Open Access Article
Open Access ArticleAssociation of total and different food-derived advanced glycation end-products with risks of all-cause and cause-specific mortality†
Changyu
Si‡
a,
Fubin
Liu‡
a,
Yu
Peng
a,
Yating
Qiao
a,
Peng
Wang
a,
Xixuan
Wang
a,
Jianxiao
Gong
a,
Huijun
Zhou
a,
Ming
Zhang
*b and
Fangfang
Song
*a
aDepartment of Epidemiology and Biostatistics, Key Laboratory of Molecular Cancer Epidemiology, Tianjin, National Clinical Research Center for Cancer, Tianjin's Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, 300060, China. E-mail: songfangfang@tmu.edu.cn
bComprehensive Management Department of Occupational Health, Shenzhen Prevention and Treatment Center for Occupational Diseases, Shenzhen, 518020, China. E-mail: mingle1981@163.com
First published on 18th January 2024
Abstract
Background: advanced glycation end-products (AGEs), formed through a series of non-enzymatic reactions, can promote inflammation and oxidative stress. Their accumulation in the body has been linked to cardiovascular disease (CVD) and cancer. However, the association of total AGEs and AGEs from different food sources with risks of all-cause, CVD, and cancer mortality is still unknown. Methods: we conducted a prospective cohort study of a nationally representative sample of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 participants from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) and NHANES 2003–2006. A food frequency questionnaire (FFQ) was utilized to calculate total and different food-derived AGE intake. Associations between various dietary AGE scores and the risk of all-cause, CVD, and cancer mortality were assessed by weighted Cox proportional hazard regression models. Results: over a median follow-up period of 27.1 years, we found that in the general population, AGE scores of both baked foods and meat were risk factors for all-cause, CVD, and cancer mortality. Specially, higher AGE scores in total and those derived from 10 of the 13 food groups were statistically associated with an increased risk of CVD mortality. Egg-, fruit-, and vegetable-derived AGE scores were positively correlated with the risk of cancer mortality. Additionally, there were positive multiplicative and additive interactions between smoking and meat-derived AGE scores on all-cause mortality. Conclusions: high amounts of AGE consumption is associated with an increased risk of CVD mortality, and meat and baked food-derived AGEs were positively linked to all-cause, CVD, and cancer mortalities. Adherence to unhealthy lifestyles, such as smoking, may increase mortality from leading causes in individuals with AGE-enriched diet habits.
124 participants from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) and NHANES 2003–2006. A food frequency questionnaire (FFQ) was utilized to calculate total and different food-derived AGE intake. Associations between various dietary AGE scores and the risk of all-cause, CVD, and cancer mortality were assessed by weighted Cox proportional hazard regression models. Results: over a median follow-up period of 27.1 years, we found that in the general population, AGE scores of both baked foods and meat were risk factors for all-cause, CVD, and cancer mortality. Specially, higher AGE scores in total and those derived from 10 of the 13 food groups were statistically associated with an increased risk of CVD mortality. Egg-, fruit-, and vegetable-derived AGE scores were positively correlated with the risk of cancer mortality. Additionally, there were positive multiplicative and additive interactions between smoking and meat-derived AGE scores on all-cause mortality. Conclusions: high amounts of AGE consumption is associated with an increased risk of CVD mortality, and meat and baked food-derived AGEs were positively linked to all-cause, CVD, and cancer mortalities. Adherence to unhealthy lifestyles, such as smoking, may increase mortality from leading causes in individuals with AGE-enriched diet habits.
Introduction
Advanced glycation end-products (AGEs), also known as advanced Maillard reaction products, are a group of complex heterogeneous compounds produced by an interaction between reducing sugars and free amino groups.1 AGEs are accumulated and bound with their receptors to cause oxidative stress and activate inflammatory signaling pathways in tissue microenvironments, thus promoting the occurrence of chronic diseases such as type-2 diabetes, obesity, metabolic syndrome, cardiovascular diseases (CVDs), cancer, and neurodegenerative diseases.2–5There are two main sources of human exposure to AGEs: exogenous AGEs from food and endogenous AGEs originating from irreversible non-enzymatic reactions of reducing sugars or reactive dicarbonyls with free amino groups,6 with the former contributing more to the human AGE pool7 and being directly related to AGE levels in circulation.8 Moreover, the dietary intake of AGEs may act as a modifiable risk factor and a potential target of future intervention for aforementioned chronic diseases. However, most of the current evidence on the relationship between dietary AGEs and mortality is limited to cancer populations,9,10 with only one Japanese study reporting an association between AGE intake and the risk of mortality in the general population.11 Notably, the content of AGEs in various foods is not the same, depending mainly on their nutritional component and cooking methods. In particular, foods rich in fat and protein can propagate and accelerate the formation of new AGEs at high temperatures.12 However, research and evidence regarding the associations of AGEs from different food sources with risks of all-cause, CVD, and cancer mortality is limited.
We hypothesized that a high intake of AGEs was associated with an increased risk of mortality. Therefore, the aim of this study was to prospectively examine the associations of dietary AGEs in total and from different food sources with risk of mortality in a nationally representative cohort, thereby providing dietary guidance and developing disease prevention strategies.
Methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES), which was unique in its combination of interviews and physical examinations, aimed at assessing the health and nutritional status of adults and children in the United States. NHANES was conducted by the National Center for Health Statistics (NCHS) and all participants received written informed consent. NHANES has been approved by the NCHS Ethics Review Board.Our study used data from different periods of NHANES including NHANES III (1988–1994), and NHANES 2003–2004 and 2005–2006, as only the participants during these periods were given the Food Frequency Questionnaire (FFQ) to obtain the frequency data of food consumption. In this analysis, participants with missing interview or examination status (n = 2765) and missing follow-up (n = 9390) data from baseline and pregnant women (n = 871) were excluded. After further exclusion of those who had missing food frequency questionnaire sample weight (n = 3354), extreme total energy intake exceeding the 500–5000 kcal range (n = 1659), and extreme total AGE intake exceeding a range of weighted mean ± three standard deviations (3SD) (n = 177), we brought 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 subjects in the study (ESI Fig. 1†).
124 subjects in the study (ESI Fig. 1†).
AGE score calculation
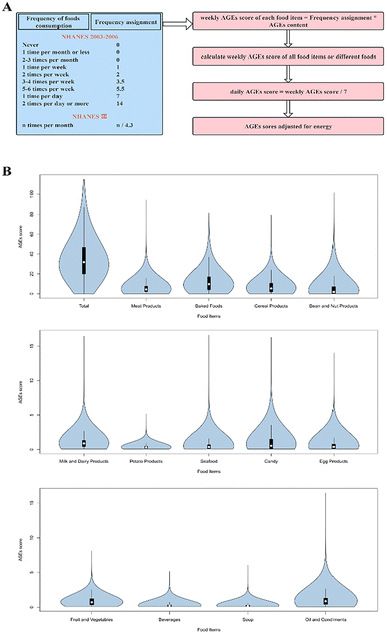
The FFQs of NHANES III and NHANES 2003–2006 have 81 and 139 items, respectively, and we took the intersection of the two for the subsequent calculation of the AGE scores. At present, there is a lack of standard and effective methods for assessing AGE intake. To accurately estimate AGE intake, we used a new database published by Scheijen et al.,13 where the ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was applied to measure the amounts of three major AGEs, including N(ε)-(carboxymethyl)lysine (CML), N(ε)-(1-carboxyethyl)lysine (CEL) and N(δ)-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1), in 190 food items. Since the NHANES FFQ does not report portion sizes and detailed cooking methods, we used the AGE score, which is a previously published strategy for assessing the intake of AGEs,14 as a reasonable alternative to the relative intake of AGEs in this study. According to the previously reported algorithm,14 responses were scored to indicate the weekly frequency of food consumption (Fig. 1A). Then, we multiplied the weekly frequency assignment by the CML, CEL, and MG-H1 contents of each food item in the AGE database.13 After that, we summed the three together as the combined AGEs to calculate the weekly AGE score of each food item, which was further added up to obtain weekly AGE scores of all foods or different food groups. Subsequently, a daily AGE score was obtained and naturally log (ln)-transformed. Ultimately, total energy intake was adjusted by residual method,15i.e., individual AGE score was calculated by adding the AGE score, corresponding to the average energy intake in the whole population to the residual, in order to remove the variation caused by total energy intake. Briefly, all food items were divided into thirteen categories, including meat and meat products, milk and dairy products, baked foods, bean and nut products, fruits and vegetables, oil and condiments, potatoes and their products, cereal and cereal products, candy, beverages, eggs, and egg products, soup and seafood.Outcome ascertainment
We used the NHANES Public-Use Linked Mortality File through December 31, 2019, which was linked by the NCHS population surveys to death certificate records from the National Death Index (NDI), a centralized database in the NCHS that records all deaths in the United States.16 We observed three outcome measures: all-cause mortality, CVD mortality, and cancer mortality. Definition for deaths followed the 10th revision of the International Statistical Classification of Diseases (ICD-10) guidelines (cancer: C00-C97; heart diseases: I00-I09, I11, I13 and I20-I51; Stroke: I60-I69).17 We defined death from CVD as a death due to heart disease or stroke. The time-to-event was calculated from the last dietary assessment to the date of death, loss to follow-up, or censorship (31 December 2019), whichever came first.Covariates assessment
Potential confounders, including age, gender, total energy intake, race/ethnicity, education, marital status, and poverty income ratio (PIR), were collected by standardized questionnaires in a household interview. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Mexican American, and others. Education was classified as below high school, high school, and college or above. PIR was used to assess family income status, with higher values indicating more family income, and was divided into ≤1.30, 1.31 to 3.50, and >3.50. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared and divided into three categories: <25 kg m−2, 25 to 30 kg m−2, >30 kg m−2.18 According to the responses to questions of whether they smoked at least 100 cigarettes during their lifetime and whether they were currently smoking, participants were classified as non-smokers, former smokers, and current smokers. Alcohol consumption was defined as none (0 g per day), moderate drinking (0.1 to 27.9 g per day for men and 0.1 to 13.9 g per day for women), and heavy drinking (≥28 g per day for men and ≥14 g per day for women).19 For NHANES 2003–2006, we classified physical activity as vigorous, moderate, and inactive on the basis of whether doing vigorous activities, or moderate activities. For NHANES III, the inactive group and the vigorous group were defined as those who did not report leisure time physical activity and those with recommended levels of physical activity,20 respectively. The moderate group was defined as neither being inactive nor meeting that recommended standard levels of physical activity. Hypertension, diabetes and dyslipidemia information were obtained from questionnaires and laboratory or examination data. In addition, the cancer history questionnaire was used to determine whether there was a history of cancer.Statistical analysis
Statistical analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC) using sample weights, strata, and primary sampling units embedded in the NHANES data, accounting for the complex, multistage, stratified, and clustered sampling design (including oversampling of certain subgroups) of NHANES.The distribution of AGE scores in total and from different food groups is illustrated in Fig. 1B and ESI Table 1.† Due to their non-normal distribution, all scores were naturally log-transformed and standardized when considered as continuous variables. We compared the baseline demographics and nutritional characteristics of participants between quartiles of total AGE scores, using Rao–Scott chi-square test for categorical variables and sampling-weighted analysis of variance (ANOVA) for continuous variables. In addition, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by performing sampling-weighted Cox proportional hazard models to examine associations of AGE scores in total and derived from different food groups as continuous and categorical variables (in quartiles), respectively, with risks of all-cause, CVD, and cancer mortality after the adjustment for covariates based on a directed acyclic graph (ESI Fig. 2†), the following covariates were included in the final model: age, gender, race, education, marital status, PIR, BMI, physical activity, smoking status, alcohol intake, cancer, and energy intake. The proportional hazards assumption was tested by the Schoenfeld residual method and held.
Secondary analyses were performed. First, after the food AGE dichotomy, population attributable risk (PAR) was used to estimate the contribution of overall and various food-derived AGE scores to all-cause, CVD, and cancer mortality to select primarily contributory food groups. Second, we performed subgroup analyses to examine the associations across strata of traditional risk factors including age, sex, BMI, smoking status, drinking, and physical activity for the AGE score in total and from primarily contributory food groups. Third, additive interaction was evaluated using the relative excess risk due to interaction (RERI) index and multiplicative interaction analyses were performed for AGE scores on the above-selected food groups and lifestyle factors.
Results
Baseline characteristics
The analysis included a total of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 participants, of which a weighted mean age (SD) at baseline was 45.60 (0.39) years and 57.5% were women. The weighted mean AGE score was 28.00, and the selected sampling-weighted distribution for baseline characteristics of study participants across quartiles of the AGEs is shown in Table 1. Participants who had the highest (Q4) total AGE scores were more likely to be elderly, male, non-Hispanic White, poorly educated, married or living with a partner, and with less family income when compared with those with the lowest (Q1) total AGE scores. Besides, they also tended to be former smokers, non-drinkers, physically active and had normal BMI and cancer history. The distribution of total energy intake was not significantly different among the different AGE score groups.
124 participants, of which a weighted mean age (SD) at baseline was 45.60 (0.39) years and 57.5% were women. The weighted mean AGE score was 28.00, and the selected sampling-weighted distribution for baseline characteristics of study participants across quartiles of the AGEs is shown in Table 1. Participants who had the highest (Q4) total AGE scores were more likely to be elderly, male, non-Hispanic White, poorly educated, married or living with a partner, and with less family income when compared with those with the lowest (Q1) total AGE scores. Besides, they also tended to be former smokers, non-drinkers, physically active and had normal BMI and cancer history. The distribution of total energy intake was not significantly different among the different AGE score groups.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 participants in NHANES 2003–2006 and NHANES III
124 participants in NHANES 2003–2006 and NHANES III
| Characteristics | Quartiles of total AGE scores | ||||
|---|---|---|---|---|---|
| Q1 (0–12.67) | Q2 (12.67–24,34) | Q3 (24.34–39.21) | Q4 (39.21–114.61) | P-value | |
| n = 2783 | n = 4749 | n = 6560 | n = 8032 | ||
| Data were expressed as weighted mean ± SD for continuous variables or weighted % for categorical variables. Differential analyses were carried out by using sampling-weighted analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables. Abbreviation: AGEs, advanced glycation end products; BMI, body mass index; PIR, poverty income ratio; SD, standard deviation. | |||||
| Age (years), mean ± SD | 43.30 ± 0.47 | 45.12 ± 0.50 | 46.13 ± 0.52 | 47.87 ± 0.62 | <0.0001 |
| Gender, % | 0.0304 | ||||
| Male | 44.52 | 45.17 | 45.05 | 50.39 | |
| Female | 55.48 | 54.83 | 54.95 | 49.61 | |
| Race/ethnicity, % | <0.0001 | ||||
| Non-hispanic white | 65.36 | 74.43 | 76.64 | 78.40 | |
| Non-hispanic black | 13.92 | 10.44 | 9.64 | 10.99 | |
| Mexican American | 10.18 | 7.97 | 5.98 | 4.31 | |
| Others | 10.54 | 7.16 | 7.74 | 6.30 | |
| Education, % | <0.0001 | ||||
| Below high school | 17.40 | 16.14 | 17.75 | 20.82 | |
| High school | 29.40 | 30.59 | 38.84 | 39.75 | |
| College or above | 53.20 | 53.27 | 43.41 | 39.43 | |
| Marital status, % | 0.0109 | ||||
| Married/living with partner | 59.32 | 62.37 | 64.59 | 65.63 | |
| Divorced/separated/widowed | 19.33 | 18.60 | 18.12 | 17.81 | |
| Never married | 21.35 | 19.03 | 17.29 | 16.56 | |
| Poverty income ratio, % | 0.0072 | ||||
| 0–1.30 | 17.74 | 18.29 | 16.58 | 20.26 | |
| 1.31–3.50 | 38.75 | 37.06 | 40.01 | 39.91 | |
| 3.51∼ | 43.51 | 44.65 | 43.41 | 39.83 | |
| Body mass index (kg m −2 ), % | <0.0001 | ||||
| <25 | 33.28 | 36.92 | 39.19 | 42.34 | |
| 25–30 | 31.44 | 33.71 | 32.60 | 31.24 | |
| >30 | 35.28 | 29.37 | 28.21 | 26.42 | |
| Physical activity, % | <0.0001 | ||||
| Vigorous | 34.42 | 36.87 | 33.98 | 34.52 | |
| Moderate | 33.30 | 36.27 | 40.36 | 40.86 | |
| Inactive | 32.28 | 26.86 | 25.66 | 24.62 | |
| Smoking status, % | 0.0028 | ||||
| Non-smoker | 48.85 | 49.46 | 50.18 | 47.93 | |
| Current smoker | 27.95 | 24.49 | 24.59 | 23.46 | |
| Former smoker | 23.20 | 26.05 | 25.23 | 28.61 | |
| Alcohol intake, % | 0.0008 | ||||
| No drinking | 72.84 | 73.74 | 75.47 | 77.56 | |
| Moderate drinking | 8.75 | 8.99 | 9.18 | 9.88 | |
| Heavy drinking | 18.41 | 17.27 | 15.35 | 12.56 | |
| Cancer history, % | 0.0184 | ||||
| Yes | 7.13 | 7.76 | 9.00 | 9.82 | |
| No | 92.87 | 92.24 | 91.00 | 90.18 | |
| Energy (kcal per day), mean ± SD | 2098.49 ± 25.02 | 2160.18 ± 27.45 | 2130.90 ± 23.02 | 2153.03 ± 24.84 | 0.2636 |
Association of total and different food-derived AGE score with risk of mortality
After a median follow-up time of 27.1 (interquartile range: 16.6–29.1) years, 7981 deaths from all causes were reported, among which 2668 were CVD-related and 1644 were cancer-related. The association of total and different food-derived AGE scores with the risks of all-cause, CVD, and cancer mortality after adjustment for potential confounding factors are shown in Fig. 2A–C and ESI Table 2.† For the total AGE score, HR values for all-cause mortality and CVD mortality were both 1.01 (95% CI: 1.00–1.02) when treated as a continuous variable. Participants with the highest AGE score had 1.86-fold higher risk of CVD mortality [HR (95% CI) = 1.86 (1.39–2.50)] in comparison to those with the lowest AGE score. For different food groups, AGE scores derived from dairy, baked, meat, and eggs were associated with an increased risk of all-cause mortality; and except for beverages, beans, and egg products, AGE scores of the remaining ten food sources had positive correlations with CVD mortality (all Ptrend values < 0.05). Moreover, AGE scores of fruits and vegetables, baked foods, meat, and eggs were related to the risk of cancer mortality (all Ptrend values < 0.05). It was worth noting that meat and baked food AGE scores were associated with an increased risk of all-cause, CVD, and cancer mortality. To be specific, as a continuous variable, the AGE score from meat products was positively associated with risks of all-cause mortality [HR (95% CI) = 1.01 (1.00–1.02)] and CVD mortality [HR (95% CI) = 1.02 (1.01–1.03)]; when treated as a categorical variable, among those inclined to consume the highest quartile of meat-derived AGEs, the risks of all-cause, CVD, and cancer mortality increased by 21% (95% CI: 1.01–1.45), 91% (95% CI: 1.35–2.70) and 31% (95% CI: 1.02–1.69), respectively. When the baked food-derived AGE score was used as a continuous variable, the HR values for all-cause, CVD, and cancer mortality were 1.01 (95% CI: 1.00–1.02), 1.02 (95% CI: 1.01–1.03), and 1.01 (95% CI: 1.00–1.02), respectively. There was also a significantly positive relationship between the Q4 [HR (95% CI) = 2.13 (1.59–2.86)] level of the AGE score of baked foods and CVD mortality, with a similar risk trend for all-cause and cancer mortality.Consequently, to pick out the food groups with the primary attribution to mortality risk, we used PAR analysis after dichotomizing lifestyle factors. Of the 13 food groups, meat, baked foods, and potatoes were considered to be the food sources of AGE score that had a major contribution to mortality, with higher PAR values (Table 2 and ESI Table 3†). The PAR of all-cause mortality increased from 2.4% when exposed to the Q4 level alone to 10.9% when exposed to a combination of Q2, Q3, and Q4 levels for the meat-derived AGE score, from 8.0% to 14.2% for baked food-derived AGE score, and from 2.0% to 13.6% for the potato-derived AGE score. For CVD mortality, the PAR for meat-, baked foods- and potato-derived AGE scores increased from 2.8% to 46.3%, 15.6% to 49.4%, and 2.2% to 42.3%, respectively. The PAR for cancer mortality also showed substantially similar results, with values increasing from 4.8% to 26.5% for meat sources, 15.2% to 29.7% for baked food sources, and 1.7% to 24.9% for potato sources. In a nutshell, as the incremental combination of AGE levels, the proportion of mortality attributable to it increased. The PAR analysis results for all-cause, CVD, and cancer mortalities by the other food groups, which were not included in subsequent analyses due to their low or negative PAR values (ESI Table 3†).
| Food group sources | N | PAR (%, 95% confidence interval) | ||
|---|---|---|---|---|
| All-cause mortality | CVD mortality | Cancer mortality | ||
| The model was adjusted for age (<65, ≥65), gender (female, male), race (white, others), education (below high school, others), poverty income ratio (0–1.3, >1.3), body mass index (<30, ≥30 kg m−2), physical activity (vigorous or moderate, inactive), smoking status (yes, no), drinking status (yes, no), marital status (never, others), energy (low, high) and cancer (yes, no). Abbreviations: AGEs, advanced glycation end products; PAR, population attributable risk; CVD, cardiovascular disease. | ||||
| Meat and meat products | ||||
| ≥Q4 | 8734 | 2.4 (0.6, 4.2) | 2.8 (0.2, 5.9) | 4.8 (0.7, 8.9) |
| ≥Q3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907 907 |
6.1 (2.5, 9.7) | 20.2 (14.3, 25.9) | 12.0 (3.9, 20.0) |
| ≥Q2 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 777 |
10.9 (3.9, 17.8) | 46.3 (36.6, 55.0) | 26.5 (11.3, 40.5) |
| Baked foods | ||||
| ≥Q4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 268 |
8.0 (5.7, 10.4) | 15.6 (11.5, 19.6) | 15.2 (10.0, 20.2) |
| ≥Q3 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 394 394 |
12.9 (8.7, 17.2) | 33.2 (26.4, 39.6) | 21.8 (12.7, 30.6) |
| ≥Q2 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 766 766 |
14.2 (6.6, 21.7) | 49.4 (38.6, 58.8) | 29.7 (13.6, 44.2) |
| Potatoes and their products | ||||
| ≥Q4 | 4850 | 2.0 (0.8, 3.3) | 2.2 (0.1, 4.4) | 1.7 (0.1, 4.0) |
| ≥Q3 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637 637 |
3.4 (0.8, 6.1) | 9.3 (4.7, 13.8) | 6.6 (0.7, 12.4) |
| ≥Q2 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 533 |
13.6 (7.0, 20.2) | 42.3 (32.5, 51.1) | 24.9 (10.9, 38.0) |
Further subgroup analyses were carried out based on traditional risk factors. A higher total AGE score was associated with an increased risk of all-cause mortality in the age ≥ 60, CVD mortality in all subgroups except the age < 60, and drinkers (all Ptrend values < 0.05; ESI Table 4†). Compared with the Q1 level, the Q4 level of meat-derived AGE score was positively associated with risk of all-cause mortality among smokers and non-drinkers, CVD mortality in all subgroups except the age < 60 and physically inactive participants, and cancer mortality in non-drinkers and physically active participants (Ptrend < 0.05; ESI Table 5†). With regard to AGE score from baked foods, the risk of all-cause mortality increased in the age ≥ 60, female, non-drinkers, and physically inactive participants, CVD mortality in all subgroups except the age < 60, drinkers, and cancer mortality among smokers (Ptrend < 0.05; ESI Table 6†). For potato-derived AGE score, the increased risk was observed in the age ≥ 60, female and non-drinkers for all-cause mortality and CVD mortality, and in physically active participants for CVD mortality. (Ptrend < 0.05; ESI Table 7†).
Interactive analysis
Based on modifiable lifestyle factors, we observed joint effects between smoking and meat-derived AGE score on the risk of all-cause mortality as well as between physical activity and meat-derived AGE score on the risk of cancer mortality (all Pinteraction < 0.05; ESI Table 5†). Furthermore, physical activity and AGE score from potato products also had a multiplicative interaction on CVD mortality (Pinteraction < 0.05; ESI Table 7†). Specifically, smokers with the highest AGE score from meat products had a 74.0% increased risk of all-cause mortality compared to non-smokers with the lowest AGE scores (Fig. 3A). Moreover, compared with physically active subjects with the lowest AGE scores, those who were physically inactive and had the highest meat- and potato-derived AGE scores suffered a 94.0% and 60.0% increased risk of cancer and CVD mortality, respectively (Fig. 3B and C, ESI Table 8†).We further performed additive interaction analysis with more biological significance after adjusting for covariates. As shown in ESI Table 9,† physical inactivity and meat-derived AGE score had an additive interaction on all-cause mortality (RERI = −0.150, P = 0.0312). Besides, there were positive additive interactions between smoking and meat-derived AGE scores on all-cause mortality (RERI = 0.152, P = 0.0107) and cancer mortality (RERI = 0.364, P = 0.0159), as well as between smoking and baked food-derived AGE score on cancer mortality (RERI = 0.465, P = 0.0032).
Discussion
In this large prospective study of a nationally representative cohort, we reported that higher AGE scores from meat and baked food sources increased the risk of mortality from all-cause, CVD, and cancer. In particular, AGE scores in total and from 10 of 13 food groups (except beverage, bean, and egg sources) were primarily associated with CVD mortality risk. Furthermore, we demonstrated a positive trend between fruits and vegetables, as well as eggs-derived AGE scores with the risk of cancer mortality. Moreover, we observed that the AGE scores in total and from three food groups (meat, dairy, and potato products), primarily attributable to CVD and cancer mortality, interacted with certain modifiable lifestyle factors (e.g. physical activity and smoking).With the rapid development of social economy and accelerated pace of life, people tend to buy fast food or convenience food with high energy content but low nutritional value,21 so that food consumption and dietary patterns have undergone striking changes, shifting from traditional diets high in grains and fiber to more westernized diets, such as excessive absorption of refined carbohydrates and immoderate consumption of red or processed meats.22 Currently, the modern diet as the predominant source of AGEs and produces a significant increase in the body's AGEs pool is now well documented.23,24 Earlier studies have explored the association between total AGE intake and the risks of CVD and cancer mortality in cancer patients. For example, the Women's Health Initiative (WHI) study referred to a published database that estimated a single CML-AGEs content using ELISA12 to assess AGEs intake and reported more post-diagnosis dietary CML intake increased the risk of mortality in breast cancer patients with a longer follow-up time of about 15.1 years.10 Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) study suggested a null association between pre-diagnosis dietary AGEs intake assessed by a combination of CML, CEL, and MG-H1 using a database based on a validated UPLC-MS method13 and all-cause mortality in colorectal cancer patients with a follow-up time of only 5.67 years.9 However, the effects of AGEs on a healthy population tend to be different from those with pre-existing conditions. The Takayama Study focused on the general population in Japan, based on an UPLC-MS method,13 and did not support a positive relationship between dietary CML intake with mortality in the general population after 14.1 years of follow-up.11 Specifically, the Japanese study observed a negative association between CML intake and all-cause, non-cancer, non-CVD mortality in males, possibly due to the protective effects of CML intake from nuts/pulses against mortality in males according to subgroup analysis by food sources. However, in the present study, subgroup analysis stratified by sex revealed that total AGE score was not associated with all-cause mortality in both sexes, but AGE scores from total, meats and baked foods increased the risk of CVD mortality in both males and females, and the potato-derived AGE score could also increase the risk of CVD mortality in females. Of note, we herein assessed the intake of combined AGEs containing CML, CEL, and MG-H1 by utilizing the UPLC-MS method in the Western population, whose cooking methods and the amount and duration of heat exposure resulting in the formation of AGEs may differ from the Japanese population, which could be the reason for the inconsistent findings. More research is warranted to clarify the association of the intake of dietary AGEs with the risk of mortality.
Several subgroup differences were found, highlighting the positive association of AGE score with CVD mortality risk in physically active people and smokers. In addition, we obtained a better handle on smoking, considered a poor lifestyle, and meat-derived AGE score had positive multiplicative and synergy additive interactions on all-cause mortality. Smoking cessation might have an active influence on CVD by reducing the concentration of AGEs.25,26 Therefore, taking well-directed intervention measures, such as giving up smoking may be helpful to avert adverse outcomes.
In addition, considering the different combined AGE values of various food items, we consequently utilized dietary questionnaires from the NHANES database to calculate the total AGEs and different food-derived AGE scores and selected three food groups primarily contributing to mortality from main causes through PAR analysis. To date, the evidence on the relationship between AGE scores derived from diverse food and mortality was scarce; moreover, the Japanese study suggested that the effects of CML on mortality might vary according to food sources. Herein, we observed that meat-derived AGE score would augment risks of all-cause, CVD, and cancer mortalities, consistent with the findings of a study suggesting that meat intake (non-seafood) was associated with an increased risk of mortality among U.S. adults.27 High consumption of cereal and potato products, especially those with high levels of refined carbohydrates and ultra-processed foods, was also associated with increased CVD mortality.28 As in our study, considering the baked foods, CVD mortality risk increased 1.13-fold in the highest quartile of the AGE score. It was worth noting that the uptake of seafood-derived AGEs was detrimental to CVD mortality, which may be due to the fact that not only fresh seafood, but also seafood cooked in different ways (such as fried or smoked fish) were involved in our food group. Likewise, although many studies have indicated that eating fatty or oily fish was strongly related to reduced mortality from CVD, it is surprising to see that evidence did not show such an assumption for fried fish or fish sandwiches, especially those commercially fried in unhealthy oils, which are often low in omega-3.29 As for egg products, in high-temperature baking such as fried eggs, the egg liquid would provide enough protein and free amino acids, and when the amino group was supplemented in large quantities, the Maillard reaction will dominate.30 These results reinforced the fact that the accumulation of AGEs due to the systematic heating and processing of foods was associated with the adverse health effects related to the diet, so the generation of AGEs in food should be avoided, and consumers can be educated on cooking methods produced by low AGEs, such as poaching, steaming, stewing, and boiling.12,31
The major extraordinary edge in this study included that, to our knowledge, this was the first longitudinal study to systematically explore the association of AGE score in total and from different food groups with risks of all-cause, CVD, and cancer mortality. This added to the growing body of evidence examining the relationship between dietary intake of AGEs and mortality in the general population. Besides, we used a validated food database based on the UPLC-MS methodology to more accurately assess the exposure levels of AGEs containing three main compounds, i.e., CML, CEL, and MG. Nonetheless, our study also has some boundaries that must be well thought out. First, this study may have recall bias due to the self-reported dietary questionnaires by participants, which may lead to misclassification of exposure. Secondly, since the food items in FFQ of NHANES III and NHANES 2003–2006 were not completely consistent, the intersection of food items in the two datasets was utilized, which may make the AGE score estimation not strictly accurate. Thirdly, due to the mixing of food items in NHANES FFQ, our classification of food was not completely precise. For instance, we did not distinguish between red meat and white meat, but both were categorized as meat products, which may have a misclassification bias when assessing the effects of AGEs on mortality. Fourthly, we cannot rule out biases due to unmeasured confounding factors, such as the possibility that participants who ate more meat and potatoes consumed less fruits. Fifthly, the levels of AGEs also rest with cooking methods, temperature, and time, which can lead to deviations in the measurement of intake of AGEs. Finally, the potentially toxic by-products of the Maillard reaction, such as 5-hydroxymethylfurfural, acrylamide, or heterocyclic aromatic amines, may have potentially additive or synergistic negative health effects on diet AGEs, whereas, we obtained no available information about these information in NHANES.
Conclusions
Our findings suggested that the intake of AGEs in total and from most food sources increased the risk of CVD mortality, and a higher intake of meat- and baked-derived AGEs was associated with higher all-cause, CVD, and cancer mortality. Moreover, smoking and meat-derived AGE scores had both positive additive and multiplicative interactions on all-cause mortality. Hence, it is crucial to abide by a healthy dietary pattern and lifestyle, such as eating less refined carbohydrates and ultra-processed foods, quitting smoking may have great public health significance in preventing adverse outcomes. However, these findings require further prospective studies to prove the role of dietary AGEs in all-cause and cause-specific mortality, as well as intervention studies targeting dietary AGEs reduction to assess the potential benefits on health-related outcomes.Author contributions
Conceptualization: Fangfang Song, Ming Zhang. Data curation: Ming Zhang, Peng Wang. Formal analysis: Changyu Si, Yu Peng, Yating Qiao. Funding acquisition: Ming Zhang, Fangfang Song. Project administration: Fangfang Song. Investigation: Changyu Si, Xixuan Wang. Methodology: Fubin Liu. Software: Xixuan Wang, Jianxiao Gong, Huijun Zhou. Supervision: Fangfang Song, Ming Zhang. Validation: Xixuan Wang. Visualization: Fubin Liu. Writing – original draft: Changyu Si, Fubin Liu. Writing – review and editing: Changyu Si, Fubin Liu, Yu Peng, Yating Qiao, Peng Wang, Xixuan Wang, Jianxiao Gong, Huijun Zhou, Ming Zhang, Fangfang Song. Drs. Song and Zhang had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.Conflicts of interest
The authors declare they have no conflict of interest.Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2500400 and 2021YFC2500401), the National Natural Science Foundation of China (81974488), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A) and Guangdong Basic and Applied Basic Research Foundation (2022A1515010436). The funding agencies had no role in study design, data collection, analysis, decision to publish, or manuscript preparation. The authors would like to thank the study participants and team members. We gratefully acknowledge the contributions of all staff who work on The National Health and Nutrition Examination Surveys.References
- J. O'Brien and P. A. Morrissey, Nutritional and toxicological aspects of the Maillard browning reaction in foods, Crit. Rev. Food Sci. Nutr., 1989, 28(3), 211–248 CrossRef PubMed.
- R. Ramasamy, S. J. Vannucci, S. S. Yan, K. Herold, S. F. Yan and A. M. Schmidt, Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation, Glycobiology, 2005, 15(7), 16R–28R CrossRef CAS PubMed.
- A. Riehl, J. Németh, P. Angel and J. Hess, The receptor RAGE: Bridging inflammation and cancer, Cell Commun. Signaling, 2009, 7, 12 CrossRef PubMed.
- N. T. Moldogazieva, I. M. Mokhosoev, T. I. Mel'nikova, Y. B. Porozov and A. A. Terentiev, Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases, Oxid. Med. Cell Longevity, 2019, 2019, 3085756 Search PubMed.
- H. Chen, L. Wu and Y. Li, et al., Advanced glycation end products increase carbohydrate responsive element binding protein expression and promote cancer cell proliferation, Mol. Cell. Endocrinol., 2014, 395(1–2), 69–78 CrossRef CAS PubMed.
- R. D. Semba, A. Ang and S. Talegawkar, et al., Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: the Energetics Study, Eur. J. Clin. Nutr., 2012, 66(1), 3–9 Search PubMed.
- V. Gill, V. Kumar, K. Singh, A. Kumar and J. J. Kim, Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health, Biomolecules, 2019, 9(12), 888 CrossRef CAS PubMed.
- J. Uribarri, W. Cai and M. Peppa, et al., Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging, J. Gerontol., Ser. A, 2007, 62(4), 427–433 CrossRef PubMed.
- Z. Mao, E. K. Aglago and Z. Zhao, et al., Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer, Nutrients, 2021, 13(12), 4435 Search PubMed.
- O. O. Omofuma, L. L. Peterson and D. P. Turner, et al., Dietary Advanced Glycation End-Products and Mortality after Breast Cancer in the Women's Health Initiative, Cancer Epidemiol., Biomarkers Prev., 2021, 30(12), 2217–2226 CrossRef CAS PubMed.
- C. Nagata, K. Wada and M. Yamakawa, et al., Dietary Intake of Nε-carboxymethyl-lysine, a Major Advanced Glycation End Product, is Not Associated with Increased Risk of Mortality in Japanese Adults in the Takayama Study, J. Nutr., 2020, 150(10), 2799–2805 Search PubMed.
- J. Uribarri, S. Woodruff and S. Goodman, et al., Advanced glycation end products in foods and a practical guide to their reduction in the diet, J. Am. Diet. Assoc., 2010, 110(6), 911–916 CrossRef PubMed.
- J. Scheijen, E. Clevers and L. Engelen, et al., Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database, Food Chem., 2016, 190, 1145–1150 CrossRef CAS PubMed.
- A. Saha, P. Poojary and L. Chan, et al., Increased odds of metabolic syndrome with consumption of high dietary advanced glycation end products in adolescents, Diabetes Metab., 2017, 43(5), 469–471 CrossRef CAS PubMed.
- W. C. Willett, G. R. Howe and L. H. Kushi, Adjustment for total energy intake in epidemiologic studies, Am. J. Clin. Nutr., 1997, 65(4 Suppl), 1220S–1228S Search PubMed ; discussion 1229S-1231S.
- Statistics NCfH, The Linkage of National Center for Health Statistics Survey Data to the National Death Index – 2019 Public-Use Linked Mortality Files (LMF): Linkage Methodology and Analytic Considerations, https://www.cdc.gov/nchs/data-linkage/mortality-public.htm, Accessed 7–11, 2021.
- G. R. Brämer, International statistical classification of diseases and related health problems. Tenth revision, World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales, 1988, 41(1), 32–36 Search PubMed.
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures, Vital and health statistics. Ser. 1, Programs and collection procedures, 1994, 32, 1–407 Search PubMed.
- S. Liangpunsakul, Relationship between alcohol intake and dietary pattern: findings from NHANES III, World J. Gastroenterol., 2010, 16(32), 4055–4060 CrossRef CAS PubMed.
- R. R. Pate, M. Pratt and S. N. Blair, et al., Physical activity and public health, A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine, J. Am. Med. Assoc., 1995, 273(5), 402–407 CrossRef CAS PubMed.
- N. I. Larson, C. L. Perry, M. Story and D. Neumark-Sztainer, Food preparation by young adults is associated with better diet quality, J. Am. Diet. Assoc., 2006, 106(12), 2001–2007 CrossRef PubMed.
- A. Han, T. Sun, J. Ming, L. Chai and X. Liao, Are the Chinese Moving toward a Healthy Diet? Evidence from Macro Data from 1961 to 2017, Int. J. Environ. Res. Public Health, 2020, 17(15), 5294 CrossRef PubMed.
- A. G. Huebschmann, J. G. Regensteiner, H. Vlassara and J. E. Reusch, Diabetes and advanced glycoxidation end products, Diabetes Care, 2006, 29(6), 1420–1432 CrossRef CAS PubMed.
- T. Goldberg, W. Cai and M. Peppa, et al., Advanced glycoxidation end products in commonly consumed foods, J. Am. Diet. Assoc., 2004, 104(8), 1287–1291 CrossRef CAS PubMed.
- S. Yamagishi, T. Matsui and K. Nakamura, Possible involvement of tobacco-derived advanced glycation end products (AGEs) in an increased risk for developing cancers and cardiovascular disease in former smokers, Med. Hypotheses, 2008, 71(2), 259–261 CrossRef CAS PubMed.
- I. D. Nicholl and R. Bucala, Advanced glycation endproducts and cigarette smoking, Cell. Mol. Biol., 1998, 44(7), 1025–1033 CAS.
- V. W. Zhong, L. Van Horn and P. Greenland, et al., Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality, JAMA Intern. Med., 2020, 180(4), 503–512 Search PubMed.
- M. Dehghan, A. Mente and X. Zhang, et al., Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study, Lancet, 2017, 390(10107), 2050–2062 CrossRef CAS PubMed.
- D. Mozaffarian and J. H. Wu, Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events, J. Am. Coll. Cardiol., 2011, 58(20), 2047–2067 CrossRef CAS PubMed.
- H. Hu, Y. Wang and M. Shen, et al., Effects of baking factors and recipes on the quality of butter cookies and the formation of advanced glycation end products (AGEs) and 5-hydroxymethylfurfural (HMF), Curr. Res. Food Sci., 2022, 5, 940–948 Search PubMed.
- G. Chen and J. S. Smith, Determination of advanced glycation endproducts in cooked meat products, Food Chem., 2015, 168, 190–195 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03945e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |