The arsenic-lowering effect of inulin-type prebiotics in end-stage renal disease: a randomized crossover trial†
Li
Li
a,
Jing
Zhao
a,
Jinxue
Wang
a,
Qianqian
Xiong
a,
Xuechun
Lin
a,
Xiaolei
Guo
a,
Fan
Peng
a,
Wangqun
Liang
b,
Xuezhi
Zuo
*c and
Chenjiang
Ying
 *a
*a
aDepartment of Nutrition and Food Hygiene, Hubei Key Laboratory of Food Nutrition and Safety, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. E-mail: yingcj@hust.edu.cn; Tel: +86-27-83650523
bDivision of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
cDepartment of Clinical Nutrition, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. E-mail: zuo1967@tjh.tjmu.edu.cn; Tel: +86-27-83662873
First published on 30th November 2023
Abstract
Background: Circulatory imbalance of trace elements is frequent in end-stage renal disease (ESRD), leading to a deficiency of essential elements and excess of toxic elements. The present study aimed to investigate whether inulin-type fructans (ITFs) could ameliorate the circulatory imbalance by modulating gut microbiota and regulating the absorption and elimination of trace elements. Methods: Peritoneal dialysis patients were enrolled in a randomized crossover trial, undergoing interventions with ITFs (10 g d−1) and maltodextrin (placebo) over a 9-month period (with a 3-month washout). The primary outcomes included essential elements Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo and potential toxic elements V, Cr, Ni, As, Cd, Ba, Tl, Pb, Th, and U in plasma. Secondary outcomes included the gut microbiome, short chain fatty acids (SCFAs), bile acids (BAs), and daily removal of trace elements through urine, dialysate and feces. Results: Among the 44 participants initially randomized, 29 completed the prebiotic, placebo or both interventions. The daily dietary intake of macronutrients and trace elements remained consistent throughout the study. The administration of 10 g d−1 ITFs significantly reduced plasma arsenic (As) by 1.03 μg L−1 (95%CI: −1.74, −0.33) (FDR-adjusted P = 0.045) down from the baseline of 3.54 μg L−1 (IQRs: 2.61–4.40) and increased the As clearance rate by urine and dialysis (P = 0.033). Positive changes in gut microbiota were also observed, including an increase in the Firmicutes/Bacteroidetes ratio (P = 0.050), a trend towards higher fecal SCFAs (P = 0.082), and elevated excretion of primary BAs (P = 0.035). However, there were no significant changes in plasma concentrations of other trace elements or their daily removal by urine, dialysis and feces. Conclusions: The daily administration of 10 g d−1 ITFs proved to be effective in reducing the circulating retention of As but demonstrated to be ineffective for other trace elements in ESRD. These sentences are ok to include but as “The clinical trial registry number is ChiCTR-INR-17013739 (https://www.chictr.org.cn/showproj.aspx?proj=21228)”.
Introduction
Circulatory imbalance of trace elements is frequent in end-stage renal disease (ESRD), which is characterized by essential element deficiency and toxic element excess.1 It has been reported that there is a deficiency of the essential elements zinc (Zn), iron (Fe), selenium (Se) and manganese (Mn) in ESRD, whereas an excess of the potential toxic elements lead (Pb), mercury (Hg), arsenic (As), cadmium (Cd), chromium (Cr), barium (Ba), nickel (Ni), uranium (U), vanadium(V) and thallium (Tl), with their levels increasing with the disease progression.2–4 The deficiency of essential trace elements mainly results from low dietary intake due to poor appetite and the need for hyperkalemia prevention.5,6 Also, extended dialysis loss may be a contributor, given that the essential trace elements are small molecules with low binding with plasma proteins and are easily lost through dialysis.7,8 For potential toxic elements, the overload is mainly due to the decreased excretion through dysfunctional kidney and the dialysis has limited effects due to their high binding with plasma proteins.9,10Trace element imbalance poses hazards for ESRD patients.9 Essential trace elements are crucial components of antioxidant enzymes, for example, Mn, copper (Cu) and Zn serve as vital cofactors for superoxide dismutase enzymes MnSOD and Cu/ZnSOD, and Se is a component of glutathione peroxidase (GPx).11–14 Deficiency in essential elements impairs the antioxidant defense system, leading to an imbalance in the redox system. Toxic elements, such as Pb, As, Cd and Hg, act as pro-oxidants, stimulating the generation of reactive oxygen species (ROS), leading to excess oxidative stress.15 The kidneys are the first target of toxicity due to their capacity to reabsorb and accumulate heavy metals.16,17 Lead induces interstitial oxidative stress, fibrosis and proximal tubular atrophy.18 Arsenic initiates kidney damage by stimulating oxidative stress, modulating cell signaling cascades, DNA methylation and histone acetylation.19 For Cd, approximately 50% of the total body stores accumulates in the kidneys, leading to tubular necrosis and glomerular filtration dysfunction.20 As mentioned above, the dysfunctional kidneys in ESRD reduce the excretion of toxic elements and exacerbate their retention, which further deteriorate the renal function, thus creating a vicious circle.9,21
Probiotic/synbiotic supplementation has been documented to improve the imbalance between oxidative stress and the antioxidant defense system, as reported by Pourrajab B. et al. in a systematic review and meta-analysis. The proposed mechanism involves the direct capture of metal ions by probiotics, preventing them from catalyzing oxidation processes.22 Microbiota-driven therapies are also promising to improve the imbalance of trace elements.23,24 For example, inulin, a β-2,1-linked polysaccharide, has been widely used and well documented in manipulating the gut microbiota.25 On one hand, inulin is fermented by the gut microbiota into short chain fatty acids (SCFAs) and lactate, reducing the luminal pH.26 The increased acidity in the intestine prevents essential trace metals from binding with phytates and oxalates, thereby increasing their intestinal absorption.27 Butyrate, which is produced during fermentation, serves as a nutrient substrate for intestinal epithelial cells, increases the intestinal surface area and allows the greater absorption of essential elements.28,29 Moreover, divalent metal transporter 1 (DMT1) in the intestinal epithelium has been reported to be upregulated by inulin.30 On the other hand, inulin stimulates symbiotic bacteria, especially Bifidobacterium and Lactobacillus, which eliminate toxic elements by directly chelating with the carboxyl group of proteins and hydroxyl group of the peptidoglycans in the cell membrane.31 In addition, accelerating the enterohepatic circulation of bile acids (BAs) by manipulating the gut microbiota has also been reported to enhance the biliary secretion of heavy metals into the intestine.31
However, existing studies are limited to animal models or the general population, lacking evidence in ESRD patients with serious gut microbiota dysbiosis and deteriorative renal function. Thus, the present study was conducted to investigate whether the prebiotic inulin-type fructans improve the deficiency of the essential trace elements Mn, iron (Fe), cobalt (Co), Cu, Zn, Se, strontium (Sr), and molybdenum (Mo), and alleviate the retention of the potential toxic elements V, Cr, Ni, As, Cd, Ba, Tl, Pb, thorium (Th), and U in ESRD patients.
Methods
Ethics
Ethical approval for the involvement of human subjects in this study was granted by the Institutional Review Board of Tongji Hospital, Huazhong University of Science and Technology (TJ-IRB20171110) and registered at the Chinese Clinical Trials Registry (ChiCTR-INR-17013739, https://www.chictr.org.cn/showproj.html?proj=21228). All participants provided informed consent in accordance with the Helsinki Declaration before the study.Subjects and randomization
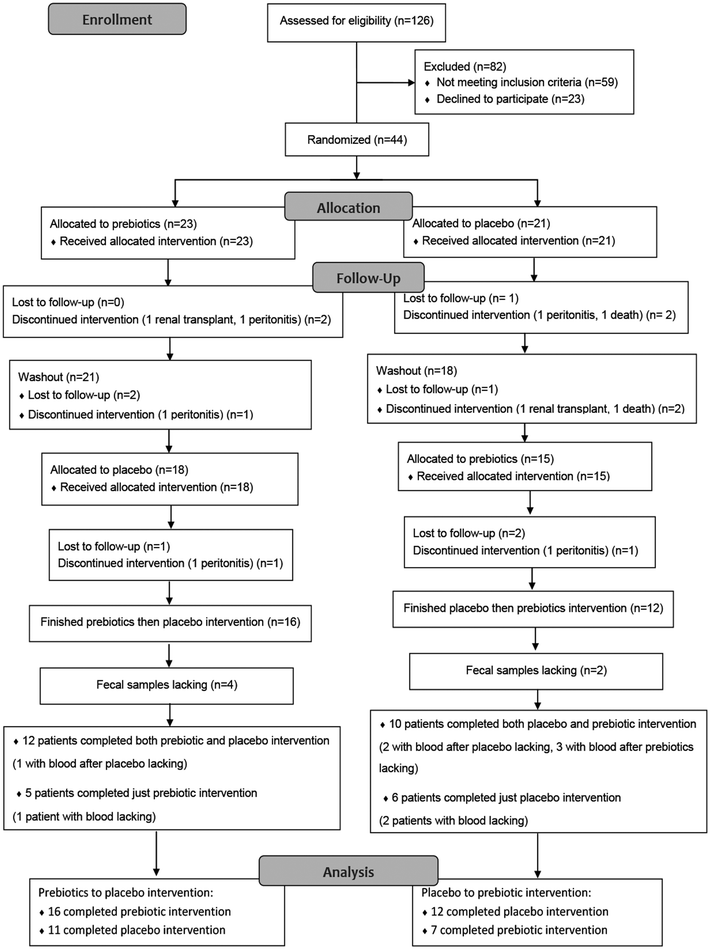
This was a randomized crossover trial with continuous ambulatory peritoneal dialysis (CAPD) patients recruited from Tongji Hospital, Wuhan. The inclusion criteria for participants were as follows: (1) received CAPD treatment for ≥3 months; (2) aged between 18 and 65 years; and (3) able to provide informed consent. Patients were excluded if: (1) diagnosed with diabetes; (2) receiving or have received antibiotic therapy ≤1 month before the study started; (3) receiving or had received radiation to the bowel or large bowel resection; (4) medically diagnosed with irritable bowel syndrome, Crohn disease, or ulcerative colitis; (5) severely malnourished (Subjective Global Assessment of >15); and (6) pregnant. In addition, the present study excluded patients definitely diagnosed with cardiovascular disease (CVD) or with obvious CVD manifestations.Forty-four eligible subjects were included, with 23 randomly assigned to sequence A (prebiotics to placebo) and 21 to sequence B (placebo to prebiotics). The allocation was determined by computer-generated random numbers and concealed from the patients and investigators. After 9-month intervention (3-month washout), 12 participants in sequence A completed both prebiotic and placebo interventions (1 with blood lacking after placebo), and 5 finished only the prebiotic intervention (1 with blood lacking after prebiotics). Ten participants in sequence B completed both the placebo and prebiotic interventions (2 with blood lacking after placebo and 3 lacking after prebiotics), and 6 finished only the placebo intervention (2 with lacking blood). At last, 22 participants with both prebiotic and placebo interventions had their fecal SCFAs and fecal BAs measured, and 29 participants completing the prebiotic, placebo or both interventions were measured for trace elements in plasma, 24 h urine and dialysate. Among the 29 participants, 24 had available feces for gut microbiota shotgun metagenomic sequencing and trace element measurements. The process of selecting eligible subjects is shown in Fig. 1.
Intervention and specimen collection
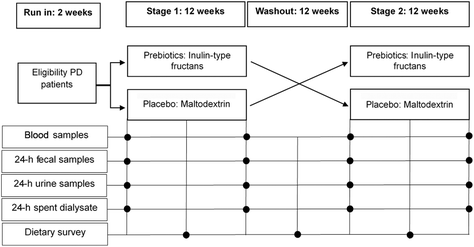
ITFs at 10 g d−1 was selected as the intervention dose, which has been reported to be well-tolerated and sufficient to modify the gut microbiota. Patients in sequence A orally consumed 10 g d−1 ITFs for 3 months, underwent a 3-month washout, and then orally consumed 10 g d−1 placebo for the last 3 months. Patients in sequence B had the opposite intervention order. A schematic presentation of the study design is shown in Fig. 2. To blind participants and investigators, the appearance, packaging and administration schedule were identical between the placebo (maltodextrin; Roquette, China) and prebiotic inulin-type fructans (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of long-chain inulin and oligofructose; Synergy1, Orafti, Chile).
50 mixture of long-chain inulin and oligofructose; Synergy1, Orafti, Chile).
Before and after each intervention, the venous blood, 24 h spent dialysate, 24 h urine and stool samples were collected and stored at −80 °C. The primary outcomes were trace element plasma concentrations, including the essential trace elements Ca, Mg, Mn, Al, Fe, Co, Cu, Zn, Se, Sr, and Mo and potential toxic elements Be, V, Cr, Ni, As, Cd, Ba, Sb, Tl, Pb, Th, and U. The secondary outcomes were trace elements in the feces, urine and dialysate, gut microbiome, fecal SCFAs, fecal BAs, and residual renal and peritoneal function.
Dietary survey and daily nutrient intake assessment
The 3-d dietary record (2 weekdays, 1 weekend day) was used to assess the daily food consumption of the participants. The participants recorded their daily food intake by weighing the raw or cooked food that they planned to eat using an electronic scale (5 kg kitchen scale; Zhongshan CAMRY) and sending photographs to a trained dietitian. The dietitian reviewed the photographs and calculated the daily macronutrient and microelement intakes based on the Chinese Food Composition Tables Standard Edition (https://www.cnsoc.org/latesachie/311911205.html). The daily dietary intake of fatty acids, including saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), was calculated using the Food Data of the U.S. Department of Agriculture (https://fdc.nal.usda.gov/fdc-app.html#/), given that this information was not available in the Chinese Food Composition Tables. The food toxic element contents in Hubei were assessed according to the report by Zhang et al.32Basic characteristics and biochemistry parameters
The demographic characteristics and medical information were obtained from electronic medical records. The biochemistry parameters, including albumin, creatinine, urea nitrogen in the blood, urine and dialysate, blood glucose and blood lipids, were measured using an auto-biochemistry analyzer machine at the Central Laboratory of Tongji Hospital. Dialysis information, including daily 1.5% and 2.5% glucose dialysate used, daily net ultrafiltration, dialysis efficiency (Kt/V), and weekly creatinine clearance rate (Ccr), were measured and calculated.Measurement of trace elements in plasma, urine, dialysates and feces
Twenty-three trace elements were measured via quadrupole inductively coupled plasma-mass spectrometry (ICP-MS; Agilent 7700x ICP-MS; Agilent Technologies, USA), using the Multi-Element Mixed Standard Solution of PerkinElmer (P/N 5183-4682, Agilent, USA) as the standard. The trace elements included Be, Mg, Al, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Mo, Cd, Sb, Ba, Tl, Pb, Th and U. Plasma specimens (100 μL) were 1: 20 diluted with 1% HNO3 and the urine and dialysate (500 μL) were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 diluted with 1% HNO3. 1 mL Milli-Q® Water and 2 mL 60% HNO3 were added to the stool samples (about 100 mg dry weight) and digested using a super microwave digestion system at a temperature of up to 220 °C, held for 20 min, and then diluted with 10 mL 2% HNO3. All the specimens were measured in triplicate, and the standard reference material 1640a (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used every 20 samples to ascertain the accuracy of the determination. The relative standard deviation (RSD) of the duplicate analysis was calculated, with the elements re-quantified when the RSD was >5%. The intra-assay and inter-assay coefficients of variations of all the metals were below 10%. For concentrations below the limit of detection (LOD), the value was set as half the LOD, and that “above the mean concentration + 3SD” was replaced with “mean concentration + 3SD”. The concentrations below LOD were 75% for plasma Al, 63% for plasma Be, and 39% for plasma Sb, and the Sb was non-detectable in 85% spent dialysate. The measured concentrations of plasma Mg and Ca were much higher than that in the multi-element mixed standard solution. Thus, 18 trace elements were included in the final analysis, with the 8 essential trace elements Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo and 10 potential toxic elements V, Cr, Ni, As, Cd, Ba, Tl, Pb, Th, and U.
5 diluted with 1% HNO3. 1 mL Milli-Q® Water and 2 mL 60% HNO3 were added to the stool samples (about 100 mg dry weight) and digested using a super microwave digestion system at a temperature of up to 220 °C, held for 20 min, and then diluted with 10 mL 2% HNO3. All the specimens were measured in triplicate, and the standard reference material 1640a (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used every 20 samples to ascertain the accuracy of the determination. The relative standard deviation (RSD) of the duplicate analysis was calculated, with the elements re-quantified when the RSD was >5%. The intra-assay and inter-assay coefficients of variations of all the metals were below 10%. For concentrations below the limit of detection (LOD), the value was set as half the LOD, and that “above the mean concentration + 3SD” was replaced with “mean concentration + 3SD”. The concentrations below LOD were 75% for plasma Al, 63% for plasma Be, and 39% for plasma Sb, and the Sb was non-detectable in 85% spent dialysate. The measured concentrations of plasma Mg and Ca were much higher than that in the multi-element mixed standard solution. Thus, 18 trace elements were included in the final analysis, with the 8 essential trace elements Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo and 10 potential toxic elements V, Cr, Ni, As, Cd, Ba, Tl, Pb, Th, and U.
Gut microbiota shotgun metagenomic sequencing
Fecal DNA was extracted using the QIAamp DNA Stool Mini Kit (QIAGEN, Germany) and fragmented to about 300 bp using Covaris M220 (Gene Company Limited, China). Paired-end sequencing was performed on an Illumina HiSeq4000 platform (Illumina Inc., San Diego, CA, USA) at Majorbio Bio-Pharm Technology Co., Ltd (Shanghai, China) with the HiSeq 3000/4000 PE Cluster Kit and HiSeq 3000/4000 SBS Kits (https://www.illumina.com). Raw sequence reads were quality-filtered, with a quality score lower than 20 and a length shorter than 50 bp discarded, and then aligned by BWA (https://bio-bwa.sourceforge.net) to the human genome (GenBank Assembly Accession: GCA_000001405.15 and RefSeq Assembly Accession: GCF_000001405. 26). With hits associated with the reads and their paired reads removed, the clean raw reads were assembled using Megahit (version 1.1.2, https://github.com/voutcn/megahit). The open reading frames (ORFs) with a length of ≥100 bp from each metagenomic sample were predicted using MetaGene (https://metagene.cb.k.u-tokyo.ac.jp/), with 95% sequence identity (90% coverage) clustered as the non-redundant gene catalog (https://www.bioinformatics.org/cd-hit/). The reads were mapped to the representative genes with 95% identity using SOAPaligner (https://soap.genomics.org.cn/). The non-redundant gene catalogs were aligned using BLASTP (Version 2.2.28+, https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the NCBI NR database for taxonomic annotations, and aligned against the Kyoto Encyclopedia of Genes and Genomes database for KEGG pathway annotation, both with an e-value cutoff of 1 × 10−5. All the raw metagenomics datasets were deposited in the NCBI Sequence Read Achieve database (Accession Number: PRJNA750234).Fecal SCFA measurement
Fecal SCFAs were measured using an ExionLC AD system equipped with an ACQUITY UPLC BEH C18 column (150 × 2.1 mm, 1.7 μm). Samples (20 mg) were diluted with 500 μL of extraction solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 4
water = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), homogenized with ultrasound for 30 min (5 °C, 40 kHz), left to stand for 30 min, and then centrifuged at 13
1), homogenized with ultrasound for 30 min (5 °C, 40 kHz), left to stand for 30 min, and then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rcf for 15 min at 4 °C. Subsequently, 20 μL supernatant was transferred to new sterile EP tubes, 20 μL of 200 mM 3NPH·HCL and 20 μL of 120 mM EDC·HCL (containing 6% pyridine) solution added, and then heated at 40 °C for 30 min to react. Finally, the reactants were diluted to 1000 μL with 50% acetonitrile aqueous solution for detection. Separation was achieved at a flow rate of 0.35 mL min−1, with water (containing 0.01% formic acid) as solvent A and acetonitrile (containing 0.01% formic acid) as solvent B. The solvent gradient varied according to the following conditions: isostatically with 10% B for 2 min; 10% to 55% B for 2–11th min; 55% to 95% B for 11–12th min; hold at 95% B from12–13th min; 95% to10% B for 13–13.1th min; and hold at 10% B from 13.1–16th min.
000 rcf for 15 min at 4 °C. Subsequently, 20 μL supernatant was transferred to new sterile EP tubes, 20 μL of 200 mM 3NPH·HCL and 20 μL of 120 mM EDC·HCL (containing 6% pyridine) solution added, and then heated at 40 °C for 30 min to react. Finally, the reactants were diluted to 1000 μL with 50% acetonitrile aqueous solution for detection. Separation was achieved at a flow rate of 0.35 mL min−1, with water (containing 0.01% formic acid) as solvent A and acetonitrile (containing 0.01% formic acid) as solvent B. The solvent gradient varied according to the following conditions: isostatically with 10% B for 2 min; 10% to 55% B for 2–11th min; 55% to 95% B for 11–12th min; hold at 95% B from12–13th min; 95% to10% B for 13–13.1th min; and hold at 10% B from 13.1–16th min.
Fecal BA quantification
Fecal BAs were measured via LC-ESI-MS/MS (ExionLC™ AD; MS, Applied Biosystems 6500 Triple Quadrupole), with a Waters Acquity UPLC HSS T3 C18 column (100 mm × 2.1 mm, i.d.: 1.8 μm). A mixed solution of 2-chloro-L-phenylalanine, cholic acid-d4, glycolithocholic acid-d4, deoxycholic acid-d4, lithocholic acid-d4, glycochenodeoxycholic acid-d4, taurocholic acid-d4, tauroursodeoxycholic-2,2, 3,4,4-d5 acid and glycodeoxycholic acid-d4, was used as the internal standard. Samples (20 mg) were diluted with 10 μL 1 μg mL−1 internal standard and 200 μL methanol, homogenized via ultrasonication, and then left to stand at −20 °C for 10 min to precipitate protein. The supernatant was extracted and evaporated to dryness and reconstituted in 100 μL 50% methanol for analysis. Mobile phase A was 5 mmol L−1 ammonium acetate with 0.01% acetic acid and mobile phase B was 100% acetonitrile with 0.01% acetic acid. The elution gradient was optimized as follows: 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 A/B at 0 min, 60
5 A/B at 0 min, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 A/B at 0.5 min, 50
40 A/B at 0.5 min, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 A/B at 4.5 min, 25
50 A/B at 4.5 min, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 A/B at 7.5 min, 5
75 A/B at 7.5 min, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 A/B at 10 min, and 95
95 A/B at 10 min, and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 A/B at 12 min.
5 A/B at 12 min.
Statistical methods
The Shapiro–Wilk test was performed to examine the normal distribution of continuous variables. Data with a normal distribution was presented as mean ± SD, otherwise presented as the median (IQRs). The baseline differences between the two intervention sequences were analyzed using independent sample t tests or Mann–Whitney U test. The daily nutrient intake during prebiotic, washout and placebo intervention was analyzed using one-way repeated-measures ANOVA. The One-Sample Wilcoxon Signed Rank Test was employed to compare the baseline plasma trace elements in the peritoneal dialysis patients in this study with those reported in the general population in Hubei or China.33,34 The mixed linear model was performed to analyze the differences in primary and secondary outcomes between prebiotic and placebo intervention (Δ prebiotics vs. Δ placebo), with changes from baseline as dependent variables; treatment, intervention period, and treatment-by-period interaction as fixed effects; subjects as random effect; and intervention sequence, age, sex, ESRD course, peritoneal dialysis (PD) course and baseline values as covariates. The multiple comparisons of plasma trace elements and gut microbiota were adjusted by the false discovery rate (FDR) recommended by Benjamini and Hochberg to control the type I error. The carry-over effects were assessed by comparing the baselines between prebiotic-pre and placebo-pre with the Friedman test. The urinary excretion rate of trace elements was calculated with the total contents of trace elements in 24 h urinary divided by plasma concentrations, and the dialysate clearance rate was calculated as the total trace elements in 24 h dialysate divided by plasma concentrations. A repeated measures correlation analysis (rmcorr) was conducted to determine the association of trace elements with the gut microbiota, fecal SCFAs, fecal BAs, and residual renal and peritoneal function using R (version 0.3.0). The statistical analyses were performed using the SPSS software (SPSS version 25; IBM Corporation), with the 2-sided P value of <0.05 set as significant.The sample size was calculated according to the effects of the prebiotics or probiotics on the plasma Zn, As, Pb and fecal SCFAs. Sixteen participants were sufficient to achieve a 16% increase in plasma Zn by synbiotics, with 90% power.23 Twenty-nine participants were sufficient to achieve 80% power to detect a 2.3 nmol L−1 reduction in blood As with probiotic intervention, as reported by Bisanz et al., with α of 0.05 using a 2 × 2 crossover design.35 In addition, according to the randomized controlled trial conducted by Tian et al., 14 participants were needed to achieve 90% power to detect 13.96 μg L−1 reduction in blood Pb with dietary fiber mixture intervention.36 Twenty participants were needed to achieve 90% power to detect about 10% increase in fecal total SCFAs with β2–1 fructan supplementation.37 The power calculations were performed using the PASS 15.0.5 software.
Results
Baseline characteristics
Forty-four patients were initially randomized, with 15 patients excluded and 29 included in the final analysis. There was no significant difference in the demographic characteristics and biochemistry parameters between patients included and excluded (ESI Table 1†). The mean age of the 29 included patients was 39.72 ± 12.23 years and their mean BMI was 20.76 ± 3.02 kg m−2. The median ESRD course was 23.67 months (IQRs: 16.87–54.69) and the median PD duration was 18.84 months (IQRs: 12.20–42.26). The PD duration was longer in the prebiotics to placebo sequence than that in the placebo to prebiotic sequence (P = 0.036). Other demographic characteristics and biochemistry parameters were comparable between the two intervention sequences (Table 1).| Variable | Total (N = 29) | Prebiotics to placeboa (N = 16) | Placebo to prebioticsa (N = 13) | P |
|---|---|---|---|---|
| a Prebiotics to placebo, participants received the prebiotics first, and then cross over to the placebo. Placebo to prebiotics, participants received the placebo first, and then cross over to the prebiotic intervention. b P values were calculated by independent-sample t test or Mann–Whitney U test. | ||||
| Age, years | 39.72 ± 12.23 | 38.60 ± 11.86 | 41.09 ± 13.03 | 0.596 |
| Sex, male/female | 15/14 | 7/9 | 8/5 | 0.340 |
| BMI, kg m−2 | 20.76 ± 3.02 | 19.88 ± 2.55 | 21.85 ± 3.30 | 0.081 |
| ESRD course, months | 23.67 (16.87–54.69) | 35.67 (19.30–59.16) | 20.19 (7.89–41.61) | 0.092 |
| PD duration, months | 18.84 (12.20–42.26) | 23.87 (16.15–57.58) | 15.65 (5.33–19.78) | 0.036 |
| Daily dialysate influent, L | 8.00 (6.00–8.00) | 8.00 (6.00–8.00) | 8.00 (6.00–8.00) | 0.619 |
| PGA, g d−1 | 72.46 ± 25.45 | 76.60 ± 25.11 | 66.48 ± 26.20 | 0.372 |
| Spent dialysate, L per 24 h | 8.36 (6.27–8.85) | 8.25 (6.27–8.89) | 8.45 (6.31–8.80) | 0.837 |
| Urine volume, L per 24 h | 0.33 (0.02–0.73) | 0.25 (0.00–0.64) | 0.48 (0.16–1.00) | 0.280 |
| Ultrafiltration, mL per 24 h | 350.00 (75.00–487.50) | 375.00 (122.50–487.50) | 350.00 (20.00–487.50) | 0.983 |
| BUN, mmol L−1 | 18.15 ± 4.45 | 18.38 ± 3.87 | 17.86 ± 5.23 | 0.762 |
| Serum creatinine, μmol L−1 | 998.24 ± 311.65 | 1022.56 ± 360.23 | 968.31 ± 250.32 | 0.649 |
| Serum HCO3, mmol L−1 | 24.86 ± 2.58 | 24.54 ± 2.67 | 25.25 ± 2.50 | 0.475 |
| rGFR, mL per min per 1.73 m2 | 4.40 (3.30–5.60) | 3.85 (3.15–6.90) | 4.40 (3.90–5.50) | 0.475 |
| Kt/V | 1.96 (1.73–2.36) | 1.95 (1.74–2.56) | 2.02 (1.66–2.36) | 0.812 |
| Ccr, L per wk per 1.73 m2 | 56.49 (48.51–71.09) | 50.62 (46.40–63.14) | 65.10 (52.05–77.71) | 0.268 |
| nPNA, g kg−1 d−1 | 0.95 ± 0.17 | 0.96 ± 0.16 | 0.93 ± 0.18 | 0.692 |
| nPCR, g kg−1 d−1 | 1.19 ± 0.29 | 1.15 ± 0.24 | 1.24 ± 0.35 | 0.409 |
| Serum albumin, g L−1 | 39.45 ± 3.54 | 39.34 ± 3.32 | 39.58 ± 3.94 | 0.859 |
| Serum prealbumin, mg L−1 | 401.34 ± 68.08 | 403.25 ± 67.68 | 399.00 ± 71.27 | 0.871 |
| Serum hemoglobin, g L−1 | 103.28 ± 20.31 | 103.19 ± 21.25 | 103.38 ± 19.94 | 0.980 |
| SBP, mmHg | 144.93 ± 25.15 | 146.31 ± 26.50 | 143.23 ± 24.34 | 0.749 |
| DBP, mmHg | 87.07 ± 15.52 | 90.44 ± 16.41 | 82.92 ± 13.84 | 0.200 |
| FBG, mmol L−1 | 5.33 (5.03–5.66) | 5.21 (4.99–6.64) | 5.34 (5.08–5.66) | 0.559 |
| TG, mmol L−1 | 1.41 (1.18–1.97) | 1.39 (1.17–1.96) | 1.42 (1.18–2.10) | 0.983 |
| TC, mmol L−1 | 4.36 ± 0.96 | 4.59 ± 1.02 | 4.08 ± 0.84 | 0.159 |
| LDL-C, mmol L−1 | 2.33 ± 0.67 | 2.41 ± 0.74 | 2.24 ± 0.58 | 0.513 |
| HDL-C, mmol L−1 | 1.02 (0.84–1.26) | 1.11 (0.86–1.31) | 0.94 (0.82–1.14) | 0.199 |
| hs-CRP, mg L−1 | 1.15 (0.60–4.60) | 0.90 (0.60–5.60) | 1.30 (0.45–4.40) | 1.000 |
Daily macronutrient and trace element intake
Throughout the study, the daily dietary energy intake was 1337.1 ± 312.9 kcal, with energy acquired from peritoneal glucose absorption at 245.4 ± 93.1 kcal d−1, resulting in a total energy of 1577.2 ± 320.8 kcal d−1. The daily dietary energy intake was significantly less than the estimated daily energy requirement of 35 kcal kg−1 for males (the P value estimated by 1-sample t test <0.001), and less than the 30 kcal kg−1 estimated for females (P = 0.001). The daily total energy acquired was still lower in male patients (P = 0.002), whereas comparable in female patients (P = 0.354). The daily dietary intake of fatty acids was 42.6 ± 16.2 g d−1, including 12.1 ± 6.2 g d−1 SFAs, 13.0 ± 5.3 g d−1 MUFAs and 16.7 ± 6.6 g d−1 PUFAs. The daily dietary fiber intake was 8.4 ± 2.7 g, which was significantly less than the recommended value of 20–25 g d−1 (P < 0.001). The dietary Fe intake was 13.5 ± 4.9 mg d−1, Zn was 7.6 ± 2.9 mg d−1, and Se was 23.0 ± 8.2 μg d−1, which was all significantly less than the recommend intake. The dietary As intake was 21.7 ± 5.9 μg d−1, Cd was 19.1 ± 6.7 μg d−1, and Pb was 24.0 ± 5.7 μg d−1. The one-way repeated-measures ANOVA showed no significant difference in the daily macronutrient and food trace element intake during the prebiotic, washout, and placebo intervention (Table 2).| Daily nutrient intake | Whole study | During prebiotics | During washout | During placebo | P |
|---|---|---|---|---|---|
| a The difference was determined using 1-factor repeated-measures ANOVA. DEI, daily energy intake; PGA, peritoneal glucose absorption; SFAs, saturated fatty acids; MUFAs, monounsaturated fatty acids; and PUFAs, polyunsaturated fatty acids. | |||||
| Dietary energy, kcal d−1 | 1337.1 ± 312.9 | 1332.6 ± 364.3 | 1276.3 ± 392.6 | 1350.4 ± 363.8 | 0.609 |
| PGA, g d−1 | 71.9 ± 26.5 | 70.4 ± 26.7 | 71.5 ± 27.4 | 72.5 ± 28.8 | 0.299 |
| Energy from PGA, kcal d−1 | 245.4 ± 93.1 | 244.1 ± 101.7 | 242.4 ± 93.2 | 246.1 ± 97.7 | 0.741 |
| Total energy, kcal d−1 | 1577.2 ± 320.8 | 1560.5 ± 381.8 | 1484.0 ± 386.1 | 1562.9 ± 357.2 | 0.709 |
| Carbohydrate, g d−1 | 190.3 ± 54.8 | 189.2 ± 65.3 | 184.7 ± 65.1 | 200.2 ± 60.9 | 0.393 |
| Carbohydrate, % DEI | 56.6 ± 6.6 | 56.3 ± 7.4 | 57.4 ± 6.6 | 59.4 ± 7.9 | 0.183 |
| Protein, g d−1 | 43.5 ± 10.0 | 43.9 ± 11.6 | 40.9 ± 12.5 | 42.3 ± 11.5 | 0.352 |
| Protein, % DEI | 13.1 ± 1.1 | 13.3 ± 1.6 | 12.9 ± 1.5 | 12.7 ± 1.7 | 0.447 |
| Fat, g d−1 | 46.6 ± 18.0 | 52.0 ± 18.3 | 42.9 ± 16.1 | 46.2 ± 23.1 | 0.167 |
| Fat, % DEI | 32.1 ± 6.0 | 32.2 ± 7.1 | 31.3 ± 6.1 | 29.8 ± 7.3 | 0.250 |
| Fatty acids, g d−1 | 42.6 ± 16.2 | 47.4 ± 16.6 | 39.2 ± 14.2 | 42.2 ± 20.8 | 0.190 |
| SFAs, g d−1 | 12.1 ± 6.2 | 13.1 ± 6.2 | 11.6 ± 6.5 | 12.0 ± 7.0 | 0.497 |
| MUFAs, g d−1 | 13.0 ± 5.3 | 14.3 ± 5.4 | 12.2 ± 5.0 | 12.8 ± 6.4 | 0.348 |
| PUFAs, g d−1 | 16.7 ± 6.6 | 19.1 ± 7.5 | 14.8 ± 4.6 | 16.7 ± 8.7 | 0.103 |
| Dietary fiber, g d−1 | 8.4 ± 2.7 | 8.4 ± 3.5 | 8.3 ± 3.8 | 8.5 ± 2.8 | 0.982 |
| Dietary Mn, mg d−1 | 3.4 ± 0.9 | 3.3 ± 1.0 | 3.5 ± 1.3 | 3.6 ± 1.2 | 0.677 |
| Dietary Fe, mg d−1 | 13.5 ± 4.9 | 13.1 ± 4.7 | 14.0 ± 8.6 | 13.8 ± 5.5 | 0.792 |
| Dietary Co, μg d−1 | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.5 ± 0.9 | 1.5 ± 1.1 | 0.600 |
| Dietary Cu, mg d−1 | 1.2 ± 1.4 | 1.0 ± 0.7 | 1.7 ± 3.2 | 1.2 ± 1.6 | 0.379 |
| Dietary Zn, mg d−1 | 7.6 ± 2.9 | 7.2 ± 2.3 | 8.2 ± 6.0 | 7.5 ± 3.3 | 0.553 |
| Dietary Se, μg d−1 | 23.0 ± 8.2 | 21.9 ± 8.4 | 24.7 ± 13.7 | 21.2 ± 7.2 | 0.645 |
| Dietary V, μg d−1 | 5.7 ± 4.0 | 5.6 ± 4.3 | 5.4 ± 3.4 | 5.1 ± 5.1 | 0.295 |
| Dietary Cr, μg d−1 | 41.9 ± 26.6 | 39.1 ± 24.4 | 36.2 ± 20.9 | 33.4 ± 13.8 | 0.412 |
| Dietary Ni, μg d−1 | 141.9 ± 90.4 | 148.1 ± 131.0 | 132.9 ± 105.7 | 124.5 ± 72.8 | 0.691 |
| Dietary As, μg d−1 | 21.7 ± 5.9 | 21.0 ± 7.2 | 21.7 ± 9.1 | 22.1 ± 7.7 | 0.946 |
| Dietary Cd, μg d−1 | 19.1 ± 6.7 | 19.0 ± 7.4 | 19.2 ± 6.2 | 18.9 ± 7.8 | 0.398 |
| Dietary Pb, μg d−1 | 24.0 ± 5.7 | 23.2 ± 7.1 | 24.1 ± 9.4 | 26.5 ± 8.6 | 0.428 |
The circulating trace elements in the peritoneal dialysis patients
Compared to the general population in Hubei or China, the essential trace elements Mn, Fe and Zn in the plasma were significantly deficient in the peritoneal dialysis patients, whereas Co, Sr and Mo were significantly excessive, with all P-values <0.001. In the case of the potential toxic elements, the plasma As was about 7-fold higher in the peritoneal dialysis patients than that in the general population in Hubei (3.54 μg L−1 (range: 1.37–14.16) vs. 0.47 μg L−1 (range: 0.23–4.63), P < 0.001) and the plasma Th was about 18-fold higher in the former than in the latter (P < 0.001), as shown in Table 3.| Trace elements | Peritoneal dialysis (n = 29) | General population in China | P |
|---|---|---|---|
| Median (min–max) | Median (min–max) | ||
| a The plasma concentration of Fe was from the study based on 1466 general population in China by Liu et al. (A. Liu, P. Xu, C. Gong, Y. Zhu, H. Zhang, W. Nie, X. Zhou, X. Liang, Y. Xu, C. Huang, X. L. Liu and J. C. Zhou, High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: a health examination center based cross-sectional study, J. Trace Elem. Med. Biol., 2020, 59, 126470). b The plasma concentrations of Mn, Co, Cu, Zn, Se, Sr and Mo were from the study on the general population in Hubei conducted by Li et al. (W. Li, X. Xu, Q. Jiang, P. Long, Y. Xiao, Y. You, C. Jia, W. Wang, Y. Lei, J. Xu, Y. Wang, M. Zhang, C. Liu, Q. Zeng, S. Ruan, X. Wang, C. Wang, Y. Yuan, H. Guo and T. Wu, Circulating metals, leukocyte microRNAs and microRNA networks: a profiling and functional analysis in Chinese adults, Environ. Int., 2022, 169, 107511). c The plasma concentrations of toxic elements Cr, V, Ni, As, Cd, Pb, Ba, Tl, Th and U were from the general population study in Hubei conducted by Li et al. (W. Li, X. Xu, Q. Jiang, P. Long, Y. Xiao, Y. You, C. Jia, W. Wang, Y. Lei, J. Xu, Y. Wang, M. Zhang, C. Liu, Q. Zeng, S. Ruan, X. Wang, C. Wang, Y. Yuan, H. Guo and T. Wu, Circulating metals, leukocyte microRNAs and microRNA networks: a profiling and functional analysis in Chinese adults, Environ. Int., 2022, 169, 107511). d P values of the difference between the peritoneal dialysis patients and general population were calculated by One-Sample Wilcoxon Signed Rank Test. | |||
Plasma Fe, mg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
0.82 (0.47–1.81) | 1.60 (1.30–2.0) | <0.001 |
Plasma Mn, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
0.50 (0.06–1.21) | 1.15 (0.55–3.80) | <0.001 |
Plasma Co, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
0.30 (0.11–2.09) | <0.0069 (<0.0069–0.0069) | <0.001 |
Plasma Cu, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
878.64 (672.13–1229.53) | 931.22 (456.52–1701.37) | 0.633 |
Plasma Zn, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
726.08 (489.19–1005.57) | 873.81 (626.16–1589.83) | <0.001 |
Plasma Se, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
62.58 (42.41–104.31) | 65.31 (37.73–151.74) | 0.524 |
Plasma Sr, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
53.05 (38.26–104.46) | 33.15 (15.11–87.39) | <0.001 |
Plasma Mo, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
12.01 (4.88–23.94) | 1.47 (0.36–22.35) | <0.001 |
Plasma V, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.24 (0.09–0.72) | 0.58 (0.25–3.18) | <0.001 |
Plasma Cr, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
3.39 (0.99–6.31) | — | — |
Plasma Ni, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.35 (0.13–10.11) | 1.63 (0.86–3.62) | 0.433 |
Plasma As, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
3.54 (1.37–14.16) | 0.47 (0.23–4.63) | <0.001 |
Plasma Cd, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.07 (0.00–0.28) | — | — |
Plasma Ba, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
11.09 (1.28–19.76) | 25.51 (13.08–52.93) | <0.001 |
Plasma Pb, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.25 (0.09–0.77) | 1.90 (0.30–20.43) | <0.001 |
Plasma Tl, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.06 (0.02–0.13) | — | — |
Plasma Th, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.07 (0.02–0.34) | 0.0038 (<0.0076–<0.0076) | <0.001 |
Plasma U, μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
0.01 (0.01–0.02) | — | — |
The effects of inulin-type fructans on trace elements
As shown in Table 4, ITFs significantly reduced the plasma As by 1.03 μg L−1 (95%CI: −1.74, −0.33) (FDR-adjusted P = 0.045) compared to the placebo. Conversely, the placebo maltodextrin significantly increased the plasma Fe in comparison with ITF intervention (290.70 μg L−1 (95%CI: 139.44, 441.97) vs. −80.87 μg L−1 (95%CI: −246.36, 84.62), FDR-adjusted P = 0.036). No significant changes were observed for the other trace elements. The baseline values of the plasma trace elements were comparable between the two intervention sequences and between prebiotics-pre and placebo-pre, as shown in ESI Table 2.†| Trace elements | Baseline (n = 29) | Δ prebiotics (n = 23) | Δ placebo (n = 23) | P |
|---|---|---|---|---|
| Median (IQRs) | Mean (95%CI) | Mean (95%CI) | ||
| a The between-group changes (Δ prebiotics vs. Δ placebo) were analyzed by the linear mixed model analysis, with changes from baseline as dependent variables; treatment, intervention period, and treatment-by-period interaction as fixed effects; subjects as a random effect; and baseline values, intervention order, age, sex, ESRD course and PD duration as covariates. | ||||
| Essential trace elements | ||||
| Plasma Mn, μg L−1 | 0.50 (0.37–0.63) | 0.05 (−0.10, 0.20) | 0.02 (−0.12, 0.17) | 0.804 |
| Plasma Fe, μg L−1 | 821.13 (568.42–927.25) | −80.87 (−246.36, 84.62) | 290.70 (139.44, 441.97) | 0.002 |
| Plasma Co, μg L−1 | 0.30 (0.24–0.48) | −0.00 (−0.11, 0.10) | 0.09 (−0.02, 0.20) | 0.214 |
| Plasma Cu, μg L−1 | 878.64 (798.81–1035.81) | −13.69 (−92.27, 64.88) | −15.13 (−87.51, 57.24) | 0.978 |
| Plasma Zn, μg L−1 | 726.08 (603.56–764.31) | −1.85 (−60.97, 57.27) | 59.16 (2.78, 115.54) | 0.140 |
| Plasma Se, μg L−1 | 62.58 (52.96–75.28) | −1.58 (−5.23, 2.07) | −1.83 (−5.30, 1.64) | 0.921 |
| Plasma Sr, μg L−1 | 53.05 (47.17–68.95) | 0.35 (−7.16, 7.86) | 10.95 (4.14, 17.76) | 0.042 |
| Plasma Mo, μg L−1 | 12.01 (9.10–16.38) | −1.10 (−2.82, 0.61) | −0.35 (−2.10, 1.40) | 0.539 |
| Potential toxic elements | ||||
| Plasma V, μg L−1 | 0.24 (0.18–0.35) | 0.06 (−0.04, 0.16) | 0.01 (−0.10, 0.12) | 0.531 |
| Plasma Cr, μg L−1 | 3.39 (1.92–4.77) | 0.10 (−0.36, 0.56) | −0.14 (−0.56, 0.28) | 0.445 |
| Plasma Ni, μg L−1 | 0.35 (0.13–6.22) | −0.14 (−0.79, 0.50) | −0.65 (−1.24, −0.06) | 0.247 |
| Plasma As, μg L−1 | 3.54 (2.61–4.40) | −0.76 (−1.26, −0.26) | 0.27 (−0.22, 0.76) | 0.005 |
| Plasma Cd, μg L−1 | 0.07 (0.04–0.09) | −0.00 (−0.03, 0.02) | 0.00 (−0.02, 0.03) | 0.631 |
| Plasma Ba, μg L−1 | 11.09 (8.68–13.45) | −0.41 (−4.19, 3.36) | 1.43 (−2.20, 5.06) | 0.481 |
| Plasma Tl, μg L−1 | 0.06 (0.04–0.07) | −0.01 (−0.03, 0.01) | 0.01 (−0.01, 0.03) | 0.195 |
| Plasma Pb, μg L−1 | 0.25 (0.14–0.32) | 0.16 (−0.15, 0.48) | 0.39 (0.03, 0.75) | 0.338 |
| Plasma Th, μg L−1 | 0.07 (0.04–0.16) | −0.03 (−0.04, −0.02) | −0.04 (−0.05, −0.03) | 0.431 |
| Plasma U, μg L−1 | 0.01 (0.01–0.01) | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.00) | 0.240 |
Concerning the daily clearance of trace elements, the 10 g d−1 ITFs significantly increased the As removal rate by urine and dialysis by 0.92 L/24 h (95%CI: 0.08, 1.77; P = 0.033), whereas a significant decrease in urine Th excretion rate was observed (P = 0.026). There were no significant changes in the daily clearance rate of the other trace elements (Table 5). The repeated measures correlation analysis showed a significantly negative association between the plasma As and daily dialysate As clearance rate (r = −0.42, P < 0.001), whereas a positive correlation between the plasma As and fecal As (r = 0.33, P = 0.012) (ESI Fig. 1†).
| Trace elements | Baseline (n = 29) | Δ prebiotics (n = 23) | Δ placebo (n = 23) | P |
|---|---|---|---|---|
| Median (IQRs) | Mean (95%CI) | Mean (95%CI) | ||
| a The between-group changes (Δ prebiotics vs. Δ placebo) were analyzed by the linear mixed model analysis, with changes from the baseline as dependent variables; treatment, intervention period, and treatment-by-period interaction as fixed effects; subjects as a random effect; and baseline values, intervention order, age, sex, ESRD course and PD duration as covariates. | ||||
| Mn | ||||
| Urinary Mn excretion rate, L per 24 h | 0.14 (0.00–0.68) | −0.63 (−2.02, 0.77) | 1.62 (−2.45, 5.68) | 0.277 |
| Dialysate Mn clearance rate, L per 24 h | −1.99 (−4.42–3.34) | 2.53 (−4.75, 9.80) | −1.67 (−8.94, 5.59) | 0.419 |
| Total Mn removal rate, L per 24 h | −1.43 (−4.33–4.11) | 5.43 (−3.87, 14.73) | 0.17 (−8.99, 9.32) | 0.415 |
| Fecal Mn, μg g−1 | 133.38 (103.61–162.42) | −7.58 (−25.96, 10.81) | −24.74 (−43.50, −5.98) | 0.198 |
| Fe | ||||
| Urinary Fe excretion rate, L per 24 h | 0.00 (0.00–0.01) | −0.00 (−0.01, 0.00) | −0.00 (−0.01, 0.00) | 0.828 |
| Dialysate Fe clearance rate, L per 24 h | 0.12 (0.06–0.18) | 0.01 (−0.03, 0.05) | −0.03 (−0.07, 0.01) | 0.174 |
| Total Fe removal rate, L per 24 h | 0.13 (0.08–0.20) | 0.00 (−0.05, 0.05) | −0.04 (−0.09, 0.01) | 0.213 |
| Fecal Fe, μg g−1 | 403.14 (269.60–2661.73) | −363.33 (−929.17, 202.52) | 147.53 (−380.82, 675.88) | 0.189 |
| Co | ||||
| Urinary Co excretion rate, L per 24 h | 0.21 (0.00–0.49) | −0.15 (−0.26, −0.04) | −0.01 (−0.11, 0.10) | 0.060 |
| Dialysate Co clearance rate, L per 24 h | 0.30 (0.11–0.64) | 0.02 (−0.12, 0.17) | 0.00 (−0.16, 0.16) | 0.832 |
| Total Co removal rate, L per 24 h | 0.57 (0.39–1.06) | −0.14 (−0.33, 0.05) | −0.10 (−0.30, 0.11) | 0.738 |
| Fecal Co, μg g−1 | 0.35 (0.23–0.43) | −0.11 (−0.17, −0.06) | −0.05 (−0.10, −0.00) | 0.103 |
| Cu | ||||
| Urinary Cu excretion rate, L per 24 h | 0.01 (0.00–0.03) | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.00) | 0.964 |
| Dialysate Cu clearance rate, L per 24 h | 0.10 (0.08–0.12) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.02) | 0.995 |
| Total Cu removal rate, L per 24 h | 0.12 (0.10–0.14) | −0.00 (−0.03, 0.02) | −0.01 (−0.04, 0.02) | 0.793 |
| Fecal Cu, μg g−1 | 32.37 (17.98–40.67) | −2.81 (−7.26, 1.64) | −2.95 (−7.17, 1.27) | 0.964 |
| Zn | ||||
| Urinary Zn excretion rate, L per 24 h | 0.07 (0.00–0.14) | 0.00 (−0.03, 0.03) | −0.02 (−0.05, 0.01) | 0.303 |
| Dialysate Zn clearance rate, L per 24 h | −0.27 (−0.38−(−0.08)) | −0.07 (−0.15, 0.01) | −0.02 (−0.10, 0.05) | 0.413 |
| Total Zn removal rate, L per 24 h | −0.18 (−0.34–(−0.01)) | −0.07 (−0.16, 0.01) | −0.05 (−0.13, 0.03) | 0.668 |
| Fecal Zn, μg g−1 | 222.99 (182.60–270.61) | 16.69 (−19.57, 52.95) | −22.14 (−56.07, 11.80) | 0.125 |
| Se | ||||
| Urinary Se excretion rate, L per 24 h | 0.04 (0.00–0.07) | −0.01 (−0.02, 0.01) | −0.00 (−0.02, 0.01) | 0.636 |
| Dialysate Se clearance rate, L per 24 h | 0.13 (0.10–0.19) | −0.02 (−0.05, 0.00) | 0.01 (−0.02, 0.03) | 0.077 |
| Total Se removal rate, L per 24 h | 0.17 (0.15–0.22) | −0.03 (−0.06, −0.00) | 0.00 (−0.02, 0.03) | 0.076 |
| Fecal Se, μg g−1 | 0.57 (0.38–0.69) | −1.14 (−3.43, 1.15) | 0.12 (−1.73, 1.97) | 0.390 |
| Sr | ||||
| Urinary Sr excretion rate, L per 24 h | 0.22 (0.00–0.51) | −0.04 (−0.14, 0.06) | 0.01 (−0.10, 0.11) | 0.497 |
| Dialysate Sr clearance rate, L per 24 h | 2.10 (1.54–3.53) | −0.17 (−1.07, 0.73) | 0.40 (−0.43, 1.23) | 0.351 |
| Total Sr removal rate, L per 24 h | 2.53 (1.83–3.52) | −0.02 (−0.82, 0.78) | 0.48 (−0.31, 1.26) | 0.372 |
| Fecal Sr, μg g−1 | 36.87 (23.95–69.21) | −8.33 (−16.81, 0.15) | −1.10 (−10.20, 7.99) | 0.243 |
| Mo | ||||
| Urinary Mo excretion rate, L per 24 h | 0.76 (0.00–3.33) | −0.49 (−1.37, 0.39) | −0.08 (−0.96, 0.80) | 0.502 |
| Dialysate Mo clearance rate, L per 24 h | 4.03 (2.74–5.01) | −0.36 (−0.97, 0.25) | 0.61 (0.05, 1.18) | 0.024 |
| Total Mo removal rate, L per 24 h | 5.70 (4.33–6.45) | −0.82 (−1.92, 0.28) | 0.40 (−0.69, 1.49) | 0.117 |
| Fecal Mo, μg g−1 | 2.43 (1.49–3.13) | −1.08 (−2.42, 0.27) | −0.04 (−1.60, 1.52) | 0.307 |
| V | ||||
| Urinary V excretion rate, L per 24 h | 0.24 (0.00–0.47) | −0.18 (−0.37, 0.01) | −0.10 (−1.08, 0.88) | 0.872 |
| Dialysate V clearance rate, L per 24 h | 0.95 (0.37–1.78) | −0.24 (−0.63, 0.15) | 0.01 (−0.36, 0.38) | 0.356 |
| Total V removal rate, L per 24 h | 1.38 (0.62–2.25) | 0.22 (−0.59, 1.03) | −0.30 (−1.26, 0.65) | 0.389 |
| Fecal V, μg g−1 | 0.28 (0.22–0.39) | −0.09 (−0.17, −0.02) | −0.02 (−0.09, 0.05) | 0.176 |
| Cr | ||||
| Urinary Cr excretion rate, L per 24 h | 0.22 (0.00–0.55) | −0.06 (−0.15, 0.03) | −0.04 (−0.14, 0.06) | 0.809 |
| Dialysate Cr clearance rate, L per 24 h | 0.51 (0.23–0.80) | 0.25 (−0.45, 0.94) | 0.49 (−0.15, 1.13) | 0.604 |
| Total Cr removal rate, L per 24 h | 0.84 (0.45–1.04) | 0.31 (−0.14, 0.77) | 0.18 (−0.34, 0.70) | 0.686 |
| Fecal Cr, μg g−1 | 1.23 (0.70–1.95) | −0.73 (−2.13, 0.66) | 1.06 (−0.47, 2.59) | 0.087 |
| Ni | ||||
| Urinary Ni excretion rate, L per 24 h | 1.16 (0.00–9.51) | −1.68 (−6.78, 3.43) | 2.52 (−2.47, 7.50) | 0.238 |
| Dialysate Ni clearance rate, L per 24 h | 5.12 (3.18–37.00) | −1.89 (−12.22, 8.45) | 4.87 (−4.83, 14.56) | 0.353 |
| Total Ni removal rate, L per 24 h | 10.23 (3.84–45.92) | −2.14 (−15.00, 10.72) | 11.19 (−2.07, 24.46) | 0.157 |
| Fecal Ni, μg g−1 | 4.63 (2.67–5.69) | −1.11 (−4.11, 1.90) | 1.05 (−2.09, 4.19) | 0.323 |
| As | ||||
| Urinary As excretion rate, L per 24 h | 1.10 (0.00–3.54) | −0.11 (−0.63, 0.41) | −0.44 (−0.94, 0.07) | 0.358 |
| Dialysate As clearance rate, L per 24 h | 4.46 (3.84–5.54) | 0.36 (−0.16, 0.88) | −0.08 (−0.61, 0.45) | 0.239 |
| Total As removal rate, L per 24 h | 6.10 (5.45–7.30) | 0.23 (−0.35, 0.81) | −0.70 (−1.33, −0.07) | 0.033 |
| Fecal As, μg g−1 | 0.14 (0.08–0.21) | −0.18 (−0.23, −0.12) | −0.14 (−0.19, −0.09) | 0.334 |
| Cd | ||||
| Urinary Cd excretion rate, L per 24 h | 1.12 (0.00–2.08) | −2.95 (−3.81, −2.10) | −2.93 (−3.76, −2.11) | 0.973 |
| Dialysate Cd clearance rate, L per 24 h | 2.04 (1.37–3.14) | −12.67 (−14.40, −10.94) | −11.18 (−13.64, −8.72) | 0.316 |
| Total Cd removal rate, L per 24 h | 3.79 (2.78–6.31) | −18.67 (−19.95, −17.38) | −18.30 (−19.61, −17.00) | 0.690 |
| Fecal Cd, μg g−1 | 0.67 (0.48–1.25) | −0.18 (−0.37, 0.01) | −0.18 (−0.36, −0.01) | 0.990 |
| Ba | ||||
| Urinary Ba excretion rate, L per 24 h | 0.08 (0.00–0.21) | 0.15 (−0.01, 0.30) | 0.05 (−0.12, 0.21) | 0.354 |
| Dialysate Ba clearance rate, L per 24 h | 0.01 (−0.01–0.04) | 2.25 (−0.96, 5.46) | 6.18 (0.42, 11.94) | 0.223 |
| Total Ba removal rate, L per 24 h | 0.08 (0.01–0.19) | 2.42 (−1.17, 6.02) | 7.10 (0.17, 14.04) | 0.216 |
| Fecal Ba, μg g−1 | 20.72 (14.40–28.51) | −1.31 (−7.29, 4.67) | −0.31 (−5.95, 5.34) | 0.805 |
| Tl | ||||
| Urinary Tl excretion rate, L per 24 h | 0.42 (0.00–0.87) | 0.51 (0.01, 1.01) | 0.12 (−0.36, 0.61) | 0.259 |
| Dialysate Tl clearance rate, L per 24 h | 1.51 (0.30–2.33) | −1.24 (−2.37, −0.11) | −0.43 (−2.07, 1.20) | 0.405 |
| Total Tl removal rate, L per 24 h | 1.88 (0.56–3.11) | −0.72 (−2.53, 1.09) | −0.40 (−3.01, 2.20) | 0.830 |
| Fecal Tl, μg g−1 | 0.02 (0.02–0.03) | −0.01 (−0.01, 0.00) | −0.00 (−0.01, 0.01) | 0.759 |
| Pb | ||||
| Urinary Pb excretion rate, L per 24 h | 1.23 (0.00–2.14) | −0.56 (−1.48, 0.36) | 0.16 (−1.10, 1.43) | 0.346 |
| Dialysate Pb clearance rate, L per 24 h | 1.32 (−0.85–12.76) | −6.58 (−8.75, −4.41) | −4.66 (−6.79, −2.53) | 0.220 |
| Total Pb removal rate, L per 24 h | 2.27 (0.36–18.40) | −6.49 (−9.66, −3.32) | −3.49 (−6.62, −0.36) | 0.179 |
| Fecal Pb, μg g−1 | 0.37 (0.22–0.48) | −0.05 (−0.13, 0.03) | −0.01 (−0.08, 0.06) | 0.416 |
| Th | ||||
| Urinary Th excretion rate, L per 24 h | 0.04 (0.00–0.18) | −0.05 (−0.11, 0.02) | 0.05 (−0.01, 0.11) | 0.026 |
| Dialysate Th clearance rate, L per 24 h | −1.19 (−2.73–0.00) | −1.08 (−1.90, −0.11) | −1.24 (−2.08, −0.41) | 0.696 |
| Total Th removal rate, L per 24 h | −1.07 (−2.62–0.15) | −1.64 (−2.41, −0.86) | −1.63 (−2.51, −0.76) | 0.991 |
| Fecal Th, μg g−1 | 0.04 (0.02–0.05) | −0.01 (−0.02, 0.00) | 0.00 (−0.01, 0.01) | 0.098 |
| U | ||||
| Urinary U excretion rate, L per 24 h | 0.39 (0.00–0.81) | −0.64 (−1.54, 0.26) | −0.03 (−1.09, 1.02) | 0.368 |
| Dialysate U clearance rate, L per 24 h | −2.06 (−4.24–1.48) | 11.44 (−8.75, 31.63) | −2.51 (−26.02, 21.00) | 0.364 |
| Total U removal rate, L per 24 h | −1.42 (−3.66–4.05) | −1.92 (−4.77, 0.93) | −2.40 (−5.23, 0.43) | 0.816 |
| Fecal U, μg g−1 | 0.04 (0.02–0.07) | −0.01 (−0.03, 0.00) | −0.01 (−0.03, −0.00) | 0.879 |
The effect of inulin-type fructans on gut microbial composition
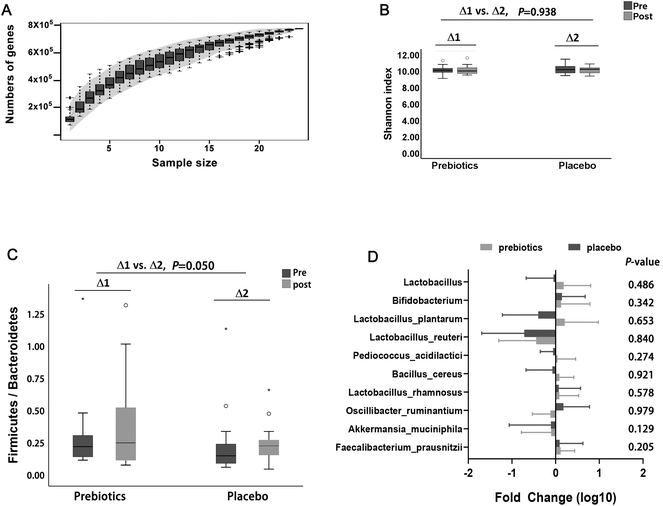
The rarefaction analysis estimating the total number of genes of the gut microbiota showed that the gene richness approached saturation in the peritoneal dialysis patients (Fig. 3A). No significant difference was observed in the α-diversity of the gut microbiota between the two interventions (P = 0.938, Fig. 3B). However, ITFs significantly increased the ratio of Firmicutes/Bacteroidetes (P = 0.050), with a quantitative reduction in the phylum-level Bacteroidetes (P = 0.139) and a quantitative increase in Firmicutes (P = 0.112) (Fig. 3C). For the symbiotic bacteria reported to increase the absorption of essential trace elements or reduce the retention of potential toxic elements, no significant changes were observed between the two interventions (Fig. 3D).The effect of inulin-type fructans on short chain fatty acids
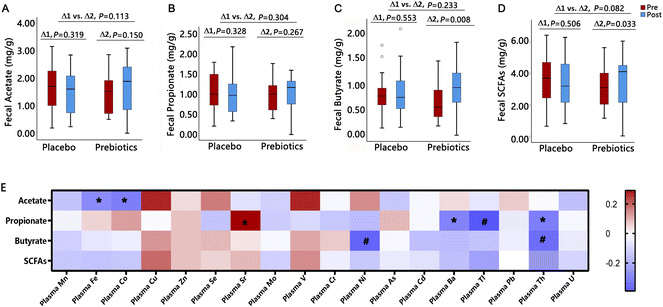
As shown in Fig. 4, the mixed linear model showed a significant increase in fecal butyrate content after ITF intervention (ΔPrebiotics = 0.22 mg g−1 (95%CI: 0.05, 0.38), P = 0.008), whereas no change was observed after the placebo (ΔPlacebo = 0.08 (95%CI: −0.10, 0.25), P = 0.553). There was an increasing tendency for total SCFAs with ITFs compared to the placebo (Δ prebiotics vs. Δ placebo, 0.44 mg g−1 (95%CI: 0.00, 0.88) vs. −0.12 mg g−1 (95%CI: −0.57, 0.33), P = 0.082). The repeated measures correlation analysis showed significantly negative associations of fecal acetate with plasma Fe (r = −0.27, P = 0.047) and Co (r = −0.30, P = 0.025), fecal propionate with plasma Tl (r = −0.39, P = 0.003) and Th (r = −0.30, P = 0.027), and fecal butyrate with plasma Ni (r = −0.36, P = 0.006) and Th (r = −0.34, P = 0.009), whereas no significant association between fecal total SCFAs and plasma trace elements.The effect of inulin-type fructans on bile acids
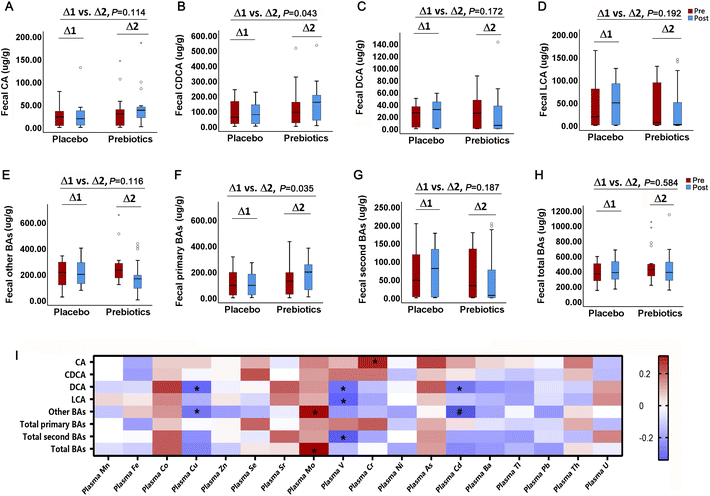
The mixed linear model showed that ITFs significantly increased the fecal excretion of chenodeoxycholic acid (CDCA) by 47.38 μg g−1 (95%CI: 1.46, 93.30) (P = 0.043) and primary BAs by 60.20 μg g−1 (95%CI: 4.51, 115.88) (P = 0.035) compared to the placebo, whereas no significant changes were observed for fecal second BAs and total BAs. The repeated measures correlation analysis showed a negative association between the total DCA and plasma Cu (r = −0.31, P = 0.021), V (r = −0.29, P = 0.028) and Cd (r = −0.28, P = 0.037), and negative correlation between total LCA and V (r = −0.32, P = 0.018), whereas no significant association between fecal total BAs and plasma trace elements, as shown in Fig. 5.The effect of inulin-type fructans on residual renal and peritoneal function
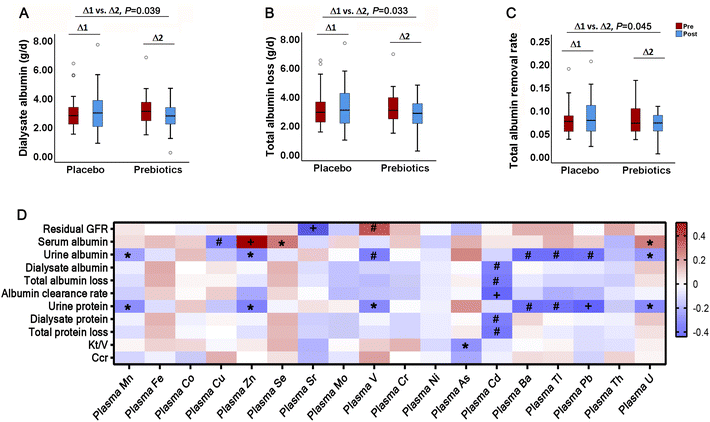
The mixed linear model showed a significant decrease in peritoneal albumin loss (P = 0.039) and total albumin loss by urine and dialysis (P = 0.033) with ITFs (Fig. 6A and B). Considering the plasma albumin, the daily albumin clearance rate was also reduced (P = 0.045) (Fig. 6C). The repeated measures correlation analysis showed that the serum albumin was negatively associated with the plasma Cu (r = −0.34, P = 0.006), whereas positively correlated with the plasma Zn (r = 0.51, P < 0.001), Se (r = 0.27, P = 0.029) and U (r = 0.26, P = 0.038). The daily albumin clearance rate was negatively associated with the plasma Cd (r = −0.40, P = 0.001). The residual GFR was negatively associated with the plasma Sr (r = −0.44, P < 0.001) and positively correlated with V (r = 0.34, P = 0.007). The dialysis adequacy Kt/V was negatively associated with the plasma As (r = −0.29, P = 0.02) (Fig. 6D).Discussion
Manipulating the gut microbiota is crucial for breaking the vicious circle between the imbalance of trace elements and disease progression in ESRD. The present study found a significant reduction in the plasma As concentration with inulin-type prebiotic intervention in peritoneal dialysis patients, with improved gut microbiota. However, no significant changes were observed in the plasma Mn, Co, Cu, Zn, Se, Sr, Mo, V, Cr, Ni, Cd, Ba, Tl, Pb, Th and U with 10 g d−1 ITF intervention over 3-months and their daily clearance by urine, dialysate and feces.The circulatory imbalance of trace elements was observed in the peritoneal dialysis patients, with deficiencies in the essential elements Mn, Fe and Zn and serious overload of the toxic elements As and Th, as reported by Gómez de Oña et al. and Jankowska et al.38,39 The deficiencies in Mn, Fe and Zn may be partly attributed to their low dietary intake, given that the daily intake of Mn, Zn and Fe was significantly less than the recommend intake for Chinese adults. The retention of toxic elements may be due to the reduction in renal clearance and the contamination of the infused dialysate. In the present study, the serious As and Th accumulation may have primarily resulted from the reduced renal excretion, given that the As and Th contents in the effluent dialysate were positive, indicating the partial elimination of As and Th through dialysis.
Inulin has been extensively documented for its role in manipulating the gut microbiota. The current study demonstrated a significant improvement in the gut microbiota composition and metabolism, including an increase in the ratio of F/B, increasing tendency of fecal SCFAs and increase in fecal primary BA excretion, which are consistent with the findings reported by Birkeland et al. and Arifuzzaman et al.40,41 The F/B ratio is a surrogate marker of gut microbiota dysbiosis, where an increase in the F/B ratio is usually observed with the alleviation of gut inflammation.42 An increase in the production of SCFAs has been reported to acidify the intestinal lumen and increase the intestinal surface area, promoting the absorption of essential trace elements.30,43 However, the essential trace elements Mn, Co, Cu, Zn, Se and Mo were not significantly affected in the present study, which is possibly attributed to the intervention dosage. A dose of 10 g d−1 of ITFs has been reported to be sufficient to significantly change the gut microbiota.44 However, in ESRD patients, their gut biochemical milieu and gut barrier are seriously disrupted by uremia toxins, antibiotics, iron supplements and phosphate binders, and aggravated by the limited consumption of fruits and vegetables due to inappetence and hyperkalemia.45,46 Therefore, the 10 g d−1 of inulin type-fructans may not have been sufficient to improve the essential trace element deficiency in ESRD patients. The increase in the plasma Fe level with maltodextrin in the present study may be due to the suitability of maltodextrin as an encapsulation carrier, given that it has been successfully used to stabilize iron supplements and iron-fortified foods.47 Although an increase in fecal BA excretion with inulin and galactooligosaccharide supplementation has been reported to reduce the toxic elements Pb and Cd, no significant changes in plasma V, Cr, Ni, Cd, Ba, Tl, Pb, Th, and U were observed in the present study.48,49 The low plasma concentrations resulting from effective dialysis clearance may make the lowering-effect of ITFs in peritoneal dialysis less apparent than in pre-dialysis and in the general population. In addition, the prebiotic dosage may be a variable factor, emphasizing the need for large-scale multicenter studies with higher intervention dosages to further evaluate the effects of ITFs on trace elements.
The prebiotic ITFs were effective in reducing the plasma As concentration. This finding is consistent with a randomized open-label pilot study, which showed protective effects against a further increase in Hg and As with probiotic yogurt.35 Arsenic is the most prevalent toxic substance in the environment, ranking above other toxic elements based on the combination of frequency, toxicity and potential for human exposure.50 The potential mechanisms underlining As reduction by ITFs may involve the improved residual kidney and peritoneal function. In ESRD, the serious As excess results from the constant dietary intake, reduced renal excretion and limited dialysis removal. In the current study, the plasma As was negatively associated with the As clearance rate by urine and dialysis, whereas positively correlated with the As fecal excretion, indicating the primary determinants of As removal by dialysis and urine on the plasma As level. This is consistent with the fact that arsenic metabolites, including arsenite (iAsIII), arsenate (iAsV), monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV), are primarily excreted through urine in humans.51 Dietary fiber supplementations have been reported to improve the residual renal and peritoneal function.52 In addition, the biologic methylation of As promotes its urine elimination, and microbiota-driven therapies have been reported to significantly increase the methylation of As.53,54 In the present study, a significant decrease in albumin loss by residual renal and peritoneum was observed with ITFs, accompanied by an increase in the clearance rate of As through the urine and dialysate, indicating the negative regulation of As clearance by dialysis and urine on the plasma As level with ITF intervention. Microbiota-driven therapies have also been reported to aggravate As excretion with feces and reduce the intestinal absorption of ingested As. Various components of the human gut microbiota, including Lactic acid bacteria, Lactobacillus strains, Faecalibacterium prausnitzii, Akkermansia muciniphila, Desulfotomaculum auripigmentum and Alkaliphilus oremlandii strain OhILAs, can biotransform and detoxify As by oxidizing, reducing, methylating, and thiolating inorganic and organic As from food and drinking water, or directly chelating As through the carboxyl group of proteins and hydroxyl group of the peptidoglycans in the bacterial cell membrane, thereby reducing the bioaccessibility of ingested As.55,56 In the present study, a quantitative increase was observed in Lactic acid bacteria and Lactobacillus strains. Biliary-intestine excretion represents another important removal route for As, and ITFs could improve the gut microbiota metabolism, accelerating the enterohepatic circulation of bile acids and promoting the biliary secretion of heavy metals in the intestine.49,57 In the present study, a significant increase in fecal primary BAs and fecal total bile acids was observed with 10 g d−1 ITFs. However, no significant change was observed in As fecal excretion compared to the placebo, indicating the need for mechanism research in animal models to further evaluate the effect of regulating the gut microbiota on trace elements.
In the present study, we assessed the effects of ITFs on the absorption and elimination of the essential trace elements Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo and potential toxic elements V, Cr, Ni, As, Cd, Ba, Tl, Pb, Th, and U and found that ITFs were effective in ameliorating the As retention in ESRD. Although no significant improvement in Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo deficiency and V, Cr, Ni, Cd, Ba, Tl, Pb, Th, and U overload was observed, the dose of 10 g ITFs did not aggravate the circulating imbalance of trace elements, that is, the ITFs were safe for ESRD patients. However, there were several limitations in the present study, as follows: (1) the sample size was relatively small; (2) the concentrations of toxic elements in the main storage tissues, including liver, kidney and bone, were not measured; (3) the oxidative stress and antioxidative enzyme activities were not measured, which can be employed to further evaluate the effects of ITFs on trace elements; and (4) the effects of medication use were difficult to evaluate and compare between the two groups, given that the relevant records about the dose and time used were incomplete in electronic medical records, and could not be clearly recalled and identified by the patients themselves.
Conclusions
In the present study, it was found that inulin-type prebiotics were effective in promoting As excretion and reducing its circulating retention, with improved gut microbiota and increased fecal primary BA excretion. However, the effects on the essential trace elements Mn, Fe, Co, Cu, Zn, Se, Sr, and Mo and potential toxic elements V, Cr, Ni, Cd, Ba, Tl, Pb, Th, and U were not significant. Thus, large-scale multicenter studies with higher intervention dosages are needed to further explore the effect of prebiotic ITFs on trace elements.Abbreviation
| ITFs | Inulin-type fructans |
| ESRD | End-stage renal disease |
| CKD | Chronic kidney disease |
| PD | Peritoneal dialysis |
| CAPD | Continuous ambulatory peritoneal dialysis |
| SCFAs | Short chain fatty acids |
| Bas | Bile acids |
| DMT1 | Divalent metal transporter 1 |
| BSHs | Bile salt hydrolases |
| rGFR | Residual glomerular filtration rate |
| Kt/V | Dialysis efficiency |
| Ccr | Creatinine clearance rate |
| BUN | Blood urea nitrogen |
| RSD | Relative standard deviation |
| LOD | Limit of detection |
| ORFs | Open reading frames |
| FDR | False discovery rate |
| RPKM | Reads per kilobase million |
| rmcorr | Repeated measures correlation analysis |
| SFAs | Saturated fatty acids |
| MUFAs | Monounsaturated fatty acids |
| PUFAs | Polyunsaturated fatty acids |
| iAsIII | Arsenite |
| iAsV | Arsenate |
| MMAV | Monomethylarsonic acid |
| DMAV | Dimethylarsinic acid |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| DCA | Deoxycholic acid |
| LCA | Lithocholic acid |
Author contributions
Conceptualization: CY; resources: CY, XZ and WL; data curation: CY and XZ; software: LL and JZ; formal analysis: LL and JZ; supervision: CY and XZ; funding acquisition: CY and XZ; validation: LL and CY; investigation: JW, QX, XL, XG and FP; visualization: LL and CY; methodology: CY, XZ, LL and JZ; writing – original draft: LL; project administration: CY; writing – review & editing: CY and XZ.Data availability statements
Raw data for this paper, including trace elements in the plasma, urine, dialysate and feces, fecal short chain fatty acids and bile acids, are available at Science Data Bank at https://www.scidb.cn/en/detail?dataSetId=6e7ad97542a04b97845a1d2f4553f4d5. Also, the raw metagenomics datasets have been deposited into NCBI Sequence Read Achieve database (PRJNA750234).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82173522 and 81673161). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
- A. Jovanovich and J. Kendrick, Personalized Management of Bone and Mineral Disorders and Precision Medicine in End-Stage Kidney Disease, Semin. Nephrol., 2018, 38, 397–409 CrossRef PubMed.
- M. Tonelli, N. Wiebe, A. Bello, C. J. Field, J. S. Gill, B. R. Hemmelgarn, D. T. Holmes, K. Jindal, S. W. Klarenbach, B. J. Manns, R. Thadhani and D. Kinniburgh, Concentrations of Trace Elements in Hemodialysis Patients: A Prospective Cohort Study, Am. J. Kidney Dis., 2017, 70, 696–704 CrossRef CAS PubMed.
- M. Tonelli, N. Wiebe, A. Bello, C. J. Field, J. S. Gill, B. R. Hemmelgarn, D. T. Holmes, K. Jindal, S. W. Klarenbach, B. J. Manns, R. Thadhani and D. Kinniburgh, Concentrations of Trace Elements and Clinical Outcomes in Hemodialysis Patients: A Prospective Cohort Study, Clin. J. Am. Soc. Nephrol., 2018, 13, 907–915 CrossRef PubMed.
- L. Shanmugam, S. R. Green, H. Radhakrishnan, T. M. Kadavanu, A. Ramachandrappa, S. R. Tiwari, A. L. Rajkumar and E. Govindasamy, Trace Elements in Chronic Haemodialysis Patients and Healthy Individuals-A Comparative Study, J. Clin. Diagn. Res., 2016, 10, 14–17 Search PubMed.
- T. Banerjee, D. C. Crews, D. S. Tuot, M. E. Pavkov, N. R. Burrows, A. G. Stack, R. Saran, J. Bragg-Gresham and N. R. Powe, Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension, Kidney Int., 2019, 95, 1433–1442 CrossRef PubMed.
- H. I. Lin, H. M. Chen, C. C. Hsu, H. J. Lin, J. J. Wang, S. F. Weng, Y. Kao and C. C. Huang, Associations between dietary patterns and stages of chronic kidney disease, BMC Nephrol., 2022, 23, 115 CrossRef CAS PubMed.
- S. Badri, S. Vahdat, M. Pourfarzam, S. Assarzadeh, S. Seirafian and S. Ataei, Potential Benefits of Selenium Supplementation in Patients with Kidney Disease, J. Res. Pharm. Pract., 2021, 10, 149–158 CrossRef CAS PubMed.
- S. Xiang, Y. Yao, Y. Wan, W. Liang, R. Meng, Q. Jin, N. Wu, F. Xu, C. Ying and X. Zuo, Comparative Study on Trace Element Excretions between Nonanuric and Anuric Patients Undergoing Continuous Ambulatory Peritoneal Dialysis, Nutrients, 2016, 8, 826 CrossRef PubMed.
- D. Glicklich, C. T. Shin and W. H. Frishman, Heavy Metal Toxicity in Chronic Renal Failure and Cardiovascular Disease: Possible Role for Chelation Therapy, Cardiol. Rev., 2020, 28, 312–318 CrossRef PubMed.
- I. D. de Souza, A. S. de Andrade and R. J. S. Dalmolin, Lead-interacting proteins and their implication in lead poisoning, Crit. Rev. Toxicol., 2018, 48, 375–386 CrossRef PubMed.
- M. Wołonciej, E. Milewska and W. Roszkowska-Jakimiec, Trace elements as an activator of antioxidant enzymes, Postepy Hig. Med. Dosw., 2016, 70, 1483–1498 CrossRef PubMed.
- A. H. Faghfouri, M. Zarezadeh, B. Aghapour, A. Izadi, H. Rostamkhani, A. Majnouni, A. Abu-Zaid, H. K. Varkaneh, Z. Ghoreishi and A. Ostadrahimi, Clinical efficacy of zinc supplementation in improving antioxidant defense system: A comprehensive systematic review and time-response meta-analysis of controlled clinical trials, Eur. J. Pharmacol., 2021, 907, 174243 CrossRef CAS PubMed.
- M. Liu, X. Sun, B. Chen, R. Dai, Z. Xi and H. Xu, Insights into Manganese Superoxide Dismutase and Human Diseases, Int. J. Mol. Sci., 2022, 23, 15893 CrossRef CAS PubMed.
- B. K. Shimada, N. Alfulaij and L. A. Seale, The Impact of Selenium Deficiency on Cardiovascular Function, Int. J. Mol. Sci., 2021, 22, 10713 CrossRef CAS PubMed.
- J. G. Paithankar, S. Saini, S. Dwivedi, A. Sharma and D. K. Chowdhuri, Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction, Chemosphere, 2021, 262, 128350 CrossRef CAS PubMed.
- M. N. Rana, J. Tangpong and M. M. Rahman, Toxicodynamics of Lead, Cadmium, Mercury and Arsenic- induced kidney toxicity and treatment strategy: A mini review, Toxicol. Rep., 2018, 5, 704–713 CrossRef CAS PubMed.
- A. P. Sanders, M. J. Mazzella, A. J. Malin, G. M. Hair, S. A. Busgang, J. M. Saland and P. Curtin, Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014, Environ. Int., 2019, 131, 104993 CrossRef CAS PubMed.
- S. Satarug, G. C. Gobe, D. A. Vesey and K. R. Phelps, Cadmium and Lead Exposure, Nephrotoxicity, and Mortality, Toxics, 2020, 8, 86 CrossRef CAS PubMed.
- F. Wan, G. Zhong, S. Wu, X. Jiang, J. Liao, X. Zhang, H. Zhang, K. Mehmood, Z. Tang and L. Hu, Arsenic and antimony co-induced nephrotoxicity via autophagy and pyroptosis through ROS-mediated pathway in vivo and in vitro, Ecotoxicol. Environ. Saf., 2021, 221, 112442 CrossRef CAS PubMed.
- Q. Zhang, C. Zhang, J. Ge, M. W. Lv, M. Talukder, K. Guo, Y. H. Li and J. L. Li, Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy, Food Funct., 2020, 11, 1856–1868 RSC.
- C. Zhu, B. Wang, L. Xiao, Y. Guo, Y. Zhou, L. Cao, S. Yang and W. Chen, Mean platelet volume mediated the relationships between heavy metals exposure and atherosclerotic cardiovascular disease risk: A community-based study, Eur. J. Prev. Cardiol., 2020, 27, 830–839 CrossRef PubMed.
- B. Pourrajab, S. Fatahi, M. H. Sohouli, M. A. Găman and F. Shidfar, The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials, Crit. Rev. Food Sci. Nutr., 2022, 62, 490–507 CrossRef CAS PubMed.
- A. Akbarzadeh, M. Taheri, B. Ebrahimi, P. Alirezaei, A. Doosti-Irani, M. Soleimani and F. Nouri, Evaluation of Lactocare® Synbiotic Administration on the Serum Electrolytes and Trace Elements Levels in Psoriasis Patients: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial Study, Biol. Trace Elem. Res., 2022, 200, 4230–4237 CrossRef CAS PubMed.
- R. Chen, H. Tu and T. Chen, Potential Application of Living Microorganisms in the Detoxification of Heavy Metals, Foods, 2022, 11, 1905 CrossRef CAS PubMed.
- R. A. Reimer, A. Soto-Vaca, A. C. Nicolucci, S. Mayengbam, H. Park, K. L. Madsen, R. Menon and E. E. Vaughan, Effect of chicory inulin-type fructan-containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial, Am. J. Clin. Nutr., 2020, 111, 1286–1296 CrossRef PubMed.
- E. Korcz, Z. Kerényi and L. Varga, Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol-lowering effects, Food Funct., 2018, 9, 3057–3068 RSC.
- C. M. Whisner and L. F. Castillo, Prebiotics, Bone and Mineral Metabolism, Calcif. Tissue Int., 2018, 102, 443–479 CrossRef CAS PubMed.
- P. S. Salvi and R. A. Cowles, Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease, Cells, 2021, 10, 1775 CrossRef CAS PubMed.
- K. Skrypnik and J. Suliburska, Association between the gut microbiota and mineral metabolism, J. Sci. Food Agric., 2018, 98, 2449–2460 CrossRef CAS PubMed.
- M. de Lima Correia Silva, P. da Graça Leite Speridião, L. M. Oyama and M. B. de Morais, Effect of fructo-oligosaccharide supplementation in soya beverage on the intestinal absorption of calcium and iron in newly weaned rats, Br. J. Nutr., 2018, 120, 1338–1348 CrossRef PubMed.
- R. M. Abdel-Megeed, Probiotics: a Promising Generation of Heavy Metal Detoxification, Biol. Trace Elem. Res., 2021, 199, 2406–2413 CrossRef CAS PubMed.
- H. Zhang, Z. Mao, K. Huang, X. Wang, L. Cheng, L. Zeng, Y. Zhou and T. Jing, Multiple exposure pathways and health risk assessment of heavy metal(loid)s for children living in fourth-tier cities in Hubei Province, Environ. Int., 2019, 129, 517–524 CrossRef CAS PubMed.
- A. Liu, P. Xu, C. Gong, Y. Zhu, H. Zhang, W. Nie, X. Zhou, X. Liang, Y. Xu, C. Huang, X. L. Liu and J. C. Zhou, High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: A health examination center based cross-sectional study, J. Trace Elem. Med. Biol., 2020, 59, 126470 CrossRef CAS PubMed.
- W. Li, X. Xu, Q. Jiang, P. Long, Y. Xiao, Y. You, C. Jia, W. Wang, Y. Lei, J. Xu, Y. Wang, M. Zhang, C. Liu, Q. Zeng, S. Ruan, X. Wang, C. Wang, Y. Yuan, H. Guo and T. Wu, Circulating metals, leukocyte microRNAs and microRNA networks: A profiling and functional analysis in Chinese adults, Environ. Int., 2022, 169, 107511 CrossRef CAS PubMed.
- J. E. Bisanz, M. K. Enos, J. R. Mwanga, J. Changalucha, J. P. Burton, G. B. Gloor and G. Reid, Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children, mBio, 2014, 5, e01580–e01514 CrossRef CAS PubMed.
- Y. Tian, Z. Pan, L. Lan, Y. Chang, T. Zhao, Z. Fu, S. Wu, T. Deng, M. Cao, W. Wang, Y. Bi, R. Yang, B. J. Yang Lee and Q. Liu, Amelioration of intestinal barrier function and reduction of blood lead level in adult women with recurrent spontaneous abortion by a novel product of dietary fiber mixture, Holofood, J. Health Popul. Nutr., 2023, 42, 63 CrossRef PubMed.
- S. T. Clarke, J. M. Green-Johnson, S. P. Brooks, D. D. Ramdath, P. Bercik, C. Avila, G. D. Inglis, J. Green, L. J. Yanke, L. B. Selinger and M. Kalmokoff, β2–1 Fructan supplementation alters host immune responses in a manner consistent with increased exposure to microbial components: results from a double-blinded, randomised, cross-over study in healthy adults, Br. J. Nutr., 2016, 115, 1748–1759 CrossRef CAS PubMed.
- C. Gómez de Oña, E. Martínez-Morillo, E. Gago González, P. Vidau Argüelles, C. Fernández Merayo and F. V. Álvarez Menéndez, Variation of trace element concentrations in patients undergoing hemodialysis in the north of Spain, Scand. J. Clin. Lab. Invest., 2016, 76, 492–499 CrossRef PubMed.
- M. Jankowska, B. Rutkowski and A. Dębska-Ślizień, Vitamins and Microelement Bioavailability in Different Stages of Chronic Kidney Disease, Nutrients, 2017, 9, 282 CrossRef PubMed.
- M. Arifuzzaman, T. H. Won, T. T. Li, H. Yano, S. Digumarthi, A. F. Heras, W. Zhang, C. N. Parkhurst, S. Kashyap, W. B. Jin, G. G. Putzel, A. M. Tsou, C. Chu, Q. Wei, A. Grier, S. Worgall, C. J. Guo, F. C. Schroeder and D. Artis, Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation, Nature, 2022, 611, 578–584 CrossRef CAS PubMed.
- E. Birkeland, S. Gharagozlian, K. I. Birkeland, J. Valeur, I. Måge, I. Rud and A. M. Aas, Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial, Eur. J. Nutr., 2020, 59, 3325–3338 CrossRef CAS PubMed.
- L. B. Olvera-Rosales, A. E. Cruz-Guerrero, E. Ramírez-Moreno, A. Quintero-Lira, E. Contreras-López, J. Jaimez-Ordaz, A. Castañeda-Ovando, J. Añorve-Morga, Z. G. Calderón-Ramos, J. Arias-Rico and L. G. González-Olivares, Impact of the Gut Microbiota Balance on the Health-Disease Relationship: The Importance of Consuming Probiotics and Prebiotics, Foods, 2021, 10, 1261 CrossRef CAS PubMed.
- A. Nath, M. A. Molnár, A. Csighy, K. Kőszegi, I. Galambos, K. P. Huszár, A. Koris and G. Vatai, Biological Activities of Lactose-Based Prebiotics and Symbiosis with Probiotics on Controlling Osteoporosis, Blood-Lipid and Glucose Levels, Medicina, 2018, 54, 98 CrossRef PubMed.
- R. L. Hughes, D. A. Alvarado, K. S. Swanson and H. D. Holscher, The Prebiotic Potential of Inulin-type Fructans: A Systematic Review, Adv. Nutr., 2021, 13, 492–529 CrossRef PubMed.
- R. Aquilani, P. Bolasco, S. Murtas, R. Maestri, P. Iadarola, C. Testa, M. L. Deiana, M. P. Esposito, R. Contu, M. Cadeddu, R. Secci and F. Boschi, Effects of a Metabolic Mixture on Gut Inflammation and Permeability in Elderly Patients with Chronic Kidney Disease: A Proof-of-Concept Study, Metabolites, 2022, 12, 987 CrossRef CAS PubMed.
- S. Tungsanga, W. Panpetch, T. Bhunyakarnjanarat, K. Udompornpitak, P. Katavetin, W. Chancharoenthana, P. Chatthanathon, N. Somboonna, K. Tungsanga, S. Tumwasorn and A. Leelahavanichkul, Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1→3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34, Int. J. Mol. Sci., 2022, 23, 2511 CrossRef CAS PubMed.
- S. Kaul, K. Kaur, N. Mehta, S. S. Dhaliwal and J. F. Kennedy, Characterization and optimization of spray dried iron and zinc nanoencapsules based on potato starch and maltodextrin, Carbohydr. Polym., 2022, 282, 119107 CrossRef CAS PubMed.
- D. Jafarpour, S. S. Shekarforoush, H. R. Ghaisari, S. Nazifi, J. Sajedianfard and M. H. Eskandari, Protective effects of synbiotic diets of Bacillus coagulans, Lactobacillus plantarum and inulin against acute cadmium toxicity in rats, BMC Complementary Altern. Med., 2017, 17, 291 CrossRef PubMed.
- Q. Zhai, J. Wang, S. Cen, J. Zhao, H. Zhang, F. Tian and W. Chen, Modulation of the gut microbiota by a galactooligosaccharide protects against heavy metal lead accumulation in mice, Food Funct., 2019, 10, 3768–3781 RSC.
- Q. Y. Chen and M. Costa, Arsenic: A Global Environmental Challenge, Annu. Rev. Pharmacol. Toxicol., 2021, 61, 47–63 CrossRef CAS PubMed.
- L. D. Garbinski, B. P. Rosen and J. Chen, Pathways of arsenic uptake and efflux, Environ. Int., 2019, 126, 585–597 CrossRef CAS PubMed.
- E. Kamal, L. A. Kaddam, A. Alagib and A. Saeed, Dietary Fibers (Gum Arabic) Supplementation Modulates Hepatic and Renal Profile Among Rheumatoid Arthritis Patients, Phase II Trial, Front. Nutr., 2021, 8, 552049 CrossRef PubMed.
- S. Bae, E. Kamynina, H. M. Guetterman, A. F. Farinola, M. A. Caudill, R. J. Berry, P. A. Cassano and P. J. Stover, Provision of folic acid for reducing arsenic toxicity in arsenic-exposed children and adults, Cochrane Database Syst. Rev., 2021, 10, Cd012649 Search PubMed.
- S. Tillmann, H. M. Awwad, A. R. Eskelund, G. Treccani, J. Geisel, G. Wegener and R. Obeid, Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression, Mol. Nutr. Food Res., 2018, 62, e1701070 CrossRef PubMed.
- G. Yan, X. Chen, S. Du, Z. Deng, L. Wang and S. Chen, Genetic mechanisms of arsenic detoxification and metabolism in bacteria, Curr. Genet., 2019, 65, 329–338 CrossRef CAS PubMed.
- E. C. G. de Matuoka, V. Monedero, M. Zúñiga, D. Vélez and V. Devesa, In Vitro Evaluation of the Protective Role of Lactobacillus StrainsAgainst Inorganic Arsenic Toxicity, Probiotics Antimicrob. Proteins, 2020, 12, 1484–1491 CrossRef PubMed.
- L. Chen, C. Li, X. Zhong, C. Lai, B. Zhang, Y. Luo, H. Guo, K. Liang, J. Fang, X. Zhu, J. Zhang and L. Guo, The gut microbiome promotes arsenic metabolism and alleviates the metabolic disorder for their mammal host under arsenic exposure, Environ. Int., 2023, 171, 107660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Table 1: Comparison of the basic characteristics and biochemistry parameters between patients excluded or included in the final analysis. Supplementary Table 2: Comparison of the baseline values of the plasma trace elements between the two intervention sequences and between prebiotics-pre and placebo-pre. Supplementary Fig. 1: Association of the arsenic plasma concentration with its daily clearance rate by urine, dialysate and feces. See DOI: https://doi.org/10.1039/d3fo01843a |
| This journal is © The Royal Society of Chemistry 2024 |