Open Access Article
Open Access ArticleCombined supplementation with hesperidin, phytosterols and curcumin decreases adiposity and improves metabolic health in ovariectomized rats†
Julio
Baudin
 ab,
Julia
Hernandez-Baixauli‡
ab,
Julia
Hernandez-Baixauli‡
 a,
Sergio
Quesada-Vázquez§
a,
Francisca
Mulero
c,
Francesc
Puiggròs
d,
Lluís
Arola
*b and
Antoni
Caimari
a,
Sergio
Quesada-Vázquez§
a,
Francisca
Mulero
c,
Francesc
Puiggròs
d,
Lluís
Arola
*b and
Antoni
Caimari
 *d
*d
aEurecat, Centre Tecnològic de Catalunya, Technological Unit of Nutrition and Health, 43204 Reus, Spain
bNutrigenomics Research Group, Department of Biochemistry and Biotechnology, Universitat Rovira i Virgili, 43007 Tarragona, Spain. E-mail: lluis.arola@urv.cat
cMolecular Imaging Unit, Spanish National Cancer Research Centre (CNIO), Madrid, Spain
dEurecat, Centre Tecnològic de Catalunya, Biotechnology Area, 43204 Reus, Spain. E-mail: antoni.caimari@eurecat.org
First published on 10th April 2024
Abstract
In recent years many women have looked for alternative therapies to address menopause. Hesperidin, phytosterols and curcumin are bioactive compounds that can ameliorate some cardiovascular risk factors associated with menopause, although there are no data concerning the effects of their combined supplementation. We used ovariectomized (OVX) rats, a postmenopausal model with oestrogen deficiency, to evaluate whether supplementation with a multi-ingredient (MI) including hesperidin, phytosterols and curcumin for 57 days would display beneficial effects against fat mass accretion and metabolic disturbances associated with menopause. Twenty OVX rats were orally supplemented with either MI (OVX-MI) or vehicle (OVX). Furthermore, 10 OVX rats orally received the vehicle along with subcutaneous injections of 17β-oestradiol biweekly (OVX-E2), whereas 10 rats were sham operated and received oral and injected vehicles (control group; SH). MI supplementation partly counteracted the fat mass accretion observed in OVX animals, which was evidenced by decreased total fat mass, adiposity index, the weight of retroperitoneal, inguinal and mesenteric white adipose tissue (MWAT) depots and MWAT adipocyte hypertrophy. These effects were accompanied by a significant decrease in the circulating levels of leptin and the mRNA levels of the fatty acid uptake-related genes Lpl and Cd36 in MWAT. These results were very similar to those observed in OVX-E2 animals. OVX-MI rats also displayed a higher lean body mass, lean/fat mass ratio, adiponectin-to-leptin ratio and insulin sensitivity than their OVX counterparts. Our findings can pave the way for using this MI formulation as an alternative therapy to manage obesity and to improve the cardiometabolic health of menopausal women.
Introduction
Menopause is a biological stage in a woman's life that is defined as the permanent cessation of menstrual cycles by reproductive ageing. The decline and gradual cessation of oestrogen production by loss of ovarian follicular activity and function1 occurring during the menopausal transition and menopause can cause a variety of vasomotor symptoms, such as hot flushes, sleep disturbances and night sweats.2–4 In addition, this ageing process may be associated with osteoporosis,5 increased body weight and a change in body fat distribution from a gynoid to an android pattern, contributing to increased abdominal fat accretion.6–8 This excess of adiposity can exacerbate the appearance of metabolic alterations such as hypercholesterolemia, hypertension and insulin resistance, increasing the risk of suffering cardiovascular diseases (CVDs),6–10 which become more evident with age and are one of the leading causes of death worldwide, accounting for 47% of deaths in women.11Hormone replacement therapy (HRT) prescription remains the most effective solution for symptoms or metabolic disturbances related to the postmenopausal state. Nevertheless, HRT prescription was challenged by the controversy over its benefits and risks derived from the unexpected findings of the Women's Ischaemia Syndrome Evaluation (WISE) study in 2002, pointing towards a higher risk of suffering CVDs and endometrial and breast cancers for some of the HRT treatments.12 Currently, the safety and main side effects depend on the type of HRT, the duration of the treatment and the woman's age at which the HRT starts after the final menstrual period occurs. Thus, it was described that the benefits are higher than the risks when HRT is prescribed to healthy women under 60 or within the first ten years of menopause.12 HRT effectively reduces vasomotor symptoms and osteoporosis.12 However, HRT has been shown to be less effective (favourable or neutral effects) in ameliorating body weight gain and reducing the risk of CVDs (coronary heart disease, thromboembolism, blood clots and stroke) and affective disorders such as dementia or Alzheimer's disease, and it is not advised for women with a medical history of breast or endometrial cancers.12
In recent years, due to controversy about the risks associated with HRT, many women have looked for alternative therapies to address menopause. Some supplements, such as natural extracts, bioactive compounds and nutraceuticals, have been used as alternative therapies to manage vasomotor symptoms and metabolic disturbances related to menopausal state. One of the best-known natural bioactive compounds with scientific evidence-based effects is the isoflavones obtained from soy, also called phytoestrogens, with genistein being the most abundant of them, accounting for 60% of the total isoflavones found in soy.13 Phytoestrogens are chemically similar to 17β-oestradiol (17β-E2), and they have been extensively studied due to their ability to bind to oestrogen receptors, producing oestrogenic effects in target organs. Genistein has twenty times more selectivity for oestrogen receptor (ER) β than for α, which is a favourable property because most of the side effects are produced through binding to ER-α.13,14 Nevertheless, there are many types of phytoestrogens, and their activity in humans seems to vary between subjects because specific intestinal bacteria and/or individual-based hepatic modifications are needed to convert a precursor into a more active compound. As an example, only 30% of the population is able to convert daidzein, a well-studied phytoestrogen of soy, to the most bioactive and absorbable isoform, called equol diadzein.15
Curcumin, hesperidin and phytosterols are other bioactive compounds from natural origin with promising effects against metabolic-related disturbances associated with menopause, although the body of scientific evidence about their effects is lower when compared to what is reported for phytoestrogens. Curcumin is a polyphenol obtained from Curcuma longa (turmeric) powdered roots commonly used as part of curry powder in curry dishes and displays well-known antioxidant and anti-inflammatory properties.16 In humans, curcumin also improves metabolic health by decreasing the surrogated marker of insulin resistance homeostasis model assessment-estimated insulin resistance (HOMA-IR).17 Additionally, in ovariectomized (OVX) rats, a well-established preclinical model to study menopause in which the ovaries are surgically removed to mimic the deficiency of circulating oestrogen levels occurring in menopause and the associated osteoporosis and metabolic disorders,18–21 curcumin supplementation ameliorated progressive bone loss and osteoporosis.22 Furthermore, Morrone et al. demonstrated that this bioactive compound displayed an anti-hypercholesterolaemic effect and reduced oxidative stress and visceral adiposity in this animal model.23
Hesperidin is a flavonoid mainly present in citrus fruits (oranges, tangerines, lemons, limes, and grapefruits), in the juices obtained from these fruits. This bioactive compound has shown protective effects in humans on the cardiovascular system because, despite its low bioavailability, it is able to modulate and reduce several biomarkers of risk factors for CVDs, such as triglycerides (TG), total cholesterol (TC), LDL-cholesterol, TNF-α, systolic blood pressure and insulin.24–26 Furthermore, mounting evidence suggests that hesperidin supplementation exerts an inhibitory effect against obesity, modulating lipid metabolism,27 and beneficial effects on bone metabolism28 were also reported in OVX rats, improving their bone mineral density (BMD).29,30 Recently, members of our group demonstrated, in humans, that supplementation with a hesperidin extract mainly rich in the 2S isomer and presented in a micronized form (M2SH) increased the bioavailability of this flavanone,31 paving the way for carrying out additional studies to evaluate whether this boosted bioavailability is translated into enhanced cardioprotective effects.
Phytosterols are phytosteroids that present a similar structure to cholesterol and that are naturally present in considerable amounts in pistachios and in oils obtained from rapeseed, wheat germ and corn, whilst legumes, cereals, fruits, and vegetables contain lower quantities. The mechanism of action of ingested phytosterols, due to their similar structure, produces a competitive exclusion of cholesterol from bile acid micelles in the intestinal tract, which reduces intestinal cholesterol reabsorption. Phytosterol supplementation is supported by the European Food Safety Authority (EFSA), which concluded that the consumption of approximately 1.5 to 2.4 g per day of phytosterols and/or stanols may reduce circulating cholesterol levels in the blood and decrease the risk of coronary heart disease.32 Xia et al. 2022 demonstrated that in postmenopausal women, consuming 2 g per day phytosterols reduced serum TC and LDL-cholesterol.33
Although supplementation with curcumin, hesperidin and phytosterol separately exert potential beneficial effects against fat mass accretion and/or menopausal-related metabolic alterations, mainly in animal models, to the best of our knowledge, the effects of combined supplementation with these three bioactive compounds have not yet been evaluated. Different studies, including some carried out by our research group,34–37 have shown that the combined administration of different bioactive compounds acting against complementary targets is a suitable strategy to tackle fatty liver and cardiometabolic risk factors. In line with the idea that better outcomes can be obtained when treatments are performed under a multifaceted approach,38 we hypothesized that a multi-ingredient (MI) supplement based on the combined supplementation of curcumin, hesperidin and pine-tree phytosterols could be an effective approach to ameliorate the multifactorial alterations related to the postmenopausal state. The aim of this study was to evaluate, in an OVX rat model, whether oral supplementation for 8 weeks with an MI supplement including hesperidin (M2SH), phytosterols and curcumin would exert beneficial effects against fat mass accretion, metabolic alterations and osteoporosis associated with menopause. The effects of this MI were compared to those produced by the administration of 17β-E2, an agent that resembles HRT, in the treatment of menopause symptoms and associated diseases.
Materials and methods
Ingredients
Lipophytol®-P (PHY; ref no.: LI0056) was kindly supplied by Lipotec S.A.U. (Gavà, Spain). This PHY is a dispersible form of microencapsulated pine tree phytosterols that facilitates their incorporation in food matrices. According to the supplier, the product contains 88% phytosterols and 10% maltodextrin.Curcumin (CUR; ref no.: EXT-887) is a lipophilic polyphenol [7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione] that was provided by NUTRIFOODS S.L.U. (Barcelona, Spain). According to the supplier, the total curcuminoid content of the dry extract was ≥95%.
Micronized 2S-hesperidin (M2SH; Cardiose®; ref no.: 1306615) was obtained from sweet orange extract (Citrus sinensis) and was kindly provided by HealthTech BioActives – HTBA – (Murcia, Spain). According to the supplier, the S-isomer content of this flavanone was 93%.
Animals, diet and treatments
All procedures related to animal experimentation were approved by the Animal Ethics Committee of the Technological Unit of Nutrition and Health of Eurecat (Reus, Spain) and the Generalitat de Catalunya (protocol code 11223). The study complied with the ARRIVE guidelines, followed the ‘Principles of Laboratory Animal Care’ and was carried out in accordance with the EU Directive 2010/63/EU for animal experiments.The animals used in this study were forty 12-week-old female Sprague-Dawley rats (ENVIGO, Indianapolis, USA). Thirty rats were bilaterally ovariectomized (OVX), and 10 rats were sham-operated (SH) at the ENVIGO facilities. The animals were fed a maintenance rat chow diet for 3 weeks to stabilize them after ovariectomy, before shipment at 15 weeks of age. At their arrival at the animal facility of Eurecat and during the whole study, rats were housed in pairs at 22 °C under a 12 h light/dark cycle (lights on at 9:00 am) and had ad libitum access to food and water. After an adaptation period of 1 week, the 30 OVX rats were distributed into three experimental groups depending on the treatment received for 8 weeks (OVX; OVX-E2; OVX-MI; n = 10 per group), and the 10 sham-operated (SH; n = 10) rats were assigned as the control group. OVX-MI was supplemented daily for 8 weeks with MI, which included three products at the following doses per body weight: PHY at 225 mg kg−1; CUR at 100 mg kg−1; and M2SH at 100 mg kg−1. The three bioactive compounds were dissolved together in low-fat condensed milk diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with water. Taking into account the content of each bioactive compound present in each ingredient, the doses of PHY, CUR and M2SH used were equivalent to the daily consumption of 1926 mg of phytosterols, 924 mg of curcumin and 905 mg of hesperidin for a 60 kg human.39 These dosages are considered acceptable and safe in the context of multi-ingredient supplementation.26,32,40,41 The SH, OVX and OVX-E2 groups were also supplemented daily with low-fat condensed milk, which was diluted 1
3 with water. Taking into account the content of each bioactive compound present in each ingredient, the doses of PHY, CUR and M2SH used were equivalent to the daily consumption of 1926 mg of phytosterols, 924 mg of curcumin and 905 mg of hesperidin for a 60 kg human.39 These dosages are considered acceptable and safe in the context of multi-ingredient supplementation.26,32,40,41 The SH, OVX and OVX-E2 groups were also supplemented daily with low-fat condensed milk, which was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with water (vehicle), for 8 weeks. Both treatments (in a volume of 0.5–0.6 mL) were administered orally by a 1 mL syringe at the same time each day (between 09:00 and 10:00 am). Five days before the beginning of the treatments, the rats were trained to lick diluted low-fat condensed milk (0.3 mL) to ensure voluntary consumption. Each rat fully ingested the daily dose of the corresponding treatment. OVX-E2 rats also received biweekly subcutaneous injections of 25 μg kg−1 17β-E2 (Sigma-Aldrich, St Louis, MO) via a carrier solution of corn oil, whereas the remaining groups received the same dose of a vehicle (corn oil).
3 with water (vehicle), for 8 weeks. Both treatments (in a volume of 0.5–0.6 mL) were administered orally by a 1 mL syringe at the same time each day (between 09:00 and 10:00 am). Five days before the beginning of the treatments, the rats were trained to lick diluted low-fat condensed milk (0.3 mL) to ensure voluntary consumption. Each rat fully ingested the daily dose of the corresponding treatment. OVX-E2 rats also received biweekly subcutaneous injections of 25 μg kg−1 17β-E2 (Sigma-Aldrich, St Louis, MO) via a carrier solution of corn oil, whereas the remaining groups received the same dose of a vehicle (corn oil).
All groups were fed a standard chow diet (Teklad Global 14% Protein Rodent Diet 2014, Harlan, Barcelona, Spain). The caloric breakdown of the diet (2.9 kcal g−1) was 20% protein, 13% fat, and 67% carbohydrates. Body weight was recorded once each week, food was renewed daily, and the food intake was documented every 7 days.
On day 57, rats were sacrificed under anaesthesia (pentobarbital sodium, 60 mg per kg body weight) after 6 h of diurnal fasting. Blood was collected by cardiac puncture, and serum was obtained by centrifugation and stored at −80 °C until analysis. The liver, caecum, kidneys, gastrocnemius and soleus muscle, the femur of the left leg and the white adipose tissue depots (retroperitoneal – RWAT-, mesenteric – MWAT- and inguinal IWAT – white adipose tissue depots) were rapidly removed, weighed, frozen in liquid nitrogen and stored at −70 °C until further analysis. The femur of the right leg was also collected and immersed in physiological serum and frozen at −20 °C until three-dimensional microcomputed tomography analysis.
Body composition analyses
Lean and fat mass measurements (in grams) were performed without anaesthesia at the beginning and at the end of the study using an EchoMRI-700™ device (Echo Medical Systems, L.L.C., Houston, USA). The measurements were performed in triplicate under ad libitum conditions and at 8:00 am, and the results were expressed as a percentage of body weight.Adiposity index
The adiposity index was calculated as the sum of the weights of the IWAT, MWAT and RWAT depot weights (in grams) and was expressed as a percentage of body weight.HOMA-IR and R-QUICKI analyses
The HOMA-IR was calculated using the following formula: (fasting glucose level – mmol L−1 × fasting insulin level – μU mL−1)/22.5.42 Insulin sensitivity was evaluated by the revised quantitative insulin sensitivity check index (R-QUICKI) using the following formula: 1/[log insulin (μU mL−1) + log glucose (mg dL−1) + log FFA (mmol l−1)].43Serum analysis
Enzymatic colorimetric kits were used to determine serum TC (995280/QCA, Barcelona, Spain), HDL-cholesterol (HDL-c) and VLDL-cholesterol + LDL-cholesterol (VLDL-c + LDL-c) (EHDL-100/Bioassay Systems, California, USA), triglycerides (992330/QCA, Barcelona, Spain), glucose (992330/QCA, Barcelona, Spain) and nonesterified free fatty acids (NEFAs) (WAKO, Neuss, Germany). To analyse the levels of 17β-E2 at baseline and progesterone at the end point, the ELISA E2 kit (CSB-E05110r/CUSABIO, Houston, USA) and the ELISA progesterone kit (CSB-E07282r/CUSABIO Houston, USA) were used, respectively. Circulating insulin levels were measured using a rat/mouse ELISA kit (10-1250-01/MERCODIA, Upssala, Sweden). Serum leptin levels were determined with a rat ELISA kit (EZRL-83K/Millipore, Barcelona, Spain), and serum adiponectin levels were quantified by a Rat Total Adiponectin/Acrp30 Quantikine ELISA kit (RRP300/R&Dsystems, Minnesota, USA). To quantify the circulating levels of the biomarkers of bone turnover procollagen I amino-terminal propeptide (PINP), osteocalcin (OC) and carboxy-terminal telopeptide of type I collagen (CTX), the PINP ELISA kit (AC-33F1, IDS, UK), the osteocalcin ELISA kit (AC-12F1, IDS, UK) and the CTX (AC-06F1, IDS, UK) were used, respectively.Oral glucose tolerance test (OGTT)
On day 43, rats were submitted to 6 h of diurnal fasting (from 08:00 am to 02:00 pm). Afterwards, blood was collected from the saphenous vein (baseline point; time 0), and immediately after, the rats were challenged with 2 g per kg body weight of glucose loaded by oral gavage. Blood samples were also collected at 15, 30, 60 and 120 minutes after the glucose load. Glucose and insulin levels at all time-points were analysed using the same kits included in the previous section.Microcomputed tomography analyses
The bone composition of the right femur was determined postmortem by three-dimensional microcomputed tomography (μCT or microCT) analysis. For this purpose, the samples were immersed in physiological serum and frozen at −20 °C. Afterwards, femur samples were fixed in 10% neutral buffered formalin. Comparable regions of interest (ROIs) consisting of the superior epiphysis, the diaphysis and the inferior epiphysis from each rat were selected for analysis. Imaging by three-dimensional microcomputed tomography was performed with a CompaCT scanner (SEDECAL, Madrid, Spain). Data were acquired with 720 projections per 360-degree scan, an integration time of 100 ms with three frames, a photon energy of 50 keV, and a current of 100 μA. The duration of the imaging time was 20 min per scan. Three-dimensional rendered images of femur samples were generated through original volumetric reconstructed images by MicroView software (GE Healthcare). The tissue mineral content (TMC), bone mineral content (BMC), tissue mineral density (TMD) and BMD values were quantified from MicroCT scans using GE MicroView software v2.2. These bone composition results were calculated as the sum of the three ROIs selected for each right femur in nine rats per group.Indirect calorimetry and activity measurements
These analyses were performed between days 37 and 42 after the beginning of the treatments using the Oxylet Pro™ System (PANLAB, Cornellà, Spain), under ad libitum conditions and with free access to water, over a period of 22 h (from 11.00 am to 09.00 am). At 09:00 am, the rats received the corresponding treatment at 09:00 am and were transferred from their cages to an acrylic box (Oxylet LE 1305 Physiocage, PANLAB). After an initial acclimatization period of 2 h, the energy expenditure (EE), respiratory quotient (RQ), fat and carbohydrate oxidation rates, locomotor activity, and number of rearings were calculated as previously described.44Analysis of total, oxidized and reduced glutathione in liver (GSH/GSSG)
Total glutathione (GSH), reduced GSH and oxidized GSH (GSSG) were analysed in the liver samples with the commercial glutathione colorimetric detection kit (EIAGSHC/INVITROGEN; Massachusetts, USA). The results are expressed as μmol g−1 of hepatic protein, which was calculated using the BCA assay method.Histological analysis
Fixed portions of MWAT (n = 8 per group) were preserved in buffered formalin (4% formaldehyde, 4 g L−1 NaH2PO4, 6.5 g L−1 Na2HPO4; pH 6.8) were cut at a thickness of 5 μm and stained with hematoxylin & eosin (H & E). MWAT images (magnification 100×) were taken with a microscope (ECLIPSE Ti; Nikon, Tokyo, Japan) and coupled to a digital sight camera (DS-Ri1, Nikon). Image analyses were performed using the ImageJ NDPI software (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij, accessed on 22 February 2024, version 1.54 g). Adipocyte area quantification in the MWAT sections of the different groups was analysed using the Adiposoft plugin.Gene expression analysis
RNA extraction, cDNA synthesis, and gene expression analyses using real-time quantitative PCR from MWAT were performed as previously described,44 using primers for the different genes obtained from Biomers.net (Ulm, Germany) and included in ESI Table 1.† Each PCR was performed at least in duplicate, and hypoxanthine guanine phosphoribosyl transferase (Hprt) and peptidylprolyl isomerase A (Ppia) were used as reference genes.Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics 28.0 (SPSS, IBM Corp. Armonk, New York, USA). Grubbs’ test was used to detect outliers, which were discarded for further analysis. The assumption of normality was determined using the Kolmogorov–Smirnov test, and the homoscedasticity among groups was evaluated using Levene's test. When one or both of these conditions were not accomplished, data were transformed to base-10 logarithm to obtain a normal distribution and/or similar variances before statistical testing. One-way ANOVA followed by Duncan's post hoc test (p value ≤0.05) was used to assess differences among the four groups in most of the variables analysed in the present study. Welch's test followed by Games-Howell's post hoc test was used when homoscedasticity was not assumed. The Kruskal–Wallis test followed by the Mann–Whitney U post hoc test was used as the nonparametric version of one-way ANOVA when the data did not follow a normal distribution. Linear relationships between key variables were tested using Pearson's correlation coefficients. Differences among groups in the circulating levels of glucose and insulin during the OGTT were analysed by repeated measures (RM-) ANOVA with time as a within-subject factor and intervention as a between-subject factor. Overall differences among groups in the area under the curve (AUC) analysis, which was calculated for the circulating levels of glucose and insulin at all study time points with the trapezoid rule using GraphPad Prism software (GraphPad Software, Inc., La Joya, CA, USA), were assessed using one-way ANOVA and Duncan's post hoc tests. The Mann–Whitney U post hoc t test was used for single statistical comparisons of oestrogen levels at baseline between the SH group (n = 10) and OVX rats (n = 30). Student's t test was also used for some single statistical comparisons to detect residual differences. Data are presented as the means ± SEM (n = 9–10). The level of statistical significance was set at bilateral 5%.Results
Ovariectomy induced oestrogen deficiency, fat mass accretion and metabolic alterations related to the postmenopausal state
After 3 weeks of recovery from surgery, before the beginning of the treatments, the efficacy of ovariectomy was evaluated by analysing the circulating levels of 17β-E2. As expected, the OVX rats displayed significantly lower levels of this hormone than their sham-operated counterparts (467 ± 124 pg mL−1vs. 183 ± 49 pg mL−1; 60.8% lower, p = 0.005, Mann–Whitney U post hoc t test).45Compared to the sham operation performed in SH rats, the removal of the ovaries in the OVX group increased food intake (Table 1) and decreased EE (Fig. 1), which accounted for the higher body weight, weight gain, fat mass percentage, MWAT and IWAT weight, adiposity index and adipocyte hypertrophy in MWAT in comparison with their SH counterparts (Table 1 and Fig. 2). Furthermore, OVX animals also showed higher cholesterol and monocyte chemoattractant protein-1 (MCP-1) circulating levels (Table 1) and a lower hepatic GSH/GSSG ratio than SH animals (Table 2). Altogether, these results indicate that OVX rats developed hallmarks of cardiometabolic alterations that can occur in the postmenopausal state, as was previously reported.46 In addition, compared with SH rats, the OVX animals showed increased circulating levels of the bone biomarkers OC and CTX-1 (for formation and resorption activity, respectively) analysed in serum (Table 3), which accounted for an increased risk of osteoporosis.47
 | ||
| Fig. 1 Effects of ovariectomy, 17β-E2 injections and MI treatment on respiratory quotient (a), carbohydrate oxidation (b), fat oxidation (c), EE (d), spontaneous locomotor activity (e) and number of rearings (f) in sham-operated (SH) and ovariectomized (OVX) rats between days 37 and 42 after the beginning of the treatments. Indirect calorimetric measurements were performed between days 37 and 42 under ad libitum conditions and over 22 h (from 11:00 am to 09:00 am). The data obtained during the first hour were discarded for final analyses. All rats were fed a standard chow diet during the measurements. Data are given as the mean ± SEM (n = 9–10). In Fig. 2d, different superscript lowercase letters (a, b) indicate significantly different mean values among groups (Kruskal–Wallis test and Mann–Whitney U post hoc test, p < 0.05). I: effect of intervention. SH: sham-operated rats, OVX: ovariectomized rats, OVX-E2: OVX rats treated with 17β-oestradiol, OVX-MI: OVX rats supplemented with the multi-ingredient. RQ: respiratory quotient; EE: energy expenditure. | ||
| SHAM | OVX | OVX-E2 | OVX-MI | Intervention effect (p value) | |
|---|---|---|---|---|---|
| Data are given as the mean ± SEM (n = 9–10). Body weight gain was calculated as the difference between the final body weight and the initial body weight. Fat mass gain was calculated as the difference between fat mass at the end of the experiment and the baseline point. Fat mass and lean mass weights (%) were calculated according to the formula (100 × fat or lean/body weight) and were expressed as a percentage of body weight. The lean/fat mass ratio was calculated as the lean mass divided by fat mass. The concentrations of serum parameters were determined at the end of the experiment. Different superscript lowercase letters (a, b, c) indicate significant different mean values (one-way ANOVA and Duncan's post hoc test. Welch test and Games-Howell post hoc test or Kruskal–Wallis test and Mann–Whitney U post hoc test, p < 0.05). I: the effect of intervention. NS: non-significant differences. SH: sham-operated rats; OVX: ovariectomized rats; OVX-E2: OVX rats treated with 17β-oestradiol; OVX-MI: OVX rats supplemented with the multi-ingredient; TG: triglycerides; MCP-1: monocyte chemoattractant protein-1; TC: total cholesterol; HDL-c: high-density lipoprotein cholesterol; VLDL-c: very-low-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol. | |||||
| Cumulative food intake (g) | 94.9 ± 1.2a | 108.4 ± 3.3b | 92.9 ± 2.9a | 106.4 ± 1.6b | I (<0.001) |
| Biometric parameters | |||||
| Initial body weight (g) | 245 ± 3a | 297 ± 5b | 289 ± 6b | 292 ± 3b | I (<0.001) |
| Final body weight (g) | 264 ± 4a | 329 ± 8b | 285 ± 5c | 323 ± 6b | I (<0.001) |
| Body weight gain (g) | 18.5 ± 3.5a | 35.1 ± 3.5b | −4.8 ± 3.2c | 30.6 ± 4.7b | I (<0.001) |
| Final fat mass (%) | 4.55 ± 0.40a | 8.63 ± 0.57b | 6.09 ± 0.70a | 6.02 ± 0.32a | I (<0.001) |
| Fat mass gain (g) | 0.2 ± 0.9a | 12.5 ± 1.1b | 2.7 ± 0.6a | 8.6 ± 0.6c | I (<0.001) |
| Final lean mass (%) | 88.3 ± 0.4a | 84.6 ± 0.6b | 85.9 ± 0.8ab | 87.0 ± 0.3a | I (<0.001) |
| Lean/fat mass ratio | 21.6 ± 2.0a | 10.3 ± 0.9b | 15.8 ± 1.7c | 14.7 ± 0.8c | I (<0.001) |
| Final total body water (%) | 73.1 ± 0.3a | 70.2 ± 0.5b | 71.2 ± 0.7b | 72.5 ± 0.5ab | I (<0.001) |
| Liver (g) | 6.91 ± 0.19 | 7.59 ± 0.29 | 7.72 ± 0.32 | 7.52 ± 0.16 | NS (0.109) |
| Caecum (g) | 2.80 ± 0.29 | 3.18 ± 0.13 | 3.51 ± 0.25 | 3.43 ± 0.15 | NS (0.122) |
| Kidneys (g) | 1.39 ± 0.03 | 1.47 ± 0.04 | 1.50 ± 0.04 | 1.42 ± 0.03 | NS (0.175) |
| Gastrocnemius (g) | 1.88 ± 0.06a | 2.29 ± 0.06b | 2.07 ± 0.11ab | 2.18 ± 0.06b | I (0.007) |
| Soleus (g) | 0.11 ± 0.00a | 0.14 ± 0.01b | 0.13 ± 0.01b | 0.14 ± 0.01b | I (0.006) |
| Serum parameters | |||||
| TG (mmol L−1) | 0.48 ± 0.06a | 0.41 ± 0.06a | 0.89 ± 0.12b | 0.46 ± 0.02a | I (<0.001) |
| MCP-1 (ng mL−1) | 6403 ± 328a | 9373 ± 844b | 9119 ± 1274ab | 9969 ± 850b | I (0.040) |
| TC (mmol L−1) | 2.38 ± 0.09a | 2.90 ± 0.07bc | 2.62 ± 0.10ab | 3.06 ± 0.16c | I (<0.001) |
| HDL-c (mmol L−1) | 1.64 ± 0.09a | 1.98 ± 0.08b | 1.56 ± 0.06a | 2.16 ± 0.12b | I (<0.001) |
| VLDL-c + LDL-c (mmol L−1) | 0.81 ± 0.05 | 0.91 ± 0.03 | 0.91 ± 0.06 | 0.81 ± 0.03 | NS (0.193) |
| Progesterone (pg mL−1) | 159 ± 18 | 101 ± 8 | 116 ± 30 | 109 ± 10 | NS (0.144) |
| SHAM | OVX | OVX-E2 | OVX-MI | Intervention effect (p value) | |
|---|---|---|---|---|---|
| Data are given as the mean ± SEM (n = 9–10). GSSG/GSH ratio was calculated as the oxidized GSH divided by reduced GSH. Different superscript lowercase letters (a, b) indicate significant different mean values (one-way ANOVA and Duncan's post hoc test, Welch test and Games-Howell post hoc test or Kruskal–Wallis test and Mann–Whitney U post hoc test, p < 0.05). I: effect of intervention. NS: non-significant differences. SH: sham-operated rats; OVX: ovariectomized rats; OVX-E2: OVX rats treated with 17β-oestradiol; OVX-MI: OVX rats supplemented with the multi-ingredient. GSH: glutathione; GSSG: oxidized glutathione. | |||||
| Hepatic parameters | |||||
| Total GSH (μmol g−1 protein) | 46.2 ± 9.9ab | 22.9 ± 3.7a | 65.6 ± 9.7b | 25.7 ± 4.9a | I (0.010) |
| Reduced GSH (μmol g−1 protein) | 39.1 ± 9.9ab | 17.5 ± 2.9a | 57.4 ± 9.8b | 15.0 ± 1.4a | I (0.001) |
| Oxidized GSH (μmol g−1 protein) | 7.08 ± 0.74 | 8.09 ± 0.95 | 8.21 ± 0.74 | 8.85 ± 0.53 | NS (0.456) |
| GSH/GSSG ratio | 6.27 ± 1.96a | 2.03 ± 0.40b | 7.88 ± 1.62a | 1.74 ± 0.20b | I (0.004) |
| SHAM | OVX | OVX-E2 | OVX-MI | Intervention effect (p value) | |
|---|---|---|---|---|---|
| Data are given as the mean ± SEM (n = 9–10). The circulating levels of the biomarkers of bone turnover in serum were determined at the end of the experiment. Microcomputed tomography analyses in femur were quantified from MicroCT scans using GE MicroView software v2.2. Different superscript lowercase letters (a, b) indicate significant different mean values (one-way ANOVA and Duncan's post hoc test, Welch test and Games-Howell post hoc test or Kruskal–Wallis test and Mann–Whitney U post hoc test, p < 0.05). I: effect of intervention. NS: non-significant differences. SH: sham-operated rats; OVX: Ovariectomized rats; OVX-E2: OVX rats treated with 17β-estradiol; OVX-MI: OVX rats supplemented with the multi-ingredient; OC: osteocalcin; CTX-1: carboxy-terminal telopeptide of type I collagen; PINP: procollagen I amino-terminal propeptide; BMD: bone mineral density; TMD: tissue mineral density; BMC: bone mineral content; TMC: tissue mineral content. | |||||
| Biometric parameters | |||||
| Femurs (g) | 1.95 ± 0.02a | 2.13 ± 0.06b | 1.93 ± 0.06a | 2.16 ± 0.06b | I (0.004) |
| Femurs (cm) | 3.73 ± 0.04 | 3.81 ± 0.03 | 3.66 ± 0.05 | 3.78 ± 0.04 | NS (0.068) |
| Serum parameters | |||||
| OC (ng mL−1) | 292 ± 11a | 377 ± 16b | 255 ± 13a | 410 ± 27b | I (<0.001) |
| CTX-1 (ng mL−1) | 14.9 ± 1.4a | 20.7 ± 1.2b | 12.7 ± 0.9a | 18.2 ± 2.3ab | I (0.004) |
| PINP (ng mL−1) | 5.85 ± 0.27a | 6.38 ± 0.40a | 4.45 ± 0.45b | 6.16 ± 0.86ab | I (0.039) |
| Microcomputed tomography analyses | |||||
| BMD (mg cc−1) | 634 ± 16 | 618 ± 21 | 666 ± 19 | 623 ± 11 | NS (0.209) |
| TMD (μg cc−1) | 19.3 ± 0.5ab | 18.3 ± 1.0a | 21.2 ± 0.9b | 19.1 ± 0.5ab | I (0.044) |
| BMC (mg) | 569 ± 5 | 591 ± 16 | 547 ± 12 | 570 ± 9 | NS (0.065) |
| TMC (μg) | 11.96 ± 0.06 | 11.94 ± 0.05 | 12.14 ± 0.05 | 11.95 ± 0.09 | NS (0.099) |
MI and 17β-E2 treatments decreased adiposity and adipocyte hypertrophy and promoted a healthier body composition
As expected, 17β-E2 administration significantly reduced the final body weight compared to the OVX animals and fully counteracted the increase in body weight gain observed in the OVX group (Table 1).48 These results could be attributed, at least in part, to the lower cumulative food intake observed in the OVX-E2 animals in comparison with their OVX counterparts (Table 1).45 MI supplementation did not attenuate the increase in body weight and body weight gain observed in OVX animals (Table 1). This lack of effect could be partly related to the similar food intake observed in the OVX-MI rats compared to the OVX group (Table 1). Remarkably, the rats that received the MI supplement or 17β-E2 showed, at the end point, a significant decrease in fat mass compared to the OVX group (Table 1). These results agree with the significant decrease in the weight of all the white adipose tissues analysed (MWAT, RWAT and IWAT, Fig. 2a–c) as well as with the lower adiposity index observed in both the OVX-MI and OVX-E2 groups in comparison with their OVX counterparts (Fig. 2d). Consequently, both the OVX-MI and OVX-E2 groups displayed a significantly lower total fat mass gain than the OVX animals (Table 1). This effect was more evident in the OVX-E2 group, which showed similar levels of fat accretion to those observed in the SH animals (Table 1). Both MI supplementation and 17β-E2 injections fully counteracted the increased MWAT average adipocyte area observed in the OVX group, showing very similar values to those observed in SH rats (Fig. 2e). An inverse pattern was reported for the number of adipocytes, which were significantly lower in the OVX group compared to the other three groups (Fig. 2f). In agreement with these results, representative MWAT histological images (H & E staining) illustrated larger adipocytes and a lower number of these cells in the OVX group (Fig. 2h) than in their SH (Fig. 2g), OVX-E2 (Fig. 2i) and OVX-MI (Fig. 2j) counterparts. Furthermore, a decrease in the percentage of larger adipocytes and an increase of smaller adipocytes were found in the MWAT of both OVX-MI and OVX-E2 animals when compared to the OVX group, showing both groups of treated rats a very similar pattern to those observed in control SH animals (Fig. 2k). Remarkably, OVX-MI rats showed a higher lean mass than OVX animals (Table 1). In line with this result, the OVX-MI group also displayed an increased lean/fat mass ratio in comparison with their OVX counterparts. Although no significant changes were found in the lean mass at the end point in the animals that received 17β-E2 (Table 1), they also displayed a significant increase in the lean/fat mass ratio when compared to the OVX group, similar to what was observed in response to MI administration (Table 1). In addition, both the OVX and OVX-E2 groups showed a significant reduction in the final total body water when compared to the SH animals, while no significant changes were found between the OVX-MI group and their SH counterparts (Table 1).MI supplementation decreased the circulating levels of leptin and increased the circulating levels of adiponectin corrected by the WAT weight and adiponectin-to-leptin ratio
In agreement with the lower final fat accretion and adiposity index shown in both groups in comparison with their OVX counterparts (Table 1 and Fig. 2d), both the OVX-E2 and OVX-MI groups showed a significant decrease in the circulating levels of leptin (Fig. 3a). Although no significant changes among groups were found in the circulating levels of adiponectin (Fig. 3b), the animals supplemented with the MI displayed a significant increase in the circulating adiponectin per g WAT ratio (Fig. 3c) as well as a higher adiponectin-to-leptin (AL) ratio than their OVX counterparts (Fig. 3d). These significant effects were not observed in response to 17β-E2 treatment (Fig. 3c and d).MI intake improved the surrogate marker of insulin sensitivity R-QUICKI
OVX rats displayed similar serum levels of glucose and insulin at baseline and after the glucose load in comparison with their SH counterparts (Fig. 4a and b, respectively). The OGTT performed on day 43 also revealed that neither 17β-E2 injections nor MI supplementation produced significant changes in the circulating levels of glucose and insulin when compared to those in the OVX group (Fig. 4a and b, respectively). | ||
| Fig. 4 Serum levels of glucose (a) and insulin (b) performed on day 43 after an OGTT (2 g kg−1 of body weight) and glucose (c), insulin (d), NEFAs (e), HOMA-IR (f), and R-QUICKI (g) at the end of the study in sham-operated (SH) and ovariectomized (OVX) rats after 8 weeks of intervention. The integrated area under the curve (AUC) was determined for glucose and insulin circulating levels using GraphPad Prism software (GraphPad Software, Inc., La Joya, CA, USA). Glucose (c), insulin (d), NEFAs (e), HOMA-IR (f) and R-QUICKI (g) at the end of the study are also shown. Data are given as the mean ± SEM (n = 9–10). In Fig. 2a and b, the statistical comparisons among groups were conducted using RM-ANOVA or two-way ANOVA, p < 0.05. In Fig. 2c–g, different superscript lowercase letters (a, b, c) indicate significantly different mean values among groups (one-way ANOVA and Duncan's post hoc test, Welch test and Games-Howell post hoc test or Kruskal–Wallis test and Mann–Whitney U post hoc test, p < 0.05). I: effect of intervention. SH: sham-operated rats, OVX: ovariectomized rats, OVX-E2: OVX rats treated with 17β-oestradiol, OVX-MI: OVX rats supplemented with the multi-ingredient. HOMA-IR: homeostasis model assessment-estimated insulin resistance; NEFAs: nonesterified fatty acids; R-QUICKI: revised quantitative insulin sensitivity check index. | ||
No changes among groups were found in the circulating levels of glucose and NEFAs at the endpoint of the study, 57 days after the beginning of the different treatments (Fig. 4c and e, respectively). However, 17β-E2 injections significantly increased the insulin levels and the surrogate marker of insulin resistance HOMA-IR in comparison with their SH and OVX-MI counterparts at the end point (Fig. 4d and f, respectively). Although no significant changes were found either in insulin levels or HOMA-IR among the SH, OVX and OVX-MI groups under one-way ANOVA, MI intervention for 57 days residually decreased HOMA-IR (26.3% lower, p = 0.043 versus OVX rats, Student's t test) and circulating insulin levels (27.5% lower, p = 0.084 versus OVX rats, Student's t test) and produced a significant increase in the R-QUICKI value compared to that in the OVX and OVX-E2 animals, which suggests an improvement in insulin sensitivity (Fig. 4g). In contrast, the OVX-E2 group displayed a significant decrease in R-QUICKI in comparison with the SH and OVX-MI animals (Fig. 4g).
MI and 17β-E2 treatments did not enhance energy expenditure, substrate oxidation or locomotor activity
No significant changes were found in RQ among the groups (Fig. 1a). Consequently, no changes in carbohydrate and fat oxidation were reported (Fig. 1b and c, respectively). Both the OVX and OVX-MI groups showed a significant decrease in EE when compared to their SH counterparts, considering both the whole period of 21 hours and the light phase (Fig. 1d). A similar pattern was observed for the animals that received 17β-E2, although the differences were not statistically significant when compared to the SH animals (Fig. 1d). There was a strong positive correlation between EE and fat oxidation (r = 0.659, p < 0.001), which was not found between EE and the oxidation of carbohydrates. This result suggests that the decreased EE found in the OVX groups compared to their SH counterparts was mainly due to their lower fat oxidation. The decreased EE observed in the OVX and OVX-MI animals cannot be attributed to lower locomotor activity since no changes were found either in this parameter (Fig. 1e) or in the number of rearings (Fig. 1f).MI supplementation did not produce the deleterious effects on HDL-c and TG observed in response to 17β-E2 treatment
17β-E2 injections residually decreased the circulating levels of TC (p = 0.043 versus OVX rats, Student's t test), although the differences between these two groups were not statistically significant according to one-way ANOVA (Table 1). This result was mainly explained by the significant decrease in HDL-c serum levels observed in OVX-E2 animals compared to their OVX counterparts (Table 1). MI intake fully counteracted the decrease in HDL-c reported in OVX-E2 rats (Table 1). Furthermore, MI supplementation slightly decreased the VLDL-c + LDL-c serum cholesterol levels in comparison with OVX rats, although the differences were not statistically significant (11% decrease; p = 0.055 versus OVX animals, Student's t test) (Table 1). In addition, the circulating TG levels in serum were significantly higher in OVX-E2 rats than in their OVX-MI counterparts (Table 1).17β-E2 treatment increased hepatic GSH levels
Significantly higher hepatic levels of total and reduced GSH were observed in OVX-E2 rats than in OVX and OVX-MI rats, attaining numerically higher levels in OVX-E2 animals than in their SH counterparts (Table 2). Although no changes among groups were found in oxidized GSH (Table 2), the animals treated with oestrogens also displayed a significant increase in the GSH/GSSG ratio, showing similar levels of this parameter as the SH rats (Table 2).17β-E2 injections fully counteracted the increase in biochemical markers of bone turnover induced by ovariectomy
The OVX and OVX-MI groups showed an increase in femur weight (Table 3) compared to that in SH and OVX-E2 animals. No significant changes were found in femur length, BMD, BMC or TMC among the groups (Table 3). Nevertheless, the OVX-E2 group displayed a significant increase in TMD when compared to their OVX counterparts (Table 3). 17β-E2 administration counteracted the increase observed in the OVX animals concerning the circulating levels of the biomarkers of bone formation and resorption activity, OC and CTX-1, an effect that was not observed after MI supplementation (Table 3). In addition, OVX-E2 animals showed a significant decrease in the circulating levels of PINP, a biomarker of bone formation, in comparison with both the SH and OVX groups (Table 3).Both MI and 17β-E2 treatments led to a downregulation of genes associated with fatty acid uptake in MWAT
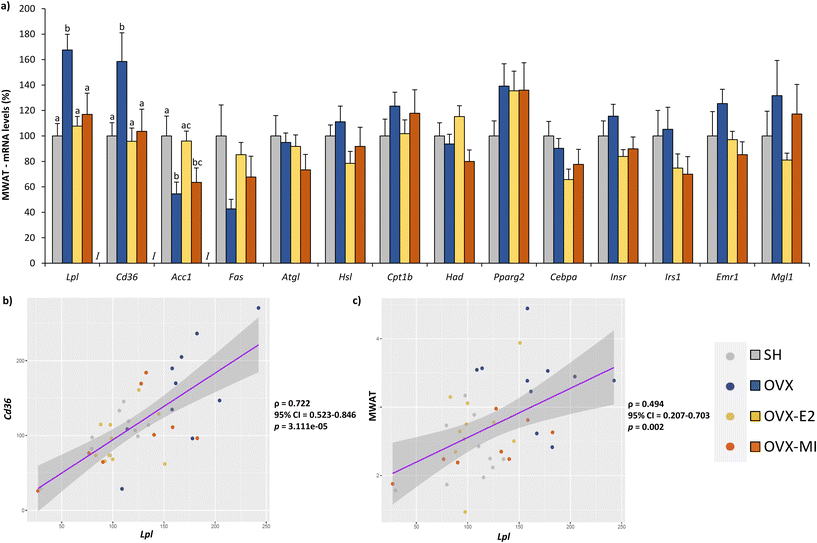
The mRNA levels of the key genes involved in lipid uptake, Lpl and Cd36, were significantly upregulated in the MWAT of the OVX group compared to their control SH counterparts (Fig. 5a). Supplementation with MI and treatment with oestradiol fully counteracted this increase (Fig. 5a). Furthermore, regarding the expression levels of Acc1, a gene involved in fatty acid synthesis, the 17β-E2 group exhibited higher mRNA levels of Acc1 compared to their OVX counterparts and displayed similar expression levels of this gene to what was observed in the SH group (Fig. 5a). Compared to their OVX counterparts, the OVX-MI and OVX-E2 groups showed no changes in the expression levels of the key genes related to lipogenesis (Fas), lipolysis (Atgl and Hsl), β-oxidation (Cpt1b and Had) and insulin signalling (Insr and Irs1) (Fig. 5a). The mRNA levels of Cd36 and Lpl showed a strong positive correlation (r = 0.722, 95% CI = 0.523–0.846, p < 0.0001) (Fig. 5b). Furthermore, MWAT weight was also positively correlated with Lpl gene expression values in this tissue (r = 0.494, 95% CI = 0.207–0.703, p = 0.002) (Fig. 5c). Although no significant changes were found among the four groups in the mRNA levels of the master regulator of adipogenesis and adipose tissue function,49Cebpa, 17β-E2 injections residually decreased the expression levels of this gene in comparison with the OVX animals (27.1% decrease; p = 0.026, Student's t test) (Fig. 5a). Furthermore, a very similar pattern of expression was observed for the pro-inflammatory gene Emr1, which encodes the F4/80 antigen, a marker of M1 classical activated macrophages,50 in both OVX-E2 (22.6% lower; p = 0.038 versus OVX animals, Student's t test) and OVX-MI (32.0% lower; p = 0.027 versus OVX animals, Student's t test), suggesting a slight anti-inflammatory effect of both treatments in MWAT (Fig. 5a).Discussion
Recently, there has been increasing interest in finding therapies that are alternative or complementary to HRT in order to address undesired menopausal symptoms and associated metabolic disorders. In this regard, several lines of research suggest that the intake of hesperidin, phytosterols and curcumin may be beneficial for the management of these complications. Nevertheless, no studies thus far have elucidated whether, in agreement with our hypothesis that more healthy effects can be obtained using ingredients acting against complementary targets, the combined supplementation of these three bioactive compounds can be a potential alternative therapy to address menopause. In this research, we found oral treatment with a MI supplement, including curcuminoids, phytosterols and hesperidin, for 57 days was able to exert beneficial effects against obesity, and metabolic alterations were observed in a preclinical model of a postmenopausal state with oestrogen deficiency, namely, OVX rats, including (1) decreased fat mass accretion, (2) diminished adipocyte size and promoted a shift towards decreased number of large adipocytes and increased number of small adipocytes in MWAT, (3) downregulated mRNA levels of key genes involved in fatty acid uptake from the bloodstream (Lpl and Cd36) in MWAT, (4) enhanced lean body mass and lean/fat mass ratio, (5) increased the AL ratio and (6) an improved R-QUICKI value, the surrogate marker for insulin sensitivity. These results confirmed our hypothesis, and interestingly, most of them were very similar or even superior to what we reported in response to 17β-E2 administration.Many women at menopause experience changes in body composition and body fat redistribution, particularly fat accretion in the abdominal region, which could lead to the appearance of metabolic disturbances, increasing the risk of metabolic syndrome (MetS) and CVDs.6,7 These effects are more pronounced in women who are overweight or obese prior to reaching the postmenopausal state.6 The lower adiposity observed in OVX-MI rats that were supplemented with the MI when compared to their OVX counterparts demonstrated herein could be attributed, at first glance, to the protective effect against obesity that has been previously reported for different polyphenols, including curcumin and hesperidin, and, to a lesser extent, for phytosterols, in both animal models and humans.23,24,27,51,52 Nevertheless, to the best of our knowledge, there are few data available concerning the anti-obesity effects of these 3 bioactive compounds administered alone or in combination in OVX rodent models. In this regard, only one study has demonstrated that the oral supplementation of curcumin for 30 days significantly decreases intestinal adiposity accumulation in OVX rats.23 The protective effects of hesperidin and phytosterols orally administered to OVX rodents against fat accretion have not previously been reported. Several studies have demonstrated that the significant body weight gain and progressive fat accretion in different body areas occurring in menopausal and postmenopausal women can be related to a decreased EE.53–55 This effect and a significant increase in food intake were observed in the present study in OVX rats, one of the best characterized and described animal models to resemble postmenopausal women, when compared to their SH counterparts. In the present study, it was plausible to speculate that supplementation with MI could partly counteract fat mass gain through the enhancement of EE and the inhibition of food intake, two very well-reported mechanisms by which different bioactive compounds can exert this beneficial effect.54 In this regard, Receno et al. showed that the prolonged intake of curcumin in aged male F344Xbn rats decreased food intake.56 Curcumin was also able to enhance EE and to stimulate thermogenesis efficiently by enhancing the activity of AMP-activated protein kinase (AMPK) and increasing ATP production.57,58 Nevertheless, the lack of effects on EE and food intake observed after treatment with this MI supplement would rule out these two mechanisms to explain the anti-obesity effects reported in OVX-MI rats.
Another potential mechanism involved in the antiobesity effects of bioactives is the regulation of lipid metabolism. The lower adiposity found in the OVX-MI group could be related to the fact that MI supplementation fully counteracted the overexpression of the Lpl and Cd36 genes in MWAT caused by ovariectomy. Cd36 encodes a cell surface receptor known as fatty acid translocase (FAT), which is responsible for long-chain fatty acid uptake from the bloodstream.59 On the other hand, although the main function of LPL is to hydrolyse the TG core of circulating TG-rich lipoproteins, tissue-specific regulation of Lpl provides a mechanism for localized control of the uptake of lipoprotein lipids that results in a physiologically appropriate distribution of lipids among tissues.59 In WAT, Lpl is a marker for adipocyte differentiation, and Lpl expression increases in parallel with cellular TG accumulation as preadipocytes differentiate, thus contributing to the growth and expansion of adipose tissue.60,61 Therefore, our results suggest that a lower fatty acid uptake and storage in white adipose tissue, evidenced by decreased MWAT gene expression levels of Cd36 and Lpl, could be one of the mechanisms to explain the lower fat mass observed in OVX-MI rats compared to their OVX counterparts. The diminished adipocyte area reported in the MWAT in response to MI supplementation, which indicated decreased adipocyte hypertrophy, would be in agreement with this idea. In agreement with the results reported here, Mosqueda-Solís et al. showed that the administration of hesperidin (100 mg per kg per day) for 8 weeks decreased the gene expression levels of Lpl in RWAT in male Wistar rats fed a hypercaloric western diet, which resulted in a nonsignificant reduction in this adipose tissue depot.62 In addition, three different studies demonstrated that curcumin supplementation attenuated the overexpression of the hepatic mRNA levels of Cd36 in a high-fat-diet-fed C57BL/6J mouse model, which was accompanied by an inhibitory effect of curcumin on hepatic lipid accumulation.63–65
The antiadiposity effect observed in the rats treated with the MI supplement was very similar to what we reported in response to 17β-E2 injections. Interestingly, both nutritional and pharmacologic treatments seemed to share a common antiadiposity mechanism, namely, decreased expression of the Lpl and Cd36 genes. The strong positive correlation found between the mRNA levels of both genes found in the present study suggests a potential coregulation mechanism, emphasizing the coordinated role of Cd36 and LPL in fatty acid uptake and storage. Furthermore, the positive correlation between Lpl mRNA levels and MWAT weight would also be in agreement with the key role of this gene in the growth and expansion of WAT. Therefore, although correlations do not imply causality, these results contribute to supporting our hypothesis.
The decreased food intake observed in OVX-E2 animals, which was previously reported,66,67 is another mechanism that helps explain the lower fat mass accretion triggered by oestradiol administration, which also produced a clear decrease in body weight, different from what was observed in response to supplementation with MI. The increased lean mass found in OVX-MI rats when compared to their OVX counterparts could explain, at least in part, why the decreased adiposity observed after MI supplementation was not accompanied by lower body weight gain. Lean mass, which was calculated by quantitative magnetic resonance, provides an accurate measurement of muscle mass.68 Therefore, the higher lean body mass observed in MI animals might also be the result of muscle mass accretion and would also contribute to the higher lean/fat mass ratio found in these rats and in OVX-E2 animals, suggesting a healthier body composition in both groups.69,70
As was previously reported, the lower adiposity found in OVX-MI and OVX-E2 rats was translated into significantly lower circulating levels of leptin, which are highly and positively related to body fat mass, in both individuals with normoweight and obesity.71–73 Although the anti-obesity effects produced by both the nutraceutical and drug treatments were not translated to increased levels of the anti-inflammatory and insulin sensitivity-related adipokine adiponectin in the bloodstream,72,73 remarkably, only MI supplementation significantly increased adiponectin plasma levels corrected by WAT weight, a surrogate marker of effective adiponectin production.74–76 Curcumin administration has been shown in vitro and in animal models of obesity and type 2 diabetes mellitus (T2DM), as well as in humans with obesity, MetS, and/or T2DM to significantly ameliorate diabetes, to increase adiponectin production by adipose tissue and to decrease the serum levels of leptin.17,76–80 Several publications have described the AL ratio as a biomarker of adipose tissue dysfunction and insulin resistance.81–83 The AL ratio has been better correlated with insulin resistance and insulin sensitivity than adiponectin, leptin, HOMA-IR and R-QUICKI and is clinically useful to identify subjects susceptible to cardiometabolic diseases.81–84 An AL ratio ≥1.0 is considered normal, while a ratio between ≥0.5 and <1.0 suggests moderate-to-medium increased risk, and a ratio <0.5 indicates a severe risk of suffering from cardiometabolic diseases.81,82 Remarkably, in this report, the OVX group exhibited an AL ratio close to 0.5, indicating a medium to high cardiometabolic risk, and this ratio was increased to 1 in response to MI supplementation.81,82 These findings strongly suggest that the combined intake of hesperidin, phytosterols and curcumin improved metabolic health in the OVX-MI group. The 27.5% and 26.3% decrease in the circulating levels of insulin and HOMA-IR, respectively, and the significant increase in the surrogate marker of insulin sensitivity R-QUICKI found in OVX-MI rats when compared to their OVX counterparts would reinforce this idea. The inclusion of curcumin in the MI supplement may explain, at least in part, the aforementioned effects observed in OVX-MI. In this regard, in humans, it was shown that after 9 months of curcumin treatment, none of the subjects were diagnosed with T2DM, and they exhibited significantly lower HOMA-IR levels,17 indicating improved insulin resistance. Additionally, hesperidin could have contributed to the insulin-sensitivity effects reported in OVX-MI rats since two studies showed significant reductions in insulin and HOMA-IR levels after the consumption of orange juice rich in hesperidin.85,86 However, these insulin-related effects were not observed in response to oestradiol injections,87 as occurred in our study in OVX-E2 animals. Altogether, our results underscore the potential of MI supplementation as a natural therapy to improve insulin sensitivity in postmenopausal women, although further randomized controlled trials are needed to shed more light on this issue.
In the present study, MI supplementation exerted slight beneficial effects on cholesterol metabolism, which were evidenced by significantly counteracting the decrease in HDL-c serum cholesterol concentrations observed in OVX-E2 animals. Different studies have reported that rats are not the most appropriate animal model for diseases related to cholesterol alterations88–90 since they efficiently eliminate excess cholesterol through bile acid synthesis, imparting a high resistance to cholesterol accumulation in the body, unlike what happens with other animal models such as Golden Syrian hamsters.88–90 This consideration (and the fact that, in this study, rats were not fed a high-fat-high-cholesterol diet) could help to explain the aforementioned results and the lower beneficial effects observed in this study compared to the one carried out by our group with Golden Syrian hamsters fed a hyperlipidic diet, in which phytosterols significantly decreased both TC and VLDL-c + LDL-c.91 Further studies carried out with OVX rats fed a high-fat-high-cholesterol diet would be useful to gain insight into the potential anti-hypercholesterolaemic effect of MI.
Many studies carried out in both OVX rodents and postmenopausal women have demonstrated that oestrogen deficiency induces bone loss and osteoporosis, which is mainly characterized by decreased BMD5,47 and a significant increase in bone turnover (both formation and resorption) markers.30,47,92 These bone-related alterations are normally counteracted or ameliorated after HRT treatment.12,93 In the present study, 17β-E2 administration counteracted the increase in the circulating levels of both bone resorption (CTX-1) and bone formation (PINP and OC) turnover markers displayed by OVX animals, an effect that was not observed in rats receiving MI supplementation. However, no significant changes in femur BMD among groups were reported, which could be attributed to the young age of the rats and to the fact that, in rats, in the short term, the femur is more resistant to bone loss and BMD-related alterations than the tibia. Therefore, further studies in which the proximal tibia is collected and analysed with rats aged between 6–9 months at the time of surgery, which have a lower bone growth rate and a significantly more pronounced alteration in trabecular bone remodelling compared to younger rats,47 would be very useful to gain more knowledge on this issue.
In agreement with what was previously described for sex steroids, such as oestrogen, which has a well-known antioxidant activity reducing the production of reactive oxygen species,94,95 in our study, 17β-E2 treatment fully counteracted the decrease in total and reduced GSH as well as in the GSSG/GSH ratio levels observed in OVX animals. The anti-inflammatory and antioxidant properties of the polyphenols curcumin and hesperidin have been well reported.17,27,96 However, in this study, we observed that MI supplementation was not able to prevent the increase in the inflammation marker MCP-1 and counteract the well-described GSH redox impairment associated with ovariectomy surgery.94,97–99 This lack of effects could be tentatively attributed, at least in part, to the lower bioavailability of curcumin, since it has been described that the intestinal absorption of curcumin is poor and there is a high rate of metabolism and rapid systemic degradation of this bioactive compound in the body.100–102 To enhance the absorption and health effects of curcumin, several studies focused on loading curcumin into capsules17,103,104 and therefore, microencapsulation would be a potential next step to take into account in further studies.
The present study has some limitations. In this regard, it would have been interesting to compare the effects of the supplementation of the three bioactive compounds included in the MI (hesperidin, phytosterols and curcumin) separately to elucidate whether the combination of these ingredients exerted a synergic or an additive effect. In addition, our molecular analyses were limited to gene expression data in MWAT, which were useful to shed light on some of the potential mechanisms by which the MI exerted the reporting effects, but they do not always match protein levels. Therefore, additional research focused on protein levels and/or activity of key players involved in lipolysis (ATGL and HSL) and/or β-oxidation (CPT1B and HAD) in MWAT would have been useful to elucidate whether the effects of the MI against fat accretion and adipocyte hypertrophy were mediated, at least in part, by the activation of these lipid catabolic pathways. Finally, immunohistochemical staining to evaluate macrophage infiltration in MWAT and molecular analyses in other tissues that play a key role in lipid and glucose metabolism, such as liver, would have been of great value to gain more insight into the beneficial effects produced by MI supplementation.
Conclusions
We demonstrated here that combined supplementation with hesperidin, phytosterols and curcumin reduced fat accretion and adipocyte hypertrophy, and improved the body composition in OVX rats. The lower mRNA levels of fatty acid uptake-related genes (Lpl and Cd36) in the visceral MWAT may have accounted for the beneficial antiadiposity effects of MI supplementation. These effects were accompanied by a significant decrease in the circulating levels of leptin, an increase in the AL ratio and an improvement in insulin sensitivity, suggesting an enhancement of the metabolic health of the MI-supplemented rats. As far as we know, this is the first study evidencing that the combination of hesperidin, phytosterols and curcumin exerted such effects and that these effects were very similar or in some cases even better to what was observed in response to oestradiol administration, a treatment that resembles one of the HRTs given to menopausal women. These results pave the way to promote the use of this MI supplement to tackle obesity and to improve the metabolic health of menopausal women. Further randomized controlled clinical trials carried out with this target population supplemented with this MI formulation would be of great value to shed more light on this issue.Abbreviations
| 17β-E2 | 17β-Oestradiol |
| Acc1 | Acetyl-CoA carboxylase 1 |
| AL ratio | Adiponectin/leptin ratio |
| ANOVA | Analysis of variance |
| Atgl | Adipose triacylglycerol lipase |
| AUC | Area under the curve |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| Cd36 | Fatty acid translocase, homologue of CD36 |
| Cebpa | CCAAT/enhancer binding protein alpha |
| Cpt1b | Carnitine palmitoyltransferase 1 beta |
| CTX-1 | Carboxy-terminal telopeptide of type I collagen |
| CUR | Curcumin |
| CVDs | Cardiovascular diseases |
| EE | Energy expenditure |
| Fas | Fatty acid synthase |
| Emr1 | EGF-like module containing, mucin-like, hormone receptor-like 1 |
| ER | Oestrogen receptor |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| Had | Hydroxyacyl-CoA dehydrogenase |
| HDL-c | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostasis model assessment-estimated insulin resistance |
| Hprt | Hypoxanthine guanine phosphoribosyl transferase |
| HRT | Hormone replacement therapy |
| Hsl | Hormone-sensitive lipase |
| Insr | Insulin receptor |
| IR | Insulin resistance |
| Irs1 | Insulin receptor substrate 1 |
| IWAT | Inguinal white adipose tissue |
| LDL-c | Low-density lipoprotein cholesterol |
| Lpl | Lipoprotein lipase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| Mgl1 | C-type lectin domain family 10, member A |
| MI | Multi-ingredient |
| MWAT | Mesenteric white adipose tissue |
| M2SH | Micronized 2-S-hesperidin |
| NEFAs | Nonesterified fatty acids |
| OC | Osteocalcin |
| OGTT | Oral glucose tolerance test |
| OVX | Ovariectomized rats |
| PHY | Phytosterol |
| PINP | Procollagen I amino-terminal propeptide |
| Pparg2 | Peroxisome proliferator-activated receptor gamma 2 |
| Ppia | Peptidylprolyl isomerase A |
| ROI | Regions of interest |
| RQ | Respiratory quotient |
| R-QUICKI | Revised quantitative insulin sensitivity check index |
| RWAT | Retroperitoneal white adipose tissue |
| SH | Sham-operated rats |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| TMC | Tissue mineral content |
| TMD | tissue mineral density |
| VLDL-c | Very-low-density lipoprotein cholesterol. |
Author contributions
Julio Baudin (JB), Francesc Puiggròs (FP), Lluís Arola (LA) and Antoni Caimari (AC) designed the research; JB, Julia Hernandez-Baixauli (JHB), Sergio Quesada-Vázquez (SQV), Francisca Mulero (FM) and AC conducted the research and analysed the data; JB and AC wrote the manuscript. Funding acquisition was conducted by FM, FP, LA and AC. All the authors read, reviewed and approved the final manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The research described here was financially supported by the Agency for Business Competitiveness of the Government of Catalonia (ACCIÓ/TECCT11-1-0012) and by Comunidad de Madrid (S2022/BMD-7403 RENIM-CM).Julio Baudin, Julia Hernandez-Baixauli and Sergio Quesada were supported by the Vicente López fellowship granted by Eurecat.
We gratefully acknowledge the help given by Dr Juan Maria Alcaide-Hidalgo and Dr Xavier Escoté, researchers at the Nutrition and Health Unit (Eurecat, Reus, Spain) to carry out the study at the animal facility and to perform the histological analyses in MWAT, respectively. We also appreciate the assistance provided by Yaiza Tobajas, Iris Triguero, Anna Antolin, Cristina Egea and Gertruda Chomiciute, laboratory technicians at the Nutrition and Health Unit (Eurecat, Reus, Spain). Moreover, we would like to thank the PhD Students Irene Bravo and David Sabador at the National Cancer Research Center (CNIO) for their support in the microcomputed tomography analyses.
We thank the companies Lipotec S.A.U. and HealthTech BioActives – HTBA – for kindly providing Lipophytol®-P (PHY; ref no.: LI0056) and Micronized 2S-Hesperidin (M2SH; Cardiose®; ref no.: 1306615), respectively.
References
- N. Santoro and J. F. Randolph, Reproductive Hormones and the Menopause Transition, Obstet. Gynecol. Clin. North Am., 2011, 38, 455–466 CrossRef PubMed.
- M. A. McNeil and S. B. Merriam, Menopause, Ann. Intern. Med., 2021, 174, ITC97–IT112 CrossRef PubMed.
- R. C. Thurston, B. D. Johnson, C. L. Shufelt, G. D. Braunstein, S. L. Berga and F. Z. Stanczyk, et al., Menopausal symptoms and cardiovascular disease mortality in the Women's Ischemia Syndrome Evaluation (WISE), Menopause, 2017, 24, 126–132 CrossRef PubMed.
- S. D. Harlow, M. Gass, J. E. Hall, R. Lobo, P. Maki and R. W. Rebar, et al., Executive Summary of the Stages of Reproductive Aging Workshop + 10: Addressing the Unfinished Agenda of Staging Reproductive Aging, J. Clin. Endocrinol. Metab., 2012, 97, 1159–1168 CrossRef CAS PubMed.
- S. Thapa, A. Nandy and E. Rendina-Ruedy, Endocrinal metabolic regulation on the skeletal system in post-menopausal women, Front. Physiol., 2022, 13, 1–10 Search PubMed.
- A. Fenton, C. Smart, L. Goldschmidt, V. Price and J. Scott, Fat mass, weight and body shape changes at menopause – causes and consequences: a narrative review, Climacteric, 2023, 26, 381–387 CrossRef CAS PubMed.
- H. Juppi, S. Sipilä, V. Fachada, M. Hyvärinen, N. Cronin and P. Aukee, et al., Total and regional body adiposity increases during menopause—evidence from a follow–up study, Aging Cell, 2022, 21, 1–17 CrossRef PubMed.
- G. M. C. Rosano, C. Vitale, G. Marazzi and M. Volterrani, Menopause and cardiovascular disease: the evidence, Climacteric, 2007, 10, 19–24 CrossRef CAS PubMed.
- E. Kapoor, M. L. Collazo-Clavell and S. S. Faubion, Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management, Mayo Clin. Proc., 2017, 92, 1552–1558 CrossRef PubMed.
- V. R. Mesch, L. E. Boero, N. O. Siseles, M. Royer, M. Prada and F. Sayegh, et al., Metabolic syndrome throughout the menopausal transition: influence of age and menopausal status, Climacteric, 2006, 9, 40–48 CrossRef CAS PubMed.
- The European Society of Cardiology. Understanding the burden of CVD. 2023. https://www.escardio.org/The-ESC/Advocacy/understanding-the-burden-of-cvd-facts-and-figures (accessed September 2023).
- J. Mehta, J. M. Kling and J. E. Manson, Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts, Front. Endocrinol., 2021, 12, 1–14 Search PubMed.
- P. Thangavel, A. Puga-Olguín, J. F. Rodríguez-Landa and R. C. Zepeda, Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases, Molecules, 2019, 24, 3892 CrossRef CAS PubMed.
- J. Sharifi-Rad, C. Quispe, M. Imran, A. Rauf, M. Nadeem and T. A. Gondal, et al., Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits, Oxid. Med. Cell. Longevity, 2021, 2021, 3268136 Search PubMed.
- A. M. P. Romani, The controversy on the beneficial effect of phytoestrogens in diabetic treatment in postmenopausal women, Biochem. Pharmacol., 2021, 190, 114619 CrossRef CAS PubMed.
- A. B. Kunnumakkara, D. Bordoloi, G. Padmavathi, J. Monisha, N. K. Roy and S. Prasad, et al., Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases, Br. J. Pharmacol., 2017, 174, 1325–1348 CrossRef CAS PubMed.
- S. Chuengsamarn, S. Rattanamongkolgul, R. Luechapudiporn, C. Phisalaphong and S. Jirawatnotai, Curcumin Extract for Prevention of Type 2 Diabetes, Diabetes Care, 2012, 35, 2121–2127 CrossRef CAS PubMed.
- A. Sophocleous and A. I. Idris, Ovariectomy/Orchiectomy in Rodents, in Methods in molecular biology, ed. A. I. Idris, Springer, New York, 3rd edn, 2019, vol. 1914, 13, pp. 261–267 Search PubMed.
- V. R. Shouza, E. Mendes, M. Casaro, A. T. F. B. Antiorio, F. A. Oliveira and C. M. Ferreira, Description of Ovariectomy Protcol in Mice, in Methods in molecular biology, ed. P. C. Guest, Springer, New York, 2nd edn, 1916, vol. 29, pp. 303–309 Search PubMed.
- S. V. Koebele and H. A. Bimonte-Nelson, Modeling menopause: The utility of rodents in translational behavioral endocrinology research, Maturitas, 2016, 87, 5–17 CrossRef CAS PubMed.
- R. Diaz Brinton, Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities, Endocrinology, 2012, 153, 3571–3578 CrossRef PubMed.
- W. K. Kim, K. Ke, O. J. Sul, H. J. Kim, S. H. Kim and M. H. Lee, et al., Curcumin protects against ovariectomy–induced bone loss and decreases osteoclastogenesis, J. Cell. Biochem., 2011, 112, 3159–3166 CrossRef CAS PubMed.
- M. D. S. Morrone, C. E. Schnorr, G. A. Behr, J. Gasparotto, R. C. Bortolin and K. da Boit Martinello, et al., Curcumin Supplementation Decreases Intestinal Adiposity Accumulation, Serum Cholesterol Alterations, and Oxidative Stress in Ovariectomized Rats, Oxid. Med. Cell. Longevity, 2016, 2016, 1–12 CrossRef PubMed.
- A. S. Khorasanian, S. T. Fateh, F. Gholami, N. Rasaei, H. Gerami and S. S. Khayyatzadeh, et al., The effects of hesperidin supplementation on cardiovascular risk factors in adults: a systematic review and dose–response meta-analysis, Front. Nutr., 2023, 10, 1177708 CrossRef PubMed.
- R. M. Valls, A. Pedret, L. Calderón-Pérez, E. Llauradó, L. Pla-Pagà and J. Companys, et al., Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study), Eur. J. Nutr., 2021, 60, 1277–1288 CrossRef CAS PubMed.
- A. Mas-Capdevila, J. Teichenne, C. Domenech-Coca, A. Caimari, J. M. Del Bas and X. Escoté, et al., Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability, Nutrients, 2020, 12, 1488 CrossRef CAS PubMed.
- H. Xiong, J. Wang, Q. Ran, G. Lou, C. Peng and Q. Gan, et al., Hesperidin: A Therapeutic Agent For Obesity, Drug Des., Dev. Ther., 2019, 13, 3855–3866 CrossRef CAS PubMed.
- A. d. C. Ortiz, S. O. M. Fideles, C. H. B. Reis, M. Z. Bellini, E. d. S. Pereira and J. P. G. Pilon, et al., Therapeutic Effects of Citrus Flavonoids Neohesperidin, Hesperidin and Its Aglycone, Hesperetin on Bone Health, Biomolecules, 2022, 12, 626 CrossRef CAS PubMed.
- D. Lim, Y. Lee and Y. Kim, Preventive Effects of Citrus unshiu Peel Extracts on Bone and Lipid Metabolism in OVX Rats, Molecules, 2014, 19, 783–794 CrossRef PubMed.
- M. N. Horcajada, V. Habauzit, A. Trzeciakiewicz, C. Morand, A. Gil-Izquierdo and J. Mardon, et al., Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats, J. Appl. Physiol., 2008, 104, 648–654 CrossRef CAS PubMed.
- A. Crescenti, A. Caimari, J. M. Alcaide-Hidalgo, R. Mariné-Casadó, R. M. Valls and J. Companys, et al., Hesperidin Bioavailability Is Increased by the Presence of 2S-Diastereoisomer and Micronization—A Randomized, Crossover and Double-Blind Clinical Trial, Nutrients, 2022, 14, 2481 CrossRef CAS PubMed.
- S. Opinion, Scientific Opinion on the substantiation of a health claim related to 3 g/day plant sterols/stanols and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 19 of Regulation (EC) No 1924/2006, EFSA J., 2012, 10, 2693 CrossRef.
- W. Xia, S. Xiang, M. Gaman, P. Jamilian, K. Prabahar and G. Du, et al., The effects of phytosterol and phytostanol supplementation on the lipid profile in postmenopausal women: A systematic review and meta–analysis of randomized controlled trials, Phyther. Res., 2022, 36, 4398–4408 CrossRef CAS PubMed.
- S. Quesada-Vázquez, A. Antolín, M. Colom-Pellicer, G. Aragonès, L. Herrero and J. M. Del Bas, et al., Reduction of Obesity and Insulin Resistance through Dual Targeting of VAT and BAT by a Novel Combination of Metabolic Cofactors, Int. J. Mol. Sci., 2022, 23, 14923 CrossRef PubMed.
- J. Companys, L. Calderón-Pérez, L. Pla-Pagà, E. Llauradó, B. A. Sandoval-Ramirez and M. J. Gosalbes, et al., Effects of enriched seafood sticks (heat-inactivated B. animalis subsp. lactis CECT 8145, inulin, omega-3) on cardiometabolic risk factors and gut microbiota in abdominally obese subjects: randomized controlled trial, Eur. J. Nutr., 2022, 61, 3597–3611 CrossRef CAS PubMed.
- S. Quesada-Vázquez, M. Colom-Pellicer, È. Navarro-Masip, G. Aragonès, J. M. Del Bas and A. Caimari, et al., Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice, Nutrients, 2021, 13, 3532 CrossRef PubMed.
- H. Yang, J. Mayneris-Perxachs, N. Boqué, J. M. del Bas, L. Arola and M. Yuan, et al., Combined Metabolic Activators Decrease Liver Steatosis by Activating Mitochondrial Metabolism in Hamsters Fed with a High-Fat Diet, Biomedicines, 2021, 9, 1440 CrossRef CAS PubMed.
- M. Suárez, N. Boqué, J. del Bas, J. Mayneris-Perxachs, L. Arola and A. Caimari, Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease, Nutrients, 2017, 9, 1052 CrossRef PubMed.
- S. Reagan–Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2008, 22, 659–661 CrossRef PubMed.
- Y. Li, A. D. Kandhare, A. A. Mukherjee and S. L. Bodhankar, Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats, Regul. Toxicol. Pharmacol., 2019, 105, 77–85 CrossRef CAS PubMed.
- V. Soleimani, A. Sahebkar and H. Hosseinzadeh, Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review, Phyther. Res., 2018, 32, 985–995 CrossRef CAS PubMed.
- D. R. Matthews, J. P. Hosker, A. S. Rudenski, B. A. Naylor, D. F. Treacher and R. C. Turner, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia, 1985, 28, 412–419 CrossRef CAS PubMed.
- G. Perseghin, A. Caumo, M. Caloni, G. Testolin and L. Luzi, Incorporation of the Fasting Plasma FFA Concentration into QUICKI Improves Its Association with Insulin Sensitivity in Nonobese Individuals, J. Clin. Endocrinol. Metab., 2001, 86, 4776–4781 CrossRef CAS PubMed.
- A. Crescenti, J. M. del Bas, A. Arola-Arnal, G. Oms-Oliu, L. Arola and A. Caimari, Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood, J. Nutr. Biochem., 2015, 26, 912–920 CrossRef CAS PubMed.
- M. M. Witte, D. Resuehr, A. R. Chandler, A. K. Mehle and J. M. Overton, Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy, Gen. Comp. Endocrinol., 2010, 166, 520–528 CrossRef CAS PubMed.
- J. Medina-Contreras, R. Villalobos-Molina, A. Zarain-Herzberg and J. Balderas-Villalobos, Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview, Mol. Cell. Biochem., 2020, 475, 261–276 CrossRef CAS PubMed.
- N. Yousefzadeh, K. Kashfi, S. Jeddi and A. Ghasemi, Ovariectomized rat model of osteoporosis: A practical guide, EXCLI J., 2020, 19, 89–107 Search PubMed.
- L. Mosquera, L. Shepherd and A. I. Torrado, Comparison of Two Methods of Estradiol Replacement: their Physiological and Behavioral Outcomes, J. Vet. Sci. Technol., 2015, 6, 276 Search PubMed.
- D. Moseti, A. Regassa and W. K. Kim, Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules, Int. J. Mol. Sci., 2016, 17, 124 CrossRef PubMed.
- L. A. Waddell, L. Lefevre, S. J. Bush, A. Raper, R. Young and Z. M. Lisowski, et al., ADGRE1 (EMR1, F4/80) Is a Rapidly-Evolving Gene Expressed in Mammalian Monocyte-Macrophages, Front. Immunol., 2018, 9, 1–14 CrossRef PubMed.
- T. M. Panknin, C. L. Howe, M. Hauer, B. Bucchireddigari, A. M. Rossi and J. L. Funk, Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials, Int. J. Mol. Sci., 2023, 24, 4476 CrossRef CAS PubMed.
- T. Vezza, F. Canet, A. M. de Marañón, C. Bañuls, M. Rocha and V. M. Víctor, Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders, Antioxidants, 2020, 9, 1266 CrossRef CAS PubMed.
- K. M. Gavin, W. M. Kohrt, D. J. Klemm and E. L. Melanson, Modulation of Energy Expenditure by Estrogens and Exercise in Women, Exerc. Sport Sci. Rev., 2018, 46, 232–239 CrossRef PubMed.
- J. C. Lovejoy, C. M. Champagne, L. de Jonge, H. Xie and S. R. Smith, Increased visceral fat and decreased energy expenditure during the menopausal transition, Int. J. Obes., 2008, 32, 949–958 CrossRef CAS PubMed.
- D. S. Day, W. S. Gozansky, R. E. Van Pelt, R. S. Schwartz and W. M. Kohrt, Sex Hormone Suppression Reduces Resting Energy Expenditure and β-Adrenergic Support of Resting Energy Expenditure, J. Clin. Endocrinol. Metab., 2005, 90, 3312–3317 CrossRef CAS PubMed.
- C. Receno, C. Liang, D. Korol, M. Atalay, K. Heffernan and T. Brutsaert, et al., Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats, Int. J. Mol. Sci., 2019, 20, 1178 CrossRef CAS PubMed.
- L. Mele, G. Bidault, P. Mena, A. Crozier, F. Brighenti and A. Vidal-Puig, et al., Dietary (poly)phenols, brown adipose tissue activation, and energy expenditure: A narrative review, Adv. Nutr., 2017, 8, 694–704 CrossRef CAS PubMed.
- S. Wang, N. Moustaid-Moussa, L. Chen, H. Mo, A. Shastri and R. Su, et al., Novel insights of dietary polyphenols and obesity, J. Nutr. Biochem., 2014, 25, 1–18 CrossRef CAS PubMed.
- H. Wang and R. H. Eckel, Lipoprotein lipase: from gene to obesity, Am. J. Physiol.: Endocrinol. Metab., 2009, 297, E271–E288 CrossRef CAS PubMed.
- P. Björntorp, M. Karlsson, H. Pertoft, P. Pettersson, L. Sjöström and U. Smith, Isolation and characterization of cells from rat adipose tissue developing into adipocytes, J. Lipid Res., 1978, 19, 316–324 CrossRef.
- P. Bjorntorp, Hormonal control of regional fat distribution, Hum Reprod., 1997, 12, 21–25 CrossRef CAS PubMed.
- A. Mosqueda-Solís, J. Sánchez, B. Reynés, M. Palou, M. P. Portillo and A. Palou, et al., Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in western diet-fed rats, Sci. Rep., 2018, 8, 15100 CrossRef PubMed.
- Y. Liu, F. Cheng, Y. Luo, Z. Zhan, P. Hu and H. Ren, et al., PEGylated Curcumin Derivative Attenuates Hepatic Steatosis via CREB/PPAR- γ /CD36 Pathway, BioMed. Res. Int., 2017, 2017, 8234507 Search PubMed.
- M. Y. Um, K. H. Hwang, J. Ahn and T. Y. Ha, Curcumin Attenuates Diet–Induced Hepatic Steatosis by Activating AMP–Activated Protein Kinase, Basic Clin. Pharmacol. Toxicol., 2013, 113, 152–157 CrossRef CAS PubMed.
- J. Mun, S. Kim, H. G. Yoon, Y. You, O. K. Kim and K. C. Choi, et al., Water Extract of Curcuma longa L. Ameliorates Non-Alcoholic Fatty Liver Disease, Nutrients, 2019, 11, 2536 CrossRef CAS PubMed.
- F. Mauvais-Jarvis, D. J. Clegg and A. L. Hevener, The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis, Endocr. Rev., 2013, 34, 309–338 CrossRef CAS PubMed.
- Y. Q. Liang, M. Akishita, S. Kim, J. Ako, M. Hashimoto and K. Iijima, et al., Estrogen receptor β is involved in the anorectic action of estrogen, Int. J. Obes., 2002, 26, 1103–1109 CrossRef CAS PubMed.
- G. Z. Taicher, F. C. Tinsley, A. Reiderman and M. L. Heiman, Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis, Anal. Bioanal. Chem., 2003, 377, 990–1002 CrossRef CAS PubMed.
- N. López, J. Sánchez, C. Picó, A. Palou and F. Serra, Dietary l-leucine supplementation of lactating rats results in a tendency to increase lean/fat ratio associated to lower orexigenic neuropeptide expression in hypothalamus, Peptides, 2010, 31, 1361–1367 CrossRef PubMed.
- A. Caimari, J. M. del Bas, N. Boqué, A. Crescenti, F. Puiggròs and E. Chenoll, et al., Heat-killed Bifidobacterium animalis subsp. Lactis CECT 8145 increases lean mass and ameliorates metabolic syndrome in cafeteria-fed obese rats, J. Funct. Foods, 2017, 38, 251–263 CrossRef CAS.
- J. Gómez-Ambrosi, J. Salvador, C. Silva, C. Pastor, F. Rotellar and M. Gil, et al., Increased cardiovascular risk markers in obesity are associated with body adiposity: Role of leptin, Thromb. Haemostasis, 2006, 95, 991–996 CrossRef PubMed.
- L. Recinella, G. Orlando, C. Ferrante, A. Chiavaroli, L. Brunetti and S. Leone, Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases, Front. Physiol., 2020, 11, 578966 CrossRef PubMed.
- S. Zhao, C. M. Kusminski and P. E. Scherer, Adiponectin, Leptin and Cardiovascular Disorders, Circ. Res., 2021, 128, 136–149 CrossRef CAS PubMed.
- R. Mariné-Casadó, C. Domenech-Coca, A. Crescenti, M. Á. Rodríguez Gómez, J. M. Del Bas and L. Arola, et al., Maternal Supplementation with a Cocoa Extract during Lactation Deeply Modulates Dams’ Metabolism, Increases Adiponectin Circulating Levels and Improves the Inflammatory Profile in Obese Rat Offspring, Nutrients, 2022, 14, 5134 CrossRef PubMed.
- A. Caimari, R. Mariné-Casadó, N. Boqué, A. Crescenti, L. Arola and J. M. del Bas, Maternal intake of grape seed procyanidins during lactation induces insulin resistance and an adiponectin resistance-like phenotype in rat offspring, Sci. Rep., 2017, 7, 12573 CrossRef PubMed.
- Y. Panahi, M. S. Hosseini, N. Khalili, E. Naimi, S. S. Soflaei and M. Majeed, et al., Effects of supplementation with curcumin on serum adipokine concentrations: A randomized controlled trial, Nutrition, 2016, 32, 1116–1122 CrossRef CAS PubMed.
- S. L. Atkin, N. Katsiki, G. Derosa, P. Maffioli and A. Sahebkar, Curcuminoids Lower Plasma Leptin Concentrations: A Meta-analysis, Phyther. Res., 2017, 31, 1836–1841 CrossRef CAS PubMed.
- S. Chuengsamarn, S. Rattanamongkolgul, B. Phonrat, R. Tungtrongchitr and S. Jirawatnotai, Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial, J. Nutr. Biochem., 2014, 25, 144–150 CrossRef CAS PubMed.
- M. Kubota, M. Shimizu, H. Sakai, Y. Yasuda, D. Terakura and A. Baba, et al., Preventive Effects of Curcumin on the Development of Azoxymethane-Induced Colonic Preneoplastic Lesions in Male C57BL/KsJ- db/db Obese Mice, Nutr. Cancer., 2012, 64, 72–79 CrossRef CAS PubMed.
- S. P. Weisberg, R. Leibel and D. V. Tortoriello, Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity, Endocrinology, 2008, 149, 3549–3558 CrossRef CAS PubMed.
- G. Frühbeck, V. Catalán, A. Rodríguez, B. Ramírez, S. Becerril and J. Salvador, et al., Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation, Nutrients, 2019, 11, 454 CrossRef PubMed.
- G. Frühbeck, V. Catalán, A. Rodríguez and J. Gómez-Ambrosi, Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk, Adipocyte, 2018, 7, 57–62 CrossRef PubMed.
- M. Inoue, M. Yano, M. Yamakado, E. Maehata and S. Suzuki, Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia, Metabolism, 2006, 55, 1248–1254 CrossRef CAS PubMed.
- G. Frühbeck, V. Catalán, A. Rodríguez, B. Ramírez, S. Becerril and J. Salvador, et al., Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome, Sci. Rep., 2017, 7, 6619 CrossRef PubMed.
- C. Ribeiro, G. Dourado and T. Cesar, Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: A randomized controlled trial, Nutrition, 2017, 38, 13–19 CrossRef CAS PubMed.
- A. C. D. Lima, C. Cecatti, M. P. Fidélix, M. A. T. Adorno, I. K. Sakamoto and T. B. Cesar, et al., Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials, J. Med. Food, 2019, 22, 202–210 CrossRef CAS PubMed.
- P. Babaei, R. Mehdizadeh, M. M. Ansar and A. Damirchi, Effects of Ovariectomy and Estrogen Replacement Therapy on Visceral Adipose Tissue and Serum Adiponectin Levels in Rats, Menopause Int., 2010, 16, 100–104 CrossRef PubMed.
- A. Dillard, N. R. Matthan and A. H. Lichtenstein, Use of hamster as a model to study diet-induced atherosclerosis, Nutr. Metab., 2010, 7, 89 CrossRef CAS PubMed.
- Z. Zhang, H. Wang, R. Jiao, C. Peng, Y. M. Wong and V. S. Y. Yeung, et al., Choosing hamsters but not rats as a model for studying plasma cholesterol-lowering activity of functional foods, Mol. Nutr. Food Res., 2009, 53, 921–930 CrossRef CAS PubMed.
- E. Bravo, A. Cantafora, A. Calcabrini and G. Ortu, Why prefer the golden Syrian hamster (Mesocricetus auratus) to the Wistar rat in experimental studies on plasma lipoprotein metabolism?, Comp. Biochem. Physiol., 1994, 107B, 347–355 CrossRef CAS.
- S. Laos, A. Caimari, A. Crescenti, J. Lakkis, F. Puiggròs and L. Arola, et al., Long-term intake of soyabean phytosterols lowers serum TAG and NEFA concentrations, increases bile acid synthesis and protects against fatty liver development in dyslipidaemic hamsters, Br. J. Nutr., 2014, 112, 663–673 CrossRef CAS PubMed.
- S. Shetty, N. Kapoor, J. Bondu, N. Thomas and T. Paul, Bone turnover markers: Emerging tool in the management of osteoporosis, Indian J. Endocrinol. Metab., 2016, 20, 846–852 CrossRef PubMed.
- J. V. Pinkerton, F. S. Aguirre, J. Blake, F. Cosman, H. Hodis and S. Hoffstetter, et al., The 2017 hormone therapy position statement of The North American Menopause Society, Menopause, 2017, 24, 728–753 CrossRef PubMed.
- M. Ulas and M. Cay, 17β-Estradiol and Vitamin E Modulates Oxidative Stress-Induced Kidney Toxicity in Diabetic Ovariectomized Rat, Biol. Trace Elem. Res., 2011, 144, 821–831 CrossRef CAS PubMed.
- P. J. Devine, S. D. Perreault and U. Luderer, Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity1, Biol. Reprod., 2012, 86, 1–10 Search PubMed.
- K. Yang, J. Chen, T. Zhang, X. Yuan, A. Ge and S. Wang, et al., Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis, Front. Immunol., 2022, 13, 949746 CrossRef CAS PubMed.
- J. F. Machi, D. da Silva Dias, S. Freitas, O. Albuquerque de Moraes, M. Barbosa da Silva and P. L. Cruz, et al., Impact of aging on cardiac function in a female rat model of menopause: role of autonomic control, inflammation, and oxidative stress, Clin. Interventions Aging, 2016, 11, 341 CrossRef CAS PubMed.
- Y. Y. Kim, S. H. Kim, S. Oh, O. J. Sul, H. Y. Lee and H. J. Kim, et al., Increased fat due to estrogen deficiency induces bone loss by elevating monocyte chemoattractant protein-1 (MCP-1) production, Mol. Cells, 2010, 29, 277–282 CrossRef CAS PubMed.
- M. Delgobo, J. P. Agnes, R. M. Gonçalves, V. W. dos Santos, E. B. Parisotto and A. Zamoner, et al., N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms, J. Nutr. Biochem., 2019, 67, 190–200 CrossRef CAS PubMed.
- L. Z. Racz, C. P. Racz, L. C. Pop, G. Tomoaia, A. Mocanu and I. Barbu, et al., Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin, Molecules, 2022, 27, 6854 CrossRef CAS PubMed.
- M. Funamoto, K. Shimizu, Y. Sunagawa, Y. Katanasaka, Y. Miyazaki and H. Kakeya, et al., Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus, J. Diabetes Res., 2019, 2019, 8208237 Search PubMed.
- X. Y. Xu, X. Meng, S. Li, R. Y. Gan, Y. Li and H. B. Li, Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives, Nutrients, 2018, 10, 1553 CrossRef PubMed.
- K. Priyadarsini, The Chemistry of Curcumin: From Extraction to Therapeutic Agent, Molecules, 2014, 19, 20091–20112 CrossRef PubMed.
- P. Neerati, R. Devde and A. K. Gangi, Evaluation of the Effect of Curcumin Capsules on Glyburide Therapy in Patients with Type–2 Diabetes Mellitus, Phyther. Res., 2014, 28, 1796–1800 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo05122f |
| ‡ Present address: Laboratory of Metabolism and Obesity, Vall d'Hebron-Institut de Recerca, Universitat Autònoma de Barcelona, Barcelona, Spain. |
| § Present address: Liver Vascular Biology Research Group, IDIBAPS Biomedical Research Institute, CIBEREHD, University of Barcelona, Spain. |
| This journal is © The Royal Society of Chemistry 2024 |