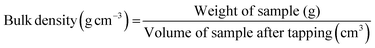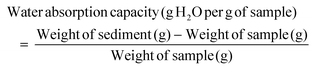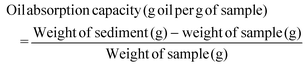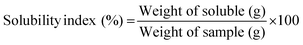Open Access Article
Open Access ArticleMelon peel flour: utilization as a functional ingredient in bakery products
Mafalda Alexandra
Silva
ab,
Tânia Gonçalves
Albuquerque
 *ab,
Rita Carneiro
Alves
*ab,
Rita Carneiro
Alves
 b,
M. Beatriz P. P.
Oliveira
b,
M. Beatriz P. P.
Oliveira
 b and
Helena S.
Costa
b and
Helena S.
Costa
 ab
ab
aDepartment of Food and Nutrition, National Institute of Health Dr. Ricardo Jorge, I.P., Av. Padre Cruz, 1649-016 Lisbon, Portugal. E-mail: tania.albuquerque@insa.min-saude.pt; Tel: +351 217519200
bREQUIMTE/LAQV, Department of Chemical Sciences, Faculty of Pharmacy, University of Porto, Porto, Portugal
First published on 10th January 2024
Abstract
Food by-products are a major concern with a direct impact on the economy, society, and environment. The valorisation of these by-products could be an advantageous approach to face the increase in food waste since it can compromise environmental health and food sustainability. On the other hand, this valorisation would allow the development of new food products with health benefits for the population. Cucumis melo L. is a highly consumed fruit all over the world since it has excellent sensory and nutritional qualities, being also a good source of bioactive compounds. However, its peel and seeds are usually discarded. The aim of this study was to evaluate the potential of melon peel flour as a functional ingredient for innovative food products. For that, two different formulations containing melon peel flour were developed (a biscuit and a muffin) by replacing a conventional flour (wheat flour) in different percentages (50% and 100%, respectively). The nutritional composition, total phenolic content, and antioxidant potential of the developed products were studied, showing a high content of fibre, high levels of phenolic compounds and good sensory acceptability. These results show that it is possible to enrich different foods with melon peel flour in order to improve their nutritional properties, contributing to improving public health, simultaneously valorising a usually rejected by-product, reducing food waste and the environmental impact.
Introduction
The large assembly of food by-products that results from fruit production and processing industries is a growing problem that leads to great management difficulties, both at economic and environmental levels. Currently, these by-products are used as fertilizers or for animal feed. However, they have high levels of dietary fibre and bioactive compounds, which gives them the potential to be used for the development/enrichment of food products with improved and beneficial properties for human health.1,2Melon (Cucumis melo L.) is a fruit widely consumed around the world, mainly due to its excellent sensory qualities. In addition, it has a rich nutritional composition and is a good source of bioactive compounds.3 Its global production generates around 8 to 20 million tons per year of by-products, which are currently discarded.4 Their valorisation meets the priorities established by the Sustainable Development Goals (SDGs) of the United Nations, which aim to promote the use of existing natural resources in a more sustainable and efficient way, meeting the concepts of a circular and green economy.5
Currently, society faces major nutritional challenges. It is estimated that the world population will continue to increase exponentially, reaching around 9.8 billion people by 2050.6 With this growth, the challenge of ensuring that the whole population gets enough safe, nutritious, and healthy food becomes ever-greater.7 The use of these by-products in the enrichment/development of new food products could also be one of the crucial strategies to combat the hunger and malnutrition that the population faces today.
Bakery products are part of the population's daily diet. However, they are generally nutritionally poor, being rich in saturated fat and sugar and poor in dietary fibre.8 A sufficient and regular intake of dietary fibre provides many health benefits, reducing the risk of developing several diseases, such as obesity, diabetes, and hypertension, among others.9–11 The dietary recommendation for dietary fibre intake is 25 g per day, for adults.12 However, the average daily intake of dietary fibre for the Portuguese population is 16.3 and 19.5 g day−1, for women and men, respectively.13 To increase the population's intake of dietary fibre, the incorporation of fruit by-products in bakery products has been studied.14–18
The objective of this study was to explore the potential use of melon peel flour, evaluating its nutritional composition, antioxidant activity and functional properties. In addition, two formulations based on melon peel flour with enhanced nutritional properties and that are organoleptically acceptable were developed.
