 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protective effect of provitamin A dietary carotenoid intake on overweight/obesity and their relation to inflammatory and oxidative stress biomarkers – a case-control study†
Natália
Koós
,
Farhad
Vahid
 and
Torsten
Bohn
and
Torsten
Bohn
 *
*
Nutrition and Health Research Group, Department of Precision Health, Luxembourg Institute of Health, Strassen, Luxembourg. E-mail: Torsten.Bohn@lih.lu
First published on 1st May 2024
Abstract
This investigation assessed associations between dietary carotenoid intake and the odds of overweight/obesity, as well as inflammatory/oxidative stress biomarkers, in 851 participants with overweight/obesity (BMI ≥25 kg m−2) and 754 normal-weight controls. A 124-item food-frequency-questionnaire (FFQ) and food composition databases were employed to estimate carotenoid intake. Binary logistic regressions assessed the association of carotenoid intake with the odds of overweight/obesity, adjusting for several potential confounders. Multiple linear regression models revealed associations between carotenoid intake and biomarkers (anthropometrics, blood lipids, inflammation, antioxidant status). Logistic regression models adjusted for various confounders and fruits and vegetables showed protective associations for provitamin A carotenoids (i.e., β-carotene + α-carotene + β-cryptoxanthin; odds ratio (OR): 0.655, p = 0.041) and astaxanthin (OR: 0.859, p = 0.017). Similarly adjusted multiple linear regressions revealed significant associations between several carotenoids and lower levels of interleukin (IL)-6, IL-1β, and TNF-α and increased IL-10 and total antioxidant capacity. Further analysis revealed that lycopene was significantly associated with increased odds of overweight/obesity (OR: 1.595, p = 0.032) in a model adjusted for various confounders and vegetables (i.e., unadjusted for fruits). A protective association between the sum of provitamin A carotenoid and astaxanthin dietary intake and the odds of having overweight/obesity was found. The findings that carotenoids other than lycopene were not or inversely associated with the odds of overweight/obesity may point toward differentiating effects of various carotenoids or their associations with different food groups. Provitamin A rich food items including fruits and vegetables appear to be a prudent strategy to reduce inflammation and the odds of having overweight/obesity.
1. Introduction
According to the World Health Organization (WHO), overweight and obesity are preventable conditions characterized by excessive adipose tissue accumulation, posing a health risk that affects 1.9 billion individuals of the adult population.1 Importantly, having overweight or obesity increases the risk of several chronic diseases, including cardiovascular disease (CVD),1–3 type 2 diabetes (T2DM),2 and some types of cancer.1,4 Consequently, high direct and indirect healthcare costs are attributable to managing overweight and its co-morbidities, placing a considerable burden on the economy, society, and the healthcare system.5,6 Of note, the obesity epidemic does not only affect developed countries. The prevalence of overweight and obesity tends to increase rapidly in lower- and middle-income countries due to changes brought about by the nutrition transition, following the adoption of westernized lifestyles.7 Therefore, investigating factors associated with the increased risk of excess weight is highly relevant to public health in many developing regions.Currently, obesity prevention and treatment strategies focus on adopting a healthier lifestyle, including diet.8–10 Increased consumption of fruits and vegetables is generally recommended,10,11 together with limiting caloric intake and simple carbohydrates and saturated fatty acid (SFA) consumption, among others.12 Fruits and vegetables are widely regarded for their health-promoting properties.13 Unfortunately, dietary intakes in the general population tend to be suboptimal, not reaching the recommended 5 daily portions.14,15 Based on a 2019 survey, only 12% and 10% of the US adult population met fruit and vegetable intake recommendations, respectively.14 Several aspects of fruits and vegetables may explain their association with improved health outcomes. Both are a rich source of micronutrients, are relatively low in calories, and provide dietary fiber along with a plethora of secondary plant metabolites that have been shown to exert biological activity.13 Among these, carotenoids are an important group of compounds constituting widespread liposoluble pigments that show the highest tissue and blood concentrations in humans among secondary plant metabolites (around 2 μM).16 Carotenoids and their circulating concentrations or their dietary intake have been associated with a lower risk of T2DM,17 metabolic syndrome,18 CVD,19 some types of cancer,20 and even total mortality.21
It has been reported that carotenoids are effective reactive oxygen species (ROS) scavengers and may also interact with transcription factors such as nuclear factor kappa B (NF-κB) or nuclear factor erythroid 2-related factor 2 (Nrf2), associated with hampered inflammation and oxidative stress, respectively.16 Obesity is now widely recognized as a condition associated with elevated oxidative stress markers and systemic inflammation,22–24 associated with several co-morbidities.25 Consequently, it seems plausible that increased dietary intake of antioxidants is associated with a lower risk of excess adiposity. Research indicated the protective role of dietary patterns with higher dietary antioxidant index (DAI) scores,26–28 while a higher dietary inflammatory index (DII) was associated with an increased risk of obesity.29–33 Carotenoids are increasingly recognized as potential actors in modulating adipocyte function. Carotenoids and their metabolites, i.e., apocarotenoids, interact with transcription factors such as retinoid X receptors (RXR), retinoic acid receptor (RARs), and peroxisome proliferator-activated receptors (PPARs).34 These have been suggested to interfere with lipid oxidation and storage processes, adipose tissue differentiation, inflammation, and oxidative stress, all related to the expansion of fat cells.34 Finally, overweight and obesity have been associated with altered gut microbiota composition, dysbiosis, and increased gut permeability,35,36 which may provide another mechanism of action for carotenoids due to their possible interaction with the gut microbiota.37,38
A few studies have demonstrated a beneficial association of carotenoids with body composition.39–42 These studies showed some inconsistency regarding the role of individual carotenoids. Furthermore, research has mostly focused on frequently consumed carotenoids such as β-carotene, leaving a gap in our understanding of the role of other carotenoids that also contribute to the total dietary intake of carotenoids, such as phytoene or phytofluene.43
In addition to their potential health benefits, carotenoid concentrations in blood have also been regarded as markers of fruit and vegetable consumption.44 Therefore, an important question to answer is to what extent carotenoid-health associations are independent of the effects of increased fruit and vegetable intake. Although observational studies generally seem to support the beneficial role of carotenoids,17,18,20 supplementation studies using individual carotenoids have, in part, reported adverse health effects, such as for smokers.45,46 Isolated carotenoids may differ in dosing, missing synergistic effects and bioavailability,47 and thus may act differently than when present in food matrices.
The present study investigated whether carotenoid intake was associated with the odds of overweight/obesity in an Iranian population, as an example of a population experiencing a rapid change in lifestyle concomitantly with an increased prevalence of obesity.48,49 We investigated the association of overweight/obesity with individual carotenoids. Additionally, we aimed to assess the role of carotenoids in these associations beyond acting as markers for the consumption of fruits and vegetables by adjusting for their intake. As a further objective, we investigated the association between carotenoid intake and several biomarkers associated with overweight/obesity, such as central obesity, blood pressure, lipid profile, glucose control, and inflammatory and antioxidant status.
2. Materials and methods
2.1 Study population
A detailed description of the methods and study design has been published previously.26,50 Briefly, this study had a case-control design based on a study population randomly selected from patients of medical centers in Arak, Iran. Three sub-centers recruited participants who were referred to the main center for performing the measurements. The case group consisted of individuals having overweight/obesity with a BMI ≥25 kg m−2. Controls were also recruited in these centers with BMI <25 kg m−2. Participants were eligible for inclusion if they met the following criteria: age 18–81 years, absence of disease, no dietary restrictions or major changes to the diet during the year preceding inclusion, no medication or supplement use at present, absence of drug addiction, filling out ≥80% of questionnaires, residency in Arak during the 5 years preceding enrolment, and additionally for women absence of pregnancy and no lactation. Written informed consent was obtained from all participants. The study was approved by the Arak University of Medical Science Ethics Committee (Ethics Committee no. IR.ARAKMU. REC.1398.094).2.2 Sociodemographic data collection
Information about participants’ age, sex, civil status, level of education, alcohol intake, current smoking habits, physical activity, and disease history (including diabetes, CVD, and hypertension) were collected by a questionnaire.2.3 Dietary and carotenoid intake
Data was collected by medical personnel trained by nutritionists. Dietary intake data was obtained via a validated 124-item food frequency questionnaire (FFQ),51 with the frequency of consumption during the previous year indicated on a five-point scale: never, daily, weekly, monthly, or annually. Pictures of typical portion sizes of food items were shown to participants to aid in specifying the amount of food intake. To account for the seasonality of food intake, the frequencies “daily” and “weekly” could be indicated for the specific season. Nutritionist IV software (First Databank, Hearst Corp., San Bruno, CA, United States of America) was used to assess average daily macro- and micronutrient intake (yearly total intake divided by 365). The software used the United States Department of Agriculture (USDA) database (https://fdc.nal.usda.gov/ accessed on May 2023). Total energy intake was calculated by adding up the energy content of consumed macronutrients using the following energy equivalents: 9 kcal g−1 fat, 4 kcal g−1 protein or carbohydrate, and 2 kcal g−1 fiber.Dietary intake (μg) of the following carotenoids was assessed: α- and β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene, phytoene, phytofluene, violaxanthin, neoxanthin, and astaxanthin. To measure intakes as accurately as possible, several databases were consulted. α- and β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene consumption was estimated by the USDA database and the USDA-NCC Carotenoid Database.52 The European Food Safety Authority database was used for astaxanthin intake assessment.53 Phytofluene, phytoene, neoxanthin, and violaxanthin were estimated using another published database.43 For analysis, carotenoids were assessed individually and further grouped into provitamin A carotenoids (α-carotene + β-carotene + β-cryptoxanthin), non-provitamin A carotenoids (lutein + zeaxanthin + lycopene + phytoene + phytofluene + violaxanthin + neoxanthin + astaxanthin) and total carotenoids (sum of all eleven measured carotenoids). Lutein and zeaxanthin could not be consistently assessed independently; thus, they were combined into a single variable.
Carotenoid intakes were adjusted for total daily energy intake and expressed as μg carotenoid per 1000 kcal consumed. This was achieved by first dividing the estimated daily carotenoid intake by the total energy consumed (kcal), which was subsequently multiplied by 1000. In this article, unless otherwise noted, all analyses were performed on energy-adjusted values.
2.4 Anthropometrics
Weight and height were measured by Seca scales (Seca GmbH & Co. KG. Hamburg, Germany) to the nearest 0.5 kg and by wall-attached tape measurement to the nearest cm, respectively. The obtained measures were then used to calculate participants’ BMI (as kg m−2). Based on the BMI, participants were assigned their case/control status, as indicated above. In addition to the BMI, another measure of body composition, the body surface area (BSA), was calculated using the Mosteller formula.54 Normal BSA was considered to be ≤1.91 m2 and ≤1.71 m2 for men and women, respectively. Waist circumference (WC) (cm) was measured by a flexible measuring tape placed horizontally around the waist at the level of the narrowest part of the abdomen. The participant was asked to stand comfortably with relaxed abdominal muscles during the measurement.2.5 Biomarker assessment
Using a sphygmomanometer, trained personnel obtained systolic and diastolic blood pressure (SBP, DBP) measurements after a 10–15-minute period of quiet sitting, following a standardized procedure.Intravenous blood samples were collected following an 8–12 hours fast. Serum samples were obtained by 10-minute whole blood centrifugation at 2000g. Samples were stored at −80 °C until analysis. All analyses were performed by accredited laboratories.
The following markers related to inflammation and oxidative status were measured: high-sensitivity C-reactive protein (hs-CRP) by enzyme-linked immunosorbent assays (ELISA, DRG Company, USA). Interleukins (IL-6, IL-1β, IL-10, and IL-4) were measured by commercially available kits (ABCAM, United Kingdom). Antioxidant and oxidative status were evaluated by Teb Pazhouhan Razi kits (Teb Pazhouhan Razi, Tehran, Iran) for total antioxidant capacity (TAC) and malondialdehyde (MDA). TNF-α was measured by commercial kits (ABCAM).
Glucose control-related biomarkers and lipid profiles were measured by ELISA, following kit instructions (Pishtazteb Co., Tehran, Iran). The following were assessed: fasting blood glucose, glycated hemoglobin (HbA1c), fasting insulin, triglycerides, cholesterol (total, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c)). The homeostatic model assessment for insulin resistance (HOMA IR) was calculated as follows: HOMA IR = (fasting insulin × fasting glucose)/405.
3. Statistical analysis
3.1 General data treatment and transformation
All statistical analyses were performed by SPSS Statistics (version 25, IBM, Chicago, IL).55 Distribution was assessed for normality by visual appraisal of Q–Q plots and Kolmogorov–Smirnov tests. A p-value <0.05 (2-sided) was accepted as statistically significant. Study population characteristics are reported as mean ± standard deviation (SD) or as median + interquartile range (IQR) for data with normal and non-normal distribution, respectively. For categorical variables, frequencies are shown. Comparisons between cases and controls were performed by non-parametric Mann–Whitney U-tests and chi-square tests for continuous and categorical variables, respectively. Between-group (case versus control) differences related to sociodemographic variables, dietary and carotenoid intake, anthropometrics, blood pressure, and markers of inflammation, oxidative stress, blood lipids, and glucose control were assessed. The same comparisons were performed based on sex stratification. Carotenoid intake was assessed both as total intake as well as adjusted for daily energy consumption (per 1000 kcal).Spearman correlations investigated correlations between pairs of carotenoids, as the normal distribution of data could not be assumed based on Kolmogorov–Smirnov tests and normality plots. Carotenoid intakes were also correlated with fruit and vegetable consumption.
For all other analyses, only energy-adjusted carotenoid intakes were used. Due to the observation that variables were not normally distributed, they were log-transformed for further analysis. Because the estimated intake of astaxanthin was 0.00 μg for several participants, the log-transformation of this variable created missing values. To overcome this issue, 0.00 μg intakes were replaced by 0.1 prior to analysis. Log-transformation of MDA created two missing values (original values = 0.00 μM). These were substituted by the lowest value above “0” following the log-transformation of the variable to preserve the sample size. No missing observations were noted for any of the variables included. Data was assessed for outlying observations, which were then evaluated against physiological plausibility. One observation for fasting insulin was substituted by the median for analysis.
3.2 Main outcomes
Sensitivity analysis was undertaken to evaluate the effect of fruit and vegetable intake separately. Sex-stratified analyses were also performed. Furthermore, the study population was regrouped using a BSA-based definition of normal weight versus overweight/obesity, as described above, i.e., it was evaluated whether a different assessment method of adiposity would substantially alter the study outcomes. Sex- and BSA-based analyses were performed on model 3 only.
4. Results
4.1 Study population and descriptive results
The study population consisted of 851 participants with overweight/obesity and 754 normal-weight controls. The sex distribution was 49.7% men and 50.3% women. Table 1 provides an overview of the sociodemographic characteristics of the study population. Based on weight status (normal or overweight/obesity according to BMI), significant differences were only noted for disease history, while between-sex differences were noted for regular physical activity (Table 1). In addition, ESI Table 1† shows study population characteristics, including sociodemographic variables in groups with overweight/obesity and normal-weight defined by BSA.| Total (n = 1605) | Overweight/obesityb (n = 851) | Normal weightb (n = 754) | p-Valuec | Male (n = 798) | Female (n = 807) | p-Valuec | |
|---|---|---|---|---|---|---|---|
| a For continuous variable(s), the median (IQR) is shown, while frequencies are displayed for categorical variables. b Groups of overweight/obese and normal weight are determined based on BMI (kg m−2), where normal-weight status is defined as BMI <25 kg m−2 and overweight/obesity is defined as BMI ≥25 kg m−2. c Two-sided p-values were derived from chi-square tests for categorical variables and Mann–Whitney U-test for continuous variables. Significant p-values (p < 0.05) are shown in bold. Abbreviations: M = male, F = female, CVD = cardiovascular disease, IQR = interquartile range. | |||||||
| Age (years) | 48.45 (19.99) | 48.45 (20.66) | 48.50 (19.03) | 0.417 | 48.95 (20.50) | 48.07 (19.66) | 0.129 |
| Sex (M/F) | 798/807 | 415/436 | 383/371 | 0.417 | — | — | |
| Education | |||||||
| Diploma/low literacy | 1070 | 571 | 499 | 0.697 | 534 | 536 | 0.832 |
| Higher than diploma | 535 | 280 | 255 | 264 | 271 | ||
| Marital status | |||||||
| Single | 273 | 143 | 130 | 0.961 | 127 | 146 | 0.207 |
| Married | 1215 | 645 | 570 | 619 | 596 | ||
| Other | 117 | 63 | 54 | 52 | 65 | ||
| Regular physical activity | |||||||
| Yes | 397 | 198 | 199 | 0.147 | 215 | 182 | 0.042 |
| No | 1208 | 653 | 555 | 583 | 625 | ||
| Smoking status | |||||||
| Yes | 261 | 138 | 123 | 0.958 | 124 | 137 | 0.435 |
| No | 1344 | 713 | 631 | 674 | 670 | ||
| History of CVD | |||||||
| Yes | 313 | 207 | 106 | <0.001 | 161 | 152 | 0.498 |
| No | 1292 | 644 | 648 | 637 | 655 | ||
| History of diabetes | |||||||
| Yes | 436 | 252 | 184 | 0.019 | 222 | 214 | 0.558 |
| No | 1169 | 599 | 570 | 576 | 593 | ||
| History of hypertension | |||||||
| Yes | 827 | 509 | 318 | <0.001 | 395 | 432 | 0.106 |
| No | 778 | 342 | 436 | 403 | 375 | ||
Table 2 provides an overview of the anthropometric, inflammatory, oxidative stress, and serum measurements. As expected, significant BMI and WC differences were observed between individuals having overweight/obesity vs. normal-weight. FBG, HbA1c, HOMA IR, triglycerides, DBP, hs-CRP, IL-6, TNF-α, and IL-4 were higher in participants with overweight/obesity, while HDL-c, IL-10, and TAC levels were higher in normal weight controls. Sex-based comparisons revealed statistically significant differences in WC, SBP, and DBP.
| Total (n = 1605) | Overweight/obesityb (n = 851) | Normal weightb (n = 754) | p-Valuec | Male (n = 798) | Female (n = 807) | p-Valuec | |
|---|---|---|---|---|---|---|---|
| a Normally distributed variables are shown as mean ± standard deviation, while median (IQR) values are shown for non-normally distributed variables. b Groups of overweight/obese and normal weight are determined based on BMI (kg m−2), where normal-weight status is defined as BMI <25 kg m−2 and overweight/obesity is defined as BMI ≥25 kg m−2. c Two-sided p-values were derived from Mann–Whitney U-tests as the normal distribution of variables could not be assumed. Significant p-values (p < 0.05) are shown in bold. Abbreviations: BMI = body mass index, WC = waist circumference, FBG = fasting blood glucose, HbA1c = glycated hemoglobin, HOMA IR = homeostatic model assessment of insulin resistance, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglycerides, SBP = systolic blood pressure, DBP = diastolic blood pressure, hs-CRP = high-sensitivity C-reactive protein, IL = interleukin, TNF-α = tumor necrosis factor α, TAC = total antioxidant capacity, MDA = malondialdehyde, IQR = interquartile range. | |||||||
| BMI (kg m−2) | 25.41 (5.23) | 27.35 (2.84) | 22.10 (1.69) | <0.001 | 25.33 (5.01) | 25.54 (5.59) | 0.342 |
| WC (cm) | 93.00 (28.35) | 105.00 (24.30) | 84.00 (18.78) | <0.001 | 103.30 (32.25) | 88.50 (20.00) | <0.001 |
| FBG (mg dL−1) | 89.00 (13.00) | 89.00 (15.00) | 88.00 (11.00) | 0.046 | 89.00 (14.00) | 89.00 (13.00) | 0.416 |
| Insulin (mIU mL−1) | 6.40 (4.7) | 6.50 (5.20) | 6.20 (4.20) | 0.155 | 6.40 (4.82) | 6.40 (4.60) | 0.408 |
| HbA1c (%) | 5.10 (2.20) | 5.10 (2.00) | 4.30 (3.60) | <0.001 | 5.10 (2.20) | 5.10 (2.20) | 0.887 |
| HOMA IR | 1.39 (1.20) | 1.44 (1.31) | 1.35 (1.05) | 0.043 | 1.38 (1.27) | 1.39 (1.11) | 0.572 |
| HDL-c (mg dL−1) | 54.00 (19.00) | 52.00 (19.00) | 55.00 (19.00) | <0.001 | 53.00 (19.25) | 54.00 (19.00) | 0.553 |
| LDL-c (mg dL−1) | 125.30 (45.30) | 126.00 (49.20) | 124.80 (43.05) | 0.139 | 125.40 (44.30) | 125.00 (48.00) | 0.782 |
| TC (mg dL−1) | 199.00 (48.00) | 199.00 (51.00) | 199.00 (46.25) | 0.199 | 198.00 (48.25) | 198.79 ± 38.17 | 0.927 |
| TG (mg dL−1) | 103.00 (75.00) | 111.00 (73.00) | 95.00 (70.75) | <0.001 | 104.00 (73.25) | 103.00 (75.00) | 0.520 |
| SBP (mmHg) | 126.00 (21.50) | 127.50 (24.50) | 124.00 (20.00) | 0.072 | 124.50 (21.50) | 127.50 (21.50) | <0.001 |
| DBP (mmHg) | 81.50 (14.50) | 82.58 ± 11.22 | 80.50 (14.00) | 0.006 | 80.74 ± 10.58 | 83.25 ± 10.74 | <0.001 |
| hs-CRP (mg dL−1) | 2.00 (1.49) | 2.15 (1.36) | 1.90 (1.55) | <0.001 | 2.10 (1.42) | 1.99 (1.48) | 0.188 |
| IL-6 (pg mL−1) | 136.00 (117.00) | 144.00 (125.00) | 121.00 (117.25) | <0.001 | 136.00 (117.00) | 130.00 (123.00) | 0.707 |
| IL-1β (pg mL−1) | 1.80 (1.80) | 1.80 (1.80) | 1.90 (1.70) | 0.420 | 1.80 (1.80) | 1.90 (1.60) | 0.348 |
| TNF-α (pg mL−1) | 24.60 (23.35) | 25.60 (24.80) | 23.30 (21.70) | <0.001 | 24.60 (23.80) | 24.60 (22.80) | 0.656 |
| IL-10 (pg mL−1) | 1.90 (1.90) | 1.90 (1.80) | 2.00 (2.00) | 0.049 | 2.00 (1.90) | 1.90 (2.00) | 0.196 |
| IL-4 (pg mL−1) | 8.80 (9.60) | 9.20 (9.30) | 8.40 (9.00) | 0.003 | 9.00 (9.60) | 8.80 (9.60) | 0.659 |
| TAC (μM) | 1.51 (1.60) | 1.39 (1.41) | 1.60 (1.56) | 0.001 | 1.51 (1.60) | 1.50 (1.60) | 0.715 |
| MDA (μM) | 2.70 (2.30) | 2.70 (2.10) | 2.80 (2.40) | 0.105 | 2.80 (2.50) | 2.70 (2.10) | 0.674 |
Dietary intake was not statistically different between men and women. However, total energy consumption and vegetable intake differed significantly between cases and controls (ESI Table 2†). Upon adjustment for total daily energy intake, median intakes of carotenoids were higher in controls than cases for α- and β-carotene, provitamin A carotenoids, lutein + zeaxanthin, violaxanthin, neoxanthin, astaxanthin, and total carotenoid intake (Fig. 1 and ESI Table 2†). Total daily carotenoid intakes (not adjusted for total energy intake) are shown in ESI Table 3.†
 | ||
| Fig. 1 Dietary intake (median + interquartile range) of carotenoids intake (μg/1000 kcal per day) of the study population (n = 1605). Groups of overweight/obese and normal weight were determined based on BMI, where normal-weight status was defined as BMI <25 kg m−2 and overweight/obesity is defined as BMI ≥25 kg m−2. Two-sided p-values were derived from nonparametric Mann–Whitney U-tests. Only statistically significant (p < 0.05) intakes (normal weight vs. overweight/obesity) are shown. For more details, p-values and non-significant results, please see ESI Table 2.† * Carotenoid intakes are adjusted for daily energy intake. ** The “total carotenoids” intakes only in this figure is reported as μg/500 kcal per day; all other ones and elsewhere in the publication are reported as μg/1000 kcal per day. | ||
4.2 Correlation between carotenoids and dietary intake
Fig. 2 and ESI Tables 4 and 5† show Spearman correlations between dietary intake and individual carotenoids. Carotenoids tended to have inverse correlations with total daily energy intake (Fig. 2). Carotenoids showed a positive association with vegetable consumption, although the strength of the correlation varied. Lycopene showed the strongest correlation with this food group (rs = 0.448, p < 0.001), which was unaltered when carotenoid intakes adjusted for energy consumption were used. Most carotenoids also showed positive correlations with fruit intake (strength of association in the range of 0.055 to 0.588), with the exception of α-carotene and astaxanthin. Similar results were obtained with energy-adjusted carotenoid intakes.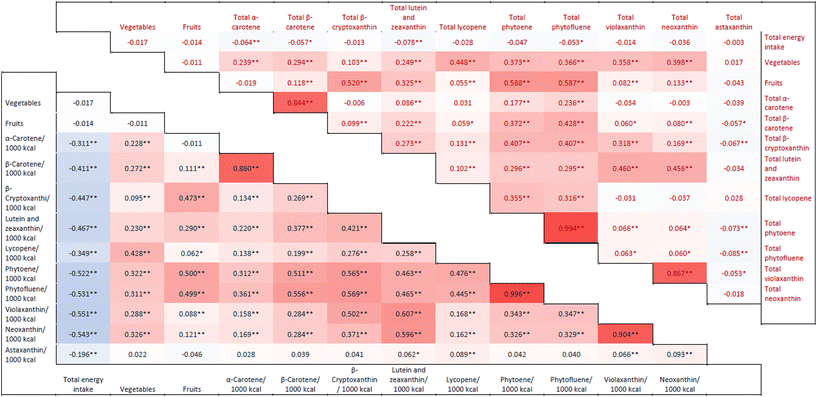 | ||
| Fig. 2 Heat Map – Spearman correlations were performed to investigate the relationship between unadjusted (total) intake of carotenoids (upper right triangle) and between carotenoids adjusted for total daily energy intake (lower left triangle). Spearman correlation coefficients are shown. For details and p-values, refer to ESI Tables 4 and 5.† Interpretation of colors: dark red = very high correlations; Salmon and coral = moderate correlations; light red = weak correlations. Blue color is used only for the correlations between total energy and carotenoids and as the intensity increases, the degree of correlation increases. * Statistically significant at p < 0.05. ** Statistically significant at p < 0.01. | ||
The strongest between-carotenoid correlations (total intake and energy-adjusted) were noted between phytoene and phytofluene (rs = 0.994 and 0.996, p < 0.001), neoxanthin and violaxanthin (rs = 0.867 and 0.904, p < 0.001), and between α- and β-carotene (rs = 0.844 and 0.860. p < 0.001) (Fig. 2 and ESI Tables 4 and 5†).
4.3 Odds of overweight/obesity and carotenoid intake
The crude models (model 1) suggested protective associations between several carotenoids and the odds of overweight/obesity (Fig. 3 and ESI Table 6†). In model 2 (adjusted for sociodemographic and lifestyle factors), a few of these associations (lutein + zeaxanthin, violaxanthin, neoxanthin) lost statistical significance. This model showed a significant reduction in OR for α- and β-carotene, provitamin A carotenoids, phytoene and phytofluene, astaxanthin, and total carotenoids (OR in the range of 0.493–0.862, all p < 0.05). After adjusting for fruit and vegetable intake (model 3), only provitamin A carotenoids and astaxanthin remained significantly associated with reduced OR of overweight/obesity (OR: 0.655, p = 0.041, and 0.859, p = 0.017, respectively).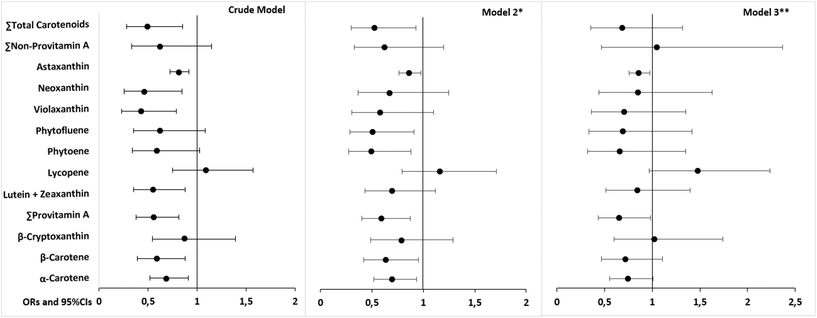 | ||
| Fig. 3 Logistic regression models (ORs and 95% CIs) for the association between dietary carotenoid intake (adjusted for daily energy intake and expressed as μg/1000 kcal) and the odds of overweight/obesity. For more details and p-values please see ESI Table 6.† Binary logistic regression analysis was performed with the odds of overweight/obesity as the dependent variable (0 = normal weight, 1 = overweight/obese). Continuous variables were log-transformed. Groups of overweight/obese and normal weight are determined based on BMI, where normal-weight status is defined as BMI <25 kg m−2 and overweight/obesity is defined as BMI ≥25 kg m−2. * Model 2 adjusted for the following variables: age (years), sex (male/female), marital status (married/single/other), education level (having diploma or low literacy/higher than diploma), smoking status (yes/no), disease history (yes/no for cardiovascular disease, diabetes, and hypertension). ** Model 3 was adjusted for the same variables as model 2 with the addition of fruit and vegetable intake (fruits (g per day) + vegetables (g per day)). Abbreviations: OR = odds ratio, CI = confidence interval. | ||
4.4 Associations between carotenoid intake and biomarkers
Only the final adjusted model (model 3) is discussed here; the crude model 1 and model 2 results are shown in ESI Tables 10 and 11,† respectively. Generally, some associations became non-significant upon adding fruit and vegetable consumption to the model, but several associations remained statistically significant. None of the carotenoids had a significant association with BMI (Tables 3–6). Only lycopene showed a positive association with WC (β = 0.017, p = 0.015). None of the carotenoids were associated with biomarkers related to glucose control (Tables 3–6). Regarding blood lipid profile, only HDL-c showed positive associations with phytoene (β = 0.049, p = 0.016), phytofluene (β = 0.046, p = 0.023), and non-provitamin A carotenoids (β = 0.056, p = 0.014). A negative association was observed between DBP and β-cryptoxanthin (β = −0.017, p = 0.022).| α-Carotene | β-Carotene | β-Cryptoxanthin | Provitamin A | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | 95% CI | |||||||||||||||||||||
| β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | |
| a Multiple linear regression models were used to assess the associations between carotenoids and a selected list of biomarkers relevant to cardiometabolic health, inflammatory status, and oxidative stress. Model 3 was adjusted for the following variables: age (years), sex (male/female), marital status (married/single/other), education level (having diploma or low literacy/higher than diploma), smoking status (yes/no), disease history (yes/no for cardiovascular disease, diabetes, and hypertension) and fruit + vegetable intake. BMI (kg m−2) was also included as an explanatory variable, in models where it was not the dependent variable. All continuous variables were log-transformed. Carotenoids were adjusted for total energy intake (and expressed as μg/1000 kcal) prior to log-transformation. b Statistically significant p-values (p < 0.05) are shown in bold. Abbreviations: BMI = body mass index, HOMA IR = homeostatic model assessment of insulin resistance, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, SBP = systolic blood pressure, DBP = diastolic blood pressure, hs-CRP = high-sensitivity C-reactive protein, IL = interleukin, TNF-α = tumor necrosis factor α, TAC = total antioxidant capacity, MDA = malondialdehyde, WC = waist circumference, FBG = fasting blood glucose, TC = total cholesterol, TG = triglycerides. | ||||||||||||||||||||||||
| BMI (kg m−2) | −0.005 | 0.223 | −0.014 | 0.003 | 0.022 | 0.015 | −0.005 | 0.430 | −0.017 | 0.007 | 0.022 | 0.015 | 0.005 | 0.530 | −0.010 | 0.020 | 0.021 | 0.015 | −0.007 | 0.224 | −0.019 | 0.004 | 0.022 | 0.015 |
| WC (cm) | −0.007 | 0.197 | −0.017 | 0.003 | 0.255 | 0.249 | −0.011 | 0.120 | −0.026 | 0.003 | 0.255 | 0.249 | −0.007 | 0.429 | −0.025 | 0.011 | 0.254 | 0.248 | −0.011 | 0.117 | −0.024 | 0.003 | 0.255 | 0.249 |
| FBG (mg dL−1) | 0.007 | 0.186 | −0.003 | 0.018 | 0.006 | −0.002 | 0.006 | 0.407 | −0.009 | 0.022 | 0.005 | −0.003 | 0.005 | 0.589 | −0.014 | 0.024 | 0.005 | −0.003 | 0.007 | 0.314 | −0.007 | 0.022 | 0.005 | −0.002 |
| Insulin (mIU mL−1) | −0.006 | 0.762 | −0.043 | 0.032 | 0.009 | 0.002 | −0.013 | 0.644 | −0.066 | 0.041 | 0.009 | 0.002 | 0.020 | 0.551 | −0.046 | 0.087 | 0.009 | 0.002 | −0.008 | 0.761 | −0.058 | 0.043 | 0.009 | 0.002 |
| HOMA IR (%) | 0.001 | 0.951 | −0.041 | 0.043 | 0.007 | −0.001 | −0.006 | 0.839 | −0.067 | 0.054 | 0.007 | −0.001 | 0.025 | 0.506 | −0.049 | 0.100 | 0.007 | 0.000 | −0.001 | 0.985 | −0.057 | 0.056 | 0.007 | −0.001 |
| HDL-c (mg dL−1) | 0.000 | 0.966 | −0.017 | 0.016 | 0.022 | 0.015 | −0.005 | 0.659 | −0.029 | 0.018 | 0.022 | 0.015 | 0.012 | 0.406 | −0.017 | 0.042 | 0.022 | 0.015 | −0.003 | 0.787 | −0.025 | 0.019 | 0.022 | 0.015 |
| LDL-c (mg dL−1) | −0.009 | 0.326 | −0.027 | 0.009 | 0.011 | 0.004 | −0.004 | 0.756 | −0.030 | 0.022 | 0.011 | 0.003 | −0.001 | 0.974 | −0.032 | 0.031 | 0.011 | 0.003 | −0.007 | 0.581 | −0.031 | 0.017 | 0.011 | 0.003 |
| TC (mg dL−1) | −0.005 | 0.389 | −0.018 | 0.007 | 0.007 | 0.000 | −0.001 | 0.952 | −0.018 | 0.017 | 0.007 | −0.001 | −0.002 | 0.869 | −0.024 | 0.020 | 0.007 | −0.001 | −0.003 | 0.765 | −0.019 | 0.014 | 0.007 | −0.001 |
| TG (mg dL−1) | −0.010 | 0.551 | −0.041 | 0.022 | 0.012 | 0.005 | −0.002 | 0.921 | −0.048 | 0.043 | 0.012 | 0.004 | −0.028 | 0.333 | −0.084 | 0.029 | 0.012 | 0.005 | −0.006 | 0.772 | −0.049 | 0.037 | 0.012 | 0.004 |
| SBP (mmHg) | −0.003 | 0.431 | −0.012 | 0.005 | 0.016 | 0.008 | −0.004 | 0.470 | −0.017 | 0.008 | 0.016 | 0.008 | −0.014 | 0.061 | −0.029 | 0.001 | 0.017 | 0.010 | −0.005 | 0.408 | −0.016 | 0.007 | 0.016 | 0.008 |
| DBP (mmHg) | −0.003 | 0.529 | −0.011 | 0.006 | 0.02 | 0.013 | −0.005 | 0.413 | −0.017 | 0.007 | 0.02 | 0.013 | −0.017 | 0.022 | −0.032 | −0.002 | 0.023 | 0.016 | −0.005 | 0.402 | −0.016 | 0.006 | 0.02 | 0.013 |
| hs-CRP (mg dL−1) | 0.026 | 0.111 | −0.006 | 0.057 | 0.03 | 0.023 | 0.036 | 0.116 | −0.009 | 0.081 | 0.03 | 0.023 | 0.011 | 0.712 | −0.045 | 0.067 | 0.029 | 0.021 | 0.035 | 0.102 | −0.007 | 0.078 | 0.03 | 0.023 |
| IL-6 (pg mL−1) | −0.057 | 0.008 | −0.100 | −0.015 | 0.031 | 0.024 | −0.058 | 0.064 | −0.118 | 0.003 | 0.029 | 0.021 | −0.070 | 0.068 | −0.145 | 0.005 | 0.029 | 0.021 | −0.064 | 0.029 | −0.121 | −0.006 | 0.03 | 0.022 |
| IL-1 β (pg mL−1) | −0.054 | 0.003 | −0.090 | −0.018 | 0.036 | 0.028 | −0.075 | 0.004 | −0.127 | −0.024 | 0.035 | 0.028 | −0.065 | 0.047 | −0.129 | −0.001 | 0.033 | 0.026 | −0.073 | 0.003 | −0.122 | −0.025 | 0.036 | 0.028 |
| TNF-α (pg mL−1) | −0.037 | 0.088 | −0.079 | 0.005 | 0.026 | 0.019 | −0.057 | 0.065 | −0.118 | 0.004 | 0.026 | 0.019 | −0.080 | 0.036 | −0.155 | −0.005 | 0.027 | 0.019 | −0.053 | 0.068 | −0.110 | 0.004 | 0.026 | 0.019 |
| IL-10 (pg mL−1) | 0.051 | 0.030 | 0.005 | 0.097 | 0.034 | 0.027 | 0.085 | 0.012 | 0.019 | 0.151 | 0.035 | 0.028 | 0.068 | 0.103 | −0.014 | 0.150 | 0.033 | 0.026 | 0.077 | 0.015 | 0.015 | 0.139 | 0.035 | 0.028 |
| IL-4 (pg mL−1) | 0.002 | 0.959 | −0.063 | 0.067 | 0.018 | 0.011 | 0.003 | 0.952 | −0.091 | 0.096 | 0.018 | 0.011 | −0.112 | 0.058 | −0.227 | 0.004 | 0.021 | 0.013 | 0.001 | 0.982 | −0.087 | 0.089 | 0.018 | 0.011 |
| TAC (μM) | 0.065 | 0.019 | 0.011 | 0.119 | 0.028 | 0.021 | 0.074 | 0.062 | −0.004 | 0.152 | 0.027 | 0.020 | 0.061 | 0.215 | −0.035 | 0.157 | 0.026 | 0.018 | 0.077 | 0.040 | 0.004 | 0.150 | 0.027 | 0.020 |
| MDA (μM) | 0.018 | 0.371 | −0.021 | 0.057 | 0.015 | 0.007 | 0.008 | 0.786 | −0.049 | 0.064 | 0.014 | 0.007 | −0.041 | 0.249 | −0.111 | 0.029 | 0.015 | 0.007 | 0.010 | 0.699 | −0.043 | 0.064 | 0.014 | 0.007 |
| Lutein + zeaxanthin | Lycopene | Phytoene | Phytofluene | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | 95% CI | |||||||||||||||||||||
| β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | |
| a Multiple linear regression models were used to assess the associations between carotenoids and a selected list of biomarkers relevant to cardiometabolic health, inflammatory status, and oxidative stress. Model 3 was adjusted for the following variables: age (years), sex (male/female), marital status (married/single/other), education level (having diploma or low literacy/higher than diploma), smoking status (yes/no), disease history (yes/no for cardiovascular disease, diabetes, and hypertension) and fruit + vegetable intake. BMI (kg m−2) was also included as an explanatory variable, in models where it was not the dependent variable. All continuous variables were log-transformed. Carotenoids were adjusted for total energy intake (and expressed as μg/1000 kcal) prior to log-transformation. b Statistically significant p-values (p < 0.05) are shown in bold. Abbreviations: BMI = body mass index, HOMA IR = homeostatic model assessment of insulin resistance, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, SBP = systolic blood pressure, DBP = diastolic blood pressure, hs-CRP = high-sensitivity C-reactive protein, IL = interleukin, TNF-α = tumor necrosis factor α, FBG = fasting blood glucose, TAC = total antioxidant capacity, MDA = malondialdehyde, WC = waist circumference, TC = total cholesterol, TG = triglycerides. | ||||||||||||||||||||||||
| BMI (kg m−2) | 0.008 | 0.287 | −0.007 | 0.022 | 0.022 | 0.015 | 0.010 | 0.096 | −0.002 | 0.022 | 0.023 | 0.016 | 0.004 | 0.692 | −0.016 | 0.025 | 0.021 | 0.014 | 0.005 | 0.614 | −0.015 | 0.026 | 0.021 | 0.015 |
| WC (cm) | −0.006 | 0.497 | −0.022 | 0.011 | 0.254 | 0.480 | 0.017 | 0.015 | 0.003 | 0.031 | 0.257 | 0.251 | −0.001 | 0.918 | −0.025 | 0.023 | 0.254 | 0.248 | −0.004 | 0.763 | −0.028 | 0.020 | 0.254 | 0.248 |
| FBG (mg dL−1) | 0.001 | 0.892 | −0.016 | 0.019 | 0.004 | −0.003 | 0.010 | 0.160 | −0.004 | 0.025 | 0.006 | −0.002 | 0.010 | 0.442 | −0.015 | 0.035 | 0.005 | −0.003 | 0.010 | 0.442 | −0.015 | 0.035 | 0.005 | −0.003 |
| Insulin (mIU mL−1) | 0.024 | 0.453 | −0.039 | 0.086 | 0.01 | 0.002 | 0.027 | 0.299 | −0.024 | 0.079 | 0.01 | 0.002 | 0.026 | 0.572 | −0.064 | 0.115 | 0.009 | 0.002 | 0.022 | 0.623 | −0.067 | 0.112 | 0.009 | 0.002 |
| HOMA IR (%) | 0.025 | 0.483 | −0.045 | 0.095 | 0.007 | 0.000 | 0.038 | 0.202 | −0.020 | 0.095 | 0.008 | 0.000 | 0.036 | 0.487 | −0.065 | 0.136 | 0.007 | 0.000 | 0.032 | 0.528 | −0.068 | 0.133 | 0.007 | 0.000 |
| HDL-c (mg dL−1) | 0.025 | 0.081 | −0.003 | 0.052 | 0.024 | 0.016 | 0.018 | 0.116 | −0.004 | 0.041 | 0.023 | 0.016 | 0.049 | 0.016 | 0.009 | 0.088 | 0.025 | 0.018 | 0.046 | 0.023 | 0.006 | 0.085 | 0.025 | 0.018 |
| LDL-c (mg dL−1) | 0.010 | 0.501 | −0.020 | 0.040 | 0.011 | 0.003 | −0.003 | 0.834 | −0.027 | 0.022 | 0.011 | 0.003 | −0.016 | 0.473 | −0.059 | 0.027 | 0.011 | 0.003 | −0.016 | 0.473 | −0.058 | 0.027 | 0.011 | 0.003 |
| TC (mg dL−1) | 0.005 | 0.652 | −0.016 | 0.025 | 0.007 | −0.001 | −0.003 | 0.744 | −0.020 | 0.014 | 0.007 | −0.001 | −0.008 | 0.608 | −0.037 | 0.022 | 0.007 | −0.001 | −0.008 | 0.616 | −0.037 | 0.022 | 0.007 | −0.001 |
| TG (mg dL−1) | −0.034 | 0.213 | −0.087 | 0.019 | 0.013 | 0.005 | 0.012 | 0.593 | −0.032 | 0.056 | 0.012 | 0.005 | −0.032 | 0.410 | −0.108 | 0.044 | 0.012 | 0.005 | −0.033 | 0.396 | −0.109 | 0.043 | 0.012 | 0.005 |
| SBP (mmHg) | −0.009 | 0.197 | −0.023 | 0.005 | 0.016 | 0.009 | 0.000 | 0.962 | −0.011 | 0.012 | 0.015 | 0.008 | 0.007 | 0.484 | −0.013 | 0.027 | 0.016 | 0.008 | 0.006 | 0.556 | −0.014 | 0.026 | 0.015 | 0.008 |
| DBP (mmHg) | −0.007 | 0.307 | −0.021 | 0.007 | 0.02 | 0.013 | −0.007 | 0.201 | −0.019 | 0.004 | 0.021 | 0.013 | −0.006 | 0.526 | −0.026 | 0.013 | 0.02 | 0.013 | −0.007 | 0.499 | −0.027 | 0.013 | 0.02 | 0.013 |
| hs-CRP (mg dL−1) | −0.030 | 0.259 | −0.083 | 0.022 | 0.029 | 0.022 | 0.015 | 0.504 | −0.029 | 0.058 | 0.029 | 0.021 | 0.027 | 0.487 | −0.049 | 0.102 | 0.029 | 0.021 | 0.031 | 0.423 | −0.044 | 0.106 | 0.029 | 0.022 |
| IL-6 (pg mL−1) | −0.002 | 0.948 | −0.073 | 0.069 | 0.027 | 0.019 | −0.073 | 0.014 | −0.131 | −0.015 | 0.03 | 0.023 | −0.010 | 0.840 | −0.112 | 0.091 | 0.027 | 0.019 | −0.030 | 0.557 | −0.132 | 0.071 | 0.027 | 0.020 |
| IL-1β (pg mL−1) | −0.038 | 0.216 | −0.098 | 0.022 | 0.031 | 0.024 | −0.036 | 0.159 | −0.085 | 0.014 | 0.032 | 0.024 | −0.032 | 0.470 | −0.118 | 0.054 | 0.031 | 0.023 | −0.048 | 0.272 | −0.134 | 0.038 | 0.031 | 0.024 |
| TNF-α (pg mL−1) | −0.056 | 0.117 | −0.127 | 0.014 | 0.026 | 0.018 | −0.100 | 0.001 | −0.157 | −0.042 | 0.031 | 0.024 | −0.041 | 0.421 | −0.142 | 0.059 | 0.024 | 0.017 | −0.043 | 0.399 | −0.144 | 0.057 | 0.025 | 0.017 |
| IL_10 (pg mL−1) | 0.077 | 0.049 | 0.000 | 0.154 | 0.034 | 0.026 | 0.035 | 0.275 | −0.028 | 0.098 | 0.032 | 0.025 | 0.066 | 0.239 | −0.044 | 0.176 | 0.032 | 0.025 | 0.068 | 0.222 | −0.041 | 0.178 | 0.032 | 0.025 |
| IL-4 (pg mL−1) | −0.037 | 0.502 | −0.146 | 0.071 | 0.019 | 0.011 | 0.028 | 0.539 | −0.061 | 0.117 | 0.019 | 0.011 | −0.054 | 0.492 | −0.210 | 0.101 | 0.019 | 0.011 | −0.058 | 0.467 | −0.213 | 0.098 | 0.019 | 0.011 |
| TAC (μM) | 0.151 | 0.001 | 0.060 | 0.241 | 0.031 | 0.024 | 0.012 | 0.759 | −0.063 | 0.086 | 0.025 | 0.018 | 0.064 | 0.334 | −0.066 | 0.193 | 0.025 | 0.018 | 0.071 | 0.280 | −0.058 | 0.201 | 0.026 | 0.018 |
| MDA (μM) | −0.017 | 0.612 | −0.083 | 0.049 | 0.014 | 0.007 | 0.019 | 0.481 | −0.035 | 0.074 | 0.014 | 0.007 | 0.034 | 0.479 | −0.060 | 0.128 | 0.014 | 0.007 | 0.037 | 0.439 | −0.057 | 0.131 | 0.014 | 0.007 |
| Violaxanthin | Neoxanthin | Astaxanthin | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||||||||
| β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | |
| a Multiple linear regression models were used to assess the associations between carotenoids and a selected list of biomarkers relevant to cardiometabolic health, inflammatory status, and oxidative stress. Model 3 was adjusted for the following variables: age (years), sex (male/female), marital status (married/single/other), education level (having diploma or low literacy/higher than diploma), smoking status (yes/no), disease history (yes/no for cardiovascular disease, diabetes, and hypertension) and fruit + vegetable intake. BMI (kg m−2) was also included as an explanatory variable, in models where it was not the dependent variable. All continuous variables were log-transformed. Carotenoids were adjusted for total energy intake (and expressed as μg/1000 kcal) prior to log-transformation. b Statistically significant p-values (p < 0.05) are shown in bold. Abbreviations: BMI = body mass index, HOMA IR = homeostatic model assessment of insulin resistance, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, SBP = systolic blood pressure, DBP = diastolic blood pressure, hs-CRP = high-sensitivity C-reactive protein, IL = interleukin, TNF-α = tumor necrosis factor α, TAC = total antioxidant capacity, MDA = malondialdehyde, WC = waist circumference, FBG = fasting blood glucose, TC = total cholesterol, TG = triglycerides. | ||||||||||||||||||
| BMI (kg m−2) | 0.002 | 0.797 | −0.016 | 0.021 | 0.021 | 0.014 | 0.009 | 0.337 | −0.009 | 0.028 | 0.022 | 0.015 | −0.001 | 0.540 | −0.005 | 0.002 | 0.021 | 0.015 |
