Open Access Article
Open Access ArticleThe impact of heat-set milk protein gel textures modified by pH on circulating amino acid appearance and gastric function in healthy female adults: a randomised controlled trial†
Amber M.
Milan
 *abc,
Giselle G. A.
Menting
*abc,
Giselle G. A.
Menting
 d,
Matthew P. G.
Barnett
d,
Matthew P. G.
Barnett
 be,
Yutong
Liu
a,
Warren C.
McNabb
ce,
Nicole C.
Roy
cef,
Scott C.
Hutchings
be,
Yutong
Liu
a,
Warren C.
McNabb
ce,
Nicole C.
Roy
cef,
Scott C.
Hutchings
 b,
Tanyaradzwa
Mungure
bg,
Mike
Weeks
b,
Siqi
Li‡
b,
Tanyaradzwa
Mungure
bg,
Mike
Weeks
b,
Siqi
Li‡
 e,
Joanne
Hort
eh,
Stefan
Calder
ij,
Greg
O'Grady
ij and
Richard F.
Mithen
ace
e,
Joanne
Hort
eh,
Stefan
Calder
ij,
Greg
O'Grady
ij and
Richard F.
Mithen
ace
aThe Liggins Institute, The University of Auckland, Auckland, New Zealand. E-mail: a.milan@auckland.ac.nz; yutong.liu@auckland.ac.nz; r.mithen@auckland.ac.nz
bAgResearch Limited, Palmerston North, New Zealand. E-mail: matthew.barnett@agresearch.co.nz; scott.hutchings@agresearch.co.nz; mike.weeks@agresearch.co.nz
cThe High-Value Nutrition National Science Challenge, Auckland, New Zealand
dWageningen University, Wageningen, Netherlands. E-mail: giselle.m.nutrition@gmail.com
eThe Riddet Institute, Palmerston North, New Zealand. E-mail: W.McNabb@massey.ac.nz; s.li2@massey.ac.nz
fDepartment of Human Nutrition, The University of Otago, Otago, New Zealand. E-mail: nicole.roy@otago.ac.nz
gThe University of Melbourne, Melbourne, Australia. E-mail: ta.mungure@unimelb.edu.au
hFood Experience and Sensory Testing Lab, Massey University, Palmerston North, New Zealand. E-mail: j.hort@massey.ac.nz
iDepartment of Surgery, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand. E-mail: greg.ogrady@auckland.ac.nz; stefan.calder@auckland.ac.nz
jAuckland Bioengineering Institute, The University of Auckland, Auckland, New Zealand
First published on 9th May 2024
Abstract
Modification of dairy proteins during processing impacts structural assemblies, influencing textural and nutritional properties of dairy products, and release and availability of amino acids during digestion. By modifying only pH, acid heat-set bovine dairy gels with divergent textural properties were developed to alter protein digestion. In vitro assay confirmed faster digestion of protein from a firm gel (pH 5.65) versus a soft gel (pH 6.55). We hypothesised that firm gel (FIRM-G; pH 5.6) would result in greater indispensable amino acid (IAA) appearance in circulation over 5 h and corresponding differences in gastric myoelectrical activity relative to soft gel (SOFT-G; pH 6.2). In a randomised, single-blind cross-over trial, healthy females (n = 20) consumed 150 g of each gel; plasma amino acid appearance was assessed over 5 hours. Iso-nitrogenous, iso-caloric gels were prepared from identical mixtures of bovine milk and whey protein concentrates; providing 17.7 g (FIRM-G) and 18.9 g (SOFT-G) of protein per serving. Secondary outcomes included gastric myoelectrical activity measured by body surface gastric mapping, glycaemic, triglyceridaemic, and subjective appetite and digestive responses. Overall plasma IAA (area under the curve) did not differ between gels. However, plasma IAA concentrations were higher, and increased more rapidly over time after SOFT-G compared with FIRM-G (1455 ± 53 versus 1350 ± 62 μmol L−1 at 30 min, p = 0.024). Similarly, total, branched-chain and dispensable amino acids were higher at 30 min with SOFT-G than FIRM-G (total: 3939 ± 97 versus 3702 ± 127 μmol L−1, p = 0.014; branched-chain: 677 ± 30 versus 619 ± 34 μmol L−1, p = 0.047; dispensable: 2334 ± 53 versus 2210 ± 76 μmol L−1, p = 0.032). All other measured parameters were similar between gels. Peak postprandial aminoacidaemia was higher and faster following ingestion of SOFT-G. Customised plasma amino acid appearance from dairy is achievable by altering gel coagulum structure using pH during processing and may have minimal influence on related postprandial responses, with implications for targeting food design for optimal health. The Clinical Trial Registry number is ACTRN12622001418763 (https://www.anzctr.org.au) registered November 7, 2022.
1. Introduction
For millennia, liquid milk and processed dairy foods have remained dietary staples across many cultures. Dairy products, such as yoghurt and cheese, undergo dairy protein coagulation through a combination of conditions including heat, pH, or enzymatic modifications (e.g., bacterial culture alteration of pH), resulting in modified structures which impact digestion1,2 and circulating nutrient appearance in humans.2,3 These dairy gels, characterised by irreversible protein coagulation, differ from liquid dairy in textural and sensory properties, providing more diet versatility for nutrient delivery.Harder, denser dairy structures, such as cheese, generally take longer to be digested than softer structures2 due to the inaccessibility of the coagulated proteins for gastric hydrolysis, as shown in vitro.4,5In vivo, denser dairy structures that slow down gastric protein digestion, such as gels compared to liquids, also prolong the associated rise in circulating amino acids.6
Gastric protein digestion of dairy products processed under a wide range of conditions has been widely studied.1,2,7 Far fewer studies directly compare similar semi-solid dairy structures or gels,4,5,8–23 with only a subset comparing matched compositions,4,8–11,21–23 and limited evidence of digestive responses in humans.3,24,25 These studies demonstrate variable structure effects. Although some support slower gastric digestion with firmer structures,21,24 others indicate that textural variation alone, such as viscosity,10 firmness, or aeration,11 has little effect. Yet, matrix effects on pepsin accessibility, such as swelling,21 extent of mechanical processing relative to structure density,4,22 and contraction of protein structure under gastric conditions8,19,22,23 (influenced by pH8,19), determine gastric digestion rate.
Dairy structure's ability to influence gastric behaviour offers an opportunity to modify nutrient responses to target health benefits for specific populations.2 For instance, foods for diabetics2 could target slower nutrient digestion to induce greater satiety through delayed gastric emptying26 while reducing postprandial lipaemia.27 In contrast, athletes, or older adults, with greater protein requirements for anabolic stimulus, could benefit from structures which accelerate protein digestion and amino acid utilisation.28–30 For both insulin resistant31 and elderly32 populations, dairy gels show beneficial insulin31 and anabolic responses32 respectively, demonstrating the potential of tailored dairy products to optimise nutrient availability.
With this goal in mind, heat-set bovine dairy protein gels with divergent textural properties33 were developed by adjusting the pH at a key step during processing.34 In that study,34 a firm gel (pH 5.65) with a 90-fold higher storage modulus (G′ of 7200 Pa; i.e., the elastic behaviour when deformed) was compared to a soft gel (pH 6.55, G′ of 138 Pa) under dynamic in vitro gastric digestion conditions. Although the soft gel initially released proteins rapidly, it quickly formed a compact structure, delaying gastric digestion. In contrast, firm gel protein breakdown was continuous, with less protein remaining in gastric digesta from 2 h.34 Despite a softer texture, intragastric compaction and higher pH of the soft gel were greater determinates of overall protein digestion kinetics in vitro.
Given these in vitro differences, we hypothesised that similar gastric digestion dynamics would be observed in humans, such that soft gel may release more protein early in digestion, but the continuous breakdown of protein from the firm gel would result in overall greater circulating indispensable (essential) amino acids (IAA). We designed a randomised controlled trial to investigate the impact of pH conditions of heat-set dairy gels (firm and soft gel) on the delivery of amino acids into the peripheral circulation. Secondly, we explored the related impacts on gastric digestion activity and potential implications for appetite regulation and digestive comfort.
2. Materials and methods
2.1 Setting
The study was conducted from November 2022 to June 2023. All participant assessments were at the University of Auckland's Maurice and Phyllis Paykel Clinical Research Unit (CRU), The Liggins Institute.2.2. Ethics approval and trial registration
Ethics approval was obtained from the Northern B Health and Disability Ethics Committees (New Zealand, 2022 EXP 13438). The clinical trial was prospectively registered at https://www.anzctr.org.au on 7 November 2022 (Trial ID: ACTRN12622001418763). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and written informed consent was obtained from all subjects.2.3 Experimental design
This study was a single-blinded, cross-over randomised controlled trial to compare the impact of consumption of a single 150 g serving of either a firm bovine dairy gel (FIRM-G, pH 5.6) or a soft bovine dairy gel (SOFT-G, pH 6.2) in dairy-tolerant female individuals. The prescribed washout between interventions was a minimum of 3 days and a maximum of 28 days.The sequence of treatment arms was randomly generated by an independent statistician and subjects were allocated by an independent researcher through a password-protected database before the first dairy gel intervention. Researchers not involved in data collection were blinded to the identity and order of the intervention the participants received, and for the duration of data analysis. Participants were not blinded to the identity of gels and could identify the gels based on appearance and texture. No sensory masking of dairy gels was used.
The primary outcome of the study was the difference in pooled plasma IAA concentration incremental area under the curve (AUC) responses between FIRM-G and SOFT-G. Secondary outcomes were concentration differences in individual circulating amino acids, glucose, insulin, triglycerides, and calcium between the FIRM-G and the SOFT-G. Other secondary outcomes were the difference in gastric myoelectrical activity by body surface gastric mapping measures (BSGM) between FIRM-G and SOFT-G. Furthermore, the secondary outcomes also included differences in subjective perception, appetite, digestive responses between FIRM-G and SOFT-G.
2.4 Intervention
Acid heat-set dairy gels were designed to have equivalent nutritional composition (Table 1), with 12.6% protein. The FIRM-G was produced at pH 5.6 while the SOFT-G was produced at pH 6.2.| Component | FIRM-G | SOFT-G |
|---|---|---|
| a Results for aspartic acid and glutamic acid may include contributions of asparagine and glutamine, respectively, converted during hydrolysis. | ||
| Estimated | ||
| pH | 5.6 | 6.2 |
| Total energy, kJ | 512 | 512 |
| Fat, g (%) | 2.7 (1.8) | 2.7 (1.8) |
| Protein, g (%) | 18.9 (12.6) | 18.9 (12.6) |
| Carbohydrates, g (%) | 5.6 (3.7) | 5.6 (3.7) |
| Lactose, g (%) | 1.1 (0.7) | 1.1 (0.7) |
| Moisture, % | 80.3 | 80.3 |
| Ash, % | 1.0 | 1.0 |
| Measured | ||
| Total energy, kJ | 506 | 506 |
| Fat, g (%) | 2.6 (1.7) | 2.6 (1.7) |
| Saturated fat, g (%) | 1.7 (1.1) | 1.8 (1.2) |
| Protein, g (%) | 17.7 (11.8) | 18.9 (12.6) |
| Carbohydrates, g (%) | 6.2 (4.1) | 5.0 (3.3) |
| Lactose, g (%) | 0.8 (0.5) | 0.8 (0.5) |
| Total sugars, g (%) | 4.4 (2.9) | 4.1 (2.7) |
| Sodium, mg | 264 | 264 |
| Moisture, % | 80.8 | 80.8 |
| Ash, % | 1.0 | 1.0 |
| Amino acid, g | 19.13 | 19.82 |
| Indispensable amino acids | ||
| Leucine | 1.83 | 1.89 |
| Lysine | 1.41 | 1.42 |
| Valine | 1.13 | 1.19 |
| Isoleucine | 0.95 | 0.99 |
| Phenylalanine | 0.87 | 0.89 |
| Threonine | 0.90 | 0.94 |
| Histidine | 0.47 | 0.49 |
| Methionine | 0.49 | 0.50 |
| Tryptophan | 0.29 | 0.28 |
| Dispensable amino acids | ||
| Glutamic acida | 3.90 | 4.05 |
| Proline | 1.74 | 1.83 |
| Aspartic acida | 1.49 | 1.54 |
| Serine | 0.72 | 0.72 |
| Tyrosine | 0.98 | 1.05 |
| Alanine | 0.86 | 0.90 |
| Arginine | 0.59 | 0.61 |
| Glycine | 0.33 | 0.35 |
| Cystine | 0.17 | 0.17 |
The methodology used to prepare dairy gels was adapted from Ji, Lee, and Anema (2016),33 who showed through rheological and microstructural characterisation that modifying the pH of heat-set dairy gels can transform soft, spoonable gels (pH 6.25–6.6) to firm, cuttable gels (pH 5.25–6.0). The gels were adapted from methods used to produce a firm (pH 5.65) and soft (pH 6.55) gel for in vitro digestion analysis, as reported by Li et al. with the following physiochemical properties: firm gel: G′ 7200 Pa, phase angle 16.2° and soft gel: G′ 138 Pa, phase angle 12.3°.
Both dairy gels were produced in the Ministry of Primary Industries accredited FoodPilot (Riddet Complex, Massey University, Palmerston North, New Zealand). Gels were composed of milk protein concentrate (80% protein, 135.2 g kg−1), whey protein concentrate (80% protein, 19.7 g kg−1), water (794.4 g kg−1), glucose powder (30.3 g kg−1), anhydrous milk fat (15.1 g kg−1), chocolate powder (3.8 g kg−1), and liquid chocolate flavour (1.6 g kg−1). The casein to whey protein ratio was 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30%. Milk protein concentrate, whey protein concentrate, and anhydrous milk fat were supplied by Fonterra Co-operative Group (Auckland, New Zealand). Glucose powder was purchased from Davis Trading Company Ltd (Auckland, New Zealand). Chocolate powder and chocolate flavour were purchased from Pacific Flavours and Ingredients Ltd (Auckland, New Zealand).
30%. Milk protein concentrate, whey protein concentrate, and anhydrous milk fat were supplied by Fonterra Co-operative Group (Auckland, New Zealand). Glucose powder was purchased from Davis Trading Company Ltd (Auckland, New Zealand). Chocolate powder and chocolate flavour were purchased from Pacific Flavours and Ingredients Ltd (Auckland, New Zealand).
Reconstitution of the dry ingredients was done in reverse osmosis water warmed up to 50 °C using a Silverson ® Laboratory mixer (Silverson, Auckland, New Zealand). The ingredients were mixed well to disperse and left for 1 h at 20 °C to fully hydrate before being homogenised with pre-warmed anhydrous milk fat (approximately 50 °C) at 15 MPa and 5 MPa (first & second stage, respectively). The homogeniser was flushed with hot water (∼55 °C) prior to use. The pressure drop over the first stage was 10 MPa and the pressure drop over the second stage was 5 MPa. The gels were formed by heat treatment following different extents of acidification (gels were not formed until the heating step was complete). The homogenised milk was acidified using food-grade glucono delta-lactone, purchased from Thermo Fisher Scientific Inc. (Auckland, New Zealand). The mixture was portioned into 500 mL screw top containers, glucono delta-lactone was added, and the mixture left at 60 °C for 40 min in a water bath to completely acidify. The FIRM-G pH after acidification was 5.6, whereas for the SOFT-G, the pH was 6.2. After acidification, the mixture was retorted in 450 mL cans at 110 °C for 20 min to complete gelation. The gels were then cooled to 20 °C over 120 min and then chilled to 4 °C over at least 12 h. Prior to serving, both dairy gels were kept at 7 °C and served in a bowl with a spoon.
Following acidification of the FIRM-G, there was a small yield loss of coagulated protein due to the lowered pH that could not be transferred into the cans for retorting. This resulted in small discrepancies in absolute measured protein and carbohydrate contents between the final products, with 1.2 g higher protein, and 1.2 g lower carbohydrate in the SOFT-G relative to the FIRM-G.
The gels were tested out to 21-day storage for microbial safety as verified by an accredited laboratory, detailed below. Rheological stability was also confirmed up to 21 days.34 Six lots of FIRM-G and SOFT-G batches were manufactured between February and June 2023 using the same ingredients. The median receiving each batch was 5 participants (interquartile range = 4–6).
2.5 Inclusion and exclusion criteria
Participants were females, 18–40 years of age, with a body mass index (BMI) between 18–30 kg m−2. The population was limited to females to reduce sex variability in circulating amino acid concentrations and responses35 and hormonal gastric emptying impacts.36Participants were ineligible if they had known bovine milk allergies. Known significant gastrointestinal disorders (e.g., celiac disease, inflammatory bowel disease), or current medication use expected to interfere with normal digestive or metabolic processes were also exclusion criteria. Participants were also ineligible if they were pregnant or breastfeeding, had known chronic disease such as diabetes, cardiovascular, cancer, renal failure, previous gastrointestinal surgery other than cholecystectomy or appendectomy, neurological conditions such as multiple sclerosis, spinal cord injury, or stroke, or self-reported alcohol intake exceeding 28 alcohol units per week.
2.6 Study procedures
Following informed consent, participants received a screening questionnaire asking about medical history to confirm eligibility. Participant ethnicity data was collected using the standard New Zealand census categories. The night prior to each clinical visit, subjects were instructed to abstain from alcohol, digestive medications, and vigorous exercise, and were provided with a standardised dinner after which they were to remain fasted from 9 pm onwards.Study participants arrived at the CRU fasted. Upon arrival, a venous cannula was inserted into an arm vein to collect fasting blood samples. In addition, the appetite and digestive comfort visual analogue scale questionnaires were completed for the fasted state. The BSGM was positioned afterwards on the participant's stomach. Participants were provided with one of the dairy gels and first consumed three spoons of the gel whereafter they responded to questionnaires on appeal, sensory characteristics, and were asked to indicate which gel they thought they were tasting (perceived identity). The remainder of the gel was eaten within 10 min. Participants were provided with 100 mL of water to rinse their mouths without swallowing. Following dairy gel ingestion, assessments were carried out at regular intervals over 5 h (blood samples, BSGM scans, and visual analogue scale questionnaires), as detailed below. Participants were hydrated with intravenous saline after drawing each blood sample.
2.7 Analysis methodology
Plasma free amino acid concentrations were measured using ultra high-performance liquid chromatography (UHPLC) to assess 23 amino acids as described previously35 with the following variation. A variable wavelength detector (Dionex, set at 280 nm) was added to the UHPLC system to quantify the concentration of tryptophan, (which autofluoresces and hence cannot be measured using fluorescence detection).
The concentration of plasma glucose (at all timepoints), triglycerides and serum calcium (hourly timepoints) and plasma high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and total cholesterol (fasting only) were measured using a Roche Cobas c311 Autoanalyser (Roche Diagnostics, Mannheim, Germany) by enzymatic colorimetric assay. Plasma insulin concentration (at all timepoints) was measured using Cobas E601 Autoanalyser (Roche Diagnostics).
Appetite, desire for specific food type, and digestive symptoms were recorded before, during and after dairy gel consumption, aligned with blood sampling intervals at 30 min intervals for the first 90 min, then hourly starting at 2 h for 5 h. Appeal and sensory scores of the gels were recorded at a single timepoint during consumption, as was perceived identity of the gel (i.e., SOFT-G or FIRM-G) to assess participant perception of unmasked gels.
Appetite was assessed as “How hungry do you feel?/How full do you feel?/How satisfied do you feel?/How much do you think you can eat now?” and anchored as (0 mm) “I not hungry/I am not full at all/I am completely empty/nothing at all” and (100 mm) “I am as hungry as I have ever been/I am totally full/I cannot eat another bite/a large amount”.
Desire for specific food type was assessed as “Would you like to eat something…sweet?/…salty?/…savoury?/…fatty?” and anchored as (0 mm) “Yes, very much” and (100 mm) “No, not at all”.
Digestive symptoms were anchored as (0 mm) “no symptom” and (100 mm) “the most severe symptom imaginable”. Symptoms included “abdominal cramps/abdominal distension/abdominal rumbling/belching/bloating/diarrhoea/faecal urgency/flatulence/gastric reflux/nausea/vomiting/digestive comfort”.
Liking was assessed as visual appeal, smell, taste, mouthfeel, aftertaste anchored as (0 mm) “bad” and (100 mm) “good”; palatability, and overall liking were anchored as (0 mm) “definitely disliked” and (100 mm) “definitely liked”. Sensory attributes were assessed as “sweetness/saltiness/fattiness/savouriness/sourness/bitterness/thickness/smoothness/mouth drying/melt in the mouth/flavour intensity” anchored as (0 mm) “not at all” and (100 mm) “extremely”.
Food safety was tested at 7, 14, and 21 days in triplicate by Eurofins ELS Ltd (Wellington, New Zealand) using International Organization for Standardization (ISO) methods 6611:2004, 16649-2, 7937 (modified), 6888-1:2021, 4833-1, and testing for the presence of Salmonella and Listeria spp. were tested using BACGene Salmonella spp. and BACGene Listeria Multiplex kit respectively. All samples were below the limit of quantification (LOQ) for ISO methods; neither Salmonella nor Listeria spp. were detected in any samples.
Outliers (amino acid data) were identified using the Q3 + 3IQR method and imputed by multiple imputation as the mean of five iterations. Values lower than the limit of quantification were imputed at 50% of the lowest measured value, where >50% of samples were detected for a participant. No other data sets were imputed.
Derived amino acid variables were calculated as the sum of the concentration of branched chain amino acids (BCAA), IAA, dispensable amino acids (DAA), and total amino acids (TAA). The incremental AUC was calculated using the trapezoidal method and corrected for baseline concentrations.
Categorical variables were analysed using a Chi-squared test. Continuous variables were analysed using Student's paired t-test or linear mixed models, with gel, time and their interaction as fixed effects, and subject as a random effect. All analyses were adjusted for multiple comparisons using a Sidak–Holm correction. Alpha was set at 0.05.
A sample size of 19 was determined based on an 80% power (β) to detect at the 5% significance level (α) an effect size of 45% difference in IAA AUC between gels. Using previously collected IAA AUC in healthy subjects, consuming 500 mL of pasteurised milk, an expected AUC was 3.7 ± 2.3 × 105 μmol min L−1.43,44 An effect size of 45%, smaller than observed difference between pasteurised and UHT milk (55%), is equivalent to the relative IAA AUC of 5.3 × 105 μmol min L−1. As the exact expected effect size was unknown, a sample size of 20 participants was set in alignment with previous studies assessing similar secondary outcomes. Recruitment targets estimated a 10% drop out rate.
3. Results
3.1 Subject characteristics
Twenty subjects completed the study. Of the 565 volunteers screened for eligibility, 25 were eligible (Fig. 1); of those excluded, the main reasons were lost contact (n = 210) or recruitment completion (n = 196). Of 24 subjects randomised, four withdrew; 20 subjects were included in the per protocol analysis.Participants’ clinical characteristics were within healthy ranges (Table 2). Participants identified as Caucasian (54%), Asian (25%), Māori (8%), Samoan (4%) and other (8%).
| Attribute | Value |
|---|---|
| a Values presented as mean ± SEM or count (percentage) as indicated across both assessments. n = 20 for all measures except blood pressure (n = 18) and ethnicity: participants could identify with more than one ethnicity group (n = 19; n = 24 ethnicity reports). BMI, body mass index; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol. | |
| Age, years | 28.9 ± 1.3 |
| Ethnicity | |
| Caucasian, n (%) | 13 (54) |
| Asian, n (%) | 6 (25) |
| Chinese, n (%) | 1 (4) |
| Indian, n (%) | 2 (8) |
| Other Asian, n (%) | 3 (13) |
| Māori, n (%) | 2 (8) |
| Samoan, n (%) | 1 (4) |
| Other, n (%) | 2 (8) |
| BMI, kg m−2 | 22.1 ± 0.6 |
| Waist circumference, cm | 70.3 ± 1.3 |
| Glucose, mmol L−1 | 5.0 ± 0.1 |
| Insulin, μU mL−1 | 7.2 ± 0.6 |
| HDL-C, mmol L−1 | 1.55 ± 0.05 |
| LDL-C, mmol L−1 | 2.39 ± 0.30 |
| Total cholesterol, mmol L−1 | 4.31 ± 0.10 |
| Triglycerides, mmol L−1 | 0.87 ± 0.04 |
| Calcium, mmol L−1 | 2.34 ± 0.01 |
| Blood pressure, mmHg | |
| Systolic | 109.6 ± 1.8 |
| Diastolic | 68.5 ± 2.2 |
Although participants were recruited based on self-described dairy tolerance, two participants self-reported as lactose intolerant. However, those participants did not report any adverse events after consuming the dairy gels.
Of the 15 participants that reported a regular menstrual cycle, the majority were in the follicular phase (day 0–14) when consuming SOFT-G (n = 9) and FIRM-G (n = 8); however, only four participants were in the follicular phase on both occasions.
3.2 Compliance and adverse events
There were three protocol deviations where participants could not complete the full 150 g serve of gel. One only consumed 77 g of SOFT-G and withdrew from the study so was excluded from analysis. Two completed 94 and 128 g of FIRM-G, respectively, and data were included in analysis.The quantity (compliance) of gel consumed by participants included in analysis was 150 ± 0 g (100%) and 146 ± 13 g (97%) for SOFT-G and FIRM-G, respectively.
There were no adverse events.
3.3 Participant perception of gels
Subjects were more likely to correctly identify SOFT-G (H0: equal frequency; χ2p = 0.025; n = 15 identified as ‘soft’) but unable to identify FIRM-G (H0: equal frequency; χ2p = 1.000; n = 10 identified as ‘firm’).Overall liking was 45 ± 6%. All other gel appeal attributes were rated between 40 and 50% aside from smell and aftertaste (67 ± 5 and 54 ± 5%, respectively). Subjects had no sensory preference for either gel (ESI Table 1†). FIRM-G was perceived as thicker (p = 0.017) and less smooth (p = 0.043) than SOFT-G; gels were not differentiated on any other measured attribute (p > 0.05 each, respectively). Gels were scored with low perception of all other flavours and attributes (<50%).
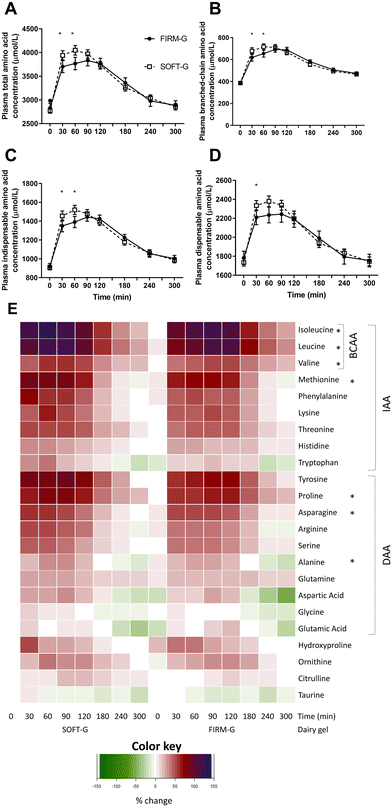
3.4 Plasma amino acids
The AUC of plasma TAA and IAA were not different between the FIRM-G and SOFT-G (TAA: p = 0.118; IAA: p = 0.430; Table 3). In contrast, the FIRM-G resulted in a smaller DAA response (AUC, p = 0.042; Table 3). TAA, IAA and DAA all responded differently between gels (gel × time interaction p = 0.010, p = 0.011 and p = 0.014, respectively; Fig. 2), showing lower concentrations at 30 and 60 min for FIRM-G relative to SOFT-G. Plasma IAA, DAA, and TAA concentrations differed in their peak concentration duration between gels, with similar patterns. Although a peak amino acid concentration was achieved by 30 min for both gels, FIRM-G sustained this peak longer, until 240 min (p = 0.806 and p = 0.005 between 30 and 180 min, and 30 and 240 min, respectively for IAA). SOFT-G peak amino acid concentrations were only sustained until 120 min (p = 1.000 and p = 0.007 between 30 min and 120 and 180 min, respectively for IAA; Fig. 2).| Amino acida | FIRM-G | SOFT-G | p-Valueb |
|---|---|---|---|
| a Values presented as means ± SEM in μmol min L−1; n = 20. b Significance analysed by Student's t-test. c TAA: total amino acids: all measured amino acids; BCAA: branched chain amino acids: isoleucine, leucine, valine; IAA: indispensable (essential) amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine; DAA: dispensable amino acids: alanine, arginine, asparagine, aspartic acid, citrulline, glutamic acid, glutamine, glycine, proline, serine, tyrosine. | |||
| Alanine | 616 ± 2104 | 8037 ± 1772 | 0.160 |
| Arginine | 4120 ± 1208 | 6522 ± 709 | 0.099 |
| Asparagine | 4105 ± 494 | 4670 ± 305 | 0.283 |
| Aspartic acid | 82 ± 68 | 52 ± 98 | 0.812 |
| Citrulline | 667 ± 254 | 983 ± 237 | 0.212 |
| Glutamic acid | 1793 ± 509 | −435 ± 787 | 0.024* |
| Glutamine | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 ± 4137 443 ± 4137 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 ± 3060 560 ± 3060 |
0.081 |
| Glycine | −3397 ± 1423 | −104 ± 1188 | 0.070 |
| Histidine | 3443 ± 775 | 4429 ± 2576 | 0.268 |
| Hydroxyproline | −31 ± 107 | 307 ± 79 | 0.007** |
| Isoleucine | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 783 ± 739 783 ± 739 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 ± 679 578 ± 679 |
0.752 |
| Leucine | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 ± 1234 999 ± 1234 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 ± 1176 959 ± 1176 |
0.393 |
| Lysine | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 935 ± 1961 935 ± 1961 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 ± 1352 308 ± 1352 |
0.538 |
| Methionine | 2576 ± 255 | 3207 ± 164 | 0.037* |
| Ornithine | 3253 ± 558 | 2854 ± 483 | 0.489 |
| Phenylalanine | 4787 ± 659 | 6216 ± 677 | 0.082 |
| Proline | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674 ± 2151 674 ± 2151 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 ± 1484 653 ± 1484 |
0.075 |
| Serine | 7696 ± 809 | 8065 ± 723 | 0.705 |
| Taurine | −1653 ± 624 | −1081 ± 646 | 0.513 |
| Threonine | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 ± 1173 065 ± 1173 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 ± 872 556 ± 872 |
0.174 |
| Tryptophan | 2361 ± 937 | 1468 ± 1112 | 0.568 |
| Tyrosine | 8591 ± 942 | 9938 ± 846 | 0.110 |
| Valine | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383 ± 1672 383 ± 1672 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 ± 1485 736 ± 1485 |
0.817 |
| Pooled | |||
| TAA | 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 ± 34 581 ± 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 043 |
398![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 957 ± 27 957 ± 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 158 |
0.118 |
| BCAA | 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 ± 7099 330 ± 7099 |
120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548 ± 6485 548 ± 6485 |
0.724 |
| IAA | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 ± 6815 332 ± 6815 |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 459 ± 6058 459 ± 6058 |
0.430 |
| DAA | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 723 ± 10 723 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 957 ± 33 957 ± 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448 448 |
0.042* |
The FIRM-G resulted in lower postprandial concentrations relative to the SOFT-G for the following amino acids; valine and proline (p < 0.05 each), and methionine (p < 0.01), all of which occurred between 30 and 60 min (ESI Fig. 1 and 2†). Of these three amino acids, only methionine showed a significantly lower AUC in the FIRM-G compared to the SOFT-G (p = 0.037, Table 3). The FIRM-G had a higher AUC for glutamic acid (p = 0.024, Table 3), whereas it had a lower AUC for hydroxyproline (p = 0.007, Table 3), compared to the SOFT-G. Although isoleucine, leucine, alanine, and asparagine also differed between the gels over time (p = 0.013, p = 0.011, p = 0.048, and p = 0.041, respectively), concentrations were not different at any specific timepoint (ESI Fig. 1 and 2†) nor were there differences in AUC (Table 3).
3.5 Clinical biochemistry
The total triglyceride, glucose, insulin, and calcium responses following gel ingestion did not differ between gels (gel × time interaction p = 0.888, p = 0.185, p = 0.150 and p = 0.198; ESI Fig. 3†).3.6 Symptom and appetite scores
No digestive symptom or appetite scores differed between gels following ingestion (ESI Table 2†). The FIRM-G was rated thicker (p = 0.017) and less smooth (p = 0.043) compared to the SOFT-G.Appetite scores changed over time (p < 0.001, each, respectively; ESI Table 2†), as did thoughts of fatty, salty, and savoury food, increasing gradually over the postprandial period (p < 0.01, each, respectively). However, thoughts of sweet food did not change from fasting (p = 0.207).
3.7 Body surface gastric mapping
BSGM data quality was successfully achieved in all cases; however, the test recording for one participant's visit was lost due to a technical problem, so the final paired dataset for the BSGM analysis was n = 19.None of the measured metrics (amplitude, Gastric Alimetry Rhythm Index, Principal Gastric Frequency) differed between gels (Table 4), either over time, or derived pharmacokinetic parameters of AUC, Cmax, and Tmax (ESI Table 3†).
| Measurea | Time | FIRM-G | SOFT-G | P valueb |
|---|---|---|---|---|
| a Data presented as mean ± SEM; n = 19. Amplitude and gastric alimetry rhythm index are adjusted for body mass index (BMI). b Significance between gels over time analysed by linear mixed model; between gels overall analysed by Student's paired t test. | ||||
| BMI-adjusted amplitude (μV) | Pre-meal | 45.4 ± 4.1 | 45.4 ± 4.9 | 0.539 |
| 0–1 h | 46.7 ± 4.5 | 48.9 ± 4.8 | ||
| 1–2 h | 45.6 ± 5.0 | 45.9 ± 3.7 | ||
| 2–3 h | 41.2 ± 4.4 | 37.4 ± 3.2 | ||
| 3–4 h | 38.0 ± 3.6 | 31.1 ± 2.0 | ||
| 4–5 h | 35.4 ± 3.8 | 29.2 ± 2.2 | ||
| Overall | 42.1 ± 3.7 | 39.9 ± 2.7 | 0.598 | |
Fed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fasted amplitude ratio fasted amplitude ratio |
— | 1.25 ± 0.08 | 1.36 ± 0.14 | 0.508 |
| Gastric alimetry rhythm index | Pre-meal | 0.42 ± 0.04 | 0.39 ± 0.05 | 0.634 |
| 0–1 h | 0.56 ± 0.04 | 0.53 ± 0.05 | ||
| 1–2 h | 0.61 ± 0.04 | 0.63 ± 0.04 | ||
| 2–3 h | 0.57 ± 0.04 | 0.55 ± 0.04 | ||
| 3–4 h | 0.56 ± 0.04 | 0.52 ± 0.04 | ||
| 4–5 h | 0.52 ± 0.06 | 0.45 ± 0.05 | ||
| overall | 0.56 ± 0.03 | 0.53 ± 0.04 | 0.367 | |
| Principal gastric frequency (cpm) | pre-meal | 2.87 ± 0.06 | 2.93 ± 0.07 | 0.627 |
| 0–1 h | 2.96 ± 0.06 | 3.02 ± 0.06 | ||
| 1–2 h | 2.91 ± 0.06 | 2.93 ± 0.05 | ||
| 2–3 h | 2.89 ± 0.05 | 2.88 ± 0.05 | ||
| 3–4 h | 2.91 ± 0.05 | 2.93 ± 0.06 | ||
| 4–5 h | 2.92 ± 0.06 | 2.95 ± 0.05 | ||
| overall | 2.91 ± 0.05 | 2.94 ± 0.05 | 0.291 | |
Amplitude, gastric alimetry rhythm index, principal gastric frequency all changed postprandially (p < 0.001 main time effect, each, respectively). Amplitude decreased relative to pre-meal by 3–4 h and 4–5 h (p = 0.002 and p < 0.001, respectively; Fig. 3). Principal Gastric Frequency peaked at 0–1 h and then dipped at 2–3 h (p = 0.003 pre-meal to 0–1 h, p = 0.001 0–1 h to 2–3 h). Postprandial Gastric Alimetry Rhythm Index was higher than baseline, returning to pre-meal by 4–5 h (p < 0.05 pre-meal to 0–1 h, 1–2 h, 2–3 h, each, respectively; p = 0.153 pre-meal to 4–5 h).
4. Discussion
Contrary to our hypothesis that FIRM-G would elicit overall greater IAA appearance in plasma (AUC) than SOFT-G, the overall response was the same between the two heat-set gels. However, the gel pH conditions, resulting in different textures, did alter plasma IAA response patterns. SOFT-G resulted in earlier peak plasma IAA, and greater BCAA concentrations at 30–60 min than FIRM-G. Despite differing aminoacidaemia, dairy gels with different textures did not differ in glycaemic, triglyceridaemic, calcaemia, subjective appetite and digestive responses and gastric myoelectrical activity. Overall, the pH during dairy gel coagulation did not influence overall plasma TAA and IAA appearance. However, a softer gel (higher pH) supported an earlier higher peak concentration after consumption, which has implications for postprandial amino acid utilisation.Despite no difference in overall plasma IAA and TAA appearance over 5 hours, SOFT-G elicited an earlier and larger peak concentration in IAA and BCAA relative to FIRM-G. These differences were reflective of higher plasma concentrations of methionine (including AUC), valine and proline after SOFT-G consumption, and earlier and higher peak concentrations of leucine, isoleucine, proline and serine relative to FIRM-G. Although the rise in circulating aminoacidaemia was sustained with FIRM-G consumption, this reflected a lower peak rather than greater response relative to SOFT-G; after 60 min aminoacidaemia was equal between gels.
Direct comparisons of semi-solid dairy product gastric protein digestion in vivo are rare.8,12,24,25 With respect to gradients of macrostructural differences, more solid dairy products are generally more resistant to gastric disintegration in vitro.2 For instance, gastric emptying is delayed with yoghurt relative to milk45 or aminoacidaemia is faster after yoghurt relative to cheese ingestion.3 Yet, although a yoghurt and cheese comparison found differences in peak aminoacidaemia, overall IAA (AUC) was uninfluenced by structure.3 Recently, whey protein gel gastric digestion was investigated in healthy humans, concluding that a firmer gel slows gastric emptying relative to a softer gel.24 In minipigs, gel pH was a more important determinant of plasma IAA concentration 8; indeed, differences in pH of semi-solid dairy products (i.e., yoghurt, cheese) is a confounding variable in most,3,8,12 but not all,24,25 comparisons. These observations further highlight the complex influence of dairy protein composition and intragastric dynamics on in vivo physiological responses.
A strength of this study was alignment to previous in vitro digestion of similar gels,34 supporting the hypothesis that the FIRM-G would result in a higher plasma IAA response (AUC). Although amino acid appearance in peripheral circulation did not differ as expected, these dynamics still likely reflect in vitro gastric behaviour predictions.34 For instance, rapid rather than cumulative in vitro gastric protein hydrolysis predicted plasma response timing. In the in vitro study, firm gel formed a softer and looser gastric chyme during digestion that was more rapidly hydrolysed and emptied.34 In contrast, soft gel showed pronounced intragastric coagulation, incorporating free proteins into a firm and compact gel chyme inaccessible to hydrolysis. The firm gel solid chyme retained less ingested protein by 120 min of digestion (21 versus 48% for soft gel) resulting in higher crude protein in the digesta emptied from the stomach between 40 to 200 min.34In vivo, a similar effect of a more firm, dense, and pepsin-resistant curd, as observed with pasteurised relative to UHT milk,46,47 reduced postprandial plasma IAA AUC,43,44 supporting the current hypothesis.
The in vitro finding that soft gel released protein faster than firm gel during the initial stage of digestion34 could explain earlier and higher peak plasma IAA and BCAA following SOFT-G ingestion in the current study. Higher proline after SOFT-G, indicating early casein digestion,48 would also support this. However, the significantly decelerated protein emptying and digestion after intragastric compaction of soft gel found in vitro was not reflected here. Nevertheless, the similar plasma IAA between gels after 60 min indicated that SOFT-G digestion rate also decelerated, but less than in vitro. This indicates different digestion dynamics between in vitro and in vivo and a less significant intragastric compaction behaviour of the SOFT-G in the present study.
There are inherent limitations of in vivo predictions from in vitro models,49 including simulation of physiological processes (e.g., small intestinal protein digestion and absorption,1,7,49 metabolic control of amino acids50) and individual variations (i.e., gastric pH, pepsin activity,51 gastric sieving, strength of gastric motility). Here, differences in gel production relative to the in vitro study may have influenced in vitro-in vivo alignment. The in vitro study gels were produced at slightly different pH, higher protein (15 versus 12.6%), energy (559 versus 506 kJ), and volume (200 versus 150 g), with no fat or added sugar. Fat causes weaker milk protein coagulation in the stomach,52 whereas meal protein,53 energy,54 volume,55 and fat56 content can all influence human gastric emptying and hormonal appetite regulation. The in vitro study used a higher soft gel pH (pH 6.55 versus 6.2 in vivo), larger portion, and higher protein, all contributing to maintaining relatively high intragastric pH during early gastric digestion, critical for intragastric compaction due to pepsin's stronger milk clotting activity (relative to proteolytic activity) that peaks at pH 6.0.8,34,57
In vitro dynamics may explain specific amino acid profiles differences between SOFT-G and FIRM-G. In vitro, soft gel was depleted of β-lactoglobulin by 120 min.34 This corresponds with rapid digestion of whey proteins,58 known to accentuate and quicken elevated plasma leucine concentrations28 partially due to proportionally more BCAA than casein. This mechanism aligns with the greater plasma BCAA following SOFT-G ingestion. Rapid aminoacidaemia from leucine59 or whey protein ingestion29 is associated with an enhanced skeletal muscle protein synthesis response.29 Hence, SOFT-G could be a suitable structured dairy format for elderly populations for whom anabolic stimulation requires adequate postprandial protein availability29 and where peak postprandial aminoacidaemia may already be delayed.35 The longer aminoacidaemia elevation with FIRM-G mirrors aminoacidaemia with casein, which results in better postprandial utilisation, inhibition of protein breakdown,60 and may be a more important determinant of dietary nitrogen utilisation and anabolic potential than high circulating peaks.48 It is unclear whether rapid aminoacidaemia29 or potential for sustained postprandial utilisation60 of SOFT-G or FIRM-G, respectively, translates into meaningful clinical benefits for sarcopenia prevention.
In this study, gastric myoelectrical activity, measured using gold-standard BSGM techniques, showed no difference in gastric function between gels, despite differing nutrient delivery. The BSGM measurement is validated to provide more robust assessments of gastric function than traditional electrogastrography, yielding meal response curves that are hypothesized to align with gastric emptying in healthy subjects.38 Although BSGM is well validated to detect pathological variations in gastric activity, meal variation or differences in gastric digestion in healthy individuals remain a novel application. Additional parameters, further to amplitude and frequency, may be required to detect meal effects on gastric myoelectrical activity; in an ex vivo rat perfusion model, these parameters were not influenced by gastric content rheological properties but gastric contraction speed and direction were.61
Satiety can be influenced by altered gastric digestion and nutrient release2 and remains a target attribute for foods designed for metabolic health62 or ageing.63 Subjective appetite scores responded as expected but were similar between gels. Others have similarly found no impact of dairy structure on self-reported appetite.64 As high-protein foods, the satiating capacity of these gels may be important. Although no adverse digestive effects were reported, the 150 g serving size (similar to other single-serve dairy products) could not be completed by three participants (one SOFT-G, two FIRM-G). While unclear whether excessive satiation was the cause, participants did not enjoy or dislike either gel disproportionately. Desire for sweet food was not suppressed by either gel, which may warrant exploration in relation to product flavour development or implications within habitual diets.
This study had limitations with respect to protein quantity. The FIRM- G provided slightly lower protein (i.e., 1.2 g lower), and compliance was poorer (i.e., 4 g lower intake). However, given that >5 g of ingested protein is required to elevate postprandial plasma amino acids,65 this discrepancy is unlikely to negate the greater peak aminoacidaemia with SOFT-G. SOFT-G also provided slightly higher carbohydrate content (i.e., 1.2 g higher). The insulinotropic effects of dairy foods impact postprandial aminoacidaemia,66 but glycaemic and triacylglyceridaemic responses to gels remained similar, suggesting a negligible impact of content differences on the study findings.
Participants could detect inherent textural differences33,34 between gels, noting that FIRM-G was thicker and less smooth than SOFT-G; this lack of blinding could have biased patient-reported outcomes. This study included only females capable of consuming dairy products. However, the negligible lactose load was below tolerable quantities for those with intolerance,67 and gels were tolerated well, suggesting generalisability to those with intolerance. The findings are likely generalisable to males, despite potential differences in fasting plasma amino acids,35 gastric emptying36 and myoelectrical activity.40 Menstrual stage was not controlled and generally differed between visits. Gastric emptying may be delayed during either follicular68 or luteal69 phases, or may be unaffected by menstrual cycle.36,69,70 Extrapolation of these study findings to populations including those with insulin resistance or the elderly requires confirmation due to influences of altered metabolic responses on gastrointestinal27,71 and postprandial nutrient dynamics35,72–75 and related health outcomes.
5. Conclusion
Heat-set dairy gels produced at different pH impacted circulating plasma amino acid profiles following ingestion. The rapid gastric protein digestion from SOFT-G (pH 6.2) observed in vitro, corresponded with a transient rapid rise in circulating IAA concentrations in humans. In contrast, FIRM-G (pH 5.6) sustained elevation of circulating amino acids, similarly reflective of in vitro gastric protein digestion dynamics. However, our predictions that aminoacidaemia would be greater with FIRM-G were rejected, highlighting the complexity of predicting in vivo curd dynamics and amino acid release rates from in vitro gastric digestion systems.Nonetheless, this study demonstrates the utility of understanding in vitro gastric dynamics to predict aspects of physiological responses to novel food formulations. Novel structures or processing conditions may be capable of bespoke nutrient delivery, without meaningful impacts on related physiological responses including gastric myoelectrical activity, digestive comfort, or appetite regulation. This study demonstrates that modifying pH during processing is a viable tool to influence textural properties and aminoacidaemia, supporting exploration of the relationship between structure and long-term health outcomes.
Author contributions
Conceptualisation, A.M.M., M.P.G.B., W.C.M., N.C.R., T.M., and R.F.M.; methodology, A.M.M., S.C., and G.O.; formal analysis, G.G.A.M. and A.M.M.; investigation, G.G.A.M., Y.L., A.M.M., M.P.G.B., and R.F.M.; software, S.C, and G.O.; resources, S.C.H., T.M., M.W., A.M.M., and G.O.; data curation, G.G.A.M., Y.L., and A.M.M.; writing—original draft preparation, G.G.A.M., S.L., and A.M.M.; writing—review and editing, M.P.G.B., Y.L., W.C.M., N.C.R., S.C.H., T.M., M.W., S.C., and G.O.; visualisation, G.G.A.M, A.M.M., S.C.; supervision, W.C.M., N.C.R., M.W., and R.F.M.; project administration, A.M.M., M.P.G.B., W.C.M., N.C.R., and R.F.M.; funding acquisition, W.C.M. and N.C.R. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
All authors report no conflicts of interest.Acknowledgements
This study was funded by the New Zealand Ministry of Business, Innovation, and Employment (MBIE) through the New Zealand Milks Mean More Endeavour Programme, grant number MAUX1803. The authors would like to thank our study participants, Charley Hurst (AgResearch), Janene Biggs, India Wallace, Gen Johnston, Juno Collins, and Dr Julia Cree (University of Auckland) for clinical support, Jing Rong and Chris Keven for technical support (University of Auckland), and Gary Radford and Dorine Le Guen (Massey University) for gel production support. We thank Dr David Everett and Dr Alistair Carr (AgResearch) for valuable critical appraisal of the manuscript.References
- A. Fardet, D. Dupont, L. E. Rioux and S. L. Turgeon, Influence of food structure on dairy protein, lipid and calcium bioavailability: A narrative review of evidence, Crit. Rev. Food Sci. Nutr., 2019, 59, 1987–2010 CrossRef CAS PubMed.
- A. I. Mulet-Cabero, A. R. Mackie, A. Brodkorb and P. J. Wilde, Dairy structures and physiological responses: a matter of gastric digestion, Crit. Rev. Food Sci. Nutr., 2020, 60, 3737–3752 CrossRef CAS PubMed.
- A. M. H. Horstman, R. A. Ganzevles, U. Kudla, A. F. M. Kardinaal, J. J. G. C. van den Borne and T. Huppertz, Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix, Int. Dairy J., 2021, 113, 104890 CrossRef CAS.
- Q. Guo, A. Ye, M. Lad, M. Ferrua, D. Dalgleish and H. Singh, Disintegration kinetics of food gels during gastric digestion and its role on gastric emptying: An in vitro analysis, Food Funct., 2015, 6, 756–764 RSC.
- D. H. Tran Do and F. Kong, Texture changes and protein hydrolysis in different cheeses under simulated gastric environment, LWT–Food Sci. Technol., 2018, 93, 197–203 CrossRef.
- F. Barbé, O. Ménard, Y. Le Gouar, C. Buffière, M.-H. Famelart, B. Laroche, S. Le Feunteun, D. Dupont and D. Rémond, The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids, Food Chem., 2013, 136, 1203–1212 CrossRef PubMed.
- G. A. A. van Lieshout, T. T. Lambers, M. C. E. Bragt and K. A. Hettinga, How processing may affect milk protein digestion and overall physiological outcomes: A systematic review, Crit. Rev. Food Sci. Nutr., 2020, 60, 2422–2445 CrossRef CAS PubMed.
- F. Barbé, O. Ménard, Y. Le Gouar, C. Buffière, M.-H. Famelart, B. Laroche, S. Le Feunteun, D. Rémond and D. Dupont, Acid and rennet gels exhibit strong differences in the kinetics of milk protein digestion and amino acid bioavailability, Food Chem., 2014, 143, 1–8 CrossRef PubMed.
- Q. Guo, A. Ye, M. Lad, D. Dalgleish and H. Singh, Behaviour of whey protein emulsion gel during oral and gastric digestion: Effect of droplet size, Soft Matter, 2014, 10, 4173–4183 RSC.
- O. Ménard, M.-H. Famelart, A. Deglaire, Y. Le Gouar, S. Guérin, C.-H. Malbert, D. Dupont, O. Ménard, M.-H. Famelart, A. Deglaire, Y. Le Gouar, S. Guérin, C.-H. Malbert and D. Dupont, Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition, Nutrients, 2018, 10, 1308 CrossRef PubMed.
- L. Lorieau, A. Halabi, A. Ligneul, E. Hazart, D. Dupont and J. Floury, Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion, Food Hydrocolloids, 2018, 82, 399–411 CrossRef CAS.
- F. Barbé, S. Le Feunteun, D. Rémond, O. Ménard, J. Jardin, G. Henry, B. Laroche and D. Dupont, Tracking the in vivo release of bioactive peptides in the gut during digestion: Mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrix fed mini-pigs, Food Res. Int., 2014, 63, 147–156 CrossRef.
- X. Fang, L.-E. Rioux, S. Labrie and S. L. Turgeon, Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion, Int. Dairy J., 2016, 56, 169–178 CrossRef CAS.
- X. Fang, L.-E. Rioux, S. Labrie and S. L. Turgeon, Disintegration and nutrients release from cheese with different textural properties during in vitro digestion, Food Res. Int., 2016, 88, 276–283 CrossRef CAS.
- L. Rinaldi, S. F. Gauthier, M. Britten and S. L. Turgeon, In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes, LWT–Food Sci. Technol., 2014, 57, 99–105 CrossRef CAS.
- T. M. Qureshi, G. E. Vegarud, R. K. Abrahamsen and S. Skeie, Angiotensin I-converting enzyme-inhibitory activity of the Norwegian autochthonous cheeses Gamalost and Norvegia after in vitro human gastrointestinal digestion, J. Dairy Sci., 2013, 96, 838–853 CrossRef CAS PubMed.
- S. Lamothe, M.-M. Corbeil, S. L. Turgeon and M. Britten, Influence of cheese matrix on lipid digestion in a simulated gastro-intestinal environment, Food Funct., 2012, 3, 724–731 RSC.
- L.-E. Rioux and S. L. Turgeon, The ratio of casein to whey protein impacts yogurt digestion in vitro, Food Dig., 2012, 3, 25–35 CrossRef CAS.
- H. J. Qazi, A. Ye, A. Acevedo-Fani and H. Singh, In vitro digestion of curcumin-nanoemulsion-enriched dairy protein matrices: Impact of the type of gel structure on the bioaccessibility of curcumin, Food Hydrocolloids, 2021, 117, 106692 CrossRef CAS.
- R. Deng, A. E. M. Janssen, F. J. Vergeldt, H. Van As, C. de Graaf, M. Mars and P. A. M. Smeets, Exploring in vitro gastric digestion of whey protein by time-domain nuclear magnetic resonance and magnetic resonance imaging, Food Hydrocolloids, 2020, 99, 105348 CrossRef CAS.
- R. Deng, M. Mars, R. G. M. Van Der Sman, P. A. M. Smeets and A. E. M. Janssen, The importance of swelling for in vitro gastric digestion of whey protein gels, Food Chem., 2020, 330, 127182 CrossRef CAS PubMed.
- M. Bayrak, J. Mata, J. K. Raynes, M. Greaves, J. White, C. E. Conn, J. Floury and A. Logan, Investigating casein gel structure during gastric digestion using ultra-small and small-angle neutron scattering, J. Colloid Interface Sci., 2021, 594, 561–574 CrossRef CAS PubMed.
- J. Floury, T. Bianchi, J. Thévenot, D. Dupont, F. Jamme, E. Lutton, M. Panouillé, F. Boué and S. Le Feunteun, Exploring the breakdown of dairy protein gels during in vitro gastric digestion using time-lapse synchrotron deep-UV fluorescence microscopy, Food Chem., 2018, 239, 898–910 CrossRef CAS PubMed.
- R. Deng, M. Mars, A. E. M. Janssen and P. A. M. Smeets, Gastric digestion of whey protein gels: A randomized cross-over trial with the use of MRI, Food Hydrocolloids, 2023, 141, 108689 CrossRef CAS.
- Q. Guo, A. Ye, M. Lad, D. Dalgleish and H. Singh, The breakdown properties of heat-set whey protein emulsion gels in the human mouth, Food Hydrocolloids, 2013, 33, 215–224 CrossRef CAS.
- L. Marciani, M. Wickham, G. Singh, D. Bush, B. Pick, E. Cox, A. Fillery-Travis, R. Faulks, C. Marsden, P. A. Gowland and R. C. Spiller, Enhancement of intragastric acid stability of a fat emulsion meal delays gastric emptying and increases cholecystokinin release and gallbladder contraction, Am. J. Physiol.: Gastrointest. Liver Physiol., 2007, 292(6), G1607–G1613 CrossRef CAS PubMed.
- C. K. Rayner, M. Samsom, K. L. Jones and M. Horowitz, Relationships of upper gastrointestinal motor and sensory function with glycemic control, Diabetes Care, 2001, 24, 371–381 CrossRef CAS PubMed.
- D. W. West, N. A. Burd, V. G. Coffey, S. K. Baker, L. M. Burke, J. A. Hawley, D. R. Moore, T. Stellingwerff and S. M. Phillips, Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise, Am. J. Clin. Nutr., 2011, 94, 795–803 CrossRef CAS PubMed.
- B. Pennings, Y. Boirie, J. M. G. Senden, A. P. Gijsen, H. Kuipers and L. J. van Loon, Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men, Am. J. Clin. Nutr., 2011, 93, 997–1005 CrossRef CAS PubMed.
- M. Dangin, C. Guillet, C. Garcia-Rodenas, P. Gachon, C. Bouteloup-Demange, K. Reiffers-Magnani, J. Fauquant, O. Ballèvre and B. Beaufrère, The rate of protein digestion affects protein gain differently during aging in humans, J. Physiol., 2003, 549, 635–644 CrossRef CAS PubMed.
- G. Pasin and K. B. Comerford, Dairy Foods and Dairy Proteins in the Management of Type 2 Diabetes: A Systematic Review of the Clinical Evidence, Adv. Nutr., 2015, 6, 245–259 CrossRef CAS PubMed.
- W. J. Hermans, C. J. Fuchs, J. Nyakayiru, F. K. Hendriks, L. H. Houben, J. M. Senden, L. J. van Loon and L. B. Verdijk, Acute Quark Ingestion Increases Muscle Protein Synthesis Rates at Rest with a Further Increase after Exercise in Young and Older Adult Males in a Parallel-Group Intervention Trial, J. Nutr., 2023, 153, 66–75 CrossRef CAS PubMed.
- Y. Ran Ji, S. K. Lee and S. G. Anema, Characterisation of heat-set milk protein gels, Int. Dairy J., 2016, 54, 10–20 CrossRef.
- S. Li, T. E. Mungure, A. Ye, S. M. Loveday, J. Cui, A. Ellis, M. Weeks and H. Singh, Intragastric restructuring dictates the digestive kinetics of heat-set milk protein gels of contrasting textures, Food Res. Int., 2024 Search PubMed (submitted).
- A. M. Milan, R. F. D'Souza, S. Pundir, C. A. Pileggi, M. P. G. Barnett, J. F. Markworth, D. Cameron-Smith and C. Mitchell, Older adults have delayed amino acid absorption after a high protein mixed breakfast meal, J. Nutr., Health Aging, 2015, 19, 839–845 CrossRef CAS PubMed.
- A. M. Caballero-Plasencia, M. Valenzuela-Barranco, J. L. Martín-Ruiz, J. M. Herrerías-Gutiérrez and J. M. Esteban-Carretero, Are there changes in gastric emptying during the menstrual cycle?, Scand. J. Gastroenterol., 1999, 34, 772–776 CrossRef CAS PubMed.
- A. Flint, A. Raben, J. E. Blundell and A. Astrup, Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies, Int. J. Obes., 2000, 24, 38–48 CrossRef CAS PubMed.
- A. A. Gharibans, T. C. L. Hayes, D. A. Carson, S. Calder, C. Varghese, P. Du, Y. Yarmut, S. Waite, C. Keane, J. S. T. Woodhead, C. N. Andrews and G. O’Grady, A novel scalable electrode array and system for non–invasively assessing gastric function using flexible electronics, Neurogastroenterol. Motil., 2023, 35(2), e14418 CrossRef CAS PubMed.
- G. O’Grady, C. Varghese, G. Schamberg, S. Calder, P. Du, W. Xu, J. Tack, C. Daker, H. Mousa, T. L. Abell, H. P. Parkman, V. Ho, L. A. Bradshaw, A. Hobson, C. N. Andrews and A. A. Gharibans, Principles and clinical methods of body surface gastric mapping: Technical review, Neurogastroenterol. Motil., 2023, 35(10), e14556 CrossRef PubMed.
- C. Varghese, G. Schamberg, S. Calder, S. Waite, D. Carson, D. Foong, W. J. Wang, V. Ho, J. Woodhead, C. Daker, W. Xu, P. Du, T. L. Abell, H. P. Parkman, J. Tack, C. N. Andrews, G. O'Grady and A. A. Gharibans, Normative Values for Body Surface Gastric Mapping Evaluations of Gastric Motility Using Gastric Alimetry: Spectral Analysis, Am. J. Gastroenterol., 2023, 118, 1047–1057 CrossRef CAS PubMed.
- S. Calder, G. Schamberg, C. Varghese, S. Waite, G. Sebaratnam, J. S. T. Woodhead, P. Du, C. N. Andrews, G. O’Grady and A. A. Gharibans, An automated artifact detection and rejection system for body surface gastric mapping, Neurogastroenterol. Motil., 2022, 34(11), e14421 CrossRef PubMed.
- R Development Core Team, R: A language and environment for statistical computing, ISBN: 3-900051-07-0, 2023.
- A. M. Milan, M. P. G. Barnett, W. C. McNabb, N. C. Roy, S. Coutinho, C. L. Hoad, L. Marciani, S. Nivins, H. Sharif, T. R. Angeli-Gordon, P. Du, A. A. Gharibans, G. O'Grady, P. Sharma, A. Shrestha and R. F. Mithen, Heat Treatment of Bovine Milk Impacts Gastric Emptying and Nutrient Appearance, in NSNZ 2022, MDPI, Basel Switzerland, 2023, vol. 18, p. 8 Search PubMed.
- A. M. Milan, M. P. G. Barnett, W. C. McNabb, N. C. Roy, S. Coutinho, C. L. Hoad, L. Marciani, S. Nivins, H. Sharif, S. Calder, P. Du, A. A. Gharibans, G. O’Grady, K. Fraser, D. Bernstein, S. Rosanowski, P. Sharma, A. Shrestha and R. F. Mithen, The impact of heat treatment of bovine milk on gastric emptying and nutrient appearance in peripheral circulation in healthy females: a randomized controlled trial comparing pasteurized and ultra-high temperature milk, Am. J. Clin. Nutr., 2024 DOI:10.1016/j.ajcnut.2024.03.002.
- S. Mahé, P. Marteau, J.-F. Huneau, F. Thuillier and D. Tomé, Intestinal nitrogen and electrolyte movements following fermented milk ingestion in man, Br. J. Nutr., 1994, 71, 169–180 CrossRef PubMed.
- A. Ye, W. Liu, J. Cui, X. Kong, D. Roy, Y. Kong, J. Han and H. Singh, Coagulation behaviour of milk under gastric digestion: Effect of pasteurization and ultra-high temperature treatment, Food Chem., 2019, 286, 216–225 CrossRef CAS PubMed.
- N. G. Ahlborn, C. A. Montoya, S. M. Hodgkinson, A. Dave, A. Ye, L. M. Samuelsson, N. C. Roy and W. C. McNabb, Heat treatment and homogenization of bovine milk loosened gastric curd structure and increased gastric emptying in growing pigs, Food Hydrocolloids, 2023, 137, 108380 CrossRef CAS.
- M. Lacroix, C. Bos, J. Léonil, G. Airinei, C. Luengo, S. Daré, R. Benamouzig, H. Fouillet, J. Fauquant, D. Tomé and C. Gaudichon, Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement, Am. J. Clin. Nutr., 2006, 84, 1070–1079 CrossRef CAS PubMed.
- T. Bohn, F. Carriere, L. Day, A. Deglaire, L. Egger, D. Freitas, M. Golding, S. Le Feunteun, A. Macierzanka, O. Menard, B. Miralles, A. Moscovici, R. Portmann, I. Recio, D. Rémond, V. Santé-Lhoutelier, T. J. Wooster, U. Lesmes, A. R. Mackie and D. Dupont, Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models?, Crit. Rev. Food Sci. Nutr., 2018, 58, 2239–2261 CrossRef CAS PubMed.
- B. Stoll and D. G. Burrin, Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers, J. Anim. Sci., 2006, 84, E60–E72 CrossRef PubMed.
- E. K. Ulleberg, I. Comi, H. Holm, E. B. Herud, M. Jacobsen and G. E. Vegarud, Human gastrointestinal juices intended for use in in vitro digestion models, Food Dig., 2011, 2, 52–61 CrossRef CAS PubMed.
- A. I. Mulet-Cabero, A. Torcello-Gómez, S. Saha, A. R. Mackie, P. J. Wilde and A. Brodkorb, Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption, Food Chem., 2020, 319, 126514 CrossRef CAS PubMed.
- A. T. Hutchison, D. Piscitelli, M. Horowitz, K. L. Jones, P. M. Clifton, S. Standfield, T. Hausken, C. Feinle-Bisset and N. D. Luscombe-Marsh, Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men, Am. J. Clin. Nutr., 2015, 102, 1574–1584 CrossRef CAS PubMed.
- J. A. L. Calbet and D. A. MacLean, Role of caloric content on gastric emptying in humans, J. Physiol., 1997, 498, 553–559 CrossRef CAS PubMed.
- J. N. Hunt and I. Macdonald, The influence of volume on gastric emptying, J. Physiol., 1954, 126, 459–474 CrossRef CAS PubMed.
- D. Gentilcore, R. Chaikomin, K. L. Jones, A. Russo, C. Feinle-Bisset, J. M. Wishart, C. K. Rayner and M. Horowitz, Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes, J. Clin. Endocrinol. Metab., 2006, 91, 2062–2067 CrossRef CAS PubMed.
- M. Yang, A. Ye, Z. Yang, D. W. Everett, E. P. Gilbert and H. Singh, Kinetics of pepsin-induced hydrolysis and the coagulation of milk proteins, J. Dairy Sci., 2022, 105, 990–1003 CrossRef CAS PubMed.
- S. Mahé, N. Roos, R. Benamouzig, L. Davin, C. Luengo, L. Gagnon, N. Gaussergès, J. Rautureau and D. Tomé, Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein, Am. J. Clin. Nutr., 1996, 63, 546–552 CrossRef PubMed.
- T. A. Churchward-Venne, N. A. Burd, C. J. Mitchell, D. W. D. West, A. Philp, G. R. Marcotte, S. K. Baker, K. Baar and S. M. Phillips, Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men, J. Physiol., 2012, 590, 2751–2765 CrossRef CAS.
- M. Dangin, Y. Boirie, C. Garcia-Rodenas, P. Gachon, J. Fauquant, P. Callier, O. Ballèvre and B. Beaufrère, The digestion rate of protein is an independent regulating factor of postprandial protein retention, Am. J. Physiol.: Endocrinol. Metab., 2001, 280, E340–E348 CrossRef CAS PubMed.
- R. G. Lentle, P. W. M. Janssen, K. Goh, P. Chambers and C. Hulls, Quantification of the effects of the volume and viscosity of gastric contents on antral and fundic activity in the rat stomach maintained ex vivo, Dig. Dis. Sci., 2010, 55, 3349–3360 CrossRef PubMed.
- T. T. Hansen, S. V. Andersen, A. Astrup, J. Blundell and A. Sjödin, Is reducing appetite beneficial for body weight management in the context of overweight and obesity? A systematic review and meta–analysis from clinical trials assessing body weight management after exposure to satiety enhancing and/or hunger reducing pro, Obes. Rev., 2019, 20, 983–997 CrossRef PubMed.
- L. M. Donini, C. Savina and C. Cannella, Eating Habits and Appetite Control in the Elderly: The Anorexia of Aging, Int. Psychogeriatr., 2003, 15, 73–87 CrossRef PubMed.
- K. M. Sanggaard, J. J. Holst, J. F. Rehfeld, B. Sandström, A. Raben and T. Tholstrup, Different effects of whole milk and a fermented milk with the same fat and lactose content on gastric emptying and postprandial lipaemia, but not on glycaemic response and appetite, Br. J. Nutr., 2004, 92, 447–459 CrossRef CAS PubMed.
- D. R. Moore, M. J. Robinson, J. L. Fry, J. E. Tang, E. I. Glover, S. B. Wilkinson, T. Prior, M. A. Tarnopolsky and S. M. Phillips, Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men, Am. J. Clin. Nutr., 2009, 89, 161–168 CrossRef CAS PubMed.
- M. Nilsson, M. Stenberg, A. H. Frid, J. J. Holst and I. M. E. Björck, Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins, Am. J. Clin. Nutr., 2004, 80, 1246–1253 CrossRef CAS PubMed.
- S. R. Hertzler, B.-C. L. Huynh and D. A. Savaiano, How Much Lactose is Low Lactose?, J. Am. Diet. Assoc., 1996, 96, 243–246 CrossRef CAS PubMed.
- I. M. Brennan, K. L. Feltrin, N. S. Nair, T. Hausken, T. J. Little, D. Gentilcore, J. M. Wishart, K. L. Jones, M. Horowitz and C. Feinle-Bisset, Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women, Am. J. Physiol.: Gastrointest. Liver Physiol., 2009, 297, G602–G610 CrossRef CAS PubMed.
- R. C. Gill, P. D. Murphy, H. R. Hooper, K. L. Bowes and Y. J. Kingma, Effect of the menstrual cycle on gastric emptying, Digestion, 1987, 36, 168–174 CAS.
- M. Horowitz, G. J. Maddern, B. E. Chatterton, P. J. Collins, O. M. Petrucco, R. Seamark and D. J. C. Shearman, The normal menstrual cycle has no effect on gastric emptying, Br. J. Obstet. Gynaecol., 1985, 92, 743–746 CrossRef CAS PubMed.
- C. K. Rayner and M. Horowitz, Physiology of the ageing gut, Curr. Opin. Clin. Nutr. Metab. Care, 2013, 16, 33–38 CrossRef PubMed.
- A. M. Milan, A. Nuora, S. Pundir, C. A. Pileggi, J. F. Markworth, K. M. Linderborg and D. Cameron-Smith, Older adults have an altered chylomicron response to a high-fat meal, Br. J. Nutr., 2016, 115, 791–799 CrossRef CAS PubMed.
- P. Sharma, S. M. Han, N. Gillies, E. B. Thorstensen, M. Goy, M. P. G. Barnett, N. C. Roy, D. Cameron-Smith and A. M. Milan, Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults, Nutrients, 2020, 12, 3529 CrossRef CAS PubMed.
- B. van Ommen, J. van der Greef, J. M. Ordovas and H. Daniel, Phenotypic flexibility as key factor in the human nutrition and health relationship, Genes Nutr., 2014, 423 CrossRef PubMed.
- J. E. Galgani, C. Moro and E. Ravussin, Metabolic flexibility and insulin resistance, Am. J. Physiol.: Endocrinol. Metab., 2008, 295, E1009–E1017 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04474b |
| ‡ Current Address: Fonterra Research and Development Centre, Dairy Farm Road, Palmerston North, New Zealand. |
| This journal is © The Royal Society of Chemistry 2024 |