Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dietary fiber supplementation increases Drosophila melanogaster lifespan and gut microbiota diversity†
Daniela
Beghelli
 a,
Laura
Giusti
b,
Lorenzo
Zallocco
a,
Laura
Giusti
b,
Lorenzo
Zallocco
 c,
Maurizio
Ronci
d,
Alessia
Cappelli
a,
Matthew G.
Pontifex
e,
Michael
Muller
e,
Claudia
Damiani
a,
Ilenia
Cirilli
c,
Maurizio
Ronci
d,
Alessia
Cappelli
a,
Matthew G.
Pontifex
e,
Michael
Muller
e,
Claudia
Damiani
a,
Ilenia
Cirilli
 f,
Silvana
Hrelia
g,
David
Vauzour
f,
Silvana
Hrelia
g,
David
Vauzour
 e,
Elena
Vittadini
e,
Elena
Vittadini
 *a,
Guido
Favia
a and
Cristina
Angeloni
g
*a,
Guido
Favia
a and
Cristina
Angeloni
g
aSchool of Biosciences and Veterinary Medicine, University of Camerino, Via Gentile III da Varano, 62032 Camerino, MC, Italy. E-mail: elenagiovanna.vittadini@unicam.it
bSchool of Pharmacy, University of Camerino, Via Gentile III da Varano, 62032 Camerino, MC, Italy
cDepartment of Pharmacy, University of Pisa, 56126 Pisa, Italy
dDepartment of Pharmacy, University G. d'Annunzio of Chieti-Pescara, Chieti, Italy
eNorwich Medical School, Faculty of Medicine and Health Sciences, University of East Anglia, Norwich NR4 7TJ, UK
fDepartment of Life and Environmental Sciences, Polytechnic University of Marche, Ancona, Italy
gDepartment for Life Quality Studies, Alma Mater Studiorum, University of Bologna, Corso d'Augusto 237, 47921 Rimini, RN, Italy
First published on 12th June 2024
Abstract
Dietary fiber has been shown to have multiple health benefits, including a positive effect on longevity and the gut microbiota. In the present study, Drosophila melanogaster has been chosen as an in vivo model organism to study the health effects of dietary fiber supplementation (DFS). DFS extended the mean half-life of male and female flies, but the absolute lifespan only increased in females. To reveal the underlying mechanisms, we examined the effect of DFS on gut microbiota diversity and abundance, local gut immunity, and the brain proteome. A significant difference in the gut microbial community was observed between groups with and without fiber supplementation, which reduced the gut pathogenic bacterial load. We also observed an upregulated expression of dual oxidase and a modulated expression of Attacin and Diptericin genes in the gut of older flies, possibly delaying the gut dysbiosis connected to the age-related gut immune dysfunction. Brain proteome analysis showed that DFS led to the modulation of metabolic processes connected to mitochondrial biogenesis, the RhoV-GTPase cycle, organelle biogenesis and maintenance, membrane trafficking and vesicle-mediated transport, possibly orchestrated through a gut–brain axis interaction. Taken together, our study shows that DFS can prolong the half-life and lifespan of flies, possibly by promoting a healthier gut environment and delaying the physiological dysbiosis that characterizes the ageing process. However, the RhoV-GTPase cycle at the brain level may deserve more attention in future studies.
1. Introduction
It has been reported that the commensal microbial community may impact a diverse range of host physiologies including regulation of immunity, metabolism, and brain functionality.1,2 The gut microbiota, the collection of bacteria, archaea, eukarya and viruses colonising the GI tract, could be considered a ‘dynamic endocrine organ’3 in which trillions of microbes interact with the innate and adaptive local and systemic immune systems.4,5 Recently, the gut microbiota has also been reported to communicate with the central nervous system via biochemical, endocrine, and neurological pathways that work bi-directionally through the so-called gut–brain axis (GBA).3,6Drosophila melanogaster has emerged as a powerful model to study the function of gut microbes,7 and its relatively simple microbial community and ease of physiological and genetic manipulation make it an ideal candidate to study complex GBA interactions.8 For instance, the adult Drosophila midgut is typically in stable contact with a symbiotic commensal community composed of 5–20 different microbial species that mainly comprise yeasts and commensal bacterial species such as acetic acid bacteria (Acetobacter, AABs) and lactic acid bacteria (Lactobacillus, LABs), together with less abundant but highly prevalent Enterobacteriaceae and Leuconostoc as major host strain-specific bacterial genera.9 Interindividual differences in the microbiota are however observed,8,10 and are purported to relate to numerous factors, including host genetics, environmental exposure, sex, age, and dietary regime.8,11 Indeed, Drosophila dietary interventions comprising probiotic and symbiotic formulations have been reported to ameliorate deficits in managing inflammation, metabolic stress and oxidative stress associated with ageing, promoting longevity through the mechanisms of the GBA.
Diets rich in dietary fiber have been shown to exert multiple health benefits, including a positive effect on longevity and boosting the gut microbiota.12 Gut microbes, by digesting fiber, produce short chain fatty acids (SCFAs) such as butyrate, propionate and acetate that affect the brain in a number of ways,13 although there may also be some connections through the immune system.14 For example, gut immune defence mechanisms and gut microbes play an important role in the immune system and inflammation by controlling what is passed into the body and what is excreted.15 The current understanding of the impact of the gut microbiota on host physiology is strictly conditioned by the technical difficulties associated with an in-depth integrated genetic analysis of both the microbes and the host.2
Whole grains and legumes are particularly rich in dietary fiber, with a significant amount contained in their brans or hulls. These terms usually refer to the outer layers of the grain or caryopsis of seeds such as cereals or legumes. Importantly, opting for cereal and legume bran utilisation instead of isolated dietary fiber provides economic and environmental advantages: the use of agricultural by-products avoids the extra costs of fiber extraction, and waste materials are recycled from an environmentally sustainable perspective. Bran and hull are excellent sources of insoluble dietary fibers such as cellulose, hemicellulose, and lignin, together with soluble raffinose, fructans, xylanes and β-glucans.16–18
A commercial syrup with a consistency like honey (MELTEC® by HIFOOD S.p.A. Parma, Italy) rich in fiber obtained from legumes and cereals is available on the market and is a clean label ingredient proposed for partial/total sugar substitution in bakery applications.19
On the basis of the above, our study aimed to assess the effect of dietary fiber supplementation (at medium and very high intake levels) on longevity, gut microbiota diversity and abundance, local gut immunity, and the brain proteome of D. melanogaster.
