Open Access Article
Open Access ArticleEfficacy and safety of galacto-oligosaccharide in the treatment of functional constipation: randomized clinical trial†
Jae-Hwan
Lee
a,
Geun-Bae
Kim
a,
Kisoo
Han
b,
Eun-Jin
Jung
c,
Hyung Joo
Suh
 de and
Kyungae
Jo
de and
Kyungae
Jo
 *e
*e
aDepartment of Animal Science and Technology, Chung-Ang University, Anseong 17546, Republic of Korea
bNeoCremar Co. Ltd, Seoul 05702, Republic of Korea
cDepartment of Food and Biotechnology, Korea University, Sejong 30019, Republic of Korea
dTransdisciplinary Major in Learning Health Systems, Graduate School, Korea University, Seoul 02841, Republic of Korea
eDepartment of Integrated Biomedical and Life Science, Graduate School, Korea University, Seoul 02841, Republic of Korea. E-mail: kyungae11@korea.ac.kr; Fax: +82-2-921-7207; Tel: +82-2-940-2764
First published on 24th May 2024
Abstract
The efficacy and safety of galacto-oligosaccharides (GOS) in treating functional constipation were evaluated in a four-week randomized, double-blind clinical trial on 63 patients who met Rome IV criteria (34 GOS, 29 placebo group). The number of bowel movements per day and changes in the shape of bowel movements in the treatment group significantly improved compared to those in the control group after four weeks. The Patient Assessment Constipation Quality of Life questionnaire showed that satisfaction with constipation significantly increased in the treatment group. The levels of Bifidobacterium sp. and Lactobacillus sp. significantly increased after four weeks of GOS treatment compared to those measured at baseline. No significant adverse drug reactions were identified in any indicator except for pulse rate. Thus, the prebiotic GOS can be safely used in foods and pharmaceuticals to alleviate symptoms of functional constipation by improving the intestinal flora.
1. Introduction
Constipation is a common digestive tract symptom. Although the reported prevalence varies depending on the specific definition used, constipation is estimated to affect 5–20% of the total population, with a higher incidence in women and the older population.1,2 Constipation can be defined as an intestinal disorder that includes various symptoms such as a decrease in the frequency of defecation, feeling that the anus is blocked or exerting excessive force during defecation, and requiring manipulations such as pressing the anus with a finger for defecation.3 Constipation itself can be secondary to a disease and medications.1 According to the Rome IV criteria, functional disorders associated with constipation are classified as functional constipation, opioid-induced constipation, functional defecation disorder, and irritable bowel syndrome (IBS) with predominant constipation.4 Constipation and its associated symptoms are often intermittent and mild, but in some cases can be chronic, difficult to treat, and debilitating.3With the development of various digestive tract function tests, many studies have recently been conducted to explore the pathophysiology of constipation, resulting in the identification of mechanisms of action of constipation medications. However, many patients with constipation continue to prefer treatments with unknown mechanisms or self-treatment regimens. In general, in cases of chronic constipation, the recommended treatment is to increase the amount of fiber in the diet or consume a fiber preparation as a supplement.5 The primary categories of drug therapy for constipation include osmotic laxatives such as magnesia, sorbitol, and polyethylene glycol; stimulant laxatives such as bisacodyl or senna; and gastrointestinal motility promoters such as tegaserod.6 However, in many cases, laxatives are associated with various serious side effects such as water and electrolyte loss, secondary aldosteronism, protein-losing gastroenteritis, osteomalacia, allergic reactions, renal failure, liver failure, lipid pneumonia, and malabsorption; therefore, their long-term use is not recommended.7,8
To improve constipation, the use of food to induce changes in the intestinal microbiota and prevent or alleviate symptoms is a safe method that can be maintained over the long term. Changes in the gut microbiota can be induced by the direct intake of probiotics, with Bifidobacterium and Lactobacillus being the most widely used probiotics in humans.9,10 In particular, galacto-oligosaccharide (GOS) is a growth-promoting substance for Bifidobacterium that inhibits the proliferation of harmful bacteria such as Escherichia coli, Enterococcus, and putrefactive bacteria in the intestine, and is therefore classified as a prebiotic.11,12 GOS is a non-digestible functional oligosaccharide produced by the β-galactosidase enzyme using lactose as the raw material.13 Intake of these prebiotics increases the composition of the intestinal microbiota relative to the growth of Bifidobacterium and Lactobacillus. Changes in the intestinal microbiota have been reported to reduce the occurrence of intestinal diseases, improve mineral absorption, and suppress the development of colorectal cancer, thereby improving overall health.14,15 Therefore, in this study, the efficacy and safety of GOS were evaluated in adults with functional constipation.
2. Materials and methods
2.1 Participants and study design
The subjects of this study were male and female adults aged between 19 and 75 years who satisfied the Rome IV diagnostic criteria for functional constipation (Table S1†). Patients who met the Rome IV diagnostic criteria for IBS (Table S2†) were excluded. This clinical trial was approved by the Institutional Bioethics Committee of Woosuk University Hospital of Oriental Medicine (IRB No. H1910-01). All subjects signed a consent form for the clinical trial after receiving a detailed explanation of the purpose and content of the study.This clinical trial aimed to evaluate the clinical efficacy and safety of GOS for chronic constipation. Seventy participants were randomly assigned to the treatment and control groups, with 35 participants in each group. Double blinding was performed until all subjects completed the trial. The treatment group orally took three capsules of GOS twice a day for four weeks within 30 min after meals; the components per capsule were 333.33 mg GOS, 58.67 mg maltodextrin, 4 mg silicon dioxide, and 4 mg magnesium stearate. The control group received three capsules of the control drug (placebo) twice daily for four weeks; the components per placebo capsule were 392 mg maltodextrin, 4 mg silicon dioxide, and 4 mg magnesium stearate.
2.2 Symptom assessment
Two weeks before drug administration, at the first (baseline) visit, written informed consent, body measurements [weight and body mass index (BMI)], dietary survey, lifestyle survey, vital signs (blood pressure, pulse, and body temperature), physical examination, and constipation symptom survey (Rome IV criteria) data were obtained.Bowel habits were assessed using the Bowel-Related Symptom Questionnaire. The number of bowel movements was calculated as the average number of bowel movements per day and the bowel movement time was calculated as the time required for one bowel movement. The degree of strain during defecation, sensation of incomplete evacuation, sensation of anorectal obstruction or blockage, and abdominal discomfort were scored on a scale of 0–10 (0 = “very mild” and 10 = “very severe”). Satisfaction with defecation was scored from 0 (very dissatisfied) to 10 (very satisfied).16 Stool morphology was measured using the Bristol Stool Form Scale (BSF) developed by Heaton et al.17 The BSF is a tool that can determine the hardness of feces by visually observing the fecal shape and characteristics. Scores of 1–2 represent constipation, scores of 3–4 represent normal stools, and scores of 5–7 represent a tendency toward diarrhea.
Health-related quality of life was assessed using the Short-Form Health Survey-36 (SF-36) tool developed by Ware and Sherbourne.18 The SF-36 consists of 36 questions from the following eight domains: physical functioning, role limitation-physical, role-emotional, vitality, mental health, social functioning, bodily pain, and general health. Each scale was calculated by converting to 100 points; the higher the score, the higher the level of health-related quality of life.
Constipation-related quality of life was measured using the Patient Assessment Constipation Quality of Life (PAC-QOL) questionnaire developed by Marquis et al.19 The PAC-QOL consists of self-reported items examining the effect of constipation on quality of life in the last two weeks, comprising 28 questions encompassing the following seven domains: symptoms related to constipation, frequency of influence on daily life, degree of influence on daily life, emotional functioning, mood, social functioning, and satisfaction felt in relation to constipation. Scores are given on a scale of 1–5, with 1 indicating “not at all”, 2 indicating “a little bit”, 3 indicating “moderately”, 4 indicating “quite a bit”, and 5 indicating “extremely”. A lower total score indicates a higher constipation-related quality of life.
2.3 Radiographic evaluation of colon transit time
Colonic transit time was examined using a radiopaque pellet in all participants before and after the start of the study. Plain abdominal radiography was performed 72 h after the patient ingested the 20 radiographic markers (M.I.Tech, Seoul, Korea).202.4 Gut microbiome analysis
Approximately 10–20 g of feces was collected from two or more different areas before and on the last day of test drug intake. The sample was placed in a collection container provided by the research team and delivered to the research team in a refrigerated state. Total DNA was extracted from the feces using a QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer instructions. Fecal microbiota were analyzed using pyrosequencing, a high-throughput analysis technique, according to amplification of the hypervariable V1–V3 region of the 16S rRNA gene using the Roche 454 GS FLX Titanium instrument (454 Life Sciences, Branford, CT, USA).21 Microbiota analysis was conducted at the Korea Research & Institute of Biomedical Science (KRIBS; Daejeon, Korea).2.5 Safety evaluation
For the safety evaluation, all adverse reactions that occurred after administration of the clinical trial drugs were recorded, and vital signs measurements (blood pressure, pulse, and body temperature), physical measurements (weight and BMI), hematology, blood chemistry assessment, urinalysis, and thyroid function tests were performed. The type and incidence of adverse reactions and their relevance to clinical trial drugs were evaluated using statistical analyses.2.6 Statistical analysis
All statistical analyses were performed using SAS Version 9.4 (SAS Institute). A two-sample t-test was conducted for comparisons of continuous variables, and Fisher's exact test for categorical variables. Statistical significance was set at p < 0.05, and all results are expressed as mean ± standard deviation.3. Results
3.1 Characteristics of test subjects
