Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A Capsicum annuum L. seed extract exerts anti-neuroexcitotoxicity in HT22 hippocampal neurons†
Ji-Yun
Kang
a,
Ji-Yeon
Gu
a,
Dong-Cheol
Baek
a,
Chang-Gue
Son
 *ab and
Jin-Seok
Lee
*ab
*ab and
Jin-Seok
Lee
*ab
aInstitute of Bioscience & Integrative Medicine, Daejeon Hospital of Daejeon University, Daejeon, Republic of Korea. E-mail: neptune@dju.ac.kr; Fax: +82-42-257-6398; Tel: +82-42-257-6397
bResearch Center for CFS/ME, Daejeon Hospital of Daejeon University, Daejeon, Republic of Korea
First published on 2nd February 2024
Abstract
The hippocampal memory deficit stands out as a primary symptom in neurodegenerative diseases, including Alzheimer's disease. While numerous therapeutic candidates have been proposed, they primarily serve to delay disease progression. Given the irreversible brain atrophy or injury associated with these conditions, current research efforts are concentrated on preventive medicine strategies. Herein, we investigated whether the extracts of Capsicum annuum L. seeds (CSE) and Capsicum annuum L. pulp (CPE) have preventive properties against glutamate-induced neuroexcitotoxicity (one of the main causes of Alzheimer's disease) in HT22 hippocampal neuronal cells. Pretreatment with CSE demonstrated significant anti-neuroexcitotoxic activity, whereas CPE did not exhibit such effects. Specifically, CSE pretreatment dose-dependently inhibited the elevation of excitotoxic elements (intracellular calcium influx and reactive oxygen species; ROS) and apoptotic elements (p53 and cleaved caspase-3). In addition, the glutamate-induced alterations of neuronal activity indicators (brain-derived neurotrophic factor; BDNF and cAMP response element-binding protein phosphorylation; CREB) were significantly attenuated by CSE treatment. We also found that luteolin is the main bioactive compound corresponding to the anti-neuroexcitotoxic effects of CSE. Our results strongly suggest that Capsicum annuum L. seeds (but not its pulp) could be candidates for neuro-protective resources especially under conditions of neuroexcitotoxicity. Its underlying mechanisms may involve the amelioration of ROS-mediated cell death and BDNF-related neuronal inactivity and luteolin would be an active compound.
Introduction
Hippocampal atrophy is a prevalent characteristic observed in patients diagnosed with Alzheimer's disease, a condition affecting an estimated 50 million individuals worldwide.1 Both patients and their families frequently report reduced health-related quality of life (HR-QoL) due to the cognitive and memory impairments associated with the disease. This situation significantly contributes to the overall burden of disease, underscoring the substantial impact of Alzheimer's disease on affected individuals and their immediate social circles.2 Memory impairment can be attributed to various environmental factors, such as repeated exposure to stress and traumatic events, leading to hippocampal neuronal loss through abnormal neurotransmission and/or immune reactions.3 Despite the approval of memantine, an N-methyl-D-aspartate (NMDA) receptor antagonist, as a medication for moderate to severe Alzheimer's disease, achieving complete recovery in the advanced stage remains elusive to date.4 Moreover, considering the elevated conversion rate of 80% from mild cognitive impairment (MCI) to Alzheimer's disease within 6 years,5 this phase is regarded as an intermediate stage, underscoring the significance of early intervention to halt the progression of dementia.On the other hand, chronic and excessive stress is widely recognized to contribute to neurodegenerative diseases, including Alzheimer's disease.6,7 The overproduction of excitatory neurotransmitters has been implicated in these stress-related pathogenic processes, in contrast to the physiological roles of excitatory neurotransmitters, such as cell differentiation and synaptic plasticity.8 Among various neurotransmitters, glutamate serves as a fundamental excitatory neurotransmitter crucial for promoting synaptic plasticity in the learning and memory formation process;9 however, excessive glutamate overflow results in neuroexcitotoxicity, a characteristic feature associated with hippocampal neuronal atrophy in dementia.10 The high influx of glutamate through ionotropic and metabotropic receptors, as well as transporters, leads to intracellular calcium (Ca2+) overload and the generation of mitochondrial reactive oxygen species (ROS). Subsequently, this condition is associated with early apoptosis or necroptosis.11 Hippocampal pyramidal neurons are particularly susceptible to excitotoxic insults, including polyamine and oxidative stress.12,13 Consequently, numerous research groups are diligently exploring potent candidates to inhibit hippocampal excitotoxicity.14,15 Recent research indicates that spicy foods, like chili pepper, may play a significant role in preventing brain diseases, and the consumption of spicy foods has been associated with decreased β-amyloid levels in the cerebrospinal fluid.16
Capsicum annuum L. is extensively consumed as a healthy food source, produced in significant quantities globally, and is recognized for its diverse nutritional and pharmacological advantages.17 However, the agricultural and horticultural sectors associated with its cultivation generate considerable waste, primarily comprising fruit components (placenta, seeds, and unused pulps) as well as plant parts (stems and leaves).18 Recently, studies on Capsicum annuum L. seeds have reported diverse pharmacological effects, including anti-obesity, antioxidant, anti-inflammatory, and glucose homeostatic regulation properties.19 Notably, the extract from Capsicum annuum L. seeds exhibited superior hippocampal ROS scavenging abilities compared to extracts from fifteen other types of seeds.20 Interestingly, certain studies have demonstrated that the seed extract from red pepper exhibits a higher antimutagenic and antioxidant capacity compared to the pulp extract.21 Additionally, flavonoids such as luteolin, quercetin, kaempferol, and rutin are abundant in Capsicum annuum L. seeds,19 and their bioactive properties in relation to hippocampal memory impairment have been partially investigated.22,23
This study aimed to investigate the neuroprotective effects of Capsicum annuum L. seed extract on HT22 hippocampal neuron cells, along with the major active compound, and explore its underlying mechanisms under glutamate-induced neuroexcitotoxicity.
Materials and methods
Materials and reagents
The following reagents were procured from various manufacturers: 2′,7′-dichlorofluorescin diacetate (DCFH-DA), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), capsaicin (cat no. M2028), ethyl alcohol, L-glutamic acid monosodium salt monohydrate (glutamate), N-acetyl-L-cysteine (NAC), N-(1-naphthyl)-ethylenediamine dihydrochloride, phosphoric acid, sodium hydroxide, tetraethyl ethylenediamine (TEMED), Trizma base, and Tween 20 (Sigma-Aldrich, St Louis, MO, USA); antibiotic antimycotic solution, Dulbecco's modified Eagle's medium (DMEM), Dulbecco's phosphate-buffered saline (DPBS), fetal bovine serum (FBS), and trypsin–ethylenediaminetetraacetic acid (EDTA) (Welgene, Daegu, Korea); capsiate (cat no. HY-N8377), luteolin (cat no. HY-N6747) and lutein (cat no. HY-N0162) (MedChemExpress, Monmouth Junction, NJ, USA); 10% ammonium persulfate solution (APS), radioimmunoprecipitation assay buffer (RIPA), and skim milk (LPS Solution, Daejeon, Korea); bovine serum albumin (BSA) (GenDEPOT, Barker, TX, USA); 4% paraformaldehyde (PFA), 10× Tris glycine buffer, and 10× Tris glycine-SDS buffer (XOGENE, Daejeon, Korea); lactate dehydrogenase (LDH) cytotoxicity assay kit and water-soluble tetrazolium salt (WST)-8 based cell viability assay kit (DoGen, Seoul, Korea); Ca2+ assay kit (BioVision, CA, USA); PRO-PREP™ protein extraction solution (iNtRON Biotechnology, Seongnam, Korea); sodium chloride and hydrochloric acid (Samchun, Seoul, Korea); methylene alcohol (Daejung Chemicals & Metals Co., Siheung, Korea); n-butanol (J.T. Baker, Mexico City, Mexico); and polyvinylidene fluoride (PVDF) membranes (Pall Co., Port Washington, NY, USA).Preparation of Capsicum annuum. L seed and pulp extracts
Capsicum annuum L. was purchased from Cheonnong Inc. (Chungcheongnam-do, Korea; code no. 02-0004-2003-20), and seeds were certified from the Korea Seed and Variety Service (registration no. 02-0004-1153) of Ministry of Agriculture, Food and Rural Affairs. The preparation of Capsicum annuum L. seed extract (CSE) and pulp extract (CPE) proceeded as follows: twenty grams of each were dried at 37 °C overnight and subsequently incubated with 200 mL of 30% ethyl alcohol in an orbital shaker for 72 hours at room temperature. The resulting mixtures were filtered through Whatman filter paper (Advantec®, Tokyo, Japan). The filtrates were concentrated using a rotary evaporator and then lyophilized, resulting in a final extraction yield (w/w) of 6% for seeds and 12.35% for pulps, respectively.Fingerprinting analysis of Capsicum annuum L. seed and pulp extracts
To ascertain the chemical compositions of CSE and CPE, high-performance liquid chromatography (HPLC) analysis was conducted. For this analysis, 100 mg of CSE or CPE was dissolved in 1 L of ethyl alcohol, and the solutions were filtered (0.45 μm). The filtrates were subjected to HPLC using a Prominence system (Shimadzu Co., Kyoto, Japan). Separation was achieved using a Capcell Pak C18 column (250 × 4.6 mm, 5 μm; Sigma-Aldrich). The mobile phase conditions were prepared as follows: (A) distilled water (1% phosphoric acid) and (B) acetonitrile. The column was eluted at a flow rate of 1.0 mL min−1 with the following gradients: 0–20 min, 5% B (isocratic); 20–40 min, 25–30% B (linear gradient); 40.1–60 min, 100% B (isocratic). A photodiode array detector was set to measure at a wavelength of 222 nm. The Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm; Sigma-Aldrich) was employed to separate the lutein. The column was eluted at a flow rate of 0.5 mL min−1. The mobile phase consisted of 5 mM ammonium formate (0.1% formic acid) and 5 mM ammonium formate in methanol (0.1% formic acid); the ratio of the mobile phase was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85. Reference peaks of CSE were identified at 29.39 min (capsiate), 46.12 min (capsaicin), 33.01 min (luteolin), and 2.71 min (lutein). Similarly, CPE peaks were detected at 29.36 min (capsiate), 46.12 min (capsaicin), and 2.73 min (lutein) retention times. The concentrations of each compound were determined as 795.49 μg g−1 (capsiate), 228.35 μg g−1 (capsaicin), 37.93 μg g−1 (luteolin), and 1.56 μg g−1 (lutein) in CSE, and 1694.11 μg g−1 (capsiate), 167.42 μg g−1 (capsaicin), and 0.14 μg g−1 (lutein) in CPE (Fig. S1A–C†).
85. Reference peaks of CSE were identified at 29.39 min (capsiate), 46.12 min (capsaicin), 33.01 min (luteolin), and 2.71 min (lutein). Similarly, CPE peaks were detected at 29.36 min (capsiate), 46.12 min (capsaicin), and 2.73 min (lutein) retention times. The concentrations of each compound were determined as 795.49 μg g−1 (capsiate), 228.35 μg g−1 (capsaicin), 37.93 μg g−1 (luteolin), and 1.56 μg g−1 (lutein) in CSE, and 1694.11 μg g−1 (capsiate), 167.42 μg g−1 (capsaicin), and 0.14 μg g−1 (lutein) in CPE (Fig. S1A–C†).
Cell culture and modeling glutamate-induced excitotoxicity
HT22 cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotic antimycotic solution. HT22 cells were incubated at 37 °C under 5% CO2. The HT22 cells were seeded at 4 × 103 cells per well into 96-well microplates and incubated for 12 h. To determine the appropriate dosage of glutamate-induced excitotoxicity, the dose-dependent (5, 10, or 20 mM) and time-dependent (4, 12, or 24 h) responses were assessed using the WST-8 assay (EZ-Cytox, DoGen, Korea). Absorbance at 450 nm was measured using a UV spectrophotometer (Molecular Devices, CA, USA).Cell viability and lactate dehydrogenase (LDH) activity assay
The HT22 cells were seeded at 4 × 103 cells per well into 96-well microplates and incubated for 12 h. To confirm the cytotoxicity, the serial doses of both CSE and CPE were evaluated for 24 h using the WST-8 assay. Considering non-cytotoxic dosage, the cells were pretreated with three doses of CSE or CPE (25, 50, and 100 μg mL−1) or a positive control (NAC, 1 mM) for 2 h before exposure to 10 mM glutamate for 24 h. In addition, the dose-dependent effects of CSE and the identified bioactive compounds of CSE (1 or 5 μM of capsiate, capsaicin, luteolin or lutein) were evaluated using the WST-8 assay under the same conditions. This anti-neuroexcitotoxic activity of CSE was supported by extracellular LDH activity (DZ-LDH, DoGen, Korea). Absorbance at 450 nm was measured using a UV spectrophotometer (Molecular Devices).Intracellular ROS production
HT22 cells were seeded at 4 × 104 cells per well into 6-well plates. Under the conditions of pretreating CSE (25, 50, or 100 μg mL−1) for 2 h and following exposure to glutamate (10 mM) for 12 h, the cells were incubated with 10 μM of DCFH-DA for 10 min at 37 °C. Intracellular ROS production was observed by green fluorescence (488 nm) using an Axiophot microscope (Carl Zeiss, Jena, Germany), and its intensity was semi-quantified using ImageJ 1.46 software (NIH, Bethesda, MD, USA).Intracellular calcium influx assay
HT22 cells were seeded at 1 × 105 cells per well into 60 mm dishes. Under the same conditions above, the intracellular Ca2+ concentration was determined using a calcium colorimetric assay kit (E-BC-K103-M, Elabscience Biotechnology Inc., Houston, Texas, USA) according to the manufacturer's protocol. Absorbance at 610 nm was measured with a UV spectrophotometer (Molecular Devices).Western blotting analysis
To analyze the apoptotic protein expression levels, the HT22 cells (seeding of 6 × 104 cells per well in a 60 mm dish, pretreating CSE for 2 h and exposing glutamate for 24 h, subsequently) were lysed using the Pro-Prep™ protein extraction solution (iNtRON Biotechnology). Concentrations of the extracted protein were determined using a bicinchoninic acid protein assay kit (Sigma-Aldrich).The cell lysates were separated by polyacrylamide gel electrophoresis and transferred to PVDF membranes. After the membranes were blocked with 5% skim milk in Tris-buffered saline (TBST; 0.05% Tween 20 in TBS) for 1 h, they were incubated with primary antibodies against p53 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, MA5-12453, Invitrogen), Bcl-2-associated X protein (BAX; 1
1000, MA5-12453, Invitrogen), Bcl-2-associated X protein (BAX; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, #2772, Cell Signaling), B-cell lymphoma 2 (Bcl-2; 1
1000, #2772, Cell Signaling), B-cell lymphoma 2 (Bcl-2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab194583, Abcam), cytochrome C (1
1000, ab194583, Abcam), cytochrome C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, #4272, Cell Signaling), pro-caspase 3 (1
1000, #4272, Cell Signaling), pro-caspase 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, sc-7272, Santa Cruz), cleaved-caspase 3 (1
1000, sc-7272, Santa Cruz), cleaved-caspase 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, #9664, Cell Signaling), brain-derived neurotrophic factor (BDNF; 1
1000, #9664, Cell Signaling), brain-derived neurotrophic factor (BDNF; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab108319, Abcam), tropomyosin receptor kinase B (TrkB; 1
1000, ab108319, Abcam), tropomyosin receptor kinase B (TrkB; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, sc-377218, Santa Cruz), cAMP response element-binding protein (CREB; 1
1000, sc-377218, Santa Cruz), cAMP response element-binding protein (CREB; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab31387, Abcam), phospho-CREB (1
1000, ab31387, Abcam), phospho-CREB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab32096, Abcam) and α-tubulin (1
1000, ab32096, Abcam) and α-tubulin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ab7291, Abcam) overnight at 4 °C. The membranes were washed and incubated with HRP-conjugated anti-mouse (1
1000, ab7291, Abcam) overnight at 4 °C. The membranes were washed and incubated with HRP-conjugated anti-mouse (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, to detect p53, pro-caspase 3, TrkB and α-tubulin) or anti-rabbit (1
5000, to detect p53, pro-caspase 3, TrkB and α-tubulin) or anti-rabbit (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, to detect BAX, Bcl-2, cytochrome C, cleaved-caspase 3, BDNF, CREB and phospho-CREB) antibodies for 45 min. The protein bands were visualized using an advanced enhanced chemiluminescence (ECL) kit and observed using the FUSION Solo System (Vilber Lourmat, Collegien, France). The intensity of the bands was semi-quantified using ImageJ 1.46 software (NIH).
5000, to detect BAX, Bcl-2, cytochrome C, cleaved-caspase 3, BDNF, CREB and phospho-CREB) antibodies for 45 min. The protein bands were visualized using an advanced enhanced chemiluminescence (ECL) kit and observed using the FUSION Solo System (Vilber Lourmat, Collegien, France). The intensity of the bands was semi-quantified using ImageJ 1.46 software (NIH).
Statistical analysis
The data are expressed as means ± standard deviations. Statistical analyses were conducted using GraphPad Prism 9 software (GraphPad, Inc., La Jolla, CA, USA). Significance was determined through one-way analysis of variance (ANOVA) followed by Dunnett's test. In all analyses, p < 0.05 was considered significant.Results
Comparative effects of CSE and CPE against neuroexcitotoxicity
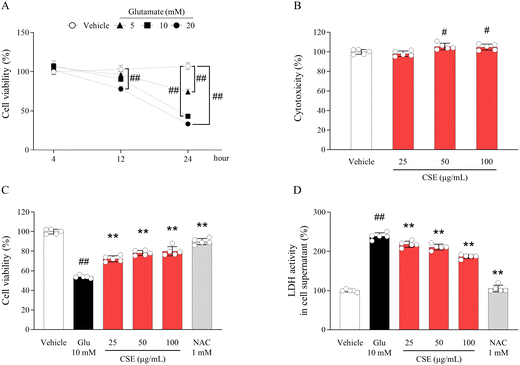
The appropriate dose and time-point for inducing neuroexcitotoxicity were determined to be 10 mM and 24 hours, conditions that resulted in approximately 50% HT22 hippocampal neuronal cell death (p < 0.01, Fig. 1A). No cytotoxicity was observed up to 100 μg mL−1 of both CSE and CPE (Fig. S2A† and Fig. 1B), and CSE exhibited statistically superior anti-neuroexcitotoxic activity to CPE (Fig. S2B†). These effects were demonstrated in a dose-dependent manner, as evidenced by cell viability and LDH activity (p < 0.01 for all doses, Fig. 1C and D). NAC, a potent antioxidant used as a positive control, had similar activity to CSE.Effects of CSE on intracellular ROS production and calcium influx
Exposure to excessive glutamate resulted in significantly higher intracellular ROS production (3.2-fold) and Ca2+ influx (2.2-fold) compared to vehicle-treated cells. Pretreatment with CSE markedly attenuated these elevations in DCFH-DA fluorescence signals, intracellular ROS production, and Ca2+ influx when compared with the glutamate-treated group (p < 0.01 for all doses, Fig. 2A–D). With the exception of the effect on Ca2+ influx, NAC also demonstrated similar effects to those observed with CSE.Effects of CSE on neuronal apoptotic molecules
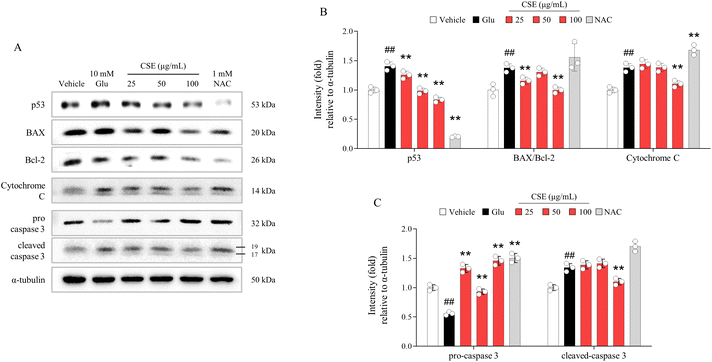
In the case of apoptotic molecules, including p53 (1.4-fold increase), the BAX/Bcl-2 ratio (1.37-fold increase), cytochrome C (1.38-fold increase), pro-caspase 3, and cleaved-caspase 3 (1.38-fold increase), notable alterations were observed in glutamate-exposed HT22 cells. These alterations were nearly completely normalized by pretreatment with CSE (p < 0.05 or p < 0.01, Fig. 3A–C). Some of the apoptotic molecules were also improved by NAC pretreatment.Effects of CSE on neuronal plasticity molecules
In line with the apoptosis-related alterations, glutamate exposure resulted in reduced neuronal plasticity, as indicated by decreased protein expression levels of BDNF, TrkB, CREB, and its phosphorylated forms. Pretreatment with CSE significantly ameliorated these diminished neuronal activities (p < 0.05 or p < 0.01, Fig. 4A and B) in comparison with glutamate-treated cells. These BDNF-promoting activities of CSE were seen even in the absence of glutamate induction (Fig. S2E and F†). In contrast to the effects of NAC on apoptotic signals, it enhanced neuronal plasticity.Effects of bioactive compounds contained in CSE
Based on non-cytotoxic doses of up to 5 μM of compounds contained in CSE (Fig. 5A), their anti-neuroexcitotoxic effects were assessed. Among the four compounds, only luteolin exhibited significant pharmacological activity in this glutamate-induced model (p < 0.01 for all doses), whereas capsiate, capsaicin, and lutein did not show such effects (Fig. 5B). Pretreatment with NAC also showed anti-neuroexcitotoxicity effects.Effects of luteolin on neuronal apoptotic and plasticity molecules
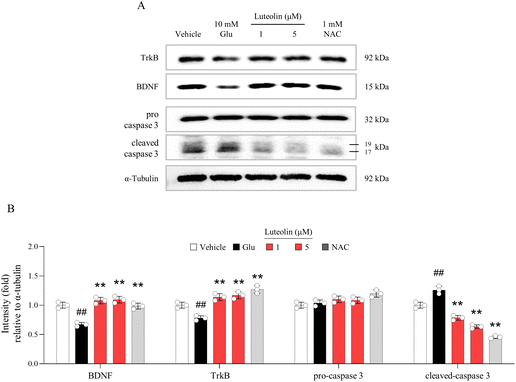
Corresponding to the pharmacological activity of CSE, luteolin also had preventive effects against glutamate-induced neuronal apoptosis and inactivity, as evidenced by altered levels of protein expression for cleaved caspase 3, BDNF, and TrkB (p < 0.01 for both doses, Fig. 6A and B). Its effects were strong even at a low dose of 1 μM as a bioactive compound of CSE, whereas 1 mM of NAC showed similar results.Discussion
Neuroexcitotoxicity is a key pathological process in several neurological disorders, including dementia and epilepsy. It is widely recognized that the excessive influx of glutamate leads to excitotoxic neuronal death and glial activation in the central nervous system.24 In 2004, memantine, an NMDA receptor antagonist, received approval from the U.S. FDA as a drug for delaying the progression of Alzheimer's disease.25 However, with the steady increase in the number of affected patients, there is a growing societal and medical focus on achieving full recovery or developing preventive medicine strategies.To assess the preventive effects of Capsicum annuum L., we employed a glutamate-exposed HT22 hippocampal neuroexcitotoxicity model commonly used for investigating the neuropharmacological activity of candidates.26,27 Due to the fact that this HT22 cell line has an important feature of lacking the NMDA receptor,28 it is difficult to evaluate the pharmacological potential of candidates compared to NMDA receptor antagonists, such as MK-801 and memantine. Therefore, we adopted NAC in this study, which is commonly used as a positive control because of its mitochondrial ROS-dependent neuroexcitotoxic feature.29 Intriguingly, our findings indicate that Capsicum annuum L. seed extracts (CSE) alleviate hippocampal excitotoxicity, in contrast to its pulp extracts (CPE) (Fig. S2D†). Indeed, the efficacy of medicinal plants or food substances can be influenced by cultural environments, including factors such as the season and cultivation region.30 Additionally, chemical compositions vary significantly among different parts of fruits (such as seeds and pulp), roots, stems, and leaves.31,32 As expected, the CSE exhibited dose-dependent pharmacological effects on neuronal excitotoxic death and LDH release activity (Fig. 1). Declarative and spatial memory impairments are significantly correlated with the degree of hippocampal atrophy, as evidenced by the reduced hippocampal volume observed in patients with mild cognitive impairment and dementia.33 Furthermore, several animal studies have indicated that kainic acid-induced excitotoxicity leads to memory deficits and temporal lobe epilepsy.34,35
Excessive excitatory neurotransmission leads to an increase in intracellular Ca2+ influx through ionotropic and metabotropic glutamate receptors. A significant earlier study has proposed associations between α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (one of the ionotropic glutamate receptors) endocytosis-mediated excitotoxicity and the decay of long-term potentiation (LTP), which is linked to memory loss, in the APP23 transgenic mouse model.36 Its oversupply depolarizes both cell and mitochondrial membranes and links to rapid neuronal death by the release of free radicals (ROS) and apoptotic signals.37 Our findings further demonstrated the depolarization of HT22 hippocampal neurons upon treatment with CSE, but not with CPE (Fig. 1B and S2A†). As anticipated, pretreatment with CSE prevented the alterations in the neuroexcitotoxic cascades, including intracellular Ca2+ overflow and ROS overproduction (Fig. 2). It is widely acknowledged that the hippocampus is particularly susceptible to oxidative stress, including reactive oxygen species (ROS), owing to its significantly lower antioxidant capacity compared to other brain regions.38,39 Consequently, the intracellular rise in ROS induced by excessive glutamate leads to ferroptosis or apoptosis in hippocampal neurons, ultimately resulting in impairments in learning and memory functions.40,41
To assess the anti-apoptotic properties of CSE, apoptosis-related molecules were screened by referencing both clinical and preclinical studies.42 In patients with Alzheimer's disease exhibiting hippocampal shrinkage, the expressions of apoptotic genes were up-regulated, especially those dependent on caspase-3.43 In the present study, pretreatment with CSE considerably normalized the alterations in apoptotic signals (Fig. 3). This caspase-3-dependent apoptosis is associated with the regulation of genes such as Bcl-2, BAX, and/or caspase-3, as well as mitochondrial cytochrome c, induced by glutamate, ultimately resulting in hippocampal neuronal cell death.44,45 Numerous animal studies have demonstrated that the neuronal loss within the hippocampal CA3 region contributes to impairments in learning and long-term spatial memory.46,47 Caspase-3 and apoptotic signal-related molecules contribute to the endocytosis of AMPA receptors and long-term depression (LTD) in hippocampal neurons. Additionally, caspase-3-induced hippocampal shrinkage plays a pivotal role in the regulation of long-term neural learning and neurogenesis within these neurons.45,48–50
Neurotrophins play a crucial role in regulating hippocampal synaptic plasticity and neurogenesis, and their depletion results in decreased neuron-to-neuron interaction.51 Spatial memory impairments were observed following hippocampal-specific deletion of BDNF, one of the key neurotrophins, which also restricted the extinction of aversive memory.52 BDNF is typically released through the phosphorylation of CREB and binds to the post-synaptic TrkB receptor. Currently, ZEB85, a full agonist of TrkB, is being considered as a potent therapeutic option for Alzheimer's disease.53 As anticipated, the reduction of neuroplasticity-related hippocampal molecules was prevented by CSE pretreatment, as demonstrated by the protein expression of CREB, BDNF, and TrkB (Fig. 4). Intriguingly, the BDNF levels were dose-dependently increased by CSE, even in the absence of glutamate-inducing conditions (Fig. S2E and D†). It is well known that BDNF concentrations in human sera or cerebrospinal fluids can be affected by the consumption of nutritional foods, such as kernel-based bread and fermented cheese.54 The signaling of BDNF to trigger long-term potentiation (LTP) in the dentate gyrus is intricately linked with hippocampal gray matter shrinkage in the aging brain.55 Moreover, the exogenous application of BDNF is correlated with an elevation in the spine density of CA1 pyramidal neurons through the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway and Ca2+ entry via the transient receptor potential canonical (TRPC) subfamily channel 3.56,57
The phytochemical composition of the seeds is dominated by flavonoid compounds such as luteolin, quercetin, kaempferol, and rutin, as well as capsaicinoids, capsinoids, and volatile compounds.19 Our data further revealed that both CSE and CPE consist of a diverse array of natural compounds, including capsiate, capsaicin, luteolin, and lutein (Fig. S1†). Additionally, we identified the active compound in CSE. Among the four constituents of CSE, luteolin exhibited the sole pharmacological activity against neuroexcitotoxicity (Fig. 5A and B). This luteolin almost completely normalized the altered neuroplasticity (BDNF and TrkB) and apoptotic-related molecules (cleaved caspase 3) as a bioactive compound of CSE (Fig. 6). Researchers have discovered that the neuroprotective effects of luteolin enhance memory function by preventing the loss of the CA1 pyramidal cell layer in an Alzheimer's disease model.23 Additionally, it protects the hippocampus from neuron injury induced by kainic acid, a substance that causes glutamatergic excitotoxicity by elevating glutamate levels, in rats.58 Hence, the results imply that luteolin could be accountable for the anti-neuroexcitotoxic effects of CSE.
Taken together, we conclude that Capsicum annum L. seeds could be a potential candidate for alleviating hippocampal neuroexcitotoxicity, and luteolin would be an active compound. Its underlying mechanism may involve regulating the ROS-mediated apoptotic pathway (caspase-3) and neuronal plasticity signaling (BDNF/TrkB/CREB). To facilitate clinical application, further studies investigating the optimal dosage need to be conducted in the future. Our findings may be the first evidence and guidance for developing the seeds of Capsicum annum L. into dietary supplements or functional foods in the future.
Author contributions
Ji-Yun Kang: conceptualization, methodology, investigation, validation, writing—original draft, writing—review, editing, and visualization. Ji-Yeon Gu and Dong-Cheal Baek: validation, methodology, and investigation. Chang-Gue Son and Jin-Seok Lee: supervision, project administration, funding acquisition, writing—review and editing, and conceptualization. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT, and Future Planning (NRF-2022R1A2C1013084 and NRF-2018R1A6A1A03025221).References
- H. Yao, Y. Araki, F. Yamashita, M. Sasaki and M. Hashimoto, Hippocampal atrophy associated with dementia risk factors and dementia, in Genetics, Neurology, Behavior, and Diet in Dementia, 2020, pp. 373–387 Search PubMed.
- C. Patterson, World alzheimer report 2018, 2018 Search PubMed.
- E. J. Kim, B. Pellman and J. J. Kim, Stress effects on the hippocampus: a critical review, Learn. Mem., 2015, 22, 411–416 CrossRef PubMed.
- O. Binvignat and J. Olloquequi, Excitotoxicity as a target against neurodegenerative processes, Curr. Pharm. Des., 2020, 26, 1251–1262 CrossRef CAS.
- M. Tábuas-Pereira, I. Baldeiras, D. Duro, B. Santiago, M. H. Ribeiro, M. J. Leitão, C. Oliveira and I. Santana, Prognosis of early-onset vs. late-onset mild cognitive impairment: comparison of conversion rates and its predictors, Geriatrics, 2016, 1, 11 CrossRef.
- N. J. Justice, The relationship between stress and Alzheimer's disease, Neurobiol. Stress, 2018, 8, 127–133 CrossRef.
- M. Ávila-Villanueva, J. Gómez-Ramírez, F. Maestú, C. Venero, J. Ávila and M. A. Fernández-Blázquez, The role of chronic stress as a trigger for the alzheimer disease continuum, Front. Aging Neurosci., 2020, 12, 561504 CrossRef.
- Y. Zhou and N. C. Danbolt, Glutamate as a neurotransmitter in the healthy brain, J. Neural Transm., 2014, 121, 799–817 CrossRef CAS PubMed.
- G. Riedel, B. Platt and J. Micheau, Glutamate receptor function in learning and memory, Behav. Brain Res., 2003, 140, 1–47 CrossRef CAS.
- E. Gemmell, H. Bosomworth, L. Allan, R. Hall, A. Khundakar, A. E. Oakley, V. Deramecourt, T. M. Polvikoski, J. T. O'Brien and R. N. Kalaria, Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias, Stroke, 2012, 43, 808–814 CrossRef.
- M. Jakaria, S.-Y. Park, M. E. Haque, G. Karthivashan, I.-S. Kim, P. Ganesan and D.-K. Choi, Neurotoxic agent-induced injury in neurodegenerative disease model: Focus on involvement of glutamate receptors, Front. Mol. Neurosci., 2018, 11, 307 CrossRef PubMed.
- T. R. Butler, R. L. Self, K. J. Smith, L. J. Sharrett-Field, J. N. Berry, J. M. Littleton, J. R. Pauly, P. J. Mulholland and M. A. Prendergast, Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors, Neuroscience, 2010, 165, 525–534 CrossRef CAS PubMed.
- J.-S. Lee, W.-Y. Kim, Y.-J. Jeon, S.-K. Lee and C.-G. Son, Aquilariae Lignum extract attenuates glutamate-induced neuroexcitotoxicity in HT22 hippocampal cells, Biomed. Pharmacother., 2018, 106, 1031–1038 CrossRef PubMed.
- Y. Shindo, R. Yamanaka, K. Hotta and K. Oka, Inhibition of mg2+ extrusion attenuates glutamate excitotoxicity in cultured rat hippocampal neurons, Nutrients, 2020, 12, 2768 CrossRef CAS PubMed.
- U. J. Kim, B. H. Lee and K. H. Lee, Neuroprotective effects of a protein tyrosine phosphatase inhibitor against hippocampal excitotoxic injury, Brain Res., 2019, 1719, 133–139 CrossRef CAS PubMed.
- D.-Y. Tian, J. Wang, B.-L. Sun, Z. Wang, W. Xu, Y. Chen, Y.-Y. Shen, H.-Y. Li, D.-W. Chen and F.-Y. Zhou, Spicy food consumption is associated with cognition and cerebrospinal fluid biomarkers of Alzheimer disease, Chin. Med. J., 2021, 134, 173–177 CrossRef CAS PubMed.
- S. K. Mandal, S. K. Rath, R. Logesh, S. K. Mishra, H. P. Devkota and N. Das, Capsicum annuum L. and its bioactive constituents: A critical review of a traditional culinary spice interms of its modern pharmacological potentials with toxicological issues, Phytother. Res., 2023, 37, 965–1002 CrossRef CAS PubMed.
- M. Yasin, L. Li, M. Donovan-Mak, Z.-H. Chen and S. K. Panchal, Capsicum Waste as a Sustainable Source of Capsaicinoids for Metabolic Diseases, Foods, 2023, 12, 907 CrossRef CAS PubMed.
- J. Echave, A. G. Pereira, M. Carpena, M. Á. Prieto and J. Simal-Gandara, in Capsicum, IntechOpen London, UK, 2020 Search PubMed.
- Y. Okada and M. Okada, Protective effects of plant seed extracts against amyloid β-induced neurotoxicity in cultured hippocampal neurons, J. Pharm. BioAllied Sci., 2013, 5, 141 CrossRef.
- K.-H. Sim and Y.-S. Han, The antimutagenic and antioxidant effects of red pepper seed and red pepper pericarp (Capsicum annuum L.), Prev. Nutr. Food Sci., 2007, 12, 273–278 CrossRef.
- G. M. Alshammari, W. H. Al-Qahtani, M. A. Alshuniaber, A. E. A. Yagoub, A. S. Al-Khalifah, L. N. Al-Harbi, M. H. Alhussain, S. A. AlSedairy and M. A. Yahya, Quercetin improves the impairment in memory function and attenuates hippocampal damage in cadmium chloride-intoxicated male rats by suppressing acetylcholinesterase and concomitant activation of SIRT1 signaling, J. Funct. Foods, 2021, 86, 104675 CrossRef CAS.
- T. Y. Lin, C. W. Lu and S. J. Wang, Luteolin protects the hippocampus against neuron impairments induced by kainic acid in rats, Neurotoxicology, 2016, 55, 48–57 CrossRef CAS PubMed.
- D. Belov Kirdajova, J. Kriska, J. Tureckova and M. Anderova, Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells, Front. Cell. Neurosci., 2020, 14, 51 CrossRef.
- S. J. Thomas and G. T. Grossberg, Memantine: a review of studies into its safety and efficacy in treating Alzheimer's disease and other dementias, Clin. Interventions Aging, 2009, 367–377 CAS.
- E.-J. Yang, G.-S. Kim, M. Jun and K.-S. Song, Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells, Food Funct., 2014, 5, 1395–1402 RSC.
- Y. Wei, D. Liu, Y. Zheng, C. Hao, H. Li and W. Ouyang, Neuroprotective effects of kinetin against glutamate-induced oxidative cytotoxicity in HT22 cells: Involvement of Nrf2 and heme oxygenase-1, Neurotoxic. Res., 2018, 33, 725–737 CrossRef CAS PubMed.
- M. He, J. Liu, S. Cheng, Y. Xing and W. Z. Suo, Differentiation renders susceptibility to excitotoxicity in HT22 neurons, Neural Regener. Res., 2013, 8, 1297 CrossRef CAS.
- S. Penugonda, S. Mare, G. Goldstein, W. A. Banks and N. Ercal, Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12, Brain Res., 2005, 1056, 132–138 CrossRef CAS PubMed.
- M. A. Eshete and E. L. Molla, Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda District, Ethiopia, J. Ethnobiol. Ethnomed., 2021, 17, 1–18 CrossRef PubMed.
- J. Lim, G. Kim, C. Mo and M. S. Kim, Design and fabrication of a real-time measurement system for the capsaicinoid content of Korean red pepper (Capsicum annuum L.) powder by visible and near-infrared spectroscopy, Sensors, 2015, 15, 27420–27435 CrossRef CAS PubMed.
- L. M. Anaya-Esparza, Z. V.-d. l. Mora, O. Vázquez-Paulino, F. Ascencio and A. Villarruel-López, Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications, Molecules, 2021, 26, 5341 CrossRef CAS PubMed.
- H. Jahn, Memory loss in Alzheimer's disease, Dialogues Clin. Neurosci., 2013, 15, 445–454 CrossRef PubMed.
- N. S. Mohd Sairazi, K. Sirajudeen, M. A. Asari, M. Muzaimi, S. Mummedy and S. A. Sulaiman, Kainic acid-induced excitotoxicity experimental model: protective merits of natural products and plant extracts, Evidence-Based Complementary Altern. Med., 2015, 2015, 972623 CrossRef.
- M. Lévesque and M. Avoli, The kainic acid model of temporal lobe epilepsy, Neurosci. Biobehav. Rev., 2013, 37, 2887–2899 CrossRef.
- Z. Dong, H. Han, H. Li, Y. Bai, W. Wang, M. Tu, Y. Peng, L. Zhou, W. He and X. Wu, Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis, J. Clin. Invest., 2015, 125, 234–247 CrossRef.
- G. E. Villalpando-Rodriguez and S. B. Gibson, Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat, Oxid. Med. Cell. Longevity, 2021, 2021, 9912436 Search PubMed.
- S. A. Austin, A. V. R. Santhanam, L. V. d'Uscio and Z. S. Katusic, Regional heterogeneity of cerebral microvessels and brain susceptibility to oxidative stress, PLoS One, 2015, 10, e0144062 CrossRef.
- Í. M. S. Santos, A. d. R. Tomé, G. B. Saldanha, P. M. P. Ferreira, G. C. G. Militão and R. M. De Freitas, Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid, Oxid. Med. Cell. Longevity, 2009, 2, 214–221 CrossRef.
- X. Wang, Z. Wang, J. Cao, Y. Dong and Y. Chen, Ferroptosis mechanisms involved in hippocampal-related diseases, Int. J. Mol. Sci., 2021, 22, 9902 CrossRef CAS.
- C. A. Massaad and E. Klann, Reactive oxygen species in the regulation of synaptic plasticity and memory, Antioxid. Redox Signaling, 2011, 14, 2013–2054 CrossRef CAS PubMed.
- U. Fischer and K. Schulze-Osthoff, Apoptosis-based therapies and drug targets, Cell Death Differ., 2005, 12, 942–961 CrossRef CAS PubMed.
- F. D. Sajan, F. Martiniuk, D. L. Marcus, W. H. Frey, R. Hite, E. Z. Bordayo and M. L. Freedman, Apoptotic gene expression in Alzheimer's disease hippocampal tissue, Am. J. Alzheimer's Dis. Other Demen., 2007, 22, 319–328 CrossRef PubMed.
- Y. Zhang and B. R. Bhavnani, Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens, BMC Neurosci., 2006, 7, 1–22 CrossRef PubMed.
- M. D'amelio, V. Cavallucci and F. Cecconi, Neuronal caspase-3 signaling: not only cell death, Cell Death Differ., 2010, 17, 1104–1114 CrossRef PubMed.
- J. B. Wilke, M. Hindermann, A. Moussavi, U. J. Butt, R. Dadarwal, S. A. Berghoff, A. K. Sarcheshmeh, A. Ronnenberg, S. Zihsler and S. Arinrad, Inducing sterile pyramidal neuronal death in mice to model distinct aspects of gray matter encephalitis, Acta Neuropathol. Commun., 2021, 9, 121 CrossRef CAS PubMed.
- K. L. Brunson, M. Eghbal-Ahmadi, R. Bender, Y. Chen and T. Z. Baram, Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8856–8861 CrossRef CAS.
- Z. Li and M. Sheng, Caspases in synaptic plasticity, Mol. Brain, 2012, 5, 1–6 CrossRef PubMed.
- M.-H. Han, S. Jiao, J.-M. Jia, Y. Chen, C. Y. Chen, M. Gucek, S. P. Markey and Z. Li, The novel caspase-3 substrate Gap43 is involved in AMPA receptor endocytosis and long-term depression, Mol. Cell. Proteomics, 2013, 12, 3719–3731 CrossRef CAS.
- S. Snigdha, E. D. Smith, G. A. Prieto and C. W. Cotman, Caspase-3 activation as a bifurcation point between plasticity and cell death, Neurosci. Bull., 2012, 28, 14–24 CrossRef CAS PubMed.
- G. Leal, C. Bramham and C. Duarte, BDNF and hippocampal synaptic plasticity, Vitam. Horm., 2017, 104, 153–195 CAS.
- S. Heldt, L. Stanek, J. Chhatwal and K. Ressler, Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories, Mol. Psychiatry, 2007, 12, 656–670 CrossRef CAS.
- C. Liu, C. B. Chan and K. Ye, 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders, Transl. Neurodegener., 2016, 5, 1–9 CrossRef.
- E. Gravesteijn, R. P. Mensink and J. Plat, Effects of nutritional interventions on BDNF concentrations in humans: a systematic review, Nutr. Neurosci., 2022, 25, 1425–1436 CrossRef CAS.
- C. S. Rex, J. C. Lauterborn, C.-Y. Lin, E. A. Kramár, G. A. Rogers, C. M. Gall and G. Lynch, Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor, J. Neurophysiol., 2006, 96, 677–685 CrossRef CAS PubMed.
- M. D. Amaral and L. Pozzo-Miller, BDNF induces calcium elevations associated with I BDNF, a nonselective cationic current mediated by TRPC channels, J. Neurophysiol., 2007, 98, 2476–2482 CrossRef CAS.
- M. Alonso, J. H. Medina and L. Pozzo-Miller, ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons, Learn. Mem., 2004, 11, 172–178 CrossRef PubMed.
- H. Wang, H. Wang, H. Cheng and Z. Che, Ameliorating effect of luteolin on memory impairment in an Alzheimer's disease model, Mol. Med. Rep., 2016, 13, 4215–4220 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: Fingerprinting analysis of CSE and CPE. Capsiate, capsaicin, luteolin, and lutein were detected in CSE (A) and CPE (B) using HPLC at a wavelength of 222 nm, and their retention time and quantification are displayed (C). Fig. S2: CSE and CPE comparative effects, as well as the effects of CSE on BDNF expression. The CPE was assessed for cytotoxicity (A) and cell viability (B) using a WST-8 assay on glutamate-induced HT22 cells. The cytotoxicity (C) and cell viability (D) of both CSE and CPE were evaluated simultaneously. The protein expression of BDNF was determined by western blotting analysis (E) in the absence of glutamate-induced conditions. Signal intensities were analyzed in proportion to α-tubulin (F). #p < 0.05 and ##p < 0.01 compared with the vehicle-treated cells; **p < 0.01 compared with the glutamate-treated cells. See DOI: https://doi.org/10.1039/d3fo04501c |
| This journal is © The Royal Society of Chemistry 2024 |