Materials and methods
Standards and reagents
All chemicals and reagents were purchased from various commercial sources and were of analytical grade. Ultrapure water from the Milli-Q system (Millipore, Bedford, MA, USA) was used.Melon peel flour preparation
In 2021, the melon samples were collected from melon production and distribution companies (Frutas A. R. Santos, Torres Vedras, Portugal and Planície Verde, Rio Maior, Portugal), mainly discarded fruits, which do not correspond to market needs in terms of size, shape, and/or colour. Initially, the melons were washed under running water, immersed in water with sodium hypochlorite and the melon peels were manually separated and cut into small pieces. After various experiments with temperatures and drying times, to achieve the desired moisture content (<10%), the melon peels were dehydrated in a food dehydrator (Lacor 69523 Pro, Spain) at 50 °C for 18 h. Then, the melon peels were homogenized in a blender (Grindomix GM200, Retsch, Haan, Germany) at 5000 rpm for 1 min and sieved (420 μm) to obtain the melon peel flour, which was subsequently stored under vacuum and protected from light.Biscuit preparation
Two types of biscuits were prepared (Fig. 1): a control biscuit and a biscuit with 50% melon peel flour instead of wheat flour. Due to the functional properties of melon peel flour, it was only possible to develop biscuits with a 50% replacement. The ingredients and the respective amounts used in both formulations are presented in Table 1. The samples were cooked in a domestic oven (Flama 1548FL, Cesar, Oliveira de Azeméis, Portugal) for 10 min at 200 °C.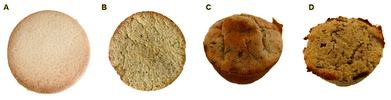 | ||
| Fig. 1 Bakery products developed. (A) Control biscuit, (B) biscuit with 50% melon peel flour, (C) control muffin, (D) muffin with 100% melon peel flour. | ||
| Ingredients (%) | Control biscuit | Biscuit with 50% melon peel flour |
|---|---|---|
| Wheat flour | 45.46 | 22.73 |
| Melon peel flour | — | 22.73 |
| Sugar | 18.18 | 18.18 |
| Butter | 18.18 | 18.18 |
| Eggs | 18.18 | 18.18 |
Muffin preparation
Two types of muffins were prepared (Fig. 1): a control muffin and a muffin with melon peel flour instead of wheat flour. Several ingredients and several recipes were tested to produce the two types of muffins, being the best ones selected, and used to prepare the samples herein studied. The final formulations (ingredients and amounts) used to produce muffins are presented in Table 2. The muffins were cooked in a domestic oven (Flama 1548FL, Cesar, Oliveira de Azeméis, Portugal) for 15 min at 180 °C.| Ingredients (%) | Control muffin | Muffin with 100% melon peel flour |
|---|---|---|
| Wheat flour | 23.72 | — |
| Melon peel flour | — | 18.05 |
| Banana | 27.67 | 29.72 |
| Soya yogurt | 19.76 | 21.23 |
| Eggs | 19.76 | 21.23 |
| Dry yeast | 0.79 | 0.85 |
| Vegetable oil | 2.37 | 2.55 |
| Chocolate | 5.93 | 6.37 |
Functional properties
The functional properties of melon peel flour, wheat flour and oat flour were determined. Oat flour is widely used in bakery products and is considered an alternative to wheat flour in the diets of celiac patients. Therefore, its functional properties were determined and compared with melon peel flour, which is gluten-free.| Dispersibility (%) = 100 − volume of settled particle (mL) |
Proximate composition analysis
The melon peel flour, biscuits, and muffins (controls and innovative products) were analysed regarding their content of moisture, ash, total protein, total fat and total dietary fibre. The available carbohydrates and energy values were calculated.23,24For quality assurance purposes, the test material FAPAS® 2496 was analysed with these methods and compared with the assigned values.
The moisture content was determined using a dry air oven (Memmert, Germany), according to the gravimetric method.25 The ash content was determined by direct incineration of the samples in a muffle furnace (525 ± 25 °C; 20 h) following the official method of AOAC 923.03.25 The protein content of the samples was determined by the Kjeldahl method, by quantifying the total nitrogen present in the analysed samples and using the block digestion system Foss Tecator 2006 Digestor and a Foss 2200 Kjeltec Auto Distillation unit (Foss, Hilleroed, Denmark).25 The total fat content was determined by the Soxhlet extraction method (Soxtec ™ 2050, Foss, Hilleroed, Denmark) with acid hydrolysis, using petroleum ether 40–60° as the extraction solvent.25 The total dietary fibre content was determined using an enzymatic–gravimetric method, according to AOAC 985.29.25
Antioxidant activity and total phenolic compounds
Sensory evaluation
The development of a new food product needs to consider sensory aspects and consumer acceptance. A hedonic test was used to evaluate the acceptability of the biscuit and muffin developed. 40 untrained panellists evaluated the acceptability of foods about their appearance, colour, odour, texture, taste, and overall acceptability (evaluation taking into account all analysed parameters) using the seven-point hedonic scale method ranging from 1 (disliked it very much), 4 (neither liked nor disliked it), to 7 (liked it very much). The panellists were also asked about their purchase intention (intention to purchase the product if commercially available) through the options “certainly would not buy it”, “probably would not buy it”, “might or might not buy it”, “probably would buy it” and “certainly would buy it”.Statistical analysis