| WC (cm) | −0.003 | 0.818 | −0.024 | 0.019 | 0.254 | 0.248 | −0.003 | 0.782 | −0.025 | 0.018 | 0.254 | 0.248 | −0.002 | 0.420 | −0.006 | 0.002 | 0.254 | 0.248 |
| FBG (mg dL−1) | −0.011 | 0.335 | −0.034 | 0.012 | 0.005 | −0.002 | −0.017 | 0.152 | −0.039 | 0.006 | 0.006 | −0.002 | −0.001 | 0.704 | −0.005 | 0.003 | 0.005 | −0.003 |
| Insulin (mIU mL−1) | 0.043 | 0.298 | −0.038 | 0.125 | 0.01 | 0.002 | 0.029 | 0.478 | −0.051 | 0.110 | 0.009 | 0.002 | 0.008 | 0.303 | −0.007 | 0.023 | 0.01 | 0.002 |
| HOMA IR (%) | 0.032 | 0.493 | −0.059 | 0.123 | 0.007 | 0.000 | 0.013 | 0.786 | −0.078 | 0.103 | 0.007 | −0.001 | 0.007 | 0.412 | −0.010 | 0.024 | 0.007 | 0.000 |
| HDL-c (mg dL−1) | 0.011 | 0.554 | −0.025 | 0.047 | 0.022 | 0.015 | 0.005 | 0.764 | −0.030 | 0.041 | 0.022 | 0.015 | 0.001 | 0.685 | −0.005 | 0.008 | 0.022 | 0.015 |
| LDL-c (mg dL−1) | 0.015 | 0.452 | −0.024 | 0.054 | 0.011 | 0.003 | 0.008 | 0.692 | −0.031 | 0.046 | 0.011 | 0.003 | 0.001 | 0.714 | −0.006 | 0.009 | 0.011 | 0.003 |
| TC (mg dL−1) | −0.002 | 0.903 | −0.029 | 0.025 | 0.007 | −0.001 | −0.008 | 0.533 | −0.035 | 0.018 | 0.007 | −0.001 | −0.001 | 0.761 | −0.006 | 0.004 | 0.007 | −0.001 |
| TG (mg dL−1) | −0.045 | 0.204 | −0.114 | 0.024 | 0.013 | 0.005 | −0.046 | 0.186 | −0.114 | 0.022 | 0.013 | 0.005 | −0.007 | 0.315 | −0.020 | 0.006 | 0.012 | 0.005 |
| SBP (mmHg) | −0.017 | 0.074 | −0.035 | 0.002 | 0.017 | 0.010 | −0.018 | 0.053 | −0.036 | 0.000 | 0.018 | 0.010 | 0.000 | 0.804 | −0.003 | 0.004 | 0.015 | 0.008 |
| DBP (mmHg) | −0.014 | 0.120 | −0.033 | 0.004 | 0.021 | 0.014 | −0.007 | 0.465 | −0.025 | 0.011 | 0.02 | 0.013 | −0.002 | 0.360 | −0.005 | 0.002 | 0.02 | 0.013 |
| hs-CRP (mg dL−1) | −0.007 | 0.851 | −0.075 | 0.062 | 0.029 | 0.021 | 0.003 | 0.921 | −0.064 | 0.071 | 0.029 | 0.021 | 0.000 | 0.989 | −0.013 | 0.013 | 0.029 | 0.021 |
| IL-6 (pg mL−1) | −0.026 | 0.581 | −0.118 | 0.066 | 0.027 | 0.020 | −0.028 | 0.555 | −0.119 | 0.064 | 0.027 | 0.020 | −0.002 | 0.784 | −0.020 | 0.015 | 0.027 | 0.019 |
| IL-1β (pg mL−1) | −0.068 | 0.091 | −0.146 | 0.011 | 0.032 | 0.025 | −0.056 | 0.159 | −0.133 | 0.022 | 0.032 | 0.024 | −0.008 | 0.272 | −0.023 | 0.007 | 0.031 | 0.024 |
| TNF-α (pg mL−1) | 0.004 | 0.933 | −0.088 | 0.096 | 0.024 | 0.017 | 0.013 | 0.775 | −0.078 | 0.104 | 0.024 | 0.017 | 0.008 | 0.382 | −0.010 | 0.025 | 0.025 | 0.017 |
| IL-10 (pg mL−1) | 0.119 | 0.019 | 0.019 | 0.219 | 0.035 | 0.027 | 0.137 | 0.007 | 0.038 | 0.236 | 0.036 | 0.029 | −0.005 | 0.570 | −0.024 | 0.013 | 0.032 | 0.024 |
| IL-4 (pg mL−1) | −0.060 | 0.403 | −0.202 | 0.081 | 0.019 | 0.011 | −0.061 | 0.391 | −0.201 | 0.079 | 0.019 | 0.011 | −0.017 | 0.209 | −0.044 | 0.010 | 0.019 | 0.012 |
| TAC (μM) | 0.229 | 0.000 | 0.112 | 0.347 | 0.034 | 0.026 | 0.273 | 0.000 | 0.157 | 0.389 | 0.038 | 0.030 | 0.004 | 0.700 | −0.018 | 0.027 | 0.025 | 0.018 |
| MDA (μM) | 0.003 | 0.953 | −0.083 | 0.088 | 0.014 | 0.007 | 0.018 | 0.676 | −0.067 | 0.103 | 0.014 | 0.007 | 0.007 | 0.403 | −0.009 | 0.023 | 0.014 | 0.007 |
| Non-provitamin A | Total carotenoids | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||||
| β | p-Value | Lower | Upper | R 2 | R 2 adj. | β | p-Value | Lower | Upper | R 2 | R 2 adj. | |
| a Multiple linear regression models were used to assess the associations between carotenoids and a selected list of biomarkers relevant to cardiometabolic health, inflammatory status, and oxidative stress. Model 3 was adjusted for the following variables: age (years), gender (male/female), marital status (married/single/other), education level (having diploma or low literacy/higher than diploma), smoking status (yes/no), disease history (yes/no for cardiovascular disease, diabetes, and hypertension) and fruit + vegetable intake. BMI (kg m−2) was also included as an explanatory variable, in models where it was not the dependent variable. All continuous variables were log-transformed. Carotenoids were adjusted for total energy intake (and expressed as μg/1000 kcal) prior to log-transformation. b Statistically significant p-values (p < 0.05) are shown in bold. Abbreviations: BMI = body mass index, HOMA IR = homeostatic model assessment of insulin resistance, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, SBP = systolic blood pressure, DBP = diastolic blood pressure, hs-CRP = high-sensitivity C-reactive protein, IL = interleukin, TNF-α = tumor necrosis factor α, TAC = total antioxidant capacity, MDA = malondialdehyde, WC = waist circumference, FBG = fasting blood glucose, TC = total cholesterol, TG = triglycerides. | ||||||||||||
| BMI (kg m−2) | 0.016 | 0.184 | −0.007 | 0.039 | 0.022 | 0.015 | −0.001 | 0.956 | −0.019 | 0.018 | 0.021 | 0.014 |
| WC (cm) | 0.018 | 0.194 | −0.009 | 0.045 | 0.255 | 0.249 | −0.004 | 0.686 | −0.026 | 0.017 | 0.254 | 0.248 |
| FBG (mg dL−1) | 0.015 | 0.305 | −0.014 | 0.043 | 0.005 | −0.002 | 0.016 | 0.163 | −0.007 | 0.039 | 0.006 | −0.002 |
| Insulin (mIU mL−1) | 0.055 | 0.281 | −0.045 | 0.156 | 0.01 | 0.002 | 0.019 | 0.657 | −0.063 | 0.100 | 0.009 | 0.002 |
| HOMA IR (%) | 0.070 | 0.223 | −0.043 | 0.183 | 0.008 | 0.000 | 0.035 | 0.456 | −0.057 | 0.127 | 0.007 | 0.000 |
| HDL-c (mg dL−1) | 0.056 | 0.014 | 0.011 | 0.100 | 0.026 | 0.018 | 0.017 | 0.367 | −0.019 | 0.053 | 0.022 | 0.015 |
| LDL-c (mg dL−1) | −0.003 | 0.901 | −0.051 | 0.045 | 0.011 | 0.003 | −0.006 | 0.758 | −0.045 | 0.033 | 0.011 | 0.003 |
| TC (mg dL−1) | −0.005 | 0.775 | −0.038 | 0.028 | 0.007 | −0.001 | −0.004 | 0.780 | −0.031 | 0.023 | 0.007 | −0.001 |
| TG (mg dL−1) | −0.022 | 0.618 | −0.107 | 0.064 | 0.012 | 0.005 | −0.008 | 0.810 | −0.078 | 0.061 | 0.012 | 0.004 |
| SBP (mmHg) | −0.001 | 0.965 | −0.023 | 0.022 | 0.015 | 0.008 | −0.006 | 0.543 | −0.024 | 0.013 | 0.015 | 0.008 |
| DBP (mmHg) | −0.016 | 0.173 | −0.038 | 0.007 | 0.021 | 0.014 | −0.011 | 0.256 | −0.029 | 0.008 | 0.021 | 0.013 |
| hs-CRP (mg dL−1) | 0.015 | 0.729 | −0.070 | 0.100 | 0.029 | 0.021 | 0.048 | 0.172 | −0.021 | 0.117 | 0.03 | 0.022 |
| IL-6 (pg mL−1) | −0.053 | 0.366 | −0.167 | 0.061 | 0.027 | 0.020 | −0.083 | 0.079 | −0.176 | 0.010 | 0.029 | 0.021 |
| IL-1β (pg mL−1) | −0.066 | 0.181 | −0.163 | 0.031 | 0.031 | 0.024 | −0.111 | 0.006 | −0.189 | −0.032 | 0.035 | 0.028 |
| TNF-α (pg mL−1) | −0.122 | 0.034 | −0.236 | −0.009 | 0.027 | 0.019 | −0.113 | 0.016 | −0.205 | −0.021 | 0.028 | 0.020 |
| IL-10 (pg mL−1) | 0.104 | 0.100 | −0.020 | 0.227 | 0.033 | 0.026 | 0.146 | 0.004 | 0.045 | 0.246 | 0.036 | 0.029 |
| IL-4 (pg mL−1) | −0.019 | 0.833 | −0.194 | 0.156 | 0.018 | 0.011 | 0.016 | 0.823 | −0.126 | 0.158 | 0.018 | 0.011 |
| TAC (μM) | 0.124 | 0.097 | −0.022 | 0.269 | 0.027 | 0.019 | 0.145 | 0.016 | 0.027 | 0.264 | 0.028 | 0.021 |
| MDA (μM) | 0.036 | 0.501 | −0.070 | 0.142 | 0.014 | 0.007 | 0.028 | 0.530 | −0.059 | 0.114 | 0.014 | 0.007 |
Several carotenoids showed statistically significant associations with markers of oxidative stress and inflammation. This was limited to interleukins, TNF-α, and TAC; no associations were significant for hs-CRP or MDA. For IL-6, IL-1β, and TNF-α, carotenoids tended to show inverse associations. IL-6 was negatively associated with α-carotene (β = −0.057, p = 0.008), provitamin A carotenoids (β = −0.064, p = 0.029), and lycopene (β = −0.073, p = 0.014). IL-1β had an inverse association with the individual provitamin A carotenoids, provitamin A carotenoids as a group, and total carotenoids (Tables 3 and 6). TNF-α was negatively associated with β-cryptoxanthin, lycopene, non-provitamin A carotenoids, and total carotenoids (βs in the range of −0.122 and −0.080, all p-values <0.05). IL-10 was positively associated with several carotenoids (Tables 3–6). None of the carotenoids were associated with IL-4 in the final model. TAC was positively associated with α-carotene, provitamin A carotenoids, lutein + zeaxanthin, violaxanthin, neoxanthin, and total carotenoids (βs in the range of 0.065–0.273, all p-values <0.05).
4.5 Sensitivity analyses
In the sensitivity analysis using BSA to categorize participants into cases and controls, β-carotene and provitamin A carotenoids showed a significant protective effect (ESI Table 7†). The sex-stratified analysis (ESI Table 8†) revealed a significant reduction in the odds of overweight/obesity associated with astaxanthin in men (OR: 0.800; p = 0.013). None of the other carotenoids showed significant associations with the odds of overweight/obesity (ESI Table 8†). ESI Table 9† displays the association between dietary intake of carotenoids and overweight/obesity based on BMI, separately adjusted either for fruits or for vegetables (sensitivity analysis). Adjusting only for fruit (but not vegetable) consumption + model 2 yielded comparable results with model 2. Adjusting for vegetable (but not fruit) intake + model 2 showed a protective effect of astaxanthin (OR: 0.862, p = 0.019) and an increased OR associated with higher intakes of lycopene (OR: 1.595, p = 0.032) (ESI Table 9†).Evaluation of the associations between carotenoid intakes and biomarker analyses in cases and controls separately suggested a general tendency for associations to be more often significant in participants with overweight/obesity (ESI Table 12†). In participants with overweight/obesity, BMI was positively associated with all individual non-provitamin A carotenoids except for lycopene as well as non-provitamin A carotenoids (grouped) (βs in the range of 0.005–0.021, p < 0.05). WC was positively associated with lycopene only in cases (β = 0.021, p = 0.009). No associations were significant for glucose-control related markers. Regarding blood lipid profile, only phytoene, phytofluene, and non-provitamin A carotenoids were associated with HDL-c concentrations in cases (β = 0.069, p = 0.018, β = 0.066, p = 0.022, and β = 0.072, p = 0.024, respectively). β-Cryptoxanthin was inversely associated with SBP (β = −0.024, p = 0.014) and DBP (β = −0.024, p = 0.020) only in controls. Pro-inflammatory biomarkers IL-6, IL-1β, and TNF-α showed negative associations primarily in the overweight/obese group (ESI Table 12†). IL-10 and TAC tended to show positive associations with several carotenoids. IL-4 was only associated with lycopene (β = 0.120, p = 0.037). These associations were almost always only significant in cases.
The sex-based analysis (ESI Table 13†) showed a significant positive association between lycopene and WC only among women (β = 0.026, p = 0.005). Additionally, a positive association between non-provitamin A carotenoids and WC also reached statistical significance among women (β = 0.039, p = 0.035). Among women, lycopene intake was also positively significantly associated with FBG (β = 0.027, p = 0.006), insulin concentrations (β = 0.071, p = 0.044), and HOMA IR (β = 0.098, p = 0.014). In contrast, violaxanthin and neoxanthin had a positive association with fasting insulin only in men (β = 0.125, p = 0.036, β = 0.118, p = 0.043, respectively). The negative association between systolic and diastolic blood pressure and β-cryptoxanthin consumption was only significant in men (β = −0.024, p = 0.038, β = −0.029, p = 0.008, respectively). The associations between IL-6, IL-1β and TNF-α and carotenoids generally showed similar directions of response in both sexes, although the associations tended to reach significance in only one of the sexes (ILs tended to be more often significant in men and TNF-α in women). The associations between lutein and zeaxanthin and IL-1β and TNF-α were only inversely associated in women. β-Cryptoxanthin showed a negative association with IL-4 in men (β = −0.223, p = 0.007).
5. Discussion
The current analysis revealed significant protective associations between the intake of provitamin A carotenoids and astaxanthin and the odds of overweight/obesity (OR: 0.655, p = 0.041, and OR: 0.859, p = 0.017, respectively), even after adjusting for fruit and vegetable consumption. Similarly, when classified based on BSA values, provitamin A as well as β-carotene were associated with lower OR for overweight/obesity (OR: 0.627, p = 0.032, and OR: 0.634, p = 0.049, respectively). Higher dietary intake of several carotenoids (α-carotene, β-carotene, β-cryptoxanthin, provitamin A carotenoids, lutein/zeaxanthin, lycopene, violaxanthin, neoxanthin, non-provitamin A carotenoids and total carotenoids) was beneficially associated with several inflammatory markers and/or TAC. Based on further stratified analyses, associations with biomarkers were more often significant in participants with overweight/obesity than in those with normal-weight. Interestingly, sensitivity analysis revealed inverse associations between lycopene intake and the odds of overweight/obesity in models adjusted for confounders and vegetable intake.A significant difference in the intake of several carotenoids was observed between cases and controls, with median intakes being higher by 669 μg d−1 for total and 605 μg d−1 for provitamin A carotenoids among normal-weight participants per 1000 kcal. Fully adjusted logistic regression models showed that a higher intake of provitamin A carotenoid and astaxanthin was significantly associated with lower odds of overweight/obesity as defined by BMI. For every 605 μg d−1 (per 1000 kcal) increase in provitamin A carotenoids, there was a 34.5% decrease (OR = 0.655) in the odds of overweight/obesity. With a BSA-based classification, provitamin A carotenoids and β-carotene showed a significant inverse association. Importantly, adjusting for fruit and vegetable intake did not alter the significant association, which may point toward effects being independent of fruit/vegetable intake.
Several epidemiological studies support a beneficial association between carotenoid intake or their tissue concentrations and overweight/obesity.39,56 Additionally, negative associations between circulating carotenoids and adiposity-related markers (such as BMI, WC, and fat mass) have also been reported.40,41,57 Some studies found a beneficial effect for β-carotene, α-carotene, lutein + zeaxanthin, lycopene, and β-cryptoxanthin,40,41 with β-carotene and α-carotene showing the strongest association.40 Previous reports have proposed a substantial effect for provitamin A carotenoids, as these may be turned into retinoids, which may interact with RXR/PPAR receptors, involved in the proliferation of adipose tissue.58,59 However, a cross-sectional analysis of PREDIMED-Plus participants, i.e., men and women with overweight/obesity (between 55 and 75 years), found beneficial associations with anthropometric measures for xanthophylls but not for carotenes and provitamin A carotenoids,57 which is in contrast with our results. This may point to additional benefits of carotenoids beyond acting as precursors for vitamin A, such as improving antioxidant status or simply acting as markers of fruits and vegetables (the authors did not adjust models for fruit/vegetables intake). In support of this, non-provitamin A carotenoids were also associated with potential health benefits other than BMI, including astaxanthin, phytoene, and phytofluene (Tables 3–6).
As for astaxanthin, one meta-analysis found no effect of astaxanthin supplementation on BMI or body weight, although beneficial effects on HDL-c and CRP levels were observed60 and astaxanthin showed to be efficacious in reducing weight gain elicited by a high-fat diet in a mouse model.61 As fish and seafood are the main sources of astaxanthin,53 likely, the benefits of consuming fish and seafood, related to lower BMI in some studies,62 are reflected in the present findings. Overall, several studies have shown beneficial associations between carotenoids and body composition-related markers, but inconsistencies exist regarding the type of carotenoids and their associations with overweight/obesity, which warrants further investigation.
Using a BMI or BSA-based assignment to normal or overweight/obese groups yielded somewhat different associations, suggesting that the method of evaluating body composition may influence the associations to a certain extent. In line with this, a cross-sectional study in middle-aged men concluded that increased consumption of β-carotene was inversely associated with BMI, while β-carotene, α-carotene, lycopene, and total carotenoid intake was associated with lower measures of WC.63 It was also reported that more direct measures of adipose tissue mass, such as by dual-energy X-ray absorptiometry, were more strongly associated with carotenoid concentrations in serum than anthropometric measures40 This may be related to the fact that adipose tissue is among the main storage sites of carotenoids,64 where they or their metabolites may interact with specific transcription factors such as PPARs/RXRs or signalling pathways relevant to adipogenesis including AMPK. Interactions with such processes can influence adipogenesis, lipogenesis, and metabolic activity, as demonstrated for several carotenoids and/or their BCO1/BCO2 conversion products.59,64 Furthermore, carotenoids may influence adipose tissue through their actions outside this tissue, such as the liver, muscles, or the brain, by stimulating mitochondrial fatty acid oxidation in muscle tissue.64
In our final model, we applied a covariate adjustment for fruit and vegetable intake to separate the effects of carotenoids from fruit and vegetable intake. Fruits and vegetables can be rich sources of dietary fiber, vitamins, minerals, and several phytochemicals13 that might act in concert to yield health benefits. Fruits and vegetables are also generally low in calories,65 which may also improve weight management.56,66 Adjusting for fruits and vegetables indeed modified some associations and should thus be considered in future investigations.
Regarding biomarkers, dietary intake of several carotenoids (all provitamin A carotenoids, lutein/zeaxanthin, lycopene, violaxanthin, neoxanthin, non-provitamin A carotenoids and total carotenoids) were associated with lower concentrations of inflammatory markers and increased antioxidant capacity, which may be important factors in the development of obesity and its co-morbidities. It has been suggested that a lower intake of dietary antioxidants, including secondary plant metabolites, may contribute to increased oxidative stress associated with obesity.67 In support of this hypothesis, studies have shown a lower dietary intake or lower blood concentrations of dietary antioxidants in overweight/obese individuals compared to normal-weight people.68,69 In the present study, several carotenoids (α-carotene, provitamin A carotenoids, lutein + zeaxanthin, violaxanthin, neoxanthin, and total carotenoids) were associated with increased TAC. A systematic review and meta-analysis of randomized controlled trials assessed the effect of carotenoid supplementation, including astaxanthin and lutein/zeaxanthin, on inflammatory markers for CRP, Il-6, and TNF-α,70 finding significant reductions in CRP and IL-6 levels, and similar results were obtained in observational studies.71 In the present study, IL-1β was negatively associated with provitamin A carotenoids as a group and individually (α- and β-carotene and β-cryptoxanthin), as well as total carotenoids, and TNF-α with β-cryptoxanthin, lycopene, non-provitamin A and total carotenoids. Some discrepancies among these results may be due to the assessment of carotenoid exposure (blood concentrations or dietary intake, with blood concentrations being expected to result in stronger associations as dietary intakes of carotenoids are moderated by bioavailability, which can vary substantially). This is a limitation of the present study, as HPLC carotenoid analyses of blood samples, similar as to many other studies72,73 were unavailable due to their large cost and labor intensity.
Furthermore, it was stated that the baseline levels of inflammatory cytokines may predict responsiveness to supplementation with carotenoids.70 In line with this, when stratified into overweight/obesity and normal weight groups, associations between carotenoid intake and reduced inflammatory cytokine concentrations (IL-6 and TNF-α) were stronger in the group having overweight/obesity vs. persons with normal BMI.
Our analysis showed intriguing results for lycopene. First, we found increased odds of overweight/obesity with increasing intake of lycopene in models adjusted for vegetable intake (ESI Table 9†). Lycopene was also the only carotenoid positively associated with WC. This association was observed among women and participants with overweight/obesity in stratified analyses as well. Additionally, among women, lycopene intake was associated with higher fasting blood glucose, insulin levels and insulin resistance. In contrast, lycopene intake was associated with reduced inflammation (IL-6 and TNF-α) in the fully adjusted model (also adjusted for fruits and vegetables), in women when stratified for sex, and also in BMI-stratified analyses (though not IL-6 in participants with overweight/obesity), indicative of a potential anti-inflammatory effect. Several epidemiological and experimental studies support a beneficial effect of lycopene (intake or blood concentrations) and tomato-products rich in lycopene on cardiovascular risk factors, metabolic syndrome, and certain types of cancer.74–76 Lycopene is acknowledged as one of the most potent antioxidants among carotenoids.76 One may, therefore expect a lower risk of conditions characterized by inflammation and oxidative stress, such as overweight and obesity.47 However, important dietary sources of lycopene, at least in a more westernized diet, include tomato products such as pasta sauces and ketchup.76 In a study of US adults, pizza and pasta were found to be the main contributors of lycopene to the diet.77 As such, a potential explanation for the discrepancy with our finding may be residual confounding from those less healthy food sources of dietary lycopene. Additionally, it was shown that people with high blood concentrations of lycopene had lower concentrations of other carotenoids, including α-carotene, β-carotene, zeaxanthin, and lutein.77 Therefore, sources of certain carotenoids, especially of lycopene, may be related to the intake of convenience foods rather than fresh fruits and vegetables. In a cross-sectional evaluation, serum carotenoid concentrations (α- and β-carotene, β-cryptoxanthin, and lutein/zeaxanthin) were associated with fresh fruit, vegetable, and juice consumption (r = 0.22), while lycopene was not (r = −0.06).41 This supports the notion that the food sources of carotenoids may differ and are important sources of bias to take into account.
When stratified by sex, the observed associations differed in part between men and women. Such differences may derive from between-sex differences in carotenoid utilization and/or specific biomarker regulation. A study in Japanese healthy adults found a protective effect for serum carotenoids (α- and β-carotene, and canthaxanthin) on measures of central adiposity only in female participants.78 Plasma concentrations of carotenoids have been described to be higher in women, irrespective of dietary intake,79,80 which may have implications for carotenoid-mediated effects. Even though we did not observe any sex-based differences in carotenoid intake (adjusted for total daily energy intake), it is plausible that this intake yielded different tissue concentrations.
The present study had several strengths worth noting. First of all, we investigated the associations of several individual dietary carotenoids with the odds of overweight/obesity. We included individual carotenoids in the analyses instead of only assessing them as groups, which provided valuable insights and allowed comparability across studies even if different individual carotenoids were chosen for analysis. In addition to only studying the odds of overweight/obesity, we included biomarkers relevant to the etiology of obesity or its complications and assessed their association with the intake of carotenoids, providing a more objective method of assessing the health status of individuals with overweight/obesity. Furthermore, we performed extensive sensitivity analyses in subgroups of the study population. Dietary information was collected via a validated FFQ, which was administered by trained personnel, improving the reliability of the data. Most importantly, in our final model, we adjusted for fruit and vegetable intake that may also explain the observed health benefits.
Some limitations of our study are inherent to the study design; most notably, the retrospective collection of data makes the results prone to recall bias. Additionally, the use of FFQs for dietary intake assessment in our study may be affected by social desirability bias (response bias). Furthermore, the cross-sectional nature of the study does not allow for drawing any conclusions regarding causality. Also the case-control design of the study may have introduced some sampling bias (selection bias) in the present findings, which prompts caution upon interpreting results. Despite the relatively large sample size, the generalizability of findings may be limited, considering that participants belonged to an Iranian urban population; however, this is a limitation inherent to many studies due to lack of local consumption patterns.81,82 In addition, dietary patterns in Iran have shifted from traditional Iranian diets towards western diets similar to the US diet.83 Finally, although several confounders were adjusted for in the models, there is a possibility for residual confounding.
6. Conclusion
Our findings suggest an association between carotenoids and the odds of overweight/obesity. Protective associations in this regard were noted for increased dietary intake of provitamin A carotenoids as a group and astaxanthin. Additionally, our study indicates that dietary antioxidant intake in the form of carotenoids was associated with reduced inflammation and increased antioxidant capacity in plasma, even after fruit and vegetable intake adjustment. Furthermore, as lycopene was in part statistically positively associated with the odds of overweight/obesity and WC, it is important to take the food source and, potentially the overall dietary pattern into account when evaluating dietary carotenoid associations with health outcomes.Author contributions
F. V. designed the study; N. K. performed the statistical analyses and drafted the manuscript; F. V., N. K., and T. B. interpreted the data; F. V. and T. B. provided expertise and oversight on the intellectual content. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express our deepest appreciation to all the participants who took part in this study.References
- WHO, Obesity and overweight, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight, (accessed 21 July, 2023).
- S. A. Keramat, K. Alam, R. H. Rana, R. Chowdhury, F. Farjana and R. Hashmi, et al., Obesity and the risk of developing chronic diseases in middle-aged and older adults: Findings from an Australian longitudinal population survey, 2009–2017, PLoS One, 2021, 16, e0260158 CrossRef CAS PubMed.
- E. Jokinen, Obesity and cardiovascular disease, Minerva Pediatr., 2015, 67, 25–32 CAS.
- S. Pati, W. Irfan, A. Jameel, S. Ahmed and R. K. Shahid, Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management, Cancers, 2023, 15, 485 CrossRef CAS PubMed.
- J. Hecker, K. Freijer, M. Hiligsmann and S. Evers, Burden of disease study of overweight and obesity; the societal impact in terms of cost-of-illness and health-related quality of life, BMC Public Health, 2022, 22, 46 CrossRef CAS PubMed.
- T. Lehnert, D. Sonntag, A. Konnopka, S. Riedel-Heller and H. H. Konig, Economic costs of overweight and obesity, Best Pract. Res., Clin. Endocrinol. Metab., 2013, 27, 105–115 CrossRef PubMed.
- B. M. Popkin and S. W. Ng, The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable, Obes. Rev., 2022, 23, e13366 CrossRef PubMed.
- A. Wirth, M. Wabitsch and H. Hauner, The prevention and treatment of obesity, Dtsch. Arztebl. Int., 2014, 111, 705–713 Search PubMed.
- M. Gargallo Fernandez Manuel, I. Breton Lesmes, J. Basulto Marset, J. Quiles Izquierdo, X. Formiguera Sala, J. Salas-Salvado and FESNADO-SEEDO consensus group, Evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults (FESNAD-SEEDO consensus document). The role of diet in obesity treatment (III/III), Nutr. Hosp., 2012, 27, 833–864 CAS.
- WHO, Obesity: Prevention and Control, https://www.who.int/health-topics/obesity#tab=tab_3, (accessed July 24, 2023).
- A. D. Smethers and B. J. Rolls, Dietary Management of Obesity: Cornerstones of Healthy Eating Patterns, Med. Clin. North Am., 2018, 102, 107–124 CrossRef PubMed.
- R. Botchlett and C. Wu, Diet Composition for the Management of Obesity and Obesity-related Disorders, J Diabetes Mellit. Metab. Syndr., 2018, 3, 10–25 Search PubMed.
- J. L. Slavin and B. Lloyd, Health benefits of fruits and vegetables, Adv. Nutr., 2012, 3, 506–516 CrossRef CAS PubMed.
- S. H. Lee, L. V. Moore, S. Park, D. M. Harris and H. M. Blanck, Adults Meeting Fruit and Vegetable Intake Recommendations - United States, 2019, Morb. Mortal. Wkly. Rep., 2022, 71, 1–9 CrossRef PubMed.
- V. Miller, S. Yusuf, C. K. Chow, M. Dehghan, D. J. Corsi and K. Lock, et al., Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study, Lancet Global Health, 2016, 4, e695–e703 CrossRef PubMed.
- A. Kaulmann and T. Bohn, Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention, Nutr. Res., 2014, 34, 907–929 CrossRef CAS PubMed.
- Y. W. Jiang, Z. H. Sun, W. W. Tong, K. Yang, K. Q. Guo, G. Liu and A. Pan, Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies, Adv. Nutr., 2021, 12, 1723–1733 CrossRef CAS PubMed.
- M. A. Beydoun, X. Chen, K. Jha, H. A. Beydoun, A. B. Zonderman and J. A. Canas, Carotenoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis, Nutr. Rev., 2019, 77, 32–45 CrossRef PubMed.
- Y. Yao, H. M. Goh and J. E. Kim, The Roles of Carotenoid Consumption and Bioavailability in Cardiovascular Health, Antioxidants, 2021, 10, 1978 CrossRef CAS PubMed.
- D. Aune, N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta and D. C. Greenwood, et al., Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies, Am. J. Clin. Nutr., 2018, 108, 1069–1091 CrossRef PubMed.
- F. Lauretani, R. D. Semba, M. Dayhoff-Brannigan, A. M. Corsi, A. Di Iorio and E. Buiatti, et al., Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI study, Eur. J. Nutr., 2008, 47, 335–340 CrossRef PubMed.
- A. Fernandez-Sanchez, E. Madrigal-Santillan, M. Bautista, J. Esquivel-Soto, A. Morales-Gonzalez, C. Esquivel-Chirino and I. Durante-Montiel, et al., Inflammation, oxidative stress, and obesity, Int. J. Mol. Sci., 2011, 12, 3117–3132 CrossRef CAS PubMed.
- P. Manna and S. K. Jain, Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies, Metab. Syndr. Relat. Disord., 2015, 13, 423–444 CrossRef CAS PubMed.
- M. S. Ellulu, I. Patimah, H. Khaza'ai, A. Rahmat and Y. Abed, Obesity and inflammation: the linking mechanism and the complications, Arch. Med. Sci., 2017, 13, 851–863 CrossRef CAS PubMed.
- S. K. Biswas, Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox?, Oxid. Med. Cell. Longevity, 2016, 2016, 5698931 Search PubMed.
- F. Vahid, D. Rahmani and S. H. Davoodi, The correlation between serum inflammatory, antioxidant, glucose handling biomarkers, and Dietary Antioxidant Index (DAI) and the role of DAI in obesity/overweight causation: population-based case-control study, Int. J. Obes., 2021, 45, 2591–2599 CrossRef CAS PubMed.
- M. Gholamalizadeh, S. Rastgoo, S. Doaei, F. Vahid, H. Malmir, N. Ashoori and A. M. Jarrahi, Index of Nutritional Quality (INQ) and the Risk of Obesity in Male Adolescents: a Case-Control Study, Biol. Trace Elem. Res., 2021, 199, 1701–1706 CrossRef CAS PubMed.
- B. Aminnejad, Z. Roumi, N. Hasanpour Ardekanizadeh, F. Vahid, M. Gholamalizadeh and N. Kalantari, et al., Association of dietary antioxidant index with body mass index in adolescents, Obes. Sci. Pract., 2023, 9, 15–22 CrossRef PubMed.
- F. Vahid, F. Bourbour, M. Gholamalizadeh, N. Shivappa, J. R. Hebert and K. Babakhani, et al., A pro-inflammatory diet increases the likelihood of obesity and overweight in adolescent boys: a case-control study, Diabetol. Metab. Syndr., 2020, 12, 29 CrossRef CAS PubMed.
- T. M. S. Oliveira, J. Bressan, A. M. Pimenta, M. A. Martinez-Gonzalez, N. Shivappa, J. R. Hebert and H. H. M. Hermsdorff, Dietary inflammatory index and prevalence of overweight and obesity in Brazilian graduates from the Cohort of Universities of Minas Gerais (CUME project), Nutrition, 2020, 71, 110635 CrossRef PubMed.
- M. Saghafi-Asl, S. Mirmajidi, M. Asghari Jafarabadi, F. Vahid, N. Shivappa, J. R. Hébert and V. Ebrahimzadeh Attari, The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity, Sci. Rep., 2021, 11, 7515 CrossRef CAS PubMed.
- F. Vahid, F. Bourbour, M. Gholamalizadeh, N. Shivappa, J. R. Hébert and K. Babakhani, et al., A pro-inflammatory diet increases the likelihood of obesity and overweight in adolescent boys: a case–control study, Diabetol. Metab. Syndr., 2020, 12, 29 CrossRef CAS PubMed.
- M. Gholamalizadeh, M. Ahmadzadeh, F. BourBour, F. Vahid, M. Ajami and N. Majidi, et al., Associations between the dietary inflammatory index with obesity and body fat in male adolescents, BMC Endocr. Disord., 2022, 22, 115 CrossRef CAS PubMed.
- M. L. Bonet, J. A. Canas, J. Ribot and A. Palou, Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity, Arch. Biochem. Biophys., 2015, 572, 112–125 CrossRef PubMed.
- Y. Sarmiento-Andrade, R. Suarez, B. Quintero, K. Garrochamba and S. P. Chapela, Gut microbiota and obesity: New insights, Front. Nutr., 2022, 9, 1018212 CrossRef PubMed.
- A. L. Cunningham, J. W. Stephens and D. A. Harris, A review on gut microbiota: a central factor in the pathophysiology of obesity, Lipids Health Dis., 2021, 20, 65 CrossRef CAS PubMed.
- H. R. Rocha, M. C. Coelho, A. M. Gomes and M. E. Pintado, Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases, Nutrients, 2023, 15, 2265 CrossRef CAS PubMed.
- A. Eroglu, I. S. Al'Abri, R. E. Kopec, N. Crook and T. Bohn, Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota, Adv. Nutr., 2023, 14, 238–255 CrossRef CAS PubMed.
- N. Yao, S. Yan, Y. Guo, H. Wang, X. Li and L. Wang, et al., The association between carotenoids and subjects with overweight or obesity: a systematic review and meta-analysis, Food Funct., 2021, 12, 4768–4782 RSC.
- C. Wang, C. W. Ling, D. Ding, Y. H. Li, W. T. Cao, X. Y. Tang and Y. M. Chen, Associations of Serum Carotenoids with DXA-Derived Body Fat and Fat Distribution in Chinese Adults: A Prospective Study, J. Acad. Nutr. Diet., 2020, 120, 985–1001 CrossRef PubMed.
- L. F. Andersen, D. R. Jacobs Jr., M. D. Gross, P. J. Schreiner, O. D. Williams and D. H. Lee, Longitudinal associations between body mass index and serum carotenoids: the CARDIA study, Br. J. Nutr., 2006, 95, 358–365 CrossRef CAS PubMed.
- E. T. Nuss, A. R. Valentine, Z. Zhang, H. J. Lai and S. A. Tanumihardjo, Serum carotenoid interactions in premenopausal women reveal alpha-carotene is negatively impacted by body fat, Exp. Biol. Med., 2017, 242, 1262–1270 CrossRef CAS PubMed.
- E. Biehler, Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to totalcarotenoid intake: Assessment in Luxembourg, J. Food Compos. Anal., 2011, 25, 56–65 CrossRef.
- F. R. Baldrick, J. V. Woodside, J. S. Elborn, I. S. Young and M. C. McKinley, Biomarkers of fruit and vegetable intake in human intervention studies: a systematic review, Crit. Rev. Food Sci. Nutr., 2011, 51, 795–815 CrossRef CAS PubMed.
- J. Blumberg and G. Block, The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland, Nutr. Rev., 1994, 52, 242–245 CrossRef CAS PubMed.
- G. S. Omenn, G. E. Goodman, M. D. Thornquist, J. Balmes, M. R. Cullen and A. Glass, et al., Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial, J. Natl. Cancer Inst., 1996, 88, 1550–1559 CrossRef CAS PubMed.
- T. Bohn, Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases, Antioxidants, 2019, 8, 179 CrossRef CAS PubMed.
- F. Pourfarzi, A. Sadjadi, H. Poustchi and F. Amani, Prevalence of overweight and obesity in Iranian population: A population-based study in northwestern of Iran, J. Public Health Res., 2021, 11, 2475 Search PubMed.
- B. Abiri, A. R. Ahmadi, S. Amini, M. Akbari, F. Hosseinpanah and S. A. Madinehzad, et al., Prevalence of overweight and obesity among Iranian population: a systematic review and meta-analysis, J. Health Popul. Nutr., 2023, 42, 70 CrossRef PubMed.
- F. Vahid, W. Rahmani, S. H. Davoodi and T. Bohn, The micronutrient content of the diet is correlated with serum glucose biomarkers and lipid profile and is associated with the odds of being overweight/obese-a case-control study, Front. Nutr., 2023, 10, 1148183 CrossRef PubMed.
- P. Mirmiran, F. H. Esfahani, Y. Mehrabi, M. Hedayati and F. Azizi, Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study, Public Health Nutr., 2010, 13, 654–662 CrossRef PubMed.
- J. M. Holden, Carotenoid Content of U.S. Foods: An Update of the Database, J. Food Compos. Anal., 1999, 12, 169–196 CrossRef CAS.
- EFSA, Opinion of the scientific panel on additives and products or substances used in animal feed on the request from the European commission on the safety of use of colouring agents in animal nutrition PART I. General principles and astaxanthin, EFSA J., 2005, 3, 1–40 Search PubMed.
- R. D. Mosteller, Simplified calculation of body-surface area, N. Engl. J. Med., 1987, 317, 1098 CAS.
- I. Corp, IBM SPSS Statistics for Windows, Version 25.0, IBM Corp., Armonk, NY, 2017 Search PubMed.
- D. S. Sartorelli, L. J. Franco and M. A. Cardoso, High intake of fruits and vegetables predicts weight loss in Brazilian overweight adults, Nutr. Res., 2008, 28, 233–238 CrossRef CAS PubMed.
- M. Marhuenda-Munoz, I. Dominguez-Lopez, K. Langohr, A. Tresserra-Rimbau, M. A. Martinez Gonzalez and J. Salas-Salvado, et al., Circulating carotenoids are associated with favorable lipid and fatty acid profiles in an older population at high cardiovascular risk, Front. Nutr., 2022, 9, 967967 CrossRef PubMed.
- M. L. Bonet, J. A. Canas, J. Ribot and A. Palou, Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity, Arch. Biochem. Biophys., 2015, 572, 112–125 CrossRef PubMed.
- T. Bohn, M. L. Bonet, P. Borel, J. Keijer, J. F. Landrier and I. Milisav, et al., Mechanistic aspects of carotenoid health benefits - where are we now?, Nutr. Res. Rev., 2021, 34, 276–302 CrossRef CAS PubMed.
- W. Xia, N. Tang, H. Kord-Varkaneh, T. Y. Low, S. C. Tan, X. Wu and Y. Zhu, The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials, Pharmacol. Res., 2020, 161, 105113 CrossRef CAS PubMed.
- S. Bhuvaneswari, et al., Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet, Process Biochem., 2010, 45, 1406–1414 CrossRef CAS.
- N. Bender, M. Portmann, Z. Heg, K. Hofmann, M. Zwahlen and M. Egger, Fish or n3-PUFA intake and body composition: a systematic review and meta–analysis, Obes. Rev., 2014, 15, 657–665 CrossRef CAS PubMed.
- I. Sluijs, J. W. Beulens, D. E. Grobbee and Y. T. van der Schouw, Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men, J. Nutr., 2009, 139, 987–992 CrossRef CAS PubMed.
- M. L. Bonet, J. Ribot, S. Galmes, F. Serra and A. Palou, Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2020, 1865, 158676 CrossRef CAS PubMed.
- B. J. Rolls, A. Drewnowski and J. H. Ledikwe, Changing the energy density of the diet as a strategy for weight management, J. Am. Diet. Assoc., 2005, 105, S98–103 CrossRef PubMed.
- L. Schwingshackl, G. Hoffmann, T. Kalle-Uhlmann, M. Arregui, B. Buijsse and H. Boeing, Fruit and Vegetable Consumption and Changes in Anthropometric Variables in Adult Populations: A Systematic Review and Meta-Analysis of Prospective Cohort Studies, PLoS One, 2015, 10, e0140846 CrossRef PubMed.
- H. K. Vincent, K. E. Innes and K. R. Vincent, Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity, Diabetes, Obes. Metab., 2007, 9, 813–839 CrossRef CAS PubMed.
- M. T. Adnan, M. N. Amin, M. G. Uddin, M. S. Hussain, M. S. Sarwar and M. K. Hossain, et al., Increased concentration of serum MDA, decreased antioxidants and altered trace elements and macro-minerals are linked to obesity among Bangladeshi population, Diabetes Metab. Syndr., 2019, 13, 933–938 CrossRef PubMed.
- H. K. Vincent, C. M. Bourguignon and A. G. Taylor, Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults, J. Hum. Nutr. Diet, 2010, 23, 20–29 CrossRef CAS PubMed.
- F. Hajizadeh-Sharafabad, E. S. Zahabi, M. Malekahmadi, R. Zarrin and M. Alizadeh, Carotenoids supplementation and inflammation: a systematic review and meta-analysis of randomized clinical trials, Crit. Rev. Food Sci. Nutr., 2022, 62, 8161–8177 CrossRef CAS PubMed.
- L. Jing, M. Xiao, H. Dong, J. Lin, G. Chen, W. Ling and Y. Chen, Serum Carotenoids Are Inversely Associated with RBP4 and Other Inflammatory Markers in Middle-Aged and Elderly Adults, Nutrients, 2018, 10, 260 CrossRef PubMed.
- E. R. Bertone, S. E. Hankinson, P. A. Newcomb, B. Rosner, W. C. Willett, M. J. Stampfer and K. M. Egan, A population-based case–control study of carotenoid and vitamin A intake and ovarian cancer (United States), Cancer, Causes Control, 2001, 12, 83–90 CrossRef CAS PubMed.
- M.-G. Deng, F. Liu, K. Wang, Y. Liang, J.-Q. Nie and J. Liu, Relationship between dietary carotenoid intake and sleep duration in American adults: a population-based study, Nutr. J., 2023, 22, 68 CrossRef CAS PubMed.
- H. M. Cheng, G. Koutsidis, J. K. Lodge, A. Ashor, M. Siervo and J. Lara, Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis, Atherosclerosis, 2017, 257, 100–108 CrossRef CAS PubMed.
- K. E. Senkus, L. Tan and K. M. Crowe-White, Lycopene and Metabolic Syndrome: A Systematic Review of the Literature, Adv. Nutr., 2019, 10, 19–29 CrossRef PubMed.
- M. Imran, F. Ghorat, I. Ul-Haq, H. Ur-Rehman, F. Aslam, M. Heydari, M. A. Shariati, E. Okuskhanova, Z. Yessimbekov, M. Thiruvengadam, M. H. Hashempur and M. Rebezov, Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders, Antioxidants, 2020, 9, 706 CrossRef CAS PubMed.
- Y. E. Zhou, M. S. Buchowski, J. Liu, D. G. Schlundt, F. A. Ukoli, W. J. Blot and M. K. Hargreaves, Plasma Lycopene Is Associated with Pizza and Pasta Consumption in Middle-Aged and Older African American and White Adults in the Southeastern USA in a Cross-Sectional Study, PLoS One, 2016, 11, e0161918 CrossRef PubMed.
- K. Suzuki, T. Inoue, R. Hioki, J. Ochiai, Y. Kusuhara, N. Ichino, K. Osakabe, N. Hamajima and Y. Ito, Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population, Clin. Nutr., 2006, 25, 780–789 CrossRef CAS PubMed.
- T. Allore, S. Lemieux, M. C. Vohl, P. Couture, B. Lamarche and C. Couillard, Correlates of the difference in plasma carotenoid concentrations between men and women, Br. J. Nutr., 2019, 121, 172–181 CrossRef CAS PubMed.
- V. Böhm, G. Lietz, B. Olmedilla-Alonso, D. Phelan, E. Reboul and D. Bánati, et al., From carotenoid intake to carotenoid blood and tissue concentrations - implications for dietary intake recommendations, Nutr. Rev., 2021, 79, 544–573 CrossRef PubMed.
- J. Yabuzaki, Carotenoids Database: structures, chemical fingerprints and distribution among organisms, Database, 2017, 2017, bax004 CrossRef PubMed.
- S. A. Arscott, in Carotenoids and Human Health, ed. S. A. Tanumihardjo, Humana Press, Totowa, NJ, 2013, pp. 3–19, DOI:10.1007/978-1-62703-203-2_1.
- W. Floor, From Quasi-Vegetarians to Quasi-Carnivores: The Changing Diet of Iranians, Ir. Stud., 2021, 54, 931–946 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo05648a |
| This journal is © The Royal Society of Chemistry 2024 |