A total of 7 out of 70 subjects dropped out of the study or terminated the trial early, leaving measurement results of a total of 63 subjects for statistical analysis. The treatment group consisted of 34 subjects, including 5 males and 29 females, with an average age of 36.41 ± 14.90 years (Table S3†). The control group consisted of 29 subjects, 4 males and 25 females, with an average age of 34.55 ± 13.55 years. There were no significant differences in the pre-trial characteristics such as demographic information, dietary intake, alcohol consumption, smoking status, and caffeinated drink intake, of the test subjects between the two groups.3.2 Symptom evaluation
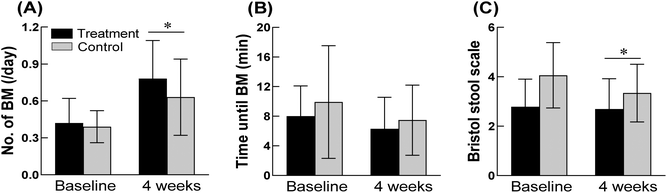
In the treatment group, the average number of defecations observed for one week increased by 0.36 times per day from 0.42 ± 0.20 at baseline to 0.78 ± 0.31 after taking GOS for four weeks (Fig. 1A). In addition, the treatment group that received GOS for four weeks showed a significant increase of 0.15 bowel movements per day compared to that of the control group (p = 0.0480). The time taken for one bowel movement was lower in the treatment group (6.29 ± 4.26 min) than that of the control group (7.47 ± 4.74 min) after four weeks, although the difference was not statistically significant (p = 0.3054; Fig. 1B). The Bristol Stool Scale score was significantly higher in the treatment group than in the control group after four weeks (4.06 ± 1.32 vs. 3.34 ± 1.17, p = 0.0283; Fig. 1C). Straining during defecation, sensation of incomplete evacuation, sensation of anorectal obstruction or blockage, and abdominal discomfort were all decreased in the treatment group compared to those of the control group, although the differences were not statistically significant (Table 1).| Treatment (n = 34), mean ± SD | Control (n = 29), mean ± SD | p-Value | ||
|---|---|---|---|---|
| SD, standard deviation. p-Values are based on Fisher's exact test (*p < 0.05). | ||||
| Straining during defecation | Baseline | 6.24 ± 2.02 | 6.66 ± 2.21 | 0.4334 |
| 4 weeks | 4.74 ± 2.65 | 5.69 ± 2.44 | 0.1449 | |
| Sensation of incomplete evacuation | Baseline | 4.79 ± 2.97 | 5.86 ± 2.64 | 0.1400 |
| 4 weeks | 3.59 ± 2.74 | 4.86 ± 2.68 | 0.0683 | |
| Sensation of anorectal obstruction or blockage | Baseline | 4.15 ± 2.80 | 4.97 ± 2.31 | 0.2149 |
| 4 weeks | 3.38 ± 2.84 | 3.83 ± 2.85 | 0.5383 | |
| Abdominal discomfort | Baseline | 4.50 ± 2.85 | 4.31 ± 2.79 | 0.7914 |
| 4 weeks | 3.41 ± 2.68 | 4.24 ± 2.23 | 0.1909 | |
| Satisfaction of defecation | Baseline | 4.62 ± 2.75 | 3.21 ± 2.24 | 0.0312* |
| 4 weeks | 5.91 ± 2.77 | 5.21 ± 2.53 | 0.2986 | |
The results of the SF-36 survey showed no significant differences between the control and GOS groups in any of the eight domains (Table 2). Physical functioning and role-physical, role-emotional, and vitality scores increased in the GOS group compared to those of the control group, although the differences were not statistically significant.
| Treatment (n = 34), mean ± SD | Control (n = 29), mean ± SD | p-Value | ||
|---|---|---|---|---|
| SD, standard deviation. p-Values are based on Fisher's exact test. | ||||
| Physical functioning | Baseline | 85.15 ± 16.67 | 84.31 ± 20.95 | 0.2084 |
| 4 weeks | 90.57 ± 21.45 | 89.66 ± 15.06 | 0.4137 | |
| Role-physical | Baseline | 86.03 ± 31.50 | 86.21 ± 24.63 | 0.9805 |
| 4 weeks | 93.81 ± 26.12 | 92.24 ± 22.26 | 0.9456 | |
| Role-emotional | Baseline | 82.35 ± 34.07 | 85.06 ± 30.32 | 0.7424 |
| 4 weeks | 92.24 ± 36.76 | 91.95 ± 26.21 | 0.9072 | |
| Vitality | Baseline | 56.03 ± 15.85 | 52.93 ± 18.00 | 0.4703 |
| 4 weeks | 60.85 ± 12.14 | 56.03 ± 14.78 | 0.3545 | |
| Mental health | Baseline | 68.47 ± 17.86 | 71.31 ± 18.24 | 0.5356 |
| 4 weeks | 72.65 ± 20.85 | 74.07 ± 13.76 | 0.7259 | |
| Social functioning | Baseline | 82.72 ± 18.97 | 89.66 ± 18.01 | 0.1441 |
| 4 weeks | 92.49 ± 20.12 | 93.53 ± 11.39 | 0.8559 | |
| Bodily pain | Baseline | 76.76 ± 22.03 | 80.95 ± 21.98 | 0.4550 |
| 4 weeks | 84.98 ± 32.48 | 86.64 ± 19.62 | 0.9848 | |
| General health | Baseline | 55.88 ± 18.44 | 59.14 ± 20.92 | 0.5140 |
| 4 weeks | 61.57 ± 22.96 | 62.59 ± 21.28 | 0.7574 | |
In the PAC-QOL questionnaire, the score for the satisfaction felt in relation to constipation was significantly improved in the treatment group (2.86 ± 1.13) compared to that of the control group (3.49 ± 0.97) after 4 weeks (Table 3). Scores for symptoms related to constipation, frequency of influence on daily life, degree of influence on daily life, emotional functioning, mood, and social functioning all decreased in the treatment group compared to those in the control group, although the differences were not statistically significant.
| Treatment (n = 34), mean ± SD | Control (n = 29), mean ± SD | p-Value | ||
|---|---|---|---|---|
| SD, standard deviation. p-Values are based on Fisher's exact test (*p < 0.05). | ||||
| Symptoms related to constipation | Baseline | 3.03 ± 0.98 | 3.17 ± 0.68 | 0.5107 |
| 4 weeks | 2.00 ± 0.81 | 2.33 ± 0.86 | 0.7284 | |
| Frequency of influence on daily life | Baseline | 2.88 ± 0.76 | 2.64 ± 0.65 | 0.1922 |
| 4 weeks | 1.74 ± 0.74 | 1.85 ± 0.66 | 0.5343 | |
| Degree of influence on daily life | Baseline | 2.48 ± 0.81 | 2.37 ± 0.89 | 0.6163 |
| 4 weeks | 1.55 ± 0.67 | 1.61 ± 0.75 | 0.7144 | |
| Emotional functioning | Baseline | 3.12 ± 0.87 | 3.06 ± 0.86 | 0.7669 |
| 4 weeks | 2.03 ± 0.79 | 2.39 ± 0.75 | 0.0685 | |
| Mood | Baseline | 3.30 ± 0.85 | 3.32 ± 0.82 | 0.9331 |
| 4 weeks | 1.81 ± 0.85 | 2.18 ± 0.94 | 0.1068 | |
| Social functioning | Baseline | 3.17 ± 0.99 | 3.23 ± 0.92 | 0.7947 |
| 4 weeks | 1.84 ± 0.81 | 2.11 ± 1.07 | 0.2561 | |
| Satisfaction felt in relation to constipation | Baseline | 4.10 ± 0.76 | 4.15 ± 0.74 | 0.7984 |
| 4 weeks | 2.86 ± 1.13 | 3.49 ± 0.97 | 0.0216* | |
3.3 Colon transit time
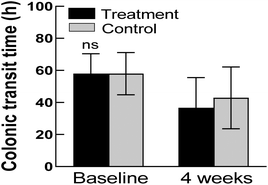
Colonic transit time was reduced by 21.41 h in the treatment group from 57.99 ± 12.37 h at baseline to 36.59 ± 18.99 h after four weeks of taking GOS (Fig. 2). In addition, the colonic transit time was decreased in the treatment group compared to that in the control group at the end of the study, although the difference was not statistically significant (p = 0.2181).3.4 Fecal evaluation
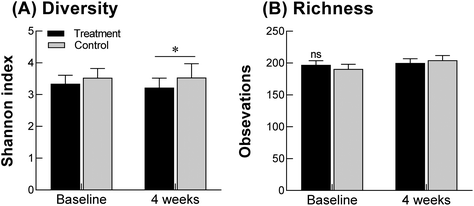
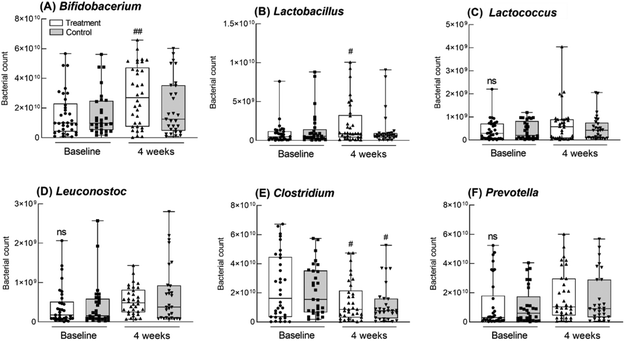
Changes in the intestinal flora were measured at the genus level (Fig. 5). In the control group, Bifidobacterium sp., as beneficial bacteria in the intestine, showed a tendency to increase after 4 weeks compared with that measured before intake, although the difference was not statistically significant. However, in the GOS group, Bifidobacterium increased significantly after intake compared to that measured before intake (p = 0.0047; Fig. 5A). In addition, Lactobacillus showed a significant increase in the GOS group after 4 weeks compared to that measured before intake (p = 0.0182, Fig. 5B). The levels of Bifidobacterium and Lactobacillus did not differ significantly between the control and GOS groups either before or after intake. There was also no significant difference in Lactococcus and Leuconostoc levels before and after intake between the control and GOS groups (Fig. 5C and D). The level of Clostridium, a harmful bacterial genus, was significantly reduced in both the control and GOS groups after intake compared to that measured before intake (p = 0.0210 and p = 0.0355, respectively; Fig. 5E); however, there was no significant difference between the two groups after four weeks of drug intake. The level of Prevotella, another harmful bacterium, did not differ significantly between the groups (Fig. 5F). Therefore, at the genus level, GOS administration significantly increased Bifidobacterium and Lactobacillus, suggesting that GOS may contribute to an increase in beneficial bacteria in the intestine that could help to improve constipation.
3.5 Safety evaluation
Subjects who were randomly assigned to the clinical trial and took the sample at least once were established as the safety set; a total of 63 subjects (34 in the treatment group and 29 in the control group) were included for the safety evaluation. Overall, two patients (5.9%) in the treatment group and three patients (10.3%) in the control group reported mild or moderate adverse effects; however, no severe adverse reactions were reported and there were no dropouts due to adverse reactions (Table S4†). Among the vital signs, pulse was significantly decreased in the treatment group (77.38 ± 11.66 beats per min) compared to that of the control group (83.59 ± 12.94 beats per min, p = 0.0498), whereas no significant differences were found between the groups in other vital signs, including systolic and diastolic blood pressure, body temperature, body weight, and BMI (Table S5†), or hematology (Table S6†) and blood chemistry (Table S7†) parameters.4. Discussion
A total of 63 adult men and women were included in this double-blind study to evaluate the efficacy and safety of GOS or placebo intake for four weeks to improve constipation. Although the participants were recruited regardless of sex, there was a high proportion of women (85%) in each group. This distribution represents a common demographic feature in other chronic constipation studies.22,23 Chronic constipation can be subdivided into various types depending on its mechanism or etiology, including slow-transit constipation, rectal outlet atresia, and constipation-type IBS.1 However, in this study, the subjects were limited to those with functional constipation according to the Rome IV criteria. According to these diagnostic criteria, functional constipation includes various causes other than organic diseases and constipation-type IBS, and thus represents various types of constipation.24 If these are classified pathophysiologically, in addition to physiological causes such as lack of fiber or water intake, bowel transit time delay-type constipation and pelvic outlet obstruction-type constipation may be mentioned. These types may overlap with each other and show various symptoms depending on the pattern or degree of colonic transit time and the type and degree of underlying pelvic exit disorders.Various methods have been proposed and several drugs have been developed to treat constipation. Since GOS is a pH-stable compound, it is not decomposed by stomach acid or enzymes secreted in the human digestive tract; therefore, most of the intake is not absorbed by the human body and is instead used by beneficial bacteria present in the small and large intestines. In addition, GOS has proven to be safe, even with long-term administration, and has shown positive effects in infants, adults, older individuals, and patients with IBS.25–31 However, the mechanism through which GOS improves functional constipation remains unclear.
In this study, GOS intake was associated with the improvement in various symptoms of constipation such as the number of bowel movements, shape of the stool, and change in the time required for one bowel movement. We further evaluated the degree of symptoms used as criteria for diagnosing functional constipation, such as excessive straining during defecation, feeling of anal blockage during defecation, feeling of residual defecation after defecation, abdominal discomfort, and satisfaction with defecation. After taking GOS for four weeks, there was a significant improvement in the number of bowel movements per day and the shape of the stool. In addition, the SF-36 and the PAC-QOL, a questionnaire specific to constipation, were used to measure the quality of life of patients with constipation.32–34 According to previous studies, pain, general health, social functioning, and mental health scores are significantly lower in individuals with constipation.34 As such, quality of life tends to decrease when symptoms of constipation are present, but then improve when the symptoms are alleviated with treatment.35 Consistent with these previous findings, we found that after taking GOS for 4 weeks, there was a significant improvement in satisfaction related to constipation and the detailed areas of the PAC-QOL questionnaire. The quality of life in patients with constipation is related to disease severity, particularly the number of bowel movements.36,37 Therefore, our results suggest that GOS can improve the frequency of defecation to result in an overall improvement of satisfaction related to constipation.
Additionally, colon transit time and pH were evaluated as indicators of constipation improvement. Previous studies have shown that colonic transit time is improved in patients with constipation following the administration of specific probiotic strains (e.g. Bifidobacterium lactis DN-173 010).38 Additionally, probiotics alone or in combination with prebiotics can decrease the intestinal pH as the levels of bacterial short-chain fatty acids (SCFAs) increase.39 In particular, GOS is known to inhibit the growth of pathogenic bacteria and to promote the growth of beneficial bacteria in the intestine because GOS is fermented by beneficial intestinal bacteria such as bifidobacteria to produce SCFAs that lower intestinal pH.25,26,28 In this study, the colonic transit time decreased by 36.9% in the GOS group compared to that measured at baseline and decreased by 14.7% in the GOS group compared to that of the control group at the end of the study, although no statistically significant difference was confirmed. In addition, fecal pH decreased in the treatment group, but this difference was also not statistically significant.
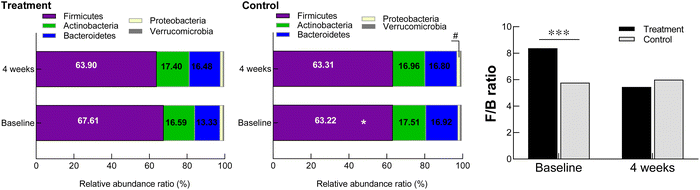
The intestinal microbiota is composed of various bacterial strains that interact with the host,40 and an imbalance in the intestinal microbiota affects intestinal homeostasis to play a role in the occurrence of constipation.41 Indigestible prebiotics such as GOS act as energy sources for beneficial bacteria in the gut and contribute to changes in the gut microbial community,42 resulting in an overall improvement in the gut microbiota composition. We found that GOS ingestion decreased the relative abundance of Firmicutes at the phylum level and the F/B ratio. An increase in Firmicutes is a major feature observed in patients with constipation,42 and an increase in the F/B ratio has also been associated with constipation.43,44
GOS intake significantly increased the abundances of Bifidobacterium sp. and Lactobacillus sp. at the genus level. Although the level of Clostridium sp., a harmful bacterium, tended to decrease after four weeks of intake, this effect was found in both the GOS and placebo groups. Lactobacilli and bifidobacteria are important components of the human gut microbiota at all ages.45 Lactobacilli are an important part of the native microbiome of humans and animals,46 which help to improve or promote lactose intolerance, intestinal peristalsis, and fecal excretion.45 Bifidobacteria are primarily found in the intestine, which help to maintain a proper balance in the human intestine and play a role in protecting against pathogens and decaying bacteria.47 The genus Bifidobacterium plays an important role in maintaining intestinal homeostasis and is closely associated with IBS, inflammatory bowel disease, and constipation.48Bifidobacterium spp. break down and ferment carbohydrates to produce SCFAs that improve the intestinal environment.49 A decrease in Bifidobacterium spp. in the intestinal microbiota has been reported among patients with constipation.50,51 Previous studies also showed that GOS intake of 1.0 to 10.0 g per day improved the intestinal environment, increased the relative abundance of bifidobacteria, and suppressed the production of harmful substances by intestinal bacteria.52–56 The intestinal microbiota of patients with constipation is unique and can be distinguished from that of healthy adults.57 Therefore, GOS, which contributes to an increase in Bifidobacterium spp. and Lactobacillus spp. in the intestinal microbiota, will help to improve constipation.
In the safety evaluation, two mild adverse events occurred in the treatment group and two mild and one moderate adverse event occurred in the control group. All of these events were confirmed to be unrelated to the sample ingestion and were completely cured. In the treatment group, the pulse rate decreased after four weeks, but there were no statistically significant changes in blood pressure, body temperature, body weight, BMI, hematology, blood chemistry, urine, and thyroid function tests for either the treatment or control group. Therefore, based on the results of this study and numerous previous studies evaluating GOS safety, GOS can be safely used in patients with constipation.58,59
In conclusion, in this clinical trial, when GOS was ingested for four weeks by patients with functional constipation, statistically significant improvements were observed in the number of bowel movements per day, shape of stool, and satisfaction with constipation. In addition, GOS showed a trend toward improving the colonic transit time and bowel habits. This functional constipation improvement was confirmed to be due to the significant promotion in the proliferation of beneficial intestinal bacteria, Bifidobacterium sp. and Lactobacillus sp., and GOS showed excellent results in terms of safety. Overall, these results suggest that GOS can be used to improve functional constipation. To verify this possibility clearly, further investigations on the correlation between GOS intake and the change in intestinal microbiota will be performed in our future research.
Author contributions
Conceptualization: Suh HJ. Data curation: Kim G. Formal analysis: Han K, Jung E. Methodology: K Jo, Lee J. Software: K Jo, Suh HJ. Validation: Lee J, Kim G. Investigation: Han K, Jung E. Writing – original draft: Lee J, Suh HJ, Jo K. Writing-review & editing: Lee J, Kim G, Han K, Jung E, Suh HJ, Jo K.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was conducted with the support of Neo Cremar Co., Ltd, South Korea.References
- M. Forootan, N. Bagheri and M. Darvishi, Chronic constipation: a review of literature, Medicine, 2018, 97, e10631 CrossRef PubMed.
- S. J. Kang, Y. S. Cho, T. H. Lee, S. E. Kim, H. S. Ryu and J. W. Kim, Medical management of constipation in elderly patients: systematic review, J. Neurogastroenterol. Motil., 2021, 27, 495–512 CrossRef PubMed.
- A. E. Bharucha and B. E. Lacy, Mechanisms, evaluation, and management of chronic constipation, Gastroenterology, 2020, 158, 1232–1249 CrossRef CAS PubMed.
- R. Sood and A. C. Ford, Diagnosis: Rome IV criteria for FGIDs – an improvement or more of the same? Nature Reviews, Gastroenterol. Hepatol., 2016, 13, 501–502 Search PubMed.
- L. R. Schiller, Chronic constipation: new insights, better outcomes?, Lancet Gastroenterol. Hepatol., 2019, 4, 873–882 CrossRef PubMed.
- S. S. C. Rao and D. M. Brenner, Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review, Am. J. Gastroenterol., 2021, 116, 1156–1181 CrossRef CAS PubMed.
- M. Portalatin and N. Winstead, Medical management of constipation, Clin. Colon Rectal Surg., 2012, 25, 12–19 CrossRef PubMed.
- A. Vilanova-Sanchez, A. C. Gasior, N. Toocheck, L. Weaver, R. J. Wood, C. A. Reck, A. Wagner, E. Hoover, R. Gagnon, J. Jaggers, T. Maloof, O. Nash, C. Williams and M. A. Levitt, Are Senna based laxatives safe when used as long term treatment for constipation in children?, J. Pediatr. Surg., 2018, 53, 722–727 CrossRef PubMed.
- A. C. Ford, E. M. Quigley, B. E. Lacy, A. J. Lembo, Y. A. Saito, L. R. Schiller, E. E. Soffer, B. M. Spiegel and P. Moayyedi, Efficacy of prebiotics, probiotics, and Synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis, Am. J. Gastroenterol., 2014, 109, 1547–1561 CrossRef PubMed.
- K. Whelan and E. M. Quigley, Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease, Curr. Opin. Gastroenterol., 2013, 29, 184–189 CrossRef PubMed.
- G. T. Macfarlane, H. Steed and S. Macfarlane, Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics, J. Appl. Microbiol., 2008, 104, 305–344 CAS.
- R. C. Martinez, H. R. Cardarelli, W. Borst, S. Albrecht, H. Schols, O. P. Gutiérrez, A. J. Maathuis, B. D. de Melo, E. Franco, C. De Martinis, E. G. Zoetendal, K. Venema, S. M. Saad and H. Smidt, Effect of galactooligosaccharides and Bifidobacterium animalis Bb-12 on growth of Lactobacillus amylovorus DSM 16698, microbial community structure, and metabolite production in an in vitro colonic model set up with human or pig microbiota, FEMS Microbiol. Ecol., 2013, 84, 110–123 CrossRef CAS PubMed.
- S. Saqib, A. Akram, S. A. Halim and R. Tassaduq, Sources of β-galactosidase and its applications in food industry, 3 Biotech, 2017, 7, 79 CrossRef PubMed.
- C. A. Brennan and W. S. Garrett, Gut microbiota, inflammation, and colorectal cancer, Annu. Rev. Microbiol., 2016, 70, 395–411 CrossRef CAS PubMed.
- A. B. Shreiner, J. Y. Kao and V. B. Young, The gut microbiome in health and in disease, Curr. Opin. Gastroenterol., 2015, 31, 69–75 CrossRef CAS PubMed.
- H. Chang, S. Myung, S. Yang, H. Jung, T. Kim, I. J. Yoon, O. R. Kwon, W. Hong, J. Kim and Y. I. Min, Effect of electrical stimulation in constipated patients with impaired rectal sensation, Int. J. Colorectal Dis., 2003, 18, 433–438 CrossRef PubMed.
- K. W. Heaton, J. Radvan, H. Cripps, R. A. Mountford, F. E. Braddon and A. O. Hughes, Defecation frequency and timing, and stool form in the general population: a prospective study, Gut, 1992, 33, 818–824 CrossRef CAS PubMed.
- J. E. Ware Jr. and C. D. Sherbourne, The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection, Med. Care, 1992, 30, 473–483 CrossRef CAS PubMed.
- P. Marquis, C. De La Loge, D. Dubois, A. McDermott and O. Chassany, Development and validation of the patient assessment of constipation quality of life questionnaire, Scand. J. Gastroenterol., 2005, 40, 540–551 CrossRef PubMed.
- M. Bouchoucha, G. Devroede, P. Arhan, B. Strom, J. Weber, P. H. Cugnenc, P. Denis and J. P. Barbier, What is the meaning of colorectal transit time measurement?, Dis. Colon Rectum, 1992, 35, 773–782 CrossRef CAS PubMed.
- Y. S. Jeon, J. Chun and B. S. Kim, Identification of household bacterial community and analysis of species shared with human microbiome, Curr. Microbiol., 2013, 67, 557–563 CrossRef CAS PubMed.
- M. Amenta, M. T. Cascio, P. Di Fiore and I. Venturini, Diet and chronic constipation. Benefits of oral supplementation with symbiotic zir fos (Bifidobacterium longum W11 + FOS Actilight), Acta Biomed., 2006, 77, 157–162 Search PubMed.
- J. Tack, S. Müller-Lissner, P. Bytzer, R. Corinaldesi, L. Chang, A. Viegas, S. Schnekenbuehl, C. Dunger-Baldauf and P. Rueegg, A randomised controlled trial assessing the efficacy and safety of repeated Tegaserod therapy in women with irritable bowel syndrome with constipation, Gut, 2005, 54, 1707–1713 CrossRef CAS PubMed.
- W. G. Thompson, G. F. Longstreth, D. A. Drossman, K. W. Heaton, E. J. Irvine and S. A. Müller-Lissner, Functional bowel disorders and functional abdominal pain, Gut, 1999, 45(Suppl. 2), II43–II47 Search PubMed.
- M. Haarman and J. Knol, Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula, Appl. Environ. Microbiol., 2005, 71, 2318–2324 CrossRef CAS PubMed.
- M. Ito, Y. Deguchi, A. Miyamori, K. Matsumoto, H. Kikuchi, K. Matsumoto, Y. Kobayashi, T. Yajima and T. Kan, Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation, Microb. Ecol. Health Dis., 1990, 3, 285–292 CrossRef.
- M. Ito, Y. Deguchi, K. Matsumoto, M. Kimura, N. Onodera and T. Yajima, Influence of galactooligosaccharides on the human fecal microflora, J. Nutr. Sci. Vitaminol., 1993, 39, 635–640 CrossRef CAS PubMed.
- E. Malinen, J. Mättö, M. Salmitie, M. Alander, M. Saarela and A. Palva, PCR-ELISA II: Analysis of Bifidobacterium populations in human faecal samples from a consumption trial with Bifidobacterium lactis Bb-12 and a galacto-oligosaccharide preparation, Syst. Appl. Microbiol., 2002, 25, 249–258 CAS.
- G. Moro, I. Minoli, M. Mosca, S. Fanaro, J. Jelinek, B. Stahl and G. Boehm, Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants, J. Pediatr. Gastroenterol. Nutr., 2002, 34, 291–295 CAS.
- J. Napoli, J. Brand-Miller and P. Conway, Bifidogenic effects of feeding infant formula containing galacto-oligosaccharides in healthy formula-fed infants, Asia Pac. J. Clin. Nutr., 2003, 12, 48 Search PubMed.
- M. M. Rinne, M. Gueimonde, M. Kalliomäki, U. Hoppu, S. J. Salminen and E. Isolauri, Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota, FEMS Immunol. Med. Microbiol., 2005, 43, 59–65 CrossRef CAS PubMed.
- J. Belsey, S. Greenfield, D. Candy and M. Geraint, Systematic review: impact of constipation on quality of life in adults and children, Aliment. Pharmacol. Ther., 2010, 31, 938–949 CrossRef CAS PubMed.
- M. C. Ruiz-López and E. Coss-Adame, Quality of life in patients with different constipation subtypes based on the Rome III criteria, Rev. Gastroenterol. Méx., 2015, 80, 13–20 Search PubMed.
- A. Wald, C. Scarpignato, M. A. Kamm, S. Mueller-Lissner, I. Helfrich, C. Schuijt, J. Bubeck, C. Limoni and O. Petrini, The burden of constipation on quality of life: results of a multinational survey, Aliment. Pharmacol. Ther., 2007, 26, 227–236 CrossRef CAS PubMed.
- H. J. Mason, E. Serrano-Ikkos and M. A. Kamm, Psychological state and quality of life in patients having behavioral treatment (biofeedback) for intractable constipation, Am. J. Gastroenterol., 2002, 97, 3154–3159 CrossRef PubMed.
- A. Glia and G. Lindberg, Quality of life in patients with different types of functional constipation, Scand. J. Gastroenterol., 1997, 32, 1083–1089 CrossRef CAS PubMed.
- H. K. Kwon, H. J. Do, H. J. Kim, S. W. Oh, Y. L. Lym, J. K. Choi, H. K. Joh, H. J. Kweon and D. Y. Cho, The impact of functional constipation on the quality of life in the elderly over 60 years, Korean J. Fam. Med., 2010, 31, 35–43 CrossRef.
- A. Agrawal, L. A. Houghton, J. Morris, B. Reilly, D. Guyonnet, N. Goupil Feuillerat, A. Schlumberger, S. Jakob and P. J. Whorwell, Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation, Aliment. Pharmacol. Ther., 2009, 29, 104–114 CrossRef CAS PubMed.
- A. Chmielewska and H. Szajewska, Systematic review of randomised controlled trials: probiotics for functional constipation, World J. Gastroenterol., 2010, 16, 69–75 Search PubMed.
- J. R. Marchesi, D. H. Adams, F. Fava, G. D. Hermes, G. M. Hirschfield, G. Hold, M. N. Quraishi, J. Kinross, H. Smidt, K. M. Tuohy, L. V. Thomas, E. G. Zoetendal and A. Hart, The gut microbiota and host health: a new clinical frontier, Gut, 2016, 65, 330–339 CrossRef PubMed.
- H. Cao, X. Liu, Y. An, G. Zhou, Y. Liu, M. Xu, W. Dong, S. Wang, F. Yan, K. Jiang and B. Wang, Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine, Sci. Rep., 2017, 7, 10322 CrossRef PubMed.
- Y. Zhao and Y. B. Yu, Intestinal microbiota and chronic constipation, SpringerPlus, 2016, 5, 1130 CrossRef PubMed.
- I. B. Jeffery, P. W. O'Toole, L. Öhman, M. Claesson, J. J. Deane, E. M. Quigley, M. Simrén and M. An, irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota, Gut, 2012, 61, 997–1006 CrossRef PubMed.
- H. Li, J. Chen, X. Ren, C. Yang, S. Liu, X. Bai, S. Shan and X. Dong, Gut microbiota composition changes in constipated women of reproductive age, Front. Cell. Infect. Microbiol., 2020, 10, 557515 CrossRef PubMed.
- L. Diop, S. Guillou and H. Durand, Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial, Nutr. Res., 2008, 28, 1–5 CrossRef CAS PubMed.
- M. R. D'Aimmo, M. Modesto and B. Biavati, Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products, Int. J. Food Microbiol., 2007, 115, 35–42 CrossRef PubMed.
- B. Biavati, M. Vescovo, S. Torriani and V. Bottazzi, Bifidobacteria: history, ecology, physiology and applications, Ann. Microbiol., 2000, 50, 117–132 Search PubMed.
- V. Grimm, C. Westermann and C. U. Riedel, Bifidobacteria-host interactions—an update on colonisation factors, BioMed Res. Int., 2014, 960826 Search PubMed.
- C. I. Rodriguez and J. B. H. Martiny, Evolutionary relationships among bifidobacteria and their hosts and environments, BMC Genomics, 2020, 21, 26 CrossRef CAS PubMed.
- C. Chassard, M. Dapoigny, K. P. Scott, L. Crouzet, C. Del'Homme, P. Marquet, J. C. Martin, G. Pickering, D. Ardid, A. Eschalier, C. Dubray, H. J. Flint and A. Bernalier-Donadille, Functional dysbiosis within the gut microbiota of patients with constipated–irritable bowel syndrome, Aliment. Pharmacol. Ther., 2012, 35, 828–838 CrossRef CAS PubMed.
- I. L. Khalif, E. M. Quigley, E. A. Konovitch and I. D. Maximova, Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation, Dig. Liver Dis., 2005, 37, 838–849 CrossRef CAS PubMed.
- Y. Bouhnik, L. Raskine, G. Simoneau, E. Vicaut, C. Neut, B. Flourié, F. Brouns and F. R. Bornet, The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose–response relation study, Am. J. Clin. Nutr., 2004, 80, 1658–1664 CrossRef CAS PubMed.
- R. Takayama, A. Watanbe, H. Yamamoto, K. Odaka, K. Okabe, N. Sakurai and Y. Aoki, Effect of a soft drink containing galacto-oligosaccharides on defecation frequency, fecal properties, and fecal microflora in healthy young women, J. Jpn. Assoc. Dietary Fiber Res., 2005, 9, 22–33 Search PubMed.
- S. Tamai, Y. Nakamura, O. Ozawa and K. Yamauchi, Effects of galactooligosaccharides intake on human fecal flora and metabolites, J. Appl. Glycosci., 1994, 41, 333–338 CAS.
- S. Tamai, K. Ohtsuka, O. Ozawa and T. Uchida, Effect of a small amount of galactooligosaccharide on fecal Bifidobacterium, J. Jpn. Soc. Nutr. Food Sci., 1992, 45, 456–460 CrossRef CAS.
- K. Umeda, A. Ikeda, R. Uchida, I. Sasahara, T. Mine, H. Murakami and K. Kameyama, Combination of poly-γ-glutamic acid and galactooligosaccharide improves intestinal microbiota, defecation status, and relaxed mood in humans: a randomized, double-blind, parallel-group comparison trial, Biosci. Microbiota, Food Health, 2023, 42, 34–48 CrossRef CAS PubMed.
- G. Parthasarathy, J. Chen, X. Chen, N. Chia, H. M. O'Connor, P. G. Wolf, H. R. Gaskins and A. E. Bharucha, Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation, Gastroenterology, 2016, 150, 367–379 CrossRef PubMed.
- T. Kobayashi, N. Yasutake, K. Uchida, W. Ohyama, K. Kaneko and M. Onoue, Safety of a novel galacto-oligosaccharide: genotoxicity and repeated oral dose studies, Hum. Exp. Toxicol., 2009, 28, 619–630 CrossRef CAS PubMed.
- D. A. Savaiano, A. J. Ritter, T. R. Klaenhammer, G. M. James, A. T. Longcore, J. R. Chandler, W. A. Walker and H. L. Foyt, Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial, Nutr. J., 2013, 12, 160 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo00999a |
| This journal is © The Royal Society of Chemistry 2024 |