Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
α acid fraction from Hop extract exerts an endothelium-derived hyperpolarization vasorelaxant effect through TRPV4 employing the feedforward mechanism of PKCα†
Paola
Di Pietro‡
a,
Emanuela
Salviati‡
b,
Antonio
Damato
c,
Valeria
Prete
a,
Angela Carmelita
Abate
a,
Pietro
Campiglia
 b,
Carmine
Vecchione
ac,
Eduardo
Sommella
b and
Albino
Carrizzo
b,
Carmine
Vecchione
ac,
Eduardo
Sommella
b and
Albino
Carrizzo
 *ac
*ac
aDepartment of Medicine Surgery and Dentistry “Scuola Medica Salernitana”, Baronissi, SA 84081, Italy. E-mail: acarrizzo@unisa.it
bDepartment of Pharmacy, University of Salerno, Fisciano, SA 84084, Italy
cIRCCS Neuromed, Istituto Neurologico Mediterraneo, Pozzilli, IS 86077, Italy
First published on 20th March 2024
Abstract
Until now, the beneficial vascular properties of Hop reported in the literature have been mainly attributed to specific compound classes, such as tannins and phenolic acids. However, the potential vascular action of a Hop subfraction containing a high amount of α or β acids remains completely understood. Therefore, this study aims to investigate the vascular effects of the entire Hop extract and to fraction the Hop extract to identify the main bioactive vascular compounds. A pressure myograph was used to perform vascular reactivity studies on mouse resistance arteries. Phytocomplex fractionation was performed on a semi-prep HPLC system and characterized by UHPLC-PDA-MS/MS coupled to mass spectrometry. Western blot analysis was performed to characterize the phosphorylation site enrolled. The entire Hop extract exerts a direct dose-dependent endothelial vascular action. The B1 subfraction, containing a high concentration of α acids, recapitulates the vascular effect of the crude extract. Its vasorelaxant action is mediated by the opening of Transient Receptor Potential Vanilloid type 4 (TRPV4), potentiated by PKCα, and subsequent involvement of endothelial small-conductance calcium-activated potassium channels (SKCa) and intermediate-conductance calcium-activated potassium channels (IKCa) that drives endothelium-dependent hyperpolarization (EDH) through heterocellular myoendothelial gap junctions (MEGJs). This is the first comprehensive investigation of the vascular function of Hop-derived α acids in resistance arteries. Overall, our data suggest that the B1 subfraction from Hop extracts, containing only α acids, has great potential to be translated into the useful armamentarium of natural bioactive compounds with cardiovascular benefits.
Introduction
Over the past 20 years, the hop, the female inflorescence of the plant Humulus lupulus L. belonging to the Cannabinaceae family, has attracted the attention of the scientific community for its beneficial properties, so scientific reports of hop polyphenols have multiplied.1 It is well-known that a significant portion of hop polyphenols is made up of higher molecular compounds such as tannins, and a small portion of the hop polyphenols consists of low molecular substances like phenolic acids (e.g., ferulic acid, flavan-3-ol monomers and flavonols such as quercetin and kaempferol),2 which are glycosidically bound to various sugars3 and part of the soft resin fraction consists of two related series, α-acids (humulone, cohumulone, and adhumulone) andβ-acids (lupulone, colupulone, and adlupulone).4 The literature shows that certain polyphenols contained in the Hop seem to be particularly active in evoking beneficial actions for human health. Xanthohumol (XN) and 8-prenylnaringenin (8-PN), known polyphenols, have been identified as multipotent bioactive compounds.5 Multiple reports have demonstrated that xanthohumol treatment is able to inhibit vascular calcification, reducing the overexpression of osteogenic transcription factors bone morphogenetic protein 2 (BMP-2) and runt-related transcription factor 2 (Runx2) and enhancing the antioxidant capacity through Nrf2 enhancement in a rat model;6 while, only a single study has demonstrated the direct vascular effect of XN on rats’ thoracic aortic ring, documenting the capability of XN to evoke a dose–response relaxation through a nitric oxide-dependent mechanism.7 On the other hand, 8-PN prevents agonist-dependent activation of Akt, ERK1/2, and Pyk2, key modulators of platelet activation, calcium mobilization, and dense granule secretion and increases cyclic nucleotides within the platelets. Recently, the combined supplementation with 8-PN or XN in diabetic mice reverses diabetes-associated oxidation in the liver and kidneys, consequently decreasing this diabetic biomarker that predisposes to cardiovascular complications.8Different from what has been reported about polyphenols, evidence about the cardiovascular effects of α and β acids is very poor. A single paper has demonstrated the anti-inflammatory and positive endothelial effects of tetrahydro-iso-alpha acids (THIAAs) from hops in combination with niacin in patients with dyslipidemia.9 Its beneficial role has been attributed to the modulation of lipid metabolism, glucose tolerance and body weight and to its protective role in liver injury by reducing oxidative stress via Nrf2-mediated gene expression.10
However, the potential vascular action of a Hop subfraction containing high amounts of α and β acids and their related molecular mechanism remains completely understood.
Here, we demonstrate for the first time, by the application of a funnel effect on the whole hop extract, defined as a series of sequential fractionations and functional characterization using whole extract chromatography-based approaches, aiming at identifying only a small number of bioactive α and β acids present in subfractions, that subfraction B1, containing only α acids, is able to evoke dose-dependent relaxation of mouse mesenteric resistance arteries through the endothelium-dependent hyperpolarization (EDH) response. Its mechanism is mediated by endothelial small-conductance calcium-activated potassium channels (SKCa) and intermediate-conductance calcium-activated potassium channels (IKCa) driven by the activation of Transient Receptor Potential Vanilloid type 4 (TRPV4), which is amplified by the feedforward mechanism of PKCα phosphorylation.
Materials and methods
Reagents
Ultra-pure water (H2O) was obtained using a Direct-8 Milli-Q system (Millipore, Milan, Italy); LC-MS grade acetonitrile (ACN) and water (H2O), methanol (CH3OH) and the additives formic acid (CH3OOH) and trifluoroacetic acid (TFA) were all purchased from Sigma-Aldrich (St Louis (MO), USA). Hop pellets (Target T90) were kindly donated by a local brewery company (AEFFE, Castel San Giorgio, Salerno, Italy). All other powders and inhibitors were purchased from Sigma-Aldrich.Hop bitter acid fractionation and characterization
Extraction, isolation and characterization of hop bitter acid fractions were performed by semi-preparative reversed liquid chromatography (semi-prep RP-HPLC) and reversed-phase ultra-high-performance liquid chromatography-tandem mass spectrometry (RP UHPLC-MS/MS) as previously optimized.11,12 Briefly, the whole phytocomplex was extracted as follows. 1 g of sample was extracted with 12.5 mL of CH3OH and kept under stirring for 15 minutes in the dark at room temperature. The operation was repeated three times. The supernatants were pooled, dried under reduced pressure, and lyophilized. The sample was solubilized in CH3OH and filtered on a 0.45 μm nylon membrane prior to injection in the LC system. The whole phytocomplex was characterized as reported by Sommella et al., 2018.13 Phytocomplex fractionation was performed on a semi-preparative HPLC LC20AP system (Shimadzu, Milan, Italy). A Luna® C18 column 250 mm × 10 mm, 5 μm was employed at a flow rate of 6 mL min−1; the mobile phase was (A) 0.1% CH3COOH in H2O and (B) 0.1% CH3COOH in ACN. Analysis was performed in gradient as follows: 0 min, 5%B; 10 min, 5–40%B; 15 min, 40–75%B; 17 min, 75–100%B; and 22 min, 100%B. Detection was performed using a photodiode array (PDA) and chromatograms were monitored at 280 and 330 nm. Three fractions were collected based on retention time windows. In order to assess the correct fractionation process, the obtained crude fractions were further characterized by UHPLC-PDA-MS/MS on a UHPLC Nexera coupled to an IT-TOF mass spectrometer (Shimadzu, Milan, Italy). A Kinetex® EVO C18 150 × 2.1 mm, 2.6 μm (Phenomenex®) column was employed at a flow rate of 0.5 mL min−1. The separation was performed with the following parameters: mobile phase was: (A) 0.1% CH3COOH in H2O v/v, (B) ACN plus 0.1% CH3COOH, gradient: 0–15 min, 5–30%B; 15–20 min, 30–70%B; 20–22 min, 70–95%B; 22–25 min, 98–98%B; and 25–30 min, 5%B. The column oven was set at 45 °C, and 2 μL of sample was injected. PDA detection parameters were: sampling rate 12 Hz, time constant 0.160 s and chromatograms were extracted at 280 and 330 nm. ESI-MS detection was performed in negative ionization mode as follows: detector, 1.60 kV; CDL (curve desolvation line), 250 °C; heater block, 250 °C; nebulizer (N2), 1.5 L min−1; drying gas, 100 kPa. MS1 150–1500 m/z; ion accumulation time, 30 ms; IT, repeat = 3. MS/MS data dependent acquisition precursor range: 150–900 m/z; peak width 3 Da, accumulation time 40 ms; CID energy, 50%; repeat = 3. The confirmation of the metabolite identity was done using high resolution MS and MS/MS spectra, the retention time of available standards, and comparison with the online database (Mass bank of North America (MoNA) and PubChem) and previous MS/MS data obtained on the same MS device.13 The raw LCMS data associated with the characterization of the hop phytocomplex and matrices are reported in the Zenodo (https://zenodo.org/) repository with the following doi: https://doi.org/10.5281/zenodo.10632144.Ex vivo vascular reactivity studies
The data that support the findings of this study are available from the corresponding authors on reasonable request. All vascular experiments were performed on second-order branches of the mesenteric arterial tree removed from male C57BL/6 mice (project no. 766/2018-PR). The vessels were placed in a pressure myograph system filled with Krebs solution at pH 7.4 at 37 °C as previously described.14 In some experiments, the endothelium was mechanically removed by inserting a tungsten wire into the lumen of the vessel and rotating it back and forth before mounting the vessel on the pressure myograph. Caution was taken to avoid endothelial damage. Some mesenteric arteries mounted on a myograph system were pre-treated for 1 h with 300 μM of the eNOS inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME) (Sigma-Aldrich; #N5751) for 30 min, before data for dose–response curves were obtained. Another experimental series was performed on vessels transfected in the presence or absence of Ca2+. Other experimental series were performed in the presence of apamin (Apa)—a potent inhibitor of ATP-type Ca2+-activated K+ channels and SKCa,—and charybdotoxin (CTx)—a potent and selective inhibitor of the voltage-gated Ca2+-activated K+ channel (Kv1.3) and BKCa channel. Other experimental series were performed using TRAM-34, a selective inhibitor of IKCa current, in the presence of H-89, a selective inhibitor of PKA or in the presence of GSK205, a selective inhibitor of the TRPV4 channel. Other experiments have been performed in the presence of the connexin mimetic peptide 40,37GAP26, a biologically active peptide with high inhibitory effects against subintimal hyperpolarization. Quantification of the vasomotor response was performed by a second individual who was blind to the genotype of the animal and/or the hypothesis that was being tested for each group. In all vascular experiments, precontraction was obtained by administering increasing doses of phenylephrine (10−9 to 10−6 M) in order to obtain a similar level of pre-contraction equal to 80% of initial KCl-induced constriction.Protein extraction and immunoblot analysis
For total protein extraction, mesenteric arteries were lysed in a buffer containing 150 mmol L−1 NaCl, 50 mmol L−1 Tris-HCl (pH 8.5), 2 mmol L−1 EDTA, 1% v/v NP-40, 0.5% w/v deoxycholate, 10 mmol L−1 NaF, 10 mM sodium pyrophosphate, 2 mmol L−1 PMSF, 2 g mL−1 leupeptin, and 2 g mL−1 aprotinin, pH 7.4. Lysates were incubated on ice for 15 min and then centrifuged at 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min at 4 °C to collect the supernatant. The protein concentration was measured using a dye-binding protein assay kit (Bio-Rad) and reading to the spectrophotometer at a wavelength of 595 nm. Immunoblotting was performed as previously described.15 Secondary antibodies (1
000g for 30 min at 4 °C to collect the supernatant. The protein concentration was measured using a dye-binding protein assay kit (Bio-Rad) and reading to the spectrophotometer at a wavelength of 595 nm. Immunoblotting was performed as previously described.15 Secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000) were purchased from Amersham Life Sciences. Bands were visualized with enhanced chemiluminescence (ECL, Amersham Life Sciences), according to the manufacturer's instructions. Immunoblotting data were analyzed using ImageJ software (Wayne Rasband, NIH, USA) to determine the density of the bands.
3000) were purchased from Amersham Life Sciences. Bands were visualized with enhanced chemiluminescence (ECL, Amersham Life Sciences), according to the manufacturer's instructions. Immunoblotting data were analyzed using ImageJ software (Wayne Rasband, NIH, USA) to determine the density of the bands.
Statistical analysis
All data are presented as mean ± SEM. For continuous variables, we used a t-test to compare 2 independent groups. When more than 2 independent groups were compared, we used 1-way ANOVA followed by the Bonferroni post hoc test. To analyze the effects of our treatments on endothelium-dependent vasorelaxation in response to increasing doses of acetylcholine, we performed a 2-way repeated-measures ANOVA with the Bonferroni post hoc test for multiple comparisons. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted with Prism statistical software (Graphpad, La Jolla CA, USA).Results
The entire Hop extract exerts a direct endothelial action
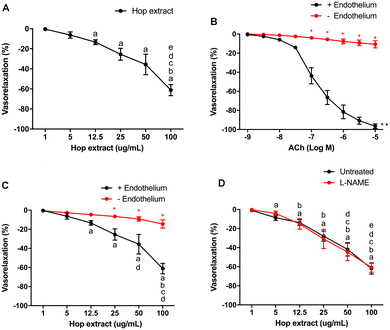
To evaluate whether the entire Hop extract exerts an effect on vascular function, we exposed ex vivo phenylephrine-preconstricted mouse mesenteric arteries—a prototype of resistance vessel involved in the modulation of blood pressure—to increasing doses (1–100 μg mL−1) of Hop extract (Fig. 1A). Hop extract evoked vasorelaxation in a dose-dependent manner, reaching about 60% of vasorelaxation, an effect completely abolished in the absence of endothelium mechanically removed, suggesting that endothelial cells represent the cellular target of the extract (Fig. 1B and C). The pre-exposure of vessels to N(ω)-nitro-L-arginine methyl ester (L-NAME), a well-characterized inhibitor of eNOS enzyme did not evoke any effects on the vasorelaxant action of Hop extract (Fig. 1D).Under calcium-free conditions Hop extract fails to modulate vascular function
In order to dissect the molecular mechanisms involved in the vasorelaxant effect of Hop extract, we performed vascular reactivity studies using several specific inhibitors of endothelial signaling pathways. Preincubation of mesenteric arteries with indomethacin, a potent active COX1/2 inhibitor, did not evoke any effect on the dose–response action driven by Hop extract (Fig. 2A). A similar condition has been detected using CAY10441, a potent and selective IP (prostacyclin) receptor antagonist, thus firmly excluding the involvement of COX-produced metabolites on its vascular effect (Fig. 2B). Considering the several shreds of evidence suggesting an involvement of calcium-sensitive potassium (KCa) channel pathways in plant derived estrogen-induced vasodilation beyond the endothelial NO16,17 and prostaglandin's vasorelaxant action,18 we have performed a vascular study using thapsigargin, a potent, non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) that plays an important role in the maintenance of the vessel tone. Under these experimental conditions Hop extract continues to evoke dose-dependent vasorelaxation (Fig. 2C). Thus, we performed a new experiment in the external free-calcium medium. Surprisingly, under external calcium-free conditions the Hop extract fails to evoke any vasorelaxant effects (Fig. 2D), thus suggesting that the compound is able to modulate the vascular tone regulating the calcium flux. In association with the effective inhibition observed in the dose–response curve graph, the analysis of vessels’ increased diameter (μm) during the assay revealed the inability to modulate the vessel tone (right graph) at each dose. These data led us to explore Ca2+-permeable cation channels expressed on the endothelial cell membrane as a possible mechanism recruited by Hop extract.Fractionation of the entire Hop extract ascribes the vascular effect to a hop fraction with a high content of α and β acids (fraction B)
To better characterize the vascular effect and narrow the field of molecules from the Hop extract that are mainly active at the vascular level, we simplified the entire Hop extract in different secondary metabolite fractions by a semi-prep HPLC strategy. Three fractions were collected based on the retention time, and thus hydrophobicity (A, B and C Fractions). The class specific fractions were further screened by UHPLC-PDA-MS/MS to characterize all metabolites present in the mixture. In particular, Hop fraction A contained hydroxycinnamic acids, flavonol-glycosides and procyanidins, fraction B was characterized by α-acids, while fraction C was rich in β-bitter acids and prenylflavonoids (Table S1†).Vascular reactivity evaluation using the three fractions obtained revealed that only the B fraction was able to recapitulate the same vasorelaxant action evoked by the entire Hop extract (Fig. 3A–C). As already observed for Hop extract, upon the assessment of the vasorelaxant capability in an external Ca2+-free medium, the B fraction completely loses its vascular action (Fig. 3D).
At this point, to investigate possible specific Ca2+ channels involved in its vascular action, we performed another experimental series in the presence of Ca2+ but using apamin (APA)—a potent inhibitor of ATP-type Ca2+-activated K+ channels and SKCa,—and charybdotoxin (CTx)—a potent and selective inhibitor of the voltage-gated Ca2+-activated K+ channel (Kv1.3) and BKCa channels. Interestingly, in the presence of Ca2+ complete medium, the specific inhibition of SKCa and BKCa was able to significantly reduce the vasorelaxant effect evoked by the B fraction (from 62% → 25%) (Fig. 4A). The measurement of the vessel lumen during the dose–response curve revealed a marked inhibition in the increase of vessel diameter (Fig. 4A, right graph).
Beyond SKCa and BKCa, the intermediate-conductance calcium-activated potassium channels (IKCa) exert a pivotal role in endothelium-dependent hyperpolarization (EDH) responses at the vascular level.19 Together with SKCa, IKCa has been shown to be localized on arterial endothelial cells, but are not present on the smooth muscle.20 Considering the specific endothelial effects of the Hop extract-derived B fraction, we have performed a vascular reactivity study using TRAM-34, a highly selective blocker of IKCa3.1. Interestingly, in the presence of TRAM-34, the B fraction significantly loses effectiveness in inducing dose-dependent vasodilation (from 60% → 35%) (Fig. 4B).
Combining the treatment with Apamin and TRAM-34, the dose–response curve of the B fraction shows a complete abolishment of its vasorelaxant properties (Fig. 4C).
This huge effect was also confirmed by the evaluation of the increased diameter showing the inability of the B fraction to induce vasorelaxation, thus confirming the involvement of SKCa and IKCa in the vascular action of the B fraction.
It is well known that endothelial hyperpolarization, initiated by the activation of two Ca2+-activated K+ (KCa), can be transmitted to myocytes through the myoendothelial microdomain signaling sites.21 These sites are characterized by heterocellular myoendothelial gap junctions (MEGJs) which are composed of connexin proteins, of which six molecules assemble to form a hexameric hemichannel.22 Thus, to selectively investigate this process we performed studies using 40,37GAP26, a biologically active peptide able to inhibit the vascular assembly of connexins Cx37 and Cx40. Of note, during the inhibition of Cx37 and Cx40 the B fraction was not able to evoke a dose–response vasorelaxation (Fig. 4D).
The additional funnel effect on the B fraction identifies the B1 subfraction as the most bioactive mixture at the vascular level
These results prompted us to deeply understand the vascular reactivity of the B fraction, so, with the goal of keeping to a minimum the number of vascularly active compounds we proceeded to a further simplification (by semi-prep liquid chromatography) into three sub-fractions called B1, B2 and B3, respectively. During the first assessment of their vascular properties, we revealed that only the B1 subfraction was able to evoke a dose-dependent vascular action, in contrast to that observed during the dose–response curves using B2 and B3 subfractions which were not able to modify the vascular tone (Fig. 5A). The evaluation of vascular diameter changes did not reveal any modification for B2 and B3, different from that we observed for the B1 fraction, which was able to reach an increase of 120 μm. Interestingly, the UHPLC-MS/MS analysis revealed that the B1 subfraction is composed of α acids such as Humulinone, Cohumulone, and Cohumulinone and their derivatives (Table 1 & Fig. 5B). | ||
| Fig. 5 Dose–response curves in ex vivo C57BL/6 mouse mesenteric arteries (A) to B1, B2 and B3 fractions and the related changes in the internal diameter expressed in μm (right graph). (B) PDA chromatogram profile (λ: 330 nm) of secondary metabolites isolated from the Hop B1 fraction with peak assignment (HRMS/MS peak annotation is reported in Table 1); (C) dose–response curves of ex vivo C57BL/6 mouse mesenteric arteries to the B1 fraction and the related changes in the internal diameter expressed in μm (right graph) before and after exposure to Tram-34; (D) before and after exposure to Tram-34 plus Apamin or (E) before and after exposure to 40,37Gap26. Values are means ± S.D., n = 5 experiments per group. Statistics were performed using two-way ANOVA. Intergroup statistics were performed by one-way ANOVA with repeated measures followed by Bonferroni's multiple comparison post hoc test. (A) *, P < 0.05 or **, P < 0.001 vs. untreated at the same concentration (as indicated by color code); (C–E) *, P < 0.05 or **, P < 0.001 vs. B1 fraction at the same concentration (as indicated by the color code); (A–E) a, P < 0.05 compared to 1 μg mL−1; b, P < 0.05 compared to 5 μg mL−1; c, P < 0.05 compared to 12.5 μg mL−1; d, P < 0.05 compared to 25 μg mL−1; e, P < 0.05 compared to 50 μg mL−1 (as indicated by the color code). | ||
| Peak | r t (min) | Compound | [M − H]− | [MS/MS] | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 4.25 | Hydro-cohumulinone | 381.1912 | 181.0541 | C20H30O7 | −1.84 |
| 2 | 5.22 | Hydro-adhumulinone | 395.2053 | 195.0698 | C21H32O7 | −2.02 |
| 3 | 6.01 | Oxy-humulinone | 393.1924 | 247.1727; 349.2028; 395.1835 | C21H30O7 | 1.27 |
| 4 | 6.67 | Oxidized Humulinone | 333.1701 | 249.0771; 205.0532; 221.0491 | C19H26O5 | −1.50 |
| 5 | 6.91 | Oxy-humulinone isomer | 393.1919 | 263.1316; 349.2034; 395.1865 | C21H30O7 | 0.01 |
| 6 | 7.04 | Oxidized Humulinone isomer | 333.1700 | 205.0540; 221.0494 | C19H26O5 | −1.30 |
| 7 | 8.10 | Humulinone | 377.1959 | 221.0743; 263.1311 | C21H30O6 | −2.92 |
| 8 | 8.36 | Cohumulone | 347.1853 | 263.0930; 219.0680; 278.1163 | C20H28O5 | −3.17 |
| 9 | 8.73 | Cohumulinone | 363.1817 | 249.1157 | C20H28O6 | 1.10 |
Re-assessing the vascular reactivity response of the B1 subfraction we demonstrate that TRAM-34 was able to significantly attenuate the vascular effect evoked by the B1 subfraction, reducing the vasodilating effect by about 25% (Fig. 5C). The concomitant treatment with TRAM-34 plus Apamin, thus blocking SKCa and IKCa, respectively, completely abolished the vasorelaxant effect evoked by the B1 subfraction (Fig. 5D). Moreover, in the presence of 40,37GAP26, the B1 subfraction completely loses its vascular properties (Fig. 5E), thus confirming the key role of MEGJs in transferring EDH to smooth muscle cells.
Transient receptor potential vanilloid type 4 (TRPV4) is required to translate the vascular effects of the B1 subfraction
One of the main activation processes leading EDH pathways depends on an increase in endothelial [Ca2+]i that drives the opening of endothelial not voltage-gated channels SKCa and IKCa.21 Recent evidence has demonstrated that the treatment with Transient Receptor Potential Vanilloid type 4 (TRPV4) agonists promotes an intracellular calcium increase and elicits potent vasodilation that can be inhibited only using TRPV4 blockers.23 To underpin the molecular mechanisms involved in the potent vascular effects evoked by the B1 subfraction, we performed vascular studies using GSK205, a potent, and selective TRPV4 antagonist with IC50s of 2 nM. During the TRPV4 inhibition, the B1 subfraction completely loses the vasorelaxant capability (Fig. 6A).TRPV4 activity can be modulated by PKA and PKCα, which, through specific phosphorylation, can determine its activity amplification.24 During the treatment with H-89, a selective PKA inhibitor, the B1 subfraction was still able to evoke a dose-dependent vasorelaxation (Fig. 6B). Conversely, using siRNA of PKCα we observed a significant abolishment of the B1 vasorelaxant response (Fig. 6C), thus suggesting that PKCα represents the specific kinase involved in the TRPV4 sensitization under α acid stimulation.
To investigate the molecular involvement of PKCα, we performed a western blot analysis on protein extract obtained from mesenteric arteries stimulated with the B1 subfraction and pretreated with the PKA inhibitor or with short interfering RNA (siRNA) PKCα. Our results revealed that the B1 subfraction treatment evokes the increase of TRPV4 phosphorylation in serine 824, which is dependent on PKCα activation, which in turn was phosphorylated in the threonine 497 activation site (Fig. 7).
 | ||
| Fig. 7 Representative immunoblots and densitometric analyses of 5 independent experiments evaluating protein levels of phospho-S824-TRPV4, phospho-T497-PKCα, and phospho-T197-PKA expression in ex vivo C57BL/6 mouse mesenteric arteries exposed to vehicle (untreated) or to the B1 subfraction before and after exposure to the PKA inhibitor, H-89 or in the presence of siRNA against PKCα. Values are means ± S.D. Statistics were performed using two-way ANOVA with Tukey's multiple comparisons. *, P < 0.05 or **, P < 0.01, *** P < 0.001. Raw data of five independent western blotting are reported in the ESI, Fig. S1.† | ||
These data demonstrate for the first time that the B1 subfraction exerts an endothelium-derived hyperpolarization vasorelaxant effect through TRPV4 employing a feedforward mechanism of PKCα.
Discussion
The main finding of the present study was the characterization of the vascular effects of the Hop extract applying the funnel approach in order to locate, among a few compounds contained in the extract, the best vasoactive molecule(s), with the potential to be translated into a useful armamentarium of natural bioactive compounds with cardiovascular benefits.It is well-known that current cardiovascular disease treatment is initially based on diet, exercise, and specific pharmacological therapy, which is able to improve vascular function, endothelial integrity, and vascular tone and reduce chronic inflammatory impairment. However, when the condition is diagnosed late, treatment is not always effective, thus forcing us to use multiple medications that greatly worsen patient compliance. For these reasons, scientific research has long focused on characterizing and identifying new natural bioactive compounds with beneficial vascular effects, since they are highly tolerated by the patient in the preventive mode and exert also an excellent function as adjuvants to drug therapy.25
Plants, as extracts or isolated pure compounds, have already been shown to play a valuable role in the prevention or treatment of lifestyle-related disorders and cardiovascular diseases.26–28 Several studies have described the beneficial effects of various natural extracts and active principles and their potential role in the improvement of the cardiovascular system, reducing oxidative stress, controlling dyslipidemia and glycemia, etc.29 On this issue, a great deal of interest has been expressed towards natural bioactive compounds as functional ingredients in the hop extract due to their various beneficial health effects reported in the literature. In the in vitro model, H. lupulus hops extract is able to exert an anti-aggregatory action reducing GPIIb/IIIa expression. This effect has been mainly attributed to Xanthohumol.30 Maliar et al. demonstrated that a methanolic extract of hop is able to inhibit the activity of thrombin and urokinase.31 The beneficial effects of hop extract and its related compounds have also been translated into an animal model of cardiovascular risk factors. In fact, in obese rodents, a diet containing a high load of isohumulones normalizes the metabolism of lipids.32 Finally, in humans, moderate and regular daily intake of hop extract seems to exert beneficial effects, increasing the antioxidant potential of high-density lipoprotein (HDL) and facilitating cholesterol efflux33,34 and reducing the inflammatory markers in men at a high cardiovascular risk.35
Although all these beneficial vascular effects have been demonstrated, the direct properties of Hop in modulating vascular tone have been poorly investigated. Only two studies have assessed the direct vascular effects of hop extract. In particular, in 2008, it was demonstrated that aqueous Humulus lupulus L. extract has a relaxant effect on endothelium intact thoracic arterial rings obtained from ovariectomized rats. Its effect is mediated by NOS activation, cyclooxygenase products and Ca2+ pathways.7 While, in 2020, Jeon SY et al. investigated the vascular property of a combination of red clover and hop extract (RHEC), demonstrating its capability to evoke vasorelaxation of rat aortic rings, suggesting once again the involvement of NO in its mechanism of action.36
To the best of our knowledge, the direct vascular effectiveness hop extract on resistance arteries has not been reported yet. Moreover, to date, the data available in the literature have focused the attention only on the polyphenolic extracts of Hop, neglecting the beneficial potential that lies in the α and β acids.
We have previously evaluated the immunomodulatory and anti-inflammatory effects of a specific Humulus lupulus L. secondary metabolite fraction. Specifically, cytofluorimetric analysis showed that a fraction containing bitter acids was able to up-regulate NKG2D and NKp44 activating receptors and enhanced the cytolytic activity of NK cells against the leukemia cell line K562.11,12,37 Moreover, a mass-spectrometry based metabolomics analysis revealed that the fraction rich in beta acids and prenylflavonoids regulates the inflammatory response in dendritic cells after stimulation with lipopolysaccharide (LPS).11
In the present work, in a similar way, we decide to investigate the possible direct action of Hop extract in mouse mesenteric arteries, which represent a prototype of resistance vessels ideal to investigate the involvement of IKCa/SKCa channels and eNOS-dependent vasodilatation, respectively, starting from the whole Hop extract and applying a funnel effect to identify the vascularly active molecule(s).
Although in the present study we have tested a low dosage of Hop extract compared to the previous literature (100 μg mL−1vs. 500 μg mL−1 (ref. 36) or 301 μg mL−1 (ref. 7)), it is important to emphasize that the range of 10–100 μg mL−1 of Hop extract used should be considered as supra-physiological. This point must be kept in mind during the future step to translational research from ex vivo to in vivo in animal experimentation and human interventions. Here, we demonstrated that Hop extract is able to evoke, in mouse resistance arteries, a dose–response vasorelaxation reaching 60% of the maximal vasorelaxant response. All the potency of its effect was found to be endothelium-dependent, but excluding the involvement of the enzyme endothelial nitric oxide synthase, since inhibition of eNOS by L-NAME did not change its efficacy at all doses. These data have led us to explore the involvement of alternative relaxing mechanisms mediated by endothelium.
Arachidonic acid (AA) can be metabolized into biologically active prostanoids including prostacyclin (PGI2) and thromboxane A2 (TXA2) through the cyclooxygenase (COX-1) and COX-2 pathways. If TXA2 mainly mediated vasocontraction, PGI2 is considered an important vascular protective mediator that leads to vasorelaxation.38 Data obtained using both indomethacin, a specific inhibitor of COXs, and the IP receptor antagonist, CAY10441, did not change the vascular reactivity evoked by Hop extract, thus excluding the prostanoid involvement in the Hop vascular effect on resistance vessels.
Thus, we decided to turn our attention to the mechanisms involved in the modulation of the internal calcium concentration, which represents a key signal involved in the modulation of vascular tone. Firstly, by inhibiting the calcium release from the endoplasmic reticulum, we did not detect any changes in the dose–response effect evoked by Hop extract, thus excluding a possible action of Hop extract on the regulation of sarcoplasmic reticulum Ca2+ pump isozymes. Differently, during external calcium deprivation, the administration of increasing doses of Hop extract completely failed to induce a dose–response vasorelaxation, driving us to focalize our attention on the endothelium derived hyperpolarization (EDH), a known mechanism involved in the maintenance of vascular homeostasis. This hypothesis was perfectly in line with the concept that from a physiological standpoint the EDH has a major functional influence on vascular smooth muscle tone in small resistance arteries but originates exclusively from endothelium stimulation, which represents the main target of the Hop extract.
Besides the entire Hop phytocomplex, class specific fractions obtained through an analytical workflow were tested for the ability to exert vascular reactivity. As a result, the vascular reactivity studies showed that only the B fraction continued to exhibit the vasorelaxant properties that recapitulate the action of the entire Hop extract efficacy. Of note is that, similarly to that observed with the entire extract, also the dose–response evoked by the B fraction, in the absence of external calcium, was completely abolished, thus enforcing our hypothesis of the involvement of EDH signaling.
To date, although the complete characterization of EDH mechanisms remains unknown, it seems to be well established that this process can be grouped into two broad categories: the classical pathway and the alternative pathway.21 The classical pathway is activated by opening two Ca2+-activated K+ (KCa) namely the small-conductance KCa (SKCa) and intermediate-conductance KCa (IKCa) that have been identified only on endothelial cells. The resulting endothelial hyperpolarization then transfers to muscle cells either through myoendothelial junctions or through the subsequent activation of K+ channels on smooth muscle cells.
The alternative pathways involve the release of diffusible endothelial factors such as epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2), which hyperpolarize myocytes by opening K+ channels.21 Both pathways depend on an increase of Ca2+ in the endothelium.
Thus, with the aim of fully characterizing the involvement of EDH in the B fraction vascular action, we have performed a sequential workflow using different specific inhibitors of KCa channels. Interestingly, the inhibition of both SKCa and IKCa, with TRAM-34 and Apamin, respectively, resulted in a complete abolishment of the vascular effect of the B fraction and its consequent inability to modulate the vessel diameter. The propagation of that hyperpolarized signal from the endothelium to the muscle cells involves specific structures namely myoendothelial gap junctions (MEGJs). MEGJs are composed of gap junctional proteins, such as connexins (Cxs), that play an important role in spreading this K+-channel-initiated hyperpolarization along the blood vessels, thus propagating the EDH signal.39 The importance of MEGJs in the EDH mechanism induced by the B fraction was confirmed by the study conducted in the presence of the inhibitor of connexins 37 and 40, GAP26, which was able to completely inhibit the vascular effects of this hop fraction.
We shifted the field to identify the only bioactive compound(s) with a direct vascular effect from the entire hop matrix and applied a second funnel effect to the B fraction. This procedure revealed that the B1 subfraction, which contains high-content α bitter acids (Humulinone, Cohumulone, Cohumulinone and derivatives) is able to recapitulate the vasorelaxant effect evoked both by the entire Hop extract and B fraction. These results suggest that the vascular action of the B fraction was mediated by the presence of these specific α acid derivatives. In support of this, the evaluation of the mechanism of action of fraction B1 was found to overlap with that exerted by fraction B, demonstrating that the opening of SKCa and IKCa and the subsequent involvement of MEGjs mediate the beneficial vascular effect of the B1 subfraction.
By confirming at this point the involvement of EDH in the mechanism of action of the B1 fraction, we sought to identify the upstream signal that was responsible for this classical activation of the EDH phenomenon. Between the multiple ion channels that modulate Ca2+ increase in endothelial cells, the ion channels of the transient receptor potential (TRP) family and inositol triphosphate receptors represent the main isoforms present in ECs.38,40 In the last decade, the TRP vanilloid 4 (TRPV4) channel has emerged as an important Ca2+-influx pathway involved in the modulation of metabolism-related diseases41,42 and in ECs obtained from resistance arteries.1,43 Interestingly, the specific endothelial TRPV4 knockout showed an increase in resting blood pressure.44
In our experimental setting, the Hop-derived B1 fraction determines the opening of TRPV4 channels, resulting in a massive increase in intracellular Ca2+ that drives the subsequent opening of SKCa and IKCa channels giving rise to the EDH phenomenon. Thereof, the pharmacological inhibition of TRPV4 by GSK205 is able to completely prevent the dose–response vasorelaxant effects evoked by the B1 fraction. This effect is mediated by PKCα, which phosphorylation in threonine 497, an activation site of the kinase, resulted abolished following TRPV4 inhibition.
Interestingly, the use of PKCα siRNA helped us to unveil the link between PKCα and TRPV4, since during the kinase silencing, we observed an abolishment of TRPV4 phosphorylation. Based on these results, we hypothesize that PKCα acts as a feedforward mechanism by which the B1 subfraction is able to stimulate the amplification of TRPV4 intracellular signaling favoring the massive increase of calcium, leading to the activation of the EDH mechanism that determines the dose–response vasorelaxation evoked by the high content α bitter acid subfraction (summary figure).
Conclusions
In summary this is the first comprehensive investigation of the vascular function of Hop-derived α acids in mouse resistance arteries. Using phytocomplex fractionation performed on a semi-preparative HPLC system and UHPLC-PDA-MS/MS coupled to mass spectrometry, we have demonstrated that the high content of the α acid fraction derived from the entire Hop extract is the most vascular bioactive fraction. Moreover, using different approaches based on both pharmacological inhibitors and small interfering RNA (siRNA) we demonstrate that the B1 subfraction is able to evoke a dose-dependent relaxation of mouse mesenteric resistance arteries through the endothelium-dependent hyperpolarization (EDH) response. Its mechanism is mediated by endothelial small-conductance calcium-activated potassium channels (SKCa) and intermediate-conductance calcium-activated potassium channels (IKCa) driven by the activation of Transient Receptor Potential Vanilloid type 4 (TRPV4), which is amplified by the feedforward mechanism of PKCα phosphorylation.Although future research will be needed to translate the beneficial effects of α acids into in vivo models of cardiovascular disease, such as eNOS or epoxide hydrolase dysfunctional models, that could be treated by EDH stimulation, there is strong evidence that in resistance arteries, subfraction B1 has great potential and could be added to the armamentarium of natural products useful for preventing the onset of cardiovascular disease.
Author contributions
P. D., E. Sa., E. S. and A. C. designed the study. P. D., E. S., A. D., A. C. A., and V. P. performed the experiments. P. D., E. Sa., E. S. and A. C. wrote the manuscript. P. C. and C. V. revised and edited the manuscript. E. S. and A. C. supervised and administered the project. All authors have revised and approved the manuscript.Conflicts of interest
The authors declare no conflict of interest.References
- C. Marziano, K. Hong, E. L. Cope, M. I. Kotlikoff, B. E. Isakson and S. K. Sonkusare, Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries, J. Am. Heart Assoc., 2017, 6(12), e007157 CrossRef PubMed.
- G. Scioli, A. Della Valle, G. Zengin, M. Locatelli, T. Tartaglia, A. Cichelli, A. Stefanucci and A. Mollica, Artisanal fortified beers: Brewing, enrichment, HPLC-DAD analysis and preliminary screening of antioxidant and enzymatic inhibitory activities, Food Biosci., 2022, 48, 1–7 CrossRef.
- P. Zanoli and M. Zavatti, Pharmacognostic and pharmacological profile of Humulus lupulus L, J. Ethnopharmacol., 2008, 116, 383–396 CrossRef CAS PubMed.
- R. Garcia-Villalba, S. Cortacero-Ramirez, A. Segura-Carretero, J. A. Martin-Lagos Contreras and A. Fernandez-Gutierrez, Analysis of hop acids and their oxidized derivatives and iso-alpha-acids in beer by capillary electrophoresis-electrospray ionization mass spectrometry, J. Agric. Food Chem., 2006, 54, 5400–5409 CrossRef CAS PubMed.
- C. Di Vito, A. Bertoni, M. Nalin, S. Sampietro, M. Zanfa and F. Sinigaglia, The phytoestrogen 8-prenylnaringenin inhibits agonist-dependent activation of human platelets, Biochim. Biophys. Acta, 2012, 1820, 1724–1733 CrossRef CAS PubMed.
- S. F. Liou, T. T. N. Nguyen, J. H. Hsu, E. Sulistyowati, S. E. Huang, B. N. Wu, M. C. Lin and J. L. Yeh, The Preventive Effects of Xanthohumol on Vascular Calcification Induced by Vitamin D(3) Plus Nicotine, Antioxidants, 2020, 9(10), 956 CrossRef CAS PubMed.
- H. Figard, C. Girard, F. Mougin, C. Demougeot and A. Berthelot, Effects of aqueous hop (Humulus Lupulus L.) extract on vascular reactivity in rats: mechanisms and influence of gender and hormonal status, Phytomedicine, 2008, 15, 185–193 CrossRef CAS PubMed.
- C. Luis, R. Costa, I. Rodrigues, A. Castela, P. Coelho, S. Guerreiro, J. Gomes, C. Reis and R. Soares, Xanthohumol and 8-prenylnaringenin reduce type 2 diabetes-associated oxidative stress by downregulating galectin-3, Porto Biomed. J., 2019, 4, e23 CrossRef.
- J. J. Lamb, V. R. Konda, A. Desai, J. S. Bland and M. L. Tripp, The Effects of Tetrahydro-iso-alpha Acids and Niacin on Monocyte-Edothelial Cell Interactions and Flow-mediated Vasodilation, Global Adv. Health Med., 2012, 1, 84–91 CrossRef PubMed.
- T. Takase, T. Toyoda, N. Kobayashi, T. Inoue, T. Ishijima, K. Abe, H. Kinoshita, Y. Tsuchiya and S. Okada, Dietary iso-alpha-acids prevent acetaldehyde-induced liver injury through Nrf2-mediated gene expression, PLoS One, 2021, 16, e0246327 CrossRef CAS.
- E. Sommella, G. Verna, M. Liso, E. Salviati, T. Esposito, D. Carbone, C. Pecoraro, M. Chieppa and P. Campiglia, Hop-derived fraction rich in beta acids and prenylflavonoids regulates the inflammatory response in dendritic cells differently from quercetin: unveiling metabolic changes by mass spectrometry-based metabolomics, Food Funct., 2021, 12, 12800–12811 RSC.
- V. Capaci, L. Monasta, M. Aloisio, E. Sommella, E. Salviati, P. Campiglia, M. G. Basilicata, F. Kharrat, D. Licastro, G. Di Lorenzo, F. Romano, G. Ricci and B. Ura, A Multi-Omics Approach Revealed Common Dysregulated Pathways in Type One and Type Two Endometrial Cancers, Int. J. Mol. Sci., 2023, 24(22), 16057 CrossRef CAS PubMed.
- E. Sommella, F. Pagano, E. Salviati, M. Chieppa, A. Bertamino, M. Manfra, M. Sala, E. Novellino and P. Campiglia, Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry, J. Sep. Sci., 2018, 41, 1548–1557 CrossRef CAS PubMed.
- G. G. Schiattarella, F. Cattaneo, A. Carrizzo, R. Paolillo, N. Boccella, M. Ambrosio, A. Damato, G. Pironti, A. Franzone, G. Russo, F. Magliulo, M. Pirozzi, M. Storto, M. Madonna, G. Gargiulo, V. Trimarco, L. Rinaldi, M. De Lucia, C. Garbi, A. Feliciello, G. Esposito, C. Vecchione and C. Perrino, Akap1 Regulates Vascular Function and Endothelial Cells Behavior, Hypertension, 2018, 71, 507–517 CrossRef CAS PubMed.
- M. R. Romano, F. Biagioni, A. Carrizzo, M. Lorusso, A. Spadaro, T. Micelli Ferrari, C. Vecchione, M. Zurria, G. Marrazzo, G. Mascio, B. Sacchetti, M. Madonna, F. Fornai, F. Nicoletti and M. D. Lograno, Effects of vitamin B12 on the corneal nerve regeneration in rats, Exp. Eye Res., 2014, 120, 109–117 CrossRef CAS.
- O. P. Mishra, R. Mishra, Q. M. Ashraf and M. Delivoria-Papadopoulos, Nitric oxide-mediated mechanism of neuronal nitric oxide synthase and inducible nitric oxide synthase expression during hypoxia in the cerebral cortex of newborn piglets, Neuroscience, 2006, 140, 857–863 CrossRef CAS PubMed.
- R. Vera, M. Galisteo, I. C. Villar, M. Sanchez, A. Zarzuelo, F. Perez-Vizcaino and J. Duarte, Soy isoflavones improve endothelial function in spontaneously hypertensive rats in an estrogen-independent manner: role of nitric-oxide synthase, superoxide, and cyclooxygenase metabolites, J. Pharmacol. Exp. Ther., 2005, 314, 1300–1309 CrossRef CAS PubMed.
- C. Hermenegildo, P. J. Oviedo, M. C. Garcia-Martinez, M. A. Garcia-Perez, J. J. Tarin and A. Cano, Progestogens stimulate prostacyclin production by human endothelial cells, Hum. Reprod., 2005, 20, 1554–1561 CrossRef CAS PubMed.
- G. J. Crane, N. Gallagher, K. A. Dora and C. J. Garland, Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery, J. Physiol., 2003, 553, 183–189 CrossRef CAS PubMed.
- S. D. Walker, K. A. Dora, N. T. Ings, G. J. Crane and C. J. Garland, Activation of endothelial cell IK(Ca) with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery, Br. J. Pharmacol., 2001, 134, 1548–1554 CrossRef CAS.
- D. X. Zhang and D. D. Gutterman, Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation, J. Cardiovasc. Pharmacol., 2011, 57, 133–139 CrossRef CAS.
- C. de Wit, M. Boettcher and V. J. Schmidt, Signaling across myoendothelial gap junctions–fact or fiction?, Cell Commun. Adhes., 2008, 15, 231–245 CrossRef CAS PubMed.
- R. Kohler, W. T. Heyken, P. Heinau, R. Schubert, H. Si, M. Kacik, C. Busch, I. Grgic, T. Maier and J. Hoyer, Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 1495–1502 CrossRef.
- M. Chen and X. Li, Role of TRPV4 channel in vasodilation and neovascularization, Microcirculation, 2021, 28, e12703 CrossRef CAS PubMed.
- A. Carrizzo, C. Izzo, M. Forte, E. Sommella, P. Di Pietro, E. Venturini, M. Ciccarelli, G. Galasso, S. Rubattu, P. Campiglia, S. Sciarretta, G. Frati and C. Vecchione, A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases, Int. J. Mol. Sci., 2020, 21, 8706 CrossRef CAS PubMed.
- A. Carrizzo, M. Ambrosio, A. Damato, M. Madonna, M. Storto, L. Capocci, P. Campiglia, E. Sommella, V. Trimarco, F. Rozza, R. Izzo, A. A. Puca and C. Vecchione, Morus alba extract modulates blood pressure homeostasis through eNOS signaling, Mol. Nutr. Food Res., 2016, 60, 2304–2311 CrossRef CAS.
- A. Carrizzo, O. Moltedo, A. Damato, K. Martinello, P. Di Pietro, M. Oliveti, F. Acernese, G. Giugliano, R. Izzo, E. Sommella, S. Migliarino, O. Piazza, C. Izzo, N. Virtuoso, A. Strianese, V. Trimarco, P. Campiglia, S. Fucile, A. Puca, B. Trimarco and C. Vecchione, New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients Via a Nitric Oxide-Dependent Mechanism, J. Am. Heart Assoc., 2020, 9, e014923 CrossRef CAS.
- R. K. Bachheti, L. A. Worku, Y. H. Gonfa, M. Zebeaman, Deepti, D. P. Pandey and A. Bachheti, Prevention and Treatment of Cardiovascular Diseases with Plant Phytochemicals: A Review, J. Evidence-Based Complementary Altern. Med., 2022, 2022, 5741198 Search PubMed.
- P. L. Freitas, J. P. N. Miranda, L. M. Franca and A. M. A. Paes, Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota, Nutrients, 2022, 14(17), 3510 CrossRef CAS PubMed.
- Y. M. Lee, K. H. Hsieh, W. J. Lu, H. C. Chou, D. S. Chou, L. M. Lien, J. R. Sheu and K. H. Lin, Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), Prevents Platelet Activation in Human Platelets, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 852362 Search PubMed.
- T. Maliar, M. Maliarova, M. Blazkova, M. Kunstek, L. Uvackova, J. Viskupicova, A. Purdesova and P. Benovic, Simultaneously Determined Antioxidant and Pro-Oxidant Activity of Randomly Selected Plant Secondary Metabolites and Plant Extracts, Molecules, 2023, 28(19), 6890 CrossRef CAS PubMed.
- Y. Miura, M. Hosono, C. Oyamada, H. Odai, S. Oikawa and K. Kondo, Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARalpha activations in C57BL/6 mice, Br. J. Nutr., 2005, 93, 559–567 CrossRef CAS.
- J. Romeo, M. Gonzalez-Gross, J. Warnberg, L. E. Diaz and A. Marcos, Effects of moderate beer consumption on blood lipid profile in healthy Spanish adults, Nutr., Metab. Cardiovasc. Dis., 2008, 18, 365–372 CrossRef CAS PubMed.
- T. Padro, N. Munoz-Garcia, G. Vilahur, P. Chagas, A. Deya, R. M. Antonijoan and L. Badimon, Moderate Beer Intake and Cardiovascular Health in Overweight Individuals, Nutrients, 2018, 10(9), 1237 CrossRef PubMed.
- G. Chiva-Blanch, E. Magraner, X. Condines, P. Valderas-Martinez, I. Roth, S. Arranz, R. Casas, M. Navarro, A. Hervas, A. Siso, M. Martinez-Huelamo, A. Vallverdu-Queralt, P. Quifer-Rada, R. M. Lamuela-Raventos and R. Estruch, Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: a randomized feeding trial, Nutr., Metab. Cardiovasc. Dis., 2015, 25, 36–45 CrossRef CAS PubMed.
- S. Y. Jeon, M. R. Kim, E. O. Lee, B. H. Jeon, J. J. Lee and Y. C. Lee, Effect of a new herbal composition comprised of red clover and hop extract on human endothelial cell damage and vasorelaxant activity, J. Food Biochem., 2020, 44, e13314 CrossRef CAS PubMed.
- E. Salviati, E. Ciaglia, E. Sommella, F. Montella, A. Bertamino, C. Ostacolo, B. Parrino, R. Rubino, C. Vecchione, A. Puca, E. Novellino and P. Campiglia, Immunomodulatory activity of Humulus lupulus bitter acids fraction: Enhancement of natural killer cells function by NKp44 activating receptor stimulation, J. Funct. Foods, 2019, 61, 103469 CrossRef CAS.
- K. Hong, E. L. Cope, L. J. DeLalio, C. Marziano, B. E. Isakson and S. K. Sonkusare, TRPV4 (Transient Receptor Potential Vanilloid 4) Channel-Dependent Negative Feedback Mechanism Regulates G(q) Protein-Coupled Receptor-Induced Vasoconstriction, Arterioscler., Thromb., Vasc. Biol., 2018, 38, 542–554 CrossRef CAS PubMed.
- K. Goto, T. Ohtsubo and T. Kitazono, Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels, Int. J. Mol. Sci., 2018, 19(1), 315 CrossRef PubMed.
- H. R. Heathcote, M. D. Lee, X. Zhang, C. D. Saunter, C. Wilson and J. G. McCarron, Endothelial TRPV4 channels modulate vascular tone by Ca(2+) -induced Ca(2+) release at inositol 1,4,5-trisphosphate receptors, Br. J. Pharmacol., 2019, 176, 3297–3317 CrossRef CAS.
- F. Wu, S. Bu and H. Wang, Role of TRP Channels in Metabolism-Related Diseases, Int. J. Mol. Sci., 2024, 25(2), 692 CrossRef CAS PubMed.
- P. Gao, L. Li, X. Wei, M. Wang, Y. Hong, H. Wu, Y. Shen, T. Ma, X. Wei, Q. Zhang, X. Fang, L. Wang, Z. Yan, G. H. Du, H. Zheng, G. Yang, D. Liu and Z. Zhu, Activation of Transient Receptor Potential Channel Vanilloid 4 by DPP-4 (Dipeptidyl Peptidase-4) Inhibitor Vildagliptin Protects Against Diabetic Endothelial Dysfunction, Hypertension, 2020, 75, 150–162 CrossRef CAS PubMed.
- P. Bagher, T. Beleznai, Y. Kansui, R. Mitchell, C. J. Garland and K. A. Dora, Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 18174–18179 CrossRef CAS PubMed.
- M. Ottolini, K. Hong, E. L. Cope, Z. Daneva, L. J. DeLalio, J. D. Sokolowski, C. Marziano, N. Y. Nguyen, J. Altschmied, J. Haendeler, S. R. Johnstone, M. Y. Kalani, M. S. Park, R. P. Patel, W. Liedtke, B. E. Isakson and S. K. Sonkusare, Local Peroxynitrite Impairs Endothelial Transient Receptor Potential Vanilloid 4 Channels and Elevates Blood Pressure in Obesity, Circulation, 2020, 141, 1318–1333 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo00058g |
| ‡ Co-first authorship. |
| This journal is © The Royal Society of Chemistry 2024 |