Open Access Article
Open Access ArticleCharacterisation and beneficial effects of a Lupinus angustifolius protein hydrolysate obtained by immobilisation of the enzyme alcalase®†
Guillermo
Santos-Sánchez
 ab,
Ivan
Cruz-Chamorro
ab,
Ivan
Cruz-Chamorro
 *ab,
José Carlos
Márquez-López
c,
Justo
Pedroche
c,
Ana Isabel
Álvarez-López
ab,
María del Carmen
Millán-Linares
*ab,
José Carlos
Márquez-López
c,
Justo
Pedroche
c,
Ana Isabel
Álvarez-López
ab,
María del Carmen
Millán-Linares
 b,
Patricia Judith
Lardone
ab and
Antonio
Carrillo-Vico
b,
Patricia Judith
Lardone
ab and
Antonio
Carrillo-Vico
 *ab
*ab
aInstituto de Biomedicina de Sevilla, IBiS/Hospital Universitario Virgen del Rocío/CSIC/Universidad de Sevilla, 41013 Seville, Spain. E-mail: vico@us.es; icruz-ibis@us.es
bDepartamento de Bioquímica Médica y Biología Molecular e Inmunología, Facultad de Medicina, Universidad de Sevilla, 41009 Seville, Spain
cDepartment of Food & Health, Instituto de la grasa, CSIC, Ctra, Utrera Km 1, 41013 Seville, Spain
First published on 15th March 2024
Abstract
Bioactive peptides have been considered potential components for the future functional foods and nutraceuticals generation. The enzymatic method of hydrolysis has several advantages compared to those of chemical hydrolysis and fermentation. Despite this fact, the high cost of natural and commercial proteases limits the commercialization of hydrolysates in the food and pharmacological industries. For this reason, more efficient and economically interesting techniques, such as the immobilisation of the enzyme, are gaining attention. In the present study, a new protein hydrolysate from Lupinus angustifolius was generated by enzymatic hydrolysis through the immobilisation of the enzyme alcalase® (imLPH). After the chemical and nutritional characterization of the imLPH, an in vivo study was carried out in order to evaluate the effect of 12 weeks treatment with imLPH on the plasmatic lipid profile and antioxidant status in western-diet-fed apolipoprotein E knockout mice. The immobilisation of alcalase® generated an imLPH with a degree of hydrolysis of 29.71 ± 2.11%. The imLPH was mainly composed of protein (82.50 ± 0.88%) with a high content of glycine/glutamine, arginine, and aspartic acid/asparagine. The imLPH-treatment reduced the amount of abdominal white adipose tissue, total plasma cholesterol, LDL-C, and triglycerides, as well as the cardiovascular risk indexes (CRI) -I, CRI-II, and atherogenic index of plasma. The imLPH-treated mice also showed an increase in the plasma antioxidant capacity. For the first time, this study demonstrates the beneficial in vivo effect of a lupin protein hydrolysate obtained with the alcalase® immobilised and points out this approach as a possible cost-effective solution at the expensive generation of the hydrolysate through the traditional batch conditions with soluble enzymes.
1. Introduction
In recent decades, the use of bioactive peptides has become a promising strategy to improve the quality of human life. Bioactive peptides, in most cases shorter than 50 residues, are normally encrypted in the parent protein and become active after the cleavage of the protein.1 They have been shown to have pleiotropic effects and benefits for health, such as antioxidants, immunomodulators, reducers of glycemic, blood pressure, and lipid levels.2,3 This fact has attracted the attention of the pharmaceutical, food, and cosmetic industries on generating bioactive peptides from animal, vegetable, marine, industrial co-products or discarded remnant protein sources.4–6 Production of bioactive peptides on an industrial scale is carried out principally through two processes (chemical or biological), with enzymatic hydrolysis being the technique most used.7 The enzymatic hydrolysis method has certain advantages in comparison to other techniques such as fermentation and chemical hydrolysis because of an improved reaction rate and the savings in cost and energy, because of the use of mild temperature and pH conditions. Furthermore, no secondary products are generated, which is a more environmentally friendly process. In addition, it has remarkable regioselectivity and food stereoselectivity and generates a high number of peptides through a process that is considered simple.7,8 However, at an industrial level, enzymatic hydrolysis has the disadvantage of being a technique with low productivity and expensive, due to the price of enzymes. For that reason, enzymatic hydrolysis carried out using immobilised enzymes could be a solution to these limitations.1 This technique, in which a two-phase system is used, one for the enzyme and the other for the product, is more economical because the enzyme can be reused and it is not necessary to stop the enzymatic reaction by means of pH or temperature, reducing the costs of the overall hydrolytic process.9Recently, we demonstrated that the hydrolysis with soluble alcalase® of lupin (Lupinus angustifolius) proteins (sLPH) generates a peptides-mixture that exert antioxidant, anti-inflammatory and lipid-lowering effects in cell-free system models,10in vitro,11 in murine models of atherosclerosis and metabolic syndrome,12–14 and in healthy subjects.15
With the objective of commercialising this protein hydrolysate within a functional food, the aims of this study were (1) to generate a hydrolysate from the lupin protein through immobilisation (imLPH) of the alcalase® enzyme, (2) to characterise the imLPH, (3) to assess its in vitro antioxidant capacity, and (4) to evaluate its antioxidant and hypolipidemic capacity in the murine model of atherosclerosis and metabolic syndrome (ApoE−/−) compared to sLPH.
2. Materials and methods
2.1. Preparation of alcalase®-glyoxyl derivatives
The enzyme alcalase® (Novozymes, Bagsvaerd, Denmark) was immobilised as previously described elsewhere, as well as its enzymatic activity.162.2. Hydrolysis of lupin protein concentrate
Lupin protein concentrate (LPC) (Frank Food Products, Twello, The Netherlands) was hydrolysed by the immobilised alcalase® in a reactor with pH and temperature-controlled conditions. LPC was previously defatted, so that the fat content did not negatively influence the hydrolytic process. The defatted sample was incubated for 45 min at pH 8 (optimal pH conditions of alcalase®). Subsequently, the suspension was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497g for 15 min and 15 mL of the collected supernatant was suspended with alcalase®-glyoxyl derivative in a relation E/S = 5.52 g immobilised enzyme per g lupin protein to reach the E/S = 0.1 UA per g protein. After 1 h of incubation, alcalase®-glyoxyl derivative was removed by filtration and the collected supernatant was named imLPH. The sLPH was generated according to ref. 10.
497g for 15 min and 15 mL of the collected supernatant was suspended with alcalase®-glyoxyl derivative in a relation E/S = 5.52 g immobilised enzyme per g lupin protein to reach the E/S = 0.1 UA per g protein. After 1 h of incubation, alcalase®-glyoxyl derivative was removed by filtration and the collected supernatant was named imLPH. The sLPH was generated according to ref. 10.
2.3. Degree of hydrolysis (DH) determination
The percentage of cleaved peptide bonds, defined as degree of hydrolysis (DH), was measured by using the trinitrobenzenesulfonic acid (TNBS) (Sigma-Aldrich, St Louis, MO, USA) method according to a previous report.17 The complete hydrolysis of a sample was carried out at 110 °C for 24 hours in 6 N HCl, and this process was used to ascertain the total count of amino groups.2.4. Analytical methods
The amounts of proteins and peptides, the moisture and ash content, and the total fiber were determined according to our previous.11 The total lipid content was determined from hydrolysed samples (100 mg) with chloroform/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) as previously described.18 Fatty acid methyl esters were produced by acid-catalysed transmethylation and analysed by gas–liquid chromatography.19 Heptadecanoic acid was used as an internal standard. The oil content was calculated as the sum of the different fatty acids.
2, v/v) as previously described.18 Fatty acid methyl esters were produced by acid-catalysed transmethylation and analysed by gas–liquid chromatography.19 Heptadecanoic acid was used as an internal standard. The oil content was calculated as the sum of the different fatty acids.
2.5. Amino acid composition of the hydrolysate
The amino acids quantification was assayed according to ref. 20. The quantification of tryptophan was performed according to ref. 21.2.6. Determination of the molecular profile of LPHs by FPLC
A Superose 12 HR 10/30 column (Amersham Pharmacia Biotech, Uppsala, Sweden), with 300 to 1 kDa resolution, was employed for gel filtration in a fast protein liquid chromatography (FPLC) system. The samples (with 1.6 mg mL−1 protein concentration) were injected using a 200 mL loop. To elution (flow rate of 0.8 mL min−1) a 50 mL of solution (0.05 M sodium phosphate buffer, 0.5 M sodium chloride, and 0.02% sodium azide (w/v); pH 7.5) was used, monitored at 280 nm with the CLARIOstar plus microplate reader (BMG Labtech, Ortenberg, Germany).2.7. In vitro antioxidant activity
2.8. Animals and experimental design
ApoE−/− mice were housed in a colony at the Instituto de Biomedicina de Sevilla (IBiS) Animal Facility with a 12 h light/dark circle (lights on at 8:00 a.m.) and under controlled temperature (22 ± 2 °C) and humidity (<55%) conditions with free access to food and water. The animals were fed a Western diet (45% calories from fat, Table S1†) (University of Granada, Granada, Spain). In this study, four independent experiments (4–5 mice per group) were carried out. When male mice were 6-week-old, they were randomly divided into three groups and intragastrically treated with: vehicle (C group), 100 mg kg−1 imLPH, or 100 mg kg−1 sLPH for 12 weeks (5 days per week). Each week, individual body weight and daily food intake were recorded. Treatment administration was adjusted weekly to the weight of the mice. When the experiment was concluded, 12 h-fasted animals were euthanized by a sodium thiopental puncture (50 mg kg−1). The blood was then obtained by cardiac puncture and centrifuged (3000g, 4 °C, 10 min) to obtain plasma, which was stored at −20 °C until use. The experimental protocol was performed under the Spanish legislation and the EU Directive 2010/63/EU for animal experiments and was approved by the Virgen Macarena and Virgen del Rocío University Hospitals ethical committee (reference 21/06/2016/105). Initial ApoE−/− mice to generate the colony were kindly gifted by Dr Antonio Ordoñez and Dr Raquel del Toro (IBiS).2.9. Plasmatic lipid profile
Plasmatic lipid profile was measured by Cobas Integra 400 analyzer (Roche Diagnostics, IN, USA) at the Estación Biológica de Doñana (EBD-CSIC, Seville, Spain). Then, the Castelli risk I, II, and the atherogenic index of plasma (AIP) [log (TG/HDL-C)] were calculated.2.10. Antioxidant status assessment
To measure plasma antioxidant status, total antioxidant capacity (TAC) assay was performed. After 5 min of incubation, the CLARIOstar plus microplate reader (BMG Labtech) was employed to measure the absorbance at 490nm. To extrapolate the data, a uric acid standard curve was used.2.11. Statistical analysis
Jeffreys's Amazing Statistics Programme (JASP v. 0.16.3, Amsterdam, The Netherlands) was used for the statistical analysis, applying a one-way ANOVA followed by Dunn's post hoc test or the mixed-effect analysis, considering a p-value ≤0.05 as significant. Data were represented as the mean ± standard deviation and graphs were drawn with the GraphPad Prism v. 8 (GraphPad Software, Boston, MA, USA).3. Results
3.1 imLPH characterization
Fig. 1A shows that the degree of hydrolysis obtained after the use of immobilised alcalase® was 29.71 ± 2.11% after 60 min, a similar value compared to the soluble enzyme. The imLPH was mainly composed of proteins (82.50%) followed by ash (13.09%) and moisture (1.98%). Furthermore, the percentage of fiber, soluble sugars, fats, and polyphenols was 1.76, 0.43, 0.16, and 0.03%, respectively. The analysis of the imLPH showed peptides between 40 and <1 kDa in size, with two main peaks at 2 and <1 kDa (Fig. 1B). Compared to sLPH, imLPH contained a higher content of protein, ash, and fats, but a lower content of moisture, fiber, soluble sugars, and polyphenols.3.2. Amino acid composition
Table 1 reports the results obtained by the analysis of the amino acid composition of imLPH and sLPH. Acid glutamic and glutamine (25.72%) amino acids were the most abundant in imLPH, followed by arginine (14.23%), aspartic acid and asparagine (9.52%), leucine (6.79%), and serine (5.77%). However, the imLPH had low levels of the sulphur amino acids methionine (0.28%) and cysteine (0.79%). Compared to sLPH, imLPH showed similar values for all amino acids except arginine (imLPH: 14.23%, sLPH: 11.60%), leucine (imLPH: 6.79%, sLPH: 8.55%) and proline (imLPH: 3.20%, sLPH: 0.75%). Furthermore, tryptophan was the least represented amino acid in sLPH (0.35%) while methionine was in imLPH (0.28%).| Amino acid | imLPH (%) | sLPH (%) |
|---|---|---|
| Glu + Gln | 25.72 ± 0.80 | 24.47 ± 0.17 |
| Arg | 14.23 ± 0.12 | 11.60 ± 0.06 |
| Asp + Asn | 9.52 ± 0.25 | 10.27 ± 0.12 |
| Leu | 6.79 ± 0.21 | 8.55 ± 0.05 |
| Ser | 5.77 ± 0.09 | 5.97 ± 0.16 |
| Lys | 4.64 ± 0.13 | 4.92 ± 0.02 |
| Phe | 4.07 ± 0.32 | 4.95 ± 0.01 |
| Tyr | 4.01 ± 0.05 | 4.42 ± 0.12 |
| Gly | 3.93 ± 0.07 | 4.52 ± 0.06 |
| Ile | 3.77 ± 0.12 | 4.45 ± 0.01 |
| Ala | 3.34 ± 0.04 | 3.89 ± 0.07 |
| Val | 3.23 ± 0.09 | 3.47 ± 0.07 |
| Thr | 3.19 ± 0.09 | 4.05 ± 0.02 |
| Pro | 3.20 ± 0.61 | 0.75 ± 0.01 |
| His | 2.56 ± 0.14 | 2.36 ± 0.01 |
| Trp | 0.95 ± 0.11 | 0.35 ± 0.02 |
| Cys | 0.79 ± 0.14 | 0.56 ± 0.19 |
| Met | 0.28 ± 0.02 | 0.44 ± 0.15 |
3.3. LPHs improve the total antioxidant capacity and have DPPH radical scavenging activity
To assess the possible antioxidant capacity of sLPH and imLPH, the TAC and DPPH assays were performed. As Fig. 2A shows, both hydrolysates were capable of increasing the TAC values in a concentration-dependent manner, with respect to the C group (p < 0.0001). Higher TAC values were observed for each concentration of sLPH compared to imLPH (Fig. 2A). Regarding the DPPH radical scavenging activity, both sLPH and imLPH were capable of increasing the % DPPH radical inhibition in comparison to the C group (Fig. 2B). In particular, sLPH increased the DPPH radical inhibition by 5.5 (p < 0.0001), 13.91 (p < 0.0001), and 36.23% (p < 0.0001) at 0.5, 1 and 2.5 mg mL−1, respectively. In the same way, imLPH increased the DPPH radical scavenging by 3.31 (p = 0.004), 8.1 (p < 0.0001), and 20.43% (p < 0.0001) at increasing concentrations. Significant between sLPH and imLPH were shown at 1 and 2.5 mg mL−1, sLPH being the one that presented the highest effects (Fig. 2B).3.4. imLPH reduces the amount of abdominal white adipose tissue without altering the gain in body weight
No differences in the body weight were found throughout the experiment between the three experimental groups (Fig. 3A and B). Furthermore, the initial body weight (IBW) (C: 20.61 ± 1.24 g; sLPH: 20.58 ± 0.92 g; imLPH: 20.32 ± 1.90 g) (Fig. 3C), the final body weight (FBW) (C: 27.49 ± 2.65 g; sLPH: 27.04 ± 2.76 g; imLPH: 27.16 ± 2.37 g) (Fig. 3D), the body weight gain (BWG) (C: 6.85 ± 2.36 g; sLPH: 6.46 ± 2.55 g; imLPH: 6.84 ± 1.10 g) (Fig. 3E), and the daily food intake (DFI) (C: 2.72 ± 0.17 g per mouse per day; sLPH: 2.66 ± 0.24 g per mouse per day; imLPH: 2.64 ± 0.17 g per mouse per day) (Fig. 3F) did not show significant differences between the groups. However, a 27.18% and 23.5% reduction in abdominal white adipose tissue was observed in sLPH-treated mice (p = 0.016) and imLPH-treated mice (p = 0.017), respectively, compared to the C group (Fig. 3G). Between the three experimental groups, no significant differences in liver weight were found (C: 100 ± 10.11%; sLPH: 101.6 ± 8.63%; imLPH: 99.85 ± 13.74%) (Fig. 3H).3.5. imLPH reduces plasma lipid concentration and cardiovascular risk
In comparison with the C group, mice treated with sLPH and imLPH showed a significant reduction in total cholesterol (TC) by ∼10 (p = 0.014), and 13% (p = 0.004), respectively (Fig. 4A). Both treatments showed a reduction in low-density lipoprotein cholesterol (LDL-C) by ∼11 (sLPH, p = 0.013), and 13% (imLPH, p = 0.005), without observing differences in high-density lipoprotein cholesterol (HDL-C) values (Fig. 4A). Furthermore, sLPH and imLPH treatment reduced the plasmatic triglycerides (TG) levels by 14.5% (p = 0.012) and 14.2% (p = 0.003), respectively, compared to the C group (Fig. 4A). In light of these results, cardiovascular risk was evaluated by calculating the indexes: CRI-I, CRI-II, and AIP. A significant decrease mediated by LPH in CRI-I (sLPH: −12%, p = 0.013; imLPH: −8%, p = 0.029), CRI-II (sLPH: −13%, p = 0.010; imLPH: −9%, p = 0.024), and AIP (sLPH: −14%, p = 0.011; imLPH: −12%, p = 0.017) was observed in treated-mice compared to the C group (Fig. 4B). There were no significant differences between both hydrolysates.3.6. imLPH increases plasma antioxidant status
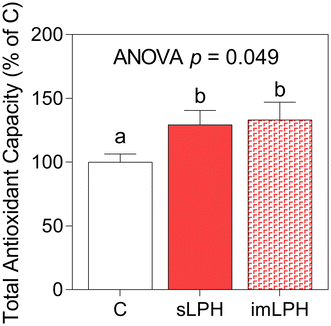
As shown in Fig. 5, plasma levels of TAC increased by 29.4% (p = 0.013) and 33.2% (p = 0.009) when mice were treated with sLPH and imLPH, respectively.4. Discussion
This is the first study to describe the generation, characterisation, and bioactive activity of a lupin protein hydrolysate obtained by immobilised alcalase® (imLPH), a technologically more efficient method for the generation of bioactive peptides. The hydrolysis by the immobilization of the alcalase® enzyme generated a hydrolysate with a high protein content, mainly of small-sized peptides (<8 kDa). Furthermore, imLPH reduced hyperlipidemia and oxidative stress in ApoE−/− mice without changes in weight parameters, demonstrating that the described effects were not due to changes in the weight of the animals.In the last decade, bioactive peptides have been amply studied to be integrated, as an ingredient, in the generation of functional foods and nutraceuticals to prevent and/or treat several chronic diseases. However, the increased cost of producing these tailor-made protein products at the industrial level and the low stability limit their commercialization.22 In this line, and due to the increase in the knowledge and interest in bioactive peptides, other cost-effective methods for the release of encrypted bioactive peptides through the use of enzymes are being used, such as the use of immobilised enzymes or ultrafiltration membranes, contributing to the economic feasibility of the process.23 In this context, immobilizing enzymes onto support materials enables a continuous operation in which enzymes can be recycled, thereby lowering processing expenses.24 Furthermore, the incorporation of fragments of the protease into the food by autolysis is solved with immobilisation of the enzyme.25
Previous results of our group demonstrated that sLPH exerts antioxidant and lipid lowering effects on in vitro systems, ApoE−/− mice, and healthy humans.2 However, the commercialisation of this sLPH as a functional food or nutraceutical is limited because of the high cost and low stability of the soluble enzymatic hydrolysis. For this reason and in order to incorporate it into the food markets, a hydrolysate obtained through the use of low-cost immobilised alcalase® was generated in the present study. It is well known that the enzyme alcalase® is an endopeptidase that generates protein hydrolysates with high small-sized peptide content that have been shown to be the most bioactive.26
The protein content of imLPH was 82%, which was higher than the protein content of the starting flour (30–40%), and similar to the previously described for sLPH (77%). Regarding the molecular weight profile of imLPH, this was similar to the one observed for sLPH. However, imLPH showed higher peaks in peptides <1 kDa and <2 kDa, while sLPH showed higher peptide content <8 kDa. This is of great interest because it is widely known that the small-size peptides are the most bioactive.26,27 In addition, the presence of smaller peptides provides imLPH with a hypoallergenic character higher than that of sLPH, since according to Zhang et al. the peptides with a MW less than 1–4 kDa had low immunoreactivity.28,29 This aspect opens up the question of the use of imLPH as a peptide source for oral tolerance in infant formula. On the other hand, the amino acid analysis showed that, in the same way that sLPH, imLPH have a good balance of amino acids, with a high content of Glu + Gln, Arg, Asp + Asn, Leu, and Ser, but low in sulphur-containing amino acids such as Met and Cys and Trp, a particular characteristic of legumes.30
Mice treated with imLPH for 12 weeks showed a reduction in the amount of abdominal white adipose tissue and plasma levels of TC, TG, and LDL-C, without changes in HDL-C levels. Furthermore, these effects were not significantly different from those of sLPH. It is widely known that elevated levels of plasma cholesterol, triglycerides, and LDL, as well as accumulation of abdominal fat, are risk factors for numerous chronic non-communicable diseases, including obesity, type II diabetes, metabolic associated fatty liver disease (MAFLD), and metabolic syndrome.31–33 Additionally, a significant reduction in the cardiosvascular risk indexes CRI I, CRI II, and AIP was observed both in imLPH and sLPH-treated mice after 12 weeks of treatment. These indexes are widely used as predictors of cardiovascular disease risk in humans and mice.34–36 Likewise, no significant differences were found between both hydrolysates, which supports the generation of the LPH through the economical use of immobilized enzyme.
On the other hand, mice treated with imLPH showed a higher plasma total antioxidant capacity compared to the control group. In this sense, the differences observed in antioxidant activity between both hydrolysates in the in vitro studies were not reflected in the in vivo study, in which there were no significant differences between both hydrolysates.
All observed effects are in agreement with the results previously reported by the sLPH,12 without observing significant differences between the imLPH and sLPH. These effects highlight imLPH as an alternative to reduce costs on an industrial scale and a potential ingredient in future nutraceuticals and functional foods for the prevention and treatment of certain chronic diseases in which the accumulation of abdominal adipose tissue, oxidative stress, and hyperlipidemia are pivotal risk factors for the initiation and development of these diseases. However, future clinical trials are necessary to shed light on the effects of imLPH on health. Furthermore, the anti-inflammatory or hypoglycemic activity of imLPH should also be evaluated in detail. In addition, applying this process on an industrial scale also for other legumes would provide an economic improvement in addition to reducing the risk of allergenicity.
Although the absence of identification of specific peptides generated after hydrolysis treatment is a limitation of this study, the primary focus of this research lies in assessing the overall bioactivity of the hydrolysate with the ultimate objective of reducing production costs at the pilot plant scale, thus enhancing the potential applicability of this product within the food market. Furthermore, protein hydrolysates have been shown to exert pleiotropic effects as a consequence of the physicochemical variability of the different peptides within the hydrolysate.
Finally, although other milk,28,37 whey,38 or chickpea22 hydrolysates have been produced by hydrolysis with immobilised alcalase® and subsequently characterised, this is the first time that the bioactivities of a hydrolysate from any species of lupin generated by an enzyme-immobilization method have been shown.
5. Conclusions
This study, which is the first to describe the generation of a hydrolysate from lupin by using immobilized alcalase®, shows that the immobilisation of the enzyme does not affect the beneficial antioxidants and lipid-lowering properties of the peptides generated. Production of the imLPH with the enzyme immobilisation method would provide an economic and industrial improvement, which would mean an advance towards the future commercialisation of LPH.Author contributions
A. C.-V.: conceptualization; resources, funding acquisition, drafting of the manuscript, supervision; I. C.-C.: conceptualization, methodology, formal analysis, drafting of the manuscript, supervision; G. S.-S.: methodology, formal analysis, drafting of the manuscript; A. I. A.-L.: methodology, formal analysis; J. C. M.-L.: methodology, formal analysis; J. P.: resources, funding acquisition; M. C. M.-L.: resources, funding acquisition; P. J. L.: resources, funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Ministerio de Economía y Competitividad, Spanish Government [AGL2012-40247-C02-01 and AGL2012-40247-C02-02]; Andalusian Government Ministry of Health PC-0111-2016-0111, PEMP-0085-2020 (co-financed with FEDER funds, call Resolution of 7 July 2021 of the General Secretary for Research, Development and Innovation in Health, which calls for grants to finance research, development and innovation in biomedicine and health sciences in Andalusia by 2021) and PAIDI Program from the Andalusian Government [CTS160]. FPU grants from the Spanish Ministerio de Educación, Cultura y Deporte, [FPU16/02339] supported G. S.-S. A postdoctoral fellowship from the Andalusian Government Ministry of Economy, Knowledge, Business, and University [DOC_00587/2020] supported I. C.-C. Andalusian Government Ministry of Health [PI-0136-2019] supported A. I. A.-L. We thank the staff from the IBiS Animal Facility for their valuable assistance.References
- P. Duffuler, K. S. Bhullar, S. C. de Campos Zani and J. Wu, Bioactive peptides: From basic research to clinical trials and commercialization, J. Agric. Food Chem., 2022, 70, 3585–3595 CrossRef CAS PubMed.
- I. Cruz-Chamorro, G. Santos-Sánchez, A. I. Álvarez-López, J. Pedroche, P. J. Lardone, A. Arnoldi, C. Lammi and A. Carrillo-Vico, Pleiotropic biological effects of Lupinus spp. protein hydrolysates, Trends Food Sci. Technol., 2023, 133, 244–266 CrossRef CAS.
- G. Santos-Sánchez, A. I. Álvarez-López, E. Ponce-España, A. Carrillo-Vico, C. Bollati, M. Bartolomei, C. Lammi and I. Cruz-Chamorro, Hempseed (Cannabis sativa) protein hydrolysates: A valuable source of bioactive peptides with pleiotropic health-promoting effects, Trends Food Sci. Technol., 2022, 127, 303–318 CrossRef.
- A. P. Shirsath and M. M. Henchion, Bovine and ovine meat co-products valorisation opportunities: A systematic literature review, Trends Food Sci. Technol., 2021, 118, 57–70 CrossRef CAS.
- J. Yongsawatdigul and A. Hamzeh, in Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products, IntechOpen, 2021 Search PubMed.
- A. Jakubczyk, M. Karaś, K. Rybczyńska-Tkaczyk, E. Zielińska and D. Zieliński, Current trends of bioactive peptides—New sources and therapeutic effect, Foods, 2020, 9, 846 CrossRef CAS PubMed.
- D. E. Cruz-Casas, C. N. Aguilar, J. A. Ascacio-Valdés, R. Rodríguez-Herrera, M. L. Chávez-González and A. C. Flores-Gallegos, Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides, Food Chem.: Mol. Sci., 2021, 3, 100047 CAS.
- S. K. Ulug, F. Jahandideh and J. Wu, Novel technologies for the production of bioactive peptides, Trends Food Sci. Technol., 2021, 108, 27–39 CrossRef CAS.
- J. Shao, G. Zhang, J. Fu and B. Zhang, Advancement of the preparation methods and biological activity of peptides from sesame oil byproducts: a review, Int. J. Food Prop., 2020, 23, 2189–2200 CrossRef CAS.
- M. d. C. Millán-Linares, M. d. M. Yust, J. M. Alcaide-Hidalgo, F. Millán and J. Pedroche, Lupine protein hydrolysates inhibit enzymes involved in the inflammatory pathway, Food Chem., 2014, 151, 141–147 CrossRef PubMed.
- I. Cruz-Chamorro, N. Álvarez-Sánchez, M. del Carmen Millán-Linares, M. del Mar Yust, J. Pedroche, F. Millán, P. J. Lardone, C. Carrera-Sánchez, J. M. Guerrero and A. Carrillo-Vico, Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes, Food Res. Int., 2019, 126, 108585 CrossRef CAS PubMed.
- G. Santos-Sánchez, I. Cruz-Chamorro, A. I. Álvarez-Ríos, N. Álvarez-Sánchez, B. Rodríguez-Ortiz, A. I. Álvarez-López, M.-S. Fernández-Pachón, J. Pedroche, F. Millán and M. d. C. Millan-Linares, Bioactive Peptides from Lupin (Lupinus angustifolius) Prevent the Early Stages of Atherosclerosis in Western Diet-Fed ApoE–/–Mice, J. Agric. Food Chem., 2022, 70, 8243–8253 CrossRef PubMed.
- G. Santos-Sánchez, I. Cruz-Chamorro, A. I. Álvarez-Ríos, J. M. Fernández-Santos, M. V. Vázquez-Román, B. Rodríguez-Ortiz, N. Álvarez-Sánchez, A. I. Álvarez-López, M. d. C. Millán-Linares and F. Millán, Lupinus angustifolius protein hydrolysates reduce abdominal adiposity and ameliorate metabolic associated fatty liver disease (MAFLD) in Western diet fed-ApoE−/− mice, Antioxidants, 2021, 10, 1222 CrossRef PubMed.
- G. Santos-Sánchez, I. Cruz-Chamorro, C. Bollati, M. Bartolomei, J. Pedroche, F. Millán, M. del Carmen Millán-Linares, A. L. Capriotti, A. Cerrato and A. Laganà, A Lupinus angustifolius protein hydrolysate exerts hypocholesterolemic effects in Western diet-fed ApoE−/− mice through the modulation of LDLR and PCSK9 pathways, Food Funct., 2022, 13, 4158–4170 RSC.
- I. Cruz-Chamorro, N. Álvarez-Sánchez, A. I. Álvarez-Ríos, G. Santos-Sánchez, J. Pedroche, F. Millán, C. Carrera-Sánchez, M. S. Fernández-Pachón, M. C. Millán-Linares and A. Martínez-López, Safety and Efficacy of a Beverage Containing Lupine Protein Hydrolysates on the Immune, Oxidative and Lipid Status in Healthy Subjects: An Intervention Study (the Lupine–1 Trial), Mol. Nutr. Food Res., 2021, 65, 2100139 CrossRef CAS PubMed.
- M. d. M. Yust, J. Pedroche, M. d. C. Millán-Linares, J. M. Alcaide-Hidalgo and F. Millán, Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase, Food Chem., 2010, 122, 1212–1217 CrossRef CAS.
- J. Adler-Nissen, Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid, J. Agric. Food Chem., 1979, 27, 1256–1262 CrossRef CAS PubMed.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- R. Garcés and M. Mancha, One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues, Anal. Biochem., 1993, 211, 139–143 CrossRef PubMed.
- M. Alaiz, J. L. Navarro, J. Girón and E. Vioque, Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate, J. Chromatogr., A, 1992, 591, 181–186 CrossRef CAS PubMed.
- M. a. M. Yust, J. Pedroche, J. Girón-Calle, J. Vioque, F. Millán and M. Alaiz, Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection, Food Chem., 2004, 85, 317–320 CrossRef CAS.
- M. del Mar Yust, J. Pedroche, M. del Carmen Millán-Linares, J. M. Alcaide-Hidalgo and F. Millán, Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase, Food Chem., 2010, 122, 1212–1217 CrossRef.
- D. Yu, Y. Sun, W. Wang, S. F. O'Keefe, A. P. Neilson, H. Feng, Z. Wang and H. Huang, Recovery of protein hydrolysates from brewer's spent grain using enzyme and ultrasonication, Int. J. Food Sci. Technol., 2020, 55, 357–368 CrossRef CAS.
- L. Y. Chew, G. T. Toh and A. Ismail, in Enzymes in food biotechnology, Elsevier, 2019, pp. 247–261 Search PubMed.
- R. DiCosimo, J. McAuliffe, A. J. Poulose and G. Bohlmann, Industrial use of immobilized enzymes, Chem. Soc. Rev., 2013, 42, 6437–6474 RSC.
- V. G. Tacias-Pascacio, R. Morellon-Sterling, E.-H. Siar, O. Tavano, A. Berenguer-Murcia and R. Fernandez-Lafuente, Use of Alcalase in the production of bioactive peptides: A review, Int. J. Biol. Macromol., 2020, 165, 2143–2196 CrossRef CAS PubMed.
- Z. Karami and B. Akbari-Adergani, Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties, J. Food Sci. Technol., 2019, 56, 535–547 CrossRef CAS PubMed.
- H. Li, J. Yang, A. Qin, F. Yang, D. Liu, H. Li and J. Yu, Milk protein hydrolysates obtained with immobilized alcalase and neutrase on magnetite nanoparticles: Characterization and antigenicity study, J. Food Sci., 2022, 87, 3107–3116 CrossRef CAS PubMed.
- Q. Zhang, Q.-H. Chen and G.-Q. He, Effect of ultrasonic-ionic liquid pretreatment on the hydrolysis degree and antigenicity of enzymatic hydrolysates from whey protein, Ultrason. Sonochem., 2020, 63, 104926 CrossRef CAS PubMed.
- A. Matemu, S. Nakamura and S. Katayama, Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score, Antioxidants, 2021, 10, 316 CrossRef CAS PubMed.
- M. F. Linton, P. G. Yancey, S. S. Davies, W. G. Jerome, E. F. Linton, W. L. Song, A. C. Doran and K. C. Vickers, The role of lipids and lipoproteins in atherosclerosis, Endotext [Internet], 2019.
- E. Buzzetti, M. Pinzani and E. A. Tsochatzis, The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD), Metabolism, 2016, 65, 1038–1048 CrossRef CAS PubMed.
- E. Ko, E. L. Yoon and D. W. Jun, Risk factors in nonalcoholic fatty liver disease, Clin. Mol. Hepatol., 2023, 29, S79 CrossRef PubMed.
- A. M. de Oliveira, A. F. S. de Freitas, M. D. de Souza Costa, M. K. da Silva Torres, Y. A. de Araujo Castro, A. M. R. Almeida, P. M. G. Paiva, B. M. Carvalho and T. H. Napoleao, Pilosocereus gounellei (Cactaceae) stem extract decreases insulin resistance, inflammation, oxidative stress, and cardio-metabolic risk in diet-induced obese mice, J. Ethnopharmacol., 2021, 265, 113327 CrossRef CAS PubMed.
- N. Oršolić, I. Landeka-Jurčević, D. Đikić, D. Rogić, D. Odeh, V. Balta, E. Perak-Junaković, S. Terzić and D. Jutrić, Effect of propolis on diet-induced hyperlipidemia and atherogenic indices in mice, Antioxidants, 2019, 8, 156 CrossRef PubMed.
- T. Kalelioglu, A. Genc, N. Karamustafalioglu and M. Emul, Assessment of cardiovascular risk via atherogenic indices in patients with bipolar disorder manic episode and alterations with treatment, Diabetes Metab. Syndr., 2017, 11, S473–S475 CrossRef PubMed.
- M. Abd El-Salam and S. El-Shibiny, Preparation, properties, and uses of enzymatic milk protein hydrolysates, Crit. Rev. Food Sci. Nutr., 2017, 57, 1119–1132 CrossRef CAS PubMed.
- T. B. Pessato, N. C. de Carvalho, O. L. Tavano, L. G. R. Fernandes, R. d. L. Zollner and F. M. Netto, Whey protein isolate hydrolysates obtained with free and immobilized Alcalase: Characterization and detection of residual allergens, Food Res. Int., 2016, 83, 112–120 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo05086f |
| This journal is © The Royal Society of Chemistry 2024 |