Open Access Article
Open Access ArticlePediococcus pentosaceus KF159 alleviates house dust mite-induced atopic dermatitis by promoting IL10 production and regulatory T cell induction
Ji-Eun
Eom
 a,
Dong-Uk
Shin
ab,
Gun-Dong
Kim
a,
Jung-Hoon
Yoon
a,
Dong-Uk
Shin
ab,
Gun-Dong
Kim
a,
Jung-Hoon
Yoon
 c,
Hee Soon
Shin
*ab and
So-Young
Lee
*ab
c,
Hee Soon
Shin
*ab and
So-Young
Lee
*ab
aFood Functionality Research Division, Korea Food Research Institute (KFRI), Wanju 55365, Republic of Korea. E-mail: hsshin@kfri.re.kr; sylee09@kfri.re.kr
bDepartment of Food Biotechnology, Korea University of Science and Technology (UST), Daejeon 34113, Republic of Korea
cDepartment of Food Science and Biotechnology, Sungkyunkwan University, Suwon 16419, Republic of Korea
First published on 10th June 2024
Abstract
Atopic dermatitis (AD) is a chronic immune disease that requires long-term management owing to its relative ease of recurrence. However, steroid treatment is limited owing to the side effects. Therefore, research on therapeutics with proven safety is required. Here, we evaluated the anti-allergic activity of the probiotic strain Pediococcus pentosaceus KF159 (PPKF159) with an ex vivo mouse model sensitized with ovalbumin (OVA) and a mouse model of AD induced by house dust mites. Changes in pathological symptoms were confirmed based on the clinical status of the AD-induced lesion site and the levels of T helper type 2 (Th2)-derived cytokines and immunoglobulin E (IgE). In addition, cell-mediated responses and related mechanisms were elucidated using various kinds of primary cells including splenocytes, mesenteric lymph nodes, Peyer's patch, and bone marrow-derived dendritic cells (BMDCs) in vitro and ex vivo. Oral administration of PPKF159 alleviated AD-like clinical symptoms such as erythema, edema, hemorrhage, and increased tissue thickness, and suppressed the production of Th2-associated cytokines and serum IgE while increasing T helper type 1 (Th1)-mediated cytokine production. PPKF159 induced tolerogenic dendritic cells (tol-DCs) by increasing the expression of ICOS-L, PD-L1, and IDO which were closely related to Treg induction in PPKF159-treated BMDCs. In addition, BMDCs and naive T cells co-cultured in the presence of PPKF159 had elevated IL10 production and increased proportions of CD4+CD25+Foxp3+ Tregs compared to the absence of PPKF159. This study showed that PPKF159 relieved AD-like clinical symptoms, modulated the Th1/Th2 immune balance, and inhibited IgE production in a mouse AD model. PPKF159 induced the transformation of dendritic cells into tolerogenic versions. These induced tol-DCs directly enhanced the production of IL10 or improved the secretion of IL10 through the induction of CD4+CD25+Foxp3+ Treg cells, thereby improving AD. These results suggest that PPKF159 can be applied as a functional food material for the treatment and prevention of AD.
Introduction
Atopic dermatitis (AD) is a chronic and recurrently occurring inflammatory skin disorder characterized by itching and eczematous skin lesions.1 It mostly occurs in areas that fold, such as the wrists, neck, face, elbows, and knees.2 Major symptoms of AD include dry and scaly skin, and excessive pruritus caused by dysfunction of the epidermal barrier.3 Most AD-related genes are highly inheritable; patients with a family history are at a higher risk of developing the disease.4 The pathophysiology of AD is associated with various other factors including skin barrier dysfunction, immune dysregulation, microbial colonization, and environmental triggers such as house dust mites.5 The epidermis serves as a physical and functional barrier that protects the body. However, AD causes the skin to become vulnerable to antigen penetration owing to defects in its barrier.6Processed antigens are presented to naive T cells which are differentiated and activated into the T helper (Th) type 2 cell phenotype.7 Th2 immune responses associate with elevated levels of cytokines such as interleukin (IL) 4, IL5, and IL13 and play an important role in AD development.8 The Th2-based immune response induces an increase in the level of immunoglobulin E (IgE) and infiltration of the major AD-effector cells, such as mast cells and eosinophils.9 IgE binds to mast cells and allergen-bound IgE causes degranulation of mast cells, releasing components such as histamine, leukotrienes and prostaglandins, upon exposure to the allergen again, which is responsible for causing allergic symptoms.9 In contrast, Tregs play a central role in maintaining immune homeostasis and inhibiting the Th2-mediated immune response to allergens.10 This improves the conditions associated with allergic diseases by an immune process that prevents inappropriate immune responses.11,12 Forkhead box P3 (Foxp3) is a transcription factor of Tregs that plays a therapeutic role in the pathogenesis of AD by contributing to the production of suppressive cytokines and the regulation of DC maturation and function and modifying the immune imbalance of excessively produced Th2.13 Thus, the induction of immune tolerance through Treg enhancement may be an important target to improve the conditions associated with allergic diseases (such as AD) caused by an enhanced Th2-mediated immune response.
Probiotics improve disease conditions by promoting beneficial functions of the intestinal microbiota and restoring its composition.14 They can be used to treat and prevent various physiological conditions and diseases including lactose intolerance, antibiotic side effects, immune system imbalance, allergies, inflammatory bowel disease, diarrhea, cystic fibrosis, and different types of cancer.15,16 In particular, it has excellent control effects against allergic diseases including allergic rhinitis, allergic asthma, food allergy, and AD.17–19 Probiotics control immune responses via various mechanisms. They can regulate maturation through contact with DCs and influence their interaction with other immune cells to polarize antigen-specific T cell responses to Th1, Th2, Th17, or regulatory T (Treg) cells.20 Additionally, probiotics contribute to the regulation of allergic hypersensitivity by modulating the Th1/Th2 immune balance and increasing Treg-mediated immune responses that suppress the Th2-mediated response.21,22Pediococcus is a probiotic that is used as the main starter microorganism for fermenting vegetables such as kimchi, meat, and dairy.23 The Pediococcus genus includes Gram-positive, catalase-negative, facultatively aerobic, and homofermentative cocci. Pediococcus pentosaceus24 is stable against acid and bile resistance and does not produce harmful metabolites (urease, indole, and phenylpyruvic acid) and enzymes involved in cancer formation (β-glucosidase and β-glucuronidase).25 In addition, P. pentosaceus shows immune-enhancing activity in an immunosuppressive model,26 a reduction in the level of pro-inflammatory cytokines and intestinal permeability in dextran sulfate sodium -induced colitis,27 and an inhibitory effect on allergens, whether it is alive or dead.28
In our preliminary in vitro studies, we found that P. pentosaceus KF159 (PPKF159) isolated from Kimchi, Korean fermented food, highly induce the production of IL10, immunosuppressive and anti-inflammatory cytokine. Therefore, in this study we investigated a probiotic PPKF159 for its immunosuppressive and anti-inflammatory effect to improve the conditions of AD. In particular, we evaluated the immune-modulating effect of PPKF159 on AD, the alleviation of AD-related symptoms including erythema, edema, hemorrhage, and thickening of skin tissue, regulation of the Th1/Th2 balance, induction of IL10 and Tregs, and the inhibitory ability of IgE in a house dust mite(HDM)-induced AD mouse model. The anti-allergic effect of P. pentosaceus strains on allergic diseases is already known, however, most of them are limited to studies on food allergy or contact dermatitis, and studies on AD are rare. Accordingly, the possibility of PPKF159 as a functional food material that can help prevent and improve AD was confirmed.
