Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The formation of sulfur metabolites during in vitro gastrointestinal digestion of fish, white meat and red meat is affected by the addition of fructo-oligosaccharides†
Núria
Elias Masiques
 a,
Els
Vossen
a,
Jo
De Vrieze
bc,
Stefaan
De Smet
a and
Thomas
Van Hecke
*a
a,
Els
Vossen
a,
Jo
De Vrieze
bc,
Stefaan
De Smet
a and
Thomas
Van Hecke
*a
aLaboratory for Animal Nutrition and Animal Product Quality, Department of Animal Sciences and Aquatic Ecology, Ghent University, Coupure Links 653, B-9000, Ghent, Belgium. E-mail: thomas.vanhecke@ugent.be; Tel: +32 9 264 90 14
bCenter for Microbial Ecology and Technology (CMET), Ghent University, Coupure Links 653, B-9000 Gent, Belgium
cCentre for Advanced Process Technology for Urban Resource Recovery (CAPTURE), P.O. Frieda Saeysstraat 1, B-9000 Gent, Belgium
First published on 27th July 2024
Abstract
The formation of sulfur metabolites during large intestinal fermentation of red meat may affect intestinal health. In this study, four muscle sources with varying heme-Fe content (beef, pork, chicken and salmon), with or without fructo-oligosaccharides (FOS), were exposed to an in vitro gastrointestinal digestion and fermentation model, after which the formation of sulfur metabolites, protein fermentation metabolites, and short (SCFA) and branched (BCFA) chain fatty acids was assessed. When FOS were present during muscle fermentation, levels of SCFA (+54%) and H2S (+36%) increased, whereas levels of CS2 (−37%), ammonia (−60%) and indole (−30%) decreased, and the formation of dimethyl sulfides and phenol was suppressed. Red meat fermentation was not accompanied by higher H2S formation, but beef ferments tended to contain 33 to 49% higher CS2 levels compared to the ferments of other muscle sources. In conclusion, there is a greater effect on sulfur fermentation by the addition of FOS to the meats, than the intrinsic heme-Fe content of meat.
1. Introduction
Meat is an important dietary source of quality protein, rich in the essential sulfur-containing amino acids cysteine and methionine, and essential micronutrients such as vitamins B6 and B12, iron, and zinc.1 In contrast, a high consumption of red and processed meat is associated with an increased epidemiological risk of developing various chronic diseases.2,3 One possible factor contributing to these associations could be heme-Fe, a distinctive component of red meat.4 Heme-Fe is better absorbed than elemental Fe, but circa 90% will still reach the colon.5 Previously, rodents consuming high levels of heme-Fe or beef (vs. chicken) demonstrated an altered large intestinal microbial composition, characterized by an expansion of sulfate-reducing bacteria and mucin-degrading bacteria, leading to increased levels of fecal sulfides.6–9 Ijssennagger et al. hypothesized that sulfide production in the large intestine would degrade the protective intestinal mucus network by reducing the disulfide bonds connecting the mucin polymers, leading to the formation of trisulfides. The resulting breaks in the mucus barrier could lead to exposure of the underlying epithelium to bacteria and toxins, which in turn could lead to inflammation.6 Preventing high sulfide concentrations in the intestine may therefore improve the mucus integrity. On the other hand, low H2S concentrations are believed to be beneficial as they protect bacteria from oxidative stress and promote the maintenance of the intestinal mucus layer.10 Dual biological effects of other sulfides have been described as well. For example, the toxicity of CS2 has been extensively demonstrated through environmental exposure, although bioregulatory effects have also been reported.11Different animal protein sources have generally a comparable amino acid profile and are characterized by an approximately equal protein digestibility.12 Sulfur-containing metabolites can be formed during the fermentation of indigestible protein in the large intestine. The fermentation of the sulfur-containing amino acids cysteine and methionine in the colon results in the formation of H2S and methanethiol, respectively.13 These molecules are metabolized into a range of sulfur metabolites by a complex interplay of chemical reactions, including methylation, oxidation and carbonation. For instance, H2S can be oxidized to sulfate or methylated to methanethiol and dimethyl sulfide.13 Vitali et al. hypothesized that CS2 may be produced by carbonation of H2S by colonic bacteria as a detoxification mechanism.14 Methanethiol can be oxidized in Fenton-type reactions to form dimethyl disulfide (DMDS) and dimethyl trisulfide (DMTS),15 which in turn could decompose into CS2.16 Since heme-Fe is a known pro-oxidant, it can be hypothesized that this compound may promote the oxidation of these sulfur metabolites. Therefore, despite similar contents of sulfur-containing amino acids, it is hypothesized that the fermentation of muscles of various animal species may still result in different levels of sulfur metabolites.
Because meat is usually consumed as part of a meal, interactions with other dietary compounds must be considered as well when studying the formation of metabolites during digestion.1 The presence of fermentable carbohydrates in the colon can limit the extent of protein fermentation.17,18 Dietary fiber is the main substrate for the colonic intestinal microbiota and will result in the production of short-chain fatty acids (SCFA), such as acetate, propionate and butyrate, thereby acidifying the intestinal contents.17 Since bacterial proteases work best at neutral pH, the formation of protein fermentation metabolites is reduced at lower pH.19 Formed protein fermentation products may also affect gut health. Ammonia levels in the colon may result in energy deficiency for colonocytes.20 Indole, generated by the bacterial metabolism of tryptophan, is thought to contribute to the maintenance of epithelial barrier functions,21 whereas phenol production during tyrosine fermentation, has been linked with an impaired barrier function in colonic epithelial cells.22 Fermentation of tyrosine or phenylalanine leads to the formation of p-cresol, which impairs colonocyte mitochondrial metabolism.23
In the present study, four muscle sources with varying heme-Fe content (beef, pork, chicken and salmon), with or without fructo-oligosaccharides (FOS), were exposed to an in vitro enzymatic gastrointestinal digestion and large intestinal fermentation model, using human fecal inocula from four healthy individuals. We hypothesized that (1) heme-Fe in red meat may favor the formation of certain sulfur metabolites, either by influencing the large intestinal microbial composition and/or activity, or by stimulating the oxidation of various sulfur metabolites. In addition, (2) the presence of FOS could modulate muscle protein fermentation and, thereby, also the formation of sulfur metabolites. Fructo-oligosaccharides were chosen in this experiment due to their ability to ferment quickly in the large intestine, leading to rapid acidification of the gut, and rapid inhibition of protein fermentation. Next to the sulfur metabolites (CS2, H2S, methanethiol, DMDS, DMTS and dimethyl tetrasulfide (DMTeS)), other markers for protein fermentation (phenol, cresol, indole, ammonia, BCFA) and SCFA (acetate, propionate, butyrate, valerate and caproate) were assessed.
2. Materials and methods
2.1 Chemicals
Enzymes used for in vitro digestion (α-amylase from hog pancreas (∼50 U mg−1; 10080), mucin from porcine stomach type II (M2378), pepsin from porcine gastric mucosa (>250 U mg−1 solid; P7000), lipase from porcine pancreas type II (10–400 U mg−1 protein; L3126), pancreatin from porcine pancreas (8 × USP specifications; P7545), and porcine bile extract (B8631)), fructo-oligosaccharides (FOS) from chicory (F8052), and standards for volatile analysis (indole [>99%, I3408], p-cresol [>99%, C85751], DMDS [>99%, 471569], and phenol [>99%, 33517]) were purchased from Merck (Diegem, Belgium).2.2 Experimental setup
Cooked muscle sources (beef, pork, chicken and salmon), with or without 20% (w/w) FOS, were exposed to an in vitro gastrointestinal digestion model, followed by large intestinal fermentation. The proportion of muscle to FOS was chosen so that the protein-to-fiber ratio was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during fermentation, considering that meat consists of about 20% protein. Samples were digested in triplicate, and the whole digestion experiment (enzymatic and fermentation) was repeated four times, each time using a different human fecal inoculum (total n = 12). Following 24 h of fermentation, sulfur metabolites (CS2, H2S, methanethiol, DMDS, DMTS and DMTeS) were analyzed, next to SCFA (acetate, propionate, butyrate, valerate and caproate) and protein fermentation metabolites (indole, phenol, cresol, ammonia, and BCFA: iso-butyrate and iso-valerate).
1 during fermentation, considering that meat consists of about 20% protein. Samples were digested in triplicate, and the whole digestion experiment (enzymatic and fermentation) was repeated four times, each time using a different human fecal inoculum (total n = 12). Following 24 h of fermentation, sulfur metabolites (CS2, H2S, methanethiol, DMDS, DMTS and DMTeS) were analyzed, next to SCFA (acetate, propionate, butyrate, valerate and caproate) and protein fermentation metabolites (indole, phenol, cresol, ammonia, and BCFA: iso-butyrate and iso-valerate).
2.3 Meat and fish samples
Beef (loin), pork (chops), chicken (breast), and salmon (filet) were purchased as fresh as possible from a local supermarket. Meats and fish were manually chopped and minced in a grinder (Omega T-12) equipped with a 3.5 mm plate, packed in anaerobic bags in equal proportions and heated in a warm water bath at 70 °C for 70 min. Thereafter, meat and fish samples were homogenized using a food processor (Moulinex DP700), vacuum packed, and stored at −80 °C until digestion.2.4 Composition of muscle sources
Muscle sources were analyzed for dry matter (ISO 1442-1973) and crude protein content (ISO 937-1978). The amino acid profile of the muscle sources was determined by HPLC on oxidized and hydrolysed samples, following the 2009/152/EC procedure. The heme-Fe content was calculated following the colorimetrical determination of hematin (heme-Fe = hematin × atomic weight Fe/molecular weight hematin), according to Hornsey.24 All analyses were performed in duplicate.2.5 Fecal inoculum collection and preparation
Fecal samples were collected from four healthy adult volunteers (24–34 years old, gender-balanced) without known gastrointestinal disorders or antibiotic treatment during the last six months. A consent form was signed prior to participation in the study. Immediately after fecal donation, phosphate buffer (0.1 M) was added to obtain a fecal slurry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/v, feces/PBS). The slurry was manually homogenized in a plastic bag and filtered through a sieve of 1 mm. The fecal inoculum was kept under continuous CO2 flushing in a water bath at 37 °C, and was used within 1 h to induce fermentation.
4 w/v, feces/PBS). The slurry was manually homogenized in a plastic bag and filtered through a sieve of 1 mm. The fecal inoculum was kept under continuous CO2 flushing in a water bath at 37 °C, and was used within 1 h to induce fermentation.
2.6 In vitro gastrointestinal digestion and fermentation
Meat and fish samples (1.5 g), with or without FOS (0.3 g), were exposed to an in vitro gastrointestinal digestion model according to Van Hecke et al. under normal conditions.25 Fructo-oligosaccharides (powder) were added directly into the digestion vessels with the meat or fish samples. The digestion vessels were incubated under continuous stirring conditions. Next, the digestion vessels were successively incubated with 2 mL of saliva (5 min, room temperature), 4 mL of gastric juice (2 h, 37 °C), 0.6 mL of bicarbonate buffer (1 M, pH 8.0), 4 mL of duodenal juice and 2 mL of bile juice (2 h, 37 °C). Finally, 7 mL of phosphate buffer (0.1 M) was added, and the digestion vessels were sealed and stored at −80 °C until the day of fermentation. The composition of the simulated digestive fluids can be found in ESI Table 1.†For the fermentation procedure, digestion vessels were defrosted at 37 °C for 1 h and subsequently flushed with N2 gas for 30 min to obtain an anaerobic environment. Afterwards, 7 mL of fecal inoculum solution was added, and 1 mL of ethane gas was injected into the flask as an internal gas standard for the measurement of H2S gas. After fermentation for 24 h at 37 °C, the gas production in the headspace and pH (Hanna Instruments, Temse, Belgium) were determined, and digestion samples were transferred into small tubes and stored at −80 °C.
Since the applied in vitro digestion model includes mucin (3 g L−1 gastric juice), which may also be a precursor for the formation of trisulfides,6 it was also investigated whether the inclusion of mucin in the digestion model would influence the formation of sulfur metabolites. For that, pork was in vitro digested without and with mucin (0 or 3 g L−1 gastric juice) as an addition to the model.
2.7 Microbial characterization of inocula
The DNA was extracted from frozen inocula solutions (−80 °C) following the method described by Vilchez-Vargas et al.26 The samples were sent to LGC Genomics, GmbH (Berlin, Germany), for Illumina amplicon sequencing of the V3–V4 region of the 16S rRNA gene of the bacterial community on the MiSeq platform with V3 chemistry (detailed analysis in the ESI†).A table containing the relative abundances of the different OTUs (operational taxonomic units), and their taxonomic assignment, was created following the amplicon data processing. The relative abundance was plotted at the phylum and family levels.
2.8 Composition of gases in the headspace
The composition of gases in the headspace (H2S, CO2, and ethane) after fermentation was analyzed by a micro gas chromatograph, equipped with 2 gas chromatographic modules with a thermal conductivity detector (3000 Micro GC; Agilent Technologies, Diegem, Belgium). Levels of H2S in the headspace of digestion vessels were quantified using a standard curve with H2S gas, and the internal standard (ethane), expressed as μmol g−1 fermented muscle. A gas bag with low, medium and high concentration of H2S was used for further quantification (1.5%, 5% and 10%, respectively).2.9 Sulfur and non-sulfur protein fermentation metabolites
Sulfur (CS2, methanethiol, DMDS, DMTS, and DMTeS) and non-sulfur (indole, phenol and cresol) protein fermentation metabolites were extracted using solid-phase micro-extraction (SPME) with a carboxen-polydimethylsiloxane coated fiber (85 μm) and Combi PAL autosampler (CTC Analytics, Zwingen, Switzerland). Analyses were performed according to Vossen et al.27 with several adjustments. Fermentation samples (2.5 mL) were defrosted at room temperature for 1 h prior to analysis and transferred into a 10 mL glass vial. The SPME fiber was exposed to each sample for 40 min at 37 °C. The fiber was then inserted into the injection port (250 °C) of the GC for sample desorption for 20 min. An empty glass vial was first analyzed every analysis day as an air blank, using the same method. The GC-MS analyses were carried out on a Trace DSQ II (Thermo, Finnigan) that was operated at 70 eV (EI + mode) with Xcalibur software (version 1.4 SR1) for data acquisition and processing. A fused silica capillary column (SLB-IL60 column) of 30 m × 0.25 mm × 0.2 μm was used (Supelco, Bellefonte, PA, USA). The temperature program was set at 40 °C for 5 min, 4.5 °C min−1 to 65 °C for 1 min and 10 °C min−1 to 270 °C, which was held for 16 min. The interface and ion source temperatures were 250 and 230 °C, respectively. The mass-to-charge ratio interval was 33–500 a.m.u. at 3.0 scans per second. Injections were carried out in splitless mode, and helium (1 mL min−1) was used as the carrier gas. Peak identification was performed by comparing the chromatograms with the National Institute of Standards and Technology (NIST) Mass Spectral Library (version 2.0, 2005) and retention time matching with external standards for indole, phenol, cresol and DMDS. Peaks were integrated for area quantification by targeting the quantification ion as follows (m/z): methanethiol 48; CS2 76; phenol 94; DMDS 94; cresol 108; indole 117; DMTS 126; DMTeS 158. Results were expressed as area under the curve (AUC) mL−1 ferment.2.10 Ammonia
Ammonia was measured spectrophotometrically in the supernatants remaining after preparation for the analysis of SCFA and BCFA, following reaction with phenol and quantified with a standard curve of ammonium chloride.282.11 Short- and branched-chain fatty acids
The SCFA (acetate, propionate, butyrate, valerate and caproate) and BCFA (iso-butyrate and iso-valerate) were measured by gas chromatography (HP 7890A, Agilent Technologies, Diegem, Belgium), equipped with a flame ionization detector and a Supelco Nukol capillary column (30 m × 0.25 mm × 0.25 μm, Sigma-Aldrich, Diegem, Belgium) as described by Gadeyne et al.29 Briefly, a 10% formic acid solution, containing 2-ethyl butanoic acid as internal standard, was added to the digestion samples. Following centrifugation (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C), the supernatant was filtered and transferred into a glass vial, followed by injection into the GC.
000g at 4 °C), the supernatant was filtered and transferred into a glass vial, followed by injection into the GC.
2.12 Statistical analysis
A mixed model ANOVA procedure (SAS Enterprise Guide 8) was used with the fixed effects ‘muscle source’ (Pm), ‘FOS’ (Pf) and their interaction term (Pm*f), and the random factor ‘fecal inoculum’. Tukey-adjusted post hoc tests were performed for all pairwise comparisons with P < 0.05 considered significant. The distribution of the residuals was evaluated to test normality and homogeneity of variance. When residuals were not normal (methanethiol, DMDS, DMTS, DMTeS, indole, phenol and cresol), an independent samples Kruskal–Wallis test with pairwise comparisons was used (SPSS Statistics 27), using the effects ‘muscle source’ (Pm), ‘FOS’ (Pf) or their combined effect (Pc) as independent variables. Significant values were adjusted by the Bonferroni correction for multiple tests with P < 0.05 considered significant.3. Results
3.1 Composition of muscle sources
Beef had a 4-fold higher heme-Fe content compared to that of pork, and a 20-fold higher level compared to that of chicken and salmon (Table 1). Salmon and pork had higher dry matter content than beef and chicken. The protein content in salmon was lower than that in the meats (16.8% vs. 21%), which was accompanied by an overall lower amino acid content. The amino acid profile was similar among muscle sources, except for lower relative levels of histidine in chicken (−21%) and salmon (−33%), and somewhat lower proline levels in chicken and salmon (−13%), all when compared to the red meats. Compared to the meat species, salmon contained +10% higher relative methionine levels, whereas relative tryptophan levels were −21% lower.| Beef | Pork | Chicken | Salmon | ||
|---|---|---|---|---|---|
| Total aromatic AA is the sum of phenylalanine, tryptophan and tyrosine. Total sulfur AA is the sum of methionine and cysteine. Total branched-chain AA is the sum of isoleucine, leucine and valine. | |||||
| Composition | |||||
| Heme-Fe | mg per 100 g | 2.06 | 0.50 | 0.12 | 0.11 |
| Dry matter | g per 100 g | 28.6 | 31.1 | 25.9 | 36.1 |
| Protein | g per 100 g | 21.2 | 20.4 | 21.4 | 16.8 |
| Alanine | g per 100 g protein | 6.02 | 5.95 | 5.79 | 5.98 |
| Arginine | g per 100 g protein | 6.66 | 7.00 | 6.73 | 5.98 |
| Aspartic acid | g per 100 g protein | 9.37 | 9.55 | 9.35 | 10.0 |
| Cysteine | g per 100 g protein | 1.05 | 1.14 | 1.09 | 1.08 |
| Glutamic acid | g per 100 g protein | 15.8 | 15.8 | 14.7 | 14.0 |
| Glycine | g per 100 g protein | 4.78 | 4.93 | 4.34 | 4.89 |
| Histidine | g per 100 g protein | 4.26 | 4.42 | 3.41 | 2.91 |
| Isoleucine | g per 100 g protein | 4.89 | 4.94 | 4.99 | 4.67 |
| Leucine | g per 100 g protein | 8.38 | 8.37 | 8.06 | 7.88 |
| Lysine | g per 100 g protein | 9.14 | 9.18 | 9.04 | 9.41 |
| Methionine | g per 100 g protein | 2.82 | 2.93 | 2.88 | 3.16 |
| Phenylalanine | g per 100 g protein | 4.16 | 4.21 | 4.06 | 4.24 |
| Proline | g per 100 g protein | 4.02 | 4.15 | 3.46 | 3.59 |
| Serine | g per 100 g protein | 3.91 | 4.01 | 3.69 | 3.79 |
| Threonine | g per 100 g protein | 4.73 | 4.77 | 4.43 | 4.63 |
| Tryptophan | g per 100 g protein | 1.02 | 1.10 | 1.10 | 0.84 |
| Tyrosine | g per 100 g protein | 3.81 | 3.83 | 3.62 | 3.75 |
| Valine | g per 100 g protein | 5.24 | 5.38 | 5.41 | 5.51 |
| TOTAL aromatic AA | g per 100 g protein | 6.02 | 5.95 | 5.79 | 5.98 |
| TOTAL branched-chain AA | g per 100 g protein | 18.5 | 18.7 | 18.5 | 18.1 |
| TOTAL sulfur AA | g per 100 g protein | 6.66 | 7.00 | 6.73 | 5.98 |
3.2 Microbial characterization of inocula
The microbial community characterization of each fecal inoculum can be found in ESI Fig. 1,† expressed as relative abundances. Overall, Firmicutes (50–76%) and Bacteroidetes (17–49%) were the most abundant phyla in all inocula, next to less abundant phyla, such as Actinobacteria (0.6–5%), Proteobacteria (0.2–0.9%) and Desulfobacterota (0–0.7%). Fecal inoculum 1 contained relatively high abundances of the Verrucomicrobiota (13%), which completely consisted of the mucin-degrading Akkermansia genus, whereas this was <1% or absent in the other fecal inocula. The abundance of the sulfate-reducing family Desulfovibrionaceae was higher in inoculum 1 (0.7%) compared to that in inoculum 2 (0.07%) and 4 (0.05%) and absent in inoculum 3. Inoculum 4 contained higher abundances of Bacteroidia (49%) compared to the other inocula (17–26%), and this mainly consisted of the genus Prevotella (39%) along with relatively low levels of Bacteroides (2%), whereas the other inocula contained relatively high levels of the genus Bacteroides (13–20%) and low Prevotella levels (<2%).3.3 Short- and branched-chain fatty acids, pH and CO2
The production of total SCFA, acetate, propionate and butyrate, was more or less similar across the different inocula, whereas ferments with fecal inoculum 4 were characterized by higher overall production of valerate (6-fold), caproate (18-fold) and BCFA (4-fold) (ESI Fig. 2†). Following 24 h of fermentation, the addition of FOS to the muscle sources generally resulted in significantly lower pH (5.6 vs. 6.9 in ferments without FOS), and a 2.5-fold increase in CO2 levels. Next, higher levels of acetate (+29%), propionate (+93%), butyrate (+64%), and valerate (+33%), along with lower levels of caproate (−43%) were observed while no effect on BCFA levels was found (Table 2). Generally, these parameters were not affected by the muscle source, except for a significantly higher pH in ferments of chicken compared to that in ferments of salmon.| Beef | Pork | Chicken | Salmon | SEM | P m | P f | P m*f | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −FOS | +FOS | −FOS | +FOS | −FOS | +FOS | −FOS | +FOS | ||||||
| AU is artificial unit, expressed as the area under the curve (AUC) of carbon dioxide (CO2)/AUC of the internal standard (ethane). SCFA = short-chain fatty acids. BCFA = branched-chain fatty acids. P-values were obtained using a mixed model ANOVA procedure with fixed effects ‘muscle source’ (Pm), ‘FOS’ (Pf) and their interaction term (Pm*f), and the random factor ‘inocula’. * indicates a significant effect of FOS within the same muscle source; letters a and b indicate statistical differences among muscle sources within the same FOS level. | |||||||||||||
| pH | 6.95ab | 5.59ab* | 6.93ab | 5.60ab* | 7.05a | 5.70a* | 6.84b | 5.52b* | 0.07 | 0.001 | 0.001 | 0.897 | |
| CO2 | AU | 9.29 | 26.9* | 9.49 | 25.6* | 7.36 | 23.8* | 10.0 | 22.3* | 0.97 | 0.294 | 0.001 | 0.280 |
| Total SCFA | μmol g−1 digest | 81.2 | 138* | 91.9 | 142* | 87.4 | 127* | 88.8 | 130* | 3.09 | 0.211 | 0.001 | 0.197 |
| Acetate | μmol g−1 digest | 38.9 | 56.2* | 44.3 | 57.7* | 41.1 | 53.3 | 43.4 | 49.2 | 1.73 | 0.407 | 0.001 | 0.245 |
| Propionate | μmol g−1 digest | 24.6 | 54.5* | 27.0 | 55.5* | 29.2 | 50.7* | 29.6 | 54.6* | 1.72 | 0.801 | 0.001 | 0.465 |
| Butyrate | μmol g−1 digest | 11.2 | 18.6* | 12.8 | 20.7* | 10.8 | 16.7 | 10.3 | 16.9 | 0.85 | 0.234 | 0.001 | 0.879 |
| Valerate | μmol g−1 digest | 6.11 | 8.19 | 7.26 | 8.19 | 6.01 | 6.47 | 5.28 | 8.76 | 0.83 | 0.765 | 0.030 | 0.657 |
| Caproate | μmol g−1 digest | 0.41 | 0.19 | 0.53 | 0.18 | 0.30 | 0.24 | 0.28 | 0.26 | 0.05 | 0.854 | 0.034 | 0.424 |
| Total BCFA | μmol g−1 digest | 7.85 | 6.81 | 8.78 | 6.44 | 7.23 | 7.15 | 7.41 | 7.28 | 0.70 | 0.994 | 0.277 | 0.767 |
| Iso-butyrate | μmol g−1 digest | 2.24 | 1.94 | 2.53 | 1.87 | 2.13 | 2.07 | 2.14 | 2.33 | 0.25 | 0.978 | 0.499 | 0.783 |
| Iso-valerate | μmol g−1 digest | 5.61 | 4.87 | 6.26 | 4.57 | 5.10 | 5.08 | 5.27 | 4.95 | 0.46 | 0.982 | 0.199 | 0.737 |
3.4 Sulfur metabolites
The sulfur metabolites H2S, CS2, DMTS and DMTeS were not detected in the gastrointestinal digests following the addition of the fecal inocula (0 h fermentation). Traces of methanethiol and DMDS were identified at 0 h fermentation, but, overall, methanethiol was >100-fold higher following 24 h of fermentation, and DMDS was >1000-fold higher in 24 h ferments without FOS. In the presence of FOS, DMDS increased 60-fold.Irrespective of treatment, after 24 h of fermentation, the application of the different fecal inocula resulted in overall similar levels of H2S, CS2, methanethiol and DMTS, whereas the application of fecal inoculum 3 resulted in a relatively higher formation of DMDS (3-fold), and fecal inoculum 4 in a higher formation of DMTeS (4-fold) compared to the other inocula (ESI Fig. 3†). The presence of mucin in the gastric juice did not alter the formation of any sulfur metabolite (ESI Fig. 4†); hence, the observed formation of sulfur metabolites did not originate from the gastric mucin applied in the digestion model.
Generally, the addition of FOS to the fermentations resulted in significantly higher H2S levels (+36%) that were unexpected, whereas CS2 levels were significantly decreased (−37%), and the formation of DMDS, DMTS and DMTeS was suppressed (Fig. 1). These effects were observed in all applied individual fecal inocula. Across all fecal inocula, concentrations of methanethiol were not significantly influenced by FOS; however, contrasting effects were observed according to the applied inocula. The presence of FOS resulted in higher methanethiol levels when inocula 2 and 4 were used, and lower levels when inocula 1 and 3 were used. Second, there was a significant effect of the muscle source on the levels of H2S, CS2 and methanethiol. When FOS were added to the muscles, a higher formation of H2S (+33%) was observed in ferments of chicken compared to that in salmon. On the other hand, among ferments without FOS, methanethiol levels were significantly higher in salmon ferments, compared to beef (8-fold) and chicken (14-fold) ferments. Irrespective of the presence of FOS, beef ferments tended to produce more CS2 compared to pork (+45%, P = 0.051), chicken (+33%, P = 0.062), and salmon ferments (+49%, P = 0.060). The levels of DMDS, DMTS and DMTeS following fermentation were not affected by the muscle source.
3.5 Non-sulfur protein fermentation metabolites
Levels of indole and cresol were detected in gastrointestinal digests following the addition of all fecal inocula (0 h fermentation), whereas phenol levels were only detected in digests with fecal inoculum 3. Following 24 h of fermentation, the formation of indole was evident from the circa 26-fold higher levels across all inocula.In contrast, there was a larger variability in the potential of the fecal inocula to form phenol and cresol. More specifically, when compared to the levels in 0 h ferments, cresol was only formed when using fecal inoculum 1 (3-fold) and inoculum 4, with a 5-fold increase in ferments without FOS and a remarkable 99-fold increase in ferments with FOS. The ability of inoculum 2 to metabolize phenol was very limited, whereas the levels in inoculum 3 increased by up to 76-fold in the absence of FOS (ESI Fig. 5†).
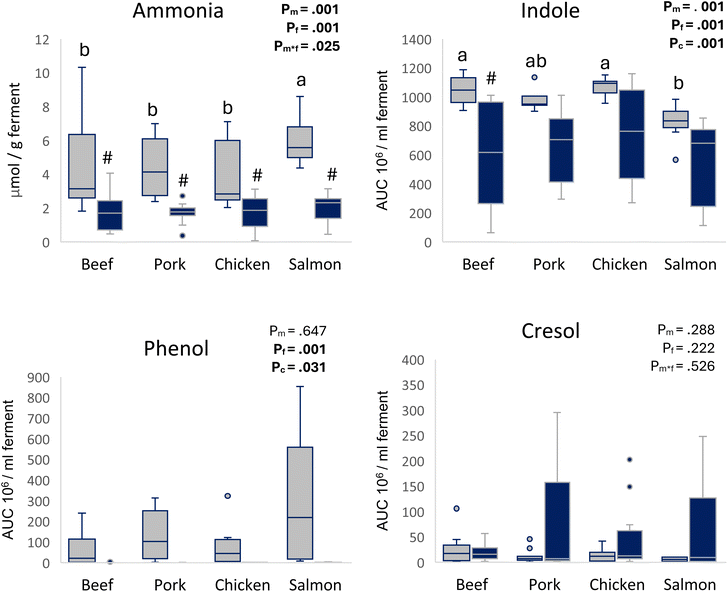
Following 24 h of fermentation, overall, the addition of FOS resulted in a significant reduction in ammonia formation (−60%), as well as indole (−30%), and completely prevented the formation of phenol (Fig. 2). The muscle source had a significant effect on ammonia and indole levels. Without the addition of FOS, salmon ferments had significantly higher ammonia levels (+45%) compared to the ferments of other muscle species. Indole levels were significantly lower for salmon compared to those for beef (−20%) and chicken (−24%). The muscle source did not affect phenol or cresol levels.
4. Discussion
Excessive colonic protein fermentation, including the generation of sulfur metabolites, may affect large intestinal health. The objective of the present study was to determine the formation of sulfur metabolites, along with other protein fermentation metabolites, during in vitro colonic fermentation of muscle sources with varying heme-Fe content, with or without the addition of FOS. We hypothesised that muscle foods high in heme-Fe would have a higher rate of formation of sulfur metabolites during fermentation, whereas the presence of FOS would decrease the rate of formation of protein-derived fermentation metabolites. Generally, the presence of FOS during fermentation of the muscle sources decreased the formation of the sulfur metabolites CS2, DMDS, DMTS, and DMTeS, and of ammonia, indole and phenol, but surprisingly resulted in increased levels of H2S. The effect of FOS addition on the levels of methanethiol depended largely on the applied inocula, showing both increases and decreases. Differences between muscle sources were milder, with lower indole levels, but higher formation of ammonia and methanethiol during fermentation of salmon compared to meat ferments, despite the lower protein content in salmon.Dietary fiber is known to reduce protein fermentation in the gut since bacteria prefer carbohydrates as an energy source.18 Carbohydrate fermentation and the subsequent production of SCFA decrease the luminal pH, creating a more acidic environment, which is unfavourable for proteolytic bacteria.30 In our model, the addition of FOS to meats and fish generally increased the production of SCFA during fermentation, accompanied by a decrease in pH, and reduced the levels of most sulfur metabolites, ammonia and indole, and suppressed the formation of phenol. In line with this, in vitro fermentation, and pig and human intervention studies have demonstrated the potential of various dietary fibers, such as inulin, FOS and arabinoxylan, to reduce intestinal levels of ammonia and phenol.31–33 In the present study, the formation of cresol was only apparent with one of the four inocula, whereby FOS addition contrastingly resulted in an unexpected increased formation of cresol. The latter was also reported when FOS were present during in vitro fermentation of tyrosine with human fecal inocula, but this was also accompanied by increased formation of phenol and indole,34 which was not observed in the present study. The previous authors attributed this increased formation of proteolytic metabolites to a higher overall abundance of bacteria when FOS were added.
Previous research has shown that the addition of FOS to faecal slurries containing cysteine suppressed H2S production by 90% upon fermentation.35 In agreement with this, a porcine in vitro fermentation model showed a rather marginal reduction of 4.5% and 12.5% for H2S and methanethiol levels, respectively, when FOS were added to the inoculum solution.36 Those metabolites were both reduced by 12% when the pigs’ diet was supplemented with inulin (1%), accompanied by reduced abundances of large intestinal Desulfovibrio.37 Contrary to these studies, the addition of FOS in our digestion model increased H2S levels compared to the fermentation of muscle sources without FOS. We hypothesize that these increased levels of H2S in FOS-supplemented ferments may be explained by either primarily higher cysteine metabolization due to higher bacterial load, lower metabolization of H2S into other sulfur metabolites, or by effects of pH on the equilibrium between H2S and its hydrosulfide anion (HS−). At physiological pH, 70% of H2S is present in its anionic form of HS−, while the remaining 30% is H2S.38 Hence, lower pH conditions, such as the ones observed in the FOS ferments, might shift the equilibrium towards a higher H2S/HS− ratio,39 and ferments without FOS, conversely, may contain higher levels of HS−. Inconclusive results on the effect of FOS were found for methanethiol levels, but the formation of CS2 was decreased and the formation of DMxS was almost completely suppressed by the addition of FOS. Since the metabolism of these compounds is intertwined, higher levels of DMxS and CS2 in ferments of muscle sources without FOS could indicate a higher formation and metabolization rate of H2S and/or methanethiol.
Heme-Fe was hypothesized to modulate the production of sulfur metabolites during in vitro large intestinal fermentation, as described in rodents by Ijssennagger et al.6 Rats consuming beef vs. chicken had increased levels of fecal CS2,40 or higher abundances of colonic Desulfovibrionaceae.7 In correspondence, our in vitro model showed CS2 levels to be significantly modulated by the muscle type, whereby beef ferments tended to contain 33 to 49% higher CS2 levels compared to ferments of the other muscle sources. Heme-Fe was hypothesized to promote the oxidation of methanethiol into DMDS and DMTS, as previously described in Fenton-type reactions,15 but the levels of these sulfur metabolites were not different following the fermentation of the muscle species. Rats consuming chicken vs. beef also did not show differences in fecal DMDS,40 and methanethiol and DMDS were not differently found in the colonic content of pigs consuming processed red meat or chicken meat within different dietary patterns.27
Whereas the protein fermentation metabolite profile was similar in ferments of the different meats, salmon ferments displayed a somewhat different profile. In the absence of FOS, salmon ferments showed higher values of methanethiol, ammonia and lower indole concentrations compared to those of most meat ferments; whereas in the presence of FOS, salmon ferments showed lower H2S levels compared to those of the chicken ferments. The higher methanethiol levels may be explained by either a higher primary formation from methionine (salmon contains higher methionine levels) or a reduced secondary metabolization of methanethiol. Indeed, methanethiol can be oxidized or methylated to DMDS and DMTS,15 but can also decompose into CS2.16 Levels of DMxS were not influenced by muscle type, and only a modest increase in CS2 was observed in the beef ferments. Previously, a more rapid degradation of methionine and total amino acid content was reported during the anaerobic fermentation of fish waste compared to pork waste, accompanied by a slower degradation of cysteine in fish waste.16 This may contribute to explaining the higher formation of methanethiol from methionine and ammonia from protein, and the lower H2S from cysteine during salmon fermentation in the present study. The lower levels of indole in salmon ferments compared to meat ferments could be explained by the lower levels of its precursor tryptophan in salmon. Since ammonia may result in energy deficiency for colonocytes20 and indole is thought to contribute to the maintenance of epithelial barrier functions,21 the relatively higher ammonia and lower indole levels in ferments of salmon compared to meats are remarkable. Interestingly, Shi et al.41 found higher levels of indole in feces of frequent chicken consumers compared to frequent pork consumers, but such outcomes are not comparable to our study.
Inter-individual variability across inocula in metabolite production was anticipated due to biological variability, as previously reported by Feng et al.42 Moreover, the habitual diet of each individual is suggested to configure the gut microbiota composition.17 This is why, although it was not the main focus of the study, the ability of each inoculum to ferment the substrates was briefly described, highlighting the importance of using individual inocula instead of pooling fecal samples.43 For instance, the higher levels of sulfate-reducing Desulfovibrio spp. in inoculum 1 were expected to be accompanied by a higher formation of sulfur metabolites, but this was not the case. In contrast, the use of inoculum 3, which was absent in Desulfovibrio spp., resulted in 3-fold higher DMDS formation, compared to the other inocula. Inoculum 4 showed high relative abundances of Prevotella (39%) and low levels of Bacteroides (2%), whereas these proportions were opposite in the other inocula, with Bacteroides being dominant (13–20%), and Prevotella less abundant (<2%). Since Bacteroides-dominated gut communities are associated with lower p-cresol levels,44 this may explain why cresol formation was abundant when using fecal inoculum 4, which was especially clear in the presence of FOS. In addition, faecal inoculum 4 had a higher capacity to produce DMTeS, BCFA as well as valerate and caproate compared to the other inocula. It is also not clear why two inocula resulted in increased methanethiol formation in the presence of FOS, whereas FOS addition reduced methanethiol formation in the presence of the other two inocula, but this may also be attributed to different microbiota compositions among individuals. In addition, microbiota changes may occur during the 24 h of fermentation.45
In vitro experiments are useful tools for studying the formation of metabolites during fermentation, but cannot reproduce the exact physiological intestinal conditions. For example, it is challenging for an in vitro system to simulate the specialized detoxification systems present in the colonic mucosa, responsible for the rapid detoxification of H2S and methanethiol to thiosulfate, and further conversion to the less toxic thiocyanate.46,47 This detoxification system is indeed disturbed in patients with ulcerative colitis46 and colorectal cancer,48 and may hence explain why heme-Fe worsens symptoms in rats with induced colitis, whereas resistant starch mitigates symptoms.49 In addition, the in vitro model does not allow absorption of metabolites in the gut, which may affect the pH of the system and microbial growth and metabolism. Since both passive and active transport systems are involved in absorption, it is challenging to simulate these processes in vitro. Therefore, our findings need to be tested and confirmed in vivo. Nevertheless, in vitro models are a useful, fast and cost-effective way to study interactions between food ingredients.
In conclusion, in vitro fermentation of the muscle sources was significantly modulated by the presence of FOS. The addition of FOS resulted in unexpectedly higher levels of H2S, whereas the formation of dimethyl sulfides was almost completely suppressed, CS2 levels were decreased, and the effects of FOS on methanethiol levels depended on the applied fecal inoculum. Red meat fermentation did not result in higher formation of sulfur metabolites, with the exception of a tendency towards higher CS2 formation in beef ferments. Compared to meat ferments, salmon ferments contained higher levels of ammonia and methanethiol, whereas indole and H2S formation was lower. Thus, the differing intrinsic contents of heme-Fe between muscle types did not seem to play a role in the fermentation metabolic profile.
Abbreviations
| AU | Artificial unit |
| BCFA | Branched-chain fatty acids |
| CO2 | Carbon dioxide |
| CS2 | Carbon disulfide |
| DMDS | Dimethyl disulfide |
| DMTeS | Dimethyl tetrasulfide |
| DMTS | Dimethyl trisulfide |
| DMxS | Dimethyl sulfides |
| FOS | Fructo-oligosaccharides |
| H2S | Hydrogen sulfide |
| Heme-Fe | Heme iron |
| HS− | Hydrosulfide anion |
| OTU | Operational taxonomic units |
| PUFA | Polyunsaturated fatty acids |
| SCFA | Short-chain fatty acids |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors acknowledge F. Dindaragil and S. Coolsaet for their assistance during the incubations and laboratory analysis. This work was funded by the Flanders Research Foundation (FWO) project G038620N, and the Special Research Fund (BOF) BOF.BAS.2022.0016.01.References
- S. De Smet and E. Vossen, Meat: The balance between nutrition and health. A review, Meat Sci., 2016, 120, 145–156 CrossRef PubMed.
- V. Bouvard, D. Loomis, K. Z. Guyton, Y. Grosse, F. E. Ghissassi, L. Benbrahim-Tallaa, N. Guha, H. Mattock and K. Straif, Carcinogenicity of consumption of red and processed meat, Lancet Oncol., 2015, 16, 1599–1600 CrossRef PubMed.
- R. Micha, G. Michas and D. Mozaffarian, Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence, Curr. Atheroscler. Rep., 2012, 14, 515–524 CrossRef CAS PubMed.
- N. Seiwert, D. Heylmann, S. Hasselwander and J. Fahrer, Mechanism of colorectal carcinogenesis triggered by heme iron from red meat, Biochim. Biophys. Acta, Rev. Cancer, 2020, 1873, 188334 CrossRef CAS PubMed.
- G. P. Young, I. S. Rose and D. J. St John, Haem in the gut. I. Fate of haemoproteins and the absorption of haem, J. Gastroenterol. Hepatol., 1989, 4, 537–545 CrossRef CAS PubMed.
- N. Ijssennagger, C. Belzer, G. J. Hooiveld, J. Dekker, S. W. C. Van Mil, M. Müller, M. Kleerebezem and R. Van der Meer, Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10038–10043 CrossRef CAS PubMed.
- T. Van Hecke, J. Vrieze, N. Boon, W. H. Vos, E. Vossen and S. De Smet, Combined Consumption of Beef–Based Cooked Mince and Sucrose Stimulates Oxidative Stress, Cardiac Hypertrophy, and Colonic Outgrowth of Desulfovibrionaceae in Rats, Mol. Nutr. Food Res., 2019, 63, 1800962 CrossRef PubMed.
- Y. Zhu, X. Lin, F. Zhao, X. Shi, H. Li, Y. Li, W. Zhu, X. Xu, C. Li and G. Zhou, Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria, Sci. Rep., 2015, 5, 15220 CrossRef CAS PubMed.
- T. Van Hecke, E. Vossen, S. Goethals, N. Boon, J. De Vrieze and S. De Smet, In vitro and in vivo digestion of red cured cooked meat: oxidation, intestinal microbiota and fecal metabolites, Food Res. Int., 2021, 142, 110203 CrossRef CAS PubMed.
- F. Blachier, M. Beaumont and E. Kim, Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin, Curr. Opin. Clin. Nutr. Metab. Care, 2019, 22, 68–75 CrossRef CAS PubMed.
- A. W. DeMartino, D. F. Zigler, J. M. Fukuto and P. C. Ford, Carbon disulfide. Just toxic or also bioregulatory and/or therapeutic?, Chem. Soc. Rev., 2017, 46, 21–39 RSC.
- S. Wen, G. Zhou, S. Song, X. Xu, J. Voglmeir, L. Liu, F. Zhao, M. Li, L. Li, X. Yu, Y. Bai and C. Li, Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish, Proteomics, 2015, 15, 3688–3698 CrossRef CAS PubMed.
- A. Tangerman, Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3366–3377 CrossRef CAS PubMed.
- B. Vitali, M. Ndagijimana, F. Cruciani, P. Carnevali, M. Candela, M. E. Guerzoni and P. Brigidi, Impact of a synbiotic food on the gut microbial ecology and metabolic profile, BMC Microbiol., 2010, 10, 4 CrossRef PubMed.
- H. W. Chin and R. C. Lindsay, Ascorbate and transition-metal mediation of methanethiol oxidation to dimethyl disulfide and dimethyl trisulfide, Food Chem., 1994, 49, 387–392 CrossRef CAS.
- R. He, X. Z. Yao, M. Chen, R. C. Ma, H. J. Li, C. Wang and S. H. Ding, Conversion of sulfur compounds and microbial community in anaerobic treatment of fish and pork waste, Waste Manage., 2018, 76, 383–393 CrossRef CAS PubMed.
- K. Korpela, Diet, Microbiota, and Metabolic Health: Trade-Off Between Saccharolytic and Proteolytic Fermentation, Annu. Rev. Food Sci. Technol., 2018, 9, 65–84 CrossRef CAS PubMed.
- N. E. Diether and B. P. Willing, Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction, Microorganisms, 2019, 7, 19 CrossRef CAS PubMed.
- M. Beaumont and F. Blachier, Amino Acids in Intestinal Physiology and Health, Adv. Exp. Med. Biol., 2020, 1265, 1–20 CrossRef CAS PubMed.
- M. Andriamihaja, A. M. Davila, M. Elou-Lawson, N. Petit, S. Delpal, F. Allek, A. Blais, C. Delteil, D. Tomé and F. Blachier, Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet, Am. J. Physiol.: Gastrointest. Liver Physiol., 2010, 299, G1030–G1037 CrossRef CAS PubMed.
- H. M. Roager and T. R. Licht, Microbial tryptophan catabolites in health and disease, Nat. Commun., 2018, 9, 3294 CrossRef PubMed.
- M. S. Gilbert, N. Ijssennagger, A. K. Kies and S. W. C. Van Mil, Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry, Am. J. Physiol.: Gastrointest. Liver Physiol., 2018, 315, G159–G170 CrossRef CAS PubMed.
- M. Andriamihaja, A. Lan, M. Beaumont, M. Audebert, X. Wong, K. Yamada, Y. Yin, D. Tomé, C. Carrasco-Pozo, M. Gotteland, X. Kong and F. Blachier, The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells, Free Radicals Biol. Med., 2015, 85, 219–227 CrossRef CAS PubMed.
- H. C. Hornsey, The colour of cooked cured pork. I. Estimation of the Nitric oxide-Haem Pigments, J. Sci. Food Agric., 1956, 7, 534–540 CrossRef CAS.
- T. Van Hecke, V. Basso and S. De Smet, Lipid and Protein Oxidation during in Vitro Gastrointestinal Digestion of Pork under Helicobacter pylori Gastritis Conditions, J. Agric. Food Chem., 2018, 66, 13000–13010 CrossRef CAS PubMed.
- R. Vilchez-Vargas, R. Geffers, M. Suárez-Diez, I. Conte, A. Waliczek, V. S. Kaser, M. Kralova, H. Junca and D. H. Pieper, Analysis of the microbial gene landscape and transcriptome for aromatic pollutants and alkane degradation using a novel internally calibrated microarray system, Environ. Microbiol., 2013, 15, 1016–1039 CrossRef CAS PubMed.
- E. Vossen, S. Goethals, J. De Vrieze, N. Boon, T. Van Hecke and S. De Smet, Red and processed meat consumption within two different dietary patterns: Effect on the colon microbial community and volatile metabolites in pigs, Food Res. Int., 2020, 129, 108793 CrossRef CAS PubMed.
- A. L. Chaney and E. P. Marbach, Modified Reagents for Determination of Urea and Ammonia, Clin. Chem., 1962, 8, 130–132 CrossRef CAS.
- F. Gadeyne, K. S. Ruyck, G. V. Ranst, N. D. Neve, B. Vlaeminck and V. Fievez, Effect of changes in lipid classes during wilting and ensiling of red clover using two silage additives on in vitro ruminal biohydrogenation, J. Agric. Sci., 2016, 154, 553–566 CrossRef CAS.
- E. A. Smith and G. T. Macfarlane, Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids, FEMS Microbiol. Ecol., 1998, 25, 355–368 CrossRef CAS.
- K. S. Swanson, C. M. Grieshop, E. A. Flickinger, L. L. Bauer, B. W. Wolf, J. Chow, K. A. Garleb, J. A. Williams and G. C. Fahey Jr., Fructooligosaccharides and Lactobacillus acidophilus modify bowel function and protein catabolites excreted by healthy humans, J. Nutr., 2002, 132, 3042–3050 CrossRef CAS PubMed.
- X. Wang, G. R. Gibson, A. Costabile, M. Sailer, S. Theis and R. A. Rastall, Prebiotic Supplementation of In Vitro Fecal Fermentations Inhibits Proteolysis by Gut Bacteria, and Host Diet Shapes Gut Bacterial Metabolism and Response to Intervention, Appl. Environ. Microbiol., 2019, 85, e02749–e02718 CAS.
- B. A. Williams, D. Zhang, A. T. Lisle, D. Mikkelsen, C. S. McSweeney, S. Kang, W. L. Bryden and M. J. Gidley, Soluble arabinoxylan enhances large intestinal microbial health biomarkers in pigs fed a red meat-containing diet, Nutrition, 2016, 32, 491–497 CrossRef CAS PubMed.
- E. A. Al Hinai, P. Kullamethee, I. R. Rowland, J. Swann, G. E. Walton and D. M. Commane, Modelling the role of microbial p-cresol in colorectal genotoxicity, Gut Microbes, 2019, 10, 398–411 CrossRef CAS PubMed.
- C. K. Yao, A. Rotbart, J. Z. Ou, K. Kalantar-Zadeh, J. G. Muir and P. R. Gibson, Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas-profiling technology, Gut Microbes, 2018, 9, 510–522 CAS.
- Y. F. Deng, X. Di Liao, Y. Wang, J. B. Liang and V. Tufarelli, Prebiotics Mitigate In Vitro Sulfur-Containing Odour Generation in Caecal Content of Pigs, Ital. J. Anim. Sci., 2015, 14, 3762 CrossRef.
- Y. F. Deng, Y. Y. Liu, Y. T. Zhang, Y. Wang, J. B. Liang, V. Tufarelli, V. Laudadio and X. D. Liao, Efficacy and role of inulin in mitigation of enteric sulfur-containing odor in pigs, J. Sci. Food Agric., 2017, 97, 2382–2391 CrossRef CAS PubMed.
- A. Giuffrè and J. B. Vicente, Hydrogen Sulfide Biochemistry and Interplay with Other Gaseous Mediators in Mammalian Physiology, Oxid. Med. Cell. Longevity, 2018, 2018, 6290931 Search PubMed.
- F. Blachier, M. Beaumont, M. Andriamihaja, A. M. Davila, A. Lan, M. Grauso, L. Armand, R. Benamouzig and D. Tomé, Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences, Am. J. Pathol., 2017, 187, 476–486 CrossRef CAS PubMed.
- T. Van Hecke, E. Vossen, S. Goethals, N. Boon, J. De Vrieze and S. De Smet, In vitro and in vivo digestion of red cured cooked meat: oxidation, intestinal microbiota and fecal metabolites, Food Res. Int., 2021, 142, 110203 CrossRef CAS PubMed.
- J. Shi, D. Zhao, F. Zhao, C. Wang, G. Zamaratskaia and C. Li, Chicken-eaters and pork-eaters have different gut microbiota and tryptophan metabolites, Sci. Rep., 2021, 11, 11934 CrossRef CAS PubMed.
- G. Feng, D. Mikkelsen, E. C. Hoedt, B. A. Williams, B. M. Flanagan, M. Morrison and M. J. Gidley, In vitro fermentation outcomes of arabinoxylan and galactoxyloglucan depend on fecal inoculum more than substrate chemistry, Food Funct., 2020, 11, 7892–7904 RSC.
- D. M. Klurfeld, C. D. Davis, R. W. Karp, E. Allen-Vercoe, E. B. Chang, B. Chassaing, G. C. Fahey Jr, B. R. Hamaker, H. D. Holscher, J. W. Lampe, A. Marette, E. Martens, S. J. O'Keefe, D. J. Rose, M. Saarela, B. O. Schneeman, J. L. Slavin, J. L. Sonnenburg, K. S. Swanson, G. D. Wu and C. J. Lynch, Considerations for best practices in studies of fiber or other dietary components and the intestinal microbiome, Am. J. Physiol. Endocrinol. Metab., 2018, 315, e1087–e1097 CrossRef CAS PubMed.
- R. Levy, A. T. Magis, J. C. Earls, O. Manor, T. Wilmanski, J. Lovejoy, S. M. Gibbons, G. S. Omenn, L. Hood and N. D. Price, Longitudinal analysis reveals transition barriers between dominant ecological states in the gut microbiome, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 13839–13845 CrossRef CAS PubMed.
- S. Tao, Y. Bai, X. Zhou, J. Zhao, H. Yang, S. Zhang and J. Wang, In Vitro Fermentation Characteristics for Different Ratios of Soluble to Insoluble Dietary Fiber by Fresh Fecal Microbiota from Growing Pigs, ACS Omega, 2019, 4, 15158–15167 CrossRef CAS PubMed.
- V. De Preter, I. Arijs, K. Windey, W. Vanhove, S. Vermeire, F. Schuit, P. Rutgeerts and K. Verbeke, Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis, Inflamm. Bowel Dis., 2012, 18, 2371–2380 CrossRef PubMed.
- J. Furne, J. Springfield, T. Koenig, E. DeMaster and M. D. Levitt, Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa, Biochem. Pharmacol., 2001, 62, 255–259 CrossRef CAS PubMed.
- S. Ramasamy, S. Singh, P. Taniere, M. J. S. Langman and M. C. Eggo, Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, 291, G288–G296 CrossRef CAS PubMed.
- R. K. Le Leu, G. P. Young, Y. Hu, J. Winter and M. A. Conlon, Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation, Dig. Dis. Sci., 2013, 58, 3475–3482 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo00928b |
| This journal is © The Royal Society of Chemistry 2024 |