Open Access Article
Open Access Article(−)-Epicatechin and colonic metabolite 2,3-dihydroxybenzoic acid, alone or in combination with metformin, protect cardiomyocytes from high glucose/high palmitic acid-induced damage by regulating redox status, apoptosis and autophagy†
Esther
García-Díez
 a,
Jara
Pérez-Jiménez
a,
Jara
Pérez-Jiménez
 ab,
María Ángeles
Martín
ab,
María Ángeles
Martín
 ab and
Sonia
Ramos
ab and
Sonia
Ramos
 *ab
*ab
aDepartment of Metabolism and Nutrition, Institute of Food Science and Technology and Nutrition (ICTAN), Consejo Superior de Investigaciones Científicas (CSIC), José Antonio Novais 10, Ciudad Universitaria, 28040, Madrid, Spain. E-mail: s.ramos@ictan.csic.es; Tel: +34 915492300
bCentro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), ISCIII, Spain
First published on 30th January 2024
Abstract
(−)-Epicatechin (EC) and a main colonic phenolic acid derived from flavonoid intake, 2,3-dihydroxybenzoic acid (DHBA), display antioxidant and antidiabetic activities. Diabetic cardiomyopathy (DCM) is one of the main causes of mortality in patients with diabetes, lacking a suitable treatment. Hyperglycaemia and dyslipidaemia are mainly responsible for oxidative stress and altered apoptosis and autophagy in cardiomyocytes during DCM. In this context, phenolic compounds could be suitable candidates for alleviating DCM, but have scarcely been investigated or their use in combination with antidiabetic drugs. This study evaluates the effects of EC, DHBA and antidiabetic drug metformin (MET), alone or all combined (MIX), on redox status, autophagy and apoptosis in H9c2 cardiomyocytes challenged with high concentrations of glucose (HG) and palmitic acid (PA). Under HG + PA conditions, EC, DHBA, MET and MIX equally improved redox status, reduced apoptosis induction and ameliorated autophagy inhibition. Mechanistically, all treatments alleviated HG + PA-induced oxidative stress by reinforcing antioxidant defences (∼40% increase in glutathione, ∼30% diminution in GPx activity and ∼15% increase in SOD activity) and reducing ROS generation (∼20%), protein oxidation (∼35%) and JNK phosphorylation (∼200%). Additionally, all treatments mitigated HG + PA-induced apoptosis and activated autophagy by decreasing Bax (∼15–25%), caspase-3 (∼20–40%) and p62 (∼20–40%), and increasing Bcl-2, beclin-1 and LC3-II/LC3-I (∼40–60%, ∼15–20%, and ∼25–30%, respectively). JNK inhibition improved protective changes to redox status, apoptosis and autophagy that were observed in EC-, DHBA- and MIX-mediated protection. Despite no additive or synergistic effects being detected when phenolic compounds and MET were combined, these results provide the first evidence for the benefits of EC and DHBA, comparable to those of MET alone, to ameliorate cardiomyocyte damage, that involve an improvement in antioxidant competence, autophagy and apoptosis, these effects being mediated at least by targeting JNK.
1. Introduction
Foods of vegetal origin are rich in phenolic compounds that have been said to exert beneficial effects on health and risk reduction for certain diseases.1–3 Flavanols, such as (−)-epicatechin (EC) and derived proanthocyanidins, are abundant in cocoa, tea, grapes, carob, and other vegetables and fruits. Once ingested, a high percentage of these flavanols is metabolized by the gut microbiota, generating different phenolic metabolites.4,5 Among the main colonic phenolic acids derived from the intake of flavanol-rich food are mono- and di-hydroxylated phenylpropionic, hydroxybenzoic and phenylacetic acids that have been detected in human serum at the micromolar level (19 to 359 nmol L−1).4–6 Thus, at present, it is assumed that to estimate the impact of phenolic compounds on health, the contribution of food polyphenols and metabolites (including low molecular weight microbial phenolic metabolites) should be considered.4,5,7Current evidence shows that colonic flavonoid-derived metabolites exhibit health-beneficial effects against different diseases, including diabetes. This is a complex metabolic disorder that constitutes a leading cause of morbidity and mortality worldwide, and it is accompanied by serious comorbidities.8 In this respect, EC and some colonic phenolic metabolites have been reported to modulate the redox status and protect the functionality of insulin-sensitive tissues within a simulated diabetic milieu.9–11 In addition, a previous study by our group demonstrated that EC and 2,3-dihydroxybenzoic acid (DHBA) under physiological conditions strengthen insulin signalling and regulate glucose and lipid metabolism in cardiac cells.12
Cardiomyopathy is a major complication of diabetes that accounts for more than half of diabetes-related morbidity and mortality cases.13 Hyperglycaemia and dyslipidaemia are among factors that contribute to the development and progression of diabetic cardiomyopathy (DCM), generating an oxidative stress milieu and dysregulating numerous cellular signalling pathways in cardiomyocytes.14–16 Accumulating evidence suggests that a complex interplay among oxidative damage, apoptosis and autophagy plays a critical role in the development and progression of DCM.14–16 Thus, in DCM, cardiomyocyte death has been associated with enhanced oxidative stress, impaired mitochondria, induced apoptosis, and dysregulated autophagy.14–16 Therefore, approaches aimed at alleviating these alterations may effectively contribute to mitigating the development and progression of DCM.
Metformin (MET) is the first-line hypoglycaemic drug in the treatment of type 2 diabetes despite its side effects, and although this is still controversial, it has been suggested to exert cardioprotective outcomes.17 In this regard, in cardiac cells, metformin has been demonstrated to improve metabolic status, to ameliorate oxidative stress and inflammation, and to inhibit myocardial apoptosis, reducing cardiac remodelling and preserving left ventricular function.17 Likewise, flavanols have recently been suggested as safe agents that contribute to ameliorating DCM through the mitigation of cardiac oxidative stress and apoptosis.15 Therefore, the combination of drugs and these natural compounds may lead to synergistic interactions and provide more efficient therapies against DCM.18 However, the potential preventive activities of EC and microbial phenolic metabolites derived from flavanol intake, alone or in combination with MET, related to oxidative stress, apoptosis and autophagy during diabetes in cardiac cells remain largely unknown.
Therefore, the aim of this study was to investigate under glucolipotoxic conditions, the effects and underlying mechanisms of EC and colonic-derived flavanol metabolite DHBA, alone or combined with MET, on redox status, autophagy and apoptosis in embryonic rat H9c2 cardiomyocytes.
2. Materials and methods
2.1. Materials and chemicals
(−)-EC (>95% purity), DHBA (≥99% purity), metformin (MET >97% purity), SP600125 (JNK inhibitor), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), D-glucose, glutathione reductase (GR), glutathione (GSH), oxidized glutathione, 5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1), nicotinamide-adenine-dinucleotidephosphate (NADPH), o-phthaldehyde (OPT), palmitic acid (PA), tert-butylhydroperoxide (t-BOOH), penicillin G and streptomycin were purchased from Sigma Chemicals (Madrid, Spain). N-Acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin caspase-3 substrate (Ac-DEVD-AMC) was obtained from BD Pharmingen (Madrid, Spain). Anti-ERK1/2 and antiphospho-ERK1/2 recognizing phosphorylated Thr202/Thy204 of ERK1/2, anti-JNK1/2 and antiphospho-JNK1/2 recognizing phosphorylated Thr183/Tyr185 of JNK1/2, anti-active cleaved caspase-3 and anti-β-actin were obtained from Cell Signalling Technology (Werfen, Barcelona, Spain). Anti-Bcl-2 (sc-7382), anti-Bax (sc-526), anti-p62 (sc-48389), anti-beclin-1 (sc-48341) and anti-LC3 (sc-398822) were purchased from Santa Cruz Biotechnology (Qimigen, Madrid, Spain). Bradford reagent and materials and chemicals for electrophoresis were obtained from BioRad (BioRad Laboratories S.A., Madrid, Spain). Cell culture dishes and cell culture media came from Falcon (Fisher Scientific, Madrid, Spain) and Cultek (Madrid, Spain), respectively. All devices to perform western blot analyses were obtained from Bio-Rad (Madrid, Spain).2.2. Cell culture and treatments
Embryonic ventricular rat-heart-derived H9c2 cardiomyoblasts (kindly provided by Prof. Dr Victoria Cachofeiro, Facultad de Medicina, UCM, Madrid, Spain and purchased from Sigma) were grown in DMEM (24.5 mM D-glucose) medium supplemented with 10% foetal bovine serum (FBS) and 50 mg L−1 antibiotics (penicillin and streptomycin). After 48 h, cells were differentiated for 6 days into adult cardiomyocytes in DMEM supplemented with 1% FBS and 10 nM retinoic acid according to a previously described method.12,19 The medium was replaced every 48 h for 6 days, and on day 7, it was changed to DMEM (5.5 mM D-glucose, normal glucose) containing 1% FBS and 2 mM glutamine.20,21 The following day, cells were preincubated for 2 h with 1 μM of EC, DHBA, or MET or a mixture of previous compounds prior to 22 h of treatment with glucose (30 mM) and PA (200 μM, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BSA–PA) and cells were harvested. A mixture of compounds (MIX) with 1 μM of each one (1 μM of EC + 1 μM of DHBA + 1 μM of MET) was added to the culture medium to test their potential additive or synergist effects. Cells exposed to normal glucose alone (5.5 mM) were regarded as a control.
1 BSA–PA) and cells were harvested. A mixture of compounds (MIX) with 1 μM of each one (1 μM of EC + 1 μM of DHBA + 1 μM of MET) was added to the culture medium to test their potential additive or synergist effects. Cells exposed to normal glucose alone (5.5 mM) were regarded as a control.
It is worth noting that the dose of EC and DHBA employed in this study is considered physiologically relevant since concentrations ranging between 0.1 and 10 μM have been found in biological fluids after the intake of foodstuffs containing flavanols, and they are considered to be within the range recommended for in vitro studies.4,6,22 Moreover, 1 μM is a dose that has been used in similar studies with natural compounds and MET in H9c2 cells.23–25 Similarly, it should be highlighted that the high concentrations of glucose and PA selected to resemble diabetic milieu in cardiac cells are widely used in the literature and could be considered as representatives of patients with diabetes.20,21
In experiments with the inhibitor, cells were preincubated with 10 μM of SP600125 for 1 h prior to 2 h of EC, DHBA or MIX treatment followed by glucose and PA challenge.
2.3. Cell viability and ROS generation analysis
Cell viability was determined by a crystal violet assay.12,26 H9c2 cells were seeded in 24-well plates (0.5 × 104 cells per well) and after 48 h differentiation was initiated and continued for 7 days. Then, the following day, cells were incubated with different treatments (24 h) and later incubated with crystal violet (0.2% in ethanol) for 20 min. Plates were rinsed with distilled water, allowed to dry, and 1% sodium dodecylsulfate was added. The absorbance of each well was measured using a microplate reader (Bio-Tek, Winooski, VT) at 570 nm.Cellular ROS generation was quantified by a DCFH assay.10,11 Briefly, after cell treatments, 5 μM of DCFH-DA was added to wells for 30 min at 37 °C. Then, cells were washed with PBS, serum-free medium was added and the fluorescence was immediately measured in a microplate reader at 485 nm/530 nm (excitation/emission wavelengths, respectively).
2.4. Mitochondrial membrane potential (MMP) measurement and caspase-3 activity analysis
Changes in MMP were analysed using the fluorescent dye JC-1.27,28 In brief, after cell treatments, 5 μM of JC-1 was added to cells and incubated for 30 min at 37 °C. Then, H9c2 cells were washed with PBS, serum-free medium was added and the fluorescence was immediately measured in a microplate reader at 590 nm (aggregate form of JC-1; excitation 535 nm/emission 590 nm) and at 530 nm (monomer form of JC-1; excitation 490 nm/emission 530 nm) to assess the shift in fluorescence.The activity of caspase-3 was measured as previously described.29 Cells were lysed (5 mM Tris [pH 8], 20 mM EDTA, and 0.5% Triton-X100), and the activity was assayed in a reaction mixture containing 20 mM of HEPES (pH 7), 10% glycerol, 2 mmol L−1 dithiothreitol, 50 μg of protein per condition, and 20 μM of Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) as a substrate. Enzymatic activity was measured at an excitation wavelength of 380 nm and emission wavelength of 440 nm in a microplate reader.
2.5. Determination of GSH content, glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities and carbonyl groups
GSH was quantified by a Hissin and Hilf fluorometric assay.10,11 The method is based on the reaction of GSH with OPT at pH 8.0, and fluorescence was measured using a microplate reader at an excitation wavelength of 340 nm and emission wavelength of 460 nm. The results for samples were referred to those for a standard curve of GSH.Determination of GPx activity is based on the oxidation of GSH by GPx, using t-BOOH as a substrate, coupled to the disappearance of NADPH by GR. The methods have been previously described,11 and the protein was measured by the Bradford reagent (Bio-Rad, Madrid, Spain).
SOD activity was assayed using a commercial kit, following manufacturer's instructions (Sigma, Madrid, Spain).11 The assay is based on the capacity of SOD to reduce superoxide anions, coupled with the disappearance of Dojindo's highly water-soluble tetrazolium salt (WST-1) to yield a dye. SOD activity was quantified by measuring the decrease in the absorbance at 440 nm in a microplate reader. Protein concentration was measured by the Bradford reagent.
Protein oxidation of cells was spectrophotometrically measured as carbonyl group content following derivatization with 2,4-dinitrophenylhydrazine by a Richert assay.10 Cell extracts were incubated with 0.2% 2,4-dinitrophenylhydrazine in 2 M HCl (samples) or 2 M HCl (blanks) for 1 h. An equal volume of 20% trichloroacetic acid was added to precipitate proteins and later centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750g for 1 min. Then, pellets were washed 3 times with ethyl acetate
750g for 1 min. Then, pellets were washed 3 times with ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), allowed to dry and resuspended in 25 mM Tris pH 9.0, 6 M urea buffer. Absorbance was measured at 360 nm in a microplate reader, and the carbonyl content was expressed as nmol mg−1 protein using an extinction coefficient of 22
1), allowed to dry and resuspended in 25 mM Tris pH 9.0, 6 M urea buffer. Absorbance was measured at 360 nm in a microplate reader, and the carbonyl content was expressed as nmol mg−1 protein using an extinction coefficient of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nmol L−1 cm−1. Protein concentration was measured by the Bradford reagent (Bio-Rad, Madrid, Spain).
000 nmol L−1 cm−1. Protein concentration was measured by the Bradford reagent (Bio-Rad, Madrid, Spain).
2.6. Preparation of cell lysates and western blot analysis
Cells were lysed in a buffer containing 25 mM HEPES (pH 7.5), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.1% Triton X-100, 200 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 μg mL−1 leupeptin and 1 mM phenylmethylsulfonyl fluoride. Then, supernatants were collected, assayed for protein concentration using the Bradford reagent, aliquoted and stored at −80 °C until used for western blot analyses.Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride filters (Merck, Madrid, Spain). Membranes were probed with the corresponding primary antibody followed by incubation with peroxide-conjugated anti-rabbit (Bethyl, Bionova Científica S.L., Madrid, Spain) or anti-mouse (Sigma, Madrid, Spain) immunoglobulin. Blots were developed with the ECL system (Amersham, Cytiva, Madrid, Spain). Normalization of the western blot was ensured by β-actin, and bands were quantified using a scanner and accompanying software.
2.7. Statistics
Prior to statistical analysis, data were tested for the homogeneity of variance by Levene's text; for multiple comparisons, one-way ANOVA was followed by the Bonferroni test when variances were homogeneous or by the Tamhane test when variances were not homogeneous. P < 0.05 was considered significant. The SPSS version 27.0 program was used.3. Results
3.1. High glucose (HG) and PA concentrations induce cell death and oxidative stress in H9c2 cardiomyocytes
Hyperglycaemia and dyslipidaemia are key factors that make an important contribution to DCM.14,15 To develop a model of diabetes induced by high concentrations of glucose and PA in cardiac cells, H9c2 cells were exposed to rising doses of glucose and PA separately for 24 h, and cell viability, ROS generation, caspase-3 activity and MMP were evaluated.Among all glucose concentrations tested in H9c2 cells for 24 h, only the two highest doses (30 and 40 mM) led to decreased MMP (measured as JC-1), while all other parameters evaluated remained unchanged (Table 1). In contrast, concentrations of PA higher than 50 μM caused cell damage, as cell viability and MMP decreased, while ROS generation and caspase-3 activity increased (Table 1). In view of these results, 30 mM of glucose was the selected dose, as it is a commonly used concentration in cellular models able to induce damage,10,30–32 and it was combined with previously tested doses of PA. Incubation of cells with 30 mM of glucose and doses of PA higher than 50 μM reduced cell viability and JC-1, as well as enhancing ROS values and caspase-3 activity (Table 1). All these data suggest that high doses of glucose and PA are able to alter redox status and induce apoptosis in H9c2 cells to simulate a situation that resembles diabetic milieu in cardiac cells.
| Crystal violet (% of control) | ROS (% of control) | Caspase-3 activity (% of control) | JC-1 (% of control) | ||
|---|---|---|---|---|---|
| HG (mM) | 0 | 99.7 ± 3.4 | 100.0 ± 3.8 | 100.0 ± 7.1 | 100.0 ± 9.2a |
| 20 | 94.7 ± 6.3 | 94.8 ± 4.5 | 99.8 ± 5.6 | 93.7 ± 8.3a | |
| 30 | 99.9 ± 6.7 | 106.2 ± 9.9 | 102.7 ± 10.8 | 79.9 ± 7.7b | |
| 40 | 100.3 ± 6.4 | 102.3 ± 3.9 | 115.2 ± 8.2 | 78.2 ± 2.6b | |
| PA (μM) | 0 | 100.7 ± 8.5a | 100.0 ± 7.4a | 100.3 ± 3.7a | 100.0 ± 4.1a |
| 50 | 101.9 ± 5.5a | 99.2 ± 5.5a | 106.4 ± 5.6a | 97.9 ± 4.4a | |
| 100 | 82.6 ± 7.5bc | 129.7 ± 5.2b | 187.9 ± 5.7b | 88.7 ± 6.2b | |
| 150 | 82.3 ± 5.7bc | 154.4 ± 8.3c | 203.3 ± 12.2b | 75.0 ± 4.8c | |
| 200 | 81.3 ± 9.6bc | 157.7 ± 12.5c | 193.4 ± 4.9b | 69.9 ± 7.1c | |
| 400 | 70.6 ± 8.2c | 177.0 ± 2.4d | 260.2 ± 13.9c | 70.8 ± 8.3c | |
| HG (30 mM) + PA | 0 | 100.2 ± 6.6a | 100.1 ± 6.5a | 100.0 ± 9.7a | 100.1 ± 5.7a |
| 50 | 97.4 ± 7.5a | 95.4 ± 4.5a | 118.5 ± 9.3c | 81.6 ± 9.6b | |
| 100 | 76.6 ± 4.6b | 129.3 ± 5.7b | 255.8 ± 9.7b | 66.1 ± 4.1c | |
| 150 | 75.8 ± 7.5b | 144.7 ± 10.4c | 260.5 ± 12.5b | 67.1 ± 9.5c | |
| 200 | 74.1 ± 7.1b | 154.9 ± 8.0c | 259.6 ± 17.8b | 67.2 ± 4.0c | |
| 400 | 66.9 ± 6.7b | 181.2 ± 8.3d | 461.0 ± 23.9c | 68.1 ± 5.5c |
Since 200 μM of PA in the presence of 30 mM of glucose provoked notable damage to H9c2 cells, it was the selected concentration to study the protective effects of EC, DHBA, MET and MIX in terms of redox status, apoptosis and autophagy. Thus, all compounds were tested separately to clearly establish the potential protective effect that could be attributed to each of them separately. Then, all compounds were combined to evaluate their desirable synergistic or additive protective effects.
3.2. Epicatechin, microbial phenolic metabolite DHBA, metformin and their mixture prevent cell death and redox imbalance induced by HG and PA in H9c2 cardiomyocytes
Oxidative stress constitutes a key factor for the development and progression of DCM.15,33,34 To evaluate the protective effect of EC (1 μM), colonic phenolic metabolite DHBA (1 μM), the first-line drug in the treatment of type 2 diabetes metformin (1 μM of MET), or a mixture of all previous compounds at the same μM dose (1 μM EC + 1 μM DHBA + 1 μM MET, MIX) on cultured H9c2 cells were submitted to glucolipotoxic conditions. For this, cells were treated for 2 h with compounds prior to 22 h of treatment with 30 mM of glucose and 200 μM of PA; cell viability, ROS generation, JC-1, GSH content, and key antioxidant enzymatic activities, such as GPx and SOD, were analysed.Pre-treatment of H9c2 cells with all compounds mentioned either individually or all combined (MIX) similarly contributed to an increase in MMP and to prevent high-glucose + PA-induced ROS generation and protein oxidation (measured as carbonyl groups), resulting in enhanced cardiac cell viability (Table 2). In addition, EC, DHBA, MET and MIX produced complete reversion to pre-stress values for GSH, GPx and SOD, while HG + PA-provoked a reduction in GSH content and an alteration in GPx and SOD activities (Table 2). These results indicate that all tested compounds alone or combined protect H9c2 cells against the redox imbalance caused by the challenge of high glucose and palmitic acid, resulting in the preservation of cellular viability.
| Crystal violet (% of control) | ROS (% of control) | JC-1 (% of control) | GSH (% of control) | GPx (% of control) | SOD (% of control) | Carbonyl groups (% of control) | |
|---|---|---|---|---|---|---|---|
| Control | 100.1 ± 4.3a | 100.0 ± 4.8a | 100.0 ± 8.7a | 100.2 ± 7.1a | 100.0 ± 6.1a | 100.4 ± 6.6a | 100.0 ± 5.7a |
| HG + PA | 78.6 ± 2.4b | 142.5 ± 5.5b | 57.7 ± 5.0b | 62.5 ± 3.7b | 127.7 ± 8.6b | 86.2 ± 1.5b | 171.2 ± 13.0b |
| EC + HG + PA | 88.1 ± 5.4c | 119.9 ± 8.7c | 74.5 ± 6.7c | 92.1 ± 5.9a | 108.9 ± 9.4ab | 96.0 ± 6.7a | 134.3 ± 9.9c |
| DHBA + HG + PA | 86.0 ± 5.1cd | 127.1 ± 5.1cd | 77.3 ± 6.6c | 102.4 ± 5.2a | 99.9 ± 11.4a | 107.7 ± 4.5a | 135.7 ± 11.1c |
| MET + HG + PA | 84.7 ± 5.8cd | 127.1 ± 8.5cd | 78.7 ± 6.8c | 111.3 ± 8.1a | 104.4 ± 8.4a | 105.2 ± 8.3a | 135.0 ± 13.3c |
| MIX + HG + PA | 82.6 ± 5.8d | 131.4 ± 7.7d | 78.2 ± 6.5c | 95.6 ± 9.8a | 97.1 ± 5.5a | 97.6 ± 8.2a | 141.6 ± 8.0c |
3.3. EC, DHBA, MET and MIX restrain downregulation of ERK and upregulation of JNK phosphorylation induced by HG and PA in cardiac H9c2 cells
ERK and JNK belong to the mitogen-activated protein kinase (MAPK) family, and in general terms, these proteins are closely related to oxidative stress, since their dysregulation is associated with cardiac dysfunction.35,36 Thus, to elucidate molecular mechanisms underlying recovery in the redox status imbalance provoked by pre-treatment of cells with EC, DHBA, MET and MIX in HG + PA-challenged cells, the phosphorylation of ERK and JNK kinases was assayed. As shown in Fig. 1A and B, HG + PA remarkably inhibited the phosphorylation of ERK, while all substances except MET induced an increase in p-ERK values; in contrast, all compounds and MIX effectively mitigated the activation of JNK caused by the HG + PA challenge (Fig. 1A and C). No compound had an effect on total ERK and JNK levels.3.4. EC, DHBA, MET and MIX alleviate HG + PA-triggered apoptosis in cardiomyocytes
Apoptosis is one of the main pathophysiological mechanisms of DCM injury,14,15 which is regulated by pro- and anti-apoptotic members of the Bcl-2 family of proteins, with caspase-3 being a reliable marker for the activation of this process.15 To further explore the potential contribution of apoptosis inhibition to the protective effect of all compounds and their mixture, levels of pro- and anti-apoptotic Bcl-2 proteins (Bax and Bcl-2, respectively), as well as cleaved caspase-3 values and activity were examined.As shown in Fig. 2, treatment of cells with HG + PA diminished Bcl-2 levels and enhanced Bax and cleaved caspase-3 values, as well as caspase-3 activity. Nevertheless, pre-treatment with EC, DHBA, MET or MIX significantly prevented the condition of apoptosis induced by HG + PA through the upregulation of Bcl-2 and downregulation of Bax, cleaved caspase-3 and caspase-3 activity. Collectively, these results suggest that all tested compounds and their mixture can prevent HG + PA-induced apoptosis through the modulation of key players involved in this type of death in H9c2 cells.
3.5. EC, DHBA, MET and MIX avoid HG + PA-inhibited autophagy in H9c2 cardiac cells
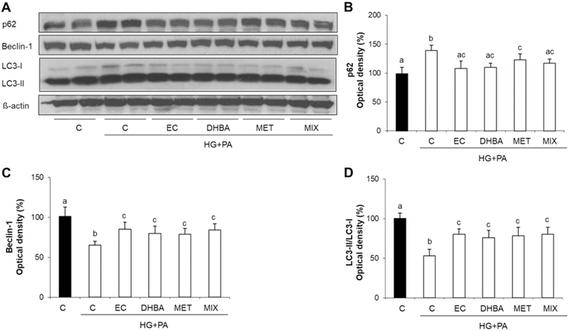
Autophagy plays a vital role in maintaining cellular homeostasis and it is frequently impaired in DCM.14 In general terms, autophagy is induced by an initial step that includes Beclin-1, which is inversely correlated with p62 and positively with LC3.16 To continue analysing potential molecular mechanisms underlying the protective effect of EC, DHBA, MET and MIX on HG + PA-induced oxidative stress and cell damage, key autophagy proteins, such as p62, Beclin-1 and LC3, were evaluated. Fig. 3 shows that the HG + PA challenge impaired autophagy by increasing p62 values and decreasing Beclin-1 levels and the LC3-II/LC3-I ratio. In contrast, values of Beclin-1 and the LC3-II/LC3-I ratio were equally enhanced, as well as p62 levels being correspondingly diminished, in cells treated with all compounds alone or with their mixture (Fig. 3A–D). All these results suggest that EC, DHBA, MET and MIX might contribute to cellular protection through restoration of the autophagy process in cardiomyocytes.3.6. EC, DHBA and MIX avert cardiomyocyte cell death, ROS generation and MMP loss via JNK
Redox balance constitutes a key aspect for correct cardiac functionality;15 in this regard, the activation of JNK has been associated with the cardiac response to stress, and it influences different processes, including cell survival and apoptosis.35 Thus, to determine the potential role of JNK in the modulation of cell viability, ROS generation and MMP preservation induced by EC, DHBA and MIX, the effect of a specific JNK inhibitor (SP600125, SP) was assayed. To this end, cardiac H9c2 cells were pre-incubated with 10 μM of SP for 1 h and treated for 2 h with EC, DHBA or MIX prior to a 22 h-HG + PA challenge.Fig. 4 shows that EC, DHBA and MIX pre-treatment partially prevented a decrease in cell viability and MMP loss, as well as an increase in ROS generation induced by HG + PA incubation. In HG + PA-challenged cells that were pre-incubated with EC, DHBA or MIX, the inhibition of JNK maintained ROS generation and MMP values at levels comparable to those of untreated cells (controls). In addition, cell viability was further improved in the presence of the JNK inhibitor; the percentage of live cells in those pre-treated with SP was higher than that of just pre-incubated EC, DHBA or MIX following submission to the HG + PA challenge, although control values were not reached.
3.7. EC, DHBA and MIX prevent cardiomyocyte apoptosis stimulation and autophagy inhibition via JNK
To further clarify the role of JNK on the protective effect induced by EC, DHBA and MIX treatment in HG + PA-challenged cells, the effect of the JNK inhibitor on apoptosis and autophagy was investigated. As shown in Fig. 4 and 5, in HG + PA-treated cells, EC, DHBA and MIX induced a decrease in Bax and cleaved caspase-3 levels, as well as in the caspase-3 activity that were further improved when cells had previously received SP. Accordingly, in HG + PA-challenged cells, Bcl-2 values induced by EC, DHBA and MIX were further enhanced by inhibiting JNK, reaching higher levels than those of controls with all tested compounds used individually or in combination (Fig. 5).In addition, cell pre-treatment with EC, DHBA or MIX partially prevented the alteration in the levels of p62 and Beclin-1, as well as in the LC3-II/LC3-I ratio induced by HG + PA incubation (Fig. 6). Accordingly, under HG + PA conditions, the inhibition of JNK in cells pre-treated with EC, DHBA or MIX further decreased p62 levels and increased Beclin-1 values and the LC3-II/LC3-I ratio in comparison to EC-, DHBA- and MIX-HG + PA-treated cells, producing similar values to those of untreated cells, except for Beclin-1 levels in MIX pre-treated cells later exposed to the HG + PA challenge.
Altogether, these results suggest the involvement of JNK in the maintenance of normal cardiac functionality through the downregulation of apoptosis and upregulation of autophagy induced by EC, DHBA and MIX.
4. Discussion
Flavonoids have been suggested to exert beneficial effects on diabetes and have drawn attention because of their safety.3,15 In addition, a rising amount of evidence suggests that the use of flavonoid combinations could lead to synergistic effects, including under pathological situations.23,26,37 Specifically, in diabetes it has been suggested that the administration of flavonoids together with antidiabetic drugs could be accompanied by improved effects in comparison with those obtained with the drug alone.25,38 However, the study of these combinations needs further investigation, especially in the case of DCM as the evidence is very scarce. The present study demonstrates, for the first time, the cardioprotective effect of epicatechin and colonic-derived flavanol metabolite DHBA in a model of DCM induced by glucolipotoxic conditions in H9c2 cardiac cultured cells. These results contribute to elucidating the molecular mechanism of action of these phenolic compounds (alone or combined with MET) and provide new evidence for their potential benefits during a simulated DCM context. Thus, beneficial activities exerted by these natural compounds, comparable to those of metformin (the first-line drug used in diabetes) contributed to prevent redox imbalance by reducing ROS overproduction, restoring altered antioxidant defences, restraining signalling pathways related to stress and apoptosis, and recovering the autophagy process.After the intake of flavanol-rich foods, the most abundant compounds in urine and blood are hydroxybenzoic acids, among other phenolic acids, which accumulate in tissues together with other untransformed phenolic compounds (pure compounds), all of them in their intact or conjugated forms due to a conjugation–deconjugation cycle.4,6,39 Moreover, the beneficial effects of a flavanol-rich diet have been related to the cumulative activities of circulating compounds.26,40 Therefore, the use of pure compounds and combinations of flavanol-derived colonic metabolites accurately resembles physiological conditions. In this regard, it should be highlighted that a realistic concentration of these phenolic compounds (1 μM) has been used, as they have been detected at low μM concentrations in biological fluids after the intake of foodstuffs containing flavanols.6,22 Accordingly, in the present study, EC and one of the most abundant colonic phenolic compounds found in biological fluids after flavanol intake, DHBA, alone or in combination with metformin, have been selected to evaluate their protective effect and potential synergistic actions against DCM.
Diabetes is a very complex metabolic disease characterized by hyperglycaemia and hyperlipidaemia, among other metabolic alterations. Different studies have shown that oxidative stress plays a crucial role in the development and progression of DCM,15,33,34 and hyperglycaemia and/or hyperlipidaemia stimulate ROS production and altered MMP.14–16 Under this pro-oxidative situation, antioxidant defences, namely GSH, GPx and SOD, are weakened and oxidized products such as carbonyl groups are accumulated in the heart.38,41 Notably, in this study, EC and DHBA effectively attenuated glucolipotoxic-induced oxidative stress by reducing ROS production and carbonyl groups and by enhancing antioxidant enzymatic (GPx and SOD) and non-enzymatic (GSH) defences. Accordingly, triterpene isolated from the stem bark of P. longifolia and an acai-supplemented diet exerted an antioxidant cardioprotective effect on cultured cells and rats, respectively, with diminished ROS generation and reinforcement of antioxidant cellular defences.25,41 Likewise, reduced oxidative stress was reported when high-glucose cardiac cells were incubated with MET (1 μM), alone or combined with triterpene,25 in agreement with the present study. Additionally, the administration of a flavanol-rich food to Zucker diabetic fatty rats, alone or combined with MET, greatly prevented ROS and carbonyl group generation, demonstrating the capacity of these phenolic compounds to ameliorate oxidative damage and protect the heart during DCM;38 indeed, an enhanced protective effect of natural compounds for some parameters related to oxidative stress has been shown when compared to metformin alone.25 Interestingly and in agreement with present results, comparable protective effects for phenolic compounds and MET regarding the recovery of cellular redox status have also been demonstrated in cardiac cells.23
Persistent oxidative stress can lead to myocardial cell apoptosis and death.14,15 Importantly, adult cardiomyocytes rarely proliferate, and the loss of cells could threaten cardiac function.14 Indeed, increased apoptosis constitutes a crucial event in the pathogenesis of DCM, and this loss of cells has been related to high mortality and morbidity in patients with DCM.14,33 Consequently, a reduction in cardiomyocyte apoptosis could constitute a good strategy for protecting cardiac cells from glucolipotoxicity.14,15 In agreement with this, EC and DHBA, alone or with MET, diminished cardiomyocyte apoptosis induced by high glucose and PA by alleviating pro-apoptotic alterations (Bax and caspase-3 levels and caspase-3 activity) and favouring anti-apoptotic modifications (Bcl-2 values). Moreover, EC and DHBA, in the presence or absence of MET, improved the mitochondrial dysfunction (loss of MMP), upregulated p-ERK, and downregulated phosphorylated JNK; remarkably, all these effects have been associated with reduced apoptosis in cardiomyocytes.15,35,36 Correspondingly, a beneficial effect by diminishing apoptosis through the downregulation of Bax and caspase-3/7 activity and upregulation of Bcl-2 has been reported in diabetic animals fed with phenolic-compound-enriched diets or treated with MET.42,43 Similar anti-apoptotic effects were demonstrated in cardiomyocytes exposed to high concentrations of glucose and/or PA incubated with different natural components, including phenolic compounds, MET or their combination.23,25,28,44,45 Importantly, GLP-1 agonist antidiabetic drugs, such as exendin-4 and liraglutide have demonstrated a cardioprotective effect by inducing similar regulatory mechanisms, that is, alleviating mitochondrial injury, oxidative stress and apoptosis, finally leading to improved cellular function.27 In addition, ERK activation and JNK inhibition have been associated with cardioprotective activity under pathological stimuli by promoting anti-apoptotic effects,35,46,47 such as enhancement of the Bcl-2/Bax ratio.
Autophagy is a conserved process essential for maintaining cellular homeostasis, and its dysregulation has been connected to the development of different diseases, including DCM.14 Indeed, in a situation of glucolipotoxicity, autophagy is suppressed,16 and several studies have demonstrated the cardioprotective effect of the activation of this biological process in agreement with present results.14,33 Importantly, the stimulation of autophagy by commonly used antidiabetic drugs, such as MET, exendin-4 and liraglutide, as well as by the natural compound resveratrol has proved beneficial in alleviating diabetes-induced cardiac dysfunction.27,43,44 Thus, Xie et al.43 reported enhanced autophagy in the hearts of diabetic mice after chronic treatment with metformin, as demonstrated by increased levels of Beclin-1 and LC3-II in comparison to diabetic mice, although still lower than those of control animals, in agreement with the present study. Indeed, it has been shown that metformin enhanced autophagic flux and reduced apoptosis, providing a cardioprotective effect during cardiomyopathy.48 However, the role of autophagy induction in the heart remains controversial, and different studies have stated a beneficial effect after inhibiting this crucial process.14,16,33 It is noteworthy that it has been suggested that during DCM, the functions of autophagy could be different depending on the causes that provoked the activation, as well as on the stage and severity of the disease.16 In this regard, it has been indicated that in cardiac cells, autophagy induction may be a compensatory response to oxidative stress, as the activation of this essential cellular process has been associated with ameliorated ROS production and apoptosis, in agreement with our results.48,49 Therefore, in the present study, the regulatory effect on autophagy exerted by EC, DHBA, MET and their combination may help as a compensatory response in protecting against cell death for maintaining normal cellular function.
The role of JNK during DCM remains elusive, and beneficial and detrimental effects have been reported after its inactivation in cardiomyocytes.35 In this regard, it has been suggested that JNK differently contributes to cardiac damage depending on the duration and severity of the stimulus; thus, sustained activation of JNK has been associated with the promotion of cardiac damage through the regulation of cellular oxidative stress-related and growth/death pathways.35 In agreement, in the presence of a JNK inhibitor, EC and DHBA, alone or in combination with MET, showed improved protection of cardiac cells against HG + PA-induced ROS overproduction, MMP loss, apoptosis induction, autophagy inhibition and diminished cell viability. Similarly, it has been reported that in cardiomyocytes, MET prevented high-glucose-induced ROS overproduction, MMP loss and reduced cell viability, with these beneficial effects being abolished upon JNK activation.45 Besides, phenolic salvianolic acid showed an anti-apoptotic effect in the hearts of diabetic rats that was improved in the presence of a JNK inhibitor by decreasing Bax and caspase-3 levels and increasing values of Bcl-2.50 Therefore, JNK regulates multiple downstream proteins, including proteins of the Bcl-2 family that modulate apoptosis, and JNK is also involved in the regulation of mitochondrial function.35 In addition, the modulation of autophagy through JNK-dependent p62 accumulation has also been reported.51 All these mechanisms indicate that the regulation of JNK during cardiac dysfunction is complex and involves several pathways, and that a role for all these proteins on the protective effect described in the present study cannot be ruled out. In this regard, it should be highlighted that AMPK is a major target of MET.17 Interestingly, the antioxidant N-acetyl cysteine (NAC) and other phenolic compounds, such as triterpene methyl-3β-hydroxylanosta-9,24-dien-21-oate (RA3), as well as compounds used in the current study (EC and DHBA) have been shown to positively regulate the phosphorylated levels of AMPK in H9c2 cells under physiological and high-glucose conditions.12,25,52 This modulatory effect was related to improved metabolic efficiency of cardiac cells.12,52 Interestingly, NAC plus metformin was more efficient than NAC alone in preserving p-AMPK levels against a high-glucose challenge,52 although a comparable effect on p-AMPK values was reported when RA3 was combined with metformin or used alone.25 Further studies are required to explore these differential effects for natural phenolic compounds alone or in combination with metformin.
Due to the lack of reports about the effects of EC and 2,3-DHBA on DCM, this study can be considered a novel and relevant approach. These results constitute a complementary approach for elucidating the molecular mechanism of action of EC and 2,3-DHBA (alone or combined with MET) and provide new evidence for the potential beneficial effects of these natural compounds within a simulated DCM context. However, this study has some limitations. Deeper insights into the molecular effect of phenolic compounds on autophagy and apoptosis would be valuable to gain further understanding about their beneficial effects on health. In addition, to further evaluate the combined use of these compounds with antidiabetic drugs, in vivo studies should be performed to improve the understanding of their dosage, bioavailability and long-term protective effect within diabetic milieu. Likewise, in the present study, metformin was selected as it is considered a first-line drug for patients with diabetes, but the potential use of other antidiabetic drugs, such as dipeptidyl peptidase-4 inhibitors, a GLP-1 agonist with demonstrated cardiovascular benefits,53,54 alone or combined with natural phenolic compounds deserves further investigation. Thus, the potential use of natural compounds as nutraceuticals or dietary supplements could also be considered, but the scientific evidence on human health benefits and safety is still lacking.
Of further interest was the ability of EC and DHBA to show comparable improvements to those of MET in many parameters assayed in this study. These findings could indicate good efficacy for EC and DHBA to overcome certain complications. Similarly, Rugerio-Escalona et al.55 have demonstrated that extracts from Hamelia patens evoked comparable effects to those of MET in diabetic rats. Thus, it should be taken into account that the benefits of EC and DHBA treatment could be related to their ability to modulate multiple signalling pathways, maybe including some not targeted by MET and/or some that have not been analysed in the present study. Importantly, it must be also cautiously considered that the lack of additive or synergistic effects found in MIX could also indicate an interaction between the natural compound and the drug, which could even lead to herbal toxicity.18,37,56 Further investigations are required to explore the molecular mechanisms of action of natural compounds, and their combinations with current antidiabetic drugs to carefully evaluate their potential interactions and ensure the lack of long-term side effects and/or toxicity. The translation of in vitro research into animals and further into human clinical trials is also challenging and requires further efforts, as well as the establishment of the optimal dosage that safely leads to the maximum therapeutic response for type 2 diabetes treatment.
5. Conclusions
EC and DHBA, alone or in combination with MET, alleviated glucolipotoxic-induced cardiac damage in cultured H9c2 cells. These compounds, alone or in combination, counteracted redox imbalance by preventing the exhaustion of antioxidant defences, ameliorated apoptosis induction by upregulating anti-apoptotic proteins (Bcl-2) and downregulating pro-apoptotic proteins (Bax and caspase-3), and improved autophagy (diminished p62 and increased Beclin-1 and LC3-II/LC3-I ratio) to contribute to the maintenance of cell functionality. However, no additive or synergistic effects were detected when natural phenolic compounds and the antidiabetic drug were combined. Further studies are warranted to elucidate in more depth the regulation of apoptosis and autophagy by these compounds. In addition, the regulation of JNK seems to play a major role in the molecular modulation involved in the preservation of normal cardiomyocyte function. Importantly, EC and DHBA, at concentrations that are not toxic to cardiac cells and are reachable through diet, showed comparable effects to those of first-line anti-diabetic drug metformin. Thus, EC and DHBA could be considered suitable candidates in the prevention of DCM, although further research is needed.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by the grant RTI2018-095059-B-I00, funded by MCIN/AEI/10.13039/501100011033/ and by “ERDF A way of making Europe”. E. G.-D. was the recipient of a contract from Comunidad de Madrid (PEJ-2020-AI/BIO-18529). Technical assistance provided by Sergio Castillo-González is gratefully acknowledged (contracted by Comunidad de Madrid, PEJ-2018-TL/SAL-10236).References
- M. A. Martin and S. Ramos, Impact of cocoa flavanols on human health, Food Chem. Toxicol., 2021, 151, 112121, DOI:10.1016/j.fct.2021.112121.
- M. Rudrapal, S. J. Khairnar, J. Khan, A. B. Dukhyil, M. A. Ansari, M. N. Alomary, F. M. Alshabrmi, S. Palai, P. K. Deb and R. Devi, Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action, Front. Pharmacol., 2022, 13, 806470, DOI:10.803389/fphar.802022.806470.
- J. Quero, I. Mármol, E. Cerrada and M. J. Rodríguez-Yoldi, Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management, Food Funct., 2020, 11, 2805–2825 RSC.
- M. Monagas, M. Urpi-Sarda, F. Sanchez-Patan, R. Llorach, I. Garrido, C. Gomez-Cordoves, C. Andres-Lacueva and B. Bartolome, Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites, Food Funct., 2010, 1, 233–253, 10.1039/c1030fo00132e.
- G. Pereira-Caro, S. Gaillet, J. L. Ordóñez, P. Mena, L. Bresciani, K. A. Bindon, D. Del Rio, J.-M. Rouanet, J. M. Moreno-Rojas and A. Crozier, Bioavailability of red wine and grape seed proanthocyanidins in rat, Food Funct., 2020, 11, 3986, 10.1039/d3980fo00350f.
- M. Urpi-Sarda, M. Monagas, N. Khan, R. Llorach, R. M. Lamuela-Raventos, O. Jauregui, R. Estruch, M. Izquierdo-Pulido and C. Andres-Lacueva, Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry, J. Chromatogr. A, 2009, 1216, 7258–7267 CrossRef CAS PubMed.
- V. M. Zamora-Gasga, E. Montalvo-González, G. Loarca-Piña, P. A. Vázquez-Landaverde, J. Tovar and S. G. Sáyago-Ayerdi, Microbial metabolites profile during in vitro human colonic fermentation of breakfast menus consumed by Mexican school children, Food Res. Int., 2017, 97, 7–14, DOI:10.1016/j.foodres.2017.1003.1038.
- International Diabetes Federation, IDF Diabetes Atlas, International Diabetes Federation, Brussels, Belgium, 10th edn, 2021, available online: https://www.diabetesatlas.org, accessed on 14 December 2023 Search PubMed.
- E. Cremonini and P. I. Oteiza, (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells, Arch. Biochem. Biophys., 2018, 646, 55–63 CrossRef CAS PubMed.
- I. Cordero-Herrera, M. A. Martín, L. Goya and S. Ramos, Cocoa flavonoids protect hepatic cells against high glucose-induced oxidative stress. Relevance of MAPKs, Mol. Nutr. Food Res., 2015, 59, 597–609 CrossRef CAS PubMed.
- D. Alvarez-Cilleros, M. A. Martín, L. Goya and S. Ramos, (-)-Epicatechin and the colonic metabolite 3,4-dihydroxyphenylacetic acid protect renal proximal tubular cell against high glucose-induced oxidative stress by modulating NOX-4/SIRT-1 signalling, J. Funct. Foods, 2018, 46, 19–28, DOI:10.1016/j.jff.2018.1004.1051.
- E. García-Díez, M. E. López-Oliva, J. Pérez-Jiménez, M. A. Martín and S. Ramos, Metabolic regulation of (−)-epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid on the glucose uptake, lipid accumulation and insulin signalling in cardiac H9c2 cells, Food Funct., 2022, 13, 5602, 10.1039/d5602fo00182a.
- M. J. Wilkinson, A. Zadourian and P. R. Taub, Heart failure and diabetes mellitus: Defining the problem and exploring the interrelationship, Am. J. Cardiol., 2019, 124, S3–S11 CrossRef CAS PubMed.
- U. Varma, P. Koutsifeli, V. L. Benson, K. M. Mellor and L. M. D. Delbridge, Molecular mechanisms of cardiac pathology in diabetes - Experimental insights, Biochim. Biophys. Acta, Mol. Basis Dis., 2018, 1864, 1949–1959 CrossRef CAS PubMed.
- F. F. Jubaidi, S. Zainalabidin, I. S. Taib, Z. A. Hamid and S. B. Budin, The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis, Int. J. Mol. Sci., 2021, 22, 5094, DOI:10.3390/ijms22105094.
- C. Ouyang, J. You and Z. Xie, The interplay between autophagy and apoptosis in the diabetic heart, J. Mol. Cell Cardiol., 2014, 71, 71–80 CrossRef CAS PubMed.
- T. Salvatore, R. Galiero, A. Caturano, E. Vetrano, L. Rinaldi, F. Coviello, A. Di Martino, G. Albanese, R. Marfella, C. Sardu and F. C. Sasso, Effects of metformin in heart failure: From pathophysiological rationale to clinical evidence, Biomolecules, 2021, 11, 1834, DOI:10.3390/biom11121834.
- J. Blahova, M. Martiniakova, M. Babikova, V. Kovacova, V. Mondockova and R. Omelka, Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus, Pharmaceuticals, 2021, 14, 806, DOI:10.3390/ph14080806.
- T. C. Karagiannis, A. J. E. Lin, K. Ververis, L. Chang, M. M. Tang, J. Okabe and A. El-Ost, Trichostatin A accentuates doxorubicin–induced hypertrophy in cardiac myocytes, Aging, 2010, 2, 659–668 CrossRef CAS PubMed.
- H. J. Wang, E. Y. Lee, S. J. Han, S. H. Kim, B.-W. Lee, C. W. Ahn, B. S. Cha and H. C. Lee, Dual pathways of p53 mediated glucolipotoxicity-induced apoptosis of rat cardiomyoblast cell: activation of p53 proapoptosis and inhibition of Nrf2-NQO1 antiapoptosis, Metab. Clin. Exp., 2012, 61, 496–503 CrossRef CAS PubMed.
- T. Ma, X. Huang, H. Zheng, G. Huang, W. Li, X. Liu, J. Liang, Y. Cao, Y. Hu and Y. Huang, SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy, Oxid. Med. Cell. Longevity, 2021, 9265016, DOI:10.9261155/9262021/9926501.
- A. Rodriguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. Dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: a double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60, 2130–2140 CrossRef CAS PubMed.
- P. V. Dludla, C. J. F. Muller, J. Louw, S. E. Mazibuko-Mbeje, L. Tiano, S. Silvestri, P. Orlando, F. Marcheggiani, I. Cirilli, N. Chellan, S. Ghoor, B. B. Nkambule, M. F. Essop, B. Huisamen and R. Johnson, The combination effect of aspalathin and Phenylpyruvic Acid-2-O-b-D-glucoside from rooibos against hyperglycemia-induced cardiac damage: An in vitro study, Nutrients, 2020, 12, 1151, DOI:10.3390/nu12041151.
- A. Garate-Carrillo, I. Ramirez-Sanchez, J. Nguyen, J. Gonzalez, G. Ceballos and F. Villarreal, Antifibrotic effects of (−)-epicatechin on high glucose stimulated cardiac fibroblasts, J. Med. Food, 2021, 24, 1177–1185 CAS.
- N. F. Sangweni, R. A. Mosa, P. V. Dludla, A. P. Kappo, A. R. Opoku, C. J. F. Muller and R. Johnson, The triterpene, methyl-3β-hydroxylanosta-9,24-dien-21-oate (RA3), attenuates high glucose-induced oxidative damage and apoptosis by improving energy metabolism, Phytomedicine, 2021, 85, 153546, DOI:10.151016/j.phymed.152021.153546.
- D. Alvarez-Cilleros, S. Ramos, L. Goya and M. A. Martín, Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction, Food Chem. Toxicol., 2018, 115, 88–97, DOI:10.1016/j.fct.2018.1003.1006.
- W. Yu, W. Zha and J. Ren, Exendin-4 and liraglutide attenuate glucose toxicity-induced cardiac injury through mTOR/ULK1-dependent autophagy, Oxid. Med. Cell. Longevity, 2018, 2018, 5396806, DOI:10.5391155/5392018/5396806.
- B. Zhang, Y. Chen, Q. Shen, G. Liu, J. Ye, G. Sun and X. Sun, Myricitrin attenuates high glucose-induced apoptosis through activating Akt-Nrf2 signaling in H9c2 cardiomyocytes, Molecules, 2016, 21, 880, DOI:10.3390/molecules21070880.
- A. Granado-Serrano, M. Martín, L. Bravo, L. Goya and S. Ramos, Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-Kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2), J. Nutr., 2006, 136, 2715–2721 CrossRef CAS PubMed.
- K. Xu, X.-F. Liu, Z.-Q. Ke, Q. Ya, S. Guo and C. Liu, Resveratrol modulates apoptosis and autophagy induced by high glucose and palmitate in cardiac cells, Cell. Physiol. Biochem., 2018, 46, 2031–2040 CrossRef CAS PubMed.
- Z. Yang, Y. Wu, L. Wang, P. Qiu, W. Zha and W. Yu, Prokineticin 2 (PK2) rescues cardiomyocytes from high glucose/high palmitic acid-Induced damage by regulating the AKT/GSK3β pathway in vitro, Oxid. Med. Cell. Longevity, 2020, 2020, 3163629, DOI:10.3161155/3162020/3163629.
- X. Ye, W. Chen, P. Tu, R. Jia, Y. Liu, Y. Li, Q. Tang, X. Zheng and Q. Chu, Food-derived cyanidin-3-O-glucoside alleviates oxidative stress: evidence from the islet cell line and diabetic db/db mice, Food Funct., 2021, 12, 11599–11610 RSC.
- Y. Chen, Y. Hua, X. Li, I. M. Arslan, W. Zhang and G. Meng, Distinct types of cell death and the implication in diabetic cardiomyopathy, Front. Pharmacol., 2020, 11, 42, DOI:10.3389/fphar.2020.00042.
- D. Zheng, L. Chen, G. Li, L. Jin, Q. Wei, Z. Liu, G. Yang, Y. Li and X. Xie, Fucoxanthin ameliorated myocardial fibrosis in STZ-induced diabetic rats and cell hypertrophy in HG-induced H9c2 cells by alleviating oxidative stress and restoring mitophagy, Food Funct., 2022, 13, 9559–9575 RSC.
- S. Javadov, S. Jang and B. Agostini, Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives, Pharmacol. Ther., 2014, 144, 202–225 CrossRef CAS PubMed.
- M. Shvedova, Y. Anfinogenova, E. N. Atochina-Vasserman, I. A. Schepetkin and D. N. Atochin, c-Jun N-Terminal Kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury, Front. Pharmacol., 2018, 9, 715, DOI:10.3389/fphar.2018.00715.
- C. Smith and A. Swart, Aspalathus linearis (Rooibos)-a functional food targeting cardiovascular disease, Food Funct., 2018, 9, 5041–5058 RSC.
- E. García-Díez, M. E. López-Oliva, A. Caro-Vadillo, F. Pérez-Vizcaíno, J. Pérez-Jiménez, S. Ramos and M.Á Martín, Supplementation with a cocoa-carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in Zucker diabetic rats, Antioxidants, 2022, 11, 432, DOI:10.3390/antiox11020432.
- C. Menendez, M. Duenas, P. Galindo, S. Gonzalez-Manzano, R. Jimenez, L. Moreno, M. J. Zarzuelo, I. Rodriguez-Gomez, J. Duarte, C. Santos-Buelga and F. Perez-Vizcaino, Vascular deconjugation of quercetin glucuronide: the flavonoid paradox revealed?, Mol. Nutr. Food Res., 2011, 55, 1780–1790 CrossRef CAS PubMed.
- C. Kay, J. de Gesso, E. Warner, H. Amin, R. de Ferrars, M. Edwards, Q. Zang, D. O'Hagan and M. O'Connell, The bioactivity of flavonoids is likely the result of cumulative low exposure to a variety of structurally similar phenolic metabolites, FASEB J., 2015 DOI:10.1096/fasebj.1029.1091_supplement.1118.1094.
- V. N. Lavorato, D. C. de Miranda, M. C. Isoldi, F. R. Drummond, L. Lopes Soares, E. C. C. Reis, M. do Carmo Gouveia Pelúzio, M. L. Pedrosa, M. E. Silva and A. J. Natali, Effects of aerobic exercise training and açai supplementation on cardiac structure and function in rats submitted to a high-fat diet, Food Res. Int., 2021, 141, 110168, DOI:10.111016/j.foodres.112021.110168.
- J. Zou, D. Sui, W. Fu, Y. Li, P. Yu, X. Yu and H. Xu, Total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack alleviate oxidative stress, inflammation and apoptosis in a rat model of diabetic cardiomyopathy, J. Funct. Foods, 2021, 76, 104319, DOI:10.101016/j.jff.102020.104319.
- Z. Xie, K. Lau, B. Eby, P. Lozano, C. He, B. Pennington, H. Li, S. Rathi, Y. Dong, R. Tian, D. Kem and M.-H. Zou, Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice, Diabetes, 2011, 60, 1770–1778 CrossRef CAS PubMed.
- B. Wang, Q. Yang, Y.-Y. Sun, Y.-F. Xing, Y.-B. Wang, X.-T. Lu, W.-W. Bai, X.-Q. Liu and Y.-X. Zhao, Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice, J. Cell. Mol. Med., 2014, 18, 1599–1611 CrossRef CAS PubMed.
- M. Hu, P. Ye, H. Liao, M. Chen and F. Yang, Metformin protects H9C2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: Role of AMPK and JNK, J. Diabetes Res., 2016, 2016, 2961954, DOI:10.2961155/2962016/2961954.
- N. H. Purcell, B. J. Wilkins, A. York, M. K. Saba-El-Leil, S. Meloche, J. Robbins and J. D. Molkentin, Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14074–14079 CrossRef CAS PubMed.
- C. Sun, G. Zeng, T. Wang, H. Ren, H. An, C. Lian, J. Liu, L. Guo and W. Li, Astragaloside IV ameliorates myocardial infarction induced apoptosis and restores cardiac function, Front. Cell Dev. Biol., 2021, 9, 671255, DOI:10.673389/fcell.672021.671255.
- H. Kanamori, G. Naruse, A. Yoshida, S. Minatoguchi, T. Watanabe, T. Kawaguchi, Y. Yamada, A. Mikami, M. Kawasaki, G. Takemura and S. Minatoguchi, Metformin enhances autophagy and provides cardioprotection in δ-sarcoglycan deficiency-induced dilated cardiomyopathy, Circ. Heart Fail., 2019, 12, e005418, DOI:10.001161/CIRCHEARTFAILURE.005118.005418.
- H. Yuan, C. N. Perry, C. Huang, E. Iwai-Kanai, R. S. Carreira, C. C. Glembotski and R. A. Gottlieb, LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection, Am. J. Physiol.: Heart Circ. Physiol., 2009, 296, H470–H479 CrossRef CAS PubMed.
- Q. Chen, T. Xu, D. Li, D. Pan, P. Wu, Y. Luo, Y. Ma and Y. Liu, JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid A intervention, Am. J. Transl. Res., 2016, 8, 2534–2548 CAS , https://www.ajtr.org/ISSN:1943-8141/AJTR0025124.
- A. Puissant, G. Robert, N. Fenouille, F. Luciano, J. P. Cassuto, S. Raynaud and P. Auberger, Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation, Cancer Res., 2010, 70, 1042–1052 CrossRef CAS PubMed.
- R. Johnson, N. F. Sangweni, S. E. Mabhida, P. V. Dludla, L. Mabasa, S. Riedel, C. Chapman, R. A. Mosa, A. P. Kappo, J. Louw and C. J. F. Muller, An in vitro study on the combination effect of metformin and N-acetyl cysteine against hyperglycaemia-induced cardiac damage, Nutrients, 2019, 11, 2850, DOI:10.3390/nu11122850.
- P. Home, Cardiovascular outcome trials of glucose-lowering medications: an update, Diabetologia, 2019, 62, 357–369, DOI:10.1007/s00125-00018-04801-00121.
- O. Schnell, L. Rydén, E. Standl, A. Ceriello and on behalf of the D&CVD EASD Study Group, Updates on cardiovascular outcome trials in diabetes, Cardiovasc. Diabetol., 2017, 16, 128, DOI:10.1186/s12933-12017-10610-y.
- C. Rugerio-Escalona, C. Ordaz-Pichardo, E. Becerra-Martinez, M. C. Cruz-López, V. E. López-y-López, A. Mendieta-Moctezuma, I. E. Maldonado-Mendoza and F. E. Jiménez-Montejo, “Diabetes and metabolism disorders medicinal plants: A glance at the past and a look to the future 2018”: Antihyperglycemic activity of Hamelia patens Jacq. extracts, Evid.-Based Complement. Altern. Med., 2018, 7926452, DOI:10.7921155/7922018/7926452.
- M. G. Campos, J. Machado, M. L. Costa, S. Lino, F. Correia and F. Maltez, Case report: Severe hematological, muscle and liver toxicity caused by drugs and artichoke infusion interaction in an elderly polymedicated patient, Curr. Drug Saf., 2018, 13, 44–50 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04039a |
| This journal is © The Royal Society of Chemistry 2024 |