Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: a systematic review of animal and human interventions†
Nima
Mohammadi
a,
Michelle
Farrell
a,
Laura
O'Sullivan
a,
Andrea
Langan
a,
Marcelo
Franchin
a,
Luciana
Azevedo
b and
Daniel
Granato
 *ac
*ac
aUniversity of Limerick, School of Natural Sciences, Faculty of Science and Engineering, Department of Biological Sciences, Bioactivity and Applications Lab, V94 T9PX Limerick, Ireland. E-mail: daniel.granato@ul.ie
bFederal University of Alfenas, In Vitro and In Vivo Nutritional and Toxicological Analysis Laboratory, Av. Jovino Fernandes Sales, 2600, Bairro Santa Clara – CEP 37133-840, Alfenas, Minas Gerais, Brazil
cHealth Research Institute, University of Limerick, V94 T9PX Limerick, Ireland
First published on 8th March 2024
Abstract
Cardiovascular diseases (CVDs) are a group of chronic health disorders prevalent worldwide that claim millions of lives yearly. Inflammation and oxidative stress are intricately associated with myocardial tissue damage, endothelial dysfunction, and increased odds of heart failure. Thus, dietary strategies aimed at decreasing the odds of CVDs are paramount. In this regard, the consumption of anthocyanins, natural pigments found in edible flowers, fruits, and vegetables, has attracted attention due to their potential to promote cardiovascular health. The main mechanisms of action linked with their protective effects on antioxidant and anti-inflammatory activities, serum lipid profile modulation, and other cardiovascular health parameters are explained and exemplified. However, little is known about the dose-dependency nature of the effects, which anthocyanin has better efficiency, and whether anthocyanin-containing foods display better in vivo efficacy than nutraceuticals (i.e., concentrated extracts containing higher levels of anthocyanins than foods). Thus, this systematic review focused on determining the effects of anthocyanin-containing foods and nutraceuticals on biomarkers associated with CVDs using animal studies and human interventions supported by in vitro mechanistic insights. Overall, the results showed that the regular consumption of anthocyanin-containing foods and nutraceuticals improved vascular function, lipid profile, and antioxidant and anti-inflammatory effects. The daily dosage, the participants’ health status, and the duration of the intervention also significantly influenced the results.
1. Introduction
Amidst the growing interest in consuming natural foods for a healthier lifestyle, an explicit focus has emerged on various components, significant nutrients (e.g., proteins, fats, and carbohydrates) and minor compounds, such as carotenoids and phenolic compounds. Although databases like the United States Department of Agriculture (USDA) and phenol-explorer provide valuable data on polyphenols, which have been extensively used and published in nutritional epidemiology, our understanding of how diet affects health remains complex due to the vast number of chemical compounds present in foods.1 While polyphenol data contribute significantly to our knowledge, there are still gaps in understanding, as many other compounds remain underexplored, highlighting the ongoing challenges in nutritional research.2Polyphenols comprise a large group of compounds derived from plants with a chemical structure containing one or more hydroxyls bonded in the benzene ring.3 There are four primary polyphenols classes: flavonoids, phenolic acids, stilbenes, and lignans.4Table 1 presents the types and sources of phenolic compounds, showcasing their notable impact on human cardiovascular health by eliminating and preventing reactive oxygen species (ROS) production, reducing blood pressure (BP), improving endothelial functioning through increased plasma epicatechin levels, and enhancing endothelial vasodilators, enhancing high-density lipoprotein cholesterol (HDL-C) production in prehypertensive individuals, reducing low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL-C), downregulating pro-inflammatory cytokines to reduce inflammation, and leading to reductions in LDL-C levels in humans.4
| Polyphenol class | Examples of sources | Examples of CVD health benefits of each class. | Ref. |
|---|---|---|---|
| Reactive oxygen species: ROS; blood pressure: BP; high-density lipoproteins: HDL-C; low-density lipoprotein: LDL-C; very low-density lipoprotein cholesterol: VLDL-C. | |||
| Flavonoids | Onions, kale, celery, spinach, broccoli, strawberries, blackberries, blueberries, purple cabbage, oranges, lemons, grapefruits, pomegranate, soy, tea, cocoa and red and white wine. | - Antioxidant activity is displayed by eliminating and preventing ROS production by donating electrons and blocking ROS-activating enzymes. | 5 |
| - Flavonoid intake can reduce BP in both men and women. | 4 | ||
| - Flavonoids can improve human endothelial functioning by increasing plasma epicatechin levels and enhancing endothelial vasodilators. | 6 | ||
| - Can improve HDL-C production in prehypertensive humans. | 7 | ||
| 8 | |||
| 9 | |||
| Phenolic acids | Strawberries, black radish, whole grain, coffee, cereals, berries, and spices. | - Phenolic acids in coffee can reduce LDL-C and VLDL-C in humans. | 4 |
| - Improvement in endothelial function in males. | 5 | ||
| 10 | |||
| 11 | |||
| Stilbenes | Red wine, berries, and grapes. | - Can reduce LDL-C levels and increase HDL-C levels in women. | 4 |
| - Reduction in inflammation by downregulation of pro-inflammatory cytokines. | 5 | ||
| 12 | |||
| 13 | |||
| Lignans | Flaxseed, grains, sunflower seed and whole bran cereals. | - Reduction in BP in hypertensive humans. | 4 |
| - Can lead to reductions in LDL-C levels in men. | 5 | ||
| 14 | |||
| 15 | |||
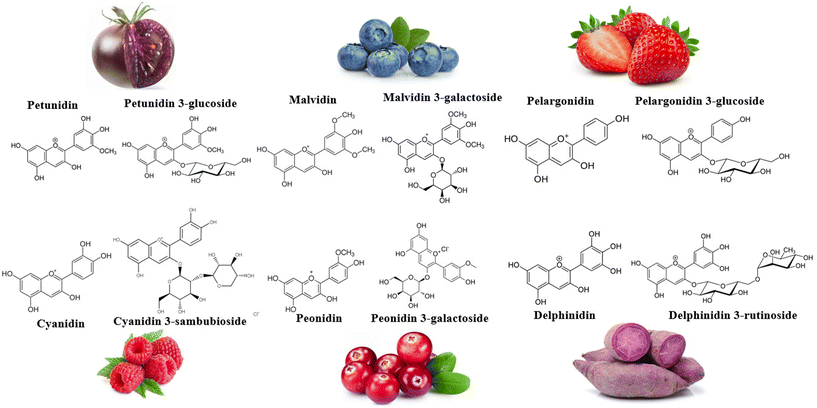
Anthocyanins (ANT), captivating the attention of public health experts, have emerged as polyphenols of nutritional interest. Comprising anthocyanidin structures such as peonidin, pelargonidin, cyanidin, petunidin, and malvidin, ANT are coupled with a sugar moiety, such as rutinose, glucose, galactose, and sambubiose (Fig. 1). Anthocyanidins’ basic structure includes an aromatic ring attached to a heterocyclic ring containing oxygen, subsequently linked to a third aromatic ring by a carbon–carbon bond. Nearly 700 ANT can be found in dark-coloured (red to blue) fruit pulps and skins/peels, vegetables, flowers, and seeds.16
The health benefits attributed to ANT are associated with their mechanisms to mitigate pro-inflammatory responses and ameliorate ROS generation in plasma and tissues, improve endothelial function, normalise the levels of circulating plasma lipids, and enhance nitric oxide (NO) production.9,17–20
Non-communicable diseases (NCDs) account for 71% of all global deaths annually, and 32% result from cardiovascular diseases (CVDs), accounting for 17.9 million lives yearly. CVDs include a variety of diseases that directly affect the heart and blood vessels, thus increasing the odds of ischemic events, clinical complications, and, eventually, death.21 One of the leading causes of CVDs is atherosclerosis, which can be conceptualised as a lipid-driven chronic inflammation of middle-sized and large arteries initiated by endothelial cell (EC) activation through modified lipids such as oxidised low-density lipoprotein cholesterol (ox-LDL). This inflammatory disease leads to lipids accumulating and hardening the arteries, forming plaque due to lipid oxidation and hyperlipidemia.22 Inflammation is a crucial contributor to atherosclerosis onset and critical to atherosclerotic plaque development.23,24 Inflammation occurs in response to tissue injury or infection. If not closely regulated, an inflammatory response, also known as a cytokine storm, can increase the odds of CVDs, including clinical symptoms and changes in blood-related biomarkers.25 Atherosclerosis occurs when the lumen of the arteries narrows due to plaque build-up; hence, blood flow is restricted. Increased plaque production harms cardiovascular health as it decreases the size of the lumen and increases vessel thickness, thus leading to unstable blood flow and, subsequently.26
Considering this scenario and the need to decrease the risk of diseases through adopting healthier eating habits, natural plants and healthy foods, such as polyphenol-containing foods, have been the subject of more in-depth studies over the past few decades. A varied and balanced diet is the critical target in the risk reduction of the onset and progression of CVDs.27 Life habits, genetics, and diet can modify CVD risk factors, including inflammation, BP, endothelial function, blood lipids, and plasma antioxidant capacity.28 Therefore, incorporating food groups that positively impact CVD-related biomarkers would be paramount to reducing CVD risk. In this context, evidence from different protocols has shown that consuming fruits and vegetables-containing ANT has an inverse association with CVDs.29,30 Such effects include reduced BP, inflammation, oxidative stress biomarkers, and signalling agents.31
Early findings associated with ANT mainly focused on their antioxidant and anti-inflammatory effects. These studies aimed to discover if the consumption of ANT was effective in modulating oxidative and inflammation-led cascades in vivo, which might be the basis for reducing the risk of CVDs.9,19 Limitations exist in the early findings of ANT and CVD. For example, in animal and clinical studies, the type and daily dosage of ANT consumed are not frequently considered, including their composition, intervention time, and target population.
Nonetheless, new hypothesis-driven research has been undertaken in different animal-based studies and human interventions to unveil how ANT-containing foods impact cardiovascular health. For example, in a systematic review and meta-analysis of prospective cohort studies, Kimble et al. concluded that the sustained consumption of ANT from dietary sources significantly reduced the risk of CVD mortality by modulating the lipid profile, increasing the plasma antioxidant capacity and decreasing the secretion of pro-inflammatory cytokines, such as interleukin-6 (IL-6).32 Still, no association was observed between the dietary intake of ANT and the risk of cerebral infarction, ischemic, and haemorrhagic stroke. In our previous reviews on the association between bioactive-rich molecules (e.g., ANT and other phenolics), consuming functional foods24,27 added with natural antioxidants may become a breakthrough in public health as the rate of CVD may be considerably decreased. Still, more relational and populational-based studies are necessary to ascertain the cause-and-effect relationship.
In comparison to previous review articles published in the past 10 years on “ANT × cardiovascular health”, the focus was primarily on the protective effects of berries on endothelial dysfunction,33 with some researchers providing a detailed examination of the mechanisms underlying the vascular protective effect of ANT. Additionally, the clearance of senile vascular endothelial cells and its implications for preventing cardiovascular diseases were investigated.34 However, none of these previous research outputs addressed dose-dependency behaviour, the effects of different ANT types, or the source of ANT consumption (e.g., nutraceutical or natural foods). To move beyond the current state-of-the-art and provide valuable and novel information to the literature, a broader and more comprehensive perspective on the effects of ANT on cardiovascular health is offered herein. Our study aims to fill these gaps by investigating the dose dependency of ANT and comparing the effectiveness of anthocyanin-containing foods with nutraceuticals. Furthermore, findings from various types of studies, including animal experiments, human interventions, and mechanistic insights from in vitro protocols, are integrated to provide a multidimensional analysis of the beneficial effects of ANT on oxidative and pro-inflammatory status, lipid profile modulation and other cardiovascular health parameters. A more nuanced understanding of the topic is offered by evaluating factors such as daily dosage, participants’ health status, and intervention time. Overall, a significant advancement in the field is presented by providing valuable insights that extend beyond the scope of previous research efforts.
2. Background on cardiovascular diseases
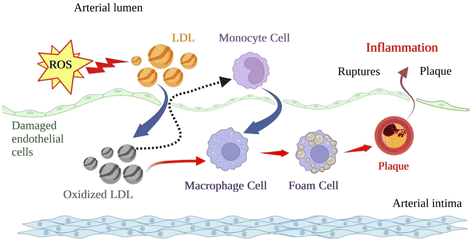
Since atherosclerosis is a major driving factor in developing CVDs, we should highlight its recognised multivariate association among vascular inflammation, endothelial dysfunction, and oxidative/nitrosative stress in multiple tissues.35,36 It is well established the impact of lipid-driven chronic inflammation on vascular function and the formation of atherosclerotic plaque through endothelial activation, monocyte and macrophage stimulation, transendothelial migration and lipid deposition with the evolvement of cytokines and chemokines (family of small cytokines with chemotactic properties) and their receptors CVD.37,38The formation of this plaque is displayed in Fig. 2. Cytokines, including interleukins (i.e., IL-6, IL-1, IL-1β), tumour necrosis factor α (TNF-α), prostaglandins, and alterations in signalling pathways (i.e., level of monocyte chemoattractant protein, MCP-1) are vital inflammatory mediators present in the blood from the onset of atherosclerosis.39 Oxidative/nitrosative stress is a condition of imbalance between forming free radicals and other reactive species and antioxidant defences. ROS and reactive nitrogen species (RNS) are highly reactive, short-lived organic and inorganic molecules that result from normal physiological metabolism in living systems. Oxygen radicals and hydrogen peroxide (H2O2) are normally formed during mitochondrial functions in biological systems since photosynthesis and aerobic respiration similarly use oxygen.40 These species are required for bodily functions such as cell homeostasis and gene expression.41
Oxidative stress and inflammation are closely interrelated and are linked to proatherogenic stimuli. Indeed, the ox-LDL, the hallmark of atherosclerotic lesions, is uptaken by unregulated scavenger macrophage receptors to form foam cells. Moreover, the central ox-LDL cellular receptor is LOX-1, which has pro-inflammatory potential in atherogenesis and is up-regulated after exposure to several pro-inflammatory factors and TNF-α. These mechanisms generate feedback loops from oxidised molecules captured by overexpressed receptors reaching the fatty streaks and atherosclerotic plaque formation (the final stage of the disease).24,42
In this sense, both oxidative stress and inflammation cause injury to cells, including endothelium.43 Endothelial dysfunction promotes a pro-inflammatory environment, as evidenced by increased endothelial expression of adhesion molecules and the imbalance of arachidonic acid metabolites and chemoattractant molecules.
Vascular inflammation forms a positive feedback loop, leading to endothelial dysfunction.44 Indeed, risk factors that can cause oxidative/nitrosative stress, inflammation, and CVDs include an unhealthy diet, inactive lifestyle, high BP, and smoking.45 This connection between vascular inflammation, endothelial dysfunction, and oxidative stress supports the clinical practice that well-established antihypertensive agents and statins are still relevant due to their antioxidant and anti-inflammation pleiotropic effects.46
Deciphering and enlightening the mechanisms to break this feedback pro-oxidant and inflammatory status, especially by diet (including nutraceuticals and functional foods, e.g. ANT), will be fundamental for developing novel therapies to mitigate the burden of atherosclerosis and other CVDs.
3. Absorption, metabolism, and excretion of dietary anthocyanins
Before describing the modulating effects of ANT in animals and humans, it is essential to provide an overview of how these bioactive compounds are absorbed, metabolised, and excreted.47–50 In general, as illustrated in Fig. 3A, studying the absorption and bioavailability of dietary ANT requires a standardised platform to characterise the chemical composition (e.g., ANT composition and levels) of the food or nutraceuticals (e.g., capsules containing ANT in a higher level compared to the natural food source) and in vitro bioactivities (e.g., antioxidant and anti-inflammatory capacities). Cell-based studies using different cells (e.g., intestine, stomach, liver, erythrocytes, and macrophages) should be performed to confirm their toxicological safety and profile at different dosages. Animal testing can be conducted to validate the selection of biomarkers and evaluate the efficacy of the food/nutraceutical in vivo. In a later stage, efficacy should be studied in human interventions to establish the cause-effect relationship and understand the existence of any dose–response behaviour.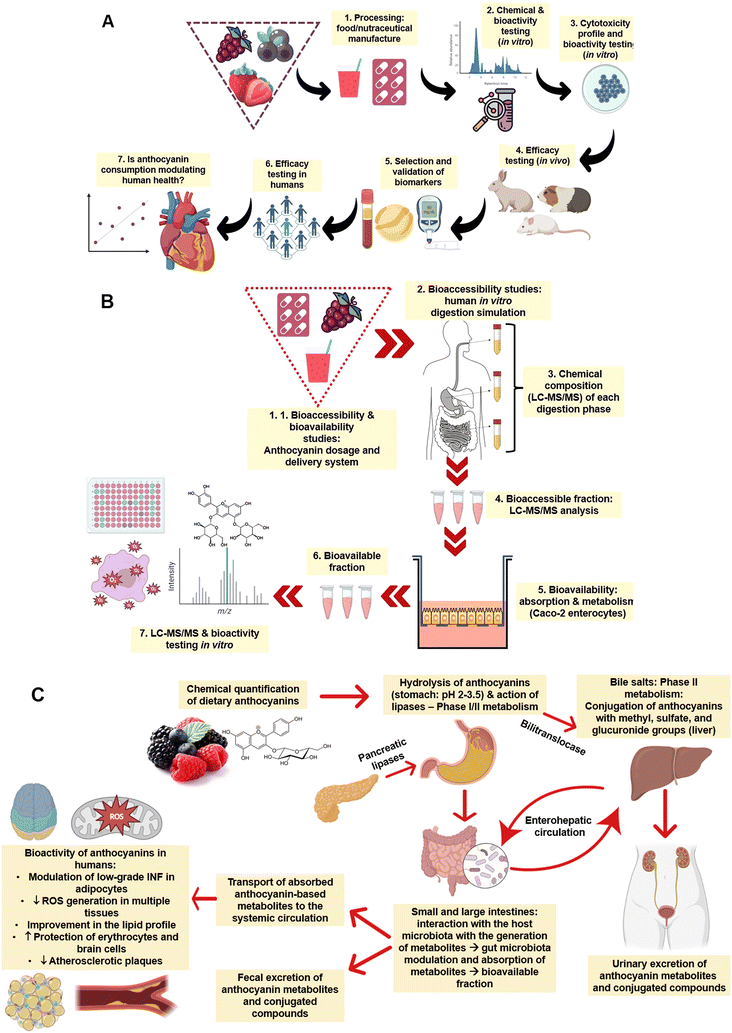 | ||
| Fig. 3 (A) General pathway from the processing of raw materials and production of nutraceuticals to the assessment of health-promoting effects of anthocyanins in vitro and in vivo (e.g., animals and humans); (B) schematic representation of in vitro bioaccessibility and bioavailability assessments of anthocyanin-rich extracts, foods, and nutraceuticals; and (C) pathways of anthocyanins absorption, distribution, metabolism, and excretion. Note: concepts are in line with those of Granato.51 | ||
In a simulated digestion system (Fig. 3B), such as the INFOGEST protocol,52 the bioaccessibility and bioavailability of ANT in foods and nutraceuticals can be studied, including the development of metabolites during each digestion phase (e.g., oral, oral + stomach, and oral + stomach + intestine). Similar conditions using temperature and enzymes can recapitulate the human physiological conditions, and different analytical techniques, such as liquid-chromatography-mass spectrometry coupled with bioactivity testing, can be utilised. This platform is crucial to mimicking and understanding the effects of each digestion phase on the metabolism and absorption of ANT.53 The interaction with human enterocytes, pharmacokinetics, the absorption rate of ANT, and the production of metabolites can also be studied using Caco-2 monolayers or other cell models, obtaining the in vitro bioavailability of such compounds.
Fig. 3C summarises the fate of ANT after oral administration in humans. This process depends on the anthocyanin's chemical structure – aglycones or glycosylated bonds with glucose, xylose, arabinose, galactose, rhamnose or rutinose. The molecular weight affects the absorption rate of ANT and, therefore, their concentration in human blood (e.g., bioavailability). Research shows that some ANT can be absorbed in the stomach (10 to 50 nM) through a bilitranslocase-mediate mechanism, but most ANT are absorbed in the small intestine.17 In the oral cavity (pH 6.6–7.1), ANT are partially degraded (<10%) with the action of hydrolases and second-stage enzymes. In the stomach (pH 2–3), because of the low pH and hydrolysis of glycosidic bonds via C-ring fission with fragments of A- and B-ring, ANT are stabilised and partially absorbed (∼10%) in a complete structure within 15–30 min. The liver plays a crucial role in the metabolism of ANT, as catechol-O-methyltransferase and sulfotransferases are responsible for anthocyanin methylation and sulfation, respectively. The remaining ANT enter the small intestine (pH 4–7) and are transformed into quinone bases, chalcones, and pseudo bases, making them more chemically unstable. ANT undergo chemical modifications because of enzymatic reactions (lactase and β-glucosidase), especially by glycosylation and decomposition into low molecular weight (e.g. phenolic acids and aldehydes) compounds.17 In the colon (pH 4–7), ANT and their metabolites formed in the stomach-small intestine axis interact with gut microbiota (e.g., biotransformation) before enterocyte absorption augmenting the abundance of beneficial phyla and modulating the ratio of Bacteroides/Firmicutes.54 More specifically, intestinal microbiota release deglycosylation enzymes, cleave the glycosylation portion, generate aglycones, and further open the ring to create different phenolic acids (such as protocatechuic acid (PCA) and vanillic acid, syringic acid, ferulic acid, and hippuric acid) or aldehydes. Overall, part of ANT uptake along the gastrointestinal tract is reduced, while part of phenolic acid may be enhanced.34 This metabolic process is able to increase the levels of short-chain fatty acids, central compounds linked to enterocyte energy metabolism, gluconeogenesis, and colonocyte health and proliferation.55 In this process, less than 3% of ANT is excreted in the urine and plasma concentration of ANT can reach 120 nmol L−1 in a couple of hours after ingestion.17
To exemplify this approach, dos Santos Lima et al. analysed the bioaccessibility and bioactivity of blackcurrant press cake after in vitro simulated digestion. They found that the bioaccessibility of total ANT increased by 136%, where the bioaccessibility of delphinidin 3-glucoside, delphinidin 3-rutinoside, C3G, and cyanidin 3-rutinoside was 198%, 128%, 112%, and 131%, respectively. Both fractions (e.g., digested and undigested) showed cellular antioxidant activity, but the undigested fraction inhibited the oxidation of glutathione and decreased intracellular ROS generation in Caco-2, HepG2, and EA·hy926 cells. In chemical assays (FRAP and DPPH), the digested sample's antioxidant activity was significantly higher (between two- and three-fold) than the undigested sample. This research highlights some interesting findings related to the bioaccessibility of ANT: only chemical assays are insufficient to have a bioactivity fingerprint of ANT, and ANT's bioaccessibility rate cannot be a predictor of their bioactivity.56
Interestingly, when dealing with an in vivo model, this blackcurrant press cake at the highest dosage (diet with 15%) exerted pre-neoplastic lesions and morphological changes in the colon of Wistar rats and caused gut bacterial dysbiosis. It is hypothesised that after digestion and fermentation, the breakdown products and individual colonic metabolites can modulate colon carcinogenesis, linked with the pro-oxidant activity of natural antioxidants at high doses. These findings point out that the pro-oxidant activities of ANT need to be deeply investigated in vivo protocols.57
Overall, insights gained from these ANT metabolic processes, besides their chemical and amount modification knowledge, underscore the necessity of finding solutions to maintain adequate levels of metabolites in plasma and target tissues while considering their nutraceutical effects in living systems. The future belongs to foodomics studies, functional food research, phytopharmaceuticals containing ANTs and components with synergistic action, and exploratory epigenetic studies.58
4. Methods and study selection: animal and human studies
The PICOS (population, intervention, comparison, outcome) shown in Tables 1 and 2 – ESI† was utilised to determine the exclusion and inclusion criteria required for the literature review. A literature search used Ebsco, EMBASE, and Web of Science. Various search terms with multiple operators, such as ‘AND’, ‘OR’, were included to narrow the search and combine keywords. The searched databases were limited to English-only papers published from 1st January 2000 to 1st January 2022. Keywords included ANT, CVDs, cardiometabolic risk, inflammation, oxidative stress, and cardiovascular health. Tables 3 and 4 – ESI† describe this database search strategy.In the first step of the screening process, all articles’ titles found in the database searches were analysed to classify them as relevant or irrelevant (excluded). Relevant articles’ abstract was then screened for eligibility based on the inclusion/exclusion criteria. The data extracted from each study included the author, year of publication, study duration, number of subjects, type of animal and their health status, intervention type, type of control, the dosage of intervention, and significant results post-intervention.
The three databases identified 1351 and 1300 animal and human manuscripts, respectively, and from that, duplicates were identified and manually excluded using EndNote Software (Version 20, 2022). Titles and abstracts of the remaining 1256 (animal) and 1209 (human) studies were assessed and reviewed to ensure inclusion/exclusion criteria eligibility. After screening the title and abstracts, 185 and 181 animal and human papers remained, respectively, and these articles were screened for eligibility by looking at the complete text, abstract, and title. Finally, 30 and 55 articles remained and met all the relevant criteria to be included in the study. Flow diagrams depicting the study selection and screening process can be seen in Fig. 1 and 2 – ESI.† Some examples of reasoning as to why full-text articles assessed were excluded include not being relevant to the topic, insufficient subjects per group and no variables of interest. The included studies underwent a rigorous cross-checking process by the authors NM and MF to ensure the accuracy and reliability of the findings. Each study underwent independent assessment. In cases where discrepancies arose, a third expert operator was consulted, and consensus-based adjustments were implemented to resolve them. Data extraction tables describing the key characteristics and outcomes of the studies included in this review can be seen in Tables 2 and 3.
| Citation | Source of anthocyanin | Subjects | Health status | Length of intervention | Dose of anthocyanins | Main outcomes |
|---|---|---|---|---|---|---|
| Apolipoprotein AI: apo-AI; apolipoprotein B: apoB; blood pressure: BP; catalase: CAT; cholesterol ester transfer protein: CETP; coronary artery disease: CAD; coronary heart disease: CHD; C-reactive protein: CRP; cyclic guanosine monophosphate: Cgmp; diastolic blood pressure: DBP; endothelin-1: ET-1; fasting blood glucose: FBG; flow mediated dilation: FMD; freeze-dried blueberries: FDB; freeze-dried blueberries: FDB; glutathione peroxidase: GPX/GSH-Px; hemoglobin A1c: HbA1c; high density lipoprotein cholesterol: HDL-C; high sensitivity C-reactive protein: hs-CRP; intercellular adhesion molecule-1: ICAM-1; interleukin: IL; intima-media thickness: IMT; lecithin-cholesterol acyltransferase: LCAT; low density lipoprotein cholesterol: LDL-C; lyophilised grape powder: LGP; mean arterial pressure: MAP; metabolic syndrome: MetS; monocyte chemoattractant protein-1: MCP-1; myeloperoxidase: MPO; nuclear factor kappa B: NF-κB; paraoxonase 1: PON1; soluble CD40 ligand: sCD40L; soluble vascular cell adhesion molecule-1: sVCAM-1; superoxide dismutase: SOD; systolic blood pressure: SBP; total antioxidant capacity: TAC; total cholesterol: TC; total oxidant status: TOS; triglycerides: TG; tumour necrosis factor-alpha: TNF-α; tumour necrosis factor RI/RII: TNF RI/TNF RII | ||||||
| 172 | FDB | 115 males and females | MetS | 6 months | - 364 mg per day of FDB. | - Increase in FMD, Cgmp and HDL-C after 364 mg per day. |
| - 182 mg per day of FDB | - Reduction in arterial stiffness after 364 mg per day. | |||||
| - 0 mg per day of FDB | - No changes in BP. | |||||
| - No modulations were observed after 182 mg per day. | ||||||
| 115 | Agraz (Vaccinium meridionale Swartz) | 40 females | MetS | 12 weeks | 75.65 mg per day | - No modulation in apoA-1 concentration, PON1 activities, MPO concentration and cholesterol efflux capacity. |
| - No changes in inflammatory markers IL-6, IL-8, TNF-α, NF-κB, IL-1β and MCP-1. | ||||||
| 170 | High and low blackcurrant juice | 66 males and females | Healthy | 6 weeks | - High: 14.3 mg per day | - No modulation in BP after both high and low juice. |
| - Low: 4 mg per day | - FMD increased after high juice. | |||||
| 81 | Açai and juçara juice extracts. | 30 males and females | Healthy | 4 weeks | - Acai juice: 221.58 mg per day | - HDL-C cholesterol increased after both juices. |
| - Jucara juice: 329.54 mg per day | - No changes in LDL-C levels. | |||||
| - TAC, CAT and GPx increased after acai juice. | ||||||
| - CAT increased after jucara juice. | ||||||
| - TOS, uric acid and SOD did not change. | ||||||
| 121 | Cranberry juice | 44 males and females | CAD | 4 weeks | 94 mg per day | - Brachial artery FMD, ICAM-1, CRP, BP, TC, LDL-C, TG's, blood glucose or markers of inflammation. |
| - Carotid femoral pulse wave velocity decreased. | ||||||
| 84 | Black rice pigment fraction | 60 males and females | CHD | 6 months | 4.32 g per day | - TAC increase. |
| - Reductions in hs-CRP, sVCAM-1, sCD40L. | ||||||
| - No modulations in levels of TC, LDL-C, TG, HDL-C, IMT, SOD, apoA-I and apoB | ||||||
| 82 | Freeze-dried black raspberry | 39 males and females | Smokers | 4 weeks | 1.23 g per day | - No effects on blood lipids such as TC, LDL-C, TG, HDL-C, SOD or LDL-C oxidation. |
| - Lipid peroxidation reduced. | ||||||
| - CAT and GPx levels increased. | ||||||
| 151 | Strawberry beverage | 34 male and female adults | Smokers | 4 weeks | 141.7 mg per day | - No observed changes in TGs, LDL-C, HDL-C, TC, apo B, DBP, apo A and hsCRP. |
| - The %FMD increased after the strawberry beverage. | ||||||
| - SBP reduced. | ||||||
| 173 | Hibiscus sabdariffa tea | 65 males and females | Hypertensive | 6 weeks | 21.12 mg per day | - Reductions in SBP, MAP and DBP. |
| 176 | FDB powder | 52 males | Diabetes | 8 weeks | 261.8 mg per day | - Reductions were observed in TG's, TC, HbA1c and LDL-C. |
| - No alterations in hsCRP, SBP, DBP and heart rate. | ||||||
| 87 | LGP | 44 females | Post-menopause | 4 weeks | 27.72 mg per day | - No observed effects in HDL-C or TC. |
| - Levels of TGs, LDL-C, Apo B, Apo E, TNF-α, CETP, F2-isoprostanes and TG's reduced. | ||||||
| - No modulations of LCAT, LDL-C oxidation and plasma cytokines IL-6 and CRP. | ||||||
| 91 | Medox capsules | 55 males and females | MetS | 4 weeks | 160 mg per day | - Considerable reductions observed in serum TG's, LDL-C. |
| - Slight decrease in serum TC, CRP and FBG. | ||||||
| - No changes in levels of HDL-C or uric acid. | ||||||
| 86 | Aronia melanocarpa extract | 47 males and females | MetS | 2 months | 300 mg per day | - TC, LDL, ET-1, TGs, SBP and DBP levels reduced. |
| - Antioxidant enzymes GPx and SOD increased. | ||||||
| - No notable changes in HDL-C, CRP, uric acid and FBG levels. | ||||||
| - Fibrinogen levels increased significantly. | ||||||
| - CAT levels unexpectedly decreased. | ||||||
| 116 | Elderberry extract (Sambucus nigra) | 52 females | Post-menopause | 12 weeks | 500 mg per day | - No modulation of inflammatory markers TNF-α, CRP, IL-6, TNF RI, TNF RII and RANTES. |
| - No changes in BP, platelet reactivity, ET-1, TC, HDL, LDL and TGs. | ||||||
| 177 | - Wine grape solids and grape seed solids in capsules. | 35 males | Healthy | 6 weeks | 118.5 mg per day | - No observed effects on FMD, BP, HDL-C, LDL-C pulse wave transit time, heart rate and large and small arterial elasticity. |
| - Platelet activation, platelet aggregation, and serum TGs were lowered. | ||||||
| 88 | Medox capsules | 118 males and females | Healthy | 3 weeks | 150 mg per day | - Pro-inflammatory chemokines IL-8, RANTES and IFNα were all reduced. |
| - Cytokines IL-4 and IL-13 reduced significantly. | ||||||
| - No modulations in CRP, HDL-C, TC or oxidised LDL. | ||||||
| 89 | Medox capsules | 40 males and females | Diabetic | 4 weeks | 320 mg per day | - Reductions observed in IL-6, IL-18 TNF-α, FBG and LDL-C. |
| - No modulations were observed in IL-8, hs-CRP, mean TG and HDL-C. | ||||||
| 114 | Medox capsules | 146 males and females | Hypercholesterolemic individuals | 24 weeks | 320 mg per day | - HDL-C levels increased, and LDL-C levels decreased. |
| - Inflammatory markers hsCRP and IL-1β reduced. | ||||||
| - No alterations in TNF-α were observed. | ||||||
| 85 | Aronia berry capsules | 49 male and female | Smokers | 12 weeks | 45.1 mg per day | - TC and LDL-C levels reduced. |
| - No modulations were observed in TG's and HDL-C levels. | ||||||
| - Inflammation and oxidative stress biomarkers were unaltered. | ||||||
| 175 | Grape juice extract capsules or grape and wine extract capsules. | 60 males and females | Hypertensive | 10 weeks | 225.8 mg per day and 21.5 mg per day respectively | - DBP and ET-1 were reduced after grape wine extract. |
| - No BP effects after grape juice extract. | ||||||
| - Platelet functions, platelet aggregation, FMD and plasma lipids were unaltered. | ||||||
| 143 | Caucasian whortleberry capsules | 80 males and females | Hyperlipidemic | 2 months | 7.35 mg per day | - Levels of TG's, TC and LDL-C reduced. |
| - HDL-C levels increased. | ||||||
| Citation | Source of anthocyanin | Subjects | Length of intervention | Intervention dose | Main outcomes |
|---|---|---|---|---|---|
| Atherogenic index: AI; blackberry anthocyanin: BLA; blueberry anthocyanin: BA; catalase: CAT; coronary risk index: CRI; cyanindin 3-glucoside: C3G; cyclic guanosine monophosphate: cGMP; diastolic blood pressure: DBP; endothelial nitric oxide synthase: eNOS; glutathione peroxidase: GPX/GSH-Px; high-density lipoprotein cholesterol: HDL-C; inducible nitric oxide synthase: Inos; interleukin: IL; low-density lipoprotein cholesterol: LDL-C; malondialdehyde: MDA; mitogen-activated protein kinases: MAPKs; monocyte chemoattractant protein-1: MCP-1; peroxisome proliferator-activated receptor-alpha: PPARα; reactive oxygen species: ROS; superoxide dismutase: SOD; systolic blood pressure: SBP; tart cherry extract: TCE; total cholesterol: TC; triglycerides: TG; tumour necrosis factor-alpha: TNF-α; very low-density lipoprotein cholesterol: VLDL-C; gallic acid equivalent: GAE. | |||||
| 73 | TCE | Thirty-five male C5BL/6J mice | 6 weeks | 60 mg per kg per day of TCE | - Leptin levels reduced. |
| - MCP-1 and adiponectin levels increased. | |||||
| - Levels of SOD increased. | |||||
| 166 | C3G | 26 male and female mice | 12 weeks | 100 mg per kg BW per day | - Reduction in aortic sinus plaque and aortic cholesterol accumulation |
| - Plasma TC, TG and non-HDL-C levels decreased. | |||||
| - Apo A-1 and plasma HDL-C concentrations increased. | |||||
| 108 | Lyophilised cornelian cherry | 40 rabbits | 60 days | 100 mg kg−1 | - LDL-C and TG decreased. |
| - Levels of HDL-C and PPARα increased. | |||||
| 69 | Anthocyanin rich black rice extract | 30 mice | 20 weeks | 300 mg kg−1 | - Plaque size reduced. |
| - Reduction in thin fibrous cap and large necrotic cap. | |||||
| - Reduction in expression of TFmRNA and iNOS. | |||||
| - Reduced TC, non-HDL-C and TG's. | |||||
| - No alteration in antioxidant capacity. | |||||
| 70 | Mulberry fruit | 48 wistar rats | 4 weeks | — | - Reduction in serum cholesterol, TC, TG and LDL-C. Increase in HDL-C levels. |
| 179 | C3G | 20 mice | 8 weeks | 2 g kg−1 | - Reduction in LDL-C and increase in HDL-C levels. |
| - Reduction in atherosclerotic lesions, superoxide, and lipid hydroperoxide levels in the aorta. | |||||
| - cGMP levels were lowered. | |||||
| - eNOS levels were higher. | |||||
| 72 | BLA and BBA | 60 male mice | 12 weeks | BLA-200 mg kg−1 food | - In both BLA and BBA reductions in serum LDL-C, TC and MDA. |
| BBA-200 mg kg−1 food | - GPx increased in both. | ||||
| - BBA lowered leptin levels, SOD activity and HDL-C. | |||||
| - Inflammatory markers IL-6, TNF-α and NFKβ expression reduced. | |||||
| - Overall, BBA reduced inflammatory markers better than BLA. | |||||
| 142 | Freeze-dried blueberry powder | 32 rats | 10 weeks | 7.7 mg per kg body weight | - SBP reduced. |
| - Positive impact on aortic dilatory response. | |||||
| - No significant differences in TC, LDL-C and HDL-C | |||||
| 71 | Extracts of Euterpe edulis | 32 wistar rats | 50 days | — | - Reduced MDA levels. |
| 168 | Saskatoon berry powder | 30 male rats | 16 weeks | 26.83 g kg−1 powder | - TC levels decreased. |
| - SBP and diastolic stiffness improved. | |||||
| 167 | Passiflora edulis peel extract | 40 rats | 20 days | 200 mg per kg bw | - Reduction in MAP and DBP. |
| - Heart rate reduced in all groups. | |||||
| - SBP reduced after 50 mg per kg bw | |||||
| 79 | Black mulberry – ethanol extract | 50 male rats | 6 weeks | Low dose – 105 mg per kg bw | - High dose lowered TC, TG, LDL-C and AI levels. |
| High dose – 210 mg per kg bw | - High dose increased activity of antioxidant enzymes SOD, GSH-Px and CAT | ||||
| 138 | Calafate | 15 male rats | 10 weeks | 350 mg kg−1 | - HDL-C increased. |
| - Reduction in AI and CRI | |||||
| 169 | Blackcurrant extract powder | 25 mice | 9 weeks | — | - Reduction in thickness of tunica media. |
| - Reduction of foam cells. | |||||
| - High expression of eNOS. | |||||
| 137 | Lypholized grape powder | Female ovariectomised guinea pigs | 12 weeks | — | - Reduced TG's and levels of VLDL-C. |
| - Reduced concentration of cholesterol in the aorta. | |||||
| 77 | Blueberry-enriched anthocyanin extract | 40 rats | 4 weeks | 80 mg kg−1, 20 mg kg−1 | - Reduction of LV fibrosis. |
| - Reduction of myocardial leukocyte infiltration | |||||
| - Reduction in pro-inflammatory cytokines IL-1β and TNF-α | |||||
| - Reduction in TLR4 protein | |||||
| 110 | Freeze-dried raspberry | 30 mice | 8 weeks | 18.18 mg GAE per kg FW. | - Decreased levels of ROS. |
| - Increased GPx activity, plasma resistin and HDL-C levels. | |||||
| - MCP-1 levels were lowered. | |||||
| 75 | Freeze-dried jaboticaba peels | 30 male rats | 10 weeks | 1727.12 mg C3G per 100 g | - CAT activity and GSH levels were high. |
| - Increased SOD activities and liver GPx. | |||||
| 112 | C3G | 60 male rats | Low dose: 200 mg C3G per kg BW | - Low dose showed a reduction in right ventricular systolic pressure. | |
| High dose: 400 mg C3G per kg BW | - High dose showed a reduction in mean pulmonary artery pressure | ||||
| - Decrease in plasma levels of IL-6 and TNF-α in both groups. | |||||
| - SOD increased in both groups. | |||||
| 140 | Concord grape juice | 20 rabbits | 96 days | 225 ml per day | - Serum cholesterol decreased |
| - Lower development of aortic atheroma and decrease in hypercholesterolemia-enhanced PA | |||||
| - Reduction in SBP and MBP. | |||||
| 135 | Sweet potato leaf powder | 72 hamsters | 6 weeks | - Reduction in plasma TC and LDL levels | |
| Reduced concentration of AI and VLDL-c concentration. | |||||
5. Effects of anthocyanin consumption on antioxidant capacity
The concept of oxidative stress and mild chronic vascular inflammation as part of hypertension and atherosclerosis pathophysiology has also been accepted.46 Oxidative stress occurs when there is an imbalance between oxidation and antioxidation, which leads to neutrophil infiltration, increased protease secretion, and production of oxidative intermediates.59ROS represents a group of unstable compounds with increased reactivity formed in organisms from O2. ROS include H2O2, superoxide anion (O2˙−), singlet oxygen (1O2), and hydroxyl radical (˙OH). In addition, cellular metabolites formed by either endogenous or exogenous nitrogen can form reactive nitrogen species (RNS), such as NO, peroxynitrite (ONOO−), and nitrite/nitrate. Free radicals can initiate multi-step chain reactions; each reaction produces a free radical that participates in the following step. As a result of this complex sequence, the target molecule loses an electron and becomes a free radical, triggering a series of reactions that damage and eventually kill the living cell.40
ROS may be generated intracellularly, extracellularly, or in specific intracellular compartments via several enzymes, such as cyclooxygenases, myeloperoxidases, cytochrome P450 monooxygenase, uncoupled nitric oxide synthase (NOS), peroxidases, a family of NADPH oxidases (NOX), lipooxygenases (LOXs), and xanthine oxidase (XO).46 XO is the source of NO, H2O2, and superoxide ions (O2˙−). Its increased activity is linked with dyslipidaemia, diabetes, and hypertension. At the same time, LOXs are dioxygenases that catalyse the hydroperoxidation of polyunsaturated fatty acids. The role of NOX is host defence, regulation of gene expression, post-translational processing of proteins, cellular signalling, and cell differentiation. Moreover, NOX dysregulation contributes to various pathological conditions, such as diabetic nephropathy, hypertension, atherosclerosis, immunosuppression, hypothyroidism, neurodegenerative disorders, and cancer.59
Oxidative stress associated with atherosclerosis can occur locally in the vessel wall or the systemic and involves multiple cell types, including endothelial cells, smooth muscle cells (SMC), immune cells, and stem/progenitor cells. Moreover, ROS and NO regulate LDL-C uptake in arterial walls, leading to the oxidation of various phospholipids and the generation of ox-LDL.60 Physiologically, there are two types of antioxidant systems in the body that can maintain the oxidant–antioxidant balance: the enzymatic system, which includes superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), and the non-enzymatic system, such as albumin, bilirubin and glutathione, the molecular regulatory mechanism by nuclear factor E2-related factor 2-anti-oxidant response elements (Nrf2/ARE)-mediated antioxidant gene expression as well as micronutrients (vitamins and minerals) and food compounds (e.g., polyphenols and carotenoids).46,59
Endogenous antioxidant enzymes reduce ROS to water by working simultaneously, firstly by SOD converting superoxide anions to hydrogen peroxide. H2O2 still damages cells because of its pro-oxidant behaviour; thus, CAT and GPx work to reduce H2O2 in water. By completing this action, the detrimental effects of ROS and the risk of CVD are ultimately reduced. SOD and CAT depend on minerals such as zinc and magnesium to function effectively; thus, an individual's nutritional status is critical regarding antioxidant status.61 Suppose the endogenous antioxidant system is not working well, or there is an overproduction of ROS. In that case, oxidative/nitrosative stress will take place, thus forming oxidation-end and immediate products associated with inflammation and endothelial dysfunction. ox-LDL is derived from oxidative stress and enhances CVD by initiating inflammation and subsequent growth/build-up of plaque in the arteries.62 Malondialdehyde (MDA) is an end-product of lipid peroxidation, whereby lipids react with oxidants such as free radicals to produce an oxidation product.63 In fact, NO modulates the vascular tone by reverting the acetylcholine's constrictive action, consequently leading toward vasorelaxation and keeping the balance of contracting factors derived from the endothelium, such as thromboxane A2 and endothelin-1.64
Apart from the direct ability to scavenge free radicals by phenolic compounds, we should hallmark their other potential antioxidant effects associated with the following mechanisms:65 (a) metal interaction (iron and/or copper chelation), (b) inhibition of ROS-producing enzymes, especially lipoxygenases, NADPH oxidase and XO, (c) inhibition of leukocyte activation. (d) Direct vasodilatory action, (e) a down-expression of inflammatory biomarkers, such as inhibition of iNOS, cyclooxygenase-2 (COX-2), and leukocyte activation and (f) inhibition of platelet aggregation.
ANT display their antioxidant activity in three main mechanisms. These include single electron transfer (SET), hydrogen atom transfer (HAT), and chelating transition metals. SET involves the movement of one electron by antioxidant (AH+) to the free radical (e.g., peroxyl radical, ROO˙), thus reducing it. HAT involves hydrogen donation from the antioxidant.66 These mechanisms allow ANT to neutralise unstable free radicals and deactivate their precursors, making them less reactive, thus inhibiting free radical production and chain reactions.67 Depending on the conditions of the system, ANT may display anti-inflammatory effects by downregulating pro-inflammatory markers such as TNF-α, IL-6, and C-reactive protein (CRP) or by promoting anti-inflammatory mediators and the formation of NO.68
5.1. Animal studies
Changes in the endogenous enzyme's activities following the consumption of an ANT-containing food or nutraceutical were reported in several studies.69–73 Nemes et al. reported an overall decrease in ROS production, and this was associated with increased SOD activity, and significant enhancement of antioxidant capacity post-consumption of tart cherry extract, rich in cyanidin 3-rutinoside (68.31 mg/100 mg) and cyanidin 3-glucoside (C3G, 29.14 mg/100 mg) in obese mice. The reduction in ROS observed results from increased SOD levels as it regulated the bioavailability of NO. NO is involved in dilating blood vessels and inhibits platelet aggregation and proliferation of vascular smooth muscle cells (VSMC), critical components at the beginning of the atherogenic process.74 Similarly, Batista75 found that when male Sprague-Dawley rats were supplemented with freeze-dried jaboticaba (Plinia jaboticaba) peel extract at 1, 2, and 4 g/100 g (1737.12 mg C3G per 100 g) for 10 weeks, SOD, CAT, and GPx activities were enhanced, but no dose-dependency behaviour was observed. Additionally, decreased lipid peroxidation in the liver and brain was observed. It is known that CAT, GPx, and SOD represent the primary defence system that aids in eliminating excess build of ROS in the body, therefore decreasing oxidative stress involved in initiating atherosclerosis.76Liu et al. analysed the impact of a blueberry extract [20 mg kg−1 and 80 mg kg−1 containing 25.7 g of total ANT per 100 g of extract, where malvidin 3-galactoside (28.11%), malvidin 3-arabinoside (16.18%), malvidin 3-glucoside (14.08%) were the main ANT] on cyclophosphamide (CTX)-induced cardiac injury in rats over four weeks. The mechanisms of CTX, which causes acute cardiotoxicity, involve an increase in ROS, cell apoptosis, and inflammation, which all contribute to the progression or development of CVD; hence, this model is analysed as a comparison. The impacts of blueberry extract on these rats include increased antioxidant enzymes, SOD, and GPx activity.77 A decrease in MDA in cardiac tissues was evident post-intervention in lowering lipid peroxidation.78
In a study conducted by Jiang et al.,79 significant increases were observed in endogenous enzymes, such as CAT (22%), GPx (26%), and SOD (16%) in male atherosclerotic rats that consumed ANT-containing mulberry extracts containing 237.5 mg C3G per g (daily dosage of 105 or 210 mg kg−1). The MDA levels also decreased by 28%, and animals’ oxidative stress levels, which contribute to atherosclerosis, reduced significantly along with the decrease in atherosclerotic lesions.79 Overall, the antioxidant action of ANT in animal trials highlights a significant increase in the activity of endogenous antioxidant enzymes. A decrease in lipid peroxidation biomarkers in blood and tissues results in an overall reduction of oxidative stress in the body. Another aspect should be highlighted: ANT-containing foods usually comprise at least two different ANT and other phenolic compounds; thus, it is impossible to attribute health benefits to a single compound. Instead, current evidence suggests that synergism between multiple ANT and other phenolics may also be partially responsible for the bioactivity.80
5.2. Human studies
Eleven of 22 studies reviewed examined the ANT's antioxidant activity. De Liz et al.81 reported increased CAT (275.1%) and GPx (15.3%) activity after daily açaí (Euterpe oleracea) juice consumption for 28 days (200 mL per day containing 115 mg of C3G and 107 mg of cyanidin 3-rutinoside). Similar results were found by Park et al. after consuming freeze-dried black raspberry (30 g per day for four weeks containing 1.23 g of total ANT), with CAT increasing by 25% and GPx by 23.5%.82 These increases are linked with improved antioxidant capacity, the ability to reduce ROS, and the mitigating damage caused by oxidative stress, thus decreasing CVD risk.83 The increase in CAT activity was significantly higher post-açaí juice consumption, even though the anthocyanin dosage was much higher in the black raspberry supplement. This shows the effects of stress, in this case, smoking, on the modulatory potential of ANT toward CVD.Considering the SOD activity, four studies observed no changes in SOD activity after ANT consumption.81,82,84,85 However, a study involving the intake of Aronia melanocarpa extract capsules (250 mg of Aronia fruit containing 45.1 mg of total ANT and 41.9 mg of proanthocyanidins) for two months in individuals with metabolic syndrome (MetS) resulted in an increase of 28.8% in SOD activity. Aronia is mainly composed of ANT, flavanols, procyanidins, and phenolic acids; hence, the increase in SOD may be attributed to multiple interactions between these bioactive compounds.86 Four studies observed no changes in ox-LDL levels and, therefore, no improvements in oxidative stress and CVD-related biomarkers.82,85,87,88
In a study comprising 60 patients (45–75 years old) with coronary heart disease (CHD), divided into placebo and a group that consumed 10 g of rice pigment fraction (4.3 g of total ANT per 10 g – unknown concentration of C3G and peonidin 3-glucose) for six months. The authors used a randomised, double-blind, placebo-controlled six-month intervention. The supplementation increased the plasma's antioxidant capacity, reducing plasma VCAM-1 and CPR plasma levels. These results show that rice pigment fraction display cardioprotective effects by improving plasma antioxidant status and inhibiting inflammatory factors. Using an open-label clinical trial comprising 40 healthy, type-II diabetic, and type-II diabetic-at-risk patients,89 320 mg ANT supplementation as a nutraceutical per day over 4 weeks was given to patients. In the diabetic patients, the secretion of pro-inflammatory biomarkers (IL-6, IL-18, and TNF-α) was downregulated, while fasting blood glucose, LDL-C, and uric acid levels were improved in type-II diabetic-at risk patients.
Uric acid was utilised in the studies throughout this review as a marker of oxidative stress. Uric acid can act as a pro-oxidant and increase XO activity, heightening the production of superoxide anions and ROS. Therefore, reducing uric acid levels would effectively lower ROS production and atherosclerosis.90 In three studies, no changes in uric acid were observed.82,86,91 The apparent difference between these reviews and Nikbakht et al. is that Nikbakht's study population was mainly men (60% of patients). It has been previously reported that men have higher uric acid levels than women, as estrogen in women can stimulate uric acid excretion.92,93
Isoprostanes, valuable biomarkers of lipid peroxidation, were measured throughout three studies based on grape powders and ANT-containing capsules. Results from these studies resulted in reductions in F2 isoprostanes and 8-iso-PGF2α.87 Conversely, a study completed on aronia berry extract observed no reduction in urinary eight isoprostanes.85 The contrast in results may be linked to the fact that Xie et al.85 conducted their study on former smokers. In contrast, Li et al.94 and Zern et al.87 investigated individuals with type-II diabetes (T2D) and postmenopausal women. It has been claimed previously that individuals with T2D and postmenopausal women have higher levels of isoprostanes than healthy individuals, which may be why the decrease was more recognised.95,96
Although the studies above show apparent discrepancies in the sample (e.g., sample size, study design and use of placebo and control groups, participant's age and gender, pre-existence of underlying health conditions, distinct life habits, such as smoking), we can highlight that the ANT consumption, both as a food or nutraceutical positively modulated the antioxidant activity and inflammation-related biomarkers in humans; despite not all biomarkers being modulated, there is still clear potential for ANT to act as natural antioxidant and anti-inflammatory agents, and reduce the risk of CVDs.
Among the studies, four foods (juice, extract, or fruit) and three different types of capsules contend extract of foods, all rich in ANT, led to positive results. A standard intervention period of four weeks was also observed in five studies, four of which displayed positive results. Finally, in all the studies that experienced positive antioxidant effects, only one was based on healthy individuals, indicating that ANT may be more effective at modulating the antioxidant status of unhealthy/diseased individuals.
6. Effects of anthocyanins consumption on inflammation
Inflammation is a protective response elicited by the innate immune system in response to the presence of pathogens, damaged cells, or toxins. The innate immune system acts as the primary defence system for the cardiovascular system. As a result, the damaged cells activate inflammatory cells and trigger inflammatory signalling pathways such as nuclear factor kappa beta (NF-κβ).37 Post activation of NF-κβ, pro-inflammatory cytokines, including TNF-α, monocyte chemoattractant protein-1 (MCP-1), adipokines, IL-6, IL-17, and IL-1β are produced.97 Cytokines are mainly secreted from the immune cells and are classified as pro-inflammatory cytokines (e.g. IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokines (e.g., IL-4 and IL-10).37,98,99A controlled inflammatory response is beneficial; however, dysregulation can harm health.100 Inflammation relies on two mechanisms of action that combine the genetic expression of cytokines and chemokines. Inflammation is a local response triggered by cellular injury, which can be identified by leucocyte infiltration, capillary dilation, and the production of a host of chemical mediators that aim to eliminate toxic agents and repair damaged tissue.101 MCP-1 is a chemokine which mediates monocyte recruitment and facilitates entry into the wall of vessels at atherosclerosis sites.102 Toll-like receptor 4 (TLR4) is a pattern recognition receptor crucial in initiating the inflammatory response and cellular metabolism and is intricately linked to tissue damage.103
Inflammation involves the response to body tissue harm or infection; there are two types: acute and chronic. Acute inflammation occurs rapidly but only for a short period, whereas chronic occurs for an extended period.98 The innate immune system, comprised of cellular defences and chemical and physical barriers, is responsible for the inflammatory responses linked to physical, infectious, or chemical challenges. When the immune system comes in contact with damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), it stimulates cells from the monocyte–macrophage lineage, thus expressing pro-inflammatory cytokines and preventing actions of anti-inflammatory genes.104
When atheroma/plaque forms due to the build-up of fat or macrophages, the macrophages activate these cytokines, promoting build-up; this build-up narrows the arteries, constricting blood flow and increasing the risk of CVD.39 Therefore, the expression of these pro-inflammatory cytokines and chemokines, which can be explained by two mechanisms, protein, and genetic expression, must be reduced to lower CVD risk.101
CRP, also analysed in different studies throughout this review, is an acute phase reactant and plays a part in CVD by affecting vascular function. CRP plays this role through various mechanisms, such as lowering NO's bioavailability, heightening adhesion molecules’ expression, and changing macrophages’ LDL-C intake.105 High plasma CRP levels are linked with the severity of atherosclerosis; when CRP comes in contact with infectious agents, it can promote inflammatory reactions in the blood vessels.106
6.1. Animal studies
Numerous studies in this review reported changes in several CVD-related inflammatory biomarkers. Thirteen of the 22 studies investigated throughout this review displayed inflammatory modulations post-anthocyanin consumption of freeze-dried extract of Euterpe edulis. Two primary ANT in the extract were C3G (9.52 g kg−1) and cyanidin 3-rutinoside (16.31 g kg−1).107 Freitas et al. reported a significant reduction in the expression of pro-inflammatory mediators, including TNF-α, IL-17, and interferon-gamma (IFN-γ) in healthy rats which received commercial mice chow combined with defatted pulp freeze-dried extract of Euterpe edulis-enriched diets for fifty days compared to the other treatment groups. Moreover, a decrease in IL-10 and IL-4 mRNA expression is evident post-consumption of the ANT-containing extract. The extract inhibited the release of inflammation mediators and positively modulated the inflammation response.71 Similar results were seen in an in vivo trial by Sozański et al.: the consumption of a 100 mg kg−1 dosage of lyophilised cornelian cherry containing higher amounts of C3G (123.5 ± 19.7 mg per 100 g) and pelargonidin 3-O-galactoside (87.9 ± 19.9 mg per 100 g) significantly reduced IL-6, which markedly increased in cholesterol-treated rabbits after 60 days.108 Additionally, there was an increase in the expression of peroxisome proliferator-activated receptor-alpha (PPARα) in the liver of the cornelian cherry-fed mice, suggesting the beneficial impact of cornelian cherry on fatty acid catabolism in a hyperlipidemic diet. Furthermore, TNF-α levels decreased considerably even below the baseline of the control rabbit, which had no inflammation induced as they were fed a standard feed with no cholesterol.109 As a result, the consumption of ANT-containing supplements in animals fed high cholesterol was compared to healthy animals.Additionally, Wu et al. examined the effect of 200 mg kg−1 supplementation of blueberry (mostly C3G and cyanidin 3-rutinoside) and blackberry extract (mostly C3G) on obese mice over 12 weeks. Similar results were reported: both anthocyanin-enriched diets attenuated the genetic expression of TNF-α, IL-6, and NF-κB. Moreover, a higher decrease in inflammatory markers was observed in blueberry than in blackberry extract-fed mice.72 This may result from different levels and ANT types in the two diets.
T2D is another risk factor for CVD, and it is associated with systemic inflammation whereby typically elevated levels of pro-inflammatory cytokines are produced. The effect of including a 5.3% freeze-dried raspberry containing an extractable polyphenolic concentration of 18.18 mg gallic acid equivalent per kg fresh weight, along with ANT identified but not quantified, including cyanidin-3-sophoroside, C3G, cyanidin-3-rutinoside, pelargonidin glucoside, and cyanidin-3-sambubioside, into the diet of obese diabetic mice was analysed over eight weeks. Proinflammatory cytokines were significantly reduced post-intervention, 71% and 64%, respectively. Furthermore, a 15% decrease in MCP-1 was also evident in the raspberry-fed animals compared to the control.110 A reduction in cytokines produced indicates the potential that raspberry ANT may facilitate their reduction through cell signalling pathways. The overall decreased risk in all three biomarkers of inflammation may show the potential cardio-protective properties of raspberries in reducing the risk of inflammation-related disorders such as atherosclerosis and CVD.
Obesity and T2D are intricately linked to inflammation and inflammation-related disorders. In an in vivo study by Nemes et al.,73 the impact of 60 mg kg−1 of ANT-containing tart cherry extract, which contains cyanidin-3-O-glucosyl-rutinoside (1.15 mg/100 mg), C3G (29.14 mg/100 mg), and cyanidin-3-O-rutinoside (68.31 mg/100 mg), on both CVD and T2D was analysed in thirty-five obese male C5BL/6J mice for six weeks. The high-fat diet was reported to increase IL-6, MCP-1, leptin, and resistin levels. However, tart cherry extract significantly reduced leptin levels and IL-6 by approximately 45% and 27%, respectively. MCP-1 levels were significantly elevated in high-fat diet (HFD) and HFD + tart cherry extract-fed mice compared to the control.73 Adiponectin, an anti-inflammatory adipokine, increased, in contrast to decreased resistin levels, an example of pro-inflammatory adipokines. Pro-inflammatory cytokines, e.g., IL-6 and TNF-α, can regulate resistin gene expression, which explains the decrease observed in this study.111 In addition, doses between 40 and 80 mg kg−1 of ANT have demonstrated beneficial effects in chronic treatment in laboratory animals and obese humans.73
Ouyang et al.112 also reported a significant decrease in pro-inflammatory mediators IL-6 and TNF-α in monocrotaline-induced rats (60 mg kg−1 of body weight) following post-C3G treatment (at doses of 200 or 400 mg kg−1 of body weight) for four weeks. IL-6 key role is to stimulate antigens to encourage the proliferation of T cells and the maturation of B cells in the immune system. As a result, systemic inflammatory factors are released, and pulmonary artery hypertension (PAH) develops. Therefore, the decrease in IL-6 indicates a reduction in the inflammatory response, inhibiting the progression of PAH.
Liu et al. investigated the impact of blueberry extracts (80 mg kg−1) on CTX-induced cardiac injury in rats over a four-week period. The rats were evenly distributed among the experimental groups, each consisting of eight individuals. These groups included the standard control group, the group treated with the extract at 80 mg kg−1, the group treated with CTX at 100 mg kg−1, the group treated with both CTX and the extract at 20 mg kg−1, and the group treated with both CTX and the extract at 80 mg kg−1.77 The total anthocyanin content of the extracted powder was 25.7 g/100 g and included a variety of ANT, such as malvidin 3-galactoside, petunidin 3-galactoside, and cyanidin 3-galactoside. It was reported that the blueberry-rich-anthocyanin extract attenuated CTX-induced increased secretion of pro-inflammatory cytokines (including IL-1β and TNF-α. The IL-10) levels, an anti-inflammatory cytokine increase, and a significant reduction of TLR4 protein was.77 Elevated TNF-α and IL-Iβ can result in cellular apoptosis and, over time, heart failure.113 IL-10 inhibits inflammation by decreasing proinflammatory cytokines’ production and preventing tissue damage.77 Moreover, the decrease observed in this study shows the effect of blueberry extract on the pathogenesis of CTX-induced inflammation and cardiac injury.
Experimental evidence portrays the impact of ANT on different biomarkers of inflammation closely linked to CVD. The consumption of ANT displays positive effects on the regulation of inflammation mediators. In conclusion, ANT show several anti-inflammatory properties in relation to sustained low-grade inflammation, which is linked to CVD. However, with the current data available, assessing the effects of intervention time, daily dosage, type of ANT given, and interaction with other polyphenols in crude extracts on inflammation markers is unrealistic.
6.2. Human studies
Four studies positively affected pro-inflammatory cytokines throughout this review.87,88,114 One study examined the effects of 3-week ANT-containing capsules (300 mg per day) on healthy individuals; results included reductions in IL-8 by 45%, IL-4 by 60% and IL-13 by 38%. Additionally, a study examining the effects of consuming lyophilised grape powder rich in polyphenols and ANT on inflammatory cytokines, plasma lipids and oxidative stress was completed on 44 pre- and post-menopausal women. The lyophilised grape powder contained 0.77 g kg−1 of total ANT.87 The lyophilised grape powder was mixed in water before consumption, each participant consumed 36 g per day lyophilised grape powder. Lyophilised grape powder reduced TNF-α in postmenopausal women after a 4-week intervention, and IL-1β decreased by 12.8% after 24-week ANT consumption. These reductions in pro-inflammatory cytokines are linked to reducing the formation and atherosclerosis development risk, thus lowering CVD risk. These four studies displayed common characteristics which may be linked to the results observed. For example, in three studies, ANT were consumed as capsules, the intervention length was four weeks, and the daily ANT dosage was ∼300 mg. These exact parameters (i.e., intervention time and daily dosage) may be beneficial in targeting pro-inflammatory cytokines.In contrast to these studies, three other interventions reviewed involving agraz extract, aronia berry, and elderberry extract displayed no positive modulations on inflammatory markers IL-6, IL-8, TNF-α, and IL-1β.85,115,116 Among these studies, two were based on food intake of ANT; they contained less than 300 mg and were undertaken for 12 weeks. Marín-Echeverri et al. investigated the effects of agraz (Vaccinium meridionale), an edible berry rich in ANT, specially cyanidin 3-galactoside, C3G, delphinidin 3-pentoside, and cyanidin 3-arabinoside. The study enrolled 40 volunteers aged between 25 and 60 in a double-blind crossover design. Over four weeks, female participants were assigned to consume either agraz or a placebo, with a 4-week washout period separating the two phases of the study. Additionally, the study found no effects on myeloperoxidase (MPO) levels, MCP-1 and NF-κB.115 MPO is a mediator of inflammation and is linked to CVD as it promotes endothelial and lipoprotein dysfunction, unstable atherosclerotic plaque, and decreased availability of NO.117 NF-κβ, which was also unaltered post-agraz consumption, is a transcription factor that regulates inflammatory cytokines. However, extensive activation can cause chronic inflammatory responses and the progression of CVD.118 Finally, MCP-1, which was unaltered in this study and the study on aronia berry capsules by Xie et al.,85 is a chemokine formed from macrophages, muscle, and endothelial cells due to arterial injury and high cholesterol. High MCP-1 levels are linked to increased cardiovascular events. All these parameters were unaffected after ANT intervention, indicating ANT are ineffective at targeting these CVD markers.85
Curtis et al.116 treated postmenopausal women with capsules of elderberry extract containing 125 mg of ANT (C3G) per capsule, totalling 500 mg per day for 12 weeks. Inflammatory markers such as tumour necrosis factor receptor I/II (TNF RI), (TNF RII) were quantified. They reported that intervention had no impact on the plasma concentrations of inflammatory biomarkers (such as TNFα, CRP, TNF receptors I and II, IL-6, and RANTES), vascular function (including endothelin-1 levels, platelet reactivity, blood pressure, and pulse), as well as plasma lipid and lipoprotein levels (including total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), all of which remained within expected physiological levels. Additionally, glucose concentrations were unaffected by the intervention.116 TNF RI and RII are receptors released by macrophages and can boost TNF-induced inflammation.119 RANTES, a chemokine that assists in employing leukocytes to sites of infection or damage, is linked with CVD risk and pathogenesis.120 However, RANTES was lowered by 15% in a study based on 300 mg per day of ANT for three weeks in healthy individuals. By effectively lowering RANTES, the recruitment of leukocytes and the risk of CVD can be reduced.88
CRP was measured in 12 studies, and only three showed a positive effect when ANT were consumed. The black rice pigment fraction containing 4320 mg of ANT, consumed by patients for six months with CHD, decreased CRP from 3.82 ± 1.82 mg L−1 to 2.55 ± 1.66 mg L−1, while the levels of these inflammatory biomarkers didn't change significantly after the same duration in the placebo group (from 3.58 ± 1.71 mg L−1 to 3.82 ± 1.96 mg L−1).84 Similarly, in two studies, decreases in CRP by 18% and 21.6% were observed after anthocyanin capsules containing 320 mg in individuals with MetS and hypercholesterolemia.91,114 By reducing CRP levels, atherogenesis and the risk of CVD would also be diminished. However, nine studies observed no effects on CRP levels after consuming ANT. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) were measured throughout this review. ICAM-1 was measured in two studies, but no modulations were observed.85,121 ICAM-1 is an endothelial adhesion molecule, it prompts leukocyte adhesion into the vascular subendothelial space, and elevated levels are linked with the development of atherosclerosis.122 VCAM was also measured in two studies; one study by Wang et al. observed a decrease in VCAM-1 after a 6-month intervention with black rice pigment fraction in individuals with CHD.180 VCAM plays a role in the adhesion and transmigration of leukocytes as part of the inflammatory process.124 Reducing such levels could lower the inflammatory process, atherosclerosis progression, and CVD. Xie et al.85 observed contrasting results and investigated the effects of daily consumption of Aronia berry ANT (500 mg) in 49 healthy adult former smokers over 12 weeks. Individuals with heart diseases such as CHD possess higher soluble vascular cell adhesion molecule-1 (sVCAM) levels.
Throughout this review, various anti-inflammatory effects of anthocyanin-containing foods and supplements were observed, the key being a reduction in inflammatory cytokines, CRP, and chemokine modulation. However, as some studies received unexpected results (i.e., no changes in biomarkers compared to the control group), an investigation is required before a factual health/nutrition claim is made.
7. Effects of anthocyanin consumption on lipid profile
Hyperlipidaemia explains the imbalance of cholesterol fractions within the body. As a result, the risk of developing CVDs is doubled compared to that of a healthy individual. It simultaneously can increase the likelihood of a cardiovascular event such as myocardial infarction. The increased build-up of plaque that LDL-C triggers can block the arteries and elevate the risk of atherosclerosis. The increase in HDL-C levels indicates a reduced risk of CVD as HDL-C function effectively removes cholesterol from the body.125An individual's lipid profile consisting of TC, LDL-C, HDL-C, and TG significantly determines the risk and progression of CVDs. Lipids are distributed in the body as lipoproteins and are composed of phospholipids, protein, TG, and unesterified cholesterol. Five essential lipoproteins are found in the blood, including VLDL-C, LDL-C, HDL-C, chylomicrons, and intermediate-density lipoproteins (IDLs).126
Levels of lipoproteins found in the blood can be decisive in cardiovascular health and the risk of CVD. High levels of TC and TG can influence and disturb blood movement and promote the narrowing of the heart vessels, which is linked to CVD risk. TGs are fat in the blood; high TG levels can be considered a marker for atherogenic lipoproteins and contribute to fatty build-up and plaque development through foam formation.127 Additionally, the ratio of TG to HDL-C is vital for CVDs.128 TC includes HDL-C and LDL-C, which are significant in cardiovascular health. Like TG, increased LDL-C, VLDL-C, or low HDL-C can lead to increased LDL oxidation with subsequent plaque accumulation and atherosclerosis.129 LDL-C is one of the vital cholesterol-carrying and atherogenic lipoproteins. Thus, the increased LDL-C, especially in the artery's intima-media, can result in thrombosis and atherosclerosis. Individuals with elevated levels of HDL-C and low non-HDL-C have a decreased risk of CVD, as HDL-HDL-C is associated with a positive impact on heart health.129,130
In contrast, increased levels of HDL-C are linked with the risk reduction of CVD, with even a slight increase of 10 mg L−1 reducing risk by 2–3%.123 This reduction is possible as HDL-C can remove extra cholesterol and fat from the blood vessels.131 Enhancement of reverse cholesterol transport (RCT) is one of the critical benefits of HDL-C. It involves transporting cholesterol from the artery walls to the liver, where it can be removed from the body. HDL-C is required to facilitate this transport, and in doing so, plaque build-up is reduced, blood flow and oxygen transport are enhanced, and atherosclerosis can be mitigated.132 Thus, increasing HDL-C levels to improve cardiovascular health and reduce CVD risk is recognised.
The atherogenic index (AI) predicts CVD and associated diseases such as dyslipidaemia and is calculated as the ratio of (TG/HDL-C).133 CRI is estimated by the ratio of TC/HDL-C.108 HDL-C is a heterogeneous group of particles that differ in size, cholesterol content, shape, and apolipoprotein composition and possess cardio-protective properties. Apolipoproteins are secreted from the liver and bind to circulating phospholipids and cholesterol, forming nascent discoid lipid-poor HDL-C particles. These immature particles trigger cholesterol efflux in subendothelial macrophages and fibroblasts via interactions with adenosine triphosphate (ATP)-cassette transporter and then finally store their cholesterol in their core. HDL-C can deliver this cholesterol load by two pathways; they can transfer it directly to the load by scavenging receptors or indirectly by moving the cholesterol load to LDL-C or very low-density lipoproteins-cholesterol (VLDL-C) particles. The liver can take up the cholesterol via the LDL receptor, and it can be broken down and excreted into the faeces as bile acids. This process, known as reverse cholesterol transfer, explains HDL-C's favourable cardioprotective impact against LDL-C.123
7.1. Animal studies
Different serum lipids levels were analysed, including triglycerides (TG), total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, atherogenic index (AI), and coronary risk index (CRI). The role of the lipid profile in the progression of CVD is demonstrated in several studies. ANT possess LDL-C-lowering effects, so their impact on lipid profiles is analysed.134Chang et al.135 reported a significant decrease in lipid profiles of Syrian hamsters fed sweet potato leaf powder for six weeks. Hamsters fed the anthocyanin-enriched diet reversed the changes in lipid biomarkers triggered by consuming a high-fat, high-cholesterol diet. The ingestion of 5% purple sweet potato leaf powder reduced the following biomarkers TC and LDL-C by 20.6% and 48%, respectively; consequently, a significant decrease was also evident in the AI of sweet potato leaf powder-fed hamsters. Overall, the sweet potato leaf powder feeding resulted in a substantial increase in faecal sterol contents for both the 0.1% and 0.2% cholesterol groups by AI decrease in LDL-C, and the increase in faecal sterol indicates that the excess accumulation of LDL-C is transported to the liver and broken down, excreted into the faeces.135,136
Similar results were observed in a trial by Yang et al.,70 whereby mulberry fruits were assigned to hyperlipidaemia rats over four weeks, and animals were given a normal diet (ND) or HFD with 5–10% mulberry freeze-dried powder. The HFD induced significant negative impacts on lipid biomarkers compared to the ND. However, post-experiment, these results were positively modulated, whereby a decrease was seen for TC, TG, LDL-C, and AI levels, 16.2%, 35.7%, 23.5%, and 43.4%, respectively. Furthermore, an increase in HDL-C levels was reported by 33%.70 Evidently, the consumption of mulberry fruit positively attenuated the changes in lipid biomarkers.
Additionally, equivalent results were evident in six studies that analysed the same biomarkers.75,79,108,110,137,138 Zern et al.137 also reported a 25% increase in free cholesterol and a 51% reduction in cholesteryl esters in animals fed with a diet containing 10 g of lyophilised grape powder per 100 g of chow containing ANT at a concentration of 0.077 g/100 g. The decrease in cholesteryl esters indicates a reduction in the accumulation of these esters in macrophages, which begin the onset of foam formation; hence, a decrease in atherosclerosis is observed.139 A reduction of CRI by 72% was also reported in a trial carried out by Guzmán & Sánchez, which indicates that the consumption of a daily dose of 350 mg kg−1 of anthocyanin-rich calafate had an overall positive impact on cardiovascular health in obesity-induced rats. The rats with diet-induced obesity displayed a significant increase in AI and C,RI, and supplementation of calafate resulted in a decrease in AI by 81% and a 62% reduction in CRI. Both AI and CRI are central indicators of CVD development; hence, the supplementation of calafate portrays some potential benefits in diet-induced obesity rats.138
Xia et al.69 also examined the effect of anthocyanin-rich extract from black rice on lipid biomarkers in relation to atherosclerosis in Apo E-deficient mice over a twenty-week trial. Consumption of anthocyanin-rich black rice extract (300 mg kg−1), containing C3G and peonidin 3-glucoside, resulted in a 60% reduction in serum TC and non-HDL-C compared to the control animals. Additionally, it increased HDL-C levels in mice, similar to the simvastatin group.69 This result is significant as it suggests that the anthocyanin-rich extract improved the lipid profile. It reduced the potential for the progression of atherosclerosis and the formation of an unstable plaque.140 The mortality risk of CVD is reduced by 15% for every 10% decrease in total cholesterol levels. Hence, the importance of lipid-lowering effects anthocyanin portrays, especially regarding.141
In contrast to these results, one study reported no significant changes in the animals’ lipid profiles after anthocyanin intervention.142 Supplementation with freeze-dried blueberries (2%), containing ANT at 11.80 mg per 100 g, for ten weeks, resulted in no significant differences in HDL-C, LDL-C, and TC levels in rats fed with either a high-fat diet HFD or an ND; this may be because of the small size analysed or the lower ANT content (7.7 mg per kg BW) compared to other trials. Regularly consuming ANT plays a crucial role in lipid-lowering, is vital for heart health, and decreases the risk of CVDs. Overall, post-intervention, an increase in HDL-C concentrations and a reduction of LDL-C, TC, TG, AI, and CRI positively reduce the overall risk factor of the progression of atherosclerosis and CVDs.
7.2. Human studies
Kianbakht et al.143 examined the effects of a 350 mg whortleberry capsule on 40 patients. Participants took one capsule every 8 hours for two months. The capsules contained approximately 2.45 mg of total ANT per capsule, with a total anthocyanin content of 86.56 ± 2.46 mg per 10 g of the extract. The study investigated the effects of this intake on TC, TG, HDL-C, and LDL-C levels in individuals with hyperlipidemia.143 Hyperlipidaemia is a risk factor for CVD and is associated with plaque build-up (e.g., atherogenic plaques) and subsequent blockages.144 This trial involved the intake of capsules containing 2.45 mg of ANT every eight hours for two months. Reductions were observed in TG, TC, and LDL-C (19.2%, 27.6%, and 26.3%, respectively). Furthermore, HDL-C levels increased by 37.5%. The reductions observed correlate to decreased blood fat build-up, risk of atherosclerosis, and subsequently, CVD development.129 Additionally, this increase in HDL-C is of considerable importance as this would enhance cholesterol removal and delay the progression of atherosclerosis.145Similar results were seen in five other studies measuring the same parameters.85–87,91,94 Two of these studies were completed on individuals with MetS and resulted in reductions in TG, TC, and LDL-C; however, there were no changes in HDL-C levels. Although the reductions observed are beneficial, HDL-C must also be modulated and increased to improve and thoroughly demonstrate anthocyanin potential. Low HDL-C levels are a primary indicator of MetS and high TG; a sufficient balance of HDL-C and LDL-C is required to target CVD effectively. For instance, people with LDL-C levels higher than 190 mg dL−1 and HDL-C lower than 40 mg dL−1 are at risk of atherosclerosis and CVDs, whereas those with LDL-C levels below 100 mg dL−1 and HDL-C above 50 mg per day are not.146 Similarly, former smokers did not alter HDL-C levels in a study based on aronia berry intake. Smoking has been linked to reduced HDL-C levels.147
Moreover, a study investigated the effects of consuming 36 grams of lyophilised grape powder containing ANT (0.77 g kg−1) or a placebo over four weeks on lipid profile modulation in premenopausal and postmenopausal women. Twenty-four women in the premenopausal stage and 20 women in the postmenopausal stage were randomly selected to participate. Results from this study included a reduction in TGs in premenopausal women by 15% and postmenopausal by 6%. Furthermore, LDL-C, apolipoprotein (apo) B, apo E, and cholesterol ester transfer protein (CETP) were reduced. Apolipoproteins are vital in transporting lipids and cholesterol between cells.148 Apolipoproteins are the only protein constituents of lipoproteins and are linked with phospholipids, TG, and cholesterol to produce lipoproteins.149 Apo B, reduced in this study, is a vital CVD risk factor found in all atherogenic particles, including VLDL-C and LDL-C. Apo B gives an exact measure of the atherogenic lipoprotein particles in the blood and is, therefore, a good marker of increased CVD risk. Hence, lowering apo B also reduces CVD risk.150 Apo E, a key component of atherogenic lipoproteins, particularly VLDL-C, and chylomicrons, directly impacts CVDs and cholesterol levels by apo E polymorphism and overall addition to lipoprotein and cholesterol levels.149 Contrasting results were seen in four other studies, whereby no alterations of apolipoproteins A1, B, or B-100 were observed.84,115,151 By reducing apolipoproteins, the extent of cholesterol and lipoproteins in the blood can be reduced, thus minimising fat and plaque build-up and, henceforth, the risk of CVDs.
CETP, which aids in the movement of cholesterol from HDL-C to particles which involve apo B, such as VLDL-C, in exchange for TG, was also reduced in this study. Accordingly, it is established that a strategy to increase HDL-C levels is the inhibition of CETP.152 CETP was lowered in this study, thus improving HDL-C and, additionally, CV health.
However, not all studies investigated in this review observed effects on lipid profiles. The primary lipid profile parameters, including TC, HDL-C, LDL-C, and TG, were unaltered in three studies involving black rice pigment fraction, black raspberry, and strawberry, thus displaying no potential in reducing CVD risk. In these studies, ANT were consumed as foods, thus suggesting that nutraceutical intake may be more effective in lipid modulation. However, results are too preliminary to draw any sound conclusions on the source of ANT to be consumed to decrease any risk of CVDs.82,84,151 We hypothesise that these modulating effects depend highly on the dosage, intervention time, types, and levels of ANT in the matrix, presence of other phenolic compounds in the food/capsule, and their bioavailability.
As a general observation of the studies, ANT consumption modulated lipid profile in vivo. Modulation of TG, TC, LDL-C, apolipoproteins, and HDL-C seem to be the main potential targets of ANT, all contributing to improved blood flow, reduced fat accumulation, and decreased possibility of CVD initiation and progression. The alterations observed allow for improved cardiovascular health and reduced risk of CVD, but a conclusive understanding of the dosage, anthocyanin type, and intervention time has yet to be discovered.
8. Effects of anthocyanin consumption on other cardiovascular health parameters
Endothelial cells play a care crucial role in the flow and maintaining the haemostatic balance. Other functions of the endothelium include the regulation of thrombosis and thrombolysis, the control of vascular tone, the growth of blood vessels and interactions between the vessel walls, platelets, and recruitment of white blood cells, such as leukocytes.153 This haemostatic balance impacts cerebral blood flow, where the impairment of oxygen availability may cause focal or global damage, leading to transient or permanent neurologic sequelae or death. Moreover, the link between ROS and brain tissue damage is highlighted here as an important pathologic mechanism of stroke. Antioxidants that remove free radicals can limit neuronal injury following stroke and are thus a potential treatment for ischemic stroke.59Hypertension is a preventable risk factor for heart failure, myocardial infarction, haemorrhagic stroke, and premature mortality. As BP exceeds 115/70 mmHg, the likelihood of the cardiovascular event occurring doubles for every increase in BP by 20/10 mmHg. Furthermore, the SBP target is ≤140 mmHg and the DBP target is ≤90 mmHg for individuals aged up to eighty.154 Short- and long-term consequences can occur due to high BP, including heart failure with preserved ejection fraction, atrial fibrillation, and valvular heart disease.155
eNOS plays a vital role in controlling and maintaining a healthy cardiovascular system. A decrease in eNOS expression and subsequent reduction in NO production leads to an enhanced risk of developing hypertension and diabetes, risk factors of CVD. NO is a crucial component for reducing the risk of CVD, as it regulates vascular tone, production, and proliferation of cells, and it is involved in platelet aggregation and leukocyte adhesion.156 eNOS is a dimer whereby one monomer has a reductase domain, and the other has an oxygenase domain, which is phosphorylated at serine 1177.157 The cofactor tetrahydrobiopterin (BH4) plays a crucial role in the redox switch as in its absence, eNOS switches to produce a cytotoxic superoxide anion instead of the production of NO; this mechanism is known as eNOS uncoupling.156
Adequate vascular function is essential to control and maintain vascular tone and is an effective indicator of cardiovascular health.158 Vascular tone is the contractile action of vascular smooth muscle cells located in the walls of small arterioles and arteries; this action determines the blood flow resistance in the body. Vascular tone is vital in BP regulation and blood circulation to the body's organs and tissues.159
Vascular function is controlled by endothelial cells, which react to factors such as hormones, platelet aggregation, and inflammation. The endothelium regulates vascular tone by releasing various vasoconstrictor and vasodilator mediators, including endothelin-1 (ET-1).160 Vascular dysfunction is a strong predictor for CVD and is linked to implicated health events in those with atherosclerosis.161 Endothelial dysfunction contributes to the pathogenesis of cardiovascular disorders and is represented by impaired vascular tone, heightened inflammatory reactions, and disrupted redox balance,162 making it a therapeutic target for both drug and nutrition interventions. For this instance, Festa et al. have shown that polyphenols from grape extracts and wine present vasodilator effects due to the increased expression and phosphorylation of eNOS and consequent NO production. This biological effect is vital in preventing vascular dysfunction, as NO production contributes to maintaining endothelial homeostasis.33
Therefore, maintaining healthy vascular function to reduce CVD risk is recognised. The production and activity of vasoconstrictor ET-1 increase during CVD and endothelial dysfunction. ET-1 has many adverse effects on CVD, including severe vasoconstriction and pro-inflammatory effects, and it encourages the formation of free radicals.161
Flow-mediated dilation (FMD) and BP investigated throughout this review also play critical roles in vascular function and CVD. FMD is a physiologically significant stimulant regulating vascular tone and blood circulation. Reductions in FMD are recognised as indicators of atherosclerosis and CVD.160 BP measures the heart's force to pump blood around the body. High BP, otherwise known as hypertension, is linked with decreased vascular function by promoting arterial stiffness and endothelial dysfunction.163 Arterial stiffness is the reduced ability of an artery to extend and contract in reaction to changes in pressure and is interlinked with CVD risk. Atherosclerosis can cause increases in the stiffness of the arteries. Therefore, arterial stiffness reduction indicates a subsequent decrease in atherosclerosis and CVD risk.164 In a 12-week trial with 66 healthy male participants, they were randomly assigned to ingest either a (poly)phenol-rich extract (116 mg, equivalent to 75 g of berries), a whole fruit powder (12 mg, equivalent to 10 g of berries), or a placebo (maltodextrin). Both the aronia berry extract containing 30 mg g−1 ANT and the whole fruit powder containing 3.6 mg g−1 ANT showed significant improvements in endothelial function, evidenced by increased FMD. The benefits were immediate, observed within 2 hours of consuming aronia extract, and persisted throughout the study. Analysis of plasma phenolic metabolites revealed heightened levels following aronia consumption. Though gut microbiota diversity remained unchanged, aronia extract fostered the growth of Anaerostipes, while whole fruit powder encouraged Bacteroides growth. These findings suggest regular consumption of aronia berries may effectively improve endothelial function and influence gut microbiota composition, potentially contributing to cardiovascular health maintenance in low-risk individuals.165
8.1. Animal studies
Ten out of twenty-two studies reported changes in endothelial and vascular function parameters. The components analysed in those studies are mainly focused on BP, heart rate (HR), aortic plaque, inducible nitric oxide synthase (iNOS), eNOS, foam cell formation, atherosclerosis lesions, platelet aggregation, and left ventricle (LV) ejection fraction.Xia et al.69 studied the effects of supplementing 300 mg kg−1 of black rice extract containing C3G and peonidin 3-glucoside, though of an unknown quantity, for 20 weeks on the cardiovascular function of Apo-E deficient mice. Results showed an 18% decrease in the aortic sinus plaque area, a reduction in transcription factor messenger ribonucleic acid (TF mRNA) and iNOS, a decrease in thin fibrous cap and larger necrotic core, and a larger collagen-1 positive area. Finally, a decrease of matrix metalloproteinase-1 (MMP-1) within the plaque was observed. The anthocyanin-rich extract positively affected plaque stability as a decrease in the expression of TF mRNA and iNOS, which are inflammatory agents, was observed.69
Similarly, Y. Wang et al.166 used Apo-E deficient mice and supplemented their diet with 100 mg of C3G per kg for twelve weeks. This study investigated the effects of adding C3G to the diet to see its protective effects against endothelial dysfunction and atherosclerosis. They found a 66% decrease in the aortic sinus plaque area and a 61% reduction in the aortic cholesterol accumulation. These results suggest that the isolated form of C3G for a shorter period led to the conclusion that nutraceuticals may be more effective than anthocyanin-rich foods. The authors conducted further analysis on the consumption of C3G in hypercholesteremia; a higher dose of 2 g of C3G per kg was consumed for eight weeks. A reduction of 54% of atherosclerosis lesions, a decrease of cyclic guanosine monophosphate (cGMP), an increase in nitrate and nitrite, and a subsequent increase in Ser1177 of eNOS were observed.166 The increased NO bioavailability and eNOS expressions reduced the risk of CVD. Although these results are promising, translating the daily dosage to humans would be prohibitive. Therefore, experimental results using animals can be utilised to better understand the effects of ANT on selected biomarkers, but any conclusions on human health are unrealistic.
The effectiveness of a single dose of 200 mg of purple passion fruit (Passiflora edulis peel extract) per kg BW in spontaneously hypertensive rats (SHR) was examined. After twenty days, mean arterial pressure (MAP), diastolic blood pressure (DBP), and systolic blood pressure (SBP) were reduced.167 Similar results were evident in an experiment by Shanmuganayagam et al.,140 in which hypercholesterolemic rabbits were given 225 mL per day of concord grape juice containing a total phenolic content of 1975 g L−1, including ANT, although the specific ANT were not identified and quantified. Positive effects were observed on SBP and DBP. Furthermore, this study showed reduced hypercholesterolemic-enhanced platelet aggregation, a critical stage in atherogenesis.140 It can be concluded that anthocyanin possesses cardio-protective properties against platelet aggregation. Similar results were evident in two other animal trials involving rats and mice, whereby improvements were reported in SBP and DBP in the overall vessel structures, suggesting the positive impact the consumption of ANT displays against endothelial dysfunction.168,169
Chang et al.135 explored the arterial occlusion time post-ingestion of sweet potato leaf powder containing ANT on FeCl3-induced thrombosis in hamsters. Still, they did not identify and quantify the type of ANT. An increase of 4.1-fold was reported in the hamsters fed 2.5% of sweet potato leaf powder, suggesting sweet potato leaf powder elongated the occlusion time and ameliorated thrombosis formation. Hence, sweet potato leaf powder displayed several cardioprotective effects and may be a potential nutritional strategy for preventing hyperlipidaemia and CVD.135
Finally, for ten weeks, Rodriguez-Mateos et al.142 studied the effects of supplementing with freeze-dried blueberries (∼19 g daily). They found that a 2% freeze-dried blueberry supplement containing 7.7 mg of total ANT per kg BW restored vasorelaxation levels to an average level, which had been elevated due to the high-fat cholesterol diet (HFCD). This may indicate that ANT can positively modulate changes in vascular function.142
In conclusion, ANT display cardioprotective impacts against endothelial dysfunction and vascular function. The most effective changes regarding plaque size were communicated when C3G was given for 60 days. As a result, it can be concluded that nutraceuticals or isolated ANT are more effective in reducing the overall risk factor for CVD. However, anthocyanin-containing foods carry out this function to a lesser extent.
8.2. Human studies
Thirteen of 22 studies investigated ANT’ effects on vascular functioning. Khan et al.170 reported that the high blackcurrant juice drink (100 mL), containing 81.5 mg of total polyphenols and 14.3 mg of total ANT in healthy individuals, led to a 1.42% increase in %FMD after 6-week consumption.170 Although a 1% increase appears relatively small, a meta-analysis conducted by Inaba et al.171 discovered that an increase as small as 1% translates to a 13% decrease in the likelihood of future cardiovascular events. Hence, this 1.42% increase portrays ANT’ potential for CVD risk reduction by targeting FMD.171Similar FMD increases were observed in two other studies involving anthocyanin-rich freeze-dried blueberries and strawberries.151,172 After six months of consuming freeze-dried blueberries containing an unknown amount of C3G, individuals with MetS experienced a 1.45% increase in FMD. The concentration of C3G was found to be 20 ± 15 nmol L−1 in serum and 33 ± 9 nmol L−1 in urine. Also, post-strawberry consumption FMD values were higher than the placebo values. Contrastingly, no changes in FMD were observed in three studies investigated throughout this review, two based on grapes and two on nutraceuticals. This may indicate that grape ANT and nutraceuticals may not be as effective towards FMD as food intake, as previously stated.
Curtis et al.172 randomly allocated 115 adults with MetS into three treatment groups: 26 g of freeze-dried blueberries (FDB), 13 g of FDB with 13 g of placebo, and 26 g of placebo alone. The anthocyanin dosages were 364 mg, 182 mg, and 0 mg, respectively. After consuming FDB, a significant increase of 0.99 pmol mL−1 in cGMP levels and a modulation of arterial stiffness were observed. Intracellular second-messenger cGMP controls various physiological events in the cardiovascular system and is vital to numerous functions, including endothelial, cardiac monocyte, vascular smooth muscle, and enhancement of vascular tone. Hence, this improvement in cGMP can be translated to enhanced vascular function and overall cardiovascular health.172 Moreover, in this study, arterial stiffness was decreased by 2.24%. Dohadwala et al.121 also observed a 0.5 m s−1 reduction of carotid-femoral pulse wave velocity by arterial stiffness measurement in a study based on 4-week consumption of 480 mL per day of double-strength cranberry juice containing 835 mg total polyphenols and 94 mg ANT. However, the study did not demonstrate significant potential in reducing CVD risk as few changes in endothelial vasodilator function, lipids, or markers of inflammation were observed throughout the study.121
When blood pressure (BP) is elevated, it can negatively impact vascular function and cardiovascular health. Considering this, McKay et al.173 conducted a study to investigate the effects of a 6-week intake of anthocyanin-rich Hibiscus sabdariffa tea, which contained a total phenolic content of 21.85 mg and 7.04 mg of ANT (a mixture of C3G, delphinidin 3-sambubioside, delphinidin 3-glucoside, and cyanidin 3-sambubioside), on the BP of hypertensive individuals. The study revealed that incorporating three servings of hibiscus tea (720 mL per day) into the diet daily for six weeks effectively reduced BP.173
Hypertension is one of the critical risk factors of CVD, accounting for 49% of heart failures, and is involved in 35% of all atherosclerotic cardiovascular actions.174 Post-tea intervention decreases in SBP, DBP, and MAP were observed (5.5%, 4.7%, and 4.0% accordingly). This dietary intervention portrayed a strong potential for preventing hypertension and thus reducing CVD risk.173 Similar results were seen in two other studies whereby reductions in SBP by 3 mmHg and DBP by 2 mmHg were observed after grape wine consumption. Additionally, SBP and DBP lowered after the aronia extract.86,175 The intervention on grape wine extract was also completed in individuals with hypertension and aronia extract in individuals with MetS; both conditions involve increased BP. Eight other studies displayed no BP modulations after anthocyanin intake; the individual's health conditions in these studies were not directly linked with high BP. This indicates that ANT may be more effective in individuals with previously heightened BP.85,116,121,151,170,175–177
A study investigated the effects of purple grape wine solids, rich in polyphenols (550 mg) and containing 18.8 mg of total ANT, alongside grape juice (250 mg) with 118.5 mg of total ANT, on the modulation of ET-1 levels, FMD, and platelet function and aggregation. ET-1 decreased by 10% after consuming the grape wine extract; no effects were seen after consuming grape juice. This result was unexpected as the grape juice contained higher levels of ANT, indicating that the vascular effects observed were based on red wine-derived polyphenols and not ANT.175 ET-1 also decreased after aronia extract intake containing 300 mg of ANT in individuals with MetS.86 The reductions in ET-1 observed translate to decreased vascular dysfunction and CVD risk. Additionally, researchers found that the grape-wine extract intervention significantly lowered 24-hour ambulatory systolic/diastolic BP compared to placebo, especially during the daytime. Endothelin-1 levels decreased by 10%, while other vascular function measures remained unchanged. Grape juice extract alone did not affect BP or vascular function. The cardiovascular benefit of grape wine extract appears to be solely related to BP reduction in healthy, mildly hypertensive subjects without affecting lipid metabolism or platelet function.175 Contrastingly, a study on grape solids containing polyphenols revealed that 550 mg of wine solids contained 18.8 mg of unidentified and unquantified ANT. Platelet aggregation in healthy individuals was reduced after two weeks.177 Platelets play an essential role in the development of CVD, with platelet aggregation taking part in the incidence and progression of thrombosis involving clotting of the veins and arteries. Thus, the reduction of platelet aggregation is crucial in preventing atherothrombotic disorders such as CVD.178 Furthermore, the soluble P-selectin on activated platelets, a CVD risk biomarker, was reduced by 5.9%.114
Throughout this review, ANT displayed several modulating effects on various parameters, contributing to healthy vascular function and improved cardiovascular health. Numerous links were observed upon investigation, including arterial stiffness being best modulated by food anthocyanin intake, FMD by grape-derived nutraceuticals, and ET-1 by nutraceuticals. Furthermore, BP reductions were most effective in individuals with previously heightened BP levels pre-intervention. Predominantly, the sustained consumption of anthocyanin-containing foods provides a promising strategy to improve vascular function and subsequent risk reduction of CVD.
9. Final remarks and trends
Knowledge of bioactive compounds, such as phenolics, and their potential benefits for reducing CVD risk has recently increased. However, additional investigations and observations are still needed to achieve a precise conclusion concerning the daily consumption amount, intervention time, and type of phenolic compound. Throughout this review, the relationship between anthocyanin-containing foods and CVD was analysed, whereby the mechanisms of actions in which anthocyanin-containing foods display their cardioprotective properties were associated with their antioxidant and anti-inflammatory effects in vivo. Additionally, the consumption of ANT tended to improve endothelial function, modulate the serum-lipids ratio (e.g., LDL/HDL), increase plasma antioxidant activity by increasing the activity of endogenous enzymes and decrease the production of oxidised products in the blood, decrease the secretion of pro-inflammatory mediators in human cells and in vivo, and improve vascular function (e.g., increasing FMD and decreasing levels BP, arterial stiffness, ET-1, and platelet aggregation). These mechanisms effectively lower CVD risk, including reducing plaque formation and atherosclerosis, improving blood flow and vascular tone, and decreasing vessel fat streak accumulation. However, the data analysis of the current literature does not enable us to conclude some crucial factors: a structure–activity relationship between the anthocyanin type and cardiovascular health could not be established. Similarly, the effects of the daily dosage, intervention length, and source of ANT (e.g., natural anthocyanin-containing foods or nutraceuticals) on CVD-related biomarkers are still puzzling. In conclusion, this systematic review highlights the need for further research in human populations, allowing deeper consideration into the ability of anthocyanin-containing foods and nutraceuticals to confirm their effectiveness as a functional dietary strategy to reduce CVD risk and increase their technological applications for functional food design.Abbreviations
| 8-iso-PGF2α | 8-iso-prostaglandin F2 alpha |
| ATP | Adenosine tri-phosphate |
| ANT | Anthocyanins |
| apoB | Apolipoprotein B |
| Apo | Apolipoprotein |
| AI | Atherogenic index |
| BP | Blood pressure |
| C3G | Cyanidin 3-glucoside |
| CVD | Cardiovascular disease |
| CAT | Catalase |
| CETP | Cholesterol ester transfer protein |
| CHD | Coronary heart disease |
| CRI | Coronary risk index |
| CRP | C-reactive protein |
| cGMP | Cyclic guanosine monophosphate |
| COX-2 | Cyclooxygenase-2 |
| CTX | Cyclophosphamide |
| DBP | Diastolic blood pressure |
| DNA | Deoxyribonucleic acid |
| eNOS | Endothelial nitric oxide synthase |
| ET-1 | Endothelin-1 |
| FMD | Flow-mediated dilation |
| GPx | Glutathione peroxidase |
| HR | Heart rate |
| HDL-C | High-density lipoprotein-cholesterol |
| HFD | High-fat diet |
| HAT | Hydrogen atom transfer |
| iNOS | Inducible nitric oxide synthase |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN-γ | Interferon-gamma |
| IL | Interleukins |
| IDLs | Intermediate-density lipoproteins |
| LV | Left ventricle |
| LOXs | Lipooxygenases |
| LDL-C | Low-density lipoprotein cholesterol |
| MDA | Malondialdehyde |
| MMP-1 | Matrix metalloproteinase-1 |
| MAP | Mean arterial pressure |
| mRNA | Messenger ribonucleic acid |
| MetS | Metabolic syndrome |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MPO | Myeloperoxidase |
| NOX | NADPH oxidases |
| NO | Nitric oxide |
| Nrf2/ARE | Nuclear factor E2-related factor 2-anti-oxidant response elements |
| NF-κβ | Nuclear factor kappa beta |
| OSI | Oxidative stress index |
| PL | Phospholipids |
| GSH-Px | Plasma glutathione peroxidase |
| PAH | Pulmonary artery hypertension |
| PPARα | Peroxisome proliferator-activated receptor-alpha |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RANTES | Regulated upon activation, normal T cell expressed and secreted |
| RCT | Reverse cholesterol transport |
| SET | Single electron transfer |
| SHR | Spontaneously hypertensive rats |
| SOD | Superoxide dismutase |
| SBP | Systolic blood pressure |
| TBARS | Thiobarbituric acid reactive substances |
| TLR4 | Toll-like receptor 4 |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TF mRNA | Transcription factor messenger ribonucleic acid |
| TG | Triglycerides |
| TNF-α | Tumour necrosis factor-alpha |
| TNF RI/TNF RII | Tumour necrosis factor receptor I/II |
| T2D | Type-2 diabetes |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VSMC | Vascular smooth muscle cells |
| VLDL-C | Very low-density lipoprotein cholesterol |
| XO | Xanthine oxidase |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the National Council for Scientific and Technological Development (CNPq) and the Research Support Foundation of the State of Minas Gerais (FAPEMIG). Flaticon (https://www.flaticon.com) is also acknowledged for the icons used in the graphical abstract and figures.References
- Y. Xu, M. Le Sayec, C. Roberts, S. Hein, A. Rodriguez-Mateos and R. Gibson, Adv. Nutr., 2021, 12, 1781–1801 CrossRef CAS PubMed.
- A.-L. Barabási, G. Menichetti and J. Loscalzo, Nat. Food, 2019, 1, 33–37 CrossRef.
- C. Vetrani, G. Costabile, M. Vitale and R. Giacco, J. Funct. Foods, 2020, 71, 104013 CrossRef CAS.
- C. G. Fraga, K. D. Croft, D. O. Kennedy and F. A. Tomás-Barberán, Food Funct., 2019, 10, 514–528 RSC.
- T. Behl, S. Bungau, K. Kumar, G. Zengin, F. Khan, A. Kumar, R. Kaur, T. Venkatachalam, D. M. Tit, C. M. Vesa, G. Barsan and D. E. Mosteanu, Biomed. Pharmacother., 2020, 130, 110714 CrossRef CAS PubMed.
- L. Ciumărnean, M. V. Milaciu, O. Runcan, S. C. Vesa, A. L. Răchisan, V. Negrean, M. G. Perné, V. I. Donca, T. G. Alexescu, I. Para and G. Dogaru, Molecules, 2020, 25(18), 4320 CrossRef PubMed.
- R. Sansone, A. Rodriguez-Mateos, J. Heuel, D. Falk, D. Schuler, R. Wagstaff, G. G. C. Kuhnle, J. P. E. Spencer, H. Schroeter, M. W. Merx, M. Kelm and C. Heiss, Br. J. Nutr., 2015, 114, 1246–1255 CrossRef CAS PubMed.
- Z. Faridi, V. Y. Njike, S. Dutta, A. Ali and D. L. Katz, Am. J. Clin. Nutr., 2008, 88, 58–63 CrossRef CAS PubMed.
- S. S. Hassellund, A. Flaa, S. E. Kjeldsen, I. Seljeflot, A. Karlsen, I. Erlund and M. Rostrup, J. Hum. Hypertens., 2013, 27, 100–106 CAS.
- S. Martínez-López, B. Sarriá, R. Mateos and L. Bravo-Clemente, Eur. J. Nutr., 2019, 58, 865–878 CrossRef PubMed.
- H. Jokura, I. Watanabe, M. Umeda, T. Hase and A. Shimotoyodome, Nutr. Res., 2015, 35, 873–881 CrossRef CAS PubMed.
- M. Naissides, J. C. L. Mamo, A. P. James and S. Pal, Atherosclerosis, 2006, 185, 438–445 CrossRef CAS PubMed.
- J. Tomé-Carneiro, M. Larrosa, M. J. Yáñez-Gascón, A. Dávalos, J. Gil-Zamorano, M. Gonzálvez, F. J. García-Almagro, J. A. Ruiz Ros, F. A. Tomás-Barberán, J. C. Espín and M. T. García-Conesa, Pharmacol. Res., 2013, 72, 69–82 CrossRef PubMed.
- T. Miyawaki, H. Aono, Y. Toyoda-Ono, H. Maeda, Y. Kiso and K. Moriyama, J. Nutr. Sci. Vitaminol., 2009, 55, 87–91 CrossRef CAS PubMed.
- L. A. T. Bloedon, S. Balikai, P. O. Szapary, L. A. T. Bloedon, D. J. Rader, P. O. Szapary, J. Chittams, J. A. Berlin, P. O. Szapary and S. C. Cunnane, J. Am. Coll. Nutr., 2008, 27, 65–74 CrossRef CAS PubMed.
- C. T. M. Kumar, S. Mondal, W. G. Prasad, G. S. Rathod, H. V. Raghu and A. Kokkiligadda, Food Chem. Adv., 2022, 1, 100088 CrossRef.
- R. Naseri, F. Farzaei, P. Haratipour, S. F. Nabavi, S. Habtemariam, M. H. Farzaei, R. Khodarahmi, D. Tewari and S. Momtaz, Front. Pharmacol., 2018, 9, 1310 CrossRef CAS PubMed.
- Y. Wang, T. Liu, Y. Xie, N. Li, Y. Liu, J. Wen, M. Zhang, W. Feng, J. Huang, Y. Guo, T. Kabbas Junior, D. Wang and D. Granato, Food Res. Int., 2022, 162, 112008 CrossRef CAS PubMed.
- J. F. Reis, V. V. S. Monteiro, R. de Souza Gomes, M. M. do Carmo, G. V. da Costa, P. C. Ribera and M. C. Monteiro, J. Transl. Med., 2016, 14, 315 CrossRef PubMed.
- X. Jiang, X. Li, C. Zhu, J. Sun, L. Tian, W. Chen and W. Bai, Crit. Rev. Food Sci. Nutr., 2019, 59, 921–946 CrossRef CAS PubMed.
- WHO, Cardiovascular diseases (CVDs), https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds), (accessed 11 June 2021).
- C. Garcia and C. N. Blesso, Free Radicals Biol. Med., 2021, 172, 152–166 CrossRef CAS PubMed.
- R. Casas, S. Castro-Barquero, R. Estruch and E. Sacanella, Int. J. Mol. Sci., 2018, 19(12), 3988 CrossRef PubMed.
- L. Azevedo, M. S. M. Serafim, V. G. Maltarollo, A. M. Grabrucker and D. Granato, Trends Food Sci. Technol., 2022, 128, 75–89 CrossRef CAS.
- T. Senoner and W. Dichtl, Nutrients, 2019, 11(9), 2090 CrossRef CAS PubMed.
- B. Li, L. Wang, W. Bai, W. Chen, F. Chen and C. Shu, in Anthocyanins, ed. B. Li, L. Wang, W. Bai, W. Chen, F. Chen and C. Shu, Springer Nature Singapore, Singapore, 2021, pp. 397–422 Search PubMed.
- D. Granato, Curr. Opin. Food Sci., 2022, 47, 100894 CrossRef CAS.
- W. M. M. Verschuren, J. M. A. Boer and E. H. M. Temme, Heart, 2022, 108(15), 1234–1239 CrossRef CAS PubMed.
- J. M. Alvarez-Suarez, F. Giampieri, S. Tulipani, T. Casoli, G. Di Stefano, A. M. González-Paramás, C. Santos-Buelga, F. Busco, J. L. Quiles, M. D. Cordero, S. Bompadre, B. Mezzetti and M. Battino, J. Nutr. Biochem., 2014, 25, 289–294 CrossRef CAS PubMed.
- D. Aune, E. Giovannucci, P. Boffetta, L. T. Fadnes, N. N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten and S. Tonstad, Int. J. Epidemiol., 2017, 46, 1029–1056 CrossRef PubMed.
- F. Gomez-Delgado, N. Katsiki, J. Lopez-Miranda and P. Perez-Martinez, Crit. Rev. Food Sci. Nutr., 2021, 61, 1651–1669 CrossRef PubMed.
- R. Kimble, K. M. Keane, J. K. Lodge and G. Howatson, Crit. Rev. Food Sci. Nutr., 2019, 59, 3032–3043 CrossRef CAS PubMed.
- J. Festa, M. Da Boit, A. Hussain and H. Singh, Mol. Nutr. Food Res., 2021, 65, 2100170 CrossRef CAS PubMed.
- Y. Dong, X. Wu, L. Han, J. Bian, C. He, E. El-Omar, L. Gong and M. Wang, Nutrients, 2022, 14(14), 2836 CrossRef CAS PubMed.
- J. N. Peoples, A. Saraf, N. Ghazal, T. T. Pham and J. Q. Kwong, Exp. Mol. Med., 2019, 51, 1–13 CrossRef CAS PubMed.
- F. Wang, Q. Yuan, F. Chen, J. Pang, C. Pan, F. Xu and Y. Chen, Front. Cell Dev. Biol., 2021, 9, 1–10 Search PubMed.
- L. Chen, H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang and L. Zhao, Oncotarget, 2018, 9, 7204–7218 CrossRef PubMed.
- S. Simantiris, A. S. Antonopoulos, C. Papastamos, G. Benetos, N. Koumallos, K. Tsioufis and D. Tousoulis, J. Clin. Lipidol., 2023, 17, 55–63 CrossRef PubMed.
- P. Ockermann, L. Headley, R. Lizio and J. Hansmann, Nutrients, 2021, 13(8), 2831 CrossRef CAS PubMed.
- B. L. Zaric, M. T. Macvanin and E. R. Isenovic, Int. J. Biochem. Cell Biol., 2023, 154, 106346 CrossRef CAS PubMed.
- T. Hussain, B. Tan, Y. Yin, F. Blachier, M. C. B. Tossou and N. Rahu, Oxid. Med. Cell. Longevity, 2016, 2016, 7432797 Search PubMed.
- D. J. Tyrrell and D. R. Goldstein, Nat. Rev. Cardiol., 2021, 18, 58–68 CrossRef CAS PubMed.
- A. J. Kattoor, N. V. K. Pothineni, D. Palagiri and J. L. Mehta, Curr. Atheroscler. Rep., 2017, 19, 42 CrossRef PubMed.
- H. N. Siti, Y. Kamisah and J. Kamsiah, Vasc. Pharmacol., 2015, 71, 40–56 CrossRef CAS PubMed.
- P. Marchio, S. Guerra-Ojeda, J. M. Vila, M. Aldasoro, V. M. Victor and M. D. Mauricio, Oxid. Med. Cell. Longevity, 2019, 2019, 8563845 Search PubMed.
- H. N. Siti, Y. Kamisah and J. Kamsiah, Vasc. Pharmacol., 2015, 71, 40–56 CrossRef CAS PubMed.
- A. C. Gonçalves, A. R. Nunes, A. Falcão, G. Alves and L. R. Silva, Pharmaceuticals, 2021, 14, 690 CrossRef PubMed.
- L. Tian, Y. Tan, G. Chen, G. Wang, J. Sun, S. Ou, W. Chen and W. Bai, Crit. Rev. Food Sci. Nutr., 2019, 59, 982–991 CrossRef CAS PubMed.
- J. Fang, Drug Metab. Rev., 2014, 46, 508–520 CrossRef CAS PubMed.
- H. Xue, Y. Sang, Y. Gao, Y. Zeng, J. Liao and J. Tan, Antioxidants, 2022, 12(1), 3 CrossRef PubMed.
- D. Granato, Biomed. Pharmacother., 2023, 165, 115155 CrossRef CAS PubMed.
- E. Martinelli, D. Granato, L. Azevedo, J. E. Gonçalves, J. M. Lorenzo, P. E. S. Munekata, J. Simal-Gandara, F. J. Barba, C. Carrillo, M. S. Riaz Rajoka and L. Lucini, Trends Food Sci. Technol., 2021, 116, 232–243 CrossRef CAS.
- D. D. Herrera-Balandrano, J. Wang, Z. Chai, X. Zhang, J. Wang, N. Wang and W. Huang, Food Biosci., 2023, 52, 102424 CrossRef CAS.
- P. Kapoor, A. Kumari, B. Sheoran, S. Sharma, S. Kaur, R. K. Bhunia, S. Rajarammohan, M. Bishnoi, K. K. Kondepudi and M. Garg, J. Cereal Sci., 2022, 104, 103433 CrossRef.
- P. Kapoor, A. Tiwari, S. Sharma, V. Tiwari, B. Sheoran, U. Ali and M. Garg, Sci. Rep., 2023, 13, 1729 CrossRef CAS PubMed.
- A. dos Santos Lima, V. G. Maltarollo, M. Araújo Vieira do Carmo, L. Cezar Pinheiro, T. Mendanha Cruz, F. Augusto Ribeiro de Barros, N. Pap, D. Granato and L. Azevedo, Food Res. Int., 2024, 182, 114099 CrossRef CAS.
- A. dos Santos Lima, R. D. Novaes, L. C. Pinheiro, L. A. de Almeida, H. S. D. Martino, A. Giusti-Paiva, N. Pap, D. Granato and L. Azevedo, Food Res. Int., 2023, 170, 112917 CrossRef CAS PubMed.
- I. Mozos, C. Flangea, D. C. Vlad, C. Gug, C. Mozos, D. Stoian, C. T. Luca, J. O. Horbańczuk, O. K. Horbańczuk and A. G. Atanasov, Biomolecules, 2021, 11(6), 811 CrossRef CAS PubMed.
- S. Feng, M. Yang, S. Liu, Y. He, S. Deng and Y. Gong, J. Intensive Med., 2023, 3(4), 313–319 CrossRef PubMed.
- M. V. de Mello Barros Pimentel, A. Bertolami, L. P. Fernandes, L. P. Barroso and I. A. Castro, Biomed. Pharmacother., 2023, 160, 114345 CrossRef CAS PubMed.
- P. Evans and B. Halliwell, Br. J. Nutr., 2001, 85, S67 CrossRef CAS PubMed.
- A. V. Poznyak, N. G. Nikiforov, A. M. Markin, D. A. Kashirskikh, V. A. Myasoedova, E. V. Gerasimova and A. N. Orekhov, Front. Pharmacol., 2021, 11, 613780 CrossRef PubMed.
- A. Ayala, M. F. Muñoz and S. Argüelles, Oxid. Med. Cell. Longevity, 2014, 2014, 1–31 CrossRef PubMed.
- L. Azevedo, M. S. M. Serafim, V. G. Maltarollo, A. M. Grabrucker and D. Granato, Trends Food Sci. Technol., 2022, 128, 75–89 CrossRef CAS.
- P. Mladěnka, L. Zatloukalová, T. Filipskỳ and R. Hrdina, Free Radical Biol. Med., 2010, 49, 963–975 CrossRef PubMed.
- D. Granato, F. Shahidi, R. Wrolstad, P. Kilmartin, L. D. Melton, F. J. Hidalgo, K. Miyashita, J. van Camp, C. Alasalvar, A. B. Ismail, S. Elmore, G. G. Birch, D. Charalampopoulos, S. B. Astley, R. Pegg, P. Zhou and P. Finglas, Food Chem., 2018, 264, 471–475 CrossRef CAS PubMed.
- R. Tsao, Nutrients, 2010, 2, 1231–1246 CrossRef CAS PubMed.
- C. C. Tangney and H. E. Rasmussen, Curr. Atheroscler. Rep., 2013, 15, 324 CrossRef PubMed.
- X. Xia, W. Ling, J. Ma, M. Xia, M. Hou, Q. Wang, H. Zhu and Z. Tang, J. Nutr., 2006, 136, 2220–2225 CrossRef CAS PubMed.
- X. Yang, L. Yang and H. Zheng, Food Chem. Toxicol., 2010, 48, 2374–2379 CrossRef CAS PubMed.
- R. de B. Freitas, D. N. Rômulo, G. M. Bianca, C. D. S. Eliziária, S. A. Murilo, G. F. Luciano, M. L. Luciana, P. Maria Do Carmo, V. G. Reggiani and V. L. João Paulo, Food Agric. Immunol., 2018, 29, 95–108 CrossRef CAS.
- T. Wu, Y. Gao, X. Guo, M. Zhang and L. Gong, Oxid. Med. Cell. Longevity, 2018, 2018, 4051232 Search PubMed.
- A. Nemes, J. R. Homoki, R. Kiss, C. Hegedus, D. D. Kovács, B. Peitl, F. Gál, L. Stündl, Z. Szilvássy and J. Remenyik, Nutrients, 2019, 11(9), 1966 CrossRef CAS PubMed.
- T. Fukai, Cardiovasc. Res., 2002, 55, 239–249 CrossRef CAS PubMed.
- Â. G. Batista, S. A. Lenquiste, C. B. B. Cazarin, J. K. da Silva, A. Luiz-Ferreira, S. Bogusz, L. Wang Hantao, R. N. de Souza, F. Augusto, M. A. Prado and M. R. Maróstica, J. Funct. Foods, 2014, 6, 450–461 CrossRef.
- H. Chen, M. Yu, M. Li, R. Zhao, Q. Zhu, W. Zhou, M. Lu, Y. Lu, T. Zheng, J. Jiang, W. Zhao, K. Xiang, W. Jia and L. Liu, Mol. Cell. Biochem., 2012, 363, 85–91 CrossRef CAS PubMed.
- Y. Liu, D. Tan, L. Shi, X. Liu, Y. Zhang, C. Tong, D. Song and M. Hou, PLoS One, 2015, 10, 1–18 Search PubMed.
- D. Del Rio, A. J. Stewart and N. Pellegrini, Nutr. Metab. Cardiovasc. Dis., 2005, 15, 316–328 CrossRef PubMed.
- Y. Jiang, M. Dai, W. J. Nie, X. R. Yang and X. C. Zeng, J. Ethnopharmacol., 2017, 200, 228–235 CrossRef CAS PubMed.
- A. E. Abdurrahim, V. C. Mazurak and L. Chen, Front. Nutr., 2023, 10 DOI:10.3389/fnut.2023.1229015.
- S. de Liz, A. L. Cardoso, C. L. K. Copetti, P. de F. Hinnig, F. G. K. Vieira, E. L. da Silva, M. Schulz, R. Fett, G. A. Micke and P. F. Di Pietro, Clin. Nutr., 2020, 39, 3629–3636 CrossRef CAS PubMed.
- E. Park, S. Cho, J. Lee, S. M. Lee, Y. Kim, M.-S. Go, Y.-J. Kim, I.-K. Jung, J. H. Auh, H.-K. Choi and J.-H. Kim, J. Funct. Foods, 2015, 16, 393–402 CrossRef CAS.
- K. Goszcz, S. J. Deakin, G. G. Duthie, D. Stewart, S. J. Leslie and I. L. Megson, Front. Cardiovasc. Med., 2015, 2, 1–22 Search PubMed.
- Q. Wang, P. Han, M. Zhang, M. Xia, H. Zhu, J. Ma, M. Hou, Z. Tang and W. Ling, Asia Pac. J. Clin. Nutr., 2007, 16, 295–301 CAS.
- L. Xie, T. Vance, B. Kim, S. G. Lee, C. Caceres, Y. Wang, P. A. Hubert, J. Y. Lee, O. K. Chun and B. W. Bolling, Nutr. Res., 2017, 37, 67–77 CrossRef CAS PubMed.
- M. Broncel, M. Koziróg, P. Duchnowicz, M. Koter-Michalak, J. Sikora and J. Chojnowska-Jezierska, Med. Sci. Monit., 2010, 16, CR28–CR34 Search PubMed.
- T. L. Zern, R. J. Wood, C. Greene, K. L. West, Y. Liu, D. Aggarwal, N. S. Shachter and M. L. Fernandez, J. Nutr., 2005, 135, 1911–1917 CrossRef CAS PubMed.
- A. Karlsen, L. Retterstøl, P. Laake, I. Paur, S. Kjølsrud-Bøhn, L. Sandvik and R. Blomhoff, J. Nutr., 2007, 137, 1951–1954 CrossRef CAS PubMed.
- E. Nikbakht, I. Singh, J. Vider, L. T. Williams, L. Vugic, A. Gaiz, A. R. Kundur and N. Colson, Inflammation Res., 2021, 70, 275–284 CrossRef CAS PubMed.
- J. Dawson and M. Walters, Br. J. Clin. Pharmacol., 2006, 62, 633–644 CrossRef CAS PubMed.
- A. Aboonabi, R. R. Meyer, A. Gaiz and I. Singh, Nutr. Res., 2020, 76, 82–93 CrossRef CAS PubMed.
- X. Zhang, C. Zhu, J. Gao, F. Mei, J. Yin, L. Bu, X. Cheng, C. Sheng and S. Qu, Lipids Health Dis., 2018, 17, 288 CrossRef CAS PubMed.
- O. M. Woodward and V. L. H. Kuhns, Int. J. Mol. Sci., 2020, 1–20 Search PubMed.
- D. Li, Y. Zhang, Y. Liu, R. Sun and M. Xia, J. Nutr., 2015, 145, 742–748 CrossRef CAS PubMed.
- S. Kaviarasan, S. Muniandy, R. Qvist and I. S. Ismail, J. Clin. Biochem. Nutr., 2009, 45, 1–8 CrossRef CAS PubMed.
- J. Helmersson, P. Mattsson and S. Basu, Clin. Sci., 2002, 102, 39–43 CrossRef CAS.
- Z. Ma, B. Du, J. Li, Y. Yang and F. Zhu, Int. J. Mol. Sci., 2021, 22(20), 11076 CrossRef CAS PubMed.
- M. N. Amin, S. A. Siddiqui, M. Ibrahim, M. L. Hakim, M. S. Ahammed, A. Kabir and F. Sultana, SAGE Open Med., 2020, 8, 1–12 Search PubMed.
- J.-M. Zhang and J. An, Int. Anesthesiol. Clin., 2007, 45, 27–37 CrossRef PubMed.
- R. Medzhitov, Nature, 2008, 454, 428–435 CrossRef CAS PubMed.
- A. M. Minihane, S. Vinoy, W. R. Russell, A. Baka, H. M. Roche, K. M. Tuohy, J. L. Teeling, E. E. Blaak, M. Fenech, D. Vauzour, H. J. McArdle, B. H. A. Kremer, L. Sterkman, K. Vafeiadou, M. M. Benedetti, C. M. Williams and P. C. Calder, Br. J. Nutr., 2015, 114, 999–1012 CrossRef CAS PubMed.
- C. M. Ballantyne and V. Nambi, Atheroscler. Suppl., 2005, 6, 21–29 CrossRef CAS PubMed.
- R. B. Dange, D. Agarwal, G. S. Masson, J. Vila, B. Wilson, A. Nair and J. Francis, Cardiovasc. Res., 2014, 103, 17–27 CrossRef CAS PubMed.
- J. M. Bennett, G. Reeves, G. E. Billman and J. P. Sturmberg, Front. Med., 2018, 5, 1–30 CrossRef PubMed.
- D. L. Cozlea, D. M. Farcas, A. Nagy, A. A. Keresztesi, R. Tifrea, L. Cozlea and E. Caraşca, Curr. Health Sci. J., 2013, 39, 225–231 CAS.
- W. K. Lagrand, C. A. Visser, W. T. Hermens, H. W. M. Niessen, F. W. A. Verheugt, G.-J. Wolbink and C. E. Hack, Circulation, 1999, 100, 96–102 CrossRef CAS PubMed.
- A. A. Novello, L. L. Da Conceição, M. M. Dos Santos Dias, L. M. Cardoso, C. A. De Castro, M. E. Ricci-Silva, J. P. Viana Leite and M. D. C. Gouveia Peluzio, J. Food Nutr. Res., 2015, 54, 101–112 CAS.
- T. Sozański, A. Z. Kucharska, A. Szumny, J. Magdalan, K. Bielska, A. Merwid-Ląd, A. Woźniak, S. Dzimira, N. Piórecki and M. Trocha, Phytomedicine, 2014, 21, 1774–1784 CrossRef PubMed.
- R. Monteiro and I. Azevedo, Mediators Inflammation, 2010, 2010(Atp Iii), 1–10, DOI:10.1155/2010/289645.
- G. D. Noratto, B. P. Chew and L. M. Atienza, Food Chem., 2017, 227, 305–314 CrossRef CAS PubMed.
- H. K. Park, M. K. Kwak, H. J. Kim and R. S. Ahima, Korean J. Intern. Med., 2017, 32, 239–247 CrossRef CAS PubMed.
- S. Ouyang, W. Chen, Z. Gaofeng, L. Changcheng, T. Guoping, Z. Minyan, L. Yang, Y. Min and J. Luo, Mol. Med. Rep., 2021, 23, 338 CrossRef CAS PubMed.
- X. Sui and C. Gao, Int. J. Mol. Med., 2014, 33, 227–233 CrossRef CAS PubMed.
- X. Zhang, Y. Zhu, F. Song, Y. Yao, F. Ya, D. Li, W. Ling and Y. Yang, Nutr. Metab., 2016, 13, 86 CrossRef PubMed.
- C. Marín-Echeverri, C. N. Blesso, M. L. Fernández, Y. Galvis-Pérez, G. Ciro-Gómez, V. Núñez-Rangel, J. C. Aristizábal and J. Barona-Acevedo, Antioxidants, 2018, 7(12), 185 CrossRef PubMed.
- P. J. Curtis, P. A. Kroon, W. J. Hollands, R. Walls, G. Jenkins, C. D. Kay and A. Cassidy, J. Nutr., 2009, 139, 2266–2271 CrossRef CAS PubMed.
- G. Ndrepepa, Clin. Chim. Acta, 2019, 493, 36–51 CrossRef CAS PubMed.
- A. Fiordelisi, G. Iaccarino, C. Morisco, E. Coscioni and D. Sorriento, Int. J. Mol. Sci., 2019, 20, 1599 CrossRef CAS PubMed.
- D. Luo, Y. Luo, Y. He, H. Zhang, R. Zhang, X. Li, W. L. Dobrucki, A. J. Sinusas, W. C. Sessa and W. Min, Am. J. Pathol., 2006, 169, 1886–1898 CrossRef CAS PubMed.
- C. Herder, W. Peeters, T. Illig, J. Baumert, D. P. V. de Kleijn, F. L. Moll, U. Poschen, N. Klopp, M. Müller-Nurasyid, M. Roden, M. Preuss, M. Karakas, C. Meisinger, B. Thorand, G. Pasterkamp and W. Koenig, PLoS One, 2011, 6, e25734 CrossRef CAS PubMed.
- M. M. Dohadwala, M. Holbrook, N. M. Hamburg, S. M. Shenouda, W. B. Chung, M. Titas, M. A. Kluge, N. Wang, J. Palmisano, P. E. Milbury, J. B. Blumberg and J. A. Vita, Am. J. Clin. Nutr., 2011, 93, 934–940 CrossRef CAS PubMed.
- M. D. Gross, S. J. Bielinski, J. R. Suarez-Lopez, A. P. Reiner, K. Bailey, B. Thyagarajan, J. J. Carr, D. A. Duprez and D. R. Jacobs, Clin. Chem., 2012, 58, 411–420 CrossRef CAS PubMed.
- K. M. Ali, A. Wonnerth, K. Huber and J. Wojta, Br. J. Pharmacol., 2012, 167, 1177–1194 CrossRef CAS PubMed.
- M. F. Troncoso, J. Ortiz-Quintero, V. Garrido-Moreno, F. Sanhueza-Olivares, A. Guerrero-Moncayo, M. Chiong, P. F. Castro, L. García, L. Gabrielli, R. Corbalán, L. Garrido-Olivares and S. Lavandero, Biochim. Biophys. Acta, Mol. Basis Dis., 2021, 1867, 166170 CrossRef CAS PubMed.
- S. Karr, Am. J. Manag. Care, 2017, 23, S139–S148 Search PubMed.
- C.-K. Lee, C.-W. Liao, S.-W. Meng, W.-K. Wu, J.-Y. Chiang and M.-S. Wu, Biomedicines, 2021, 9, 985 CrossRef CAS PubMed.
- B. G. Talayero and F. M. Sacks, Curr. Cardiol. Rep., 2011, 13, 544–552 CrossRef PubMed.
- C. H. Tejera, J. Minnier, S. Fazio, M. M. Safford, L. D. Colantonio, M. R. Irvin, V. Howard, N. A. Zakai and N. Pamir, Am. J. Prev. Cardiol., 2021, 7, 100198 CrossRef PubMed.
- R. Zhou, G. A. Stouffer and S. C. Smith, J. Cardiovasc. Pharmacol. Ther., 2021, 26, 533–549 CrossRef CAS PubMed.
- D. Orozco-Beltran, V. F. Gil-Guillen, J. Redon, J. M. Martin-Moreno, V. Pallares-Carratala, J. Navarro-Perez, F. Valls-Roca, C. Sanchis-Domenech, A. Fernandez-Gimenez, A. Perez-Navarro, V. Bertomeu-Martinez, V. Bertomeu-Gonzalez, A. Cordero, M. Pascual de la Torre, J. L. Trillo, C. Carratala-Munuera, S. Pita-Fernandez, R. Uso, R. Durazo-Arvizu, R. Cooper, G. Sanz, J. M. Castellano, J. F. Ascaso, R. Carmena and M. Tellez-Plaza, PLoS One, 2017, 12, e0186196 CrossRef PubMed.
- C. E. Kosmas, I. Martinez, A. Sourlas, K. V. Bouza, F. N. Campos, V. Torres, P. D. Montan and E. Guzman, Drugs Context, 2018, 7, 1–9 Search PubMed.
- S. Farrer, Adv. Prev. Med., 2018, 2018, 1–9 CrossRef PubMed.
- X. Zhu, L. Yu, H. Zhou, Q. Ma, X. Zhou, T. Lei, J. Hu, W. Xu, N. Yi and S. Lei, Lipids Health Dis., 2018, 17, 37 CrossRef PubMed.
- S. Baumgartner, E. Bruckert, A. Gallo and J. Plat, Atherosclerosis, 2020, 311, 116–123 CrossRef CAS PubMed.
- H. H. Chang, Y. C. Lan, S. D. Chung and C. T. Chien, Life, 2021, 11(8), 802 CrossRef CAS PubMed.
- H. H. Wang, G. Garruti, M. Liu, P. Portincasa and D. Q. H. Wang, Ann. Hepatol., 2017, 16, s27–s42 CrossRef CAS PubMed.
- T. L. Zern, K. L. West and M. L. Fernandez, J. Nutr., 2003, 133, 2268–2272 CrossRef CAS PubMed.
- C. Guzmán and R. Sánchez, Funct. Foods Health Dis., 2021, 11, 512–521 Search PubMed.
- A. G. Groenen, B. Halmos, A. R. Tall and M. Westerterp, Crit. Rev. Biochem. Mol. Biol., 2021, 56, 426–439 CrossRef CAS PubMed.
- D. Shanmuganayagam, T. F. Warner, C. G. Krueger, J. D. Reed and J. D. Folts, Atherosclerosis, 2007, 190, 135–142 CrossRef CAS PubMed.
- A. L. Gould, J. E. Rossouw, N. C. Santanello, J. F. Heyse and C. D. Furberg, Circulation, 1998, 97, 946–952 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, A. Ishisaka, K. Mawatari, A. Vidal-Diez, J. P. E. Spencer and J. Terao, Br. J. Nutr., 2013, 109, 1746–1754 CrossRef CAS PubMed.
- S. Kianbakht, B. Abasi and F. Hashem Dabaghian, Phyther. Res., 2014, 28, 432–436 CrossRef CAS PubMed.
- R. H. Nelson, Primary Care, 2013, 40, 195–211 CrossRef PubMed.
- E. A. Fisher, J. E. Feig, B. Hewing, S. L. Hazen and J. D. Smith, Arterioscler. Thromb. Vasc. Biol., 2012, 32, 2813–2820 CrossRef CAS PubMed.
- W. Hao and A. Friedman, PLoS One, 2014, 9, e90497 CrossRef PubMed.
- Y. He, Q. Zeng, X. Li, B. Liu and P. Wang, PLoS One, 2013, 8, e57089 CrossRef CAS PubMed.
- D. M. Figueroa, E. M. Gordon, X. Yao and S. J. Levine, in Mechanisms and Manifestations of Obesity in Lung Disease, ed. R. A. Johnston and B. T. Suratt, Academic Press, 2018, pp. 301–326 Search PubMed.
- J. E. Eichner, Am. J. Epidemiol., 2002, 155, 487–495 CrossRef PubMed.
- J. H. Contois, J. P. McConnell, A. A. Sethi, G. Csako, S. Devaraj, D. M. Hoefner and G. R. Warnick, Clin. Chem., 2009, 55, 407–419 CrossRef CAS PubMed.
- L. Huang, D. Xiao, X. Zhang, A. K. Sandhu, P. Chandra, C. Kay, I. Edirisinghe and B. Burton-Freeman, J. Nutr., 2021, 151, 1517–1526 CrossRef PubMed.
- M. V. Holmes and G. D. Smith, Nat. Rev. Cardiol., 2017, 14, 635–636 CrossRef CAS PubMed.
- P. Rajendran, T. Rengarajan, J. Thangavel, Y. Nishigaki, D. Sakthisekaran, G. Sethi and I. Nishigaki, Int. J. Biol. Sci., 2013, 9, 1057–1069 CrossRef CAS PubMed.
- L. C. Saiz, J. Gorricho, J. Garjón, M. C. Celaya, J. Erviti and L. Leache, Cochrane Database Syst. Rev., 2018, 7, CD010315 Search PubMed.
- F. D. Fuchs and P. K. Whelton, Hypertension, 2020, 75, 285–292 CrossRef CAS PubMed.
- N. Tran, T. Garcia, M. Aniqa, S. Ali, A. Ally and S. M. Nauli, Am. J. Biomed. Sci. Res., 2022, 15, 153–177 CAS.
- P. L. Huang, Trends Endocrinol. Metab., 2009, 20, 295–302 CrossRef CAS PubMed.
- A. V. Bisconti, E. Cè, S. Longo, M. Venturelli, G. Coratella, S. Shokohyar, R. Ghahremani, S. Rampichini, E. Limonta and F. Esposito, Front. Physiol., 2019, 10, 1–12 Search PubMed.
- W. F. Jackson, Hypertension, 2000, 35, 173–178 CrossRef CAS PubMed.
- S. A. Phillips, D. K. Andaku, R. G. Mendes, F. R. Caruso, R. Cabiddu, R. B. Jaenisch, R. Arena and A. Borghi-Silva, Braz. J. Cardiovasc. Surg., 2017, 32, 125–135 Search PubMed.
- F. Böhm and J. Pernow, Cardiovasc. Res., 2007, 76, 8–18 CrossRef PubMed.
- H. J. Sun, Z. Y. Wu, X. W. Nie and J. S. Bian, Front. Pharmacol., 2020, 10, 1–15 Search PubMed.
- T. Maruhashi, J. Soga, N. Fujimura, N. Idei, S. Mikami, Y. Iwamoto, A. Iwamoto, M. Kajikawa, T. Matsumoto, N. Oda, S. Kishimoto, S. Matsui, H. Hashimoto, Y. Aibara, F. M. Yusoff, T. Hidaka, Y. Kihara, K. Chayama, K. Noma, A. Nakashima, C. Goto, H. Tomiyama, B. Takase, T. Kohro, T. Suzuki, T. Ishizu, S. Ueda, T. Yamazaki, T. Furumoto, K. Kario, T. Inoue, S. Koba, K. Watanabe, Y. Takemoto, T. Hano, M. Sata, Y. Ishibashi, K. Node, K. Maemura, Y. Ohya, T. Furukawa, H. Ito, H. Ikeda, A. Yamashina and Y. Higashi, J. Am. Heart Assoc., 2018, 7, e008588 CrossRef CAS PubMed.
- M. Cecelja and P. Chowienczyk, JRSM Cardiovasc. Dis., 2012, 1, 1–10 CrossRef PubMed.
- G. Istas, E. Wood, M. Le Sayec, C. Rawlings, J. Yoon, V. Dandavate, D. Cera, S. Rampelli, A. Costabile, E. Fromentin and A. Rodriguez-Mateos, Am. J. Clin. Nutr., 2019, 110, 316–329 CrossRef PubMed.
- Y. Wang, Y. Zhang, X. Wang, Y. Liu and M. Xia, J. Nutr., 2012, 142, 1033–1037 CrossRef CAS PubMed.
- B. J. Lewis, K. A. Herrlinger, T. A. Craig, C. E. Mehring-Franklin, Z. DeFreitas and C. Hinojosa-Laborde, J. Nutr. Biochem., 2013, 24, 1359–1366 CrossRef CAS PubMed.
- R. du Preez, S. Wanyonyi, P. Mouatt, S. K. Panchal and L. Brown, Nutrients, 2020, 12(4), 931 CrossRef CAS PubMed.
- K. Horie, H. Maeda, N. Nanashima and I. Oey, Molecules, 2021, 26(21), 5459 CrossRef PubMed.
- F. Khan, S. Ray, A. M. Craigie, G. Kennedy, A. Hill, K. L. Barton, J. Broughton and J. J. F. Belch, Free Radical Biol. Med., 2014, 72, 232–237 CrossRef CAS PubMed.
- Y. Inaba, J. A. Chen and S. R. Bergmann, Int. J. Cardiovasc. Imaging, 2010, 26, 631–640 CrossRef PubMed.
- P. J. Curtis, V. Van Der Velpen, L. Berends, A. Jennings, M. Feelisch, A. M. Umpleby, M. Evans, B. O. Fernandez, M. S. Meiss, M. Minnion, J. Potter, A. M. Minihane, C. D. Kay, E. B. Rimm and A. Cassidy, Am. J. Clin. Nutr., 2019, 109, 1535–1545 CrossRef PubMed.
- D. L. McKay, C. Y. O. Chen, E. Saltzman and J. B. Blumberg, J. Nutr., 2010, 140, 298–303 CrossRef CAS PubMed.
- M. E. A. M. van Kleef and W. Spiering, Eur. J. Prev. Cardiol., 2017, 24, 36–43 CrossRef PubMed.
- R. Draijer, Y. de Graaf, M. Slettenaar, E. de Groot and C. I. Wright, Nutrients, 2015, 7, 3138–3153 CrossRef CAS PubMed.
- K. S. Stote, M. M. Wilson, D. Hallenbeck, K. Thomas, J. M. Rourke, M. I. Sweeney, K. T. Gottschall-Pass and A. R. Gosmanov, Curr. Dev. Nutr., 2020, 4(4), 1–10 Search PubMed.
- L. A. J. Van Mierlo, P. L. Zock, H. C. M. Van Der Knaap and R. Draijer, J. Nutr., 2010, 140, 1769–1773 CrossRef CAS PubMed.
- S. P. Jackson, Blood, 2007, 109, 5087–5095 CrossRef CAS PubMed.
- L.-Y. Wang, Y. Wang, D.-S. Xu, K.-F. Ruan, Y. Feng and S. Wang, J. Ethnopharmacol., 2012, 143, 347–354 CrossRef CAS PubMed.
- Q. Wang, P. Han, M. Zhang, M. Xia, H. Zhu, J. Ma, M. Hou, Z. Tang and W. Ling, Asia Pac. J. Clin. Nutr., 2007, 16(1), 295–301 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04579j |
| This journal is © The Royal Society of Chemistry 2024 |