Open Access Article
Open Access ArticleFunctional features of the exopolysaccharide extracts produced by Lactiplantibacillus strains isolated from table olives†
Elio
López-García
 *a,
Ana
Marín-Gordillo
b,
Marina
Sánchez-Hidalgo
*a,
Ana
Marín-Gordillo
b,
Marina
Sánchez-Hidalgo
 c,
Javier
Ávila-Román
c,
Verónica
Romero-Gil
d,
Alejandra
Bermúdez-Oria
a,
Antonio
Benítez-Cabello
a,
Antonio
Garrido-Fernández
a,
Francisco
Rodríguez-Gómez
a and
Francisco Noé
Arroyo-López
a
c,
Javier
Ávila-Román
c,
Verónica
Romero-Gil
d,
Alejandra
Bermúdez-Oria
a,
Antonio
Benítez-Cabello
a,
Antonio
Garrido-Fernández
a,
Francisco
Rodríguez-Gómez
a and
Francisco Noé
Arroyo-López
a
aInstituto de la Grasa (CSIC), Carretera de Utrera Km 1. Campus Universitario Pablo de Olavide. Building 46, 41013, Seville, Spain. E-mail: elopez@ig.csic.es
bTechnological Applications for Improvement of the Quality and Safety in Foods. R&D Division, Avda. Diego Martín Barrio 10. Second Floor, 41013, Seville, Spain
cDepartment of Pharmacology, Faculty of Pharmacy. University of Seville, 41012, Seville, Spain
dDepartment of Food Science and Technology. University of Cordoba, Carretera Madrid-Cádiz Km 396A. Darwin Building, 14071, Cordoba, Spain
First published on 24th January 2024
Abstract
This study evaluates the functional characteristics of the exopolysaccharide (EPS) extracts produced by various strains of Lactiplantibacillus pentosus (LPG1, 119, 13B4, and Lp13) and Lactiplantibacillus plantarum (Lp15) isolated from table olives. None of the EPS crude extracts showed cytotoxicity when administered to THP-1 human macrophage cells at dosages ranging from 6.25 to 50 μg mL−1. Many exhibited anti-inflammatory properties (reduction of pro-inflammatory cytokines TNF-α and IL-6 production) and antioxidant activity (reduction of ROS%) when macrophages were stimulated with Escherichia coli lipopolysaccharide. Notably, the EPS extract produced by the L. pentosus LPG1 strain had the best results corroborated by western blot immune analysis for differential expression of COX-2, Nrf-2, and HO-1 proteins, with the most significant antioxidant and anti-inflammatory response observed at a dosage of 50 μg mL−1. Chemical analysis revealed that the EPS extract produced by this strain contains a heteropolymer composed of mannose (35.45%), glucose (32.99%), arabinose (17.93%), xylose (7.48%), galactose (4.03%), rhamnose (1.34%), and fucose (0.77%). Finally, we conducted response surface methodology to model the EPS extract production by L. pentosus LPG1 considering pH (3.48–8.52), temperature (16.59–33.41 °C) and salt concentration (0.03–8.77% NaCl) as independent variables. The model identified linear effects of salt and pH and quadratic effects of salt as significant terms. The maximum EPS extract production (566 mg L−1) in a synthetic culture medium (MRS) was achieved at pH 7.5, salt 7.0%, and a temperature of 20 °C. These findings suggest the potential for novel applications for the EPS produced by L. pentosus LPG1 as nutraceutical candidates for use in human diets.
1. Introduction
Exopolysaccharides (EPSs) are non-toxic, natural, and biodegradable biopolymers with high molecular weight produced by microorganisms. They are released into the surrounding environment and adhere to bacterial cells, forming a capsule. EPSs are formed by linear or branched long chains of repeating saccharide units or their derivatives.1 Based on their biosynthesis mechanisms and chemical composition, EPSs can be categorised into two distinct groups: homopolysaccharides (containing only one type of monosaccharide) and heteropolysaccharides (including at least two different types of monosaccharides in their composition).2 EPSs can be used as gelling or flocculant agents with commercial applications in the food and pharmaceutical sectors.3Lactobacilli are well-known for their ability to form EPS, and their final metabolites are typically recognised as safe, thanks to the “food-grade” status of lactic acid bacteria (LAB). EPSs produced by these microorganisms play a crucial role in developing fermented foods, enhancing their rheological and gustatory properties. Additionally, EPSs derived from these microorganisms can create functional foods that benefit consumers. Numerous studies have demonstrated that EPSs from certain LAB strains can contribute to the prevention of human diseases. These EPSs exhibit various health-promoting properties, including antioxidant, antiviral, antiulcer, anticancer, immunomodulatory, bifidogenic, and cholesterol-lowering features.1,3
Many species of Lactobacilli, including Lactiplantibacillus pentosus and Lactiplantibacillus plantarum, have been shown to produce EPS.4 These two microorganisms are particularly significant within the LAB species as they play a crucial role in governing the fermentations of table olives.5 Table olives are a prominent fermented vegetable with an annual worldwide production of 3 million tons, especially relevant in the Mediterranean Basin.6 EPSs in bacteria cells defend against environmental stresses, facilitating adhesion/colonisation and stabilising the biofilms on the olive epidermis. Dominguez-Manzano et al. (2012) proved that certain Lactiplantibacillus strains can grow on olive epidermis, forming biofilms in a population exceeding 10 million CFU g−1, with EPS enveloping microbial cells.7
However, there is limited research on producing EPSs by microorganisms isolated from table olive fermentations and on the applications of purified EPSs obtained from table olive fermentations that are inoculated with LAB starters. Sánchez et al. (2006) studied the EPSs produced by L. pentosus LPS26, isolated from directly brined green olives.8 They also investigated the cultural conditions that influence EPS production. In a separate study, Zhu et al. (2018) explored the potential applications of isolated EPS crude extracts obtained directly from industrial table olive fermentations in preventing and treating pig diarrhoea caused by enterotoxigenic Escherichia coli.9 The same researchers further examined the functional properties of EPSs isolated from pilot plant table olive fermentations, which were inoculated with various L. pentosus and yeast strains.10 Their findings indicated that EPSs produced during olive fermentation could disrupt the attachment of opportunist pathogens to animal intestinal epithelial cells, underscoring their intriguing properties.
This study evaluates the cell cytotoxicity and functional features of the EPS crude extract produced in a synthetic and well-standardised culture medium by various Lactiplantibacillus strains isolated from table olives. Subsequently, we modelled the production of the EPS extract with the most favourable functional properties, considering environmental variables (such as pH, temperature, and salt) that typically play a pivotal role in table olive fermentations.
2. Materials and methods
2.1. Microorganisms assayed
Four L. pentosus (LPG1, 119, 13B4, and Lp13) and one L. plantarum (Lp15) strains were assayed in the present study. All of them were previously isolated from table olive fermentations and identified by molecular methods.11,12 Before experiments, one colony of each strain was transferred onto 100 mL of Man-Rogosa and Sharpe (MRS, Becton & Dickinson Company, Sparks, USA) broth medium and incubated for 18 h at 37 °C without agitation.2.2. Production, extraction, and purification of the EPS crude extract
For all strains assayed, 200 mL of MRS broth was used as the culture medium for EPS production. The bacterial inoculum (1%, w/v) was added to the synthetic medium and incubated at 37 °C for 72 h under static conditions. After the incubation, the bacterial culture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 4 °C, and the culture supernatant was carefully separated from the cell pellet. Then, the extraction of EPS crude extract from the supernatant was carried out using the methodology described by Sánchez et al. (2006) with slight modifications.8 Briefly, the supernatant of each strain was treated with 10% (w/v) trichloroacetic acid (TCA) and incubated at 4 °C for 2 h under gentle agitation, and then centrifuged at 10
000 rpm for 30 min at 4 °C, and the culture supernatant was carefully separated from the cell pellet. Then, the extraction of EPS crude extract from the supernatant was carried out using the methodology described by Sánchez et al. (2006) with slight modifications.8 Briefly, the supernatant of each strain was treated with 10% (w/v) trichloroacetic acid (TCA) and incubated at 4 °C for 2 h under gentle agitation, and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C for protein removal. The supernatant was recovered by adding 2 mL of cold absolute ethanol for every 1 mL of supernatant, followed by an overnight incubation at 4 °C. After this, the precipitated EPS extracts were recovered by centrifugation at 10
000 rpm for 15 min at 4 °C for protein removal. The supernatant was recovered by adding 2 mL of cold absolute ethanol for every 1 mL of supernatant, followed by an overnight incubation at 4 °C. After this, the precipitated EPS extracts were recovered by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C, resuspended in ultrapure water and dialysed in Slide-A-Lyzer™ dialysis cassettes of 10 kDa molecular-mass cut-off with 12 mL of capacity (Thermo Fisher Scientific Inc., Madrid, Spain) against 4 L of distilled water at 4 °C for 2 days with three water changes per day. EPS extract was frozen, lyophilised, weighed, and stored at room temperature (25 °C) until subsequent tests.
000 rpm for 15 min at 4 °C, resuspended in ultrapure water and dialysed in Slide-A-Lyzer™ dialysis cassettes of 10 kDa molecular-mass cut-off with 12 mL of capacity (Thermo Fisher Scientific Inc., Madrid, Spain) against 4 L of distilled water at 4 °C for 2 days with three water changes per day. EPS extract was frozen, lyophilised, weighed, and stored at room temperature (25 °C) until subsequent tests.
2.3. Cell culture
The human monocytic leukaemia THP-1 cell line (ATCC® Number TIB-202) was used in the present study for functional assays. It was grown in RPMI-1640 medium (GIBCO®, Thermo Fisher Scientific, Ireland) containing 10% heat-inactivated fetal bovine serum, 100 μg mL−1 streptomycin, and 100 U mL−1 penicillin. Cells were grown in a humidified atmosphere at 37 °C containing 5% CO2. To obtain human macrophages, THP-1 cells were seeded at a density of 104 cells per well into 96-well plates (100 μL per well) in the presence of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich®, St Louis, MO, USA) obtaining a final concentration of 8 nM, and incubated in a humidified atmosphere (5% CO2) for 72 h at 37 °C.2.4. Cell viability assay
In vitro cytotoxicity of the EPS crude extracts was quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Calbiochem®, Germany) dye uptake assay using macrophages obtained from transformed THP-1 cells.13 The medium was removed after the transformation period. Then, cells were washed twice with phosphate saline buffer (PBS, 4 °C) and incubated for 24 h with different concentrations of the EPS crude extracts (0, 6.25, 12.5, 25, 50, and 100 μg mL−1), which were freshly prepared in culture medium.Controls were incubated in a fresh medium containing dimethyl sulfoxide (DMSO, up to 1% v/v), which did not affect cell viability. Subsequently, the cells were washed with PBS before adding 100 μL of 0.25 mg mL−1 MTT solution into each well and incubated for 4 h. DMSO and 0.1 M glycine buffer (pH 10.5) were used to dissolve the formazan crystals. Afterwards, the absorbance was measured at 550 nm using a Multiskan EX microplate reader (Labsystems, Thermo Scientific, USA).
2.5. Antioxidant activity
The intracellular production of Reactive Oxygen Species (ROS) was detected using the 2′,7′-dichlorodihydrofluorescein diacetate DCFDA assay kit (Abcam®, Cambridge, UK). 104 cells per well were seeded into a 96-well black plate in the presence of 8 nM of phorbol 12-myristate 13-acetate (PMA). The medium was removed after transformation, and cells were washed twice with ice-cold PBS. Pretreatment of cells with the bacterial EPS extracts (6.25, 25.0, or 50.0 μg mL−1) and dexamethasone (Dex) as an internal control (0.39 μg mL−1) was performed for 1 h. Subsequently, the intracellular ROS production was stimulated by adding Escherichia coli lipopolysaccharide (LPS) (1 μg mL−1) for 24 h. Unstimulated (control) and stimulated (LPS) were incubated with a growth medium containing DMSO (1% v/v). Afterwards, the supernatants were discarded. According to the manufacturer's instructions, DCFDA (100 μL, 20 μM) was added to each well for 45 min at 37 °C. Fluorescence was measured at 485 nm for excitation and 535 nm for emission using a fluorescence plate reader (Sinergy HT, Biotek®, Bad Friedrichshall, Germany).2.6. Antiinflamatory activity
THP-1 cells were seeded in 96-well plates at 104 cells per well in the presence of 8 nM PMA. After differentiation, the medium was removed, and cells were washed with ice-cold PBS and pre-treated with the different bacterial EPS extracts (12.5, 25.0, and 50.0 μg mL−1) and Dex (as positive reference compound at 0.39 μg mL−1), which was maintained for 1 h at 37 °C with 5% CO2. Then, cells were exposed to LPS stimulation (1 μg ml−1) for 24 h. The activity of pro-inflammatory cytokines IL-6 and TNFα (Diaclone® GEN-PROBE, France) was measured using specific ELISA kits, essentially according to the manufacturer's recommendation. The absorbance was measured at 450 nm using a microplate reader (Labsystems® Multiskan EX, Thermo Scientific, USA).2.7. Inmunoblotting detection
Macrophages were obtained by incubating THP1-cells (8 × 105 cells per well) with 8 nM PMA in 6-well plates (2 mL per well) for 72 h. Afterwards, the medium was discarded, and cells were washed twice with PBS at 4 °C and then pre-treated with the EPS extract produced by LPG1 strain (12.5, 25.0 or 50.0 μg mL−1) or vehicle (DMSO 1%) for 1 h. Then, LPS stimulation was carried out for 24 h. Cells were lysed in ice-cold lysis buffer containing a cocktail of protease inhibitors (5 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), 50 mM Tris-HCl pH 7.5, 8 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM EDTA, 0.01 mg mL−1 pepstatin, 0.01 mg mL−1 leupeptin, 0.01 mg mL−1 aprotinin, and 250 mM NaCl (40 μL per sample) and incubated for 30 min on ice. Cell homogenates were then centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 5 min to discard cell debris and DNA, and protein content in the supernatants was determined using the Bradford method.14 Identical aliquots containing equal protein content (50 μg) were separated into an acrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. The specific primary antibodies incubated were used as follows: rabbit anti-Nrf2 (1
000g at 4 °C for 5 min to discard cell debris and DNA, and protein content in the supernatants was determined using the Bradford method.14 Identical aliquots containing equal protein content (50 μg) were separated into an acrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. The specific primary antibodies incubated were used as follows: rabbit anti-Nrf2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), rabbit anti-HO-1 (1
1000), rabbit anti-HO-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) (Cell Signaling, Danvers, MA, USA), and rabbit anti-COX-2 (1
1000) (Cell Signaling, Danvers, MA, USA), and rabbit anti-COX-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000; Cayman Chemical, Michigan, USA). Each membrane was washed 3 times for 10 min and incubated with the secondary horseradish peroxidase-linked anti-rabbit (Sigma-Aldrich, St Louis, MO, USA). Anti-β-actin antibody was used as house-keeping loading-control to normalise protein expression. Immunodetection was carried out by using a chemiluminescence light-detecting kit (Super-Signal West Pico Chemiluminescent Substrate, Pierce®, IL, USA), and densitometry analysis was quantified by a Scientific Imaging Systems (Biophotonics Image J Analysis Software; National Institute of Mental Health, Bethesda, MD, USA).
3000; Cayman Chemical, Michigan, USA). Each membrane was washed 3 times for 10 min and incubated with the secondary horseradish peroxidase-linked anti-rabbit (Sigma-Aldrich, St Louis, MO, USA). Anti-β-actin antibody was used as house-keeping loading-control to normalise protein expression. Immunodetection was carried out by using a chemiluminescence light-detecting kit (Super-Signal West Pico Chemiluminescent Substrate, Pierce®, IL, USA), and densitometry analysis was quantified by a Scientific Imaging Systems (Biophotonics Image J Analysis Software; National Institute of Mental Health, Bethesda, MD, USA).
2.8. Monosaccharide composition analysis of the EPS crude extract
The monosaccharide analysis of the EPS sample produced by the LPG1 strain in the MRS broth medium was determined from duplicated extracts. Initially, a trifluoroacetic acid (TFA) hydrolysis (2 N TFA at 121 °C for 1 h) was performed before reduction, acetylation, and gas chromatography analysis, following the method described by Englyst & Cummings (1984).15 Inositol was used as an internal standard for this analysis. Calibration was performed using a series of standard solutions of L-arabinose, D-xylose, D-mannose, D-glucose, D-galactose, L-rhamnose, and D-fucose (Sigma-Aldrich®, St Louis, MO, USA). The chromatographic analysis was conducted under the conditions described by Lama-Muñoz et al. (2012).16 A chromatograph-equipped model Hewlett–Packard 5890 series II with a 30 m × 0.25 mm fused silica capillary column (SP-2330 from Supelco, Bellefonte, PA) was used. The oven temperature program involved an initial 7 min at 180 °C and then a gradual increase to 220 °C at a rate of 3 °C min−1 (for 15 min). Helium was the carrier gas, flowing at a 1 mL min−1 rate. The injector temperature was set at 250 °C, and the FID detector temperature was maintained at 300 °C, with a split ratio of 1/100. The results for each specific sugar were expressed as a percentage of the total sugar content, whilst the total sugar content was expressed as a percentage of the total EPS lyophilised weight.2.9 Modelling EPS extract production
The production of the EPS extract by the LPG1 strain was modelled by response surface methodology (RMS). Table 1 presents the experimental conditions included in the central composite design, which consisted of 16 runs covering the pH range of 3.48–8.52, temperature range of 16.59–33.41 °C, and salt concentration range of 0.03 to 8.77% NaCl. Data analysis was performed using Design-Expert v.12 software (StatEase software, Minneapolis, USA). It involved a first sequential sum of squares (Type I), which indicates the significance of higher-order polynomial terms and ensures that the model was not aliased. Once the proposed model was fitted, an ANOVA was conducted, and significant (or necessary for hierarchical performance) terms were selected (p ≤ 0.05). Subsequently, the model's coefficients, standard errors, and confidence limits were estimated. The model's fit was assessed through lack of fit estimation and adjusted R-squared. Finally, numerical and graphical optimisation was performed.| Run | Salt (%NaCl) | pH | Temperature (°C) | mg L−1 EPS extract |
|---|---|---|---|---|
| a Duplicated experiments. | ||||
| 1 | 1.80 | 4.50 | 30.00 | 272.4 |
| 2 | 1.80 | 4.50 | 20.00 | 440.2 |
| 3 | 7.00 | 4.50 | 20.00 | 293.6 |
| 4 | 8.77 | 6.00 | 25.00 | 0.0 |
| 5 | 4.40 | 8.52 | 25.00 | 458.9 |
| 6 | 7.00 | 7.50 | 30.00 | 356.9 |
| 7 | 4.40 | 6.00 | 16.59 | 320.3 |
| 8 | 7.00 | 4.50 | 30.00 | 276.2 |
| 9a | 4.40 | 6.00 | 25.00 | 353.9 |
| 10a | 4.40 | 6.00 | 25.00 | 346.4 |
| 11 | 0.03 | 6.00 | 25.00 | 546.4 |
| 12 | 7.00 | 7.50 | 20.00 | 566.4 |
| 13 | 1.80 | 7.50 | 20.00 | 498.1 |
| 14 | 4.40 | 6.00 | 33.41 | 363.1 |
| 15 | 1.80 | 7.50 | 30.00 | 434 |
| 16 | 4.40 | 3.48 | 25.00 | 247.9 |
The modelling assays were conducted in 100 mL MRS broth adjusted to the different salt and pH levels as determined by experimental design. The culture media were inoculated with the LPG1 strain at a 5 log10 CFU mL−1 concentration and incubated at the corresponding temperature for 7 days. After this incubation, EPS crude extract extraction and purification from the culture medium were carried out as previously described.
3. Results and discussion
3.1. In vitro functional assays of the EPS crude extracts produced by the Lactiplantibacillus strains
EPS samples from the five Lactiplantibacillus strains were produced in a synthetic growth medium without additional sugar addition. This was done to obtain sufficient EPS extracts for subsequent in vitro tests, as described below. Among the strains, those that exhibited the highest production of EPS extracts in MRS at 37 °C after 3 days were Lp13 (470.0 ± 51.6 mg L−1) and LPG1 (469.0 ± 57.1 mg L−1), without significant differences. Following closely was the strain 13B4 (430.1 ± 26.5 mg L−1), followed by 119 (385.1 ± 23.6 mg L−1). Notably, Lp15 (300.1 ± 6.9 mg L−1) produced the significantly lowest EPS extract amount among the microorganisms assayed.
Table 2 shows the viability values obtained for THP-1 macrophages following administration of all EPS crude extracts produced by the Lactiplantibacillus strains at concentrations ranging from 6.25 to 100 μg mL−1, as determined by the MTT assay. Sample stocks were prepared in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 2500 μg mL−1. None of the tested EPS extracts exhibited cytotoxicity in the range of 6.25 to 50 μg mL−1, while only concentrations of 100 μg L−1 resulted in a slight reduction in the viability (17.0–28.9%) of human THP-1 macrophage cells.
1) at 2500 μg mL−1. None of the tested EPS extracts exhibited cytotoxicity in the range of 6.25 to 50 μg mL−1, while only concentrations of 100 μg L−1 resulted in a slight reduction in the viability (17.0–28.9%) of human THP-1 macrophage cells.
| EPS (μg mL−1) | |||||
|---|---|---|---|---|---|
| Strain | 6.25 | 12.5 | 25 | 50 | 100 |
| LPG1 | 100.3 ± 5.7 | 105.9 ± 3.5 | 102.0 ± 7.3 | 114.6 ± 13.4 | 71.1 ± 9.8 |
| 119 | 123.8 ± 7.5 | 101.0 ± 7.8 | 106.0 ± 2.3 | 102.1 ± 3.0 | 72.7 ± 3.7 |
| 13B4 | 118.6 ± 6.9 | 109.2 ± 7.0 | 113.1 ± 8.9 | 99.1 ± 2.4 | 83.0 ± 3.7 |
| Lp15 | 116.5 ± 3.8 | 100.5 ± 2.7 | 112.6 ± 6.6 | 110.1 ± 4.0 | 77.4 ± 2.0 |
| Lp13 | 113.9 ± 5.5 | 114.7 ± 4.0 | 110.3 ± 7.7 | 119.3 ± 4.1 | 82.4 ± 2.0 |
| (%) | 0.125 | 0.25 | 0.5 | 1 | 2 |
| Vehicle (DMSO) | 115.5 ± 4.8 | 99.4 ± 2.7 | 100.3 ± 3.7 | 94.3 ± 0.7 | 77.0 ± 1.5 |
Consequently, concentrations below 50 μg mL−1 were chosen for subsequent functional experiments. Sharma et al. (2020) also reported the absence of cytotoxicity in mammalian epithelial cell lines when exposed to EPS produced by L. paraplantarum KM1, even at concentrations higher than 500 μg mL−1.17 The non-toxic effect was noticed for the EPS produced by L. helveticus LZ-R-5 on the viability of the macrophage RAW264.7 cells within the tested concentrations (50–400 μg mL−1).18 Similar results were also obtained for the EPS produced by L. pentosus LZ-R-17, which did not exhibit cytotoxicity in RAW264.7 cells in the 50–400 μg mL−1 range.19 All these data show that olive EPS crude extracts are safe for consumption and, thus, could be formulated in foods.
Fig. 1 shows the pro-inflammatory TNF-α and IL-6 production in THP-1 macrophages after administration (12.5–50 μg mL−1) of all EPS crude extracts produced by the Lactiplantibacillus strains assayed in this work. Results were compared to the stimulation of macrophages with E. coli-LPS, which produces a significant pro-inflammatory response and IL-6 and TNF-α production compared to unstimulated control cells (p < 0.001). As expected, the anti-inflammatory reference drug Dex caused a marked inhibition of TNF-α and IL-6 (p < 0.001) levels induced by LPS. As can be deduced from Fig. 1, many of the EPS crude extracts assayed showed an anti-inflammatory effect, producing a statistical reduction in the production of TNF-α with respect to the LPS control. Pretreatment of cells with EPS-LPG1, EPS-119, and EPS-Lp13 significantly reduced TNF-α levels at all the tested concentrations. As regards the pretreatments with EPS-13B4 and EPS-Lp15 only showed a significant reduction at 12.5 and 25 μg mL−1 (Fig. 1A). Furthermore, pretreatment with EPS-LPG1 and EPS-119 also statistically reduced IL-6 levels at all tested concentrations, and only the concentration of 50 μg mL−1 of EPS-13B4, EPS-Lp15, and EPS-Lp13 was able to decrease the IL-6 levels (Fig. 1B) significantly. Although many functional features of Lactobacilli EPS are reported, especially immunomodulatory properties are drawing much attention. Nikolic et al. (2012) also showed that purified EPS obtained from L. paraplantarum BGCG11 at 100 μg mL−1 doses produced an anti-inflammatory response in peripheral blood mononuclear cells.20 Murofushi et al. (2015) reported that EPS produced by L. plantarum N14 decreased the production of pro-inflammatory cytokines.21 Min et al. (2020) noticed that L. plantarum YW11 produced an EPS that reduced the production of diverse pro-inflammatory cytokines (among them TNF-α and IL-6) in a mouse model induced with 5% dextran sulphate sodium.22 On the contrary, You et al. (2020) showed that EPS produced by L. pentosus LZ-R-17 increased the production of TNF-α and IL-6 in the 50–200 μg mL−1 range, suggesting an immune-modulatory but not anti-inflammatory activity.19 Thus, data obtained in this work showed the great anti-inflammatory effects of much of the EPS crude extracts obtained from the Lactiplantibacillus strains assayed in this work, especially LPG1.
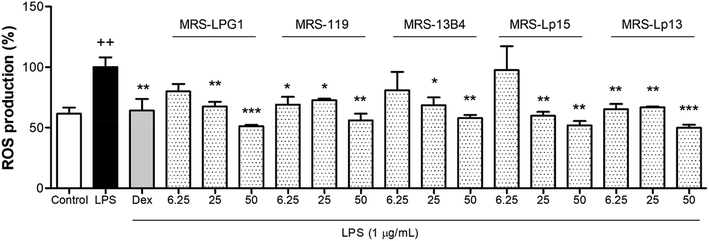
Fig. 2 illustrates the production of intracellular ROS levels in human LPS-stimulated THP-1 macrophage cells after administration of all EPS crude extracts produced by the Lactiplantibacillus in a range of concentrations of 6.25–50 μg mL−1, measured using the DCFDA method. ROS are undesirable by-products of aerobic metabolism synthesised by the cell under environmental stresses. ROS production increased significantly in the LPS treatment compared to the control (p < 0.01), which showed 57.3% of the baseline ROS levels. Dex compound (0.39 μg mL−1) reduced ROS production to levels similar to those of the control group (p < 0.01). A major reduction of ROS% was obtained for all strains at a dosage of 50 μg L−1, which was statistically different from the ROS% production obtained in THP-1 cells after administration of LPS. Specifically, the treatment using EPS-LPG1 caused a significant decrease in ROS production at 25 and 50 μg mL−1 (p < 0.01 and p < 0.001), which achieved a reduction close to 30% and 50% in ROS levels, respectively. There is plenty of well-described evidence of the antioxidant activity of EPSs synthesised by Lactobacillus species, such as the EPSs produced by L. helveticus MB2–1, L. acidophilus P185, L. plantarum S123, L. plantarum RO30, L. plantarum MC5, L. casei NA-2, and L. pentosus.23–29 Data obtained in this work showed the great antioxidant activity of many of the EPS crude extracts obtained from Lactiplantibacillus strains, especially LPG1.
3.2. WB immune assay for the EPS crude extract produced by L. pentosus LPG1
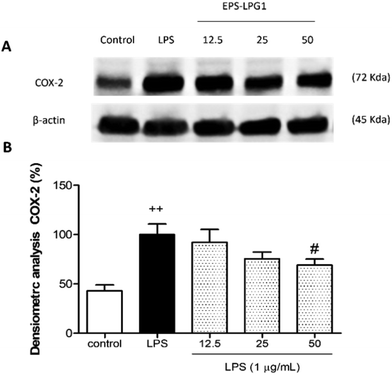
To confirm the potential anti-inflammatory mechanism of action of the sample LPG1-EPS that showed the most capacity to reduce ROS production, TNF-α, and IL-6 levels, the expression of the pro-inflammatory enzyme COX-2 was evaluated by western blotting analysis. THP-1 macrophages were pre-treated with different sample concentrations (12.5, 25, and 50 μg mL−1) for 1 h and then stimulated with LPS (1 μg mL−1). As presented in Fig. 3, the control group showed a 43% COX-2 expression level. LPS stimulation induced a significant increase in COX-2 protein levels in THP-1 macrophages in comparison with control cells (p < 0.01) (Fig. 3B). Pretreatment with LPG1-EPS notably caused a reduction of 24.6% and 31.0% at 25 and 50 μg mL−1, respectively, being stronger this effect at 50 μg mL−1 (p = 0.06).It is well established that oxidative stress plays an important role in chronic inflammation, promoting its maintenance over time. In this regard, LPS activates both cellular oxidative stress and inflammatory processes. To identify whether EPS-LPG1 could substantially attenuate LPS-induced oxidative stress through regulation of the Nrf2-dependent antioxidant signalling pathway, HO-1 protein and Nrf2 expressions were also determined by western blotting. As presented in Fig. 4, LPS stimulation did not significantly affect the expression of Nrf2 when compared to control cells. The pretreatment with EPS-LPG1 was notably able to up-regulate Nrf2 protein levels at 25 and 50 μg mL−1 (p = 0.057, p < 0.05, respectively) in comparison with the LPS group (Fig. 4B). Further, these results were correlated with a statistical increase in the Nrf2 target gene, HO-1, at 25 and 50 μg mL−1 (p = 0.057, p < 0.05, respectively) (Fig. 4C).
COX-2 is a bifunctional enzyme that catalyses the first step in the biosynthesis of prostaglandins, prostacyclins, and thromboxanes. Diverse stimuli, such as bacterial LPS, induce its expression. For this reason, this enzyme has been the focus of attention for developing anti-inflammatory compounds. On the contrary, heme oxygenase-1 (HO-1) is a Nrf2-regulated gene that prevents vascular inflammation. HO-1 activity produces and inhibits apoptosis and oxidative damage, with significant reductions in inflammatory events and the production of inflammatory cytokines. As can be easily deduced from Fig. 3, the maximum decrease in the production of COX-2 enzyme was obtained when 50 μg L−1 of EPS-LPG1 crude extract was administered to the human macrophages. In addition, maximum output of Nrf-2 proteins was obtained at 50 μg mL−1 of EPS-LPG1, which was also correlated with a high production of HO-1 enzyme, statistically different from values obtained for experiments with exclusively bacterial-LPS. All these data confirm the potent anti-inflammatory and antioxidant effects of the EPS crude extract produced by LPG1. Wang et al. (2020) reported that an acidic EPS produced by L. plantarum JLAU103 inhibited the production of COX-2 in RAW264.7 macrophages activated by LPS, exerting immunomodulatory activity at 60–80 μg mL−1.30 Recently, Cao et al. (2023) also noticed that EPS crude extract obtained from Bacillus velezensis SN-1 increased the expression of antioxidant genes HO-1 in mice's liver through the Nrf2/ARE signalling pathway.31
3.3. Monosaccharide composition of the EPS crude extract produced by L. pentosus LPG1
The EPS extract produced by the LPG1 strain in MRS broth medium without additional sugar exhibited a composition primarily consisting of various monosaccharides. They included D-mannose (35.45 ± 2.02%), D-glucose (32.99 ± 2.66%), L-arabinose (17.93 ± 2.68%), D-xylose (7.48 ± 3.74%), D-galactose (4.03 ± 0.45%), L-rhamnose (1.34 ± 1.34%), and D-fucose (0.77 ± 0.77%). As a result, the EPS-LPG1 crude extract was identified as a soluble heteropolymer, with the sugars representing 27.57% of the total lyophilised weight of the EPS sample. The rest could be proteins, DNA, acids, etc.Oleksy & Klewicka (2018) pointed out that heteropolysaccharides produced by Lactobacillus strains showed significant structural diversity, even within the same species.1 For instance, L. pentosus LPS26, isolated from directly brined green table olives, produced a capsular polymer of two distinct EPSs. The first EPS had a high molecular weight (1.9 × 106 Da) and consisted of glucose and rhamnose. The second EPS had a lower molecular weight (3.3 × 104 Da) and was composed of glucose and mannose.8 Similarly, the EPS crude extract from L. pentosus B8, isolated from Sichuan pickles, comprised two EPSs. The first had a low molecular weight (1.12 × 104 Da) and was composed of mannose and glucose, and the second had a high molecular weight (1.78 × 105 Da). It consisted of mannose, glucose, and galactose.29 In another study by Wang et al. (2021), the EPS sample produced by L. pentosus YY-122 had a molecular weight of 5.9 × 104 Da. It contained glucose, mannose, glucosamine, galactose, and rhamnose.32
On the other hand, the EPS produced by L. pentosus LZ-R-17 in fermented milk had a molecular weight of 1.20 × 106 Da and was primarily composed of galactose and glucose.19 In the current study, in addition to the glucose and galactose monosaccharides, the EPS extract produced by the LPG1 strain was found to have high proportions of mannose and arabinose and lower proportions of xylose, rhamnose and fucose. These findings are consistent with those obtained by Zhu et al. (2018), who also detected the presence of rhamnose, galactose, glucose, and arabinose in the sugar composition of the EPS samples isolated from industrial brine table olive fermentations.9
3.4. Optimisation of the EPS crude extract production by L. pentosus LPG1
It is widely recognised that environmental factors and cultural conditions can determine the yield of EPS production by LAB, as discussed by Oleksy & Klewicka (2018).1 Therefore, we aimed to optimise EPS extract production with the most desirable functional properties as a function of pH (ranging from 3.48 to 8.52), temperature (16.59–33.41 °C) and salt concentrations (0.03–8.77% NaCl). These parameters encompass the typical environmental variations encountered during table olive fermentations.Table 1 shows the EPS crude extract production results in the MRS broth medium by the strain LPG1 across various experimental conditions. EPS production ranged from non-detectable levels in treatment 4 (8.77% NaCl, pH 6.0, temperature 25 °C) to a peak of 566.4 mg L−1 in treatment 7 (7.0% NaCl, pH 7.50, temperature 20 °C). Notably, treatment 4 exhibited no growth of the LPG1 strain, resulting in no EPS production.
The specific model suggested by the sequential sum of squares (Type III) analysis was cubic. Following a backward selection of variables, the retained terms were the linear and quadratic effect of salt and the linear effect of pH (as shown in Table 3). No statistically significant terms for temperature were retained in the model. Consequently, the equation predicting the production of the EPS crude extract by LPG1 strain as a function of actual factors was as follows:
| EPS-LPG1 = 243.68–74.21 × Salt + 47.93 × pH + 7.15 × Salt2 |
| Source | Sum of squares | df | Mean square | F value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902.793 902.793 |
3 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300.9309 300.9309 |
8.69508528 | 0.0031 | Significant |
| Salt | 7821.29118 | 1 | 7821.29118 | 1.87341735 | 0.1984 | |
| pH | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590.6118 590.6118 |
1 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590.6118 590.6118 |
16.9084201 | 0.0017 | |
| pH2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374.4907 374.4907 |
1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374.4907 374.4907 |
3.92214716 | 0.0732 | |
| Residual | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923.6714 923.6714 |
11 | 4174.87922 | |||
| Lack of Fit | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 895.5464 895.5464 |
10 | 4589.55464 | 163.184165 | 0.0609 | Not significant |
| Pure error | 28.125 | 1 | 28.125 | |||
| Cor total | 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826.464 826.464 |
14 |
Sánchez et al. (2006) previously reported that controlling the pH was necessary for enhancing EPS production by L. pentosus LPS26, and they observed an increase in EPS synthesis as temperature decreased.8 Our study also found pH significantly influenced EPS crude extract production by LPG1 strain, with higher pH values leading to increased EPS production. However, in contrast, we did not observe any significant influence of temperature on EPS production. Additionally, salt concentration was identified as an important environmental variable affecting EPS production by this microorganism.
Using the model, we can optimise the production of the EPS crude extract based on environmental variables. Regarding the individual desirability profile after numerical optimisation at 25 °C (Fig. 5), salt reached the highest value at 1.8%. It decreased as its concentration was higher, with a slight curvature due to its quadratic term (Fig. 5A). Conversely, desirability increased linearly as pH was more elevated (Fig. 5B).
 | ||
| Fig. 5 Desirability evolution as a function of salt (A) and pH (B) as deduced from the numerical optimisation. | ||
Considering both variables simultaneously, Fig. 6 illustrates contour lines representing desirability after optimisation, where pH and salt levels are maintained within the model's range while maximising EPS crude extract production. The highest desirability (0.774) was achieved at 1.8% salt and pH 7.5. This result may be valid within the temperature range due to the absence of the effect of this variable. The predicted EPS extract production at this numerical optimum (1.8% salt and 7.5 pH) was 492.74 mg L−1, using an MRS medium containing 20 g L−1 glucose. In a recent study by Zhao & Liang (2023), RSM was employed to identify the optimal conditions for L. plantarum MC5 EPS production considering various environmental variables.27 They achieved a maximum EPS production of 345.98 mg L−1 at an inoculum size of 4.0%, an incubation time of 30 h, an initial pH of 6.4, and a temperature of 34 °C. Sánchez et al. (2006) reported a maximum EPS production of 514 mg L−1 by L. pentosus LPS26, isolated from table olives.8 This high production was observed at pH 6.0, a suboptimal growth temperature of 20 °C, and a glucose concentration of 30 g L−1 in the medium. It is worth noting that other Lactobacillus strains have been identified as even more prolific EPS producers. For instance, Lactobacillus helveticus MB2-1 achieved a maximum EPS production yield of 753 mg L−1, but when cultured in a medium supplemented with 80 g L−1 lactose and 20 g L−1 soya peptone.23Lactobacillus reuteri LB121 reached an impressive EPS production yield of 9800 mg L−1 in MRS supplemented with sucrose.33L. pentosus B8 exhibited an EPS production yield of 1401 mg L−1 in an MRS culture medium supplemented with 40 g L−1 sucrose at 30 °C.29 Additionally, L. pentosus KS-27, isolated from sucuk, produced 991 mg L−1 of EPS in an MRS medium supplemented with 100 g L−1 sucrose.34 Finally, L. pentosus LZ-R17 produced 185.20 mg L−1 of crude EPS when cultured in a pasteurised milk medium.19
 | ||
| Fig. 6 Desirability contour lines for optimisation of EPS produced by L. pentosus LPG1 strain as a function of pH and salt concentration for a temperature fixed at 20.5 °C. | ||
4. Conclusions
L. pentosus LPG1 is a valuable producer of heteropolysaccharides with high functional properties. The production yield of the EPS crude extract can be effectively controlled by manipulating variables such as pH and salt concentration. Remarkably, achieving substantial EPS production within the typical ranges encountered during table olive fermentations is feasible. This achievement presents a promising opportunity to employ the strain LPG1 as a starter culture in the table olive fermentation. Furthermore, the application of purified EPSs produced by this strain in olive brines holds significant potential for various applications in human nutrition. Future research will be prioritised to study the rheological and functional features of this EPS crude extract added to food matrices and deeper characterisation of its structure, which is crucial for understanding its functionality.Author contributions
Conceptualisation, F.N.A.-L. and F.R.-G.; methodology, E.L.-G., A.M.-G., J.A.-R., A.B.-C, V.R.-G., and A.B.-O.; software, M.S.-H. and A.G.-F.; formal analysis, M.S.-H., J.A.-R., V.R.-G., A.B.-C., A.G.-F., F.R.-G. and F.N.A.-L.; resources, F.N.A.-L.; data curation, M.S.-H., F.R.-G., A.G.-F., and F.N.A.-L.; writing—original draft preparation, E.L.-G., M.S.-H., A.G.-F., F.R.-G., and F.N.A.-L.; writing—review and editing, E.L.-G., M.S.-H., A.G.-F., F.R.-G, and F.N.A.-L.; supervision, F.N.A.-L.; project administration, F.N.A.-L.; funding acquisition, F.N.A.-L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author(s) declare financial support was received for this article's research, authorship, and publication. This research was funded through the TOBE project (RTI2018–100883-B-I00, MCIU/AEI/FEDER, UE). ELG and VRG thank the Spanish Ministry of Science and Innovation for their FPI contract (PRE2019-087812) and Postdoctoral Contract (FJC2019-040605-I), respectively. ABC thanks the Junta de Andalucia for his postdoctoral contract PAIDI2020-00162.References
- M. Oleksy and E. Klewicka, Exopolysaccharides produced by Lactobacillus sp. Biosynthesis and applications, Crit. Rev. Food Sci. Nutr., 2018, 58(3), 450–462 CAS.
- J. Angelin and M. Kavitha, Exopolysaccharides from probiotic bacteria and their health potential, Int. J. Biol. Macromol., 2020, 1(162), 853–865, DOI:10.1016/j.ijbiomac.2020.06.190.
- N. Kaur and P. Dey, Bacterial exopolysaccharides as emerging bioactive macromolecules: from fundamentals to applications, Res. Microbiol., 2023, 174, 104024 CrossRef CAS PubMed.
- M. Loeffler, J. Hilbig, L. Velasco and J. Weiss, Usage of in situ exopolysaccharide-forming lactic acid bacteria in food production: Meat products—A new field of application?, Compr. Rev. Food Sci. Food Saf., 2020, 19, 2932–2954 CrossRef CAS PubMed.
- A. Hurtado, C. Requant, A. Bordons and N. Rozes, Lactic acid bacteria from fermented olives, Food Microbiol., 2012, 31, 1–8, DOI:10.1016/j.fm.2012.01.006.
- International Olive Oil Council [IOC]. (2021). Key figures on the world market for table olives. Madrid, Spain: International Olive Council. https://www.internationaloliveoil.org/wp-content/uploads/2021/12/114-OT-2021.pdf (accessed July 2023).
- J. Dominguez-Manzano, C. Olmo-Ruiz, J. Bautista-Gallego, F. N. Arroyo-López, A. Garrido-Fernández and R. Jiménez-Díaz, Biofilm formation on abiotic and biotic surfaces during Spanish-style Green table olive fermentations, Int. J. Food Microbiol., 2012, 157, 230–238 CrossRef CAS PubMed.
- J. I. Sánchez, B. Martínez, R. Guillén, R. Jiménez-Díaz and A. Rodríguez, Culture conditions determine the balance between two different exopolysaccharides produced by Lactobacillus pentosus, LPS26, Appl. Environ. Microbiol., 2006, 72, 7485–7502 CrossRef PubMed.
- Y. Zhu, G. González-Ortin, R. Jiménez-Díaz, M. Pérez-Trujillo, T. Parella, P. López-Colom and S. M. Martín-Orúe, Exopolysaccharides from olive brines could reduce the adhesión of ETEC K88 to intestinal epithelial cells, Food Funct., 2018, 9, 3884 RSC.
- Y. Zhu, G. González-Ortiz, A. Benítez-Cabello, B. Calero-Delgado, R. Jiménez-Díaz and S. M. Martín-Orúe, Using starter cultures in table olive fermentation can modulate the antiadhesive properties of brine exopolysaccharides against enterotoxigenic Escherichia coli, Food Funct., 2019, 10, 3738 RSC.
- A. León-Romero, Selección de cepas de Lactobacillus pentosus con propiedades potencialmente probióticas, aisladas de fermentaciones de aceitunas. Estudio de sus propiedades tecnológicas. PhD. Thesis. University of Seville, 2014 Search PubMed.
- A. Benitez-Cabello, B. Calero-Delgado, F. Rodríguez-Gómez, A. Garrido-Fernández, R. Jiménez-Díaz and F. N. Arroyo-López, Biodiversity and multifunctional features of lactic acid bacteria isolated from table olive biofilms, Front. Microbiol., 2019, 10, 836 CrossRef PubMed.
- K. Priti, A. Nagarajan and P. D. Uchil, Analysis of cell viability by the BTT assay, Cold Spring Harb. Protoc., 2018, 2018(6), 469–471, DOI:10.1101/pdb.prot095505.
- M. M. Bradford, A rapid and sensitive method for quantitating microgram quantities of protein utilising the principle of protein-dye binding, Anal. Biochem., 1976, 72(1–2), 248–254, DOI:10.1016/0003-2697(76)90527-3.
- H. Englyst and J. H. Cummings, Simplified method for measuring total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates, Analyst, 1984, 109(7), 937–942, 10.1039/AN9840900937.
- A. Lama-Muñoz, G. Rodríguez-Gutiérrez, F. Rubio-Senent and J. Fernández-Bolaños, Production characterisation and isolation of neutral and pectic oligosaccharides with low molecular weights from olive by-products thermally treated, Food Hydrocolloids, 2012, 28(1), 92–104 CrossRef.
- K. Sharma, N. Sharma, S. Handa and S. Pathania, Purification and characterisation of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity, J. Genet. Eng. Biotechnol., 2020, 18, 56 CrossRef PubMed.
- X. You, Z. Li, K. Ma, C. Zhang, X. Chen, G. Wang, L. Yang, M. Dong, X. Rui, Q. Zhang and W. Li, Structural characterisation and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5, Carbohydr. Polym., 2020, 235, 115977 CrossRef CAS PubMed.
- X. You, L. Yang, X. Zhao, K. Ma, X. Chen, C. Zhang, G. Wang, M. Dong, X. Rui, Q. Zhang and W. Li, Isolation purification, characterisation and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir, Int. J. Biol. Macromol., 2020, 158, 408–419 CrossRef CAS PubMed.
- M. Nikolic, P. López, I. Strahinic, A. Súarez, M. Kojic, M. Fernández-García, L. Topisirovic, N. Golic and P. Ruas-Madiedo, Characterisation of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics, Int. J. Food Microbiol., 2012, 158, 155–162 CrossRef CAS PubMed.
- Y. Murofushi, J. Villena, K. Morie, P. Kanmani, M. Tohno, T. Shimazu, A. Hisashi, S. Yoshihito, K. Hashiguchi, T. Saito and H. Kitazawa, The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum, N14, Mol. Immunol., 2015, 64(1), 63–75 CrossRef CAS PubMed.
- Z. Min, H. Xiaona, T. Aziz, Z. Jian and Y. Zhennai, Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and alleviate inflammatory bowel disease symptoms, Acta Biochim. Pol., 2020, 67(4), 485–493 CAS.
- W. Li, L. Juan, X. Rui, J. Yu, W. Tang, X. Chen, M. Jiang and M. Dong, Production of exopolysaccharides by Lactobacillus helveticus MB2–1 and its functional characteristics in vitro, LWT–Food Sci. Technol., 2014, 59, 732–739 CrossRef CAS.
- K. A. El Ghany, E. A. Elhafez, R. A. Hamouda, H. Mahrous, F. A. H. Ahmed and H. A. Hamza, Evaluation of antioxidant and antitumor activities of Lactobacillus acidophilus bacteria isolated from Egyptian infants, Int. J. Pharmacol., 2014, 10, 282–288 Search PubMed.
- M. Saleem, S. Malik, H. M. Mehwish, M. M. Ali, N. Hussain, M. Khurshid, M. S. Riaz Rajoka and Y. Chen, Isolation and functional characterisation of exopolysaccharide produced by Lactobacillus plantarum, S123 isolated from traditional Chinese cheese, Arch. Microbiol., 2021, 203, 3061–3070 CrossRef CAS PubMed.
- E. A. Elmansy, E. M. Elkady, M. S. Asker, A. M. Abdou, N. A. Abdallah and S. K. Amer, Exopolysaccharide produced by Lactoplantibacillus plantarum RO30 isolated from Romi cheese: characterisation, antioxidant and burn healing activity, World J. Microbiol. Biotechnol., 2022, 38, 245 CrossRef CAS PubMed.
- X. Zhao and Q. Liang, Optimisation, probiotic characteristics, and rheological properties of exopolysaccharides from Lactiplantibacillus plantarum MC5, Molecules, 2023, 28, 2463 CrossRef CAS PubMed.
- X. Xu, Y. Qiao, Q. Peng, B. Shi and V. P. Dia, Antioxidant and immunomodulatory properties of partially purified exopolysaccharide from Lactobacillus casei isolated from Chinese northeast sauerkraut, Immunol. Invest., 2022, 51, 748–765 CrossRef CAS PubMed.
- G. Jiang, R. Li, J. He, J. Chen, Z. Xu, B. Zheng, Y. Yang, Z. Xia and Y. Tian, Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus, B8 Isolated from Sichuan Pickle, Foods, 2022, 15, 2327 CrossRef PubMed.
- J. Wang, X. Fang, T. Wu, L. Fang, C. Liu and W. Min, In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus plantarum JLAU103 on RAW264.7 macrophages, Int. J. Biol. Macromol., 2020, 156, 1308–1315 CrossRef CAS PubMed.
- C. Cao, Y. Bian, W. Cang, J. Wu and R. Wu, Structural characterisation and hepatoprotective activity of exopolysaccharide from Bacillus velezensis SN-1, J. Sci. Food Agric., 2023, 103, 738–749 CrossRef CAS PubMed.
- M. Wang, W. Zhou, Y. Yang, J. Xing, X. Xu and Y. Lin, Potential prebiotic properties of exopolysaccharides produced by a novel Lactobacillus strain, Lactobacillus pentosus YY-112, Food Funct., 2021, 12, 9456 RSC.
- S. Badel, T. Bernardi and P. Michaud, New perspectives for Lactobacilli exopolysaccharides, Biotechnol. Adv., 2011, 29, 54–66 CrossRef CAS PubMed.
- A. Kamiloglu, Functional and technological characterisation of lactic acid bacteria isolated from Turkish dry-fermented sausage (sucuk), Braz. J Microbiol., 2022, 53(2), 959–968 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04223e |
| This journal is © The Royal Society of Chemistry 2024 |