The statistical analyses of data were performed using Microsoft Office Excel® 2010. Results are expressed as mean ± standard deviation (SD). Values presented in the tables are the average values of three individual samples (n = 3). For multiple comparisons of normally distributed data, parametric one-way analysis of variance (ANOVA) followed by the Tukey test was used. A value of p < 0.05 was considered significant.Results and discussion
Functional properties
The functional properties of food are extremely important as they help to characterise the structural quality, nutritional value, and consumer acceptability.29 The functional properties of the wheat, oat and melon peel flours are presented in Table 3. Melon peel flour had a lower bulk density value (0.46 ± 0.01 g cm−3) than wheat and oat flours. This result is similar to the bulk density (0.40 ± 0.00 g cm−3) reported by Farida et al. (2022).30 However, it is lower than the values reported by Adegunwa et al. (2019) for plantain and watermelon rind composite flour (0.52–0.75 g cm−3).22 The bulk density (g cm−3) of flour is measured without the influence of any compression and depends on the particle size and moisture content.31,32 Higher bulk densities are desirable to reduce storage and transport costs, as this is an indication that the flour has a lower space requirement. On the other hand, flours with lower bulk densities may be advantageous in the preparation of weaning food formulations.33| Parameters | Wheat flour | Oat flour | Melon peel flour |
|---|---|---|---|
| Values are the average of three individual samples (n = 3), expressed as mean ± standard deviation. Mean values with different superscript letters within a row are significantly different (p < 0.05). WAC, water absorption capacity. OAC, oil absorption capacity. | |||
| Bulk density (g cm−3) | 0.75 ± 0.02a | 0.60 ± 0.01b | 0.46 ± 0.01c |
| WAC (g H20 per g sample) | 1.75 ± 0.08c | 2.93 ± 0.15b | 14.34 ± 0.49a |
| OAC (g oil per g sample) | 4.83 ± 0.29a | 4.78 ± 0.19a | 5.00 ± 0.43a |
| Dispersibility (%) | 74.83 ± 0.29a | 58.17 ± 0.76b | 14.67 ± 0.58c |
| Solubility (%) | 7.75 ± 0.13b | 5.77 ± 0.08c | 15.93 ± 1.06a |
| Swelling power (g per g sample) | 5.32 ± 0.06c | 6.07 ± 0.01b | 15.01 ± 0.26a |
The hydration properties of dietary fibres are related to several factors such as the chemical structure of the constituent polysaccharides, porosity, and particle size, among others. The chemical properties of fibre and its origin (food source) are strongly related to the ability of dietary fibre to retain water. For example, dietary fibre obtained from fruit juice by-products has a higher affinity than those derived from cereals.34
Dispersibility of flour measures its ability to be reconstituted in water, and the greater the dispersibility, the better the flour reconstitutes in water. The dispersibility value for melon peel flour was 14.67%, the lowest value of the three flours analysed. However, it is considered high when compared to plantain and watermelon rind composite flour (0.89–1.70%).22
The water absorption capacity represents the amount of water that a flour sample can retain after being subjected to an external force (pressure or centrifugation). Melon peel flour obtained the highest value for water absorption capacity (14.34 ± 0.49 g H2O per g sample), indicating that it has a greater affinity with water. These values are high compared to other flours extracted from fruit by-products like umbu peel flours (3.74–3.95 g H2O per g) and pequi peel flours (3.74–3.98 g H2O per g).35,36 High water absorption values are important for applications such as functional ingredients in bakery products as they prevent water loss during cooking.30 This parameter is also linked to the flour's ability to produce a viscoelastic dough, which makes it easier to shape and stretch.29 However, as the water absorption capacity of melon peel flour is high, it is more difficult to obtain a cohesive and uniform dough, as well as to integrate all the ingredients used in a recipe. This can be a challenge when developing foods with high percentages of melon peel flour.
In addition to the water absorption capacity, the oil absorption capacity of the flour is also important and consists of the amount of oil that the flour can retain after being subjected to centrifugation.34 Although melon peel flour presented the highest value for oil absorption capacity (5.00 ± 0.43 g oil per g sample), no significant differences were found between the values of the remaining flours analysed. The oil absorption capacity of melon peel flour is almost 3 times lower than its water absorption capacity. However, for wheat and oat flours, the opposite was observed. Both flours have a greater absorption capacity for oil than for water. This is in line with Farida et al. (2022), who also found a greater water absorption capacity than oil absorption capacity for melon peel flour. High oil absorption capacity is desired in baked foods, as fat can act as a flavour retainer and improve the flavour of the food.37,38 Both the water and oil absorption capacities of flour depend on many factors related to proteins (amino acid composition, conformation, surface polarity, hydrophobicity), which may justify the found differences.38
The swelling power of flours refers to their ability to absorb water and increase in volume. It is an important property that affects the quality and functionality of flours in various applications. Flours with high starch content generally have a greater swelling capacity, especially when there is more branched amylopectin. The constituents of starch varies greatly depending on the plant source from which it is derived, which may explain the differences in the swelling capacity of flour from different (plant) sources and different species.29 The swelling power of melon peel flour was 15.01 ± 0.26 g g−1 sample, higher than the values observed for wheat and oat flour. Melon peel flour has a high swelling power compared to banana peel flour (3.62–7.34 g g−1 sample) and cassava flour (10.32–12.04 g g−1 sample).39,40 Adegunwa et al. (2019) found that temperature has an influence on increasing the swelling power of plantain and watermelon rind composite flour.22 Abou-Arab et al. (2017), who studied the effect of drying methods (microwave-drying, solar-drying and air oven-drying), observed that all the methods studied increased the swelling capacity of the citrus peel powder compared to the fresh sample.41
The solubility of melon peel flour was 15.93%, a lower value than that obtained by Farida et al. (2022) (22.65%). Its solubility is considered low compared to values obtained for plantain and watermelon rinsed composite flour (22.85–27.49%) and for pequi peel flours (16.7–19.8%), but high compared to plantain flour (6.93%).22,36,42
Proximate composition
The results regarding the nutritional composition of the melon peel flour are presented in Table 4. With the results obtained, it is possible to verify that the main constituents of the melon peel flour are dietary fibre and available carbohydrates, 50 and 24 g per 100 g, respectively. Melon peel flour can be considered a good source of dietary fibre, due to its high content. This result also indicates that the melon peel flour produced can be nutritionally better than wheat and whole wheat flour, when comparing their dietary fibre contents (3 and 10.6 per 100 g, respectively).43 This is because, generally, the peel of the fruit has higher levels of dietary fibre than the pulp or grains.| Components | Melon peel flour | Control biscuit | Biscuit with 50% melon peel flour | Control muffin | Muffin with 100% melon peel flour |
|---|---|---|---|---|---|
| Values are average of three individual samples (n = 3), expressed as mean ± standard deviation. Mean values with different superscript letters within a row are significantly different (p < 0.05). NCF, nitrogen conversion factor. | |||||
| Energy (kJ (kcal)) | 940 (227) | 1946 (464)a | 1737 (416)b | 1064 (255)c | 685 (165)d |
| Moisture (g) | 8.71 ± 0.1 | 7.17 ± 0.0a | 9.25 ± 0.1b | 44.3 ± 0.1c | 58.6 ± 0.2d |
| Ash (g) | 10.2 ± 0.0 | 0.49 ± 0.0a | 2.97 ± 0.0b | 1.81 ± 0.0c | 2.76 ± 0.0d |
| Total protein (g) (NCF = 6.25) | 6.91 ± 0.1 | 7.69 ± 0.2a | 7.18 ± 0.0a | 6.68 ± 0.2b | 7.91 ± 0.1b |
| Total fat (g) | 0.51 ± 0.0 | 19.5 ± 0.0a | 18.9 ± 0.1b | 11.7 ± 0.2c | 7.23 ± 0.1d |
| Available carbohydrates (g) | 23.7 ± 2.2 | 63.6 ± 0.4a | 47.0 ± 0.8b | 26.0 ± 0.1c | 10.5 ± 0.8d |
| Total dietary fibre (g) | 50.0 ± 2.0 | 1.53 ± 0.3a | 14.7 ± 0.6b | 9.56 ± 0.5c | 13.0 ± 0.6d |
| Salt (g) | 3.67 ± 0.0 | 0.14 ± 0.0a | 0.99 ± 0.1b | 0.12 ± 0.0c | 0.64 ± 0.0d |
On the other hand, the dietary fibre content of melon peel flour can also be considered high compared to other fruit peel flours, namely pomegranate peel flour (17.53 g per 100 g), pequi peel flours (39.79–43.32 g per 100 g), and prickly pear peel flour (33 g per 100 g).16,36,44 Regarding the protein content obtained for melon peel flour (6.91 g per 100 g), it is considered low when compared to wheat and whole wheat flour (12 and 15.1 gper 100 g, respectively).43 However, when compared with other fruit peel flours, it appears that melon peel flour has higher values than prickly pear peel flour (3.58 g per 100 g), pequi peel flours (3.25–3.26 g per100 g), pumpkin peel flour (3.68 g per 100 g) and pomegranate peel flour (0.70 g per 100 g).16,18,36,44 Furthermore, melon peel flour had a lower fat content than wheat and whole wheat flours (1.7 and 2.73 g per 100 g, respectively), pumpkin peel flour (3.53 g per 100 g) and prickly pear peel flour (2.12 g per 100 g). However, compared to pequi peel flour (0.17–0.32 g per 100 g) and pomegranate peel flour (0.40 g per 100 g), it is slightly higher.16,18,36,43,44 It also has a low moisture content (8.71 g per 100 g), as recommended, since high levels contribute to a greater proliferation of microorganisms and chemical reactions, making preservation difficult.45
Table 4 also shows the results of the nutritional composition of the biscuits and muffins (controls and innovative products) developed. The control biscuit is characterized by its poor nutritional quality, namely due to its low dietary fibre (1.53 ± 0.3 g per 100 g) and high available carbohydrate contents (63.6 ± 0.4 g per 100 g). By replacing wheat flour with 50% melon peel flour, the dietary fibre content increased to 14.7 ± 0.6 g per 100 g. Since the dietary reference intake for adults for fibre is 25 g day−1, one portion of the innovative biscuit (35 g) can contribute to 21% of the recommended needs, while the biscuit control only contributes to 2% of the recommended needs. Moreover, according to Regulation (EC) No. 1924/2006 on nutrition and health claims, the developed biscuit can be considered high in fibre (>6 g per 100 g).46
For the developed muffin, the same was observed. The full replacement of wheat flour by melon peel flour increased the fibre content from 9.56 ± 0.5 g per 100 g to 13.0 ± 0.6 g per 100 g. Therefore, the muffin developed can be considered high in fibre according to the same regulation (>6 g per 100 g).46 Furthermore, one portion of the developed muffin (48 g) can contribute to 25% of the recommended needs. In addition to dietary fibre, replacing wheat flour with melon peel flour allowed an increase in protein content from 6.68 ± 0.2 g per 100 g (control muffin) to 7.91 ± 0.1 g per 100 g (muffin with 100% melon peel flour), and the reduction in energy value from 255 kcal (control muffin) to 165 kcal (muffin with 100% melon peel flour).
Antioxidant activity
Antioxidant activity was determined using two different methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) and ferric reducing antioxidant power (FRAP) assays. The results obtained for the melon peel flour, biscuits, and muffins (controls and innovative products) are shown in Table 5.| Melon peel flour | Control biscuit | Biscuit with 50% melon peel flour | Control muffin | Muffin with 100% melon peel flour | |
|---|---|---|---|---|---|
| Values are average of three individual samples (n = 3), expressed as mean ± standard deviation. Mean values with different superscript letters within a row are significantly different (p < 0.05).a TE, Trolox equivalents.b GAE, gallic acid equivalents. | |||||
| DPPH˙ (mg TEa per 100 g) | 246.8 ± 10.98 | 10.2 ± 0.39a | 53.3 ± 2.73b | 39.8 ± 3.73c | 43.5 ± 2.38c |
| FRAP (mg TEA per 100 g) | 7490.8 ± 189.0 | 323.7 ± 63.8a | 1727.7 ± 89.7b | 1049.9 ± 60.4c | 1475.1 ± 87.2d |
| Total phenolics (mg GAEb per 100 g) | 1709.6 ± 162.7 | 147.3 ± 7.6a | 456.5 ± 28.0b | 412.1 ± 32.4c | 712.7 ± 57.0d |
The DPPH˙ assay is considered a standard colorimetric method, easy to apply and routinely used to evaluate the free radical scavenging potential of an antioxidant molecule.47 In the DPPH˙ method, melon peel flour had an antioxidant potential of 246.8 ± 10.98 mg TE per 100 g. The incorporation of melon peel flour into the biscuits allowed a ∼5-fold increase in their antioxidant potential (from 10.2 to 53.3 mg TE per 100 g). In the case of the developed muffin, no significant differences (p < 0.05) were observed between the antioxidant potential of the control and the innovative muffin (Table 5).
The FRAP assay has been widely used in food science. Compared to other assays that only measure free radical inhibition, the FRAP assay directly measures antioxidants in a sample.48 This assay measures the reduction, by antioxidants, of the ferric ion binding complex (Fe3+) to the ferrous complex (Fe2+), in an acidic medium.49 For this assay, the content obtained for melon peel flour was 7490.8 ± 189.0 mg TE per 100 g. Similar to what was observed with the DPPH˙ method, the incorporation of melon peel flour into the biscuit also allowed an increase of approximately 5 times in antioxidant power, resulting in a content of 1727.7 mg TE per 100 g for the biscuit developed. For the muffin with melon peel flour, an increase in its antioxidant power was also observed, resulting in a food product with 1475.1 mg TE per 100 g.
Many studies associate the accumulation of free radicals with some diseases, such as cancer and neurodegenerative and cardiovascular diseases. Antioxidants are compounds that can prevent and stabilize damage caused by these free radicals by donating electrons to damaged cells. Although biological systems have innate antioxidant defense mechanisms, these may be inefficient. Therefore, a dietary intake of antioxidants is very important to help protect cells from damage caused by free radicals.50,51
Total phenolic compounds
The total phenolic content of melon peel flour and the developed bakery products (controls and innovative products) are presented in Table 5. Melon peel flour presented a value of 1709.6 mg GAE per 100 g, for the total phenolic content, a value higher than those reported by Farida et al. (2020), 694.78–1271.39 mg GAE per 100 g.30 The content obtained in this study is very similar to that of prickly pear peel flour (1710 mg GAE per 100 g) reported by Parafati et al. (2020) and is higher than those of pomegranate peel flour (1387 ± 3.04 mg GAE per 100 g) and pumpkin peel flour (93.40 ± 0.69 mg GAE per 100 g).16,44,52 However, our values are lower compared to those reported by Feizy et al. (2020) for watermelon peel flour (2473.45 mg GAE per 100 g).53In the case of the developed biscuit, the utilization of melon peel flour promoted a total phenolic content of 456.5 ± 28.0 mg GAE per 100 g. This incorporation allowed a ∼3-fold increase in the content of phenolic compounds. Concerning the developed muffin, a total phenolic content of 712.7 ± 57.0 mg GAE per 100 g was obtained. By replacing wheat flour by melon peel flour, a 1.7-fold increase in the total phenolics was observed.
Phenolic compounds are secondary metabolites produced by plants, whose intake has been associated with a reduction in the incidence of some diseases such as cancer, diabetes, and cardiovascular diseases.54–56 Interest in phenolic compounds has been increasing over time, and epidemiological studies that suggest a relationship between the consumption of foods rich in phenolic compounds and disease prevention highlight the importance of a diet rich in these types of compounds.