2. Materials and methods
2.1. Drosophila melanogaster husbandry
After eclosion, D. melanogaster (CantonS strain) were separated under FlyNap (Carolina Biological Supply, Burlington, USA) anaesthesia according to the sex (males: M and females: F) as previously reported.20 Flies were reared in plastic tubes containing standard media (Formula 4-24®, Carolina Biological Supply, Burlington, USA) and yeast (Saccharomyces cerevisiae), considered the control diet (CTRL), and were allowed to grow under a 12 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light–dark cycle at 25 °C and 60% (±5%) relative humidity.
12 h light–dark cycle at 25 °C and 60% (±5%) relative humidity.
2.2. Dietary fiber supplementation
Dietary fiber supplementation was implemented by the inclusion of a commercial fiber syrup (MELTEC®, HIFOOD, Parma, Italy) obtained from corn (Zea mays) dextrin and seed coats of chickpeas (testa of Cicer arietinum seed).19 MELTEC® composition was about 66% (g fiber per 100 g sample) dietary fiber content [of which, 27.4 g per 100 g was high molecular weight dietary fiber and 35.86 g per 100 g was low molecular weight dietary fiber], 9% (g per 100 g sample) carbohydrates (with sugars <0.5% g per 100 g sample), fats < 0.2% (g per 100 g sample), 0.2% (g per 100 g sample) proteins, and a moisture content of ≈25% (g water per 100 g sample).19 In the present study, the MELTEC® syrup was solubilized in water at two different concentrations (0.25% or 1% w/v: M0.25% or M1%, respectively) and added or not (CTRL diet received only water) to soak the standard medium (Formula 4–24®; Carolina Biological, Burlington, NC, USA). The ingredients of the standard medium (as reported by the manufacturer) are as follows: oat flour, soy flour, wheat flour, other starches, dibasic calcium phosphate, calcium carbonate, citric acid, niacinamide, riboflavin, sodium chloride, sodium iron pyrophosphate, sucrose, thiamine, mononitrate, emulsifier preservatives, mould inhibitor, and food colouring.The concentrations M0.25% and M1%, relative to the fiber content present in Formula 4-24® (which we cannot publish as it is proprietary to the supplier), represent an increase of approximately 40% and 160% in fiber content compared to control flies, representing medium fiber and very high fiber intakes,21 and if the fly weight is scaled to that of 70 kg men, these concentrations would correspond to 12.5 or 50.0 g of dietary fiber per day in a human diet.
2.3. Drosophila melanogaster longevity assay
A total of nearly 1200 male and female flies were used for the evaluation of the effect of DFS on life span. Flies were randomly divided into 3 groups (N = 200 per sex per group): CTRL, M0.25% and M1%, and flies were transferred into vials containing fresh food every 3–4 days. The number of dead flies was counted each time, and it was repeated until all flies died.2.4. Body weight determination
To assess the effect of DFS on body weight gain, flies were weighed on days 3, 15, and 30. Briefly, 20 flies (4 replicates) in each group of flies reared for the longevity assay were transferred into empty tubes to be weighed on an electronic scale and then they were put back in their experimental tubes. The mean body weights of the flies in each group/age were calculated.2.5. Microbial 16S rRNA extraction and sequencing
For the evaluation of DFS on the gut microbiota, flies were fed with different concentrations of experimental diets alongside a control diet for 15 days from birth. Microbial DNA was then isolated from five gut pools (10 whole gut pools per group per sex), using a JetFlex Genomic DNA Purification Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Briefly, prior to dissecting, flies were anesthetized with FlyNap and their body surfaces were sterilized with 70% ethanol for 1 min and rinsed twice with sterile PBS. The foreguts and midguts were dissected with special care to reduce contamination and were kept on ice until sampling was completed. The pooled guts were successively homogenized with sterile 0.5 mm wide glass beads (Bertin) for 30 s at 6800 rpm in an automatic tissue homogenizer (Precellys 24, Bertin).22 The quantity of DNA was assessed using a Nanodrop 1000 spectrophotometer (Thermo Scientific). The V3–V4 regions of bacterial 16S rDNA were amplified using bacterial/archaeal degenerate primers 515F/806R, and 16S rRNA amplicon sequencing was performed with an Illumina NovaSeq 6000 PE250. Sequence analysis was performed using Uparse software (Uparse v7.0.1001), using all the effective tags. Sequences with ≥97% similarity were assigned to the same OTUs. A representative sequence for each OTU was screened for further annotation. For each representative sequence, Mothur software was used against the SSUrRNA database of SILVA Database 1.38. Information on the abundance of OTUs was normalised using a standard sequence number corresponding to the sample with the least sequences. Alpha-diversity was assessed using both Chao1 and Shannon H diversity indices whilst beta diversity was assessed using the Bray–Curtis distance. Statistical significance was determined by Kruskal–Wallis or Permutational Multivariate Analysis of Variance (PERMANOVA). Comparisons at the phylum, family and genus levels were made using DESeq2 combined with a false discovery rate (FDR) approach used to correct for multiple testing (q < 0.05).2.6. Gut RNA extraction and analysis of mRNA levels by reverse transcriptase polymerase chain reaction
For the evaluation of the DFS effect on gut anti-microbicidal gene expression, further 1200 male and female flies were lifelong supplemented (M0.25% or M1%, respectively) with the fiber syrup alongside the control. The gut pools were collected on days 15, 30 and 45 in female flies, whereas on days 15 and 30 in male flies (four pools of 10 guts at each time point). RNA was extracted from the whole gut pools of flies using the RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany), and to measure the yield and purity of RNA, a NanoVue spectrophotometer (GE Healthcare, Milano, Italy) was used. Only samples with ratios A260/A280 > 1.8 were used. For each sample, 1 μg of total RNA was reverse transcribed (RT) to obtain cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer's instructions. The subsequent polymerase chain reaction (PCR) was performed in a total volume of 10 μL containing 2 μL of RNAse free dH2O, 2.5 μL (12.5 ng) of cDNA, 5 μL SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories), and 0.5 μL (500 nM) of each primer. The primers used were purchased from Sigma-Aldrich and are reported in ESI Table 1.†Rpl32 was used as the reference gene.2.7. Brain proteomic analysis
For proteomic analysis, Drosophila head samples (three replicates of 15 heads each) collected on day 45 in female flies were resuspended in rehydration solution (7 M urea, 2 M thiourea, 4% CHAPS, 60 mM dithiothreitol and 0.002% bromophenol blue) using a microtube pestle and sonicated for 1 min 3 times in an ultrasonic bath. After sonication (1 min, 2 times), homogenates were allowed to rehydrate for 1 h at room temperature (RT) with occasional stirring. Thereafter, the solution was centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min at RT. The protein concentration of the resulting supernatant was determined using the Pierce 660 protein assay (Thermo Fisher Scientific, Waltham, MA, USA). BSA was used as a standard. 2DE was carried out as previously described.23 Briefly, 75 μg of proteins were filled up to 350 μL in rehydration solution. Immobiline IPG BlueStrips (SERVA Electrophoresis, GmbH, Heidelberg, Germany) of 18 cm with a linear gradient (pH 3–10) were rehydrated overnight in the sample and then transferred to the Ettan IPGphor 2 (GE Healthcare Europe, Uppsala, Sweden) for isoelectrofocusing (IEF). The second dimension (Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis; SDS-PAGE) was carried out by transferring the proteins to 12% polyacrylamide, running at 45 mA per gel and 14 °C for about 7 h, using the Protean® Plus Dodeca Cell (BioRad). The gels were stained with ruthenium II tris(bathophenanthroline disulfonate) and tetrasodium salt (Cyanagen Srl, Bologna, Italy) (RuBP). ImageQuant LAS4010 (GE Healthcare) was used for the acquisition of images. The analysis of images was performed using SameSpot software (v4.1, TotalLab; Newcastle Upon Tyne, UK).
000g for 20 min at RT. The protein concentration of the resulting supernatant was determined using the Pierce 660 protein assay (Thermo Fisher Scientific, Waltham, MA, USA). BSA was used as a standard. 2DE was carried out as previously described.23 Briefly, 75 μg of proteins were filled up to 350 μL in rehydration solution. Immobiline IPG BlueStrips (SERVA Electrophoresis, GmbH, Heidelberg, Germany) of 18 cm with a linear gradient (pH 3–10) were rehydrated overnight in the sample and then transferred to the Ettan IPGphor 2 (GE Healthcare Europe, Uppsala, Sweden) for isoelectrofocusing (IEF). The second dimension (Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis; SDS-PAGE) was carried out by transferring the proteins to 12% polyacrylamide, running at 45 mA per gel and 14 °C for about 7 h, using the Protean® Plus Dodeca Cell (BioRad). The gels were stained with ruthenium II tris(bathophenanthroline disulfonate) and tetrasodium salt (Cyanagen Srl, Bologna, Italy) (RuBP). ImageQuant LAS4010 (GE Healthcare) was used for the acquisition of images. The analysis of images was performed using SameSpot software (v4.1, TotalLab; Newcastle Upon Tyne, UK).
Gel spots were excised and in vitro digested as reported by Giusti et al. in 2018.24 Samples were analysed by LC-MS/MS using an UltiMate3000 RSLCnano chromatographic system coupled to an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), operating in positive ionization mode, equipped with a nanoESI source (EASY-Spray NG). Peptides were loaded on a PepMap100 C18 pre-column cartridge (5 μm particle size, 100 Å pore size, 300 μm i.d. × 5 mm length, Thermo Fisher Scientific, Waltham, MA, USA) and subsequently separated on an EASY-Spray PepMap RSLC C18 column (2 μm particle size, 100 Å pore size, 75 μm i.d. × 15 cm length, Thermo Fisher Scientific, Waltham, MA, USA) at a flow rate of 300 nL min−1 and a temperature of 40 °C using 0.1% FA in water (eluent A) and 99.9% ACN and 0.1% FA (eluent B). Chromatographic separation was achieved by a two-step linear gradient from 5% to 30% eluent B in 40 min, and from 30% to 55% in 5 min followed by an increase to 90% in one minute, for a total runtime of 56 min.
Precursor (MS1) survey scans were recorded in the Orbitrap at resolving powers of 240 K (at m/z 200). Data-dependent MS/MS (MS2) analysis was performed in top speed mode with a 3 s cycle time, during which the most abundant multiple-charged (2+–5+) precursor ions detected within the range of 375–1500 m/z were selected for HCD activation in order of abundance and detected in the ion trap at a rapid scan rate after fragmentation using 30% normalized collision energy. Quadrupole isolation with a 1.6 m/z isolation window was used, and dynamic exclusion was enabled for 60 s after a single scan. Automatic gain control targets and maximum injection times were standard and auto for MS1 and 150% and 70 for MS2. For MS2, the signal intensity threshold was 5.0 × 103, and the option “Injection Ions for All Available Parallelizable Time” was set.
Raw data were directly loaded in PEAKS Studio Xpro software (Bioinformatics Solutions Inc., Waterloo, Canada) using the ‘correct precursor only’ option. The mass lists were searched against the Uniprot/SwissProt database (downloaded January 2022) restricted to fruit fly taxonomy to which a list of common contaminants was appended (42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757 searched entries). Non-specific cleavage was allowed at one end of the peptides, with a maximum of 2 missed cleavages and 2 variable PTMs per peptide. 10 ppm and 0.5 Da were set as the highest error mass tolerance values for precursors and fragments, respectively. −10lg
757 searched entries). Non-specific cleavage was allowed at one end of the peptides, with a maximum of 2 missed cleavages and 2 variable PTMs per peptide. 10 ppm and 0.5 Da were set as the highest error mass tolerance values for precursors and fragments, respectively. −10lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P threshold for PSMs was manually set to 35. The FDR was <0.1%.
P threshold for PSMs was manually set to 35. The FDR was <0.1%.
2.8. Statistical analysis
Survival curves were prepared using a Kaplan–Meier survival estimator and analysed using the OASIS2 software.25 The values relative to RT-PCR (female guts) and body weights (male and female weights, separately considered) are represented as means ± SEM and two-way ANOVA analysis was used to compare differences among groups followed by Tukey's test (Prism 8.4.2, GraphPad software, San Diego, CA). The values of p < 0.05 were considered statistically significant.For proteomic experiments, the spot volume ratios between the different conditions (CTRL and DFS) were calculated using the average spot normalized volume of three biological replicates. Comparison analysis was performed using one-way ANOVA. Spots that exhibited both the p-value and q value of <0.05 were taken into consideration for further protein identification. Bioinformatics analysis of the identified proteins was carried out using Metascape software.
3. Results
3.1. Dietary fiber supplementation impacts the lifespan of Drosophila melanogaster
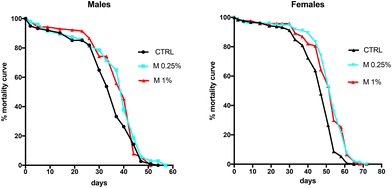
To examine the effect of lifelong dietary fiber supplementation (DFS) on the lifespan of flies, male and female Drosophila were reared on a standard diet supplemented with different DFS concentrations (M0.25% and M1%) (Fig. 1). DFS influenced the half-life of both male (CTRL vs. M0.25%: p < 0.002; CTRL vs. M1%: p < 0.01) and female flies (CTRL vs. M0.25% or M1%: p < 0.0001), although the absolute lifespan was increased in female flies (about 11% longer in flies supplemented with both M0.25% and M1%).3.2. Impact of dietary fiber supplementation on body weight
Caloric restriction has been previously reported to increase the lifespan of Drosophila melanogaster.26,27 The weight of flies was, therefore, monitored over time to rule out that the observed effect on longevity was not due to a decrease in food intake and energy induced by high fibre supplementation. Whilst the body weight significantly increased during ageing (age effect: p < 0.0028 and 0.0001, in female and male flies, respectively), it did not significantly fluctuate across the experimental groups (effect of supplementation: p = n.s.; Fig. 2). The only exception is represented by male flies supplemented with M1% that, on day 30, showed a significant increase in their body weight vs. CTRL flies of the same age (interaction of age × supplementation: p < 0.0151; Fig. 2). Therefore, the improvement of the mean lifespan observed both in male and female flies was not related to the caloric restriction (CR) effect,28 fiber, but rather to other mechanisms as hypothesised below.3.3. Dietary fiber supplementation affects the gut microbiota
Using 16S rRNA gene sequencing, differences in microbial diversity among control (n = 4), DFS 0.25% (n = 4) and DFS 1% (n = 4) fed flies were first analysed. The relative abundances (log) of beneficial or pathogenic microbes are reported in ESI Fig. 1–3.† Although not reaching significance, flies receiving DFS had a dose-dependent higher diversity (p-value: 0.23638) and a lower microbiota richness (p-value: 0.55093) as assessed by the Shannon H diversity index and the Chao1 index, respectively (Fig. 3A). A significant difference in the gut microbial community was observed between groups based on the Bray–Curtis distance; P < 0.05 by PERMANOVA (Fig. 3B and C). Subsequently, differential abundance in bacterial groups that could be driving this separation was tested by means of the DESeq2 algorithm. At the genus level, we detected 6 bacteria which were strongly modulated (DSEq2 q < 0.05). Among those, a 2.9-fold and 4.2-fold increase in Asaia (q = 0.041) and Alloprevotella (q = 0.049) in DFS groups were observed, whilst the abundances of Bacillus, Pseudomonas, Listeria and Enterococcus were all strongly decreased (−4 to −6-fold decrease) in this group when compared to the control (Table 1).| Genus | log2FC | P values | FDR |
|---|---|---|---|
| g__Bacillus | −6.5323 | 1.2 × 10−6 | 6.21 × 10−5 |
| g__Pseudomonas | −4.4395 | 0.000349 | 0.009086 |
| g__Listeria | −4.7008 | 0.001469 | 0.025465 |
| g__Enterococcus | −3.4396 | 0.003066 | 0.039861 |
| g__Asaia | 2.9055 | 0.003991 | 0.041509 |
| g__Alloprevotella | 4.2581 | 0.005674 | 0.049171 |
3.4. Dietary fiber supplementation influences the gut immune defence mechanisms in female Drosophila melanogaster
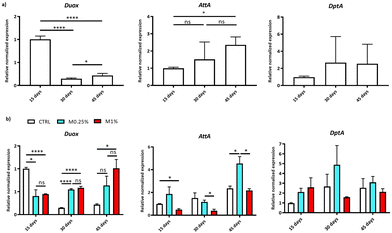
To better explore, at the molecular level, the mechanisms underlying the life-span-extending effect of DFS, the expressions of Duox and antimicrobial peptides (AMPs) Attacin A and Diptericin A (AttA and DptA, respectively) genes related to gut immune defence mechanisms were investigated. Given that DFS was more effective in increasing the longevity of female flies, mRNA level analysis was limited to samples taken from female Drosophila supplemented or not (CTRL) with M0.25% or M1%. Data were normalised vs. the gene expressions of CTRL flies on day 15.The modulation of the gut immune responses concomitant with ageing was observed in CTRL. The two AMPs considered were found to increase (in particular AttA, age effect: p < 0.0001) while the Duox (p < 0.0001) gene underwent significant downregulation (Fig. 4a).3 In contrast, supplemented flies did not show a Duox decreasing expression trend with age, whereas the different AMP coding genes, regardless of age, were particularly upregulated (the effect of supplementation is represented in Fig. 4b). The p-values are summarized in ESI Table 3† for each main effect and their interactions.
3.5. Dietary fiber supplementation modulates brain protein expression in aged female Drosophila melanogaster
The brains of 45-day-old female flies supplemented with M0.25% were analysed by proteomic analysis. Since DFS had comparable effects on increasing longevity at both supplementation levels, only the M0.25% concentration was considered. The list of the identified proteins together with their MW, pI, peptides and coverage values of MS/MS, ratios and p values are reported in the ESI (ESI Table 2†).Twenty-five spots were differentially expressed (p < 0.05) between CTRL and M0.25% groups, of which 18 spots showed an increase in expression, whereas 7 were reduced after M0.25% supplementation (Fig. 5).
The brain proteins significantly modulated by supplementation were analysed using Metascape Resource (Reactome gene set analysis)29 to unveil the metabolic pathways in which they exert their function. Mitochondrial biogenesis (log10(P) −5.11), the RhoV GTPase cycle (log10(P) −4.61), organelle biogenesis and maintenance (log10(P) −4.09), membrane trafficking (log10(P) −3.04), vesicle-mediated transport (log10(P) −3.03), signalling by Rho GTPases (log10(P) −2.90) and the citric acid cycle and respiratory electron transport (log10(P) −2.46) were the metabolic processes in which these proteins (whose expression was modulated by fiber supplementation) were involved (Fig. 6).
4. Discussion
In this study, the effect of long-term supplementation of a commercial syrup (MELTEC®) rich in fiber on the life span and gut modulation of D. melanogaster was investigated due to the widely acknowledged positive impacts of dietary fibers on health.Fibre supplementation (at both M0.25% and M1% concentrations) significantly extended the half-life of female and male flies, although the absolute life span was only extended in female flies.
Such observed effects may not be attributed to a potential CR mechanism, as no change in the body weight of flies or food intake was associated with high fiber supplementation.30
DFS (at both M0.25% and M1% concentrations) also affected the gut microbiota diversity after 15 days of supplementation, with a significant difference in the gut microbial community and an elevated bacterial load of g. Asaia and g. Alloprevotella in comparison with the control group, suggesting a prebiotic action of DFS. The presence of these two bacteria is novel in D. melanogaster even if the acetic acid bacterium Asaia has been previously found in sugar-feeding insects of phylogenetically distant genera and orders.31
Asaia has previously been recognised as a natural effector for mosquito (in Anopheles stephensi and An. gambiae) immune priming.32 The presence of Asaia in D. melanogaster may therefore be underlying the immune modulatory effects observed following DFS intake, although further studies would be necessary to establish causality.
In addition, a significant increase in the abundance of Alloprevotella has been previously reported in young healthy people receiving a short-term dietary fiber intervention (4 days).33
However, we also observed the presence of pathogenic microbes (Table 1) in our flies and, if on one side it is well known that the gut microbiota of Drosophila is greatly influenced by microbes in the environment, especially food,34,35 DFS mainly impacts the absolute quantity of gut microbes rather than the overall gut microbiota composition, however reducing the pathogenic species and increasing the beneficial ones.
It is well known that, in the midguts of Drosophila melanogaster, the proliferative capability of intestinal stem cells (ISCs) is very high;36 likewise the integrity of the intestinal epithelium is strictly influenced by gut microbes, both transient pathogens and commensal bacteria. Gut microbes, together with stem cell activity (leading to gut epithelium renewal), and the gut immune responses give rise to a complex network that is the basis of intestinal homeostasis. Indeed, to resist an infection, the gut epithelium needs both to mount an efficient immune response to eliminate the hazard and replace the injured enterocytes, destroyed by bacteria or the same immune responses, through ISC proliferation.37
In old flies, immune senescence alters the healthy microbiota, which, in turn, triggers aberrant epithelium renewal and barrier dysfunction.36
In our study, the presence of dietary fiber may have contributed to the selection of the healthy gut microbiota (g. Asaia and Alloprevotella) that outcompetes the pathogenic bacteria for available resources, stimulating exploitative competition or have created an unfavourable gut environment for the undesired bacteria.3,14
Indeed, 15-day old flies supplemented with DFS, besides the described modulation of the microbiota, also showed a downregulation of Duox together with a different modulation of AMPs. This finding is probably the expression of targeted immunosurveillance in the gut environment through the action of AMPs in shaping the microbiota.38
In aged flies, the primary innate immune response of Duox tends to physiologically decrease,3 while in the present study, a significantly increased expression of Duox and AttA was observed following supplementation.
It is plausible that dietary fibers may therefore have promoted higher immune responses, thus impacting the homeostasis of the intestine and the longevity of flies. Indeed, AttA acts not only as an immune effector conferring defence upon systemic infections39 but also regulates multiple aspects of host metabolism, thus adding an additional role of AttA in gut homeostasis.40
Consistent with our observations, Si et al. (2018)41 found several AMP-encoded genes upregulated on days 30 or 50 in flies lifelong supplemented with glucomannan hydrolysate. Likewise, Westfall et al. (2018)3 observed an increase in AMP expression that was dramatically benefitted by individual probiotic, prebiotic and synbiotic formulation supplementations in aging flies. These authors also found an increase in the expression of Duox during ageing in the groups that received different types of supplementations.
In D. melanogaster, the control of the expression of AMPs in the body is due to two major signalling pathways: immune deficiency (Imd) and Toll. The Imd pathway acts all along the gut, and gut microbes, by stimulating NF-κB-regulated immune responses of the immune deficiency pathway, induce the production of AMPs, in particular AttA and AttD.38
Physiologically, old flies show higher levels of Imd pathway activity, consistent with their increased bacterial loads.36,42 Our data also confirm increasing trends in AMP expression in CTRL groups as their age increases, but an even higher AMP expression was also observed in the supplemented flies that may have controlled, more efficiently, this overload of commensal bacteria.42 As hypothesised by Buchon (2013), one of the key roles of the immune response in the gut is to maintain a tight microbial balance to avoid an exacerbated proliferation of intestinal stem cells.37
A higher AMP expression in ageing flies correlates with an increase in oxidative stress43 or could be influenced by changing hormonal signaling,44 but it is noteworthy that AMP expression is also influenced by the insulin/IGF-1 signalling pathway45 which, alone, could explain the impact of fiber supplementation on AMP overexpression in ageing Drosophila.
In the midgut of flies, however, there exists a second main inducible antimicrobial effector that is represented by reactive oxidative species (ROS) whose production is induced by Duox, a member of the NADPH oxidase family.36
Besides direct antimicrobial activity, ROS can also act as signalling messengers in tissue repair and wound healing.46 In insects, it has been demonstrated that Duox-dependent ROS production could be induced by both pathogenic and commensal bacteria and it is involved in multiple aspects of gut homeostasis. For instance, Duox participates in ISC activation or in the redox-dependent regulation of signalling pathways and cross-linking of the peritrophic matrix.37,47
However, healthy ageing is favoured by optimal NADPH oxidase activity, with ROS production being helpful not only in eliminating pathogens but also in managing the bacteria.48 The immune senescence that characterises ageing could cause the commensal expansion that, by promoting an increased concentration of ROS, determines excessive ISC proliferation with the loss of tissue homeostasis.49
Broderick et al.,34 in 2014, showed that differences in feeding, by modulating the microbiota, induce different gene expressions. Even if 53% of the upregulated genes were involved in the Imd pathway, there were also other modulated genes involved in signalling and metabolism, thus unveiling an unexpected connection between the microbiota and host functions.
It has already been demonstrated that an imbalance of the gut microbiota, through the GBA, can impair the brain function. Indeed, the increased permeability of the gut barrier could permit the entry of antigens, reducing the availability of useful metabolites for the brain such as SCFAs and neurotransmitters and increasing (neuro)inflammation.50 However, diet has always proved to play a key role in maintaining the health of the intestinal barrier.51
Fiber-rich diets are being studied as a possible approach for modulating the microbiota in order to reduce inflammation, induce neuroprotection and improve brain functions.3,52
In our study, the brain proteome of 45-day-old flies shows twenty-five differently expressed proteins between M0.25% and CTRL flies and these proteins belong to different metabolic processes such as mitochondrial biogenesis, the RhoV GTPase cycle, organelle biogenesis and maintenance, membrane trafficking, vesicle-mediated transport, signalling by Rho GTPase and the citric acid cycle and respiratory electron transport.
Among the signalling pathways and/or metabolic processes that were modified by fiber supplementation, the modulation of brain mitochondrial biogenesis (8 of the 29 modulated proteins were involved) in M0.25% supplemented flies vs. CTRL seems interesting given the importance of mitochondria in the ageing process.53 Indeed, an impairment of functional and anatomical integrity of the mitochondria represents a hallmark of aging in Drosophila. In addition, this dysfunction represents an important factor in the increased risk of disease and death associated with ageing, and it is accompanied by elevated ROS production.3,54,55
Westfall et al. (2018)3 observed that a new probiotic and symbiotic formulation extended longevity in D. melanogaster through mechanisms of gut–brain communication that reduced, inter alia, loss of mitochondrial complex integrity.
Interestingly, mitochondrial biogenesis has been shown to induce synaptic plasticity in neurons.56 In our study, fiber supplementation modulated not only the aforementioned process (mitochondrial biogenesis) but also membrane trafficking (5 proteins out of 25 proteins involved) and signalling by Rho GTPase (4 proteins out of 25 proteins involved), all involved in synaptic plasticity maintenance. The promising ‘theory of microbiome-driven synaptic plasticity’ postulated that through neurotransmitters, neuronal electrophysiology, and immune mediators which interact with both the central and enteric nervous systems and signalling cascades, the microbiome–gut–brain axis can induce synaptic plasticity and long-term changes to the physical and functional neuronal structures.57,58
Finally, in our study, fiber supplementation also modulated some brain proteins involved in the RhoV GTPase cycle (12/25 proteins). RhoV, important during development for maintaining E-cadherin at adherens junctions,59 activates the JNK and p38 MAPK cascades, essential for cell survival and immune response regulation.60 The modulation of immune responses in the intestines of M0.25% flies may relate to the central Rhov GTPase cycle modulation. This cycle, controlling tight junctions, could affect brain cell communications, membrane trafficking and vesicle-mediated transport, favouring neuroplasticity in flies fed with DFS. More research on the roles and interactions of RhoV GTPase is essential, given its potential for synaptic plasticity and cell adhesion in various cell types, including neurons and enterocytes.
5. Conclusions
In the present study, lifelong dietary fiber supplementation improved the half-life of both sexes of D. melanogaster and promoted longevity in female flies, by simultaneously affecting the gut microbiota, local gut immunity and brain proteome. Even if most of the work in this field has been performed in animal models, and further experiments, especially those with gnotobiotic or axenic flies, are needed to confirm the effect of DFS, our data show that a diet rich in fiber could modulate the gut environment, with positive effects on the brain proteome revealing a combinatorial effect on various triggers of ageing including the basic signalling pathway and mitochondria biogenesis.However, it was found that the modulation of the RHOV GTPase cycle in the brain is an element of innovation and in our opinion, its involvement during aging could serve as a potential target of the microbiota–GBA communication axis which could be an area of exploration for future research studies.
Author contributions
All authors contributed to this work significantly. Conceptualization: D.B. and C.A.; methodology: D.B., D.V., L.G., C.D., and C.A.; software: D.B., L.G., M.R., A.C., and L.Z.; formal analysis: D.B., D.V., A.C., C.D., I.C., M.R., and L.Z.; resources: D.B., D.V., and L.G.; data curation: L.G, A.C., D.V., C.D. C.A., L.Z., and D.B.; writing – original draft preparation: D.B.; writing – review & editing: L.G., C.A., D.V., E.V., C.D., G.F., and S.H. All authors have read and agreed to the published version of the manuscript.Data availability
The datasets supporting this article have been uploaded as part of the ESI.† Other data that support the findings of this study will be made available upon request to the corresponding author.Conflicts of interest
There are no conflicts to declare.References
- E. K. Kim and E.-J. Choi, Pathological roles of MAPK signaling pathways in human diseases, Biochim. Biophys. Acta, Mol. Basis Dis., 2010, 1802, 396–405 CrossRef CAS PubMed.
- S. C. Shin, S.-H. Kim, H. You, B. Kim, A. C. Kim, K.-A. Lee, J.-H. Yoon, J.-H. Ryu and W.-J. Lee, Drosophila Microbiome Modulates Host Developmental and Metabolic Homeostasis via Insulin Signaling, Science, 2011, 334, 670–674 CrossRef CAS PubMed.
- S. Westfall, Longevity extension in Drosophila through gut-brain communication, Sci. Rep., 2018, 8, 8362 CrossRef PubMed.
- A. L. Kau, P. P. Ahern, N. W. Griffin, A. L. Goodman and J. I. Gordon, Human nutrition, the gut microbiome and the immune system, Nature, 2011, 474, 327–336 CrossRef CAS PubMed.
- A. Chakrabarti, L. Geurts, L. Hoyles, P. Iozzo, A. D. Kraneveld, G. La Fata, M. Miani, E. Patterson, B. Pot, C. Shortt and D. Vauzour, The microbiota–gut–brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice, Cell. Mol. Life Sci., 2022, 79, 80 CrossRef CAS PubMed.
- Q. Aziz, J. Doré, A. Emmanuel, F. Guarner and E. M. M. Quigley, Gut microbiota and gastrointestinal health: current concepts and future directions: Gut microbiota and gastrointestinal health, Neurogastroenterol. Motil., 2013, 25, 4–15 CrossRef CAS PubMed.
- X. Liu, J. J. Hodgson and N. Buchon, Drosophila as a model for homeostatic, antibacterial, and antiviral mechanisms in the gut, PLoS Pathog., 2017, 13, e1006277 CrossRef PubMed.
- G. Han, H. J. Lee, S. E. Jeong, C. O. Jeon and S. Hyun, Comparative Analysis of Drosophila melanogaster Gut Microbiota with Respect to Host Strain, Sex, and Age, Microb. Ecol., 2017, 74, 207–216 CrossRef PubMed.
- W. B. Ludington and W. W. Ja, Drosophila as a model for the gut microbiome, PLoS Pathog., 2020, 16, e1008398 CrossRef PubMed.
- K. M. White, M. K. Matthews, R. C. Hughes, A. J. Sommer, J. S. Griffitts, P. D. Newell and J. M. Chaston, A Metagenome-Wide Association Study and Arrayed Mutant Library Confirm Acetobacter Lipopolysaccharide Genes Are Necessary for Association with Drosophila melanogaster, G3: Genes, Genomes, Genet., 2018, 8, 1119–1127 CrossRef CAS PubMed.
- G. Han, H. J. Lee, S. E. Jeong, C. O. Jeon and S. Hyun, Comparative Analysis of Drosophila melanogaster Gut Microbiota with Respect to Host Strain, Sex, and Age, Microb. Ecol., 2017, 74, 207–216 CrossRef PubMed.
- A. Bhanja, P. P. Sutar and M. Mishra, Inulin–A polysaccharide: Review on its functional and prebiotic efficacy, J. Food Biochem., 2022, 46(12), 1–13 CrossRef PubMed.
- D. Ríos-Covián, P. Ruas-Madiedo, A. Margolles, M. Gueimonde, C. G. de los Reyes-Gavilán and N. Salazar, Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health, Front. Microbiol., 2016, 7, 185 Search PubMed.
- Ö. C. O. Umu, K. Rudi and D. B. Diep, Modulation of the gut microbiota by prebiotic fibres and bacteriocins, Microb. Ecol. Health Dis., 2017, 28, 1348886 CrossRef PubMed.
- M. G. Rooks and W. S. Garrett, Gut microbiota, metabolites and host immunity, Nat. Rev. Immunol., 2016, 16, 341–352 CrossRef CAS PubMed.
- A. Zitterman, in Encyclopedia of Food Sciences and Nutrition, Elsevier, 2003, pp. 1844–1850 Search PubMed.
- A. Fardet, New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre?, Nutr. Res. Rev., 2010, 23, 65–134 CrossRef CAS PubMed.
- A. Nartea, A. Kuhalskaya, B. Fanesi, O. L. Orhotohwo, K. Susek, L. Rocchetti, V. Di Vittori, E. Bitocchi, D. Pacetti and R. Papa, Legume byproducts as ingredients for food applications: Preparation, nutrition, bioactivity, and techno–functional properties, Compr. Rev. Food Sci. Food Saf., 2023, 22, 1953–1985 CrossRef CAS PubMed.
- A. Carcelli, A. Albertini, E. Vittadini and E. Carini, A fibre syrup for the sugar reduction in fruit filling for bakery application, Int. J. Gastron. Food Sci., 2022, 28, 100545 CrossRef.
- S. Piegholdt, G. Rimbach and A. E. Wagner, The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster, FASEB J., 2016, 30, 948–958 CrossRef CAS PubMed.
- T. M. Barber, S. Kabisch, A. F. H. Pfeiffer and M. O. Weickert, The Effects of the Mediterranean Diet on Health and Gut Microbiota, Nutrients, 2023, 15, 2150 CrossRef CAS PubMed.
- M. V. Mancini, C. Damiani, A. Accoti, M. Tallarita, E. Nunzi, A. Cappelli, J. Bozic, R. Catanzani, P. Rossi, M. Valzano, A. Serrao, I. Ricci, R. Spaccapelo and G. Favia, Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing, BMC Microbiol., 2018, 18, 126 CrossRef CAS PubMed.
- D. Beghelli, L. Zallocco, M. C. Barbalace, S. Paglia, S. Strocchi, I. Cirilli, V. Marzano, L. Putignani, G. Lupidi, S. Hrelia, L. Giusti and C. Angeloni, Pterostilbene Promotes Mean Lifespan in Both Male and Female Drosophila Melanogaster Modulating Different Proteins in the Two Sexes, Oxid. Med. Cell. Longevity, 2022, 2022, 1–21 CrossRef PubMed.
- L. Giusti, C. Angeloni, M. Barbalace, S. Lacerenza, F. Ciregia, M. Ronci, A. Urbani, C. Manera, M. Digiacomo, M. Macchia, M. Mazzoni, A. Lucacchini and S. Hrelia, A Proteomic Approach to Uncover Neuroprotective Mechanisms of Oleocanthal against Oxidative Stress, Int. J. Mol. Sci., 2018, 19(8), 2329 CrossRef PubMed.
- J.-S. Yang, H.-J. Nam, M. Seo, S. K. Han, Y. Choi, H. G. Nam, S.-J. Lee and S. Kim, OASIS: Online Application for the Survival Analysis of Lifespan Assays Performed in Aging Research, PLoS One, 2011, 6, e23525 CrossRef CAS PubMed.
- A. Metaxakis and L. Partridge, Dietary Restriction Extends Lifespan in Wild-Derived Populations of Drosophila melanogaster, PLoS One, 2013, 8, e74681 CrossRef CAS PubMed.
- Y. Gao, C. Zhu, K. Li, X. Cheng, Y. Du, D. Yang, X. Fan, U. Gaur and M. Yang, Comparative proteomics analysis of dietary restriction in Drosophila, PLoS One, 2020, 15, e0240596 CrossRef CAS PubMed.
- K. T. Howitz, K. J. Bitterman, H. Y. Cohen, D. W. Lamming, S. Lavu, J. G. Wood, R. E. Zipkin, P. Chung, A. Kisielewski, L.-L. Zhang, B. Scherer and D. A. Sinclair, Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan, Nature, 2003, 425, 191–196 CrossRef CAS PubMed.
- Y. Zhou, B. Zhou, L. Pache, M. Chang, A. H. Khodabakhshi, O. Tanaseichuk, C. Benner and S. K. Chanda, Metascape provides a biologist-oriented resource for the analysis of systems-level datasets, Nat. Commun., 2019, 10, 1523 CrossRef PubMed.
- J. Zheng, R. Mutcherson and S. L. Helfand, Calorie restriction delays lipid oxidative damage in Drosophila melanogaster, Aging Cell, 2005, 4, 209–216 CrossRef CAS PubMed.
- E. Crotti, C. Damiani, M. Pajoro, E. Gonella, A. Rizzi, I. Ricci, I. Negri, P. Scuppa, P. Rossi, P. Ballarini, N. Raddadi, M. Marzorati, L. Sacchi, E. Clementi, M. Genchi, M. Mandrioli, C. Bandi, G. Favia, A. Alma and D. Daffonchio, Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders, Environ. Microbiol., 2009, 11, 3252–3264 CrossRef CAS PubMed.
- A. Cappelli, C. Damiani, M. V. Mancini, M. Valzano, P. Rossi, A. Serrao, I. Ricci and G. Favia, Asaia Activates Immune Genes in Mosquito Eliciting an Anti-Plasmodium Response: Implications in Malaria Control, Front. Genet., 2019, 10, 836 CrossRef CAS PubMed.
- T. Tian, X. Zhang, T. Luo, D. Wang, Y. Sun and J. Dai, Effects of Short-Term Dietary Fiber Intervention on Gut Microbiota in Young Healthy People, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2021, 14, 3507–3516 CrossRef PubMed.
- N. A. Broderick, N. Buchon and B. Lemaitre, Microbiota-Induced Changes in Drosophila melanogaster Host Gene Expression and Gut Morphology, mBio, 2014, 5, e01117–e01114 CrossRef PubMed.
- C. Ren, P. Webster, S. E. Finkel and J. Tower, Increased Internal and External Bacterial Load during Drosophila Aging without Life-Span Trade-Off, Cell Metab., 2007, 6, 144–152 CrossRef CAS PubMed.
- A. Bonfini, X. Liu and N. Buchon, From pathogens to microbiota: How Drosophila intestinal stem cells react to gut microbes, Dev. Comp. Immunol., 2016, 64, 22–38 CrossRef CAS PubMed.
- N. Buchon, N. A. Broderick and B. Lemaitre, Gut homeostasis in a microbial world: insights from Drosophila melanogaster, Nat. Rev. Microbiol., 2013, 11, 615–626 CrossRef CAS PubMed.
- M. A. Hanson and B. Lemaitre, New insights on Drosophila antimicrobial peptide function in host defense and beyond, Curr. Opin. Immunol., 2020, 62, 22–30 CrossRef CAS PubMed.
- A. Marra, M. A. Hanson, S. Kondo, B. Erkosar and B. Lemaitre, Drosophila Antimicrobial Peptides and Lysozymes Regulate Gut Microbiota Composition and Abundance, mBio, 2021, 12, e00824–e00821 CrossRef CAS PubMed.
- N. A. Broderick, Friend, foe or food? Recognition and the role of antimicrobial peptides in gut immunity and Drosophila –microbe interactions, Philos. Trans. R. Soc., B, 2016, 371, 20150295 CrossRef PubMed.
- Y. Si, X. Liu, K. Ye, A. Bonfini, X. Y. Hu, N. Buchon and Z. Gu, Glucomannan Hydrolysate Promotes Gut Proliferative Homeostasis and Extends Life Span in Drosophila melanogaster, J. Gerontol., Ser. A, 2019, 74(10), 1549–1556 CrossRef CAS PubMed.
- H.-Y. Lee, S.-H. Lee, J.-H. Lee, W.-J. Lee and K.-J. Min, The role of commensal microbes in the lifespan of Drosophila melanogaster, Aging, 2019, 11, 4611–4640 CrossRef CAS PubMed.
- G. N. Landis, D. Abdueva, D. Skvortsov, J. Yang, B. E. Rabin, J. Carrick, S. Tavaré and J. Tower, Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 7663–7668 CrossRef CAS PubMed.
- M. Spinazzi, A. Casarin, V. Pertegato, L. Salviati and C. Angelini, Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells, Nat. Protoc., 2012, 7, 1235–1246 CrossRef CAS PubMed.
- I. Eleftherianos and J. C. Castillo, Molecular Mechanisms of Aging and Immune System Regulation in Drosophila, Int. J. Mol. Sci., 2012, 13(8), 9826–9844 CrossRef CAS PubMed.
- M. T. Juarez, R. A. Patterson, E. Sandoval-Guillen and W. McGinnis, Duox, Flotillin-2, and Src42A Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in Drosophila, PLoS Genet., 2011, 7, e1002424 CrossRef CAS PubMed.
- S.-H. Kim and W.-J. Lee, Role of DUOX in gut inflammation: lessons from Drosophila model of gut-microbiota interactions, Front. Cell. Infect. Microbiol., 2014, 3, 1–12 Search PubMed.
- R. M. Jones, L. Luo, C. S. Ardita, A. N. Richardson, Y. M. Kwon, J. W. Mercante, A. Alam, C. L. Gates, H. Wu, P. A. Swanson, J. D. Lambeth, P. W. Denning and A. S. Neish, Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species: Symbiotic lactobacilli stimulate gut epithelial proliferation, EMBO J., 2013, 32, 3017–3028 CrossRef CAS PubMed.
- A. Ayyaz and H. Jasper, Intestinal inflammation and stem cell homeostasis in aging Drosophila melanogaster, Front. Cell. Infect. Microbiol., 2013, 3, 1–8 Search PubMed.
- G. D. C. Á. Alpino, G. A. Pereira-Sol, M. D. M. E. Dias, A. S. D. Aguiar and M. D. C. G. Peluzio, Beneficial effects of butyrate on brain functions: A view of epigenetic, Crit. Rev. Food Sci. Nutr., 2024, 2022, 1–10 Search PubMed.
- M. T. Pereira, M. Malik, J. A. Nostro, G. J. Mahler and L. P. Musselman, Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture, Dis. Models Mech, 2018, 11, dmm034520 CrossRef CAS PubMed.
- T. M. Cantu-Jungles, H. E. Rasmussen and B. R. Hamaker, Potential of Prebiotic Butyrogenic Fibers in Parkinson's Disease, Front. Neurol., 2019, 10, 663 CrossRef PubMed.
- A. P. Gureev, E. A. Shaforostova and V. N. Popov, Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways, Front. Genet., 2019, 10, 435 CrossRef CAS PubMed.
- P. D'Aquila, D. Bellizzi and G. Passarino, Mitochondria in health, aging and diseases: the epigenetic perspective, Biogerontology, 2015, 16, 569–585 CrossRef PubMed.
- M. Rera, R. I. Clark and D. W. Walker, Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 21528–21533 CrossRef CAS PubMed.
- M. P. Mattson, K. Moehl, N. Ghena, M. Schmaedick and A. Cheng, Intermittent metabolic switching, neuroplasticity and brain health, Nat. Rev. Neurosci., 2018, 19, 81–94 CrossRef PubMed.
- A. Glinert, S. Turjeman, E. Elliott and O. Koren, Microbes, metabolites and (synaptic) malleability, oh my! T he effect of the microbiome on synaptic plasticity, Biol. Rev., 2022, 97, 582–599 CrossRef CAS PubMed.
- K. Berding, C. Carbia and J. F. Cryan, Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis, Exp. Biol. Med., 2021, 246, 796–811 CrossRef CAS PubMed.
- H. G. Tay, Y. W. Ng and E. Manser, A Vertebrate-Specific Chp-PAK-PIX Pathway Maintains E-Cadherin at Adherens Junctions during Zebrafish Epiboly, PLoS One, 2010, 5, e10125 CrossRef PubMed.
- A. Symons, S. Beinke and S. C. Ley, MAP kinase kinase kinases and innate immunity, Trends Immunol., 2006, 27, 40–48 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table 1: List of primers for PCR. Table 2: List of identified proteins together with their MW, pI, peptides and coverage values of MS/MS, ratios and p values. Table 3: List of p-values obtained for each main effect and their interaction in the two-way ANOVA analysis of the results of the RT-PCR assay. Fig. 1–3: Relative abundances (log) of beneficial or pathogenic microbes (family, genus and phylum, respectively). Fig. 4: Sample images relative to the 2DE gels (CTRL vs. M0.25%). See DOI: https://doi.org/10.1039/d4fo00879k |
| This journal is © The Royal Society of Chemistry 2024 |