melville, NY, USA) was utilized at the University of Connecticut Advanced Light Microscopy Facility at a magnification of 20×.2.7 En Face analysis of the aorta
As previously mentioned,26 isolated aortas were fixed in 10% formalin, cleared of surrounding tissue, opened lengthwise, and rinsed with 60% isopropanol. Lipid accumulation was visualized by staining with 0.5% Oil Red O (ORO) in isopropanol. Stained aorta images were taken in 1× PBS using a Samsung nx1000 with a 60 mm lens on a tripod. The plaque percentage was calculated by quantifying the lesion area relative to the total aorta area using Image J software.272.8 Splenocyte isolation and lipopolysaccharide (LPS) stimulation
Splenic cells were collected from mice on experimental diets for 16 weeks, as we previously described.28 The splenocytes were then plated at a concentration of 4.0 × 106 cells per well of a 12-well plate and treated with or without 500 ng ml−1 of LPS (MilliporeSigma, St Louis, MO, USA) for 20 hours and then RNA was extracted for gene analysis.2.9. Statistical analysis
An unpaired t-test was conducted to determine statistically significant differences between groups using GraphPad Prism 10 (GraphPad Software, La Jolla, CA, USA). All data were deemed statistically significant when the P-value was less than 0.05. Data are presented as mean ± SEM.3. Results
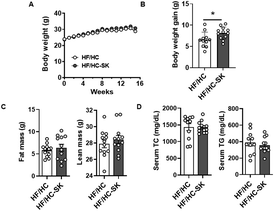
3.1. Dietary sugar kelp consumption did not alter body weight gain, body composition, and serum TG and TC concentrations in Ldlr KO mice fed an atherogenic diet
Throughout the 16 weeks on the atherogenic diet, there were no significant differences in body weight between the HF/HC and HF/HC-SK groups (Fig. 1A). However, the body weight gain, i.e., the difference between initial and final body weight, was significantly higher in the HF/HC-SK group than in the HF/HC control (Fig. 1B), although food consumption was similar in both groups (data not shown). The fat and lean mass of mice were not significantly altered by sugar kelp consumption (Fig. 1C).Serum TG and TC concentrations were not significantly different between the two groups after 16 weeks of feeding (Fig. 1D). The consumption of sugar kelp for 6 weeks did not change plasma TG levels (ESI Fig. S1A†); however, at 12 weeks, sugar kelp consumption elicited trends toward an increase in both plasma TG and TC levels compared to the HF/HC control group (ESI Fig. S1B†).
3.2. Dietary sugar kelp consumption had minor effects on metabolic rates, energy expenditure, and physical activity in Ldlr KO mice fed an atherogenic diet
We previously found that sugar kelp consumption increased metabolic rates, energy expenditure, and physical activity in DIO mice.16 Therefore, we explored if sugar kelp consumption would elicit similar effects in Ldlr KO mice fed an atherogenic diet. We did not observe significant differences in average oxygen consumption rates (VO2), average carbon dioxide production rates (VCO2), respiratory exchange ratio (RER), energy expenditure, and physical activity during the dark (Fig. 2A & B) and light cycles (data not shown) between the control and sugar kelp-fed mice. There was a trend toward a decrease in wheel count in the HF/HC-SK group compared with the HF/HC control during the dark and light cycles (data not shown).3.3. Dietary sugar kelp consumption did not attenuate liver steatosis in Ldlr KO mice fed an atherogenic diet
Studies suggest that Ldlr KO mice may be a suitable model for MASLD, as they have elevated blood TG and TC levels due to their inability to clear LDL cholesterol from the bloodstream, increasing lipid accumulation in the liver.29,30 Therefore, we explored the potential impact of sugar kelp consumption on hepatic steatosis in Ldlr KO mice fed an atherogenic diet.The consumption of sugar kelp did not significantly change liver weight, TG, and TC compared with the HF/HC control group (Fig. 3A & B). The expression level of genes involved in cholesterol metabolism, such as sterol regulatory element-binding factor 2 (Srebf2) and of β-Hydroxy β-methylglutaryl-CoA (Hmg-CoA) reductase (Hmgcr), were not significantly altered by sugar kelp consumption (Fig. 3C). Furthermore, the expression of lipogenic genes, including sterol regulatory element-binding factor 1 (Srebf1), acetyl-CoA carboxylase alpha (Acaca), fatty acid synthase (Fasn), and stearoyl-CoA desaturase 1 (Scd1), was not significantly different between groups (Fig. 3D). However, the expression level of microsomal triglyceride transfer protein (Mttp) was markedly increased in HF/HC-SK, and the mRNA level of apolipoprotein B (ApoB) showed a trend toward an increase in HF/HC-SK compared to the HF/HC control group (Fig. 3E), although these gene changes did not elicit any significant changes in circulating lipoprotein levels.
3.4. Sugar kelp consumption aggravated hepatic inflammation without changing liver injury in Ldlr KO mice fed an atherogenic diet
We next explore the impact of dietary sugar kelp consumption on hepatic inflammation, a critical factor in the progression from simple steatosis to NASH. The hepatic expression levels of pan-macrophage markers, including adhesion G protein-coupled receptor E1 (Adgre1) and cluster of differentiation 68 (Cd68), were significantly increased in the HF/HC-SK group compared to the HF/HC control (Fig. 4A). A significant increase in the ADGRE1-positive area was observed in the liver of HF/HC-SK-fed mice (Fig. 4B). Moreover, a hepatic mRNA level of M1 pro-inflammatory markers, such as tumor necrosis factor (Tnf), was significantly induced by the consumption of sugar kelp without significantly altering M2 anti-inflammatory markers, such as mannose receptor C-type 1 (Mrc1) and arginase 1 (Arg1) (Fig. 4C). Despite increased hepatic inflammation observed in the HF/HC-SK group, serum ALT activity, a hallmark of liver injury, was not significantly different between the HF/HC-SK and HF/HC control mice (Fig. 4D).3.5. Dietary sugar kelp supplementation did not attenuate atherosclerosis in Ldlr KO mice fed an atherogenic diet
We performed an En Face analysis to assess the effect of dietary sugar kelp consumption on atherogenesis. The percentages of ORO-positive areas between the HF/HC-SK and HF/HC control groups were not significantly different (Fig. 5A & B). We next explored the potential impact of sugar kelp consumption on the reactivities of splenocytes to LPS, as splenocytes have been implicated in the progression of atherosclerosis and hepatic inflammation.31 When splenocytes isolated from HF/HC-SK-fed mice were stimulated by LPS ex vivo, Tnf and Il1b mRNA expression was not significantly altered compared with those from the HF/HC control mice (Fig. 5C).3.6. The consumption of sugar kelp did not induce iodine toxicity in atherogenic diet-fed Ldlr KO mice
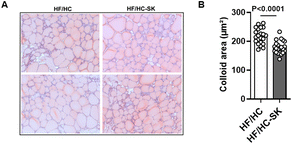
To evaluate whether the consumption of sugar kelp induces iodine toxicity, we measured an area of colloid, the space within the thyroid follicles where the hormone synthesis occurs (Fig. 6A & B). The consumption of sugar kelp significantly decreased the colloid area compared to the control group, indicating no iodine toxicity evidenced by less colloid goiter in Ldlr KO mice compared to its control.4. Discussion
We previously reported that sugar kelp consumption exerts anti-obesity, anti-inflammatory, and hypolipidemic effects in DIO mice.16 Several animal models, such as leptin-deficient mice (ob/ob) or leptin receptor-deficient mice (db/db) under chow diet feeding, have been proposed as appropriate animal models for MASLD.32Ldlr KO mice have also been proposed as a proper model for investigating not only atherosclerosis but MASLD, as they exhibit increased levels of TG and TC in the blood and oxidized LDL-induced hepatic inflammation and liver damage.29,32 Thus, the present study explored whether dietary sugar kelp consumption could prevent atherosclerosis and MASLD in Ldlr KO. We found that in Ldlr KO fed an atherogenic diet, dietary sugar kelp supplementation did not elicit anti-obesity and lipid-lowering effects. Also, consuming sugar kelp did not prevent atherosclerosis development in Ldlr KO mice. Therefore, the present study demonstrates that sugar kelp consumption did not attenuate the development of MASLD and atherosclerosis in Ldlr KO mice fed an atherogenic diet.Contrary to our previous study in DIO mice,16 sugar kelp consumption did not elicit anti-obesity, lipid-lowering, or hepatoprotective effects in the present study. There are several possibilities to explain the discrepancy. First, our last study used male C57BL/6J mice, a well-established mouse model for DIO,33 but the present study employed Ldlr KO mice, which are more suitable for atherosclerosis. Second, the two studies utilized different diets. The previous study used an obesogenic high-fat/high-sucrose/high-cholesterol diet to induce obesity and NASH.34 However, the present study utilized an atherogenic high-fat/high-cholesterol diet to cause the development of atherosclerosis35 (Vcam1).18 Therefore, those two possibilities may not be major reasons for the discrepancy between our previous study and the current study. Another possibility may be related to the dissimilar bioactivity of two batches of sugar kelp due to the different composition of each batch.36 Particularly, the composition of fucoidan in each sugar kelp may contribute to the discrepancy due to its health benefits that exert lipid-lowering and anti-inflammatory effects.37,38 Several studies have shown the lipid-lowering effects of fucoidan, a polysaccharide in brown seaweed. For example, intraperitoneally-administrated 50 mg kg−1 of fucoidan extracted from Fucus vesiculosus every 3 days decreased the activation of Sterol regulatory element-binding protein-1c (SREBP-1c), a transcription factor for lipogenic genes, and SREBP-2, a master regulator of cholesterol metabolism, in poloxamer-407-induced hyperlipidemic mice.39 Additionally, 5% (wt/wt) supplementation of fucoidan from Cladosiphon okamuranus in the HF diet suppressed the mRNA expression of Srebf1c in apoprotein E-deficient mice, another common mouse model of atherosclerosis.40 The content of fucoidan varies from about 2% to 55% of the dry weight of sugar kelp depending on seasonal and environmental factors.41,42 According to the study, the minimum amount of orally consumed fucoidan that exerts beneficial effects was 100 mg kg−1 conducted by Wang et al.18 Additionally, we did not observe an anti-atherogenic property of sugar kelp in the present study, although Wang et al.18 reported the protective role of fucoidan against atherosclerosis development in Ldlr KO mice fed an atherogenic diet. According to the study, orally administered 100 mg kg−1 fucoidan extracted from Laminaria Japonica attenuated atherosclerosis in Ldlr KO mice, by lowering the aortic mRNA levels of pro-inflammatory mediators, such as Tnf, Il1b, intracellular adhesive molecule 1 (Icam1), and vascular cellular adhesive molecule 1. Therefore, we speculated that the sugar kelp used in the current study might not contain enough fucoidan to elicit lipid-lowering and athero-protective effects in Ldlr KO mice. Our results emphasize the importance of identifying bioactive compounds in seaweed, including sugar kelp and utilizing them to standardize the quality of the seaweed products.
We further investigated the effects of sugar kelp on hepatic inflammation in Ldlr KO mice fed the atherogenic diet. The atherogenic diet, rich in saturated fat and cholesterol, leads to liver inflammation by activating inflammatory signaling pathways and releasing pro-inflammatory cytokines.7,10 It is well-known that soluble fibers have lipid-lowering and anti-atherogenic effects. However, sugar kelp contains mostly insoluble fiber, which may explain why we did not see the reduction of atherosclerotic lesions by sugar kelp. The present study also found that sugar kelp consumption increased hepatic inflammation but did not increase liver injury markers in Ldlr KO mice fed the atherogenic diet. It may be possible that the elevated hepatic inflammation in the sugar kelp-fed mice may be attributed to the presence of potential heavy metals, such as lead and mercury, known for their hepatotoxic properties.43 The sugar kelp used in this study contained 173 ppb of lead and 56.9 ppb of mercury. The consumption of brown seaweed has been associated with various health benefits due to the bioactive compounds therein.44 However, concerns exist about consuming whole seaweeds and seaweed-based products due to the potential heavy metal contents.45 The toxic effects of heavy metals, such as lead and mercury, can lead to the activation of nuclear factor kappa B (NF-κB), producing inflammatory cytokines, such as IL-1β, TNF-α, IL-6, and IL-8, and inducible nitric oxide synthase (iNOS) in the liver of adult male Wistar albino rats and Institute of Cancer Research (ICR) male mice.46,47 The increased hepatic inflammation, although it was insufficient to induce liver injury, emphasizes the need to establish regulations related to seaweed production and quality control.
Sugar kelp is a rich source of iodine like other seaweeds, with concentrations varying depending on environmental factors, such as iodine levels in seawater, where it is cultivated.48 The consumption of large amounts of iodine-rich seaweed has been associated with iodine toxicity, particularly in vulnerable populations, such as pregnant women and individuals with thyroid disorders.49,50 Also, an acute iodine intake, even in healthy adults, can lead to a disruption in thyroid hormone production, induce hyperthyroidism or hypothyroidism, and develop colloid goiter, depending on individual susceptibility.51 In the present study, the consumption of sugar kelp did not induce iodine toxicity, evidenced by a decreased area of colloid compared to its control. Even though iodine toxicity was not observed in the current study, standardizations that control the levels of iodine in brown seaweed products are required to prevent possible iodine toxicity.
5. Conclusions
In summary, our findings indicate that consuming sugar kelp did not elicit a protective effect against MASLD and atherosclerosis in Ldlr KO mice fed an atherogenic diet. We speculate that the sugar kelp used in the current study might have an insufficient amount of fucoidan, contributing to the lack of lipid-lowering or hepatoprotective and anti-atherogenic effects. Moreover, increased hepatic inflammation with sugar kelp consumption implies a future study that unravels the possible hepatotoxicity of heavy metals in brown seaweed. Lastly, we observed that the consumption of sugar kelp did not promote iodine toxicity. Our findings emphasize the need for regulations in brown seaweed products to prevent heavy metal contamination. It is also crucial to standardize brown seaweed products to achieve consistent levels of iodine and other bioactive compounds, ensuring that all products exert consistent biological effects.Author contributions
Conceptualization, methodology: Ji-Young Lee, Young-Ki Park; data curation: Hyungryun Jang, Hayoung Woo, Hyunju Kang, Olivia Corvino, Mi-Bo Kim; writing – original draft preparation: Hyungryun Jang; writing – review and editing: Ji-Young Lee, Young-Ki Park; funding acquisition: Young-Ki Park; All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.References
- A. A. Farooqui, Role of Dyslipidemia in Atherosclerosis, Stroke Revisited: Dyslipidemia in Stroke, 2021, pp. 3–14 Search PubMed.
- S. Merat, F. Casanada, M. Sutphin, W. Palinski and P. D. Reaven, Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet, Arterioscler., Thromb., Vasc. Biol., 1999, 19, 1223–1230 CrossRef CAS PubMed.
- D. A. Chistiakov, A. A. Melnichenko, V. A. Myasoedova, A. V. Grechko and A. N. Orekhov, Mechanisms of foam cell formation in atherosclerosis, J. Mol. Med., 2017, 95, 1153–1165 CrossRef CAS PubMed.
- D. Tousoulis, E. Oikonomou, E. K. Economou, F. Crea and J. C. Kaski, Inflammatory cytokines in atherosclerosis: current therapeutic approaches, Eur. Heart J., 2016, 37, 1723–1732 CrossRef CAS PubMed.
- J. Deer, J. Koska, M. Ozias and P. Reaven, Dietary models of insulin resistance, Metabolism, 2015, 64, 163–171 CrossRef CAS PubMed.
- I. C. Simoes, A. Karkucinska-Wieckowska, J. Janikiewicz, S. Szymanska, M. Pronicki, P. Dobrzyn, M. Dabrowski, A. Dobrzyn, P. J. Oliveira and H. Zischka, Western diet causes obesity-induced nonalcoholic fatty liver disease development by differentially compromising the autophagic response, Antioxidants, 2020, 9, 995 CrossRef CAS PubMed.
- E. Buzzetti, M. Pinzani and E. A. Tsochatzis, The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD), Metabolism, 2016, 65, 1038–1048 CrossRef CAS PubMed.
- S. S. Choi and A. M. Diehl, Hepatic triglyceride synthesis and nonalcoholic fatty liver disease, Curr. Opin. Lipidol., 2008, 19, 295–300 CrossRef CAS PubMed.
- Z. Chen, R. Tian, Z. She, J. Cai and H. Li, Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, Free Radicals Biol. Med., 2020, 152, 116–141 CrossRef CAS PubMed.
- J. A. Del Campo, P. Gallego and L. Grande, Role of inflammatory response in liver diseases: Therapeutic strategies, World J. Hepatol., 2018, 10, 1 CrossRef PubMed.
- M. M. Stefaniak, M. Gudjónsdóttir, G. Marteinsdottir, S. Omarsdottir, E. Bravo, O. E. Sigurjónsson and K. Kristbergsson, Determination of bioactive properties of food grade extracts from Icelandic edible brown seaweed sugar kelp (Saccharina latissima) with in vitro human cell cultures (THP-1), Funct. Foods Health Dis., 2019, 9, 1–15 Search PubMed.
- S.-y. Kang, E. Kim, I. Kang, M. Lee and Y. Lee, Anti-diabetic effects and anti-inflammatory effects of Laminaria japonica and Hizikia fusiforme in skeletal muscle: in vitro and in vivo model, Nutrients, 2018, 10, 491 CrossRef PubMed.
- H. A. Jung, S. E. Jin, B. R. Ahn, C. M. Lee and J. S. Choi, Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264. 7 macrophages, Food Chem. Toxicol., 2013, 59, 199–206 CrossRef CAS PubMed.
- A. O′sullivan, Y. O′callaghan, M. O′grady, B. Queguineur, D. Hanniffy, D. Troy, J. Kerry and N. O′brien, In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland, Food Chem., 2011, 126, 1064–1070 CrossRef.
- Y. Lee and J.-Y. Lee, in Dietary Interventions in Liver Disease, Elsevier, 2019, pp. 91–99 Search PubMed.
- M.-B. Kim, Y. Lee, M. Bae, H. Kang, S. Hu, T. X. Pham, J.-Y. Lee and Y.-K. Park, Sugar kelp (Saccharina latissima) inhibits hepatic inflammation and fibrosis in a mouse model of diet-induced nonalcoholic steatohepatitis, J. Nutr. Biochem., 2021, 97, 108799 CrossRef CAS PubMed.
- B. Lafeuille, É. Tamigneaux, K. Berger, V. Provencher and L. Beaulieu, Variation of the Nutritional Composition and Bioactive Potential in Edible Macroalga Saccharina latissima Cultivated from Atlantic Canada Subjected to Different Growth and Processing Conditions, Foods, 2023, 12, 1736 CrossRef CAS PubMed.
- X. Wang, L. Pei, H. Liu, K. Qv, W. Xian, J. Liu and G. Zhang, Fucoidan attenuates atherosclerosis in LDLR-/-mice through inhibition of inflammation and oxidative stress, Int. J. Clin. Exp. Pathol., 2016, 9, 6896–6904 CAS.
- A. Khan, K. Waqar, A. Shafique, R. Irfan and A. Gul, in Omics Technologies and Bio-Engineering, ed. D. Barh and V. Azevedo, Academic Press, 2018, pp. 431–448, DOI:10.1016/B978-0-12-804659-3.00018-X.
- J. F. Bentzon and E. Falk, Atherosclerotic lesions in mouse and man: is it the same disease?, Curr. Opin. Lipidol., 2010, 21, 434–440 CrossRef CAS PubMed.
- J. W. Knowles and N. Maeda, Genetic modifiers of atherosclerosis in mice, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 2336–2345 CrossRef CAS PubMed.
- T. P. Carr, C. J. Andresen and L. L. Rudel, Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts, Clin. Biochem., 1993, 26, 39–42 CrossRef CAS PubMed.
- H. E. Rasmussen, K. R. Blobaum, Y.-K. Park, S. J. Ehlers, F. Lu and J.-Y. Lee, Lipid extract of Nostoc commune var. sphaeroides Kutzing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells, J. Nutr., 2008, 138, 476–481 CrossRef CAS PubMed.
- T. X. Pham, Y. Lee, M. Bae, S. Hu, H. Kang, M. B. Kim, Y. K. Park and J. Y. Lee, Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes, Br. J. Nutr., 2019, 121, 748–755 CrossRef CAS PubMed.
- Y. Lee, T. X. Pham, M. Bae, S. Hu, E. O'Neill, O. K. Chun, M. J. Han, S. I. Koo, Y. K. Park and J. Y. Lee, Blackcurrant (Ribes nigrum) Prevents Obesity–Induced Nonalcoholic Steatohepatitis in Mice, Obesity, 2019, 27, 112–120 CrossRef CAS PubMed.
- C. S. Ku, B. Kim, T. X. Pham, Y. Yang, C. J. Wegner, Y.-K. Park, M. Balunas and J.-Y. Lee, Blue-green algae inhibit the development of atherosclerotic lesions in apolipoprotein E knockout mice, J. Med. Food, 2015, 18, 1299–1306 CrossRef CAS PubMed.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- T. X. Pham, Y. Lee, M. Bae, S. Hu, H. Kang, M.-B. Kim, Y.-K. Park and J.-Y. Lee, Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes, Br. J. Nutr., 2019, 121, 748–755 CrossRef CAS PubMed.
- V. Bieghs, P. J. Van Gorp, K. Wouters, T. Hendrikx, M. J. Gijbels, M. van Bilsen, J. Bakker, C. J. Binder, D. Lütjohann and B. Staels, LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease, PLoS One, 2012, 7, e30668 CrossRef CAS PubMed.
- S. Subramanian, L. Goodspeed, S. Wang, J. Kim, L. Zeng, G. N. Ioannou, W. G. Haigh, M. M. Yeh, K. V. Kowdley and K. D. O'Brien, Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice, J. Lipid Res., 2011, 52, 1626–1635 CrossRef CAS PubMed.
- S. Potteaux, H. Ait-Oufella and Z. Mallat, Role of splenic monocytes in atherosclerosis, Curr. Opin. Lipidol., 2015, 26, 457–463 CrossRef CAS PubMed.
- D. Jahn, S. Kircher, H. M. Hermanns and A. Geier, Animal models of NAFLD from a hepatologist's point of view, Biochim. Biophys. Acta, Mol. Basis Dis., 2019, 1865, 943–953 CrossRef CAS PubMed.
- S. Collins, T. L. Martin, R. S. Surwit and J. Robidoux, Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics, Physiol. Behav., 2004, 81, 243–248 CrossRef CAS PubMed.
- M.-B. Kim, Y. Lee, M. Bae, H. Kang, T. X. Pham, S. Hu, J.-Y. Lee and Y.-K. Park, Comprehensive characterization of metabolic, inflammatory and fibrotic changes in a mouse model of diet-derived nonalcoholic steatohepatitis, J. Nutr. Biochem., 2020, 85, 108463 CrossRef CAS PubMed.
- S. S. Ghosh, S. Righi, R. Krieg, L. Kang, D. Carl, J. Wang, H. D. Massey, D. A. Sica, T. W. Gehr and S. Ghosh, High fat high cholesterol diet (western diet) aggravates atherosclerosis, hyperglycemia and renal failure in nephrectomized LDL receptor knockout mice: role of intestine derived lipopolysaccharide, PLoS One, 2015, 10, e0141109 CrossRef PubMed.
- C. Monteiro, H. Li, N. Diehl, J. Collén, S. Heinrich, K. Bischof and I. Bartsch, Modulation of physiological performance by temperature and salinity in the sugar kelp Saccharina latissima, Phycol. Res., 2021, 69, 48–57 CrossRef CAS.
- J. Zhao, Q. Cao, M. Xing, H. Xiao, Z. Cheng, S. Song and A. Ji, Advances in the study of marine products with lipid-lowering properties, Mar. Drugs, 2020, 18, 390 CrossRef CAS PubMed.
- E. Apostolova, P. Lukova, A. Baldzhieva, P. Katsarov, M. Nikolova, I. Iliev, L. Peychev, B. Trica, F. Oancea and C. Delattre, Immunomodulatory and anti-inflammatory effects of fucoidan: A review, Polymers, 2020, 12, 2338 CrossRef CAS PubMed.
- J. Park, M. Yeom and D.-H. Hahm, Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation, J. Pharmacol. Sci., 2016, 131, 84–92 CrossRef CAS PubMed.
- T. Yokota, K. Nomura, M. Nagashima and N. Kamimura, Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoEshl mice deficient in apolipoprotein E expression, J. Nutr. Biochem., 2016, 32, 46–54 CrossRef CAS PubMed.
- A. Bruhn, T. Janicek, D. Manns, M. M. Nielsen, T. J. S. Balsby, A. S. Meyer, M. B. Rasmussen, X. Hou, B. Saake and C. Göke, Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—seasonal variation and impact of environmental factors, J. Appl. Phycol., 2017, 29, 3121–3137 CrossRef CAS PubMed.
- S. L. Holdt and S. Kraan, Bioactive compounds in seaweed: functional food applications and legislation, J. Appl. Phycol., 2011, 23, 543–597 CrossRef CAS.
- K. Renu, R. Chakraborty, H. Myakala, R. Koti, A. C. Famurewa, H. Madhyastha, B. Vellingiri, A. George and A. V. Gopalakrishnan, Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)-induced hepatotoxicity–A review, Chemosphere, 2021, 271, 129735 CrossRef CAS PubMed.
- B. Pradhan, P. P. Bhuyan, S. Patra, R. Nayak, P. K. Behera, C. Behera, A. K. Behera, J.-S. Ki and M. Jena, Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective, Biocatal. Agric. Biotechnol., 2022, 39, 102242 CrossRef CAS.
- P. Cherry, C. O'Hara, P. J. Magee, E. M. McSorley and P. J. Allsopp, Risks and benefits of consuming edible seaweeds, Nutr. Rev., 2019, 77, 307–329 CrossRef PubMed.
- D. M. Badary, Folic acid protects against lead acetate-induced hepatotoxicity by decreasing NF-κB, IL-1β production and lipid peroxidation mediataed cell injury, Pathophysiology, 2017, 24, 39–44 CrossRef PubMed.
- J. Lee, S. J. Lee and K. T. Lim, Preventive effects of ZPDC glycoprotein (24 kDa) on hepatotoxicity induced by mercury chloride in vitro and in vivo, Cell Biochem. Funct., 2014, 32, 520–529 CrossRef CAS PubMed.
- National Food Institute, Technical University of Denmark, Denmark, M. Sá Monteiro, J. Sloth, S. Holdt and M. Hansen, Analysis and risk assessment of seaweed, EFSA J., 2019, 17, e170915 Search PubMed.
- T. Michikawa, M. Inoue, T. Shimazu, N. Sawada, M. Iwasaki, S. Sasazuki, T. Yamaji and S. Tsugane, Seaweed consumption and the risk of thyroid cancer in women, Eur. J. Cancer Prev., 2012, 21, 254–260 CrossRef CAS PubMed.
- M. Zimmermann and F. Delange, Iodine supplementation of pregnant women in Europe: a review and recommendations, Eur. J. Clin. Nutr., 2004, 58, 979–984 CrossRef CAS PubMed.
- C. T. Trinh, A review of the pathology, diagnosis and management of colloid goitre, Eur. Endocrinol., 2020, 16, 131 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01037j |
| This journal is © The Royal Society of Chemistry 2024 |