Sensory evaluation
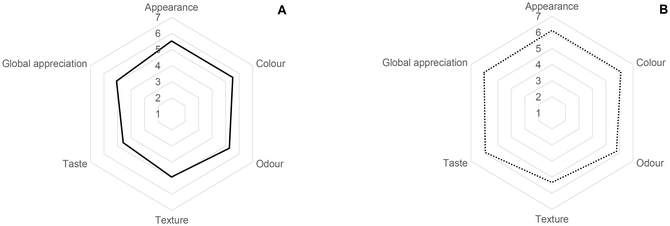
Sensory analysis is extremely important for the food industry and can be used, for example, in the development of new products, reformulation of products already established on the market, and identification of consumer preferences for a particular product, among others. The sensory analysis scores for appearance, colour, odour, texture, taste and overall acceptability of enriched muffins and biscuits obtained from a panel of untrained tasters are shown in Fig. 2.Regarding the results obtained for the muffin developed, most panellists gave a rating of 6 “liked it” to the attributes of appearance, colour, and odour. On the other hand, the texture and flavour were classified with the score 5 “like it slightly”, by 43% and 35% of the panellists, respectively. The flavour parameter obtained the lowest rating (4.6). This may be related to the fact that no added sugars were used in its formulation. In the case of the biscuit, most of the panellists assigned a score of 6 “liked it” to all evaluated parameters. The appearance and colour of the biscuit were the parameters that most pleased the panellists, with 65% answering “liked it”. The colour of the food product has a vital role in sensory analysis for consumers. Through the score attributed by the panellists to this parameter, the use of green-peel melons in the formulations, which gives a slightly greenish tone to the biscuits, may not constitute a rejection factor for consumers.
The results obtained for the overall acceptability were quite satisfactory, in the case of the biscuit developed with 50% melon peel flour, since 53% of the participants classified it as 6 “liked it” and 12 participants classified it as “liked it very much”. On the other hand, the overall acceptability of the muffin with 100% melon peel flour was classified with a score of 5 “liked it slightly”. However, the number of individuals who “liked it” and “liked it very much” was 8 and 5, respectively. It should be noted that the muffin is entirely made from melon peel flour, without the use of any other type of flour. In this sense, the results obtained also seem to be quite satisfactory.
The results described above agree with the results obtained for the question related to the purchasing potential of the developed products. The biscuit recorded the best scores with 53% of panellists responding that they “probably would buy it” and 35% responding that they “certainly would buy it”. Regarding the muffin, 15 of the 40 tasters described that they “might or might not buy it” and 10 tasters responded that they “probably would buy it”.
In general, the results obtained by the sensory analysis were quite satisfactory for both developed products based on melon peel flour, with a slight preference among the tasters for the biscuit enriched with 50% melon peel flour.
Conclusions
The melon peel flour has a very interesting nutritional composition. It can be considered rich in dietary fibre and a good source of total phenolic compounds. The integration of high quantities of melon peels in food products allowed the development of two formulations high in dietary fibre and rich in phenolic compounds, contributing to enhancing their nutritional quality. Given the importance of dietary fibre for health and its high content in melon peel flour, the use of this by-product is an excellent option to enrich food products with dietary fibre and contribute to increasing their intake. It was possible to develop a muffin using only melon peel flour, without wheat flour, without added sugar, lactose-free and gluten-free, which was well accepted by the tasters. With these good results, it is believed that this flour could be a potential option to replace wheat flour, increasing the supply of this type of product for the population looking for healthier options and individuals who are gluten intolerant. This work also demonstrates that it is possible to reduce the economic, social and environmental impacts of these by-products that are until now discarded, contributing to a more sustainable production in the food industry, improving public health and meeting the Sustainable Development Goals of the United Nations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Foundation for Science and Technology (FCT) under the projects Food4DIAB (EXPL/BAA-AGR/1382/2021) and UIDB/50006/2020 and by AgriFood XXI I&D&I project (NORTE-01-0145-FEDER-000041) co-financed by the European Regional Development Fund (ERDF), through the NORTE 2020 (Programa Operacional Regional do Norte 2014/2020). Rita C. Alves thanks FCT for the CEECIND/01120/2017 contract.References
- C. Gowe, Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives, Food Sci. Qual. Manag., 2015, 45, 47–61 Search PubMed.
- R. C. Fierascu, E. Sieniawska, A. Ortan, I. Fierascu and J. Xiao, Fruits by-products – A source of valuable active principles. A short review, Front. Bioeng. Biotechnol., 2020, 8, 319 CrossRef PubMed.
- M. A. Silva, T. G. Albuquerque, R. C. Alves, M. B. P. P. Oliveira and H. S. Costa, Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods?, Trends Food Sci. Technol., 2020, 98, 81–89 CrossRef.
- P. M. Rolim, L. M. J. Seabra and G. R. Macedo, Melon by-products: Biopotential in human health and food processing, Food Rev. Int., 2020, 36, 1 CrossRef.
- United Nations, Transforming our world: the 2030 Agenda for Sustainable Development, 2015 Search PubMed.
- United Nations, Department of Economic and Social Affairs Population Division, World Population Prospects the 2017 Revision, 2017 Search PubMed.
- Food and Agriculture Organization (FAO), IFAD and UNICEF, WFP, WHO, The state of food security and nutrition in the world - Urbanization, agrifood systems transformation and healthy diets across the rural-urban continuum, The Lancet Diabetes and Endocrinology, Rome, 2023 Search PubMed.
- J. Hughes, V. Vaiciurgis and S. Grafenauer, Flour for home baking : A cross-sectional analysis of supermarket products emphasising the whole grain opportunity, Nutrients, 2020, 12, 2058 CrossRef CAS PubMed.
- J. Montonen, P. Knekt, R. Järvinen, A. Aromaa and A. Reunanen, Whole-grain and fiber intake and the incidence of type 2 diabetes, Am. J. Clin. Nutr., 2003, 77, 622–629 CrossRef CAS PubMed.
- L. P. L. van de Vijver, L. M. C. van den Bosch, P. A. van den Brandt and R. A. Goldbohm, Whole-grain consumption, dietary fibre intake and body mass index in the Netherlands cohort study, Eur. J. Clin. Nutr., 2009, 63, 31–38 CrossRef CAS PubMed.
- P. Du, K. Luo, Y. Wang, Q. Xiao, J. Xiao and Y. Li, et al., Intake of dietary fiber from grains and the risk of hypertension in late midlife women: Results from the SWAN study, Front. Nutr., 2021, 8, 730205 CrossRef PubMed.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre, EFSA J., 2010, 8, 1462 Search PubMed.
- C. Lopes, D. Torres, A. Oliveira, M. Severo, V. Alarcão, S. Guiomar, et al., Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015–2016: Relatório de resultados, vol. 112. 2017. Available from: https://www.ian-af.up.pt.
- P. Naknaen, T. Itthisoponkul, A. Sondee and N. Angsombat, Utilization of watermelon rind waste as a potential source of dietary fiber to improve health promoting properties and reduce glycemic index for cookie making, Food Sci. Biotechnol., 2016, 25, 2 Search PubMed.
- L. H. Ho and N. W. B. A. Latif, Nutritional composition, physical properties, and sensory evaluation of cookies prepared from wheat flour and pitaya (Hylocereus undatus) peel flour blends, Cogent Food Agric., 2016, 2, 1136369 Search PubMed.
- T. Ismail, S. Akhtar, M. Riaz and A. Ismail, Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies, Int. J. Food Sci. Nutr., 2014, 65, 6 CrossRef PubMed.
- L. C. R. Reis, E. M. P. Facco, M. Salvador, S. H. Flôres and A. O. Rios, Characterization of orange passion fruit peel flour and its use as an ingredient in bakery products, J. Culin. Sci. Technol., 2020, 18, 3 Search PubMed.
- S. A. Saewan and S. S. George, Preparation of pumpkin pulp and peel flour and study their impact in the biscuit industry, J. Biol. Agric. Health, 2020, 10, 6 Search PubMed.
- J. C. Wang and J. E. Kinsella, Functional properties of novel proteins: alfalfa leaf protein, J. Food Sci., 1976, 41, 2 Search PubMed.
- K. D. Kulkarni, D. N. Kulkarni and U. M. Ingle, Sorghum malt-based weaning food formulations: preparation, functional properties, and nutritive value, Food Nutr. Bull., 1991, 13, 4 Search PubMed.
- I. Mateos-Aparicio, C. Mateos-Peinado and P. Rupérez, High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean, Innovative Food Sci. Emerging Technol., 2010, 11, 3 CrossRef.
- M. O. Adegunwa, I. O. Oloyede, L. A. Adebanjo and E. O. Alamu, Quality attribute of plantain (Musa paradisiaca) sponge-cake supplemented with watermelon (Citrullus lanatus) rind flour, Cogent Food Agric., 2019, 5, 1 Search PubMed.
- H. Greenfield and D. A. T. Southgate, Food composition data - Production, management and use, FAO Publis., Rome, 2nd edn, 2003 Search PubMed.
- Regulation (EU), No 1169/2011 of the European Parliament and of the Council of 25 October 2011, Off. J. Eur. Union., 2011, L 304, 18–63 Search PubMed.
- AOAC, Official methods of analysis of AOAC international, AOAC Inter., USA, 2000 Search PubMed.
- T. G. Albuquerque, F. Santos, A. Sanches-Silva, M. B. Oliveira, A. C. Bento and H. S. Costa, Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: Potential health benefits, Food Chem., 2016, 193, 187 CrossRef CAS PubMed.
- K. Thaipong, U. Boonprakob, K. Crosby, L. Cisneros-Zevallos and B. D. Hawkins, Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts, J. Food Compos. Anal., 2006, 19, 669 CrossRef CAS.
- F. L. Song, R. Y. Gan, Y. Zhang, Q. Xiao, L. Kuang and H. B. Li, Total phenolic contents and antioxidant capacities of selected chinese medicinal plants, Int. J. Mol. Sci., 2010, 11, 6 Search PubMed.
- C. G. Awuchi, Proximate composition and functional properties of different grain flour composites for industrial applications, Int. J. Food Sci., 2019, 2, 1 Search PubMed.
- B. D. Farida, S. Aoun, A. Cherifi and D. Lynda, Melon peel powder: Phytochemical screening, antioxidant contents, functional properties food application, J. Food Sci. Res., 2022, 7, 2 Search PubMed.
- C. Imoisi, J. U. Iyasele, U. C. Michael and E. E. Imhontu, The effects of watermelon rind flour on the functional and proximate properties of wheat bread, J. Chem. Soc. Niger., 2020, 45, 5 Search PubMed.
- M. Hasmadi, M. Noorfarahzilah, H. Noraidah, M. K. Zainol and M. H. A. Jahurul, Functional properties of composite flour: A review, Food Res., 2020, 4, 6 Search PubMed.
- S. Y. Giami and D. A. Bekebain, Proximate composition and functional properties of raw and processed full–fat fluted pumpkin (Telfairia occidentalis) seed flour, J. Sci. Food Agric., 1992, 59, 3 CrossRef.
- M. Elleuch, D. Bedigian, O. Roiseux, S. Besbes, C. Blecker and H. Attia, Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review, Food Chem., 2011, 124, 411 CrossRef CAS.
- L. B. Cangussu, P. Fronza, A. S. Franca and L. S. Oliveira, Chemical characterization and bioaccessibility assessment of bioactive compounds from umbu (Spondias tuberosa a.) fruit peel and pulp flours, Foods, 2021, 10, 2597 CrossRef CAS PubMed.
- D. P. Leão, A. S. Franca, L. S. Oliveira, R. Bastos and M. A. Coimbra, Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products, Food Chem., 2017, 225, 146 CrossRef PubMed.
- O. O. Onabanjo, I. O. Olayiwola, M. O. Adegunwa, D. A. Ighere, A. O. Dave-Omore and N. S. Abaku, Functional and pasting characteristic of wheat, yellow maize and beniseed composite flour, Trends Appl. Sci. Res., 2020, 15, 3 Search PubMed.
- P. Kaushal, V. Kumar and H. K. Sharma, Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeonpea (Cajanus cajan) flour and their blends, LWT – Food Sci. Technol., 2012, 48, 1 CrossRef.
- S. K. S. A. Bakar, N. Ahmad and F. Jailani, Chemical and functional properties of local banana peel flour, J. Food Nutr. Res., 2018, 6, 8 Search PubMed.
- E. Eriksson, K. Koch, C. Tortoe, P. T. Akonor and E. Baidoo, Physicochemical, functional and pasting characteristics of three varieties of cassava in wheat composite flours, Br. J. Appl. Sci. Technol., 2014, 4, 11 Search PubMed.
- E. A. Abou-Arab, M. H. Mahmoud and F. M. Abu-Salem, Functional properties of citrus peel as affected by drying methods, Am. J. Food Technol., 2017, 12, 3 Search PubMed.
- G. L. Arueya and O. Akande, Development and characterization of dumpling dough with “optimal” dietary fibre ratio using Ofada rice (Oryza Sativa L) and unripe plantain (Musa Paradisiaca AAB) fruit, Integr. Food Nutr. Metab., 2018, 5, 4 Search PubMed.
- U.S. Department of Agriculture, Agricultural Research Service, 2020, FoodData Central, Available from: https://fdc.nal.usda.gov/fdc-app.html#/.
- L. Parafati, C. Restuccia, R. Palmeri, B. Fallico and E. Arena, Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation, Foods, 2020, 9, 1189 CrossRef CAS PubMed.
- M. B. Soquetta, F. S. Stefanello, K. D. M. Huerta, S. S. Monteiro, C. S. Rosa and N. N. Terra, Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa), Food Chem., 2016, 199, 471 CrossRef CAS PubMed.
- Regulation (EC) N, 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods, Off. J. Eur. Union, 2007, L 404, 10–25 Search PubMed.
- K. Mishra, H. Ojha and N. K. Chaudhury, Estimation of antiradical properties of antioxidants using DPPH- assay: A critical review and result, Food Chem., 2012, 130, 4 CrossRef.
- B. L. Halvorsen, K. Holte, M. C. W. Myhrstad, I. Barikmo, E. Hvattum and S. F. Remberg, et al., A systematic screening of total antioxidants in dietary plants, J. Nutr. Am. Soc. Nutr., 2002, 132, 3 Search PubMed.
- I. F. F. Benzie and J. J. Strain, The Ferric Reducing Ability of Plasma (FRAP) as a measure of “‘Antioxidant Power’”: The FRAP assay, Anal. Biochem., 1996, 239, 70 CrossRef CAS PubMed.
- M. M. Rahman, M. B. Islam, M. Biswas and A. A. H. M. Khurshid, In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh, BMC Res. Notes, 2015, 8, 621 CrossRef PubMed.
- I. Liguori, G. Russo, F. Curcio, G. Bulli, L. Aran and D. Della-Morte, et al., Oxidative stress, aging, and diseases, Clin. Interventions Aging, 2018, 13, 757 CrossRef CAS PubMed.
- A. Hussain, T. Kausar, A. Din, M. A. Murtaza, M. A. Jamil, S. Noreen, H. ur Rehman, H. Shabbir and M. A. Ramzan, Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima), J. Food Process. Preserv., 2021, 45, e15542 CAS.
- J. Feizy, S. Ahmadi and M. Jahani, Antioxidant activity and mineral content of watermelon peel, J. Food Bioprocess Eng., 2020, 3, 1 Search PubMed.
- J. Sharifi-Rad, V. Seidel, M. Izabela, M. Monserrat-Mequida, A. Sureda and V. Ormazabal, et al., Phenolic compounds as Nrf2 inhibitors: potential applications in cancer therapy, Cell Commun. Signaling, 2023, 21, 89 CrossRef CAS PubMed.
- D. P. Farias, F. F. Araújo, I. A. Neri-Numa and G. M. Pastore, Antidiabetic potential of dietary polyphenols: A mechanistic review, Food Res. Int., 2021, 145, 110383 CrossRef PubMed.
- M. Lutz, E. Fuentes, F. Ávila, M. Alarcón and I. Palomo, Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases, Molecules, 2019, 24, 366 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |