Open Access Article
Open Access ArticleHexane insoluble fraction from purple rice extract improves steatohepatitis and fibrosis via inhibition of NF-κB and JNK signaling
Aya
Naiki-Ito
 *a,
Ranchana
Yeewa
ab,
Kuang
Xiaochen
a,
Weerakit
Taychaworaditsakul
ab,
Taku
Naiki
a,
Hiroyuki
Kato
a,
Yuko
Nagayasu
a,
Teera
Chewonarin
*a,
Ranchana
Yeewa
ab,
Kuang
Xiaochen
a,
Weerakit
Taychaworaditsakul
ab,
Taku
Naiki
a,
Hiroyuki
Kato
a,
Yuko
Nagayasu
a,
Teera
Chewonarin
 *b and
Satoru
Takahashi
*b and
Satoru
Takahashi
 a
a
aDepartment of Experimental Pathology and Tumor Biology, Nagoya City University Graduate School of Medical Sciences, 1-Kawasumi, Mizuho-cho, Mizuho-ku 467-8601, Nagoya, Japan. E-mail: ayaito@med.nagoya-cu.ac.jp; Fax: +81-52-842-0817; Tel: +81-52-853-8156
bDepartment of Biochemistry, Faculty of Medicine, Chiang Mai University, 110 Intravaroros Rd., Sripoom, Muang, Chiang Mai 50200, Thailand. E-mail: teera.c@cmu.ac.th; Fax: +66-53-894031; Tel: +66-53-949437#218
First published on 13th July 2024
Abstract
Nonalcoholic fatty liver disease (NAFLD) is fatty liver mainly related to metabolic syndrome. NAFLD with inflammation and hepatocellular damage is defined as nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma. We have previously reported that a hexane insoluble fraction from an anthocyanin-rich purple rice ethanolic extract (PRE-HIF) can suppress prostate carcinogenesis. However, the extract's effect on NASH has not yet been established. In the present study, we investigated the chemopreventive effect of a PRE-HIF on NASH and liver fibrosis using a connexin 32 (Cx32) dominant negative transgenic (Cx32ΔTg) rat NASH model. Seven-week-old male Cx32ΔTg rats were fed a control diet, a high-fat diet (HFD), or an HFD with 1% PRE-HIF and intraperitoneal administration of dimethylnitrosamine for 17 weeks. Histological findings of NASH such as fat deposition, lobular inflammation, hepatocyte ballooning injury, and bridging fibrosis were observed in the HFD group but not in the control group, and all histological parameters were significantly improved by PRE-HIF treatment. Corresponding to the histological changes, increased expression of inflammatory cytokine mRNAs (TNF-α, IL-6, IL-18, IFN-γ, IL-1β, TGF-β1, TIMP1, TIMP2, COL1A1), along with and activation of nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK) signaling were observed in the HFD group, which was significantly decreased by PRE-HIF. The number and area of hepatic precancerous glutathione S-transferase placental form-positive foci tended to be decreased by PRE-HIF. These results indicate that intake of purple rice as a dietary supplement may reduce steatohepatitis, liver injury, and fibrosis in NASH by inactivation of NF-κB or JNK.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is increasing in incidence and is recognized as the most common chronic liver disease (CLD) that presents with histological or imaging fatty deposits in the liver. A recent meta-analysis reported that the global prevalence of NAFLD was 38.2% (33.72–42.89) in 2016–2019, representing an increase of about 50% compared with 25.3% (21.59–29.33) in 1990–2006.1 This recent spread of NAFLD is closely related to an increase in the incidence of metabolic syndrome, including obesity and type 2 diabetes. The regional prevalence rates were higher in Latin America (44.4%) and North Africa and the Middle East (36.5%) than in the Asia-Pacific region (28.0%), Western Europe (25.1%), and East Asia (29.7%), indicating a shift in the global disease burden of NAFLD from North American and European to North African and Middle Eastern, Asian, and Latin American regions.2 NAFLD is classified as nonalcoholic fatty liver (NAFL), which is simple fatty liver and rarely progresses, and nonalcoholic steatohepatitis (NASH) with inflammation and hepatocyte injury. As with other forms of CLD, progressive inflammation in NASH carries the risk of developing liver cirrhosis or hepatocellular carcinoma (HCC). Interestingly, the prevalence of NAFLD, as well as deaths from NAFLD-related HCC, has increased more rapidly than other CLD-related liver cancer deaths.2 Therefore, interventions are needed to reduce the development of NAFLD and the progression from NAFL to NASH.The two-hit theory, in which NAFL progresses to NASH, is a well-known mechanism involved in the pathogenesis of NAFLD.3 However, since there are cases of NASH that do not progress through NAFL, the concept of the “multiple parallel hits hypothesis” has been proposed.4 In other words, interactions between the liver and other organs such as adipose tissue and the intestinal tract contribute to the development of steatohepatitis, hepatotoxicity, and fibrosis; increased insulin resistance,5 abnormal adipocytokine secretion from adipose tissue,6 and increased short-chain fatty acids production from gut microbiota,7,8 and persistent inflammation and oxidative stress in the liver.9
The importance of oxidative stress in the progression of NASH is supported by our previous studies. Connexin 32 (Cx32) dominant negative transgenic (Cx32ΔTg) rats, which lack the main hepatocyte gap junction protein, were highly susceptible to hepatocarcinogenesis as compared with wild-type rats.10,11 In our recent studies using this transgenic rat model, Cx32ΔTg rats also had higher sensitivity to NASH induction by a high-fat diet (HFD) and combined administration of dimethylnitrosamine (DMN), and had increased levels of inflammatory cytokines and reactive oxygen species (ROS), resulting in more advanced NASH, liver fibrosis, and hepatocarcinogenesis than in wild-type rats.12,13 Nuclear factor (NF)-κB and inflammatory cytokines such as tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β1 were involved in NASH progression in Cx32ΔTg rats,12 and luteolin and lactoferrin, which have antioxidant properties, inhibited these factors, resulting in suppression of NASH.13,14
Purple rice (Oryza sativa L. indica) is a pigmented rice commonly cultivated and consumed in Northern Thailand.15 Purple rice has attracted attention as a functional food containing abundant bioactive substances. Among them, anthocyanins have been reported to be active components of purple rice with various biological properties such as anti-inflammatory,16,17 anti-microbial,16 antioxidant,18,19 and anti-tumor effects,20,21 We recently reported that a hexane insoluble fraction of purple rice ethanolic extract (PRE-HIF), which is rich in anthocyanins and has inhibitory effects against testosterone-induced benign prostatic hyperplasia in a rat model,22,23 prostate carcinogenesis in a transgenic rat model of prostate adenocarcinoma, and tumor growth of human and rat castration-resistant prostate cancers.24 Considering the involvement of oxidative stress and inflammation in the pathogenesis of NASH, we hypothesized that PRE-HIF inhibits NASH progression.
In the present study, we attempted to examine the chemopreventive effect of PRE-HIF on NASH and fibrosis using a Cx32ΔTg–HFD–DMN NASH model.
2. Materials and methods
2.1. Chemicals
The glutinous purple rice variety, Kum Doi Saket (Oryza sativa L. indica), was purchased from the Purple Rice Research Unit, Chiang Mai University, Thailand. PRE-HIF was extracted as described previously and used in this study.23 HFD-60, a HFD (60 kcal% of fat), and DMN were purchased from Oriental BioService, Inc. (Kyoto, Japan) and Tokyo Kasei Kogyo Co. Ltd (Tokyo, Japan), respectively.2.2 Generation of Cx32ΔTg rats
The production and screening of Cx32ΔTg rats were performed as previously described in detail.25,26 Rats were housed in plastic cages lined with hardwood chips in an air-conditioned specific pathogen-free animal room at 22 ± 2 °C and 50% humidity with a 12 h/12 h light–dark cycle. All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medical Sciences (no. H30M-60, approved on February 22, 2019).2.3. Animal experiments
The experimental design is illustrated in Fig. 1. Thirty-two 7-week-old male Cx32ΔTg rats received HFD and 8 received a control diet (AIN-93M, Oriental BioService, Inc.) for 17 weeks. Starting 5 weeks later, DMN was intraperitoneally administered 6 times every 2 weeks at doses of 15 mg kg−1 for the first and second doses, 10 mg kg−1 for the third and fourth doses, and 5 mg kg−1 for the fifth and sixth doses. HFD-treated rats were randomly divided into 2 groups: a 1% PRE-HIF supplied group (HFD + PRE) and a non-treated group (HFD). During the experiment, rats were weighed once a week and food intake was measured twice a week. Seventeen weeks after the start of the experiment, all rats were sacrificed under deep anesthesia and blood samples were collected from the abdominal aorta. Liver, kidney, and visceral fat surrounding the spermatic duct were resected and weighed.2.4. Histological analysis of the liver
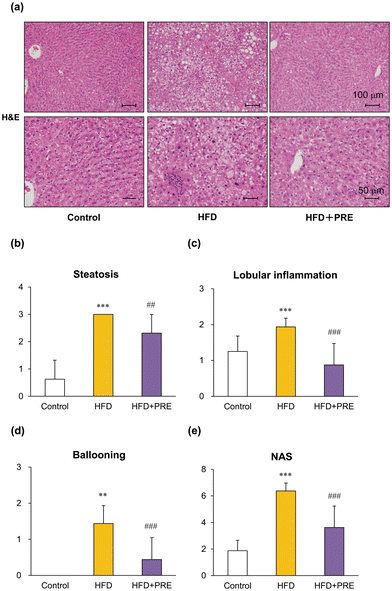
The left lateral lobes of each liver were sliced 3–4 mm thick and 10% formalin-fixed paraffin-embedded (FFPE) specimens (2–3 μm thick) were used for histological evaluation; FFPE sections were stained with hematoxylin and eosin (H&E) or azan. They were also immunohistochemically stained with antibodies against α-smooth muscle actin (α-SMA; Agilent Technologies, Santa Clara, CA, USA, Cat# M0851, RRID:AB_2223500) or glutathione S-transferase placental form (GST-P; MBL International, Woburn, MA, USA, Cat# 311-H, RRID:AB_591790). The degree of steatohepatitis and fibrosis was quantitatively assessed by the NAFLD activity score (NAS) as previously described.14,27 Briefly, NAS represents a sum of 3 scores namely, severity of steatosis (0–3), lobular inflammation (0–2), and hepatocyte ballooning (0–3). NAS and scores for fibrosis (0–4) were evaluated by 3 experienced pathologists (A.N.-I., H.K., and S.T.). The average value of GST-P positive foci greater than 80 μm in diameter in all areas of a liver section were measured using an image analyzer (Keyence, Osaka, Japan).2.5. RNA extraction, cDNA preparation, and quantitative real-time PCR
Total RNA was isolated from frozen tissues collected from the left lateral lobe of the liver using Isogen (Isogen, Nippon Gene Co., Ltd, Tokyo, Japan) and converted to cDNA using PrimeScript™ RT Master Mix (Takara). The cDNA template was subjected to quantitative real-time PCR (qRT-PCR) using TB Green™ Premix Ex Taq™ II (Takara) and detected by AriaMx Real-Time PCR System (g8830a, Agilent Technologies). The primers used are listed in Table 1. Gapdh mRNA levels were used as internal controls.| Gene | Forward | Reverse |
|---|---|---|
| Tnf-α | ACTGAACTTCGGGGTGATCG | GCTTGGTGGTTTGCTACGAC |
| Il-1β | AGCTGAAAGCTCTCCACCTC | GTGCCGTCTTTCATCACACAG |
| Il-6 | TCCGGAGAGGAGACTTCACA | ACAGTGCATCATCGCTGTTC |
| Ifn-γ | GCATTCATGAGCATCGCCAA | AGATTCTGGTGACAGCTGGTG |
| Tgf-β1 | GTCAACTGTGGAGCAACACG | TTCCGTCTCCTTGGTTCAGC |
| Il-18 | TGACAAAAGAAACCCGCCTG | TCACAGATAGGGTCACAGCC |
| Timp1 | GTAAAGCCTGTAGCTGTGCC | CCACAGCGTCGAATCCTTTG |
| Timp2 | CCAAAGCAGTGAGCGAGAAG | TCGATGTCCTTGTCAGGTCC |
| Col1a1 | TGACGCATGGCCAAGAAGA | CGTGCCATTGTGGCAGATAC |
| Gapdh | GCATCCTGCACACCAACTG | GCCTGCTTCACCACCTTGTT |
2.6. Western blotting
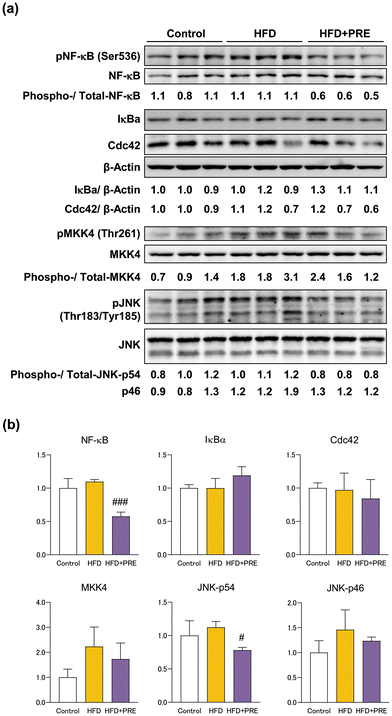
Proteins from frozen tissues collected from the left lateral lobe of the liver were extracted as previously outlined.12 The antibodies used were against the following antigens: Cdc42 (Cell Signaling Technology, Danvers, MA, Cat# 2466, RRID:AB_2078082), NF-κB (Cell Signaling Technology Cat# 8242, RRID:AB_10859369), phosphorylated (p) NF-κB (Ser536) (Cell Signaling Technology Cat# 3031, RRID:AB_330559), MKK4 (Cell Signaling Technology Cat# 9152, RRID:AB_330905), pMKK4 (Thr261) (Cell Signaling Technology Cat# 9151, RRID:AB_330889), JNK (Cell Signaling Technology Cat# 9252, RRID:AB_2250373), pJNK (Thr183/Tyr185) (Cell Signaling Technology Cat# 9251, RRID:AB_331659); and β-actin (Sigma-Aldrich, St Louis, MI, USA, Cat# A5316, RRID:AB_476743). Anti-β-actin was used at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution and all other antibodies at 1
5000 dilution and all other antibodies at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. ImageJ software ver.1.53 (National Cancer Institute Bethesda, MD, USA, RRID:SCR_003070) was used to quantify band intensity.
1000. ImageJ software ver.1.53 (National Cancer Institute Bethesda, MD, USA, RRID:SCR_003070) was used to quantify band intensity.
2.7. Statistical analysis
Data are presented as the mean ± standard deviation (SD), and differences between groups were compared by one-way ANOVA with Dunnett's test using Graph Pad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA, RRID:SCR_002798). Significance was considered at P < 0.05.3. Results
3.1. PRE-HIF suppresses steatohepatitis in Cx32ΔTg rats
No toxic changes were observed with PRE-HIF administration in a previous study using a rat prostate cancer model.24 In the present study, intake of PRE-HIF did not affect body weight or weight of the liver, visceral fat, and kidneys in the Cx32ΔTg–HFD–DMN NASH model (Table 2). The HFD groups tended to gain weight compared with the Control group, but the difference was not significant. The HFD had more calories per weight than the Control diet, but the intake was lower. As a result, the caloric intake of the Control, HFD, and HFD + PRE groups was 70.9, 70.7, and 73.6 kcal per rat per day, respectively, which was not significantly different, and this may explain why there was no significant difference in body weight. No histological changes were observed in the kidneys of either group (data not shown). In the liver, combined HFD and DMN administration induced diffuse fat deposition and neutrophil infiltration, and both changes were significantly more advanced than in the Control group. Hepatocyte ballooning was observed in the HFD group but not in the control group (Fig. 2a). PRE-HIF significantly decreased the area of steatosis and clusters of neutrophils as well as hepatocyte ballooning injury (Fig. 2b–d). Taken together, these results indicate that the histological grade of NASH represented by the NAS was significantly higher in the HFD group than in the control group and was inhibited by PRE-HIF treatment (Fig. 2e).| No. of rats | Body (g) | Liver | Kidney | Fat | Food intake (g per rat per day) | Calorie (kcal per 100 g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | |||||
| HFD, high fat diet; PRE, 1% hexane insoluble fraction from a purple rice ethanolic extract. | ||||||||||
| Control | 8 | 547.6 ± 40.6 | 14.59 ± 1.21 | 2.67 ± 0.14 | 2.58 ± 0.14 | 0.47 ± 0.03 | 11.21 ± 2.59 | 2.04 ± 0.39 | 19.26 ± 2.77 | 368.0 |
| HFD | 16 | 577.6 ± 36.9 | 14.99 ± 1.35 | 2.59 ± 0.14 | 2.62 ± 0.13 | 0.45 ± 0.03 | 13.85 ± 3.01 | 2.39 ± 0.44 | 14.35 ± 2.06 | 492.6 |
| HFD + PRE | 16 | 590.1 ± 52.0 | 15.18 ± 1.94 | 2.57 ± 0.15 | 2.61 ± 0.25 | 0.44 ± 0.03 | 16.05 ± 3.56 | 2.71 ± 0.46 | 15.01 ± 2.21 | 487.7 |
3.2. PRE-HIF decreases liver fibrosis in Cx32ΔTg rats
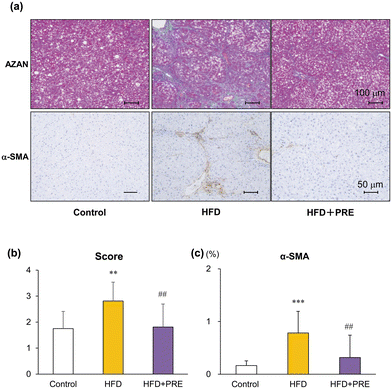
Azan staining and α-SMA immunohistochemical staining were performed to analyze liver fibrosis (collagen fibers) and activation of hepatic stellate cells (HSC)s, respectively. As previously reported, bridging fibrosis and sometimes liver cirrhosis due to complete surrounding by fibrous bands was induced by a HFD and DMN treatment (Fig. 2a). Histological fibrosis scores and α-SMA-positive areas were increased in the HFD group compared with the control group and was significantly reduced in the PRE-HIF treated group (Fig. 3a–c). These results suggest that PRE-HIF inhibits progression to liver fibrosis in NASH.3.3. PRE-HIF tends to decrease induction of preneoplastic lesions in Cx32ΔTg rats
To investigate the potential chemopreventive effect of PRE-HIF on NASH-associated hepatocarcinogenesis, the formation of hepatic preneoplastic foci, i.e., GST-P positive foci was evaluated. The number and area of GST-P positive foci tended to be increased by HFD treatment and decreased by PRE-HIF treatment, although not significantly (Fig. 4a–c). The mRNA expression level of brain expressed, X-linked 1 (Bex1), which shows gene upregulation in hepatic neoplastic lesions including GST-P positive foci and HCC,13 was significantly elevated in the HFD group as compared with the control group. The upregulation of Bex1 in HFD was blocked by PRE-HIF treatment (Fig. 4d).3.4. PRE-HIF downregulates inflammatory cytokines in liver of the Cx32ΔTg rats
In previous studies, inflammatory cytokines associated with inflammation (TNF-α, IL-6, IL-18, IFN-γ, IL-1β) and fibrosis (TGF-β1, TIMP1, TIMP 2, COL1A1) were shown to be involved in the inflammatory and fibrotic progression of NASH, respectively.12,14 Therefore, we next quantified PRE-HIF induced changes in their mRNA expression levels by qRT-PCR. HFD induced upregulation of these cytokines, with significant upregulation of TNF-α and IL-18. PRE-HIF significantly downregulated all cytokines (Fig. 5). These results indicates that PRE-HIF contributes to the suppression of NASH and liver fibrosis through reduction of cytokine production.3.5. PRE-HIF inhibit NF-κB and JNK signaling in liver of the Cx32ΔTg rats
NF-κB and JNK/SAPK signaling play an important role in NASH progression.13,28–30 In the present study, we examined changes in the expression of proteins associated with these signals upon PRE-HIF administration. Western blotting showed that protein expression of pNF-κB and pJNK was significantly decreased by PRE-HIF administration (Fig. 6). These results suggest that inactivation of NF-κB and JNK/SAPK is involved in the inhibitory effect of PRE-HIF on NASH progression.4. Discussion and conclusion
NASH is a chronic and potentially progressive liver lesion that can lead to irreversible cirrhosis and HCC. Therefore, intervention with active ingredients derived from foods that can be consumed on a daily basis could be an effective treatment. This study focused on scientific evidence regarding the functions of purple rice, particularly the anthocyanin-rich hexane-insoluble fraction, PRE-HIF. The effect of PRE-HIF on histological features of NASH, such as fat deposition, hepatocellular injury, inflammation, and fibrosis were quantified using a human NASH scoring system.27 Cyanidin 3-glucoside (C3G) and peonidin 3-glucoside (P3G) are the main anthocyanins in PRE-HIF,23 and C3G exhibited a greater inhibitory effect on cell proliferation in prostate cancer cells.24 C3G and P3G are among the most widely known anthocyanins in pigmented foods, and have anti-oxidant and anti-inflammatory effects. NASH progresses due to oxidative stress, including inflammation, ROS production, and mitochondrial dysfunction. Neutrophil infiltration is a histologic hallmark of NASH, with the ability to release ROS and proteases, causing tissue damages that may contribute to amplification of the inflammatory response and fibrosis.31 In fact, patients with NASH have poor conditions for the removal of ROS due to decreased activity of the antioxidant enzymes, glutathione and catalase,32 and impaired mitochondrial function.33 In this study, neutrophil infiltration in the liver lobule was significantly decreased in the HFD-PRE treated group. In addition, a previous study indicated that P3G inhibits steatosis by decreasing ROS.34 These details suggest that the inhibitory effect of PRE-HIF on NASH is due to inhibition of oxidative stress. Other notable effects of C3G include suppression of inflammatory cytokines;35,36 C3G inhibited expression of TNF-α, IL-6, IL-8, and Il-1β in lipopolysaccharide-stimulated THP-1 macrophage cells.35 The inhibitory effects of C3G on TNF-α, IL-6, IL-8, and Il-1β were also observed in vivo and led to improvement of acute lung injury.36 A case-control study and intervention study also indicated that intake of anthocyanins (320 mg daily) reduced IL-18 and IL-1β, which were increased in NAFLD patients, via inhibition of NLRP3 inflammasome.37 In the present study, anthocyanin-rich PRE-HIF downregulated not only inflammation-related cytokines, such as TNF-α, IL-6, IL-8, and IL-1β, but also the fibrosis-related cytokines TGF-β, TIMP1, and TIMP2. The levels of these cytokines correlated with the histological progression of NASH, suggesting that PRE suppressed steatohepatitis and liver fibrosis by suppressing their expression.Liver fibrosis is regarded as one of the most serious complications of NASH. In a common NASH model in which rodents are fed a HFD or methionine-choline deficient diet, steatosis and steatohepatitis are relatively easy to induce, but the model does not readily lead to definite liver fibrosis over a short duration.19,38 In contrast, advanced liver fibrotic lesions such as bridging fibrosis and cirrhosis were inducible at 17 weeks in HFD- and DMN-treated Cx32ΔTg rats, suggesting that the Cx32ΔTg rat NASH model may be useful for analyzing the effects and mechanisms of test substances on fibrosis in NASH.12,14 In NASH, as in other forms of chronic hepatitis-associated liver fibrosis, the key effector cell is the HSC. HSCs are activated by inflammatory cytokines such as TNF-α and TGF-β, transform into α-SMA-positive myofibroblast phenotype, and are involved in fibrogenesis in the liver.39 Indeed, liver fibrosis was significantly more advanced in the HFD group than in the control group, accompanied by an increase in inflammatory cytokine expression and α-SMA-positive HSCs in the Cx32ΔTg rat model. PRE-HIF decreased α-SMA-positive HSCs and improved the liver fibrosis score in the NASH model. This is the first report of the inhibitory effect of PRE-HIF on NASH-related liver fibrosis. A previous study indicated that C3G can inhibit activation of HSCs and attenuate carbon tetrachloride-induced liver fibrosis in mice, and that protocatechuic acid, a metabolite of C3G suppresses TNF-α and IL-6 levels in HSCs in vitro.40 Therefore, activated HSCs are implicated in the progression of liver fibrosis due to NASH and other diseases, which can be suppressed by anthocyanin-rich PRE-HIF. In addition to HSC activity, an imbalance in the expression of matrix metalloproteinase (MMP) and TIMPs, which are responsible for the extracellular matrix, after activation of HSCs, may also contribute to progression of liver fibrosis.41 The downregulation of TIMPs by anthocyanin-rich PRE-HIF in the rat NASH model in this study and the upregulation of MMP-9 by anthocyanins extracted from blueberries in the mouse carbon tetrachloride-induced liver fibrosis model42 were both considered to be phenomena that work to inhibit liver fibrosis.
Inflammatory cells and cytokines are both involved in the pathogenesis of NASH. The inflammatory pathways involving JNK and NF-κB are potent mediators of inflammation and, therefore, may be targets for the chemoprevention of NASH.30,43 The levels of phosphorylated JNK and NF-κB in the liver were significantly reduced by PRE-HIF, contributing to the inhibition of NASH progression. C3G has been reported to exhibit anti-inflammatory effects in osteoarthritis44 and to alleviate periodontitis by modulating endoplasmic reticulum stress45 by inhibition of NF-κB or JNK signaling pathways. Based on this and previous studies, the inhibition of JNK and NF-κB signaling and NASH progression by PRE may be mainly due to C3G.
In conclusion, intake of PRE-HIF as a dietary supplement may suppress the progression of NASH and liver fibrosis through inactivation of NF-κB and JNK signaling without obvious adverse effects. PRE-HIF inhibited hepatic fat deposition as well as liver injury and fibrosis in NASH, suggesting that it may be useful as a functional food for a broad population.
Author contributions
AN-I, TC and ST conceived and designed the experiments and supervised the project. AN-I, RY, KX, WT, TN, HK, and YN performed research. AN-I, RY, and KX acquired and analyzed the data. AN-I, RY, TC, and ST interpreted the data, AN-I wrote the first draft of the manuscript and all authors commented on the manuscript. All authors read and approved the final manuscript.Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| Bex1 | Brain expressed, X-linked 1 |
| CLD | Chronic liver disease |
| Cx32 | Connexin 32 |
| Cx32ΔTg | Cx32 dominant negative transgenic |
| DMN | Dimethylnitrosamine |
| FFPE | Formalin-fixed paraffin-embedded |
| GST-P | Glutathione S-transferase placental form |
| HCC | Hepatocellular carcinoma |
| H&E | Hematoxylin and eosin |
| HFD | High-fat diet |
| HSC | Hepatic stellate cells |
| JNK | c-Jun N-terminal kinase |
| MMP | Matrix metalloproteinase |
| NAFL | Nonalcoholic fatty liver |
| NAFLD | Non-alcoholic fatty liver disease |
| NAS | Non-alcoholic fatty liver disease activity score |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor-κB |
| P3G | Peonidin 3-glucoside |
| PRE-HIF | Hexane insoluble fraction of purple rice ethanolic extract |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-α |
| TGF-β1 | Transforming growth factor-β1. |
Data availability
The data generated or analysed during this study are included in this published article and its ESI. Any other data sets that support the findings of this study are available on request from the corresponding author [A. N.-I.].Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 19K07509 to A. N.-I.; and Research Grant of the Princess Takamatsu Cancer Research Fund Grant Number 20-25225 to A. N.-I.References
- Z. M. Younossi, P. Golabi, J. M. Paik, A. Henry, C. Van Dongen and L. Henry, The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review, Hepatology, 2023, 77, 1335–1347 CrossRef PubMed.
- J. M. Paik, K. Kabbara, K. E. Eberly, Y. Younossi, L. Henry and Z. M. Younossi, Global burden of NAFLD and chronic liver disease among adolescents and young adults, Hepatology, 2022, 75, 1204–1217 CrossRef CAS PubMed.
- C. P. Day and O. F. James, Steatohepatitis: a tale of two “hits”?, Gastroenterology, 1998, 114, 842–845 CrossRef CAS PubMed.
- H. Tilg and A. R. Moschen, Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis, Hepatology, 2010, 52, 1836–1846 CrossRef CAS PubMed.
- E. Bugianesi, A. Gastaldelli, E. Vanni, R. Gambino, M. Cassader, S. Baldi, V. Ponti, G. Pagano, E. Ferrannini and M. Rizzetto, Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms, Diabetologia, 2005, 48, 634–642 CrossRef CAS PubMed.
- K. L. Donnelly, C. I. Smith, S. J. Schwarzenberg, J. Jessurun, M. D. Boldt and E. J. Parks, Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease, J. Clin. Invest., 2005, 115, 1343–1351 CrossRef CAS PubMed.
- I. Cho, S. Yamanishi, L. Cox, B. A. Methe, J. Zavadil, K. Li, Z. Gao, D. Mahana, K. Raju, I. Teitler, H. Li, A. V. Alekseyenko and M. J. Blaser, Antibiotics in early life alter the murine colonic microbiome and adiposity, Nature, 2012, 488, 621–626 CrossRef CAS PubMed.
- A. R. Moschen, S. Kaser and H. Tilg, Non-alcoholic steatohepatitis: a microbiota-driven disease, Trends Endocrinol. Metab., 2013, 24, 537–545 CrossRef CAS PubMed.
- F. Bessone, M. V. Razori and M. G. Roma, Molecular pathways of nonalcoholic fatty liver disease development and progression, Cell. Mol. Life Sci., 2019, 76, 99–128 CrossRef CAS PubMed.
- A. Naiki-Ito, M. Asamoto, T. Naiki, K. Ogawa, S. Takahashi, S. Sato and T. Shirai, Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat, Toxicol. Pathol., 2010, 38, 280–286 CrossRef CAS PubMed.
- A. Naiki-Ito, H. Kato, M. Asamoto, T. Naiki and T. Shirai, Age-dependent carcinogenic susceptibility in rat liver is related to potential of gap junctional intercellular communication, Toxicol. Pathol., 2012, 40, 715–721 CrossRef CAS PubMed.
- A. Naiki-Ito, H. Kato, T. Naiki, R. Yeewa, Y. Aoyama, Y. Nagayasu, S. Suzuki, S. Inaguma and S. Takahashi, A novel model of non-alcoholic steatohepatitis with fibrosis and carcinogenesis in connexin 32 dominant-negative transgenic rats, Arch. Toxicol., 2020, 94, 4085–4097 CrossRef CAS PubMed.
- H. Sagawa, A. Naiki-Ito, H. Kato, T. Naiki, Y. Yamashita, S. Suzuki, S. Sato, K. Shiomi, A. Kato, T. Kuno, Y. Matsuo, M. Kimura, H. Takeyama and S. Takahashi, Connexin 32 and luteolin play protective roles in non-alcoholic steatohepatitis development and its related hepatocarcinogenesis in rats, Carcinogenesis, 2015, 36, 1539–1549 CAS.
- Y. Aoyama, A. Naiki-Ito, K. Xiaochen, M. Komura, H. Kato, Y. Nagayasu, S. Inaguma, H. Tsuda, M. Tomita, Y. Matsuo, S. Takiguchi and S. Takahashi, Lactoferrin Prevents Hepatic Injury and Fibrosis via the Inhibition of NF-kappaB Signaling in a Rat Non-Alcoholic Steatohepatitis Model, Nutrients, 2021, 14, 42 CrossRef PubMed.
- B. S. Sivamaruthi, P. Kesika and C. Chaiyasut, Anthocyanins in Thai rice varieties: distribution and pharmacological significance, Int. Food Res. J., 2018, 25, 2024–2032 CAS.
- T. Pattananandecha, S. Apichai, S. Sirilun, J. Julsrigival, K. Sawangrat, F. Ogata, N. Kawasaki, B. Sirithunyalug and C. Saenjum, Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand, Molecules, 2021, 26, 5234 CrossRef CAS PubMed.
- E. T. Callcott, C. L. Blanchard, P. Snell and A. B. Santhakumar, The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans, Food Funct., 2019, 10, 8230–8239 RSC.
- R. Chatthongpisut, S. J. Schwartz and J. Yongsawatdigul, Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells, Food Chem., 2015, 188, 99–105 CrossRef CAS PubMed.
- J. Tanaka, T. Nakanishi, K. Ogawa, K. Tsuruma, M. Shimazawa, H. Shimoda and H. Hara, Purple rice extract and anthocyanidins of the constituents protect against light-induced retinal damage in vitro and in vivo, J. Agric. Food Chem., 2011, 59, 528–536 CrossRef CAS PubMed.
- R. Summart and T. Chewonarin, Purple rice extract supplemented diet reduces DMH- induced aberrant crypt foci in the rat colon by inhibition of bacterial beta-glucuronidase, Asian Pac. J. Cancer Prev., 2014, 15, 749–755 CrossRef PubMed.
- S. Wongjaikam, R. Summart and T. Chewonarin, Apoptosis induction in colon cancer cell lines and alteration of aberrant crypt foci in rat colon by purple rice (Oryza sativa L. var. glutinosa) extracts, Nutr. Cancer, 2014, 66, 690–699 CrossRef PubMed.
- C. Kiriya, R. Yeewa, C. Khanaree and T. Chewonarin, Purple rice extract inhibits testosterone-induced rat prostatic hyperplasia and growth of human prostate cancer cell line by reduction of androgen receptor activation, J. Food Biochem., 2019, 43, e12987 CrossRef PubMed.
- R. Yeewa, W. Sakuludomkan, C. Kiriya, C. Khanaree and T. Chewonarin, Attenuation of benign prostatic hyperplasia by hydrophilic active compounds from pigmented rice in a testosterone implanted rat model, Food Funct., 2020, 11, 1585–1598 RSC.
- R. Yeewa, A. Naiki-Ito, T. Naiki, H. Kato, S. Suzuki, T. Chewonarin and S. Takahashi, Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism, Nutrients, 2020, 12, 558 CrossRef CAS PubMed.
- M. Asamoto, N. Hokaiwado, T. Murasaki and T. Shirai, Connexin 32 dominant-negative mutant transgenic rats are resistant to hepatic damage by chemicals, Hepatology, 2004, 40, 205–210 CrossRef CAS PubMed.
- H. Kato, A. Naiki-Ito, T. Naiki, S. Suzuki, Y. Yamashita, S. Sato, H. Sagawa, A. Kato, T. Kuno and S. Takahashi, Connexin 32 dysfunction promotes ethanol-related hepatocarcinogenesis via activation of Dusp1-Erk axis, Oncotarget, 2016, 7, 2009–2021 CrossRef PubMed.
- D. E. Kleiner, E. M. Brunt, M. Van Natta, C. Behling, M. J. Contos, O. W. Cummings, L. D. Ferrell, Y. C. Liu, M. S. Torbenson, A. Unalp-Arida, M. Yeh, A. J. McCullough and A. J. Sanyal, Design and validation of a histological scoring system for nonalcoholic fatty liver disease, Hepatology, 2005, 41, 1313–1321 CrossRef PubMed.
- T. D. Challa, S. Wueest, F. C. Lucchini, M. Dedual, S. Modica, M. Borsigova, C. Wolfrum, M. Bluher and D. Konrad, Liver ASK1 protects from non-alcoholic fatty liver disease and fibrosis, EMBO Mol. Med., 2019, 11, e10124 CrossRef CAS PubMed.
- X. Song, J. Sun, H. Liu, A. Mushtaq, Z. Huang, D. Li, L. Zhang and F. Chen, Lycopene Alleviates Endoplasmic Reticulum Stress in Steatohepatitis through Inhibition of the ASK1-JNK Signaling Pathway, J. Agric. Food Chem., 2024, 72, 7832–7844 CrossRef CAS PubMed.
- Y. Yu, Y. Liu, W. An, J. Song, Y. Zhang and X. Zhao, STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis, J. Clin. Invest., 2019, 129, 546–555 CrossRef PubMed.
- P. Kubes and W. Z. Mehal, Sterile inflammation in the liver, Gastroenterology, 2012, 143, 1158–1172 CrossRef CAS PubMed.
- M. Marí, A. Morales, A. Colell, C. García-Ruiz and J. C. Fernandez-Checa, in Studies on Hepatic Disorders, ed. E. Albano and M. Parola, Springer International Publishing, Cham, 2015, pp. 279–308, DOI:10.1007/978-3-319-15539-5_12.
- P. Perez-Matute, L. Perez-Martinez, J. R. Blanco and J. A. Oteo, Role of mitochondria in HIV infection and associated metabolic disorders: focus on nonalcoholic fatty liver disease and lipodystrophy syndrome, Oxid. Med. Cell. Longevity, 2013, 2013, 493413 CAS.
- R. Hao, S. Shan, D. Yang, H. Zhang, Y. Sun and Z. Li, Peonidin-3-O-Glucoside from Purple Corncob Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Mitochondrial and Lysosome Functions to Reduce Oxidative Stress and Inflammation, Nutrients, 2023, 15, 372 CrossRef CAS PubMed.
- X. Hao, R. Guan, H. Huang, K. Yang, L. Wang and Y. Wu, Anti-inflammatory activity of cyanidin-3-O-glucoside and cyanidin-3-O-glucoside liposomes in THP-1 macrophages, Food Sci. Nutr., 2021, 9, 6480–6491 CrossRef CAS PubMed.
- X. Yan, L. Wu, B. Li, X. Meng, H. Dai, Y. Zheng and J. Fu, Cyanidin-3-O-glucoside attenuates acute lung injury in sepsis rats, J. Surg. Res., 2015, 199, 592–600 CrossRef CAS PubMed.
- X. Zhu, X. Lin, P. Zhang, Y. Liu, W. Ling and H. Guo, Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study, Clin. Res. Hepatol. Gastroenterol., 2022, 46, 101843 CrossRef CAS PubMed.
- K. Yamaguchi, L. Yang, S. McCall, J. Huang, X. X. Yu, S. K. Pandey, S. Bhanot, B. P. Monia, Y. X. Li and A. M. Diehl, Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis, Hepatology, 2008, 47, 625–635 CrossRef CAS PubMed.
- Y. A. Lee, M. C. Wallace and S. L. Friedman, Pathobiology of liver fibrosis: a translational success story, Gut, 2015, 64, 830–841 CrossRef CAS PubMed.
- X. Jiang, T. Shen, X. Tang, W. Yang, H. Guo and W. Ling, Cyanidin-3-O-beta-glucoside combined with its metabolite protocatechuic acid attenuated the activation of mice hepatic stellate cells, Food Funct., 2017, 8, 2945–2957 RSC.
- M. Parola and M. Pinzani, Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues, Mol. Aspects Med., 2019, 65, 37–55 CrossRef CAS PubMed.
- J. Sun, Y. Wu, C. Long, P. He, J. Gu, L. Yang, Y. Liang and Y. Wang, Anthocyanins isolated from blueberry ameliorates CCl(4) induced liver fibrosis by modulation of oxidative stress, inflammation and stellate cell activation in mice, Food Chem. Toxicol., 2018, 120, 491–499 CrossRef CAS PubMed.
- S. L. Friedman, B. A. Neuschwander-Tetri, M. Rinella and A. J. Sanyal, Mechanisms of NAFLD development and therapeutic strategies, Nat. Med., 2018, 24, 908–922 CrossRef CAS PubMed.
- H. P. Tu, C. Y. Kuo, M. M. Fu, Y. T. Chin, C. Y. Chiang, H. C. Chiu, Y. J. Hsia and E. Fu, Cyanidin-3-O-glucoside downregulates ligation-activated endoplasmic reticulum stress and alleviates induced periodontal destruction in rats, Arch. Oral Biol., 2022, 134, 105313 CrossRef CAS PubMed.
- T. Wongwichai, P. Teeyakasem, D. Pruksakorn, P. Kongtawelert and P. Pothacharoen, Anthocyanins and metabolites from purple rice inhibit IL-1beta-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-kappaB and ERK/MAPK pathway, Biomed. Pharmacother., 2019, 112, 108610 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |