A synbiotic formulation of Lactobacillus reuteri and inulin alleviates ASD-like behaviors in a mouse model: the mediating role of the gut–brain axis†
Chuanchuan
Wang‡
ab,
Weixuan
Chen‡
 a,
Yishan
Jiang
a,
Xiao
Xiao
a,
Qianhui
Zou
a,
Jiarui
Liang
a,
Yu
Zhao
a,
Qianxu
Wang
a,
Tian
Yuan
bc,
Rui
Guo
*ab,
Xuebo
Liu
a,
Yishan
Jiang
a,
Xiao
Xiao
a,
Qianhui
Zou
a,
Jiarui
Liang
a,
Yu
Zhao
a,
Qianxu
Wang
a,
Tian
Yuan
bc,
Rui
Guo
*ab,
Xuebo
Liu
 *a and
Zhigang
Liu
*a and
Zhigang
Liu
 *ab
*ab
aLaboratory of Functional Chemistry and Nutrition of Food, College of Food Science and Engineering, Northwest A&F University, Yangling, Shaanxi 712100, China. E-mail: zhigangliu@nwafu.edu.cn
bNorthwest A&F University Shenzhen Research Institute, Shenzhen, Guangdong 518000, China
cShaanxi Key Laboratory of Natural Products & Chemical Biology, College of Chemistry & Pharmacy, Northwest A&F University, Yangling, Shaanxi 712100, China
First published on 5th December 2023
Abstract
Autism Spectrum Disorder (ASD), a complex neurodevelopmental disorder marked by social communication deficits and repetitive behaviors, may see symptom amelioration through gut microbiota modulation. This study investigates the effects of a synbiotic – specifically a probiotic amplified by prebiotic supplementation – on ASD-like mouse model's social deficiencies. This model was established via valproic acid injection into pregnant females. Post-weaning, male progeny received daily synbiotic treatment, a combination of Lactobacillus reuteri (L. reuteri) and inulin, for four weeks. Results indicated that the synbiotic rectified social impairments and attenuated inflammatory cytokine expressions in the brain. Moreover, synbiotic intervention protected gut barrier integrity and altered the gut microbiota composition, enhancing the butyrate-producing Bifidobacterium abundance. The synbiotic elevated metabolites such as butyrate and 3-hydroxybutyric acid (3-HB), alongside upregulated genes associated with 3-HB synthesis in the colon and liver, and brain receptors. Conclusively, the synbiotic combination of L. reuteri and inulin mitigated ASD-related social impairments, partially via their regulatory effect on the gut–brain axis.
Introduction
Autism Spectrum Disorder (ASD), commonly referred to as autism, is a multifaceted neurodevelopmental disorder that threatens children's health. The disorder is typified by a spectrum of symptoms including social impairment, repetitive behavior, and cognitive deficits, along with heightened anxiety-like behavior.1,2 ASD arises from a complex interplay of genetic and environmental factors. The main environmental factors contributing to ASD are exposure to drugs during pregnancy, autoimmune diseases, microbial infections, and dysbiosis of the maternal gut microbiota due to dietary choices during the prenatal and postnatal periods.3 Nonetheless, the precise pathogenic mechanisms underlying the disorder remain poorly understood, and efficacious treatments are currently lacking.Recently, attention has been drawn toward dietary strategies, such as probiotics, prebiotics, and synbiotics, to potentially restore neurodevelopmental disorders. Synbiotics, being a combination of probiotics and prebiotics, provide both probiotic and prebiotic properties, with the main aim of prebiotic supplementation being able to enhance the survival and colonization rates of probiotic microorganisms in the gastrointestinal tract.4 Through the gut–brain axis mechanism, the influence of diet on the central nervous system function can be explained. Although the pathogenesis of ASD remains unclear, mounting evidence suggests that the microbiota–gut–brain axis plays a significant role in regulating the brain function.5 Studies have observed a noteworthy difference in the gut microbiota composition of children with ASD compared to normal children, with the abundance of beneficial bacteria in the gut of children with ASD being notably lower than that of normal children.6 The levels of short-chain fatty acids (SCFAs), particularly propionate, butyrate, and acetate, in the feces of ASD patients, are significantly reduced, indicating a reduction in the microbiota's fermentative capacity.7 SCFAs have been demonstrated to enhance the expression of tight junction proteins (occludin, claudin-5, ZO-1), which protect the integrity of the gut barrier, enter the bloodstream via the gut epithelium and cross the blood–brain barrier, ultimately influencing the central nervous system function.8 Hence, modulating the gut microbiota's structure and metabolite production may serve as a promising strategy to ameliorate neurodevelopmental disorders such as ASD.
Maternal obesity induces ASD-like behaviors in offspring, and intervention with L. reuteri in the mothers was found to rescue the social impairment capacity of offspring with ASD.9 Supplementation of L. reuteri in ASD mice can alleviate synaptic plasticity and social impairment through the vagus nerve in ASD mice.10 These findings suggest that the probiotic L. reuteri is an effective nutritional intervention for attenuating social impairment in ASD. Furthermore, supplementation with the prebiotic inulin has been shown to increase the relative abundance of beneficial bacteria and the content of SCFAs in the feces of ASD mice, preventing their cognitive deficits and impairment and social deficits.11 However, the mechanism of the effect of synbiotics on autistic social deficits remains unclear. In this study, we hypothesized that supplementation with synbiotics could alter the gut microbiota and enhance the formation of the metabolite SCFAs, thereby mitigating neurodevelopmental disorders in ASD.
Materials and methods
Animal experiment
Male and female C57BL/6J mice, aged 7 weeks, were procured from Jicui Yaokang Biological Technology Co., Ltd (Jiangsu, China). The mice were housed under standard conditions in Northwest A&F University's animal facility. The animals were maintained under a strict 12-hour light/dark cycle (8:00 a.m. to 8:00 p.m.) and provided with ad libitum access to water. Additionally, they were adaptively fed for one week and maintained at a temperature of 22 ± 2 °C with a humidity level of 50 ± 15%. All experimental procedures were carried out following the Guide for the Care and Use of Laboratory Animals: Eighth Edition (ISBN-10: 0-309-15396-now4) and were approved by the animal ethics committee of Northwest A&F University (protocol number N81803231).Probiotic culture
The probiotic L. reuteri C501, extracted from a healthy male volunteer's fecal sample, was obtained from Zhuhai Yihetech Biotechnology Co., Ltd. The probiotic L. reuteri C501 was cultured in MRS broth at 37 °C for more than 24 h for obtaining resuscitated bacterial powder. The revived bacterial solution was inoculated in the new MRS broth and cultured at 37 °C for more than 24 h for passage. The probiotic culture was centrifuged, the supernatant was discarded, washed with phosphate-buffered saline (PBS), centrifuged, the supernatant was discarded, and the bacterial precipitates were suspended in PBS again. The bacterial content was determined by plate counting and stored at 4 °C for later use. Two days later, the plates were counted and the concentration of the bacterial solution was diluted to 109 CFU mL−1. To ensure the activity of probiotics, the bacteria should be cultivated regularly every week.Dosage information
After adaptive feeding, one male and two female mice were mated in a co-cage, and vaginal suppositories were observed twice a day. On the day when the vaginal plugs were observed, female mice were kept in a single cage and recorded as day 0 of pregnancy, and fed a growth and reproduction diet (Medicience Biomedical Co., Ltd, Jiangsu, China). Female mice were randomly divided into two groups: the control group and the ASD modeling group. The ASD model was constructed by using i.p. 500 mg kg−1 VPA (≥98%, V298968, Aladdin Bio-Chem Technology Co., Ltd, Shanghai, China) at 12.5 days of pregnancy in the ASD modeling group of female mice. Before injection, the solution was dissolved in 0.9% saline and the injection dose was 100 μL. The control group of female mice was injected with 100 μL of saline.12–14 Offspring mice were weaned 21 days after birth, and the offspring mice were kept in separate cages, and only male mice were kept for follow-up intervention experiments, and the offspring mice were guaranteed to come from more than 3 different female mice.Offspring male mice were weaned and given probiotics or an equal volume of PBS by i.g., and fed water with or without prebiotics for 4 weeks. The synbiotic intervention was the simultaneous intervention of probiotics and prebiotics. The intervention dose of probiotic L. reuteri was 100 μL (∼1 × 108 CFU per mouse per day), while the control group received the same amount of PBS.10 Prebiotic inulin was administered at 1% w/v water intake (S11143, Yuanye Bio-Technology Co., Ltd, Shanghai, China), and the water was changed every 2 days.
The weight of the mice was recorded in detail every week. The mice were fed a maintenance diet during the intervention period. The maintenance diet was purchased from China Jiangsu Medicine Biomedical. Fecal samples were collected and the behavior of the mice was assessed when the mice were 8–9 weeks old, and tissue and serum were collected from the sacrificed mice after behavioral analysis. Samples were stored in a −80 °C refrigerator for future use.
Growth curve
The whole genome information of L. reuteri C501 was obtained by the whole genome sequencing technique, where CAZy analysis was performed to obtain its corresponding carbohydrate-active enzymes, and the glycoside hydrolase that could hydrolyze the carbon source was screened to complete the prediction based on the specific prebiotic for the strain.The best prebiotic of the strains was screened using in vitro growth curves. The strain was transferred to MRS medium and cultured at 37 °C for more than 24 h. The predicted prebiotic was used as the sole carbon source, GAM was used as a blank control, and sucrose was used as a positive control. 50 μL of bacterial solution was first added to the well plate, and then 300 μL of carbon source was added, and the specific formulation is shown in Table S1.† A growth curve tester was used to determine the strain's growth curve in the medium with the corresponding prebiotic as a substrate, and then the effect of the prebiotic was determined to screen out the dominant prebiotic and determine the composition of the synbiotic. The growth curves of different concentrations of the optimal prebiotic were analyzed to determine the optimal dose of the prebiotic.
The strains were transferred in MRS liquid medium and incubated at 37 °C for more than 24 h. Different multiples of dilutions were used and plates were coated to determine the concentration of this supernatant. Dilutions were performed according to the concentration of the bacterial solution, with different concentrations of the best carbon source obtained above as the only carbon source, 50 μL of the bacterial solution was added to the well plate, followed by 300 μL of the carbon source, as shown in Table S1.† The growth curves of the strains with different concentrations in the medium with the best prebiotic as the substrate were determined, which in turn determined the optimal dose of the probiotic.
Behavioral experiments
During the second 10 min, sociability was assessed. An empty wire cup (empty) was placed in the left chamber and a wire cup containing an age- and sex-matched stranger mouse was placed in the right chamber (mouse 1). The subject was placed in the middle chamber and allowed to explore freely. The time the subjects interacted (sniffed) with empty or mouse 1 was recorded using the tracking software SuperMaze. The sociability interaction index was calculated as (time spent interacting with mouse 1 − time spent interacting with empty)/(time spent interacting with mouse 1 + time spent interacting with empty).
During the third 10 min, the preference for social novelty was evaluated. A wire cup with a second stranger mouse (mouse 2) was placed in the left chamber and a wire cup with mouse 1 was placed in the right chamber. The subject was placed in the middle chamber and allowed to explore freely. The amount of time the subject spent interacting with mouse 1 or mouse 2 was recorded. The social novelty interaction index was calculated as (time spent interacting with mouse 2 − time spent interacting with mouse 1)/(time spent interacting with mouse 2 + time spent interacting with mouse 1). The experimental instruments were wiped with 75% alcohol before testing on mice.
Repetitive self-grooming behavior
The self-grooming test was performed as previously described to assess repetitive behavior.16 To adapt to the environment, the mice were placed into a clean cage (25 cm × 18 cm × 16 cm) alone and moved freely for 10 min. The time spent grooming was then recorded for 10 min (paw licking, body grooming, or scratching). The experimental instruments were wiped with 75% alcohol before testing on mice.L. reuteri quantification in feces
As described previously, bacterial quantification was performed by quantitative PCR.10 The feces were placed on ice and quickly weighed to a weight of about 0.18 g to 0.2 g. Genomic DNA was extracted from the fecal samples using a TIANamp Stool DNA Kit (Tiangen Biochemical Technology Co., Ltd, Beijing, China), according to the manufacturer's protocol. The concentration of DNA was determined using a NanoDrop 2000/2000C (Thermo Fisher Scientific; Waltham, MA, USA), and then diluted to the same concentration with enzyme-free water. General bacterial primers were used as endogenous reference gene primers, and the target gene L. reuteri primers were quantified by qPCR using 2× SYBR Green qPCR Master Mix (Suzhou Hengyu Biotechnology Co., Ltd, Jiangsu, China). The primers are provided in Table S2.†qRT-PCR analysis
As previously described,17 total RNA was extracted from the brain, colon, and liver tissue using the BIOZOL reagent (Hangzhou Bioer Technology Co., Zhejiang, China). RNA concentrations were determined using a NanoDrop 2000/2000C (Thermo Fisher Scientific; Waltham, MA, USA), diluted to the same concentration with enzyme-free water. RNA was reverse transcribed into cDNA using the UEIris RT mix with DNase (Suzhou Hengyu Biotechnology Co., Ltd, Jiangsu, China). After reverse transcription, the samples were diluted 5-fold with enzyme-free water, and then the reaction system was prepared with 2× SYBR Green qPCR Master Mix (Suzhou Hengyu Biotechnology Co., Ltd, Jiangsu, China) for quantitative analysis. The expression of GAPDH was used as an endogenous control, and relative gene expression was calculated with the 2−ΔΔCt method. ΔCt represents the disparity in threshold cycle (Ct) values between the gene of interest and a reference gene for each sample. Subsequently, ΔΔCt is determined by subtracting the ΔCt of a control or reference sample from the ΔCt of the sample under analysis. Gene primer sequences are provided in Table S2.†Fecal and serum SCFA level analyses
As previously described,18 the concentration of SCFAs in the feces and serum was detected using gas chromatography. The standard curve was constructed with the standard mixture of different concentrations of SCFAs. Briefly, 0.1 mL serum or 0.15–0.20 g feces was homogenized with 1 mL of MilliQ water and 0.15 mL of 50% H2SO4 (w/w). The mixture was added with 1.6 mL of diethyl ether, and the samples were incubated on ice for 20 min. Centrifugation was performed at 8000 rpm for 5 min to obtain the supernatant, and filtration was carried out using a 0.2 μm filter (Branch Billion Lung Experimental Equipment Co., Ltd, Tianjin, China) into clear GC vials. The standards of SCFAs included acetate, A116173; propionate, P110445; butyrate, B11se0438 (Aladdin Bio-Chem Technology Co., Ltd, Shanghai, China). The concentration of SCFAs in the feces and serum was analyzed using a GC-2014C gas chromatograph (Shimadzu Corporation, Kyoto, Japan), equipped with a DB-FFAP capillary column (Agilent Technologies, Wilmington, DE, USA) and flame ionization detector.Nissl staining
Half of the brain tissue of the mice was collected, immersed in 4% paraformaldehyde solution, and paraffin-embedded. The tissue was cut into 5 μm sections. The tissue sections were placed at 37 °C overnight in advance. According to the manufacturer's instructions, the tissue sections were then stained with Nissl staining solution (Beyotime Biotechnology, Shanghai, China). Tissue sections were observed using an optical microscope (Olympus, Tokyo, Japan). ImageJ (National Institutes of Health, MD, USA) can be used for quantitative analysis of the number of Nissl positive cells.Immunofluorescence staining
As previously reported, BDNF was subjected to immunofluorescence staining.11 Brain sections were placed at 37 °C overnight. The sections were dewaxed and rehydrated by soaking in xylene and gradient ethanol, and then washed with PBS for 4 min × 5 times. After immersing in 0.5% Triton X-100 for 15 min, the sections were washed with PBS for 3 min × 6 times. The sections were heated in a 100 °C water bath in the repair solution sodium citrate for 15 min, cooled to room temperature, and washed with PBS for 5 min × 6 times. Then the sections were immersed in 3% H2O2 for 15 min to block endogenous peroxidase and washed with PBS for 3 min × 5 times. The water was removed by drying and incubation was performed using goat serum at room temperature for 20 min for sealing non-specific sites. Then, the sections were wiped dry and sliced with a primary antibody (anti-BDNF, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, cat# ab108319, Abcam, Cambridge, UK) incubated overnight at 4 °C. On the second day, the sections were rinsed with PBS for 3 min × 6 times, wiped dry, incubated with a secondary antibody at 25 °C for 2 h (goat anti-rabbit IgG (H + L) Alexa Fluor 594, AB0151, Abways Technology, Shanghai, China), rinsed with PBS for 3 min × 6 times, and sealed with a DAPI anti-fluorescence quencher. The sections were observed under a fluorescence microscope (Olympus, Tokyo, Japan). Quantification of the mean fluorescence intensity (AU) of BDNF in the cortical regions was performed using ImageJ.
500, cat# ab108319, Abcam, Cambridge, UK) incubated overnight at 4 °C. On the second day, the sections were rinsed with PBS for 3 min × 6 times, wiped dry, incubated with a secondary antibody at 25 °C for 2 h (goat anti-rabbit IgG (H + L) Alexa Fluor 594, AB0151, Abways Technology, Shanghai, China), rinsed with PBS for 3 min × 6 times, and sealed with a DAPI anti-fluorescence quencher. The sections were observed under a fluorescence microscope (Olympus, Tokyo, Japan). Quantification of the mean fluorescence intensity (AU) of BDNF in the cortical regions was performed using ImageJ.
Hematoxylin and eosin (H&E) staining and Alcian Blue staining
As described previously,19 the colon tissues of the mice were collected after the mice were sacrificed, immersed in a 4% paraformaldehyde solution, and paraffin-embedded. The tissue was cut into 5 μm sections. The tissue sections were stained with H&E. As described previously,20 Alcian Blue staining has often been used to observe the goblet cell number. Then tissue sections were stained with an Alcian Blue reagent test kit (Xinle Biotechnology, Shanghai, China), according to the manufacturer's instructions. The colon tissue sections were observed with an optical microscope. The length of the crypt (μm) and the thickness of the muscles (μm) were measured using ImageJ by H&E staining. ImageJ can be used for quantitative analysis of goblet cells by alcian blue staining.Metabolomic assay
High-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to perform HM350 targeted quantification of 350 metabolites in the sample. Mouse blood was collected from mouse eyes and placed in a 37 °C water bath (3000 rpm, 10 min) before centrifugation. Serum samples (50 μL) were taken into centrifuge tubes, and HM350 mixed standard solutions were serially diluted to prepare standard samples. Samples and standards were extracted with the HM350 release agent and analyzed by MS onboard. Metabolomic assays were performed on an LC-MS QTRAP 6500+ (SCIEX) mass spectrometer equipped with a BEH C18 column (2.1 mm × 10 cm, 1.7 μm, Waters).16S rRNA sequencing analysis
Feces (0.1 g) were collected before behavioral testing and immediately stored at −80 °C until DNA extraction. The sample DNA was isolated, and 30 ng DNA samples and the corresponding PCR reaction systems were selected to amplify the V3–V4 region of the 16S rDNA gene. Agencourt AMPure XP magnetic beads were used to purify PCR-amplified products and dissolved in elution buffer to complete library construction. The fragment range and concentration of the library were checked using the Agilent 2100 Bioanalyzer. Qualified libraries were sequenced on the HiSeq platform according to the size of the inserted fragments. The filtered clean data were used for later analysis. Sequence splicing was performed using FLASH software (Fast Length Adjustment of Short reads, v1.2.11), and the paired reads were spliced into a sequence through overlapping relationships to obtain tags of hypervariable regions. The software USEARCH (v7.0.1090) was used to cluster the spliced tags into OTUs (OperationalTaxonomic Units), compared with the database, and species annotation. Based on the OTU and annotation results, species difference analyses between groups were performed by the Kruskal test. R (v3.1.1) Venn Diagram package was employed to generate the Venn diagram. R (v3.2.1) mixOmics package was chosen for partial least squares discriminant analysis (PLS-DA) analysis. R (v3.1.1) gplots package was used to form a heatmap. R (v3.4.1) was used to make species histograms.Sequence data availability
The whole-genome sequence of L. reuteri C501 of the samples was uploaded in the NCBI database and can be found under accession number CP128357-CP128359.Statistical analysis
Data processing was performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) One-way ANOVA was performed to determine significant differences between groups. A post hoc analysis was then performed using Tukey's multiple comparison test. Data were expressed as mean ± SEM, and p < 0.05 was considered statistically significant.Results
Effect of different prebiotics on the growth of probiotics in vitro
Studies have shown that probiotics can use prebiotics as carbon and energy sources through glycoside hydrolases (GHs).21,22 CAZy analysis with whole genome sequencing revealed that L. reuteri C501 contains endo-inulinase (EC 3.2.1.7) and exo-inulinase (EC 3.2.1.80), and the glycoside hydrolases of GH32 were involved in the hydrolysis of prebiotic inulin; and L. reuteri C501 was found to use exo-beta-1,3-glucanase (EC 3.2.1.58) and endo-beta-1,3-glucanase (EC 3.2.1.39) of GH55, and endo-1,3(4)-beta-glucanase (EC 3.2.1.6) glycoside hydrolases of GH16 to hydrolyze the prebiotic β-glucan.After the initial screening of prebiotics, inulin (INU) and β-glucan (β-GLU) were derived as prebiotics for the probiotic L. reuteri. The screening of the best carbon source was carried out by measuring the growth curves of different carbon sources. Three different sources of β-glucan were used to exclude the source of β-GLU: β-GLU1 was from highland barley, β-GLU2 was from oats, and β-GLU3 was from yeast. It was found that the probiotic L. reuteri with inulin added to the medium grew better (Fig. S1A–E†). The above results indicated that inulin was selected as the dominant prebiotic for L. reuteri, and therefore, synbiotics were formed from inulin and L. reuteri.
Growth curves were measured in vitro under 109 CFU mL−1 bacterial solution, and the optimal concentration of prebiotic inulin was determined to be 20 g L−1 (Fig. S2A†). When the optimal concentration of probiotic inulin was 20 g L−1, the growth curve of L. reuteri C501 was measured by adding the probiotic with different concentrations. In vitro tests showed that the optimal gradient of the probiotic was 2 × 108 CFU mL−1 (Fig. S3A†).
Effects of synbiotic treatment on behavioral impairment in VPA-induced ASD mice
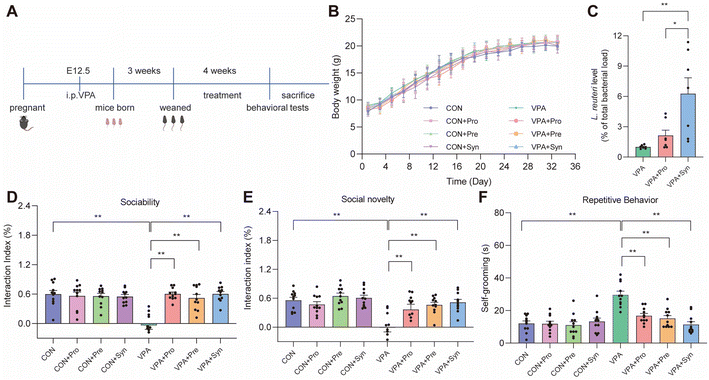
To investigate the effects of synbiotics on the behavioral impairment of ASD mice, an experimental process was followed, as shown in Fig. 1A. During the intervention period, there was no difference in body weight change in ASD mice treated with probiotics, prebiotics, and synbiotics (Fig. 1B). We found a significantly elevated level of L. reuteri in the synbiotic treatment compared to the probiotic treatment in ASD mice (p < 0.05) (Fig. 1C).A key feature of ASD is abnormalities in social interactions. To investigate the effects of synbiotics on social behaviors in ASD mice, a three-chamber social test was conducted. ASD mice showed significant social deficits (p < 0.01), including sociability (Fig. 1D and S4A†) and social novelty (Fig. 1E and S4B†). Probiotics, prebiotics, and synbiotics significantly alleviated this deficiency (p < 0.01) (Fig. 1D and E and Fig. S4A and B†). Another key characteristic of ASD is repetitive stereotyped movements. The effect of synbiotics on repetitive stereotyped movements was evaluated by the self-grooming test in ASD mice. Compared with the control group, the ASD mice showed significantly increased grooming time during the self-grooming test (p < 0.01) (Fig. 1F). This alteration was prevented by treatment (Fig. 1F). These results indicated that probiotics, prebiotics, and synbiotics all reversed social deficits and repetitive behaviors in ASD mice.
Effects of synbiotic treatment on neuronal damage and neuroinflammatory responses in VPA-induced ASD mice
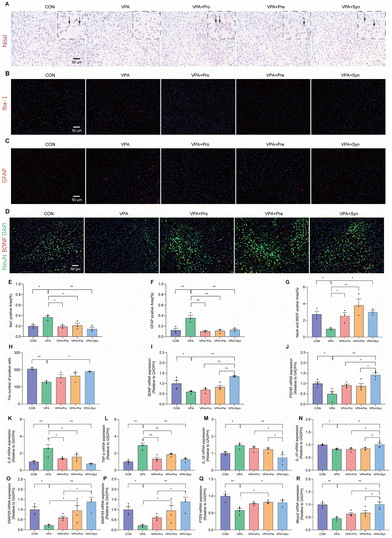
To determine the effect of synbiotic treatment on neuronal loss in ASD mice, Nissl staining, Iba-1 staining, GFAP staining, and co-staining with NeuN (as a neuronal marker) and BDNF were conducted to observe the changes in the cortical neurons in the mouse cortex (Fig. 2A–H). Nissl staining showed that the number of Nissl positive cells in the cortical region of ASD mice was notably reduced compared with the control group (p < 0.01). This change was mitigated by synbiotic treatment (p < 0.05) (Fig. 2A and H). Furthermore, to investigate the effects of synbiotics on neurotrophic factor expressions and neuroinflammation in the ASD-like mouse brain, the immunofluorescence co-staining of NeuN and BDNF was conducted in the mouse cortex (Fig. 2D). It was found that the ASD mice had a significantly reduced mean fluorescence intensity of NeuN and BDNF in the cortex, compared to the control mice (p < 0.01) (Fig. 2G). However, this reduction was significantly prevented by prebiotic, probiotic, and synbiotic treatments in ASD mice (p < 0.01) (Fig. 2D and G). ASD mice had significantly greater mean fluorescence intensities of Iba-1 (p 0.05) and GFAP (p 0.01) than the control mice in the cortex (p 0.01) (Fig. 2B, C, E and F). However, the increase in ASD mice was significantly prevented by prebiotic, probiotic, and synbiotic treatments (p 0.01) (Fig. 2D and G). It was also found that the mRNA expression levels of BDNF and the post-synaptic density protein (PSD-95) in the cortex of ASD mice were lower than those of the control mice (p < 0.05) (Fig. 2I and J), and synbiotic intervention markedly restored the expression levels of these two genes (p < 0.01) (Fig. 2I and J). In addition, the intervention effect of synbiotics on ASD mice was significantly increased than that of probiotics and prebiotics (p < 0.05) (Fig. 2I and J). Thus, synbiotic treatment restored neuronal loss in the brains of ASD mice.To evaluate the effect of synbiotic treatment on neuroinflammatory responses in ASD mice, the expression levels of cortical inflammatory cytokine genes (IL-6, TNF-α, IL-1β, and IL-10) were measured. Compared with the control group, the mRNA expression of the pro-inflammatory cytokines IL-6, TNF-α, and IL-1β was significantly increased (p < 0.05) (Fig. 2K–M), while the anti-inflammatory cytokine IL-10 was significantly decreased in the cortex of ASD mice (p < 0.05) (Fig. 2N). Synbiotic treatment significantly reversed the expression level of inflammatory cytokines in ASD mice (p < 0.05) (Fig. 2K–N). Furthermore, compared with probiotics and prebiotics, synbiotics significantly reduced IL-1β overexpression in the cortex of ASD mice (p < 0.05) (Fig. 2M), and significantly enhanced the expression of the anti-inflammatory cytokine IL-10 (p < 0.05) (Fig. 2N).
To assess the effect of synbiotic treatment on social-related genes in the cerebral cortex of ASD mice, the expression levels of cortical ASD-related genes (Shank3, SNAP25, PTEN, and Mecp2) were evaluated (Fig. 2O–R). ASD mice showed significantly decreased mRNA expressions of Shank3, SNAP25, PTEN, and Mecp2 in the cortex compared with the control group (p < 0.05), which was alleviated by synbiotic treatment (p < 0.05) (Fig. 2O–R). These results suggest that synbiotic intervention rescued the expression of brain inflammation and social-related genes in ASD mice.
Effects of synbiotic treatment on gut barrier integrity damage and inflammatory responses in VPA-induced ASD mice
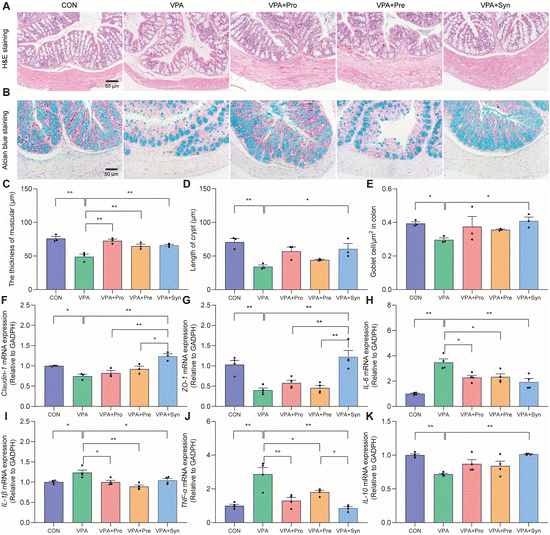
To investigate the effect of synbiotics on gut barrier integrity damage in ASD mice, H&E staining and Alcian blue staining were used for analysis. H&E staining showed severe pathological changes such as a decreased thickness of muscles and damaged crypts in ASD mice (p < 0.01) (Fig. 3A). Synbiotics significantly inhibited colon damage in ASD mice (p < 0.01) (Fig. 3C and D). The number of goblet cells was measured by Alcian blue staining (Fig. 3B). The reduction of goblet cells was significantly mitigated by synbiotic treatment in the colons of ASD mice (p < 0.01) (Fig. 3E). The mRNA expressions of tight junction proteins Claudin-1 and ZO-1 in ASD mice significantly lowered than those in the control mice (p < 0.01) (Fig. 3F and G), and the change could be reversed by synbiotic intervention. These results indicated that synbiotic intervention attenuated colon damage in ASD mice.To study the effect of synbiotics on the gut inflammatory responses of ASD, the mRNA expression of inflammatory cytokines in colon tissues was detected by qRT-PCR. Compared with the control group, the expressions of pro-inflammatory cytokines IL-6, IL-1β and TNF-α were significantly increased in ASD mice (p < 0.05) (Fig. 3H–J). The mRNA expression of these genes was significantly down-regulated by synbiotic intervention in the colon tissues. At the same time, synbiotics significantly elevated the mRNA expression of the anti-inflammatory cytokine IL-10 in ASD mice (p < 0.01) (Fig. 3K). These results demonstrated that the synbiotic intervention prevented gut inflammatory responses in ASD mice.
Effects of synbiotic treatment on gut microbiota diversity in VPA-induced ASD mice
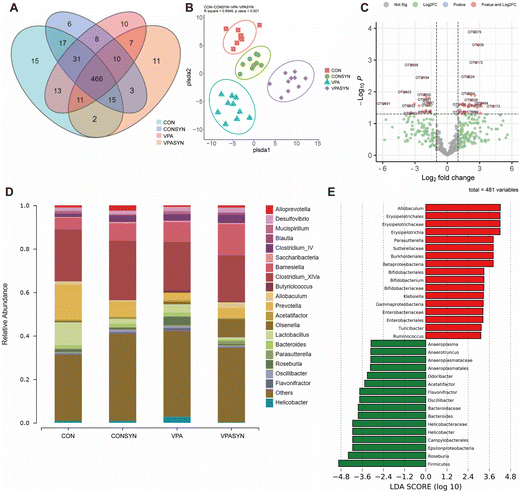
To investigate the effects of synbiotic treatment on the gut microbiota diversity of ASD mice, gut microbiota was detected by 16S rRNA sequencing analysis in the feces (Fig. 4). The Venn diagram results showed that there were 15 specific OTUs in the control group and 10 specific OTUs in the VPA group. There were 11 specific OTUs in the VPA + Syn group (Fig. 4A). PLS-DA was used to distinguish the differences in the composition of bacteria between different groups. The results indicated that the samples were clearly distinguished between the four groups. There was a separation between the diversity of the control mice and ASD mice. The diversity of the synbiotic intervention group was also different from that of the VPA group (R square = 0.8949, p < 0.001, Fig. 4B). The correlations between the OTUs and the coordinates of microbial communities revealed that Desulfovibrio, Clostridiales, and Porphyromonadaceae, among others, had the most significant impact on the distribution of these OTUs (P < 0.001, Table S3†). Volcano plots were generated to show the 26 OTUs that differed between the CON and VPA groups (Fig. S5A†), and the 56 OTUs that differed between the VPA and VPA + Syn groups are marked with red dots (Fig. 4C). At the genus level, the mice in the VPA group had increased enrichment of Roseburia (associated with gastrointestinal disease) and decreased enrichment of Allobaculum (butyrate-producing) compared to the control group, a change that was restored by the synbiotic intervention (Fig. 4D). Significantly different species were analyzed by Linear Discriminant Analysis (LDA) for microbiota that had a significant role in different groups. Consistent with this, the synbiotic intervention increased the abundance of the butyrate-producing bacteria Allobaculum, Bifidobacterium, and Parasutterella and decreased the abundance of the harmful bacteria Odoribacter and Roseburia compared to the ASD mice (Fig. S5B† and Fig. 4E). These results showed that synbiotics altered the gut microbiota composition of ASD mice.Effects of synbiotic treatment on metabolites in VPA-induced ASD mice
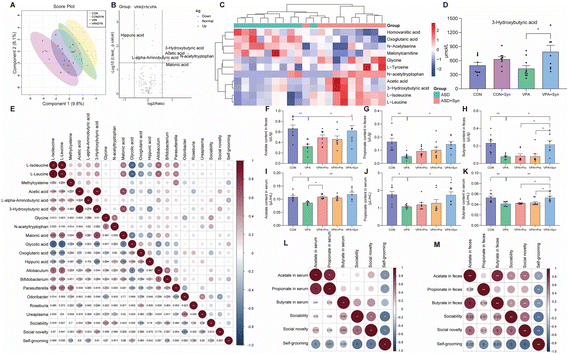
To investigate the effect of synbiotics on serum metabolites in ASD, high-performance liquid chromatography-tandem mass spectrometry was employed to analyze serum metabolites. PLS-DA was used to reflect the differences in composition between the different groups in serum metabolites, and the larger the distance between the groups indicated the greater the differences between them. The results showed a distinct separation between the CON group mice and VPA group mice, and the VPA + Syn group also exhibited a different diversity than the VPA group (Fig. 5A). A volcano plot was used to display the difference in the data between the VPA and VPA + Syn groups, and applying certain screening conditions, metabolites that were significantly differentially expressed could be screened. The results showed that compared with the VPA group mice, the synbiotic intervention upregulated the contents of 3-hydroxybutyric acid (3-HB), N-acetyltryptophan, malonic acid, acetic acid, and L-alpha-aminobutyric acid, and downregulated the content of hippuric acid (Fig. 5B). The heatmap analysis revealed that the metabolite content in the VPA group mice was reduced compared with that in the CON group mice, and this change was restored after synbiotic intervention (Fig. 5C). To further elucidate the effects of synbiotics on serum metabolic disorders in ASD, the content of metabolites was analyzed in serum (Fig. S6†). In ASD mice, synbiotic treatment significantly increased the level of 3-HB (p < 0.05) (Fig. 5D). These results indicated that compared with the CON group, the VPA group mice had serum metabolic disorders, which were reversed by synbiotic intervention, and differences were found in the content of 3-HB in the serum of the VPA and VPA + Syn groups.To investigate the effect of synbiotic treatment on SCFA formation in ASD mice, SCFA content in the feces and serum was determined. Compared with the control group, the contents of acetate, propionate, and butyrate in the feces and serum of ASD mice were significantly reduced. In addition, the content of SCFAs was significantly increased by synbiotic treatment in ASD mice (p < 0.05) (Fig. 5F–K). However, the content of butyrate in ASD mice under the synbiotic intervention was significantly higher than that under the probiotic intervention and prebiotic intervention (p < 0.05) (Fig. 5H and K). The mRNA expression levels of SCFA receptors, including GPR41 and GPR43, were significantly elevated by the treatment of synbiotics in ASD mice (p < 0.05) (Fig. S7†). These results indicate that the synbiotic intervention restored the production of SCFAs in ASD mice, especially the butyrate levels.
Spearman correlation analysis was conducted to elucidate the correlation between the metabolite levels, bacterium abundance, and behavior (Fig. 5E). The abundance of butyrate-producing Bifidobacterium was negatively correlated with self-grooming in mice (p = 0.05, r = −0.32), while the harmful bacteria abundance of Odoribacter was negatively correlated with social novelty (p = 0.003, r = −0.465). In addition, the level of 3-HB was positively correlated with social novelty (p = 0.046, r = 0.326) (Fig. 5E). And the level of serum butyrate was significantly positively correlated with sociability (p = 0.05, r = 0.37) and social novelty (p = 0.033, r = 0.404) (Fig. 5L). Similarly, the level of fecal butyrate showed a significant positive correlation with sociability (p = 0.026, r = 0.419) and social novelty (p = 0.001, r = 0.575) and a significant negative correlation with self-grooming (p = 0.007, r = −0.497) (Fig. 5M). These results suggest that metabolites and gut microbiota are related to behavior.
Effects of synbiotic treatment on the 3-HB synthesis gene in VPA-induced ASD mice
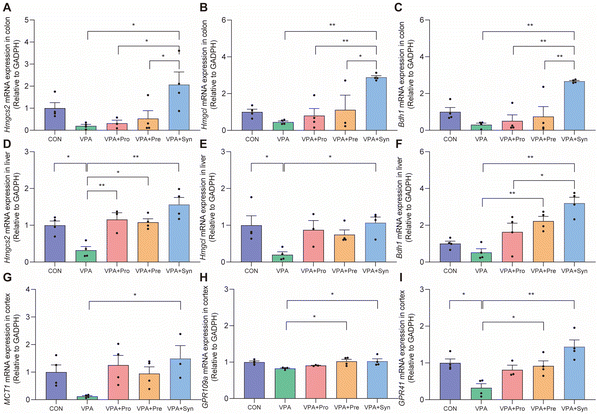
We speculated that synbiotics may ameliorate the social deficits of ASD by producing 3-HB in the intestine and liver. Thus, the mRNA expression levels of Hmgcs2, Hmgcl, and Bdh1 in the colon and liver were detected (Fig. 6A–F). Hmgcs2, Hmgcl, and Bdh1 are enzymes that synthesize 3-HB, among which Hmgcs2 is the main rate-limiting ketogenic enzyme. The results showed that the expression levels of Hmgcs2 and Hmgcl in the ASD liver were significantly lower than those in the control group (p < 0.05) (Fig. 6D and E). The synbiotic intervention significantly increased the mRNA expression levels of Hmgcs2, Hmgcl, and Bdh1 in the colon and liver (p < 0.05) (Fig. 6A–F). Synbiotics significantly increased MCT1 mRNA expression levels in the brains of ASD mice (p < 0.05) (Fig. 6G). It was found that the synbiotic intervention significantly restored the expression levels of 3-HB receptors GPR109a and GPR41 in the cortex (p < 0.05) (Fig. 6H and I). These results indicate that synbiotic intervention rescued the expression of enzymes that synthesize 3-HB in the colon and liver, as well as the receptor for 3-HB in the brains in ASD mice.Discussion
In this study, the impact of synbiotics on social dysfunction and repetitive behavior in offspring with ASD induced by intraperitoneal injection of VPA during maternal pregnancy was investigated. Synbiotics were composed of inulin and L. reuteri C501. The findings showed that synbiotics effectively reversed social deficits and repetitive behavior, neuronal damage, expression of social-related genes, and neuroinflammatory factors in the offspring of ASD mice induced by VPA during maternal pregnancy. Additionally, it was found that synbiotic intervention restored gut morphology, alleviated gut inflammation, increased the production of gut microbial metabolite SCFAs and the expression levels of their receptors, and altered the structure of the gut microbiota in ASD mice, which may be due to the important role of butyric acid. Furthermore, it was discovered that synbiotic intervention significantly upregulated the content of 3-HB in serum, increased the expression of synthetic enzymes for 3-HB synthesis in the colon and liver, and increased the expression levels of 3-HB receptors in the brain. Synbiotics relieve social deficits and repetitive behavior caused by neuronal damage and inflammatory responses in ASD offspring, which may be associated with the changes in metabolites including butyric acid and 3-HB.ASD is a neurodevelopmental disorder in which the initial symptoms appear in early childhood and persist into adulthood. The core symptoms of ASD are social dysfunction and stereotyped repetitive behaviors.1 This study demonstrated that ASD mice exhibited social dysfunction in the three-chamber social test, as they did not show significant differences in exploring objects compared to the control mice (Fig. 1), which is consistent with the literature. Furthermore, through the self-grooming test, ASD mice exhibited significant repetitive behaviors (Fig. 1), indicating that the ASD mouse model was successfully constructed through prenatal exposure to VPA. Concurrently, it was found that synbiotics significantly reversed social dysfunction and repetitive behaviors in ASD mice (Fig. 1). Nissl staining was used to observe the state of neurons, and it was observed that Nissl bodies in the cytoplasm of neurons were significantly reduced after stimulation. This study also found that compared to the normal group, the number of Nissl-positive cells in the cortical area of ASD mice was greatly reduced, indicating that neuronal cells were damaged (Fig. 2). BDNF, the most abundant neurotrophic factor in the body, is directly regulated by neural activity and is involved in mammalian brain activity, including neuron differentiation and growth, synapse formation and plasticity, and cognitive function.23 Studies have reported neuropathological abnormalities in the postmortem brains of ASD patients, including changes in neuron size and density, and abnormal distribution of developing neurons.24 In this study, we found that the cortical BDNF immunofluorescence intensity and the mRNA expression levels of BDNF and PSD-95 were significantly reduced in ASD mice, and these were significantly reversed by synbiotic intervention (Fig. 2). Therefore, social dysfunction and repetitive behaviors in ASD mice by synbiotics were mitigated may be based on their modulatory effects on neuronal function.
Social dysfunction in ASD mice may be accompanied by dysregulation expression of social-related genes. Shank3 knockout mice exhibit extensive behavioral abnormalities, such as repetitive grooming behavior, social deficits, and anxiety. Moreover, early genetic recovery of wild-type Shank3 rescued behavioral abnormalities.25 The role of SNAP25 in ASD was demonstrated; conditional knockout of the Snap25 gene in mice results in social deficits and increased stereotypical motor behavior.26 PTEN mutations are considered a pathological factor in ASD, and studies found that VPA-induced ASD mice exhibited decreased expression of PTEN in the hippocampus and cortex, suggesting that PTEN may be an important potential therapeutic target for ASD.27 It was demonstrated that hippocampal Mecp2 knockdown produces behavioral abnormalities associated with autistic-like features in rats.28 In this study, the expression levels of Shank3, SNAP25, PTEN, and Mecp2 genes were significantly reduced in the brains of ASD mice, which were significantly increased by synbiotic intervention (Fig. 2). It has been suggested that inflammation can alter social behavior by directly affecting neuronal activity in the central nervous system.29 ASD patients showed neuroinflammatory responses, and hyperplasia of astrocytes and microglia in the cortex and cerebellum.30 In this study, we found that the mRNA expression levels of inflammatory cytokines IL-6, TNF-α, and IL-1β were significantly increased and the mRNA expression levels of the anti-inflammatory factor IL-10 were significantly decreased in the brains of ASD mice, which were alleviated by synbiotic intervention (Fig. 2). Therefore, we hypothesized that synbiotics may ameliorate social dysfunction in ASD mice by reversing the expression of social-related genes and inflammatory cytokines to regulate neuronal activity in the brain.
Clinical studies have shown that ASD patients are often accompanied by gastrointestinal symptoms.31 Consistent with previous research, this study found that ASD mice showed gut barrier damage and inflammation. Compared with the control group mice, ASD mice had impaired gut tissue morphology, including reduced muscle thickness, crypt damage, and reduced goblet cell numbers (Fig. 3). The mRNA expression levels of colonic tight junction proteins Claudin-1 and ZO-1 were significantly decreased (Fig. 3). The expression levels of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α were significantly increased, while the mRNA expression levels of the anti-inflammatory cytokine IL-10 were significantly reduced (Fig. 3). Moreover, recent research indicated that synbiotics consisting of inulin and L. reuteri RC-14 may alleviate ASD behavioral symptoms in young male BTBR mice and promote gut health.32 Consistent with these findings, synbiotic intervention significantly reversed gut damage in ASD mice.
Research indicated that the potential mechanism of ASD neurodevelopment may involve the combined effects of gut inflammatory responses and gut microbiota.33 A previous study showed differences in the composition of the gut microbiota between children with ASD and those without, with a significant decrease in the abundance of beneficial bacteria in the gut of ASD children compared to normal children.6 Previous reports showed that the probiotic L. reuteri MM4-1A regulates the gut microbiota composition in ASD mice.10 The relative abundance of Allobaculum was significantly reduced in DMSO-treated BTBR mice compared with the control mice.34 It was found that the gut microbiota of ASD children was disordered and the relative abundance of Bifidobacterium was significantly reduced.35 At the genus level, the relative abundance of Parasutterella in ASD children was significantly lower than that of the control group.36 This study found that synbiotics regulated the composition of gut microbiota in ASD mice by increasing the abundance of butyrate-producing bacteria, such as Allobaculum, Bifidobacterium, and Parasutterella. At the genus level, the relative abundance of Odoribacter was higher in children with ASD compared to the control group.37 The abundance of Roseburia was increased in ASD children compared with the control group, and Roseburia was associated with gastrointestinal symptoms associated with ASD.38 The synbiotic intervention significantly reduced the abundance of harmful bacteria in ASD mice, such as Odoribacter and Roseburia (Fig. 4). Overall, synbiotics may increase the content of butyrate by increasing the abundance of butyrate-producing bacteria, which in turn decreases neuroinflammation and social dysfunction in the brains of ASD mice.
Previous research has shown that prebiotics, probiotics, and synbiotics can inverse behavioral deficits and gut health in BTBR mice.32 However, the mechanism remains unclear. Many studies have emphasized that SCFAs are important constituents of the microbiota–gut–brain axis.8,39 SCFAs are mainly composed of acetic acid, propionic acid, butyric acid, and valeric acid. This study discovered that the levels of acetate, propionate, and butyrate in ASD mice were significantly decreased compared to the control group (Fig. 5). However, there was evidence indicating that the level of propionate was significantly elevated in ASD mice.40 There was also evidence suggesting that decreased levels of acetate, propionate, and butyrate in the offspring of obese mothers may cause social and cognitive impairment.11 Therefore, changes in propionate levels in ASD are somewhat controversial. In this study, synbiotics significantly increased the levels of propionate in the feces and serum of ASD mice (Fig. 5). Studies had shown that modulation of the gut microbiota, especially bacteria-producing butyrate, may be a potential therapeutic strategy for modulating gastrointestinal-related symptoms such as constipation and diarrhea in ASD.41 Consistent with previous studies, this study found that synbiotics significantly increased the level of butyrate in the feces and serum of ASD mice (Fig. 5). Meanwhile, synbiotics significantly increased the mRNA expression levels of SCFA receptors GPR43 and GPR41 (Fig. S7†). SCFAs can protect gut health by maintaining gut barrier integrity and suppressing inflammatory responses, and regulate neural homeostasis in the brain by passing through the blood–brain barrier via MCTs.42 It is speculated that synbiotics may increase the abundance of butyrate-producing bacteria in the gut, thereby increasing butyrate content in the intestine and serum, which can enter the brain through the BBB and alleviate social impairment in ASD mice.
In mammals, the primary ketone-producing organ is the liver. However, current research suggests that HMGCS2, the first rate-limiting enzyme in ketone production, is expressed in the liver and several extrahepatic tissues, such as the colon.43 Furthermore, the ability of the colon to produce ketones depends on the amount of butyrate produced. The colon can also synthesize ketones. The results indicated that supplementation with synbiotics significantly upregulates the serum level of ketone body 3-HB in ASD mice (Fig. 5). Hmgcs2, Hmgcl, and Bdh1 are the enzymes that synthesize 3-HB.44,45 It has been reported that synbiotics increased the expression of synthetic enzymes for 3-HB synthesis in the colons and livers of ASD mice (Fig. 6). The synthesised ketone body 3-HB can be transported from the blood to the brain via MCTs and enters neurons, upregulating BDNF expression.46,47 Research showed that the receptors for 3-HB are GPR41 and GPR109a.48 The results indicated that synbiotics significantly increased the mRNA expression levels of MCT1, GPR41, and GPR109a in the brains of ASD mice (Fig. 6). It is speculated that supplementing with synbiotics may increase the abundance of butyrate-producing bacteria and butyrate levels in the colon or serum, possibly increasing ketone body 3-HB levels through Hmgcs2 in the colon or liver, which then enter the brain via MCTs, and 3-HB binds with its receptors GPR41 and GPR109a in the brain, ultimately attenuating social deficits, repetitive behaviors, neuronal damage, and neuroinflammatory responses in ASD mice. However, for the probiotic and prebiotic therapy groups, we have observed favorable effects in enhancing cognition and ameliorating neuroinflammation as well, though the mechanisms involved may be more intricate. These therapeutic modalities may impact brain function through alternative metabolites or pathways, extending beyond merely 3-HB metabolism. Overall, prebiotic and probiotic interventions have both demonstrated significant efficacy. However, given the overall superior beneficial outcome of the synbiotic approach compared to the two individual treatments, we have not delved further into the underlying mechanisms. There are mechanistic similarities among the three approaches, but due to the synergistic interplay of probiotics and prebiotics, as well as their combined impact on the entire gut microbiota, the mechanisms of synbiotics are expected to be more intricate but also meaningful.
Conclusions
In summary, we observed that the synbiotic formulation, comprising inulin and L. reuteri C501, significantly mitigated VPA-induced social behavior impairment in offspring. These effects can be possibly attributed to the synbiotic's mediating effects on the gut microbiota composition and metabolite production, particularly the generation of butyrate. However, the current study does bear certain limitations. Primary among these is that the principal sources of 3-HB are the colon and liver, and the specific signaling pathways linking metabolite receptors to neuroprotective functions remain somewhat obscure. In future investigations, employing fecal microbiota transplantation and metabolite receptor knockout mice may provide insights into these intricate mechanisms. Additionally, it will be critical to establish the appropriate clinical dosage of the synbiotics. Despite these limitations, the current study's findings propose a novel strategy for ASD nutritional intervention, underscoring the potential therapeutic value of synbiotic formulations.Author contributions
Conception and design of research: Z.L., C.W., X.L. and R.G.; performed the experiments: C.W., W.C., Y.J. and Y.Z.; analyzed the data: C.W., W.C., X.X. and J.L.; interpretation of the results of experiments: Z.L., C.W., W.C., Q.W. and R.G.; prepared the figures: C.W., W.C. and Q.Z.; drafted the manuscript: Z.L., C.W., W.C., T.Y. and X.L. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was financially supported by the following grants. Zhigang Liu was funded by the Shenzhen Central Government Guides Local Science and Technology Development Fund Projects (2021Szvup119) and the Key Research and Development Plan of Shaanxi Province (2023-YBNY-184). Tian Yuan was funded by the National Natural Science Foundation of China (No. 82103842) and the Guangdong Basic and Applied Basic Research Foundation (2021A1515110813). Rui Guo was funded by the Regional Consolidated Fund-Youth Fund Project in Guangdong Province (2022A1515110717) and the China Postdoctoral Science Foundation (2022M72261). Additionally, the authors would like to thank Zhuhai Yihetech Biotechnology Co. Ltd for providing the probotic strain, and the instrument shared platform of the College of Food Science & Engineering of Northwest A&F University for the assistance with regard to the gas chromatography analysis platform (Ms. Yuan Zhou).References
- T. Takumi, K. Tamada, F. Hatanaka, N. Nakai and P. F. Bolton, Behavioral neuroscience of autism, Neurosci. Biobehav. Rev., 2020, 110, 60–76 CrossRef PubMed.
- L. L. Orefice, J. R. Mosko, D. T. Morency, M. F. Wells, A. Tasnim, S. M. Mozeika, M. Ye, A. M. Chirila, A. J. Emanuel, G. Rankin, R. M. Fame, M. K. Lehtinen, G. Feng and D. D. Ginty, Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models, Cell, 2019, 178, 867–886 CrossRef CAS PubMed.
- C. Lord, M. Elsabbagh, G. Baird and J. Veenstra-Vanderweele, Autism spectrum disorder, Lancet, 2018, 392, 508–520 CrossRef PubMed.
- J. Suez, N. Zmora, E. Segal and E. Elinav, The pros, cons, and many unknowns of probiotics, Nat. Med., 2019, 25, 716–729 CrossRef CAS PubMed.
- C. R. Martin, V. Osadchiy, A. Kalani and E. A. Mayer, The Brain-Gut-Microbiome Axis, Cell. Mol. Gastroenterol. Hepatol., 2018, 6, 133–148 CrossRef PubMed.
- R. Zou, F. Xu, Y. Wang, M. Duan, M. Guo, Q. Zhang, H. Zhao and H. Zheng, Changes in the Gut Microbiota of Children with Autism Spectrum Disorder, Autism Res., 2020, 13, 1614–1625 CrossRef PubMed.
- M. Lombardi and J. Troisi, Gut Reactions: How Far Are We from Understanding and Manipulating the Microbiota Complexity and the Interaction with Its Host? Lessons from Autism Spectrum Disorder Studies, Nutrients, 2021, 13, 3492 CrossRef PubMed.
- Y. P. Silva, A. Bernardi and R. L. Frozza, The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication, Front. Endocrinol., 2020, 11, 25 CrossRef PubMed.
- S. A. Buffington, G. V. Di Prisco, T. A. Auchtung, N. J. Ajami, J. F. Petrosino and M. Costa-Mattioli, Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring, Cell, 2016, 165, 1762–1775 CrossRef CAS PubMed.
- M. Sgritta, S. W. Dooling, S. A. Buffington, E. N. Momin, M. B. Francis, R. A. Britton and M. Costa-Mattioli, Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder, Neuron, 2019, 101, 246–259 CrossRef CAS PubMed.
- X. Liu, X. Li, B. Xia, X. Jin, Q. Zou, Z. Zeng, W. Zhao, S. Yan, L. Li, S. Yuan, S. Zhao, X. Dai, F. Yin, E. Cadenas, R. H. Liu, B. Zhao, M. Hou, Z. Liu and X. Liu, High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis, Cell Metab., 2021, 33, 923–938 CrossRef CAS PubMed.
- A. M. Tartaglione, S. Schiavi, G. Calamandrei and V. Trezza, Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder, Neuropharmacology, 2019, 159, 107477 CrossRef CAS PubMed.
- C. Nicolini and M. Fahnestock, The valproic acid-induced rodent model of autism, Exp. Neurol., 2018, 299, 217–227 CrossRef CAS PubMed.
- A. Taleb, W. Lin, X. Xu, G. Zhang, Q. G. Zhou, M. Naveed, F. Meng, K. Fukunaga and F. Han, Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment, Biomed. Pharmacother., 2021, 137, 111322 CrossRef CAS PubMed.
- A. I. Lim, T. McFadden, V. M. Link, S. J. Han, R. M. Karlsson, A. Stacy, T. K. Farley, D. S. Lima-Junior, O. J. Harrison, J. V. Desai, M. S. Lionakis, H. Y. Shih, H. A. Cameron and Y. Belkaid, Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring, Science, 2021, 373, eabf3002 CrossRef CAS PubMed.
- C. Tai, C. W. Chang, G. Q. Yu, I. Lopez, X. Yu, X. Wang, W. Guo and L. Mucke, Tau Reduction Prevents Key Features of Autism in Mouse Models, Neuron, 2020, 106, 421–437 CrossRef CAS PubMed.
- Z. Liu, X. Dai, H. Zhang, R. Shi, Y. Hui, X. Jin, W. Zhang, L. Wang, Q. Wang, D. Wang, J. Wang, X. Tan, B. Ren, X. Liu, T. Zhao, J. Wang, J. Pan, T. Yuan, C. Chu, L. Lan, F. Yin, E. Cadenas, L. Shi, S. Zhao and X. Liu, Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment, Nat. Commun., 2020, 11, 855 CrossRef CAS PubMed.
- Q. Liu, Y. Xi, Q. Wang, J. Liu, P. Li, X. Meng, K. Liu, W. Chen, X. Liu and Z. Liu, Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer's disease mouse model via regulating the gut microbiota-brain axis, Brain, Behav., Immun., 2021, 95, 330–343 CrossRef CAS PubMed.
- S. Yan, R. Shi, L. Li, S. Ma, H. Zhang, J. Ye, J. Wang, J. Pan, Q. Wang, X. Jin, X. Liu and Z. Liu, Mannan Oligosaccharide Suppresses Lipid Accumulation and Appetite in Western-Diet-Induced Obese Mice Via Reshaping Gut Microbiome and Enhancing Short-Chain Fatty Acids Production, Mol. Nutr. Food Res., 2019, 63, e1900521 CrossRef PubMed.
- X. Zhang, Q. Zou, B. Zhao, J. Zhang, W. Zhao, Y. Li, R. Liu, X. Liu and Z. Liu, Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders, Redox Biol., 2020, 32, 101535 CrossRef CAS PubMed.
- Z. Ba, Y. Lee, H. Meng, P. M. Kris-Etherton, C. J. Rogers, Z. T. Lewis, D. A. Mills, E. J. Furumoto, M. L. Rolon and J. A. Fleming, Matrix effects on the delivery efficacy of Bifidobacterium animalis subsp. lactis BB-12 on fecal microbiota, gut transit time, and short-chain fatty acids in healthy young adults, Msphere, 2021, 6, e00084–e00021 CrossRef CAS PubMed.
- V. Lombard, H. Golaconda Ramulu, E. Drula, P. M. Coutinho and B. Henrissat, The carbohydrate-active enzymes database (CAZy) in 2013, Nucleic Acids Res., 2014, 42, D490–D495 CrossRef CAS PubMed.
- H. Park and M. M. Poo, Neurotrophin regulation of neural circuit development and function, Nat. Rev. Neurosci., 2013, 14, 7–23 CrossRef CAS PubMed.
- M. Varghese, N. Keshav, S. Jacot-Descombes, T. Warda, B. Wicinski, D. L. Dickstein, H. Harony-Nicolas, S. De Rubeis, E. Drapeau, J. D. Buxbaum and P. R. Hof, Autism spectrum disorder: neuropathology and animal models, Acta Neuropathol., 2017, 134, 537–566 CrossRef CAS PubMed.
- T. C. Jaramillo, Z. Xuan, J. M. Reimers, C. O. Escamilla, S. Liu and C. M. Powell, Early Restoration of Shank3 Expression in Shank3 Knock-Out Mice Prevents Core ASD-Like Behavioral Phenotypes, eNeuro, 2020, 7, 332–319 CrossRef PubMed.
- H. Yang, M. Zhang, J. Shi, Y. Zhou, Z. Wan, Y. Wang, Y. Wan, J. Li, Z. Wang and J. Fei, Brain-Specific SNAP-25 Deletion Leads to Elevated Extracellular Glutamate Level and Schizophrenia-Like Behavior in Mice, Neural Plast., 2017, 2017, 4526417 Search PubMed.
- U. Mahmood, S. Ahn, E. J. Yang, M. Choi, H. Kim, P. Regan, K. Cho and H. S. Kim, Dendritic spine anomalies and PTEN alterations in a mouse model of VPA-induced autism spectrum disorder, Pharmacol. Res., 2018, 128, 110–121 CrossRef PubMed.
- M. Choi, S. Y. Ko, J. Y. Seo, D. G. Kim, H. Lee, H. Chung and H. Son, Autistic-like social deficits in hippocampal MeCP2 knockdown rat models are rescued by ketamine, BMB Rep., 2022, 55, 238–243 CrossRef CAS PubMed.
- M. D. Reed, Y. S. Yim, R. D. Wimmer, H. Kim, C. Ryu, G. M. Welch, M. Andina, H. O. King, A. Waisman, M. M. Halassa, J. R. Huh and G. B. Choi, IL-17a promotes sociability in mouse models of neurodevelopmental disorders, Nature, 2020, 577, 249–253 CrossRef CAS PubMed.
- S. M. Matta, E. L. Hill-Yardin and P. J. Crack, The influence of neuroinflammation in Autism Spectrum Disorder, Brain, Behav., Immun., 2019, 79, 75–90 CrossRef PubMed.
- B. O. McElhanon, C. McCracken, S. Karpen and W. G. Sharp, Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis, Pediatrics, 2014, 133, 872–883 CrossRef PubMed.
- J. E. Nettleton, T. Klancic, A. Schick, A. C. Choo, N. Cheng, J. Shearer, S. L. Borgland, J. M. Rho and R. A. Reimer, Prebiotic, Probiotic, and Synbiotic Consumption Alter Behavioral Variables and Intestinal Permeability and Microbiota in BTBR Mice, Microorganisms, 2021, 9, 1833 CrossRef CAS PubMed.
- M. De Angelis, R. Francavilla, M. Piccolo, A. De Giacomo and M. Gobbetti, Autism spectrum disorders and intestinal microbiota, Gut Microbes, 2015, 6, 207–213 CrossRef CAS PubMed.
- J. Liu, C. Liu, Z. Gao, L. Zhou, J. Gao, Y. Luo, T. Liu and X. Fan, GW4064 Alters Gut Microbiota Composition and Counteracts Autism-Associated Behaviors in BTBR T + tf/J Mice, Front. Cell. Infect. Microbiol., 2022, 12, 911259 CrossRef CAS PubMed.
- Y. Wang, N. Li, J.-J. Yang, D.-M. Zhao, B. Chen, G.-Q. Zhang, S. Chen, R.-F. Cao, H. Yu, C.-Y. Zhao, L. Zhao, Y.-S. Ge, Y. Liu, L.-H. Zhang, W. Hu, L. Zhang and Z.-T. Gai, Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder, Pharmacol. Res., 2020, 157, 104784 CrossRef CAS PubMed.
- X. Ding, Y. Xu, X. Zhang, L. Zhang, G. Duan, C. Song, Z. Li, Y. Yang, Y. Wang, X. Wang and C. Zhu, Gut microbiota changes in patients with autism spectrum disorders, J. Psychiatr. Res., 2020, 129, 149–159 CrossRef PubMed.
- M. Zhang, W. Ma, J. Zhang, Y. He and J. Wang, Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China, Sci. Rep., 2018, 8, 13981 CrossRef PubMed.
- P. Vernocchi, M. V. Ristori, S. Guerrera, V. Guarrasi, F. Conte, A. Russo, E. Lupi, S. Albitar-Nehme, S. Gardini, P. Paci, G. Ianiro, S. Vicari, A. Gasbarrini and L. Putignani, Gut Microbiota Ecology and Inferred Functions in Children With ASD Compared to Neurotypical Subjects, Front. Microbiol., 2022, 13, 871086 CrossRef PubMed.
- B. Dalile, L. Van Oudenhove, B. Vervliet and K. Verbeke, The role of short-chain fatty acids in microbiota-gut-brain communication, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 461–478 CrossRef PubMed.
- Q. Kong, B. Wang, P. Tian, X. Li, J. Zhao, H. Zhang, W. Chen and G. Wang, Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation, Food Funct., 2021, 12, 2591–2604 RSC.
- S. Liu, E. Li, Z. Sun, D. Fu, G. Duan, M. Jiang, Y. Yu, L. Mei, P. Yang, Y. Tang and P. Zheng, Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder, Sci. Rep., 2019, 9, 287 CrossRef PubMed.
- B. Abdellatif, C. McVeigh, G. Bendriss and A. Chaari, The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders, Int. J. Mol. Sci., 2020, 21, 4159 CrossRef CAS PubMed.
- N. Camarero, C. Mascaro, C. Mayordomo, F. Vilardell, D. Haro and P. F. Marrero, Ketogenic HMGCS2 Is a c-Myc target gene expressed in differentiated cells of human colonic epithelium and down-regulated in colon cancer, Mol. Cancer Res., 2006, 4, 645–653 CrossRef CAS PubMed.
- N. J. Jensen, H. Z. Wodschow, M. Nilsson and J. Rungby, Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases, Int. J. Mol. Sci., 2020, 21, 8767 CrossRef CAS PubMed.
- S. Nishitani, A. Fukuhara, I. Tomita, S. Kume, J. Shin, Y. Okuno, M. Otsuki, H. Maegawa and I. Shimomura, Ketone body 3-hydroxybutyrate enhances adipocyte function, Sci. Rep., 2022, 12, 10080 CrossRef CAS PubMed.
- M. P. Mattson, K. Moehl, N. Ghena, M. Schmaedick and A. Cheng, Intermittent metabolic switching, neuroplasticity and brain health, Nat. Rev. Neurosci., 2018, 19, 63–80 CrossRef CAS PubMed.
- P. Puchalska and P. A. Crawford, Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics, Cell Metab., 2017, 25, 262–284 CrossRef CAS PubMed.
- J. Mierziak, M. Burgberger and W. Wojtasik, 3-Hydroxybutyrate as a Metabolite and a Signal Molecule Regulating Processes of Living Organisms, Biomolecules, 2021, 11, 402 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo02663a |
| ‡ These authors have contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2024 |