Open Access Article
Open Access ArticleA red-fleshed apple rich in anthocyanins improves endothelial function, reduces inflammation, and modulates the immune system in hypercholesterolemic subjects: the AppleCOR study†
Anna
Pedret
 a,
Judit
Companys
b,
Lorena
Calderón-Pérez
b,
Elisabet
Llauradó
a,
Laura
Pla-Pagà
b,
Patricia
Salamanca
a,
Berner-Andree
Sandoval-Ramírez
a,
Úrsula
Catalán
a,
Sara
Fernández-Castillejo
a,
Silvia
Yuste
a,
Judit
Companys
b,
Lorena
Calderón-Pérez
b,
Elisabet
Llauradó
a,
Laura
Pla-Pagà
b,
Patricia
Salamanca
a,
Berner-Andree
Sandoval-Ramírez
a,
Úrsula
Catalán
a,
Sara
Fernández-Castillejo
a,
Silvia
Yuste
 c,
Alba
Macià
c,
Laia
Gutiérrez-Tordera
de,
Mónica
Bulló
def,
Jordi
Camps
g,
Núria
Canela
h,
Rosa Maria
Valls
*a,
Laura
Rubió-Piqué
c,
Alba
Macià
c,
Laia
Gutiérrez-Tordera
de,
Mónica
Bulló
def,
Jordi
Camps
g,
Núria
Canela
h,
Rosa Maria
Valls
*a,
Laura
Rubió-Piqué
 c,
Maria José
Motilva‡
i and
Rosa
Solà‡
aj
c,
Maria José
Motilva‡
i and
Rosa
Solà‡
aj
aUniversitat Rovira i Virgili, Facultat de Medicina i Ciències de la Salut, Functional Nutrition, Oxidation and Cardiovascular Diseases Group (NFOC-Salut), Reus, Spain. E-mail: rosamaria.valls@urv.cat
bEurecat, Centre Tecnològic de Catalunya, Unitat de Nutrició i Salut, Reus, 43204, Spain
cFood Technology Department, XaRTA-TPV, Agrotecnio Center, Escola Tècnica Superior d'Enginyeria Agrària, University of Lleida, Lleida, Catalonia, Spain
dDepartment of Biochemistry and Biotechnology, Faculty of Medicine and Health Sciences, University RoviraiVirgili (URV), 43201 Reus, Spain
eInstitute of Health Pere Virgili-IISPV, University Hospital Sant Joan, 43202 Reus, Spain
fCIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III, 28029 Madrid, Spain
gUnitat de Recerca Biomèdica, Hospital Universitari Sant Joan, IISPV, Universitat Rovira i Virgili, Reus, Spain
hEurecat, Centre Tecnològic de Catalunya, Centre for Omic Sciences (COS), Joint Unit Universitat Rovira i Virgili-EURECAT, Reus 43204, Spain
iInstituto de Ciencias de la Vid y del Vino (CSIC, Gobierno de la Rioja, Universidad de La Rioja), Logroño, Spain
jHospital Universitari Sant Joan de Reus, Reus, Spain
First published on 16th May 2024
Abstract
The study determines the sustained and acute effects of a red-fleshed apple (RFA), rich in anthocyanins (ACNs), a white-fleshed apple (WFA) without ACNs, and an infusion from Aronia melanocarpa (AI) with an equivalent content of ACNs as RFA, on different cardiometabolic risk biomarkers in hypercholesterolemic subjects. A randomized, parallel study was performed for 6 weeks and two dose–response studies were performed at the baseline and after intervention. At 6 weeks, RFA consumption improved ischemic reactive hyperemia and decreased C-reactive protein and interleukine-6 compared to WFA consumption. Moreover, at 6 weeks, AI decreased P-selectin compared to WFA and improved the lipid profile. Three products reduced C1q, C4 and Factor B, and RFA and AI reduced C3. Although both RFA and AI have a similar ACN content, RFA, by a matrix effect, induced more improvements in inflammation, whereas AI improved the lipid profile. Anti-inflammatory protein modulation by proteomic reduction of the complement system and immunoglobulins were verified after WFA, AI and RFA consumption.
1. Introduction
Apples (Malus domestica) are one of the most consumed fruits around the world,1 thanks to their capacity to grow under different climatic circumstances, pleasant organoleptic characteristics and low market price.2 In addition, apples have always been linked with a healthy image, and their health effects are well-known by the entire population. Recent reviews of observational studies stated that apple consumption significantly decreased the risk of cardiovascular diseases (CVD), cardiovascular death, type 2 diabetes mellitus, and all-cause mortality.3 Apples’ bioactive compounds with healthy effects include phenolic compounds (PCs), soluble fibre (pectin), phytosterols, triterpenes, vitamins, and other trace elements.4PCs are one of the most studied dietary bioactive compounds related to healthy effects. Apple phenolics comprise several classes of PCs including hydroxycinnamic acids, flavan-3-ols, dihydrochalcones, anthocyanins (ACNs) and flavonols.5 Nevertheless, the composition and concentration of PCs differ between apple varieties and also depend on the weather, season, geographical distribution, and maturity of the fruit at the time of harvest.6
Recently, ACNs present predominantly in fruits have attracted scientific interest for their potential beneficial effects on cardiometabolic diseases (CMDs) in humans.7 ACNs belong to the bioactive family of flavonoids and are responsible for the red to dark purple pigmentation in many fruits, seeds, flowers, and plants.8 The dietary intake of ACNs is inversely associated with the CVD risk in both European and US populations.9 In this sense, ACNs decrease LDL cholesterol (LDL-C) and increase HDL cholesterol (HDL-C) in normo- and hyperlipidaemic subjects,10 modulate low-grade inflammation,11 and exhibit reactive oxygen species (ROS)-scavenging activities.12
Since the last decade, novel apple varieties with commercial interest have arisen.13 Although some apple varieties with red peel contain small amounts of ACNs, new emerging biofortified red-fleshed apples (RFA) have been developed through the use of traditional breeding (not genetically modified) with enhanced content of ACNs in their flesh, which could have added health properties compared to common white-fleshed apples (WFA). The main differences in the PC content between new RFA cultivars versus common WFA are related not only to the enhanced content of ACNs but also to the higher content of dihydrochalcones in RFA and the higher content of flavan-3-ols in WFA.5
The health effects of RFA have been demonstrated in animal studies, showing a reduction in aortic thickness,14 and in inflammatory biomarkers, beneficial modulations in the complement system and the colonic microbiota15 and protective effects against colon carcinogenesis.16 However, the literature on the beneficial effects of RFA in humans is scarce. A recent study evaluating the effects of RFA in healthy subjects observed a beneficial modulation of immune function associated with differences in faecal microbiota compared to common WFA.17 Thus, the hypothesis arises that RFA rich in ACN consumption could generate anti-inflammatory effects in the CMD context. In addition, it is unknown whether the bioavailability of ACNs after acute ingestion leads to acute effects of CMDs and whether these can be optimized by sustained CAN consumption.
The present study is framed in the AppleCOR project aimed to evaluate the CMD protective effects and mechanisms of action, by proteome and protein post-translational modification analysis, of RFA rich in ACNs, the variety Redlove, produced by biofortified traditional breeding programmes (not genetically modified).
In particular, the present study aims to determine the sustained and acute effects of a RFA, rich in ACNs, compared with those of a common WFA without ACNs, on different CMD risk biomarkers in hypercholesterolemic subjects. Another arm consuming a cold infusion from Aronia melanocarpa fruit (AI) was also included, which provided matched content and profile of ACNs as the RFA but without the apple matrix. This study would contribute towards taking a step forward in expanding the knowledge about the possible mechanisms of action and the effect of ACNs on metabolic pathways by studying the modulation of the plasma proteome profile and protein post-translational modifications.
2. Materials and methods
2.1 Experimental design
A randomized, parallel, clinical trial was performed (ESI Fig. 1†). Participants were randomly assigned to one of the three intervention groups: RFA, WFA, or AI. The daily amount of apple snacks provided to participants was 80 g day−1 and for AI was 1 L day−1, to be consumed in one or several doses along with meals. RFA and WFA were administered in individual daily seal plastic containers and Aronia was administered as a daily powder bag to be prepared by volunteers to obtain the AI (ESI Fig. 2†).Nested within the sustained consumption study, a sub-sample of volunteers performed two acute dose–response postprandial studies for 6 h, one at the baseline and the other after 6 weeks of sustained consumption. This design would allow us to evaluate the bioavailability and the influence of the sustained consumption on the acute effects of RFA, WFA, or AI. The daily dose of the intervention products during the acute dose–response postprandial studies was administered all at once and changes in the outcomes were recorded in the postprandial state. Participants were randomly allocated to the three intervention groups by a computerized random number generator made by an independent statistician. PROC PLAN (SAS 9.2, Cary, NC: 83 SAS Institute Inc.) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 allocation using random block sizes of 2, 4, and 6 was used (ESI Fig. 1†).
1 allocation using random block sizes of 2, 4, and 6 was used (ESI Fig. 1†).
After enrolment and following a 1-week run-in period on a regular diet (maintained dietary habits based on nutritionist recommendations), volunteers started the intervention trial. During the intervention period, volunteers were instructed to also maintain their lifestyle, physical activity, and dietary habits, and completely refrain from consuming ACN-rich foods (berries, grapefruit, plums, figs, pomegranate, green and red apples, avocado, black olives, red and black beans, red wine and mushrooms) and to avoid eating functional foods intended to decrease cholesterol levels. During the sustained study, volunteers attended 4 visits (every 2 weeks) at the Eurecat Human Nutrition Unit (Reus, Spain). The two acute dose–response postprandial studies were performed at Visit 1 and Visit 4, and volunteers stayed at Eurecat from 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 am to 02
00 am to 02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 pm, and received a light meal before leaving. For the sustained study, blood samples at fasting conditions, 24-hour urine and faeces samples were collected at Visit 1 and Visit 4.
00 pm, and received a light meal before leaving. For the sustained study, blood samples at fasting conditions, 24-hour urine and faeces samples were collected at Visit 1 and Visit 4.
For the acute dose–response postprandial study, blood samples were obtained at the baseline, 2 h, 4 h, and 6 h. Urine samples for the dose–response study were collected at the baseline (24-hours urine), and at the following post-prandial periods: 0–3 h, 3–6 h, and 6–24 h. Samples were stored at −80 °C in the Biobanc of HUSJ (biobanc.reus@iispv.cat) until required for batched analyses.
The adherence of the volunteers to their dietary habits through the study was assessed by a 3-day food record at Visit 1 and Visit 4. In addition, in all visits, volunteers responded to a checking questionnaire on the frequency of consumption of foods rich in ACNs and functional foods and/or supplements to reduce cholesterol. At each visit, subjects also underwent a physical examination by a general practitioner, and completed a Physical Activity Questionnaire Class AF,18 and anthropometric measurements were recorded. Moreover, plastic and seal containers for RFA and WFA and the daily dose bag of aronia powder were returned after the intervention by volunteers to assure their compliance.
2.2 Study population
Subjects from the general population were recruited by means of news in social networks, newspapers, and tableaux advertisements in the Hospital Universitari Sant Joan (HUSJ)-Eurecat, Reus, Spain, between January 2019 and May 2019. Out of the 179 subjects assessed for eligibility, 121 (70 females and 51 males) hypercholesterolemic individuals, according to current guidelines,19 were recruited. Inclusion criteria were age ≥18, LDL-c levels ≥115 mg dL−1 and willingness to provide informed consent before the initial screening visit. Exclusion criteria were: LDL-c levels <115 and ≥190 mg dL−1 or with hypolipemiant treatment (drugs and functional foods); or with hypoglycemia treatment or type 1 and type 2 diabetes mellitus diagnosed; Body Mass Index (BMI) ≥ 35 kg m−2; triglyceride (TG) levels ≥350 mg dL−1; anaemia (haemoglobin ≤13 g dL−1 in men and ≤12 g dL−1 in women); diagnosis of intestinal disorders such as Crohn's disease, ulcerative colitis, coeliac disease and irritable bowel syndrome; fructose and/or sorbitol and/or gluten intolerance; use of antioxidants supplements; pregnant or intending to become pregnant; to be in the breast-feeding period; chronic alcoholism; smoking; current or past participation in a clinical trial or consumption of a research product in the 30 days prior to inclusion in the study; and failure to follow the study guidelines.Participants signed informed consent before they participated in the study, which was approved by the Clinical Research Ethical Committee of Institut d'Investigació Sanitària Pere Virgili (S033/04Nov2016), Reus, Spain. The protocol and trial were conducted in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines of the International Conference of Harmonization (GCP ICH) and were reported as the CONSORT criteria. The trial was registered at ClinicalTrials.gov Identifier: NCT03795324.
2.3 Intervention products
For the AppleCOR project, two different apple varieties were selected: the Redlove (a RFA variety biofortified with ACN in its flesh) and the Granny Smith (a WFA variety used as an ACN-free control). Both apple varieties were provided by NUFRI S.A.T. (Mollerussa, Lleida, Spain). To increase the useful life, obtain good shelf stability and, at the same time, minimize changes in the PC content of apples, the freeze-dried snack format was selected for the nutritional intervention. RFA and WFA were administered in individual daily seal plastic containers which were refrigerated (2 °C) until their use for the study (ESI Fig. 2†). The detailed preparation process of the freeze-dried apple snacks is reported in our previous study.20Aronia fruit was selected as it is a rich source of cyanidin-3-O-galactoside and cyanidin-O-arabinoside, which are the main ACNs in RFA. Aronia fruit powder commercially available (Aronia Pulver, BIOJOY, Nuremberg, Germany) was used to prepare a daily cold-water infusion by the volunteers. An infusion prepared with 50 g of Aronia fruit powder and 1 L of mineral water (Bezoya mineral water, Calidad Pascual, Aranda de Duero, Burgos, Spain) satisfactorily allowed to provide an equivalent daily dose of ACN compounds present in RFA. The volunteers were instructed to mix the Aronia fruit powder with water, to energetically homogenize the mixture in a glass bottle for 3 min and to filter with a cloth filter, placing the filtered infusion in a light protected bottle ready to be consumed along the day. To prepare and consume 1 L day−1 AI cold infusion, volunteers were provided with the required tools and instructions (ESI Fig. 2†).
Daily doses of 80 g of WFA and RFA snacks and 1 L of AI provide 0 mg day−1, 34.5 mg day−1 and 37.4 mg day−1 of total ACNs, respectively. RFA, WFA snacks and the AI were analysed for phenol characterization using liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS), as recently published.21 The phenolic composition and the macronutrients and other phytochemical compounds of the three intervention products are shown in ESI Tables 1 and 2,† respectively.
2.4 Biomarkers of apple and AI intake: biological phenolic metabolites
To evaluate the compliance for each intervention (RFA, WFA and AI) during the sustained study, the phenol biological metabolites were used as intake biomarkers and analysed in urine and plasma samples at Visit 1 and Visit 4. The phenolic metabolites were determined by UPLC-MS/MS as described in our previous study.212.5 Anthropometric measurements and blood pressure
Anthropometric data were obtained with participants wearing lightweight clothing and no shoes. Waist circumference (WC) was measured at the umbilicus using a 150 cm anthropometric steel measuring tape. Body weight and composition were obtained by using a calibrated scale (Tanita SC 330-S; Tanita Corp., Barcelona, Spain). Height was measured using a wall-mounted stadiometer (Tanita Leicester Portable; Tanita Corp., Barcelona, Spain). Body mass index (BMI) was calculated as the ratio between measured weight (kg)/and the square of height (m).Systolic (SBP) and diastolic blood pressure (DBP) were measured twice after 2–5 minutes of respite, with the patient in a seated position, with one-minute interval between, by using an automatic sphygmomanometer (OMRON HEM-907; Peroxfarma, Barcelona, Spain). The mean values were used for statistical analyses. Office pulse pressure (PP), which represents the force that the heart generates each time it contracts, was determined by the difference between SBP and DBP.22 Blood pressure and PP were measured in the sustained consumption study (Visit 1 and Visit 4) and in both dose–response studies (baseline, 2 h, 4 h and 6 h).
2.6 Lipid profile and glucose
The lipid profile and glucose were measured under fasting conditions in the sustained consumption study (Visit 1 and Visit 4) and in both dose–response studies (baseline, 2 h, 4 h and 6 h).Total cholesterol, HDL-c, TG, Apolipoprotein A-1 (ApoA1) and Apolipoprotein B-100 (ApoB100) were measured in serum by standardized enzymatic automated methods in a Cobas Mira Plus autoanalyzer (Beckman Coulter-Synchron, Galway, Ireland). LDL-c was calculated by using the Friedewald formula.
Serum glucose and insulin concentrations were measured by standardized methods in a Cobas Mira Plus autoanalyzer (Roche Diagnostics Systems, Madrid, Spain).
2.7 Endothelial function
The endothelial-dependent vasomotor function was measured as ischemic reactive hyperaemia (IRH) using a Laser-Doppler linear Periflux 5000 flowmeter (Perimed AB, Järfälla, Stockholm, Sweden). Patients were at rest for 15–20 min before the test. Measurements were performed with the patient lying in the supine position in a room with stable temperature (20–22 °C). Calculations were performed by using the formula: IRH = ((PUtd − PUt0)/(PUt0) × 100) and results were expressed as arbitrary units (AU). The IRH value of the area under the curve (AUC) was calculated using Microsoft Excel for pharmacokinetic functions. Measurements were performed at the baseline and after 6 weeks of intervention at the sustained study, and at the baseline, 2 h, 4 h, and 6 h after ingestion of the single dose of the corresponding intervention product at both dose–response studies.Serum endothelin type 1 concentrations were determined by using an ELISA kit (R&D Systems, Minneapolis, USA).
2.8 Inflammation and complement system biomarkers
Serum high-sensitive C-reactive protein (hs-CRP) was determined by high-sensitivity immunoturbidimetric methods on a Cobas Mira Plus autoanalyzer (Roche Diagnostics Systems, Madrid, Spain).Interleukin-10 (IL-10), interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), intercellular adhesion molecule (ICAM), vascular adhesion molecule (VCAM) and P-selectin were analysed in plasma by Multiplex Assays (MILLIPLEX MAP Human High Sensitivity T Cell Panel, Millipore, Madrid, Spain and MILLIPLEX MAP Human Cardiovascular Disease (CVD) Magnetic Bead Panel 2, Millipore, Madrid, Spain).
C1q, C3, C4 and Factor B were determined in plasma samples by using an Immunology Multiplex Assay (MILLIPLEX Human Complement Panel 2, Millipore, Madrid, Spain).
2.9 Oxidation biomarkers
Plasma oxidized LDL (ox-LDL) was determined by an ELISA (Mercodia AB, Uppsala, Sweden). 8-Isoprostane concentrations were determined by mass spectrometry in urine samples.PON1 protein concentration was measured in serum using an in-house indirect ELISA with rabbit polyclonal antibodies, against the synthetic peptide CRNHQSSYQTRLNALREVQ, which is a sequence specific for mature PON1.23
PON1-associated paraoxonase and arylesterase catalytic activities were measured in serum using two different substrates: paraoxon and phenylacetate, respectively. Paraoxonase activity was analyzed by measuring the rate of hydrolysis of paraoxon at 412 nm and 37 °C in a 0.05 mmol L−1 glycine buffer, pH = 10.5, with 1 mmol L−1 CaCl2. The arylesterase activity was measured at 270 nm in a 9 mM Tris-HCl buffer, pH 8.0, and supplemented with 0.9 mM CaCl2. Activities were expressed as U L−1. Specific activities were calculated as the ratio between the activity and the PON1 protein concentration and expressed as U mg−1 PON1 protein.23
2.10 Statistical analyses
The parametricity of the variables was examined and logarithmic transformation of the variables was performed if required. Differences in the baseline characteristics among groups were assessed by an ANOVA test. Differences among treatments were assessed by an ANCOVA test adjusted by age and sex. Imputation by multiple regression analyses has been performed when imputed data were less than 20% of the total values, and in the case of the postprandial set when also basal values were available. The relationship among variables was assessed by Pearson's and/or Spearman's correlation tests.2.11 Sample size and power analysis
The sample size of the study was calculated assuming a 0.50 mmol L−1 (approximately 15%) post-intervention difference of LDL-c and a 0.72 mmol L−1 standard deviation (SD), with α = 0.05 and 1 − β = 0.08. Thus, a minimum of 22 participants were required. However, the sample size was expanded to 40 participants for arm, (in total 120 subjects) to ensure that even with volunteers’ dropouts during the intervention, the statistical power to find significant results could be maintained.2.12 Proteomic and phosphoproteomic analysis
The proteomic and phosphoproteomic analyses were performed on a subsample of 30 volunteers, with 10 subjects for each intervention group.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min. Supernatants were collected for protein precipitation with the addition of 10% TCA/acetone. The protein pellets were resuspended in 6 M urea/50 mM ammonium bicarbonate (ABC) and quantified by Bradford's method.
000g for 15 min. Supernatants were collected for protein precipitation with the addition of 10% TCA/acetone. The protein pellets were resuspended in 6 M urea/50 mM ammonium bicarbonate (ABC) and quantified by Bradford's method.
Protein digestion: 550 μg of total protein were reduced with 4 mM 1.4-dithiothreitol (DTT) for 1 h at 37 °C and alkylated with 8 mM iodoacetamide (IAA) for 30 min at 25 °C in the dark. Afterwards, samples were overnight digested (pH 8.0, 37 °C) with sequencing-grade trypsin/Lys-C (Thermo Fisher Scientific, CA, USA) at an enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein ratio of 1
protein ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Digestion was quenched by acidification with 1% (v/v) formic acid and each sample was divided in two: 50 μg of initial total protein were used for proteomics analysis and 500 μg were used for phosphosproteomics. Before peptide TMT labelling and phosphopeptide enrichment, samples were desalted on an Oasis HLB SPE column (Waters, Massachusetts, USA).
50. Digestion was quenched by acidification with 1% (v/v) formic acid and each sample was divided in two: 50 μg of initial total protein were used for proteomics analysis and 500 μg were used for phosphosproteomics. Before peptide TMT labelling and phosphopeptide enrichment, samples were desalted on an Oasis HLB SPE column (Waters, Massachusetts, USA).
Complete information on the next steps of the proteomic and phosphoproteomic analyses: peptide 11-plex TMT labelling, nanoLC-(Orbitrap)MS/MS analysis, protein identification/quantification, phosphopeptide enrichment and statistical analysis, are detailed in the ESI† section and previously described.15,24
3. Results
3.1 Study subjects
Of the 179 subjects assessed for eligibility, finally 121 of them (68%, 70 women and 51 men) received allocated intervention in either RFA (n = 40), WFA (n = 41), or AI (n = 40) groups. For the acute dose–response postprandial studies, out of the 29 allocated participants, one discontinued the intervention from the beginning, and 2 were lost for the second dose–response study. Thus, 29 participants (12 in the RFA, 8 in the WFA, and 9 in the AI groups) completed the first dose–response study, and 26 participants (9 in the RFA, 8 in the WFA, and 9 in the AI groups) the second one (see CONSORT flowchart of the study in ESI Fig. 3†). No differences in baseline characteristics were observed among the intervention groups (Table 1).| Variable | WFA (n = 41) | AI (n = 40) | RFA (n = 40) | P |
|---|---|---|---|---|
| Data expressed as mean ± standard deviation, or percentages. SBP, systolic blood pressure; DBP, diastolic blood pressure; pulse pressure = SBP-DBP; BMI, body mass index (weight/(height in meters)2); pl, plasma; LDL, low density lipoproteins; HDL, high density lipoproteins.a Median (25th–75th percentiles). AU, arbitrary units: 0–1, inactive; 2–3, very low activity; 4–5, low activity; 6–11, moderately active; > or ≥12, very active. P for ANOVA with logarithmic transformation for triglycerides. | ||||
| Age, years | 49.8 ± 13.6 | 49.6 ± 13.3 | 46.7 ± 16.3 | 0.566 |
| Females, % | 67.5 | 50 | 55.3 | 0.287 |
| SBP, mm Hg | 127 ± 16.7 | 127 ± 14.4 | 132 ± 16.5 | 0.285 |
| DPB, mm Hg | 76 ± 11.1 | 77 ± 10.1 | 79 ± 9.7 | 0.317 |
| Pulse pressure | 51 ± 12.1 | 50 ± 10.4 | 53 ± 13.1 | 0.605 |
| Weight, kg | 68.7 ± 12.4 | 71.8 ± 11.1 | 74.2 ± 11.6 | 0.123 |
| BMI, kg m−2 | 24.6 ± 3.2 | 26.3 ± 4.5 | 26.3 ± 3.8 | 0.078 |
| Waist circumference, cm | 86.9 ± 11.5 | 89.3 ± 9.6 | 90.9 ± 9.1 | 0.253 |
| Waist/height, cm | 0.52 ± 0.06 | 0.54 ± 0.06 | 0.54 ± 0.06 | 0.281 |
| Conicity index | 1.24 ± 0.09 | 1.25 ± 0.07 | 1.26 ± 0.07 | 0.836 |
| Glucose, pl, mg/dL | 91 ± 11.3 | 93 ± 6.0 | 92 ± 8.4 | 0.506 |
| Cholesterol, pl, mg/dL | ||||
| Total | 220 ± 48 | 223 ± 26 | 213 ± 54 | 0.611 |
| LDL | 145 ± 25.9 | 144 ± 20.3 | 147 ± 19.6 | 0.835 |
| HDL | 60.0 ± 17.1 | 60.0 ± 16.4 | 53.8 ± 16.6 | 0.143 |
| Triglyceridesa, pl, mg/dL | 82 (60–117) | 81 (64–108) | 87 (58–128) | 0.836 |
| Physical activity, AU | 4.34 ± 2.24 | 5.10 ± 1.68 | 4.35 ± 1.95 | 0.146 |
No changes were observed in physical activity throughout the study in any treatment group. Changes in total energy intake, macronutrients, fibre, and alcohol intake after 6 weeks of intervention are presented in ESI Table 3.† PUFA (as % of energy intake) increased after WFA treatment (P = 0.015), reaching significant differences between treatments (P = 0.022). For all other dietary components, no inter-treatment differences were observed throughout the study. Fibre consumption was significantly lower after WFA (P = 0.036) and after AI (P = 0.012).
3.2 Phenolic metabolites as biomarkers of dietary adherence
To evaluate the compliance for each intervention (RFA, WFA and AI) during the sustained study, the plasma and urine phenol metabolites were used as intake biomarkers (ESI Tables 4 and 5†). The targeted metabolomic analysis of plasma and urine samples showed that the anthocyanin peonidin-3-O-galactoside was identified as an intake biomarker of RFA and AI as sources of anthocyanins. In addition, phloretin-2′-O-glucuronide was identified as an intake biomarker of both apple snacks (RFA and WFA). Good compliance by volunteers was reflected in the significant increase of these phenolic metabolites’ concentrations in 24 h-urine and plasma samples after the sustained intervention. The level of adherence was acceptable for all products as the consumption was >80%.The 3 intervention products provided in the study were well tolerated by all volunteers and no adverse events were reported.
3.3 Anthropometric measurements and blood pressure
No changes were observed in anthropometric measurements after 6 weeks of sustained intervention (ESI Table 6†).3.4 Lipid profile and glucose
AI significantly decreased glucose levels after 6 weeks of intervention but no changes were observed in glucose, insulin, or HOMA index among the treatments (ESI Table 12†).
Decreases in glucose were observed at 6 h after WFA and at 2 h after AI treatment in the baseline postprandial study. Insulin increased significantly at 2 h in both postprandial studies after AI and RFA treatments compared to its baseline.
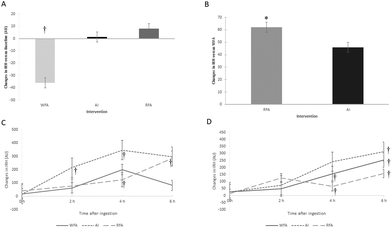
3.5 Endothelial function
3.6 Inflammation biomarkers
3.7 Oxidative stress biomarkers
3.8 Complement molecules
C1q, C4 and Factor B decreased after 6 weeks of three treatments with no differences between them. Moreover, C3 decreased after AI and RFA treatments with no differences in the inter-treatments (ESI Table 27†).A summary of all the results obtained from the sustained study after WFA, AI and RFA treatments on CMD biomarkers is detailed in Table 2.
| Parameters | WFA | AI | RFA | |
|---|---|---|---|---|
| Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; VLDL-c, very low density lipoprotein cholesterol; TG, triglycerides; NEFA, non-esterified fatty acids, Apo A1, lipoprotein A1; Apo B100, lipoprotein B100; IRH, ischemic reactive hyperemia; hsCRP, high sensitive C reactive protein; IL-10, interleukin 10; IL-6, interleukin 6; TNF-alpha, tumor necrosis factor alpha; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cellular adhesion molecule-1; oxLDL, oxidized LDL; PON1; paraxonase-1; Arylest raw, arylesterase activity of paraoxonase-1 raw; C1q, complement component C1q; C3, complement component C3; C4, complement component C4. | ||||
| Blood pressure | SBP (mmHg) | NS | NS | NS |
| DBP (mmHg) | ↓ (vs. baseline) | NS | NS | |
| PP (mmHg) | NS | NS | ↓ (vs. baseline) | |
| Lipid profile | Total cholesterol (mg dL−1) | NS | ↓ (vs. baseline) | NS |
| HDL-c (mg dL−1) | NS | NS | ||
| LDL-c (mg dL−1) | NS | ↓ (vs. baseline) | NS | |
| VLDL-c (mg dL−1) | NS | NS | NS | |
| Total chol/HDL ratio (mg dL−1) | NS | ↓ (vs. baseline) | NS | |
| LDL-c/HDL-c ratio (mg dL−1) | NS | NS | NS | |
| TG (mg dL−1) | NS | ↓ (vs. baseline) | NS | |
| NEFA (mmol L−1) | NS | NS | NS | |
| ApoA1 (mg dL−1) | ↓ (vs. baseline) | ↓ (vs. baseline) | ↓ (vs. baseline) | |
| ApoB100 (mg dL−1) | NS | NS | NS | |
| ApoA1/ApoB ratio | NS | NS | ↓ (vs. baseline) | |
| Glucose and insulin resistance | Glucose (mg dL−1) | NS | ↓ (vs. baseline) | NS |
| Insulin (μU mL−1) | NS | NS | NS | |
| HOMA-IR | NS | NS | NS | |
| Endothelial function | IRH (AU) | ↓ (vs. baseline) | NS | ↑ (vs. WFA) |
| Endothelin-1 (pg mL−1) | ↑ (vs. basline) | ↑ (vs. baseline) | ↑ (vs. baseline) | |
| Inflammation markers | hsCRP (mg dL−1) | NS | NS | ↓ (vs. WFA) |
| IL-10 (pg mL−1) | NS | NS | NS | |
| IL-6 (pg mL−1) | NS | NS | ↓ (vs. baseline and WFA) | |
| TNF-alpha (pg mL−1) | NS | NS | NS | |
| P-selectin (ng mL−1) | NS | ↓ (vs. baseline and WFA) | NS | |
| ICAM-1 (μg mL−1) | NS | ↓ (vs. baseline) | NS | |
| VCAM-1 (μg mL−1) | NS | NS | NS | |
| Oxidation markers | oxLDL (U L−1) | NS | NS | NS |
| PON1 mass (mg dL−1) | NS | NS | NS | |
| PON raw (U L−1) | NS | NS | ↑ (vs. baseline and WFA) | |
| Arylest raw (U L−1) | NS | NS | NS | |
| PON specific (U mg−1 prot) | NS | NS | NS | |
| Arylest specific (U mg−1 prot) | NS | NS | NS | |
| Complement markers | C1q (mg dL−1) | ↓ (vs. baseline) | ↓ (vs. baseline) | ↓ (vs. baseline) |
| C3 (mg dL−1) | NS | ↓ (vs. baseline) | ↓ (vs. baseline) | |
| C4 (mg dL−1) | ↓ (vs. baseline) | ↓ (vs. baseline) | ↓ (vs. baseline) | |
| Factor B (mg dL−1) | ↓ (vs. baseline) | ↓ (vs. baseline) | ↓ (vs. baseline) | |
| Cholesterol efflux (%, AU) | NS | NS | ↓ (vs. baseline and WRF) | |
3.9 Phenolic compliance biomarkers and CMD biomarkers
Multivariate linear regression analyses were performed with CMD biomarkers as dependent variables and the principal phenolic compliance biomarkers as independent variables. The models also included age and gender as independent variables. Significant results for the sustained study are shown in Table 3.| CMD biomarkers | Compliance biomarker | B | SE | Beta | p | 95% CI |
|---|---|---|---|---|---|---|
| Abbreviations: CMD, cardiometablic disease; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; Apo A1, lipoprotein A1; Apo B100, lipoprotein B100; C3, complement component C3; hsCRP, high sensitive C reactive protein. | ||||||
| HDL | ||||||
| Peonidin glucuronide (urine) | AI | 0.053 | 0.026 | 0.284 | 0.043 | 0.002 to 0.105 |
| LDL/HDL ratio | ||||||
| Peonidin arabinoside (urine) | AI | −0.196 | 0.077 | −0.386 | 0.017 | −0.353 to −0.038 |
| Total cholesterol/HDL ratio | ||||||
| Peonidin arabinoside (urine) | AI | −0.146 | 0.057 | −0.368 | 0.016 | −0.262 to −0.030 |
| Apo A1/ApoB100 ratio | ||||||
| Peonidin arabinoside (urine) | AI | 0.135 | 0.055 | 0.362 | 0.021 | 0.022 to 0.248 |
| C3 | ||||||
| Peonidin galactoside (plasma) | AI and RFA | −1.613 | 0.756 | −0.367 | 0.041 | −3.154 to −0.072 |
| hsPCR | ||||||
| Phloretin xylosyl glucoside (urine) | RFA (exclusive) | −1.086 | 0.472 | −0.639 | 0.061 | −2.240 to 0.069 |
Briefly, significant relationships were observed between different AI compliance biomarkers (peonidin-O-glucuronide and peonidin-3-O-arabinoside) and sustained lipid biomarkers (HDL-c; β = 0.053, P = 0.043, LDL-c/HDL-c ratio; β = −0.196, P = 0.017, total cholesterol/HDL-c ratio; β = −0.146, P = 0.016 and ApoA1/ApoB100 ratio; β = 0.135, P = 0.021). Moreover, a significant trend relationship was observed between phloretin-O-xylosyl glucoside, an exclusive biomarker of RFA consumption, and hs-CRP (β = −1.086, P = 0.061). Finally, peonidin-3-O-galactoside, a common biomarker of AI and RFA, shows an inverse relationship with C3 (β = −1.613, P = 0.041).
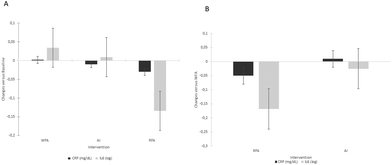
No significant relationships were observed for acute dose–response postprandial variables.
3.10 Proteomics and phosphoproteomics
3.10.1.1 Proteome modulation after the RFA, WFA and AI treatments. Table 4 shows the results of the significantly up- or downregulated proteins expressed after the RFA, WFA, and AI treatments (baseline vs. final).
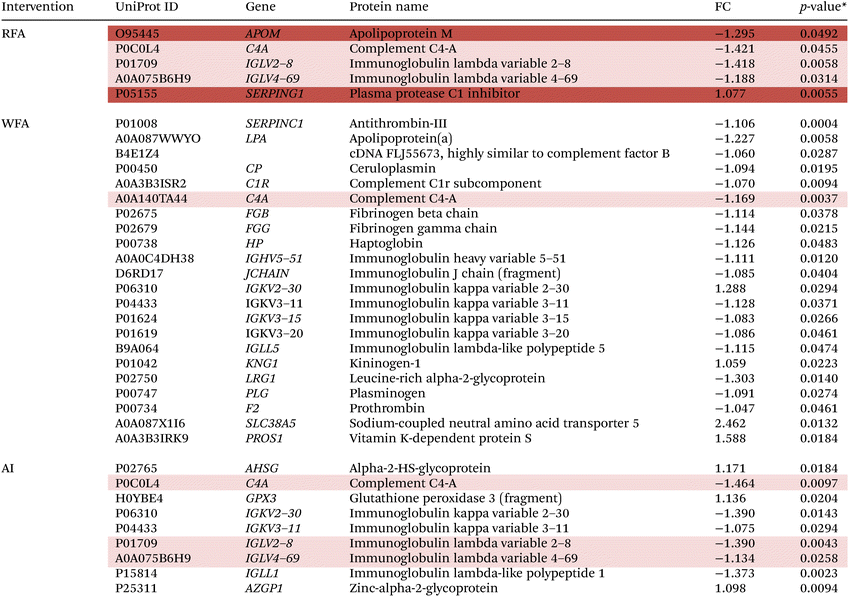
When comparing final values to baseline values after the RFA treatment, there was a decrease of four differentially expressed proteins: APOM, C4A, IGLV2–8 and IGLV4–69, and an increase of SERPING1 protein.
WFA treatment produced modulations in 22 proteins, 18 proteins were downregulated and 4 proteins were upregulated. SERPINC1, LPA, cDNA FLJ55673, CP, C1R, C4A, FGB, FGG, HP, IGHV5–51, JCHAIN, IGKV3–11, IGKV3–15, IGKV3–20, IGLL5, LRG1 and PLG were downregulated and IGKV2–30, KNG1, F2 SLC38A5 and PROS1 were upregulated after WFA treatment.
After AI treatment, 9 proteins were modulated. C4A, IGKV2–30, IGKV3–11, IGLV2–8, IGLV4–69 and IGLL1 were downregulated and AHSG, GPX and AZGP1 were upregulated.
Three proteins were found to be commonly modulated after RFA and AI treatments: C4A, IGLV2–8, and IGLV4–69. Moreover, C4A is the only commonly modulated protein after the RFA and WFA treatments.
Otherwise, two other proteins were found to be uniquely modulated after RFA: APOM and SERPING1. ESI Fig. 4† shows the Venn diagram with the intersections of proteins differentially expressed after RFA, WFA, and AI interventions.
No inter-treatment differences were observed between the 3 interventions either at the baseline or at the end of the intervention.
After the RFA treatment, we observed a significant increase in the phosphorylation of CDK5R2, RASAL1 and FAM3D and a decrease in the phosphorylation of FGA and EXOSC3.
After the WFA treatment, only one phosphopeptide was modulated, decreasing the phosphorylation of MYO15B protein.
Finally, after AI treatment, we observed a significant increase in the phosphorylation of KRT80 and a decrease in EXOSC3.
One peptide sequence (VTLGLIR) was found to be commonly modulated after the RFA and AI treatments, decreasing the phosphorylation of EXOSC3.
Otherwise, four other peptide sequences were found to be uniquely modulated after RFA decreasing FGA or increasing phosphorylation of CDK5R2, RASAL1 and FAM3D.
Significant phosphorylated peptide sequences, their corresponding proteins, their molecular functions, their biological processes, their p-values, the phosphorylated FCs, and the serine phosphorylation sites for the RFA, WFA and AI treatments are detailed in Table 5.
| Intervention | Peptide sequence | UniProt ID | Gene | Protein name | Molecular function | Biological process | p | Phosphorylation FC (basal versus final) | Phosphorylation site |
|---|---|---|---|---|---|---|---|---|---|
| FC, fold change. *A value of p < 0.05 was considered statistically significant. The peptide sequences marked in light orange are common between red apple and aronia. The peptide sequences marked in red are unique for red apple. | |||||||||
| RFA | ESSSHHPGIAEFPSR | P02671 | FGA | Fibrinogen alpha chain | Extracellular matrix structural constituent. Metal ion binding. Signaling receptor binding. Structural molecule activity. | Adaptative immune response. Blood coagulation, common pathway, fibrin clot formation. Cell–matrix adhesion. Fibrinolysis. Induction of bacterial agglutination. Innate immune response. Negative regulation of blood coagulation, common pathway. Negative regulation of extrinsic apoptotic signalling pathway via death domain receptor. Plasminogen activation. Platelet aggregation. Positive regulation of ERK1 and ERK2 cascade. Positive regulation of exocytosis. Positive regulation of heterotypic cell–cell adhesion. Positive regulation of peptide hormone secretion. Positive regulation of protein secretion. Positive regulation of substrate adhesion-dependent cell spreading. Positive regulation of vasoconstriction. Protein polymerization. Protein-containing complex assembly. Response to calcium ion. | 0.0293 | −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055 055 |
S2(phospho) |
| LSPQMLR | Q13319 | CDK5R2 | Cyclin-dependent kinase 5 activator 2 | Actin binding. Cyclin-dependent protein serine/threonine kinase activator activity. Lipid binding. Protein kinase binding. | Axon guidance. Brain development. Cerebellum development. Hippocampus development. Layer formation in cerebral cortex. Neuron migration. Positive regulation of calcium ion-dependent exocytosis. | 0.0255 | 9891 | S2(phospho)–M5(oxidation) | |
| AKSSSLNVR | O95294 | RASAL1 | RasGAP-activating-like protein 1 | Metal ion binding. Phospholipid binding. | Cell differentiation. Cellular response to calcium ion. Intracellular signal transduction. Negative regulation of Ras protein signal transduction. Positive regulation of dendrite extension. Regulation of GTPase activity. Signal transduction. | 0.0162 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 507 |
S3(phospho)–S4(phospho) | |
| SYMSFSMKTIR | Q96BQ1–C9J5Z5–C9IZW7 | FAM3D | Protein FAM3D | Cytokine activity | Negative regulation of glucagon secretion. Negative regulation of insulin secretion. | 0.0364 | 2732 | S6(phospho)–T9(phospho) | |
| VTLGLIR | Q9NQT5 | EXOSC3 | Exosome complex component RRP40 | 3′-5′-Exoribonuclease activity. RNA binding | CUT catabolic process. DNA deamination. Exonucleolytic catabolism of deadenylated mRNA. Isotype switching. Nuclear polyadenylation-dependent rRNA and Trna catabolic process. Nuclear-transcribed mRNA catabolic process. Polyadenylation-dependent snoRNA 3′-end processing. Positive regulation of isotype switching. RNA catabolic process. RNA processing. rRNA processing. U4 snRNA 3′-end processing. | 0.0159 | −6018 | T2(phospho) | |
| WFA | ENFSHPYYLR | A0A286YF23 | MYO15B | Unconventional myosin-XVB | Actin binding. ATP binding. | 0.0134 | −2528 | S4(phospho)–Y7(phospho)–Y8(phospho) | |
| AI | TAASRSGLSKAPSR | Q6KB66 | KRT80 | Keratin, type II cytoskeletal 80 | Structural constituents of skin epidermis. | Intermediate filament organization. Keratinization. | 0.0315 | 8164 | T1(phospho)–S4(phospho)–S6(phospho) |
| VTLGLIR | Q9NQT5 | EXOSC3 | Exosome complex component RRP40 | 3′-5′-Exoribonuclease activity. RNA binding. | CUT catabolic process. DNA deamination. Exonucleolytic catabolism of deadenylated mRNA. Isotype switching. Nuclear polyadenylation-dependent rRNA and Trna catabolic process. Nuclear-transcribed mRNA catabolic process. Polyadenylation-dependent snoRNA 3′-end processing. Positive regulation of isotype switching. RNA catabolic process. RNA processing. rRNA processing. U4 snRNA 3′-end processing. | 0.0441 | −5342 | T2(phospho) | |
For WFA, hemostasis (FDR: 7.12 × 10−3), metabolism of proteins (FDR: 1.84 × 10−1), immune system (FDR: 1.34 × 10−1), disease (FDR: 8.58 × 10−1) and signal transduction (FDR: 5.74 × 10−1) were the main relevant top-level reactome pathways. Moreover, the formation of the fibrin clot and the intrinsic pathway of fibrin clot formation were the hemostasis reactome pathways with the highest number of hits.
For AI, immune system (FDR: 5.69 × 10−1), metabolism of proteins (FDR: 7.47 × 10−1), development biology (FDR: 6.31 × 10−1), metabolism of RNA (FDR: 8.38 × 10−1) and gene expression (FDR: 9.92 × 10−1) were the main relevant top-level reactome pathways. Moreover, initial triggering of complement and regulation of complement cascade were the immune system reactome pathways with the highest number of hits.
A global protein-to-protein interaction network was constructed for the significantly modified total proteome and phosphoproteome (ESI Fig. 5†). 11 of the proteins that are modified during the interventions are part of the complement and coagulation cascade KEGG pathways (1.94; FDR: 2.36 × 10−16).
3.11 Correlations between proteomic and phosphoproteomic variables and inflammatory markers
The significant proteins modified after the three interventions (by proteome and phosphoproteome analyses) were correlated with the inflammatory markers with significant changes during the intervention, since the results obtained for inflammatory markers are one of the most relevant results in the present study, especially due to its clinical translation (ESI Table 33†). The difference of IGLV2–8 protein at 6 weeks was directly related to the difference of hs-CRP at 6 weeks (R = 0.567, P = 0.001). Moreover, the difference of IGLV4–69 at 6 weeks was directly related to the difference of IL-6 at 6 weeks (R = 0.416, P = 0.022). Otherwise, the ESSSHHPGIAEFPSR peptide phosphorylation at 6 weeks was inversely related to the difference of IL-6 at 6 weeks (R = −0.419, P = 0.029).4. Discussion
In this study, we observed effective health–promoting results of either ACNs through an infusion or through whole apple against cardiometabolic risk biomarkers. The present study verified that daily consumption of RFA, containing 34.5 mg of ACNs (of which 30.9 mg were cyanidin-3-O-galactoside), for 6 weeks improved arterial vasodilation assessed by IRH and produced anti-inflammatory effects decreasing hs-CRP and IL-6 compared to WFA with no content in ACNs. The arterial vasodilatation assessed by IRH was also increased at 6 h postprandial after RFA ingestion at the beginning of the study and compared to its baseline and at 6 h postprandial after three treatments at the end of the study and compared to its baseline. Moreover, AI, containing 37.4 mg of ACNs (of which 28.6 were cyanidin-3-O-galactoside), decreased P-selectin (an index of inflammation) compared with WFA. AI daily consumption had a marked impact on the lipid profile decreasing total cholesterol, LDL-c, total cholesterol/HDL ratio and TG after 6 weeks of intervention. Finally, the daily consumption of the three products evaluated reduced complement factors such as C1q, C4, and Factor B. These results were observed by proteomics and phosphoproteomic analyses and verified through multiplex, observing a reduction of the complement system proteins after the consumption of the three products, and being the complement and coagulation cascade pathways – the main signalling pathways modified.In the frame of the AppleCOR project, our previous work in hypercholesterolaemic rats showed CMD protective effects after 6 weeks of diet supplementation with the same tested products (RFA, WFA and AI). Specifically, a significant reduction in the aorta thickness, an improvement in kidney function in females, a decrease in insulin plasma concentration in males (both apples)14 and an anti-inflammatory effect were reported through the complement system by both apple consumption and reduced hs-CRP only by RFA.15 In this sense, our previous results in rats showed that regardless of the ACN content, the apple diet supplementation (RFA and WFA) achieved the reduction of inflammatory proteins.
Our present results showed that after 6 weeks of AI ingestion, the lipid profile was significantly improved compared with its baseline, whereas no significant and relevant changes were observed after the intake of WFA or RFA. However, different systematic reviews have indicated that the consumption of ACNs is linked to an improvement in the lipid profile.25,26 Recently, in line with our results, a randomized controlled trial with barberry, rich in ACNs, showed that sustained barberry consumption improves the lipid profile, decreasing plasma levels of TG, total cholesterol, LDL-c, non-HDL-c, and total cholesterol/HDL-c ratio in subjects with cardiovascular risk factors.27 We hypothesized that both products rich in ACNs (RFA and AI) would modulate the lipid profile similarly; however, RFA, with practically the same concentrations of ACNs as AI, did not manage to improve the lipid profile. Thus, the complexity of the food matrix where ACNs are present could play an important role. In this regard, AI is a simple source of solubilized ACNs which favours their bioavailability and RFA is a complex matrix where the ACNs are linked to fibre. Specifically, pectin, a soluble fibre present in apples, has been shown to affect flavonoid absorption due to the binding interactions between fibre and flavonoids.28 These differences in the food matrix could affect the bioaccessibility, bioavailability, and ultimately the effect of ACNs on the lipid profile. Subsequently, our group recently demonstrated a clear apple matrix effect between the RFA and AI, reporting a higher bioavailability and excretion of ACN after AI intake compared to RFA.21 These results emphasize the significance and complexity of PC interactions within other food components and confirm the findings of other studies using different food matrices,29 reporting that mainly dietary fibre may induce a disadvantageous impact on the bioavailability of flavonoids acting as entrapping agents.
In line with our results, a recent meta-analysis of randomized controlled trials (RCTs) evaluating the effect of dietary PCs on selected markers of cardiometabolic health concluded that purified food PC extracts improved the lipid profile more than the consumption of whole PC rich foods.30
In the present study, we used IRH, as a non-invasive method to assess endothelial function. IRH reduction reflex impaired flow-mediated vasodilation, which is considered an initial indicator of atherosclerosis and is associated with increased carotid intima-media thickness and left ventricular hypertrophy.31 In our study, increases in IRH after RFA intervention were observed both after a single dose and after sustained consumption. After 6 weeks of RFA consumption, IRH increased compared to WFA. At 6 h postprandial after RFA ingestion, IRH increased compared to its baseline in both studies, at the beginning and at the end of the sustained study. At the end of the sustained study, IRH also increased at 6 h postprandial and versus its baseline after WFA and AI. Thus, an optimization of the vasodilatory effect by an acute postprandial intake was stated, suggesting a beneficial consequence of sustained consumption of each three products.
ACN-rich foods or ACN extract consumption (acute or sustained) has previously been demonstrated to improve vascular reactivity, specifically endothelial function determined by flow-mediated dilatation.32
In the present study we were able to demonstrate that RFA, rich in ACNs, improved the endothelial function after a sustained consumption and compared to WFA, even so, we could not demonstrate that AI (with a similar content of ACN) improved it significantly. This may be because, apart from ACNs, the RFA has a different PC profile than AI, with higher concentrations of some other PCs (159 mg of other PCs vs. 61.7 mg), such as other flavonoids. In this sense, other flavonoids have also been linked to an improvement in endothelial function.33 Thus, other PCs present in the RFA may have exerted a synergistic effect with ACNs by improving endothelial function, which was therefore not achieved with AI consumption. Otherwise, this synergistic effect is also apparent when the WFA, despite having a high concentration of PCs (197 mg), not only fails to improve endothelial function, but also decreases IRH after sustained consumption. Therefore, to achieve the beneficial effects on endothelial function, the combination of ACNs plus other PCs was probably necessary and presented as an apple matrix.
The anti-inflammatory effect of ACN supplementation by reducing TNF-α, IL-6, and hs-CRP was stated by a meta-analysis.26 Our results are in line with this meta-analysis, since both the RFA and the AI managed to improve the inflammatory status by reducing the different inflammation markers evaluated. RFA decreased hs-CRP and IL-6 compared to WFA and AI decreased P-selectin compared to WFA and ICAM-1 compared to the baseline. The anti-inflammatory effect of RFA has previously been demonstrated by our group since in our previous study with hypercholesterolaemic rats we also observed a decrease in CRP by proteomic analysis of the heart tissue after the consumption of RFA.15 In contrast, a published study assessing the effects of a red apple on the lipid profile and CRP compared to a green apple, found no significant effects on any of the analysed parameters.17 However, it should be considered this study17 was a cross-over study where the intervention lasted 2 weeks followed by a one-week washout and a further two-week crossover period. So, maybe two weeks were not sufficient to modulate inflammation through CRP; however, the 6 weeks that our intervention lasts are sufficient to produce the effects on CRP. Taken together, our results suggest that the incorporation of red-flesh-coloured apples in our diet can alleviate inflammatory markers compared to the common WFA.
Molecules related to the complement system were modulated after all treatments (WFA, AI and RFA). Complement C1q, C4 and factor B proteins were reduced after WFA, AI and RFA compared to the baseline. Complement C3 was also reduced after AI and RFA compared to the baseline. These results are replicated through the plasma proteomic analyses, where downregulation of complement C4 protein was identified after all the treatments.
In addition, with the analysis of the major relevant biological pathways carried out in the present study, with the differentially expressed proteins (by proteomics and phosphoproteomics) after the RFA, WFA and AI interventions, it has been identified that the immune system is one of the most relevant biological pathways implicated in the three products. Specifically, the main immune system pathways involved are: regulation of complement cascade, activation of C3 and C5, complement cascade and initial triggering of complement. In this sense, the proteins involved in these pathways are: C4A, SERING1 (for RFA), C1R, LRG1, C4A, PROS1, F2, FGB, FGG, HP (for WFA) and C4A, IGLL1 (for AI).
The complement system is a multifaceted protein network of the immune defense system, being important in the activation of the innate and adaptative immune response. It consists of membrane-bound and soluble proteins working in cascades of step-by-step protease activation.34 Activation of the complement system can occur through different pathways, known as the classical pathway (includes C1qrs, C2, and C4 components), the alternative pathway (includes C3 components, factor B, and properdin) and the lecithin pathway (including but not limited to the mannan-binding lectin). The observation that the complement components are elevated or decreased does not allow us to identify a specific pathological situation, but it does indicate that the immune system is involved in the pathological process that affects the individual.35
Complement components C3 and C4 were previously extensive associated with CVD, metabolic syndrome, and type 2 diabetes.36 Thus, increased levels of serum C3 and C4 have been related to obesity and insulin resistance. Moreover, increased activation of C1q induced the biosynthesis of inflammatory cytokines and chemokines in the adipose tissue, resulting in adipose tissue inflammation.36
Fat ingestion has been postulated as a possible inductor of components of the complement system.37 A randomised, double-blind, intervention study demonstrated that dietary medium-chain saturated fatty acids from milk downregulated genes related to the complement system towards a decreased inflammatory state of the adipose tissue.37 Although some studies relate a possible effect of PCs on the inhibitory effect on all complement pathways,38 the literature is still limited. In this sense, a downregulation of proteins involved in the complement system has been previously demonstrated by proteomic analysis after RFA and WFA in hypercholesterolemic rats supporting effects and possible mechanisms of action of the apple.15 Therefore, we corroborate that the effect on the complement system could not only be exclusive to ACNs but also to other PCs present in the matrices of the three products studied. Complement components can rise due to inflammation, rising even earlier than other markers such as CRP.39
As we previously mentioned, the results of the proteomic analyses showed that WFA, AI or RFA cause the modulation of plasma proteins. Some of the proteome changes observed are common to the three interventions, such as the downregulation of complement C4, other changes coincide between RFA and AI, such as immunoglobulin lambda variable 2–8 and 4–69, and others are specific to each treatment. In the present study, many of the changes observed in the three intervention groups are related to immunoglobulins. The effect that different foods rich in PCs can have on the expression of plasma immunoglobulins has previously been defined.40 In addition, our results are in line with a recent study in humans where the healthy effect of red apples was tested against green apples demonstrating that the consumption for two weeks of the two varieties of apples produces changes in the expression of immunoglobulin coding genes.17 At the same time, with the results of the correlations made between the proteins changed after the three interventions and the main inflammation markers modified during the intervention, we observe that IGLV2–8 protein was directly related to hs-CRP serum concentrations and IGLV4–69 was directly related to IL-6 serum concentrations, indicating a possible anti-inflammatory role of immunoglobulin variables which should be further studied.
Thus, our results suggest that the consumption of WFA, AI, and RFA for 6 weeks can improve immune responses through the modulation of the expression of proteins of the complement system, immunoglobulins and CRP.
Concerning the phosphoproteomic results, one of the most noticeable changes observed is the decrease in phosphorylation of the ESSSHHPGIAEFPSR peptide sequence of the fibrinogen alpha chain protein. Fibrinogen has been linked to the adaptative and innate immune response, blood coagulation and inflammation.41 Otherwise, with the results of the correlations made between phosphoproteomic changes after the three interventions and the main inflammation markers modified during the intervention, we observe that the ESSSHHPGIAEFPSR peptide phosphorylation was inversely related to IL-6 serum concentrations, also indicating the possible role of the phosphorylation of this peptide on inflammation. The proteome and phosphoproteomic changes and these correlation results open new standpoints about which target molecules are involved in the anti-inflammation effects of RFA or AI. Even so, more studies are needed to corroborate the specific function of the results obtained on phosphorylation.
The study has strengths and limitations. As a strength, the participants’ diets were supervised during the entire study, and evading ACN-rich foods and avoiding eating functional foods intended to improve cholesterol levels were given as dietary recommendations to all the participants, which is of special interest in nutritional randomized controlled trials (RCTs) because these guidelines would limit confounding between other dietary compounds and the dietary intervention. The general dietary recommendations were standardized for all the intervention groups to reduce the potential confounding effects of other dietary components beyond the intervention foods. Moreover, in most previous studies, only a single food component or isolated pure flavonoids were used, and very scarce studies have evaluated more than one matrix component with ACN-rich foods, being one of the main strengths of our study.
Another important strength is that this study constitutes the first human RCT that assessed compliance markers of RFA (phloretin-O-xylosyl glucoside), AI (peonidin-O-glucuronide and peonidin-3-O-arabinoside) and peonidin-3-O-galactoside, a common biomarker of AI and RFA and observed associations with CMD biomarkers (sustained lipid biomarkers, CRP and C3, respectively), and thus, these results add robustness to our study. Moreover, in the present study, the human clinical results have been complemented with proteomic human analysis, which also demonstrated a modulation of the immune system by both the complement system and of the immunoglobulins after all treatments, thus giving more strength to the results. In addition, of great value is that the results in the present human study replicate the results found in hypercholesterolaemic rats, in which a decrease in inflammation and an improvement of the immune system were also observed by proteomic analysis.
One limitation is the inability to assess potential interactions between the interventions and other dietary components. The fact that participants were hypercholesterolaemic individuals limits the extrapolation of the results to the general population. Whether different or added effects would have been detected over longer periods is unknown, but longer intervention may affect the compliance of the volunteers.
5. Conclusions
In conclusion, several CMD biomarkers are modulated in response to the consumption of ACN-biofortified apple (RFA) and Aronia ACN-rich infusion (AI) containing similar concentration and composition of ACNs but differing in the food matrix. The diet supplementation during 6 weeks with RFA improves endothelial function, with AI improving the lipid profile and both of them reducing inflammation. The finding that RFA induces more improvements in inflammation than AI could be due to the synergy of ACNs with other bioactive compounds present in the apple matrix. In contrast, the diet supplementation with AI resulted in a better modulation of the lipid profile, compared with RFA, probably related to the higher ACN bioavailability. Moreover, our results suggest that the consumption of WFA, AI, and RFA for 6 weeks can improve immune responses through the modulation of the expression of proteins of the complement system and immunoglobulins and changes in the complement and coagulation cascade biological pathways. Taken together, the present study supports previous human evidence related to the beneficial effects of ACNs on ameliorating the risk of lifestyle-associated metabolic disorders and reinforces the interest in the newly emerging biofortified RFA cultivars as an interesting and attractive source of bioactive phenols with added health properties compared to common WFA.Author contributions
Conceptualization: Motilva MJ, Solà R, Valls RM and Pedret A; formal analysis: Canela N, Pedret A, Catalán Ú and Fernández-Castillejo S; funding acquisition: Motilva MJ, Solà R, Valls RM and Pedret A; methodology: Pedret A, Companys J, Calderón-Pérez L, Llauradó E, Pla-Pagà L, Salamanca P, Sandoval-Ramírez BA, Catalán Ú, Fernández-Castillejo S, Yuste S, Macià A, Gutiérrez-Tordera L, Bulló M, Camps J, Canela N, Valls RM, Rubió-Piqué L, Motilva MJ and Solà R; writing – original draft: Pedret A, Solà R and Fernández-Castillejo S; review and editing: Pedret A, Companys J, Calderón-Pérez L, Llauradó E, Pla-Pagà L, Salamanca P, Sandoval-Ramírez BA, Catalán Ú, Fernández-Castillejo S, Yuste S, Macià A, Gutiérrez-Tordera L, Bulló M, Camps J, Canela N, Valls RM, Rubió-Piqué L, Motilva MJ and Solà R.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This Applecor study was funded by Ministerio de Economía, Industria y Competitividad, the Agencia Estatal de Investigación (AEI), and the European Regional Development Fund (ERDF) (grant number: AppleCOR Project, AGL2016-76943-C2-2-R). The NFOC-Salut group is a consolidated research group of Generalitat de Catalunya, Spain (2014 SGR 873, 2017 SGR 522 and 2021 SGR817). We also want to thank the Calidad Pascual company, Bezoya (Aranda de Duero, Burgos, Spain) which provided the mineral water necessary for the preparation of the AI.References
- USDA, United States Department of Agriculture Foreign Agricultural Service, Fresh Apples, Grapes, and Pears: World Markets and Trade, 2023 Search PubMed.
- N. Wang, S. Jiang, Z. Zhang, H. Fang, H. Xu, Y. Wang and X. Chen, Malus sieversii: the origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples, Hortic. Res., 2018, 5, 70–81 CrossRef PubMed.
- B. A. Sandoval-Ramírez, Ú. Catalán, L. Calderón-Pérez, J. Companys, L. Pla-Pagà, I. A. Ludwig, M. P. Romero and R. Solà, The effects and associations of whole-apple intake on diverse cardiovascular risk factors. A narrative review, Crit. Rev. Food Sci. Nutr., 2020, 60, 3862–3875 CrossRef PubMed.
- K. W. Lee, Y. J. Kim, D. O. Kim, H. J. Lee and C. Y. Lee, Major phenolics in apple and their contribution to the total antioxidant capacity, J. Agric. Food Chem., 2003, 51, 6516–6520 CrossRef CAS PubMed.
- D. Bars-Cortina, A. Macià, I. Iglesias, M. P. Romero and M. J. Motilva, Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties, J. Agric. Food Chem., 2017, 65, 1684–1696 CrossRef CAS PubMed.
- M. Kalinowska, A. Bielawska, H. Lewandowska-Siwkiewicz, W. Priebe and W. Lewandowski, Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties, Plant Physiol. Biochem., 2014, 84, 169–188 CrossRef PubMed.
- R. Mattioli, A. Francioso, L. Mosca and P. Silva, Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases, Molecules, 2020, 25, 3809–3851 CrossRef CAS PubMed.
- B. A. Sandoval-Ramírez, Ú. Catalán, S. Fernández-Castillejo, L. Rubió, A. Macià and R. Solà, Anthocyanin Tissue Bioavailability in Animals: Possible Implications for Human Health. A Systematic Review, J. Agric. Food Chem., 2018, 66, 11531–11543 CrossRef PubMed.
- X. Wang, Y. Ouyang, J. Liu, M. Zhu, G. Zhao, W. Bao and F. B. Hu, Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies, Br. Med. J., 2014, 29, 349–363 Search PubMed.
- T. C. Wallace, M. Slavin and C. L. Frankenfeld, Systematic Review of Anthocyanins and Markers of Cardiovascular Disease, Nutrients, 2016, 8, 32–45 CrossRef PubMed.
- A. Basu, M. Rhone and T. J. Lyons, Berries: emerging impact on cardiovascular health, Nutr. Rev., 2010, 68, 168–177 CrossRef PubMed.
- C. Del Bo’, D. Martini, M. Porrini, D. Klimis-Zacas and P. Riso, Berries and oxidative stress markers: an overview of human intervention studies, Food Funct., 2015, 6, 2890–2917 RSC.
- J. Bonany, C. Brugger, A. Buehler, J. Carbó, S. Codarin, F. Donati, G. Echeverria, S. Egger, W. Guerra, C. Hilaire, I. Höller, I. Iglesias, K. Jesionkowska, D. Konopacka, D. Kruczyńska, A. Martinelli, C. Petiot, S. Sansavini, R. Stehr and F. Schoorl, Preference mapping of apple varieties in Europe, Food Qual. Prefer., 2014, 32, 317–329 CrossRef.
- S. Yuste, I. A. Ludwig, M. P. Romero, C. Piñol-Felis, Ú. Catalán, A. Pedret, R. M. Valls, S. Fernández-Castillejo, M. J. Motilva, A. Macià and L. Rubió, Metabolic Fate and Cardiometabolic Effects of Phenolic Compounds from Red-Fleshed Apple in Hypercholesterolemic Rats: A Comparative Study with Common White-Fleshed Apple. The AppleCOR Study, Mol. Nutr. Food Res., 2021, 65(10), e2001225 CrossRef PubMed.
- Ú. Catalán, A. Pedret, S. Yuste, L. Rubió, C. Piñol, B. A. Sandoval-Ramírez, J. Companys, E. Foguet, P. Herrero, N. Canela, M. J. Motilva and R. Solà, Red-Fleshed Apples Rich in Anthocyanins and White-Fleshed Apples Modulate the Aorta and Heart Proteome in Hypercholesterolaemic Rats: The AppleCOR Study, Nutrients, 2022, 14, 1047–1070 CrossRef PubMed.
- D. Bars-Cortina, A. Martínez-Bardají, A. Macià, M. J. Motilva and C. Piñol-Felis, Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model, J. Nutr. Biochem., 2020, 83, 108418 CrossRef CAS PubMed.
- M. P. G. Barnett, W. Young, K. Armstrong, D. Brewster, J. M. Cooney, S. Ellett, R. V. Espley, W. Laing, P. Maclean, T. McGhie, G. Pringle, N. C. Roy and L. R. Ferguson, A Polyphenol Enriched Variety of Apple Alters Circulating Immune Cell Gene Expression and Faecal Microbiota Composition in Healthy Adults: A Randomized Controlled Trial, Nutrients, 2021, 13, 1092–1110 CrossRef CAS PubMed.
- C. Vallbona, E. Roura, M. Violan and J. Alegre, Guía de prescripció d’exercici físic per a la salut (PEFS), 2007 Search PubMed.
- F. Mach, C. Baigent, A. L. Catapano, K. C. Koskinas, M. Casula, L. Badimon, M. J. Chapman, G. G. De Backer, V. Delgado, B. A. Ference, I. M. Graham, A. Halliday, U. Landmesser, B. Mihaylova, T. R. Pedersen, G. Riccardi, D. J. Richter, M. S. Sabatine, M.-R. Taskinen, L. Tokgozoglu, O. Wiklund, E. S. D. Group, C. Mueller, H. Drexel, V. Aboyans, A. Corsini, W. Doehner, M. Farnier, B. Gigante, M. Kayikcioglu, G. Krstacic, E. Lambrinou, B. S. Lewis, J. Masip, P. Moulin, S. Petersen, A. S. Petronio, M. F. Piepoli, X. Pintó, L. Räber, K. K. Ray, Ž. Reiner, W. F. Riesen, M. Roffi, J.-P. Schmid, E. Shlyakhto, I. A. Simpson, E. Stroes, I. Sudano, A. D. Tselepis, M. Viigimaa, C. Vindis, A. Vonbank, M. Vrablik, M. Vrsalovic, J. L. Zamorano, J.-P. Collet, K. C. Koskinas, M. Casula, L. Badimon, M. John Chapman, G. G. De Backer, V. Delgado, B. A. Ference, I. M. Graham, A. Halliday, U. Landmesser, B. Mihaylova, T. R. Pedersen, G. Riccardi, D. J. Richter, M. S. Sabatine, M.-R. Taskinen, L. Tokgozoglu, O. Wiklund, S. Windecker, V. Aboyans, C. Baigent, J.-P. Collet, V. Dean, V. Delgado, D. Fitzsimons, C. P. Gale, D. Grobbee, S. Halvorsen, G. Hindricks, B. Iung, P. Jüni, H. A. Katus, U. Landmesser, C. Leclercq, M. Lettino, B. S. Lewis, B. Merkely, C. Mueller, S. Petersen, A. S. Petronio, D. J. Richter, M. Roffi, E. Shlyakhto, I. A. Simpson, M. Sousa-Uva, R. M. Touyz, D. Nibouche, P. H. Zelveian, P. Siostrzonek, R. Najafov, P. van de Borne, B. Pojskic, A. Postadzhiyan, L. Kypris, J. Špinar, M. L. Larsen, H. S. Eldin, M. Viigimaa, T. E. Strandberg, J. Ferrières, R. Agladze, U. Laufs, L. Rallidis, L. Bajnok, T. Gudjónsson, V. Maher, Y. Henkin, M. M. Gulizia, A. Mussagaliyeva, G. Bajraktari, A. Kerimkulova, G. Latkovskis, O. Hamoui, R. Slapikas, L. Visser, P. Dingli, V. Ivanov, A. Boskovic, M. Nazzi, F. Visseren, I. Mitevska, K. Retterstøl, P. Jankowski, R. Fontes-Carvalho, D. Gaita, M. Ezhov, M. Foscoli, V. Giga, D. Pella, Z. Fras, L. P. de Isla, E. Hagström, R. Lehmann, L. Abid, O. Ozdogan, O. Mitchenko and R. S. Patel, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk, Eur. Heart J., 2020, 41, 111–188 CrossRef PubMed.
- S. Yuste, I. A. Ludwig, L. Rubió, M. P. Romero, A. Pedret, R. M. Valls, R. Solà, M. J. Motilva and A. Macià, In vivo biotransformation of (poly)phenols and anthocyanins of red-fleshed apple and identification of intake biomarkers, J. Funct. Foods, 2019, 55, 146–155 CrossRef CAS.
- A. Macià, M. P. Romero, S. Yuste, I. Ludwig, A. Pedret, R. M. Valls, P. Salamanca, R. Solà, M. José Motilva and L. Rubió, Phenol metabolic fingerprint and selection of intake biomarkers after acute and sustained consumption of red-fleshed apple versus common apple in humans. The AppleCOR study, Food Chem., 2022, 384, 132612–132624 CrossRef PubMed.
- A. L. Siu, K. Bibbins-Domingo, D. Grossman, L. C. Baumann, K. W. Davidson, M. Ebell, F. A. R. García, M. Gillman, J. Herzstein, A. R. Kemper, A. H. Krist, A. E. Kurth, D. K. Owens, W. R. Phillips, M. G. Phipps and M. P. Pignone, Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement, Ann. Intern. Med., 2015, 163, 778–786 CrossRef PubMed.
- S. Fernández-Castillejo, A. I. García-Heredia, R. Solà, J. Camps, M. C. López de la Hazas, M. Farràs, A. Pedret, Ú. Catalán, L. Rubió, M. J. Motilva, O. Castañer, M. I. Covas and R. M. Valls, Phenol-enriched olive oils modify paraoxonase-related variables: A randomized, crossover, controlled trial, Mol. Nutr. Food Res., 2017, 61, 10 Search PubMed.
- A. Pedret, Ú. Catalán, L. Rubió, I. Baiges, P. Herrero, C. Piñol, R. Rodríguez-Calvo, N. Canela, S. Fernández-Castillejo, M. J. Motilva and R. Solà, Phosphoproteomic Analysis and Protein-Protein Interaction of Rat Aorta GJA1 and Rat Heart FKBP1A after Secoiridoid Consumption from Virgin Olive Oil: A Functional Proteomic Approach, J. Agric. Food Chem., 2021, 69, 1536–1554 CrossRef CAS PubMed.
- L. P. Yang, W. H. Ling, Z. C. Du, Y. M. Chen, D. Li, S. Z. Deng, Z. M. Liu and L. L. Yang, Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Adv. Nutr., 2017, 8, 684–693 CrossRef CAS PubMed.
- K. Shah and P. Shah, Effect of Anthocyanin Supplementations on Lipid Profile and Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Cholesterol, 2018, 8450793–8450805 Search PubMed.
- H. Emamat, A. Zahedmehr, S. Asadian and J. Nasrollahzadeh, The effect of barberry (Berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: a randomized controlled trial, BMC Complementary Med. Ther., 2022, 22, 59–68 CrossRef CAS PubMed.
- A. E. Quirós-Sauceda, H. Palafox-Carlos, S. G. Sáyago-Ayerdi, J. F. Ayala-Zavala, L. A. Bello-Perez, E. Álvarez-Parrilla, L. A. De La Rosa, A. F. González-Córdova and G. A. González-Aguilar, Dietary fiber and phenolic compounds as functional ingredients: interaction and possible effect after ingestión, Food Funct., 2014, 5, 1063–1072 RSC.
- S. Kamiloglu, M. Tomas, T. Ozdal and E. Capanoglu, Effect of food matrix on the content and bioavailability of flavonoids, Trends Food Sci. Technol., 2021, 117, 15–33 CrossRef CAS.
- T. Kiyimba, P. Yiga, M. Bamuwamye, P. Ogwok, B. Van der Schueren and C. Matthys, Efficacy of Dietary Polyphenols from Whole Foods and Purified Food Polyphenol Extracts in Optimizing Cardiometabolic Health: A Meta-Analysis of Randomized Controlled Trials, Adv. Nutr., 2023, 14, 270–282 CrossRef CAS PubMed.
- R. M. Bruno, T. Gori and L. Ghiadoni, Endothelial function testing and cardiovascular disease: focus on peripheral arterial tonometry, Vasc. Health Risk Manage., 2014, 10, 577–584 Search PubMed.
- L. Fairlie-Jones, K. Davison, E. Fromentin and A. M. Hill, The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials, Nutrients, 2017, 9, 908–931 CrossRef PubMed.
- R. M. Valls, A. Pedret, L. Calderón-Pérez, E. Llauradó, L. Pla-Pagà, J. Companys, A. Moragas, F. Martín-Luján, Y. Ortega, M. Giralt, L. Rubió, N. Canela, F. Puiggrós, A. Caimari, J. M. Del Bas, L. Arola and R. Solà, Hesperidin in orange juice improves human endothelial function in subjects with elevated blood pressure and stage 1 hypertension: A randomized, controlled trial (Citrus study), J. Funct. Foods, 2021, 85, 104646–104655 CrossRef CAS.
- R. Lubbers, M. F. van Essen, C. van Kooten and L. A. Trouw, Production of complement components by cells of the immune system, Clin. Exp. Immunol., 2017, 188, 183–194 CrossRef CAS PubMed.
- N. S. Merle, S. E. Church, V. Fremeaux-Bacchi and L. T. Roumenina, Complement System Part I - Molecular Mechanisms of Activation and Regulation, Front. Immunol., 2015, 6, 262–292 Search PubMed.
- J. M. Moreno-Navarrete and J. M. Fernández-Real, The complement system is dysfunctional in metabolic disease: Evidences in plasma and adipose tissue from obese and insulin resistant subjects, Semin. Cell Dev. Biol., 2019, 85, 164–172 CrossRef CAS PubMed.
- J. C. Matualatupauw, M. Bohl, S. Gregersen, K. Hermansen and L. A. Afman, Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects, Int. J. Obes., 2017, 41, 1348–1354 CrossRef CAS PubMed.
- A. R. C. de Souza, T. L. de Oliveira, P. D. Fontana, M. C. Carneiro, M. L. Corazza, I. J. de Messias Reason and L. Bavia, Phytochemicals and Biological Activities of Burdock (Arctium lappa L.) Extracts: A Review, Chem. Biodivers., 2022, 19(11), e202200615 CrossRef CAS PubMed.
- N. R. Sproston and J. J. Ashworth, Role of C-Reactive Protein at Sites of Inflammation and Infection, Front. Immunol., 2018, 9, 754 CrossRef PubMed.
- P. Ruiz-Iglesias, M. Massot-Cladera, F. J. Pérez-Cano and M. Castell, Influence of Diets Enriched with Flavonoids (Cocoa and Hesperidin) on the Systemic Immunity of Intensively Trained and Exhausted Rats, Biomolecules, 2022, 12(12), 1893 CrossRef CAS PubMed.
- J. J. De Vries, C. J. M. Snoek, D. C. Rijken and M. P. M. De Maat, Effects of Post-Translational Modifications of Fibrinogen on Clot Formation, Clot Structure, and Fibrinolysis: A Systematic Review, Arterioscler., Thromb., Vasc. Biol., 2020, 40, 554 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo05114e |
| ‡ Senior authors. |
| This journal is © The Royal Society of Chemistry 2024 |