Materials and methods
Preparation of Pediococcus pentosaceus KF159 (PPKF159)
Probiotic strain PPKF159 was isolated from kimchi, a traditional Korean fermented food and was identified as Pediococcus pentosaceus by 16S rRNA gene sequence analysis. PPKF159 was deposited at the Korean Culture Center of Microorganisms (KCCM) under deposit number KCCM11674P. A purified colony was obtained using Lactobacilli MRS agar (BD Difco Co., USA) and pre-cultivated twice at 37 °C for 24 h. The resulting colonies were aerobically incubated in Lactobacilli MRS broth (BD Difco Co., USA) at 37 °C for 24 h. Cultured cells were collected by centrifugation at 4000g at 4 °C for 15 min and washed with sterile phosphate-buffered saline (PBS). Pelleted cells were frozen at −80 °C and desiccated in a freeze-dryer (FDCF-12003, Operon, Korea). Lyophilized PPKF159 was stored at −80 °C for further study.Animal studies
Animal experiments were conducted in accordance with the Guidelines for Care and Use of Laboratory Animals of the Korea Food Research Institutional Animal Care and Use Committee (KFRI IACUC), and animal experiments were approved by the KFRI IACUC (approval numbers: KFRI-M-18032 and KFRI-M-19002). Five-week-old female BALB/c mice (18–20 g) were purchased from Orient Bio Inc. (Gyeonggi, Korea) and acclimatized for one week by dividing them into a group of five mice per cage at 23 ± 2 °C, relative humidity of 50 ± 5%, 12 h light–dark cycle, and free access to food (2918C, Envigo, UK) and water. Mice were divided into the following groups: naïve group, HDM-induced AD group, PPKF159 2 × 108-treated group, PPKF159 2 × 109-treated group, and a group treated with 2.5 mg kg−1 dexamethasone. Changes in body weight were measured once per week. Daily oral administration of PPKF159 at 2 × 108 CFU per head per day, 2 × 109 CFU per head per day, or PBS was performed for each group three weeks before the induction of AD until the induction was complete. Atopic dermatitis-like skin lesions in the ears of BALB/c mice were induced using a previously established method.29 The induction of experimental AD involved stripping the surfaces of both ears thrice using surgical tape (Sinsin Pharm, Korea). Twenty microliters of 1% (w/v) dinitrochlorobenzene (DNCB, Sigma Aldrich, USA) dissolved in an acetone/olive oil solution (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) olive oil = 1
olive oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was subsequently applied to the surface of each ear. Two days later, the ear tape stripping procedure was repeated, and 20 μL of 10 mg mL−1 HDM extract (Dermatophagoides farinae, GREER source materials, USA) was applied. The tape stripping and DNCB/HDM extract application procedures were repeated once a week for eight weeks. The experimental schedule for establishing the HDM-induced AD mouse model is shown in Fig. 2A.
3) was subsequently applied to the surface of each ear. Two days later, the ear tape stripping procedure was repeated, and 20 μL of 10 mg mL−1 HDM extract (Dermatophagoides farinae, GREER source materials, USA) was applied. The tape stripping and DNCB/HDM extract application procedures were repeated once a week for eight weeks. The experimental schedule for establishing the HDM-induced AD mouse model is shown in Fig. 2A.
Effects of PPKF159 on Th2-biased immune response ex vivo
Five-week-old female BALB/c mice (18–20 g) were sensitized twice with a mixture of ovalbumin (OVA; Sigma-Aldrich, USA) and Imject alum adjuvant (Thermo Scientific, USA) by intraperitoneal injection at 1-week intervals after acclimatization. The spleen (SPL) and mesenteric lymph nodes (mLNs) were collected from OVA-sensitized Th2-biased mice 1 week after sensitization. Isolation and culture were performed using RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 50 μM β-mercaptoethanol. Isolated cells (1 × 107 cells per mL) were obtained from the flat bottom of a sterilized syringe piston. Single SPL cells were collected and treated with red blood cell lysis buffer (Sigma-Aldrich, USA). The cell suspension was centrifuged (1500 rpm for 5 min at 4 °C) and washed twice with RPMI medium. PPKF159 was prepared as live or heat-treated cells by measuring the number of viable cells and co-culture at 2 × 106 cells or 2 × 107 cells with SPL (or mLN) single cells (2 × 106 cells per well) in a 48-well culture plate. Th2-stimulation of cells was performed by the addition of OVA (100 μg mL−1) and incubated at 37 °C for 72 h in a humidified 5% CO2 incubator. Culture supernatants were used for enzyme-linked immunosorbent assays (ELISAs).Measurement of the severity of AD-like skin lesions
Ear thickness and clinical scores were measured once a week after 24 h of mite extract application. Ear thickness was measured using a spline micrometer (Mitutoyo Corp., Japan). The clinical symptoms of each mouse were scored as 0 (no symptoms), 1 (mild), 2 (moderate), or 3 (severe), depending on the degree of erythema, edema, and bleeding on the ear surface.30 Scoring was assessed by three independent observers, and the average score for each group was taken as the final score. The excised ears from each group were fixed in 10% (v/v) paraformaldehyde (JUNSEI, Japan) and embedded in paraffin. Samples were stained with hematoxylin and eosin (H&E) at the Kyungpook National University Hospital (Daegu, Korea). The examination of epidermal thickening and histological analysis were performed using a clinical microscope (Olympus BX40; Olympus, JAPAN).Immunoglobulin and cytokine levels
Mesenteric lymph nodes or SPLs were obtained from AD mice eight weeks after induction. Isolated SPL and mLN were prepared by single-celling using the same procedure as that used for the ex vivo experiments. Single cells (5 × 106 cells per well) were cultured with mites (100 μL mL−1) in 24-well culture plates and incubated at 37 °C for 72 h in a humidified 5% CO2 incubator. Culture supernatants were used for ELISA. In addition, the immunological response of AD mice was confirmed by measuring IgE and cytokine levels in serum and ear tissues. Ear tissue was homogenized in ice-cold saline (PBS) containing 0.5% (v/v) Tween 20, and the homogenate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min at 4 °C. The total protein level in the homogenate was quantified using a Bio-Rad protein assay kit, based on Bradford protein assay (Bio-Rad, USA) and subsequently used for ELISA. The levels of IL4, IL5, IL6, IFNγ, IL10, IgE (BD Biosciences, USA), IL13, and TSLP (R&D systems, USA) were measured using an ELISA kit according to the manufacturer's instructions.
000 rpm for 20 min at 4 °C. The total protein level in the homogenate was quantified using a Bio-Rad protein assay kit, based on Bradford protein assay (Bio-Rad, USA) and subsequently used for ELISA. The levels of IL4, IL5, IL6, IFNγ, IL10, IgE (BD Biosciences, USA), IL13, and TSLP (R&D systems, USA) were measured using an ELISA kit according to the manufacturer's instructions.
Isolation and culturing of murine bone marrow-derived dendritic cells (BMDCs)
Bone marrow (BM) cells were isolated from the femurs and tibias of 7-week-old female BALB/c mice. The bones were rinsed with PBS and cut using scissors. The needle of a 1 mL syringe was inserted into the bone, and 1 mL of RPMI-1640 medium was injected into it. A total of 2 × 106 BM cells were seeded into Petri dishes containing 10 mL of complete RPMI-1640 supplemented with 10% (v/v) FBS, 100 U ml−1 penicillin, 100 μg mL−1 streptomycin, and 50 μM β-mercaptoethanol in the presence of 20 ng ml−1 murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Sigma, USA). Cells were incubated for 8 d at 37 °C, and 10 mL of fresh medium was added on day 3. On day 6, half of the culture supernatant was discarded and 10 mL of fresh medium containing 10 ng mL−1 murine GM-CSF and recombinant mouse IL4 (BD Biosciences, USA) was added. On day 8, immature DCs were harvested, and CD11C+ differentiation was confirmed using flow cytometry and taken for subsequent experiments. The harvested DCs were seeded in a 12-well plate at a density of 2 × 106 cells per well. The cells were either unstimulated or stimulated with PPKF159 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 cells to bacteria ratio) or lipopolysaccharide (LPS) (200 ng mL−1) for 24 h at 37 °C. The culture supernatant was collected for cytokine determination using ELISA. Stimulated DCs were harvested and analyzed by real-time quantitative PCR (RT-qPCR).
5 cells to bacteria ratio) or lipopolysaccharide (LPS) (200 ng mL−1) for 24 h at 37 °C. The culture supernatant was collected for cytokine determination using ELISA. Stimulated DCs were harvested and analyzed by real-time quantitative PCR (RT-qPCR).
BMDC/CD4+CD62L+ T cell co-cultures
CD4+CD62L+ T cells were isolated from the spleens (SPL) of wild-type mice using the EasySepTM mouse CD4+CD62L+ T cell isolation kit (StemCell, Canada). Isolated CD4+ T cells (2 × 106 cells per well) were co-cultured with PPKF159-treated BMDCs (2 × 105 cells per well) in the presence of soluble anti-CD28 in an anti-CD3 coated 6-well plate for four days at 37 °C. Cell culture was performed using RPMI-1640 medium supplemented with 10% (v/v) FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 50 μM β-mercaptoethanol, and 1% (v/v) GlutaMAX (Gibco, USA). The expression level of CD4+CD25+Foxp3+ T cells was investigated using flow cytometry, and IL10 was measured in the supernatant using ELISA.RNA extraction and real-time quantitative PCR
Total RNA from in vitro-cultured BMDCs was extracted using an RNeasy mini kit (QIAZEN, Germany). Quantitative real-time PCR was performed with Rotor-Gene Q (QIAZEN, Germany) using the Rotor-Gene SYBR Green PCR kit (QIAZEN, Germany). The primers for IL10 (forward: 5′-GCTCTTACTGACTGGCATGAG-3′, reverse: 5′-CGCAGCTCTAGGAGCATGTG-3′), ICOSL (forward: 5′-GACTGAAGTCGGTGCAATGGT-3′, reverse: 5′-TGGGTTTTCGATTTGCCAATAGA-3′), PD-L1(CD274) (forward: 5′-TGCGGACTACAAGCGAATCACG-3′, reverse: 5′-CTCAGCTTCTGGATAACCCTCG-3′), IDO (forward: 5′-AGGCTGGCAAAGAATCTCCT-3′, reverse: 5′-AATGACAAACTCACGGACTGG-3′), CD80 (forward: 5′-ACCCCCAACATAACTGAGTCT-3′, reverse: 5′-TTCCAACCAAGAGAAGCGAGG-3′), and GAPDH (forward: 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′) were synthesized by Cosmogenetech co, Ltd (Seoul, Korea). The data were normalized to the housekeeping gene, GAPDH. The data was calculated as the difference from the value of untreated DCs (control) in BMDCs, which was set as 1.Flow cytometry analysis
In vitro examination was conducted on naive CD4+ T cells isolated from the spleens of wild-type mice and cultured with stimulated BMDCs for four days at 37 °C. The Fc receptors were blocked for 15 min at 4 °C, and the cell surface proteins were stained with anti-mouse CD4-PE and anti-mouse CD25-FITC (Biolegend, USA) for 30 min at room temperature (RT) in the dark using flow cytometry staining buffer (Invitrogen, USA). To test the increase in the levels of Tregs, CD4+ T cells were fixed using a forkhead box protein 3 (Foxp3) fix/perm buffer set (BioLegend, USA) and stained with an anti-mouse/rat Foxp3-APC (eBioscience, USA). The samples were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, USA). The data was analyzed using CytExpert software 2.4 (Beckman Coulter, USA).Statistical analysis
All data were analyzed using GraphPad Prism 10.1 software (San Diego, CA, USA) and statistically evaluated using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test to compare multiple groups. The values for in vitro and in vivo data are expressed as the mean ± standard error (SE). A statistical p-value < 0.05 was considered to be statistically significant (* < 0.05, ** < 0.01, *** < 0.001).Results
Immunomodulatory effects of PPKF159
PPKF159 were cultured with isolated cells from the spleen (SPL) and mesenteric lymph node (mLN) of OVA-sensitized mice to confirm the immunomodulatory effect of PPKF159 on the Th2-predominant immune response. Th2-cytokine levels in the cultured supernatants were measured using ELISA. Th2-associated cytokines were not secreted without OVA stimulation. However, Th2 cytokine secretion significantly increased following OVA stimulation. In contrast, IL4, IL5, and IL13 significantly decreased in the supernatant from SPL (and mLN) that was co-cultured with PPKF159 in live and dead cells (Fig. 1). The anti-allergy effect of PPKF159 was confirmed to result in an overall decrease in Th2-related cytokines in a concentration-dependent manner, regardless of whether PPKF159 was alive or dead. Among them, the production of IL5 in the culture supernatant of mLN did not significantly decrease with heat-treated cells, whereas it significantly decreased in both culture supernatant of SPL and mLN when treated with live PPKF159. As a result, live PPKF159 showed a significant reduction in all three types of Th2-related cytokines in culture supernatants of SPL and mLN.Symptomatic relief in dust mite-induced AD mice treated with PPKF159
The severity of erythema, edema, and bleeding on the ear surface worsened, and the epidermis thickened as the induction progressed in mice treated with HDM alone (sham group) (Fig. 2B and C). In contrast, the lymphocyte infiltration into the ear epidermis tissue was reduced in the PPKF159-administered group and the thickness of the epidermis was significantly lower than that of the sham group as a result of pathological observation of ear epidermal tissue through H&E staining and microscopic analysis, (Fig. 2D). In addition, oral administration of PPKF159 resulted in significantly reduced skin thickness and clinical scores (erythema, edema, and hemorrhage) at the 8th week of disease induction and the degree of improvement was similar in both groups (2 × 108 and 2 × 109 CFU per head per day) (Fig. 2E and F).Effect of PPKF159 on the production of IgE in serum
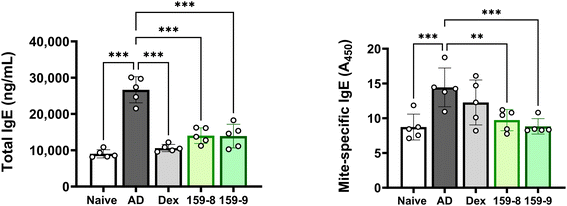
The release of total IgE and mite-specific IgE was measured using ELISA to investigate the effect of orally administered PPKF159 on serum IgE levels in an AD model. Total IgE and mite-specific IgE levels were dramatically elevated in the sham group compared with no treatment group as determined by ELISA. In contrast, a significant reduction in total IgE and mite-specific IgE levels was observed in the serum of orally administered PPKF159 groups (2 × 108 and 2 × 109 CFU per head per day) (Fig. 3).Effect of PPKF159 on cytokine production in the ear skin of AD mice
To determine whether PPKF159 can improve AD skin, ear tissues, where the disease was mainly induced, were homogenized and cytokine levels were measured using ELISA. The ability to regulate Th1/Th2 balance was measured by Th2 cell-derived IL4 and IL5 production, Th1 cell-derived IFNγ production, and the IFNγ/IL4 ratio. IL4 and IL5 levels significantly increased in the ear tissue of the AD group compared to the naive group (Fig. 4). In contrast, IL4 and IL5 levels were dramatically inhibited in ear tissue from the PPKF159-treatment group in a dose-dependent manner (Fig. 4A and D). In addition, the levels of IFNγ (a typical Th1 cytokine) were greatly enhanced compared to those in the AD group; therefore, the IFNγ/IL4 ratio increased (Fig. 4B and C). In addition, IL6 (which is known to be involved in the increase of Th2 cytokines) and thymic stromal lymphopoietin (TSLP) which are reported to aggravate the disease by increasing the Th2 immune response31 were measured. The levels of IL6 and TSLP significantly increased in the ear tissue of the sham group but significantly decreased in the PPKF159-treated group (Fig. 4E and F).Effect of PPKF159 on anti-inflammatory cytokine IL10 production
IL10 downregulates the Th2 response in AD as an anti-inflammatory cytokine that inhibits excessive T-cell responses and prevents chronic inflammation and tissue damage.32,33 IL10 levels increased in the ear tissue, SPL, and mLN after oral administration of PPKF159 (Fig. 5A). This observation was reproduced by in vitro experiments. IL10 levels significantly increased when PPKF159 was co-cultured with SPL, mLN, and Peyer's patch (PP) isolated from normal mice (Fig. 5B).Effect of PPKF159 on the induction of tolerogenic dendritic cell
Tolerogenic dendritic cells (tol-DCs) and Tregs are representative cells that secrete the anti-inflammatory cytokine IL10 and alleviate AD. As a result of co-culturing PPKF159 and BMDCs, PPKF159-treated DCs showed significantly increased levels of IL10 compared to those in DCs that were not treated with PPKF159 (Fig. 6A and B) and also showed elevated expression of tol-DC markers such as inducible T-cell co-stimulator ligand (ICOS-L), programmed cell death ligand (CD274), and indoleamine 2,3-dioxygenase (IDO) (Fig. 6D–F). Additionally, the expression of the co-stimulatory molecule CD80 was suppressed in BMDCs co-cultured with PPKF159 compared to BMDCs co-cultured with LPS (Fig. 6C). Taken together, the results of increased IL10 production, elevated expression of tol-DC-related factors, and decreased expression of costimulatory molecules suggest that PPKF159 induces tol-DC differentiation.Effect of PPKF159 on the induction of CD4+CD25+Foxp3+ regulatory T cells
A population of CD4+CD25+Foxp3+ cells was analyzed to confirm whether tol-DCs induced by PPKF159 were involved in Treg differentiation. The proportion of CD4+CD25+Foxp3+ cells was higher in PPKF159 BMDCs (T cells co-cultured with PPKF159-treated DCs) than that in the CON (T cell culture group alone) or BMDCs (T cells co-cultured with PPKF159-untreated DCs) (Fig. 7A). Moreover, IL10 levels were elevated in the co-culture supernatants of PPKF159 BMDC and T cells (Fig. 7B). These results demonstrate that PPKF159 may alleviate AD via the induction of CD4+CD25+Foxp3+Tregs and IL10.Discussion
A balance between Th1 and Th2 cells is important for immune regulation based on the induction and tolerance of immune diseases.34 Atopic dermatitis is a condition wherein the Th1/Th2 balance is impaired, and Th2 cells are one of the most important cell types associated with AD development among the various immune cells involved in allergic responses.35 In particular, AD is mainly characterized by Th2-mediated cytokine production such as IL4, IL5, and IL13, and enhanced serum IgE levels.36 IL4 induces the production of IgE by activating B cells,37 IL13 is important for Th2 cell differentiation and IgE production, and IL5 is important for the development, recruitment, and proliferation of eosinophils.32,38 Serum levels of IgE and inflammation-related cells (including mast cells, eosinophils, and lymphocytes) are significantly increased in the serum of patients with AD.39 In particular, mast cell activation occurs owing to the binding of IgE antibodies to the receptors on its surface; this results in the release of inflammatory mediators such as histamine, which leads to an allergic reaction.40According to a number of reviews and meta-analyses, probiotics prevent and improve allergic diseases by ameliorating Th2-dominant conditions, thereby restoring appropriate Th1/Th2 balance and reducing serum IgE levels.14,41 Consistent with these reports, in this study, administration of PPKF159 (2 × 108 and 2 × 109 CFU per head per day) effectively attenuated the allergic response in the HDM-induced mouse model. However, no dose-dependence was observed. Several studies have reported on the effective concentration of probiotics, indicating that the effective dose varies depending on the diseases and specific strain. According to review article on dose–response of probiotics in human studies,42 while some diseases including antibiotic associated diarrhea and high blood pressure show positive correlation between probiotics concentration and health benefits, there is no clear dose–response observed in AD. Most experimental studies on probiotics for AD have typically been conducted at a concentration of 107,108,109, or 1010 CFU.41 Studies conducted at wide range of concentrations, representing a difference of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 or more between the lowest and highest concentration, such as L. rhamnosus RHT3201 and CJLP133,43,44 have shown a dose-dependent pattern of efficacy. However, even in the cases of RHT3201 and CJLP133, similar to PPKF159, higher concentrations above 108 or 109 CFU were not accompanied by an increase in activity. Therefore, further research with oral administration at wide range of concentrations is needed to establish a clear dose–response relationship of PPKF159.
10 or more between the lowest and highest concentration, such as L. rhamnosus RHT3201 and CJLP133,43,44 have shown a dose-dependent pattern of efficacy. However, even in the cases of RHT3201 and CJLP133, similar to PPKF159, higher concentrations above 108 or 109 CFU were not accompanied by an increase in activity. Therefore, further research with oral administration at wide range of concentrations is needed to establish a clear dose–response relationship of PPKF159.
Although probiotics refer to live microorganisms that provide health benefits, many studies show that dead organisms can have various biological effects.45 Many studies using live, dead, and metabolites of microorganisms are reported to improve allergic diseases.21,34,46,47 Dead organisms also have immunomodulatory activity, and administration of heat-inactivated L. sakei probio 65 reduces the levels of IL4, tumor necrosisα, and IgE by promoting FOXP3 expression.21 In addition, the ingestion of probiotic metabolites or dead bacteria has the advantage of being relatively safe from some pathology that may occur when patients with severe immunodeficiency ingest live probiotics, and they are relatively easy to standardize and convenient for distribution with a long shelf life.45 A probiotic mixture (L. casei, L. plantarum, L. rhamnosus, and B. lactis) called Duolac ATP suppresses the production of IgE and modulates the Th1/Th2 balance by inducing differentiation of CD4+Foxp3+T cells.34 Live probiotics affect the composition of intestinal microorganisms and activate intestinal movement by peristalsis, competitively bind with pathogenic microorganisms for nutrients and receptor-binding sites, inhibit the growth of pathogens through the secretion of antibacterial peptides, and regulate the host's immune response.14,16 Metabolites such as short-chain fatty acids (SCFAs) produced by commensal and probiotic bacteria, including Lactobacillus spp. and Bifidobacterium spp. modulate the host's immune response.48 In particular, acetate reduces epithelial production of pro-Th2 cytokines such as TSLP through GPR43 signaling in the epithelium.49 TSLP is found in high concentrations in the epidermis of AD patients,50 and it plays an important role in the exacerbation of AD symptoms51 by promoting inflammation and itching, contributing to barrier alteration,52 and promoting the Th2 immune response. Butyrate SCFA promotes CD103+ DCs to produce retinoic acid through GPR109A signaling, thereby increasing Treg differentiation by DC-derived retinoic acid which affects the allergy protection effect.49 We conducted a comparative activity test on live and dead bacteria in vitro and performed an in vivo study on live bacteria that significantly suppressed Th2-related cytokines in both SPL and mLN. PPKF159 restored the Th1/Th2 balance by inhibiting excessively secreted Th2 cytokines such as IL4, IL5, and IL13, thereby enhancing the levels of IFNγ, a typical Th1-mediated cytokine. Additionally, oral administration of PPKF159 significantly reduced IgE levels. PPKF159 also inhibits the secretion of IL6 and TSLP, and this response is considered to contribute to the consequent suppression of the Th2 response. Therefore, this modulated function may have acted as a factor to alleviate AD through the living PPKF159 strain themselves or metabolites such as SCFAs produced by PPKF159 acting in the body.
IL10 is an anti-inflammatory cytokine that downregulates Th2 responses in AD.31 It is involved in limiting excessive T-cell responses and preventing chronic inflammation and tissue damage.52 It is produced by various types of immune cells (T cells, B cells, macrophages, dendritic cells, natural killer (NK) cells, and innate lymphoid cells), and IL10 production by different cell types may be functionally different.53,54 Many studies show that AD is alleviated by increasing the anti-inflammatory cytokine IL10.34,46,47,55–57 IL10 production increases in the AD-induced group compared to the normal group, and IL10 is increased to a greater degree when Lactobacillus paracasei KBL382 is orally administered, resulting in Th2 cytokine (such as IL4, IL5, and IL13), IgE, and TSLP inhibitory activity to alleviate AD.46 Additionally, IL10 increases in the AD model induced by epidermal sensitization with OVA compared to the normal group but IL4, IL13, TSLP, and IgE were suppressed as IL10 increased through oral administration of Ruminococcus gnavus.56 Similarly, our study showed that treatment with PPKF159 increased IL10 production in in vitro and in vivo (Fig. 5 and 6). Oral administration of PPKF159 alleviated pathological symptoms by promoting the secretion of anti-inflammatory cytokine IL10 and inhibiting Th2-derived cytokines, TSLP (Fig. 4) and IgE (Fig. 3). Therefore, PPKF159 contributed to the improvement of AD by promoting IL10, which has anti-inflammatory properties. IL10 is produced by various immune cells, and Tregs are representative cells characterized by IL10 production.58
Tregs play an important role in maintaining immune homeostasis by controlling immune responses10,13 and can be divided into natural Foxp3+ Treg cells (nTreg) and inducible Foxp3+ or Foxp3− Treg cells (iTreg).10,11 Among these, type I regulatory (Tr1) cells, which belong to a subset of Foxp3− iTregs, and Foxp3+ Treg cells are all associated with IL10 production.11,59,60 Both types of Tregs play an important role in maintaining immune tolerance; IL-10 expressed by Tr1 cells plays an important role in Tr1 cell-mediated immune suppression, but Tr1 cells are characterized by their suppressive function without constitutive Foxp3 expression.59 CD4+CD25+ Tregs are essential for the regulation of immune tolerance and inhibit the differentiation of naive CD4 T cells into Th2 cells.10 Probiotics prevent inflammatory diseases by inducing tol-DCs,47,57 Tregs,21,34,46 and Bregs55 to promote IL10 secretion. Microorganisms including Lactobacillus paracasei KBL382,46Ruminococcus gnavus,56 Duolac ATP,34 and Lactobacillus sakei Probio 6521 exert their anti-allergic effects by inducing Foxp3+Treg cells. Oral administration of Weissella cibaria WIKIM28 reduced AD-like skin lesions and IgE and Th2 cytokine production by inducing CD4+CD25+Foxp3+ Treg differentiation along with increased IL10 production.55 In addition, the WIKIM28 strain promotes Treg differentiation through the induction of dendritic cells with tolerant properties.47 IL10 produced by tol-DCs (a dendritic cell with maturation-resistant characteristics) is a prerequisite for the differentiation of Tregs to acquire tolerance.61 Tol-DCs are associated with tolerance rather than immunity and induce Treg development.62 Treg activation induces IL10 production and stimulation of a tol-DC phenotype.63 In addition, IL10 secretion is associated with high ICOSL expression on the surface of Foxp3+ Treg cells, and high expression of CD274 and ICOSL is more effective in inducing Tregs with IL10.64 Furthermore, tol-DCs expressing IDO contribute to the inhibition of T-cell responses, induction of tolerance, and promotion of Treg cell development.65Lactobacillus sakei WIKIM30 treated DCs potentially mediate tol-DCs by increasing IL10 production and the expression of tol-DCs (ICOSL and CD274) in a probiotic study that ameliorates AD.57 In similar, our results confirmed that PPKF159 induces Foxp3+Treg and IL10 production by using BMDCs. We also showed that PPKF159-treated BMDCs increased the expression of tol-DC phenotypes including ICOSL, IDO, and CD274 compared to BMDCs not treated with PPKF159 (Fig. 6). Further studies are needed to confirm the reproducibility of treg expression in in vivo experiments, to study the mechanism of other IL10-producing cells, and to confirm the anti-allergic activity of the metabolites produced by PPKF159 or the components of the cells themselves.
Conclusions
Our results demonstrated that PPKF159 isolated from kimchi increases Foxp3+Treg induction and IL10 production through the induction of tol-DCs, which in turn alleviate AD-like symptoms by inhibiting the Th2 response and regulating the Th1/Th2 balance. These findings suggest that PPKF159 can be used as a dietary supplement to alleviate allergic diseases including AD.Author contributions
Conceptualization: So-Young Lee and Hee Soon Shin; formal analysis: Ji-Eun Eom; funding acquisition: Hee Soon Shin; investigation: Ji-Eun Eom and Dong-Uk Shin; project administration: So-Young Lee; validation: Gun-Dong Kim and Jung-Hoon Yoon; writing-original draft: Ji-Eun Eom and So-Young Lee; review and editing: So-Young Lee and Ji-Eun Eom. All authors have read and approved the final manuscript.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
The article was supported by the Main Research Program (E0210202-04) of the Korean Food Research Institute (KFRI) and funded by the Korean Ministry of Science and ICT. Also, this research was supported by ‘Regional Innovation Mega Project’ program (2023-DD-UP-0031) through the Korea Innovation Foundation funded by Ministry of Science and ICT.References
- T. Bieber, Atopic dermatitis, N. Engl. J. Med., 2008, 358, 1483–1494 CrossRef CAS PubMed.
- D. Rudikoff and M. Lebwohl, Atopic dermatitis, Lancet, 1998, 351, 1715–1721 CrossRef CAS PubMed.
- H. Saeki, M. Furue, F. Furukawa, M. Hide, M. Ohtsuki, I. Katayama, R. Sasaki, H. Suto and K. Takehara, Guidelines for management of atopic dermatitis, J. Dermatol., 2009, 36, 563–577 CrossRef PubMed.
- K. C. Barnes, An update on the genetics of atopic dermatitis: scratching the surface in 2009, J. Allergy Clin. Immunol., 2010, 125, 16–29.e11 CrossRef CAS PubMed.
- D. Y. Leung, M. Boguniewicz, M. D. Howell, I. Nomura and Q. A. Hamid, New insights into atopic dermatitis, J. Clin. Invest., 2004, 113, 651–657 CrossRef CAS PubMed.
- R. Agrawal and J. A. Woodfolk, Skin barrier defects in atopic dermatitis, Curr. Allergy Asthma Rep., 2014, 14, 433–433 CrossRef PubMed.
- R. S. Geha, H. H. Jabara and S. R. Brodeur, The regulation of immunoglobulin E class-switch recombination, Nat. Rev. Immunol., 2003, 3, 721–732 CrossRef CAS PubMed.
- B. Homey, M. Steinhoff, T. Ruzicka and D. Y. Leung, Cytokines and chemokines orchestrate atopic skin inflammation, J. Allergy Clin. Immunol., 2006, 118, 178–189 CrossRef CAS PubMed.
- S. J. Galli and M. Tsai, IgE and mast cells in allergic disease, Nat. Med., 2012, 18, 693–704 CrossRef CAS PubMed.
- K. V. Poojary, Y.-c. M. Kong and M. A. Farrar, Control of Th2-Mediated Inflammation by Regulatory T Cells, Am. J. Pathol., 2010, 177, 525–531 CrossRef CAS PubMed.
- I. S. Choi, Immune tolerance by induced regulatory T cells in asthma, Allergy, Asthma Immunol. Res., 2012, 4, 113–115 CrossRef CAS PubMed.
- M. Noval Rivas and T. A. Chatila, Regulatory T cells in allergic diseases, J. Allergy Clin. Immunol., 2016, 138, 639–652 CrossRef CAS PubMed.
- N. Fyhrquist, S. Lehtimäki, K. Lahl, T. Savinko, A.-M. Lappeteläinen, T. Sparwasser, H. Wolff, A. Lauerma and H. Alenius, Foxp3+ Cells Control Th2 Responses in a Murine Model of Atopic Dermatitis, J. Invest. Dermatol., 2012, 132, 1672–1680 CrossRef CAS PubMed.
- H. Hardy, J. Harris, E. Lyon, J. Beal and A. D. Foey, Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology, Nutrients, 2013, 5, 1869–1912 CrossRef CAS PubMed.
- M. Colombo, N. P. A. Castilho, S. D. Todorov and L. A. Nero, Beneficial properties of lactic acid bacteria naturally present in dairy production, BMC Microbiol., 2018, 18, 219 CrossRef CAS PubMed.
- V. P. Singh, J. Sharma and S. Babu, Rizwanulla and A. Singla, Role of probiotics in health and disease: a review, JPMA, J. Pak. Med. Assoc., 2013, 63, 253–257 Search PubMed.
- M. Jin, S. Lee, Y. A. Choi, H. J. Jang, S. W. Lee, P. H. Park, T. Y. Shin, M. C. Rho, Y. H. Jang and S. H. Kim, Lactococcus lactis KR-050L extract suppresses house dust mite induced-atopic skin inflammation through inhibition of keratinocyte and mast cell activation, J. Appl. Microbiol., 2019, 126, 230–241 CrossRef CAS PubMed.
- J. Lee, J. Bang and H. J. Woo, Effect of orally administered Lactobacillus brevis HY7401 in a food allergy mouse model, J. Microbiol. Biotechnol., 2013, 23, 1636–1640 CrossRef CAS PubMed.
- E. J. Choi, M. Iwasa, K. I. Han, W. J. Kim, Y. Tang, Y. J. Hwang, J. R. Chae, W. C. Han, Y. S. Shin and E. K. Kim, Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model, Nutrients, 2016, 8, 146 CrossRef PubMed.
- S.-Y. Chang, H.-J. Ko and M.-N. Kweon, Mucosal dendritic cells shape mucosal immunity, Exp. Mol. Med., 2014, 46, e84 CrossRef CAS PubMed.
- J. Y. Kim, B. K. Park, H. J. Park, Y. H. Park, B. O. Kim and S. Pyo, Atopic dermatitis-mitigating effects of new Lactobacillus strain, Lactobacillus sakei probio 65 isolated from Kimchi, J. Appl. Microbiol., 2013, 115, 517–526 CrossRef CAS PubMed.
- T. A. Smith-Norowitz and M. H. Bluth, Probiotics and diseases of altered IgE regulation: A short review, J. Immunotoxicol., 2016, 13, 136–140 CrossRef CAS PubMed.
- M. S. Shin, S. K. Han, J. S. Ryu, K. S. Kim and W. K. Lee, Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23–2 isolated from Kimchi, J. Appl. Microbiol., 2008, 105, 331–339 CrossRef CAS PubMed.
- C. M. A. P. Franz, A. Endo, H. Abriouel, C. A. V. Reenen, A. Gálvez and L. M. T. Dicks, in Lactic Acid Bacteria:Biodiversity and Taxonomy, ed. W. H. Holzapfel and B. J. B. Wood, 2014, pp. 359–376 Search PubMed.
- H.-J. Shin, H.-J. Choi, D.-W. Kim, C.-S. Ahn, Y.-G. Lee, Y.-K. Jeong and W. H. Joo, Probiotic Potential of Pediococcus pentosaceus BCNU 9070, J. Life Sci., 2012, 22, 1194–1200 CrossRef.
- H.-K. Kwon, W.-R. Jo and H.-J. Park, Immune-enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC-ON89A) in CY-induced immunosuppressed model, BMC Complementary Altern. Med., 2018, 18, 75 CrossRef PubMed.
- X. Bian, L. Yang, W. Wu, L. Lv, X. Jiang, Q. Wang, J. Wu, Y. Li, J. Ye, D. Fang, D. Shi, K. Wang, Q. Wang, Y. Lu, J. Xie, J. Xia and L. Li, Pediococcus pentosaceus LI05 alleviates DSS-induced colitis by modulating immunological profiles, the gut microbiota and short-chain fatty acid levels in a mouse model, Microb. Biotechnol., 2020, 13, 1228–1244 CrossRef CAS PubMed.
- T. Masuda, M. Kimura, S. Okada and H. Yasui, Pediococcus pentosaceus Sn26 Inhibits IgE Production and the Occurrence of Ovalbumin-Induced Allergic Diarrhea in Mice, Biosci., Biotechnol., Biochem., 2010, 74, 329–335 CrossRef CAS PubMed.
- M. J. Bae, S. Lim, D. S. Lee, K. R. Ko, W. Lee and S. Kim, Water soluble extracts from Actinidia arguta, PG102, attenuates house dust mite-induced murine atopic dermatitis by inhibiting the mTOR pathway with Treg generation, J. Ethnopharmacol., 2016, 193, 96–106 CrossRef PubMed.
- J. D. Ohmen, J. M. Hanifin, B. J. Nickoloff, T. H. Rea, R. Wyzykowski, J. Kim, D. Jullien, T. McHugh, A. S. Nassif and S. C. Chan, et al., Overexpression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions, J. Immunol., 1995, 154, 1956–1963 CrossRef CAS.
- I. H. Heijink, E. Vellenga, P. Borger, D. S. Postma, J. G. de Monchy and H. F. Kauffman, Interleukin-6 promotes the production of interleukin-4 and interleukin-5 by interleukin-2-dependent and -independent mechanisms in freshly isolated human T cells, Immunology, 2002, 107, 316–324 CrossRef CAS PubMed.
- E. B. Brandt and U. Sivaprasad, Th2 Cytokines and Atopic Dermatitis, J. Clin. Cell. Immunol., 2011, 2(3), 110 Search PubMed.
- S. Schülke, Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses, Front. Immunol., 2018, 9, 455 CrossRef PubMed.
- H. W. Kim, R. Hong, E. Y. Choi, K. Yu, N. Kim, J. Y. Hyeon, K. K. Cho, I. S. Choi and C. H. Yun, A Probiotic Mixture Regulates T Cell Balance and Reduces Atopic Dermatitis Symptoms in Mice, Front. Microbiol., 2018, 9, 2414 CrossRef PubMed.
- E. H. Sohn, S. A. Jang, C. H. Lee, K. H. Jang, S. C. Kang, H. J. Park and S. Pyo, Effects of korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice, J. Ginseng Res., 2011, 35, 479–486 CrossRef PubMed.
- H. J. Kim, J. Baek, J. R. Lee, J. Y. Roh and Y. Jung, Optimization of Cytokine Milieu to Reproduce Atopic Dermatitis-related Gene Expression in HaCaT Keratinocyte Cell Line, Immune Network, 2018, 18, e9 CrossRef PubMed.
- G. K. Hershey, M. F. Friedrich, L. A. Esswein, M. L. Thomas and T. A. Chatila, The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor, N. Engl. J. Med., 1997, 337, 1720–1725 CrossRef CAS PubMed.
- D. Simon, L. R. Braathen and H. U. Simon, Eosinophils and atopic dermatitis, Allergy, 2004, 59, 561–570 CrossRef CAS PubMed.
- J. H. Yang, E. Lee, B. Lee, W. K. Cho, J. Y. Ma and K. I. Park, Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma(-)Induced Proinflammatory Chemokine Production In Vitro, Nutrients, 2018, 10(7), 806 CrossRef PubMed.
- K. D. Stone, C. Prussin and D. D. Metcalfe, IgE, mast cells, basophils, and eosinophils, J. Allergy Clin. Immunol., 2010, 125, S73–S80 CrossRef PubMed.
- A. Xie, A. Chen, Y. Chen, Z. Luo, S. Jiang, D. Chen and R. Yu, Lactobacillus for the treatment and prevention of atopic dermatitis: Clinical and experimental evidence, Front. Cell. Infect. Microbiol., 2023, 13, 1137275 CrossRef CAS PubMed.
- A. C. Ouwehand, A review of dose-responses of probiotics in human studies, Benefic. Microbes, 2017, 8, 143–151 CAS.
- S. H. Lee, J. M. Yoon, Y. H. Kim, D. G. Jeong, S. Park and D. J. Kang, Therapeutic effect of tyndallized Lactobacillus rhamnosus IDCC 3201 on atopic dermatitis mediated by down-regulation of immunoglobulin E in NC/Nga mice, Microbiol. Immunol., 2016, 60, 468–476 CrossRef CAS PubMed.
- T. J. Won, B. Kim, Y. Lee, J. S. Bang, E. S. Oh, J. S. Yoo, K. E. Hyung, J. Yoon, S. Hwang, E. S. Park, S. Y. Park and K. W. Hwang, Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice, Cell. Immunol., 2012, 277, 49–57 CrossRef CAS PubMed.
- C. A. Adams, The probiotic paradox: live and dead cells are biological response modifiers, Nutr. Res. Rev., 2010, 23, 37–46 CrossRef CAS PubMed.
- W. K. Kim, Y. J. Jang, D. H. Han, K. Jeon, C. Lee, H. S. Han and G. Ko, Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota, Gut Microbes, 2020, 12, 1–14 CrossRef PubMed.
- S. K. Lim, M. S. Kwon, J. Lee, Y. J. Oh, J. Y. Jang, J. H. Lee, H. W. Park, Y. D. Nam, M. J. Seo, S. W. Roh and H. J. Choi, Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice, Sci. Rep., 2017, 7, 40040 CrossRef CAS PubMed.
- H. J. Park, S. W. Lee and S. Hong, Regulation of Allergic Immune Responses by Microbial Metabolites, Immune Network, 2018, 18, e15 CrossRef PubMed.
- J. Tan, C. McKenzie, P. J. Vuillermin, G. Goverse, C. G. Vinuesa, R. E. Mebius, L. Macia and C. R. Mackay, Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways, Cell Rep., 2016, 15, 2809–2824 CrossRef CAS PubMed.
- V. Soumelis, P. A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, K. Smith, D. Gorman, S. Zurawski, J. Abrams, S. Menon, T. McClanahan, R. de Waal-Malefyt Rd, F. Bazan, R. A. Kastelein and Y. J. Liu, Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP, Nat. Immunol., 2002, 3, 673–680 CrossRef CAS PubMed.
- S. R. Wilson, L. Thé, L. M. Batia, K. Beattie, G. E. Katibah, S. P. McClain, M. Pellegrino, D. M. Estandian and D. M. Bautista, The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch, Cell, 2013, 155, 285–295 CrossRef CAS PubMed.
- W. Peng and N. Novak, Pathogenesis of atopic dermatitis, Clin. Exp. Allergy, 2015, 45, 566–574 CrossRef CAS PubMed.
- M. T. Rasquinha, M. Sur, N. Lasrado and J. Reddy, IL-10 as a Th2 Cytokine: Differences Between Mice and Humans, J. Immunol., 2021, 207, 2205–2215 CrossRef CAS PubMed.
- C. K. Wong, C. Y. Ho, F. W. S. Ko, C. H. S. Chan, A. S. S. Ho, D. S. C. Hui and C. W. K. Lam, Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma, Clin. Exp. Immunol., 2001, 125, 177–183 CrossRef CAS PubMed.
- M.-J. Bae, H.-K. Kim, S. Lim, S.-Y. Lee, H. S. Shin, J.-E. Kim, S.-H. Im and S. Kim, Lactobacillus pentosus KF340 alleviates house dust mite-induced murine atopic dermatitis via the secretion of IL-10-producing splenic B10 cells, J. Funct. Foods, 2016, 26, 258–267 CrossRef CAS.
- J. R. Ahn, S. H. Lee, B. Kim, M. H. Nam, Y. K. Ahn, Y. M. Park, S. M. Jeong, M. J. Park, K. B. Song, S. Y. Lee and S. J. Hong, Ruminococcus gnavus ameliorates atopic dermatitis by enhancing Treg cell and metabolites in BALB/c mice, Pediatr. Allergy Immunol., 2022, 33, e13678 CrossRef CAS PubMed.
- M. S. Kwon, S. K. Lim, J. Y. Jang, J. Lee, H. K. Park, N. Kim, M. Yun, M. Y. Shin, H. E. Jo, Y. J. Oh, S. W. Roh and H. J. Choi, Lactobacillus sakei WIKIM30 Ameliorates Atopic Dermatitis-Like Skin Lesions by Inducing Regulatory T Cells and Altering Gut Microbiota Structure in Mice, Front. Immunol., 2018, 9, 1905 CrossRef PubMed.
- Y. Chen, V. K. Kuchroo, J. Inobe, D. A. Hafler and H. L. Weiner, Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis, Science, 1994, 265, 1237–1240 CrossRef CAS PubMed.
- G. Chao and O. SangKon, in Regulatory T Cells, ed. H. Xuehui, IntechOpen, Rijeka, 2022, ch. 1., DOI:10.5772/intechopen.106852.
- N. Cools, P. Ponsaerts, V. F. Van Tendeloo and Z. N. Berneman, Regulatory T cells and human disease, Clin. Dev. Immunol., 2007, 2007, 89195 Search PubMed.
- A. Wakkach, N. Fournier, V. Brun, J. P. Breittmayer, F. Cottrez and H. Groux, Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo, Immunity, 2003, 18, 605–617 CrossRef CAS PubMed.
- S. Yoo and S.-J. Ha, Generation of Tolerogenic Dendritic Cells and Their Therapeutic Applications, Immune Network, 2016, 16, 52–60 CrossRef PubMed.
- U. Luckey, T. Schmidt, N. Pfender, M. Romer, N. Lorenz, S. F. Martin, T. Bopp, E. Schmitt, A. Nikolaev, N. Yogev, A. Waisman, T. Jakob and K. Steinbrink, Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance, J. Allergy Clin. Immunol., 2012, 130, 781–797 CrossRef CAS PubMed.
- X. Gao, L. Zhao, S. Wang, J. Yang and X. Yang, Enhanced inducible costimulator ligand (ICOS-L) expression on dendritic cells in interleukin-10 deficiency and its impact on T-cell subsets in respiratory tract infection, Mol. Med., 2013, 19, 346–356 Search PubMed.
- A. L. Mellor and D. H. Munn, IDO expression by dendritic cells: tolerance and tryptophan catabolism, Nat. Rev. Immunol., 2004, 4, 762–774 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |