Open Access Article
Open Access ArticleComparative evaluation of enriched formula milk powder with OPO and MFGM vs. breastfeeding and regular formula milk powder in full-term infants: a comprehensive study on gut microbiota, neurodevelopment, and growth
Botian
Chen†
 ab,
Qiong
Jia†
b,
Zekun
Chen
c,
Yanxia
You
b,
Yanpin
Liu
d,
Junying
Zhao
d,
Lijun
Chen
*d,
Defu
Ma
ab,
Qiong
Jia†
b,
Zekun
Chen
c,
Yanxia
You
b,
Yanpin
Liu
d,
Junying
Zhao
d,
Lijun
Chen
*d,
Defu
Ma
 *a and
Yan
Xing
*b
*a and
Yan
Xing
*b
aSchool of Public Health, Peking University Health Science Center, 38 Xueyuan Road, Haidian District, Beijing 100191, China. E-mail: madefu@bjmu.edu.cn; Fax: +86-10-82801519; Tel: +86-10-82801743
bDepartment of Pediatrics, Peking University Third Hospital, No.49 North Garden Rd., Haidian District, Beijing 100191, China. E-mail: yxsxz@outlook.com; Tel: +86 10 82267705
cVanke School of Public Health, Tsinghua University, Beijing 100084, China
dNational Engineering Research Center of Dairy Health for Maternal and Child, Beijing Sanyuan Foods Co. Ltd., Beijing 100163, China. E-mail: chenlijun@sanyuan.com.cn
First published on 15th January 2024
Abstract
This study investigated the non-inferiority of feeding term healthy infants with enriched formula milk powder containing 1,3-dioleoyl-2-palmitoylglycerol (OPO) and milk fat globular membrane (MFGM), compared to breast milk, in terms of the formation of gut microbiota, neurodevelopment and growth. Infants were divided into three groups: breast milk group (BMG, N = 50), fortified formula group (FFG, N = 17), and regular formula group (RFG, N = 12), based on the feeding pattern. Growth and development information was collected from the infants at one month, four months, and six months after the intervention. Fecal samples were collected from infants and analyzed for gut microbiota using 16S ribosomal DNA identification. The study found that at the three time points, the predominant bacterial phyla in FFG and BMG were Proteobacteria, Firmicutes, and Bacteroidetes, which differed from RFG. The abundance of Bifidobacterium in the RFG was lower than the FFG (one month, p = 0.019) and BMG (four months, p = 0.007). The abundance of Methanoprebacteria and so on (genus level) are positively correlated with bone mineral density (BMD) of term infants, and have the potential to be biomarkers for predicting BMD. The abundance of beta-galactosidase, a protein that regulates lactose metabolism and sphingoid metabolism, was higher in FFG (six months, p = 0.0033) and BMG (one month, p = 0.0089; four months, p = 0.0005; six months, p = 0.0005) than in the RFG group, which may be related to the superior bone mineral density and neurodevelopment of infants in the FFG and BMG groups than in the RFG group. Our findings suggest that formula milk powder supplemented with OPO and MFGM is a viable alternative to breastfeeding, providing a practical alternative for infants who cannot be breastfed for various reasons.
Introduction
For newborns, breast milk is considered the gold standard of infant nutrition as it provides all the necessary nutrients for growth and development during the first few months after birth.1 However, not all newborns can be exclusively breastfed due to various reasons, and many infants require partial or complete reliance on different types of formula milk powder.2 Currently, the global rate of exclusive breastfeeding for infants under six months is around 40%, with only 37% of infants under six months in low- and middle-income countries being exclusively breastfed.3 Furthermore, infant formula primarily derived from cow's milk has significant differences in nutritional composition compared to breast milk, especially in terms of unique nutrients present in breast milk.4,5 In the face of this serious problem, it is particularly important to develop a formula that is similar to the main nutrients found in breast milk.The fat content in breast milk accounts for approximately 3–5%, but it provides over 50% of the energy required for infants.6,7 The lipids in human milk include triglycerides (98%), cholesterol (0.5%), phospholipids (0.8%), and fat-soluble vitamins. The majority of these lipids (87%) exist in the form of fat globules with a diameter of 4–5 micrometers, composed of triglycerides, cholesterol esters, polar phospholipids, cholesterol, enzymes, proteins, and glycoproteins, forming a non-polar core.7,8 Previous studies have indicated that the lipids in breast milk have functions such as promoting the absorption of fat-soluble vitamins and protecting the gastrointestinal tract.9–11 Triglycerides can provide energy and essential fatty acids for the growth and development of infants.12 Fatty acids have various functions, including immune modulation, antimicrobial activity, and antiviral properties, making them one of the most important components in human milk fat, aside from triglycerides.13 Based on the importance of breast milk fat in the composition of breast milk and its important role, this study focused on some of the main components of breast milk fat.
1,3-Dioleoyl-2-palmitoylglycerol (OPO) is one of the most abundant triglycerides in breast milk, formed by the esterification of glycerol with two molecules of oleic acid and one molecule of palmitic acid.14 Previous research has shown that OPO promotes calcium ion absorption, facilitates the growth of bifidobacteria in the infant gut, and supports neurodevelopment.15,16 Phospholipids and cholesterol play crucial roles in the development of the infant's brain and nervous system, influencing the growth of nerve cells and cognitive function.17 Milk fat globular membrane (MFGM) is a natural lipid particle present in human milk, composed of three layers of phospholipids and rich in glycoproteins.18 MFGM has been found to contribute to the promotion of infant neurodevelopment and the growth of bifidobacteria.19 The addition of OPO and MFGM to formula milk powder can make it more similar to breast milk.20 Based on the results of the OPO and MFGM studies and the consensus that breast milk is the gold standard for infant feeding, we hypothesized that the addition of OPO and MFGM, which are more closely related to breast milk, would result in the formation of early intestinal microbiota, and the neurodevelopment and growth of infants more similar to the natural state of breastfeeding.
The aim of this study was to investigate the non-inferiority of feeding term healthy infants with enriched formula milk powder containing OPO and MFGM, compared to breast milk, in terms of the formation of gut microbiota, neurodevelopment, and growth. Additionally, we aimed to evaluate the differences between the enriched formula milk powder and regular formula milk powder in these aspects. This will facilitate the development of more suitable and nutritionally comprehensive formulas for infants who cannot be breastfed for various reasons.
Materials & methods
Study design and subjects
The present study is a prospective, randomized controlled trial conducted at the Department of Obstetrics and Gynecology and Pediatrics of Peking University Third Hospital from March 2020 to May 2022. All study participants met the inclusion and exclusion criteria, which were as follows: (1) full-term infants (gestational age ≥37 weeks); (2) birth weight ranging from 2500–4500 grams; (3) mothers with no history of diabetes or hypertension during pregnancy; (4) infants without cognitive impairments or abnormalities within 2 days of hospitalization; (5) infants with no history of asphyxia (Apgar score ≥3 at 1 minute); (6) infants who had not received antibiotics within one month after birth; (7) infants with no severe allergic reactions to dairy products and no serious conditions such as intracranial hemorrhage or necrotizing colitis; (8) guardians voluntarily signing an informed consent form and committing to complete systematic follow-up. The study has obtained approval from the Medical Science Research Ethics Committee of Peking University Third Hospital (approval number: M2019285). Prior to the inclusion of infants in the study, written informed consent was obtained from their parents.To demonstrate clinically significant differences in the relative abundance of Bifidobacterium between groups, with a power of 80% and an alpha value of 0.05, we estimated that at least 9 infants would be required per group.21
Based on the infants’ natural feeding methods, they were divided into the exclusive breastfeeding group and the formula milk group. The standard control group was the breastfeeding group (BMG), where infants were fed breast milk. In the formula milk group, newborns were randomly assigned, using a single-blind method (parents unaware of the type of formula), to the fortified formula group (FFG) [fed fortified formula milk powder containing OPO (3.4 grams/100 grams) and MFGM (350 milligrams/100 grams)] and the regular formula group (RFG) [fed regular formula milk powder without OPO and MFGM]. The fortified and regular formulas are nutritionally comparable. Both formulas are milk-based and contain 281 kJ/100 mL energy, 0.54 grams/100 kJ protein, and 1.23 grams/100 kJ fat per 100 mL of formula.
Collection of infant information and feces
In the process of subject enrollment, information such as gender, gestational age, birth weight, birth length, birth head circumference, and other details of infants in each group were collected. Additionally, demographic data related to the parents were also collected, including their age, education level, per capita monthly income, pregnancy complications, and other relevant information.The infants’ weight, length, head circumference, and other growth and development data were collected at 1 month, 4 months, and 6 months after intervention, following either breastfeeding or formula feeding. At 4 and 6 months after intervention, the Children Neuropsychological and Behavior Scale-Revision 2016 (CNBS-R2016)22 and the infants’ bone density information were gathered.
The CNBS underwent a re-standardization in 2016 and was renamed CNBS-R2016.23 It demonstrated sufficient reliability.24 CNBS-R2016 consists of five separate subscales, namely Gross Motor, Personal-Social, Language, Fine Motor, and Adaptive Behavior. The intelligence age is the average of the five separate subscales. The developmental quotient (DQ) is calculated using the formula (intelligence age/actual month age) × 100. An infant with a DQ score greater than 130 is considered excellent, scores between 110–129 indicate good development, scores within 80–109 suggest medium development, scores between 70–79 indicate critically low development, and scores below 70 indicate a developmental disorder.22
Fecal samples were collected at 1 month, 4 months, and 6 months after feeding. Parents collected the samples at home and placed them in stool tubes with preservation solution. The tubes were stored at −20 °C before being transferred to the hospital's refrigerated facility. All samples in the hospital were stored below −80 °C until further analysis.
Construction and sequencing of DNA isolation and amplification library
The SDS method was employed to extract total genomic DNA from the samples. Specific primer pairs (515F & 806R) and barcodes were used to amplify the 16S rRNA genes in the distinct regions (16S V3-V4). Each PCR mixture contained 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of each primer, 10 ng of target DNA, and the cycling conditions comprised an initial denaturation step at 98 °C for 1 minute, followed by 30 cycles at 98 °C (10 seconds), 50 °C (30 seconds), and 72 °C (30 seconds), with a final extension of 5 minutes at 72 °C.For DNA detection, the PCR products were mixed with an equal volume of 1× loading buffer (containing SYB green) and subjected to electrophoresis on a 2% agarose gel. The PCR products were then combined in equal proportions, and the resulting mixture was purified using the Qiagen Gel Extraction Kit (Qiagen, Germany) following the manufacturer's instructions.
The sequencing libraries were generated using the NEBNext® Ultra™ II DNA Library Prep Kit (Cat No. E7645), as per the manufacturer's guidelines. The quality of the library was assessed using the Qubit® 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform, generating 250 bp paired-end reads.
Bioinformatics analysis
After barcoding and fragmentation, the sample DNA is processed using FLASH (Version 1.2.11, https://ccb.jhu.edu/software/FLASH/) to merge the fragmented sequences into paired-end reads. To obtain high-quality sequence reads, fastp (Version 0.20.0) is employed, which performs quality filtering and trimming to produce clean tags. These clean tags are then compared against the Silva reference database (https://www.arbsilva.de/) for 16S ribosomal RNA using Vsearch (Version 2.15.0) to identify and remove chimera sequences. This process results in obtaining effective tags that represent reliable sequences.The effective tags are further processed using the deblur module in QIIME2 software (Version QIIME2-202006) for denoising and to generate initial Amplicon Sequence Variants (ASVs). By default, deblur employs the DADA2 algorithm for this task. ASVs with an abundance less than 5 are subsequently filtered out from the dataset.
To assign taxonomic information to the ASVs, species annotation is performed using the QIIME2 software. The Silva Database is utilized as the reference database for this annotation. To facilitate downstream analyses, multiple sequence alignment of the ASVs is performed using QIIME2 software.
Following the preprocessing steps, subsequent analyses of alpha diversity (within-sample diversity) and beta diversity (between-sample diversity) are performed based on the output normalized data. These analyses provide insights into the diversity and composition of the microbial communities under investigation.
Further, to study the functions of the communities in the samples and find out the different functions of the communities in the different groups, the PICRUSt2 software (Version 2.1.2-b) was used for function annotation analysis (using the 16S rRNA gene sequencing data obtained to infer the metagenomes).
Statistical analysis
All statistical analyses were performed using R statistical software version 4.1.3. The baseline characteristics and clinical outcomes of study participants were expressed as mean ± SD of continuous variables and frequency of categorical variables.Richness and uniformity of the communities in the sample, alpha diversity was calculated from Shannon in QIIME2. Beta diversity was calculated based on weighted unifrac distances in QIIME2. Principal Coordinate Analysis (PCoA) was performed to obtain principal coordinates and visualize differences of samples in complex multi-dimensional data. A matrix of weighted or unweighted unifrac distances among samples obtained previously was transformed into a new set of orthogonal axes, where the maximum variation factor was demonstrated by the first principal coordinate, and the second maximum variation factor was demonstrated by the second principal coordinate, and so on. The three-dimensional PCoA results were displayed using QIIME2 package, while the two-dimensional PCoA results were displayed using ade4 package and ggplot2 package in R software (Version 2.15.3). To study the significance of the differences in community structure between groups, the adonis and anosim functions in the QIIME2 software were used to do analysis. To find out the significantly different species at each taxonomic level (Phylum, Class, Order, Family, Genus, Species), the R software (Version 3.5.3) was used to do Kruskal–Wallis test. Spielman correlation analysis is used to analyze correlations between clinical data and gut microbiota (genus level). According to the type of variable, Kruskal–Wallis test (If the data conform to a normal distribution and the variance is the same, One-Way ANOVA is used) or Pearson Chi-square test were used. Then, pairwise test for multiple comparisons was performed using Bonferroni's p-adjustment method or LSD method according to the number of samples. Statistical significance was assumed at p < 0.05.
Results
Demographic and clinical information
A total of 81 infants who met the inclusion and exclusion criteria were included in the study. A total of 169 infant fecal samples were collected at 1 month, 4 months, and 6 months after the intervention. One participant each from the FFG and RFG groups were lost to follow-up. Eventually, a total of 79 infants were included in the study, there were 50 participants in the BMG group, 12 in the RFG group, and 17 in the FFG group. The study flow is illustrated in Fig. 1.There were no statistically significant differences in baseline characteristics (gestational age, sex, birth weight in grams, birth length in centimeters, birth head circumference in centimeters, maternal age in years, pregnancy conditions, mode of delivery, maternal and paternal educational levels, average monthly family income, presence of twins, and pregnancy-related diseases) among the three groups (Table 1).
| BMG(n = 50) | RFG(n = 12) | FFG(n = 17) | P-value | |
|---|---|---|---|---|
| BMG: breast milk group; RFG: regular formula group; FFG: fortified formula group. According to the type of variable, Kruskal–Wallis test (if the data conform to a normal distribution and the variance is the same, one-way ANOVA is used) or Pearson Chi-square test were used. | ||||
| Gestational age (weeks) | 39.26(1.31) | 38.50(1.24) | 38.71(1.10) | 0.0936 |
| Sex | 0.7207 | |||
| Male | 24(48.0%) | 7(58.3%) | 7(41.2%) | |
| Female | 26(52.0%) | 5(41.7%) | 10(58.8%) | |
| Birth weight (g) | 3235(336.0) | 3023.3(334.3) | 3300.6(501.7) | 0.8530 |
| Birth length (cm) | 49.64(1.60) | 49.08(1.73) | 48.71(1.90) | 0.1282 |
| Birth head circumference (cm) | 33.63(3.21) | 33.79(1.36) | 30.62(5.96) | 0.2877 |
| Mother's age (year) | 32.36(3.42) | 31.33(3.31) | 33.29(3.77) | 0.3511 |
| Pregnancy mode | 0.5115 | |||
| Conceive Spontaneously | 44(88.0%) | 9(75.0%) | 15(88.2%) | |
| Assisted conception | 6(12.0%) | 3(25.0%) | 2(11.8%) | |
| Mode of delivery | 0.1269 | |||
| Eutocia | 34(68.0%) | 5(41.7%) | 8(47.1%) | |
| Cesarean section | 16(32.0%) | 7(58.3%) | 9(52.9%) | |
| Maternal education level | 1.0000 | |||
| High school and below | 0(0.0%) | 0(0.0%) | 0(0.0%) | |
| Undergraduate and above | 50(100.0%) | 12(100.0%) | 17(100.0%) | |
| Father's education level | 0.7112 | |||
| High school and below | 2(4.0%) | 0(0.0%) | 1(5.9%) | |
| Undergraduate and above | 48(96.0%) | 12(100.0%) | 16(52.9%) | |
| Average monthly household income | 0.5318 | |||
≤10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 yuan 000 yuan |
11(22.0%) | 1(8.3%) | 4(23.5%) | |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 yuan 000 yuan |
39(78.0%) | 11(91.7%) | 13(76.5%) | |
| Twins | 0.0699 | |||
| No | 49(98.0%) | 10(83.3%) | 15(88.2%) | |
| Yes | 1(2.0%) | 2(16.7%) | 2(11.8%) | |
| Pregnancy disease | 0.1390 | |||
| No | 23(46.0%) | 8(66.7%) | 12(70.6%) | |
| Yes | 27(54.0%) | 4(33.3%) | 5(29.4%) | |
In terms of growth and development, there were no significant differences in height, weight, and head circumference among the three groups at the three time points. The growth patterns of the FFG group were similar to the BMG group and superior to the RFG group. Although not statistically significant, the FFG and BMG groups demonstrated faster rates of increase in length and weight compared to the RFG group during the intervention period. Additionally, there were no statistically significant differences in bowel movement frequency and gastrointestinal tolerance among the three groups at each time point (Table 2).
| BMG(n = 50) | RFG(n = 12) | FFG(n = 17) | P-value | |
|---|---|---|---|---|
| BMG: breast milk group; RFG: regular formula group; FFG: fortified formula group; T1: after one month of intervention; T2: after four months of intervention; T3: after six months of intervention. The analysis was performed using Kruskal–Wallis test (if the data conform to a normal distribution and the variance is the same, one-way ANOVA is used). | ||||
| Weight (g) | ||||
| T1 | 4702.8(1256.9) | 4463.6(424.3) | 4333.6(692.0) | 0.6421 |
| T2 | 7565.1(2080.6) | 7406.3(945.1) | 7254.6(1437.0) | 0.9424 |
| T3 | 8700.7(2267.9) | 7986.0(1005.5) | 8428.3(405.0) | 0.6444 |
| Length (cm) | ||||
| T1 | 55.20(2.45) | 55.14(2.39) | 53.29(4.03) | 0.1402 |
| T2 | 64.50(3.45) | 64.40(2.72) | 64.30(3.10) | 0.8015 |
| T3 | 68.57(2.34) | 67.54(2.23) | 68.43(1.66) | 0.6460 |
| Head circumference (cm) | ||||
| T1 | 36.88(2.38) | 36.68(1.42) | 37.41(6.03) | 0.8280 |
| T2 | 42.33(3.32) | 42.38(2.56) | 42.96(4.15) | 0.8569 |
| T3 | 42.69(1.45) | 42.80(0.84) | 43.42(1.63) | 0.9150 |
| Stool frequency (times/day) | ||||
| T1 | 3.15(0.74) | 3.45(0.69) | 2.71(0.83) | 0.0652 |
| T2 | 2.07(0.66) | 2.13(0.83) | 2.00(0.71) | 0.9170 |
| T3 | 1.96(0.71) | 2.20(0.45) | 1.83(0.41) | 0.5422 |
| Gastrointestinal tolerance | ||||
| T1 | 3.65(0.66) | 3.84(0.55) | 3.54(0.77) | 0.7600 |
| T2 | 4.33(0.47) | 4.31(0.44) | 4.08(0.58) | 0.3284 |
| T3 | 4.51(0.44) | 4.75(0.31) | 4.42(0.56) | 0.4399 |
In the aspect of neurodevelopment, there were no significant differences among the three groups after one month of feeding. After 4 months of feeding, the FFG group gross motor skills and developmental quotient (DQ) scores closer to the BMG group, both of which were superior to the RFG group, but the differences were not statistically significant. After 6 months of feeding, there were no significant differences in motor skills among the three groups. Regarding bone density growth, the FFG group outperformed the BMG and RFG groups, although the differences were not statistically significant (Table 3).
| BMG (n = 50) | RFG (n = 12) | FFG (n = 17) | P-value | |
|---|---|---|---|---|
| BMG: breast milk group; RFG: regular formula group; FFG: fortified formula group; BMD: bone mineral density; T1: after one month of intervention; T2: after four months of intervention; T3: after six months of intervention. The analysis was performed using Kruskal–Wallis test (if the data conform to a normal distribution and the variance is the same, one-way ANOVA is used). | ||||
| T1 | ||||
| Neurodevelopment | 4.85(1.05) | 4.82(1.08) | 4.71(0.73) | 0.9915 |
| T2 | ||||
| Gross motor | 4.41(0.92) | 3.70(0.76) | 4.32(0.72) | 0.1735 |
| Fine motor | 3.64(0.66) | 3.60(0.55) | 3.82(0.46) | 0.5783 |
| Adaptive behavior | 3.50(0.52) | 3.40(0.96) | 3.68(0.87) | 0.7947 |
| Language | 4.33(0.64) | 4.20(0.84) | 4.50(0.50) | 0.5993 |
| Personal-social | 4.45(0.69) | 4.40(0.55) | 4.45(0.52) | 0.9827 |
| Intelligence age | 3.64(0.66) | 3.60(0.55) | 3.82(0.46) | 0.5783 |
| Developmental quotient | 100.16(8.80) | 96.7(7.29) | 101.92(6.10) | 0.3718 |
| T3 | ||||
| Gross motor | 6.20(0.89) | 6.50(1.21) | 6.50(1.10) | 0.7065 |
| Fine motor | 6.20(0.66) | 6.63(0.63) | 6.25(0.42) | 0.5612 |
| Adaptive behavior | 6.60(0.48) | 6.50(0.71) | 6.25(0.42) | 0.2646 |
| Language | 6.45(0.69) | 6.50(0.58) | 6.83(0.75) | 0.5288 |
| Personal-social | 6.30(1.02) | 6.50(1.68) | 6.67(0.93) | 0.4789 |
| Intellectual age | 6.35(0.54) | 6.53(0.74) | 6.50(0.58) | 0.7710 |
| Developmental quotient | 105.11(9.06) | 104.95(9.15) | 103.97(9.09) | 0.8573 |
| Percent change in BMD | 12.88(16.56) | 13.00(10.10) | 31.50(34.60) | 0.3993 |
Characteristics of the gut microbiota in each feeding pattern
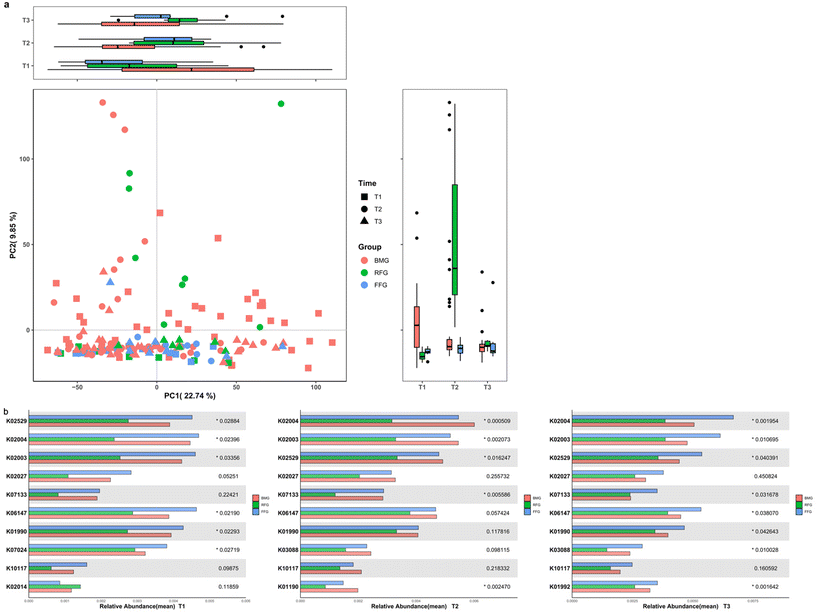
Principal axis analysis of beta diversity showed that the gut microbiota community structure of the FFG group was more similar to the BMG group. After one month of intervention, the differences between the FFG and BMG groups were significant in PCoA1 (p = 0.0004) and PCoA2 (p = 0.0026). After four months of intervention, the difference between the RFG and BMG groups in PCoA2 (p = 0.0026) was significant; the difference between the RFG and FFG groups in PCoA2 (p = 0.0134) was significant; there were no significant differences between the three groups at other time points (Fig. 2a). Alpha diversity of infant gut microbiota was measured using the Shannon index. There were no significant differences in microbial diversity among the three groups after one month and six months of intervention. However, at the four-month intervention, the microbial diversity in the RFG (p = 0.0034) group was significantly higher than that in the BMG group (Fig. 2b).At the phylum level, the gut microbiota composition of the FFG and BMG groups was very similar, with the major phyla being Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria, which was consistent across all three time points. In the RFG group, the major phyla included Proteobacteria, Firmicutes, Actinobacteria, and Verrucomicrobia (Fig. 3a). At the genus level, the gut microbiota composition of the FFG and BMG groups was also very similar, with the dominant genera being Bifidobacterium, followed by Escherichia-Shigella, Veillonella and Bacteroides. While in the RFG group, Klebsiella was the dominant genus, followed by Veillonella, Bifidobacterium and then Escherichia-Shigella. Over time, the RFG group gradually approached the FFG and BMG groups (with Bifidobacterium becoming the dominant genus), but there was still a difference at six months (Fig. 3b).
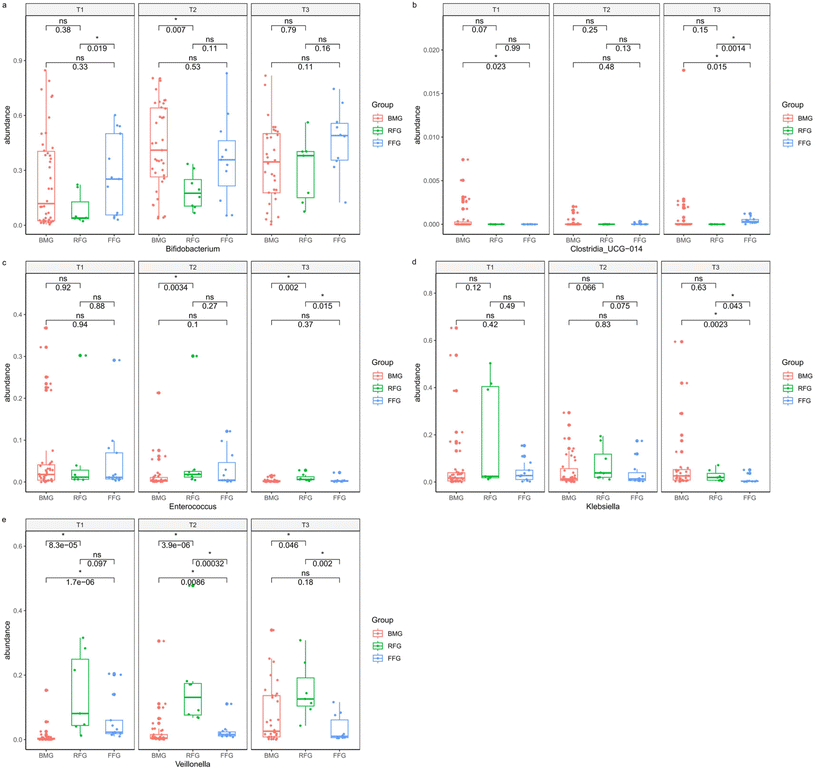
After one month of intervention, the abundance of Bifidobacterium (genus levels) in the FFG group was higher than the RFG group (p = 0.019), while there was no significant difference in the abundance of Bifidobacterium (genus levels) between the FFG and BMG groups (p = 0.33). After four months of intervention, there was still no significant difference in the abundance of Bifidobacterium (genus levels) between the FFG and BMG groups (p = 0.53), but the abundance in the RFG group was significantly lower than in the BMG group (p = 0.007). At six months of intervention, there were no significant differences in the abundance of Bifidobacterium (genus levels) among the three groups (Fig. 4a). After one month of intervention, the abundance of Clostridia_UCG-014 (genus levels) in the FFG group was higher than in the BMG group (p = 0.023). After four months of intervention, we did not find any significant differences in the abundance of Clostridia_UCG-014 (genus levels) among the three groups, and after six months of intervention, it in the FFG group was lower than in the BMG group (p = 0.015) and the RFG group (p = 0.0014) (Fig. 4b). After one month of intervention, we did not find any significant differences in the abundance of Enterococcus (genus levels). After four months of intervention, it in the RFG group was higher than in the BMG group (p = 0.0034). At six months of intervention, the abundance of Enterococcus (genus levels) in the RFG group was higher than in the BMG group (p = 0.002) and the FFG group (p = 0.015) (Fig. 4c). After one month and four months of intervention, we did not find any statistical differences in the abundance of Klebsiella (genus levels) among the three groups. After six months of intervention, the abundance of Klebsiella (genus levels) in the FFG group was lower than in the BMG group (p = 0.0023) and the RFG group (p = 0.043) (Fig. 4d). After one month of intervention, the abundance of Veillonella (genus levels) in the BMG group was lower than in the FFG group (p < 0.001) and RFG group (p < 0.001). After four months of intervention, the abundance of Veillonella (genus levels) in the BMG group was still lower than in the FFG group (p = 0.0086) and the RFG group (p < 0.001), and the abundance of Veillonella (genus levels) in the FFG group was lower than in the RFG group (p < 0.001). At six months of intervention, the abundance of Veillonella (genus levels) in the RFG group was higher than in the BMG group (p = 0.046) and the FFG group (p = 0.002) (Fig. 4e).
Potential biomarkers for clinical indicators in each feeding pattern
We found some gut microbes that have potential as biomarkers. We found that the head circumference in one-month-old term infants was positively correlated with the abundance of Sutterella and Allisonella (genus levels). Futhermore, head circumference of four-month-old infants was positively correlated with the abundance of Akkermansia and Catabacter (genus levels). While head circumference was negatively correlated with the abundance of Bilophila (genus levels) in 6-month-old infants. Therefore, it suggested that these genera of bacteria may have potential as biomarkers for predicting head circumference growth. The abundance of Methanobrevibacter, Parasutterella and so on (genus levels) was positively correlated with the bone mineral density of term infants, and also had the potential to be biomarkers for predicting the bone mineral density of infants (Fig. 5).Differences in functional prediction between groups in each feeding pattern
After one month of intervention, the differences between the FFG and BMG groups were significant in PCoA1 (p = 0.0146) and PCoA2 (p = 0.0004); the difference between the RFG and BMG groups was significant in PCoA2 (p = 0.0002). After four months of intervention, the differences between the FFG and BMG groups in PCoA1 (p = 0.0384) was significant; the differences between the RFG and BMG groups in PCoA1 (p = 0.0238) and PCoA2 (p = 0.001) were significant; and the FFG was also different from the RFG in PCoA2 (p = 0.0002). There were no significant differences between the three groups at other time points (Fig. 6a). And, we found that at three time points, the top ten proteins in relative abundance of the three groups had partial overlap. We found that the relative abundance of these proteins in BMG and FFG groups was almost higher than that in RFG group at all three time points (Fig. 6b).We found that after one month of intervention, the abundance of beta-galactosidase in the BMG group was higher than the RFG group (p = 0.0089), while there was no significant difference in the abundance of beta-galactosidase between the FFG and BMG groups (p = 0.1622). After four months of intervention, there was still no significant difference in the abundance of beta-galactosidase between the FFG and BMG groups (p = 0.0622), and the abundance in the RFG group was still significantly lower than in the BMG group (p = 0.0005). At six months of intervention, the abundance of beta-galactosidase in the RFG group was lower than the FFG group (p = 0.0033) and the BMG group (p = 0.035) (Fig. 7a). After one month of intervention, the abundance of sucrose-6-phosphatase in the FFG group was higher than the RFG group (p = 0.0133) and the BMG group (p = 0.0232) (Fig. 7b). After one month of intervention, the abundance of ABC-2 type transport system ATP-binding protein in the RFG group was lower than the FFG group (p = 0.0064) and the BMG group (p = 0.0152). At six months of intervention, the abundance of this protein in the RFG group was still lower than the FFG group (p = 0.0144) (Fig. 7c). The abundance of ABC-2 type transport system permease protein in the RFG group was lower than the FFG group after one month (p = 0.0261) and six months (p = 0.0002) of intervention. And the abundance of this protein in the RFG group was lower than the BMG group after six months (p = 0.0022) of intervention (Fig. 7d). The abundance of putative ABC transport system ATP-binding protein in the RFG group was lower than the FFG group (T1: p = 0.0097, T2: p = 0.0048) and the BMG group (T1: p = 0.0238, T2: p = 0.0003) after one month and four months of intervention (Fig. 7e). The abundance of putative ABC transport system permease protein in the RFG group was lower than the FFG group (T1: p = 0.0092, T2: p = 0.0035, T3: p = 0.0003) and the BMG group (T1: p = 0.0099, T2: p < 0.0001, T3: p = 0.0488) in each time point (Fig. 7f). The abundance of LacI family transcriptional regulator in the RFG group was lower than the FFG group (T1: p = 0.008, T2: p = 0.0303, T3: p = 0.0108) in each time point and was lower than the BMG group (p = 0.0036) after four months of intervention (Fig. 7g).
Discussion
16S ribosomal DNA identification was employed in this study to perform a longitudinal analysis of the gut microbiota composition in full-term infants at multiple time points. We found that the fecal microbiota of the FFG group, fed with fortified formula milk powder containing OPO and MFGM, was similar in terms of species and abundance to the BMG group. Both groups were dominated by Bifidobacterium. In contrast, the RFG group, fed with regular formula milk powder, showed inconsistent gut microbiota composition compared to the other two groups. At one month of intervention, the RFG group exhibited a dominance of Klebsiella, although the subsequent abundance of Bifidobacterium gradually increased and became dominant, it remained lower than the other two groups. Additionally, the FFG group fed with fortified formula milk powder showed similarities to the BMG group in terms of growth and development (including weight and length gain), surpassing the RFG group (though statistically insignificant). Moreover, in terms of neurodevelopment, the FFG group exhibited greater similarity to the BMG group and outperformed the RFG group. Our study also found that Parasutterella and other bacteria (genus level) may have biomarker potential for bone mineral density indicators, but further proof is needed in the future. Regarding the proteins related to metabolic function, we found that the relative abundance of beta-galactosidase, which is associated with lactose metabolism and sphingolipid metabolism, and sucrose-6-phosphatase, which is associated with starch and sucrose metabolism, was higher in BMG and FFG groups than in RFG group. In addition, many transporters in the top ten abundant proteins, such as ABC-2 type transport system ATP-binding protein, were also higher in BMG group and FFG group than in RFG group.Consistent with previous studies,25,26 our research identified Bifidobacterium, Escherichia-Shigella, and Klebsiella as the dominant genera in the gut microbiota of term infants. Compound 2′-fucosyllactose (2′-FL) is a digestion product of OPO, which directly induces the adherence and proliferation of Bifidobacterium and Lactic acid bacteria in the gut, and/or inhibits other competing bacteria.17 From a metabolic standpoint, this could be attributed to the fortified infant formula containing MFGM modifying the metabolic performance of neonates, favoring the utilization of fat and protein, and altering the gut microbiota to resemble that of breastfed infants,27,28 consistent with the findings of Yao et al.29 Our study also found that infants fed with OPO and MFGM formula maintained higher levels of Bifidobacterium in their gut microbiota at one, four, and six months of intervention (similar to the breastfed group) compared to infants fed with regular formula. This finding aligns with the studies conducted by Schmelzle et al.30 and Zhao et al.19 Previous research has suggested that higher abundance of Bifidobacterium is associated with a lower risk of obesity, allergies, and autistic regression.31 The use of fortified formula milk powder containing OPO and MFGM may potentially reduce the incidence of these diseases. Moreover, prior studies have shown that the abundance of Clostridia_UCG-014 is lower in the gut of breastfed infants and higher in formula-fed infants.32,33 Similarly, our study found that the abundance of Clostridia_UCG-014 in the gut microbiota of infants fed with OPO and MFGM formula was lower than that of infants fed with regular formula, resembling that of breastfed infants. Furthermore, previous studies have found that the abundance of Klebsiella in the intestinal microbiota of breastfed infants is lower compared to formula-fed infants, where Klebsiella levels are higher.34 In our study, we found that the abundance of Klebsiella in the intestinal microbiota of infants fed with OPO and MFGM formula was lower compared to the regular formula-fed group, and closer to the breastfed group. Therefore, feeding infants with OPO and MFGM formula can promote a gut microbiota composition that closely resembles that of breastfed infants, which is more favorable for infant health.
MFGM provides a significant amount of cholesterol, which is an essential component of all cell membranes. It influences the development of myelin phospholipids in the central and peripheral nervous systems.35 Our study found that the addition of OPO and MFGM in formula milk powder has a certain promotional effect on the development of gross motor skills, fine motor skills, adaptive behavior, and language abilities in four-month-old infants (closer to the state of breastfeeding), consistent with the findings of Gázquez et al.36 However, unlike previous studies, our research suggests that the impact of OPO and MFGM on infant neurodevelopment occurs earlier, possibly because our study subjects were full-term infants, not preterm infants. Studies by Litamanovitz et al.37 and Kennedy et al.38 demonstrated that formula milk powder enriched with OPO can promote increased bone mineral density in infants, which aligns with our findings. We observed that the consumption of formula milk powder containing OPO and MFGM improved infant bone density more than regular formula milk powder and breast milk. The reason behind this is that during triglyceride digestion, fatty acids esterified at the sn-1 and sn-3 positions are released, while those esterified at the sn-2 position remain intact.39 After digestion, free palmitic acid molecules solidify in the intestine due to their higher melting temperature and form insoluble and poorly digestible complexes with dietary minerals such as calcium.40 Compared to standard palm oil, OPO is not hydrolyzed during digestion, thus reducing its binding with calcium ions, increasing calcium absorption,41,42 and enhancing bone strength.37,38 Higher bone density during infancy is beneficial for overall growth and development. However, despite the observed improvement in infant bone density with formula milk powder containing OPO and MFGM, our study found no significant promotion in terms of height, weight, and head circumference among full-term infants compared to previous research findings,43 possibly due to limitations in sample size.
Galactose is a major nutrient in normal newborn infants and serves as a substrate for energy production and fuel storage and a regulator of carbohydrate assimilation.44 In addition to the basic role of providing infant energy, Yuan et al.‘s study found that galactose has a role in promoting chondrogenic differentiation and cartilage matrix formation.45 Our study found that feeding OPO and MFGM supplemented formula can effectively increase the abundance of beta-galactosidase in the intestines of infants, and beta-galactosidase (Lactose galactohydrolase and Galactan galactohydrolase) can hydrolyze lactose and galactoglycan into galactose, which greatly improves the absorption of galactose in infants.46 We think this may be one of the reasons for the high bone density of infants fed OPO and MFGM added formula. In addition, studies have found that galactose is beneficial for diseases that affect the brain, and we speculate that galactose may also have a role in promoting neural development in infants.47 In addition, beta-galactosidase is also involved in sphingolipid metabolism (beta-D-galactosyl-1,4-beta-D-glucosylceramide galactohydrolase) through the breakdown of lactosylceramide. Sphingolipid is abundant in the brain and is important for the development of the nervous system.48 Our study found that infants fed OPO and MFGM added formula had some better neurodevelopment, similar to breastfed infants, which may be due to the greater amount of sphingolipid produced by breakdown in their intestines.
Our study has some limitations. Firstly, we did not conduct longer-term follow-ups, such as during the toddler period, making it difficult to evaluate the long-term effects of formula milk powder supplemented with OPO and MFGM on growth and development. Secondly, the sample size of our study was small. Although there are statistical differences in many results on infant intestinal microbiota, infant growth and development, and infant neurodevelopment among the three groups in this study, some results are not statistically significant. What's more, if studies with a larger sample size can be conducted, we can better understand whether the lack of statistical difference in some results is due to the limited sample size. In addition, as a study with practical significance (which can provide formula milk powder more suitable for infant growth and development and neurodevelopment for infants who cannot be breastfed for various reasons), a larger sample size study can provide more clear evidence and guide the production of formula milk powder suitable for infants. In addition, the subjects of this study were only full-term infants, and we hope that there will be other studies in the future to reveal whether the application of OPO and MFGM fortified formula in premature infants has similar results as this study. However, despite this limitation, we still found several statistically significant differences, which to some extent increases the credibility of our study. We hope that future research with larger sample sizes can confirm our findings.
Conclusion
To sum up, our research findings demonstrate that fortified formula milk powder with OPO and MFGM as a substitute for breastfeeding can support infant growth and neurodevelopment on par with breastfed infants. Furthermore, the intestinal microbiota composition of infants fed with formula containing OPO and MFGM closely resembles that of breastfed infants. However, it is important to note that these results should be further validated through large-scale randomized controlled trials.Data availability statement
The datasets used to analyze for this study can be found in the National Center of Biotechnology Information (NCBI) with program ID PRJNA922711 (https://www.ncbi.nlm.nih.gov/bioproject/922711).Statement of ethics
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Peking University Third Hospital Medical Science Research Ethics Committee (protocol code M2019285 and date of approval: 5 March 2020). Written informed consent was obtained from parents before the infant was included in the study.Author contributions
B. C.: investigation, methodology, formal analysis, data curation, writing – original draft; Q. J.: investigation, writing – review & editing; Z. C.: investigation, data curation; Y. Y.: investigation, visualization; Y. L.: investigation, data curation; J. Z.: investigation; L. C.: project administration; D. M.: conceptualization, supervision, writing – review & editing; Y. X.: funding acquisition, methodology, project administration, writing – review & editing. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was financially supported by National Key Research and Development Program of China (2021YFC2700700 and 2021YFC2700705), Natural Science Foundation of Beijing, China (No. S170003), Peking University Third Hospital Incubation Fund for Youth (No. BYSYFY2021014), Peking University Third Hospital Research Fund for Clinical cohort construction (No. BYSYDL2022008), and China International Medical Foundation (No. Z-2019-41-2101-01).References
- A. Walker, Breast milk as the gold standard for protective nutrients, J. Pediatr., 2010, 156, S3–S7 CrossRef CAS PubMed.
- R. Akkerman, M. M. Faas and P. de Vos, Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation, Crit. Rev. Food Sci. Nutr., 2019, 59, 1486–1497 CrossRef CAS.
- C. G. Victora, R. Bahl, A. J. Barros, G. V. França, S. Horton, J. Krasevec, S. Murch, M. J. Sankar, N. Walker and N. C. Rollins, Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect, Lancet, 2016, 387, 475–490 CrossRef.
- C. R. Martin, P. R. Ling and G. L. Blackburn, Review of Infant Feeding: Key Features of Breast Milk and Infant Formula, Nutrients, 2016, 8, 279 CrossRef.
- Y. R. Tahboub, A. M. Massadeh, N. A. Al-Sheyab, D. El Shrafat and I. A. Nsserat, Levels of Trace Elements in Human Breast Milk in Jordan: a Comparison with Infant Formula Milk Powder, Biol. Trace Elem. Res., 2021, 199, 4066–4073 CrossRef CAS PubMed.
- R. Jenness, The composition of human milk, Semin. Perinatol., 1979, 3, 225–239 CAS.
- R. G. Jensen, Lipids in human milk, Lipids, 1999, 34, 1243–1271 CrossRef CAS.
- C. Lopez, Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure, Curr. Opin. Colloid Interface Sci., 2011, 16, 391–404 CrossRef CAS.
- D. Ramiro-Cortijo, P. Singh, Y. Liu, E. Medina-Morales, W. Yakah, S. D. Freedman and C. R. Martin, Breast Milk Lipids and Fatty Acids in Regulating Neonatal Intestinal Development and Protecting against Intestinal Injury, Nutrients, 2020, 12, 534 CrossRef PubMed.
- A. D. George, S. Burugupalli, S. Paul, T. Mansell, D. Burgner and P. J. Meikle, The Role of Human Milk Lipids and Lipid Metabolites in Protecting the Infant against Non-Communicable Disease, Int. J. Mol. Sci., 2022, 23, 7490 CrossRef CAS.
- A. L. Morrow and A. Dawodu, Fatty Acids and Fat-Soluble Vitamins in Breast Milk: Physiological Significance and Factors Affecting Their Concentrations, Nestle Nutr. Inst. Workshop Ser., 2019, 90, 57–67 Search PubMed.
- S. S. Chassen, K. Zemski-Berry, S. Raymond-Whish, C. Driver, J. C. Hobbins and T. L. Powell, Altered Cord Blood Lipid Concentrations Correlate with Birth Weight and Doppler Velocimetry of Fetal Vessels in Human Fetal Growth Restriction Pregnancies, Cells, 2022, 11, 3110 CrossRef CAS PubMed.
- M. Marounek, E. Skrivanová and V. Rada, Susceptibility of Escherichia coli to C2-C18 fatty acids, Folia Microbiol., 2003, 48, 731–735 CrossRef CAS PubMed.
- L. Esteban, M. J. Jiménez, E. Hita, P. A. González, L. Martín and A. Robles, Production of structured triacylglycerols rich in palmitic acid at sn-2 position and oleic acid at sn-1,3 positions as human milk fat substitutes by enzymatic acidolysis, Biochem. Eng. J., 2011, 54, 62–69 CrossRef CAS.
- R. Closa-Monasterolo, N. Ferré, G. Castillejo-DeVillasante, V. Luque, M. Gispert-Llaurado, M. Zaragoza-Jordana, S. Theis and J. Escribano, The use of inulin-type fructans improves stool consistency in constipated children. A randomised clinical trial: pilot study, Int. J. Food Sci. Nutr., 2017, 68, 587–594 CrossRef CAS.
- S. Yaron, D. Shachar, L. Abramas, A. Riskin, D. Bader, I. Litmanovitz, F. Bar-Yoseph, T. Cohen, L. Levi, Y. Lifshitz, R. Shamir and R. Shaoul, Effect of high β-palmitate content in infant formula on the intestinal microbiota of term infants, J. Pediatr. Gastroenterol. Nutr., 2013, 56, 376–381 CrossRef CAS.
- R. Mozzi and S. Buratta, in Handbook of Neurochemistry and Molecular Neurobiology: Neural Lipids, ed. A. Lajtha, G. Tettamanti and G. Goracci, Springer US, Boston, MA, 2009, pp. 39–58, DOI:10.1007/978-0-387-30378-9_3.
- Q. Yuan, H. Gong, M. Du, T. Li and X. Mao, Milk fat globule membrane supplementation to obese rats during pregnancy and lactation promotes neurodevelopment in offspring via modulating gut microbiota, Front. Nutr., 2022, 9, 945052 CrossRef.
- J. Zhao, W. Yi, B. Liu, Y. Dai, T. Jiang, S. Chen, J. Wang, B. Feng, W. Qiao, Y. Liu, H. Zhou, J. He, J. Hou and L. Chen, MFGM components promote gut Bifidobacterium growth in infant and in vitro, Eur. J. Nutr., 2022, 61, 277–288 CrossRef CAS.
- N. J. Andreas, B. Kampmann and K. Mehring Le-Doare, Human breast milk: A review on its composition and bioactivity, Early Hum. Dev., 2015, 91, 629–635 CrossRef CAS.
- Human Microbiome Project Consortium, Structure, function and diversity of the healthy human microbiome, Nature, 2012, 486, 207–214 CrossRef.
- C. Jin, Children Neuropsychological and Behavior Scale Revision 2016, Beijing Publishing House, Beijing, 2016, vol. 37 Search PubMed.
- J. J. Zhang, Z.M. Gao, H Xue, C. R. Zhang, Y Zeng and YY Mao, The study of developmental diagnostic scale of children aged 0–4 years, Chin. J. Child. Health Care, 1997, 144–147 CAS.
- S. Chen, J. Zhao, X. Hu, L. Tang, J. Li, D. Wu, T. Yan, L. Xu, M. Chen, S. Huang and Y. Hao, Children neuropsychological and behavioral scale-revision 2016 in the early detection of autism spectrum disorder, Front. Psychiatry, 2022, 13, 893226 CrossRef.
- S. González, M. Selma-Royo, S. Arboleya, C. Martínez-Costa, G. Solís, M. Suárez, N. Fernández, C. G. de Los Reyes-Gavilán, S. Díaz-Coto, P. Martínez-Camblor, M. C. Collado and M. Gueimonde, Levels of Predominant Intestinal Microorganisms in 1 Month-Old Full-Term Babies and Weight Gain during the First Year of Life, Nutrients, 2021, 13, 2412 CrossRef.
- E. Bezirtzoglou, A. Tsiotsias and G. W. Welling, Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH), Anaerobe, 2011, 17, 478–482 CrossRef.
- X. He, M. Parenti, T. Grip, M. Domellöf, B. Lönnerdal, O. Hernell, N. Timby and C. M. Slupsky, Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: a randomized controlled trial, Sci. Rep., 2019, 9, 339 CrossRef.
- M. Cavaletto, A. Givonetti and C. Cattaneo, The Immunological Role of Milk Fat Globule Membrane, Nutrients, 2022, 14, 4574 CrossRef CAS PubMed.
- M. Yao, E. L. Lien, M. R. Capeding, M. Fitzgerald, K. Ramanujam, R. Yuhas, R. Northington, J. Lebumfacil, L. Wang and P. A. DeRusso, Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity, J. Pediatr. Gastroenterol. Nutr., 2014, 59, 440–448 CrossRef CAS.
- H. Schmelzle, S. Wirth, H. Skopnik, M. Radke, J. Knol, H. M. Böckler, A. Brönstrup, J. Wells and C. Fusch, Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides, J. Pediatr. Gastroenterol. Nutr., 2003, 36, 343–351 CAS.
- A. Rivière, M. Selak, D. Lantin, F. Leroy and L. De Vuyst, Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut, Front. Microbiol., 2016, 7, 979 Search PubMed.
- S. Rautava, Early microbial contact, the breast milk microbiome and child health, J. Dev. Origins Health Dis., 2016, 7, 5–14 CrossRef CAS.
- B. Zhu, S. Zheng, K. Lin, X. Xu, L. Lv, Z. Zhao and J. Shao, Effects of Infant Formula Supplemented With Prebiotics and OPO on Infancy Fecal Microbiota: A Pilot Randomized Clinical Trial, Front. Cell. Infect. Microbiol., 2021, 11, 650407 CrossRef CAS.
- G. Casaburi, R. M. Duar, H. Brown, R. D. Mitchell, S. Kazi, S. Chew, O. Cagney, R. L. Flannery, K. G. Sylvester, S. A. Frese, B. M. Henrick and S. L. Freeman, Metagenomic insights of the infant microbiome community structure and function across multiple sites in the United States, Sci. Rep., 2021, 11, 1472 CrossRef CAS PubMed.
- B. Koletzko, Human Milk Lipids, Ann. Nutr. Metab., 2016, 69(Suppl 2), 28–40 Search PubMed.
- A. Gázquez, M. Sabater-Molina, I. Domínguez-López, M. Sánchez-Campillo, N. Torrento, J. Tibau, J. A. Moreno-Muñoz, M. Rodríguez-Palmero, M. C. López-Sabater and E. Larqué, Milk fat globule membrane plus milk fat increase docosahexaenoic acid availability in infant formulas, Eur. J. Nutr., 2023, 62, 833–845 Search PubMed.
- I. Litmanovitz, K. Davidson, A. Eliakim, R. H. Regev, T. Dolfin, S. Arnon, F. Bar-Yoseph, A. Goren, Y. Lifshitz and D. Nemet, High Beta-palmitate formula and bone strength in term infants: a randomized, double-blind, controlled trial, Calcif. Tissue Int., 2013, 92, 35–41 CrossRef CAS.
- K. Kennedy, M. S. Fewtrell, R. Morley, R. Abbott, P. T. Quinlan, J. C. Wells, J. G. Bindels and A. Lucas, Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization, Am. J. Clin. Nutr., 1999, 70, 920–927 CrossRef CAS PubMed.
- Y. Iwasaki and T. Yamane, Enzymatic synthesis of structured lipids, Adv. Biochem. Eng. Biotechnol., 2004, 90, 151–171 CrossRef CAS.
- D. M. Small, The effects of glyceride structure on absorption and metabolism, Annu. Rev. Nutr., 1991, 11, 413–434 CrossRef CAS PubMed.
- V. P. Carnielli, I. H. Luijendijk, J. B. Van Goudoever, E. J. Sulkers, A. A. Boerlage, H. J. Degenhart and P. J. Sauer, Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance, J. Pediatr. Gastroenterol. Nutr., 1996, 23, 553–560 CrossRef CAS.
- A. Lucas, P. Quinlan, S. Abrams, S. Ryan, S. Meah and P. J. Lucas, Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants, Arch. Dis. Child. Fetal Neonatal Ed., 1997, 77, F178–F184 CrossRef CAS.
- L. Shen, W. Huang, X. Xu, L. Wang, Q. Wang, S. Li and X. Yuan, Stool Saponified Fatty Acid, Behavior, Growth, and Stool Characteristics in Infants Fed a High-OPO Formula: A Randomized, Double-Blind Clinical Trial, Front. Pediatr., 2021, 9, 712201 CrossRef.
- R. M. Kliegman and J. W. Sparks, Perinatal galactose metabolism, J. Pediatr., 1985, 107, 831–841 CrossRef CAS.
- Z. Yuan, S. Liu, W. Song, Y. Liu, G. Bi, R. Xie and L. Ren, Galactose Enhances Chondrogenic Differentiation of ATDC5 and Cartilage Matrix Formation by Chondrocytes, Front. Mol. Biosci., 2022, 9, 850778 CrossRef CAS.
- L. Lu, L. Guo, K. Wang, Y. Liu and M. Xiao, β-Galactosidases: A great tool for synthesizing galactose-containing carbohydrates, Biotechnol. Adv., 2020, 39, 107465 CrossRef CAS PubMed.
- A. I. Coelho, G. T. Berry and M. E. Rubio-Gozalbo, Galactose metabolism and health, Curr. Opin. Clin. Nutr. Metab. Care, 2015, 18, 422–427 CrossRef CAS PubMed.
- G. van Echten-Deckert and T. Herget, Sphingolipid metabolism in neural cells, Biochim. Biophys. Acta, 2006, 1758, 1978–1994 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |