Open Access Article
Open Access ArticleIron-saturated bovine lactoferrin: a promising chemopreventive agent for hepatocellular carcinoma†
Hury Viridiana
Hernández-Galdámez
a,
Samia
Fattel-Fazenda‡
a,
Teresita N. J.
Flores-Téllez
b,
Mario Alejandro
Aguilar-Chaparro
a,
Jonathan
Mendoza-García
a,
Lidia C.
Díaz-Fernández
a,
Eunice
Romo-Medina
a,
Yesennia
Sánchez-Pérez
c,
Jaime
Arellanes-Robledo
 d,
Mireya
De la Garza
a,
Saúl
Villa-Treviño
*a and
Carolina
Piña-Vázquez
d,
Mireya
De la Garza
a,
Saúl
Villa-Treviño
*a and
Carolina
Piña-Vázquez
 *a
*a
aDepartamento de Biología Celular, Centro de Investigación y de Estudios Avanzados del IPN (CINVESTAV-IPN), CDMX, Mexico. E-mail: svilla@cinvestav.mx; carolina.pina@cinvestav.mx
bCancer Research UK Manchester Institute, The University of Manchester, Alderley Park, SK10 4TG Macclesfield, UK
cInstituto Nacional de Cancerología (INCan), Subdirección de Investigación Básica, CDMX, Mexico
dLaboratorio de Enfermedades Hepáticas, Instituto Nacional de Medicina Genómica, Ciudad de México, México. Dirección de Cátedras, Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT), Ciudad de México, Mexico
First published on 1st April 2024
Abstract
Hepatocellular carcinoma (HCC) is a tumor with minimal chance of cure due to underlying liver diseases, late diagnosis, and inefficient treatments. Thus, HCC treatment warrants the development of additional strategies. Lactoferrin (Lf) is a mammalian multifunctional iron-binding glycoprotein of the innate immune response and can be found as either a native low iron form (native-Lf) or a high iron form (holo-Lf). Bovine Lf (bLf), which shares many functions with human Lf (hLf), is safe for humans and has several anticancer activities, including chemotherapy boost in cancer. We found endogenous hLf is downregulated in HCC tumors compared with normal liver, and decreased hLf levels in HCC tumors are associated with shorter survival of HCC patients. However, the chemoprotective effect of 100% iron saturated holo-bLf on experimental hepatocarcinogenesis has not yet been determined. We aimed to evaluate the chemopreventive effects of holo-bLf in different HCC models. Remarkably, a single dose (200 mg kg−1) of holo-bLf was effective in preventing early carcinogenic events in a diethylnitrosamine induced HCC in vivo model, such as necrosis, ROS production, and the surge of facultative liver stem cells, and eventually, holo-bLf reduced the number of preneoplastic lesions. For an established HCC model, holo-bLf treatment significantly reduced HepG2 tumor burden in xenotransplanted mice. Finally, holo-bLf in combination with sorafenib, the advanced HCC first-line treatment, synergistically decreased HepG2 viability by arresting cells in the G0/G1 phase of the cell cycle. Our findings provide the first evidence suggesting that holo-bLf has the potential to prevent HCC or to be used in combination with treatments for established HCC.
1. Introduction
Liver cancer is the sixth most common neoplasm and the third most lethal, with more than 780![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths per year worldwide.1 Hepatocellular carcinoma (HCC) accounts for more than 80% of primary liver cancers.2 Major risk factors for HCC include HBV and HCV infection, diabetes, excess alcohol consumption and metabolic liver disease, particularly nonalcoholic fatty liver disease. Early HCC stage is amenable for potential curative treatment, such as local ablation, surgical resection and liver transplantation; unfortunately, only 20 to 30% of patients are eligible for such interventions since most of them have already reached an advanced cancer stage at the first HCC diagnosis.3 Of note, despite its side effect profile and poor improvement in overall survival (OS) of less than three months, sorafenib has been the first-line systemic treatment.4 Consequently, HCC has limited options for curative strategies due to underlying liver diseases, late diagnosis and inefficient treatments.
000 deaths per year worldwide.1 Hepatocellular carcinoma (HCC) accounts for more than 80% of primary liver cancers.2 Major risk factors for HCC include HBV and HCV infection, diabetes, excess alcohol consumption and metabolic liver disease, particularly nonalcoholic fatty liver disease. Early HCC stage is amenable for potential curative treatment, such as local ablation, surgical resection and liver transplantation; unfortunately, only 20 to 30% of patients are eligible for such interventions since most of them have already reached an advanced cancer stage at the first HCC diagnosis.3 Of note, despite its side effect profile and poor improvement in overall survival (OS) of less than three months, sorafenib has been the first-line systemic treatment.4 Consequently, HCC has limited options for curative strategies due to underlying liver diseases, late diagnosis and inefficient treatments.
Thus, as with most human malignancies, HCC progression needs to be challenged using different strategies. Cancer chemoprevention, defined as the use of pharmacological agents, either natural or synthetic, is a strategy to delay, reverse, suppress or prevent disease pathogenesis, such as cancer.5 Lactoferrin (Lf) is an 80 kDa iron-binding multifunctional glycoprotein of the innate immune response that is mainly found in milk and other mammalian exocrine secretions. It can be iron free, containing either less than 5% (apo-Lf) or 100% of iron (holo-Lf), the saturated form. Native Lf, which is secreted under physiological conditions, contains 10 to 20% iron saturation; however, in inflammatory or infected microenvironments, holo-Lf prevails because of the high iron concentration.6
Bovine lactoferrin (bLf) has been classified as a human Lf (hLf) bioequivalent because of the high sequence homology that shares many functions. It has been involved in several protective activities, such as antioxidant, immunomodulatory, antimicrobial and anticancer activities.6 Several studies have demonstrated that bLf is tolerated and has no toxicity in humans; moreover, it has been approved by both the USA Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) as a dietary supplement in food products.7,8 Interestingly, the chemopreventive effects of bLf have been demonstrated in several animal models bearing different tumor types, including lung, tongue, esophagus, and colorectal cancer, showing that bLf has been effective in inhibiting growth, metastasis, and tumor-associated angiogenesis, as well as potentiating chemotherapy.6 Clinical trials in patients with colorectal polyps revealed that native-bLf has chemopreventive potential.9,10 bLf binds specifically to the asialoglycoprotein receptor (ASGPR), which is expressed in the hepatocyte membrane and has been found to be conserved in HCC biopsies.11 Therefore, it is feasible to propose that HCC might be an attractive target of the antitumoral effects of bLf. For instance, native-bLf has been shown to have chemopreventive effects when it is simultaneously and daily administered either for several weeks or two weeks before a hepatocarcinogenic agent.12–14
Most research on the anticancer capabilities of bLf has been performed by using native-bLf, and it has been proposed that iron saturation in Lf is irrelevant for its anticancer effects.15 In contrast, other studies have demonstrated that iron saturation does affect the anticancer capabilities of bLf. In vitro studies have shown divergent results in breast cancer cell lines. MDA-MB-231 and MCF-7 cells showed more sensitivity to the cytotoxic effects of native-bLf than holo-bLf,16 or MDA-MB-231, MCF-7, T-47D and Hs578T cells were more sensitive to holo-bLf, followed by native-bLf and apo-bLf.17 In human glioblastoma cells, holo-bLf was found to be more effective in inducing anti-migratory activity than the native one, both at the cellular and molecular levels.18 Furthermore, in vivo experiments have shown that holo-bLf is effective in inhibiting tumorigenesis and increasing the chemotherapy capability to eliminate EL-4 lymphoma, while bLf saturated with lower levels of iron failed to synergize with chemotherapy to eradicate tumors.19 Although the exact mechanism by which holo-bLf surpasses the less saturated bLf form in vivo is unknown, the above data strongly suggest that holo-bLf has potential as a therapeutic agent.16
Based on the anticancer capability of native-bLf, some studies have shown its chemopreventive effects on rodent models of diethylnitrosamine (DEN)-induced hepatocarcinogenesis;12 however, the chemopreventive effects of holo-bLf on hepatocarcinogenesis have not yet been investigated. Therefore, it is plausible to determine whether the anticancer capability of holo-bLf is more effective than that of native-bLf. The aim of this investigation was to evaluate the chemopreventive effects of holo-bLf on HCC progression at multiple levels by challenging both in vivo and in vitro HCC models. Additionally, the simultaneous effect of holo-bLf and sorafenib on an in vitro HCC model was also investigated. The present study introduces the first exploration of holo-bLF as a chemopreventive agent in HCC, advancing our understanding of bLf anticancer effect, and providing a novel avenue for advanced HCC treatment research.
2. Materials and methods
2.1 Design of this work
Our study is organized in three phases, utilizing both in vivo and in vitro methodologies. In the initial phase, we evaluated the effectiveness of two forms of bLf (native and holo) as chemopreventive agents during the early stages of hepatocarcinogenesis (from 2 to 30 days) using an in vivo model of chemical hepatocarcinogenesis in Fischer-344 rats. bLf was administered as a single dose prior to exposure to the first carcinogen DEN. The analytical assessments included the quantification of necrosis, ROS lipid peroxidation, stem cell markers onset, and preneoplastic lesions. The second phase focused on the effect of holo-bLf on established HCC. First, we used an in vitro model with HCC cell lines (HepG2 and Hep3B) and determined the cell viability after treatment with holo-bLf. Subsequently, a xenotransplantation model with hepG2 cells was used to test the effect of holo-bLf on in vivo tumor growth. The third phase of the study investigated the in vitro combination effect of holo-bLf with sorafenib: first-line treatment vs. advanced HCC treatment. Viability assays were also conducted for that purpose, and alterations in the cell cycle were evaluated. This comprehensive and integrated approach ensures a thorough understanding of the effects of holo-bLf as a chemopreventive agent for HCC at multiple levels: early hepatocarcinogenesis, established HCC and as a combinational approach with first-line therapy for HCC.2.2 Reagents and antibodies
Bovine lactoferrin (97.01% purity) in the iron-poor form native-bLf, 20% iron-saturated, was purchased from NutriScience (Trumbull, USA). Reagents including DEN (N0756, St Louis, Missouri USA), 2-acetylaminofluorene (2-AAF, A7015, St Louis, Missouri USA), and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, D6883, St Louis, Missouri, USA) were purchased from Sigma Chemical Co. (St Louis, Missouri, USA). The AldeRed ALDH Detection Assay kit (01700, Vancouver, British Columbia, Canada) and anti-CD44v6 (AB2080 Temecula, CA, USA) were purchased from Millipore. Antibodies against CD90 (202508, San Diego CA, USA) and CD45 (202205 San Diego, CA, USA) were from BioLegend; anti-CD133 was from GeneTex (GTX12295, San Antonio, TX, USA); and anti-CD24 was from BD Pharmingen (562104, Franklin Lakes, NJ, USA).2.3 hLf gene (LTF) expression analysis in HCC patient cohorts from public databases
The UCSC Xena browser tool (https://xenabrowser.net/) was used to download hLf gene (LTF) expression in the HCC cohort of The Cancer Genome Atlas (TCGA) based upon data generated by Research Network: https://www.cancer.gov/tcga (TCGA-LIHC) and in the normal liver tissue cohort of The Genotype Tissue Expression (GTEx) database (https://www.gtexportal.org/home/). The Mann–Whitney test was used to compare both expression levels. The GSE136247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and GSE113996 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113996) cohorts were downloaded from the Gene Expression Omnibus (GEO) at (https://www.ncbi.nlm.nih.gov/geo/) and analyzed using paired t tests. Kaplan–Meier plots were generated for the TCGA-LIHC cohort using KM plot (https://kmplot.com/analysis/index.php?p=background) to calculate the cutoff value and for data download. The log-rank (Mantel–Cox) test was used to compare the differences in survival curves.
20 and GSE113996 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113996) cohorts were downloaded from the Gene Expression Omnibus (GEO) at (https://www.ncbi.nlm.nih.gov/geo/) and analyzed using paired t tests. Kaplan–Meier plots were generated for the TCGA-LIHC cohort using KM plot (https://kmplot.com/analysis/index.php?p=background) to calculate the cutoff value and for data download. The log-rank (Mantel–Cox) test was used to compare the differences in survival curves.
2.4 Preparation of iron-binding protein
Holo-bLf was produced by dissolving native-bLf in 40 mM Tris/20 mM sodium bicarbonate buffer (pH 7.4) to have a final protein concentration of 200 μM. It was saturated with iron by adding 400 μM ferric chloride and incubated at 4 °C overnight with agitation. Unbound iron was removed by dialysis using SnakeSkin™ Dialysis Tubing (pore size was 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) (Thermo Scientific Cat. 68100, Rockford Illinois, USA), with water as the dialysis buffer, with six changes for 36 h.21 The protein was concentrated by ultrafiltration in an Amicon® ultra centrifugal filter 30 kDa (Millipore, Cat. UFC903008, Tullagreen, Carrigtwohill, Co Cork Ireland) and stored at −20 °C. Protein concentrations were determined by the Bradford micromethod.22 The iron concentration was determined by the IRON-TPTZ colorimetric method (Spinreact). Holo-bLf resulted in an Fe saturation ≤90%.
000 Da) (Thermo Scientific Cat. 68100, Rockford Illinois, USA), with water as the dialysis buffer, with six changes for 36 h.21 The protein was concentrated by ultrafiltration in an Amicon® ultra centrifugal filter 30 kDa (Millipore, Cat. UFC903008, Tullagreen, Carrigtwohill, Co Cork Ireland) and stored at −20 °C. Protein concentrations were determined by the Bradford micromethod.22 The iron concentration was determined by the IRON-TPTZ colorimetric method (Spinreact). Holo-bLf resulted in an Fe saturation ≤90%.
2.5 Animal procedures
All experiments were performed under the Institutional Animal Care and Use Committee Guidelines and according to protocol No. 0168-15, approved by the Committee for the Care and Use of Laboratory Animals (CICUAL) of CINVESTAV-IPN. Animals were obtained from the Unit for Production of Experimental Laboratory Animals (UPEAL CINVESTAV, Mexico City, Mexico). Animals had free access to food, standard diet (PMI Feeds Inc., Laboratory Diet) and water. They were maintained in a holding room under controlled conditions of a 12 h light/dark cycle, 50% relative humidity and 21 °C.2.6 Hepatocarcinogenesis model and bLf administration
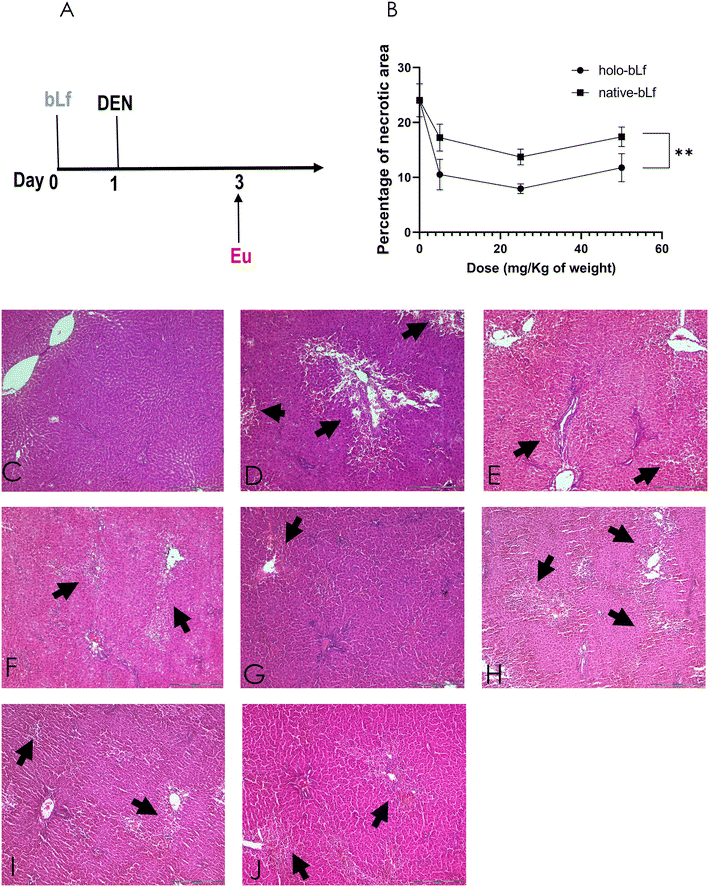
A modified version of the resistant hepatocyte protocol for inducing hepatocarcinogenesis was used. Briefly, male Fischer-344 rats weighing ∼200 g were intragastrically administered 200 mg kg−1 DEN once, followed by intragastric administration of 20 mg kg−1 2-AAF on days 3, 4, and 5, as previously reported (manuscript in preparation).8 The bLf was intragastrically administered once 24 h before DEN (Fig. 2A). Rats were euthanized by isoflurane anesthesia and exsanguination, and pieces of liver were frozen in liquid nitrogen for cryopreservation and stored at −75 °C for further analysis. Other pieces were fixed in 4% formalin and embedded in paraffin for histological analysis.2.7 Necrosis quantification
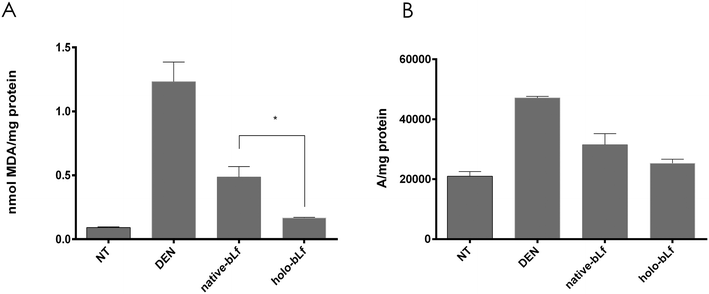
The liver tissue was treated as previously described by Macias-Perez et al. 2013.23 The extent of necrosis was quantified in 10 fields/rat at 100X using an Olympus IX70 microscope with the AnalySIS Opty Soft Imaging System GmbH 3.00 (Olympus Europa GmbH, Hamburg, Germany). Necrotic areas were defined as acellular regions adjacent to cells with pale pink cytoplasm, nuclear dissolution (karyolysis), and/or nuclear fragmentation (karyorrhexis), cellular debris and inflammatory infiltrates.24 Using this definition, the necrotic areas were calculated by quantifying the normal area (not necrotic nor acellular) and subtracting it from the total area in the microscopic field.23,252.8 Lipid peroxidation determination
Fifty milligrams of frozen liver were processed as previously described.26 Briefly, tissue was homogenized in 1 ml of a buffer containing 100 mM Tris, 150 mM NaCl and 1 mM Phenylmethylsulfonyl fluoride (PMSF) at pH 7.4. Subsequently, 50 μl of the total homogenate was combined with 30 μl of a solution comprising 150 mM Tris pH 7.4 and 300 μl of 0.4% thiobarbituric acid (TBA) dissolved in 20% acetic acid at pH 3. The mixture was homogenized and was incubated at 100 °C for 1 hour until complete evaporation of the liquid. Following this, the samples were placed on ice for 10 min, and 200 μl of a solution containing 1.2% KCl and 500 μl of pyridine/butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) was added. Tubes were the centrifugated at 6000g for 15 min at 4 °C. Duplicate transfers of 200 μl of the resulting supernatant were made to a 96-well plate, and absorbance measurements were conducted at a wavelength of 532 nm using a plate reader Thermo Scientific Additionally, a 1
15) was added. Tubes were the centrifugated at 6000g for 15 min at 4 °C. Duplicate transfers of 200 μl of the resulting supernatant were made to a 96-well plate, and absorbance measurements were conducted at a wavelength of 532 nm using a plate reader Thermo Scientific Additionally, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution of the initial homogenate was performed to determine protein concentration using the Bradford method. The results were expressed with respect to malondialdehyde (MDA) by using the MDA extinction coefficient (E = 1.56 × 105) as nmol of MDA per mg of total protein.
10 dilution of the initial homogenate was performed to determine protein concentration using the Bradford method. The results were expressed with respect to malondialdehyde (MDA) by using the MDA extinction coefficient (E = 1.56 × 105) as nmol of MDA per mg of total protein.
2.9 ROS quantification
Fifty milligrams of frozen liver were treated as previously described.27 Breafly, tissue was homogenized in 1 ml of a buffer comprising 100 mM Tris, 150 mM NaCl and 1 mM PMSF at pH 7.4 buffer. Subsequently, 40 μl of the homogenate were added into a 96 well plate, and mixed with 10 μl of 100 μM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and 50 μl of 150 mM TRIS at pH 7.4. The plate was protected from light and incubated 1 h at room temperature, after that, fluorescence was read at 480 nm excitation/515 nm emission using a TECAN GENious plate reader. Fluorescence was corrected by subtracting blanks in each experiment and autofluorescence of each lysate.2.10 Flow cytometry analysis
On day six after cancer induction, rats were anesthetized under isoflurane and perfused using a modified two-step collagenase perfusion procedure, and then, liver nonparenchymal cells were separated by centrifugation cycles.28 Then, isolated cells were incubated at 4 °C for 20 min either with the primary antibodies anti-CD133 (GeneTex Cat. GTX12295), anti-CD44v6 (Millipore Cat. AB2080), anti-CD90 Alexa Fluor® 647 (BioLegend Cat. 202508), anti-CD24 PE (BD Pharmingen Cat. 562104), or anti-CD45 FITC (BioLegend Cat. 202205). Cells incubated with anti-CD133 and anti-CD44v6 were then washed with 2% FBS in PBS and incubated with secondary FITC-labeled anti-rabbit (goat anti-rabbit IgG FITC, Jackson, Cat. 111-095-045, Baltimore Pike, West Grove, PA, USA) for 20 min at 4 °C. Cells were washed once, resuspended in 2% fetal bovine serum (FBS) in PBS and then analyzed. Dead cells were stained either with propidium iodine or blue trypan according to fluorochrome compatibility. To detect aldehyde dehydrogenase-positive cells (ALDH+), an AldeRed ALDH detection assay kit was used (Millipore cat. SCR150, Temecula, CA USA) according to the manufacturer's instructions. Cells were analyzed using the BD LSRFortessa™ X-20 cell analyzer, and data were analyzed using BD ModFITT LT v2.0 software.2.11 γ-Glutamyl transpeptidase (GGT) staining and preneoplastic lesion quantification
Liver preneoplastic lesions were stained to detect the activity of the GGT enzyme, a well-known HCC and altered hepatic foci marker, in 20 μm-thick frozen tissue sections, as previously described.23,29 Then, GGT-positive (GGT+) foci were captured and quantified using AnalySIS Opty Soft Imaging System GmbH 3.00 software (Olympus Europa GmbH, Hamburg, Germany).2.12 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HepG2 and Hep3B cells were obtained from ATCC, genotyped for authentication and tested for mycoplasma contamination using MycoAlert™ PLUS (Cat. LT07-701, Lonza, Rockland, ME, USA). Cells from passages 20 to 30 were used for the experiments. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS in 5% CO2 at 37 °C.Cell viability was determined by MTT assay (Cat. M6494 Invitrogen, Willow Creek Road, CA, USA). Briefly, cells at the exponential growth phase were subcultured and seeded in a 96-well plate (5 × 105 per well). After 48 h of incubation, the culture medium was replaced with fresh medium containing holo-bLF and/or sorafenib (S-8502, LC Laboratories, Woburn, MA, USA). Doses of both holo-bLf and sorafenib were selected based on previous reports.30–32 For the combination experiments, the sorafenib concentration was 3 or 5 μM, and that of holo-bLf was 50 μM. After 24, 48 or 72 h, cell viability was determined following the manufacturer's protocol. The combination index of sorafenib plus holo-bLf treatment was calculated by CompuSyn software (https://www.combosyn.com/index.html), as previously reported.33
2.13 Holo-bLf treatment of the xenograft mouse model
HCC xenografts were established in six-week-old female NOD-scid IL2Rγnull (NSG™) mice by the subcutaneous inoculation of 5 × 106 HepG2 cell suspension in 200 μl of 10% FBS in DMEM in the flank region. After two weeks, the tumors reached 0.5 cm3, and the mice were randomly assigned to two groups (n = 7 mice per group). Using a cannula, animals were orally administered vehicle (MQ water) or 200 mg kg−1 holo-bLf every other day for 20 days. At the end of the experiment, the mice were euthanized by an anesthesia overdose of ketamine/xylazine; then, the tumors were collected, weighed and stored at −75 °C for further analysis.2.14 Cell cycle assay
HepG2 cells (1 × 106) were treated with either holo-bLf and/or sorafenib for 24 h, following the protocol described in the previous section. Then, the cells were detached with PBS-EDTA (0.48 mM) and fixed with 70% ethanol at −20 °C for 24 h. After that, the cells were washed twice and processed with a BD Cycletest™ Plus DNA Kit according to the manufacturer's protocol. Samples were read in a BD FACSCanto II cytometer with FACSDiva v.1.1 software. Data were analyzed using BD ModFITT LT v2.0 software.2.15 Statistical analysis
Data were analyzed using GraphPad Prism software 6.01. All experiments were performed in triplicate. The results are presented as the mean ± standard deviation (SD) or standard error of the mean (SEM). Statistical significance was determined by analysis of variance (ANOVA), Tukey's multiple comparisons test and Student's t test with p < 0.05.3. Results
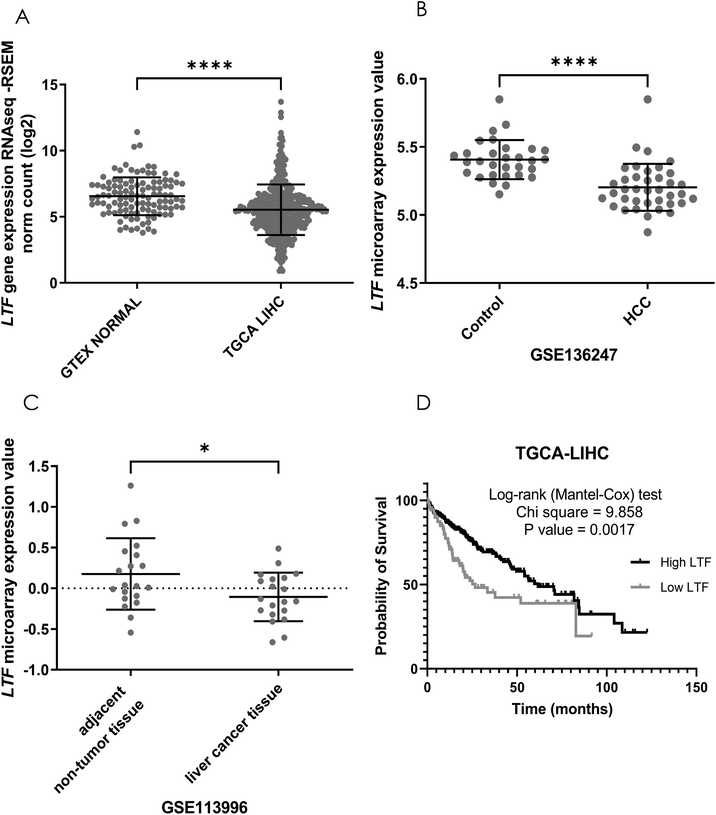
3.1 hLf gene (LTF) expression is downregulated in HCC tissue
To explore whether Lf has a natural effect on patients HCC progression, we first analyzed the hLf gene (LTF) expression profile in HCC patients by comparing LTF gene expression in normal liver tissue with HCC biopsies from the Genotype-Tissue Expression (GTEx) database and from The Cancer Genome Atlas database (TCGA-LIHC), respectively. LTF expression was significantly (p < 0.0001) higher in normal liver than in HCC biopsies (Fig. 1A). We also compared LTF gene expression in HCC adjacent tissues versus tumor tissue of two GEO cohorts (GSE136247 and GSE113996), confirming that LTF expression was also higher in adjacent tissue than in tumor samples (Fig. 1B and C). To evaluate the prognostic value of tumoral LTF expression in HCC patients, we generated Kaplan–Meier curves of LTF gene expression using data from the TCGA-LIHC cohort. We found a difference in the overall survival probability. Samples with lower LTF expression exhibited significantly (p = 0.0017) shorter survival than those with higher LTF expression (Fig. 1D). This evidence suggests that endogenous Lf has a protective effect against HCC progression. Furthermore, it has been reported that bLf is a bioequivalent of hLf,6 and the consumption of bLf increases the hLf serum concentration in patients.10 Thus, it is not unreasonable to determine the potential of bLf as a chemopreventive agent in HCC in vivo models.3.2 Holo-bLf is more efficient than native-bLf in preventing DEN-induced damage and ROS production during the early stages of rat hepatocarcinogenesis
Then, we determined the effectiveness of the two forms of bLf, namely, holo-bLf and native-bLf, to prevent the early hepatocarcinogenesis stages. For this purpose, we used a modified experimental model of hepatocarcinogenesis,34,35 as shown in Fig. 4A. After a single dose of either holo-bLf or native-bLf was administered to animals before cancer initiation with DEN (Fig. 2A), two variables were evaluated. The first is liver cell necrosis, a phenomenon that plays a key role in promoting early changes associated with liver carcinogenesis induced by DEN.36 Thus, necrosis was evaluated 48 h after DEN administration either with or without pretreatment with a single dose of holo-bLf or native-bLf (Fig. 2B–J). Holo-bLf was able to significantly (p = 0.0086) prevent DEN-produced necrosis better than native-bLf (Fig. 2B). The dose of 25 mg kg−1 was selected for further experiments.Since DEN metabolism increases reactive oxygen species (ROS) production and elevated oxidative stress correlates with increased malignancy,37,38 we determined oxidative stress by measuring two parameters, namely, lipid peroxidation and ROS production, 48 h after DEN administration either with or without pretreatment with holo-bLf or native-bLf (Fig. 3). The effect of bLf on lipid peroxidation was evaluated by measuring malondialdehyde (MDA), the main metabolite of lipid peroxidation.26 Holo-bLf pretreatment significantly (p = 0.0294) reduced lipid peroxidation more than native-bLf (Fig. 3A). ROS measurement showed no significant difference between holo-bLf and native-bLf pretreatments; however, there was a tendency for holo-bLf pretreatment to perform moderately better in reducing ROS levels (Fig. 3B). This result showed that pretreatment with a single dose of holo-bLf was more efficient than that of native-bLf in preventing DEN-induced necrosis and ROS production. Based on this evidence, we decided to use only holo-bLf hereinafter.
3.3 Holo-bLf decreases the surge in stem cell markers during hepatocarcinogenesis
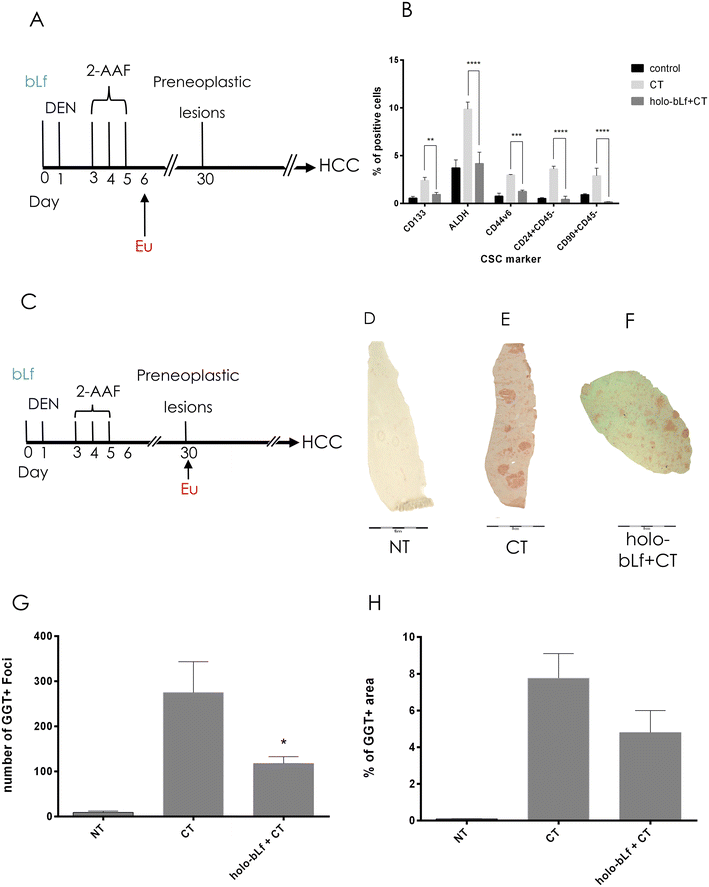
Carcinogenic schemes based on DEN and 2-acetylaminofluorene (2AAF) are proposed to rely on their capability to induce local tissue damage and proliferative repair that expand the cell population susceptible to undergo malignant transformation.39 Such populations are nonparenchymal cells known as facultative liver stem or progenitor cells (LSPCs), which drive liver compensatory regeneration.39 Importantly, inhibition of LSPC proliferation in chronically injured mouse livers significantly reduces HCC development in several models.40–42 Therefore, we determined the effect of holo-bLf on some of the cells that emerge after carcinogenic induction using well-known stem cell markers, such as CD133, ALDH, CD90, CD24, and CD44v6.43 One day after the last carcinogenic insult (Fig. 4A), positive cells increased, but pretreatment with bLf prevented this phenomenon by reducing the percentage of ALDH+-, CD133-, and CD24-positive cells to normal levels, except for CD90, which diminished significantly more than the control (p = 0.001133) (Fig. 4B).3.4 A single dose of holo-bLf before cancer initiation prevents the appearance of preneoplastic lesions
To determine whether holo-bLf has the capability to prevent the appearance of preneoplastic lesions, we administered a single oral dose of holo-bLf before liver cancer initiation. Rats then received the carcinogenic treatment and were sacrificed 30 days after initiation (Fig. 4C). GGT histochemistry analysis was performed to detect preneoplastic lesions in liver tissue (Fig. 4D–F). The results showed a significant (p = 0.0444) reduction in the number of preneoplastic lesions (Fig. 4G). A no significant tendency toward a reduction in the percentage of preneoplastic area was observed (Fig. 4H). This result suggests that holo-bLf has a chemopreventive effect on early HCC stages.3.5 Holo-bLf reduces tumor burden in a xenograft mouse model
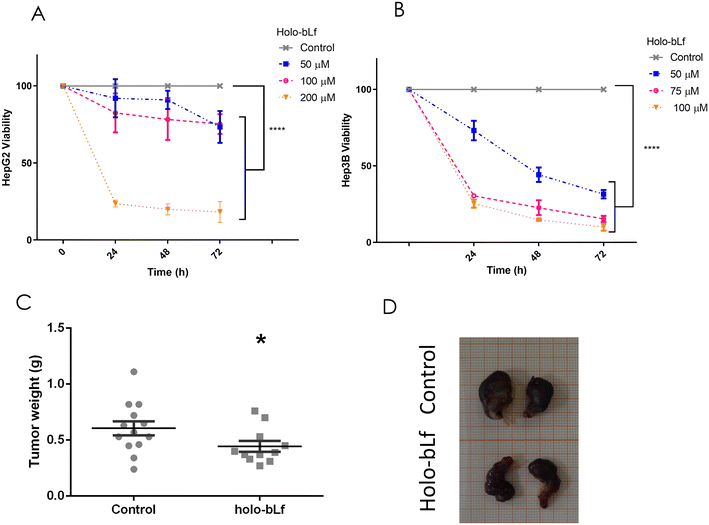
To determine the effect of holo-bLf on an established HCC tumor, its effect on two HCC cell lines, HepG2 and Hep3B, was evaluated. Holo-bLf was able to decrease the viability of HepG2 cells by 68% during the first 24 h and by 82% after 72 h of treatment with the higher dose of 200 μM (Fig. 5A), while Hep3B viability was decreased by 75% within 24 h and up to 90% after 72 h with the higher dose of 100 μM (Fig. 5B). Next, we xenotransplanted 5 × 106 HepG2 cells in NSG immunodeficient mice. Fifteen days later, when tumors reached 0.5 cm3, holo-bLf was orally administered every other day for 20 days. 200 mg kg−1 dose of holo-bLf was chosen based on Li et al. report (Li, Li et al. 2017).67 Mice were sacrificed, and tumor weights were recorded (Fig. 5C and D). The results showed that holo-bLf alone was able to reduce the tumor burden in xenotransplanted mice by 26.7% compared with the vehicle group (p = 0.0301) (Fig. 5C). This result proposes that holo-bLf has the ability to decrease established HCC tumors.3.6 Holo-bLf and sorafenib synergistically inhibit the viability of HepG2 cells in vitro
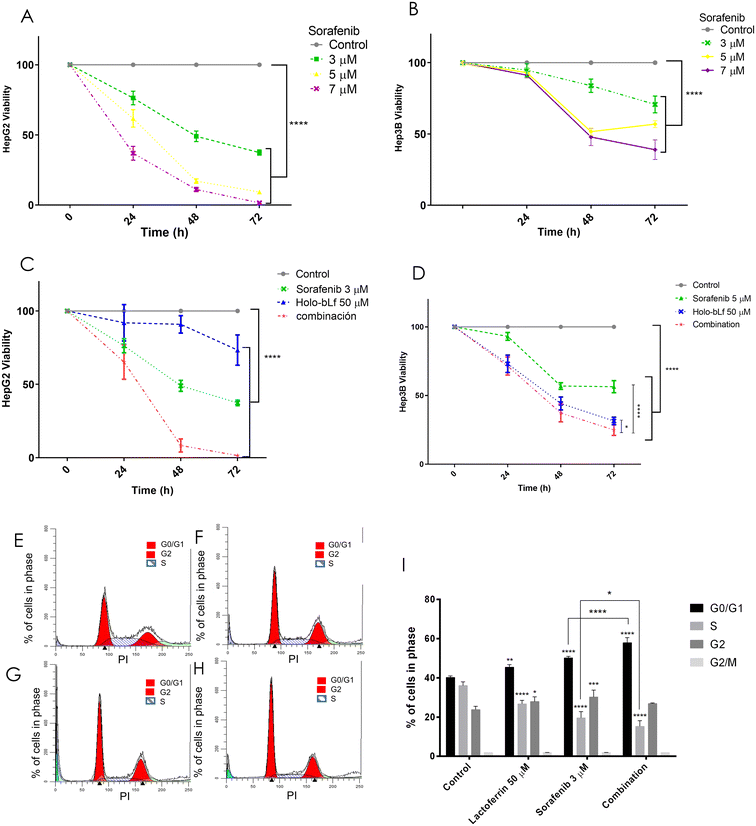
Lactoferrin has been used in combination with several chemotherapeutics and has been shown to improve their antitumoral effects in different cancers,44,45 while sorafenib is the first-line treatment for advanced HCC, despite its modest increase in OS and considerable side effects.4 Here, we aimed to test the effect of different doses of sorafenib and holo-bLf and to determine either their synergistic or additive effect using their lowest antitumoral doses on the viability of HepG2 and Hep3B cells. The combination of a sublethal dose of holo-bLf (Fig. 5A) and sorafenib (Fig. 6A), namely, 50 μM and 3 μM, respectively,46 decreased HepG2 cell viability by 33% at 24 h, 91.4% at 48 h and 98.8% at 72 h. The combination index (CI) for 48 and 72 h was 0.54 and 0.41, respectively (Fig. 6C). This evidence showed that a synergistic interaction between both compounds induced a cytotoxic effect that decreased the viability of HepG2 cells.47 The Hep3B cells exhibited a comparable trend, demonstrating a statistically significant difference between the combined effect and the individual compound effects, albeit without reaching additive or synergistic interactions (Fig. 6B and D).3.7 Holo-bLf in combination with sorafenib induces the arrest of HepG2 cells in G0/G1 phase of the cell cycle
bLf has been previously shown to induce cell cycle arrest in different cancer cell types.6 To investigate a possible mechanism involved in the decrease in cell viability induced by the synergistic effect of holo-bLf and sorafenib, we investigated whether the cell cycle was affected. HepG2 cells were incubated with holo-bLf and/or sorafenib for 24 h, and then the cells were analyzed by flow cytometry to determine cell cycle progression. HepG2 cells treated with holo-bLf showed a higher percentage of cells in G0/G1 (113% ± 0.82) and a lower percentage of cells in S phase (74% ± 1.80) when compared with controls. Sorafenib treatment increased the percentage of cells in G0/G1 (124.87% ± 0.78) and decreased that in S phase (54.42% ± 3.20), alongside an increase in G2 phase (126.93% ± 3.50) when compared with controls. The combination of both compounds increased the number of cells in G0/G1 by 143.99% ± 2.63, while the number of cells in S phase decreased to 42.41% ± 2.85 when compared with controls (Fig. 6E–H). This result clearly shows that both holo-bLf and sorafenib induce the arrest of HepG2 cells in the G0/G1 phase of the cell cycle when they are separately administered. Importantly, the combined administration induced a stronger increase in cell arrest in G0/G1 phase (Fig. 6I). Thus, these results provide evidence supporting the synergistic action of holo-bLf and sorafenib in reducing the viability of HepG2 cells, indicating that holo-bLf contributes to cell cycle arrest, thereby enhancing the cytotoxicity beyond that achieved by sorafenib alone.4. Discussion and conclusions
We performed an in silico analysis of tumor samples from three HCC patient cohorts, showing that the human LTF gene is downregulated in tumor tissue compared to healthy individual liver samples or adjacent tissue. This finding is consistent with the fact that Lf is highly expressed in noncancerous cell lines compared to cancer cell lines.48 We also found that TCGA-LIHC HCC patients with higher LFT expression had significantly better survival than those with low LFT expression, which strongly suggests that hLf might protect against liver cancer in humans. It has been reported that bLf is bioequivalent to hLf;6 moreover, bLf consumption increases the hLf serum concentration in patients.10 This evidence, along with our in silico, in vivo and in vitro findings, points to a prospective chemopreventive role of bLf in humans and as a potential compound for combinational therapy for HCC patients.bLf has been previously proven to be a chemopreventive agent in experimental HCC models; however, for the first time, we evaluated the chemopreventive effectiveness of a different iron-saturated form of bLf on HCC progression. The capability of holo-bLf and native-bLf to lessen the early alterations induced by the carcinogen DEN was evaluated, showing that holo-bLf was more effective than native-bLf in reducing DEN-induced necrosis. A plausible explanation for this phenomenon is its greater digestive stability, resulting in higher bioavailability of holo-bLf than that of either native-bLf or apo-bLf.49 Another possibility is that the molecular conformation caused by iron binding might result in differential receptor recognition and possibly in the activation of different signaling pathways downstream, such as that of the immune response.18 Further research is needed to validate these proposals.
Resistant hepatocyte models have been used for decades for the study of HCC progression.34 This model uses DEN, a well-known hepatocarcinogen, and one of the byproducts of DEN metabolism is reactive oxygen species (ROS), which cause a procarcinogenic increase in oxidative stress.38 Additionally, it has been proposed that necrosis induced by DEN plays a key role in the early stages of experimental hepatocarcinogenesis, probably by stimulating compensatory cell proliferation.36 Cell proliferation is required for the induction of resistant hepatocytes (referred to as initiated hepatocytes resistant to the mitostatic 2AAF effect) during initiation by carcinogens such as DEN.36 DEN is a procarcinogen that needs to be activated by cytochrome P450 isoforms, such as CYP1A1/2, CYP2B1/2, and CYP2E1, in the rat liver.50,51 Native bLf has the ability to reduce MeIQx-induced CYP1A2 levels,12 7,12-dimethylbenz[a]anthracene (DMBA)-induced CYP1A1 levels,52 and able to suppress alcohol-induced liver injury-induced overexpression of cytochrome P450 2E1 (CYP2E1).53 Therefore, based on the temporality of holo-bLf exposure in this study, it is not unreasonable to propose that holo-bLf might inhibit cytochrome P450 isoforms that activate DEN metabolism and, as a result, reduce DEN activation. Previous studies suggest that reducing hepatocyte cell death and compensatory proliferation, as long as oxidative stress has a pronounced beneficial effect, protects against carcinogenesis.54 Thus, holo-bLf might work as a chemopreventive agent that blocks DEN activation, inhibiting cancer initiation, granting this would need experimental confirmation.
Another mechanism might be associated with the antioxidant role of bLf. During carcinogenesis, ROS might activate protumorigenic signaling, enhance cell survival and proliferation, and drive DNA damage and genetic instability.55 We have previously reported a close correlation between the induction of ROS-derived liver lipoperoxidation and the appearance of preneoplastic lesions.38 On the other hand, the antioxidant potential of bLf has been widely documented;52,56,57 however, it has been mostly attributed to the iron sequestering capability of native- or apo-bLf, since iron is a well-known pro-oxidant that induces ROS production through the Fenton reaction.58 Therefore, we verified that iron-saturated bLf did not increase ROS production beyond the increase produced by DEN. Notably, we found that holo-bLf decreased ROS production and has a tendency to reduce lipid peroxidation beyond the effect induced by native-bLf. Thus, iron content does not appear to affect the antioxidant effect of bLf in our experimental model. Supporting this evidence, it has been reported that bLF has ROS-scavenging capability and protects DNA from direct oxidative damage in vitro, independently of its iron saturation degree.59 In a hamster buccal pouch carcinogenesis model, native-bLF increases its antioxidant capability by elevating the GSH/GSSG ratio and GPx activity in the liver.60 Such antioxidant mechanisms could be unrelated to Lf iron content and therefore contribute to the antioxidant activity of holo-bLf observed in our model, an intriguing phenomenon that needs further confirmation.
In our in vivo model, holo-bLf reduced the number of preneoplastic lesions but did not modify their total area, the hypothesis is that holo-bLf pretreatment was able to reduce the number of initiated hepatocytes but did not significantly affect their proliferation. This phenomenon might be due to the unique dose of holo-bLf that animals received 24 h before DEN administration, which was only able to block cancer initiation, but by the time that subsequent alterations appeared, such as increased proliferation of initiated cells, the active form of holo-bLf was either decreased or absent. Further studies using repeated doses of holo-bLf during and after cancer initiation might reveal the potential of holo-bLf to block the proliferation of initiated cells and, as a consequence, reduce the total area of preneoplastic lesions. This hypothesis is supported by the evidence that holo-bLf was able to reduce the proliferation of established HCC both in vitro and in vivo. Additionally, the effect of holo-bLf on initiated cells could be explained based on the HCC model used. The original model of the resistant hepatocyte relies on the proliferative stimuli given by a partial hepatectomy after the promoter carcinogen 2AAF.34,35 The model used in this study is a modified version, where 2AAF is administered 48 h after DEN. Closing the time frame between DEN and 2AAF apparently replaces the need for an extra proliferative stimulus, since such stimulus is most likely given exclusively by DEN-associated necrosis.36 Thus, it is plausible to propose that by reducing DEN-associated necrosis, holo-bLf was able to lessen liver-initiated cells.
This above proposal is also supported by the evidence that bLf diminished the emergence of a subpopulation of liver stem cells early in the carcinogenesis process. The role of cancer stem cells in established cancers has been extensively documented, but their presence during the early carcinogenesis stages has barely been investigated. Rats subjected to severe liver damage and a blockade of hepatocyte proliferation, such as those subjected to the resistant hepatocyte model, present short-lived and highly proliferating cells expressing both cholangiocyte- and hepatocyte-specific markers, as well as the embryonic liver marker AFP.61 This phenomenon is called a ductular reaction, and the cells are named oval cells. Recently, by lineage tracing experiments in mice, it was confirmed that such cells are derived from biliary epithelial cells and act as facultative liver stem/progenitor cells (LSPCs), which promote compensatory proliferation.62,63 LSPCs constitute a cell subpopulation susceptible to malignant transformation, and it has been demonstrated that they give rise to HCC tumors.39,64 However, how early these cells have a definitive commitment toward malignant transformation has not yet been clarified, with the earliest time reported being 5 months.64 In our investigation, we analyzed the LSPC subpopulation very early, i.e., one day postcarcinogenic treatment, using a wide panel of CSC markers and discovered that holo-bLf was able to significantly reduce LSPCs by identifying CD133 and one CD44 isoform (CD44v6). Although we did not investigate their commitment degree toward malignant transformation at the early carcinogenesis stage, it is probable that some of those LSPCs have already started their transformation since all carcinogenic insults have already acted, to eventually, namely, several months, progress toward HCC. The relevance of this subpopulation during carcinogenesis has been evidenced by some recent studies showing that by diminishing the LSPC surge, carcinogenesis might be prevented.41,42,65
Our investigation represents the first report showing that Lf inhibits established HCC tumors in vivo. The dose of bLf chosen was within the low range of in vivo doses used by other authors;19,66 specifically, a 200 mg kg−1 dose of holo-bLf was chosen based on the findings of Li et al.67 Previous studies have reported that bLf doses up to 4 g kg−1 are safe by demonstrating no to cause any toxicological lesions in male F344 rat organs.68 In fact, for infants aged 0–6 months, bLF intake is set by the EFSA at 200 mg per kg body weight and 1.2 g day−1.69 Of note, in our investigation, the tumors were reduced by 26.7%; similarly, a previous report showed that holo-bLf diminished the tumor burden by 37% in a mouse model of 4T1 breast cancer cells.44 Immunomodulation is considered to play a major role in Lf tumor suppression activity, mainly through stimulation of NK cells and CD4+ and CD8+ T cells.6 We used an extremely immunodeficient mouse strain named NOD-scid IL2Rγnull (NSG™), which lacks mature T cells, B cells, and NK cells; they are “nonleaky” and produce defective DCs.70 Therefore, our results also demonstrate an immune independence of T cells, B cells, and NK cells for holo-bLf in vivo antitumoral activity against HCC cells.
Sorafenib is the first-line treatment for advanced HCC patients, despite its modest impact on survival and toxicity. Because of its adverse effects, it has been necessary to combine sorafenib with other drugs to lower its dose and to improve tolerability without losing effectiveness4 To determine whether the combination of holo-bLf and sorafenib has an additive or synergistic effect on HCC cell viability, we determined the combination index (CI) using the software CompuSyn (https://www.combosyn.com). This calculation is based on the Chou–Talalay theory used to assess the statistical significance of combination experiments,71 and this method is currently accepted for calculating synergy and additivity.47,72 CI < 1 indicates synergism, CI = 1 indicates an additive effect, and CI > 1 indicates antagonism. The combination indices we obtained for HepG2 cells were CI = 0.54 after 48 h and CI = 0.41 after 72 h (Fig. 6C); therefore, we concluded that the effect of the combination of holo-bLf and sorafenib on in vitro HepG2 cell viability was synergistic. Such a combined effect on Hep3B cells was moderated with the tested doses, although significantly different from holo-bLf or sorafenib alone. Sorafenib is a multikinase inhibitor, and one of its targets is the Ras-Raf-MAPK axis; however, it has been reported that its inhibitory activity prompts the compensatory activation of PI3K-Akt-mTOR signaling. On the other hand, bLf decreases the phosphorylation of Akt and mTOR in several models.17,73 Therefore, we can hypothesize that the combination of holo-bLf and sorafenib acts synergistically because they simultaneously inhibit the Ras-Raf-MAPK and PI3K-Akt-mTOR axes.
Here, we also found that holo-bLf and sorafenib either individually or in combination induced G0/G1 cell cycle arrest. The effect of holo-bLf on the arrest of cancer cell lines in G0/G1 phase is in line with its effect shown in other cancers.74,75 Sorafenib is reported to cause cell cycle arrest in several cancer cell lines, including HepG2; coincidentally, our investigation showed that it arrested the cell cycle in G0/G1 phase.76,77
In conclusion, to our knowledge, this is the first investigation reporting the chemopreventive effect of holo-bLf on HCC. The results suggest that Holo-bLf outperforms native-bLf as a chemopreventive agent in the early stage of an in vivo HCC model. Holo-bLf moderately diminishes the tumor burden in established HCC models, whereas in vitro, it has a synergic effect when used in combination with sorafenib. Therefore, our investigation suggests that holo-bLf is a promising molecule to prevent HCC and to challenge advanced HCC in combination with sorafenib. Finally, our investigation encourages deeper mechanistic studies of the chemopreventive action of holo-bLf on HCC progression.
Author contributions
Villa-Treviño, Saúl and Piña-Vázquez, Carolina: Conceptualization, funding acquisition; supervision, formal analysis, writing – review & editing. Sánchez-Pérez Yesennia: Resources and supervision. Hernández-Galdámez, Hury Viridiana: Investigation; methodology, visualization, formal analysis, writing – original draft. Fattel-Fazenda, Samia, Aguilar-Chaparro Mario Alejandro, Mendoza-García Jonathan, Díaz-Fernández, Lidia, Romo-Medina Eunice: Methodology. Flores-Téllez, Teresita N. J.: Software, formal analysis, review & editing. De la Garza, Mireya: Conceptualization and resources. Arellanes-Robledo, Jaime: Writing – review & editing.Abbreviations
| HCC | Hepatocellular carcinoma |
| Lf | Lactoferrin |
| holo-Lf | Iron saturated Lf |
| DEN | Diethylnitrosamine |
| ROS | Reactive oxygen species |
| ALDH | Aldehyde dehydrogenase |
| TCGA | The cancer genome atlas |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| OS | Overall survival |
| bLf | Bovine Lf |
| hLf | Human Lf |
| FDA | USA food and drug administration |
| ASGPR | Asialoglycoprotein receptor |
| 2-AAF | 2-Acetylaminofluorene |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| PMSF | Phenylmethylsulfonyl fluoride |
| TBA | Thiobarbituric acid |
| MDA | Malonyldialdehyde |
| AHF | Altered hepatic foci |
| GGT | γ-Glutamyl transpeptidase |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| HBSS | Hanks’ balanced salt solution |
| LTF | hLf gene |
| GTEx | The genotype tissue expression |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMEM | Dulbecco's Modified Eagle's medium |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| ANOVA | Analysis of variance |
| LSPCs | Liver stem or progenitor cells |
| CT | Carcinogenic treatment |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| AFP | Alpha fetoprotein |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Alejandro Cruz Hernandez, Víctor Manuel Ortiz Santiago, Clara Hernandez Chavez, Maria Asunción Cabañas Cortes, and Victor Hugo Rosales for assisting in the development of this project. We thank the Unit for Production of Experimental Laboratory Animals (UPEAL CINVESTAV, Mexico City, Mexico), especially to Benjamín Emmanuel Chávez Álvarez, Carlos Giovanni Sam Miranda, and Felipe Cruz Martinez. Supported by CONAHCYT Mexico Grants, No. 2015-01-599, 53358 and for CPV EPM2022(3)-3837627. CONAHCYT did not participate in the study design, data collection, analysis, interpretation, writing of the report, or in the decision to submit the manuscript for publication.References
- J. Ferlay, M. Laversanne, M. Colombet, L. Mery, M. Piñeros, I. A. Znaor and F. Bray, Global Cancer Observatory: Cancer Today, Lyon, France: International Agency for Research on Cancer, https://gco.iarc.fr/today, (accessed April 3, 2020) Search PubMed.
- H. B. El-Serag and K. L. Rudolph, Hepatocellular carcinoma: epidemiology and molecular carcinogenesis, Gastroenterology, 2007, 132, 2557–2576 CrossRef CAS PubMed.
- J. D. Yang, P. Hainaut, G. J. Gores, A. Amadou, A. Plymoth and L. R. Roberts, A global view of hepatocellular carcinoma: trends, risk, prevention and management, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 589–604 CrossRef PubMed.
- C. Akateh, S. M. Black, L. Conteh, E. D. Miller, A. Noonan, E. Elliott, T. M. Pawlik, A. Tsung and J. M. Cloyd, Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma, World J. Gastroenterol., 2019, 25, 3704–3721 CrossRef PubMed.
- L. K. Penny and H. M. Wallace, The challenges for cancer chemoprevention, Chem. Soc. Rev., 2015, 44, 8836–8847 RSC.
- A. Cutone, L. Rosa, G. Ianiro, M. S. Lepanto, M. C. Bonaccorsi di Patti, P. Valenti and G. Musci, Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action, Biomolecules, 2020, 10(3), 456 CrossRef CAS PubMed.
- EFSA, Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on bovine lactoferrin, EFSA J., 2012, 2811 Search PubMed.
- A. M. Rulis , Agency Response Letter GRAS Notice No. GRN 000077.
- M. Iigo, D. B. Alexander, J. Xu, M. Futakuchi, M. Suzui, T. Kozu, T. Akasu, D. Saito, T. Kakizoe, K. Yamauchi, F. Abe, M. Takase, K. Sekine and H. Tsuda, Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine, BioMetals, 2014, 27, 1017–1029 CrossRef CAS PubMed.
- T. Kozu, G. Iinuma, Y. Ohashi, Y. Saito, T. Akasu, D. Saito, D. B. Alexander, M. Iigo, T. Kakizoe and H. Tsuda, Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial, Cancer Prev. Res., 2009, 2, 975–983 CrossRef CAS PubMed.
- D. Trere, L. Fiume, L. B. De Giorgi, G. Di Stefano, M. Migaldi and M. Derenzini, The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells, Br. J. Cancer, 1999, 81, 404–408 CrossRef CAS PubMed.
- K. Fujita, T. Ohnishi, K. Sekine, M. Iigo and H. Tsuda, Down-regulation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)-induced CYP1A2 expression is associated with bovine lactoferrin inhibition of MeIQx-induced liver and colon carcinogenesis in rats, Jpn. J. Cancer Res., 2002, 93, 616–625 CrossRef CAS PubMed.
- R. R. Hegazy, D. F. Mansour, A. A. Salama, R. F. Abdel-Rahman and A. M. Hassan, Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats, Pharmacol. Rep., 2019, 71, 879–891 CrossRef CAS PubMed.
- M. M. Mohammed, G. Ramadan, M. K. Zoheiry and N. M. El-Beih, Antihepatocarcinogenic activity of whey protein concentrate and lactoferrin in diethylnitrosamine-treated male albino mice, Environ. Toxicol., 2019, 34, 1025–1033 CrossRef CAS PubMed.
- J. S. Wolf, G. Li, A. Varadhachary, K. Petrak, M. Schneyer, D. Li, J. Ongkasuwan, X. Zhang, R. J. Taylor, S. E. Strome and B. W. O'Malley Jr., Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo, Clin. Cancer Res., 2007, 13, 1601–1610 CrossRef CAS PubMed.
- J. A. Gibbons, J. R. Kanwar and R. K. Kanwar, Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer, BMC Cancer, 2015, 15, 425 CrossRef PubMed.
- Y. Zhang, A. Nicolau, C. F. Lima and L. R. Rodrigues, Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells, Nutr. Cancer, 2014, 66, 1371–1385 CrossRef CAS PubMed.
- A. Cutone, B. Colella, A. Pagliaro, L. Rosa, M. S. Lepanto, M. C. Bonaccorsi di Patti, P. Valenti, S. Di Bartolomeo and G. Musci, Native and iron-saturated bovine lactoferrin differently hinder migration in a model of human glioblastoma by reverting epithelial-to-mesenchymal transition-like process and inhibiting interleukin-6/STAT3 axis, Cell. Signalling, 2020, 65, 109461 CrossRef CAS PubMed.
- J. R. Kanwar, K. P. Palmano, X. Sun, R. K. Kanwar, R. Gupta, N. Haggarty, A. Rowan, S. Ram and G. W. Krissansen, ‘Iron-saturated’ lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy, Immunol. Cell Biol., 2008, 86, 277–288 CrossRef CAS PubMed.
- J. P. Cerapio, A. Marchio, L. Cano, I. Lopez, J. J. Fournie, B. Regnault, S. Casavilca-Zambrano, E. Ruiz, A. Dejean, S. Bertani and P. Pineau, Global DNA hypermethylation pattern and unique gene expression signature in liver cancer from patients with Indigenous American ancestry, Oncotarget, 2021, 12, 475–492 CrossRef PubMed.
- R. Xiao and W. S. Kisaalita, Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin, Microbiology, 1997, 143(Pt 7), 2509–2515 CAS.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- J. R. Macias-Perez, O. Beltran-Ramirez, V. R. Vasquez-Garzon, M. E. Salcido-Neyoy, P. A. Martinez-Soriano, M. B. Ruiz-Sanchez, E. Angeles and S. Villa-Trevino, The effect of caffeic acid phenethyl ester analogues in a modified resistant hepatocyte model, Anti-Cancer Drugs, 2013, 24, 394–405 CrossRef CAS PubMed.
- S. A. Elmore, D. Dixon, J. R. Hailey, T. Harada, R. A. Herbert, R. R. Maronpot, T. Nolte, J. E. Rehg, S. Rittinghausen, T. J. Rosol, H. Satoh, J. D. Vidal, C. L. Willard-Mack and D. M. Creasy, Recommendations from the INHAND Apoptosis/Necrosis Working Group, Toxicol. Pathol., 2016, 44, 173–188 CrossRef CAS PubMed.
- G. Revilla, N. Al Qtaish, P. Caruana, M. Sainz-Ramos, T. Lopez-Mendez, F. Rodriguez, V. Paez-Espinosa, C. Li, N. F. Vallverdu, M. Edwards, A. Moral, J. I. Perez, J. C. Escola-Gil, J. L. Pedraz, I. Gallego, R. Corcoy, M. V. Cespedes, G. Puras and E. Mato, Lenvatinib-Loaded Poly(lactic-co-glycolic acid) Nanoparticles with Epidermal Growth Factor Receptor Antibody Conjugation as a Preclinical Approach to Therapeutically Improve Thyroid Cancer with Aggressive Behavior, Biomolecules, 2023, 13(11), 1647 CrossRef CAS PubMed.
- J. A. Buege and S. D. Aust, Microsomal lipid peroxidation, Methods Enzymol., 1978, 52, 302–310 CAS.
- R. Cathcart, E. Schwiers and B. N. Ames, Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay, Anal. Biochem., 1983, 134, 111–116 CrossRef CAS PubMed.
- L. Riccalton-Banks, R. Bhandari, J. Fry and K. M. Shakesheff, A simple method for the simultaneous isolation of stellate cells and hepatocytes from rat liver tissue, Mol. Cell. Biochem., 2003, 248, 97–102 CrossRef CAS PubMed.
- A. M. Rutenburg, H. Kim, J. W. Fischbein, J. S. Hanker, H. L. Wasserkrug and A. M. Seligman, Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity, J. Histochem. Cytochem., 1969, 17, 517–526 CrossRef CAS PubMed.
- Y. G. Chung, E. Tak, S. Hwang, J. Y. Lee, J. Y. Kim, Y. Y. Kim, G. W. Song, K. J. Lee and N. Kim, Synergistic effect of metformin on sorafenib in in vitro study using hepatocellular carcinoma cell lines, Ann. Hepatobiliary Pancreat. Surg., 2018, 22, 179–184 CrossRef PubMed.
- R. Jiang and B. Lonnerdal, Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways, Biochem. Cell Biol., 2017, 95, 99–109 CrossRef CAS PubMed.
- J. C. Wei, F. D. Meng, K. Qu, Z. X. Wang, Q. F. Wu, L. Q. Zhang, Q. Pang and C. Liu, Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1, Acta Pharmacol. Sin., 2015, 36, 241–251 CrossRef CAS PubMed.
- N. Zhang, J. N. Fu and T. C. Chou, Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: experimental design and data analysis using the combination index method, Am. J. Cancer Res., 2016, 6, 97–104 CAS.
- D. Solt and E. Farber, New principle for the analysis of chemical carcinogenesis, Nature, 1976, 263, 701–703 CrossRef CAS.
- E. Semple-Roberts, M. A. Hayes, D. Armstrong, R. A. Becker, W. J. Racz and E. Farber, Alternative methods of selecting rat hepatocellular nodules resistant to 2-acetylaminofluorene, Int. J. Cancer, 1987, 40, 643–645 CrossRef CAS PubMed.
- T. S. Ying, D. S. Sarma and E. Farber, Role of acute hepatic necrosis in the induction of early steps in liver carcinogenesis by diethylnitrosamine, Cancer Res., 1981, 41, 2096–2102 CAS.
- M. R. McLoughlin, D. J. Orlicky, J. R. Prigge, P. Krishna, E. A. Talago, I. R. Cavigli, S. Eriksson, C. G. Miller, J. A. Kundert, V. I. Sayin, R. A. Sabol, J. Heinemann, L. O. Brandenberger, S. V. Iverson, B. Bothner, T. Papagiannakopoulos, C. T. Shearn, E. S. J. Arner and E. E. Schmidt, TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11408–11417 CrossRef CAS PubMed.
- Y. Sanchez-Perez, C. Carrasco-Legleu, C. Garcia-Cuellar, J. Perez-Carreon, S. Hernandez-Garcia, M. Salcido-Neyoy, L. Aleman-Lazarini and S. Villa-Trevino, Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis, Cancer Lett., 2005, 217, 25–32 CrossRef CAS PubMed.
- L. Zhu, D. Finkelstein, C. Gao, L. Shi, Y. Wang, D. Lopez-Terrada, K. Wang, S. Utley, S. Pounds, G. Neale, D. Ellison, A. Onar-Thomas and R. J. Gilbertson, Multi-organ Mapping of Cancer Risk, Cell, 2016, 166, 1132–1146 CrossRef CAS PubMed.
- R. A. Davies, B. Knight, Y. W. Tian, G. C. Yeoh and J. K. Olynyk, Hepatic oval cell response to the choline-deficient, ethionine supplemented model of murine liver injury is attenuated by the administration of a cyclo-oxygenase 2 inhibitor, Carcinogenesis, 2006, 27, 1607–1616 CrossRef CAS PubMed.
- B. Knight, J. E. Tirnitz-Parker and J. K. Olynyk, C-kit inhibition by imatinib mesylate attenuates progenitor cell expansion and inhibits liver tumor formation in mice, Gastroenterology, 2008, 135, 969–979 CrossRef CAS PubMed.
- K. P. Lee, J. H. Lee, T. S. Kim, T. H. Kim, H. D. Park, J. S. Byun, M. C. Kim, W. I. Jeong, D. F. Calvisi, J. M. Kim and D. S. Lim, The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 8248–8253 CrossRef CAS PubMed.
- T. N. Flores-Tellez, S. Villa-Trevino and C. Pina-Vazquez, Road to stemness in hepatocellular carcinoma, World J. Gastroenterol., 2017, 23, 6750–6776 CrossRef CAS PubMed.
- X. Sun, R. Jiang, A. Przepiorski, S. Reddy, K. P. Palmano and G. W. Krissansen, “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice, BMC Cancer, 2012, 12, 591 CrossRef CAS PubMed.
- A. Varadhachary, J. S. Wolf, K. Petrak, B. W. O'Malley Jr., M. Spadaro, C. Curcio, G. Forni and F. Pericle, Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy, Int. J. Cancer, 2004, 111, 398–403 CrossRef CAS PubMed.
- T. Morisaki, M. Umebayashi, A. Kiyota, N. Koya, H. Tanaka, H. Onishi and M. Katano, Combining celecoxib with sorafenib synergistically inhibits hepatocellular carcinoma cells in vitro, Anticancer Res., 2013, 33, 1387–1395 CAS.
- K. R. Roell, D. M. Reif and A. A. Motsinger-Reif, An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines, Front. Pharmacol., 2017, 8, 158 CrossRef PubMed.
- E. Hoedt, S. Hardiville, C. Mariller, E. Elass, J. P. Perraudin and A. Pierce, Discrimination and evaluation of lactoferrin and delta-lactoferrin gene expression levels in cancer cells and under inflammatory stimuli using TaqMan real-time PCR, BioMetals, 2010, 23, 441–452 CrossRef CAS PubMed.
- F. J. Troost, J. Steijns, W. H. Saris and R. J. Brummer, Gastric digestion of bovine lactoferrin in vivo in adults, J. Nutr., 2001, 131, 2101–2104 CrossRef CAS PubMed.
- J. J. Espinosa-Aguirre, J. Rubio, I. Lopez, R. Nosti and J. Asteinza, Characterization of the CYP isozyme profile induced by cyclohexanol, Mutagenesis, 1997, 12, 159–162 CrossRef CAS PubMed.
- L. Verna, J. Whysner and G. M. Williams, N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation, Pharmacol. Ther., 1996, 71, 57–81 CrossRef CAS PubMed.
- P. V. Letchoumy, K. V. Mohan, J. J. Stegeman, H. V. Gelboin, Y. Hara and S. Nagini, In vitro antioxidative potential of lactoferrin and black tea polyphenols and protective effects in vivo on carcinogen activation, DNA damage, proliferation, invasion, and angiogenesis during experimental oral carcinogenesis, Oncol. Res., 2008, 17, 193–203 CrossRef PubMed.
- D. Li, Q. He, H. Yang, Y. Du, K. Yu, J. Yang, X. Tong, Y. Guo, J. Xu and L. Qin, Daily Dose of Bovine Lactoferrin Prevents Ethanol-Induced Liver Injury and Death in Male Mice by Regulating Hepatic Alcohol Metabolism and Modulating Gut Microbiota, Mol. Nutr. Food Res., 2021, 65, e2100253 CrossRef PubMed.
- A. Wree, C. D. Johnson, J. Font-Burgada, A. Eguchi, D. Povero, M. Karin and A. E. Feldstein, Hepatocyte-specific Bid depletion reduces tumor development by suppressing inflammation-related compensatory proliferation, Cell Death Differ., 2015, 22, 1985–1994 CrossRef CAS PubMed.
- J. N. Moloney and T. G. Cotter, ROS signalling in the biology of cancer, Semin. Cell Dev. Biol., 2018, 80, 50–64 CrossRef CAS PubMed.
- N. A. Abdel Baky, A. H. Al-Najjar, H. A. Elariny, A. S. Sallam and A. A. Mohammed, Pramipexole and Lactoferrin ameliorate Cyclophosphamide-Induced haemorrhagic cystitis via targeting Sphk1/S1P/MAPK, TLR-4/NF-kappaB, and NLRP3/caspase-1/IL-1beta signalling pathways and modulating the Nrf2/HO-1 pathway, Int. Immunopharmacol., 2022, 112, 109282 CrossRef CAS PubMed.
- O. S. Mohamed, N. A. Abdel Baky, M. M. Sayed-Ahmed and A. H. Al-Najjar, Lactoferrin alleviates cyclophosphamide induced-nephropathy through suppressing the orchestration between Wnt4/beta-catenin and ERK1/2/NF-kappaB signaling and modulating klotho and Nrf2/HO-1 pathway, Life Sci., 2023, 319, 121528 CrossRef CAS PubMed.
- H. Shoji, S. Oguchi, K. Shinohara, T. Shimizu and Y. Yamashiro, Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells, Pediatr. Res., 2007, 61, 89–92 CrossRef PubMed.
- Y. Ogasawara, M. Imase, H. Oda, H. Wakabayashi and K. Ishii, Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: a possible role in protection against oxidative DNA damage, Int. J. Mol. Sci., 2014, 15, 1003–1013 CrossRef PubMed.
- K. V. Chandra Mohan, R. Kumaraguruparan, D. Prathiba and S. Nagini, Modulation of xenobiotic-metabolizing enzymes and redox status during chemoprevention of hamster buccal carcinogenesis by bovine lactoferrin, Nutrition, 2006, 22, 940–946 CrossRef PubMed.
- C. J. Hindley, G. Mastrogiovanni and M. Huch, The plastic liver: differentiated cells, stem cells, every cell?, J. Clin. Invest., 2014, 124, 5099–5102 CrossRef PubMed.
- J. O. Russell, W. Y. Lu, H. Okabe, M. Abrams, M. Oertel, M. Poddar, S. Singh, S. J. Forbes and S. P. Monga, Hepatocyte-Specific beta-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes, Hepatology, 2019, 69, 742–759 CrossRef CAS PubMed.
- A. Raven, W. Y. Lu, T. Y. Man, S. Ferreira-Gonzalez, E. O'Duibhir, B. J. Dwyer, J. P. Thomson, R. R. Meehan, R. Bogorad, V. Koteliansky, Y. Kotelevtsev, C. Ffrench-Constant, L. Boulter and S. J. Forbes, Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration, Nature, 2017, 547, 350–354 CrossRef CAS PubMed.
- G. He, D. Dhar, H. Nakagawa, J. Font-Burgada, H. Ogata, Y. Jiang, S. Shalapour, E. Seki, S. E. Yost, K. Jepsen, K. A. Frazer, O. Harismendy, M. Hatziapostolou, D. Iliopoulos, A. Suetsugu, R. M. Hoffman, R. Tateishi, K. Koike and M. Karin, Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling, Cell, 2013, 155, 384–396 CrossRef CAS PubMed.
- Y. W. Zheng, T. Tsuchida, T. Shimao, B. Li, T. Takebe, R. R. Zhang, Y. Sakurai, Y. Ueno, K. Sekine, N. Ishibashi, M. Imajima, T. Tanaka and H. Taniguchi, The CD133+ CD44+ precancerous subpopulation of oval cells is a therapeutic target for hepatocellular carcinoma, Stem Cells Dev., 2014, 23, 2237–2249 CrossRef CAS PubMed.
- Y. Aoyama, A. Naiki-Ito, K. Xiaochen, M. Komura, H. Kato, Y. Nagayasu, S. Inaguma, H. Tsuda, M. Tomita, Y. Matsuo, S. Takiguchi and S. Takahashi, Lactoferrin Prevents Hepatic Injury and Fibrosis via the Inhibition of NF-kappaB Signaling in a Rat Non-Alcoholic Steatohepatitis Model, Nutrients, 2021, 14(1), 42 CrossRef PubMed.
- H. Y. Li, M. Li, C. C. Luo, J. Q. Wang and N. Zheng, Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model, J. Agric. Food Chem., 2017, 65, 10464–10472 CrossRef CAS PubMed.
- S. Tamano, K. Sekine, M. Takase, K. Yamauchi, M. Iigo and H. Tsuda, Lack of chronic oral toxicity of chemopreventive bovine lactoferrin in F344/DuCrj rats, Asian Pac. J. Cancer Prev., 2008, 9, 313–316 Search PubMed.
- N. EFSA, Panel on Dietetic Products and Allergies, Scientific Opinion on bovine lactoferrin, EFSA J., 2012, 10, 2701 CrossRef.
- L. D. Shultz, B. L. Lyons, L. M. Burzenski, B. Gott, X. Chen, S. Chaleff, M. Kotb, S. D. Gillies, M. King, J. Mangada, D. L. Greiner and R. Handgretinger, Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells, J. Immunol., 2005, 174, 6477–6489 CrossRef CAS PubMed.
- T. C. Chou, Drug combination studies and their synergy quantification using the Chou-Talalay method, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
- D. Duarte and N. Vale, Evaluation of synergism in drug combinations and reference models for future orientations in oncology, Curr. Res. Pharmacol. Drug Discov., 2022, 3, 100110 CrossRef PubMed.
- X. X. Xu, H. R. Jiang, H. B. Li, T. N. Zhang, Q. Zhou and N. Liu, Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin, J. Dairy Sci., 2010, 93, 2344–2350 CrossRef CAS PubMed.
- E. Damiens, I. El Yazidi, J. Mazurier, I. Duthille, G. Spik and Y. Boilly-Marer, Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells, J. Cell. Biochem., 1999, 74, 486–498 CrossRef CAS PubMed.
- Y. Xiao, C. L. Monitto, K. M. Minhas and D. Sidransky, Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells, Clin. Cancer Res., 2004, 10, 8683–8686 CrossRef CAS PubMed.
- A. A. Bahman, M. S. I. Abaza, S. I. Khoushiash and R. J. Al-Attiyah, Sequencedependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action, Int. J. Mol. Med., 2018, 42, 1695–1715 CAS.
- B. Yurdacan, U. Egeli, G. Guney Eskiler, I. E. Eryilmaz, G. Cecener and B. Tunca, Investigation of new treatment option for hepatocellular carcinoma: a combination of sorafenib with usnic acid, J. Pharm. Pharmacol., 2019, 71, 1119–1132 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo05184f |
| ‡ Deceased. |
| This journal is © The Royal Society of Chemistry 2024 |